Analysis of hot trends in research on the association between vitamin D and cardiovascular disease
- Department of Pediatrics, The Second Xiangya Hospital, Central South University, Changsha, China
Objective: Vitamin D deficiency is the most common nutrient deficiency. Numerous studies suggest that vitamin D is an independent risk factor for cardiovascular disease. The objective is to visualize the research hotspots and evolution trends of the correlation between vitamin D and cardiovascular disease by using multivariate statistics and social network analysis techniques and to compare adult research with that of children in this field.
Methods: (Vitamin D [MeSH Major Topic]) AND (cardiovascular disease [MeSH Major Topic]) were retrieved from the PubMed database by time period. The bibliographic items co-occurrence matrix builder (BICOMB) was adopted to extract high-frequency subject terms and establish the core subject term co-occurrence matrix. With the Netdraw function of Ucinet 6.0 software, the social network of core subject terms was completed.
Results: Before 2010, there was a slow increase in the number of research papers covering all age groups in this field (157, 54, 84, and 211 papers were published in stages 1–4, respectively). From 2010 to 2020, there were 1,423 papers retrieved, showing a significantly increased research heat. The overall development trend of the research on the association between vitamin D and cardiovascular disease in children is similar to that in all age groups. From 2010 to 2020, 122 related papers were published (while before 2009, there were only 43 papers in all), presenting a good overall development trend. The social network analysis of core subject terms showed gradually increased correlations between research hotspots, from the early studies limited on the physiological function of vitamin D in cardiovascular diseases, to the role of vitamin D in the comorbidities of various cardiovascular diseases and its value as an intervention measure. Researches on the association between vitamin D and cardiovascular disease has a good overall development trend. Study of the mechanisms and the role of vitamin D in the common co-morbidities of cardiovascular disease and its therapeutic value will be the focus of future research.
1. Introduction
Vitamin D is a lipid steroid hormone with complex physiological functions. Of all nutritional deficiencies, vitamin D deficiency is the most common. Vitamin D deficiency has become one of the global public health problems. It is estimated that approximately 30% of children and 60% of adults worldwide suffer from vitamin D deficiency and insufficiency respectively (1). Vitamin D is considered an independent risk factor for cardiovascular disease (2–4). Vitamin D deficiency can lead to the occurrence of vascular calcification, calcium and phosphorus metabolism disorders (it may contribute to the progression of vascular calcification), impaired hemodynamic variables (further resulting in peripheral arterial diseases such as endothelial abnormalities, hypertension, ischemic cardiomyopathy, cardiomyopathy, myocardial hypertrophy, myocardial infarction, and atherosclerosis), insulin-resistant metabolic syndrome, and a further increase in the risk of hypertension and cardiovascular disease-related mortality (5). In addition to as a means of preventing and treating rickets, vitamin D has been tried in a variety of disease interventions, including cardiovascular disease (6). Researchers can read reviews and meta-literatures to understand the current state of research in this field, including the relevance of vitamin D to cardiovascular disease, mechanistic theories, and the predictive and interventional value of vitamin D in the onset and regression of cardiovascular disease (2, 5, 6). This paper attempts to provide a realistic, visual and longitudinal picture of the distribution and evolution of research hotspots in this field using multivariate statistics and social network analysis techniques (an important method in bibliometrics) to inform researchers in establishing research directions.
2. Materials and methods
2.1. Literature database search
All publications retrieved in the PubMed database using relevant subject terms were analyzed.
2.2. Principles for establishing core subject terms
The main subject terms with a word frequency greater than 0.5% were used as core subject terms for analysis.
2.3. Search strategy
2.3.1. Subject terms
(Vitamin D [MeSH Major Topic]) AND (cardiovascular disease [MeSH Major Topic])
2.3.2. Staged retrieval
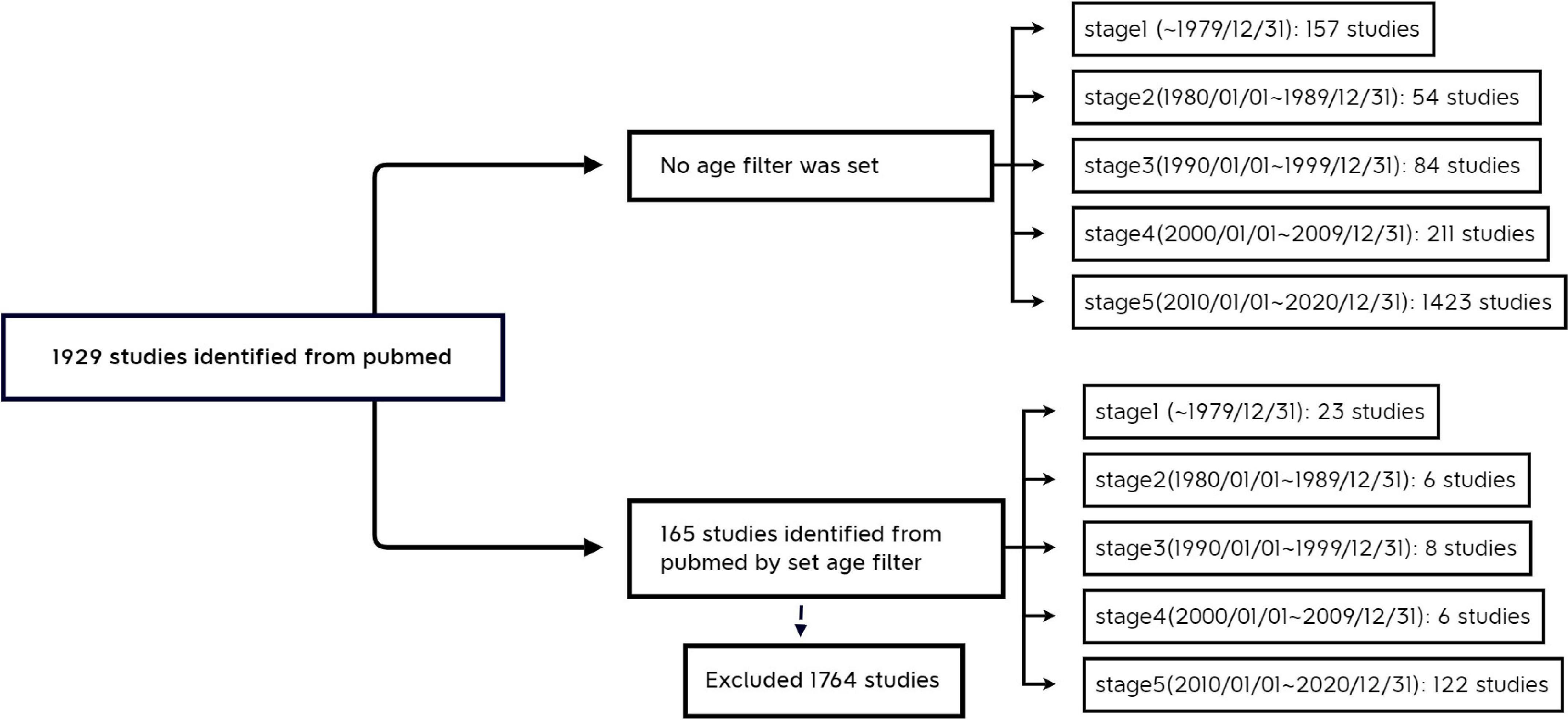
The retrieval periods were classified according to natural chronology. The first stage was before 31 December 1979 (given the limited number of documents included in the PubMed database in the early years, the period before 1979/12/31 is classified as the same stage); the retrieval period for the second stage was (“1980/01/01”[Date-Publication]]: “1989/12/31”[Date-Publication]); the retrieval period for the third stage was (“1990/01/01”[Date-Publication]: “1999/12/31”[Date-Publication]); the retrieval period for the fourth stage was (“2000/01/01”[Date-Publication]: “2009/12/31”[Date-Publication]); and the retrieval period for the fifth stage was (“2010/01/01”[Date-Publication]: “2020/12/31”[Date-Publication]).
2.3.3. Study objects
2.3.3.1. Association between vitamin D and cardiovascular disease
No age filter was set.
2.3.3.2. Study on the association between vitamin D and cardiovascular disease in children
“Child: birth–18 years” [MeSH Terms] OR “Newborn: birth–1 month” [MeSH Terms] OR “Infant: birth–23 months” [MeSH Terms] OR “Infant: 1–23 months” [MeSH Terms] OR “Preschool Child: 2–5 years” [MeSH Terms] OR “Child: 6–12 years” [MeSH Terms] OR “Adolescent: 13–18 years” [MeSH Terms] (Figure 1).
2.4. Multivariate statistical and social network analysis methods
The PubMed database search hits were imported into the Bibliographic Items Co-occurrence Matrix Builder (BICOMB) (7), and high-frequency subject terms were extracted from all subject terms at each stage of development and core subject terms were established to build a core subject term co-occurrence matrix. A social network of the co-occurrence matrix was drawn using the Netdraw function of Ucinet 6.0 software. In the social network diagram, the closer the nodes are to the center, the more central the term is in the whole social network; the larger the nodes are, the higher the frequency of the words; the thicker the line between the core words, the higher the degree of association between the two core words.
3. Results
3.1. General information of the retrieved literature
3.1.1. Vitamin D AND cardiovascular disease
We searched the PubMed database and selected subject terms with a word frequency greater than 0.5% as the core subject terms. For the time period before 1979, 157 publications were retrieved, with 336 main subject terms; the lowest core subject term frequency was 2, and 41 subject terms were analyzed. A total of 54 publications from 1980 to 1989 were retrieved, with 527 main subject terms; the lowest core subject term frequency was 2, and there were 25 subject terms included in the analysis. From 1990 to 1999, 84 publications were retrieved, with 343 main subject terms; the lowest core subject term frequency was 2, and 46 subject terms were included in the analysis. For the time period from 2000 to 2009, 211 publications were retrieved, with 879 main subject terms; the lowest core subject term frequency was 5, and 34 subject terms were included in the analysis. For the time period from 2010 to 2020, 1,423 publications were found, with 5,822 main subject terms; the lowest core subject term frequency was 30, and 27 subject terms were included in the analysis (Figure 2 and Table 1).
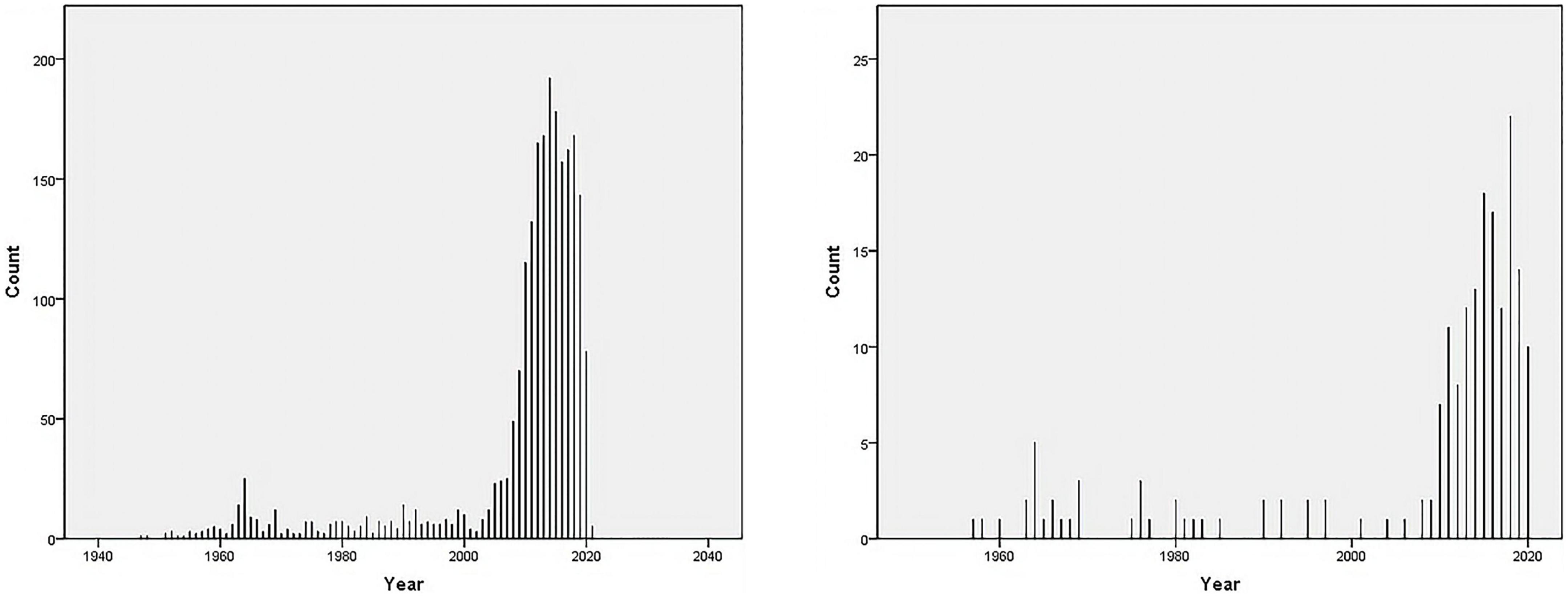
Figure 2. Number of publications on “vitamin D AND cardiovascular disease” in the PubMed database as of December 2020. (A) Vitamin D [MeSH Major Topic] AND cardiovascular disease [MeSH Major Topic]. (B) Vitamin D [MeSH Major Topic] AND cardiovascular disease [MeSH Major Topic], “Child: birth-18 years” [MeSH Terms] OR “Newborn: birth-1 month” [MeSH Terms] OR “Infant: birth-23 months” [MeSH Terms] OR “Infant: 1–23 months” [MeSH Terms] OR “Preschool Child: 2–5 years” [MeSH Terms] OR “Child: 6–12 years” [MeSH Terms] OR “Adolescent: 13–18 years” [MeSH Terms].
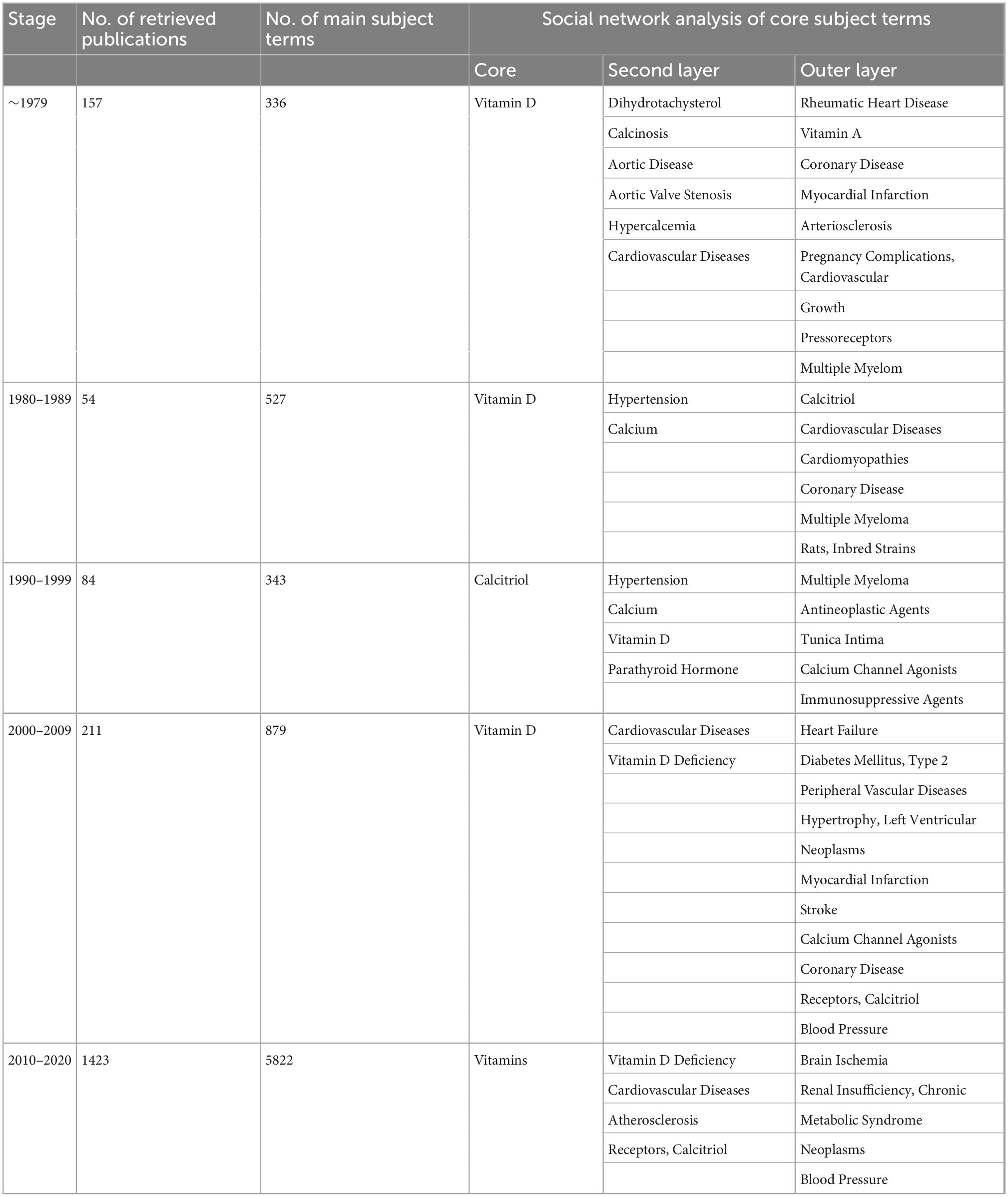
Table 1. Literature analysis of “vitamin D AND cardiovascular disease” in PubMed as of December 2020.
3.1.2. Vitamin D AND cardiovascular disease (0–18 years)
We searched the PubMed database and selected subject terms with a word frequency greater than 0.5% as the core subject terms. For the time period before 1979, 23 publications were retrieved, with 41 main subject terms; the lowest core subject term frequency was 1, and 25 subject terms were analyzed. A total of 6 publications from 1980 to 1989 were retrieved, with 16 main subject terms; the lowest core subject term frequency was 1, and there were 12 subject terms included in the analysis. From 1990 to 1999, 8 publications were retrieved, with 27 main subject terms; the lowest core subject term frequency was 1, and 17 subject terms were included in the analysis. For the time period from 2000 to 2009, 6 publications were retrieved, with 27 main subject terms; the lowest core subject term frequency was 1, and 19 subject terms were included in the analysis. For the time period from 2010 to 2020, 122 publications were retrieved, with 489 main subject terms; the lowest core subject term frequency was 3, and 23 subject terms were included in the analysis (Figure 2 and Table 2).
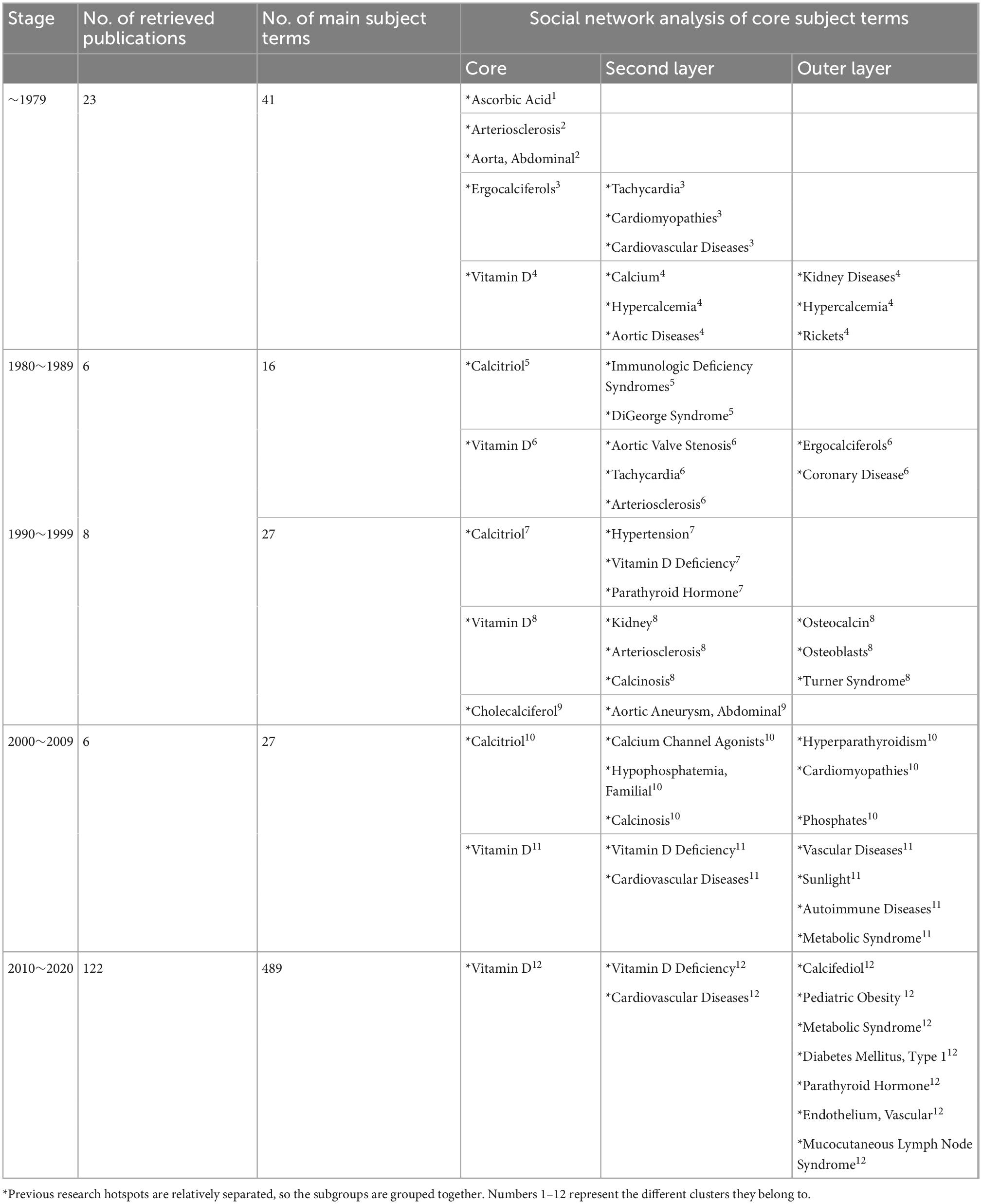
Table 2. Literature analysis of “vitamin D AND cardiovascular disease (0–18 years)” in PubMed as of December 2020.
3.2. Social network analysis of the core subject terms
3.2.1. Vitamin D AND cardiovascular disease
In the first stage (∼1979), “vitamin D” is at the core of the network, with a node area much larger than the other core subject nodes and closely linked to the other subject terms. The terms “dihydrotachysterol,” “calcinosis,” and “aortic diseases” have larger node areas in the second tier. There are relatively thicker lines between “calcinosis,” “aortic diseases,” and “vitamin D.” “Cardiovascular diseases,” “aortic valve stenosis,” and “hypercalcemia” are also in the second tier but have smaller node areas. In the outer layer, the terms “rheumatic heart disease,” “vitamin A,” “coronary disease,” “myocardial infarction,” and “arteriosclerosis” are closely linked to the core term “vitamin D,” while “pregnancy complications, cardiovascular,” “growth,” “pressoreceptors,” and “multiple myeloma” are weakly associated with “vitamin D” (Figure 3A and Table 1).
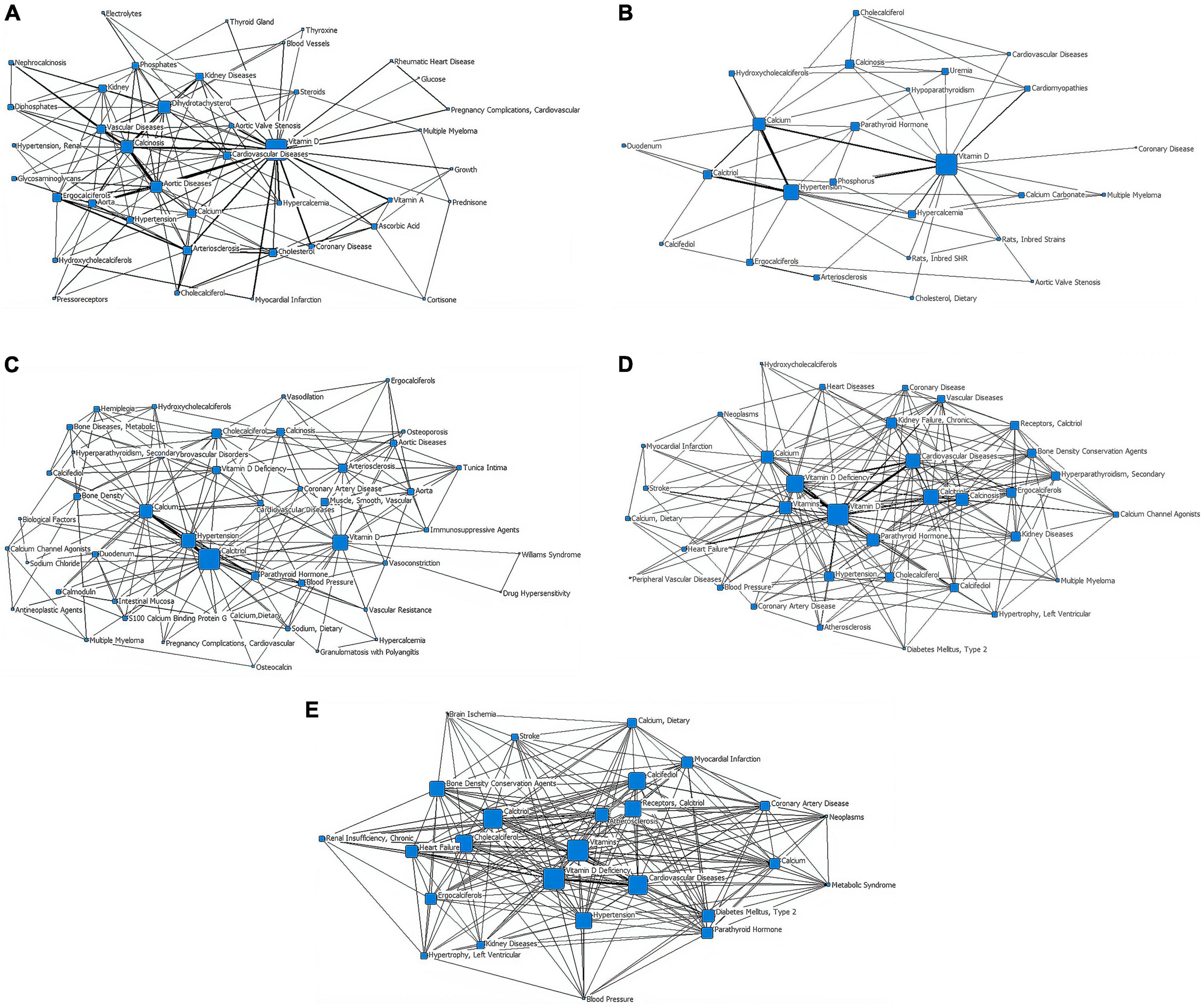
Figure 3. Social network of core subject terms of “vitamin D AND cardiovascular disease” in the PubMed database before December 2020. (A) 1979. (B) 1980-1989. (C) 1990-1999. (D) 2000-2009. (E) 2010-2020.
In the second stage (1980–1989), “vitamin D” remains at the heart of the network, with the largest node area and is strongly connected to other nodes around it. The core subject terms of the second tier are “hypertension” and “calcium,” both of which are closely related to “vitamin D.” The lines between “calcium,” “calcitriol,” and “hypertension” are all thick, forming sub-centers of the study. “Cardiomyopathies” and “cardiovascular diseases” are in the outer layer of the network, with a thicker line between “cardiomyopathies” and “vitamin D.” “Rats, Inbred Strains” appears in the outer network where “multiple myeloma” still exists (Figure 3B and Table 1).
In the third stage (1990–1999), “calcitriol” is the most central term with the largest node area, closely related to the secondary core subject terms of “calcium,” “hypertension,” and “parathyroid hormone.” The “vitamin D,” “hypertension,” and “calcium” nodes are large and closely related to the periphery and are presented as three sub-study centers. “Multiple myeloma” remains in the outer layer, but its link with “antineoplastic agents” appears. “Tunica intima,” “calcium channel agonists,” “immunosuppressive agents” exist in the outer layer of the network (Figure 3C and Table 1).
In the fourth stage (2000–2009), “vitamin D” is located at the very core of the network, with the largest node area and closer links to the surrounding nodes. “Cardiovascular diseases” and “vitamin d deficiency” are located in the second layer of the network diagram. “Vitamin D,” “vitamin D deficiency,” and “cardiovascular diseases” are linked by thicker lines. “Heart failure” is located further out in the network, but has a thicker line and closer relationship with “vitamin D.” “Diabetes mellitus, type 2,” “peripheral vascular diseases,” “hypertrophy, left ventricular,” “neoplasms,” “stroke,” “receptors, calcitriol” start to enter the outermost layer of the network. “Myocardial infarction,” which disappeared from the network in stages 2 and 3, reappears in this layer (Figure 3D and Table 1).
Stage 5 (2010–2020), in which the “vitamins” are located at the very heart of the network and have the largest node area, “Receptors, calcitriol” moves from the previous outer layer into the second layer and became one of the core subject terms here, with a larger node area. Core subject terms in this layer also include “cardiovascular diseases,” “vitamin D deficiency,” and “atherosclerosis.” The line between “cardiovascular diseases” and “vitamin D deficiency” is thicker. “Brain ischemia,” “renal insufficiency (chronic),” “metabolic syndrome” appear in the outer layer of the network (Figure 3E and Table 1).
3.2.2. Vitamin D AND cardiovascular disease (0–18 years)
In the first stage (∼1979), the distribution of subject terms is more dispersed, converging into three categories and one isolated point. “Ascorbic acid” is the isolated point. “Arteriosclerosis” and “aorta, abdominal” are one category. “Ergocalciferols,” “cardiomyopathies,” “cardiovascular diseases,” “tachycardia” are as a group. The other subject headings are centered on the term “vitamin D.” The lines between “vitamin D” and “calcium” and “hypercalcemia” are the thickest (Figure 4A and Table 2).
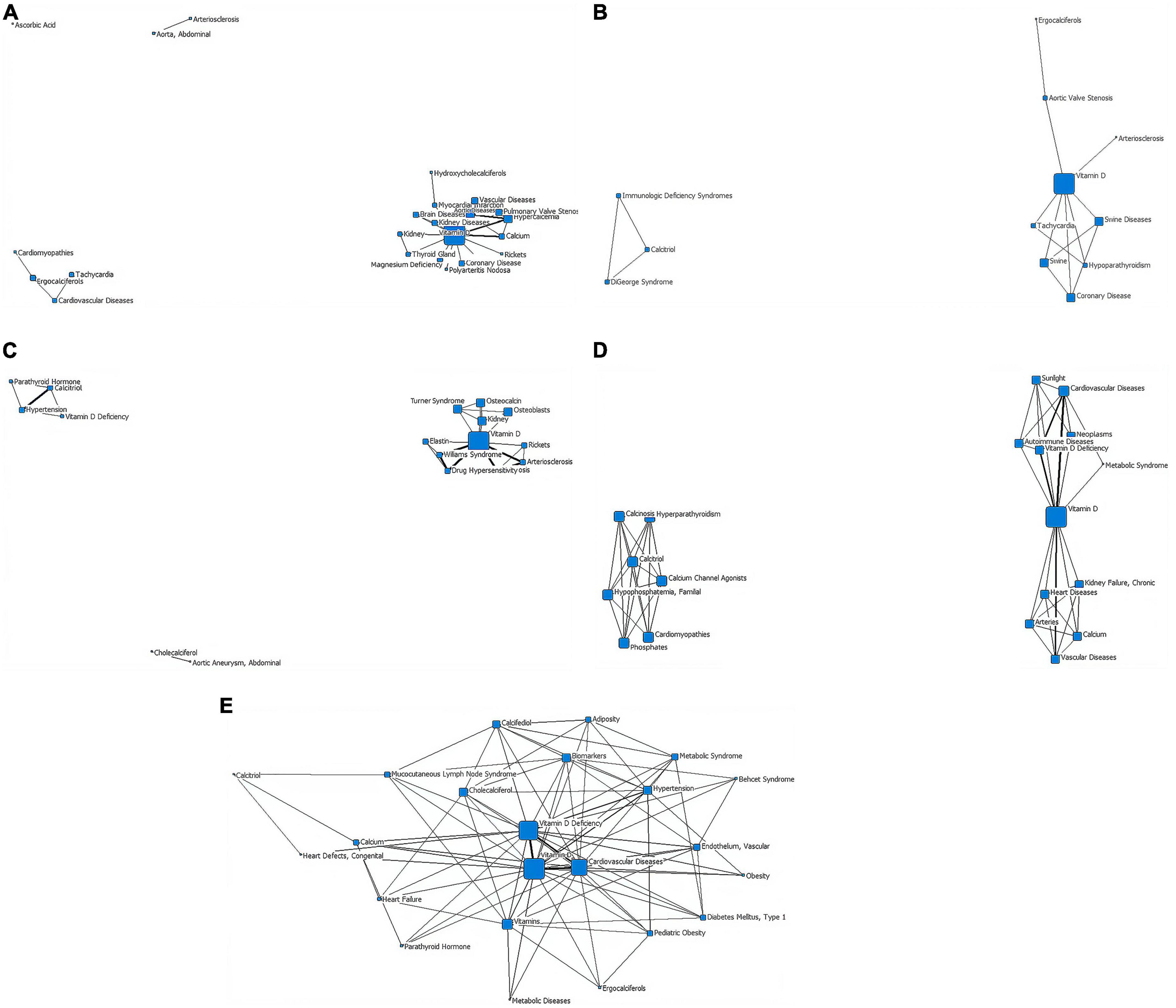
Figure 4. Social network of core subject terms of “vitamin D AND cardiovascular disease (0-18y)” in the PubMed database before December 2020. (A) 1979. (B) 1980-1989. (C) 1990-1999. (D) 2000-2009. (E) 2010-2020.
In the second stage (1980–1989), research in this period converges into two categories. The first category is composed of the terms “calcitriol,” “immunologic deficiency syndromes,” and “DiGeorge syndrome,” with a triangular distribution in the network diagram. The second group, centered on “vitamin D,” has the largest nodal area and is associated upwards with “aortic valve Stenosis” and “arteriosclerosis” and downwards with “tachycardia” and “hypoparathyroidism”. The node area for “coronary disease” in this network is also relatively large. No cross-linking between the upper and lower branches of the second layer of the network (Figure 4B and Table 2).
In the third stage (1990–1999), research in this period converges into three main categories. The first group consists of “cholecalciferol” and “aortic aneurysm, abdominal”. The second group consists of “calcitriol,” “hypertension,” “vitamin D deficiency,” and “parathyroid hormone.” In the third category, “vitamin D” is the core term, the largest node area and the hub of the other terms, with a thicker line between “calcinosis” and “arteriosclerosis”. The nodes for “kidney,” “osteoblasts,” “osteocalcin,” “Turner syndrome,” and “Williams syndrome” (a developmental anomaly caused by the deletion of the proximal end of the long arm (7q11.23) of chromosome 7, which is mainly manifested by cardiovascular abnormalities, growth retardation, behavioral and psychological abnormalities, endocrine abnormalities, etc.) are also relatively large in the third category of the network (Figure 4C and Table 2).
In the fourth stage (2000–2009), research converges into two categories. The first group is centered on “calcitriol” and is associated with “calcium channel agonists,” “hypophosphatemia, familial,” and “calcinosis,” each with a similar node size. The second category is centered on “vitamin D” (it has a node area significantly larger than the other nodes) and with the thickest lines between “vitamin D deficiency,” “cardiovascular diseases,” and “vascular diseases.” The second type of network has no direct connection between the upper and lower parts of the subject terms. “Autoimmune diseases,” “sunlight,” “metabolic syndrome” are in the outer layer of the web (Figure 4D and Table 2).
In the fifth stage (2010–2020), the volume of literature increases and is clustered into a type of network diagram, centered on “vitamin D,” which has the largest node area and a triangular association with the second tier of subject terms “cardiovascular diseases” and “vitamin D deficiency.” “Pediatric obesity,” “diabetes mellitus (Type 1),” “endothelium, Vascular,” and “mucocutaneous lymph node syndrome” start to appear in the outer layer (Figure 4E and Table 2).
4. Discussion
With the help of bibliometric methods, we analyzed the evolving trends in the research on the association between vitamin D and cardiovascular disease and summarized the studies for the whole age group and for childhood respectively.
In terms of the growth of the literature, the results of the study with an all-age population showed that the literature in this field grew slowly before 2010 (157, 54, 84, and 211 publications that met the screening criteria with 336, 527, 343, and 879 main subject terms in stages 1–4, respectively), but after 2010 the research activity increased significantly, with 1,423 publications that met the screening criteria and 5,822 main subject terms from 2010 to 2020. The number of publications that met the screening criteria from 2010 to 2020 was 1,423, with 5,822 main subject terms, which was a good overall development. The results of the bibliometric analysis on children showed that the overall trend of research on the association between vitamin D and cardiovascular disease in children was similar to that of all-age studies, i.e., it entered an accelerated phase after 2010 (122 papers from 2010 to 2020, compared with 43 papers before 2009), but there were far fewer studies in children than in the all-age population over the same period (122/1423 in the last decade), and the acceleration time lagged behind that of all-age studies (Figure 1), suggesting that adult studies in this area are more mature and the results can be used as a reference for children’s studies.
Social network analysis of core subject terms showed that early all-age studies (stages 1–3) focused on the mechanisms of classical calcium and phosphorus metabolism regulated by vitamin D and cardiovascular diseases associated with these mechanisms, such as atherosclerosis and hypertension (although cardiovascular complications of pregnancy, growth problems in children and multiple myeloma have also been addressed). In stages 4–5, the research focus gradually extended to the exploration of the mechanisms of vitamin D in co-morbidities of cardiovascular disease (e.g., tumors, stroke, cerebral ischemia, and diabetes). The literature on the association between vitamin D and cardiovascular disease in children at stages 1–4 was few in number, with a lack of linkage between research hotspots and a multipolar distribution. In the last decade, there were a marked increase in the number of studies in this field and a strengthening of the links between research hotspots, with a growing interest in cardiovascular co-morbidities and a broadening of the scope, such as rickets, kidney disease, childhood obesity, diabetes and Kawasaki disease (also known as Mucocutaneous lymph node syndrome, is a childhood disease with systemic vasculitis as the main lesion, may lead to coronary artery dilation and coronary aneurysms). The overall trend was positive.
Vitamin D has been shown to be involved in blood pressure regulation, smooth muscle cell and endothelial cell physiological processes (8–11). Both the serum 25-(OH)D concentration and the incidence of cardiovascular disease exhibit geographic and seasonal fluctuations, suggesting a possible association between vitamin D and cardiovascular disease (12–16). Coronary artery disease (CAD), heart failure (HF), and atrial fibrillation (AF) are the three most common cardiovascular diseases and studies have confirmed that vitamin D deficiency is a very common co-morbidity of these three diseases (17, 18). Numerous studies have shown that vitamin D is associated with cardiovascular disease and its common co-morbidities such as nephropathy and metabolic syndrome (2–4, 12, 19–33). Vitamin D supplementation is no longer limited to the prevention and treatment of rickets, but has also been tried for the treatment of such diseases. Effective UV exposure, vitamin D fortification and food fortification are important means of preventing vitamin D deficiency (34–42). However, findings on the role of vitamin D in the treatment of cardiovascular disease and its co-morbidities appear to be inconsistent. Several teams have reported an inverse J or U curve effect between serum 25-(OH)D levels and cardiovascular disease risk and mortality, and have hypothesized that the adverse effect is associated with hypercalcemia (43–57). Some studies have also found no significant association between vitamin D concentrations and markers of subclinical atherosclerosis (48). The threshold at which serum 25-(OH)D increases cardiovascular mortality varies widely across studies (12.5–100 nmol/L) (49, 57). It is worth noting that in many previous study designs, limited by sample size and observation time, it was generally considered that dose-response analyses for vitamins were graded into 3–5 levels and then graded for disease association assessment, with cases of too low or too high vitamin D usually being considered extreme values (not included in the analysis). However, since changes in disease risk or treatment response are continuous rather than jumping, this design may lead to incomplete analytical conclusions. This may partly explain the conflicting results of previous studies on vitamin D and disease. Serum 25-(OH)D concentrations and blood pressure measurements were performed in 1074 Peruvian adolescents aged 13–15 years and found an association between uprightness intolerance and vitamin D deficiency in children (58). Children with vitamin D deficiency had elevated diastolic blood pressure and the degree of deficiency was positively correlated with blood pressure; Serum 25-(OH)D concentration showed a U-shaped relationship with systolic blood pressure, and an inverse J-shaped relationship with diastolic blood pressure and mean arterial pressure (58). It is speculated that vitamin D deficiency in childhood is associated with hypertension in adulthood. Conclusions for a U-shaped or inverse J-shaped effect of vitamin D have also been found in other studies of extraskeletal effects of vitamin D (59). A clear definition of nutritional criteria for vitamin D is the cornerstone of clinical intervention and scientific research. Research evidence suggests that for optimal skeletal effects of vitamin D, adult serum 25-(OH)D concentrations should be >50 nmol/L. From an overall health perspective, 25-(OH)D levels ≥75 nmol/L are considered ideal by the American Geriatrics Society, the Endocrine Society, the National Osteoporosis Foundation and the International Osteoporosis Foundation (60). Most national, regional and international scientific societies have published standards for vitamin D nutritional status and guidelines for prevention and treatment, but diagnostic and treatment recommendations for adolescents are often formulated with reference to data from adults or infants (61). In recent years, researchers have also begun to pay attention to and explore the optimal vitamin D threshold for achieving different extraskeletal effects (62–66). Studies have demonstrated that vitamin D supplementation in well-nourished individuals does not provide significant cardiovascular benefits (6). Therefore, the use of vitamin D in the management of cardiovascular disease requires a ‘cautious’ approach and individualization of doses. The design of studies relating vitamin D to the development and regression of cardiovascular disease should follow the principle of non-linear curves of biological responses to nutrients, i.e., as intake increases to what is generally considered to be the appropriate range, the risk decreases; beyond this range, pharmacological or toxic effects begin to emerge and the risk rises again, showing a U- or J-shaped dose-risk relationship. the left half of the U-shaped curve provides a clearer picture of the nutrient’s The left half of the U-curve gives a clearer picture of the health benefits of nutrients, with the benefits rising in an s-shaped curve as intake increases, but the dose-effect curve flattens out when intake is too small or too large. Nutrient studies often assume that a change in nutrient status will lead to a certain outcome, and as vitamin D absorption, conversion and biological effects vary between individuals studied, it is important to focus on the changes in nutritional status experienced by the study participants to reduce bias in the results of the study (51). We did not discuss the relationship between vitamin D level and arterial stiffness, which is associated with cardiovascular risk, morbidity, and mortality, this is the limitation of the study (67).
Based on the results of this study, research into the association between vitamin D and cardiovascular disease, particularly the mechanisms of association, and the identification of the role of vitamin D in common co-morbidities of cardiovascular disease and its value as a therapeutic tool, will be a focus of future research. Standardized vitamin D testing methods, long-term follow-up of large samples, and intervention strategies will provide a more reliable evidence-based basis for the prevention and treatment of vitamin D deficiency and related diseases.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XL: preparing the tables and figures and drafting the manuscript. CWe: drafting the manuscript, reviewing and editing, conceptualization, and revising. FW and CWa: reviewing and editing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Peroni D, Trambusti I, Di Cicco M, Nuzzi G. Vitamin D in pediatric health and disease. Pediatr Allergy Immunol. (2020) 31(Suppl. 24):54–7. doi: 10.1111/pai.13154
2. Chang S, Lee H. Vitamin D and health - the missing vitamin in humans. Pediatr Neonatol. (2019) 60:237–44. doi: 10.1016/j.pedneo.2019.04.007
3. Zendehdel A, Arefi M. Molecular evidence of role of vitamin D deficiency in various extraskeletal diseases. J Cell Biochem. (2019) 120:8829–40. doi: 10.1002/jcb.28185
4. Maggio M, De Vita F, Lauretani F, Ceda G, Volpi E, Giallauria F. Vitamin D and endothelial vasodilation in older individuals: data from the PIVUS study. J Clin Endocrinol Metab. (2014) 99:3382–9. doi: 10.1210/jc.2014-1536
5. Wang J, Zhou J, Robertson G, Lee V. Vitamin D in vascular calcification: a double-edged sword. Nutrients. (2018) 10:652. doi: 10.3390/nu10050652
6. Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards J. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol. (2022) 18:96–110. doi: 10.1038/s41574-021-00593-z
7. Lei C, Wei L, Lei Y, Han Z, Yuefang H, Yingna H, et al. Development of a text mining system based on the co-occurrence of bibliographic items in literature databases. Data Anal Knowl Discov. (2008) 24:70–5.
8. Kelishadi R, Ardalan G, Motlagh M, Shariatinejad K, Heshmat R, Poursafa P, et al. National report on the association of serum vitamin D with cardiometabolic risk factors in the pediatric population of the Middle East and North Africa (MENA): the CASPIAN-III Study. Nutrition. (2014) 30:33–8. doi: 10.1016/j.nut.2013.05.018
9. Caraba A, Crişan V, Romoşan I, Mozoş I, Murariu M. Vitamin D status, disease activity, and endothelial dysfunction in early rheumatoid arthritis patients. Dis Markers. (2017) 2017:5241012. doi: 10.1155/2017/5241012
10. Hammer Y, Soudry A, Levi A, Talmor-Barkan Y, Leshem-Lev D, Singer J, et al. Effect of vitamin D on endothelial progenitor cells function. PLoS One. (2017) 12:e0178057. doi: 10.1371/journal.pone.0178057
11. Latic N, Erben R. Vitamin D and cardiovascular disease, with emphasis on hypertension, atherosclerosis, and heart failure. Int J Mol Sci. (2020) 21:6483. doi: 10.3390/ijms21186483
12. Savastio S, Pozzi E, Tagliaferri F, Degrandi R, Cinquatti R, Rabbone I, et al. Vitamin D and cardiovascular risk: which implications in children. Int J Mol Sci. (2020) 21:3536. doi: 10.3390/ijms21103536
13. Hauger H, Laursen R, Ritz C, Mølgaard C, Lind M, Damsgaard C. Effects of vitamin D supplementation on cardiometabolic outcomes in children and adolescents: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. (2020) 59:873–84. doi: 10.1007/s00394-019-02150-x
14. Mirhosseini N, Vatanparast H, Kimball S. The association between serum 25(OH) D status and blood pressure in participants of a community-based program taking vitamin D supplements. Nutrients. (2017) 9:1244. doi: 10.3390/nu9111244
15. Rosecrans R, Dohnal J. Seasonal vitamin D changes and the impact on health risk assessment. Clin Biochem. (2014) 47:670–2. doi: 10.1016/j.clinbiochem.2014.02.004
16. Kroll M, Bi C, Garber C, Kaufman H, Liu D, Caston-Balderrama A, et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS One. (2015) 10:e0118108. doi: 10.1371/journal.pone.0118108
17. Cosentino N, Campodonico J, Milazzo V, De Metrio M, Brambilla M, Camera M, et al. Vitamin D and cardiovascular disease: current evidence and future perspectives. Nutrients. (2021) 13:3603. doi: 10.3390/nu13103603
18. Acharya P, Dalia T, Ranka S, Sethi P, Oni O, Safarova M, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. (2021) 5:bvab124. doi: 10.1210/jendso/bvab124
19. Al Khalifah R, Alsheikh R, Alnasser Y, Alsheikh R, Alhelali N, Naji A, et al. The impact of vitamin D food fortification and health outcomes in children: a systematic review and meta-regression. Syst Rev. (2020) 9:144. doi: 10.1186/s13643-020-01360-3
20. Gil Á, Plaza-Diaz J, Mesa M. Vitamin D: classic and novel actions. Ann Nutr Metab. (2018) 72:87–95. doi: 10.1159/000486536
21. Zmijewski M. Vitamin D and human health. Int J Mol Sci. (2019) 20:145. doi: 10.3390/ijms20010145
22. Bouillon R, Marcocci C, Carmeliet G, Bikle D, White J, Dawson-Hughes B, et al. Skeletal and extraskeletal actions of Vitamin D: current evidence and outstanding questions. Endocr Rev. (2019) 40:1109–51. doi: 10.1210/er.2018-00126
23. Wimalawansa S. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol. (2018) 175:60–81. doi: 10.1016/j.jsbmb.2016.09.016
24. Uwaezuoke S. Vitamin D deficiency and anemia risk in children: a review of emerging evidence. Pediatric Health Med Ther. (2017) 8:47–55. doi: 10.2147/PHMT.S129362
25. Rosas-Peralta M, Holick M, Borrayo-Sánchez G, Madrid-Miller A, Ramírez-Árias E, Arizmendi-Uribe E. Dysfunctional immunometabolic effects of vitamin D deficiency, increased cardiometabolic risk, potential epidemiological alert in America. Endocrinol Diabetes Nutr. (2017) 64:162–73. doi: 10.1016/j.endien.2017.04.006
26. Caprio M, Infante M, Calanchini M, Mammi C, Fabbri A. Vitamin D: not just the bone, evidence for beneficial pleiotropic extraskeletal effects. Eat Weight Disord. (2017) 22:27–41. doi: 10.1007/s40519-016-0312-6
27. Kaul A, Gläser S, Hannemann A, Schäper C, Nauck M, Felix S, et al. Vitamin D is associated with cardiopulmonary exercise capacity: results of two independent cohorts of healthy adults. Br J Nutr. (2016) 115:500–8. doi: 10.1017/S000711451500464X
28. Sezer S, Tutal E, Bal Z, Uyar M, Bal U, Cakir U, et al. Differential influence of vitamin D analogs on left ventricular mass index in maintenance hemodialysis patients. Int J Artif Organs. (2014) 37:118–25. doi: 10.5301/ijao.5000289
29. Kim S, Choi H, Lee J, Kim D, Oh Y, Kim Y, et al. Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease. J Ren Nutr. (2014) 24:20–5. doi: 10.1053/j.jrn.2013.07.003
30. Van Ballegooijen A, Visser M, Cotch M, Arai A, Garcia M, Harris T, et al. Serum vitamin D and parathyroid hormone in relation to cardiac structure and function: the ICELAND-MI substudy of AGES-Reykjavik. J Clin Endocrinol Metab. (2013) 98:2544–52. doi: 10.1210/jc.2012-4252
31. McNally J, Doherty D, Lawson M, Al-Dirbashi O, Chakraborty P, Ramsay T, et al. The relationship between vitamin D status and adrenal insufficiency in critically ill children. J Clin Endocrinol Metab. (2013) 98:e877–81. doi: 10.1210/jc.2013-1126
32. Kaur G, Singh J, Kumar J. Vitamin D and cardiovascular disease in chronic kidney disease. Pediatr Nephrol. (2019) 34:2509–22. doi: 10.1007/s00467-018-4088-y
33. Mozos I, Marginean O. Links between vitamin D deficiency and cardiovascular diseases. Biomed Res Int. (2015) 2015:109275. doi: 10.1155/2015/109275
34. Pilz S, März W, Cashman K, Kiely M, Whiting S, Holick M, et al. Rationale and plan for vitamin D food fortification: a review and guidance paper. Front Endocrinol. (2018) 9:373. doi: 10.3389/fendo.2018.00373
35. Patseadou M, Haller D. Vitamin D in adolescents: a systematic review and narrative synthesis of available recommendations. J Adolesc Health. (2020) 66:388–407. doi: 10.1016/j.jadohealth.2019.08.025
36. Weaver C, Gordon C, Janz K, Kalkwarf H, Lappe J, Lewis R, et al. The national osteoporosis foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. (2016) 27:1281–386. doi: 10.1007/s00198-015-3440-3
37. Tonnesen R, Schwarz P, Hovind P, Jensen L. Physical exercise associated with improved BMD independently of sex and vitamin D levels in young adults. Eur J Appl Physiol. (2016) 116:1297–304. doi: 10.1007/s00421-016-3383-1
38. Saggese G, Vierucci F, Prodam F, Cardinale F, Cetin I, Chiappini E, et al. Vitamin D in pediatric age: consensus of the Italian pediatric society and the Italian society of preventive and social pediatrics, jointly with the italian federation of pediatricians. Ital J Pediatr. (2018) 44:51. doi: 10.1186/s13052-018-0488-7
39. Brandão-Lima P, Santos B, Aguilera C, Freire A, Martins-Filho P, Pires L. Vitamin D food fortification and nutritional status in children: a systematic review of randomized controlled trials. Nutrients. (2019) 11:2766. doi: 10.3390/nu11112766
40. Wilson L, Tripkovic L, Hart K, Lanham-New S. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. Proc Nutr Soc. (2017) 76:392–9. doi: 10.1017/S0029665117000349
41. Laing E, Lewis R. New concepts in vitamin D requirements for children and adolescents: a controversy revisited. Front Horm Res. (2018) 50:42–65. doi: 10.1159/000486065
42. Marwaha R, Dabas A. Interventions for prevention and control of epidemic of vitamin D deficiency. Indian J Pediatr. (2019) 86:532–7. doi: 10.1007/s12098-019-02857-z
43. Norman P, Powell J. Vitamin D and cardiovascular disease. Circ Res. (2014) 114:379–93. doi: 10.1161/CIRCRESAHA.113.301241
44. Holick M. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18:153–65. doi: 10.1007/s11154-017-9424-1
45. Pilz S, Verheyen N, Grübler M, Tomaschitz A, März W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. (2016) 13:404–17. doi: 10.1038/nrcardio.2016.73
46. Pérez-Hernández N, Aptilon-Duque G, Nostroza- Hernández M, Vargas-Alarcón G, Rodríguez-Pérez J, Blachman-Braun R. Vitamin D and its effects on cardiovascular diseases: a comprehensive review. Korean J Intern Med. (2016) 31:1018–29. doi: 10.3904/kjim.2015.224
47. Zittermann A. The biphasic effect of vitamin D on the musculoskeletal and cardiovascular system. Int J Endocrinol. (2017) 2017:3206240. doi: 10.1155/2017/3206240
48. Mozos I, Jianu D, Gug C, Stoian D. Links between high-sensitivity C-reactive protein and pulse wave analysis in middle-aged patients with hypertension and high normal blood pressure. Dis Markers. (2019) 2019:2568069. doi: 10.1155/2019/2568069
49. Zittermann A. Vitamin D status, supplementation and cardiovascular disease. Anticancer Res. (2018) 38:1179–86. doi: 10.21873/anticanres.12338
50. Grübler M, März W, Pilz S, Grammer T, Trummer C, Müllner C, et al. Vitamin-D concentrations, cardiovascular risk and events - a review of epidemiological evidence. Rev Endocr Metab Disord. (2017) 18:259–72. doi: 10.1007/s11154-017-9417-0
51. Heaney R. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev. (2014) 72:48–54. doi: 10.1111/nure.12090
52. Grant W, Boucher B, Al Anouti F, Pilz S. Comparing the evidence from observational studies and randomized controlled trials for nonskeletal health effects of vitamin D. Nutrients. (2022) 14:3811. doi: 10.3390/nu14183811
53. Zhou A, Selvanayagam J, Hyppönen E. Non-linear mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur Heart J. (2022) 43:1731–9. doi: 10.1093/eurheartj/ehab809
54. Emerging Risk Factors Collaboration/Epic-Cvd/Vitamin D Studies Collaboration. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: observational and mendelian randomisation analyses. Lancet Diabetes Endocrinol. (2021) 9:837–46.
55. Jani R, Mhaskar K, Tsiampalis T, Kassaw N, González M, Panagiotakos D. Circulating 25-hydroxy-vitamin D and the risk of cardiovascular diseases. Systematic review and meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. (2021) 31:3282–304. doi: 10.1016/j.numecd.2021.09.003
56. Judd S, Morgan C, Panwar B, Howard V, Wadley V, Jenny N, et al. Vitamin D deficiency and incident stroke risk in community-living black and white adults. Int J Stroke. (2016) 11:93–102. doi: 10.1177/1747493015607515
57. Tomson J, Emberson J, Hill M, Gordon A, Armitage J, Shipley M, et al. Vitamin D and risk of death from vascular and non-vascular causes in the Whitehall study and meta-analyses of 12,000 deaths. Eur Heart J. (2013) 34:1365–74. doi: 10.1093/eurheartj/ehs426
58. Tomaino K, Romero K, Robinson C, Baumann L, Hansel N, Pollard S, et al. Association between serum 25-hydroxy vitamin D levels and blood pressure among adolescents in two resource-limited settings in Peru. Am J Hypertens. (2015) 28:1017–23. doi: 10.1093/ajh/hpu264
59. Maddock J, Zhou A, Cavadino A, Kuźma E, Bao Y, Smart M, et al. Vitamin D and cognitive function: a mendelian randomisation study. Sci Rep. (2017) 7:13230.
60. Cashman K, Ritz C, Kiely M. Odin collaborators. improved dietary guidelines for vitamin D: application of individual participant data (IPD)-level meta-regression analyses. Nutrients. (2017) 9:469. doi: 10.3390/nu9050469
61. Alonso M, Mantecón L, Santos F. Vitamin D deficiency in children: a challenging diagnosis. Pediatr Res. (2019) 85:596–601. doi: 10.1038/s41390-019-0289-8
62. Raj S, Coffin S. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis. (2013) 55:425–33. doi: 10.1016/j.pcad.2012.11.004
63. Lips P, Cashman K, Lamberg-Allardt C, Bischoff-Ferrari H, Obermayer-Pietsch B, Bianchi M, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European calcified tissue society. Eur J Endocrinol. (2019) 180:23–54. doi: 10.1530/EJE-18-0736
64. Roth D, Abrams S, Aloia J, Bergeron G, Bourassa M, Brown K, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci. (2018) 1430:44–79. doi: 10.1111/nyas.13968
65. Perna L, Schöttker B, Holleczek B, Brenner H. Serum 25-hydroxyvitamin D and incidence of fatal and nonfatal cardiovascular events: a prospective study with repeated measurements. J Clin Endocrinol Metab. (2013) 98:4908–15. doi: 10.1210/jc.2013-2424
66. Taylor S. Vitamin D in toddlers, preschool children, and adolescents. Ann Nutr Metab. (2020) 76(Suppl. 2):30–41. doi: 10.1159/000505635
Keywords: vitamin D, cardiovascular disease, visualization studies, bibliometric analysis, bibliographic items co-occurrence matrix builder
Citation: Luo X, Wu F, Wang C and Wen C (2023) Analysis of hot trends in research on the association between vitamin D and cardiovascular disease. Front. Nutr. 9:1073698. doi: 10.3389/fnut.2022.1073698
Received: 18 October 2022; Accepted: 28 December 2022;
Published: 13 January 2023.
Edited by:
Manfred Eggersdorfer, University of Groningen, NetherlandsReviewed by:
William B. Grant, Sunlight Nutrition and Health Research Center, United StatesIoana Mozos, Victor Babes University of Medicine and Pharmacy, Romania
Copyright © 2023 Luo, Wu, Wang and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Wen,  chuanwen@csu.edu.cn
chuanwen@csu.edu.cn
 Xuemei Luo
Xuemei Luo  Feifeng Wu
Feifeng Wu Cheng Wang
Cheng Wang Chuan Wen
Chuan Wen