Technological interventions in improving the functionality of proteins during processing of meat analogs
- 1Institute of Tropical Agriculture and Food Security, Universiti Putra Malaysia (UPM), Seri Kembangan, Malaysia
- 2Department of Livestock Products Technology, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India
- 3Division of Veterinary Medicine, Faculty of Veterinary Sciences and Animal Husbandry, Sher-e-Kashmir University of Agricultural Sciences and Technology of Jammu, Jammu, India
- 4Department of Livestock Products Technology, College of Veterinary and Animal Sciences, Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut, India
- 5Department of Animal Science, Faculty of Agriculture, Universiti Putra Malaysia, Seri Kembangan, Malaysia
- 6Department of Companion Animal Medicine and Surgery, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Seri Kembangan, Malaysia
- 7Department of Applied Animal Science, College of Animal Life Sciences, Kangwon National University, Chuncheon-si, South Korea
- 8Halal Products Research Institute, Putra Infoport, Universiti Putra Malaysia, Seri Kembangan, Malaysia
Meat analogs have opened a new horizon of opportunities for developing a sustainable alternative for meat and meat products. Proteins are an integral part of meat analogs and their functionalities have been extensively studied to mimic meat-like appearance and texture. Proteins have a vital role in imparting texture, nutritive value, and organoleptic attributes to meat analogs. Processing of suitable proteins from vegetable, mycoproteins, algal, and single-cell protein sources remains a challenge and several technological interventions ranging from the isolation of proteins to the processing of products are required. The present paper reviews and discusses in detail various proteins (soy proteins, wheat gluten, zein, algal proteins, mycoproteins, pulses, potato, oilseeds, pseudo-cereals, and grass) and their suitability for meat analog production. The review also discusses other associated aspects such as processing interventions that can be adapted to improve the functional and textural attributes of proteins in the processing of meat analogs (extrusion, spinning, Couette shear cell, additive manufacturing/3D printing, and freeze structuring). ‘
1 Introduction
During the last two decades, the plant-based meat analogs sector has recorded tremendous growth, attributed mainly to their inherent merits associated with sustainability prospects, nutritive quality, improved food safety, food availability, ethical/animal welfare issues associated with their production process as compared to conventional meat and increasing trend of vegetarianisms (1–5). The EAT-Lancet commission also recommended for reduction in animal-source protein and dairy products intake with a more health diet comprising plant-based proteins such as legumes, nuts, and beans (6) for the betterment of the environment and public health (7). Meat analogs with organoleptic attributes similar to meat and can be promising alternatives for high-quality protein diets of the future with existing, already strained natural resources (8, 9). Animal production utilizes about 83% of the total farmland and make up to 56-58% of total emission but only cater to 37% of total protein and 18% of total calories globally (10).
The increasing numbers of start-ups and food processing companies are focusing on producing higher variations and varieties of meat alternatives by introducing increasing numbers of plant-based analogs for sale, such as burger patties, sausages, and minced meat alternatives. According to an estimate by OECD (11), the plant-based meat analogs had an estimated market value of 4.8 billion USD in 2019 which is expected to reach 6.8 billion USD by the year 2025 with a 7.1% growth rate. Worldwide, Europe has the largest market share of above fifty percent for plant-based meat analogs (51.5%), followed by North America with a share of 26.8%, and Asia Pacific (11.8%). About 6.3% market is in Latin America and 3.6% is in the Middle East and Africa (11, 12).
Meat and meat products are excellent source of highly bioavailable animal proteins and important minerals and have relatively higher digestibility and biological and nutritive values. However, meat and meat products generally have lower storage stability and require efforts in terms of preservation (13, 14) and are often associated with negative consumer perception mainly due to health issues related to the consumption of red and processed meat (9, 15–17) and animal welfare or ethical issues during livestock rearing and slaughtering (15). The association of meat consumption with certain diseases and animal welfare issues has led to an impetus for the development of protein-rich alternatives such as meat analogs and cell-based products (18).
Vegetable proteins form a crucial component of plant-based meat analog production. The carbon and water footprints of these vegetable proteins are significantly lower than those of animal proteins obtained from the slaughter of animals which further has animal welfare, ethical and religious issues. Blue water refers to the volume of fresh water evaporated from the global blue water resources (surface water and ground water) (19). The carbon footprint (g CO2e/kg protein), blue water footprint (L water/kg protein), and land-use footprint (m2 year/kg protein) of raw ingredients of vegetable origin, such as isolated soy protein are about 2-6, 38-205 and 6-8 respectively, that are much lower than that of beef production, 174-184, 1,607 and 1,310-1,311 values, respectively (20). In addition to the environmental merits, plant proteins are more economic/cheaper and can be processed into various meat alternatives with desirable nutritional and organoleptic properties (5).
This has necessitated the accurate selection of proteins or their suitable combinations as a paramount focus area for production of meat analogs/alternatives. The quality and functionality of plant protein have a critical role in the functional, nutritional and structural attributes of meat analogs (4).
2 Protein sources
Proteins from a wide range of sources such as soy, wheat, rice, legume, mushroom, pseudocereals, leaves, oilseeds, fungi and single-cell proteins (SCP) are used for the development of meat analogs. Recently there has been escalated focus on the utilization of protein sources from single-cell protein (SCP)/edible unicellular microorganisms/microbial protein (21), duckweed, seaweed (22), oilseed proteins and oilseed co-products (23), pseudocereals such as Amaranth and Quinoa grains (24), cauliflower, eggplant, jackfruits for processing of meat analogs (25). These have a high potential for sustainability, nutritive value (higher protein content, sulfur-containing amino acids), and price advantages (26, 27) which can be utilized after suitable technological interventions. Leaves from Alfalfa, grass, sugarcane, the mulberry bush, and tobacco has been extracted for their potential application in the development of plant-based meat analogs (27). Other proteins of good potential having good protein solubility in alkaline medium, gelling, foaming, and water absorption are chia seed, pumpkin seeds, hemp, and potato proteins and can also be used in the preparation of plant-based meat analogs (28).
2.1 Legume proteins
Soybean due to its cheapness, easy availability, nutritive value (protein digested corrected amino acid (PDCAA) score of 1.0; biological value comparable to conventional meat), functional (emulsifier, chewability, texture, stabilizers, water retention), satiating properties, and organoleptic attributes, makes it the single largest source of vegetable protein used in the preparation of meat analogs (29). Texturized soy protein (TSP) also known as texturized vegetable protein (TVP), or soy meat has 50% protein content which decreases upon rehydration. It is widely accepted as a meat replacer by vegetarians and health-conscious consumers (30). Products like tofu, tempeh, and yuba have become quite popular these days (31). Glycinin, β- and γ-conglycinin, 7S-globulin, lectins, lipoxygenase, and β-amylase are major soy proteins. Soy leghemoglobin, a heme pigment is now increasingly being used for meat analogs preparation for imparting a ‘bloody appearance’ of meat heme proteins (32). Food structure and thus, the texture is largely governed by proteins, polysaccharides, and/or their interactions. Stanojevic et al. (33) reported that the incorporation of inulin carbohydrates in tofu improved its hardness and gel strength through the hydrophobic interaction of soy saponin with carbohydrates. This interaction affected the foam-forming ability and altered its elastic behavior.
Legumes such as pea, lentils, mung beans, chickpeas, and lupin beans are also gaining importance in meat analog preparations. These legumes have poor digestibility compared to soy proteins, but with suitable technological interventions, these proteins might offer complementary desired structural and processing characteristics for developing meat analogs (34). Legume/pulse proteins (beans, peas, lentils, and chickpeas) are increasingly being used to produce gluten-free, low-allergen vegetable protein-based meat analogs with improved textural, nutritional, and organoleptic attributes (35). The legume seeds contain higher protein contents (20-35%) than other plant proteins, are a rich source of dietary fiber, vitamins, minerals, and are low in saturated fats (36).
Pulse proteins have a better amino acid profile than cereal proteins and have a comparable concentration of lysine, leucine, and phenylalanine to soy protein. Lupin beans are rich in protein content (46%) and had the least undesirable non-nutrients (23). Other less common legumes such as chickpea, faba bean, and mung bean proteins are also used for the development of meat analogs (34). Buhler et al. (37) observed the improved water-holding capacity and protein solubility of faba bean protein concentrate; having similar attributes to the soy protein concentrate. Chickpea, a commonly consumed legume has a positive effect on color (similar to meat due to the presence of carotenoids) and good textural and water holding and oil binding properties (38). Chickpea protein isolates had higher water and oil-binding properties than soy protein isolates (39).
Globulins form the major storage proteins in pulse seeds (70-80%). The major fractions of globulin are legumin (11S) and vicilin (7S) with convicilin as a minor fraction (35). Comparatively lower gelling strength of pea proteins (except mung bean and chickpea) could be overcome by suitable processing technology such as the incorporation of hydrocolloids or polysaccharides, less refined protein-rich ingredients, ultrasonication, starch enrichment, dehulling, dry fractionation, milling, air classification, alcohol (ethanol or isopropanol) washings (40, 41).
Zhu et al. (42) explored the processing of pea protein and its effect on the functionality suitability for the production of meat analogs developed from such proteins. Two separation processes, namely dry separation which consisted of ultrafine milling, air pre-classification, and electromagnetic separation, and wet separation done by isoelectric point precipitation were employed to obtain pea proteins. A marked variation in the purity of pea proteins was obtained by dry separation (72.3 g/100 g) and by wet separation (89.2 g/100 g) (42). Functional properties like emulsification and foamability were better in pea proteins obtained by the wet process while water absorption was superior in dry-separated pea protein isolates. Authors noted better natural structure, and solubility of pea protein isolates obtained by the dry method, formulating porous and soft meat analogs. In contrast, pea protein isolates obtained by the wet method had better amino acid content near to the recommended level. These pea protein isolates as well as wheat gluten have great potential in the development of meat analogs (43).
The interfacial and rheological properties of pea protein isolates were improved by soy soluble polysaccharides (SSPS) which facilitated the rearrangement and reconnection of modified pea protein particles. SSPS consists of a galacturonan backbone of homogalacturonan (a-1,4-galacturonan) and rhamnogalacturonan (repeating units of a-1,2-rhamnose and a-1,4-galacturonic acid) branched by b-1,4-galactan and a-1,5-arabinan chains (44). SSPS has a pectin-like structure and emulsifying property due to the presence of glycoproteins (45). Zhan et al. (45) reported a significant reduction in hydrophobicity and increased emulsion stability of pea protein isolates by adding freeze-dried soy soluble polysaccharides (SSPS). The functionality of pulse proteins can also be improved or modified by technological interventions such as methods of extraction. Protein-rich faba bean flour prepared by dry-fractionation was reported to exhibit superior functionality, protein solubility (85% at pH 7), foaming capacity, and gelling ability compared to isolate produced through isoelectric precipitation or acid extraction (46).
Similarly, Malik and Saini (47) observed improvement in emulsifying, foaming properties, and oil binding capacity of sunflower protein isolates by heat processing at pH 4-5 due to minimum denaturation of proteins at this pH. Not only the purified proteins but even their partially fractioned components contribute to their functional properties. The partially fractioned ingredients of sunflower such as polysaccharides, oil macromolecules as well as proteins exhibited good emulsifying potential comparable to commercially used proteins (48).
The application of ultrasound technology has also been explored for improving the functionality of proteins as high-intensity ultrasound treatment changes protein isolates’ secondary and tertiary structures, thus altering its gel strength. The ultrasonication at below 10°C for 5-30 min (probe-20 kHz, 25% amplitude with power output-500 W and ultrasonic intensity-58-61 W/cm2; probe-40 kHz with power output-500 W and ultrasonic intensity-0.5 W/cm2) of sunflower isolates resulted in the unfolding of proteins, exposing its hydrophobic groups leading to non-covalent interactions between denatured protein molecules (49). These non-covalent interactions reinforce the colloidal network during cooling (49) and promote a meat-like texture.
Rapeseed proteins have good foaming, emulsification, and gelation properties, and thus are being increasingly utilized in plant-based sausage analogs. Transglutaminase, polysaccharides like gum arabica, and chemical modifications such as acetylation or succinylation have been successfully employed to increase the gelation of rapeseed (canola) proteins (50). Li et al. (50) studied the effect of gum Arabic and pH on rapeseed protein isolate emulsion (3% w/v) and observed a stable emulsion with a particle size of 314 nm and absolute zeta potential value of −44.3 ± 0.5 mV at 1% gum Arabic and at pH 8. Authors (50) attributed it to a significant increase in β- sheet and a decrease in β-antiparallel due to the interaction between rapeseed protein isolates and gum Arabic mainly via changes in hydrogen bonds.
2.2 Wheat gluten
Wheat gluten, a byproduct of the wheat starch industry, owing to its visco-elastic properties, is commonly used as traditional cereal protein for preparing meat analogs for providing structural and fibrous attributes. Wheat gluten has excellent binding properties, baking performance, viscosity, swelling, leavening properties, and dough preparation attributes due to the formation of viscoelastic polymeric networks capable of retaining gases in it (51). It improves the nutritional quality of meat analogs (a rich source of glutamine) and is used as a binder and structural agent. Moreover, the structural characteristics and cross-linking in meat analogs are also affected by various processing conditions such as isolation of specific proteins, protein modification, pressure, temperature, chemical pretreatment, or presence of alkali, salts, or polyphenols (52–54). The incidences of gluten allergy and celiac diseases have forced food technologists to search for a suitable replacement for wheat gluten by modifying properties of other proteins similar to wheat gluten and cross-linking by fermentation, hydrolysis, or application of transglutaminase, phenolic compounds, and organosulfur compounds (55–57).
2.3 Zein
Zein is a major storage protein in maize. It forms viscoelastic networks above its glass transition temperature (the temperature at which a hard glassy structure change to a rubbery state). Its inability to be drawn into long fibers or sheets at room temperature is the greatest hurdle for food technologists to employ zein in the preparation of meat analogs (58). Mattice and Marangoni (59) developed thin strips or ribbons of zein by extruding zein-ethanol solutions of which the ethanol was removed later by air drying. The self-assembled zein networks had comparable rheological behavior to chicken breast, thus presenting an opportunity for zein in plant-based food structuring. The intermolecular β-sheet structure has the potential to improve viscoelasticity, hence improving cohesiveness and proper mouthfeel in the developed meat analogs (60). Erickson et al. (60) proposed atomistic modeling of peptide aggregation and β-sheet structuring for improving the viscoelasticity of corn zein. The authors observed that peptide sequences without proline had a higher level of β-sheet structuring.
The β-sheet structure, visco-elastic dough with improved mixing stability was developed with rice flour and zein (above 5% w/w level) for the development of gluten-free dough sheets capable of forming long noodle strands (58). Zein, although lacking certain essential amino acids, has great potential in meat analog developments due to its fibrous nature. Further, the enzymatic and chemical alterations of zein, such as hydrolysis, deamidation, and cross-linking, could be very useful tools in improving the functionality of zein protein for its wider application in the preparation of meat analogs (61).
2.4 Rice protein
Recently rice protein is gaining importance in the development of gluten-free meat analogs. As compared to soy protein, rice protein has a milder flavor and bland taste. Further rice proteins are also having hypoallergic and hypocholesterolemic effects (62). Rice protein consists of albumin (30.9%), globulin (24.9%), glutenin (32.5%), and prolamin (11.6%) (63). It is a rich source of arginine, cysteine, histidine, methionine, and valine amino acids. The lysine (3.8%) and leucine (8.2%) amino acid content are higher than wheat (64). Rice protein had higher nutritional quality, true digestibility, and biological value as compared to soy protein isolates (65), hence recommended for infants and the elderly (58). Rice bran is a rich source of dietary fiber (27.6-33.3%) and its incorporation in meat analogs could improve the nutritional as well as functional properties of the developed meat analogs (66, 67).
The full replacement of rice protein during the extrusion is challenging due to its low porosity (68). The incorporation of isolated rice protein (10%) along with isolated soy protein (40%) and wheat gluten (40%) and corn protein (10%) was observed to improve the textural properties (integrity index, chewiness, and degree of texturization) and decrease the formation of the fibrous structure upon extrusion. The raw materials were extruded at 140°C barrel temperature, 250 rpm screw speed, 100g/min feed rate, and 40% moisture. Xiao et al. (67) noted enhanced intermolecular hydrogen bonds leading to increased hardness and tensile force at temperature (<170°C) and increasing screw speed up to 280 r/min during the extrusion of rice bran-added meat analogs. Increasing moisture content was reported to a lower hardness and tensile force values during the extrusion of rice bran-added meat analogs (67). Red yeast rice, prepared by culturing rice with Monascus purpureus yeast strains, improve the color, high moisture, protein, and fat content but with significantly (p < 0.05) decreased shrinkage and cooking yield of the plant-based meat analogs as compared to beef patties (69).
2.5 Mycoproteins
Mycoproteins obtained from edible fungi such as obtained from Fusarium graminearum is gaining popularity in the development of novel meat analogs due to their comparable nutritive value to meat proteins (such as fiber source, polyunsaturated fatty acids, minerals, vitamins), texture (formation of flaky or fibrous texture) sensory attributes (meaty flavor, taste), sustainability and high digestibility, which can be further improved by using egg albumen proteins (4). Mycoproteins are considered a sustainable source of complete nutrition (essential amino acids, vitamins, carotene, minerals, and carbohydrates) with 10-20 times less land requirement and 10 times lower carbon emission than beef production, and 4 times lower carbon emission than chicken production (70). Mycoprotein has a biological value similar to milk proteins, and on average, 100 g of dry matter of mycoprotein contains 45% protein, 13% fat, 10% carbohydrates, and 25% fiber on average (71). As compared to other common plant proteins, mycoprotein contains a higher weight-percentage protein content. The fiber present in fungal cell walls is mostly made up of β-glucan (up to 75%) and chitin; consequently, forming a ‘fibrous chitin-glucan matrix’ (71). The mycoprotein is classified under the food category ‘high in fiber food’ by the European Commission (72).
2.6 Mushroom protein
With proper incorporation of gelling and thickening agents, good-quality meat analogs can be prepared by using mushrooms as a source of protein and nutrition. Arora et al. (73) prepared good quality mushroom-based sausage analogs by using carrageenan and xantham gum as binding agents and reported significantly improved textural attributes, emulsion stability, and decreased purge loss upon the incorporation of 0.8% carrageenan concentration. Kumar et al. (74) developed analog meat nuggets by incorporating mushrooms into texturized soy protein and wheat gluten (74–76).
2.7 Pseudocereals
Quinoa seeds, categorized under pseudocereals can also be used as a binder or extender replacers in place of texturized vegetable proteins during the preparation of meat products for improving binding, water retention, and cooking yield. Quinoa protein exhibit emulsifying, foaming, and water absorption properties similar to soy protein, and shows good gelation at a low pH in the presence of divalent salt addition (77).
2.8 Potato protein
The potato processing industry generates a huge amount of potato proteins that can be utilized to improve the functionality of non-functional plant proteins such as zein fractions. At present, a lot of potato protein goes to waste and their proper utilization in food is considered a sustainable and economically beneficial option (78). Commercial preparations such as Quorn® vegan nuggets, Gardien-seven-grain crispy tender, Beyond meat-sausages, and Impossible foods-impossible burgers are made with potato proteins as an ingredient (79). Potato proteins have good emulsifying capacity, foaming, and gelation properties. Patatin is the major potato protein that undergoes denaturation (destruction of tertiary structure and forming random polypeptide chain) at a lower temperature (55-75°C) and forms a good gel network (80). Glusac et al. (81) obtained a stable emulsion with potato proteins and zein which remained stable for 30 days. The oil-in-water type emulsion was stabilized by the potato proteins solubilized in the aqueous phase with the help of cross-link formed by enzymatic action by tyrosinase enzyme obtained from Bacillus megaterium and zein solubilized in the oil phase. The enzymatic-assisted cross-link formation between potato protein-zein resulted in 10-fold larger particle size in the form of covalent aggregates, 40-fold higher viscosity, and a 2-fold increase in hardness value as compared to control emulsion and has the potential to be used in meat analogs preparations (81). For improving nutritive value such as dietary fiber, antioxidants, vitamins, and ash contents, a suitable combination of raw ingredients is recommended (82).
2.9 Single-cell protein
Single-cell protein is harvested from microalgae, bacteria, and fungi cultivated on food-grade substrate, beverages, or food industry wastes by solid-state or submerged fermentation (8). Algae can be grown on a wide pH range (up to 11) whereas bacteria and yeast grow at a slightly neutral to acidic medium (pH 5-7). To harness the vast potential of protein from aquatic sources (seaweeds, and single-cell-protein), the proper extraction technology for their separation and purification is needed (83). The high content of nucleic acid (up to 16% dry weight in bacterial cells) in SCP is associated with their rapid growth. The nucleic acid content in SCP should be controlled as these nucleic acid metabolized into uric acid in the human body, thus increasing the risk of kidney stones and gout (84). It can be decreased by suitable alteration of growing conditions, and proper extraction protocols such as endogenous RNAase, alkaline hydrolysis, chemical treatment, and autolysis.
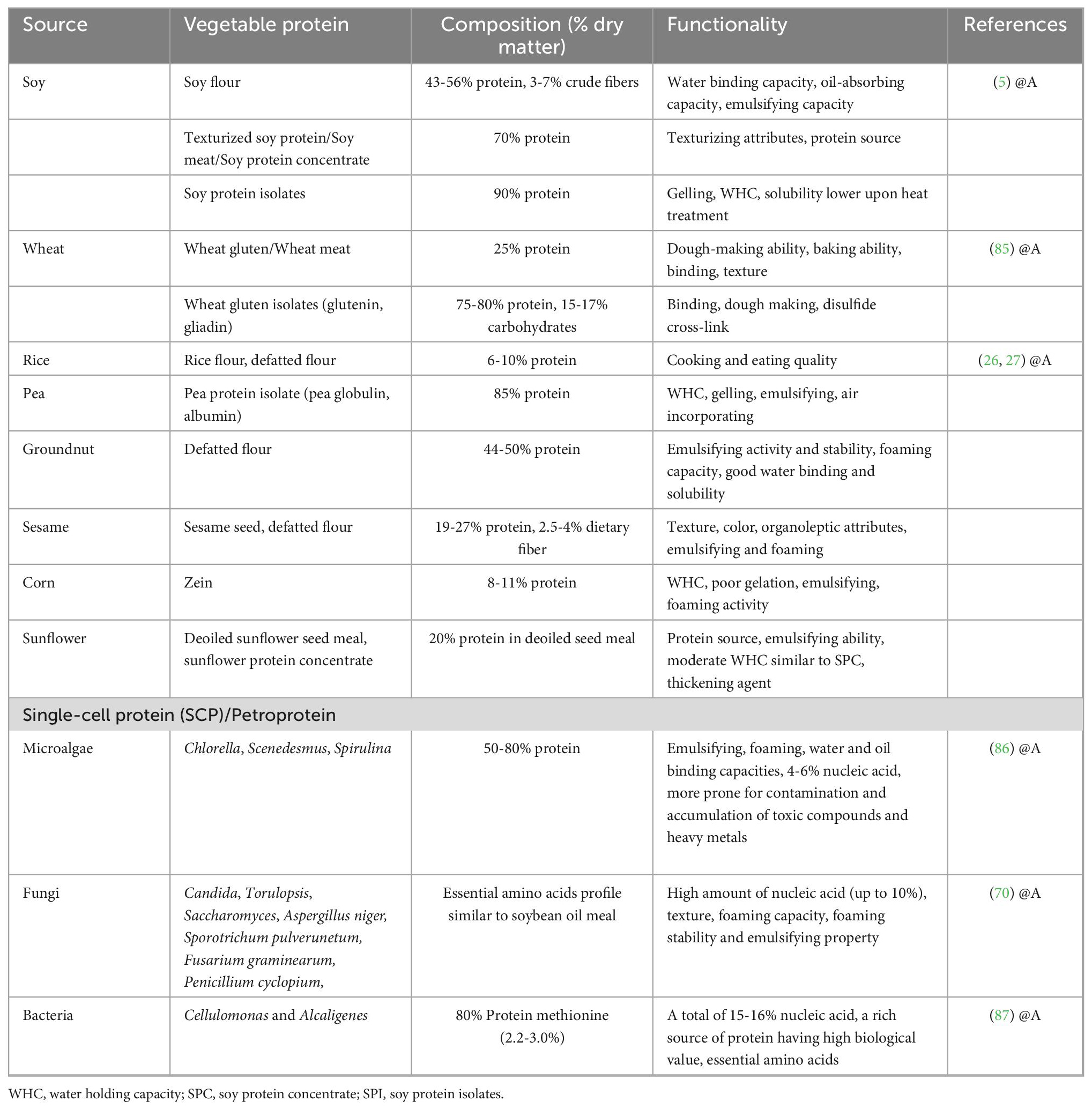
Commonly used major proteins used in the preparation of meat analogs are presented in Table 1.
3 Protein extraction
Herbaceous (lucerne, leaves from agro-industrial crops such as cassava, sugarcane, and trees) and aquatic biomass (aquatic plants such as duckweed, seaweed, macro, and microalgae) are explored for ensuring sustainable food supply due to their high protein content, higher yield, efficient protein conversion and availability on large scale (88). Protein extraction mainly focuses on the extraction of soluble proteins from green biomass. In leaves, rubisco (ribulose-1,5-biphosphate carboxylase/oxygenase) forms the major portion of soluble proteins (83). Improving protein extraction yield from comparatively sustainable aquatic biomass (microalgae, seaweeds, aquatic plants) is the present focus of the food industry by exploring and employing a range of technologies such as tissue disruption, protein solubilization and fractionation, protein precipitation and purification and concentration (83).
The protein extraction from microalgae is quite challenging due to the presence of pigments and polysaccharides in protein due to cell disruption, hence requiring a proper purification process. Similarly, during the extraction of protein from seaweed, polysaccharides and phenolics are present as co-extracted compounds and interact with seaweed proteins (89). The microalgae cell wall can be broken by bead milling, high-pressure homogenization, or by a combination of various physical treatments (such as microwave, ultrasonication, autoclaving, grinding, and osmotic shock), enzymatic treatment, or by chemical treatments (58, 90, 91). Protein extraction is performed by solubilization of proteins in an alkaline solution followed by isoelectric precipitation at pH 3-5. Other methods applied for protein extraction are precipitation with solvent, adsorption, and ultrafiltration (89). Depending upon the plant source and extraction process, the leaf protein concentrate form about 40-60% of the total leaf protein (92). The application of enzymatic mixtures degrades the structural components of the cell wall and better releases soluble components containing proteins.
Protein fractionation from seaweeds is a very complicated process due to the presence of phenolic compounds (chemically bind to proteins) and cell wall mucilage/neutral polysaccharides (reduce mass transfer). The seaweed protein extraction is done by drying the biomass followed by alkaline extraction, enzymic digestion, osmotic shock, or high shear grinding (93). However, the processing of fresh seaweeds was observed to improve extraction yield, increase protein concentration, and higher total essential amino acids (94).
The functionality of a protein depends upon the extraction protocol such as exposure to high temperature or high alkaline pH could denature proteins, consequently reducing their functionality. Non-hydrolyzed or partially hydrolyzed large proteins are preferred for gelling and textural functions (95), whereas hydrolyzed small proteins have good emulsifying properties (96). An increase in protein functionality has also been observed in the presence of impurities such as charged sugars (97).
The protein recovery methods have a direct impact on the functionality and quality of plant protein such as albumins retention in pea protein obtained by ultrafiltration and dialysis, whereas globulins retention under alkaline-isoelectric precipitation and micellar precipitation (98). Alkaline extraction also significantly impacts pea protein configuration by exposing hydrophobic regions with higher gel strength, whereas salt-dialysis prevents protein folding leading to a coarser structure and soft gel (98). Pea protein concentrate forms a fine elastic gel with albumin fraction F forming a weak gel due to low purity, whereas globulin-rich fraction forms a fine elastic gel. The globulin-rich fraction and albumin fraction of pea protein might be used as an alternative to whey protein isolates (99).
Figure 1 summarized the various protein extraction process.
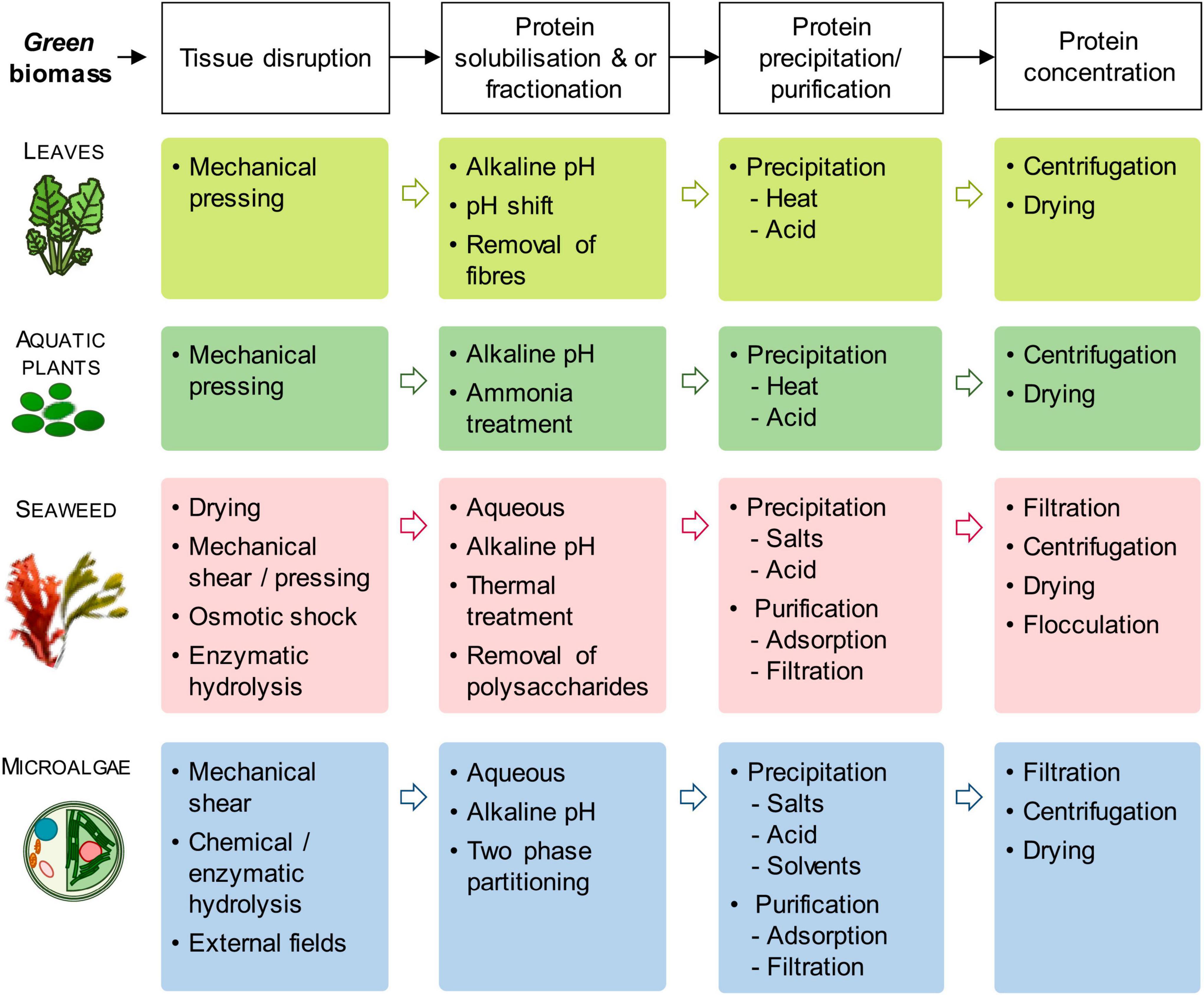
Figure 1. Generic protein extraction process from green biomass (top line) and an overview of extraction methods for each biomass group. [Adopted from Tenorio et al. (83)] under Creative Commons CC-BY-NC-ND license).
The protein separation is based on the variations in structural, functional, or chemical properties between the target compound/protein and other proteins in the mixture. These variations can be in size, shape, molecular weight, density, specific gravity, charge, solubility, charge distribution, metal binding, ligand binding, posttranslational modifications, and specific structures or sequences (100). Membrane technologies viz., microfiltration, ultrafiltration and nanotechnologies are increasingly used as energy-efficient and green technologies for the separation and purification of bioactive compounds (101, 102).
Tags are specific fusion peptides or protein sequences that are used to improve the efficacy of the protein purification process by determining interactions between proteins and immobilized ligands (103). Tags can be a whole enzyme, a small polypeptide chain or a protein domain and interacts with a range of substrates such as metal chelators, carbohydrates, antibodies, and small molecules, thus enhancing the yield, purity, and recovery of associated counterparts (104). Further tag-assisted protein purification can have potential to produce purified proteins at commercial scale if the issues of design and application of tagged fusion proteins are properly addressed (105). For a successful tagging protocol, it should provide end protein products with high yield, purity and should preserve their natural structure and functionality.
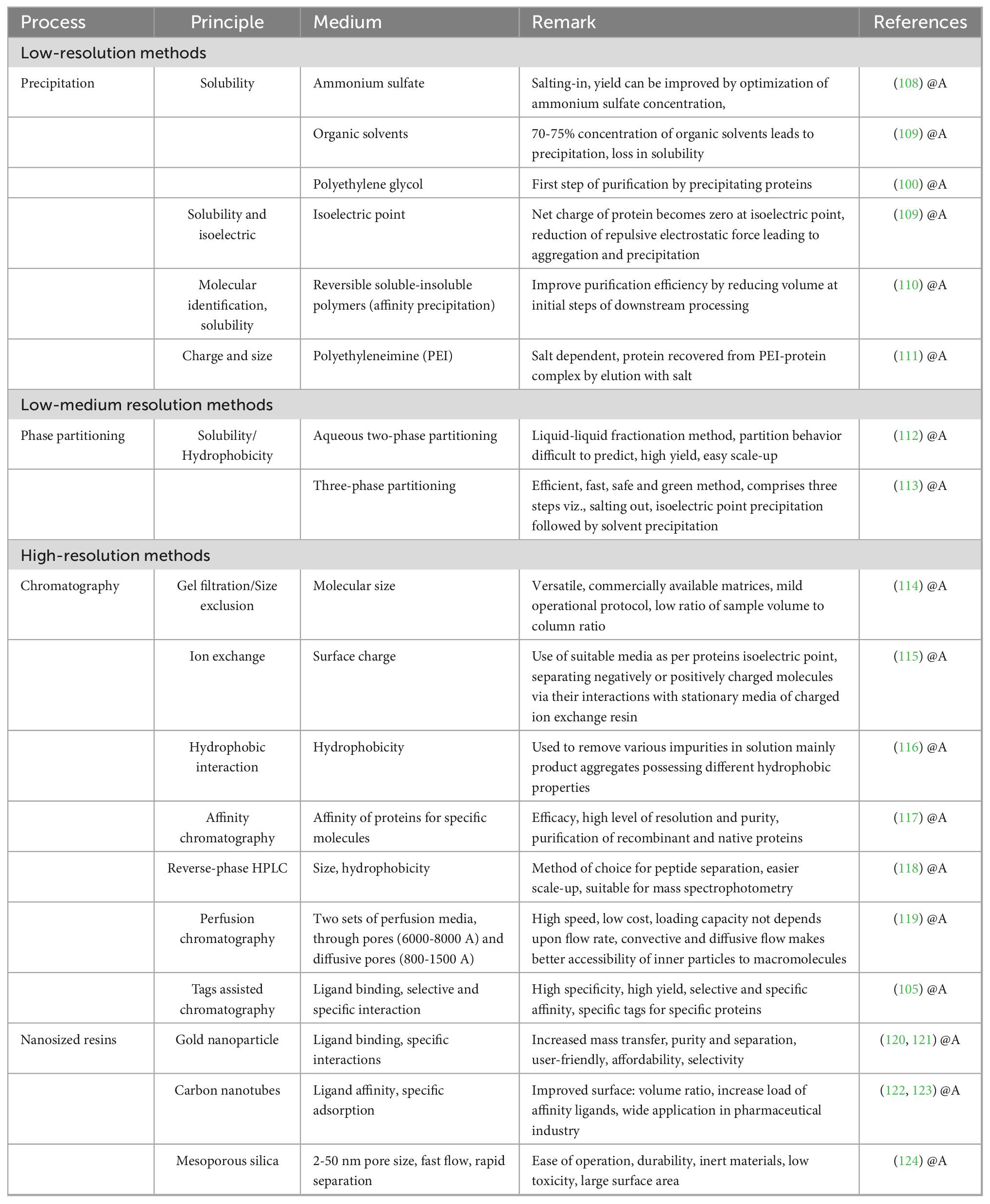
The application of nano-sized resins in chromatography column has resulted in a significant increase in process rate, resolution, and purity of final products. It reduces the time of the experiments and increases mass transfer, decreases the loading capacity of the column, and increases the surface-to-volume ratio and load of the affinity ligands (105). Further with nanofabrication, the mechanical strength of resins can be significantly improved, consequently reducing the total cost of separation or purification of desired compounds (106, 107). Various methods of protein purification are summarized in Table 2.
4 Improving functionality of plant proteins
Protein ingredients used in the production of meat analogs are among the essential aspects for product characteristics and differentiation. Essential aspects defining the structure and function relationship of protein include hydration and solubility properties, emulsification and foaming properties, flavor binding, viscosity, gelation, texturization, and dough formation (125). The functionality of proteins, on the other hand, maybe further altered by processing, resulting in new conceivable uses for the proteins (126). Other factors to consider include protein cultivar type, extraction methods, and drying methods, which influence the functional ability of plant protein ingredients (127). The fractions of albumin and globulin in plant proteins vary with cultivars and genotypes and various cultivars and genotypes have different components; thus, demonstrating variations in their functional properties (128). For example, water-soluble fraction of red lentil Firat and green lentil Pul II was reported to vary significantly, with red lentil protein concentrate had a higher water-solubility as compared to their green lentil counterparts (129, 130). Other functional parameters observed in lentils to varies with cultivars were gel-forming ability, oil absorption capacity, foaming stability, and foaming capacity (131). The protein concentration, molecular conformations of different types of proteins, types, and aggregation state play an important role in determining its functionality. The presence of other ingredients in proteins such as fibers, polysaccharides, lipids and minerals have impact on functionality (127, 132).
The water-holding capacity of protein isolates is usually greater than water-holding capacity than their corresponding flour. The presence of some non-protein ingredients such as fibers, lipids, and starch granules act as barriers to water penetration such lentil protein isolates demonstrated higher water-holding capacity than lentil flours, mainly attributed to lower lipid content and smaller particle size (133). Similar is the case with chickpea protein isolates had higher oil holding capacity (2.1-4.0 g/g) as compared to their corresponding flour (1.1-1.2 g/g) (134).
The functionality of plant proteins could be significantly modified by the application of suitable processing technologies. The solubility of proteins can be modified by heat, acid- or alkali-induced denaturation, hydrolysis, and heat-labile proteins can be enzymatically hydrolyzed to improve their solubility, heat stability and lowering allergenicity (135, 136), The proteins harvested by processing entire organisms such as algal proteins, mycoproteins, and insect proteins show a high level of heterogenicity (137). During extraction and processing, plant proteins are often undergoing severe heat, shear, or solvent extraction processes. These processes result in denatured and cross-linking or even hydrolysis of proteins. The functional and nutritional properties of oilseed proteins is affected by the extraction conditions (138).
Heat-induced denaturation of proteins leads to their aggregation via hydrophobic interactions, hydrogen bonds, and disulfide bonds. Heating of proteins at low or high from isoelectric pH or low ionic strength results in the filamentous structure of a fine-stranded aggregates with a string-of-beads shaped structure. Further, this aggregation becomes more random at moderate or high ionic strengths resulting in particulate aggregates (139, 140). With the addition of salt, the filamentous aggregates form physical entanglement networks, thus masking electrostatic repulsion. Hydrophobic interactions induce network formation in solutions of filamentous aggregates and on their gel formation, other forces also consolidate the network, resulting in further improving gel strength (139), homogenous cold-set gels are transparent (140). Particulate or heterogeneous gels have a turbid appearance and low water-holding capacity (141).
Globular proteins have non-polar regions on their surface which improve adsorption to oil-water or air-water interfaces, consequently improving emulsifying and foaming potential. Albumins from peas and chickpeas have lower flexibility and hydrophobicity leading to overall lower emulsifying properties (142, 143). The albumins of kidney beans exhibit good foamability and emulsifying properties resulting in their good solubility, lower molecular weight, and better molecular flexibility, however, these kidney beans are unable to form a stable foam at neutral pH as compared to globulins (144). Under optimum ionic strength and heating conditions during food processing, the individual units of 7S and 11S globulins undergo disaggregation-unfolding-reaggregation resulting in improving their functionality viz., solubility, gelation, emulsification, and foaming (145).
There are some challenges in the utilization of plant proteins such as allergenicity to some peoples, off-flavor, presence of antinutrients (such as glucosinolates in rapeseeds) which can be ameliorated by proper selection and incorporation levels, implementing suitable extraction protocols (cold-pressed, microwave-assisted), use of a proper solvent such as ethanol, microbial fermentation, plant-breeding and other processing technologies (146–149). The plant functionality could be improved by proper technological interventions.
4.1 High-pressure processing
High-pressure processing (HPP) reduces the functionality of plant proteins reducing particle size due to cavitation and fragmentation of macromolecules. The reduction in particle size was observed in hazelnut, and lentil proteins upon high-pressure processing up to 150 MPa (150). Over-pressing above 150 MPa results in a decrease in protein solubility such as lentil and hazelnut proteins (151). The appropriate pressure depends upon the protein types and solvent, with a pressure drop leading to an increase in particle size due to the denaturation of proteins under mechanical forces while dropping the pressure and higher temperature at the homogenizer valve (152). The particle size reduction of proteins leads to higher emulsifying and foaming properties owing to rapid movements of proteins toward the air-water interface whereas decreased foaming properties could be due to the lower intermolecular interaction and higher unfolding of proteins.
4.2 Extrusion
The high temperature applied during extrusion results in the disintegration of hydrogen bonds, thus the unfolding of proteins. The stabilization of extended protein networks is stabilized by increased protein aggregation formed by α-helices, β-sheet, non-covalently bonded β-turn or anti-parallel β-sheet structures via breaking of intermolecular disulfide bonds and formation of new intermolecular bonds (153, 154). Further, high temperature and mechanical pressure also destroy the anti-nutrients, thereby improving the digestibility of plant proteins and availability of amino acid. Chickpea flour had the highest PDCAAS (protein digestibility corrected amino acid score) and protein digestibility upon extrusion as compared to cooking or baking process (2, 155). Similarly higher in vitro digestibility of extruded soybean protein concentrate was reported by (156) as compared to uncooked samples.
4.3 Sonication
Sonication also modifies the functionality of plant protein functionality via localized hydrodynamic shearing of the native protein particles due to cavitation, microjet and violent agitation effect, and thermal degradation due to exposure to high temperature (157). Sonication of protein aggregates was observed to break into smaller particles and a better uniform distribution under the exposure to high-intensity ultrasound effect (158). Similar findings were also reported by Xiong et al. (159) upon sonication of pea protein isolates (5% w/v) solution at 30, 60, and 90% at 20kHz for 30 min. This particle size reduction upon sonication leads to a lower in intermolecular association which further increases more sulfhydryl (SH) groups and hydrophobic regions to be exposed (159). The reduction in the size of the protein aggregates due to the breaking of non-covalent interactions is accompanied by increasing its solubility (2). The increased protein solubility was reported for 1% w/w wheat and soy protein isolates solution (20 kHz frequency, 95% amplitude, 2 min) and 0.5% w/v walnut protein isolate (200, 400, and 600 W for 15 and 30 min) (160).
However, in some cases, sonication with long duration and low power or intense sonication was observed to form larger aggregates of proteins (160) such as 5-fold increase in size of buckwheat protein isolates upon sonication at 100% amplitude for 10 min (161). This could be due to the increase in particle sizes of protein aggregates due to the disruption of microstructure of protein due to sonication leading to swelling of the protein particles in an aqueous medium or self-assembly of unfolded regions of proteins via hydrophobic interactions (161). The authors also observed an increase in reactive content and sulfhydryl (SH) groups, with lower disulfide (SS) bonds due to exposure of SH contents buried inside and breaking SS bonds due to cavitation effects under sonication. Overall, the sonication of buckwheat proteins was observed to alter secondary structure resulting in improved digestibility. Sonication also induces structural changes in plant proteins due to the disintegration of non-covalent bonds, consequently disrupting the secondary structure and partially denaturing the tertiary and quaternary structure without having any significant impact on the primary structure (162).
4.4 Chemical modifications
Various chemical modifications by pH change, glycation, and enzymatic action also can induce structural changes in proteins, thus altering their functionality. Derivatization comprises a chemical modification in which reactive side chains in the protein structures are chemically changed to the desired level so to alter their physiochemical attributes and functional characteristics. The amine target groups for derivatization are amino, carboxyl, disulfide, indole, phenolic, imidazole, SH, thioether and guanidine (145). Glycation can be performed chemically by Maillard reaction or by cross-linking enzymes such as laccase and transglutaminase. Maillard reaction is preferred over other chemical methods as it occurs naturally and spontaneously during food processing (163). This non-enzymatic browning occurs via a series of non-enzymatic processes involving the carbonyl groups of reducing carbohydrates and free amino groups of protein. To get the desired yield, sensory, and functional properties of glycated proteins, various factors such as time, temperature, water activity, the concentration of reactants, and pH (164).
5 Processing of proteins
Various technologies interventions viz., thermo-extrusion, spinning, and cross-linking are used to impart fibrous texture to meat analogs, and a range of nutrients such as vitamins, flavorings, and minerals are added to make the product equivalent to meat in texture, appearance, and taste. The amino acid sequence is considered a major factor in determining the functionality of plant proteins and can be further improved by applying suitable processing technologies. The nutritional profile of meat analogs can be easily modified to make them healthier (essential amino acids, lower saturated lipids, cholesterol-free) and better for the planet (165). Chiang et al. (166) observed that fibrous meat analogs have the highest content of plant proteins (20-50%), followed by vegetable lipids (up to 5%), starch (2-30%), and miscellaneous minor ingredients. Polymerization of wheat gluten has a direct effect on the product quality during the extrusion and development of meat analogs. Wheat gluten polymerization is affected by thermal and mechanical treatment, with disulfide bonds playing a major role under reducing and non-reducing conditions. Shear rate at 50/sec did not have any perceivable effect on the covalent bond formation (167). Schreuders et al. (168) contemplated the rheological properties of plant protein blends for the preparation of meat analogs with texture maps. Meat analog blends were prepared with pea protein isolates, wheat gluten, and soy protein isolates. In the pea protein-wheat gluten blend, pea protein had a lower strain and elasticity value, whereas soy protein-wheat gluten blends were more elastic and rigid than wheat gluten-pea protein blends (168).
Plant proteins are texturized and processed to make them nutritionally (digestibility, bioavailability), functionally and structurally similar to meat for better acceptance among consumers and their utilization in the preparation of meat analogs at an industrial scale. This step of structuring plant proteins into matrices and their textural and meat-like appearance has a significant effect on the acceptance of meat analogs (169). The texture of plant proteins is affected by the composition and processing conditions. The processing of plant proteins is required to transform these proteins into materials resembling meat in texture, mouthfeel, flavor, and taste (153).
The phase-separation of protein-water systems into water-rich and protein-rich phase is the first step in fiber formation during processing (170). These fibers are further elongated into long strands with the application of shear force, thus leading to an anisotropic and fibrous mass. This phase separation could be further improved by adding a polymeric compound such as carbohydrates or hydrocolloids due to thermodynamic incompatibility as the repulsive forces between various polymers lead to their mutual exclusion (171).
Water has a plasticizer effect on the protein network and affects its rheology (172). During processing, the protein matrix transforms into a set and stable matrix with good mechanical properties needed for meat analog development. The hydrophobic content, cysteine, and charge-bearing amino acids content in a protein affect the stabilization of the protein matrix by forming covalent bonds or by non-covalent interactions (173). The formation of covalent bonds leads to the transition of the protein matrix into a coagulated and thermoset mass.
Various processing technologies used for modifications of vegetable proteins into meat analogs are described in the following sections-
5.1 Extrusion cooking
Extrusion technology is used for compressing and reshaping of raw materials by passing this in between dies of desirable shapes and sizes to develop food products with cross-section forms with different shapes and varieties (174). Extrusion is used for texturizing and structuring thermoplastic proteins into anisotropic and fibrous mass for utilization in the production of meat analogs (175). Extrusion cooking consists of a process of continuous mixing, shearing, heating, and forming a mass with one or several screws within a heated barrel. Hot extrusion is done at temperatures above 100°C leading to a cooking effect (structural and chemical changes) depending upon various factors such as particle size, moisture and protein content of raw ingredients, extrusion rate, barrel temperature, and pressure applied.
During the preparation of texturized vegetable protein (TVP) from defatted soy meal, textural attributes of the extrudates contributed to the disulfide bonds, hydrogen bonds, and hydrophobic interactions in the barrel or cooling zone, with more covalent bonds formed under extreme temperature and pressure (176). Upon increasing temperature during the extrusion of texturized vegetable protein from the optimum level (such as 150°C-160°C), a lowered texturization due to protein degradation with small pits appearing on surfaces and brown color was noticed (176). A further increasing temperature above had a detrimental effect on protein, resulting in unstable extrudates.
This technology has great potential in texturing the vegetable proteins by either increasing vegetable protein content or starch content during extrusion cooking as well as by suitable adjustment of processing parameters (system parameters, process parameters, and product parameters), die configuration, pressure, and temperature, screw length to diameter ratio, screw speed, moisture content, and extrusion rate (177). The protein quality and types and pre-treatment of raw materials also have an effect on conformational changes and textural attributes of vegetable proteins. The size constraints of end products, high energy, and high cost of extrusion are some challenges faced by the extrusion industry, and food technologists are working to overcome these challenges. The higher energy consumption in the extrusion process while producing meat analogs may dampen the sustainability merits associated with these products.
The overall quality and texturization are affected by the extrusion parameters (overall effect of shear force, temperature, and pressure), leading to denaturation, aggregation, the association of proteins, lipid oxidation, carbohydrate degradation, and complex conformational changes caused by interactions (molecular conformation, cross-linking) among vegetable protein, lipid, carbohydrate, and other molecules in the extrudate (45). These changes affect the texture, color, and shape of the extrudate. Moisture addition has lubricating and plasticizing effects, decreasing viscosity and the force needed to move material at high barrel temperature resulting in improved texturization of vegetable proteins.
The incorporation of 10% wheat starch into soy protein isolates (SPI) was noted in improving texture extrudates (178). This texturization is attributed to the increasing porous structures, protein network, and increased air cell size. Increased moisture in extrudates has been reported to improve the fibrous structure of vegetable proteins during extrusion. The increased die configuration/diameter was reported to hasten the extrusion process and increased the integrity index (a representative of the degree of texturization more for 8 cm die configuration than 5 cm die configuration), whereas higher water injection rate had a deteriorative effect on it (179).
Li et al. (180) reported a significant increase in gluten polymerization and improved textural attributes of wheat gluten proteins upon alkali treatment. The wheat gluten extrusion under alkaline conditions conferred a more fibrous microstructure due to the compact gluten network. The alkali addition (sodium carbonate- 0.1-1.0%; sodium bicarbonate- 0.1-1.5%, and sodium hydroxide- 0.1-0.5%) increased the dehydroalanine-derived cross-linking between dehydroalanine and lanthionine and decreased the cystine content leading to a compact and more fibrous structure (180). The resultant wheat gluten extrudates had higher hardness followed by reduced hardness, and higher resilience and chewiness upon increasing alkaline concentration. The decrease in free sulfhydryl content (SH) showed that dehydroalanine-derived cross-linking was less crucial than disulfide cross-linking. Thus, a desired level of alkali is required for improving the textural structures, functional properties (rehydration time and water absorption) and mouthfeel of wheat gluten with more porosity and puffed structure and a higher level of alkali concentration could result in the inhibition of the fibrous structure of gluten extrudate.
Pietsch et al. (181) studied the effect of process conditions (screw and die condition) on wheat gluten polymerization during high moisture extrusion cooking and reported that polymerization conditions were affected by process conditions in the screw section such as temperature at extruder exit and extruder pressure at extruder exit and specific mechanical energy (SME) inputs. The authors noted a significant change in gluten polymerization upon 90-160°C extruder temperature leading to a perceivable anisotropic structure of gluten protein, whereas specific mechanical energy (SME) of inputs and barrel pressure in the investigated range (1.5-3.5 MPa and 32-206 kJ/kg SME) have any significant effect on the wheat gluten polymerization. Screw speed within a limit positively affects the texturization of vegetable proteins. A screw speed of 250-350 rpm was reported to improve the texturization of peanut proteins, whereas increasing the speed further to 450 rpm caused poor texture with a weak fibrous structure (182).
5.1.1 Low moisture extrusion
Low moisture extrusion is commonly used in the food industry with a moisture content of below 30-32% on a wet basis. Low moisture extrusion has a shorter die and a higher temperature (183). Due to the application of high temperatures and shear force, low moisture extrusion has a profound impact on the quality characteristics of the extruded product such as expansion and microstructure (184). By proper selection of processing conditions and feed composition, these quality characteristics can be altered to the desired level. Beck et al. (183) observed increased specific mechanical energy inputs, bulk densities, and air cell densities during low moisture extrusion of pea proteins and pea fiber-fortified rice starch blends as compared to pure rice starch. At higher protein content (42%), a decreased sectional expansion index was recorded due to the distribution of protein and starch in thin layers within the extrudate (183).
5.1.2 High moisture extrusion
High moisture extrusion is widely used for texturizing non-meat proteins for the production of meat analogs (185). High moisture extrusion cooking is characterized by lesser mechanical energy dissipation and higher heat transfer via a larger barrel surface (186). The mass is processed into a dense with nil or minimally expanded strand and immediately cooled down to 100°C prior to its release into the atmosphere. It helps to prevent evaporation of water, expansion, and disruption into small pieces (187). With the help of twin-screw extruders consisting of a long-heated barrel and cooling die channel to cook, form, and solidify the strand, it is possible to prepare mass with moisture content up to 75% during the development of meat analogs (188). In the extruder barrel, two corotating intermeshing screws mix water into this product leading to a paste-like product (186). During this process, heat transfer takes place from barrel walls, and dissipating mechanical energy increase temperatures of 120-160°C with pressure increase in the barrel (188, 189). The elongated barrel improves the retention time of the mass and increases the barrel surface; consequently, improving heat transfer. Upon reaching the mass to die, the mass flows a laminar flow through the cooling channel, cools down, and forms a solid anisotropic structure (187).
High moisture extrusion cooking is commonly used to produce meat analogs by producing desirable texture/fibrous structure to vegetable proteins at a high temperature needed to denature vegetable proteins by the shearing force of screw rotation inside the barrel leading to the unfolding of peptide bonds and the destruction of the three-dimensional structure of proteins resulting in the formation of cross-links of hydrogen, amide, and disulfide bonds between denatured proteins (177).
High moisture extrusion has a long cooling die, facilitating higher texturization, density, elasticity, and retention of nutrients at a lower temperature. The high moisture extrudates had post-processing challenges such as lower aroma, taste, and higher storage cost, thus requiring suitable technological interventions. Protein texturization in dry extrusion (20-40% moisture) caused by superheated vapor and a sudden pressure drop inside the viscoelastic melt facilitates the production of puffing while passing through the die (153, 154) whereas wet extrusion (50-70% moisture) leads to fibrous structure similar to meat.
Wolz and Kulozik (190) reported that by suitable high moisture extrusion processing, protein aggregates of desirable attributes can be formed such as microparticulate in whey proteins extruded with the help of co-rotating twin-screw, aggregate size determined by specific mechanical energy input (SME) which further determined by mass flow and screw speed. The size of aggregates (protein-protein interaction) and micro-particle formation without significant denaturation of proteins during high moisture extrusion is determined by the thermal folding of proteins followed by aggregation (190). Under high moisture extrusion processing of soy, wheat, and pea protein aggregates, disulfide bond formation plays a significant role in determining texture or aggregates (173).
The reaction rate of gluten proteins is affected by barrel temperature, moisture content, and shear force. During low moisture extrusion of wheat gluten, increasing moisture content from 20 to 40% and increasing shear force (mainly for degradation reactions) were observed significantly increase the reaction rate under a closed cavity rheometer (191). With the improvement in the design of extruders and the upgradation of equipment, it became possible to apply some novel and emerging technologies in extrusion, such as supercritical fluid (SCF) extrusion, by utilizing supercritical carbon dioxide gas as a replacement for steam.
A supercritical fluid is a form of dense, compressed gas that has high mass transfer, penetration, and lower viscosity similar to the gas phase at the same time exhibiting solvent power, high density, and decreased surface tension similar to the liquid phase above critical temperature and pressure (13, 14). Supercritical carbon dioxide (SC-CO2) application preserved the nutritive value of extrudates, especially heat-labile vitamins and micronutrients, due to its low critical temperature (Tc-31°C). Extrudates with better conformation, smoother surface, and uniform cellular structure are obtained by supercritical fluid extrusion due to increased nucleation and decreased gas diffusion (192). Combining the extruder with a 3D printer has great potential in designing and producing plant-based meat analogs with desirable (193).
The overview of high-moisture extrusion process of peanut protein from the aspect of the energy input orders and amount are presented in Figure 2.
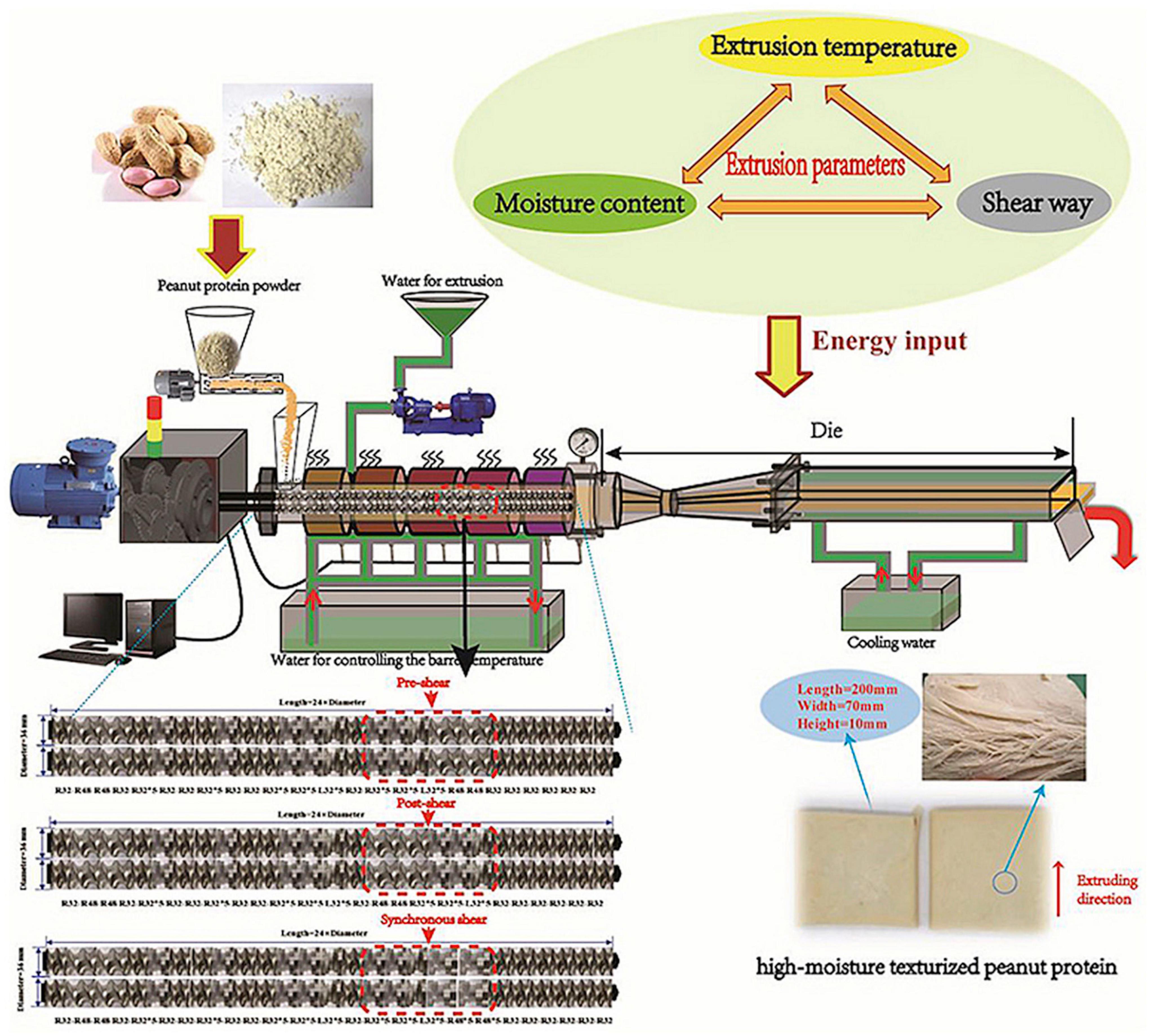
Figure 2. A new insight into the high-moisture extrusion process of peanut protein: from the aspect of the energy input orders and amount [Adapted from Zhang et al. (194)].
“Reprinted from Journal of Food Engineering, 264, Zhang, J., Liu, L., Jiang, Y., Faisal, S., & Wang, Q., A new insight into the high-moisture extrusion process of peanut protein: From the aspect of the orders and amount of energy input, 109668, Copyright (2020), with permission from Elsevier.”
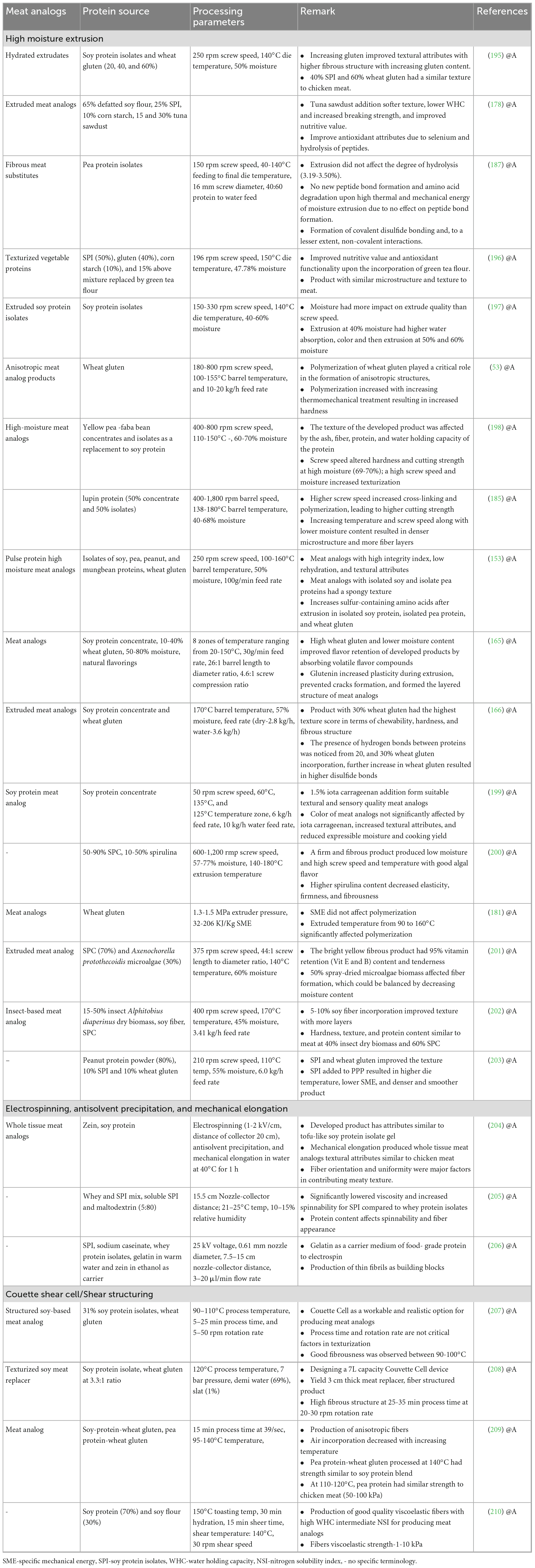
Various recent processing technologies used for formulating meat analogs are presented in Table 3.
5.2 Spinning
This technology is explored as a novel method for producing ultrathin fibers of vegetable proteins for the development of structured anisotropic meat analogs. The electrospinning process and fiber characteristics are influenced by solution properties such as surface tension, viscosity, and conductivity, and spinnerets operating parameters such as spinneret-collector distance, humidity, and temperature (211). The considerable quantity of waste generation, the requirement of low pH, high salt concentration, and the utilization of chemical additives make this process quite complicated.
Electrospinning of fibers comprises ejecting a polymer solution from a spinneret at high voltage (1-2 kV/cm) toward a collector under electrostatic repulsion. While in the air, its bending becomes unstable and stretches into thin microfibers. Mattice and Marangoni (59) explored the possibility of producing low-cost zein protein-based meat analogs by using electrospinning, antisolvent precipitation, and mechanical elongation technologies. Authors (59, 204) produced ultrathin zein fibers with comparatively lower cost and lower consumption of ethanol by using a 25% (w/w) zein solution in 70:30 ethanol in water loaded in stainless steel spinneret fixed with a 16-gauge syringe at a 0.5 mL/h flow rate by infusion pump operated at 20 kV and maintaining 20 cm distance from collector to spinneret. An instantaneous fibrous network of zein protein was prepared by antisolvent precipitation by incorporating water in excess directly into the ethanol-zein solution. Mechanical elongation of zein fibers was accomplished by dispersing zein in water and incubating at 40°C for 1h for network formation.
5.3 Couette shear cell
This is a novel technology based on flow-induced structuring/texturization of plant proteins by using a shear cell consisting of a cone-plate rheometer consisting of a stationary top cone and a rotating bottom cone (208). In these Shear Cells, proteins are aligned and produce well-defined fibrous structures upon shear force and heat application. The scalability, batch processing, and variation in shear rate throughout the Shear Cell with increasing distance between radius and cone remain major hurdles in the industrialization of this technology for the production of meat analogs. Couette or concentric cylinder shear cells are used to texturize vegetable proteins by shear bindings, formation of layered structures, and their orientation toward shear flow under the effect of shear-induced concentration fluctuations.
Couette cells (CC) are based on the concept of concentric cylinder rheometers and are used for texturing plant proteins by shear force. Krintiras et al. (208) scaled up the laboratory scale CC with a similar basic principle of viscous fluid flow between two surfaces, moving tangentially relative to one another. The CC consists of 4 main parts viz., an outer stationary cylinder which can be axially displaced with housing and removable lid to access inner half material, an inner rotating cylinder connected to the shaft via a rheodrive unit to control the angular velocity of rotating inner cylinder. The sampling material as plant proteins was placed in the shearing zone, a space between outer and inner cylinders. The processing conditions (pressure, torque response, and specific mechanical energy) are regulated by the rheodrive unit. Steam is used as a heating medium, and a pressure of 7 bar should be maintained throughout the processing. Dekkers et al. (212) developed a fibrous structure of a pectin/soy protein isolate blend under shear-induced structuring. The elongated pectin fibers were oriented toward the direction of shear force, with the length of the fiber depending upon the temperature and pectin concentration.
5.4 Additive manufacturing/3D printing
Three-dimensional/3 D printing (3DP) technology, also known as additive manufacturing (AM), rapid manufacturing, rapid prototyping, freedom fabrication, and solid free-form fabrication (SFE), is a novel technology that has vast potential in creating instrumental change in food and agricultural sector (193). It is based on additive manufacturing to develop products from digital Computer-Aided Design (CAD) software. By utilizing this technology, plant proteins can be given desired fibrous and complex muscle-like shapes.
The preparation of high-quality meat analog warrants the utilization of appropriate ingredients through the application of advanced 3D technology to mimic the functional properties of conventional meat without negatively affecting the product features (213). It is possible to develop tailored animal protein-based structures and products with extraordinary flexibility in geometric designs, flavors, and textures, and customized nutrition. Production of 3D printed products requires a reduction in particle size and dilution of flavor, thus reducing the value of premium meat products. Alternatively, this technology could be a better option for utilizing low-value tough meat cuts and meat trimmings (214).
This technology is very energy efficient and sustainable due to the efficient utilization of raw materials with minimum or no waste in the process, easier compositional and nutritional control of the developed product, utilization of novel and exotic plant proteins, saving labor and quick, and supply chain management with transportation by shifting the production facility near to the consumption area (215, 216). For getting the desired efficiency of food bioprinting with high precision, it is required to have proper knowledge of printing parameters and other processing parameters such as rheological attributes, particle size, nozzle characteristics, speed, printing distance, multi-nozzle printers, and post-processing operations such as baking, steaming and frying (193, 215).
During the preparation of meat analogs, protein powder is mixed with water to form a paste, and this paste is structured to a meat-like texture layer by layer by a 3D printer (217). Enzymatic treatment of plant proteins is used to increase cross-link and provide good texture to the product. Recently NovaMeat ® has applied 3D printing technology to develop plant-based analogs of beef steaks, meatballs, burgers and nuggets by using micro-extrusion technology. The developed products had textural and sensory attributes equivalent to conventional products. The Spanish start-up claimed to formulate the biggest cell-based prototype in the world. The company has prepared a hybrid meat analog by mixing mammalian adipose cells with a biocompatible plant-based scaffold.
Shahbazi et al. (213) constructed 3D-printed reduced-fat meat analogs containing a highly porous structure by using reduced-fat soy-based emulsion gels. The authors reported the effect of biosurfactants on the crystalline structure and fibrous sensation of the constructs by reducing the friction coefficients. The reduced-fat meat analogs printed by dodecenyl succinylated inulin and ethyl (hydroxyethyl) cellulose had finer resolution as compared with the product formulated with acetylated and octenyl succinic anhydride modified starches (213).
5.5 Freeze structuring/Freeze alignment
The meat-like fibrous texture can be achieved by freezing the plant protein emulsion/slurry subsequently followed by ice-crystal formation. These ice crystals formed help in the development of a well-aligned and unique porous microstructure of plant proteins overlapped in a layered sheet like animal muscles (218, 219). The alignment of protein can be controlled by the proper direction of heat removal, temperature, and rate of freezing. The heat removal from a well-mixed protein solution results in the formation of an isotropic structure. However, if the heat is removed in one direction without mixing, it results in the formation of anisotropic structures. The frozen product is freeze-dried (by utilizing the principle of sublimation) to get the sheet-like structure containing parallel orientation of the proteins. Further, these aligned sheets of proteins are connected by applying a cohesive fibrous product (27, 219).
Plant-protein-based meat analogs similar in texture and sensory attributes to meat were prepared by using freeze alignment (43). The authors (43) achieved successful texturization of a mixture of pea protein and wheat protein (3:1) by (a)- Freezing the protein solution to produce ice crystals that are aligned perpendicular to the cooling surface, (b)- Development of parallel ice crystals zone and entrapping of protein molecules, (c)- Formation of elongated fiber without changing the moisture: protein ratio, (d)- Removal of water/moisture by freeze drying, thus leaving a rigid fibrous mass of protein, (e)- Rehydration of fibrous proteins with an aqueous solution containing fats, color, flavor, and stabilizers. However, the proper control and monitoring of several freezing conditions in this process remain a major hindrance at present (27).
6 Prospects and conclusion
The issue of texture and flavor of plant-based meat analogs is quite challenging, and various innovative processing technologies are increasingly being used for texturizing plant proteins similar to the fibrous texture of meat. The typical texture and flavor of the meat results from a very complex process of pH-mediated post-mortem glycolytic and enzymatic changes, comprising numerous compounds and each having its typical role in imparting texture, flavor, and appearance to meat. Further research is needed to explore various protein molecules and their potential role in fabricating the texture and flavor of plant proteins similar to their meat counterparts.
To impart proper meat-like appearance, texture, and sensory attributes, plant proteins are processed into fibrous or fibril texture and added with flavorings, binders, hydrocolloids, colorants, vitamins, and minerals. This high degree of processing and incorporation of a range of ingredients leads to various issues about food safety, permissible limits, clean labeling, efficiency and sustainability, cost, and risk of lack of consumers’ confidence in these products. The processing of plant proteins at higher temperatures and pressure could decrease the nutritive value by degrading heat-labile nutrients and warrants compensatory incorporation of these nutrients as Vit B1, Vit C, flavonoids, phenolics, changing the economic dimension of the production of plant-based meat analogs.
The plant-based meat analog sector is exploring, designing innovative technologies (such as electrospinning, three-dimensional/four-dimensional (3D/4D) food printing) and applying already available technologies (such as extrusion, shear pressing, mechanical elongation, and antisolvent extraction) in refining and improving texturizing of plant proteins at a lower environmental cost with suitable combinations of various plant proteins, the application of suitable carbohydrates, hydrocolloids or other non-protein sources. Although these technologies have achieved a significant technological breakthrough in producing fibrous texture and fibrils like meat, their proper popularization and scale-up are needed. Figure 3 represents various aspects of improving the functionality of plant proteins during the development of meat analogs.
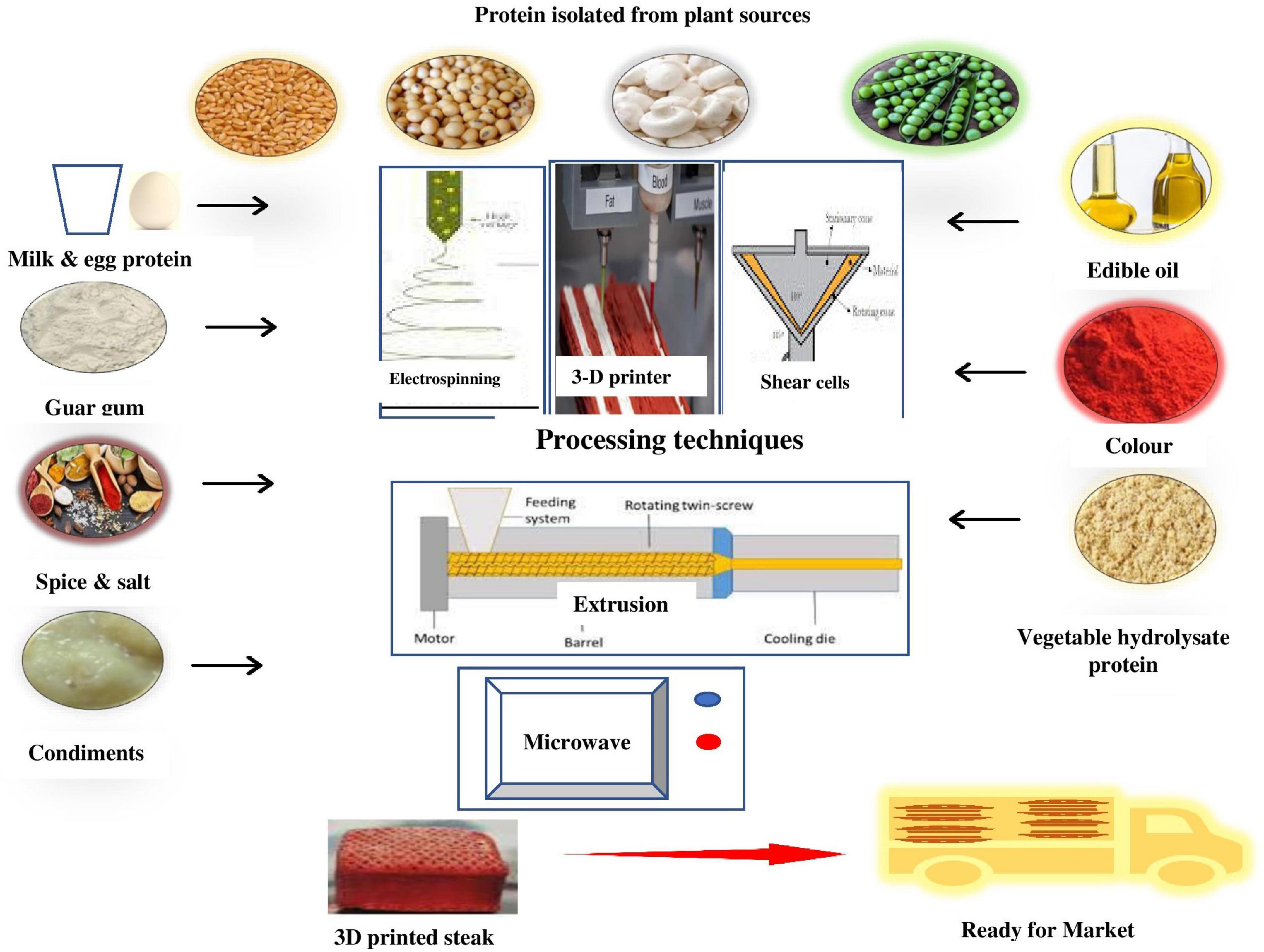
Figure 3. Various aspects of improving the functionality of plant proteins through the development of meat analogs.
Harvesting vast non-conventional protein sources such as oilseed co-products, minor cereals, pseudocereals, microalgae, fungi, and leaves have great potential in ensuring sustainability in the food sector. Leaf protein, oilseed industry co-products, microalgae, and single-cell proteins (SCP) have vast potential and, if harnessed suitably, could solve the issue of sustainability and food security to a great extent in the near future. Improving protein functionality, such as water binding capacity, gelling, and emulsifying properties are also crucial for structuring the plant-based meat analogs.
There is a need to scale up other promising innovative technologies such as 3D/4D food printing, Couvette Shear Cell, and electrospinning. There is a focus on improving the overall process efficiency of the extrusion and fiber formation by lowering energy requirements, minimum or no waste generation, and ease of operations.
Author contributions
NS, S-JL, and AS: conceptualization. PK, MA, AV, PU, NM, AA, and MH: writing—original draft. UK, NS, S-JL, and AS: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (2022R1F1A10750021122182102130101) and Himalayan Bioresource Mission, Department of Biotechnology, Government of India, New Delhi (No. BT/PR45189/NER/95/1895/2022).
Acknowledgments
PK was thankful to the Indian Council of Agricultural Research, New Delhi, India, for providing the Netaji-Subhas ICAR International Fellowship for pursuing his doctoral study at Universiti Putra Malaysia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kumar P, Mehta N, Malav O, Verma A, Umraw P, Kanth M. The structure of meat analogs. Encycl Food Chem. (2019) 3:105–9. doi: 10.1016/B978-0-08-100596-5.21705-8
2. Nasrabadi M, Doost A, Mezzenga R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. (2021) 118:106789. doi: 10.1016/J.FOODHYD.2021.106789
3. Kumar P, Sharma N, Sharma S, Mehta N, Verma A, Chemmalar S, et al. In-vitro meat: a promising solution for sustainability of meat sector. J Anim Sci Technol. (2021) 63:693–724. doi: 10.5187/jast.2021.e85
4. Sharma M, Kaur S, Kumar P, Mehta N, Umaraw P, Ghosh S. Development, prospects, and challenges of meat analogs with plant-based alternatives. In: Singh A, Kirun Patruni, Singh V editors. Recent Advances in Food Biotechnology. Singapore: Springer Nature Singapore (2022). p. 275–99. doi: 10.1007/978-981-16-8125-7_14
5. Kumar P, Chatli M, Mehta N, Singh P, Malav O, Verma A. Meat analogues: health promising sustainable meat substitutes. Crit Rev Food Sci Nutr. (2017) 57:923–32. doi: 10.1080/10408398.2014.939739
6. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the anthropocene: the EAT–lancet commission on healthy diets from sustainable food systems. Lancet. (2019) 393:447–92. doi: 10.1016/S0140-6736(18)31788-4
7. Aiking H, de Boer J. The next protein transition. Trends Food Sci Technol. (2020) 105:515–22. doi: 10.1016/J.TIFS.2018.07.008
8. Kumar P, Mehta N, Abubakar A, Verma A, Kaka U, Sharma N, et al. Potential alternatives of animal proteins for sustainability in the food sector. Food Rev Int. (2022) 1–26. doi: 10.1080/87559129.2022.2094403
9. Kumar P, Abubakar A, Verma A, Umaraw P, Nizam M, Mehta N, et al. New insights in improving sustainability in meat production: opportunities and challenges. Crit Rev Food Sci Nutr. (2022) 1–29. doi: 10.1080/10408398.2022.2096562
10. Poore J, Nemecek T. Reducing food’s environmental impacts through producers and consumers. Science. (2018) 360:987–92. doi: 10.1126/science.aaq0216
11. OECD, Food and Agriculture Organization of the United Nations. OECD- FAO Agricultural Outlook 2021–2030. Organisation for Economic Co-operation and Development. (2021). Available online at: https://doi.org/10.1787/19428846-en (accessed August 15, 2022).
12. Bryant C, Szejda K, Parekh N, Desphande V, Tse B. A survey of consumer perceptions of plant-based and clean meat in the USA, India, and China. Front Sustain Food Syst. (2019) 3:11. doi: 10.3389/fsufs.2019.00011
13. Awad A, Kumar P, Ismail-Fitry M, Jusoh S, Aziz M, Sazili A. Green extraction of bioactive compounds from plant biomass and their application in meat as natural antioxidant. Antioxidants. (2021) 10:1465. doi: 10.3390/antiox10091465
14. Awad A, Kumar P, Ismail-Fitry M, Jusoh S, Ab Aziz M, Sazili A. Overview of plant extracts as natural preservatives in meat. J Food Process Preserv. (2022) 46:e16796. doi: 10.1111/jfpp.16796
15. Kumar P, Abubakar A, Ahmed M, Hayat M, Kaka U, Pateiro M, et al. Pre-slaughter stress mitigation in goats: prospects and challenges. Meat Sci. (2023) 195:109010. doi: 10.1016/j.meatsci.2022.109010
16. Masset G, Vieux F, Verger E, Soler L, Touazi D, Darmon N. Reducing energy intake and energy density for a sustainable diet: a study based on self-selected diets in French adults. Am J Clin Nutr. (2014) 99:1460–9. doi: 10.3945/ajcn.113.077958
17. Gallus S, Bosetti C. Meat consumption is not tobacco smoking. Int J Cancer. (2016) 138:2539–40. doi: 10.1002/ijc.30010
18. Bhat Z, Kumar S, Bhat H. In vitro meat: a future animal-free harvest. Crit Rev Food Sci Nutr. (2017) 57:782–9. doi: 10.1080/10408398.2014.924899
19. Mehta N, Kumar P, Verma A, Umaraw P, Ranjan R. Missing the real culprit? Insight on the water footprint of the meat industry. Fleishwirtschaft Int. (2021) 2:54–8.
20. Thrane M, Paulsen PV, Orcutt M, Krieger T. Chapter 2-soy protein: impacts, production, and applications. In: Nadathur SR, Wanasundara JPD, Scanlin L editors. Sustainable Protein Sources. Amsterdam: Elsevier (2017). p. 23–45. doi: 10.1016/B978-0-12-802778-3.00002-0
21. Hadi J, Brightwell G. Safety of alternative proteins: technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein. Foods. (2021) 10:1226. doi: 10.3390/foods10061226
22. Yesuraj D, Deepika C, Ravishankar G, Ranga Rao A. Seaweed-based recipes for food, health-food applications, and innovative products including meat and meat analogs. In: Ranga Rao A, Ravishankar GA editors. Sustainable Global Resources of Seaweeds. (Vol. 2), Cham: Springer International Publishing (2022). p. 267–92. doi: 10.1007/978-3-030-92174-3_14
23. Kurek M, Onopiuk A, Pogorzelska-Nowicka E, Szpicer A, Zalewska M, Półtorak A. Novel protein sources for applications in meat-alternative products—insight and challenges. Foods. (2022) 11:957. doi: 10.3390/foods11070957
24. Trocino A, Zomeño C, Birolo M, Di Martino G, Stefani A, Bonfanti L, et al. Impact of pre-slaughter transport conditions on stress response, carcass traits, and meat quality in growing rabbits. Meat Sci. (2018) 146:68–74. doi: 10.1016/j.meatsci.2018.07.035
25. Delshadi R, Bahrami A, Tafti A, Barba F, Williams L. Micro and nano-encapsulation of vegetable and essential oils to develop functional food products with improved nutritional profiles. Trends Food Sci Technol. (2020) 104:72–83. doi: 10.1016/j.tifs.2020.07.004
26. Kyriakopoulou K, Keppler J, van der Goot A. Functionality of ingredients and additives in plant-based meat analogues. Foods. (2021) 10:600. doi: 10.3390/foods10030600
27. Singh M, Trivedi N, Enamala M, Kuppam C, Parikh P, Nikolova M, et al. Plant-based meat analogue (PBMA) as a sustainable food: a concise review. Eur Food Res Technol. (2021) 247:2499–526. doi: 10.1007/s00217-021-03810-1
28. López D, Ingrassia R, Busti P, Wagner J, Boeris V, Spelzini D. Effects of extraction pH of chia protein isolates on functional properties. LWT. (2018) 97:523–9. doi: 10.1016/J.LWT.2018.07.036
29. Kumar P, Sharma M, Abubakar A, Nizam bin Hayat M, Ahmed M, Kaka U, et al. Soybean: sustainability issues. In: Reference Module in Food Science. Amsterdam: Elsevier (2023). doi: 10.1016/B978-0-12-823960-5.00021-4
30. Kumar M, Tomar M, Punia S, Dhakane-Lad J, Dhumal S, Changan S, et al. Plant-based proteins and their multifaceted industrial applications. LWT. (2022) 154:112620. doi: 10.1016/J.LWT.2021.112620
31. Zhang T, Dou W, Zhang X, Zhao Y, Zhang Y, Jiang L, et al. The development history and recent updates on soy protein-based meat alternatives. Trends Food Sci Technol. (2021) 109:702–10. doi: 10.1016/j.tifs.2021.01.060
32. Sha L, Xiong Y. Plant protein-based alternatives of reconstructed meat: science, technology, and challenges. Trends Food Sci Technol. (2020) 102:51–61. doi: 10.1016/j.tifs.2020.05.022
33. Stanojevic S, Barać M, Pešić M, Vucelic-Radovic BV. Protein composition and textural properties of inulin-enriched tofu produced by hydrothermal process. LWT. (2020) 126:109309. doi: 10.1016/J.LWT.2020.109309
34. Kyriakopoulou K, Dekkers B, van der Goot A. Chapter 6-plant-based meat analogues. In: Galanakis CM editor. Sustainable meat Production and Processing. (2019). p. 103–26. doi: 10.1016/B978-0-12-814874-7.00006-7
35. Vatansever S, Tulbek C, Riaz N. Low- and high-moisture extrusion of pulse proteins as plant-based meat ingredients: a review. Cereal Foods World. (2020) 65. doi: 10.1094/CFW-65-4-0038
36. Doss A, Esther A, Rajalakshmi R. Influence of UV-B treatment on the accumulation of free phenols and tannins in the legumes of Abrus precatorius L. and Vigna mungo (L.) Hepper. Phytomed Plus. (2022) 2:100189. doi: 10.1016/J.PHYPLU.2021.100189
37. Bühler J, Dekkers B, Bruins M, van der Goot A. Modifying Faba bean protein concentrate using dry heat to increase water holding capacity. Foods. (2020) 9:1077. doi: 10.3390/foods9081077
38. Fiorentini M, Kinchla A, Nolden A. Role of sensory evaluation in consumer acceptance of plant-based meat analogs and meat extenders: a scoping review. Foods. (2020) 9:1334. doi: 10.3390/foods9091334
39. Jones O. Recent advances in the functionality of non-animal-sourced proteins contributing to their use in meat analogs. Curr Opin Food Sci. (2016) 7:7–13. doi: 10.1016/J.COFS.2015.08.002
40. do Carmo C, Silventoinen P, Nordgård C, Poudroux C, Dessev T, Zobel H, et al. Is dehulling of peas and faba beans necessary prior to dry fractionation for the production of protein- and starch-rich fractions? Impact on physical properties, chemical composition and techno-functional properties. J Food Eng. (2020) 278:109937. doi: 10.1016/J.JFOODENG.2020.109937
41. Wang Y, Guldiken B, Tulbek M, House J, Nickerson M. Impact of alcohol washing on the flavour profiles, functionality and protein quality of air classified pea protein enriched flour. Food Res Int. (2020) 132:109085. doi: 10.1016/J.FOODRES.2020.109085
42. Zhu H, Tang H, Cheng Y, Li Z, Tong L. Potential of preparing meat analogue by functional dry and wet pea (Pisum sativum) protein isolate. LWT. (2021) 148:111702. doi: 10.1016/j.lwt.2021.111702
43. Yuliarti O, Kiat Kovis T, Yi N. Structuring the meat analogue by using plant-based derived composites. J Food Eng. (2021) 288:110138. doi: 10.1016/j.jfoodeng.2020.110138
44. Nakamura A, Furuta H, Maeda H, Takao T, Nagamatsu Y. Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Biosci Biotechnol Biochem. (2002) 66:1301–13. doi: 10.1271/bbb.66.1301
45. Zhang J, Liu L, Jiang Y, Shah F, Xu Y, Wang Q. High-moisture extrusion of peanut protein-/carrageenan/sodium alginate/wheat starch mixtures: effect of different exogenous polysaccharides on the process forming a fibrous structure. Food Hydrocoll. (2020) 99:105311. doi: 10.1016/j.foodhyd.2019.105311
46. Vogelsang-O’Dwyer M, Petersen I, Joehnke M, Sørensen J, Bez J, Detzel A, et al. Comparison of Faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: techno-functional, nutritional and environmental performance. Foods. (2020) 9:322. doi: 10.3390/foods9030322
47. Malik M, Saini C. Improvement of functional properties of sunflower protein isolates near isoelectric point: application of heat treatment. LWT. (2018) 98:411–7. doi: 10.1016/J.LWT.2018.09.009
48. Karefyllakis D, Octaviana H, van der Goot A, Nikiforidis CV. The emulsifying performance of mildly derived mixtures from sunflower seeds. Food Hydrocoll. (2019) 88:75–85. doi: 10.1016/J.FOODHYD.2018.09.037
49. Malik M, Saini C. Rheological and structural properties of protein isolates extracted from dephenolized sunflower meal: effect of high intensity ultrasound. Food Hydrocoll. (2018) 81:229–41. doi: 10.1016/J.FOODHYD.2018.02.052
50. Li Q, Wang Z, Dai C, Wang Y, Chen W, Ju X, et al. Physical stability and microstructure of rapeseed protein isolate/gum Arabic stabilized emulsions at alkaline pH. Food Hydrocoll. (2019) 88:50–7. doi: 10.1016/J.FOODHYD.2018.09.020
51. Shewry P, Halford N, Belton P, Tatham A. The structure and properties of gluten: an elastic protein from wheat grain. Philos Trans R Soc London Ser B Biol Sci. (2002) 357:133–42.
52. Dangi P, Chaudhary N, Khatkar B. Rheological and microstructural characteristics of low molecular weight glutenin subunits of commercial wheats. Food Chem. (2019) 297:124989. doi: 10.1016/J.FOODCHEM.2019.124989
53. Pietsch V, Werner R, Karbstein H, Emin M. High moisture extrusion of wheat gluten: relationship between process parameters, protein polymerization, and final product characteristics. J Food Eng. (2019) 259:3–11. doi: 10.1016/J.JFOODENG.2019.04.006
54. Girard A, Awika J. Effects of edible plant polyphenols on gluten protein functionality and potential applications of polyphenol–gluten interactions. Compr Rev Food Sci Food Saf. (2020) 19:2164–99. doi: 10.1111/1541-4337.12572
55. Yano H. Recent practical researches in the development of gluten-free breads. NPJ Sci Food. (2019) 3:7. doi: 10.1038/s41538-019-0040-1
56. Keppler J, Schwarz K, van der Goot A. Covalent modification of food proteins by plant-based ingredients (polyphenols and organosulphur compounds): a commonplace reaction with novel utilization potential. Trends Food Sci Technol. (2020) 101:38–49. doi: 10.1016/J.TIFS.2020.04.023
57. Puerta P, Garzón R, Rosell C, Fiszman S, Laguna L, Tárrega A. Modifying gluten-free bread’s structure using different baking conditions: impact on oral processing and texture perception. LWT. (2021) 140:110718. doi: 10.1016/J.LWT.2020.110718
58. Jeong S, Kim H, Lee S. Rheological and secondary structural characterization of rice flour-zein composites for noodles slit from gluten-free sheeted dough. Food Chem. (2017) 221:1539–45. doi: 10.1016/J.FOODCHEM.2016.10.139
59. Mattice K, Marangoni A. Evaluating the use of zein in structuring plant-based products. Curr Res Food Sci. (2020) 3:59–66. doi: 10.1016/J.CRFS.2020.03.004
60. Erickson D, Dunbar M, Hamed E, Ozturk O, Campanella O, Keten S, et al. Atomistic modeling of peptide aggregation and β-sheet structuring in corn zein for viscoelasticity. Biomacromolecules. (2021) 22:1856–66. doi: 10.1021/acs.biomac.0c01558
61. Glusac J, Fishman A. Enzymatic and chemical modification of zein for food application. Trends Food Sci Technol. (2021) 112:507–17. doi: 10.1016/j.tifs.2021.04.024
62. Detchewa P, Prasajak P, Phungamngoen C, Sriwichai W, Naivikul O, Moongngarm A. Substitution of rice flour with rice protein improved quality of gluten-free rice spaghetti processed using single screw extrusion. LWT. (2022) 153:112512. doi: 10.1016/j.lwt.2021.112512
63. Chanput W, Theerakulkait C, Nakai S. Antioxidative properties of partially purified barley hordein, rice bran protein fractions and their hydrolysates. J Cereal Sci. (2009) 49:422–8. doi: 10.1016/j.jcs.2009.02.001
64. Kumar Singh A, Singh R, Subramani R, Kumar R, Wankhede DP. Molecular approaches to understand nutritional potential of coarse cereals. Curr Genomics. (2016) 17:177–92. doi: 10.2174/1389202917666160202215308
65. Han S, Chee K, Cho S. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. (2015) 172:766–9. doi: 10.1016/j.foodchem.2014.09.127
66. Dang T, Vasanthan T. Modification of rice bran dietary fiber concentrates using enzyme and extrusion cooking. Food Hydrocoll. (2019) 89:773–82. doi: 10.1016/j.foodhyd.2018.11.024
67. Xiao Z, Jiang R, Huo J, Wang H, Li H, Su S, et al. Rice bran meat analogs: relationship between extrusion parameters, apparent properties and secondary structures. LWT. (2022) 163:113535. doi: 10.1016/j.lwt.2022.113535
68. Lee J, Choi I, Han J. Construction of rice protein-based meat analogues by extruding process: effect of substitution of soy protein with rice protein on dynamic energy, appearance, physicochemical, and textural properties of meat analogues. Food Res Int. (2022) 161:111840. doi: 10.1016/j.foodres.2022.111840
69. Bakhsh A, Lee E, Bakry A, Rathnayake D, Son Y, Kim S, et al. Synergistic effect of lactoferrin and red yeast rice on the quality characteristics of novel plant-based meat analog patties. LWT. (2022) 171:114095. doi: 10.1016/j.lwt.2022.114095
70. Hashempour-Baltork F, Khosravi-Darani K, Hosseini H, Farshi P, Reihani S. Mycoproteins as safe meat substitutes. J Clean Prod. (2020) 253:119958. doi: 10.1016/j.jclepro.2020.119958
71. Finnigan T, Wall B, Wilde P, Stephens F, Taylor S, Freedman M. Mycoprotein: the future of nutritious nonmeat protein, a symposium review. Curr Dev Nutr. (2019) 3:nzz021. doi: 10.1093/cdn/nzz021
72. European Commission. A Farm to Fork Strategy-For a Fair, Healthy and Environmentally-Friendly Food System. (2020). Available online at: https://www.eufic.org/en/food-production/article/the-eu-farm-to-forkstrategy-can-we-make-the-european-food-system-healthier-and-sustainable (accessed August 15, 2022).
73. Arora B, Kamal S, Sharma V. Effect of binding agents on quality characteristics of mushroom based sausage analogue. J Food Process Preserv. (2017) 41:e13134. doi: 10.1111/jfpp.13134
74. Kumar P, Sharma B, Kumar R. Optimization of the level of mushroom in analogue meat nuggets. J Meat Sci. (2011) 7:53–5.
75. Kumar P, Sharma B, Kumar R, Kumar A. Optimization of the level of wheat gluten in analogue meat nuggets. Indian J Vet Res. (2012) 21:54–9.
76. Kumar P, Kumar R. Product profile comparison of analogue meat nuggets versus chicken nuggets. Fleischwirtschaft Int J Meat Prod Meat Process. (2011) 1:72–4.
77. Kaspchak E, de Oliveira M, Simas F, Franco C, Silveira J, Mafra M, et al. Determination of heat-set gelation capacity of a quinoa protein isolate (Chenopodium quinoa) by dynamic oscillatory rheological analysis. Food Chem. (2017) 232:263–71. doi: 10.1016/J.FOODCHEM.2017.04.014
78. Schut J. Analyzing Potato Protein Isolates for Their Potential in Developing Meat Analogues. (2020). Available online at: http://lup.lub.lu.se/student-papers/record/9016701 (accessed August 15, 2022).
79. Beniwal A, Singh J, Kaur L, Hardacre A, Singh H. Meat analogs: protein restructuring during thermomechanical processing. Compr Rev Food Sci Food Saf. (2021) 20:1221–49. doi: 10.1111/1541-4337.12721
80. Boukid F. Plant-based meat analogues: from niche to mainstream. Eur Food Res Technol. (2021) 247:297–308. doi: 10.1007/s00217-020-03630-9
81. Glusac J, Davidesko-Vardi I, Isaschar-Ovdat S, Kukavica B, Fishman A. Gel-like emulsions stabilized by tyrosinase-crosslinked potato and zein proteins. Food Hydrocoll. (2018) 82:53–63. doi: 10.1016/J.FOODHYD.2018.03.046
82. van der Weele C, Feindt P, van der Goot A, van Mierlo B, van Boekel M. Meat alternatives: an integrative comparison. Trends Food Sci Technol. (2019) 88:505–12. doi: 10.1016/J.TIFS.2019.04.018
83. Tenorio A, Kyriakopoulou K, Suarez-Garcia E, van den Berg C, van der Goot A. Understanding differences in protein fractionation from conventional crops, and herbaceous and aquatic biomass – consequences for industrial use. Trends Food Sci Technol. (2018) 71:235–45. doi: 10.1016/J.TIFS.2017.11.010
84. Bux F, Chisti Y. Algae Biotechnology: Products and Processes. 1st ed. Cham: Springer (2016). p. 344. doi: 10.1007/978-3-319-12334-9
85. Multari S, Licciardello C, Caruso M, Martens S. Monitoring the changes in phenolic compounds and carotenoids occurring during fruit development in the tissues of four citrus fruits. Food Res Int. (2020) 134:109228. doi: 10.1016/j.foodres.2020.109228
86. Usda. Dietary guidelines advisory committee reports. Nutr Today. (2015) 20:8–15. doi: 10.1097/00017285-198505000-00002
87. Kołodziejczak K, Onopiuk A, Szpicer A, Poltorak A. Meat analogues in the perspective of recent scientific research: a review. Foods. (2021) 11:105. doi: 10.3390/foods11010105
88. Van Krimpen M, Bikker P, der Meer I, der Peet-Schwering C, Vereijken J. Cultivation, Processing and Nutritional Aspects for pigs and Poultry of European Protein Sources as Alternatives for Imported Soybean Products. Lelystad: Wageningen UR Livestock Research (2013).
89. Safi C, Ursu A, Laroche C, Zebib B, Merah O, Pontalier P, et al. Aqueous extraction of proteins from microalgae: effect of different cell disruption methods. Algal Res. (2014) 3:61–5. doi: 10.1016/J.ALGAL.2013.12.004
90. Postma P, Pataro G, Capitoli M, Barbosa M, Wijffels R, Eppink M, et al. Selective extraction of intracellular components from the microalga Chlorella vulgaris by combined pulsed electric field–temperature treatment. Bioresour Technol. (2016) 203:80–8. doi: 10.1016/J.BIORTECH.2015.12.012
91. Al-Zuhair S, Ashraf S, Hisaindee S, Al Darmaki N, Battah S, Svistunenko D, et al. Enzymatic pre-treatment of microalgae cells for enhanced extraction of proteins. Eng Life Sci. (2017) 17:175–85. doi: 10.1002/elsc.201600127
92. Bals B, Dale B, Balan V. Recovery of leaf protein for animal feed and highvalue uses. In: CV Stevens editor. Biorefinery Co-Products: Phytochemicals, Primary Metabolites and Value-Added Biomass Processing. Hoboken, NJ: Wiley Online Library (2012). p. 179–97. doi: 10.1002/9780470976692.ch9
93. Harnedy P, FitzGerald R. Extraction of protein from the macroalga Palmaria palmata. LWT Food Sci Technol. (2013) 51:375–82. doi: 10.1016/j.lwt.2012.09.023
94. Angell A, Paul N, de Nys R. A comparison of protocols for isolating and concentrating protein from the green seaweed Ulva ohnoi. J Appl Phycol. (2017) 29:1011–26. doi: 10.1007/s10811-016-0972-7
95. Hettiarachchy N, Kannan A, Schäfer C, Wagner G. Gelling of plant based proteins. In: Bröckel U, Meier W, Wagner G editors. Product Design and Engineering. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA (2013). p. 221–45. doi: 10.1002/9783527654741.ch8
96. Lam R, Nickerson M. Food proteins: a review on their emulsifying properties using a structure–function approach. Food Chem. (2013) 141:975–84. doi: 10.1016/j.foodchem.2013.04.038
97. Schwenzfeier A, Wierenga P, Gruppen H. Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour Technol. (2011) 102:9121–7. doi: 10.1016/j.biortech.2011.07.046
98. Yang J, Zamani S, Liang L, Chen L. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll. (2021) 117:106678. doi: 10.1016/j.foodhyd.2021.106678
99. Kornet R, Penris S, Venema P, van der Goot A, Meinders M, van der Linden E. How pea fractions with different protein composition and purity can substitute WPI in heat-set gels. Food Hydrocoll. (2021) 120:106891. doi: 10.1016/j.foodhyd.2021.106891
100. Labrou N. Protein purification: an overview. In: Labrou N editor. Protein Downstream Processing. Methods in Molecular Biology. Totowa, NJ: Humana Press (2014). p. 3–10. doi: 10.1007/978-1-62703-977-2_1
101. Kumar P, Sharma N, Ranjan R, Kumar S, Bhat Z, Jeong D. Perspective of membrane technology in dairy industry: a review. Asian Australas J Anim Sci. (2013) 26:1347–58. doi: 10.5713/ajas.2013.13082
102. Castro-Muñoz R, Ruby-Figueroa R. Membrane technology for the recovery of high-added value compounds from meat processing coproducts. In: Galanakis CM editor. Sustainable Meat Production and Processing. Amsterdam: Elsevier (2019). p. 127–43. doi: 10.1016/B978-0-12-814874-7.00007-9
103. Yang X, Pistolozzi M, Lin Z. New trends in aggregating tags for therapeutic protein purification. Biotechnol Lett. (2018) 40:745–53. doi: 10.1007/s10529-018-2543-2
104. Beloborodov S, Bao J, Krylova S, Shala-Lawrence A, Johnson P, Krylov S. Aptamer facilitated purification of functional proteins. J Chromatogr B Analyt Technol Biomed Life Sci. (2018) 1073:201–6. doi: 10.1016/J.JCHROMB.2017.12.024
105. Mahmoudi Gomari M, Saraygord-Afshari N, Farsimadan M, Rostami N, Aghamiri S, Farajollahi M. Opportunities and challenges of the tag-assisted protein purification techniques: applications in the pharmaceutical industry. Biotechnol Adv. (2020) 45:107653. doi: 10.1016/j.biotechadv.2020.107653
106. Adachi T, Umboh M, Nemoto T, Major Z. Mechanical properties of epoxy resins filled with nano-silica particles. In: Matveenko VP, Krommer M, Belyaev AK, Irschik H editors. Dynamics and Control of Advanced Structures and Machines. Cham: Springer International Publishing (2017). p. 225–34. doi: 10.1007/978-3-319-43080-5_25
107. Nweke M, Rathore A, Bracewell D. Lifetime and aging of chromatography resins during biopharmaceutical manufacture. Trends Biotechnol. (2018) 36:992–5. doi: 10.1016/J.TIBTECH.2018.01.001
108. Wingfield P. Protein precipitation using ammonium sulfate. Curr Protoc Protein Sci. (2016) 84:F.1–3. doi: 10.1002/0471140864.psa03fs84
109. Novák P, Havlíček V. Protein extraction and precipitation. In: Ciborowski P, Silberring J editors. Proteomic Profiling and Analytical Chemistry: The Crossroads. Amsterdam: Elsevier (2016). p. 51–62. doi: 10.1016/B978-0-444-63688-1.00004-5
110. Ding Z, Cao X. Affinity precipitation of human serum albumin using a thermo-response polymer with an L-thyroxin ligand. BMC Biotechnol. (2013) 13:109. doi: 10.1186/1472-6750-13-109
111. Simpson R. Precipitation of proteins by polyethylenimine. Cold Spring Harb Protoc. (2006) 2006:pdb.prot4312. doi: 10.1101/pdb.prot4312
112. Iqbal M, Tao Y, Xie S, Zhu Y, Chen D, Wang X, et al. Aqueous two-phase system (ATPS): an overview and advances in its applications. Biol Proced Online. (2016) 18:18. doi: 10.1186/s12575-016-0048-8
113. Liu Z, Yu D, Li L, Liu X, Zhang H, Sun W, et al. Three-phase partitioning for the extraction and purification of polysaccharides from the immunomodulatory medicinal mushroom Inonotus obliquus. Molecules. (2019) 24:403. doi: 10.3390/molecules24030403
114. Ó’Fágáin C, Cummins P, O’Connor B. Gel-filtration chromatography. In: Walls D, Loughran S editors. Protein Chromatography. Methods in Molecular Biology. Totowa, NJ: Humana Press (2017). p. 15–25. doi: 10.1007/978-1-4939-6412-3_2
115. Chakraborty A, Puja R, Bose K. Protein purification by ion exchange chromatography. In: Bose K editor. Textbook on Cloning, Expression and Purification of Recombinant Proteins. Singapore: Springer Nature Singapore (2022). p. 173–98. doi: 10.1007/978-981-16-4987-5_7
116. McCue J. Chapter 25 theory and use of hydrophobic interaction chromatography in protein purification applications. Methods Enzymol. (2009) 463:405–14. doi: 10.1016/S0076-6879(09)63025-1
117. Fujita-Yamaguchi Y. Affinity chromatography of native and recombinant proteins from receptors for insulin and IGF-I to recombinant single chain antibodies. Front Endocrinol. (2015) 6:166. doi: 10.3389/fendo.2015.00166
118. Josic D, Kovac S. Reversed-phase high performance liquid chromatography of proteins. In: Coligan JE editor. Current Protocols in Protein Science. Hoboken, NJ: Wiley Online (2010). p. 61. doi: 10.1002/0471140864.ps0807s61
119. Schure M, Moran R. Size exclusion chromatography with superficially porous particles. J Chromatogr A. (2017) 1480:11–9. doi: 10.1016/j.chroma.2016.12.016
120. Selvaprakash K, Chen Y. Functionalized gold nanoparticles as affinity nanoprobes for multiple lectins. Colloids Surf B Biointerfaces. (2018) 162:60–8. doi: 10.1016/J.COLSURFB.2017.11.022
121. Liu S, Haller E, Horak J, Brandstetter M, Heuser T, Lämmerhofer M. Protein A- and protein G-gold nanoparticle bioconjugates as nano-immunoaffinity platform for human IgG depletion in plasma and antibody extraction from cell culture supernatant. Talanta. (2019) 194:664–72. doi: 10.1016/J.TALANTA.2018.10.079
122. Jahanban-Esfahlan A, Roufegarinejad L, Jahanban-Esfahlan R, Tabibiazar M, Amarowicz R. Latest developments in the detection and separation of bovine serum albumin using molecularly imprinted polymers. Talanta. (2020) 207:120317. doi: 10.1016/J.TALANTA.2019.120317
123. Yang Z, Chen J, Wang J, Zhang Q, Zhang B. Self-driven BSA surface imprinted magnetic tubular carbon nanofibers: fabrication and adsorption performance. ACS Sustain Chem Eng. (2020) 8:3241–52. doi: 10.1021/acssuschemeng.9b06829
124. Salimi K, Usta D, Koçer I, Çelik E, Tuncel A. Highly selective magnetic affinity purification of histidine-tagged proteins by Ni 2+ carrying monodisperse composite microspheres. RSC Adv. (2017) 7:8718–26. doi: 10.1039/C6RA27736E
125. Bohrer B. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci Hum Wellness. (2019) 8:320–9. doi: 10.1016/J.FSHW.2019.11.006
126. Kyriakopoulou K, Keppler J, van der Goot A, Boom R. Alternatives to meat and dairy. Annu Rev Food Sci Technol. (2021) 12:29–50. doi: 10.1146/annurev-food-062520-101850
127. Ma K, Greis M, Lu J, Nolden A, McClements D, Kinchla A. Functional performance of plant proteins. Foods. (2022) 11:594. doi: 10.3390/foods11040594
128. Singhal A, Karaca A, Tyler R, Nickerson M. Pulse proteins: from processing to structure-function relationships. In: Goyal AK editor. Grain Legumes. London: Intech Open (2016). p. 55. doi: 10.5772/64020
129. Boye J, Aksay S, Roufik S, Ribéreau S, Mondor M, Farnworth E, et al. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int. (2010) 43:537–46. doi: 10.1016/J.FOODRES.2009.07.021
130. Boye J, Zare F, Pletch A. Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int. (2010) 43:414–31. doi: 10.1016/J.FOODRES.2009.09.003
131. Aydemir L, Yemenicioĝlu A. Potential of Turkish Kabuli type chickpea and green and red lentil cultivars as source of soy and animal origin functional protein alternatives. LWT Food Sci Technol. (2013) 50:686–94. doi: 10.1016/J.LWT.2012.07.023
132. Berghout J, Boom R, van der Goot A. Understanding the differences in gelling properties between lupin protein isolate and soy protein isolate. Food Hydrocoll. (2015) 43:465–72. doi: 10.1016/J.FOODHYD.2014.07.003
133. Aryee A, Boye J. Comparative study of the effects of processing on the nutritional, physicochemical and functional properties of lentil. J Food Process Preserv. (2017) 41:e12824. doi: 10.1111/jfpp.12824
134. Kaur M, Singh N. Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars. Food Chem. (2007) 102:366–74. doi: 10.1016/J.FOODCHEM.2006.05.029
135. Butré C, Wierenga P, Gruppen H. Effects of ionic strength on the enzymatic hydrolysis of diluted and concentrated whey protein isolate. J Agric Food Chem. (2012) 60:5644–51. doi: 10.1021/jf301409n
136. Jeewanthi R, Lee N, Paik H. Improved functional characteristics of whey protein hydrolysates in food industry. Korean J Food Sci Anim Resour. (2015) 35:350–9. doi: 10.5851/kosfa.2015.35.3.350
137. Yi L, Lakemond C, Sagis L, Eisner-Schadler V, van Huis A, van Boekel M. Extraction and characterisation of protein fractions from five insect species. Food Chem. (2013) 141:3341–8. doi: 10.1016/j.foodchem.2013.05.115
138. Moure A, Sineiro J, Domínguez H, Parajó J. Functionality of oilseed protein products: a review. Food Res Int. (2006) 39:945–63. doi: 10.1016/J.FOODRES.2006.07.002
139. Bryant C, Julian McClements D. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci Technol. (1998) 9:143–51. doi: 10.1016/S0924-2244(98)00031-4
140. Ako K, Nicolai T, Durand D, Brotons G. Micro-phase separation explains the abrupt structural change of denatured globular protein gels on varying the ionic strength or the pH. Soft Matter. (2009) 5:4033–41.
141. Loveday S. Food proteins: technological, nutritional, and sustainability attributes of traditional and emerging proteins. Annu Rev Food Sci Technol. (2019) 10:311–39. doi: 10.1146/annurev-food-032818-121128
142. Lu B, Quillien L, Popineau Y. Foaming and emulsifying properties of pea albumin fractions and partial characterisation of surface-active components. J Sci Food Agric. (2000) 80:1964–72. doi: 10.1002/1097-0010(200010)80:133.0.CO;2-J
143. Papalamprou E, Doxastakis G, Kiosseoglou V. Chickpea protein isolates obtained by wet extraction as emulsifying agents. J Sci Food Agric. (2010) 90:304–13. doi: 10.1002/jsfa.3816
144. Mundi S, Aluko R. Physicochemical and functional properties of kidney bean albumin and globulin protein fractions. Food Res Int. (2012) 48:299–306. doi: 10.1016/J.FOODRES.2012.04.006
145. Sim S, Srv A, Chiang J, Henry C. Plant proteins for future foods: a roadmap. Foods. (2021) 10:1967. doi: 10.3390/foods10081967
146. Fetzer A, Herfellner T, Stäbler A, Menner M, Eisner P. Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed rapeseed press cake. Ind Crops Prod. (2018) 112:236–46. doi: 10.1016/J.INDCROP.2017.12.011
147. Lücke F, Fritz V, Tannhäuser K, Arya A. Controlled fermentation of rapeseed presscake by Rhizopus, and its effect on some components with relevance to human nutrition. Food Res Int. (2019) 120:726–32. doi: 10.1016/J.FOODRES.2018.11.031
148. Ferrero R, Soto-Maldonado C, Weinstein-Oppenheimer C, Cabrera-Muñoz Z, Zúñiga-Hansen M. Antiproliferative rapeseed defatted meal protein and their hydrolysates on MCF-7 breast cancer cells and human fibroblasts. Foods. (2021) 10:309. doi: 10.3390/foods10020309
149. Náthia-Neves G, Alonso E. Valorization of sunflower by-product using microwave-assisted extraction to obtain a rich protein flour: recovery of chlorogenic acid, phenolic content and antioxidant capacity. Food Bioprod Process. (2021) 125:57–67. doi: 10.1016/j.fbp.2020.10.008
150. Saricaoglu F. Application of high-pressure homogenization (HPH) to modify functional, structural and rheological properties of lentil (Lens culinaris) proteins. Int J Biol Macromol. (2020) 144:760–9. doi: 10.1016/J.IJBIOMAC.2019.11.034
151. Saricaoglu F, Gul O, Besir A, Atalar I. Effect of high pressure homogenization (HPH) on functional and rheological properties of hazelnut meal proteins obtained from hazelnut oil industry by-products. J Food Eng. (2018) 233:98–108. doi: 10.1016/J.JFOODENG.2018.04.003
152. Porto B, Tribst A, Cristianini M. Dynamic high pressure effects on biopolymers: polysaccharides and proteins. In: Grumezescu AM, Holban AM editors. Biopolymers for food design. Cambridge, MA: Academic Press (2018). p. 313–50.
153. Samard S, Gu B, Ryu G. Effects of extrusion types, screw speed and addition of wheat gluten on physicochemical characteristics and cooking stability of meat analogues. J Sci Food Agric. (2019) 99:4922–31. doi: 10.1002/jsfa.9722
154. Samard S, Ryu G. Physicochemical and functional characteristics of plant protein-based meat analogs. J Food Process Preserv. (2019) 43:e14123. doi: 10.1111/jfpp.14123
155. Nosworthy M, Medina G, Franczyk A, Neufeld J, Appah P, Utioh A, et al. Thermal processing methods differentially affect the protein quality of Chickpea (Cicer arietinum). Food Sci Nutr. (2020) 8:2950–8. doi: 10.1002/fsn3.1597
156. Omosebi M, Osundahunsi O, Fagbemi T. Effect of extrusion on protein quality, antinutritional factors, and digestibility of complementary diet from quality protein maize and soybean protein concentrate. J Food Biochem. (2018) 42:e12508. doi: 10.1111/jfbc.12508
157. O’Sullivan J, Park M, Beevers J, Greenwood R, Norton I. Applications of ultrasound for the functional modification of proteins and nanoemulsion formation: a review. Food Hydrocoll. (2017) 71:299–310. doi: 10.1016/j.foodhyd.2016.12.037
158. Arzeni C, Martínez K, Zema P, Arias A, Pérez O, Pilosof A. Comparative study of high intensity ultrasound effects on food proteins functionality. J Food Eng. (2012) 108:463–72. doi: 10.1016/j.jfoodeng.2011.08.018
159. Xiong T, Xiong W, Ge M, Xia J, Li B, Chen Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res Int. (2018) 109:260–7. doi: 10.1016/j.foodres.2018.04.044
160. O’Sullivan J, Murray B, Flynn C, Norton I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. (2016) 53:141–54. doi: 10.1016/J.FOODHYD.2015.02.009
161. Jin J, Okagu O, Yagoub A, Udenigwe C. Effects of sonication on the in vitro digestibility and structural properties of buckwheat protein isolates. Ultrason Sonochem. (2021) 70:105348. doi: 10.1016/j.ultsonch.2020.105348
162. Gharibzahedi S, Smith B. The functional modification of legume proteins by ultrasonication: a review. Trends Food Sci Technol. (2020) 98:107–16. doi: 10.1016/j.tifs.2020.02.002
163. Kutzli I, Weiss J, Gibis M. Glycation of plant proteins via maillard reaction: reaction chemistry, technofunctional properties, and potential food application. Foods. (2021) 10:376. doi: 10.3390/foods10020376
164. Oliver C, Melton L, Stanley R. Creating proteins with novel functionality via the maillard reaction: a review. Crit Rev Food Sci Nutr. (2006) 46:337–50. doi: 10.1080/10408690590957250
165. Guo Z, Teng F, Huang Z, Lv B, Lv X, Babich O, et al. Effects of material characteristics on the structural characteristics and flavor substances retention of meat analogs. Food Hydrocoll. (2020) 105:105752. doi: 10.1016/j.foodhyd.2020.105752
166. Chiang J, Loveday S, Hardacre A, Parker M. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. (2019) 19:100102. doi: 10.1016/J.FOOSTR.2018.11.002
167. Pietsch V, Karbstein H, Emin M. Kinetics of wheat gluten polymerization at extrusion-like conditions relevant for the production of meat analog products. Food Hydrocoll. (2018) 85:102–9. doi: 10.1016/j.foodhyd.2018.07.008
168. Schreuders F, Sagis L, Bodnár I, Erni P, Boom R, van der Goot A. Mapping the texture of plant protein blends for meat analogues. Food Hydrocoll. (2021) 118:106753. doi: 10.1016/j.foodhyd.2021.106753
169. Sun C, Ge J, He J, Gan R, Fang Y. Processing, quality, safety, and acceptance of meat analogue products. Engineering. (2021) 7:674–8. doi: 10.1016/j.eng.2020.10.011
170. Tolstoguzov V. Texturising by phase separation. Biotechnol Adv. (2006) 24:626–8. doi: 10.1016/J.BIOTECHADV.2006.07.001
171. Tolstoguzov V. Some thermodynamic considerations in food formulation. Food Hydrocoll. (2003) 17:1–23. doi: 10.1016/S0268-005X(01)00111-4
172. Ubbink J, Dupas-Langlet M. Rheology of carbohydrate blends close to the glass transition: temperature and water content dependence of the viscosity in relation to fragility and strength. Food Res Int. (2020) 138:109801. doi: 10.1016/j.foodres.2020.109801
173. Pietsch V, Bühler J, Karbstein H, Emin M. High moisture extrusion of soy protein concentrate: influence of thermomechanical treatment on protein-protein interactions and rheological properties. J Food Eng. (2019) 251:11–8. doi: 10.1016/j.jfoodeng.2019.01.001
174. Kumar P, Verma A, Kumar D, Umaraw P, Malav O. Meat snacks: a novel technological perspective. 1 ed. In: Galanakis CM editor. Innovations in Traditional Foods. Sawston: Woodhead Publishing (2019). p. 293–321. doi: 10.1016/B978-0-12-814887-7.00011-3
175. Ubbink J, Muhialdin B. Protein physical state in meat analogue processing. Curr Opin Food Sci. (2022) 45:100822. doi: 10.1016/j.cofs.2022.100822
176. Zhang J, Ying D, Wei Y, Zhang B, Su X, Li S. Thermal transition and decomposition properties of pH- and phosphate-induced defatted soybean meals. J Therm Anal Calorim. (2017) 128:699–706. doi: 10.1007/s10973-016-5991-8
177. Ryu G. Extrusion cooking of high-moisture meat analogues. In: Ganjyal GM editor. Extrusion cooking. Amsterdam: Elsevier Inc (2020). p. 205–24. doi: 10.1016/B978-0-12-815360-4.00007-9
178. Cho S, Ryu G. Effects on quality characteristics of extruded meat analog by addition of tuna sawdust. J Korean Soc Food Sci Nutr. (2017) 46:465–72. doi: 10.3746/jkfn.2017.46.4.465
179. Gu B, Ryu G. Effects of extrusion variables and die configuration on physicochemical characteristics of texturized soy protein isolate. J Korean Soc Food Sci Nutr. (2019) 48:1405–11. doi: 10.3746/jkfn.2019.48.12.1405
180. Li T, Guo X, Zhu K, Zhou H. Effects of alkali on protein polymerization and textural characteristics of textured wheat protein. Food Chem. (2018) 239:579–87. doi: 10.1016/j.foodchem.2017.06.155
181. Pietsch V, Emin M, Schuchmann H. Process conditions influencing wheat gluten polymerization during high moisture extrusion of meat analog products. J Food Eng. (2017) 198:28–35. doi: 10.1016/j.jfoodeng.2016.10.027
182. Zhang J, Liu L, Jiang Y, Faisal S, Wei L, Cao C, et al. Converting peanut protein biomass waste into “double green” meat substitutes using a high-moisture extrusion process: a multiscale method to explore a process for forming a meat-like fibrous structure. J Agric Food Chem. (2019) 67:10713–25. doi: 10.1021/acs.jafc.9b02711
183. Beck S, Knoerzer K, Arcot J. Effect of low moisture extrusion on a pea protein isolate’s expansion, solubility, molecular weight distribution and secondary structure as determined by Fourier transform infrared spectroscopy (FTIR). J Food Eng. (2017) 214:166–74. doi: 10.1016/J.JFOODENG.2017.06.037
184. Brennan M, Derbyshire E, Tiwari B, Brennan C. Ready-to-eat snack products: the role of extrusion technology in developing consumer acceptable and nutritious snacks. Int J Food Sci Technol. (2013) 48:893–902. doi: 10.1111/ijfs.12055
185. Palanisamy M, Franke K, Berger R, Heinz V, Töpfl S. High moisture extrusion of lupin protein: influence of extrusion parameters on extruder responses and product properties. J Sci Food Agric. (2019) 99:2175–85. doi: 10.1002/jsfa.9410
186. Wild F. Manufacture of meat analogues through high moisture extrusion. In: Reference Module in Food Science. Amsterdam: Elsevier (2016). doi: 10.1016/B978-0-08-100596-5.03281-9
187. Osen R, Toelstede S, Eisner P, Schweiggert-Weisz U. Effect of high moisture extrusion cooking on protein-protein interactions of pea (Pisum sativum L.) protein isolates. Int J Food Sci Technol. (2015) 50:1390–6. doi: 10.1111/ijfs.12783
188. Bouvier J, Campanella O. The generic extrusion process. Extrusion Processing Technology: Food and Non-Food Biomaterials. Hoboken, NJ: Wiley Online Library (2014). p. 393–464.
189. MacDonald R, Pryzbyszewski J, Hsieh F. Soy protein isolate extruded with high moisture retains high nutritional quality. J Agric Food Chem. (2009) 57:3550–5. doi: 10.1021/jf803435x
190. Wolz M, Kulozik U. System parameters in a high moisture extrusion process for microparticulation of whey proteins. J Food Eng. (2017) 209:12–7. doi: 10.1016/J.JFOODENG.2017.04.010
191. Emin M, Quevedo M, Wilhelm M, Karbstein H. Analysis of the reaction behavior of highly concentrated plant proteins in extrusion-like conditions. Innov Food Sci Emerg Technol. (2017) 44:15–20. doi: 10.1016/j.ifset.2017.09.013
192. Chauvet M, Sauceau M, Baillon F, Fages J. Mastering the structure of PLA foams made with extrusion assisted by supercritical CO 2. J Appl Polym Sci. (2017) 134:45067. doi: 10.1002/app.45067
193. Jandyal M, Malav O, Mehta N, Chatli M, Kumar P, Wagh RV, et al. 3D printing of meat: a new frontier of food from download to delicious: a review. Int J Curr Microbiol Appl Sci. (2021) 10:2095–111.
194. Zhang J, Liu L, Jiang Y, Faisal S, Wang Q. A new insight into the high-moisture extrusion process of peanut protein: from the aspect of the orders and amount of energy input. J Food Eng. (2020) 264:109668. doi: 10.1016/J.JFOODENG.2019.07.015
195. Park J, Chatpaisarn A, Ryu G. Effects of gluten and moisture contents on texturization of extruded soy protein isolate. J Korean Soc Food Sci Nutr. (2017) 46:473–80. doi: 10.3746/jkfn.2017.46.4.473
196. Ma X, Gu B, Ryu G. Optimization of extrusion variables for improving the qualities of textured vegetable protein with green tea using response surface methodology. Food Eng Prog. (2018) 22:1–8. doi: 10.13050/foodengprog.2018.22.1.1
197. Yeob G, Gi-Hyung R. Effects of moisture content and screw speed on physical properties of extruded soy protein isolate. J Korean Soc Food Sci Nutr. (2017) 46:751–8. doi: 10.3746/JKFN.2017.46.6.751
198. Ferawati F, Zahari I, Barman M, Hefni M, Ahlström C, Witthöft C, et al. High-moisture meat analogues produced from yellow Pea and Faba bean protein isolates/concentrate: effect of raw material composition and extrusion parameters on texture properties. Foods. (2021) 10:843. doi: 10.3390/foods10040843
199. Palanisamy M, Töpfl S, Aganovic K, Berger R. Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT. (2018) 87:546–52. doi: 10.1016/j.lwt.2017.09.029
200. Grahl S, Palanisamy M, Strack M, Meier-Dinkel L, Toepfl S, Mörlein D. Towards more sustainable meat alternatives: how technical parameters affect the sensory properties of extrusion products derived from soy and algae. J Clean Prod. (2018) 198:962–71. doi: 10.1016/J.JCLEPRO.2018.07.041
201. Caporgno M, Böcker L, Müssner C, Stirnemann E, Haberkorn I, Adelmann H, et al. Extruded meat analogues based on yellow, heterotrophically cultivated Auxenochlorella protothecoides microalgae. Innov Food Sci Emerg Technol. (2020) 59:102275. doi: 10.1016/J.IFSET.2019.102275
202. Smetana S, Larki N, Pernutz C, Franke K, Bindrich U, Toepfl S, et al. Structure design of insect-based meat analogs with high-moisture extrusion. J Food Eng. (2018) 229:83–5. doi: 10.1016/J.JFOODENG.2017.06.035
203. Zhang J, Liu L, Liu H, Yoon A, Rizvi S, Wang Q. Changes in conformation and quality of vegetable protein during texturization process by extrusion. Crit Rev Food Sci Nutr. (2019) 59:3267–80. doi: 10.1080/10408398.2018.1487383
204. Mattice K, Marangoni A. Comparing methods to produce fibrous material from zein. Food Res Int. (2020) 128:108804. doi: 10.1016/j.foodres.2019.108804
205. Kutzli I, Gibis M, Baier S, Weiss J. Electrospinning of whey and soy protein mixed with maltodextrin – influence of protein type and ratio on the production and morphology of fibers. Food Hydrocoll. (2019) 93:206–14. doi: 10.1016/J.FOODHYD.2019.02.028
206. Nieuwland M, Geerdink P, van den Eijnden P, Henket JTMM, Langelaan MLP, Stroeks N, et al. Reprint of “food-grade electrospinning of proteins.”. Innov Food Sci Emerg Technol. (2014) 24:138–44. doi: 10.1016/J.IFSET.2014.07.006
207. Krintiras G, Göbel J, Van Der Goot A, Stefanidis G. Production of structured soy-based meat analogues using simple shear and heat in a couette cell. J Food Eng. (2015) 160:34–41. doi: 10.1016/J.JFOODENG.2015.02.015
208. Krintiras G, Diaz J, van der Goot A, Stankiewicz A, Stefanidis G. On the use of the couette cell technology for large scale production of textured soy-based meat replacers. J Food Eng. (2016) 169:205–13. doi: 10.1016/j.jfoodeng.2015.08.021
209. Schreuders F, Dekkers B, Bodnár I, Erni P, Boom R, van der Goot A. Comparing structuring potential of pea and soy protein with gluten for meat analogue preparation. J Food Eng. (2019) 261:32–9. doi: 10.1016/J.JFOODENG.2019.04.022
210. Geerts M, Dekkers B, van der Padt A, van der Goot A. Aqueous fractionation processes of soy protein for fibrous structure formation. Innov Food Sci Emerg Technol. (2018) 45:313–9. doi: 10.1016/J.IFSET.2017.12.002
211. Moomand K, Lim L. Effects of solvent and n-3 rich fish oil on physicochemical properties of electrospun zein fibres. Food Hydrocoll. (2015) 46:191–200. doi: 10.1016/J.FOODHYD.2014.12.014
212. Dekkers B, Nikiforidis CV, van der Goot A. Shear-induced fibrous structure formation from a pectin/SPI blend. Innov Food Sci Emerg Technol. (2016) 36:193–200. doi: 10.1016/j.ifset.2016.07.003
213. Shahbazi M, Jäger H, Chen J, Ettelaie R. Construction of 3D printed reduced-fat meat analogue by emulsion gels. Part II: printing performance, thermal, tribological, and dynamic sensory characterization of printed objects. Food Hydrocoll. (2021) 121:107054. doi: 10.1016/J.FOODHYD.2021.107054
214. Bhat Z, Morton J, Kumar S, Bhat H, Aadil R, Bekhit A. 3D printing: development of animal products and special foods. Trends Food Sci Technol. (2021) 118:87–105. doi: 10.1016/j.tifs.2021.09.020
215. Dick A, Bhandari B, Prakash S. 3D printing of meat. Meat Sci. (2019) 153:35–44. doi: 10.1016/J.MEATSCI.2019.03.005
216. Liu Z, Zhang M, Bhandari B, Wang Y. 3D printing: printing precision and application in food sector. Trends Food Sci Technol. (2017) 69:83–94. doi: 10.1016/J.TIFS.2017.08.018
217. Dankar I, Haddarah A, Omar F, Sepulcre F, Pujolà M. 3D printing technology: the new era for food customization and elaboration. Trends Food Sci Technol. (2018) 75:231–42. doi: 10.1016/J.TIFS.2018.03.018
218. Grabowska K, Tekidou S, Boom R, van der Goot A. Shear structuring as a new method to make anisotropic structures from soy–gluten blends. Food Res Int. (2014) 64:743–51. doi: 10.1016/j.foodres.2014.08.010
Keywords: non-traditional proteins, extrusion, electrospinning, couette shear cell, bioprinting
Citation: Kumar P, Sharma N, Ahmed MA, Verma AK, Umaraw P, Mehta N, Abubakar AA, Hayat MN, Kaka U, Lee S-J and Sazili AQ (2022) Technological interventions in improving the functionality of proteins during processing of meat analogs. Front. Nutr. 9:1044024. doi: 10.3389/fnut.2022.1044024
Received: 14 September 2022; Accepted: 02 December 2022;
Published: 19 December 2022.
Edited by:
Anubhav Pratap-Singh, The University of British Columbia, CanadaReviewed by:
Dominic Agyei, University of Otago, New ZealandGi Hyung Ryu, Kongju National University, South Korea
Copyright © 2022 Kumar, Sharma, Ahmed, Verma, Umaraw, Mehta, Abubakar, Hayat, Kaka, Lee and Sazili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neelesh Sharma, ✉ drneelesh_sharma@yahoo.co.in; Sung-Jin Lee, ✉ sjlee@kangwon.ac.kr; Awis Qurni Sazili, ✉ awis@upm.edu.my
 Pavan Kumar
Pavan Kumar Neelesh Sharma
Neelesh Sharma Muideen Adewale Ahmed1
Muideen Adewale Ahmed1  Akhilesh K. Verma
Akhilesh K. Verma Pramila Umaraw
Pramila Umaraw Nitin Mehta
Nitin Mehta Ubedullah Kaka
Ubedullah Kaka Sung-Jin Lee
Sung-Jin Lee Awis Qurni Sazili
Awis Qurni Sazili