- 1College of Acupuncture, Moxibustion and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Clinical Research Center for Acupuncture and Moxibustion in Sichuan province, Chengdu, China
- 3Sichuan Province Building Hospital, Chengdu, China
- 4Center for Brain Imaging, School of Life Sciences and Technology, Xidian University, Xi’an, China
- 5Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 6Chengdu Integrated Traditional Chinese Medicine and Western Medicine Hospital, Chengdu, China
Background: Menstrual migraine without aura (MMoA) refers to a specific type of migraine that is associated with the female ovarian cycle. It is particularly serious and has brought huge life pressure and mental burden to female patients. Acupuncture has been commonly used to prevent migraines and relieve concomitant emotional disorders; however, the physiological mechanism underlying this intervention remains unclear. This study aimed to use resting-state functional magnetic resonance imaging (rsfMRI) to investigate whether acupuncture can modulate brain function and if the potential influence on brain activity correlates with improving emotional symptoms in MMoA patients.
Methods: Overall, 44 patients were randomly divided into a true acupuncture (TA) group and the sham acupuncture (SA) group. Patients underwent rsfMRI before and after 3-month treatment, the amplitude of low-frequency fuctuations (ALFF) and regional homogeneity (ReHo) in rsfMRI were calculated. Zung self-rating anxiety scale (SAS), Zung self-rating depression scale (SDS), frequency of migraine attacks, visual analog scale, and intensity of the migraine were used for evaluate the clinical effect. The clinical changes of variables were also used to further assess the correlation with brain activity in MMoA patients.
Results: After acupuncture treatment, the emotional symptoms of both groups of patients improved, and the clinical symptoms of migraine were alleviated. The major finding of our study was that patients with MMoA showed lower ALFF value in the left anterior cingulate and the value was positively correlated with the decreases in the SAS and SDS scores. In the SA group, common brain regions responded both in ALFF and regional homogeneity values mainly in the insula, and no significant correlations were observed between brain regions and clinical variables.
Conclusions: These results indicated that both two acupuncture treatments were helpful in treating migraine and could improve emotion symptoms. TA had a relatively better effect in reducing the frequency of migraine attack than SA. The two therapies have different modulation effects as TA regulates emotional disorders by modulating the frontal-limbic regions, and SA may modulate pain perception through the placebo effect on insula and by indirectly regulating emotional disorders. These findings provided evidence that acupuncture is a complementary and alternative therapy to relieve clinical symptoms in female patients with migraines and could help enhance clinical diagnosis and treatment.
Clinical Trial Registration: [http://www.chictr.org.cn/index.aspx], identifier [ChiCTR-IOR-15006648. Registered 23 June 2015].
Introduction
Migraine is a common neurological disorder and is considered to be the second-largest contributor to worldwide disability (Karikari et al., 2018). More than 50% of female patients with migraines report that their symptoms are associated with menstruation, and the proportion of female migraineurs is two to three times that of male migraineurs (Vetvik and MacGregor, 2017). According to the third edition of the International Classification of Headache Disorders (ICHD-3), women who report that their migraines are associated with their menses are said to have menstrual migraine without aura (MMoA) (Pavlović et al., 2015). Attacks during menstruation tend to be longer and are accompanied by more severe nausea than attacks that occur outside the menstrual cycle (No Author, 2018).
The high prevalence of migraine-related emotional disorders, such as depression and anxiety, is often overlooked. An epidemiological study suggested that patients with chronic migraine had a higher risk of major depression, anxiety, or suicidal behavior than subjects without migraine (Trejo-Gabriel-Galan et al., 2018). MMoA as a special subtype of migraine is more likely to induce or exacerbate many symptoms associated with various emotional disorders that are affected by the cyclic rise and fall of estrogen (Warnock et al., 2017). The coexistence of emotional disorders and migraines alters the quality of life and increases the burden and disability associated with migraine (Liao et al., 2020). However, specific therapeutic guidelines for depression and anxiety in migraine are unavailable. The use of psychoactive drugs increases headache frequency and susceptibility to medication overuse and disability (Pompili et al., 2009; Peck et al., 2015). Conversely, caution is needed regarding the use of migraine preventive drugs, such as flunarizine and beta-blockers, as they may be contraindicated in the presence of emotional disorders (Asmundson and Katz, 2009). Therefore, using non-pharmacological treatment strategies aimed to manage both migraine and comorbid psychiatric disorders are essential.
Acupuncture, a treatment modality of traditional Chinese medicine (TCM), has a long history in China and is increasingly being adopted in the West as a complementary and alternative preventive treatment for migraine attacks and to relieve pain. The latest randomized clinical trials (RCTs) suggest that acupuncture is an effective therapy for reducing the severity of pain and prevention of frequent attacks in migraineurs (Wang et al., 2015; Zhao et al., 2017; Xu et al., 2020). Furthermore, a systematic review showed that acupuncture is superior to analgesic medications in alleviating average headache intensity in patients with MMoA (Yang et al., 2020). Studies have also shown that acupuncture is efficacious for various types of depressive disorders (Smith et al., 2018). A retrospective cohort study proved that acupuncture could reduce the risk of depression and anxiety during the long-term follow-up period in patients with migraine (Liao et al., 2020). However, the potential regulatory effects behind the beneficial effects of acupuncture on MMoA have not yet been fully elucidated. In light of the risk of comorbid disorders, an investigation of the neural mechanisms behind acupuncture effects may aid in the development of effective treatment options that can be applied clinically.
Neuroimaging approaches, which provide feasible, efficient, and non-invasive tools for investigating the central mechanisms of acupuncture and are a global trend in acupuncture research (Zhao et al., 2014), have been used to measure structural and functional brain changes in patients with migraine (Schwedt and Dodick, 2009). In this study, the amplitude of low-frequency fluctuations (ALFFs) and regional homogeneity (ReHo) methods were used to compare the blood oxygen level-dependent (BOLD) signals in the brains of patients with MMoA during the resting-state. ALFF and ReHo methods are two important methods for depicting the various characteristics of resting-state fMRI signals. ALFF measures the intensity of neural activity at the single-voxel level, while ReHo measures the neural synchronization of a given voxel with its neighboring voxels (Cui et al., 2014). ALFF can directly demonstrate the BOLD signal and reflect spontaneous fluctuations in the voxels under the resting state (Zang et al., 2007). ReHo is based on a data-driven approach and thus requires no prior knowledge and has good test-retest reliability (Zuo et al., 2013). ALFF and ReHo analysis has been used to study migraine in our previous study (Liu et al., 2015), and other diseases like premenstrual syndrome (Pang et al., 2020), Alzheimer’s disease (Li et al., 2020a), and Parkinson’s disease (Shen et al., 2020), among others.
Hence, we hypothesized that, compared to that of sham acupuncture, the true acupuncture modulates emotion-affected dysfunctional brain regions in MMoA patients. In this study, we aimed to use rsfMRI to investigate whether acupuncture can modulate brain function and if the potential influence on brain activity correlates with improving emotional symptoms in MMoA patients.
Materials and Methods
This trial was performed at the Hospital of Chengdu University of TCM. The study was registered with the Chinese Clinical Trial Registry (Identifier: ChiCTR-IOR-15006648), performed in accordance with the principles of the Declaration of Helsinki, and approved by the ethics committee at the Hospital of Chengdu University of TCM. The sample size calculation of a neuroimaging study is different from that of a classic randomized controlled clinical trial. Power analyses for neuroimaging studies rely on assumptions about BOLD signal amplitude, smoothness, brain location, and other factors that render principled priori designations difficult (Yu et al., 2020). According to a previous neuroimaging study (Desmond and Glover, 2002), a minimum sample size (n = 20) should be used in order to obtain 80% power with an error threshold of 0.002 at a single-voxel level. Considering a conservative dropout rate of 20% and incomplete data due to severe head movement during the MRI scan, the sample size in this study was increased to 25 participants per group; therefore, a sample size of 50 patients with MMOA was determined.
Participants
Fifty eligible patients were equally allocated into the TA group and SA group (25 in each group). One patient in the TA group and five patients in the SA group dropped out during the study because of non-compliance with treatment schedule or inability to be contacted. In total, 44 patients who received acupuncture therapies were included in the final analysis of clinical outcome measures. All participants provided written, informed consent to participate after the experimental procedures were fully explained, and they were informed that they could stop participating at any time. All patients were enrolled from the outpatient clinic of the Departments of Neurology and Gynecology in two clinical centers: (1) the Hospital of Chengdu University of TCM; and (2) Chengdu Integrated TCM & Western Medicine Hospital. Recruitment took place from July 2015 to August 2018. The diagnosis of MMoA was established according to the ICHD-3 beta criteria (No Author, 2013). Participants who met all the following inclusion criteria were included in the study: (1) female, 18 -50 years of age, right-handed; (2) migraine attacks outside of the menstrual cycle did not exceed six per month; (3) a history MMoA for 6 months or more; (4) a stable 28 (±7)-day menstrual cycle; and (5) Zung self-rating anxiety scale (SAS)/Zung self-rating depression scale (SDS) score >50. Patients with any of the following conditions were excluded: (1) neurological diseases, immunodeficiency, bleeding disorders, or allergies; (2) MRI contraindications, such as claustrophobia; (3) any prophylactic headache medication or any acupuncture treatment during the last 3 months; alcohol or drug abuse; (4) pregnancy, lactation, or plans to become pregnant within 6 months; (5) Zung self-rating anxiety/depression scale score >50; and (6) psychiatric disorders, such as schizoaffective disorder, schizophrenia, organic mental disorder, psychotic features coordinated or uncoordinated with mood or bipolar disorder.
Study Design
The total observation period in this study was three menstrual cycles for each patient. Patients who met the inclusion criteria were randomly assigned to the true acupuncture (TA) and sham acupuncture (SA) groups in a 1:1 ratio. All patients were asked to document their symptoms in headache diaries, and the outcome measurement was completed both for the baseline and 4, 8, and 12 weeks after randomization. Additionally, each patient underwent functional magnetic resonance imaging (fMRI) examinations at the baseline and at end of the treatment period. After scanning, all participants reported that they had not experienced any headaches or migraines and remained awake during the procedure.
Intervention
In this study, traditional Chinese acupuncture was used and treatments were manipulated by two specialized acupuncturists with at least 5 years of training and 3 years of experience. They implemented acupuncture therapy in both groups in turns. The acupoints were selected according to traditional classic and systematic reviews of ancient and modern literature of acupuncture for MMoA upon several consensus meetings with experts based on experiences from our previous study (Wang et al., 2018). Patients in the TA group were given treatments that used the following acupoints: GB20 (Fengchi), GB8 (Shuaigu), PC6 (Neiguan), SP6 (Sanyinjiao), and LR3 (Taichong). The non-acupoints chosen were used in our previous studies for migraine prophylaxis (Zhao et al., 2017; Table 1).
All acupoints were punctured bilaterally using single-use stainless steel filiform needles (Hwato Needles, Sino-foreign Joint Venture Suzhou Hua Tuo Medical Instruments Co., China), 25–40 mm in length and 0.25–0.30 mm in diameter.
The depths of the inserted needles differed but were approximately 0.5–1.5 cm. Needles were twisted and rotated (90°< amplitude < 180°) at a frequency of 1–2 Hz. Stimulation was repeated one to three times to achieve the Deqi sensation (a sensation of soreness, numbness, distention, or radiating that indicates effective needling) in the TA group; subjects in the SA group did not experience Deqi. Each group’s treatment consisted of 27 or 27 ± 6 sessions according to changes in their menstrual cycle for 28 days or 28 ± 7 days, and each session lasted for 30 min. Interventions were performed 1 week before menses, once every other day for a total of three treatments. A total of two treatments were administered at the onset of menses and twice a week for the rest of the time (Table 2).
Outcome Measures in Clinical Efficacy
The clinical outcomes were measured every 4 weeks, and the measurement parameters included SAS, SDS, the frequency of migraine attacks (defined as the number of migraine separated by pain free intervals of at least 48 h), visual analog scale (VAS) score of 0–10, and the intensity of migraines on a scale of 0–3. All patients were required to keep a headache diary records every 4 weeks after inclusion. The headache diary documented the time of migraine onset, duration, frequency, and severity (evaluated using the VAS).
Functional Magnetic Resonance Imaging Data Acquisition
Magnetic resonance imaging was performed during the periovulatory phase (days 12–16 of the menstrual cycle). All patients with MMoA were migraine-free for at least 72 h at the time of the MRI scan. MRI data were acquired using a GE Discovery MR750 3.0 T system with an eight-channel, phased-array head coil (General Electric, Milwaukee, WI, United States). The functional images were obtained with a single-shot gradient-echo echoplanar imaging sequence with the following parameters: repetition time = 2,000 ms; echo time = 25 ms; flip angle = 90°, field of view = 240 mm × 240 mm, data matrix = 64 × 64, slice thickness = 3 mm, and voxel-size = 3.44 mm × 3.44 mm × 4 mm. During the whole functional scan, all participants were instructed to keep their eyes closed and stay awake during the entire session.
Data Analysis
Clinical Data Analysis
SPSS version 23.0 software (SPSS, Chicago, IL, United States) was used for demographic analysis. The independent-sample t-test was used to compare all demographic characteristics between the two groups. P-values less than 0.05 false discovery rate (FDR) were considered to be statistically significant. Continuous variables were presented as means (standard deviation) with 95% confidence interval (CI). For continuous variables, a paired-t test was applied for within-group comparisons, two-sample t-tests were applied for two-group comparisons. Treatment effects including SAS, SDS, VAS, frequency of migraine attack every 4 weeks, and intensity of migraines every 4 weeks were evaluated using a repeated-measures analysis of variance (ANOVA) model with a between-subjects factor THERAPY (levels: TA and SA) and a within-subjects repeated measures factor TIME (levels: baseline, 1–4, 5–8, and 9–12 weeks). P value < 0.05 was considered as statistically significant.
Magnetic Resonance Imaging Data Preprocessing
The ALFF value for each voxel was calculated by taking the average of the square root of the power spectrum from 0.01–0.08 Hz (Zang et al., 2007). The ReHo value for each voxel was obtained by calculating Kendall’s coefficient of concordance within a cubic cluster size of 27 voxels (Zang et al., 2004). We compared the changes of ALFF and ReHo differences (post-treatment minus pre-treatment) within the group by using paired t-test. Results were assumed to be statistically significant at p < 0.05 after false discovery rate (FDR) correction within the whole brain. fMRI image processing was carried out using Statistical Parametric Mapping 12 (SPM121) and Data Processing and Analysis for Brain Imaging toolbox version 2.3 (DPABI v. 2.32) software (Yan et al., 2016). The first ten volumes of individual fMRI data were discarded, and slice timing and realignment correction were performed for the remaining images. Any participant with maximum head movement greater than 2.0 mm translation or more than 2.0° rotation was not included. The individual fMRI images were then spatially normalized to the standard template and re-sampled to a 3 mm × 3 mm × 3 mm voxel size. Then, in the regression step, we used multiple regression to model the time-varying BOLD signal in each voxel, including the Friston 24 motion parameters (Friston et al., 1996), cerebrospinal and white matter signals. Afterward, the linear trends were regressed and a band-pass filter was applied at 0.01∼0.08 Hz. Finally, given that resting-state activity is sensitive to minor head movement, we calculated the mean frame-wise displacement (FD) to further determine the comparability of head movement across groups (TA: 0.14 ± 0.06; SA: 0.13 ± 0.04; mean ± SD, p = 0.52).
Correlation Between Amplitude of Low-Frequency Fluctuations/Regional Homogeneity Values and Clinical Variables
To investigate the correlation between ALFF/ReHo values and clinical variables, Pearson correlation analyses were performed in a voxel-wise manner using DPABI V2.3 software. The correlation analysis was adjusted for the same covariates as those controlled for in the between-group tests. The demographic characteristics of the patients (including age, height, weight) were used as covariates. The statistical threshold was set at p < 0.05 (FDR corrected) to explore the most significant correlations among MR voxels.
Results
Participants
The demographic characteristics of all subjects are summarized in Table 3. We found no statistical differences between the TA group and the SA group in terms of their age, height, weight, SAS, SDS, frequency of migraine attacks, VAS score, or intensity of the migraine (all p > 0.05).
Clinical Outcomes
Comparison within each group, both the TA and SA group showed significant decreases in the SAS, SDS, and VAS scores and intensity of migraine after 12 weeks of treatment (p < 0.05). And there was no difference between the two groups in the improvement of clinical symptoms. The frequency of attacks was significantly lower in the TA group than in the SA group (p < 0.05). Furthermore, no significant interaction effect was observed between the two groups by analysis of variance for repeated measures in terms of the SAS, SDS, frequency of migraine attack, VAS score every 4 weeks and the intensity of migraines at the end of treatment (all p > 0.05) (Table 4).
Neuroimaging Results
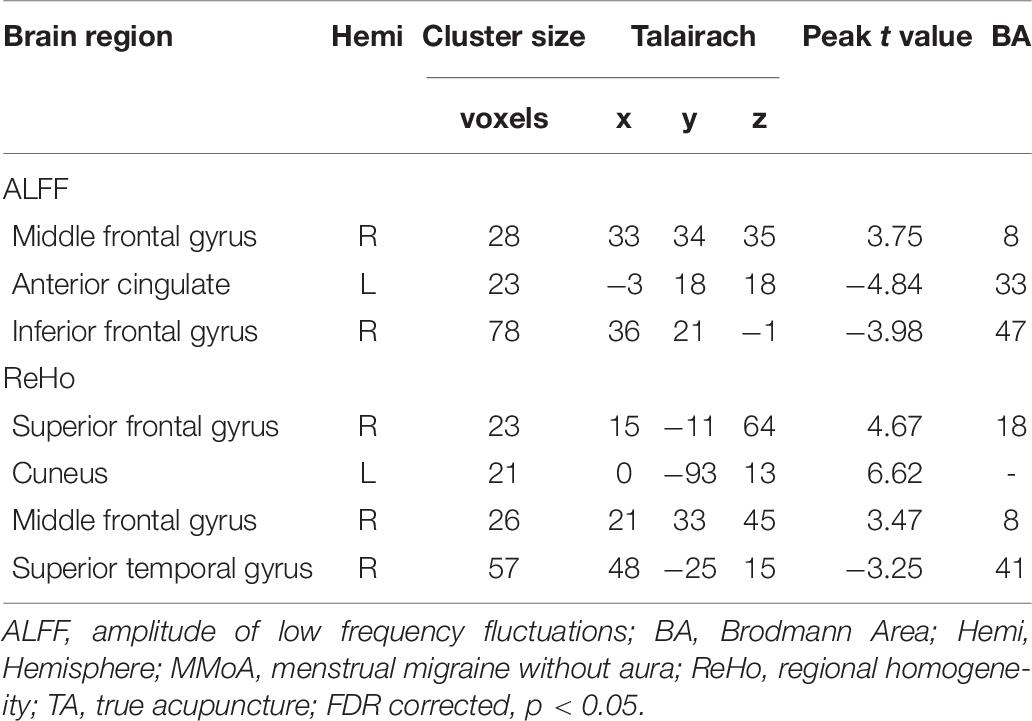
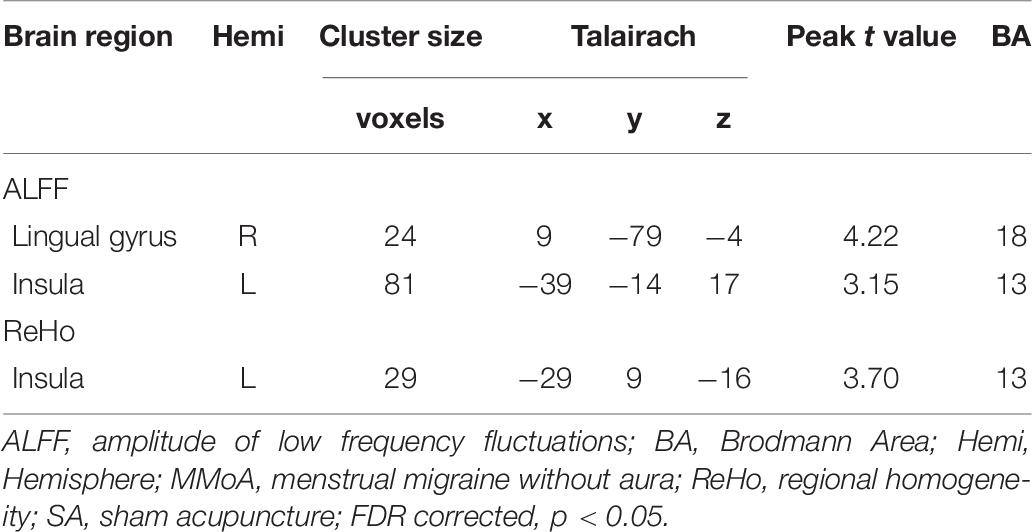
At the baseline, ALFF value in the right precuneus was positively correlated with SDS in the patients with MMoA (r = 0.33, p < 0.05) (Figure 1 and Table 5), and ALFF value in the right middle temporal gyrus (MTG) was negatively correlated with VAS (r = 0.29, p < 0.05) (Figure 2 and Table 5). After TA treatment, MMoA patients showed higher ALFF value in the right middle frontal gyrus (MFG); lower ALFF values were observed in the left anterior cingulate (ACC) and right inferior frontal gyrus (Figure 3 and Table 6). Regarding the ReHo values, we found higher ReHo values in the right superior frontal gyrus, left cuneus, and right MFG, while the right superior temporal gyrus showed a lower ReHo value in MMoA patients (Figure 3 and Table 6). After SA treatment, higher ALFF values were observed in the right lingual gyrus and left insula (Figure 4 and Table 7), and left insula showed a higher ReHo value in patients with MMoA (Figure 4 and Table 7). Furthermore, the correlation result showed that the altered ALFF value in the left ACC was positively correlated with the decreases in the SAS scores (r = 0.49, p < 0.05) and SDS scores in the TA group (r = 0.41, p < 0.05) (Figure 5). And no significant correlations were observed between brain regions and clinical variables in the SA group.
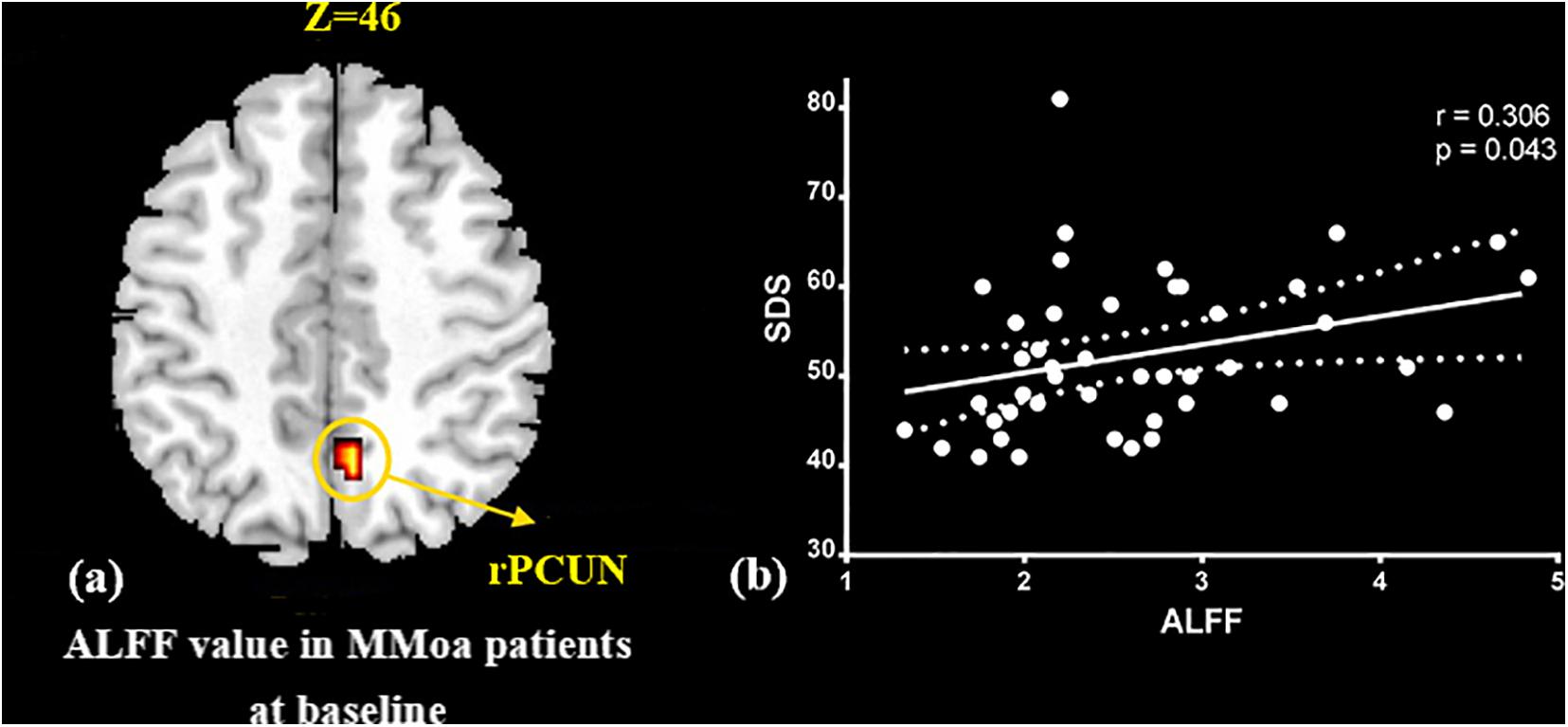
Figure 1. Correlation analysis between ALFF value in precuneus and SDS in the MMoA patients (p < 0.05, FDR corrected). (a) Brain region used for the correlation analysis shown. (b) ALFF in the precuneus was positively correlated with SDS. PCUN, precuneus; R, right; SDS, Zung self-rating depression scale.
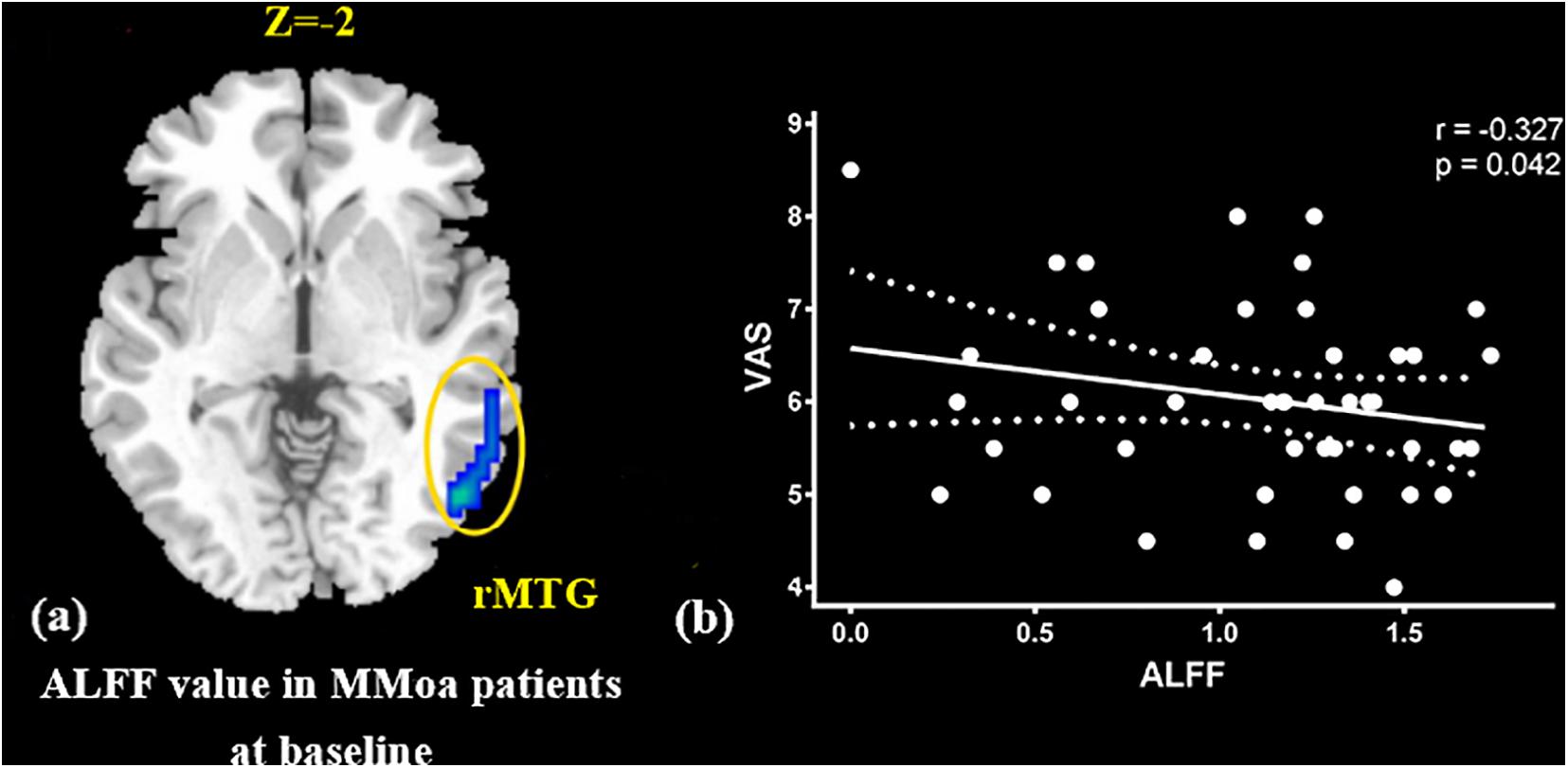
Figure 2. Correlation analysis between ALFF value in MTG and VAS in the MMoA patients (p < 0.05, FDR corrected). (a) Brain region used for the correlation analysis shown. (b) ALFF in the MTG was positively correlated with VAS. MTG, middle temporal gyrus; R, right; VAS, visual analog scale.
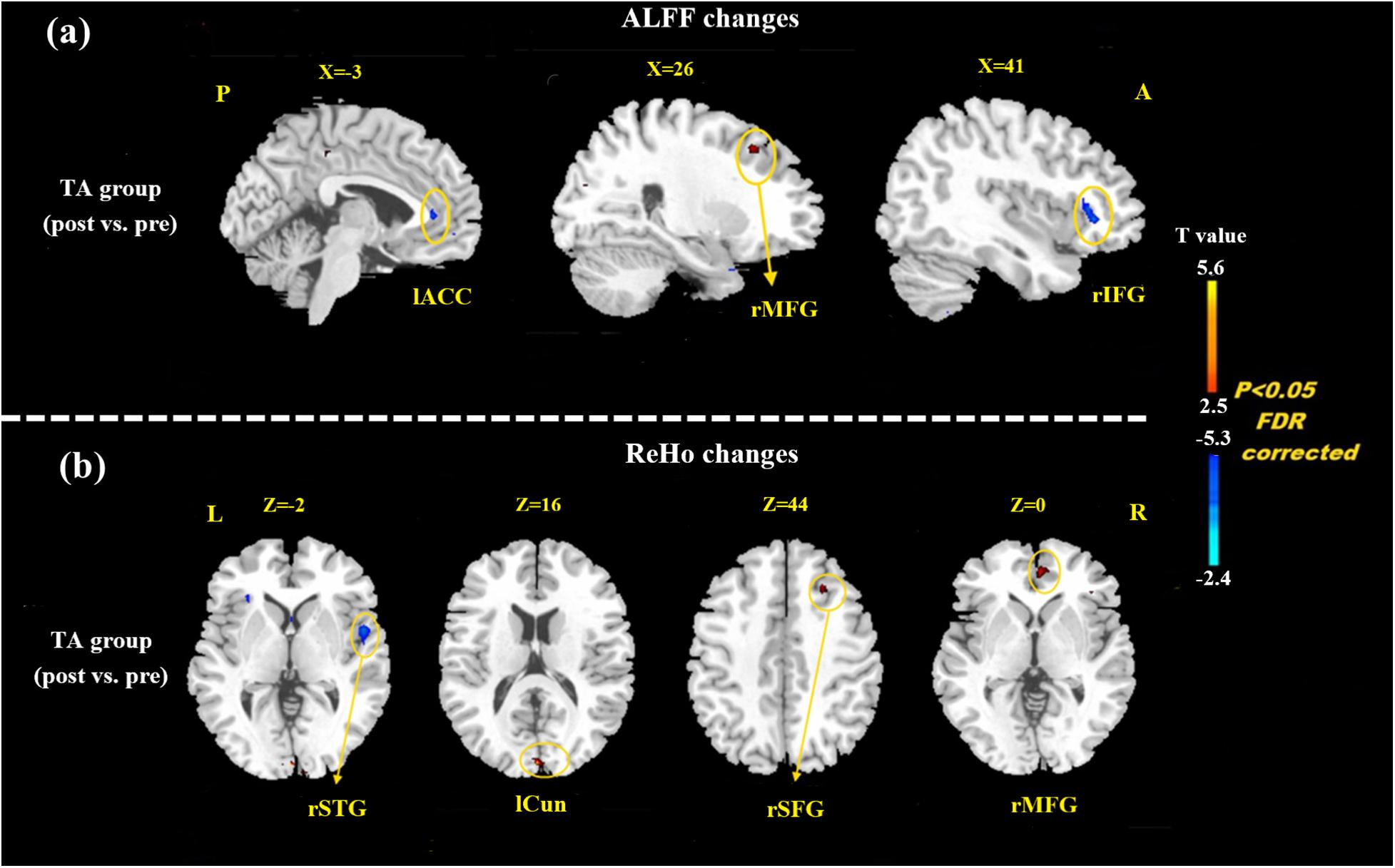
Figure 3. Resting state ALFF and ReHo changes of MMoA patients in true acupuncture group (p < 0.05, FDR corrected). (a) Brain regions showed altered ALFF value in MMoA patients after true acupuncture treatment. (b) Brain regions showed altered ReHo value in MMoA patients after true acupuncture treatment. Warm colors indicate ALFF and ReHo increases after true acupuncture group; cool colors indicate ALFF and ReHo decreases after true acupuncture group. A, anterior; ACC, anterior cingulate; Cun, cuneus; IFG, inferior frontal gyrus; L, left; MFG, middle frontal gyrus; P, posterior; R, right; SFG, superior frontal gyrus; STG, superior temporal gyrus; TA, true acupuncture.
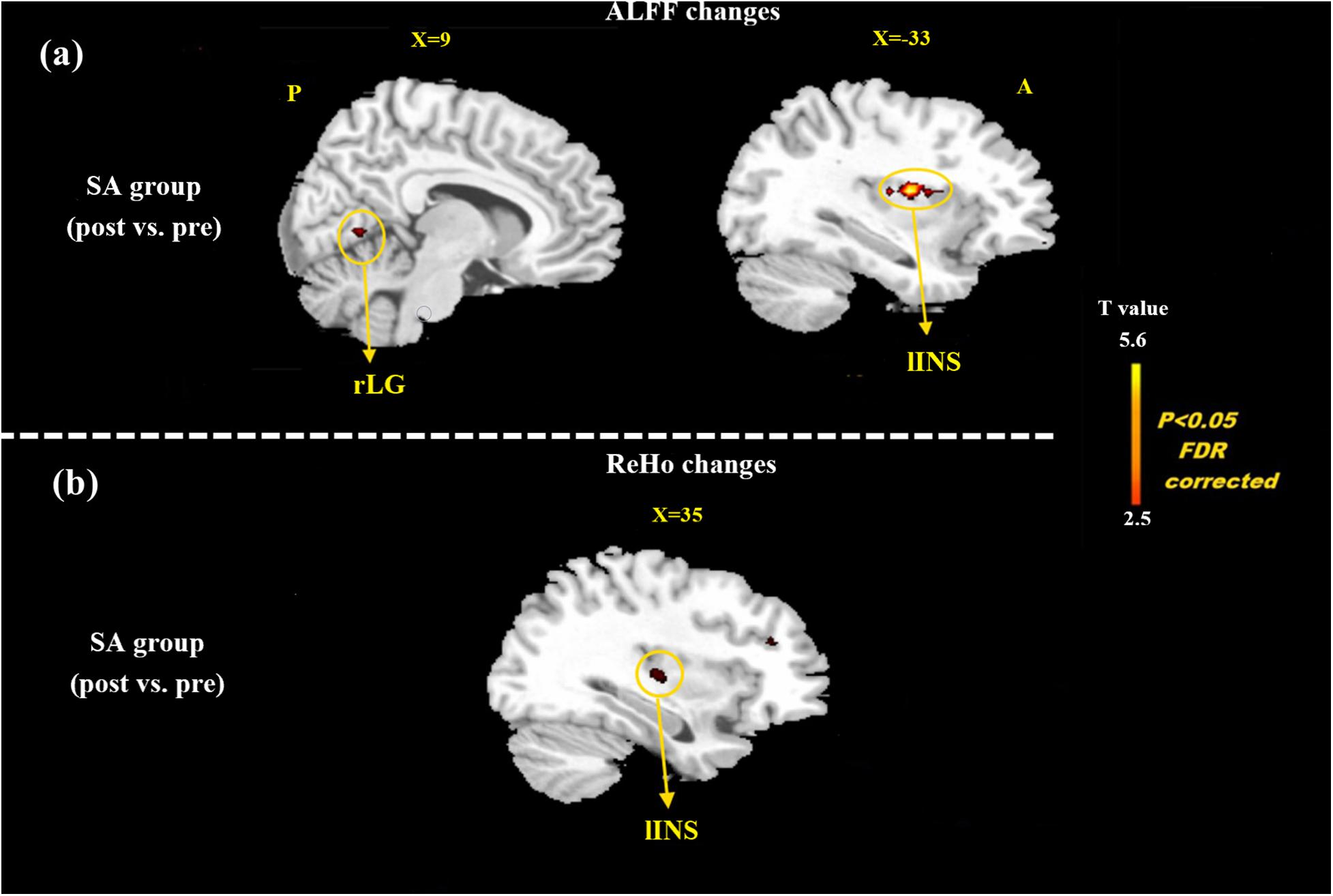
Figure 4. Resting state ALFF and ReHo changes of MMoA patients in sham acupuncture group (p < 0.05, FDR corrected). (a) Brain regions showed altered ALFF value in MMoA patients after sham acupuncture treatment. (b) Brain regions showed altered ReHo value in MMoA patients after sham acupuncture treatment. Warm colors indicate ALFF and ReHo increases after sham acupuncture group; cool colors indicate ALFF and ReHo decreases after sham acupuncture group. A, anterior; INS, insula; LG, lingual gyrus; P, posterior; SA, sham acupuncture.
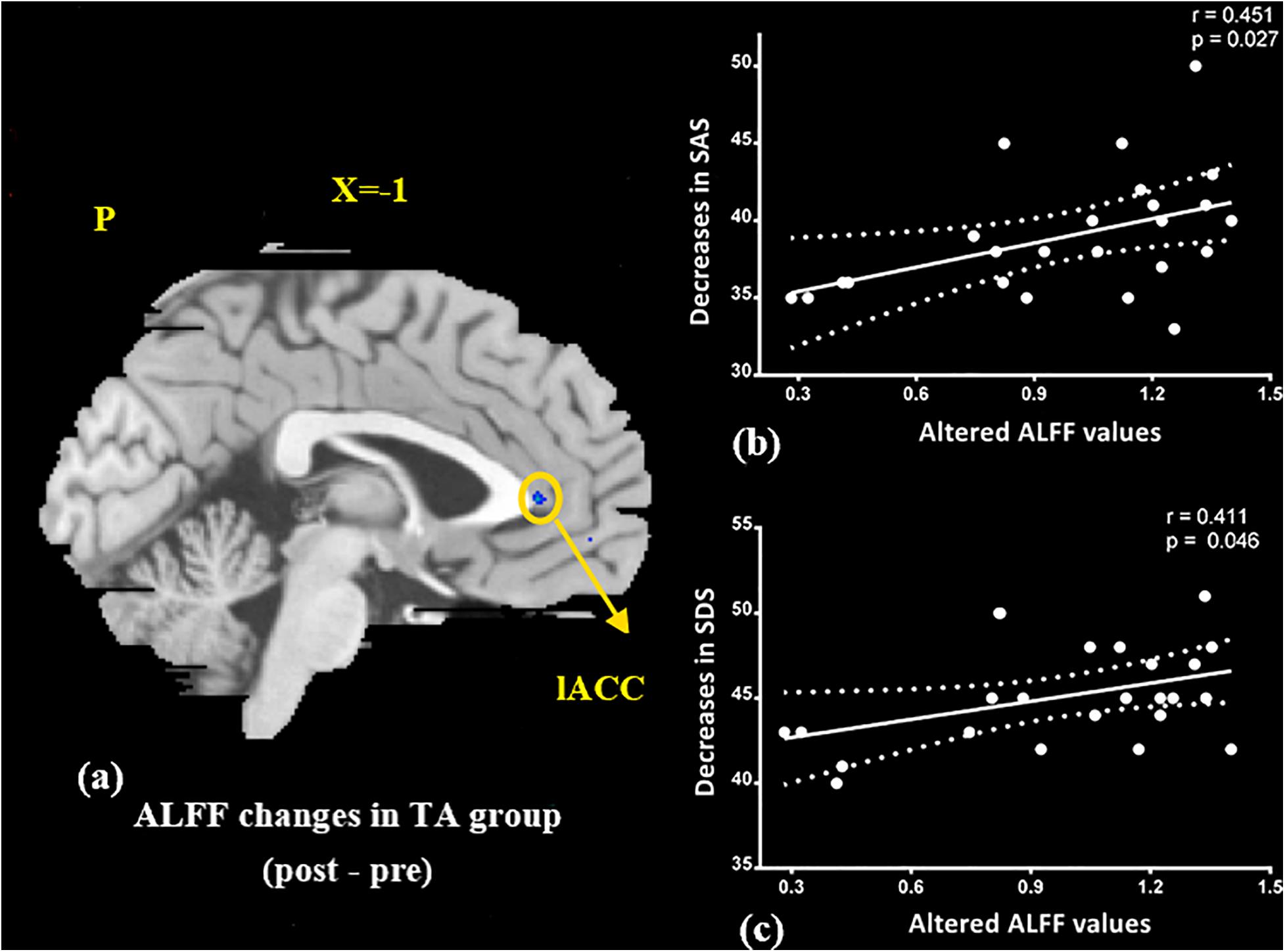
Figure 5. Correlation analysis between altered ALFF values in ACC and improvements in the SAS, SDS in the TA group (p < 0.05, FDR corrected). (a) Brain region used for the correlation analysis shown. (b) ALFF in the ACC was positively correlated with SAS. (c) ALFF in the ACC was positively correlated with SDS. ACC, anterior cingulate; L, left; P, posterior; SAS, Zung self-rating anxiety scale; SDS, Zung self-rating depression scale; TA, true acupuncture.
Discussion
In this study, we focused on the regulatory effects of acupuncture in regulating the emotional disorders in patients with MMoA using neuroimaging. At the baseline, we found the ALFF value in the precuneus was positively correlated with SDS scores in the patients with MMoA (p < 0.05). The precuneus is a core region that is responsible for baseline brain activity and participates in functions, such as intrinsic ongoing mental processes and fundamental cognitive social functions (Pereira-Pedro and Bruner, 2016). Furthermore, the precuneus is associated with interoceptive and emotional processing with widely distributed networks sharing connectivity with many brain regions in the frontal, temporal, occipital, and parietal cortices (Kitada et al., 2014). Several neuroimaging studies (Greicius et al., 2007; Sun et al., 2018; Zhu et al., 2018) have reported that depression is associated with abnormalities in precuneus at the resting state. Based on the findings of positive correlation between SDS scores and ALFF in the precuneus, it appears that depressive symptoms may affect the precuneus. The precuneus then enhances its core action on maintaining baseline brain activity, which suggests that the precuneus plays a crucial role in emotional processing of patients with MMoA. The MTG is an associative multisensory area that plays a role in assigning affective tone to short-term memories relating to pain. It also processes visual, olfactory, and auditory sensations (Olson et al., 2007; Asari et al., 2008). These results are in line with those of previous fMRI studies that showed functional alterations in the temporal lobe of patients with migraine (Absinta et al., 2012; Zhao et al., 2013). Our previous fMRI study in female patients with migraine found alterated nodal centrality in the MTG (Liu et al., 2012), and this suggests that long-term and high-frequency headache attacks may lead to pathological cortical network reorganization in female patients with migraine. The correlation analysis results showed that the ALFF values in the MTG were negatively correlated with VAS scores (p < 0.05), and this suggest that MTG excitability in form of sensitization during the resting state may contribute to the severity of headache.
Based on the clinical outcomes of this study, both TA and SA helped treat migraine after 12 weeks of therapy (p < 0.05). Both treatments remarkably improved the emotional states and alleviated the clinical symptoms of migraine (VAS score and intensity of pain). These results are in line with those of our previous RCT report on migraine (Zhao et al., 2017) and those of previous studies (Smith et al., 2018; Liao et al., 2020). Furthermore, TA was more effective than SA in controlling the frequency of migraine attack, and this has been supported by the findings of several other RCTs (Li et al., 2012; Zhao et al., 2014; Wang et al., 2015).
The clinical effects of SA are similar to those of TA in terms of SAS, SDS, VAS score, and intensity of pain; therefore, we inferred that the clinical effects of SA may partly result from non-specific physiological effect experienced during needling or a placebo effect that originates from frequent patient-acupuncture practitioner interactions (Zhao et al., 2017). Non-specific effects are mostly thought to be due to psychobiological processes triggered by the overall therapeutic context (Finniss et al., 2010). Kong et al. (2006) found that after placebo acupuncture treatment, subjective pain-rating reduction on the placebo-treated side was significantly greater than on the control side, and this demonstrated that the non-specific effect or placebo effect may significantly contribute to the analgesic effect observed during acupuncture (Kong et al., 2013). Additionally, the invasive treatment techniques are almost always perceived by patients as profoundly meaningful, and this could contribute to symptom reduction (Kaptchuk et al., 2006). Taken together, acupuncture is an effective treatment that can improve the emotional state and relieve pain in patients with MMoA, and the current study demonstrates that TA has a relatively better effect in reducing the frequency of migraine attack than SA.
Based on the resting-state fMRI results, regions of the brain that usually respond to TA treatment in terms of both ALFF and ReHo values, were mainly in the frontal-limbic regions, that are associated with regulation of emotions. Previous neuroimaging studies (Fournier et al., 2013; Guo et al., 2015; Li et al., 2020b) have amassed substantial evidence regarding functional abnormalities in fronto-limbic structures. These could be a neurobiological basis for the pathophysiology and maintenance of emotional disorders (Phillips and Kupfer, 2013). Dysfunction in the frontal-limbic regions may reflect impaired high-order frontal regulatory effects on the areas of the limbic system that are related to emotions, and this may lead to mood dysregulation in patients with MMoA. Acupuncture can modulate the activity of cortical and subcortical regions involved in emotional processing (Zhang et al., 2012). The altered ALFF values in the ACC were positively correlated with reductions in the SAS and SDS scores (p < 0.05) after TA treatment. ACC is considered to be implicated in both affective and cognitive-attentional dimensions of pain and plays a deterministic role in pain modulation and analgesia (May, 2008).
In our previous neuroimaging studies, we verified that migraineurs showed a significant decrease in ALFF in the ACC (Xue et al., 2013) and showed aberrant functional connectivity involving the ACC (Xue et al., 2012; Yuan et al., 2013). The results of our previous study (Zhao et al., 2014) also suggested that alterations in ReHo values in the ACC might be the mechanism of acupuncture treatment in patients with migraine. In the present study, acupuncture-induced reduction in emotional state was positively associated with decreased average ALFF values in the ACC, which demonstrated that TA treatment could alleviate emotional disorders by modulating emotion-affected dysfunctional regions. Common brain regions that respond to SA treatment both in terms of ALFF and ReHo values are mainly in the insula, and this indicates that SA activates alterations in spontaneous brain activity, resulting in enhanced synchronization. The insula is a major site for processing emotions, and it is also involved in processing sensory discriminative aspects of pain perception (Duerden and Albanese, 2013). Functional imaging experiments have reported that the insular networks were altered in patients with migraine (Maleki et al., 2012), and our team further discovered abnormal patterns of functional connectivity in the insula (Xue et al., 2012; Yuan et al., 2013). However, Wager et al. (2004) found that the placebo effect was achieved through the insula’s regulation of the brain’s sensitivity to pain. Our findings demonstrate that the insula was activated by acupuncture stimulation, and it plays an important role in placebo modulation of pain perception. The insula cortex also plays a reciprocal role in emotions and pain-related emotions. In the current study, the SA group showed significant reductions in the SAS and SDS scores after treatment (p < 0.05). We speculated that SA may modulate the perception of pain in patients with MMoA through the insula and indirectly regulate pain-related emotions.
Conclusion
Our findings reveal that dysfunctions in the precuneus and the frontal-limbic regions may lead to mood dysregulation in patients with MMoA. Furthermore, temporal pole excitability as sensitization may contribute to the severity of headaches. Both TA and SA treatments remarkably improved the emotional states and alleviated the clinical symptoms of migraine. Although there was no difference between the two groups in the improvement of clinical symptoms, but we found that TA had a relatively better effect in controlling the frequency of migraine attacks than SA. Moreover, the effects of the two acupuncture methods on brain activity were significantly different. TA treatment might have the potential effect of alleviating emotional disorders by modulating the frontal-limbic regions, and SA may modulate the perception of pain in patients with MMoA through the placebo effect of the insula and by indirectly regulating emotional disorders.
Limitation
This study has several limitations. First, because hormonal testing was not carried out, we were unable to compare the hormone levels of patients in the two groups. Considering the important role of hormones in MMoA, hormonal assay should be introduced in future studies to explore the influence of hormones on neuroimaging results. Second, to explore the central mechanisms underlying acupuncture for the treatment of MMoA, we chose not to include a group of healthy subjects as controls at baseline, and this may have prevented us from exploring the pathogenic mechanisms of MMoA in detail. Third, we did not have an index to access and quantify expectations during acupuncture treatment sessions. Further studies need to quantify patients’ expectations and explore the effect of acupuncture on the clinical efficacy and physiological mechanism of some non-specific factors during long-term acupuncture treatment.
Data Availability Statement
The raw data supporting the conclusions of this article can be obtained on reasonable request from the corresponding author. Requests to access the datasets should be directed to LZ, zhaoling@cdutcm.edu.cn.
Ethics Statement
The studies involving human participants were reviewed and approved by Sichuan Traditional Chinese Medicine Regional Ethics Review Committee (approval number: 2015KL-004). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YTZ and LZ: conception and study design. ZWW, JRD, WY, YZ, HQL, and DHL: data collection or acquisition. YTZ, TX, and XW: statistical analysis. YTZ and ZWW: drafting the manuscript work. LZ: revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81973962 and 82004486), the National Natural Foundation for Excellent Youth Fund (81722050), the Department of Science and Technology of Sichuan Province (20ZDYF1199 and 21MZGC0224), and the First-Class Disciplines Development supported by Chengdu University of Traditional Chinese Medicine (CZYJC1901).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
ACC, anterior cingulate; ALFF, amplitude of low-frequency fluctuation; BOLD, blood oxygen level-dependent; CI, confidence interval; fMRI, functional magnetic resonance imaging; ICHD-3, third edition of the International Classification of Headache Disorders; MFG, middle frontal gyrus; MMoA, menstrual migraine without aura; MTG, middle temporal gyrus; RCT, randomized clinical trial; ReHo, regional homogeneity; SA, sham acupuncture; SAS, Zung self-rating anxiety scale; SDS, Zung self-rating depression scale; TA, true acupuncture; TCM, traditional Chinese medicine; VAS, visual analog scale.
Footnotes
References
Absinta, M., Rocca, M. A., Colombo, B., Falini, A., Comi, G., and Filippi, M. (2012). Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia 32, 109–115. doi: 10.1177/0333102411431334
Asari, T., Konishi, S., Jimura, K., Chikazoe, J., Nakamura, N., and Miyashita, Y. (2008). Right temporopolar activation associated with unique perception. NeuroImage 41, 145–152. doi: 10.1016/j.neuroimage.2008.01.059
Asmundson, G. J., and Katz, J. (2009). Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depres. Anxiety 26, 888–901. doi: 10.1002/da.20600
Cui, Y., Jiao, Y., Chen, Y. C., Wang, K., Gao, B., Wen, S., et al. (2014). Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 63, 749–760. doi: 10.2337/db13-0519
Desmond, J. E., and Glover, G. H. (2002). Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J. Neurosci. Methods 118, 115–128. doi: 10.1016/s0165-0270(02)00121-8
Duerden, E. G., and Albanese, M. C. (2013). Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum. Brain Map. 34, 109–149. doi: 10.1002/hbm.21416
Finniss, D. G., Kaptchuk, T. J., Miller, F., and Benedetti, F. (2010). Biological, clinical, and ethical advances of placebo effects. Lancet 375, 686–695. doi: 10.1016/s0140-6736(09)61706-2
Fournier, J. C., Keener, M. T., Almeida, J., Kronhaus, D. M., and Phillips, M. L. (2013). Amygdala and whole-brain activity to emotional faces distinguishes major depressive disorder and bipolar disorder. Bipolar. Disorders 15, 741–752. doi: 10.1111/bdi.12106
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S., and Turner, R. (1996). Movement-related effects in fMRI time-series. Magn. Reson. Med. 35, 346–355. doi: 10.1002/mrm.1910350312
Greicius, M. D., Flores, B. H., Menon, V., Glover, G. H., Solvason, H. B., Kenna, H., et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429–437. doi: 10.1016/j.biopsych.2006.09.020
Guo, W., Liu, F., Yu, M., Zhang, J., Zhang, Z., Liu, J., et al. (2015). Decreased regional activity and network homogeneity of the fronto-limbic network at rest in drug-naive major depressive disorder. Aus. New Zealand J. Psychiatry 49, 550–556. doi: 10.1177/0004867415577978
No Author. (2018). Headache classification committee of the international headache society (ihs) the international classification of headache disorders, 3rd edition. Cephalalgia 38, 1–211. doi: 10.1177/0333102417738202
No Author. (2013). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33, 629–808. doi: 10.1177/0333102413485658
Kaptchuk, T. J., Stason, W. B., Davis, R. B., Legedza, A. R., Schnyer, R. N., Kerr, C. E., et al. (2006). Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ 332, 391–397. doi: 10.1136/bmj.38726.603310.55
Karikari, T. K., Charway-Felli, A., Hã¶glund, K., Blennow, K., and Zetterberg, H. (2018). Commentary: Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the global burden of disease study 2015. Front. Neurol. 9:201. doi: 10.3389/fneur.2018.00201
Kitada, R., Sasaki, A. T., Okamoto, Y., Kochiyama, T., and Sadato, N. (2014). Role of the precuneus in the detection of incongruency between tactile and visual texture information: a functional MRI study. Neuropsychologia 64, 252–262. doi: 10.1016/j.neuropsychologia.2014.09.028
Kong, J., Gollub, R. L., Rosman, I. S., Webb, J. M., Vangel, M. G., Kirsch, I., et al. (2006). Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J. Neurosci. 26, 381–388. doi: 10.1523/jneurosci.3556-05.2006
Kong, J., Spaeth, R., Cook, A., Kirsch, I., Claggett, B., Vangel, M., et al. (2013). Are all placebo effects equal? Placebo pills, sham acupuncture, cue conditioning and their association. PLoS One 8:e67485. doi: 10.1371/journal.pone.0067485
Li, Y., Zheng, H., Witt, C. M., Roll, S., Yu, S. G., Yan, J., et al. (2012). Acupuncture for migraine prophylaxis: a randomized controlled trial. CMAJ 184, 401–410. doi: 10.1503/cmaj.110551
Li, Z., Li, K., Luo, X., Zeng, Q., Zhao, S., Zhang, B., et al. (2020a). Distinct brain functional impairment patterns between suspected non-alzheimer disease pathophysiology and alzheimer’s disease: a study combining static and dynamic functional magnetic resonance imaging. Front. Aging Neurosci. 12:550664. doi: 10.3389/fnagi.2020.550664
Li, Z., Liu, W., Xiao, C., Wang, X., Zhang, X., Yu, M., et al. (2020b). Abnormal white matter microstructures in Parkinson’s disease and comorbid depression: a whole-brain diffusion tensor imaging study. Neurosci. Lett. 735:135238. doi: 10.1016/j.neulet.2020.135238
Liao, C. C., Liao, K. R., Lin, C. L., and Li, J. M. (2020). Long-Term effect of acupuncture on the medical expenditure and risk of depression and anxiety in migraine patients: a retrospective cohort study. Front. Neurol. 11:321. doi: 10.3389/fneur.2020.00321
Liu, J., Zhao, L., Lei, F., Zhang, Y., Yuan, K., Gong, Q., et al. (2015). Disrupted resting-state functional connectivity and its changing trend in migraine suffers. Hum. Brain Map. 36, 1892–1907. doi: 10.1002/hbm.22744
Liu, J., Zhao, L., Li, G., Xiong, S., Nan, J., Li, J., et al. (2012). Hierarchical alteration of brain structural and functional networks in female migraine sufferers. PLoS One 7:e51250. doi: 10.1371/journal.pone.0051250
Maleki, N., Linnman, C., Brawn, J., Burstein, R., Becerra, L., and Borsook, D. (2012). Her versus his migraine: multiple sex differences in brain function and structure. Brain 135, 2546–2559. doi: 10.1093/brain/aws175
May, A. (2008). Chronic pain may change the structure of the brain. Pain 137, 7–15. doi: 10.1016/j.pain.2008.02.034
Olson, I. R., Plotzker, A., and Ezzyat, Y. (2007). The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130, 1718–1731. doi: 10.1093/brain/awm052
Pang, Y., Liao, H., Duan, G., Feng, Z., Liu, H., Zou, Z., et al. (2020). sanyinjiao Regulated aberrant amygdala functional connectivity in premenstrual syndrome via electro-acupuncture stimulation at acupoint(SP6). Gynecol. Endocrinol. 2020, 1–5. doi: 10.1080/09513590.2020.1855633
Pavlović, J. M., Stewart, W. F., Bruce, C. A., Gorman, J. A., Sun, H., Buse, D. C., et al. (2015). Burden of migraine related to menses: results from the AMPP study. J. Headache Pain 16:24. doi: 10.1186/s10194-015-0503-y
Peck, K. R., Smitherman, T. A., and Baskin, S. M. (2015). Traditional and alternative treatments for depression: implications for migraine management. Headache 55, 351–355. doi: 10.1111/head.12521
Pereira-Pedro, A. S., and Bruner, E. (2016). Sulcal pattern, extension, and morphology of the precuneus in adult humans. Ann. Anatomy 208, 85–93. doi: 10.1016/j.aanat.2016.05.001
Phillips, M. L., and Kupfer, D. J. (2013). Bipolar disorder diagnosis: challenges and future directions. Lancet 381, 1663–1671. doi: 10.1016/s0140-6736(13)60989-7
Pompili, M., Di Cosimo, D., Innamorati, M., Lester, D., Tatarelli, R., and Martelletti, P. (2009). Psychiatric comorbidity in patients with chronic daily headache and migraine: a selective overview including personality traits and suicide risk. J. Headache Pain 10, 283–290. doi: 10.1007/s10194-009-0134-2
Schwedt, T. J., and Dodick, D. W. (2009). Advanced neuroimaging of migraine. Lancet. Neurol. 8, 560–568. doi: 10.1016/s1474-4422(09)70107-3
Shen, Y. T., Yuan, Y. S., Wang, M., Zhi, Y., Wang, J. W., Wang, L. N., et al. (2020). Dysfunction in superior frontal gyrus associated with diphasic dyskinesia in Parkinson’s disease. NPJ Parkinson’s Dis. 6:30. doi: 10.1038/s41531-020-00133-y
Smith, C. A., Armour, M., Lee, M. S., Wang, L. Q., and Hay, P. J. (2018). Acupuncture for depression. Cochrane Database Syst. Rev. 3:CD004046. doi: 10.1002/14651858.CD004046.pub4
Sun, H., Luo, L., Yuan, X., Zhang, L., He, Y., Yao, S., et al. (2018). Regional homogeneity and functional connectivity patterns in major depressive disorder, cognitive vulnerability to depression and healthy subjects. J. Affect. Disord. 235, 229–235. doi: 10.1016/j.jad.2018.04.061
Trejo-Gabriel-Galan, J. M., Aicua-Rapún, I., Cubo-Delgado, E., and Velasco-Bernal, C. (2018). Suicide in primary headaches in 48 countries: a physician-survey based study. Cephalalgia 38, 798–803. doi: 10.1177/0333102417714477
Vetvik, K. G., and MacGregor, E. A. (2017). Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet. Neurol. 16, 76–87. doi: 10.1016/s1474-4422(16)30293-9
Wager, T. D., Rilling, J. K., Smith, E. E., Sokolik, A., Casey, K. L., Davidson, R. J., et al. (2004). Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303, 1162–1167. doi: 10.1126/science.1093065
Wang, X., Zhao, Y. Y., Cai, L. Y., Yang, Z. Y., Zhou, M. Y., Ren, Y. L., et al. (2018). Exploration on characteristics of modern acupuncture and moxibustion treatment of menstrual migraine based on data mining technology. Chin Archiv. Trad. 36, 423–427.
Wang, Y., Xue, C. C., Helme, R., Da Costa, C., and Zheng, Z. (2015). Acupuncture for Frequent migraine: a randomized, patient/assessor blinded, controlled trial with one-year follow-up. Evid. Based compl. Alternat. Med. 2015:920353. doi: 10.1155/2015/920353
Warnock, J. K., Cohen, L. J., Blumenthal, H., and Hammond, J. E. (2017). Hormone-related migraine headaches and mood disorders: treatment with estrogen stabilization. Pharmacotherapy 37, 120–128. doi: 10.1002/phar.1876
Xu, S., Yu, L., Luo, X., Wang, M., Chen, G., Zhang, Q., et al. (2020). Manual acupuncture versus sham acupuncture and usual care for prophylaxis of episodic migraine without aura: multicentre, randomised clinical trial. BMJ 368:m697. doi: 10.1136/bmj.m697
Xue, T., Yuan, K., Cheng, P., Zhao, L., Zhao, L., Yu, D., et al. (2013). Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed. 26, 1051–1058. doi: 10.1002/nbm.2917
Xue, T., Yuan, K., Zhao, L., Yu, D., Zhao, L., Dong, T., et al. (2012). Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One 7:e52927. doi: 10.1371/journal.pone.0052927
Yan, C. G., Wang, X. D., Zuo, X. N., and Zang, Y. F. (2016). DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14, 339–351. doi: 10.1007/s12021-016-9299-4
Yang, M., Du, T., Long, H., Sun, M., Liang, F., and Lao, L. (2020). Acupuncture for menstrual migraine: a systematic review. BMJ Sup. Palliat. Care 2020:24. doi: 10.1136/bmjspcare-2019-002024
Yu, S., Dong, X., Sun, R., He, Z., Zhang, C., Chen, M., et al. (2020). Effect of acupuncture and its influence on cerebral activity in patients with persistent asthma: study protocol for a randomized controlled clinical trial. Trials 21:406. doi: 10.1186/s13063-020-04319-w
Yuan, K., Zhao, L., Cheng, P., Yu, D., Zhao, L., Dong, T., et al. (2013). Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J. Pain 14, 836–844. doi: 10.1016/j.jpain.2013.02.010
Zang, Y., Jiang, T., Lu, Y., He, Y., and Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. NeuroImage 22, 394–400. doi: 10.1016/j.neuroimage.2003.12.030
Zang, Y. F., He, Y., Zhu, C. Z., Cao, Q. J., Sui, M. Q., Liang, M., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91. doi: 10.1016/j.braindev.2006.07.002
Zhang, Z. J., Wang, X. M., and McAlonan, G. M. (2012). Neural acupuncture unit: a new concept for interpreting effects and mechanisms of acupuncture. Evid. Based Compl. Altern. Med. 2012:429412. doi: 10.1155/2012/429412
Zhao, L., Chen, J., Li, Y., Sun, X., Chang, X., Zheng, H., et al. (2017). The long-term effect of acupuncture for migraine prophylaxis: a randomized clinical trial. JAMA Int. Med. 177, 508–515. doi: 10.1001/jamainternmed.2016.9378
Zhao, L., Liu, J., Dong, X., Peng, Y., Yuan, K., Wu, F., et al. (2013). Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J. Headache Pain 14:85. doi: 10.1186/1129-2377-14-85
Zhao, L., Liu, J., Zhang, F., Dong, X., Peng, Y., Qin, W., et al. (2014). Effects of long-term acupuncture treatment on resting-state brain activity in migraine patients: a randomized controlled trial on active acupoints and inactive acupoints. PLoS One 9:e99538. doi: 10.1371/journal.pone.0099538
Zhu, J., Lin, X., Lin, C., Zhuo, C., and Yu, Y. (2018). Selective functional dysconnectivity of the dorsal-anterior subregion of the precuneus in drug-naive major depressive disorder. J. Affect. Disord. 225, 676–683. doi: 10.1016/j.jad.2017.08.084
Keywords: acupuncture, menstrual migraine without aura, emotional disorders, pain, fMRI
Citation: Zhang Y, Wang Z, Du J, Liu J, Xu T, Wang X, Sun M, Wen Y, Li D, Liao H, Zhao Y and Zhao L (2021) Regulatory Effects of Acupuncture on Emotional Disorders in Patients With Menstrual Migraine Without Aura: A Resting-State fMRI Study. Front. Neurosci. 15:726505. doi: 10.3389/fnins.2021.726505
Received: 17 June 2021; Accepted: 10 September 2021;
Published: 04 October 2021.
Edited by:
Man Li, Huazhong University of Science and Technology, ChinaReviewed by:
Dahua Yu, Inner Mongolia University of Science and Technology, ChinaQing Gao, University of Electronic Science and Technology of China, China
Boyi Liu, Zhejiang Chinese Medical University, China
Copyright © 2021 Zhang, Wang, Du, Liu, Xu, Wang, Sun, Wen, Li, Liao, Zhao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Zhao, zhaoling@cdutcm.edu.cn
†These authors have contributed equally to this work
 Yutong Zhang
Yutong Zhang Ziwen Wang1,2†
Ziwen Wang1,2† Jixin Liu
Jixin Liu Xiao Wang
Xiao Wang Ling Zhao
Ling Zhao