- Department of Neurology and Tianjin Neurological Institute, Tianjin Medical University General Hospital, Tianjin, China
Guillain-Barré syndrome (GBS) is a rare postoperative complication that is sometimes characterized by serious motor weakness and prolonged weaning from mechanical ventilation. Although the exact nature of the relationship between GBS and the surgical procedure is still unclear, there is a clear increased incidence of GBS in post-surgical patients compared to non-surgical patients. GBS after surgery is unique in several ways. The course of post-surgical GBS unfolds more rapidly than in other situations where GBS develops, the condition is often more severe, and respiratory muscles are more commonly involved. Prompt diagnosis and appropriate treatment are essential, and the condition can worsen if treated inappropriately. Postoperative sedation, intubation, and restraint use make the diagnosis of GBS difficult, as the onset of symptoms of weakness or numbness in those contexts are not obvious. GBS is often misdiagnosed, being attributed to other postoperative complications, and subsequently mishandled. The lack of relevant information further obscures the clinical picture. We sought to better understand post-surgical GBS by performing an analysis of the relevant literature, focusing on clearly documenting the clinical characteristics, diagnosis, and management of GBS that emerges following surgery. We underscore the importance of physicians being aware of the possibility of GBS after major surgery and of performing a variety of laboratory clinical investigations early on in suspected cases.
Introduction
Guillain-Barré syndrome (GBS) is the most common acute paralytic neuropathy worldwide; it presents primarily as symmetric ascending motor weakness (1, 2). Most often in GBS, the onset of weakness is preceded by a bacterial or viral infection, leading to stimulation of the immune system. Among the antecedent factors that have been associated with GBS, the bacterium Campylobacter jejuni is the most common infectious agent associated with GBS (3, 4). Following infection, the acute progression of limb weakness peaks within 4 weeks; 25% of patients may develop respiratory insufficiency and autonomic disturbances. Even with timely and adequate treatment, up to 20% of GBS patients remain unable to walk, and up to 5% of them will die sooner than their healthy cohort (5).
Although GBS is a typical post-infection illness, in most patients with post-surgical GBS, no viral or bacterial infection is mentioned in their medical history, and the only clearly identified antecedent event is the operation. Several reviews and case reports (1, 6–12) suggest the surgical procedure itself is the triggering event for GBS. However, the evidence supporting this notion is meager. Although the exact mechanism underlying post-surgical GBS remains unclear, it is believed that having surgery significantly increases the risk for GBS (6–8). Surgeons may be unfamiliar with post-surgical GBS, as its incidence is low. Thus, they may attribute any weakness or numbness in limbs after surgery to other postoperative complications, leading to the patient being misdiagnosed and treated incorrectly.
In this review of the literature, we describe the clinical characteristics, diagnosis, and management of this syndrome with an emphasis on these factors as they relate to patients that acquired GBS after surgery. Table 1 summarizes the relevant articles on which this review is based.
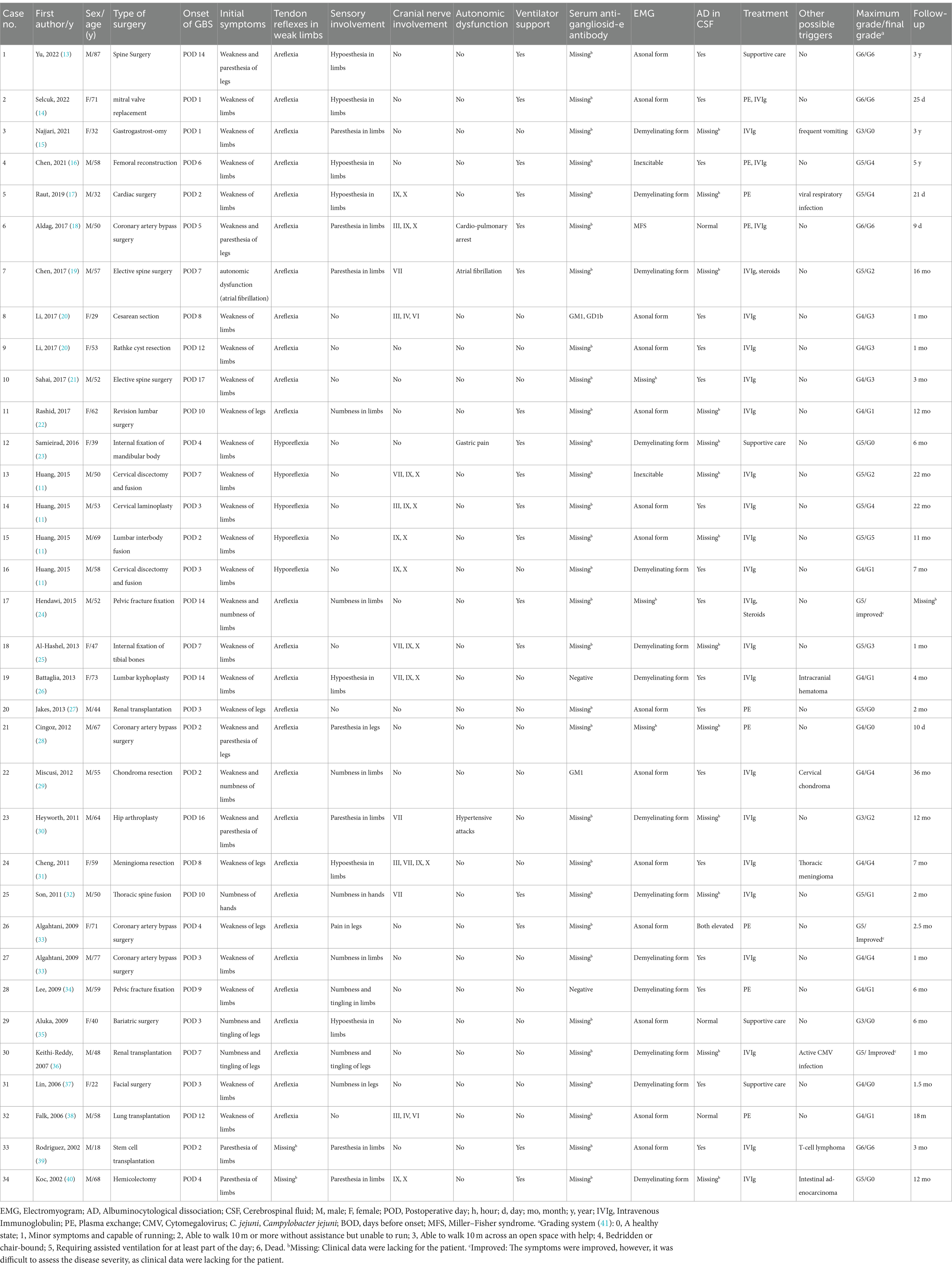
Table 1. Clinical characteristics of 34 cases published within the past two decades (2002–2022) that developed Guillain-Barré syndrome after surgery.
Retrieval strategy
The studies cited in this review were retrieved through an electronic search of the MEDLINE/PubMed and Embase datasets. The following keyword combinations were used to preliminarily select the articles to be evaluated: (“Guillain-Barre syndrome” or “GBS” or “acute inflammatory demyelinating polyneuropathy” or “AIDP” or “acute motor axonal neuropathy” or “AMAN” or “acute motor sensory axonalneuropathy” or “AMSAN”) AND (“surgery” or “operation” or “post-operative” or “post-surgical”). Most of the elected studies were published between 2002 and 2022. Only papers in English were reviewed. Articles were selected for their relevance, with a preference for new papers. Some other relevant papers known by the authors were also included.
Epidemiology
Post-surgical GBS is a rare neurological disease. Its exact incidence is unclear, because rates reported in the literature vary due to differences in the numbers of patients studied. For example, in one case series of 63 GBS patients, 6 cases (9.5%) were diagnosed with GBS within 6 weeks after surgery (8). This indicated that the relative risk of developing GBS was 13.1 times higher than the incidence in the study population, which translated to an incidence of GBS of 4.5 per 100,000 surgeries. In a study of 69 GBS patients, 4 cases (5.8%) developed GBS within 6 weeks of surgery (7). This means that the relative risk of developing GBS was 6 times higher than the incidence in the general population, which translated to an incidence of 6.28 per 100,000 operations. In a study of 36 GBS patients, 7 patients (19.4%) developed GBS following surgery (10), which was a greater percentage than that observed in the two aforementioned studies (7, 8). Finally, in a 2015 retrospective study of spinal surgery patients, Huang and colleagues observed that the incidence of GBS after surgery was up to 51.5 per 100,000 surgeries (11). Although the incidences of post-surgical GBS varied widely in these studies, they indicate that the risk of developing GBS after surgery is significantly increased. Although surgery may increase the incidence of GBS, the pathological process is still unclear.
Subtypes of GBS and post-surgical GBS
The most common subtypes of GBS are the demyelinating form (i.e., acute inflammatory demyelinating polyneuropathy); the axonal form (i.e., acute motor axonal neuropathy, acute motor-sensory axonal neuropathy); and its variant form [i.e., Miller-Fisher syndrome (MFS)]. All three forms of GBS have been reported to occur following surgery (see Table 1). However, differentiation between the three GBS subtypes is not easy during the early stage of the disease. Although distinguishing the three subtypes has no diagnostic value, it is important to do so because each has a different prognosis and treatment strategy.
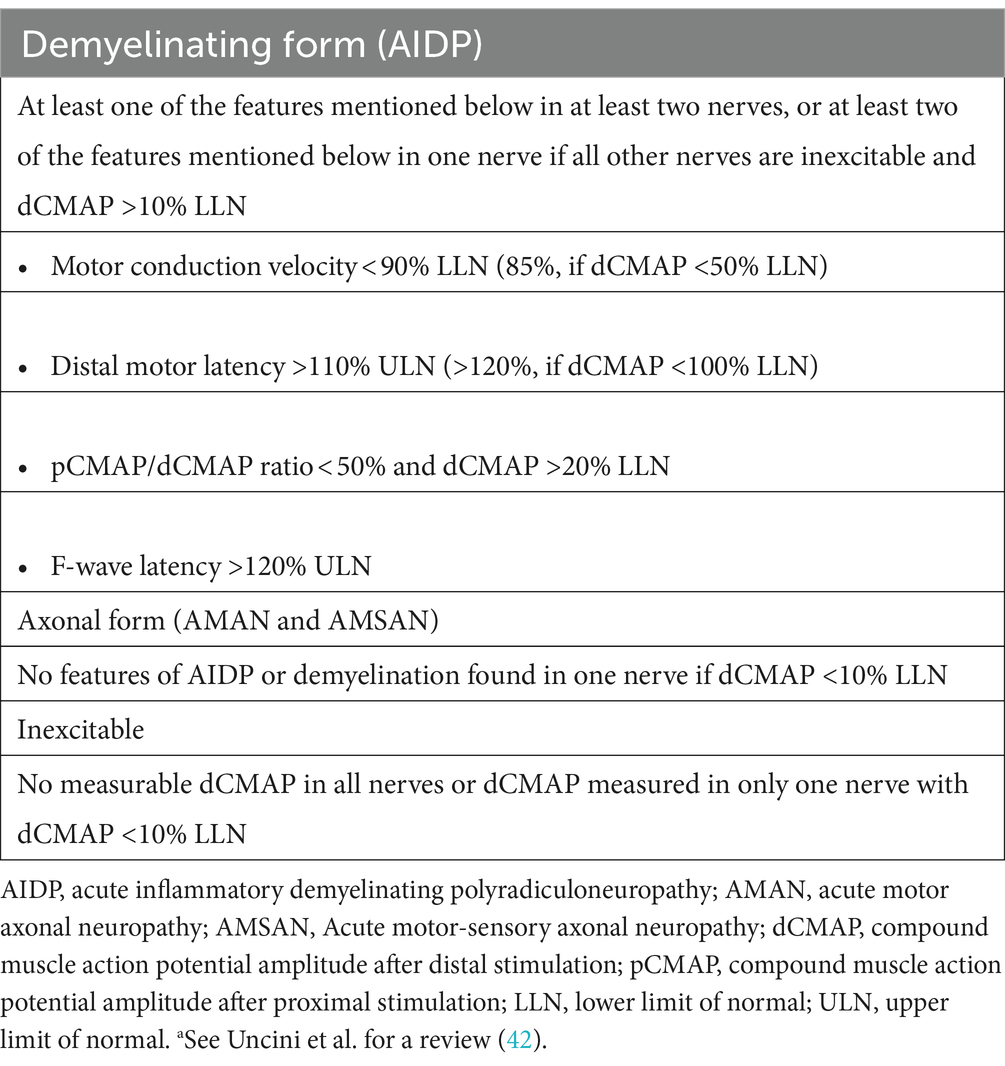
The demyelinating form is a sensorimotor subtype of GBS, which is characterized primarily by paresis and mild sensory dysfunction. The axonal form is characterized by more rapid paresis, presence of more frequent cranial nerve deficits, and autonomic dysfunction. MFS is characterized by ophthalmoplegia, areflexia, and ataxia. Classification into demyelinating or axonal forms of GBS is mainly based on electrophysiological studies and subsequently supported by the presence or absence of specific anti-ganglioside antibodies (Tables 2, 3). With regard to post-surgical GBS, the axonal subtype is more common than the demyelinating subtype (10, 43, 44). This is consistent with the study of Staff and colleagues, who observed that nerve biopsies from 21 patients with post-surgical neuropathies showed more axonal degeneration than segmental demyelination (43). Of the 34 patients analyzed in the present study, 14 had the demyelinating subtype of GBS, 14 had the axonal subtype, and 1 had MFS. In the remaining 4 patients, however, it was difficult to determine which GBS subtype they had, as clinical data were lacking for these patients. Since the subtype in 5 of the 34 patients (14.7%) was indeterminable, it may bias somewhat the subtype analysis.
Pathogenesis
Although more and more cases of post-surgical GBS have been documented after various types of surgical procedures, its underlying mechanism remains unclear. As the occurrence of GBS is either spatially or temporally separated from the operation, or both, a disruption of the inflammatory response or an autoimmune response is hypothesized to play a role in post-surgical GBS (43, 44). This is consistent with the finding that some patients with post-surgical GBS have elevated serum anti-ganglioside antibodies (20, 29), in the absence of viral or bacterial infection in their medical history. Also, nerve biopsies of 21 patients with non-traumatic post-surgical neuropathy showed endoneurial macrophage (CD-68 positive) infiltration and increased epineurial perivascular lymphocytic (CD45+ positive cells) inflammation of the nerves (43), suggesting that an immune response might play a role in surgery-associated neuropathies. However, in post-surgical GBS cases published over the past two decades, 7 of 34 patients developed neuropathy within 3 days after surgery (11, 14, 15, 17, 28, 29, 39). This three-day time interval between surgery and GBS onset is shorter than the expected time needed for an immune response to develop.
Thus, the simplest hypothesis for the GBS-surgery association could be that a nonspecific mechanism is responsible. In all likelihood, then, neuropathy may develop as a result of a complex interaction between various factors, such as a stress response, the surgical procedure itself, the primary comorbid disease, anesthesia, subclinical exogenous infections, and genetic factors. All of these factors, or a combination of them, can induce alterations in immune tolerance, predisposing T-cells to become dysregulated (43, 45–47). Although what triggers the immune attack on nerves after surgery is yet to be elucidated, it is evident from these studies that an immune-mediated mechanism may play an important role in patients with post-surgical GBS.
Diagnosis
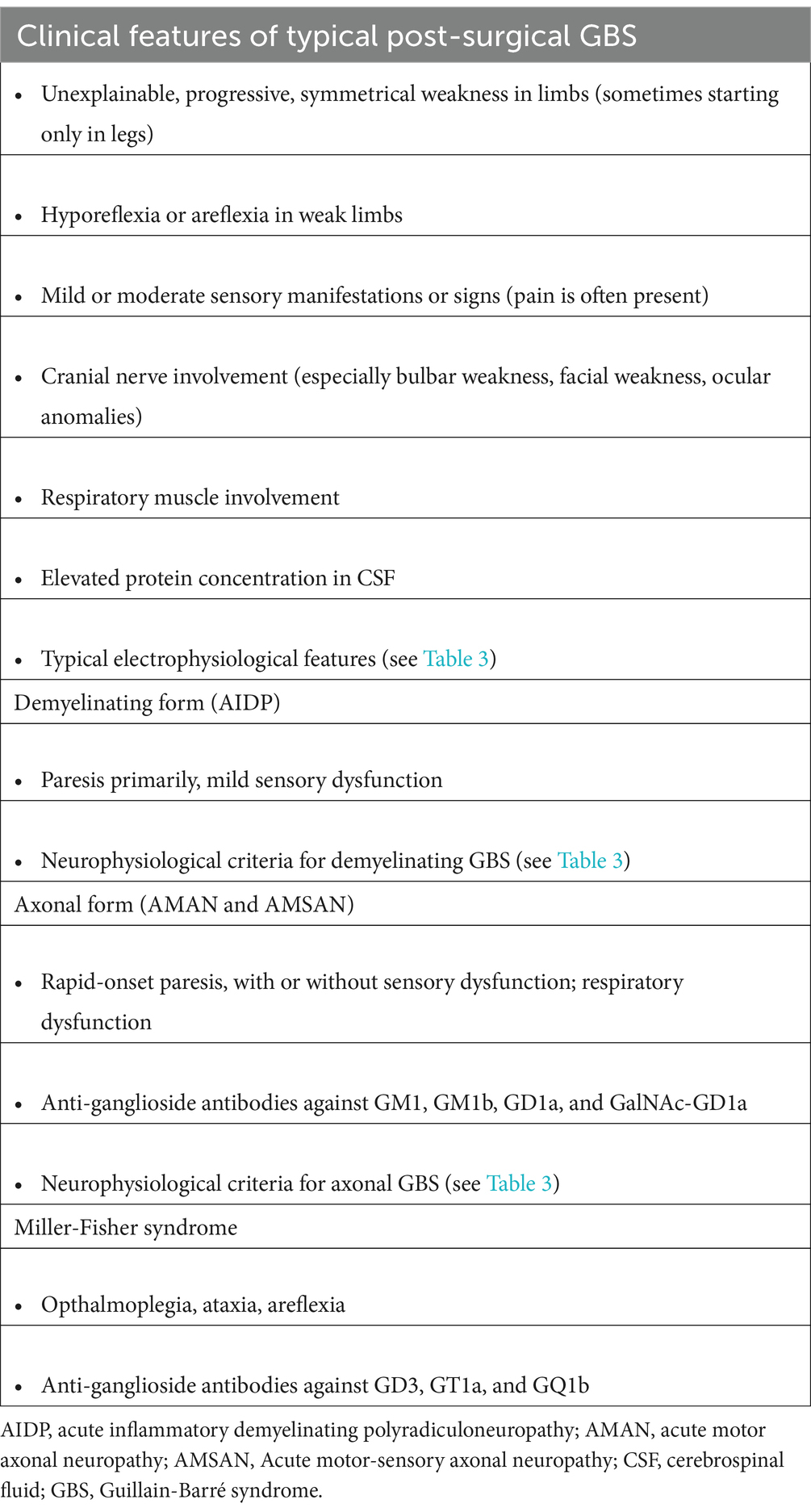
As with any patient with GBS, patients with surgery-related GBS are diagnosed largely based on clinical features (48, 49). In the surgical setting and considering the patient’s comorbidities, establishing a definitive diagnosis remains challenging because GBS symptoms significantly overlap with those resulting from other complications after surgery. Consistent with the European Academy of Neurology/Peripheral Nerve Society diagnostic criteria for GBS, the typical symptoms of post-surgical GBS are progressive, relatively symmetric bilateral muscle weakness in the extremities, and the presence of hyporeflexia or areflexia in the weak limbs at a minimum (50). In addition, some patients may present with mild to moderate peripheral sensory symptoms (e.g., numbness, paresthesia, pain).
When a patient exhibits unexplainable progressive muscle weakness or sensory symptoms in the limbs after surgery, GBS should be considered. Usually, weakness starts in the distal lower extremities, but, in some cases, it can start in the hands. Cranial nerve deficits may also be present, resulting in swallowing difficulties, facial weakness, or ocular anomalies. Weakness in the respiratory muscles can lead to the need for mechanical ventilation and prolonged weaning failure after surgery. Clinicians needs to bear in mind, however, that the clinical presentation of GBS can vary in regard to the involvement and severity of weakness, sensory symptoms, cranial nerve deficits, and respiratory dysfunction, which make it more difficult to diagnose. The typical clinical features of post-surgical GBS are summarized in Table 2.
Additional tests and examinations may be helpful to confirm the diagnosis or to exclude the possibility that other complications might be caused by something else. In GBS patients, cerebrospinal fluid (CSF) examination typically reveals albuminocytologic dissociation in the second week after symptom onset. Electromyography is also a mainstay of clinical investigations and is helpful for determining whether the disease is an axonal or demyelinating neuropathy.
Clinical characteristics and disease course
To better appreciate the clinical course of post-surgical GBS, we compiled and analyzed the clinical data of cases published over the past 20 year. In total, we identified 34 patients, who met the diagnostic criteria of GBS and developed a peripheral neuropathy within 6 weeks of a surgical procedure and outside of the immediate post-operative period (<6 weeks and > 1 day before first symptoms) (8). These 34 patients comprise an exhaustive list of all post-surgical GBS patients reported in the literature from 2002 to 2022 (11, 13–40). Details of the clinical presentation in these patients are provided in Table 1. Analysis of these cases revealed that a wide spectrum of different surgical procedures can precede the onset of GBS, including spinal, cranial, cardiac, orthopedic, abdominal, and transplant surgery. The median time interval between surgical procedure and onset of neuropathy was 6.6 days (range, 1–17 days). The mean age of patients was 53.6 years (range, 18–87). The male-to-female gender ratio was 1.8 (22 men and 12 women).
All patients reported an acute or subacute onset of symptoms. Rapid progressive weakness of the limbs was the main clinical feature. The initial symptoms were limb weakness, numbness, paresthesia, or a combination of these symptoms. These symptoms could initially occur distally, but eventually they spread proximally. The bulbar nerves were often affected; the facial and ocular motor nerves were less often affected. Respiratory insufficiency occurred in 17 of the 34 cases (50.0%), requiring artificial ventilation. Sensory involvement was present in 23 patients (67.6%), which was described as paresthesia, hypoesthesia, hyperesthesia, numbness, and pain. Autonomic dysfunction occurred in 11.8% of the cases, sometimes as the first symptom. It led to cardiac arrhythmia, urine retention, hypertension, and gastric pain. Neuropathy severity was quantified at the nadir of symptoms and signs using the GBS disability score; the median score was 4.5 (range, 6–3), indicating confinement to bed or chair. Following intravenous immunoglobulin (IVIg), or plasma exchange (PE) treatment, or both, most patients experienced clinical improvement, albeit in different degrees; after these treatments, the median GBS disability score was 2.0 (range, 5–0). Despite timely immunotherapy, of the 34 patients, 11 (32.4%) were unable to walk without assistance, and 4 died (11.8%). Clearly, post-surgical GBS remains a severe disease, and better treatment is needed for some patients.
Assessment scales
Patients with post-surgical GBS have highly diverse clinical manifestations and courses. Thus, assessment scales would be useful not only to detect GBS but also to monitor its progression. In this regard, the Peripheral Neuropathy Measures Outcome Study investigated a variety of assessment scales, with the goal of identifying an instrument capable of detecting GBS early in its course, reliably assessing disease severity, and capturing subtle disease-associated clinical changes. In that study, the research group reached consensus recommendations for the GBS Disability Scale and the Rasch-built Overall Disability Scale to measure disability, and the Medical Research Council (MRC) sum score and the new Rasch-built MRC score to measure muscle strength (51).
The GBS Disability Scale assesses functional status; its scores range from 0 (healthy) to 6 (dead) (41). The Rasch-built Overall Disability Scale assesses GBS patients on 24 items, grading each from 0 (unable to perform) to 2 (able to perform without difficulty) (52). The MRC sum score is a summation of MRC grades (range, 0–5) for performance of six pairs of muscles of the upper and lower limbs. The sum score ranges from 60 (normal strength) to 0 (total paralysis) (53). The Rasch-built MRC Scale assesses GBS patients on 12 items, grading each from 3 (normal strength) to 0 (paralysis) (54).
Additional clinical investigations for GBS
Lumbar puncture
A main feature of GBS is albuminocytological dissociation. This condition is defined by as an elevated CSF protein level but normal white blood cell count in the CSF. Of the 21 cases in the present analysis that had CSF data, 17 (80.9%) had albuminocytological dissociation. CSF analysis was not performed in 13 cases because a postoperative back wound precluded a spinal tap, or the patients declined the procedure.
CSF analysis is not necessary for diagnosing GBS. It is often performed in patients suspected of having GBS and is primarily performed to rule out other causes of muscle weakness rather than to confirm the GBS diagnosis. In the first week after onset of weakness, albuminocytologic dissociation occurred in approximately 50% of patients, although this percentage increased to no more than 80% in the third week (55). If CSF protein levels are normal, the diagnosis of GBS cannot be excluded, and repeat lumbar punctures are usually not recommended. Some patients with GBS or patients receiving IVIg exhibit a mild increase in CSF cell counts. Other differential diagnoses should be taken into account when the CSF cell count is more than 50 cells per microliter (3).
Electrophysiological examination
Electrophysiological analyses play an important role in the diagnosis of GBS and discriminating between axonal and demyelinating subtypes. It is sometimes difficult to establish the proper diagnosis of GBS and the correct classification of GBS subtypes in the early phase of the disease, especially in atypical cases. Electrophysiological analyses can confirm subclinical abnormalities in peripheral neuropathy and aid the clinical diagnosis. To avoid the pitfalls due to reversible conduction failure and length-dependent reduction of compound muscle action potential amplitude, serial electrophysiological studies can help distinguish the GBS subtypes (56).
To acquire sufficient information, the electrophysiological examination usually includes nerve conduction studies (NCS) involving multisite stimulation. Records of F-waves and H-reflexes should be acquired in at least four motor nerves and three sensory nerves (5, 57). NCS abnormalities tend to be remarkable as the disease progresses, peaking 2 weeks after onset of muscle weakness (42). Although this examination can be performed at earlier stages of the disease course, NCS findings might still be normal, and F-waves might have prolonged latency or reduced persistence at this stage.
The electrophysiological characteristics of the affected nerves depend on the GBS subtype (3, 5). The nerves of patients with the demyelinating subtype exhibit prolonged distal motor latency, reduced nerve conduction velocity, prolonged F-wave latency, increased temporal dispersion, and conduction block. The nerves of patients with the axonal subtype exhibit decreased motor and/or sensory nerve amplitudes. Clinicians should pay attention to patients with reversible nerve conduction failure caused by impaired conduction at the node of Ranvier, as these patients are often misdiagnosed as having demyelinating instead of axonal GBS. The nerves of patients with MFS exhibit abnormal sensory conduction. Evoked sensory nerve amplitudes initially decrease and then return to normal with clinical improvement. Serial NCS might be helpful to reliably classify these GBS subtypes (58, 59).
Testing for serum anti-ganglioside antibodies
Gangliosides are important cell membrane components in peripheral nerves. Serum anti-ganglioside antibodies have been found in patients exposed to Campylobacter jejuni and Mycoplasma pneumoniae, as well as in patients not exposed to these pathogens. Interestingly, most of these antibodies are associated with a specific subtype of GBS (3, 60). Testing for these antibodies can be helpful in the diagnosis and sub-classification of GBS. IgG autoantibodies to GM1, GM1b, GD1a, and GalNac-GD1a are frequently found in patients with acute motor axonal neuropathy or its axonal GBS variants, whereas IgG autoantibodies to GD3, GT1a, and GQ1b are often found in ophthalmoplegia and MFS. In contrast, an association between serum auto-antibodies and the demyelinating form of GBS has not been clearly established (61). Conclusions of axonal GBS or MFS based on anti-ganglioside antibodies should be made cautiously, however, because these tests have limited negative or positive predictive value (3). Although anti-ganglioside antibodies participate in the pathogenesis of GBS, their roles in accurately diagnosing GBS have not been confirmed. More research in this area is needed.
Differential diagnosis
Due to varying degrees of dysfunction and different distribution of deficits in sensory and motor nerves, the clinical presentation of GBS varies. Also, numerous perioperative complications, such as infection, herniated disk, spinal cord infarct, metabolic dysfunction, etc., could mimic GBS symptoms. Sometimes, post-surgical GBS can be difficult to diagnose, especially in patients with atypical symptoms. Differential diagnosis of GBS depends on the clinician recognizing the localization of lesions in the nervous system (muscles, neuromuscular junction, peripheral nerves, spinal cord, brainstem, or conus lesion). Laboratory investigations (e.g., to identify electrolyte disturbances), CSF examination, spinal cord MRI, and electrophysiological examination are recommended to identify other causes of GBS-like symptoms. The presence of distal sensory nerve deficits supports a GBS diagnosis. However, if no sensory involvement exists, differential diagnoses such as myasthenia gravis, acute myopathy, or electrolyte disturbance should be excluded. If paralysis develops abruptly after spine surgery, an MRI of the spine should be performed and spinal cord compression should be ruled out.
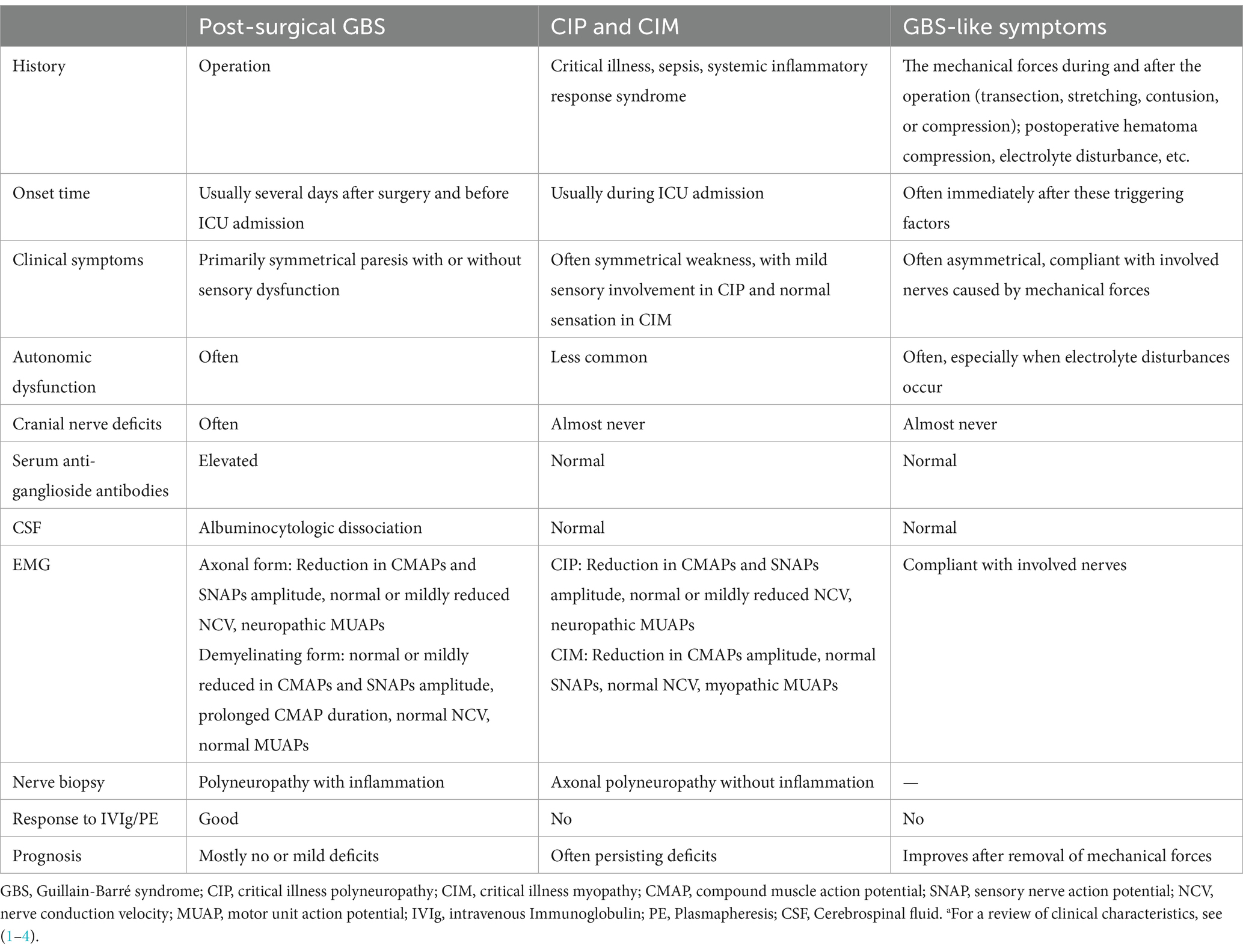
The existence of post-surgical GBS highlights the importance of considering critical illness polyneuropathy (CIN) and critical illness myopathy (CIM) in the differential diagnosis. In patients with CIN and CIM, cranial nerve involvement leading to extraocular motor dysfunction, bifacial weakness, or swallowing difficulties are uncommon (62–64). The degree of sensory nerve involvement tends to be mild in CIN and normal in CIM. Albuminocytological dissociation in CSF and anti-ganglioside antibodies in serum are almost never observed in CIN and CIM. Myopathic motor unit potentials on needle electromyography can be found in CIM. Finally, CIN and CIM do not generally respond to IVIg and/or PE. Despite its clinical features, in a critical illness setting, it remains a diagnostic challenge to diagnose GBS early in the course of disease. Table 4 summarizes the characteristics of post-surgical GBS, critical illness polyneuropathy, critical illness myopathy, and GBS-like symptoms.
Treatment
Post-surgical GBS is a severe neurologic complication after surgery. Once the diagnosis is established, treatment should be administered as soon as possible before irreversible nerve injury occurs. Timely hospitalization is recommended. Treatment of post-surgical GBS is similar to that of non-surgical-associated GBS and usually involves general care and immunotherapy.
General care
Even with adequate treatment, up to 5% of GBS patients die from complications. Until their condition begins to stabilize, all GBS patients should be hospitalized for observation. Multidisciplinary care is needed to prevent and treat serious complications. Respiratory insufficiency is more likely to occur in cases with rapid disease progression, facial and/or bulbar palsy, or severe weakness at hospital admission (64, 65). In the present study, 17 of the 34 cases (50.0%) with post-surgical GBS experienced respiratory insufficiency. Thus, clinicians need to pay attention to patients’ pulmonary function. In general, predictors of ventilator support include hypercarbia, hypoxemia, and a vital capacity of less than 15 mL/kg (66).
Although autonomic dysfunction occurs throughout the course of GBS, it mainly occurs during the progressive phase of the disease. Autonomic instability, such as cardiac arrhythmia, blood pressure instability, and gastrointestinal dysfunction, have been reported in the literature (18, 19, 23, 30), and it is sometimes a serious problem that can lead to sudden death. Thus, all patients at high risk for respiratory failure or autonomic dysfunction should be carefully monitored and transferred to an intensive care unit as soon as possible. Among the cohort of 34 cases we analyzed, 10 (29.4%) experienced choking while drinking, indicating that swallowing needs to be assessed early on so that patients at high risk of aspiration pneumonia can be identified and monitored. During the progressive phase, irritating sensory nerve symptoms described as paresthesia, numbness, pain, and tingling occurred in 16 of the 34 post-surgical GBS patients (47.1%). Early recognition of these symptoms is essential. Treatment with opioids, gabapentin, or carbamazepine improve sensory symptoms, but glucocorticoids have not (67). Other possible complications, such as urinary retention and deep-vein thrombosis, require careful management.
Immunotherapy
Starting immunotherapy early on speeds recovery and improves prognosis. Although a definitive diagnosis of GBS is difficult early in the disease course, empirical treatment with IVIg or PE should be initiated, if GBS is suspected and sufficient evidence exists to support its tentative diagnosis.
PE therapy improves symptoms when given during the first 4 weeks after disease onset (68). Its effects, however, tends to be largest when treatment is applied within the first 2 weeks of symptom onset. PE is thought to nonspecifically remove circulating antibodies and complement factors. The usual empirical regimen is a total exchange of five plasma volumes over a period of 2 weeks (68). Among patients who are still able to walk, however, two plasma exchange sessions aids in amelioration of motor deficits more rapidly than does no PE.
Another form of immunotherapy given to GBS patients is IVIg. IVIg is given at a dose of 0.4 g/kg daily for 5 consecutive days (or 1 g/kg daily for 2 days) (69). IVIg is thought to neutralize neurotoxic antibodies and inhibit the autoantibody-mediated complement cascade. Because of large variations in the pharmacokinetics of IVIg across individuals, patients who have a smaller increase in serum IgG level after IVIg administration might have a poor outcome. There is no evidence to support a second course of IVIg is beneficial for patients who have a poor prognosis, or who continue to deteriorate, the International Guillain-Barré Syndrome Outcome Study indicates the need for treatment trials with other immune modulators in patients severely affected by GBS (70).
Since results from a randomized controlled trial (69) showed that IVIg is as effective as PE in patients who are unable to walk independently, IVIg has replaced PE as the preferred choice of treatment for GBS patients in many hospitals because of its minor adverse effects and greater convenience. However, patients with acute motor axonal neuropathy might have a better outcome when treated with PE than IVIg (71). The administration of PE followed by a course of IVIg does not confer significantly better therapeutic results than application of either PE or IVIg alone. After correcting for known prognostic factors, van Koningsveld et al. observed that treatment composed of methylprednisolone and IVIg conferred short-term therapeutic benefits for some GBS patients (72). However, overall this combination therapy did not result in significantly better outcomes than IVIg alone. Moreover, prednisolone or methylprednisolone failed to significantly improve long-term outcome (73).
Prognosis
Although post-surgical GBS is potentially reversible, even when treated with standard therapy, patient outcome varies substantially. If left untreated, patients with post-surgical inflammatory neuropathy can either improve spontaneously or deteriorate (43). Recovery is associated with multiple factors (74–77), such as age, symptoms at nadir, type of therapy, primary disease, etc. In adults, severity of disease at nadir, such as being bedbound or requiring ventilation support, is usually considered to be a poor prognostic factor (78). Patients who can still walk 2 weeks after onset are likely to improve with or without treatment, but they may be left with some permanent neurologic impairment (79–81). Most improvement occurs during the first year after onset, but patients can recover further after this period. Even if they experience good functional recovery, many patients who survive GBS have to change their lifestyle (82).
Conclusion
Post-surgical GBS is a rare but serious neurologic complication after surgery that can mimic various GBS-like symptoms. Its characteristic symptoms are rapidly progressive muscle weakness and areflexia, often accompanied by dysphagia, facial paralysis, or respiratory failure. When there is unexplained symmetrical weakness after a major surgical procedure, GBS should be considered. Early diagnosis and timely therapy are imperative to prevent further neurological damage. Electrophysiological examination of peripheral nerves and CSF analysis can aid the clinical diagnosis. Treatment with IVIg or PE can improve symptoms.
Author contributions
XL: Formal analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. CZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Natural Science Foundation of Tianjin Education Commission (2020KJ159) and Tianjin Key Medical Discipline (Specialty) Construction Project. The sponsors played no role in the study design, data collection, and analysis, or decision to submit the article for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shahrizaila, N, Lehmann, HC, and Kuwabara, S. Guillain-Barre syndrome. Lancet. (2021) 397:1214–28. doi: 10.1016/S0140-6736(21)00517-1
2. Laman, JD, Huizinga, R, Boons, GJ, and Jacobs, BC. Guillain-Barre syndrome: expanding the concept of molecular mimicry. Trends Immunol. (2022) 43:296–308. doi: 10.1016/j.it.2022.02.003
3. van den Berg, B, Walgaard, C, Drenthen, J, Fokke, C, Jacobs, BC, and van Doorn, PA. Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. (2014) 10:469–82. doi: 10.1038/nrneurol.2014.121
4. Leonhard, SE, van der Eijk, AA, Andersen, H, Antonini, G, Arends, S, Attarian, S, et al. An international perspective on preceding infections in Guillain-Barre syndrome: the Igos-1000 cohort. Neurology. (2022) 99:e1299–313. doi: 10.1212/WNL.0000000000200885
5. Willison, HJ, Jacobs, BC, and van Doorn, PA. Guillain-Barre syndrome. Lancet. (2016) 388:717–27. doi: 10.1016/S0140-6736(16)00339-1
6. Rudant, J, Dupont, A, Mikaeloff, Y, Bolgert, F, Coste, J, and Weill, A. Surgery and risk of Guillain-Barre syndrome: a French Nationwide epidemiologic study. Neurology. (2018) 91:e1220–7. doi: 10.1212/WNL.0000000000006246
7. Sipila, JO, and Soilu-Hanninen, M. The incidence and triggers of adult-onset Guillain-Barre syndrome in southwestern Finland 2004-2013. Eur J Neurol. (2015) 22:292–8. doi: 10.1111/ene.12565
8. Gensicke, H, Datta, AN, Dill, P, Schindler, C, and Fischer, D. Increased incidence of Guillain-Barre syndrome after surgery. Eur J Neurol. (2012) 19:1239–44. doi: 10.1111/j.1468-1331.2012.03730.x
9. Gong, Q, Liu, S, Liu, Y, Yao, J, Fu, X, Xiao, Z, et al. Guillain-Barre syndrome triggered by surgery in a Chinese population: a multicenter retrospective study. BMC Neurol. (2021) 21:40. doi: 10.1186/s12883-021-02067-1
10. Yang, B, Lian, Y, Liu, Y, Wu, BY, and Duan, RS. A retrospective analysis of possible triggers of Guillain-Barre syndrome. J Neuroimmunol. (2016) 293:17–21. doi: 10.1016/j.jneuroim.2016.02.003
11. Huang, SL, Qi, HG, Liu, JJ, Huang, YJ, and Xiang, L. A rare complication of spine surgery: Guillain-Barre syndrome. World Neurosurg. (2015) 84:697–701. doi: 10.1016/j.wneu.2015.04.045
12. Wakerley, BR, and Yuki, N. Infectious and noninfectious triggers in Guillain-Barre syndrome. Expert Rev Clin Immunol. (2013) 9:627–39. doi: 10.1586/1744666X.2013.811119
13. Tu, WC, Chang, ST, Huang, CH, Cheng, YY, and Hsu, CS. Guillain-Barre syndrome with respiratory failure following spine surgery for incomplete cervical cord injury: a case report and literature review. Medicina (Kaunas). (2022) 58:1063–72. doi: 10.3390/medicina58081063
14. Selcuk, E, and Koksal, C. Fatal autonomic dysfunction due to Guillain-Barre syndrome after cardiac surgery. Tex Heart Inst J. (2022) 49:e207439–42. doi: 10.14503/THIJ-20-7439
15. Najjari, K, Gouravani, M, Talebpour, M, Kor, F, Iranmanesh, M, and Zabihi, MH. Gastric lumen obstruction after open Vbg and Guillain-Barre syndrome following Revisional surgery: a case report. Obes Surg. (2021) 31:3324–6. doi: 10.1007/s11695-021-05351-8
16. Chen, J, Ma, JX, Zuo, CH, Zhang, Q, Chen, HT, and Ma, XL. Severe Guillain-Barre syndrome after surgery for multiple fractures: a rare case report with a 5-year follow-up and a brief review of the literature. BMC Musculoskelet Disord. (2021) 22:8. doi: 10.1186/s12891-020-03864-4
17. Raut, MS, Hanjoora, VM, Chishti, MA, and Tewari, R. Guillain-Barre syndrome after cardiac surgery: diagnostic dilemma. Gen Thorac Cardiovasc Surg. (2019) 67:1087–8. doi: 10.1007/s11748-018-1002-2
18. Aldag, M, Albeyoglu, S, Ciloglu, U, Kutlu, H, and Ceylan, L. Miller-fisher syndrome after coronary artery bypass surgery. Cardiovasc J Afr. (2017) 28:e4–5. doi: 10.5830/CVJA-2017-033
19. Chen, EY, Stratton, C, Mercer, B, Hohler, A, Tannoury, TY, and Tannoury, C. Guillain-Barre syndrome after elective spinal surgery. J Am Acad Orthop Surg. (2017) 25:587–93. doi: 10.5435/JAAOS-D-16-00572
20. Li, X, Xiao, J, Ding, Y, Xu, J, Li, C, He, Y, et al. Clinical and electrophysiological features of post-traumatic Guillain-Barre syndrome. BMC Neurol. (2017) 17:142. doi: 10.1186/s12883-017-0919-x
21. Sahai, N, Hwang, KS, and Emami, A. Guillain-Barre syndrome following elective spine surgery. European spine J: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Res Society. (2017) 26:6–8. doi: 10.1007/s00586-016-4577-2
22. Rashid, A, Kurra, S, and Lavelle, W. Guillain-Barre syndrome after revision lumbar surgery: a case report. Cureus. (2017) 9:e1393. doi: 10.7759/cureus.1393
23. Samieirad, S, Khajehahmadi, S, Tohidi, E, and Barzegar, M. Unusual presentation of Guillain-Barre syndrome after mandibular fracture treatment: a review of the literature and a new case. Journal of Oral Maxillofacial Surgery: Official J American Association Oral Maxillofacial Surgeons. (2016) 74:129.e1–6. doi: 10.1016/j.joms.2015.09.011
24. Hendawi, T, and Zavatsky, JM. Guillain-Barre syndrome after pelvic fracture fixation: a rare cause of postoperative paralysis. Spine. (2015) 40:E372–4. doi: 10.1097/BRS.0000000000000779
25. Al-Hashel, JY, John, JK, and Vembu, P. Unusual presentation of Guillain-Barre syndrome following traumatic bone injuries: report of two cases. Medical Princ Pract: Int J Kuwait University, Health Sci Centre. (2013) 22:597–9. doi: 10.1159/000348797
26. Battaglia, F, Sevy, A, Moyse, E, and Roche, PH. Guillain-Barre syndrome following severe head trauma and spine surgery. Rev Neurol. (2013) 169:166–8. doi: 10.1016/j.neurol.2012.04.010
27. Jakes, AD, Jani, P, and Bhandari, S. Case report: Guillain-Barre syndrome following renal transplantation--a diagnostic dilemma. Nephron Clin Pract. (2013) 124:239–42. doi: 10.1159/000358087
28. Cingoz, F, Tavlasoglu, M, Kurkluoglu, M, and Sahin, MA. Guillain-Barre syndrome after coronary artery bypass surgery. Interact Cardiovasc Thorac Surg. (2012) 15:918–9. doi: 10.1093/icvts/ivs367
29. Miscusi, M, Curra, A, Della Rocca, C, Missori, P, and Petrozza, V. Acute motor-sensory axonal neuropathy after cervical spine surgery. J Neurosurg Spine. (2012) 17:82–5. doi: 10.3171/2012.4.SPINE11932
30. Heyworth, BE, Fabricant, PD, Pizzurro, MM, Beksac, B, and Salvati, EA. Guillain-Barre syndrome mimicking nerve injury after Total hip arthroplasty. HSS J: Musculoskeletal J Hospital for Special Surgery. (2011) 7:286–9. doi: 10.1007/s11420-011-9201-8
31. Cheng, J, Kahn, DE, and Wang, MY. The acute motor-sensory axonal neuropathy variant of Guillain-Barre syndrome after thoracic spine surgery. J Neurosurg Spine. (2011) 15:605–9. doi: 10.3171/2011.8.SPINE1159
32. Son, DW, Song, GS, Sung, SK, and Kim, SH. Guillain-Barre syndrome following spinal fusion for thoracic vertebral fracture. J Korean Neurosurgical Society. (2011) 50:464–7. doi: 10.3340/jkns.2011.50.5.464
33. Algahtani, H, Moulin, DE, Bolton, CF, and Abulaban, AA. Guillain-Barre syndrome following cardiac surgery. Difficult diagnosis in the intensive care unit. Neurosciences. (2009) 14:374–8.
34. Lee, MC, Campbell, R, and Born, C. Guillain-Barre syndrome after failed pelvic fracture fixation. J Trauma. (2009) 67:E132–5. doi: 10.1097/TA.0b013e31804a7fc0
35. Aluka, KJ, Turner, PL, and Fullum, TM. Guillain-Barre syndrome and Postbariatric surgery polyneuropathies. JSLS: J Society of Laparoendoscopic Surgeons. (2009) 13:250–3.
36. Keithi-Reddy, SR, Chakravarthi, RM, Hussaini, SM, Venkatapuram, RR, and Murthy, JM. Cytomegalovirus disease with Guillain-Barre syndrome in a cadaver renal allograft recipient: cause or coincidence. Int Urol Nephrol. (2007) 39:967–70. doi: 10.1007/s11255-007-9197-7
37. Lin, TM, Lee, SS, Lin, RT, Lai, CS, and Lin, SD. Guillain-Barre syndrome following facial bone fracture. J Plast Reconstr Aesthet Surg. (2006) 59:543–6. doi: 10.1016/j.bjps.2005.10.017
38. Falk, JA, Cordova, FC, Popescu, A, Tatarian, G, and Criner, GJ. Treatment of Guillain-Barre syndrome induced by cyclosporine in a lung transplant patient. J Heart Lung Transplant. (2006) 25:140–3. doi: 10.1016/j.healun.2005.06.012
39. Rodriguez, V, Kuehnle, I, Heslop, HE, Khan, S, and Krance, RA. Guillain-Barre syndrome after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. (2002) 29:515–7. doi: 10.1038/sj.bmt.1703412
40. Koc, M, Ozalp, N, and Zulfikaroglu, B. Major surgery with Guillain-Barre syndrome: a case report. J Int Med Res. (2002) 30:601–4. doi: 10.1177/147323000203000609
41. Hughes, RA, Newsom-Davis, JM, Perkin, GD, and Pierce, JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. (1978) 2:750–3. doi: 10.1016/S0140-6736(78)92644-2
42. Uncini, A, Ippoliti, L, Shahrizaila, N, Sekiguchi, Y, and Kuwabara, S. Optimizing the Electrodiagnostic accuracy in Guillain-Barre syndrome subtypes: criteria sets and sparse linear discriminant analysis. Clin Neurophysiol. (2017) 128:1176–83. doi: 10.1016/j.clinph.2017.03.048
43. Staff NPEngelstad, J, Klein, CJ, Amrami, KK, Spinner, RJ, Dyck, PJ, et al. Post-surgical inflammatory neuropathy. Brain: J Neurol. (2010) 133:2866–80. doi: 10.1093/brain/awq252
44. Huang, C, Zhang, Y, Deng, S, Ren, Y, and Lu, W. Trauma-related Guillain-Barre syndrome: systematic review of an emerging concept. Front Neurol. (2020) 11:588290. doi: 10.3389/fneur.2020.588290
45. Westfall, S, Caracci, F, Zhao, D, Wu, QL, Frolinger, T, Simon, J, et al. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav Immun. (2021) 91:350–68. doi: 10.1016/j.bbi.2020.10.013
46. Ackerman, RS, Luddy, KA, Icard, BE, Pineiro Fernandez, J, Gatenby, RA, and Muncey, AR. The effects of anesthetics and perioperative medications on immune function: a narrative review. Anesth Analg. (2021) 133:676–89. doi: 10.1213/ANE.0000000000005607
47. Yang, T, Velagapudi, R, and Terrando, N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. (2020) 21:1319–26. doi: 10.1038/s41590-020-00812-1
48. Wakerley, BR, Uncini, A, and Yuki, NGroup GBSC, Group GBSC. Guillain-Barre and Miller Fisher Syndromes--New Diagnostic Classification. Nat Rev Neurol. (2014) 10:537–44. doi: 10.1038/nrneurol.2014.138
49. Leonhard, SE, Mandarakas, MR, Gondim, FAA, Bateman, K, Ferreira, MLB, Cornblath, DR, et al. Diagnosis and Management of Guillain-Barre Syndrome in ten steps. Nat Rev Neurol. (2019) 15:671–83. doi: 10.1038/s41582-019-0250-9
50. van Doorn, PA, Van den Bergh, PYK, Hadden, RDM, Avau, B, Vankrunkelsven, P, Attarian, S, et al. European academy of neurology/peripheral nerve society guideline on diagnosis and treatment of Guillain-Barre syndrome. Eur J Neurol. (2023) 30:3646–74. doi: 10.1111/ene.16073
51. Vanhoutte, EK, Faber, CG, Merkies, IS, and Ssg, PN. 196th Enmc international workshop: outcome measures in inflammatory peripheral neuropathies 8-10 February 2013, Naarden, the Netherlands. Neuromuscular disor: NMD. (2013) 23:924–33. doi: 10.1016/j.nmd.2013.06.006
52. van Nes, SI, Vanhoutte, EK, van Doorn, PA, Hermans, M, Bakkers, M, Kuitwaard, K, et al. Rasch-built overall disability scale (R-Ods) for immune-mediated peripheral neuropathies. Neurology. (2011) 76:337–45. doi: 10.1212/WNL.0b013e318208824b
53. Kleyweg, RP, van der Meche, FG, and Schmitz, PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve. (1991) 14:1103–9. doi: 10.1002/mus.880141111
54. Vanhoutte, EK, Faber, CG, van Nes, SI, Jacobs, BC, van Doorn, PA, van Koningsveld, R, et al. Modifying the Medical Research Council grading system through Rasch analyses. Brain: J neurology. (2012) 135:1639–49. doi: 10.1093/brain/awr318
55. Fokke, C, van den Berg, B, Drenthen, J, Walgaard, C, van Doorn, PA, and Jacobs, BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain: J neurology. (2014) 137:33–43. doi: 10.1093/brain/awt285
56. Uncini, A, Manzoli, C, Notturno, F, and Capasso, M. Pitfalls in Electrodiagnosis of Guillain-Barre syndrome subtypes. J Neurol Neurosurg Psychiatry. (2010) 81:1157–63. doi: 10.1136/jnnp.2010.208538
57. Jacobson, RD, and Glantz, M. Electrodiagnostic testing for diagnosing polyneuropathy. JAMA. (2021) 326:1966–7. doi: 10.1001/jama.2021.19286
58. Rajabally, YA, Durand, MC, Mitchell, J, Orlikowski, D, and Nicolas, G. Electrophysiological diagnosis of Guillain-Barre syndrome subtype: could a single study suffice? J Neurol Neurosurg Psychiatry. (2015) 86:115–9. doi: 10.1136/jnnp-2014-307815
59. Uncini, A, and Kuwabara, S. Electrodiagnostic criteria for Guillain-Barre syndrome: a critical revision and the need for an update. Clin Neurophysiol. (2012) 123:1487–95. doi: 10.1016/j.clinph.2012.01.025
60. Yuki, N, and Hartung, HP. Guillain-Barre syndrome. N Engl J Med. (2012) 366:2294–304. doi: 10.1056/NEJMra1114525
61. Koike, H, Fukami, Y, Nishi, R, Kawagashira, Y, Iijima, M, Katsuno, M, et al. Ultrastructural mechanisms of macrophage-induced demyelination in Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. (2020) 91:650–9. doi: 10.1136/jnnp-2019-322479
62. Schefold, JC, Wollersheim, T, Grunow, JJ, Luedi, MM, Z'Graggen, WJ, and Weber-Carstens, S. Muscular weakness and muscle wasting in the critically ill. J Cachexia Sarcopenia Muscle. (2020) 11:1399–412. doi: 10.1002/jcsm.12620
63. Kress, JP, and Hall, JB. Icu-acquired weakness and recovery from critical illness. N Engl J Med. (2014) 370:1626–35. doi: 10.1056/NEJMra1209390
64. Shah, N, Shrivastava, M, Kumar, S, and Nagi, RS. Supervised, individualised exercise reduces fatigue and improves strength and quality of life more than unsupervised home exercise in people with chronic Guillain-Barre syndrome: a randomised trial. J Physiother. (2022) 68:123–9. doi: 10.1016/j.jphys.2022.03.007
65. Walgaard, C, Lingsma, HF, Ruts, L, Drenthen, J, van Koningsveld, R, Garssen, MJ, et al. Prediction of respiratory insufficiency in Guillain-Barre syndrome. Ann Neurol. (2010) 67:781–7. doi: 10.1002/ana.21976
66. Green, C, Baker, T, and Subramaniam, A. Predictors of respiratory failure in patients with Guillain-Barre syndrome: a systematic review and Meta-analysis. Med J Aust. (2018) 208:181–8. doi: 10.5694/mja17.00552
67. Burakgazi, AZ, and Hoke, A. Respiratory muscle weakness in peripheral neuropathies. J peripheral nervous system: JPNS. (2010) 15:307–13. doi: 10.1111/j.1529-8027.2010.00293.x
68. Liu, J, Wang, LN, and McNicol, ED. Pharmacological treatment for pain in Guillain-Barre syndrome. Cochrane Database Syst Rev. (2015) 2019:CD009950. doi: 10.1002/14651858.CD009950.pub3
69. Chevret, S, Hughes, RA, and Annane, D. Plasma exchange for Guillain-Barre syndrome. Cochrane Database Syst Rev. (2017) 2:CD001798. doi: 10.1002/14651858.CD001798.pub3
70. Hughes, RA, Swan, AV, and van Doorn, PA. Intravenous immunoglobulin for Guillain-Barre syndrome. Cochrane Database Syst Rev. (2014) 2019:CD002063. doi: 10.1002/14651858.CD002063.pub6
71. Walgaard, C, Jacobs, BC, Lingsma, HF, Steyerberg, EW, van den Berg, B, Doets, AY, et al. Second intravenous immunoglobulin dose in patients with Guillain-Barre syndrome with poor prognosis (Sid-Gbs): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. (2021) 20:275–83. doi: 10.1016/S1474-4422(20)30494-4
72. Dada, MA, and Kaplan, AA. Plasmapheresis treatment in Guillain-Barre syndrome: potential benefit over Ivig in patients with axonal involvement. Therapeutic Apheresis Dialysis: Official Peer-Review J Int Society for Apheresis, Japanese Society for Apheresis, Japanese Society for Dialysis Therapy. (2004) 8:409–12. doi: 10.1111/j.1526-0968.2004.00183.x
73. van Koningsveld, R, Schmitz, PI, Meche, FG, Visser, LH, Meulstee, J, van Doorn, PA, et al. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain-Barre syndrome: randomised trial. Lancet. (2004) 363:192–6. doi: 10.1016/S0140-6736(03)15324-X
74. Hughes, RA, Brassington, R, Gunn, AA, and van Doorn, PA. Corticosteroids for Guillain-Barre syndrome. Cochrane Database Syst Rev. (2016) 10:CD001446. doi: 10.1002/14651858.CD001446.pub5
75. Yamagishi, Y, Suzuki, H, Sonoo, M, Kuwabara, S, Yokota, T, Nomura, K, et al. Markers for Guillain-Barre syndrome with poor prognosis: a multi-center study. J peripheral nervous system: JPNS. (2017) 22:433–9. doi: 10.1111/jns.12234
76. Walgaard, C, Lingsma, HF, Ruts, L, van Doorn, PA, Steyerberg, EW, and Jacobs, BC. Early recognition of poor prognosis in Guillain-Barre syndrome. Neurology. (2011) 76:968–75. doi: 10.1212/WNL.0b013e3182104407
77. Witsch, J, Galldiks, N, Bender, A, Kollmar, R, Bosel, J, Hobohm, C, et al. Long-term outcome in patients with Guillain-Barre syndrome requiring mechanical ventilation. J Neurol. (2013) 260:1367–74. doi: 10.1007/s00415-012-6806-x
78. van Koningsveld, R, Steyerberg, EW, Hughes, RA, Swan, AV, van Doorn, PA, and Jacobs, BC. A clinical prognostic scoring system for Guillain-Barre syndrome. Lancet Neurol. (2007) 6:589–94. doi: 10.1016/S1474-4422(07)70130-8
79. Wijdicks, EF, and Klein, CJ. Guillain-Barre syndrome. Mayo Clin Proc. (2017) 92:467–79. doi: 10.1016/j.mayocp.2016.12.002
80. Verboon, C, Harbo, T, Cornblath, DR, Hughes, RAC, van Doorn, PA, Lunn, MP, et al. Intravenous immunoglobulin treatment for mild Guillain-Barre syndrome: an international observational study. J Neurol Neurosurg Psychiatry. (2021) 92:1080–8. doi: 10.1136/jnnp-2020-325815
81. Goedee, HS, and Rajabally, YA. To treat or not to treat mild Guillain-Barre syndrome: limited evidence for but still none against. J Neurol Neurosurg Psychiatry. (2021) 92:1027–8. doi: 10.1136/jnnp-2021-326848
Keywords: Guillain-Barré syndrome, surgery, postoperative complication, retrospective study, management
Citation: Li X and Zhang C (2024) Guillain-Barré syndrome after surgery: a literature review. Front. Neurol. 15:1368706. doi: 10.3389/fneur.2024.1368706
Edited by:
Pei Shang, Mayo Clinic, United StatesReviewed by:
Giovanna Squintani, Integrated University Hospital Verona, ItalyKoike Haruki, Saga University, Japan
Copyright © 2024 Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Zhang chaozhang@tmu.edu.cn
 Xiaowen Li
Xiaowen Li Chao Zhang*
Chao Zhang*