- 1College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
- 2Division of Pediatric Neurology, Department of Pediatrics, Prince Sultan Medical Military City, Riyadh, Saudi Arabia
- 3Division of Genetic and Metabolic Medicine, Department of Pediatrics, Prince Sultan Medical Military City, Riyadh, Saudi Arabia
The neurological complications of coronavirus disease 2019 (COVID-19) can range from simple tremors and dystonia to features of encephalopathy. Toll-like receptor 7 (TLR7) belongs to a family of innate immune receptors responsible for viral RNA detection (such as SARS-CoV-2) and immune response initiation. TLR7 loss of function variants have been previously reported as genetic risk factors for severe COVID-19 infection in young patients with no comorbidities. In this case, we report a pediatric patient who developed severe long-term neurological deterioration following his COVID-19 infection. Presenting first to the clinic with episodic dystonia and finger spasticity, the patient’s condition rapidly deteriorated with a significant drop in the Glasgow Coma Scale (GCS). Despite improvement following initial treatment with rituximab and intravenous immunoglobulin, the patient’s symptoms relapsed, and GCS further dropped to 3/15. Serial brain magnetic resonance imaging scans revealed diffuse parenchymal atrophy, ventricular enlargement, and spinal cord thickening. Autoimmune investigations were negative but clinical whole genome sequencing prioritized four gene variants, the most significant of which was a novel frameshift null variant of the X chromosomal TLR7 gene (c.1386_1389dup, p.[His464Ilefs*7]). This case illustrates a role for TLR7 in long-term COVID-19 complications and highlights that TLR7 deficiency in the future may be addressed as a therapeutic measure.
1 Introduction
Beginning as a local outbreak in China, the coronavirus disease 2019 (COVID-19) pandemic grew to have profound global impact, lasting over 2 years and afflicting more than 600 million people worldwide (1). Although most patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represent asymptomatic or mild cases (~80%), some may develop severe pneumonia (~15%) or life-threatening acute respiratory distress syndrome (ARDS) (~5%) (2). Well-established risk factors that predispose patients to more severe forms of COVID-19 include sex (male), advanced age (greater than 60 years), and previous comorbidity (namely hypertension and diabetes) (3). Critical disease, however, has also been reported in previously healthy young patients and, thus, it is likely that genetic factors mediating viral entry or underlying a host’s immune response may be at play. These factors may also help explain the long-term effects or complications some patients develop after infection resolution. Recent evidence suggests that variants of the X-linked Toll-like receptor 7 (TLR7) gene may help predict COVID-19 severity and penetrance in younger patients (4, 5). Herein, we report a novel, likely pathogenic variant of TLR7 in a pediatric patient with severe neurological deterioration following COVID-19 infection. We also comprehensively review any reported literature on TLR7 variants in COVID-19 patients.
2 Case presentation
The patient is a previously healthy 10-year-old boy, born to consanguineous parents in a twin pregnancy, who presented to the pediatric clinic of his home city with ataxia, left arm dystonia, and brief, episodic neck flexion to the left side. The patient’s past medical history was uneventful, except for a moderate COVID-19 infection with respiratory symptoms like cough and fever that resolved within two weeks prior to the clinic visit. There are no similar symptoms in the patient’s twin brother, and he is not known to have any medical illnesses. Family history revealed that the parents were first-degree cousins and had no history of similar problems of seizures or autoimmune diseases in the family (see Supplementary Figure S1 for pedigree). The patient was administered sodium valproate, along with one course of prophylactic intravenous immunoglobulin (IVIG) for suspected auto-immune encephalitis. He was subsequently discharged, but no improvement was observed. In fact, his symptoms progressed over two weeks to difficulty in speech and swallowing, tongue involvement, and drooling of saliva, and the patient was admitted to the hospital. On physical exam, spastic tone and brisk reflexes without clonus were noted. Comprehensive laboratory assessments, including basic labs, liver function test (LFT), C-reactive protein (CRP), coagulation profile, ammonia and lactate were within normal range. Notably, thyroglobulin antibodies were increased 4-fold (16.5 IU/mL), raising suspicion of Hashimoto’s encephalitis. However, when antithyroid peroxidase antibodies and anti-thyroglobulin antibodies were repeated in our hospital, both antibodies were negative. Brain computed tomography (CT) and magnetic Resonance Imaging (MRI) scans were performed, but the results were unremarkable (see Figure 1A). Electroencephalogram (EEG) and sleep graphs revealed abnormal time-logged periodic complex with sharp theta paroxysm seen every 4–5 s, suggesting progressive myoclonic epilepsy as a possible differential. Nevertheless, when given multiple anti-epileptic drugs including Clonazepam (0.5 mg PO TID), Carbidopa-Levodopa (75 mg AM, 50 mg noon, 75 mg PM, PO, TID), Benzotropine (2 mg PO OD) and Baclofen (5 mg PO TID), there was still no improvement.
Therefore, for further evaluation, the patient was transferred to the Pediatric Intensive Care Unit (PICU) in our hospital. On re-examination, the patient continued to exhibit frequent dystonic repetitive movements now associated with tachycardia and desaturation. Excessive drooling, along with spasticity in both upper and lower limbs, hyperreflexia across all tendons, and positive clonus, were also noted. The Glasgow Coma Scale (GCS) score was 8/15. To address the patient’s deteriorating vital signs and secure the airway, intubation was deemed necessary. The intervention notably improved the tachycardia. Furthermore, the administration of a Midazolam infusion (4 mic/kg/min) and fentanyl (2 mic/kg/h) effectively halted the dystonic episodes, resulting in a temporary improvement in the GCS score to 11/15. To confirm the placement of the endotracheal tube, a chest X-ray was taken, which showed significant perihilar peri-bronchial wall thickening and interstitial infiltrates, thought to be a sequelae of his moderate COVID-19 infection. Blood, urine, respiratory, and cerebrospinal fluid (CSF) cultures were obtained, but the results were negative one week later. In light of the absence of infectious markers, the suspicion of autoimmune encephalitis (AE) following a prior COVID-19 infection was raised. Nevertheless, the autoimmune serology panel was negative for dsDNA, ANA, cANCA, and pANCA antibodies with normal levels of anti-MPO (1.39) and anti-PR3 (2.67) antibodies were detected (see Supplementary Table S1).
On routine examination five days after the patient’s intubation, 3 mm sluggish pupils were noted bilaterally and GCS had decreased again to 8/15. The patient exhibited hypertonia in both upper and lower limbs, and the gag and cough reflexes remained intact. Another EEG was performed, revealing diffuse slowing of electrical activity. Brain MRI was repeated the following day, demonstrating new development of anterior and superior bifrontal periventricular, deep and subcortical T2 and fluid-attenuated inversion recovery (FLAIR) white matter high signal intensity, focal atrophic changes and compensatory widening of the ventricular system (see Figures 1B–D). The visualized upper cervical spinal cord also showed faint high T2 signal intensity within the spinal cord. These findings are suggestive of progressive multifocal leukoencephalopathy (PML) with brainstem and spinal cord involvement in addition to mild brain volumetric loss. Results of the CSF analysis three days later revealed high CSF total protein (0.37 mmol/L), high CSF IgG/albumin ratio (2.67), low serum albumin levels (26 g/L), and the detection of a CSF albumin oligoclonal band, collectively suggesting an active inflammatory state that may contribute to the patient’s declining condition (see Supplementary Table S2). A blood sample was taken and sent for whole exome sequencing (WES). For the next two weeks, the patient was given one course of Rituximab (490 mg and 356 mg), three courses of IVIG (>27 g) and received a two-day steroid pulse therapy.
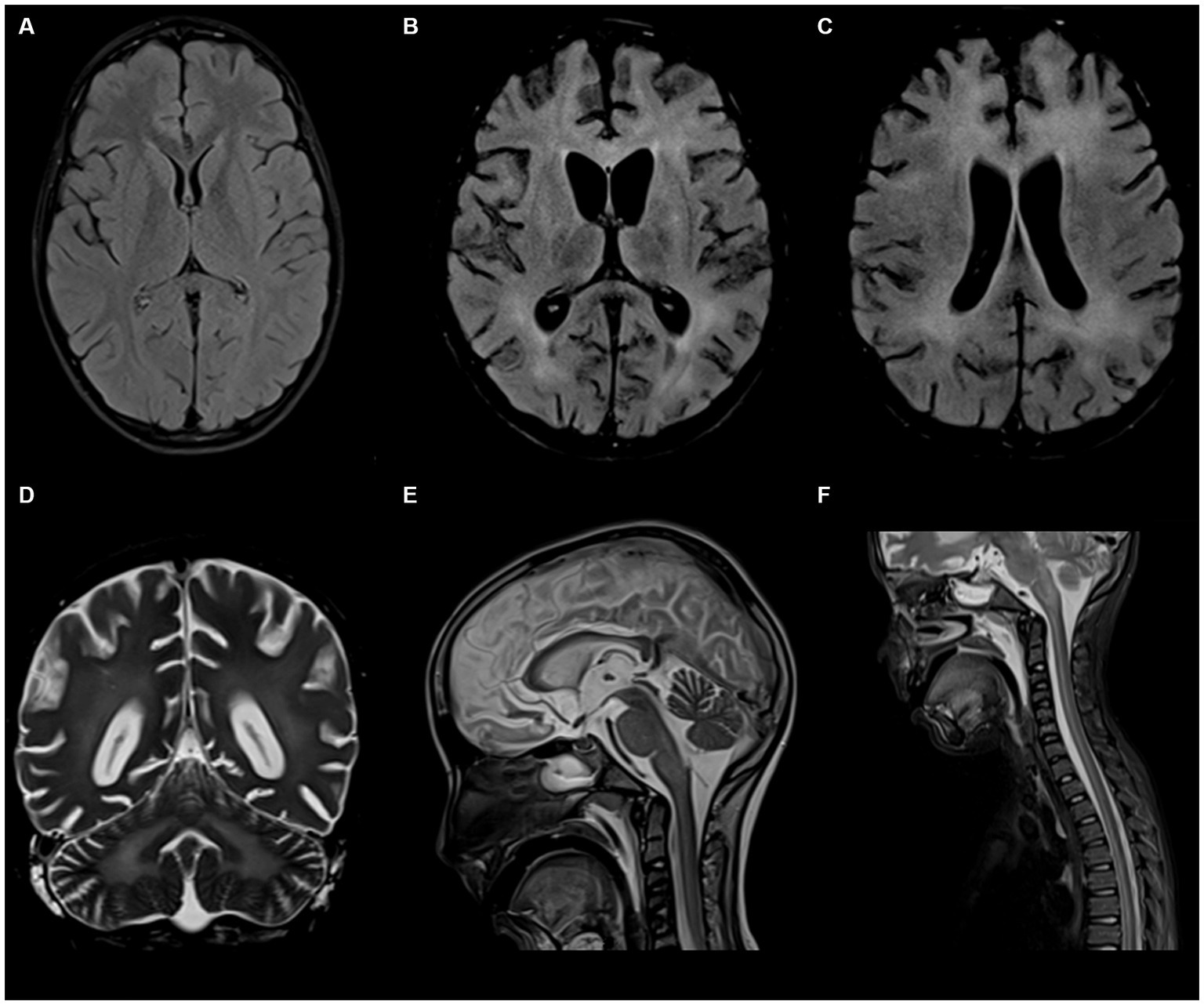
Figure 1. Brain and spinal cord magnetic resonance imaging (MRI) scans of the 10-year-old patient. (A) Represents the first MRI taken of the patient after his initial presentation: unremarkable with no sign of acute or remote brain insult. (B–D) Represent the second set of MRIs taken approximately 5 weeks since the patient’s initial visit: abnormal symmetrical T2 and FLAIR white matter high signal intensities are noted in the middle cerebellar peduncles, pons, and cortices with focal atrophic changes and compensatory ventricular enlargement. (E,F) Represent contrast-enhanced MRIs of brain and spinal cord: abnormal diffuse T2 signal intensities involving the central and anterior cord extending down to the conus medullaris.
Prior to immunotherapy, the patient had spiked a fever of 38.6°C, which resolved. Following immunotherapy treatment and vital stability, he was discharged from the PICU with a central line. However, four days later, he was readmitted with a low-grade fever of 38.1°C. Blood and urine cultures were negative, while the respiratory culture was positive for Methicillin-resistant Staphylococcus aureus (MRSA). The patient was given tazocin and vancomycin was added after nine days. However, his symptoms persisted, with recurrent low-grade fevers, tachypnea, and desaturation despite multiple changes of antibiotics. The patient required re-intubation. Subsequent extubation attempts proved unsuccessful, even in the absence of any respiratory infection.
Within a month, the patient’s GCS significantly deteriorated to 3/15. Pupils were fixed and dilated at 6 mm, the gag reflex disappeared, and the corneal reflex exhibited minimal response. Clonus, which was previously present, was now absent. WES results returned negative, revealing no clinically relevant variants related to the described phenotype. This ruled out the differential diagnosis of progressive myoclonic epilepsy as well as other potential metabolic or mitochondrial diseases that could account for the patient’s symptoms. Two weeks later, with no improvement and the absence of the corneal reflex, a buccal swab sample was sent for whole genome sequencing (WGS). No further escalation of immune therapy occurred, and only symptomatic treatment for seizures was provided. Brain MRI indicated further worsening of the diffuse brain parenchymal atrophy with resultant ventricular enlargement while spinal MRI revealed abnormal diffuse T2 signal intensities involving the central and anterior cord extending down to the conus medullaris (see Figures 1E,F). Brain multi-voxel MR spectroscopy findings were further suggestive of an inflammatory or demyelinating process post-COVID-19 infection (see Figure 2).
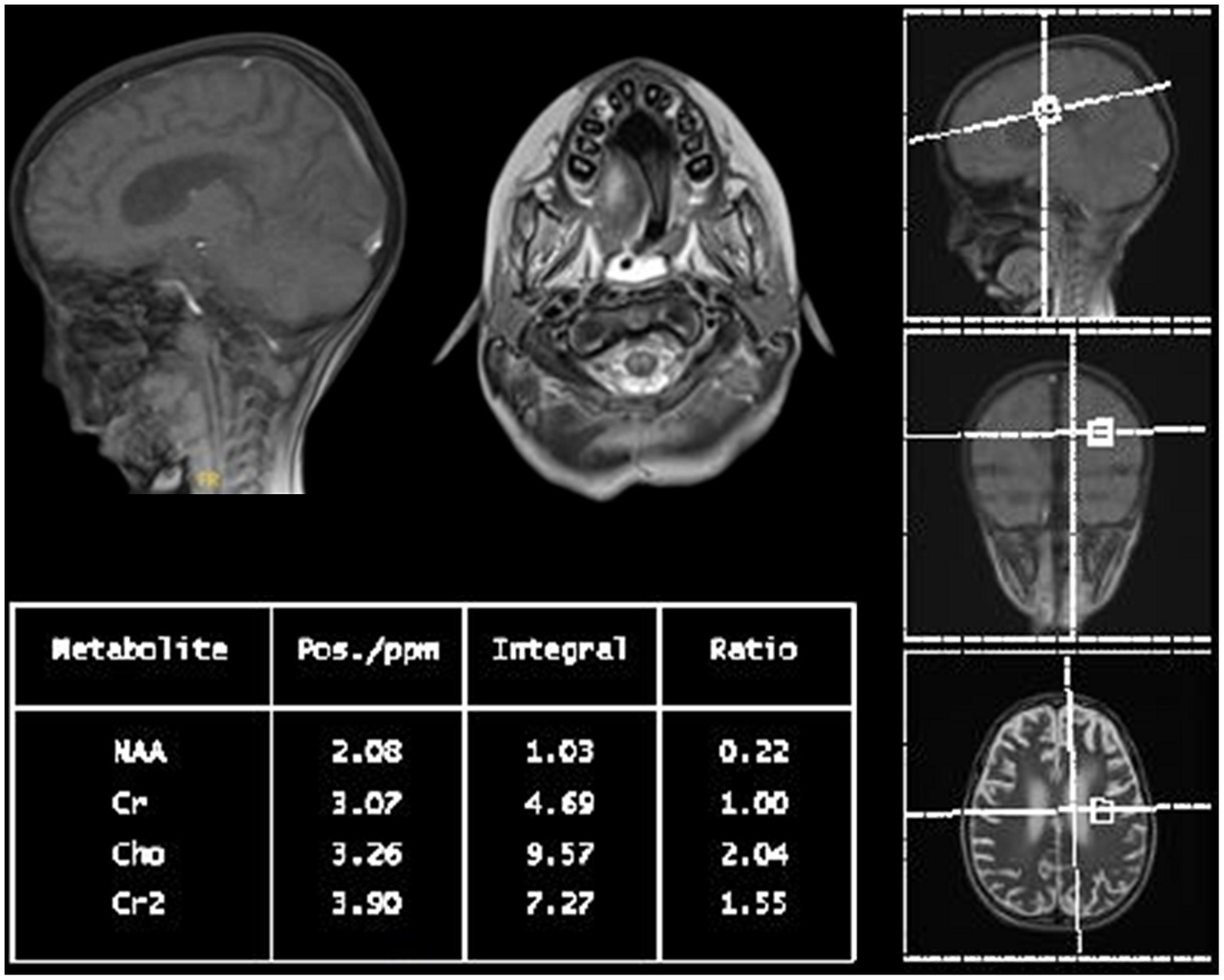
Figure 2. Multi-voxel MR spectroscopy of the patient: short and long TE at the basal ganglia, peritrigonal white matter, and cerebellar peduncles with increased choline to creatinine ratio and a decreased N-acetylaspartate to creatine ratio suggest an underlying inflammatory or demyelinating process.
The clinical WGS one month later prioritized four gene variants. A hemizygous, likely pathogenic variant of the TLR7 gene was detected (chrX:12886892, NM_016562.4: c.1386_1389dup; p.His464Ilefs*7) consistent with the genetic diagnosis of X-linked recessive COVID-19-related immunodeficiency type 74. Three additional variants were identified: homozygous, likely pathogenic variant of MEFV Innate Immunity Regulator, Pyrin (MEFV) gene (chr16:3243257, NM_000243.3:c.2230G > T; p.Ala744Ser), consistent with the genetic diagnosis of autosomal recessive Familial Mediterranean Fever; homozygous, variant of uncertain significance of Sodium Voltage-Gated Channel Alpha Subunit 5 (SCN5A) gene (chr3:38604004, NM_001099404.2:c.1598G > A; p.Arg533His), suggesting autosomal dominant Sick Sinus Syndrome type 1; and lastly heterozygous variant of uncertain significance of Signal Transducer and Activator of Transcription 3 (STAT3) gene (chr17:42337519, NM_139276.3:c.713A > C; p.Glu238Ala), indicating possible autosomal dominant infantile-onset multisystem autoimmune disease. The pathogenicity and clinical impact of the variants in each of these genes are detailed in Table 1. Thus, a diagnosis of neurological deterioration post-COVID-19 due to TLR7 deficiency was made.
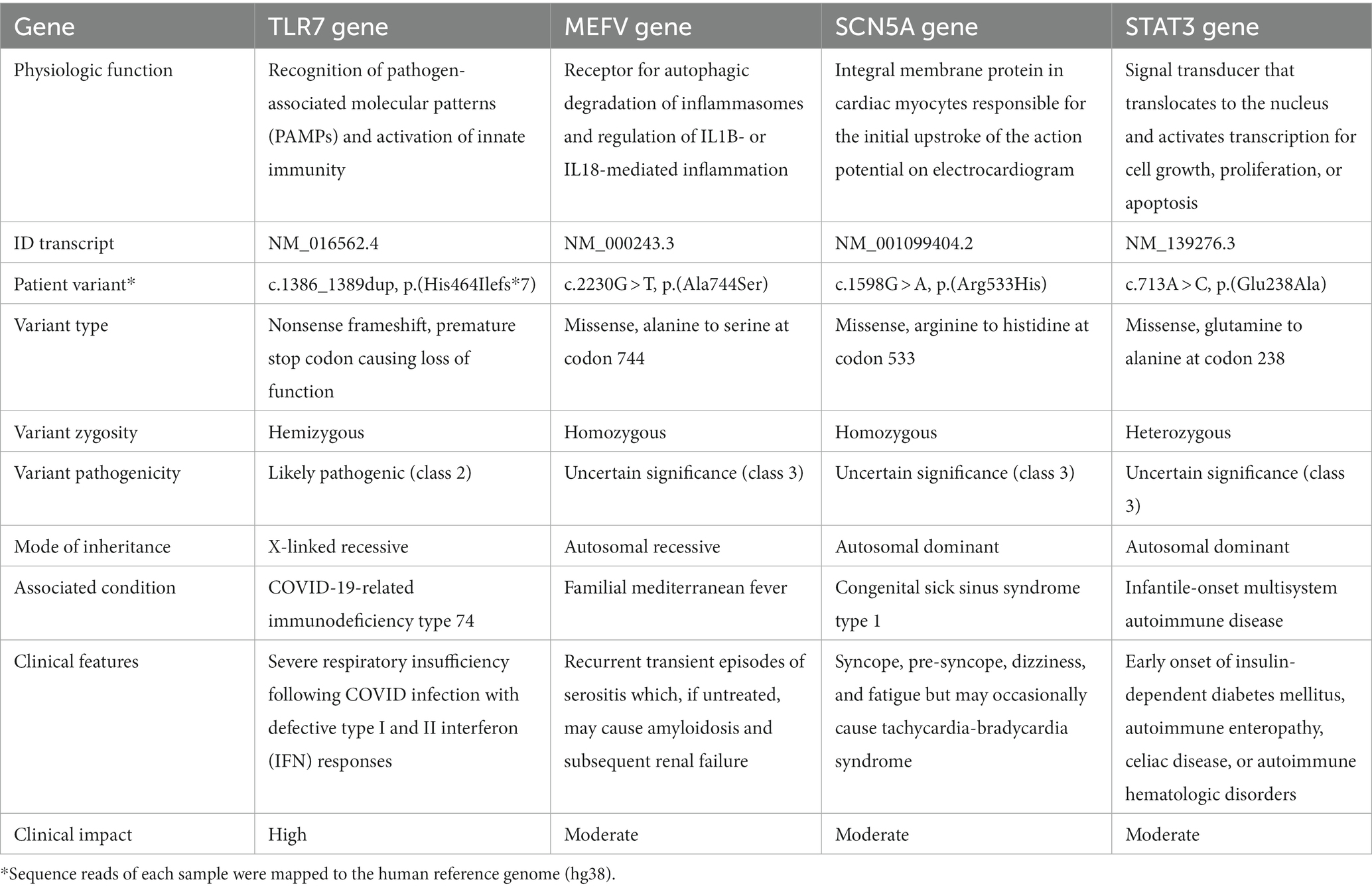
Table 1. Summary of pathogenicity, type, and clinical presentation of the major genetic variants detected.
Unfortunately, the prognosis for TLR7 deficiency remains challenging, as there is currently no specific treatment available. The patient was repeatedly admitted to the ICU, without significant improvement. His neurological symptoms, including reduced deep tendon reflexes and increased dystonia, have progressively worsened. The patient complained of constitutional symptoms due to recurring fevers and infections which were symptomatically treated. Two months later, despite stable vital signs and physical tests, the patient suffered a cardiac arrest. He was resuscitated and has been in static condition since. The patient exhibits no spontaneous breathing and remains ventilator-dependent. A concise but thorough timeline of all investigations and the clinical course of the patient is available in Supplementary Figure S2.
3 Discussion
In this report, we describe a pediatric case of severe neurological deterioration following COVID-19 where a novel hemizygous loss of function variant in TLR7 was identified. The patient presented with dystonia and finger spasticity that rapidly progressed to dysarthria and hyperreflexia despite his infection being mild and resolved, as reported by his parents. To the best of our knowledge, the variant reported in this case has not yet been described on the gnomAD (6) or ClinVar databases (7).
TLRs comprise a family of dimeric protein receptors primarily located on the surface or inside of immune cells (8). TLRs play a crucial role in the recognition of specific molecular patterns on pathogens and subsequent activation of the innate immune system. Thus, a deficiency or defect in TLR function would suggest an increased risk of infection (9). TLR7, an endosomal receptor, has been demonstrated to recognize single-stranded RNA viruses such as SARS-CoV-2 (10). Upon dimerization, TLR7 initiates a MyD88-dependent cascade magnifying the secretion of pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-23, interferon-alpha (IFN-α, a type I IFN), and IFN-γ (a type II IFN) (11). Although different strands of SARS-COV-2 have been identified, the sequence in which they activate TLR-7 is strikingly similar. This may be attributed to the GU-rich sequences found on the viral antigen (12). Recent evidence highlights an emerging role for TLR7 signaling in the pathogenesis and antiviral response against COVID-19 (10). In parallel, TLR7 deficiency or loss of function is being investigated in cases of severe infection or complications of COVID-19.
In addition to reports of fatigue or generalized weakness following COVID-19, some patients may develop pulmonary (e.g., respiratory failure), cardiovascular (e.g., myocardial infarction), or neurological (e.g., encephalitis or stroke) complications (13–15). Although the exact pathophysiology underlying the severity and long-term effects of COVID-19 is not yet known, specific patient attributes, including genetic factors, may help explain why these complications arise. For instance, in a case–control study of a total 285 participants, it was found that COVID-19 patients with a single nucleotide polymorphism (rs3853839) in TRL7 were at higher risk of poor clinical outcome (5). The G/G genotype, in particular, was associated with the greatest decrease in patient WBC count and increase in serum ferritin and D-dimer levels (p < 0.001) suggesting underlying immunopathology. Although no significant difference in mortality between genotypes was found, around 74.0% of patients with the G/G genotype were hospitalized and 22.4% developed cardiac complications (5). Therefore, it was concluded that TLR7 polymorphisms represent a significant genetic risk factor and a possible biomarker for predicting COVID-19 outcomes. Screening for rare TLR7 variants and their use as genomic biomarkers was also recently proposed in a prospective study by Solanich et al. (4). Two out of the fourteen COVID-19 patients included in this study had deleterious TLR7 missense variants (c.644A > G; p.[Asn215Ser]) and (c.2797 T > C; p.[Trp933Arg]). Functional testing on peripheral blood mononuclear cells (PBMC) derived from these patients confirmed impaired type I and II IFN responses further suggesting the impact of TLR7 loss of function on COVID-19 outcome (4).
The significant findings and outcomes of reported literature on TLR7 variants in COVID-19 patients are summarized in Table 2. Among those that discussed pediatric patients, only one was found to closely relate to our study: a 7-year-old boy with confirmed COVID-19 infection who developed neurological complications (ataxia-telangiectasia) (20). Both patients were born to consanguineous parents, had no significant family history, and were found to have hemizygous mutations in the TLR7 gene. Brain MRI scans also revealed that both patients had varying degrees of ventricular enlargement and atrophy of brain parenchyma (20). However, several key differences must be highlighted. Compared to our patient, the 7-year-old boy was a known case of hyper IgM syndrome and had an ataxia-telangiectasia mutated (ATM) gene mutation that more likely explains his symptoms. Moreover, despite having an impaired type I IFN response consistent with TLR7 deficiency, his variant (c.1114C > A, p.Leu 372Met) was discovered to be hypomorphic (20). The variant detected in our patient (c.1386_1389dup, p.[His464Ilefs*7]), on the other hand, resulted in truncation of the TLR7 gene and complete loss of function which may allude to the difference in presentation severity.
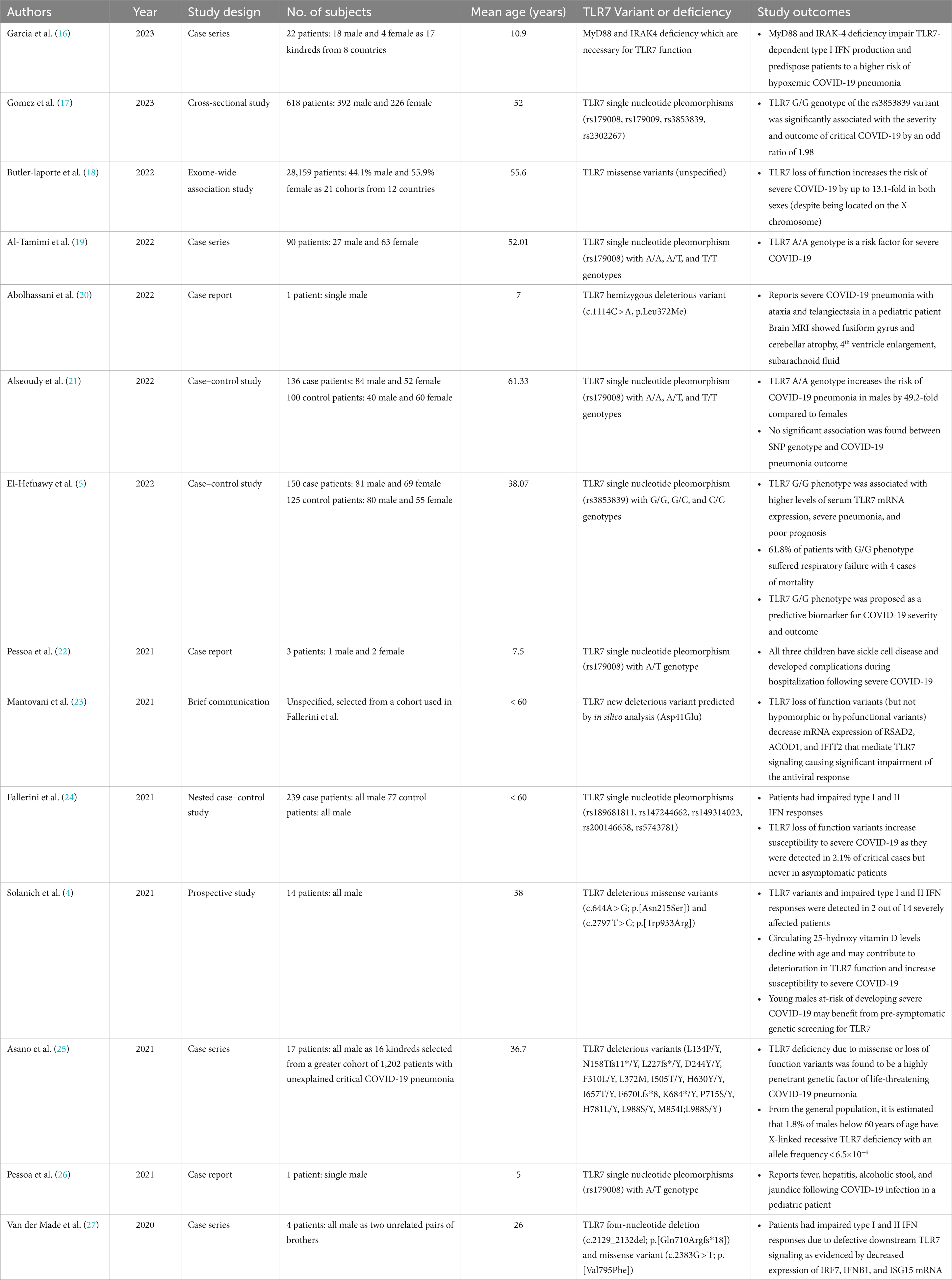
Table 2. Summary of reported literature on COVID-19 patients with detected TLR7 variants or deficiency (ordered by publication year).
This study has several limitations. First, no PBMC sample was available to test and confirm the functional impact of the novel TLR7 mutation detected in our patient. However, the frameshift variant we observed resulted in a downstream premature stop codon of 7 amino acids removing more than 10% of the transcript (last 10% transcript position 12,888,342). The truncated region is critical to proper TLR7 function, and its loss is a known mechanism of X-linked COVID-19-related immunodeficiency-74 (28). Moreover, this frameshift mutation was classified as a likely pathogenic (class 2) null variant based on American College of Medical Genetics and Genomics (ACMG) recommendations (29), further suggesting that the genetic findings in our patient are unlikely due to chance. Second, the pathogenicity or clinical significance of MEFV, SCN5A, and STAT3 were not entirely determined. Although they do not appear responsible for the patient’s initial presentation or neurological deterioration, firm conclusions on the degree of their involvement cannot be drawn. Lastly, more studies are needed to validate the results in this case. Our current understanding of the association and critical role of TLR7 in COVID-19 is still expanding and future research may help shed light on possible applications in preventive screening or targeted therapy.
4 Conclusion
In summary, we have identified a novel frameshift null variant of the X chromosomal TLR7 gene (c.1386_1389dup, p.[His464Ilefs*7]) in a pediatric patient with neurological deterioration following COVID-19 infection. Previous reports on COVID-19 patients with TLR7 variants conclude that TLR7 deficiency is an important risk factor for disease severity. However, our preliminary findings also suggest TLR7 loss of function may contribute to neurological complications post-COVID-19, especially in young previously healthy patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Prince Sultan Military Medical City (PSMMC) IRB Committee (IRB-Psmmc-934). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by– product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
ANE: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MA-R: Investigation, Methodology, Writing – original draft. FP-Z: Investigation, Methodology, Writing – original draft. KH: Project administration, Supervision, Writing – review & editing. AA: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1268035/full#supplementary-material
References
1. WHO. WHO coronavirus (COVID-19) dashboard. World Health organization (2023). https://covid19.who.int/
2. Mustafa, S, Bashir, MS, Wali, A, Alasmari, FA, and Samman, MA. Clinical laboratory and radiological findings in COVID-19 patients with variable severity: king Fahad Medical City experience. J Community Med Public Health. (2023) 7:280. doi: 10.29011/2577-2228.100280
3. Statsenko, Y, al Zahmi, F, Habuza, T, Almansoori, TM, Smetanina, D, Simiyu, GL, et al. Impact of age and sex on COVID-19 severity assessed from radiologic and clinical findings. Front Cell Infect Microbiol. (2021) 11:777070. doi: 10.3389/fcimb.2021.777070
4. Solanich, X, Vargas-Parra, G, van der Made, CI, Simons, A, Schuurs-Hoeijmakers, J, Antolí, A, et al. Genetic screening for TLR7 variants in Young and previously healthy men with severe COVID-19. Front Immunol. (2021) 12:719115. doi: 10.3389/fimmu.2021.719115
5. El-Hefnawy, SM, Eid, HA, Mostafa, RG, Soliman, SS, Omar, TA, and Azmy, RM. COVID-19 susceptibility, severity, clinical outcome and toll-like receptor (7) mRNA expression driven by TLR7 gene polymorphism (rs3853839) in middle-aged individuals without previous comorbidities. Gene Rep. (2022) 27:101612. doi: 10.1016/j.genrep.2022.101612
6. Karczewski, KJ, Francioli, LC, Tiao, G, Cummings, BB, Alföldi, J, Wang, Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. (2020) 581:434–43. doi: 10.1038/s41586-020-2308-7
7. Landrum, MJ, Lee, JM, Benson, M, Brown, GR, Chao, C, Chitipiralla, S, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. (2018) 46:D1062–7. doi: 10.1093/nar/gkx1153
8. Zhang, Z, Ohto, U, Shibata, T, Krayukhina, E, Taoka, M, Yamauchi, Y, et al. Structural analysis reveals that toll-like receptor 7 is a dual receptor for guanosine and single-stranded RNA. Immunity. (2016) 45:737–48. doi: 10.1016/j.immuni.2016.09.011
9. Mantovani, S, Oliviero, B, Varchetta, S, Renieri, A, and Mondelli, MU. TLRs: Innate Immune Sentries against SARS-CoV-2 Infection. Int J Mol Sci. (2023) 24:8065. doi: 10.3390/ijms24098065
10. Liu, Z-M, Yang, M-H, Yu, K, Lian, Z-X, and Deng, S-L. Toll-like receptor (TLRs) agonists and antagonists for COVID-19 treatments. Front Pharmacol. (2022) 13:989664. doi: 10.3389/fphar.2022.989664
11. Bao, M, and Liu, Y-J. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell. (2013) 4:40–52. doi: 10.1007/s13238-012-2104-8
12. Forsbach, A, Samulowitz, U, Völp, K, Hofmann, HP, Noll, B, Tluk, S, et al. Dual or triple activation of TLR7, TLR8, and/or TLR9 by single-stranded oligoribonucleotides. Nucleic Acid Ther. (2011) 21:423–36. doi: 10.1089/nat.2011.0323
13. Vakili, K, Fathi, M, Pezeshgi, A, Mohamadkhani, A, Hajiesmaeili, M, Rezaei-Tavirani, M, et al. Critical complications of COVID-19: a descriptive meta-analysis study. Rev Cardiovasc Med. (2020) 21:433–42. doi: 10.31083/j.rcm.2020.03.129
14. Jakubec, P, Fišerová, K, Genzor, S, and Kolář, M. Pulmonary complications after COVID-19. Life (Basel). (2022) 12:12030357. doi: 10.3390/life12030357
15. Dangayach, NS, Newcombe, V, and Sonnenville, R. Acute neurologic complications of COVID-19 and Postacute sequelae of COVID-19. Crit Care Clin. (2022) 38:553–70. doi: 10.1016/j.ccc.2022.03.002
16. García-García, A, Pérez de Diego, R, Flores, C, Rinchai, D, Solé-Violán, J, Deyà-Martínez, À, et al. Humans with inherited MyD88 and IRAK-4 deficiencies are predisposed to hypoxemic COVID-19 pneumonia. J Exp Med. (2023) 220. doi: 10.1084/jem.20220170
17. Martínez-Gómez, LE, Martinez-Armenta, C, Medina-Luna, D, Ordoñez-Sánchez, ML, Tusie-Luna, T, Ortega-Peña, S, et al. Implication of myddosome complex genetic variants in outcome severity of COVID-19 patients. J Microbiol Immunol Infect. (2023) 56:939–50. doi: 10.1016/j.jmii.2023.06.002
18. Butler-Laporte, G, Povysil, G, Kosmicki, JA, Cirulli, ET, Drivas, T, Furini, S, et al. Exome-wide association study to identify rare variants influencing COVID-19 outcomes: results from the host genetics initiative. PLoS Genet. (2022) 18:e1010367. doi: 10.1371/journal.pgen.1010367
19. Tamimi, Z, ee, A, and Jasim, A. Effect of toll-like receptor 7 gene polymorphism and ABO blood groups on the severity of COVID-19 patients. Acta Inform Med. (2022) 30:191–5. doi: 10.5455/aim.2022.30.191-195
20. Abolhassani, H, Vosughimotlagh, A, Asano, T, Landegren, N, Boisson, B, Delavari, S, et al. X-linked TLR7 deficiency underlies critical COVID-19 pneumonia in a male patient with Ataxia-telangiectasia. J Clin Immunol. (2022) 42:1–9. doi: 10.1007/s10875-021-01151-y
21. Alseoudy, MM, Elgamal, M, Abdelghany, DA, Borg, AM, el-Mesery, A, Elzeiny, D, et al. Prognostic impact of toll-like receptors gene polymorphism on outcome of COVID-19 pneumonia: a case-control study. Clin Immunol. (2022) 235:108929. doi: 10.1016/j.clim.2022.108929
22. Pessoa, NL, Diniz, LMO, Andrade, AS, Kroon, EG, Bentes, AA, and Campos, MA. Children with sickle cell disease and severe COVID-19 presenting single nucleotide polymorphisms in innate immune response genes – a case report. EJHaem. (2022) 3:199–202. doi: 10.1002/jha2.325
23. Mantovani, S, Daga, S, Fallerini, C, Baldassarri, M, Benetti, E, Picchiotti, N, et al. Rare variants in toll-like receptor 7 results in functional impairment and downregulation of cytokine-mediated signaling in COVID-19 patients. Genes Immun. (2022) 23:51–6. doi: 10.1038/s41435-021-00157-1
24. Fallerini, C, Daga, S, Mantovani, S, Benetti, E, Picchiotti, N, Francisci, D, et al. Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. elife. (2021) 10. doi: 10.7554/eLife.67569
25. Asano, T, Boisson, B, Onodi, F, Matuozzo, D, Moncada-Velez, M, Maglorius Renkilaraj, MRL, et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. (2021) 6. doi: 10.1126/sciimmunol.abl4348
26. Pessoa, NL, Bentes, AA, de Carvalho, AL, de Souza Silva, TB, Alves, PA, de Sousa Reis, EV, et al. Case report: hepatitis in a child infected with SARS-CoV-2 presenting toll-like receptor 7 Gln11Leu single nucleotide polymorphism. Virol J. (2021) 18:180. doi: 10.1186/s12985-021-01656-3
27. van der Made, CI, Simons, A, Schuurs-Hoeijmakers, J, van den Heuvel, G, Mantere, T, Kersten, S, et al. Presence of genetic variants among Young men with severe COVID-19. JAMA. (2020) 324:663–73. doi: 10.1001/jama.2020.13719
28. Kniffin, C, and Rasmussen, S. Immunodeficiency 74, COVID19-related, x-linked; IMD74 OMIM (2020).
29. Richards, S, Aziz, N, Bale, S, Bick, D, das, S, Gastier-Foster, J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
Keywords: COVID-19, SARS-CoV-2, TLR7, hemizygous, neurological deterioration, immunodeficiency, genetics, case report
Citation: Noor Eddin A, Al-Rimawi M, Peer-Zada F, Hundallah K and Alhashem A (2023) Novel TLR7 hemizygous variant in post-COVID-19 neurological deterioration: a case report with literature review. Front. Neurol. 14:1268035. doi: 10.3389/fneur.2023.1268035
Edited by:
Hong Ni, Children's Hospital of Soochow University, ChinaReviewed by:
Masako Kinoshita, National Hospital Organization Utano National Hospital, JapanMateus Castro, University of São Paulo, Brazil
Copyright © 2023 Noor Eddin, Al-Rimawi, Peer-Zada, Hundallah and Alhashem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Noor Eddin, neddin.ahmed@gmail.com
 Ahmed Noor Eddin
Ahmed Noor Eddin Mohammed Al-Rimawi
Mohammed Al-Rimawi Feham Peer-Zada
Feham Peer-Zada Khalid Hundallah
Khalid Hundallah Amal Alhashem
Amal Alhashem