- 1Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa, Genoa, Italy
- 2IRCCS Ospedale Policlinico San Martino, Genoa, Italy
- 3Department of Health Sciences (DISSAL), University of Genoa, Genoa, Italy
Introduction: Idiopathic normal pressure hydrocephalus (INPH) is a neurological disorder that is potentially reversible and clinically characterized by a specific triad of symptoms, including gait disturbance, cognitive disorders, and urinary incontinence. In INPH assessment, the most commonly used test is the Timed Up and Go test (TUG), but a more comprehensive assessment would be necessary. The first aim of the present study is to verify the sensitivity of a protocol with both clinical and instrumental outcome measures for gait and balance in recognizing INPH patients. The second aim is to verify the most important spatio-temporal parameters in INPH assessment and their possible correlations with clinical outcome measures.
Methods: Between January 2019 and June 2022, we evaluated 70 INPH subjects. We assessed balance performances with the Berg Balance Scale (BBS), Short Physical Performance Battery (SPPB), and TUG, both single (ST) and dual task (DT). We also performed an instrumental gait assessment with the GAITRite electronic walkway system, asking the patients to walk on the carpet for one minute at normal speed, fast speed, and while performing a dual task. We compared the results with those of 20 age-matched healthy subjects (HS).
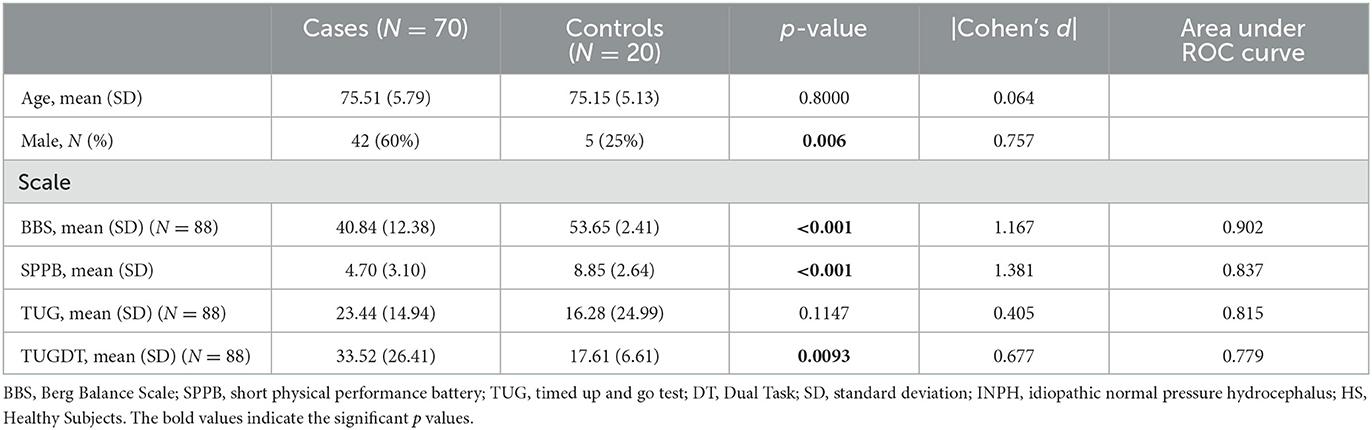
Results: INPH patients obtained statistically significant lower scores at the BBS, SPPB, and TUG DT but not at the TUG ST, likely because the DT involves cognitive factors altered in these subjects. Concerning instrumental gait evaluation, we found significant differences between HS and INPH patients in almost all spatio-temporal parameters except cadence, which is considered a relevant factor in INPH guidelines. We also found significant correlations between balance outcome measures and gait parameters.
Discussion: Our results confirm the usefulness of BBS and suggest improving the assessment with SPPB. Although the TUG ST is the most commonly used test in the literature to evaluate INPH performances, it does not identify INPH; the TUG DT, instead, might be more useful. The GAITRite system is recognized as a quick and reliable tool to assess walking abilities and spatio-temporal parameters in INPH patients, and the most useful parameters are stride length, stride width, speed, and the percentage of double support. Both clinical and instrumental evaluation may be useful in recognizing subjects at risk for falls.
1. Introduction
Idiopathic normal pressure hydrocephalus (INPH) is a neurological disorder characterized by ventricular dilation visible by brain imaging and normal cerebrospinal fluid (CSF) pressure during lumbar puncture. Clinically, it is characterized by a specific triad of symptoms including gait disturbance, cognitive disorders, and urinary incontinence (1, 2). INPH manifests during adult life as an insidiously progressive, chronic disorder that lacks an identifiable antecedent cause (3).
Due to the limited knowledge of pathophysiological mechanisms, non-specific symptoms, and the high prevalence of comorbidities, INPH is largely underdiagnosed (4). A group of Norway researchers highlighted that ~5% of dementia diagnoses were caused by INPH (3).
In this disease, the accumulation of CSF is the cause of the symptoms (5), and its withdrawal may lead to an improvement in all the symptoms (6, 7). A large multicenter study reports improvements in the gait domain in 77% of the patients, 63% in the domains of neuropsychology, 56% in balance, and 66% in urinary continence, after CSF shunt surgery (8). Therefore, INPH diagnosis is very important since it is considered a neurological disorder potentially reversible in which a gait disorder and dementia can improve to a complete remission of symptoms especially if detected and treated early (9–13).
In the INPH guidelines, a diagnosis is probable whether the patients present a suggestive clinical history with an insidious onset, a minimum duration of at least 3 months, no other disorders causing the symptoms, ventricular enlargement evidenced at brain imaging study (CT or MRI), CSF opening pressure in the range of 5–18 mm Hg (or 70–245 mm H2O) as determined by a lumbar puncture and the presence of gait or balance disturbance (3, 14), and at least one other area of impairment in cognition, urinary symptoms, or both.
With respect to gait/balance, at least two of the following should be present:
1. Decreased step height.
2. Decreased step length.
3. Decreased cadence.
4. Increased trunk sway during walking.
5. Widened standing base.
6. Toes turned outward on walking.
7. Retropulsion (spontaneous or provoked).
8. En bloc turning (turning requiring three or more steps for 180 degrees).
9. Impaired walking balance, as evidenced by two or more corrections out of eight steps on tandem gait testing.
Patients with INPH manifest some or all the classic clinical symptoms in a variable way, but often the first disorder is represented by gait disorders (15). In most studies, walking impairment is evaluated just by clinical observation or based on the patient's impression of improvement, but both are subjective measures, therefore prone to bias (10, 16–18). In the literature, the most used test is the timed up and go test (TUG) (19, 20), which mainly investigates dynamic balance, but, as mentioned, INPH symptomatology is very complex, and the TUG may not be sufficient to capture improvements in the other domains, both motor and cognitive (21). The availability of objective outcome measures is very important in every disease. INPH is even more relevant to assess the improvements after CSF subtraction to predict the possible results after shunt surgery. For a comprehensive evaluation, it is necessary to find clinical and instrumental outcome measures that may help physicians in performing an objective and correct evaluation of INPH subjects and evaluating the possible improvements after CSF subtraction. For this reason, in our movement analysis laboratory, we evaluated the gait and balance of INPH patients with a protocol of both clinical and instrumental outcome measures. The first aim of the present study is to confirm the sensitivity of clinical and instrumental outcome measures for gait and balance in recognizing INPH patients. The second aim is to verify the most important spatio-temporal parameters in INPH assessment and their possible correlations with clinical outcome measures.
2. Materials and methods
This retrospective study was based on data obtained from patients attending the multidisciplinary outpatient clinic for the diagnosis and treatment of INPH at the IRCCS Ospedale Policlinico San Martino of Genoa, Italy. The study was conducted according to the guidelines of the Declaration of Helsinki.
The inclusion criteria included those as follows: confirmed clinical and neuroradiological diagnosis of INPH (3); adults; ability to walk without support for at least 25 m; Short Physical Performance Battery (SPPB) scoring between 2 and 10; and ability to sign informed consent.
The exclusion criteria included those as follows: other forms of neurological disorders; vestibular affections; psychiatric, cardiovascular, and lung disorders or severe arthropathic changes in the lower limbs; and inability to perform the TUG.
Between January 2019 and June 2022, we evaluated 70 subjects affected by INPH, who met all the inclusion criteria. All patients underwent an MRI with stroke volume evaluation, and after recording a detailed medical history, a complete neurosurgical, neurological, and physical examination was performed. A single examiner was responsible for the assessment of all subjects. All subjects underwent an evaluation by means of clinical scales and instrumental gait evaluation.
We also dispose data from a control group of 20 healthy age-matched subjects (HS).
2.1. Clinical outcome measures
Balance performances have been evaluated with the Berg Balance Scale (BBS), SPPB, and TUG for both single and dual tasks.
Particularly, the TUG is a test widely used to assess the possible improvements after CSF withdrawal (tap test) in INPH patients. It involves rising from a seated position, walking 3 m, turning around, walking back, and sitting back down. This test is a simple and quick measure of functional mobility and has excellent reliability among healthy older adults and populations with different medical diagnoses (22). In elderly persons, the mean (95% confidence interval) TUG time for individuals between 60 and 90 years of age is lower than 10 s (23, 24). The cognitive task in TUG DT, in our protocol, a serial-3 subtraction task, involves sustained attention, information processing speed, and working memory abilities (21, 25). Previous studies document that the time difference between dual- and single-task TUG is a valid marker of frailty and falls (21, 26).
The BBS, a 14-item objective test, is a sensitive scale to detect subtle balance impairment and fall risk that has been validated in people affected by neurological disorders (27–30) and has also been used in the assessment of disability in patients affected by INPH (19, 31, 32). The total score ranges from 0 to 56, but a score below 45 is indicative of imbalance and a great risk of falls (33, 34).
SPPB is a widely used instrument to quantify balance disorders and gait impairment in elderly people, in many different neurological diseases (28, 29, 35–38), and in INPH patients (39). It is a composite measure assessing walking speed, standing balance, and sit-to-stand performance, which has been shown to have a high level of validity, reliability, and responsiveness in measuring physical function (40). The total score ranges from 0 to 12, and a score below 10 is associated with a risk of falls (41, 42).
2.2. Instrumental evaluation
We performed an instrumental gait assessment by means of the GAITRite electronic walkway system, which is a 7-m-long electronic portable walkway able to measure the temporal and spatial gait parameters. The electronic walkway is non-invasive and does not require any attachments to the subject under investigation.
As the subject ambulates across the walkway, the pressure exerted by the feet onto the walkway activates the sensors. Our patients were asked to walk on the carpet for 1 min at normal speed (normal walk, NW), at fast speed, but not running (fast walk, FW), and at normal speed enunciating aloud all possible words beginning with a chosen letter (dual task, DT). Patients always had a researcher walking alongside them as a safeguard.
PKMAS is software in conjunction with the GAITRite system, providing information about spatial and temporal parameters of the objects in contact with the walkway surface.
As seen in the literature regarding the most used parameters (43–46), we focused our attention on the following: stride length, stride width, stride time, velocity, and cadence. We also checked for the different percentages of the gait cycle time: stance, swing, single support, and total double support.
Stride length is the distance from the heel of one foot to the following heel of the same foot (cm). Stride width is the distance between a line connecting the two ipsilateral foot heel contacts (the stride) and the contralateral foot heel contact between those events and is measured perpendicular to the stride (cm). Stride time and gait cycle time are the period of time from the first contact of one foot to the following first contact of the same foot (s). Stance time is the period of time when the foot is in contact with the ground (s). Stance percentage is the stance time presented as a percentage of the gait cycle time. Swing time is the period of time when the foot is not in contact with the ground (s). Swing percentage is swing time presented as a percentage of the gait cycle time. Single support time is the period of time when only the current foot is in contact with the ground (s). Single support percentage is single support time presented as a percentage of gait cycle time. Total double support time is the sum of all periods of time when both feet are in contact with the ground during the stance phase (s). Total double support percentage is total double support time presented as a percentage of the gait cycle time. Velocity is obtained after dividing the sum of all stride lengths, by the sum of all stride time (cm/s). Cadence is the number of footfalls minus one, divided by the ambulation time (steps/min).
2.3. Statistical analysis
The sample size for the control group was calculated based on a two-sample means test, fixing power to 0.90 and alpha to 0.05. The sample size was assessed for the evaluations under study (stride length, double sup, velocity, and stride width), fixing the number of cases and mean (SD) of the evaluations based on the observed values among cases and setting the effect sizes based on preliminary results on a subgroup of available controls. The sample of controls was designed to be comparable to cases in terms of age (age categories).
We verified that cases and controls were comparable for age by performing the two-sample t-test, subsequently, we compared the two groups in terms of stride length, double sup, velocity, stride width, BBS, SPPB, and TUG using t-test, and we calculated the Cohen's d to describe the standardized mean difference of an effect (47). We also calculated Pearson's correlation coefficients between balance outcome measures and gait parameters among cases (48).
A two-sided α of <0.05 was considered to be statistically significant. All statistical analyses were performed using Stata version 16.0 (Stata Corporation, College Station, TX, USA).
3. Results
We analyzed the data of 70 INPH patients attending our movement analysis laboratory.
The mean age was 75.5 ± 5.8 years (men 60%, women 40%).
We also confronted the scores with a group of age-matched healthy subjects (75.1 ± 5.1 years), free of neurological comorbidities, who attended our outpatient service for osteoporosis management. The exclusion criteria were severe osteoporosis, neurological disorders, fractures, or previous surgery at lower limbs or who were not able to walk without support for at least 25 m; we also included caregivers and carers who met the same criteria.
Table 1 provides a summary of clinical outcome measures.
We found a significant difference between INPH patients and HS in all scales (p < 0.05) except TUG ST.
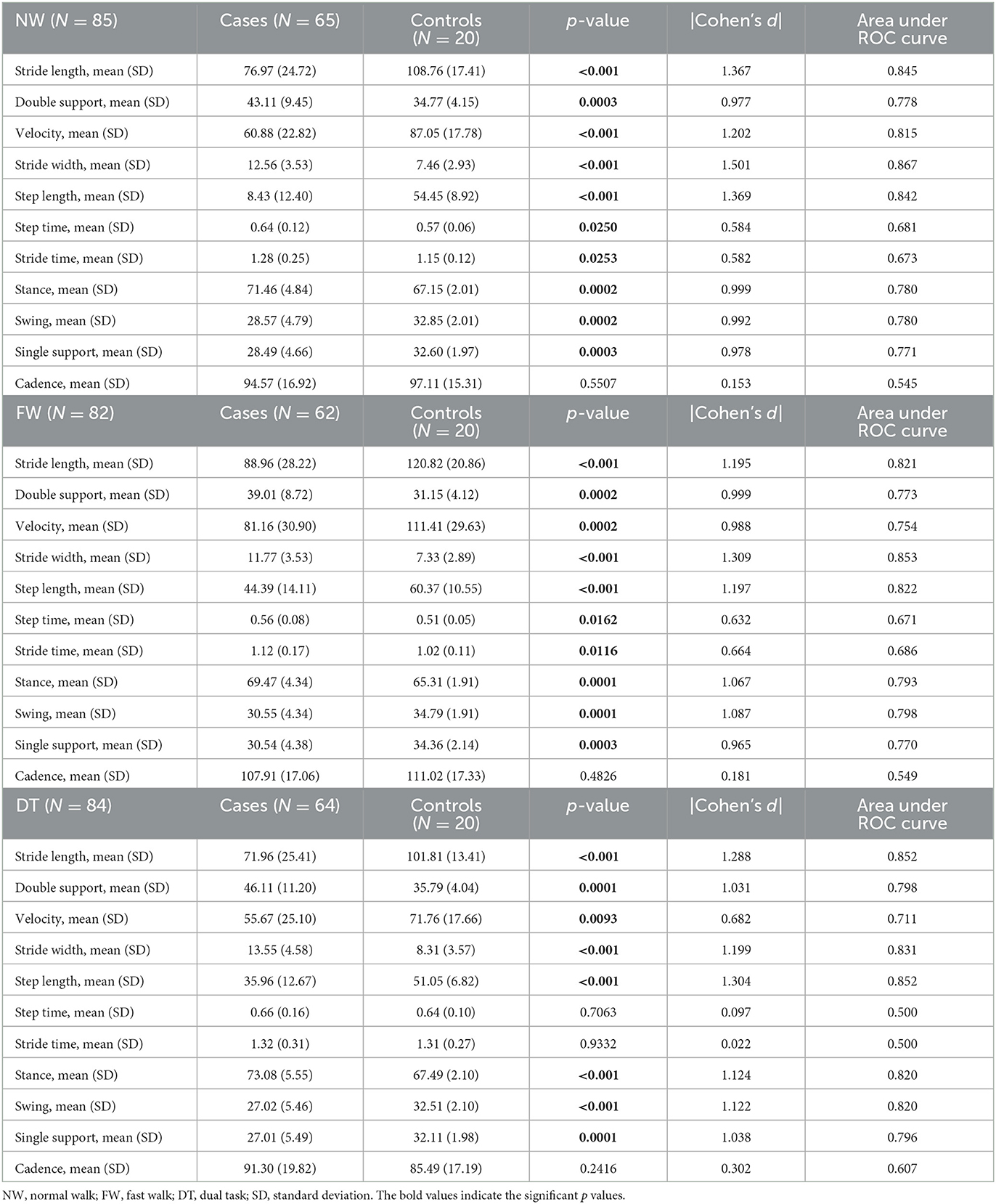
Table 2 provides a summary of the instrumental gait assessment.
A total of five patients were excluded from the analysis because they were not able to walk on the carpet for a minute. The other three patients were not able to modify the speed at the FW, and one patient was excluded from the analysis because he refused to perform the DT.
At the NW and FW, we found significant differences between INPH patients and HS in all spatio-temporal parameters except for cadence.
At DT, we found significant differences in all parameters except for step, stride time, and cadence.
Large effect size based on Cohen's d (d ≥ 0.8) was observed for stride length (NW: d = 1.367, FW: d = 1.195, DT: d = 1.288), double support (NW: d = 0.977; FW: d = 0.999; DT: d = 1.031), velocity (NW: d = 1.202; FW: d = 0.988), stride width (NW: d = 1.501; FW: d = 1.309; DT: d = 1.199), step length (NW: d = 1.369, FW: d = 1.197; DT: d = 1.304), stance (NW: d = 0.999, FW: d = 1.067; DT: d = 1.124), swing (NW: d = 0.992; FW: d = 1.087; DT: d = 1.122), and single support (NW: d = 0.978; FW: d = 0.965; DT: d = 1.038).
Regarding the correlations between clinical outcome measures and instrumental spatio-temporal parameters, see Figure 1.
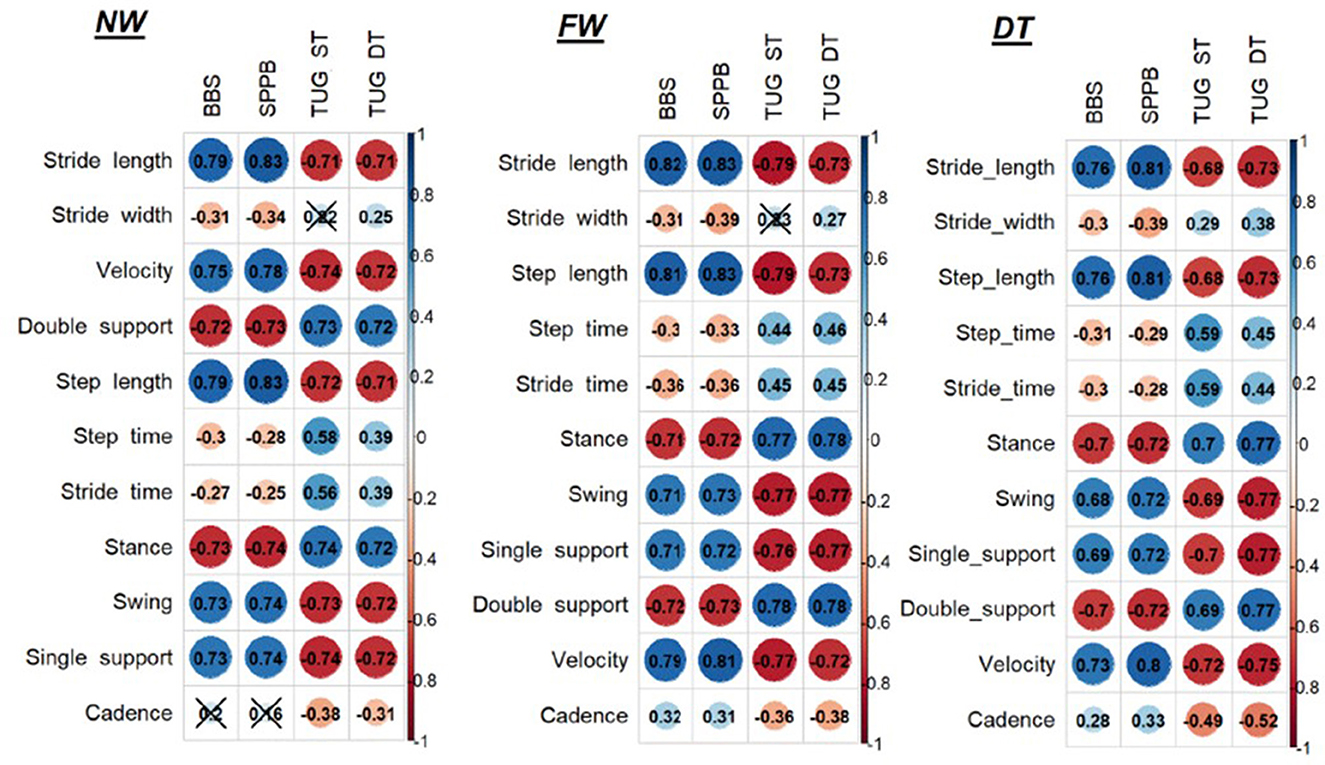
Figure 1. Correlations between clinical outcome measures and instrumental spatio-temporal parameters. NW, Normal walk; FW, Fast walk; DT, Dual task; BBS, Berg Balance Scale; SPPB, Short Physical Performance Battery; TUG, Timed up and go test; ST, Single task.
At the NW and FW, we found that stride length and velocity have a high significant correlation with BBS and SPPB and a high negative correlation with TUG both ST and DT (p < 0.001); stride width has only a low negative correlation with BBS and SPPB (p < 0.05). Concerning the percentages of gait phases, we found a high significant correlation between all the parameters: the percentage of double support and stance showed a high negative correlation with BBS and SPPB (p < 0.001), a high positive correlation with TUG ST and DT (p < 0.001), while the percentage of swing and single support present high positive correlation with BBS and SPPB (p < 0.001) and high negative correlation with TUG both ST and DT (p < 0.001).
At DT, we found that stride length has a high positive correlation with BBS and SPPB (p < 0.001), high negative correlation with TUG DT (p < 0.001), and moderate to high negative correlation with TUG ST (p < 0.001); velocity has a high positive correlation with BBS and SPPB and a negative high correlation with TUG both ST and DT (p < 0.001); stride width has only low negative correlation with BBS and SPPB (p < 0.05). Concerning the percentages of gait phases, we found a high negative correlation between double support percentage with BBS and SPPB, high positive correlation with TUG DT, and moderate to high positive correlation with TUG ST (p < 0.001); stance presents high negative correlation with BBS and SPPB (p < 0.001), high positive correlation with TUG ST and DT (p < 0.001); swing has moderate to high positive correlation with BBS, high correlation with SPPB, moderate to high negative correlation with TUG ST, and high negative correlation with TUG DT (p < 0.001); single support has a moderate to high positive correlation with BBS, high correlation with SPPB, and high negative correlation with TUG ST and DT (p < 0.001).
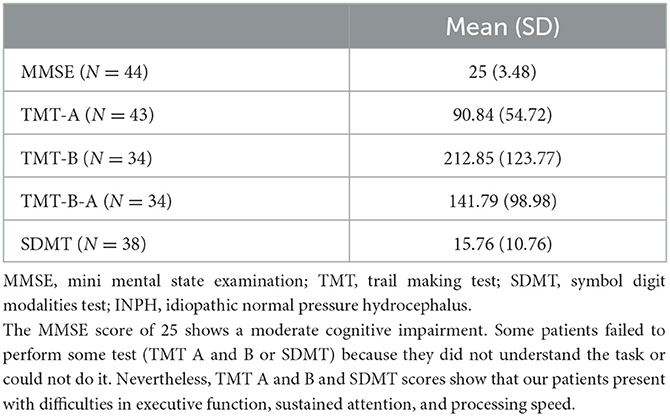
The cognitive evaluation performed on a subgroup of 44 subject is presented in Table 3. The MMSE score of 25 shows a moderate cognitive impairment, while the scores at the TMT both A and B and at SDMT show an impairment in the executive function, sustained attention and processing speed. Unfortunately, we cannot draw conclusions because we do not dispose of the data of the same control group.
4. Discussion
All our patients reported walking impairment, imbalance, and cognitive disorders such as memory loss and difficulty in attention or concentration. At the clinical evaluation, they presented a mean score lower than 45 at the BBS, a score lower than 10 at the SPPB, and they took more than 20 s at the TUG ST, which are related to imbalance and risk of falls (20, 34, 41, 42, 49, 50). Lower scores on the SPPB have been shown to be predictive of an increased risk of falling, loss of independence in activities of daily living, decreased mobility, and disability (39–41). This may lead to important considerations to achieve in their treatment, such as the adoption of a cane or a walker to improve walking stability and safety.
It is hence not surprising to find significant differences between HS and INPH persons in the balance outcome measures since these subjects often complain imbalance and frequent falls.
BBS and SPPB are confirmed as useful outcome measures for a rapid and reliable clinical assessment of balance (27, 51–53) and also in INPH assessment.
The TUG is a test widely used in INPH and other various neurological pathologies (19, 20, 43, 54, 55). Particularly, in INPH, it has been used as a marker of the efficacy of CSF subtraction. The international guideline for the prevention of falls in frail elderly individuals recommends TUG as a screening tool for increased risk of falls. Previous studies (20, 55, 56) have suggested that elderly individuals scoring 20 s or more on the TUG have a significantly higher risk for falls and recommend this test as a reliable and simple quantitative examination tool for evaluating improvement in gait disturbance and physical performance after the tap test or shunt surgery in INPH. However, our results suggest that the assessment of dynamic balance through the TUG ST test does not show significant differences between INPH subjects and the control group. This may depend on the fact that the control group was constituted of persons of an age at risk of falling due to their frailty because of sarcopenia, osteoporosis, and multiple comorbidities (57–63). Nevertheless, previous studies reported that TUG ST performances could be influenced by age, sex, body height (21, 23, 64), lower limb strength, walking speed (65), and cognitive variables (66, 67), while dual-task tests might have an added value for fall prediction than single-task tests (26, 68–71). Executive function, sustained attention, information processing speed, and functional mobility may contribute to DT performances (21, 25), as well as attention shifting and working memory (72, 73). The TUG DT, in fact, manages to capture significant differences with respect to the healthy sample because the cognitive factor, predominant in the INPH, influences the evaluation (21, 72). Furthermore, previous research showed that the time difference in performances on TUG DT and ST is a valid predictor of frailty and falls (26, 66).
Our results suggest the usefulness of BBS and SPPB in INPH assessment, while the TUG ST is not the better test to perform. To include a double task is important to recognize these patients, given the presence of both motor and cognitive difficulties in INPH.
Concerning instrumental gait evaluation, we found significant differences between healthy subjects and INPH patients in almost all spatio-temporal parameters. Cadence is considered a relevant parameter in INPH assessment, so much so that it is included in the guidelines for the probable diagnosis of INPH (3). However, our results suggest that cadence is not able to detect differences with the control group of HS, and it is hence not suitable to consider it in these subjects' assessments. Instead, reduced stride length and wide standing base, which are also included in the INPH diagnosis guidelines, in our sample are significantly different with respect to the control group, confirming that the most relevant spatio-temporal parameters to consider in INPH subjects are stride length, stride width, and speed; furthermore, we found significant differences between the two groups in the percentage of swing, single and double support phases. All these parameters are related to imbalance. Studies investigating gait impairment and imbalance suggest that double support percentage is considered a stabilizing factor during normal gait in the elderly (15, 44). It is known that faller patients walk with shorter steps, longer stance phase, and a shorter swing phase than the non-fallers (44), and shorter steps result in reduced gait speed. This indicates that the faller patients may use a more conservative and cautious gait strategy to maintain dynamic balance by reducing gait velocity and taking shorter steps to prevent falls; the faller patients may increase the double support period to stabilize their inefficient gait control (44).
The fact that all gait parameters in INPH subjects have strong correlations with all balance scales, both static and dynamic, confirms what is already known, i.e., that patients with imbalance should reduce walking speed and stride length, increasing the double support to increase stability, thus compromising the fluidity of the gait. The stride width, particularly, has been generally considered as a balance-related gait parameter (74), and INPH subjects present a wider base with respect to the healthy group even though there is no correlation with balance outcome measures. This pattern has been also interpreted as a cautious strategy to maintain balance and prevent falls (44). These correlations are similar in all tasks (NW, FW, and DT), suggesting the utility of the GAITRite system in evaluating these patients and the added value of a comprehensive assessment using different gait paradigms.
Concluding, in INPH motor assessment it is important to evaluate both balance and gait performances and BBS and SPPB are confirmed as fast and simple tests accurately evaluating the functional impairment of these patients. Although the TUG ST is the most used test in literature to evaluate INPH performances, it does not show significant differences between INPH subjects and the control group, and this may be explained because it could be influenced by age, sex, lower limb strength, and walking speed while dual-task tests might have an added value since executive function, sustained attention, information processing speed, and functional mobility are involved.
The GAITRite system is a quick and reliable tool to assess walking abilities and spatio-temporal parameters also in INPH patients. Even though decreased cadence is considered a contributing parameter to the diagnosis of INPH, we found no significant differences compared to the HS group. We suggest that the most useful parameters to consider are stride length, stride width, speed, and the percentage of double support. Both clinical and instrumental evaluation may be useful in recognizing subjects at risk for falls. Our results are encouraging to verify the usefulness of this combined assessment before and after CSF withdrawal to identify subjects who can benefit the most, and, therefore, candidates for the ventricular shunt intervention, thus helping the clinicians in the operative decision.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LM was responsible for research design, data analysis, and wrote the manuscript. MPo was responsible for the statistical analysis. CS, GB, LV, and OC performed clinical or instrumental evaluations. FC, AM, LP, SM, and DDC performed all clinical and instrumental evaluations and contributed to the data interpretation. SC performed the cognitive evaluations. EP contributed to the gait analysis. MPa and CT revised the manuscript. PF, PM, and GZ being expert neurosurgeons, recruited the subjects, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Ligurian INPH study group
Giulia Biasotti1, 2, Oscar Crisafulli1, Salvatore Fede2, Paolo Merciadri2, Elisa Pelosin1, Elena Saretti1, 2, Cristina Schenone1, 2 and Lucilla Vestito1, 2.
1Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa, Italy.
2IRCCS Ospedale Policlinico San Martino, Genoa, Italy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gavrilov GV, Gaydar BV, Svistov DV, Korovin AE, Samarcev IN, Churilov LP, et al. Idiopathic normal pressure hydrocephalus (Hakim-Adams syndrome): clinical symptoms, diagnosis and treatment. Psychiatr Danub. (2019) 31:737–44.doi: 10.1002/GPS.4847
2. Mongin M, Hommet C, Mondon K. [Normal pressure hydrocephalus: a review and practical aspects]. Rev Med Interne. (2015) 36:825–33. doi: 10.1016/j.revmed.2015.08.001
3. Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PMcL. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. (2005) 57(suppl_3):S2-4–16. doi: 10.1227/01.NEU.0000168185.29659.C5
4. Pyykkö OT, Nerg O, Niskasaari HM, Niskasaari T, Koivisto AM, Hiltunen M, et al. Incidence, comorbidities, and mortality in idiopathic normal pressure hydrocephalus. World Neurosurg. (2018) 112:e624–31. doi: 10.1016/j.wneu.2018.01.107
5. Kanemoto H, Mori E, Tanaka T, Suehiro T, Yoshiyama K, Suzuki Y, et al. Cerebrospinal fluid amyloid beta and response of cognition to a tap test in idiopathic normal pressure hydrocephalus: a case-control study. Int Psychoger. (2021) 1–9. doi: 10.1017/S1041610221000661
6. Fowler JB, De Jesus O, Mesfin FB. Ventriculoperitoneal shunt. In: StatPearls. StatPearls Publishing (2022). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK459351/ (accessed February 15, 2023).
7. Lee SM, Kwon KY. Clinical tips in diagnosing idiopathic normal pressure hydrocephalus: a new concept beyond the cerebrospinal fluid tap test. J Integr Neurosci. (2021) 20:471–5. doi: 10.31083/j.jin2002050
8. Klinge P, Hellström P, Tans J, Wikkelsø C. One-year outcome in the European multicentre study on iNPH. Acta Neurol Scand. (2012) 126:145–53. doi: 10.1111/j.1600-0404.2012.01676.x
9. Allali G, Laidet M, Armand S, Saj A, Krack P, Assal F. Apathy in idiopathic normal pressure hydrocephalus: a marker of reversible gait disorders. Int J Geriatr Psychiatry. (2018) 33:735–42. doi: 10.1002/gps.4847
10. Belotti F, Pertichetti M, Muratori A, Migliorati K, Panciani PP, Draghi R, et al. Idiopathic normal pressure hydrocephalus: postoperative patient perspective and quality of life. Acta Neurochir. (2022) 164:2855–66. doi: 10.1007/s00701-022-05275-x
11. Das S, Montemurro N, Ashfaq M, Ghosh D, Sarker AC, Khan AH, et al. Resolution of papilledema following ventriculoperitoneal shunt or endoscopic third ventriculostomy for obstructive hydrocephalus: a pilot study. Medicina. (2022) 58:281. doi: 10.3390/medicina58020281
12. Martin CMH. The “reversible” dementia of idiopathic normal pressure hydrocephalus. Consult Pharm. (2006) 21:888–903. doi: 10.4140/TCP.n.2006.888
13. Schniepp R, Trabold R, Romagna A, Akrami F, Hesselbarth K, Wuehr M, et al. Walking assessment after lumbar puncture in normal-pressure hydrocephalus: a delayed improvement over 3 days. J Neurosurg. (2017) 126:148–57. doi: 10.3171/2015.12.JNS151663
14. Fisher S, Ottenbacher KJ, Goodwin JS, Graham JE, Ostir GV. Short physical performance battery in hospitalized older adults. Aging Clin Exp Res. (2009) 21:445–52. doi: 10.1007/BF03327444
15. Lim Y-H, Ko P-W, Park K-S, Hwang SK, Kim S-H, Han J, et al. Quantitative gait analysis and cerebrospinal fluid tap test for idiopathic normal-pressure hydrocephalus. Sci Rep. (2019) 9:16255. doi: 10.1038/s41598-019-52448-3
16. Kobayashi E, Kanno S, Kawakami N, Narita W, Saito M, Endo K, et al. Risk factors for unfavourable outcomes after shunt surgery in patients with idiopathic normal-pressure hydrocephalus. Sci Rep. (2022). 12:13921. doi: 10.1038/s41598-022-18209-5
17. Messerer M, Blanchard M, Papadimitriou K, Vandenbulcke A, Rutz D, Beaud V, et al. Impact of subjective evaluations in predicting response to ventriculoperitoneal shunt for idiopathic normal pressure hydrocephalus. World Neurosurg. (2022) 166:e741–9. doi: 10.1016/j.wneu.2022.07.087
18. Nikaido Y, Urakami H, Okada Y, Akisue T, Kawami Y, Ishida N, et al. Rehabilitation effects in idiopathic normal pressure hydrocephalus: a randomized controlled trial. J Neurol. 270:357–68. doi: 10.1007/s00415-022-11362-x
19. Gallagher R, Marquez J, Osmotherly P. Clinimetric properties and minimal clinically important differences for a battery of gait, balance, and cognitive examinations for the tap test in idiopathic normal pressure hydrocephalus. Neurosurgery. (2019) 84:E378–84. doi: 10.1093/neuros/nyy286
20. Yamada S, Ishikawa M, Miyajima M, Nakajima M, Atsuchi M, Kimura T, et al. Timed up and go test at tap test and shunt surgery in idiopathic normal pressure hydrocephalus. Neurol Clin Pract. (2017) 7:98. doi: 10.1212/CPJ.0000000000000334
21. Tang PF, Yang HJ, Peng YC, Chen HY. Motor dual-task Timed Up & Go test better identifies prefrailty individuals than single-task Timed Up & Go test. Geriatr Gerontol Int. (2015) 15:204–10. doi: 10.1111/ggi.12258
22. Herman T, Giladi N, Hausdorff JM. Properties of the “timed up and go” test: more than meets the eye. Gerontology. (2011) 57:203–10. doi: 10.1159/000314963
23. Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. (2006) 29:64–8. doi: 10.1519/00139143-200608000-00004
24. Svinøy OE, Hilde G, Bergland A, Strand B. Timed up and go reference values for older adults with and without noncommunicable diseases and arthritis. Osteoarthritis Cartilage. (2020) 28:S361–2. doi: 10.1016/j.joca.2020.02.564
25. Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci. (2008) 63:1335–43. doi: 10.1093/gerona/63.12.1335
26. Lundin-Ohson L, Lars Nyberg R, Gustafson Y. Attention, frailty, and falls: the effect of a manual task on basic mobility. J Am Geriatr Soc. (1998) 46:758–61. doi: 10.1111/j.1532-5415.1998.tb03813.x
27. Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. (2008) 88:559–66. doi: 10.2522/ptj.20070205
28. Mori L, Signori A, Prada V, Pareyson D, Piscosquito G, Padua L, et al. Treadmill training in patients affected by Charcot–Marie–Tooth neuropathy: results of a multicenter, prospective, randomized, single-blind, controlled study. Eur J Neurol. (2020) 27:280–7. doi: 10.1111/ene.14074
29. Mori L, Prada V, Signori A, Pareyson D, Piscosquito G, Padua L, et al. Outcome measures in the clinical evaluation of ambulatory Charcot-Marie-Tooth 1A subjects. Eur J Phys Rehabil Med. (2019) 55:47–55. doi: 10.23736/S1973-9087.18.05111-0
30. Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W. Validating the Berg Balance Scale for patients with Parkinson's disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil. (2005) 86:789–92. doi: 10.1016/j.apmr.2004.11.005
31. Nikaido Y, Kajimoto Y, Akisue T, Urakami H, Kawami Y, Kuroda K, et al. Dynamic balance measurements can differentiate patients who fall from patients who do not fall in patients with idiopathic normal pressure hydrocephalus. Arch Phys Med Rehabil. (2019) 100:1458–66. doi: 10.1016/j.apmr.2019.01.008
32. Nikaido Y, Urakami H, Akisue T, Okada Y, Katsuta N, Kawami Y, et al. Associations among falls, gait variability, and balance function in idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg. (2019) 183:105385. doi: 10.1016/j.clineuro.2019.105385
33. Lima CA, Ricci NA, Nogueira EC, Perracini MR. The Berg Balance Scale as a clinical screening tool to predict fall risk in older adults: a systematic review. Physiotherapy. (2018) 104:383–94. doi: 10.1016/j.physio.2018.02.002
34. Park SH, Lee YS. The diagnostic accuracy of the Berg Balance Scale in predicting falls. West J Nurs Res. (2017) 39:1502–25. doi: 10.1177/0193945916670894
35. Farag I, Sherrington C, Hayes A, Canning CG, Lord SR, Close JC, et al. Economic evaluation of a falls prevention exercise program among people With Parkinson's disease. Mov Disord. (2016) 31:53–61. doi: 10.1002/mds.26420
36. Motl RW, Learmonth YC, Wójcicki TR, Fanning J, Hubbard EA, Kinnett-Hopkins D, et al. Preliminary validation of the short physical performance battery in older adults with multiple sclerosis: secondary data analysis. BMC Geriatrics. (2015) 15:157. doi: 10.1186/s12877-015-0156-3
37. Palumbo P, Palmerini L, Bandinelli S, Chiari L. Fall risk assessment tools for elderly living in the community: can we do better? PLoS ONE. (2015) 10:e0146247. doi: 10.1371/journal.pone.0146247
38. Taricco M, Dallolio L, Calugi S, Rucci P, Fugazzaro S, Stuart M, et al. Impact of adapted physical activity and therapeutic patient education on functioning and quality of life in patients with postacute strokes. Neurorehabil Neural Repair. (2014) 28:719–28. doi: 10.1177/1545968314523837
39. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. (1994) 49:M85–94. doi: 10.1093/geronj/49.2.M85
40. Treacy D, Hassett L. The short physical performance battery. J Physiother. (2018) 64:61. doi: 10.1016/j.jphys.2017.04.002
41. Lauretani F, Ticinesi A, Gionti L, Prati B, Nouvenne A, Tana C, et al. Short-physical performance battery (SPPB) score is associated with falls in older outpatients. Aging Clin Exp Res. (2019) 31:1435–42. doi: 10.1007/s40520-018-1082-y
42. Welch SA, Ward RE, Beauchamp MK, Leveille SG, Travison T, Bean JF. The Short Physical Performance Battery (SPPB): a quick and useful tool for fall risk stratification among older primary care patients. J Am Med Dir Assoc. (2021) 22:1646–51. doi: 10.1016/j.jamda.2020.09.038
43. Bower K, Thilarajah S, Pua Y-H, Williams G, Tan D, Mentiplay B, et al. Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. J Neuroeng Rehabil. (2019) 16:3. doi: 10.1186/s12984-018-0478-4
44. Kwon MS, Kwon YR, Park YS, Kim JW. Comparison of gait patterns in elderly fallers and non-fallers. Technol Health Care. (2018) 26:S427–36. doi: 10.3233/THC-174736
45. Lai Y-R, Lien C-Y, Huang C-C, Lin W-C, Chen Y-S, Yu C-C, et al. Clinical disease severity mediates the relationship between stride length and speed and the risk of falling in Parkinson's disease. J Pers Med. (2022) 12:92. doi: 10.3390/jpm12020192
46. Paquin M-H, Duclos C, Lapierre N, Dubreucq L, Morin M, Meunier J, et al. The effects of a strong desire to void on gait for incontinent and continent older community-dwelling women at risk of falls. Neurourol Urodyn. (2020) 39:642–9. doi: 10.1002/nau.24234
47. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. (2013) 4:863. doi: 10.3389/fpsyg.2013.00863
48. Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. (2012) 24:69–71.
49. Lusardi MM, Fritz S, Middleton A, Allison L, Wingood M, Phillips E, et al. Determining risk of falls in community dwelling older adults: a systematic review and meta-analysis using posttest probability. J Geriatr Phys Ther. (2017) 40:1–36. doi: 10.1519/JPT.0000000000000099
50. Park SH. Tools for assessing fall risk in the elderly: a systematic review and meta-analysis. Aging Clin Exp Res. (2018) 30:1–16. doi: 10.1007/s40520-017-0749-0
51. Fisher CM. The clinical picture in occult hydrocephalus. Clin Neurosurg. (1977) 24:270–84. doi: 10.1093/neurosurgery/24.CN_suppl_1.270
52. Mijnarends DM, Meijers JMM, Halfens RJG. Borg St, Luiking YC, Verlaan S, et al. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: a systematic review. J Am Med Dir Assoc. (2013) 14:170–8. doi: 10.1016/j.jamda.2012.10.009
53. Miranda-Cantellops N, Tiu TK. Berg Balance Testing. StatPearls. (2022). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK574518/ (accessed January 4, 2023).
54. Sundström N, Rydja J, Virhammar J, Kollén L, Lundin F, Tullberg M. The timed up and go test in idiopathic normal pressure hydrocephalus: a Nationwide Study of 1300 patients. Fluids Barriers CNS. (2022) 19:4. doi: 10.1186/s12987-021-00298-5
55. Engel DC, Pirpamer L, Hofer E, Schmidt R, Brendle C. Incidental findings of typical iNPH imaging signs in asymptomatic subjects with subclinical cognitive decline. Fluids Barriers CNS. (2021) 18:37. doi: 10.1186/s12987-021-00268-x
56. Nakajima M, Yamada S, Miyajima M, Ishii K, Kuriyama N, Kazui H, et al. Guidelines for management of idiopathic normal pressure hydrocephalus (third edition): endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurol Med Chir. (2021) 61:63–97. doi: 10.2176/nmc.st.2020-0292
57. Carneiro JA, Cardoso RR, Durães MS, Guedes MCA, Santos FL, Costa FMD, et al. Frailty in the elderly: prevalence and associated factors. Rev Bras Enferm. (2017) 70:747–52. doi: 10.1590/0034-7167-2016-0633
58. Coughlan T, Dockery F. Osteoporosis and fracture risk in older people. Clin Med. (2014) 14:187–91. doi: 10.7861/clinmedicine.14-2-187
59. Rodrigues F, Domingos C, Monteiro D, Morouço P. A review on aging, sarcopenia, falls, and resistance training in community-dwelling older adults. Int J Environ Res Public Health. (2022) 19:874. doi: 10.3390/ijerph19020874
60. Zhang X, Huang P, Dou Q, Wang C, Zhang W, Yang Y, et al. Falls among older adults with sarcopenia dwelling in nursing home or community: a meta-analysis. Clin Nutr. (2020) 39:33–9. doi: 10.1016/j.clnu.2019.01.002
61. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2019) 10:485–500. doi: 10.1002/jcsm.12411
62. Zhang C, Dong X, Ding M, Chen X, Shan X, Ouyang H, et al. Executive control, alerting, updating, and falls in cognitively healthy older adults. Gerontology. (2020) 66:494–505. doi: 10.1159/000509288
63. Paltamaa J, Sarasoja T, Leskinen E, Wikström J, Mälkiä E. Measures of physical functioning predict self-reported performance in self-care, mobility, and domestic life in ambulatory persons with multiple sclerosis. Arch Phys Med Rehabil. (2007) 88:1649–57. doi: 10.1016/j.apmr.2007.07.032
64. Kenny RA, Coen RF, Frewen J, Donoghue OA, Cronin H, Savva GM. Normative values of cognitive and physical function in older adults: findings from the irish longitudinal study on ageing. J Am Geriatr Soc. (2013) 61:S279–90. doi: 10.1111/jgs.12195
65. Beauchet O, Annweiler C, Assal F, Bridenbaugh S, Herrmann FR, Kressig RW, et al. Imagined Timed Up & Go test: a new tool to assess higher-level gait and balance disorders in older adults? J Neurol Sci. (2010) 294:102–6. doi: 10.1016/j.jns.2010.03.021
66. Chen HY, Tang PF. Factors contributing to single- and dual-task timed “Up & Go” test performance in middle-aged and older adults who are active and dwell in the community. Phys Ther. (2016) 96:284–92. doi: 10.2522/ptj.20140292
67. Pondal M, del Ser T. Normative data and determinants for the timed “up and go” test in a population-based sample of elderly individuals without gait disturbances. J Geriatr Phys Ther. (2008) 31:57–63. doi: 10.1519/00139143-200831020-00004
68. Verghese J, Buschke H, Viola L, Katz M, Hall C, Kuslansky G, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. (2002) 50:1572–6. doi: 10.1046/j.1532-5415.2002.50415.x
69. Zijlstra A, Ufkes T, Skelton DA, Lundin-Olsson L, Zijlstra W. Do dual tasks have an added value over single tasks for balance assessment in fall prevention programs? A mini-review. Gerontology. (2008) 54:40–9. doi: 10.1159/000117808
70. Mori E, Ishikawa M, Kato T, Kazui H, Miyake H, Miyajima M, et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir. (2012) 52:775–8. doi: 10.2176/nmc.52.775
71. Zhang Q, Zhao X, Liu H, Ding H. Frailty as a predictor of future falls and disability: a four-year follow-up study of Chinese older adults. BMC Geriatr. (2020) 20:388. doi: 10.1186/s12877-020-01798-z
72. Hall CD, Echt, KV, Wolf, SL, Rogers, WA,. Cognitive Motor Mechanisms Underlying Older Adults' Ability to Divide Attention While Walking. (2011). Available online at: https://academic.oup.com/ptj/article/91/7/1039 (accessed January 19, 2023).
73. Mannarelli D, Pauletti C, Currà A, Marinelli L, Corrado A, Chiaie RD, et al. The cerebellum modulates attention network functioning: evidence from a cerebellar transcranial direct current stimulation and attention network test study. Cerebellum. (2019) 18:457–68. doi: 10.1007/s12311-019-01014-8
Keywords: hydrocephalus, normal pressure, walking, balance, gait analysis, outcome
Citation: Mori L, Collino F, Marzi A, Pellegrino L, Ponzano M, Chiaro DD, Maestrini S, Caneva S, Pardini M, Fiaschi P, Zona G, Trompetto C and Ligurian INPH Study (2023) Useful outcome measures in INPH patients evaluation. Front. Neurol. 14:1201932. doi: 10.3389/fneur.2023.1201932
Received: 11 April 2023; Accepted: 11 July 2023;
Published: 07 August 2023.
Edited by:
Shinji Kawabata, Osaka Medical and Pharmaceutical University, JapanReviewed by:
Ville Leinonen, University of Eastern Finland, FinlandShigenori Kanno, Tohoku University, Japan
Ho-Won Lee, Kyungpook National University, Republic of Korea
Copyright © 2023 Mori, Collino, Marzi, Pellegrino, Ponzano, Chiaro, Maestrini, Caneva, Pardini, Fiaschi, Zona, Trompetto and Ligurian INPH Study. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Collino, federica.collino93@gmail.com
 Laura Mori
Laura Mori Federica Collino1,2*
Federica Collino1,2* Marta Ponzano
Marta Ponzano Stefano Caneva
Stefano Caneva Gianluigi Zona
Gianluigi Zona Carlo Trompetto
Carlo Trompetto