- 1The Second Clinical Medical College, Zhejiang Chinese Medical University, Zhejiang, Hangzhou, China
- 2Department of Neurology, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Zhejiang, Hangzhou, China
Background: The impact of high-sensitivity C-reactive protein (hs-CRP) as a biomarker of inflammation on the prognosis of stroke patients remains controversial, this study was conducted to evaluate the prognostic value of hs-CRP levels for patients with stroke.
Methods: PubMed, Web of Science, Embase, and Cochrane Library databases were searched from inception to October 28, 2022. Outcome measures were all-cause mortality, recurrent stroke, and poor prognosis. The relationship between the highest versus lowest levels of hs-CRP or per unit increment and outcomes as measured by risk ratio (RR) and corresponding 95% confidence intervals (CI).
Results: A total of 39 articles were eligible for meta-analysis. High hs-CRP levels at admission were associated with mortality among patients with acute ischemic stroke (AIS) [RR = 3.84, 95% CI (2.41 ~ 6.111); p < 0.001], risk of recurrent stroke [RR = 1.88, 95%CI (1.41 ~ 2.52); p < 0.001], and poor prognosis [RR = 1.77, 95% CI (1.59 ~ 1.97); p < 0.001]. The risk ratios for the association of per unit increase in hs-CRP levels with mortality, risk of recurrent stroke, and poor prognosis were as follows, respectively: 1.42 [95% CI (1.19–1.69); p < 0.001], 1.03 [95% CI (1.01–1.04); p = 0.003], and 1.27 [95% CI (1.10–1.47); p = 0.001]. For hemorrhagic stroke (HS), the risk ratios (RR) for the highest versus the lowest (reference) category of hsCRP or per unit increment to all-cause mortality were 4.36 [95% CI (1.38–13.73); p = 0.012] and 1.03 [95% CI (0.98–1.08); p = 0.238].
Conclusion: Hs-CRP levels are strongly associated with mortality, risk of stroke recurrence and poor prognosis in stroke patients. Therefore, hs-CRP levels may contribute to the prognosis prediction of these patients.
1. Introduction
Stroke is the second leading cause of death following ischemic heart disease, accounting for 11.6% of total deaths (1). Particularly, ischemic stroke (IS) makes up around 87% of stroke cases (2). In China, the burden of stroke data in 2020 revealed that the number of deaths related to stroke reached a staggering 2.3 million (3). The wide application of modern secondary prevention therapy may be counterbalanced by a high risk of further vascular events in stroke survivors (4) which has become an increasing burden on public health worldwide. Therefore, it is of great significance to explore key factors affecting the prognosis of patients with stroke to formulate appropriate treatment regimens and optimize healthcare for these patients.
The pathogenesis of stroke mainly includes oxidative stress and inflammation. Inflammatory factors can not only induce cell death responsible for functional injury (5), but also underlie the development of atherosclerosis by regulating macrophages, cytokines, and leukocyte adhesion molecules to induce endothelial dysfunction, plaque formation and rupture, platelet aggregation, and thrombosis (6, 7). Therefore, some inflammatory cytokines are investigated as predictors of functional outcomes after stroke (8).
High-sensitivity C-reactive protein (hs-CRP), which is synthesized and secreted by liver cells, is considered a non-specific biomarker of inflammation (9). Previous meta-analyses have shown that hs-CRP can be used to predict the prognosis of patients with COVID-19 (10), type 2 diabetes (11), or coronary artery disease (12), and they also indicate that the level of hs-CRP is an independent risk factor for different types of stroke (13). However, its use as a biomarker to predict patient prognosis after stroke remains controversial. Zeng et al. (14) showed that a high level of hs-CRP level was an independent predictor of adverse clinical outcomes in patients with stroke. Zhang et al. (15) pointed out that the risk of recurrent stroke in patients with IS increased by 22% with a per unit increase in the level of hs-CRP. However, some studies suggest that elevated hs-CRP levels do not seem to independently affect the outcome in patients with stroke (16).
Furthermore, previous meta-analyses have only focused on the impact of hs-CRP on the mortality (17) or stroke recurrence (18) prognosis of ischemic stroke patients, without considering the overall mortality prognosis of hemorrhagic stroke patients, and lacking analysis of poor outcomes based on mRS scores. Therefore, the present study aims to comprehensively evaluate the prognostic value of hs-CRP levels for patients with stroke by performing a meta-analysis to investigate the correlation between hs-CRP levels with recurrent stroke, mortality, and poor prognosis in patients with ischemic or hemorrhagic stroke.
2. Methods
Databases PubMed, Web of Science, Embase, and Cochrane Library were searched from inception to October 28, 2022. The following search terms were used: “C-reactive protein or high-sensitivity CRP or hs-CRP or hsCRP” and “stroke or intracerebral hemorrhage or TIA or CVA or Brain Vascular Accident or Cerebrovascular Accident or Apoplexy” and “observational or cohort or case–control or cross-sectional or follow up or prospective or retrospective.” The literature search was independently done by two researchers, and discussion among them was undertaken to settle disagreements if any. Reference lists of relevant studies were retrieved to conduct a sensitive search. The literature search strategy is presented in Supplemental methods. This study has been registered in PROSPERO (CRD42023389330).
2.1. Eligibility criteria
Inclusion criteria: (1) Adult patients diagnosed with stroke (ischemic stroke or hemorrhagic stroke) participated in studies, (2) baseline hs-CRP levels were measured after symptom onset or at the time of admission (before treated), (3) patients with IS were followed up for no less than 3 months, (4) risk ratio (RR) or odds ratio (OR) with a 95% confidence interval (CI) for prognostic indicators were provided in studies, or sufficient data were available to calculate RR or OR with 95% CI, and (5) observational studies were included in the meta-analysis.
Exclusion criteria: (1) conference abstracts, case reports, letters, reviews, and animal experiments were excluded; (2) studies that were not published in English were excluded; (3) duplicate publications or articles whose full texts were not available were excluded; (4) studies investigating endpoints other than death, poor prognosis (as assessed by the mRS Scale), or recurrent stroke were excluded.
2.2. Data collection and quality assessment
Information extracted from eligible studies included first author, year of publication, country, study type, sample size, age, sex, type of stroke, cut-off value of hs-CRP, adjusted factors, duration of follow-up, and outcome measures. The quality of included studies was evaluated using the Newcastle-Ottawa Scale (NOS) within three categories: selection, comparability, and exposure. A study can be scored on a scale of 0 to 9, with a score of 6 or higher indicating high quality.
2.3. Statistical analysis
All statistical analyses were performed using STATA software (version 15.0, STATA, College Station, TX, United States). Dichotomous and continuous variables were both expressed as RR with corresponding 95% CI to evaluate the correlation of hs-CRP levels with the prognosis in stroke patients. The Q test and I2 statistics were used to assess the heterogeneity across the included studies. If significant heterogeneity existed between studies (I2 ≥ 50%, p < 0.10), a random-effects model was applied; otherwise, a fixed-effects model was used. Subgroup analysis was conducted to find the source of heterogeneity. Sensitivity analysis was used to assess the stability of pooled results. Begg’s test and Egger’s test were done to evaluate publication bias, with a value of p <0.05 indicating statistical significance.
3. Results
3.1. Literature search and screening results
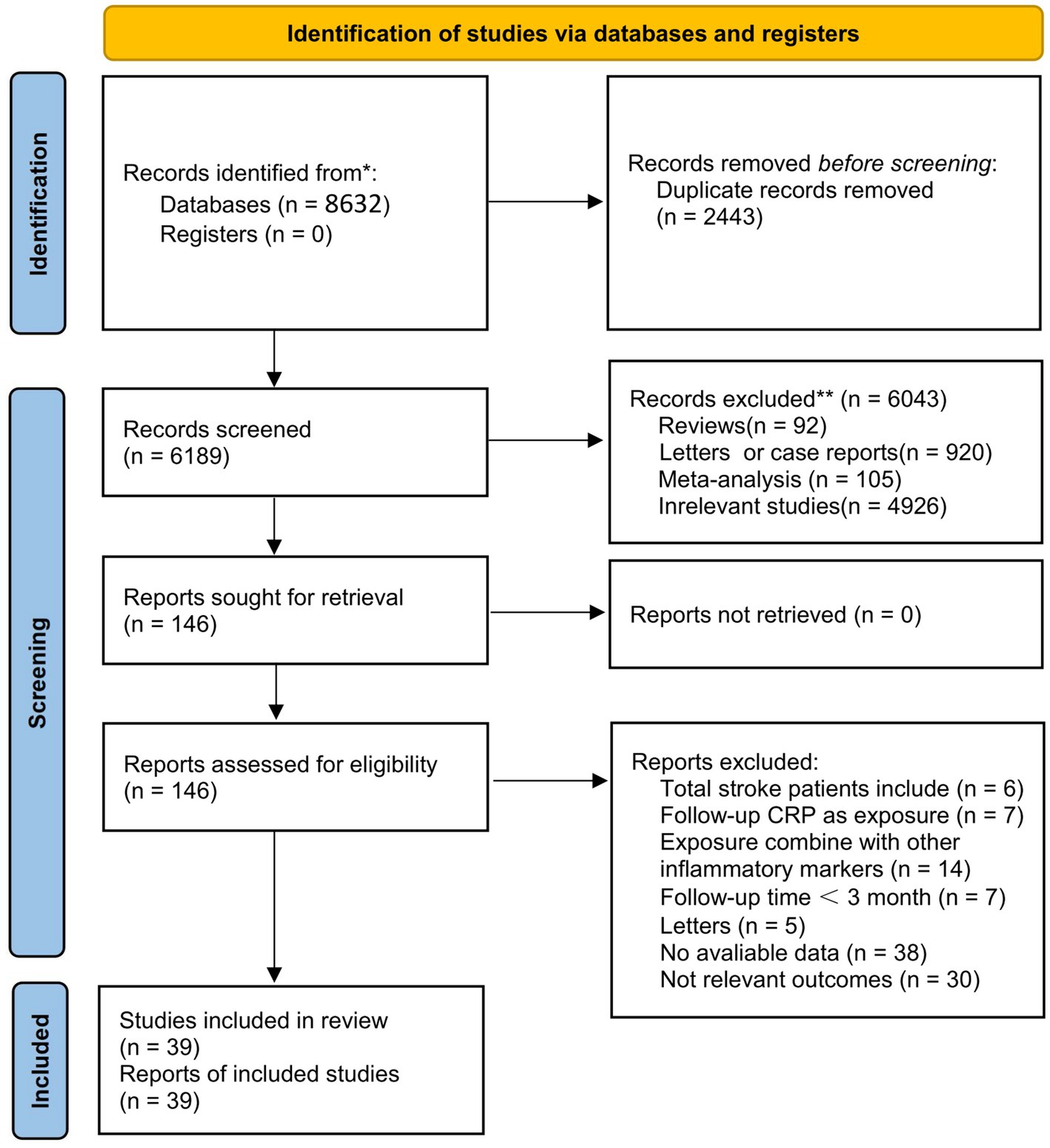
A literature search in databases yielded 8,632 articles in total. Among them, 2,443 articles were removed for duplicate publication. Screening of titles and abstracts excluded irrelevant articles such as meta-analyses, reviews, and animal experiments. Then, 146 full-text articles were reviewed. Finally, 39 articles met the criteria for inclusion in the meta-analysis (Figure 1).
3.2. Basic characteristics of included studies
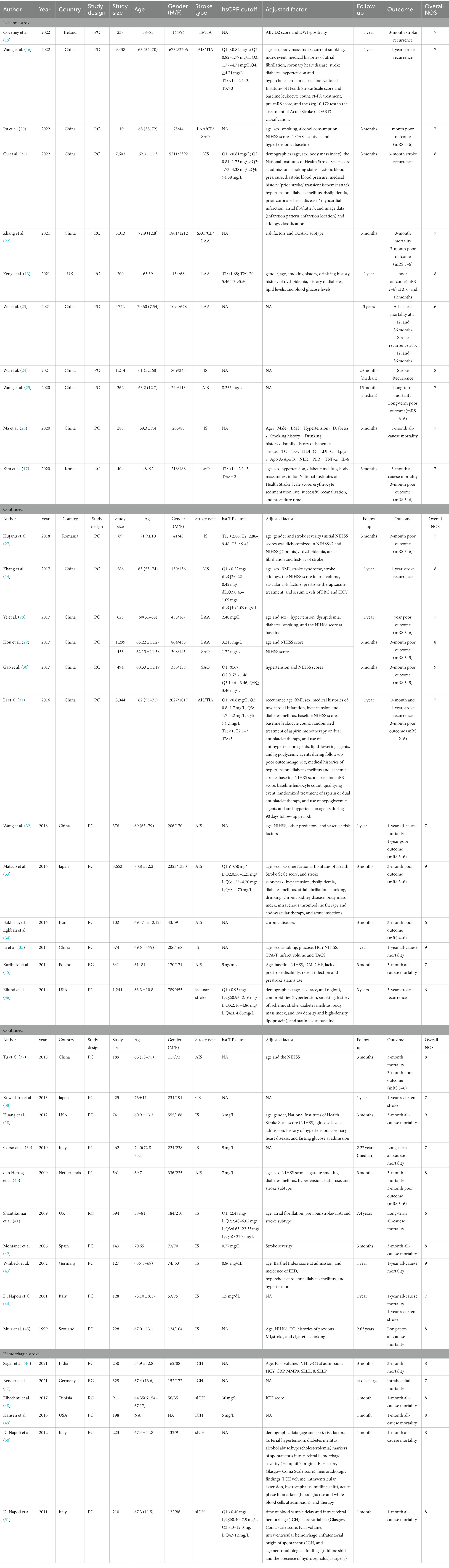
Table 1 presents the basic characteristics of included studies. Among 39 studies, 23, 10, and 15 articles, respectively, reported mortality, recurrent stroke, and poor prognosis among patients with stroke. Participants with IS were included in 33 studies (26 prospective cohort studies and 7 retrospective cohort studies) and those with HS were included in 6 studies (4 prospective cohort studies and 2 retrospective cohort studies). The sample size in studies investigating IS ranged from 89 to 9,438, amounting to 41,175 participants in total (27,140 men and 14,035 women), with the duration of follow-up ranging from 3 months to 7.4 years. Among these studies, some (n = 2) stratified IS into large artery atherosclerosis (LAA), cardiogenic embolism (CE), small-artery occlusion (SAO), etc. However, some studies only investigated one specific subtype of ischemic stroke: LAA (n = 4), SAO (n = 2), and CE (n = 1). The subtype of HS that was more frequently investigated by the included studies was intracerebral hemorrhage (ICH), with the sample size ranging from 91 to 329. As for NOS scores, included studies were awarded from 6 to 9, indicating that they had moderate or high quality.
3.3. Prognosis of patients with ischemic stroke
3.3.1. Relationship between hs-CRP levels and all-cause mortality
Ten studies (16, 22, 23, 26, 39–44) involving 3,663 patients in total reported an association between high levels of hs-CRP and mortality. There was significant heterogeneity among studies (I2 = 71.0%; p < 0.01), and thus a random-effects model was utilized. Meta-analysis showed that the risk of death increased to 384% among patients who had high hs-CRP levels upon admission, compared with those with low hs-CRP levels [RR = 3.84, 95% CI (2.41 ~ 6.111); p < 0.001; Figure 2A].
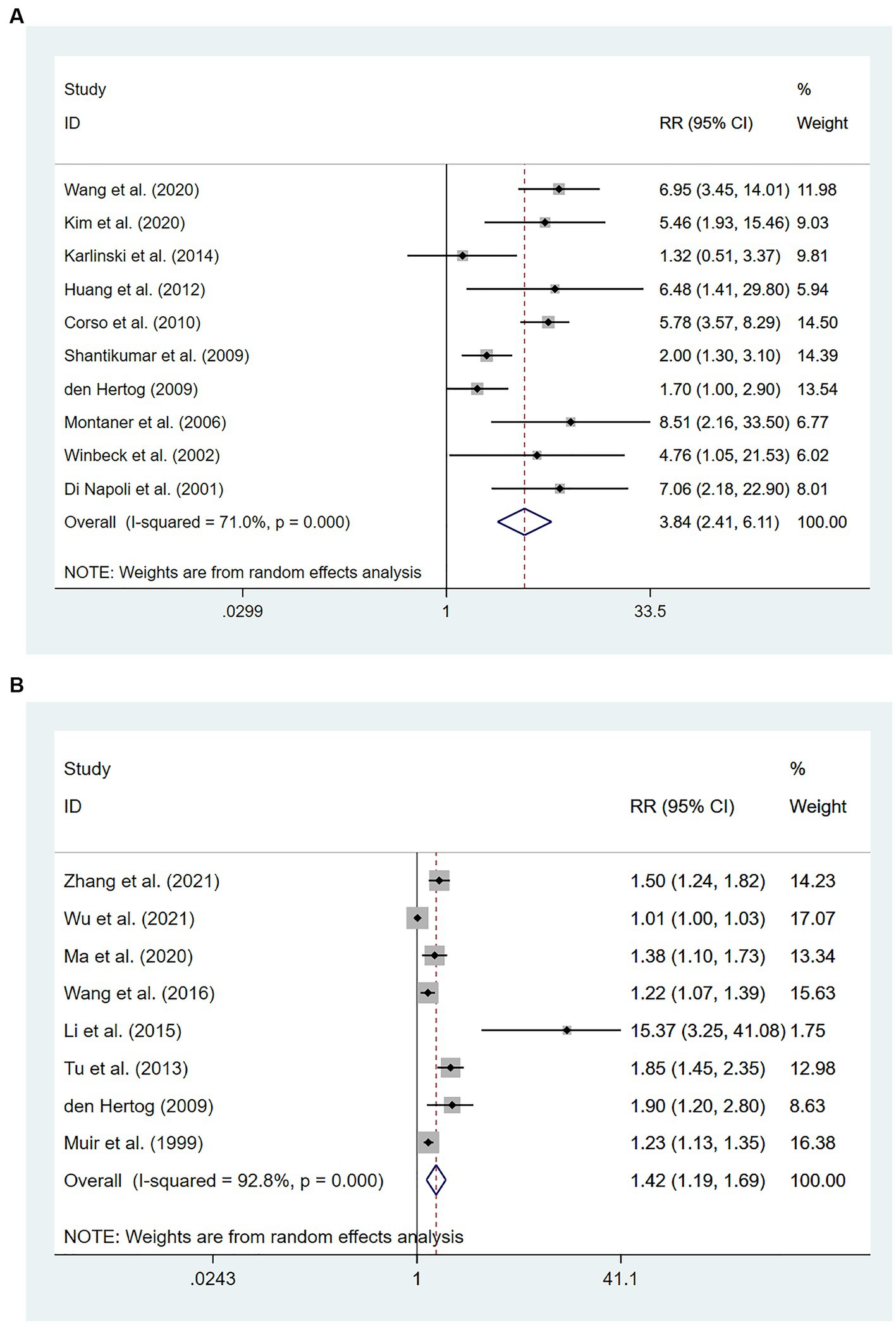
Figure 2. Forest plots showing risk ratios with 95% confidence intervals of all-cause mortality in ischemic stroke patients (A) the highest versus the lowest C-reactive protein level category; (B) per 1-SD rise in loge-hsCRP level.
Eight studies (21, 31, 32, 35–37, 44, 45) with 6,801 patients in total revealed an association between per unit increase in hs-CRP levels and mortality. Heterogeneity among studies was significant (I2 = 92.8%; p < 0.01), and thus a random-effects model was utilized. Meta-analysis showed that per unit increase in the level of hs-CRP was associated with an increased risk of death in patients with IS [RR = 1.42, 95% CI (1.19 ~ 1.69); p < 0.001; Figure 2B].
3.3.2. Relationship between hs-CRP levels and recurrent stroke
Six studies (15, 19, 24, 26, 38, 39) involving 201,743 patients reported an association between high levels of hs-CRP and the risk of recurrent stroke. There was significant heterogeneity among studies of interest (I2 = 71.1%; p < 0.01), and thus a random-effects model was used for the meta-analysis. The risk of recurrent stroke in patients with high hs-CRP levels upon admission was 188% of that in patients with low hs-CRP levels [RR = 1.88, 95%CI (1.41 ~ 2.52); p < 0.001; Figure 3A].
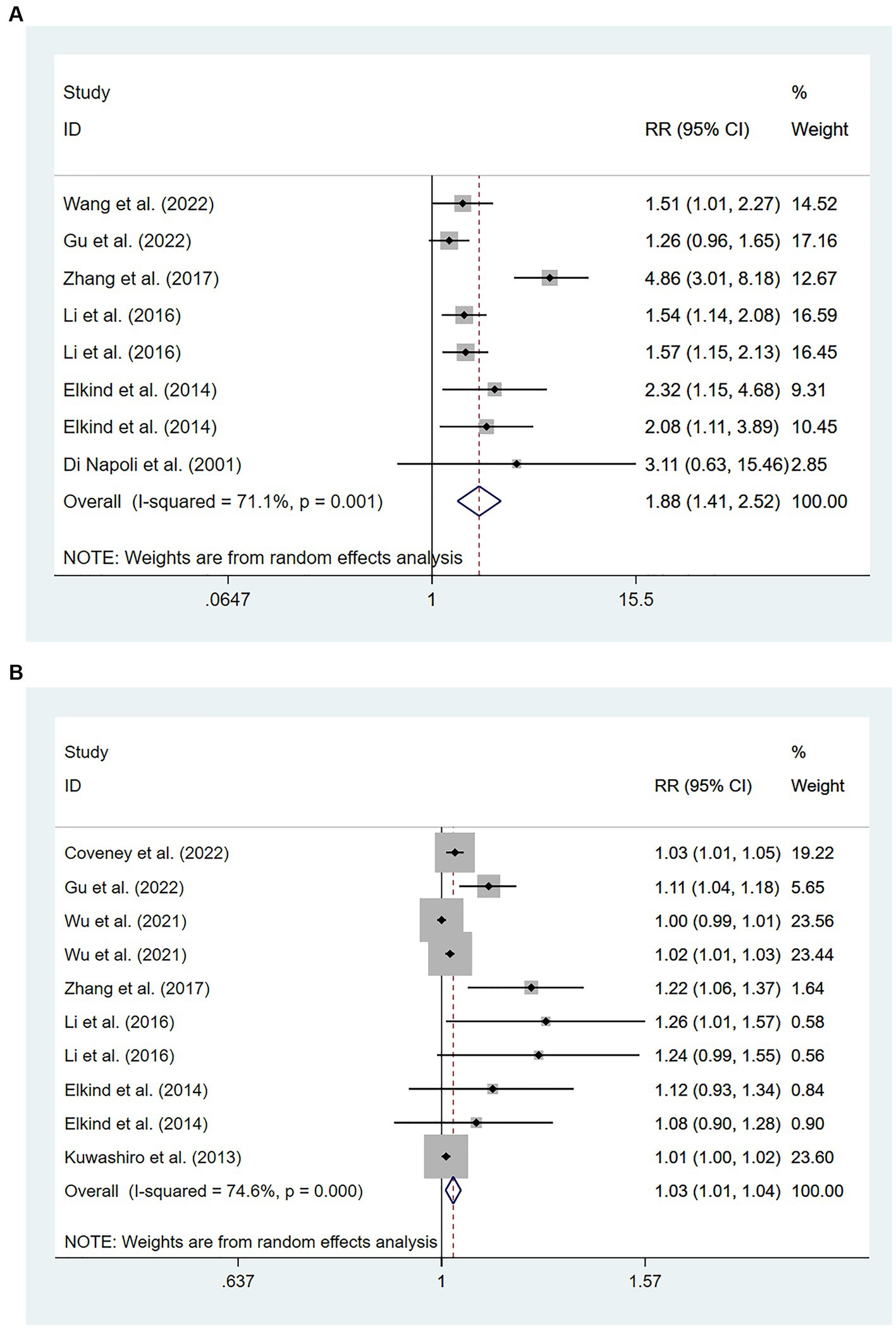
Figure 3. Forest plots showing risk ratios with 95% confidence intervals of stroke recurrence in ischemic stroke patients (A) the highest versus the lowest C-reactive protein level category; (B) per 1-SD rise in loge-hsCRP level.
Eight studies (15, 19, 24, 25, 27, 28, 35, 38) involving 15,826 patients showed an association between per unit increase in the level of hs-CRP and the risk of recurrent stroke. Heterogeneity among studies was significant (I2 = 74.6%; p < 0.01), and thus a random-effects model was used. Meta-analysis showed that the risk of recurrent stroke increased by 3% for each unit increase in hs-CRP levels [RR = 1.03, 95% CI (1.01 ~ 1.04); p = 0.003; Figure 3B].
3.3.3. Relationship between hs-CRP levels and poor prognosis
Ten cohort studies involving a total of 11,184 patients (14, 20, 24, 29, 30, 33, 34, 41, 44, 46) evaluated the association between high levels of hs-CRP and poor prognosis in patients with AIS. Figure 4A showed that the incidence of poor prognosis in patients with high hs-CRP levels was 177% of that in patients with low hs-CRP levels [RR = 1.77, 95% CI (1.59 ~ 1.97); p < 0.001; Figure 4A]. There was no significant heterogeneity among these studies (I2 = 0%; p = 0.894).
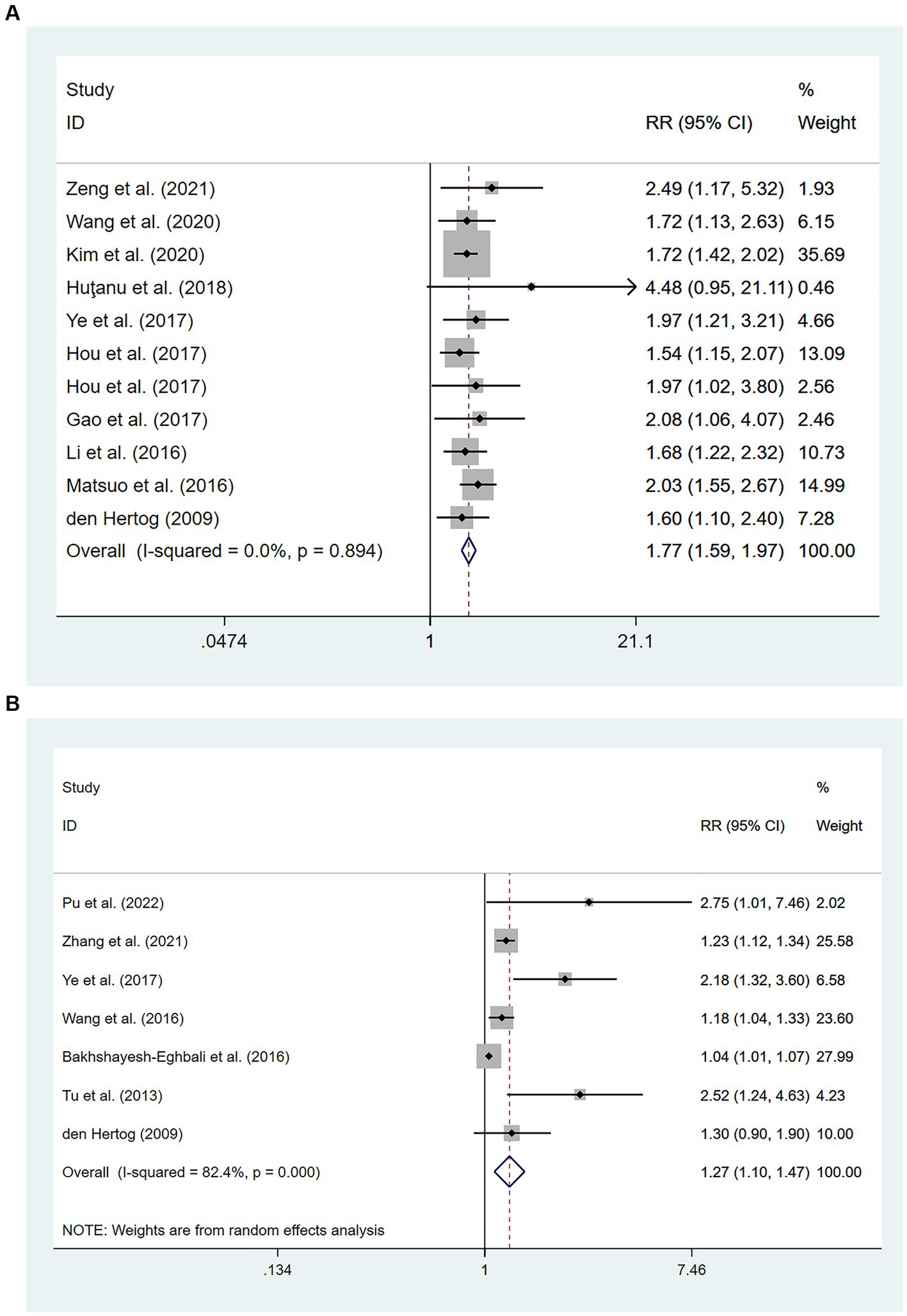
Figure 4. Forest plots showing risk ratios with 95% confidence intervals of poor outcome in ischemic stroke patients (A) the highest versus the lowest C-reactive protein level category; (B) per 1-SD rise in loge-hsCRP level.
Seven studies (31–33, 44, 45, 47, 48) with a total of 4,985 patients reported an association between per unit increase in the level of hs-CRP and poor prognosis. Meta-analysis showed that the risk of poor prognosis increased by 27% for each unit increases in hs-CRP levels [RR = 1.27, 95% CI (1.10 ~ 1.47); p = 0.001; Figure 4B], and there was significant heterogeneity among these studies (I2 = 82.4%; p < 0.01) (Figure 4).
3.4. Relationship between hs-CRP levels and all-cause mortality in patients with hemorrhagic stroke
Participants with HS were included in six studies (49–54) involving 1,301 patients. Of these studies, four (51–54) involving 722 patients found that high hs-CRP levels at the time of admission were associated with an increased risk of all-cause mortality compared with low hs-CRP levels [RR = 4.36, 95% CI (1.38 ~ 13.73); p = 0.012; Figure 5A]. Significant heterogeneity was found between these studies (I2 = 87.1%; p < 0.01).
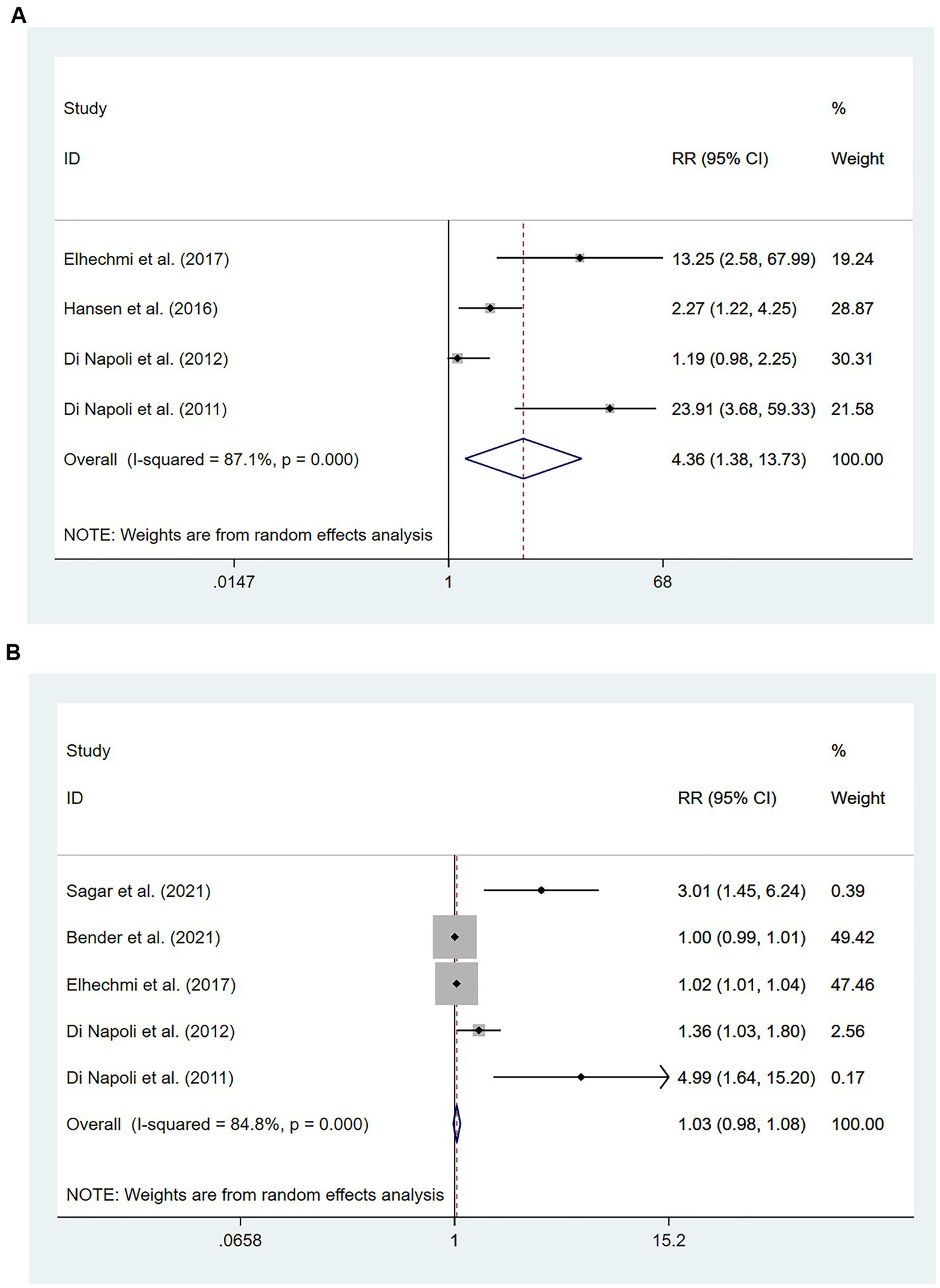
Figure 5. Forest plots showing risk ratios with 95% confidence intervals of mortality in hemorrhagic stroke patients (A) the highest versus the lowest C-reactive protein level category; (B) per 1-SD rise in loge-hsCRP level.
Five studies (49–51, 53, 54) involving 1,103 patients investigated the relationship between per unit increase in the level of hs-CRP and all-cause mortality. Meta-analysis revealed no statistical significance in the association between per unit increase in the level of hs-CRP and all-cause mortality among patients with HS [RR = 1.03, 95% CI (0.98 ~ 1.08); p = 0.238; Figure 5B]. Significant heterogeneity was observed between these studies (I2 = 84.8%; p < 0.01) (Figure 5).
3.5. Subgroup analysis
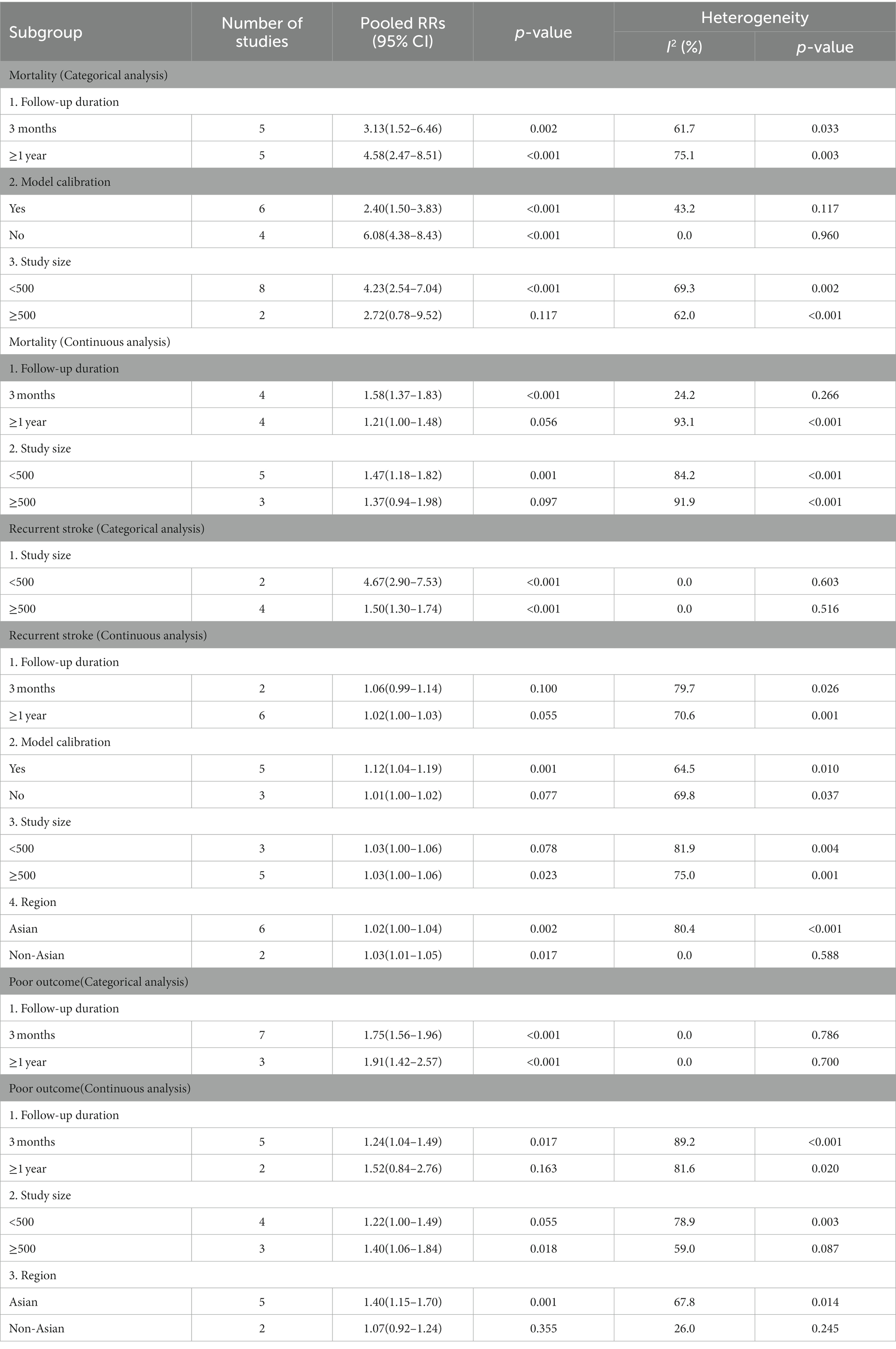
Subgroup analyses of patients with ischemic stroke were performed based on three outcome measures (Table 2). In the subgroup analysis where the level of hs-CRP was defined as a categorical variable, the correlation between high hs-CRP levels and mortality was found to be remarkable in models undergoing calibration [RR = 2.40, 95% CI (1.50 ~ 3.83); p < 0.001] or not [RR = 6.08, 95% CI (4.38 ~ 8.43); p < 0.001], with intragroup heterogeneity reduced (I2 = 43.2%, p = 0.117; I2 = 0.0%, p = 0.960). The subgroup analysis based on sample size showed that the correlation of hs-CRP levels with recurrent stroke and poor prognosis was not significantly affected by sample size. The correlation between hs-CRP and mortality was observed in the subgroup with a small sample size [RR = 4.23, 95% CI (2.54 ~ 7.04); p < 0.001]. Intragroup heterogeneity was significantly reduced regarding the risk of recurrent stroke (≥500: I2 = 0%, p = 0.561; <500: I2 = 0%, p = 0.603). Subgroup analysis based on the duration of follow-up also demonstrated that the correlation of hs-CRP levels with mortality and poor prognosis was not significantly affected by the duration of follow-up.
In the subgroup analysis where the level of hs-CRP was defined as a continuous variable, the fact that hs-CRP levels were associated with all-cause mortality 3 months: [RR = 1.58, 95% CI (1.37 ~ 1.83); p < 0.001] and poor prognosis 3 months: [RR = 1.24, 95% CI (1.04 ~ 1.49); p = 0.017] was observed only in the subgroup followed up for 3 months where small heterogeneity pertaining to mortality was found (I2 = 24.2%; p = 0.266). Region-based subgroup analysis demonstrated that there was a poor correlation between hs-CRP levels and recurrent stroke whether in the subgroup of Asia [RR = 1.02, 95% CI (1.00 ~ 1.04); p = 0.017] and the subgroup of non-Asian regions [RR = 1.03, 95% CI (1.01 ~ 1.05), p = 0.002]. The heterogeneity regarding recurrent stroke and poor prognosis was significantly reduced in the non-Asian subgroup (I2 = 0.00%, p = 0.588; I2 = 26.0%, p = 0.245).
3.6. Sensitivity analysis and publication bias
Sensitivity analysis was conducted regarding all outcome measures investigated in the present study, and no significant changes were found in analysis results after removing included studies one by one, indicating good stability of results (Supplementary Figures S1–S3).
According to Begg’s and Egger’s test, publication bias was found across studies (p < 0.05, Supplementary Figures S6–S11) except for studies investigating mortality and the risk of recurrent stroke (p ≥ 0.05, Supplementary Figures S3–S5). After the trim-and-fill method was employed to adjust for publication bias (Figures 6–7), no statistical significance existed regarding the correlation of per unit increase in the level of hs-CRP with the risk of recurrent stroke [RR = 1.05, 95% CI (0.995 ~ 1.035); p = 0.147; Figure 6B] and poor prognosis [RR = 1.160, 95% CI (0.998 ~ 1.384); p = 0.053; Figure 6C] among patients with IS. Similar results were noted in the analysis on the correlation of hs-CRP levels and mortality among patients with HS [RR = 2.64, 95% CI (0.874 ~ 7.978); p = 0.085; Figure 7A; RR = 1.43,95% CI (0.919 ~ 2.210), p < 0.001; Figure 7B], suggesting that publication bias did not affect our findings.
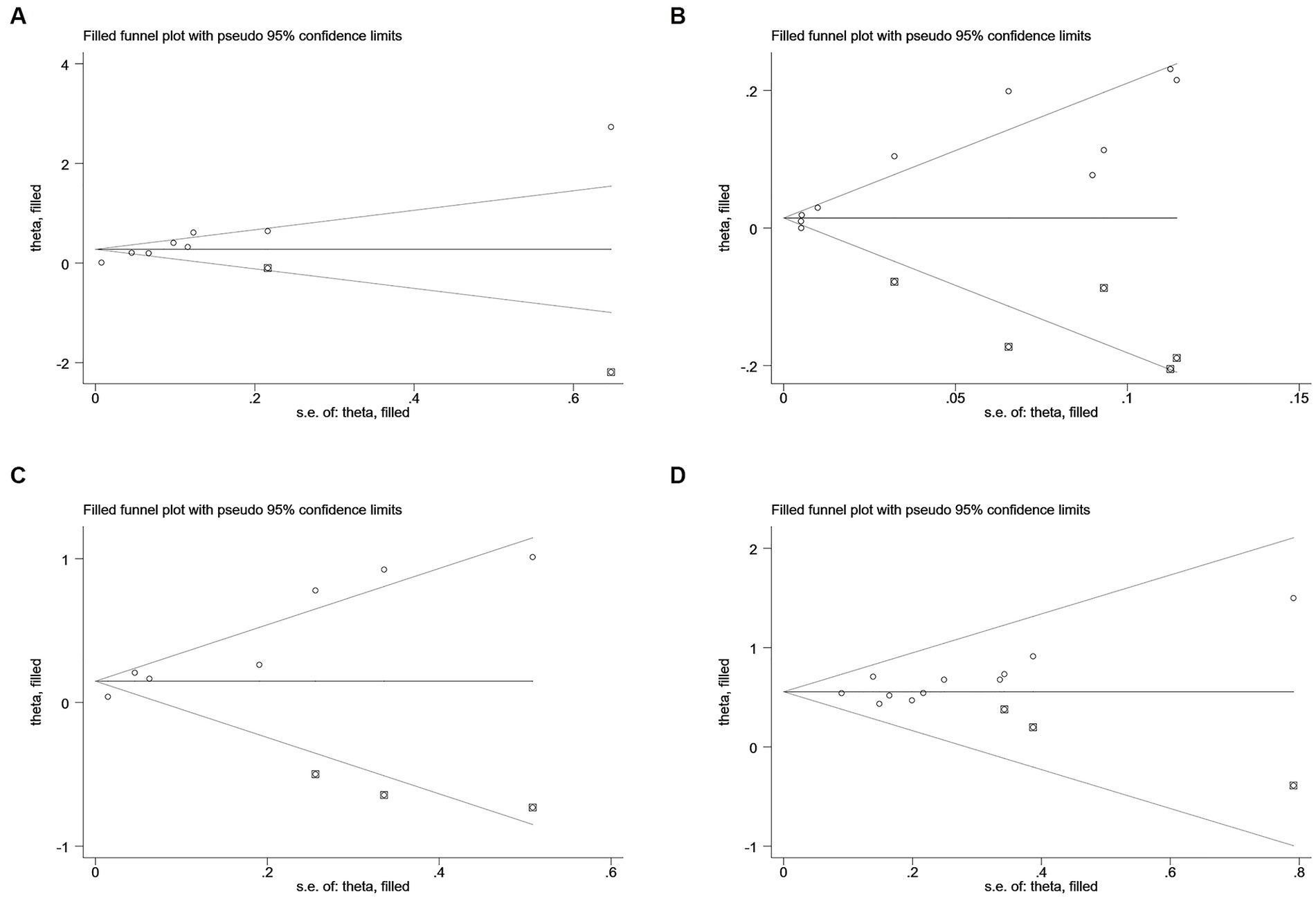
Figure 6. Funnel plots of (A) hsCRP (defined as per 1-SD increment) and mortality, (B) hsCRP (defined as per 1-SD increment) and recurrent stroke, (C) hsCRP (defined as per 1-SD increment) and poor outcome, and (D) hsCRP (defined as the highest versus the lowest) and poor outcome in ischemic stroke.
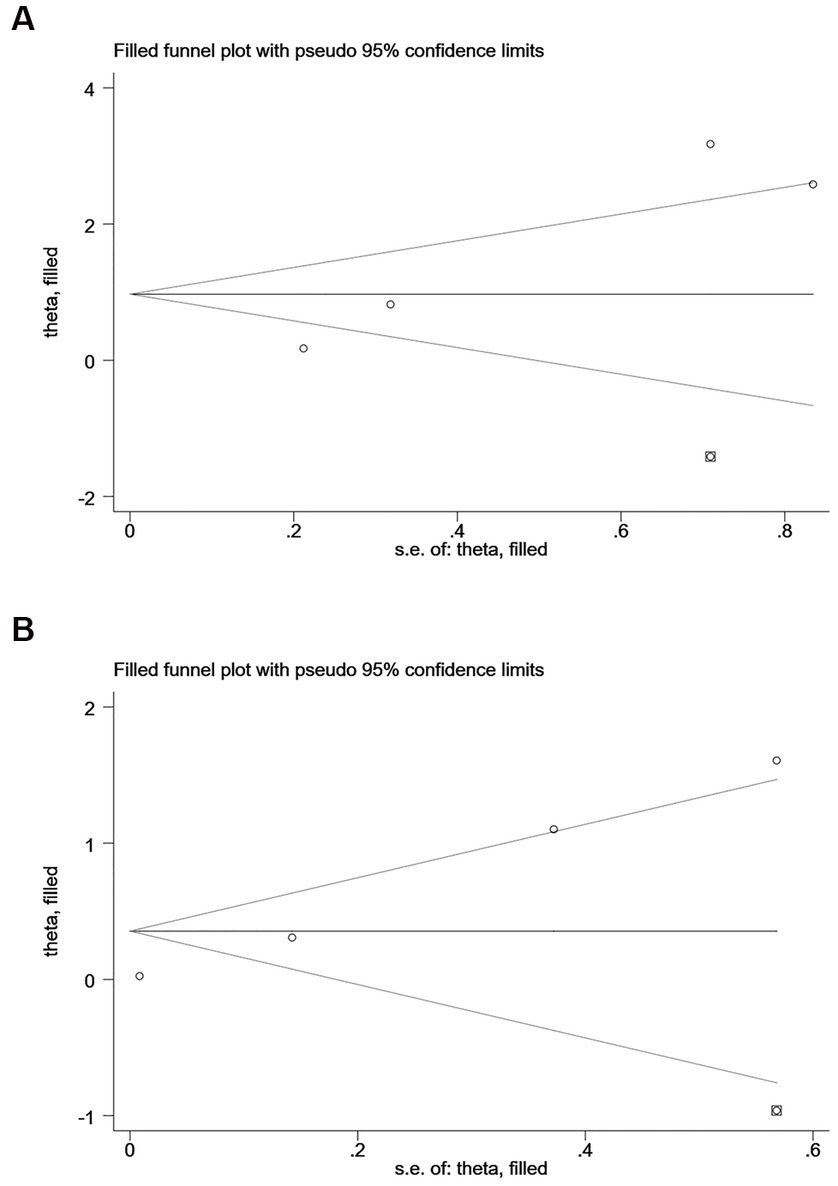
Figure 7. Funnel plots of (A) hsCRP (defined as the highest versus the lowest) and mortality, (B) hsCRP (defined as per 1-SD increment) and mortality in hemorrhagic stroke.
4. Discussion
The meta-analysis of 39 studies contributed to a comprehensive investigation for the first time into the correlation of hs-CRP levels with mortality, recurrent stroke, and poor prognosis after stroke. Compared to patients with IS who had low hs-CRP levels upon admission, those with high hs-CRP levels were more prone to death, recurrent stroke, and poor prognosis. The same trend was noted in patients with HS, given their mortality. Per unit increase in the level of hs-CRP was associated with mortality, recurrent stroke, and poor prognosis among patients with IS, while it was not related to mortality among patients with HS.
In this study, the prognostic ability of hs-CRP levels in patients with IS was confirmed. However, results contrary to those in previous studies were obtained in the analysis of continuous variables regarding HS and mortality (49). Among the included studies, there was a significant difference between the inpatient mortality investigated by Bender et al. (50) and the duration of the follow-up (≥1 month) investigated by other included studies. The removal of the study by Bender et al. (50) produced results consistent with previous studies [RR = 1.65, 95% CI (1.05 ~ 2.59); p = 0.03; Supplementary Figure S12]. In addition, longitudinal analyses of different clinical outcomes were performed in the present study. A strong correlation of hs-CRP levels with outcome measures was found only when it came to mortality among patients with ischemic or hemorrhagic stroke [RR = 3.84, 95% CI (2.41 ~ 6.11), p < 0.001]; [RR = 4.36, 95% CI (1.38 ~ 13.73), p = 0.012], suggesting that the level of hs-CRP as a prognostic indicator of mortality may have greater clinical significance than that of recurrent stroke and poor prognosis. Evidence has shown that early recurrence after stroke is an independent factor for increased risk of death (55). By increasing the risk of recurrent stroke (19, 39), elevated hs-CRP levels are associated with the death of patients after stroke (56), strengthening the significance of hs-CRP levels in predicting mortality.
The activation process of the immune response for ischemic or hemorrhagic stroke is similar to the inflammatory response (57). After a stroke, neuronal cells die or are damaged, releasing damage-associated molecular patterns (DAMPs) that trigger local inflammation in the damaged brain area. The release of inflammatory factors increases the permeability of the blood–brain barrier (BBB), causing infiltration of peripheral immune cells into the lesion, which induces an inflammatory cascade and causes secondary brain injury (58). Inflammation is a key driving force in the development of atherosclerosis (59), which leads to stroke through a variety of mechanisms including plaque rupture, thrombosis, embolism, and hemodynamic impairment (6). Currently, there is ample evidence to support that reducing hs-CRP levels through anti-inflammatory interventions can improve stroke prognosis. Canakinumab (60), an anti-inflammatory drug, has been shown to significantly reduce the risk of recurrent cardiovascular events, including stroke, in patients with a history of myocardial infarction and elevated levels of hs-CRP. Statins have also been found to improve clinical prognosis in the same manner (61). In conclusion, there is a piece of clear evidence that anti-inflammatory interventions may improve the prognosis of stroke patients. However, further investigations are needed to elucidate the underlying mechanisms, which have significant implications for the specific treatment of stroke. Our study differs from previous research (16) in that blood samples were collected after stroke onset but before treatment initiation, reflecting mainly the impact of acute-phase pro-inflammatory cytokines on prognosis. This provides additional evidence for the important role of anti-inflammatory therapy in the acute phase of stroke.
This meta-analysis revealed the correlation between hs-CRP levels and the prognosis of patients with stroke, but results should be interpreted with caution, given the great heterogeneity across included studies with no single study found in the sensitivity analysis to reduce the heterogeneity. Subgroup analyses were performed to disclose the source of heterogeneity. The heterogeneity in view of the mortality among patients with IS in the models undergoing calibration or not was reduced, respectively, (I2 = 43.2%, p = 0.117; I2 = 0.0%, p = 0.960), possibly indicating that model calibration contributed to the heterogeneity regarding mortality. When the level of hs-CRP was defined as a continuous variable, small heterogeneity pertaining to mortality was only observed within the subgroup of 3-month follow-up (I2 = 24.2%; p = 0.266), which may be related to the difference in follow-up duration between studies lasting for over 1 year. In addition, the sample size may be a potential source of heterogeneity regarding recurrent stroke (≥500: I2 = 0%, p = 0.561; < 500: I2 = 0%, p = 0.603). However, the heterogeneity in poor prognosis was only significantly reduced within the non-Asian subgroup (I2 = 26.0%, p = 0.245), which may be related to regional publication preferences. There may be a certain correlation between prognosis and stroke subtypes, but this was not investigated in the present study due to insufficient data, emphasizing the need for additional research.
There are some limitations to this study. First, factors that were adjusted such as autoimmune diseases or chronic inflammatory diseases among participants during the research process were not realized across included studies, which may be an important source of heterogeneity across studies. Second, per unit increase in hs-CRP levels was found to not be associated with poor prognosis and recurrent stroke after the trim-and-fill method was applied, calling for negative results to be reported in future research to avoid overestimating the clinical significance of hs-CRP. Third, the general development trend instead of the panorama of inflammation is reflected by hs-CRP levels due to complicated inflammation mechanisms, which is coupled with a single measurement of hs-CRP levels performed in each included study. Therefore, whether the dynamic changes in hs-CRP levels can provide additional prognostic significance remains to be determined.
Taken together, higher hs-CRP levels upon admission are associated with poor prognosis after stroke, including ischemic and hemorrhagic stroke. Elevated hs-CRP levels may further increase the recurrence and mortality of cerebral infarction or cerebral hemorrhage in patients with stroke. Moreover, the level of hs-CRP upon admission is a good prognostic biomarker for patients with stroke during the follow-up of 3 months.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
BH and LC: conceptualization. LC: methodology and writing original draft preparation. LC, MW, CY, and YW: formal analysis and investigation. BH: writing review and editing, funding acquisition, resources, and supervision. LC, MW, CY, YW, and BH commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82104762).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1199814/full#supplementary-material
References
1. Feigin, VL, Stark, BA, Johnson, CO, Roth, GA, Bisignano, C, Abady, GG, et al. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Barthels, D, and Das, H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol basis Dis. (2020) 1866:165260. doi: 10.1016/j.bbadis.2018.09.012
3. Tu, WJ, Zhao, Z, Yin, P, Cao, L, Zeng, J, Chen, H, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. (2023) 6:e231455. doi: 10.1001/jamanetworkopen.2023.1455
4. Boulanger, M, Béjot, Y, Rothwell, PM, and Touzé, E. Long-term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: systematic review and Meta-analysis. J Am Heart Assoc. (2018) 7:e007267. doi: 10.1161/JAHA.117.007267
5. Sekerdag, E, Solaroglu, I, and Gursoy-Ozdemir, Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr Neuropharmacol. (2018) 16:1396–415. doi: 10.2174/1570159X16666180302115544
6. Hansson, GK . Inflammatory mechanisms in atherosclerosis. J thrombosis and haemostasis: JTH. (2009) 7:328–31. doi: 10.1111/j.1538-7836.2009.03416.x
7. Wu, L, Xiong, X, Wu, X, Ye, Y, Jian, Z, Zhi, Z, et al. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front Mol Neurosci. (2020) 13:28. doi: 10.3389/fnmol.2020.00028
8. Sabir Rashid, A, Huang-Link, Y, Johnsson, M, Wetterhäll, S, and Gauffin, H. Predictors of early neurological deterioration and functional outcome in acute ischemic stroke: the importance of large artery disease, hyperglycemia and inflammatory blood biomarkers. Neuropsychiatr Dis Treat. (2022) 18:1993–2002. doi: 10.2147/NDT.S365758
9. Devaraj, S, Singh, U, and Jialal, I. The evolving role of C-reactive protein in atherothrombosis. Clin Chem. (2009) 55:229–38. doi: 10.1373/clinchem.2008.108886
10. Huang, I, Pranata, R, Lim, MA, Oehadian, A, and Alisjahbana, B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. (2020) 14:175346662093717. doi: 10.1177/1753466620937175
11. Tian, R, Tian, M, Wang, L, Qian, H, Zhang, S, Pang, H, et al. C-reactive protein for predicting cardiovascular and all-cause mortality in type 2 diabetic patients: a meta-analysis. Cytokine. (2019) 117:59–64. doi: 10.1016/j.cyto.2019.02.005
12. Mani, P, Puri, R, Schwartz, GG, Nissen, SE, Shao, M, Kastelein, JJP, et al. Association of Initial and Serial C-reactive protein levels with adverse cardiovascular events and death after acute coronary syndrome: a secondary analysis of the VISTA-16 trial. JAMA Cardiol. (2019) 4:314–20. doi: 10.1001/jamacardio.2019.0179
13. Zhou, Y, Han, W, Gong, D, Man, C, and Fan, Y. Hs-CRP in stroke: a meta-analysis. Clin Chim Acta. (2016) 453:21–7. doi: 10.1016/j.cca.2015.11.027
14. Zeng, Q, Zeng, Y, Slevin, M, Guo, B, Shen, Z, Deng, B, et al. C-reactive protein levels and clinical prognosis in LAA-type stroke patients: a prospective cohort study. Biomed Res Int. (2021) 2021:1–8. doi: 10.1155/2021/6671043
15. Zhang, YB, Yin, Z, Han, X, Wang, Q, Zhang, Z, and Geng, J. Association of circulating high-sensitivity C-reactive protein with late recurrence after ischemic stroke. Neuroreport. (2017) 28:598–603. doi: 10.1097/WNR.0000000000000806
16. Karlinski, M, Bembenek, J, Grabska, K, Kobayashi, A, Baranowska, A, Litwin, T, et al. Routine serum C-reactive protein and stroke outcome after intravenous thrombolysis. Acta Neurol Scand. (2014) 130:305–11. doi: 10.1111/ane.12227
17. Yu, B, Yang, P, Xu, X, and Shao, L. C-reactive protein for predicting all-cause mortality in patients with acute ischemic stroke: a meta-analysis. Biosci Rep. (2019) 39:BSR20181135. doi: 10.1042/BSR20181135
18. McCabe, JJ, O'Reilly, E, Coveney, S, Collins, R, Healy, L, McManus, J, et al. Interleukin-6, C-reactive protein, fibrinogen, and risk of recurrence after ischaemic stroke: systematic review and meta-analysis. Eur Stroke J. (2021) 6:62–71. doi: 10.1177/2396987320984003
19. Gu, HQ, Yang, KX, Lin, JX, Jing, J, Zhao, XQ, Wang, YL, et al. Association between high-sensitivity C-reactive protein, functional disability, and stroke recurrence in patients with acute ischaemic stroke: a mediation analysis. EBioMedicine. (2022) 80:104054. doi: 10.1016/j.ebiom.2022.104054
20. Hou, D, Liu, J, Feng, R, Gao, Y, Wang, Y, and Wu, J. The role of high-sensitivity C-reactive protein levels in functional outcomes in patients with large-artery atherosclerosis and small-artery occlusion. Neurol Res. (2017) 39:981–7. doi: 10.1080/01616412.2017.1358937
21. Li, YM, and Liu, XY. Serum levels of procalcitonin and high sensitivity C-reactive protein are associated with long-term mortality in acute ischemic stroke. J Neurol Sci. (2015) 352:68–73. doi: 10.1016/j.jns.2015.03.032
22. Montaner, J, Fernandez-Cadenas, I, Molina, CA, Ribó, M, Huertas, R, Rosell, A, et al. Poststroke C-reactive protein is a powerful prognostic tool among candidates for thrombolysis. Stroke. (2006) 37:1205–10. doi: 10.1161/01.STR.0000217744.89208.4e
23. Winbeck, K, Poppert, H, Etgen, T, Conrad, B, and Sander, D. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke. (2002) 33:2459–64. doi: 10.1161/01.STR.0000029828.51413.82
24. Li, J, Zhao, X, Meng, X, Lin, J, Liu, L, Wang, C, et al. High-sensitive C-reactive protein predicts recurrent stroke and poor functional outcome: subanalysis of the Clopidogrel in high-risk patients with acute nondisabling cerebrovascular events trial. Stroke. (2016) 47:2025–30. doi: 10.1161/STROKEAHA.116.012901
25. Coveney, S, McCabe, JJ, Murphy, S, Belton, O, Gray, C, Cassidy, T, et al. Dose-dependent Association of Inflammatory Cytokines with carotid atherosclerosis in transient Ischaemic attack: implications for clinical trials. Cerebrovasc Dis. (2022) 51:178–87. doi: 10.1159/000517739
26. Di Napoli, M, Papa, F, and Bocola, V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke. (2001) 32:917–24. doi: 10.1161/01.STR.32.4.917
27. Wu, M, Zhang, X, Chen, J, Zha, M, Yuan, K, Huang, K, et al. A score of low-grade inflammation for predicting stroke recurrence in patients with ischemic stroke. J Inflamm Res. (2021) 14:4605–14. doi: 10.2147/JIR.S328383
28. Kuwashiro, T, Sugimori, H, Ago, T, Kuroda, J, Kamouchi, M, and Kitazono, T. Predictive role of C reactive protein in stroke recurrence after cardioembolic stroke: the Fukuoka stroke registry. BMJ Open. (2013) 3:e003678. doi: 10.1136/bmjopen-2013-003678
29. Wang, L, Wu, L, Lang, Y, Wu, D, Chen, J, Zhao, W, et al. Association between high-sensitivity C-reactive protein levels and clinical outcomes in acute ischemic stroke patients treated with endovascular therapy. Ann Transl Med. (2020) 8:1379. doi: 10.21037/atm-20-3820
30. Huţanu, A, Iancu, M, Bălaşa, R, Maier, S, and Dobreanu, M. Predicting functional outcome of ischemic stroke patients in Romania based on plasma CRP, sTNFR-1, D-dimers, NGAL and NSE measured using a biochip array. Acta Pharmacol Sin. (2018) 39:1228–36. doi: 10.1038/aps.2018.26
31. Tu, WJ, Zhao, SJ, Liu, TG, Yang, DG, and Chen, H. Combination of high-sensitivity C-reactive protein and homocysteine predicts the short-term outcomes of Chinese patients with acute ischemic stroke. Neurol Res. (2013) 35:912–21. doi: 10.1179/1743132813Y.0000000228
32. Zhang, XG, Xue, J, Yang, WH, Xu, XS, Sun, HX, Hu, L, et al. Inflammatory markers as independent predictors for stroke outcomes. Brain behav. (2021) 11:e01922. doi: 10.1002/brb3.1922
33. Ye, Z, Zhang, Z, Zhang, H, Hao, Y, Zhang, J, Liu, W, et al. Prognostic value of C-reactive protein and homocysteine in large-artery atherosclerotic stroke: a prospective observational study. J Stroke Cerebrovasc Dis. (2017) 26:618–26. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.016
34. Gao, Y, Liu, J, Wang, W, Gao, C, Yu, C, Liu, S, et al. An elevated high-sensitivity C-reactive protein level is associated with unfavorable functional outcomes of small-artery occlusion in patients without diabetes. Eur Neurol. (2017) 78:48–55. doi: 10.1159/000477929
35. Wu, Q, Cui, J, Xie, Y, Wang, M, Zhang, H, Hu, X, et al. Outcomes of ischemic stroke and associated factors among elderly patients with large-artery atherosclerosis: a hospital-based follow-up study in China. Front Neurol. (2021) 12:642426. doi: 10.3389/fneur.2021.642426
36. Muir, KW, Weir, CJ, Alwan, W, Squire, IB, and Lees, KR. C-reactive protein and outcome after ischemic stroke. Stroke. (1999) 30:981–5. doi: 10.1161/01.STR.30.5.981
37. Ma, Z, Yue, Y, Luo, Y, Wang, W, Cao, Y, and Fang, Q. Clinical utility of the inflammatory factors combined with lipid markers in the diagnostic and prognostic assessment of ischemic stroke: based on logistic regression models. J Stroke Cerebrovasc Dis. (2020) 29:104653. doi: 10.1016/j.jstrokecerebrovasdis.2020.104653
38. Elkind, MS, Luna, JM, McClure, LA, Zhang, Y, Coffey, CS, Roldan, A, et al. C-reactive protein as a prognostic marker after lacunar stroke: levels of inflammatory markers in the treatment of stroke study. Stroke. (2014) 45:707–16. doi: 10.1161/STROKEAHA.113.004562
39. Wang, Y, Li, J, Pan, Y, Wang, M, Meng, X, and Wang, Y. Association between high-sensitivity C-reactive protein and prognosis in different periods after ischemic stroke or transient ischemic attack. J Am Heart Assoc. (2022) 11:e025464. doi: 10.1161/JAHA.122.025464
40. Huang, Y, Jing, J, Zhao, XQ, Wang, CX, Wang, YL, Liu, GF, et al. High-sensitivity C-reactive protein is a strong risk factor for death after acute ischemic stroke among Chinese. CNS Neurosci Ther. (2012) 18:261–6. doi: 10.1111/j.1755-5949.2012.00296.x
41. Kim, S, Yi, HJ, Lee, DH, and Sung, JH. Association of High-sensitivity C-reactive protein with patient prognosis following mechanical Thrombectomy for acute ischemic stroke. Curr Neurovasc Res. (2020) 17:402–10. doi: 10.2174/1567202617666200517110949
42. Corso, G, Bottacchi, E, Brusa, A, Benedetto, MD, Giardini, G, Lia, C, et al. Is there a prognostic role for C-reactive protein in ischemic stroke? Acta Neurol Scand. (2010) 122:209–16. doi: 10.1111/j.1600-0404.2009.01288.x
43. Shantikumar, S, Grant, PJ, Catto, AJ, Bamford, JM, and Carter, AM. Elevated C-reactive protein and long-term mortality after ischaemic stroke: relationship with markers of endothelial cell and platelet activation. Stroke. (2009) 40:977–9. doi: 10.1161/STROKEAHA.108.525105
44. den Hertog, HM, van Rossum, JA, van der Worp, HB, van Gemert, HM, de Jonge, R, Koudstaal, PJ, et al. C-reactive protein in the very early phase of acute ischemic stroke: association with poor outcome and death. J Neurol. (2009) 256:2003–8. doi: 10.1007/s00415-009-5228-x
45. Wang, C, Gao, L, Zhang, ZG, Li, YQ, Yang, YL, Chang, T, et al. Procalcitonin is a stronger predictor of long-term functional outcome and mortality than high-sensitivity C-reactive protein in patients with ischemic stroke. Mol Neurobiol. (2016) 53:1509–17. doi: 10.1007/s12035-015-9112-7
46. Matsuo, R, Ago, T, Hata, J, Wakisaka, Y, Kuroda, J, Kuwashiro, T, et al. Plasma C-reactive protein and clinical outcomes after acute ischemic stroke: a prospective observational study. PLoS One. (2016) 11:e0156790. doi: 10.1371/journal.pone.0156790
47. Pu, Y, Li, S, Wang, L, Fang, B, and Bai, X. Association between high-sensitivity C-reactive protein and prognosis of patients with acute cerebral infarction. Neuropsychiatr Dis Treat. (2022) 18:1771–8. doi: 10.2147/NDT.S376440
48. Bakhshayesh-Eghbali, B, Roudbary, SA, Basir Jafari, S, Nabizadeh, SP, Naderi-Asrami, N, and Sohrabnejad, R. Ability of serum C-reactive protein and white blood cell cout in predicting acute schemic stroke. A short -term follow-up study. Caspian J Intern Med. (2016) 7:206–10.
49. Sagar, R, Kumar, A, Verma, V, Yadav, AK, Raj, R, Rawat, D, et al. Incremental accuracy of blood biomarkers for predicting clinical outcomes after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2021) 30:105537. doi: 10.1016/j.jstrokecerebrovasdis.2020.105537
50. Bender, M, Naumann, T, Uhl, E, and Stein, M. Early serum biomarkers for intensive care unit treatment within the first 24 hours in patients with intracerebral hemorrhage. J Neurol Surg A Cent Eur Neurosurg. (2021) 82:138–46. doi: 10.1055/s-0040-1716516
51. Elhechmi, YZ, Hassouna, M, Chérif, MA, Ben Kaddour, R, Sedghiani, I, and Jerbi, Z. Prognostic value of serum C-reactive protein in spontaneous intracerebral hemorrhage: when should we take the sample? J Stroke Cerebrovasc Dis. (2017) 26:1007–12. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.129
52. Hansen, BM, Morgan, TC, Betz, JF, Sundgren, PC, Norrving, B, Hanley, DF, et al. Intraventricular extension of Supratentorial intracerebral hemorrhage: the modified Graeb scale improves outcome prediction in Lund stroke register. Neuroepidemiology. (2016) 46:43–50. doi: 10.1159/000442575
53. Di Napoli, M, Godoy, DA, Campi, V, Masotti, L, Smith, CJ, Parry Jones, AR, et al. C-reactive protein in intracerebral hemorrhage: time course, tissue localization, and prognosis. Neurology. (2012) 79:690–9. doi: 10.1212/WNL.0b013e318264e3be
54. Di Napoli, M, Godoy, DA, Campi, V, del Valle, M, Piñero, G, Mirofsky, M, et al. C-reactive protein level measurement improves mortality prediction when added to the spontaneous intracerebral hemorrhage score. Stroke. (2011) 42:1230–6. doi: 10.1161/STROKEAHA.110.604983
55. Khanevski, AN, Bjerkreim, AT, Novotny, V, Naess, H, Thomassen, L, Logallo, N, et al. Recurrent ischemic stroke: incidence, predictors, and impact on mortality. Acta Neurol Scand. (2019) 140:3–8. doi: 10.1111/ane.13093
56. Elkind, MS, Tai, W, Coates, K, Paik, MC, and Sacco, RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med. (2006) 166:2073–80. doi: 10.1001/archinte.166.19.2073
57. Shi, K, Tian, DC, Li, ZG, Ducruet, AF, Lawton, MT, and Shi, FD. Global brain inflammation in stroke. Lancet Neurol. (2019) 18:1058–66. doi: 10.1016/S1474-4422(19)30078-X
58. Jayaraj, RL, Azimullah, S, Beiram, R, Jalal, FY, and Rosenberg, GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. (2019) 16:142. doi: 10.1186/s12974-019-1516-2
59. Moore, KJ, Sheedy, FJ, and Fisher, EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. (2013) 13:709–21. doi: 10.1038/nri3520
60. Ridker, PM, Everett, BM, Thuren, T, MacFadyen, JG, Chang, WH, Ballantyne, C, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
Keywords: high-sensitivity C-reactive protein, prognosis, meta-analysis, ischemic stroke, hemorrhagic stroke
Citation: Chen L, Wang M, Yang C, Wang Y and Hou B (2023) The role of high-sensitivity C-reactive protein serum levels in the prognosis for patients with stroke: a meta-analysis. Front. Neurol. 14:1199814. doi: 10.3389/fneur.2023.1199814
Edited by:
Jean-charles Sanchez, University of Geneva, SwitzerlandReviewed by:
Piotr Sobolewski, Jan Kochanowski University, PolandWen-Jun Tu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Chen, Wang, Yang, Wang and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bonan Hou, Houbonan1015@gmail.com
 Liuting Chen
Liuting Chen Min Wang1
Min Wang1 Bonan Hou
Bonan Hou