- 1Center of Cerebrovascular Diseases, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China
- 2Rehabilitation Medicine Center and Institute of Rehabilitation Medicine, West China Hospital, Sichuan University, Chengdu, China
- 3Key Laboratory of Rehabilitation Medicine in Sichuan Province, Chengdu, China
Background: The triglyceride-glucose index (TGI), a reliable surrogate indicator of insulin resistance (IR), has been proven to be a predictor of the incidence of ischemic stroke. The role of TGI in predicting the outcomes of stroke patients remains controversial. Susceptibility to IR-related diseases varies among patients of different ages. The study aims to evaluate the predictive value of TGI levels on clinical outcomes of patients with ischemic stroke of different ages.
Method: This was a retrospective cohort study including patients with ischemic stroke in the Department of Neurology at West China Hospital. TGI was calculated as ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2]. The patients were subdivided into 3 tertiles according to TGI levels. Multivariate logistic regression analyses were conducted to estimate the association between TGI levels and post-stroke outcomes among the whole patients, younger patients (<65), and older patients (>=65). The outcomes included death and unfavorable functional outcome (modified Rankin scale score 3–6) at 3 and 12 months after stroke.
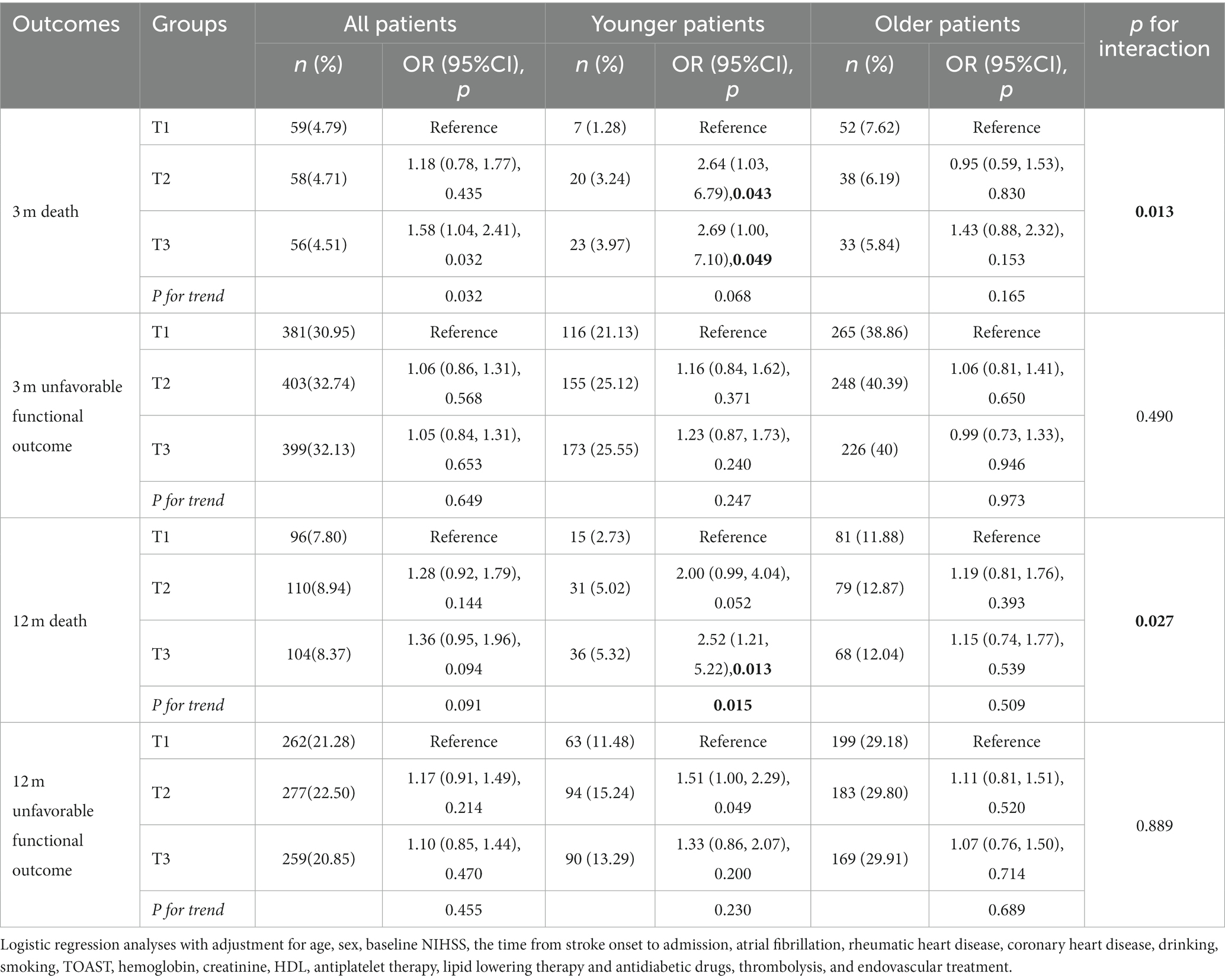
Results: A total of 3,704 patients (men, 65.08%, mean age, 61.44 ± 14.15; women 34.92%, mean age, 65.70 ± 13.69) were enrolled in this study. TGI levels were not associated with 3 month or 12 month death in the whole patients. Patients with higher TGI levels (T2 and T3) had a higher risk of 3 month death than those had lower TGI levels (T1) in the younger group (T2 vs. T1: OR 2.64, 95% CI 1.03–6.79, p = 0.043; T3 vs. T1: OR 2.69, 95% CI 1.00–7.10, p = 0.049) but not in the older group. Additionally, Kaplan–Meier estimate analysis illustrated that the 12 month death risk was significantly higher in the group with the highest TGI among younger patients (p for log-rank test = 0.028) but not among older patients. There was an interactive effect between TGI and age on 3 month death (p for interaction = 0.013) and 12 month death (p for interaction = 0.027). However, TGI was not associated with unfavorable functional outcome at 3 month or 12 month after stroke.
Conclusion: Elevated TGI independently predicts death at 3 months and 12 months in patients under 65 with ischemic stroke. Regulating TGI is expected to be an approach to enhance prognosis in young individuals affected by ischemic stroke.
1. Introduction
Stroke is a significant cause of death and long-term disability representing approximately 10% of global deaths (1). Ischemic stroke comprises more than 80% of all stroke cases and becomes a growing burden on the global healthcare system (2). Previous studies have identified several risk factors playing a crucial role in predicting patient prognosis, such as age, sex, atrial fibrillation, hypertension, and congenital heart disease (3). Moreover, certain blood biomarkers serve as valuable prognostic indicators for post-stroke death risk. These biomarkers include inflammatory factors (e.g., tumor necrosis factor-α, soluble tumor necrosis factor receptor) and blood coagulation biomarkers (e.g., von Willebrand factor, platelet count) (4). Adequate risk stratification facilitates the implementation of effective secondary preventive measures and enhances the quality of life for individuals with stroke (3). Nevertheless, modifying many of these factors is challenging, resulting in limited effects on improving outcomes. Therefore, a crucial requirement exists for identifying modifiable predictors that can help identify patients with poor outcomes and optimize the management of individuals with ischemic stroke.
Insulin resistance (IR), which is characterized by reduced insulin sensitivity, could lead to an insufficient physiological response to a normal amount of insulin. Strong evidence has demonstrated the pivotal role of IR in the pathogenesis of stroke, particularly through embolism and atherosclerosis (5–9). The hyperinsulinemic-euglycemic clamp is the gold standard test for measuring IR; however, its widespread application in clinical settings is challenging due to its high cost and complex detection process (10). Recent accumulating evidence suggests that the triglyceride glucose index (TGI), derived from triglyceride and glucose levels, is a promising surrogate marker for IR (11–13). Multiple studies have confirmed the strong association between TGI and the hyperinsulinemic-euglycemic clamp (9), and it has even demonstrated superior performance to the homeostasis model assessment of insulin resistance (HOMA-IR) for evaluating IR (12, 14).
Recent epidemiological research has provided substantial evidence that supported the relationship between IR and an elevated risk of stroke (15–18). Several prior studies found that it might serve as a predictor for the occurrence of acute ischemic stroke (19–22). However, there is a scarcity of studies investigating the role of TGI in predicting prognosis among individuals following ischemic stroke, and the results obtained thus far have been inconsistent (23–26).
According to a previous study, younger individuals with elevated TGI levels may be more vulnerable to diseases and death associated with IR (27). We hypothesized that age might influence the predictive effect of TGI on the clinical outcome after a stroke, serving as a significant interactive factor contributing to the controversy observed in previous studies. However, no evidence was found to support the influence of age on the association between TGI and the prognosis of ischemic stroke. Consequently, this comprehensive retrospective cohort study aimed to examine the predictive value of TGI levels in determining the clinical outcomes of patients with ischemic stroke. Moreover, it sought to explore the underlying influence of age on the association between TGI and stroke prognosis, marking the first investigation of its kind.
2. Method
2.1. Study population
This study is based on the Chengdu Stroke Registry that included patients with stroke at West China Hospital from 1 January 2012 to 31 December 2018. The inclusion criteria included (1) diagnosed with acute ischemic stroke according to the 1989 criteria of the WHO based on clinical characteristics and imaging manifestations (28); (2) ≥18 years old; and (3) time from onset to admission within 7 days. The exclusion criteria included (1) loss of follow-up information; and (2) inconclusive diagnosis of ischemic stroke.
2.2. Data collection
We collected patient information in terms of demographic data, vascular risk factors, baseline National Institute of Health Stroke Scale (NIHSS), subgroup of stroke according to Trial of ORG 10172 in Acute Stroke Treatment (TOAST) (29), in-hospital medications and therapy after admission, laboratory tests and follow-up information. The vascular risk factors consisted of a history of hypertension, diabetes, atrial fibrillation, rheumatic heart disease, coronary heart disease, stroke, a history of regular smoking or drinking (30–33). Severe stroke was defined as an NIHSS score higher than 15 (34).
Blood sampling was conducted within 24 h after admission to make the data representative and comparable. TGI was calculated as ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2] (35).
Follow-up information was obtained from telephone interviews conducted by trained physicians at 3 and 12 months after admission.
2.3. Outcome measures
The primary outcome was 3 month death from any cause. The secondary outcomes included 3 month unfavorable functional outcomes, 12 month death, and 12 month unfavorable functional outcomes. Unfavorable functional outcomes were defined as an mRS score of 3 to 6, including moderate to severe disability that require partial or full assistance with daily living, or death (36).
2.4. Statistical analysis
The baselines were presented as the younger group and the older group according to the cutoff age of 65 years old. Continuous variables are described as the means with standard deviations (SDs) or medians with the interquartile range (IQR). Categorical variables are described as numbers and percentages.
Multivariate logistic regression models were utilized to calculate odds ratios (ORs) and their 95% confidence intervals (CIs) of TGI as a categorical variable, using the first TGI tertile as the reference predicting different outcomes in all patients, the younger patients, and the older patients. The variables included in multivariate logistic regression were those with statistical differences in univariate analysis and important factors that had an impact on outcomes in clinical practice. To examine the interactive effect of TGI and age on outcomes, P for trend was calculated.
Then, we used Kaplan–Meier curves to predict the association between TGI and 12 month death. Log-rank test was conducted to test differences in death rates. Finally, a cubic spline curve was described to show the dose–response correlation between TGI and death in all patients, young patients, and old patients, with the 50th percentile of the TGI distribution serving as the reference (37).
To examine the interactive effect of other factors on the association between TGI and 3 month death, subgroup analysis was also performed in mild and severe stroke, male and female patients, patients with and without atrial fibrillation, rheumatic heart disease, coronary heart disease, diabetes, hypertension, cardioembolic stroke subtype, lipid lowering therapy, antidiabetic drugs, and cancer. P for interaction was derived from the likelihood-ratio test. All the data were analyzed using Stata version 15.0 software (STATA Corporation, College Station, TX). A two-sided p value <0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
After excluding 268 patients without laboratory values and 480 patients without information on follow-up, 3,704 participants were available for the final analysis. The baseline characteristics of the young and old patients are shown in Tables 1, 2, respectively.
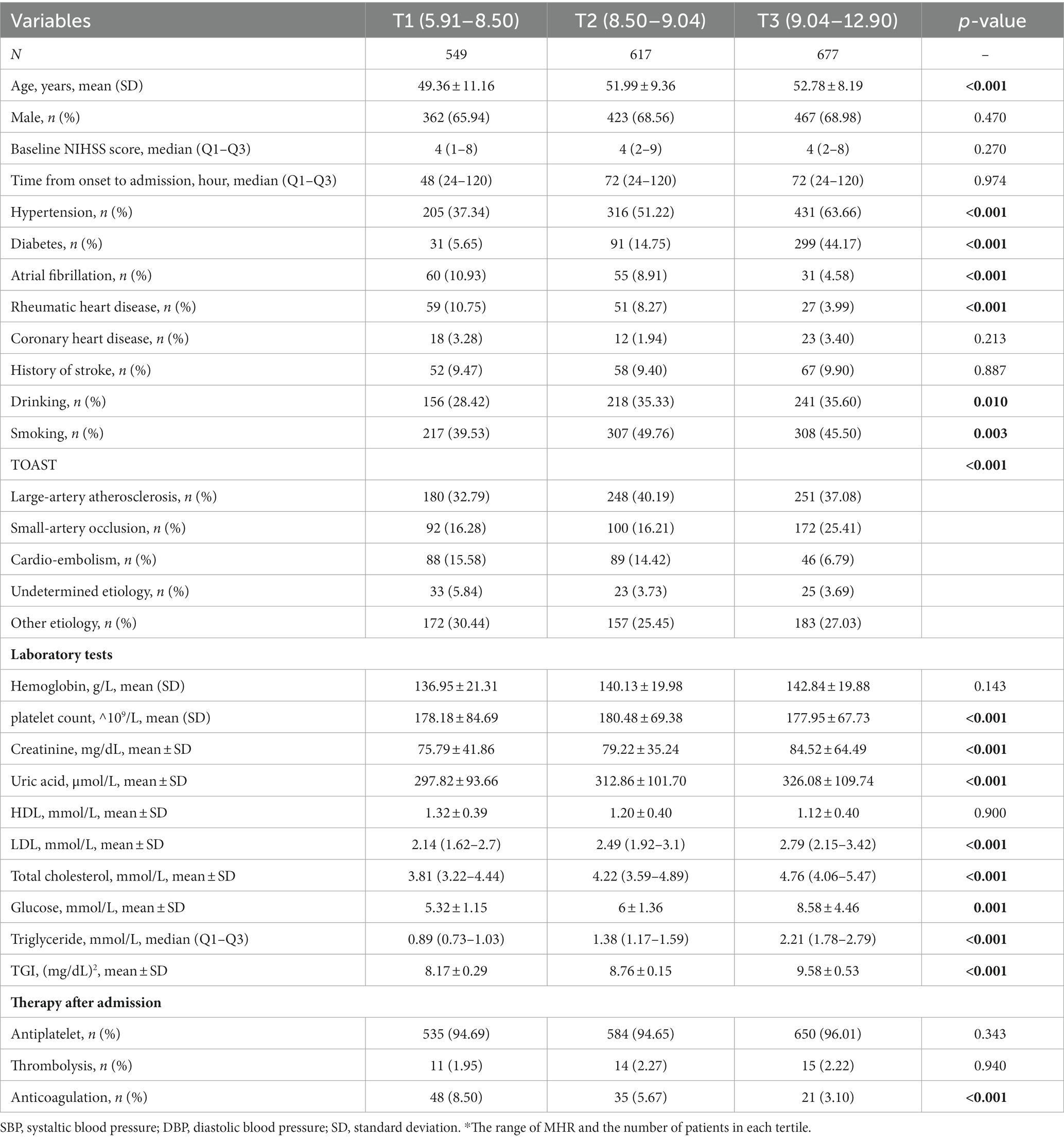
Table 1. Baseline characteristics of included patients grouped by TGI level in patients younger than 65 years old.
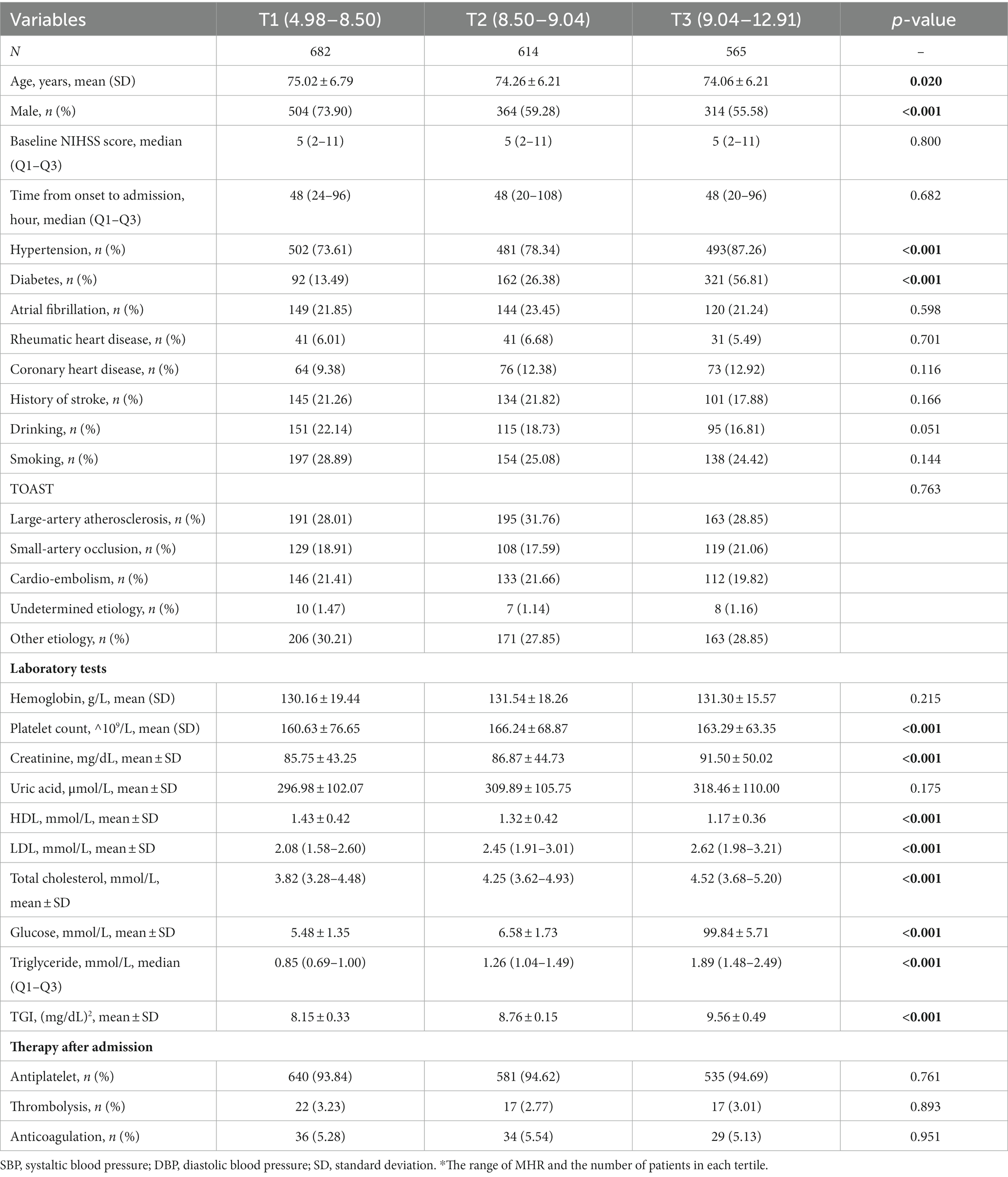
Table 2. Baseline characteristics of included patients grouped by low and high TGI group in patients older than 65 years old.
Among them, 1843 patients were younger than 65 years, with a median (IQR) TGI of 8.79 (8.42, 9.26). The TGI tertiles were divided into T1 (5.91–8.50), T2 (8.50–9.04), and T3 (9.04–12.90). Compared with T1 (49.36), the mean age in the T2 (51.99) and T3 groups (52.78) was older (p < 0.001). Additionally, 1861 patients were older than 65 years, with a median (IQR) TGI of 8.72 (8.34, 9.14). The mean ages in the T2 (74.26) and T3 (74.06) groups were slightly lower than that in the T1 group (75.02, p = 0.020).
In the younger group, patients with higher TGI tended to have more history of drinking (p = 0.010) and more comorbidities, including hypertension, diabetes, atrial fibrillation, and rheumatic heart disease (all p < 0.001). Based on TOAST classification, patients with higher TGI had a higher proportion of large-artery atherosclerosis but a lower proportion of cardioembolism subtype (p < 0.001). For laboratory serum tests, patients with higher TGI tended to have higher levels of creatinine, uric acid, LDL, total cholesterol, glucose, and triglycerides (all p = <0.001). Patients with higher TGI use less anticoagulation after admission (p < 0.001).
In the older group, patients with elevated TGI tended to have a lower proportion of males (p < 0.001) and more comorbidities, including hypertension and diabetes (both p < 0.001). Patients with different TGI levels have similar constituent structures of TOAST classification and similar therapy. The characteristics of laboratory tests were similar to those of the younger group, except that patients with higher TGI tended to have lower HDL levels (p < 0.001) but did not have higher hemoglobin or uric acid levels.
3.2. The predictive role of TGI on different outcomes in whole patients and different ages
3.2.1. TGI and 3 month outcomes
To explore the effect of TGI in predicting outcomes in patients with different ages, logistic regression analysis was conducted for TGI tertiles using T1 as the reference in all, young, and old patients. Interactive analysis was utilised to explore the interactive effect between TGI and age (Table 3).
Table 3 shows that in the whole patients, TGI levels were not associated with 3 month death. While in the younger group, elevated TGI (T2, T3) was associated with a higher risk of 3 month death (T2 vs. T1: OR 2.64, 95% CI 1.03–6.79, p = 0.043; T3 vs. T1: OR 2.69, 95% CI 1.00–7.10, p = 0.049). In older patients, the ORs and 95% CIs for the T2 and T3 groups compared with the T1 group were not significant (T2 vs. T1: OR 0.95, 95% CI 0.59–1.53, p = 0.830; T3 vs. T1: OR 1.43, 95% CI 0.88, 2.32, p = 0.153). The difference between the young and old groups was significant according to the interactive analysis (p for interaction = 0.013).
Supplementary Figure S1 describes a cubic spline curve illustrating the association between TGI levels and adjusted OR for 3 month death in all patients, young patients, and old patients.
Additionally, the logistic regression subgroup analysis is shown in Supplementary Table S1. Factors including stroke severity, sex, atrial fibrillation, rheumatic heart disease, coronary heart disease, diabetes, hypertension, cardioembolic subtype, lipid lowering therapy, antidiabetic drugs, and cancer had no effect on the association between TGI and 3 month death (all p for interaction>0.05).
The results also showed that TGI levels were not significantly associated with 3 month unfavorable functional outcomes in both younger and older groups (p > 0.05).
3.2.2. TGI and 12 month outcomes
Similar results were found for 12 month death. An association was observed in younger patients (T3 vs. T1: OR = 2.52, 95% CI 1.21–5.22, p = 0.013). Conversely, in the older group, no association was presented between TGI and 12 month death (p > 0.05). According to the interactive analysis, the significant difference between the groups was observed (p for interaction = 0.027).
These results were consistent with the Kaplan–Meier estimate analysis in all patients (Figure 1A), younger patients (Figure 1B), and older patients (Figure 1C). In the younger group, the 12 month death risk was higher in the group with high TGI (p for log-rank test = 0.028). While TGI was not significantly associated with 12 month death in the general people or the older people (p for the log-rank test = 0.691 and 0.979, respectively).
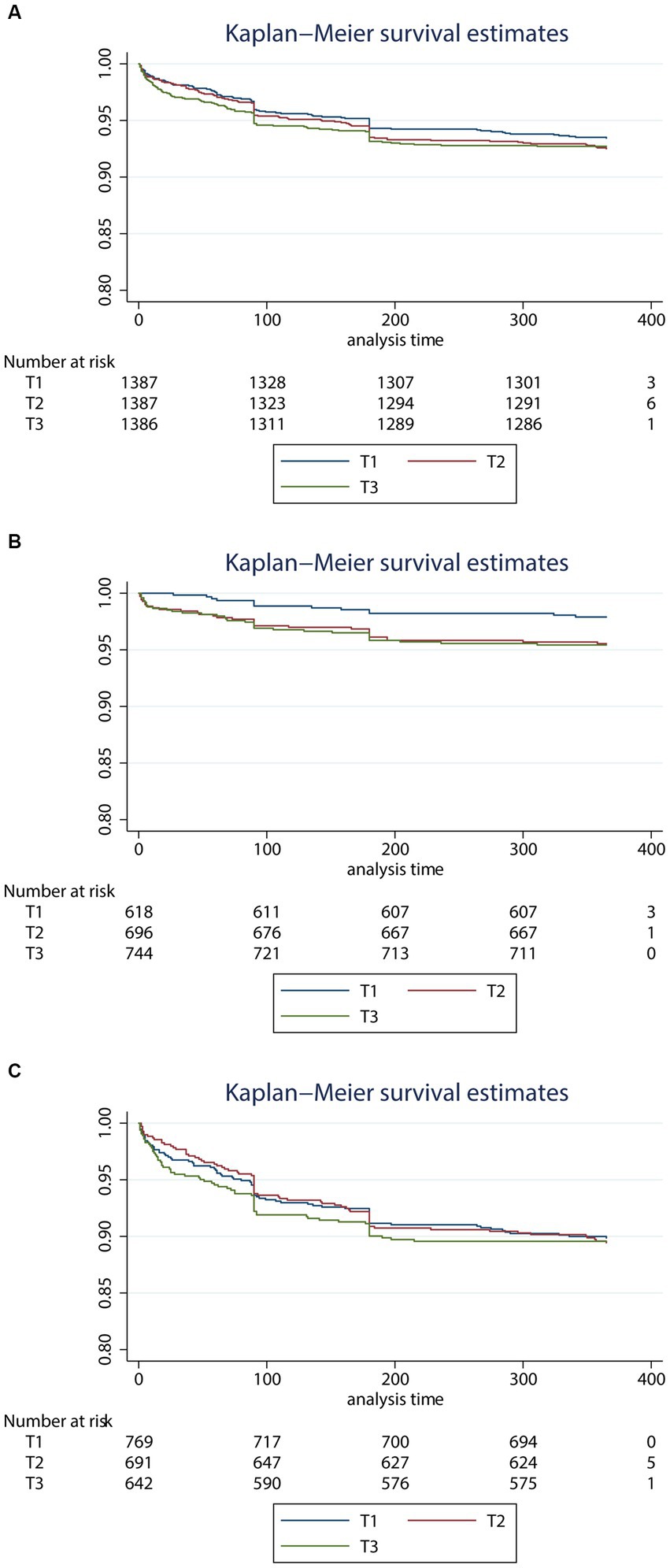
Figure 1. Kaplan–Meier survival estimates curve for the risk of 3 month death according to the level of triglyceride glucose index levels in all patients (A), younger patients (B), and older patients (C). TGI, triglyceride-glucose index. (A) All patients, (B) younger patients, and (C) older patients.
As shown in Supplementary Figure S2, a linear trend was observed for predicting 12 month death in the younger patients (p for trend = 0.012). For all patients and older patients, no significant linear association was observed (p for trend >0.05). In addition, no association was observed from interactive analysis between age and TGI on 12 month unfavorable functional outcome (p for interaction >0.05, Table 3).
4. Discussion
In this study, we explored the prognostic value of TGI levels among patients with ischemic stroke. We found that the association between TGI levels and the risk of death was mainly significant in patients younger than 65 years old. Younger patients with higher TGI levels exhibited an increased risk of death at 3 months and 12 months after stroke.
Although TGI might be associated with the prognosis of patients with stroke, the underlying mechanism is yet to be elucidated. Hyperglycemia in stroke patients predicts an unfavorable outcome, attributed to factors such as large infarct volume, hemorrhagic transformation, and recanalization failure (38–40). Hypertriglyceridemia contributes to early neurological deterioration and death primarily through atherosclerosis and cerebral vessel damage (41, 42). TGI, which may represent both hyperglycemia and hypertriglyceridemia, had better prognostic value than a single measurement alone. IR, as indicated by TGI, contributes to the development of more severe strokes and an increased risk of post-stroke death through various mechanisms. These mechanisms include proinflammatory cytokines induced by altered adiponectin signaling after stroke (43), and activation of hemostasis and a procoagulant tendency, leading to aggravated microvascular obstruction and cerebral ischemia (44).
Previous research has investigated the effect of TGI in selecting people at early risk of future stroke who are seemingly healthy (19–21). However, limited data are available regarding the association between TGI and death or functional outcomes in individuals with ischemic stroke, and the results are inconsistent (23–26). For example, high TGI was associated with a unfavorable functional outcome in Toh EMS’s study (24) but not in Zhou’s study (23). Yang et al. held a different opinion from others, reporting that TGI could not predict death in nondiabetic acute ischemic stroke patients (25). We propose that the discrepancy can be attributed, in part, to the interactive effect of TGI and other factors on stroke prognosis.
Studies have reported a greater susceptibility to IR among younger patients (45), and those with elevated TGI levels in this age group have a higher risk of IR-related diseases and death (27). Ma et al. discovered that patients below 70 years of age with elevated TGI levels exhibited an increased risk of cardiovascular and cerebrovascular diseases, including stroke, type 2 diabetes mellitus, and acute coronary syndrome, while no significant association was observed in older patients (46). Wang et al. suggested that a higher level of TGI in patients below 65 years of age with cardiovascular diseases may indicate an elevated risk of all-cause death (47). To explore the impact of age on the association between TGI and clinical outcome after stroke, we categorized the study population into older and younger groups. Interestingly, we observed a significant relationship between TGI and the risk of death at 3 months and 12 months in the younger group, while no such association was found in the older group. An interactive effect of age and TGI levels on the risk of death after stroke was observed. Additionally, a potential dose–response relationship between TGI and 12 month death was derived in younger patients based on our findings.
Therefore, we proposed that the role of IR in death may be significant in younger patients and that TGI was useful in predicting the prognosis in younger individuals with ischemic stroke. They may benefit from monitoring and treating IR.
There are several reasonable explanations for the effect of age on the association between TGI and the outcome. First, previous study suggested that younger individuals were more susceptible to IR (45), and had elevated TGI levels (45, 46, 48–50). On the contrary, an inverse association was observed in older patients: patients who were older had lower TGI levels, which was consistent with a previous study that explored the association between TGI and cardiovascular disease (48). For older individuals, TGI might not reflect IR the same as for younger individuals, probably because of more confounding factors that influence TGI measurement, such as prevalence of various chronic consumptive diseases and the subsequent malnutrition and decreased lipid reversal (51).
Moreover, based on the baseline information, higher TGI is associated with a lower proportion of heart disease, and associated with more large-artery atherosclerosis and less cardio-embolism subtype of stroke only in the younger group. Thus, for younger patients in our study, higher TGI reflected more severe atherosclerosis and worse vascular condition. A previous study has shown that for younger stroke patients, large-artery atherosclerotic subtype of stroke was a valuable predictor of worse outcomes including recurrence of vascular events, death, and unfavorable functional outcomes (52). Subgroup analysis based on etiologic stroke subtypes may help further explain our results.
Additionally, the predictive effect of TGI on functional outcome in individuals with stroke is still controversial. In our study, TGI might have had only a limited effect on unfavorable functional outcome in younger patients, which was different from the result regarding death. This was consistent with Zhou’s study (23) but opposite to Toh’s (24), and Lin’s (53) studies. Another study, which evaluated IR by HOMA-IR, confirmed that IR was associated with death, recurrence, and unfavorable functional outcome (an mRS score of 3 to 6) but not dependence (an mRS score of 3 to 5) in nondiabetic patients after stroke (54). Zhou interpreted that the different results concerning functional outcomes may result from the diversity of follow-up times, study design and race (23).
Possible explanations for the association between IR and death other than unfavorable outcomes are that IR is associated with the recurrence of ischemic stroke or cardiovascular disease in patients with stroke (25, 55), which is much more lethal than the first stroke. Nevertheless, some studies present opposite results, confirming that IR was associated with functional outcomes of poststroke patients (54, 56). However, they evaluated IR using HOMA-IR but not TGI, and the differences in IR marker measurements might account for the different findings. Additionally, stroke severity, the location of the lesion and diabetes condition also influenced functional outcomes (53, 57). Finally, the small sample size may also lead to controversial results. Further study based on a larger survey sample is needed to validate the results.
Significantly, TGI is a routinely measured and modifiable indicator to reflect the risk of post-stroke death compared with other markers. We propose suppression of IR and improvement of hyperglycemia or hypertriglyceridemia as new therapeutic strategies in patients with ischemic stroke to improve the prognosis. Since our study found that TGI has a greater impact on the outcome of younger patients, hyperglycemia and hypertriglyceridemia should be strictly controlled to prevent a higher risk of death, especially among young stroke patients. Pioglitazone, an insulin-sensitizing agent, as reported by several studies, is promising for reducing cardiovascular events in patients with ischemic stroke (58, 59). Other antidiabetic drugs, such as insulin, have been proven to inhibit the neuronal damage by suppressing elevated glucose levels within 48 h after ischemic stroke (60). Using these methods to regulate TGI, including taking drugs and modifying lifestyle, is expected to contribute to a better prognosis in post-stroke patients.
In this study, we explored the association between TGI and the outcomes of patients with ischemic stroke. This is the first study to analyze age as an interactive factor of the effect of TGI on the prognosis of ischemic stroke. The novelty and significance of this study were that we explored the possible interactive effect between age and TGI on the outcome of stroke. Besides, we explored the underlying reasons for the results that TGI affected post-stroke death but had few effects on post-stroke unfavorable functional outcomes for the first time. Although this was a retrospective study, inclusion bias and retrospective bias were adequately avoided with propensity-weighted analysis, continuous prospective follow-up, and a large sample size. Nevertheless, this study has several limitations. First, due to the low proportion of patients receiving reperfusion therapy (107 cases, 2.89%), including thrombolysis and endovascular treatment (MT), no further subgroup analysis by type of reperfusion therapy was performed. Previous studies have shown the association between TGI levels and the outcomes of patients undergoing thrombolysis (24, 53), while there were no studies on the effect of TGI on the outcomes in patients who underwent MT. According to Merlino’s study, stress hyperglycemia is strictly and independently associated with futile recanalization in patients undergoing MT (61). Thus, further study focus on the predictive value of TGI on the outcome of patients treated with MT is promising. Second, the median (Q1–Q3) time from stroke onset to blood sampling for TGI assessment is 88 (40–160) hours in the younger and 64 (40–136) hours in the older. Further subgroup analysis according to the time from onset to sampling is useful to detect the robustness of TGI as a serum marker. Fixing the time from onset to sampling or taking multiple blood samples from the same patient to observe the dynamic changes in serological indicators could make the results more convincing. Third, the retrospective nature of this study leads to inevitably residual or unmeasured confounding factors which may influence the prognosis, such as infarct size, etiological type, antithrombotic treatment at the admission and the baseline mRS socre. Besides, test for serum insulin, HOMA-IR, and hyperinsulinemic-euglycemic clamp help to measure IR in patients, which depends on further prospective studies.
5. Conclusion
Elevated TGI independently predicts death at 3 months and 12 months in patients under 65 with ischemic stroke. Therefore, TGI can be utilized as a supplementary test for prognosis assessment in young patients with ischemic stroke, while regulating TGI holds promise as an approach to enhance prognosis in young individuals affected by ischemic stroke.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Biomedical Research Ethics Committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SZ and LW conceived and designed the research. RL and LL participated in analyzing the data and wrote the article. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant nos. 81974208, 82202793, and 81501951) and Natural Science Foundation of Sichuan Province (Grant No. 2020YFC2008500, 2022NSFSC0715, and 2023NSFSC1495).
Acknowledgments
We gratefully acknowledge Chengdu Stroke Registry for providing information of patients with acute ischemic stroke in the Department of Neurology at West China Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1198487/full#supplementary-material
References
1. Feigin, VL, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
2. Barthels, D, and Das, H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol basis Dis. (2020) 1866:165260. doi: 10.1016/j.bbadis.2018.09.012
3. Diener, HC, and Hankey, GJ. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol. (2020) 75:1804–18. doi: 10.1016/j.jacc.2019.12.072
4. Foschi, M, Padroni, M, Abu-Rumeileh, S, Abdelhak, A, Russo, M, D’Anna, L, et al. Diagnostic and prognostic blood biomarkers in transient ischemic attack and minor ischemic stroke: an up-to-date narrative review. J Stroke Cerebrovasc Dis. (2022) 31:106292. doi: 10.1016/j.jstrokecerebrovasdis.2021.106292
5. Ding, PF, Zhang, HS, Wang, J, Gao, YY, Mao, JN, Hang, CH, et al. Insulin resistance in ischemic stroke: mechanisms and therapeutic approaches. Front Endocrinol. (2022) 13:1092431. doi: 10.3389/fendo.2022.1092431
6. Muniyappa, R, Chen, H, Montagnani, M, Sherman, A, and Quon, MJ. Endothelial dysfunction due to selective insulin resistance in vascular endothelium: insights from mechanistic modeling. Am J Physiol Endocrinol Metab. (2020) 319:E629–46. doi: 10.1152/ajpendo.00247.2020
7. Jolugbo, P, and Ariëns, RAS. Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke. (2021) 52:1131–42. doi: 10.1161/STROKEAHA.120.032810
8. Di Pino, A, and DeFronzo, RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. (2019) 40:1447–67. doi: 10.1210/er.2018-00141
9. Love, KM, and Liu, Z. DPP4 activity, hyperinsulinemia, and atherosclerosis. J Clin Endocrinol Metab. (2021) 106:1553–65. doi: 10.1210/clinem/dgab078
10. Park, SE, Park, CY, and Sweeney, G. Biomarkers of insulin sensitivity and insulin resistance: past, present and future. Crit Rev Clin Lab Sci. (2015) 52:180–90. doi: 10.3109/10408363.2015.1023429
11. Guerrero-Romero, F, Simental-Mendía, LE, González-Ortiz, M, Martínez-Abundis, E, Ramos-Zavala, ḾG, Hernández-González, SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
12. Vasques, AC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. (2011) 93:e98–e100. doi: 10.1016/j.diabres.2011.05.030
13. Locateli, JC, Lopes, WA, Simões, CF, de Oliveira, GH, Oltramari, K, Bim, RH, et al. Triglyceride/glucose index is a reliable alternative marker for insulin resistance in south American overweight and obese children and adolescents. J Pediatr Endocrinol Metab. (2019) 32:1163–70. doi: 10.1515/jpem-2019-0037
14. Mohd Nor, NS, Lee, SJ, Bacha, F, Tfayli, H, and Arslanian, S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr Diabetes. (2016) 17:458–65. doi: 10.1111/pedi.12303
15. Deng, XL, Liu, Z, Wang, C, Li, Y, and Cai, Z. Insulin resistance in ischemic stroke. Metab Brain Dis. (2017) 32:1323–34. doi: 10.1007/s11011-017-0050-0
16. Wieberdink, RG, Koudstaal, PJ, Hofman, A, Witteman, JCM, Breteler, MMB, and Arfan Ikram, M. Insulin resistance and the risk of stroke and stroke subtypes in the nondiabetic elderly. Am J Epidemiol. (2012) 176:699–707. doi: 10.1093/aje/kws149
17. Cai, X, Hu, J, Zhu, Q, Wang, M, Liu, S, Dang, Y, et al. Relationship of the metabolic score for insulin resistance and the risk of stroke in patients with hypertension: a cohort study. Front Endocrinol. (2022) 13:1049211. doi: 10.3389/fendo.2022.1049211
18. Chen, W, Wang, S, Lv, W, and Pan, Y. Causal associations of insulin resistance with coronary artery disease and ischemic stroke: a Mendelian randomization analysis. BMJ Open Diabetes Res Care. (2020) 8:e001217. doi: 10.1136/bmjdrc-2020-001217
19. Zhao, Y, Sun, H, Zhang, W, Xi, Y, Shi, X, Yang, Y, et al. Elevated triglyceride-glucose index predicts risk of incident ischaemic stroke: the rural Chinese cohort study. Diabetes Metab. (2021) 47:101246. doi: 10.1016/j.diabet.2021.101246
20. Wang, A, Wang, G, Liu, Q, Zuo, Y, Chen, S, Tao, B, et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol. (2021) 20:46. doi: 10.1186/s12933-021-01238-1
21. Feng, X, Yao, Y, Wu, L, Cheng, C, Tang, Q, and Xu, S. Triglyceride-glucose index and the risk of stroke: a systematic review and dose-response meta-analysis. Horm Metab Res. (2022) 54:175–86. doi: 10.1055/a-1766-0202
22. Xu, W, Zhao, H, Han, X, Liu, J, Li, H, Sun, J, et al. Relationship between early-onset stroke and triglyceride-glucose index among young Chinese adults. Lipids Health Dis. (2023) 22:3. doi: 10.1186/s12944-023-01773-8
23. Zhou, Y, Pan, Y, Yan, H, Wang, Y, Li, Z, Zhao, X, et al. Triglyceride glucose index and prognosis of patients with ischemic stroke. Front Neurol. (2020) 11:456. doi: 10.3389/fneur.2020.00456
24. Toh, EMS, Lim, AYL, Ming, C, Yeo, LLL, Sia, CH, Tan, BWQ, et al. Association of triglyceride-glucose index with clinical outcomes in patients with acute ischemic stroke receiving intravenous thrombolysis. Sci Rep. (2022) 12:1596. doi: 10.1038/s41598-022-05467-6
25. Yang, X, Wang, G, Jing, J, Wang, A, Zhang, X, Jia, Q, et al. Association of triglyceride-glucose index and stroke recurrence among nondiabetic patients with acute ischemic stroke. BMC Neurol. (2022) 22:79. doi: 10.1186/s12883-022-02588-3
26. Zhang, B, Liu, L, Ruan, H, Zhu, Q, Yu, D, Yang, Y, et al. Triglyceride-glucose index linked to hospital mortality in critically ill stroke: an observational multicentre study on eICU database. Front Med. (2020) 7:591036. doi: 10.3389/fmed.2020.591036
27. Sharif, S, et al. Mediation analysis of the relationship between type 2 diabetes and cardiovascular events and all-cause mortality: findings from the SMART cohort. Diabetes, Obesity Metab. (2019) 21:1935–43. doi: 10.1111/dom.13759
28. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA project principal investigators. J Clin Epidemiol. (1988) 41:105–14. doi: 10.1016/0895-4356(88)90084-4
29. Adams, HP, Bendixen, BH, Kappelle, LJ, Biller, J, Love, BB, Gordon, DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
30. Whelton, PK, Carey, RM, Aronow, WS, Collins, KJ, Dennison Himmelfarb, C, DePalma, S, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2018) 138:e426–83. doi: 10.1161/CIR.0000000000000597
31. Classification and Diagnosis of Diabetes. Standards of medical Care in Diabetes-2019. Diabetes Care. (2019) 42:S13–28. doi: 10.2337/dc19-S002
32. Wang, D, Liu, M, Hao, Z, Tao, W, Lin, S, Zhang, S, et al. Features of acute ischemic stroke with rheumatic heart disease in a hospitalized Chinese population. Stroke. (2012) 43:2853–7. doi: 10.1161/STROKEAHA.112.670893
33. Knuuti, J, Wijns, W, Saraste, A, Capodanno, D, Barbato, E, Funck-Brentano, C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
34. Mazya, MV, Lees, KR, Collas, D, Rand, VM, Mikulik, R, Toni, D, et al. IV thrombolysis in very severe and severe ischemic stroke: results from the SITS-ISTR registry. Neurology. (2015) 85:2098–106. doi: 10.1212/WNL.0000000000002199
35. Simental-Mendía, LE, Rodríguez-Morán, M, and Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. (2008) 6:299–304. doi: 10.1089/met.2008.0034
36. van Swieten, JC, Koudstaal, PJ, Visser, MC, Schouten, HJ, and van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1988) 19:604–7. doi: 10.1161/01.STR.19.5.604
37. Desquilbet, L, and Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. (2010) 29:1037–57. doi: 10.1002/sim.3841
38. Chamorro, Á, et al. Glucose modifies the effect of endovascular thrombectomy in patients with acute stroke. Stroke. (2019) 50:690–6. doi: 10.1161/STROKEAHA.118.0237691161/STROKEAHA.118.023769
39. Capes, SE, Hunt, D, Malmberg, K, Pathak, P, and Gerstein, HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. (2001) 32:2426–32. doi: 10.1161/hs1001.096194
40. Bruno, A, Levine, SR, Frankel, MR, Brott, TG, Lin, Y, Tilley, BC, et al. Admission glucose level and clinical outcomes in the NINDS RT-PA stroke trial. Neurology. (2002) 59:669–74. doi: 10.1212/WNL.59.5.669
41. Deng, Q, Li, S, Zhang, H, Wang, H, Gu, Z, Zuo, L, et al. Association of serum lipids with clinical outcome in acute ischaemic stroke: a systematic review and meta-analysis. J Clin Neurosci. (2019) 59:236–44. doi: 10.1016/j.jocn.2018.09.003
42. Choi, KH, Park, MS, Kim, JT, Chang, J, Nam, TS, Choi, SM, et al. Serum triglyceride level is an important predictor of early prognosis in patients with acute ischemic stroke. J Neurol Sci. (2012) 319:111–6. doi: 10.1016/j.jns.2012.04.018
43. Harada, S, Fujita-Hamabe, W, and Tokuyama, S. Ischemic stroke and glucose intolerance: a review of the evidence and exploration of novel therapeutic targets. J Pharmacol Sci. (2012) 118:1–13. doi: 10.1254/jphs.11R04CR
44. Ozkul, A, Turgut, ET, Akyol, A, Yenisey, C, Kadikoylu, G, et al. The relationship between insulin resistance and hypercoagulability in acute ischemic stroke. Eur Neurol. (2010) 64:201–6. doi: 10.1159/000319196
45. Yang, J, Tang, YD, Zheng, Y, Li, C, Zhou, Q, Gao, J, et al. The impact of the triglyceride-glucose index on poor prognosis in nondiabetic patients undergoing percutaneous coronary intervention. Front Endocrinol. (2021) 12:710240. doi: 10.3389/fendo.2021.710240
46. Ma, X, Dong, L, Shao, Q, Cheng, Y, Lv, S, Sun, Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. (2020) 19:31. doi: 10.1186/s12933-020-01006-7
47. Wang, L, Wang, Y, Liu, R, Xu, L, Zhong, W, Li, L, et al. Influence of age on the association between the triglyceride-glucose index and all-cause mortality in patients with cardiovascular diseases. Lipids Health Dis. (2022) 21:135. doi: 10.1186/s12944-022-01738-3
48. Luo, E, Wang, D, Yan, G, Qiao, Y, Liu, B, Hou, J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. (2019) 18:150. doi: 10.1186/s12933-019-0957-3
49. Zhang, Y, Ding, X, Hua, B, Liu, Q, Gao, H, Chen, H, et al. High triglyceride-glucose index is associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Nutr Metab Cardiovasc Dis. (2020) 30:2351–62. doi: 10.1016/j.numecd.2020.07.041
50. Lee, EY, Yang, HK, Lee, J, Kang, B, Yang, Y, Lee, SH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. (2016) 15:155. doi: 10.1186/s12944-016-0324-2
51. Schuetz, P, Seres, D, Lobo, DN, Gomes, F, Kaegi-Braun, N, and Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet. (2021) 398:1927–38. doi: 10.1016/S0140-6736(21)01451-3
52. Redfors, P, Jood, K, Holmegaard, L, Rosengren, A, Blomstrand, C, and Jern, C. Stroke subtype predicts outcome in young and middle-aged stroke sufferers. Acta Neurol Scand. (2012) 126:329–35. doi: 10.1111/j.1600-0404.2012.01653.x
53. Lin, SF, Hu, HH, Chao, HL, Ho, BL, Chen, CH, Chan, L, et al. Triglyceride-glucose index and intravenous thrombolysis outcomes for acute ischemic stroke: a multicenter prospective-cohort study. Front Neurol. (2022) 13:737441. doi: 10.3389/fneur.2022.737441
54. Jing, J, Pan, Y, Zhao, X, Zheng, H, Jia, Q, Mi, D, et al. Insulin resistance and prognosis of nondiabetic patients with ischemic stroke: the ACROSS-China study (abnormal glucose regulation in patients with acute stroke across China). Stroke. (2017) 48:887–93. doi: 10.1161/STROKEAHA.116.015613
55. Li, S, Yin, C, Zhao, W, Zhu, H, Xu, D, Xu, Q, et al. Homeostasis model assessment of insulin resistance in relation to the poor functional outcomes in nondiabetic patients with ischemic stroke. Biosci Rep. (2018) 38:330. doi: 10.1042/BSR20180330
56. Ago, T, Matsuo, R, Hata, J, Wakisaka, Y, Kuroda, J, Kitazono, T, et al. Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology. (2018) 90:e1470–7. doi: 10.1212/WNL.0000000000005358
57. Nam, KW, Kang, MK, Jeong, HY, Kim, TJ, Lee, EJ, Bae, J, et al. Triglyceride-glucose index is associated with early neurological deterioration in single subcortical infarction: early prognosis in single subcortical infarctions. Int J Stroke. (2021) 16:944–52. doi: 10.1177/1747493020984069
58. Lee, M, Saver, JL, Liao, HW, Lin, CH, and Ovbiagele, B. Pioglitazone for secondary stroke prevention: a systematic review and meta-analysis. Stroke. (2017) 48:388–93. doi: 10.1161/STROKEAHA.116.013977
59. Kernan, WN, Viscoli, CM, Furie, KL, Young, LH, Inzucchi, SE, Gorman, M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. (2016) 374:1321–31. doi: 10.1056/NEJMoa1506930
60. Harada, S, Fujita, W, Shichi, K, and Tokuyama, S. The development of glucose intolerance after focal cerebral ischemia participates in subsequent neuronal damage. Brain Res. (2009) 1279:174–81. doi: 10.1016/j.brainres.2009.05.014
Keywords: triglyceride-glucose index, insulin resistance, acute ischemic stroke, age, prognosis, death
Citation: Liu R, Li L, Wang L and Zhang S (2023) Triglyceride-glucose index predicts death in patients with stroke younger than 65. Front. Neurol. 14:1198487. doi: 10.3389/fneur.2023.1198487
Edited by:
Giovanni Merlino, Udine University Hospital, ItalyReviewed by:
Matteo Foschi, Azienda Unità Sanitaria Locale (AUSL) della Romagna, ItalyFedra Kuris, Santa Maria della Misericordia University Hospital, Italy
Copyright © 2023 Liu, Li, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Wang, wanglu0823@scu.edu.cn; Shihong Zhang, zsh9@hotmail.com
†These authors have contributed equally to this work and share first authorship
 Ruishan Liu
Ruishan Liu Lijuan Li
Lijuan Li Lu Wang
Lu Wang Shihong Zhang
Shihong Zhang