- The First Affiliated Hospital of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
Background: Juvenile myasthenia gravis (JMG) is a rare autoimmune disease that has so far only been described in small cohort studies. We defined the clinical characteristics, management, and outcomes of JMG patients over the past 22 years.
Methods: A search of PubMed, EMBASE, and web of science (1/2000–2/2022) identified all English language and human studies of JMG. The population was patients diagnosed with JMG. Outcomes included the history of myasthenic crisis, autoimmune comorbidity, mortality, and treatment outcome. Data extraction was performed by independent reviewers. And we performed a pooled reanalysis of all published data in the included studies and compared with other studies of adult cohorts.
Results: We identified 11 articles describing 1,109 patients diagnosed between 2006 and 2021. JMG occurred in 60.4% of female patients. The mean age at presentation was 7.38 years old, and 60.6% of the patients had ocular symptoms as the first clinical manifestation. The most common initial presentation was ptosis, which occurred in 77.7% patients. AchR-Ab positive accounted for 78.7%. 641 patients received thymus examination, found to have thymic hyperplasia in 64.9% and thymoma in 2.2%. Autoimmune comorbidity was found in 13.6% and the most common one is thyroid disease (61.5%). First-line therapy, including pyridostigmine and steroids, was initiated in 97.8 and 68.6%, respectively. Six patients resolved spontaneously without treatment. Thymectomy was performed in 45.6%. 10.6% of patients had a history of myasthenic crisis. Completely stable remission was achieved in 23.7% and mortality was reported in 2 studies, which reported 8 deaths.
Conclusion: JMG is a rare disease with a relatively benign course, and differs from adult MG in some clinical features. The treatment regimen guideline for children is still not well-established. There is a need for prospective studies to properly evaluate treatment regimes.
Background
Juvenile myasthenia gravis (JMG) is an autoimmune disorder that leads to dysfunction of acetylcholine receptors (AchR), defined as myasthenia gravis in children younger than 18 years of age (1). It is unclear whether the pathogenesis of JMG is the same as that of adults. While clinical phenotypes are similar to adults (2), JMG and adult MG still have many different characteristics, such as symptoms, clinical severity, antibody titer, and thymus histology (3). Current practice is taken from treatment guidelines for adult MG or individual experience, and it still has no standardized treatment guidelines (4, 5).
We performed a systematic review and meta-analysis to determine the clinical features, treatment, and outcomes of patients with JMG over the last 22 years.
Methods
Search strategy
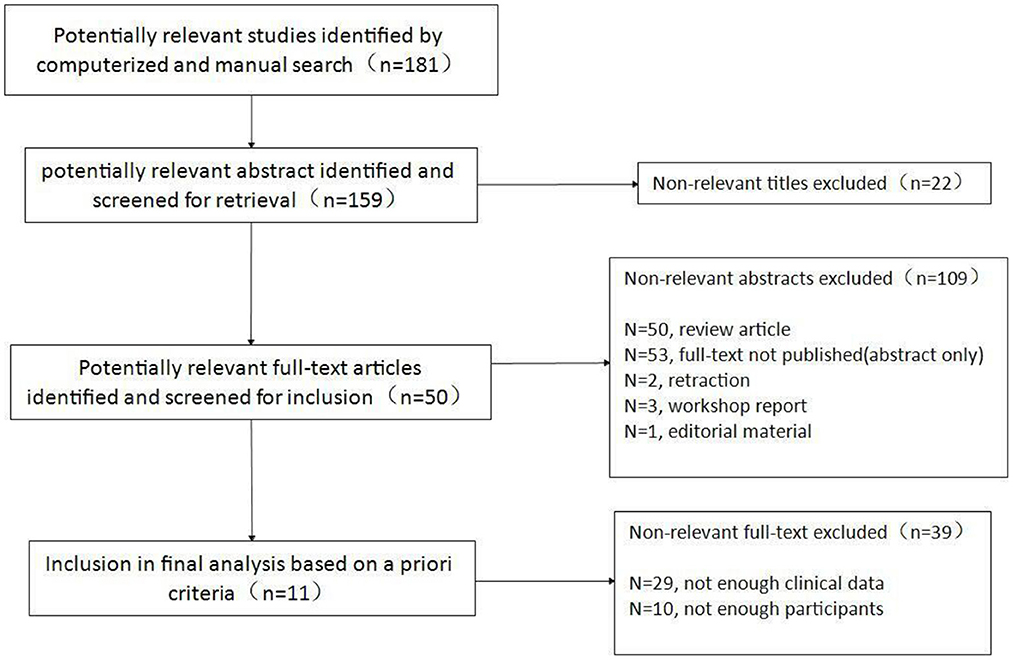
PubMed, EMBASE, and Web of science were searched using the search topic “juvenile myasthenia gravis”. Given that the earlier articles did not collect enough clinical data, articles published between January 2000 and February 2022 were included. Limiters of human studies, English language, non-case reports and non-case series studies were applied. This search yielded 181 studies. The titles and abstracts for the identified manuscripts were evaluated per the inclusion/exclusion criteria described below. Reference lists for relevant review articles were searched manually for additional studies. In addition, topic experts were contacted to determine if any additional studies or unpublished data could be identified. The titles and abstracts for all included publications were assessed by two independent reviewers, resulting in 11 full-text articles (6–16), which were subsequently evaluated in further details.
Inclusion/exclusion criteria
Studies were evaluated using inclusion criteria, as follows:
(1) Patients diagnosed with JMG (clinical presentation consistent with JMG as well as elevated serum antibodies, positive stimulatory test, or response to a trial of therapy);
(2) Onset < 18 years of age;
(3) Intervention of standard treatment;
(4) Collected enough clinical data including the age of onset, sex, symptom, serology, and treatment outcome.
(5) Patients >10.
The decision for the inclusion of each study was made independently by 2 independent reviewers. Disagreements between reviewers were resolved by consensus. Data extraction focused on study methodology, population, intervention, results. Extracted data included: age of onset (pre-pubertal ≤ 12 years, post-pubertal>12 years), sex, symptom of onset, seropositivity, results for repetitive nerve stimulation (RNS) and single fiber electromyography (SFEMG), thymic pathology, myasthenic crisis, autoimmune comorbidity, generalization, treatment and treatment outcome. Data extraction was performed using standardized tables, which were subsequently corroborated and synthesized.
Risk of bias was assessed using the criteria described in the Newcastle-Ottawa Scale by two authors independently. For all cohort studies, the assessments included methods of selecting exposure and non-exposure cases, comparability between groups, and evaluation of outcome incidents. This process was performed independently by two investigators. Any disagreement between investigators was resolved by reconciliation and/or discussion with a third investigator (Results shown in Supplementary file 1).
We performed a pooled reanalysis of all published data in the included studies and compared with other studies of adult cohorts. Because of heterogeneity between studies, all data are presented as the proportion (n/N [%]) of the total number of patients with a certain characteristics and analyzed with 95% confidence intervals (95% CI). Heterogeneity between studies was calculated for all reported variables using the I2 statistical tests by Stata 16.0 (Supplementary file 1).
Results
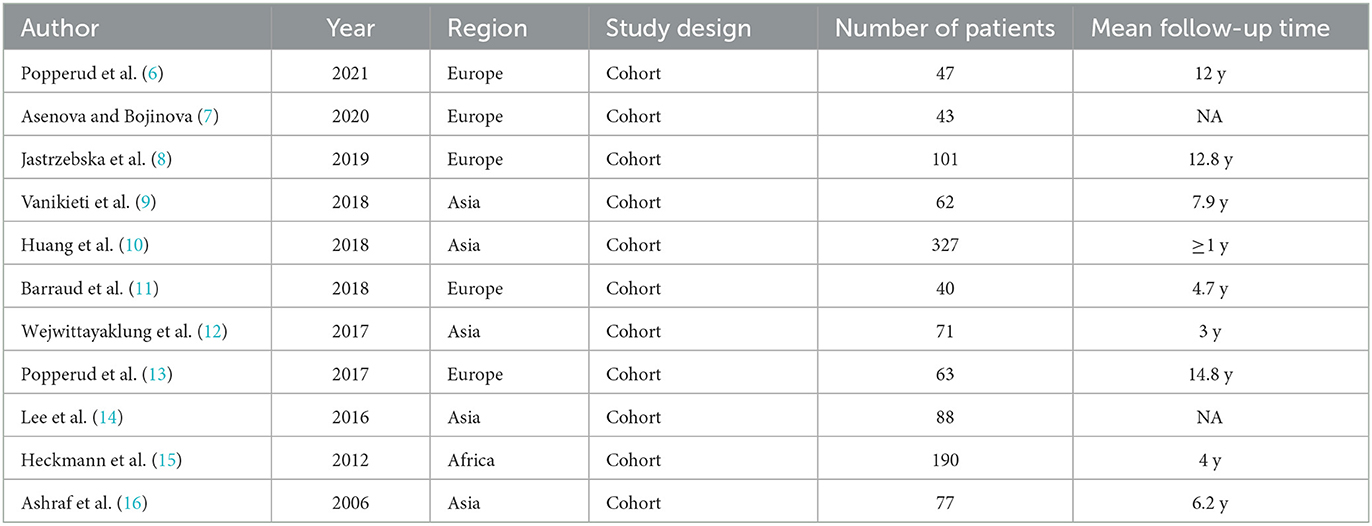
Our search identified 181 articles, of which 11 studies remained after screening (Figure 1), covering 1,109 patients diagnosed between 2006 and 2021. All studies were retrospective studies (Table 1). The number of patients in each study varied between 40 and 327. Participants were followed for a period ranging from 0.2 to 67 years. Heterogeneity calculations for the data reported in pooled study results revealed significant heterogeneity (I2>50%) for 24 of the 40 study parameters (Supplementary file 1).
Patient characteristics
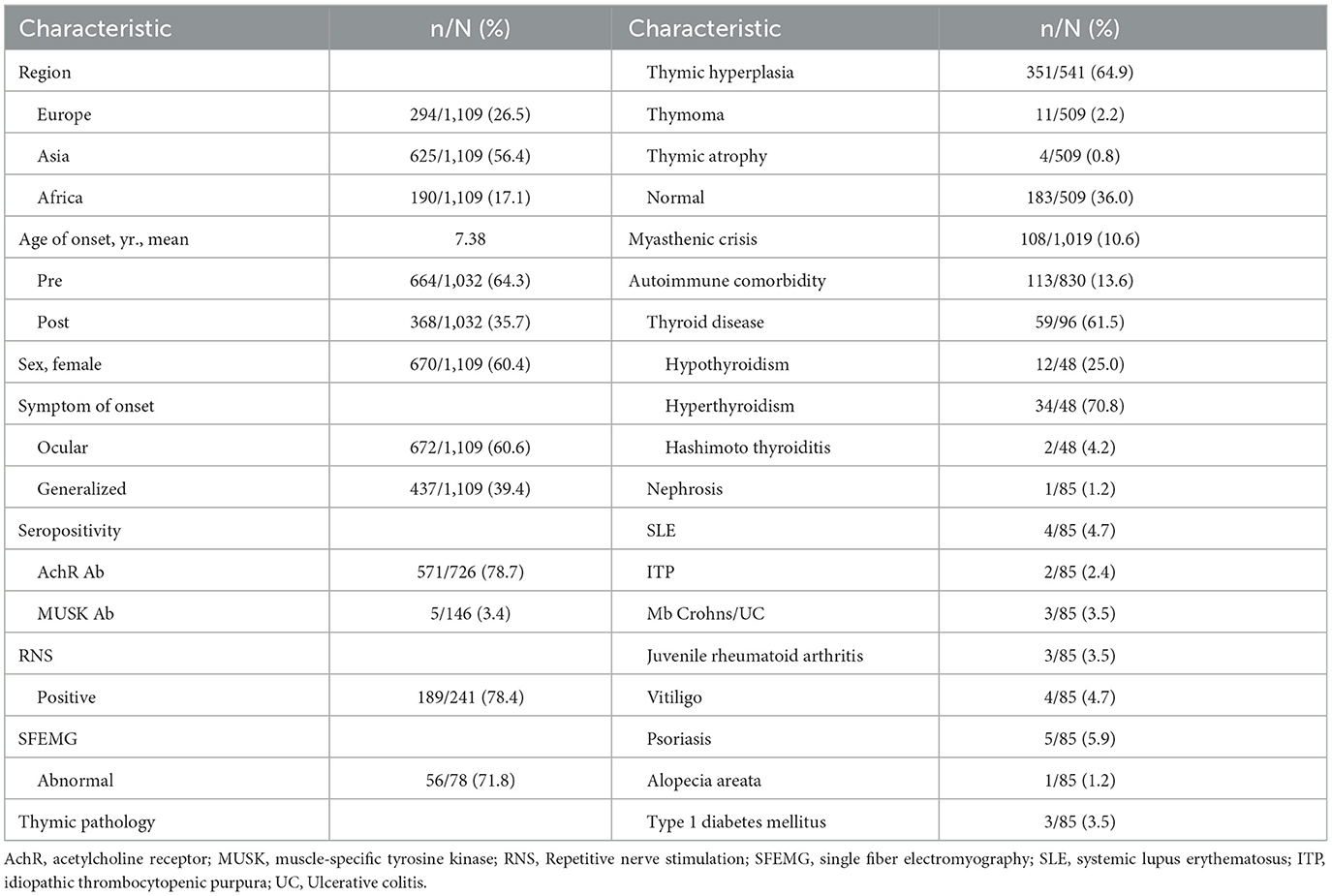
These patients were from three main regions, including Asia (56.4%), Europe (26.5%) and Africa (17.1%). The majority of patients were female (n = 670, 60.4%; 95% CI 55–72%). The mean age at presentation was 7.38 years (ranging from 1 to 216 months). It is reported that 664 (64.3; 95% CI 44–77%) patients had pre-pubertal onset, and 638 patients (35.7%) had post-pubertal onset (Table 2).
Clinical characteristics
Ocular symptoms were the first clinical manifestation of JMG in 672 of 1,109 patients (60.6%; 95% CI 31–82%), including ptosis, diplopia, and strabismus. And 437 of 1,109 patients (39.4%) presented with generalized symptoms at first. The most common initial presentation was ptosis, which occurred in 422 of 543 patients (77.7%; 95%CI 65–85%). At presentation, 64 of 769 patients (8.3%) had limb weakness and 55 of 732 patients (7.5%) had bulbar weakness. Seven of 492 patients (0.1%) developed respiratory muscle weakness.
In these researches, 571 of 726 (78.7%; 95% CI 65–85%) patients with antibody status were AchR-Ab positive. Among 146 cases of muscle-specific kinase (MUSK) antibody test results, 5 cases (3.4%) were positive. RNS test showed a positive decrement in 189 of 241 (78.4%; 95% CI 56–88%) patients. And SFEMG was abnormal in 56 of 78 (71.8%) patients.
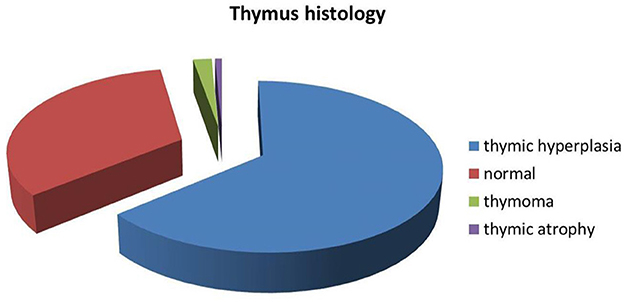
In these researches, 541 patients underwent computed tomography (CT)/magnetic resonance imaging (MRI)/ thymus biopsy to investigate thymic pathology. The most common histopathology was thymic hyperplasia which was detected in 351 of 541 (64.9%; 95% CI 41–76%) patients. Thymic atrophy was the rarest histopathology, found in 4 of 509 (0.8%) patients; 11 of 509 (2.2%; 95% CI 0–3%) patients had thymoma and 183 of 509 (36.0%; 95% CI 19–65%) patients had a normal thymus (Figure 2).
In these researches, 113 of 830 patients (13.6%; 95% CI 8–24%) were found to have other autoimmune diseases; thyroid disease was the most common (n = 59, 61.5%; 95% CI 22–90%). Hyperthyroidism was found in 34 of 48 (70.8%) patients with thyroid disease, while hypothyroidism was found in 12 of 48 (25.0%) patients. And 2 patients had hashimoto thyroiditis. Other autoimmune diseases included nephrosis (1/85), systemic lupus erythematosus (SLE) (4/85), idiopathic thrombocytopenic purpura (ITP) (2/85), Mb Crohns/ Ulcerative colitis (UC) (3/85), juvenile rheumatoid arthritis (3/85), vitiligo (4/85) psoriasis (5/85), alopecia areata (1/85), type 1 diabetes mellitus (3/85) (Figure 3).
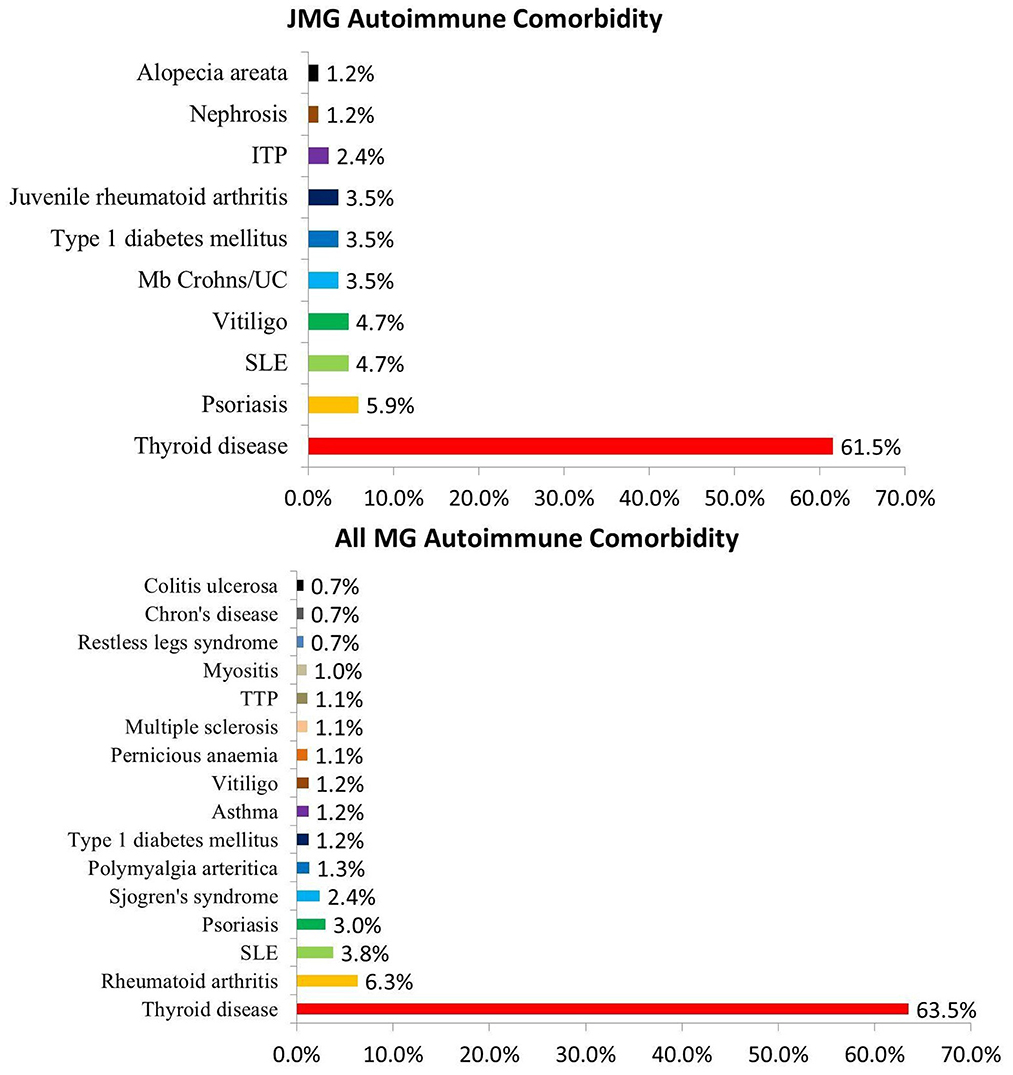
Figure 3. Proportion of autoimmune comorbidity in JMG and MG. Abbreviations: SLE, systemic lupus erythematosus; ITP, idiopathic thrombocytopenic purpura; TTP, Thrombotic thrombocytopenic purpura; UC, Ulcerative colitis.
Treatment and outcome
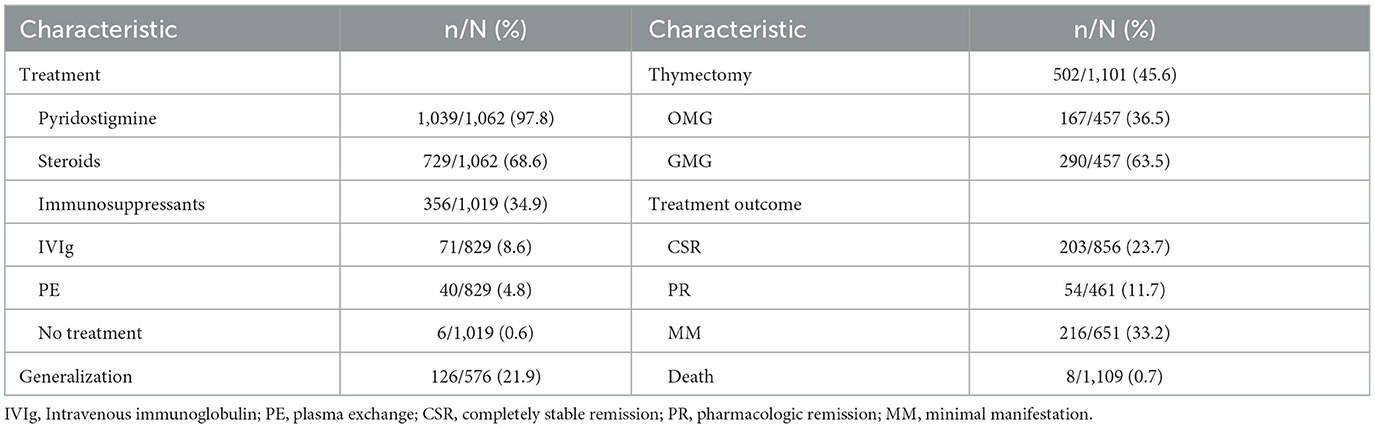
Almost all patients were treated with pyridostigmine (97.8%; 95% CI 97–100%), except for patients in spontaneous remission (n = 6, 0.6%) without any treatment and 17 patients who received other medications first. First-line therapy, consisting of steroids, was initiated in 729 of 1,062 (68.6%; 95% CI 41–74%) patients; and 356 of 1,019 (34.9%; 95% CI 15–38%) patients were treated with other immunosuppressants, including azathioprine, cyclophosphamide, cyclosporine, methotrexate, mycophenolate mofetil, and rituximab. There were 741 patients reported combination treatment (Figure 4). Intravenous immunoglobulin (IVIg) was given to 71 of 829 (8.6%; 95% CI 1–23%) patients during acute exacerbation or crisis, while plasmapheresis (PE) was given to 40 of 829 (4.8%; 95% CI 1–13%) patients. Thymectomy was performed in 502 of 1,101 (45.6%; 95% CI 19–56%) patients. Among these patients, 167 of 457 (36.5%; 95% CI 0–39%) patients were pure ocular MG and 290 of 457 (63.5%; 95% CI 61–100%) patients were generalized MG (Table 3).
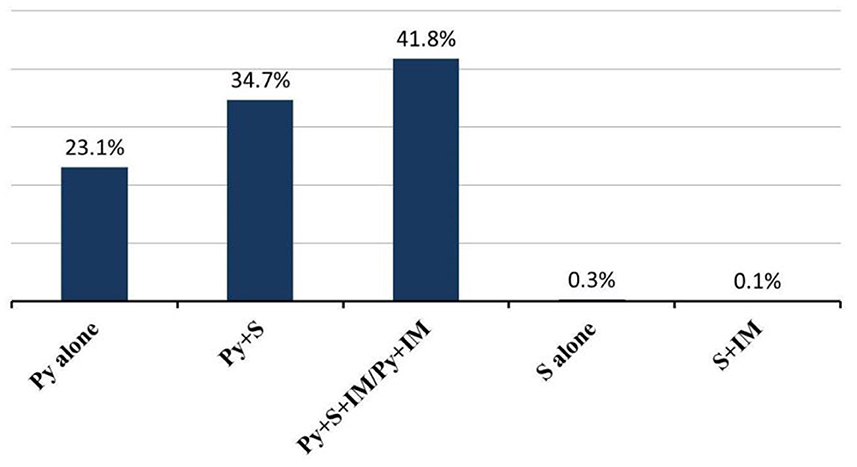
Figure 4. Combination treatment in JMG patients. Abbreviations: Py, pyridostigmine; S, steroids; IM, Immunosuppressants.
Treatment response is shown in Table 3. Mortality was reported in 2 studies, which reported 8 deaths (0.7%). Six of them died from myasthenic crisis, and the other two died from unrelated reasons-−1of HIV and 1 of ischaemic heart disease. Completely stable remission was achieved in 203 of 856 (23.7%; 95% CI 19–33%) patients, pharmacologic remission in 54 of 461 (11.7%) patients, and minimal manifestation in 216 of 651 (33.2%) patients.
In these researches, 108 of 1,019 (10.6%; 95% CI 4–16%) patients had a history of myasthenic crisis, and most of them were treated successfully with PE or IVIg. And there were 6 deaths in the course of the crisis.
126 of 576 (21.9%; 95% CI 14–44%) patients evolved into generalized MG during the follow-up time. Five studies (9–14) reported time to generalization (ranging from 1 month to 14 years), and there were 55 of 65 (84.6%) patients evolved into generalized MG within 2 years.
Discussion
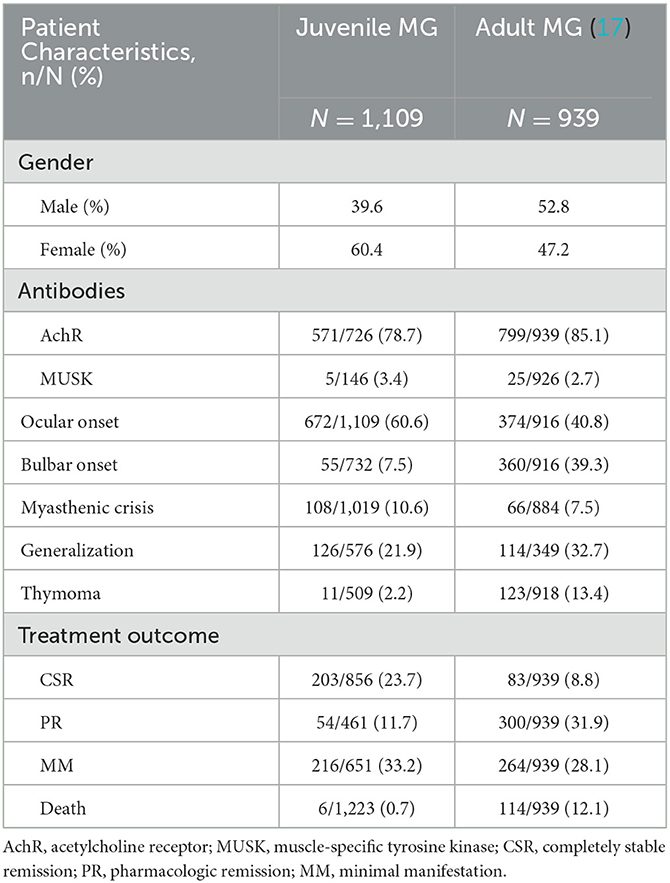
Our data show that juvenile myasthenia gravis is a rare autoimmune disease with more common ocular symptoms, and a relatively benign course, and a better prognosis than the adult disease (17). Although the data were not comprehensive enough, it can still summarize some clinical features of JMG.
In our review, a greater proportion of JMG patients had pre-pubertal onset. And pre-pubertal patients tended to have more ocular presentations (14, 15). Seronegative cases were also seen more frequently among them (8, 11). Generally, pre-pubertal JMG patients responded better to treatment than post-pubertal patients (8).
To better show the differences in clinical features between JMG and adult MG, we made a comparison with a large cohort of adult MG (17) in Table 4.
This review shows a female preponderance in the juvenile MG group, but not in the adult group. The fact is that estrogen reduces the expression of the autoimmune regulator gene in the thymus of young women, resulting in increased release of autoreactive T cells (18, 19). Seropositivity was almost similar to that observed in adults, both AchR and MUSK, similar to other studies (8, 16, 20). MUSK antibody positive is rare in both JMG and adult MG. Ocular symptoms were more common in the JMG group (9), but bulbar symptoms were less. The incidence of myasthenic crisis was similar in the two groups. However, the mortality rate of adult MG was significantly higher than that of JMG. It suggests that the cause of death in myasthenia gravis is not solely related to crisis. Thymoma can also lead to serious complications leading to death (19, 21). And thymoma is less common in JMG than in adults. This is one of the reasons for the low mortality in JMG. Consequently, the number of children achieving completely stable remission was also significantly higher than that of adults (9).
In our review, generalization of symptoms occurred in 21.9% of JMG patients. And the rate is lower than in the adult group, even much lower than in other study, where it reaches 50–80% (22, 23). This may explain the higher prevalence of isolated ocular MG among the juvenile population.
Juvenile MG has a relatively benign course. However, the treatment regimen guideline for children is not well-established. And current practice has been taken from adult guidelines and expert opinion based on individual experience (5, 24, 25). Typically, cholinesterase inhibitors and steroids are used first-line as symptomatic treatment in JMG (4, 26–28). Other immunosuppressants are the second-line therapy for both generalized MG and uncontrolled ocular MG when they failed to respond to anticholinesterase inhibitor therapy (12). Intravenous immunoglobulin (IVIG) and plasma exchange (PE) have also been used as maintenance therapy (29). Compared with the JMG group, adult patients more often received combinations of steroids and other immunosuppressive drugs, also IVIg, (Figure 5) but even so a lower proportion achieved completely stable remission.
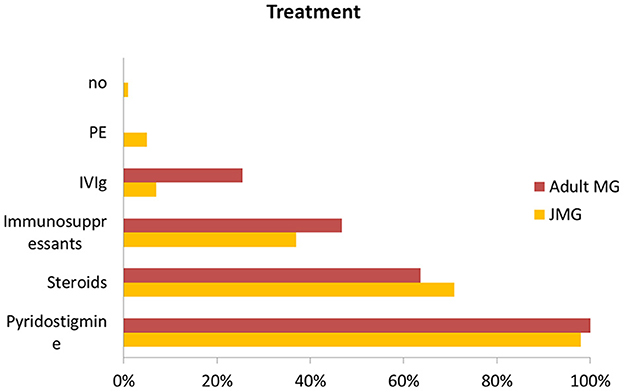
Figure 5. Comparison of treatment with JMG and adult MG. Abbreviations: IVIg, Intravenous immunoglobulin; PE, plasma exchange.
In the JMG group, ocular MG and generalized MG have different prognosis. OMG typically has a higher remission rate than GMG (10, 15), and a lower incidence of myasthenic crisis (12). Most OMG patients could achieve remission with pyridostigmine alone, while GMG patients need combination treatment to achieve remission (12).
Autoimmune comorbidity is a known feature in patients with myasthenia gravis, and also in those with juvenile-onset (13, 30, 31). We also collected some data from other studies of MG patients in combination with other autoimmune diseases, and made statistics in Figure 3 (18, 32–35). 1,863 of 18,337 MG patients (10.2%) were found to have other autoimmune diseases, the incidence similar to the JMG group (13.6%). Thyroid disease was the most common autoimmune comorbidity in MG patients (6), also in the JMG group. There was no clear trend toward clustering in other autoimmune diseases (Figure 3).
Thymectomy is an important treatment option for adults with myasthenia gravis when medical therapy is refractory, but remains controversial in the juvenile myasthenia gravis population. Although several recent studies have shown the benefits of thymectomy in JMG patients (6, 36, 37), they are all retrospective and have limitations. And it was reported that some patients presented a crisis after thymectomy, requiring hospitalization and ventilatory support (38).
In our review, 41.8% of JMG patients performed thymectomy, and 36.5% of them were pure ocular MG. Despite the lack of prospective studies evaluating thymectomy in JMG, it is generally accepted that thymectomy is considered as part of the initial management of JMG patients with abnormal thymus pathology, such as thymoma and thymic hyperplasia. Meanwhile, thymectomy can also perform in AchR-Ab positive generalized JMG patients and JMG patients who do not respond well to medication (3). There is still a lack of large prospective cohort studies of JMG patients to standardize the use of thymectomy for JMG.
Conclusion
Our systematic review and meta-analysis found that JMG has some distinctive clinical features, such as better treatment outcome and lower generalization compared with adult. Nevertheless, it also has limitations. All studies were retrospective, introducing selection bias. JMG is a rare disease, and evidence-based guidelines are lacking. To standardize treatment guidelines, future prospective multicenter studies are needed for the best treatment outcome.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
QJ and YL designed the study. YL and QK conducted the literature search, collected, analyzed, interpreted the data, and drafted the first manuscript. HL, BL, and JL interpretation of data and revision of manuscript for intellectual content. QJ analysis and interpretation of data and revision of the manuscript for intellectual content. All authors have read and approved the final version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (81904133).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1119294/full#supplementary-material
References
1. Lacomis D. What's in the neuromuscular junction literature? J Clin Neuromuscul Dis. (2020) 21:195–204. doi: 10.1097/CND.0000000000000285
2. Castro D, Derisavifard S, Anderson M, Greene M, Iannaccone S. Juvenile myasthenia gravis: a twenty-year experience. J Clin Neuromuscul Dis. (2013) 14:95–102. doi: 10.1097/CND.0b013e318253a48e
3. O'Connell K, Ramdas S, Palace J. Management of juvenile myasthenia gravis. Front Neurol. (2020) 11:743. doi: 10.3389/fneur.2020.00743
4. Munot P, Robb SA, Niks EH, Palace J, Ews group. 242nd ENMC international workshop: diagnosis and management of juvenile myasthenia gravis Hoofddorp, the Netherlands, 1–3 March 2019. Neuromuscul Disord. (2020) 30:254–64. doi: 10.1016/j.nmd.2020.02.001
5. Liew WK, Kang PB. Update on juvenile myasthenia gravis. Curr Opin Pediatr. (2013) 25:694–700. doi: 10.1097/MOP.0b013e328365ad16
6. Popperud TH, Gul KA, Brunborg C, Olaussen RW, Abrahamsen TG, Osnes LT, et al. Thymectomy in juvenile myasthenia gravis is safe regarding long term immunological effects. Front Neurol. (2021) 12:596859. doi: 10.3389/fneur.2021.596859
7. Asenova A, Bojinova V. Juvenile myasthenia gravis - clinical course and serologic tests. Comptes Rendus De L Academie Bulgare Des Sciences. (2020) 73:1759–66. doi: 10.7546/CRABS.2020.12.17
8. Jastrzebska A, Jastrzebski M, Ryniewicz B, Kostera-Pruszczyk A. Treatment outcome in juvenile-onset myasthenia gravis. Muscle Nerve. (2019) 59:549–54. doi: 10.1002/mus.26445
9. Vanikieti K, Lowwongngam K, Padungkiatsagul T, Visudtibhan A, Poonyathalang A. Juvenile ocular myasthenia gravis: presentation and outcome of a large cohort. Pediatr Neurol. (2018) 87:36–41. doi: 10.1016/j.pediatrneurol.2018.06.007
10. Huang X, Li Y, Feng H, Chen P, Liu W. Clinical characteristics of juvenile myasthenia gravis in southern China. Front Neurol. (2018) 9:77. doi: 10.3389/fneur.2018.00077
11. Barraud C, Desguerre I, Barnerias C, Gitiaux C, Boulay C, Chabrol B, et al. Clinical features and evolution of juvenile myasthenia gravis in a French cohort. Muscle Nerve. (2018) 57:603–9. doi: 10.1002/mus.25965
12. Wejwittayaklung B, Likasitwattanakul S, Sanmaneechai O. Juvenile myasthenia gravis: a longitudinal study from Thailand. Southeast Asian J Trop Med Public Health. (2017) 48:225–31.
13. Popperud TH, Boldingh MI, Rasmussen M, Kerty E. Juvenile myasthenia gravis in Norway: Clinical characteristics, treatment, and long-term outcome in a nationwide population-based cohort. Eur J Paediatr Neurol. (2017) 21:707–14. doi: 10.1016/j.ejpn.2017.04.003
14. Lee HN, Kang HC, Lee JS, Kim HD, Shin HY, Kim SM, et al. Juvenile myasthenia gravis in Korea: subgroup analysis according to sex and onset age. J Child Neurol. (2016) 31:1561–8. doi: 10.1177/0883073816666206
15. Heckmann JM, Hansen P, van Toorn R, Lubbe E, van Rensburg EJ, Wilmshurst J, et al. The characteristics of juvenile myasthenia gravis among South Africans. Samj South Afr Med J. (2012) 102:532–6. doi: 10.7196/SAMJ.5390
16. Ashraf VV, Taly AB, Veerendrakumar M, Rao S. Myasthenia gravis in children: a longitudinal study. Acta Neurol Scand. (2006) 114:119–23. doi: 10.1111/j.1600-0404.2006.00646.x
17. Cortes-Vicente E, Alvarez-Velasco R, Segovia S, Paradas C, Casasnovas C, Guerrero-Sola A, et al. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology. (2020) 94:e1171–80. doi: 10.1212/WNL.0000000000008903
18. Dragin N, Bismuth J, Cizeron-Clairac G, Biferi MG, Berthault C, Serraf A, et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest. (2016) 126:1525–37. doi: 10.1172/JCI81894
19. Punga AR, Maddison P, Heckmann JM, Guptill JT, Evoli A. Epidemiology, diagnostics, and biomarkers of autoimmune neuromuscular junction disorders. Lancet Neurol. (2022) 21:176–88. doi: 10.1016/S1474-4422(21)00297-0
20. Yan C, Zhao R, Song J, Feng X, Xi J, Luo S, et al. Comparison of anti-acetylcholine receptor profiles between Chinese cases of adult- and juvenile-onset myasthenia gravis using cell-based assays. J Neuroimmunol. (2020) 349:577403. doi: 10.1016/j.jneuroim.2020.577403
21. Verwijst J, Westerberg E, Punga AR. Cancer in myasthenia gravis subtypes in relation to immunosuppressive treatment and acetylcholine receptor antibodies: a Swedish nationwide register study. Eur J Neurol. (2021) 28:1706–15. doi: 10.1111/ene.14730
22. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. (2008) 37:141–9. doi: 10.1002/mus.20950
23. Elrod RD, Weinberg DA. Ocular myasthenia gravis. Ophthalmol Clin North Am. (2004) 17:275–309. doi: 10.1016/j.ohc.2004.05.014
24. Evoli A. Acquired myasthenia gravis in childhood. Curr Opin Neurol. (2010) 23:536–40. doi: 10.1097/WCO.0b013e32833c32af
25. Heckmann JM, Owen EP, Little F. Myasthenia gravis in South Africans: racial differences in clinical manifestations. Neuromuscul Disord. (2007) 17:929–34. doi: 10.1016/j.nmd.2007.07.002
26. Finnis MF, Jayawant S. Juvenile myasthenia gravis: a paediatric perspective. Autoimmune Dis. (2011) 2011:404101. doi: 10.4061/2011/404101
27. Chiang LM, Darras BT, Kang PB. Juvenile myasthenia gravis. Muscle Nerve. (2009) 39:423–31. doi: 10.1002/mus.21195
28. Fisher K, Shah V. Pediatric ocular myasthenia gravis. Curr Treat Options Neurol. (2019) 21:46. doi: 10.1007/s11940-019-0593-y
29. Liew WK, Powell CA, Sloan SR, Shamberger RC, Weldon CB, Darras BT, et al. Comparison of plasmapheresis and intravenous immunoglobulin as maintenance therapies for juvenile myasthenia gravis. JAMA Neurol. (2014) 71:575–80. doi: 10.1001/jamaneurol.2014.17
30. Gui M, Luo X, Lin J, Li Y, Zhang M, Zhang X, et al. Long-term outcome of 424 childhood-onset myasthenia gravis patients. J Neurol. (2015) 262:823–30. doi: 10.1007/s00415-015-7638-2
31. Fang F, Sveinsson O, Thormar G, Granqvist M, Askling J, Lundberg IE, et al. The autoimmune spectrum of myasthenia gravis: a Swedish population-based study. J Intern Med. (2015) 277:594–604. doi: 10.1111/joim.12310
32. Green JD, Barohn RJ, Bartoccion E, Benatar M, Blackmore D, Chaudhry V, et al. Epidemiological evidence for a hereditary contribution to myasthenia gravis: a retrospective cohort study of patients from North America. BMJ Open. (2020) 10:e037909. doi: 10.1136/bmjopen-2020-037909
33. Chou CC, Huang MH, Lan WC, Kong SS, Kuo CF, Chou IJ, et al. Prevalence and risk of thyroid diseases in myasthenia gravis. Acta Neurol Scand. (2020) 142:239–47. doi: 10.1111/ane.13254
34. Lin YP, Iqbal U, Nguyen PA, Islam MM, Atique S, Jian WS, et al. The concomitant association of thyroid disorders and myasthenia gravis. Transl Neurosci. (2017) 8:27–30. doi: 10.1515/tnsci-2017-0006
35. Chen YL, Yeh JH, Chiu HC. Clinical features of myasthenia gravis patients with autoimmune thyroid disease in Taiwan. Acta Neurol Scand. (2013) 127:170–4. doi: 10.1111/j.1600-0404.2012.01693.x
36. Ng WC, Hartley L. Effectiveness of thymectomy in juvenile myasthenia gravis and clinical characteristics associated with better outcomes. Neuromuscul Disord. (2021). doi: 10.1016/j.nmd.2021.09.013
37. Madenci AL, Li GZ, Weil BR, Zurakowski D, Kang PB, Weldon CB, et al. The role of thymectomy in the treatment of juvenile myasthenia gravis: a systematic review. Pediatr Surg Int. (2017) 33:683–94. doi: 10.1007/s00383-017-4086-3
Keywords: juvenile myasthenia gravis, clinical features, treatment outcome, autoimmune comorbidity, systematic review, meta-analysis
Citation: Lin Y, Kuang Q, Li H, Liang B, Lu J, Jiang Q and Yang X (2023) Outcome and clinical features in juvenile myasthenia gravis: A systematic review and meta-analysis. Front. Neurol. 14:1119294. doi: 10.3389/fneur.2023.1119294
Received: 08 December 2022; Accepted: 17 February 2023;
Published: 08 March 2023.
Edited by:
Giuseppe Vita, University of Messina, ItalyReviewed by:
Maria Pia Giannoccaro, University of Bologna, ItalyTrine Haug Popperud, Oslo University Hospital, Norway
Diana Castro, Neurology Rare Disease Center, United States
Copyright © 2023 Lin, Kuang, Li, Liang, Lu, Jiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qilong Jiang, jiangqilong5119@gzucm.edu.cn; Xiaojun Yang, yangxiaojun@gzucm.edu.cn
 Yangtao Lin
Yangtao Lin Qianjin Kuang
Qianjin Kuang Bo Liang
Bo Liang