- 1University of Colorado School of Medicine, Aurora, CO, United States
- 2Department of Pediatrics, Section of Neurology, University of Colorado School of Medicine, Children's Hospital Colorado, Aurora, CO, United States
- 3Department of Pediatrics, University of Colorado School of Medicine, Children's Hospital Colorado, Aurora, CO, United States
- 4Division of Epilepsy and Clinical Neurophysiology, Department of Neurology, Boston Children's Hospital, Boston, MA, United States
- 5Program in Neuroimmunology, Department of Neurology, Boston Children's Hospital, Boston, MA, United States
- 6Department of Child Neurology, Weill Cornell Medicine, New York-Presbyterian Hospital, New York, NY, United States
- 7Department of Pediatrics and Child Neurology (Co-appointment), Baylor College of Medicine, Houston, TX, United States
Background: Febrile-infection related epilepsy syndrome (FIRES) is a rare epilepsy syndrome in which a previously healthy individual develops refractory status epilepticus in the setting of a preceding febrile illness. There are limited data regarding detailed long-term outcomes. This study aims to describe the long-term neuropsychological outcomes in a series of pediatric patients with FIRES.
Methods: This is a retrospective multi-center case series of pediatric patients with a diagnosis of FIRES treated acutely with anakinra who had neuropsychological testing at least 12 months after status epilepticus onset. Each patient underwent comprehensive neuropsychological evaluation as part of routine clinical care. Additional data collection included the acute seizure presentation, medication exposures, and outcomes.
Results: There were six patients identified with a median age of 11.08 years (IQR: 8.19–11.23) at status epilepticus onset. Anakinra initiation was a median of 11 days (IQR: 9.25–13.50) after hospital admission. All patients had ongoing seizures and none of the patients returned to baseline cognitive function with a median follow-up of 40 months (IQR 35–51). Of the five patients with serial full-scale IQ testing, three demonstrated a decline in scores over time. Testing results revealed a diffuse pattern of deficits across domains and all patients required special education and/or accommodations for academic learning.
Conclusions: Despite treatment with anakinra, neuropsychological outcomes in this series of pediatric patients with FIRES demonstrated ongoing diffuse neurocognitive impairment. Future research will need to explore the predictors of long-term neurocognitive outcomes in patients with FIRES and to evaluate if acute treatment interventions improve these outcomes.
Introduction
New-onset refractory status epilepticus (NORSE) is a clinical presentation in which previously healthy individuals develop refractory status epilepticus without a clear structural, toxic, or metabolic cause. Febrile infection-related epilepsy syndrome (FIRES) is a subset of NORSE that is preceded by febrile illness 24 hours to 2 weeks prior to the onset of status-epilepticus (1). Children with FIRES will present after a febrile illness with new seizures that rapidly progress to refractory or super refractory status epilepticus that can last for several weeks despite treatment with at least two intravenous antiseizure medications and continuous infusions (2, 3). Limited published literature to date suggests that of patients who survive the acute phase, nearly all have ongoing drug-resistant epilepsy with the majority not returning to prior baseline function (4, 5).
No etiology is identified in the majority of FIRES cases, but there is growing evidence that there may be an immune-mediated process following an initial infection (6). A preceding febrile illness can trigger intrathecal overproduction and release of proinflammatory cytokines, which activates mechanisms of innate immunity in the central nervous system (7). This immune activation increases neuronal excitability, leading to epileptogenesis (8). Status epilepticus itself can trigger proinflammatory processes and neuroinflammation which further promotes neuronal hyperexcitability and triggers ongoing seizures (9, 10). This hypothesized immune activation has led to the use of immune therapies such as steroids, intravenous immunoglobulin (IVIG), and plasma exchange in the treatment of FIRES, though typically with low response rates (11). One particular proinflammatory cytokine, interleukin-1 beta (IL-1β) has been implicated in experimental models of status epilepticus (12). Anakinra is an interleukin-1 receptor antagonist (IL-1Ra) used in FIRES that has been shown to reduce seizures in the acute phase (5, 6, 13) and is recommended for consideration in the treatment of patients with FIRES (14, 15).
Published data regarding the long-term neurocognitive outcomes in patients with FIRES is limited. Despite reports of acute benefit with anakinra, there are no prior studies describing long-term cognitive outcomes in this population. This study aims to describe the long-term neuropsychological and seizure outcomes in a series of pediatric patients with FIRES treated with anakinra.
Materials and methods
This is a multicenter retrospective case series of six patients with a diagnosis of FIRES treated with anakinra. Treating physicians identified patients with FIRES onset between December 2015 and December 2019. Inclusion criteria included patients < 18 years old with a diagnosis of FIRES treated with anakinra in the acute hospital admission with available neuropsychological testing at least one year after refractory status epilepticus onset. Study data were collected and managed using REDCap electronic data capture tools hosted at University of Colorado School of Medicine (16). Data collected included past medical and family history, lab and imaging results during the acute hospital course, as well as seizure frequency, antiseizure medications and other treatments (i.e., neuro-modulatory therapy) during the acute hospital course, and at each follow-up neuropsychological testing time point. Neuropsychological testing was completed as part of routine clinical care across three academic medical centers. Testing measures varied by institution, age of the patient at the time of assessment, and ability to complete measures. For analysis, tests were grouped into neuropsychological domains, and scores described between and within categories. The Colorado multiple institutional review board (COMIRB) approved this study.
Descriptive data analysis included frequencies and percentages for categorical variables. Continuous variables are reported in median and interquartile ranges. A pre-determined sample size was not calculated due to descriptive nature of study and small number of available patients.
Results
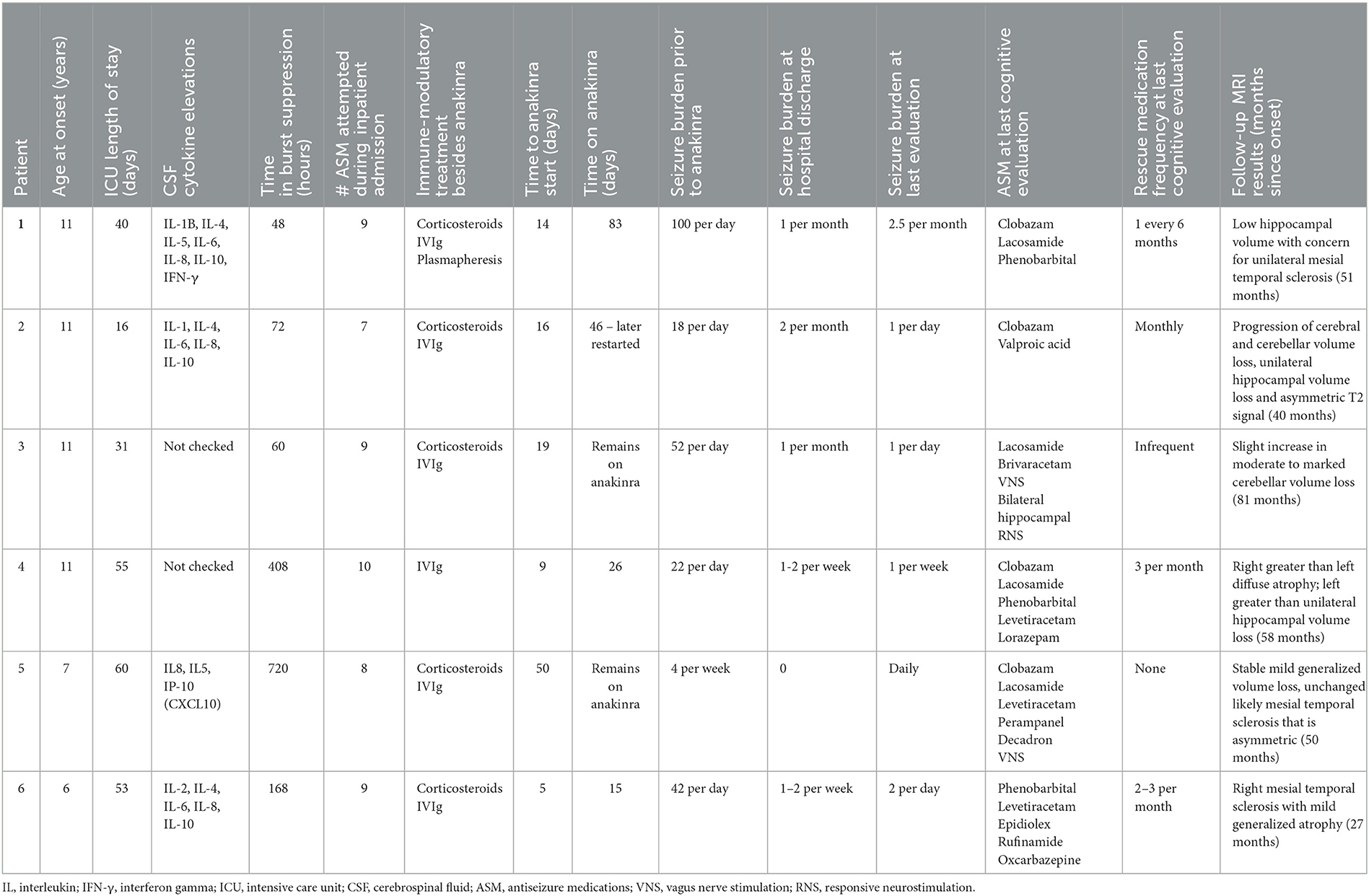
There were six patients identified with a median age of 11.08 years (Interquartile range (IQR) 8.19–11.23) at status epilepticus onset. All patients met the diagnostic criteria for FIRES with a preceding fever starting at least 24 hours prior to status epilepticus onset. Initial brain MRI was normal in three patients and non-specific inflammation such as T2/FLAIR hyperintensities occurred in three patients. Completion of genetic testing occurred in five patients and was negative or non-diagnostic in all these children. All patients had autoimmune antibody testing completed in cerebrospinal fluid (CSF), as well as in serum in five patients. This was negative in all patients except for one with a low-titer serum glutamic acid decarboxylase 65 (GAD65) (0.09 nmol/L; normal < 0.02 nmol/L) only after treatment with IVIg. Four children had CSF cytokine testing, which were all elevated (Table 1). During the acute phase, all patients received treatment with pentobarbital and obtained burst-suppression on EEG. The median duration of burst suppression was 120 hours (IQR 63–348). All patients received treatment with intravenous immunoglobulins (IVIg) and five patients received corticosteroids. These treatments were prior to anakinra in four patients and concurrent with anakinra in two. The median time to initiation of anakinra was 11.0 days (IQR 9.25–13.50). One patient received treatment with tocilizumab following anakinra; this case was previously published and is included in this series to report additional long-term outcome data (17). Five patients received treatment with phenobarbital during the acute period. There were ongoing seizures at last follow-up for all patients with variable frequency ranging from multiple per day to monthly. All patients continued to take a median of 3.5 (IQR 2–5) daily antiseizure medications at the time of their last neuropsychological evaluation. Two patients also received treatment with neuromodulation (vagus nerve stimulation or responsive neurostimulation). Brain MRI results in the long-term follow-up phase revealed a combination of hippocampal volume loss, including mesial temporal sclerosis, and / or diffuse cerebral or cerebellar volume loss (Table 1). Additional information regarding specific cytokine testing results, duration of treatment with anakinra, antiseizure medication exposure, and seizure outcomes are available in Table 1.
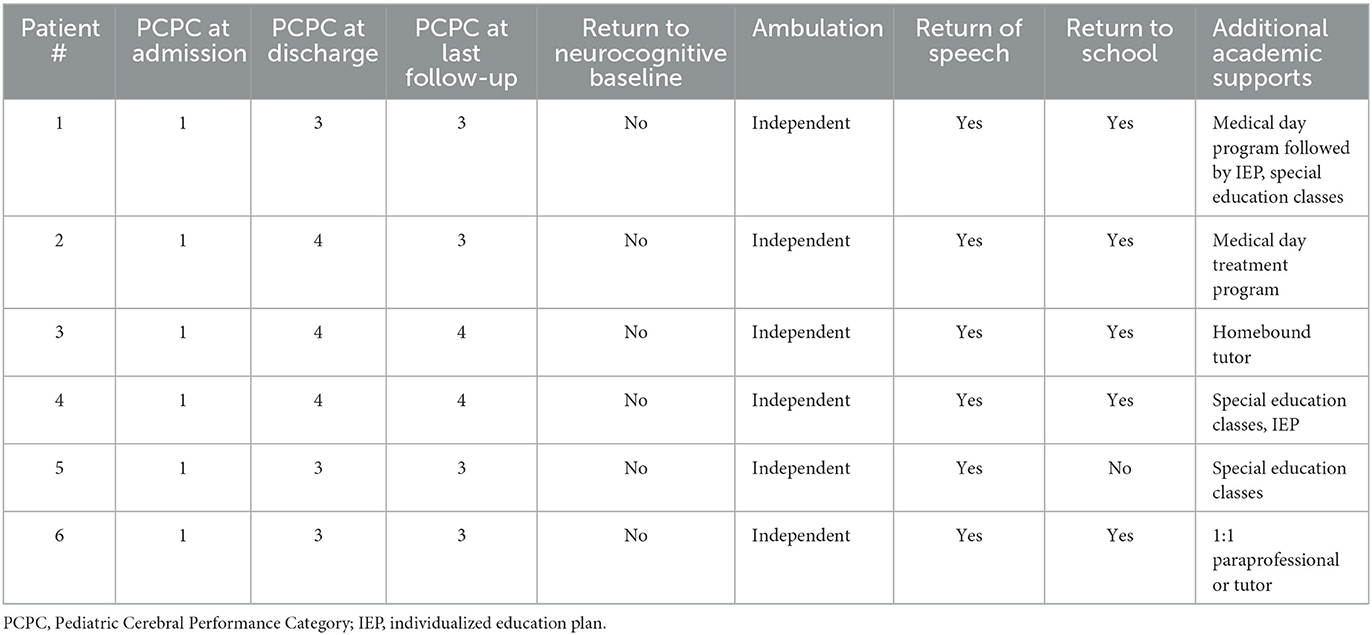
Patients had serial neuropsychological testing repeated between 1 and 4 times after status epilepticus onset with a median follow-up of 40 months (IQR 35–51 months). The testing measures completed for each patient during follow-up are available in Supplementary Table 1. None of the six patients returned to baseline functioning after onset of FIRES, according to physician report and pediatric cerebral performance category (PCPC). PCPC was normal for all patients prior to FIRES onset with all patients demonstrating decline in function to a score of moderate to severe disability at last follow-up (Table 2). There was one patient (patient 2) who had improvement in PCPC score over time with a change from the category of severe disability to moderate disability. This patient also had an improvement in full-scale IQ score from initial testing (Figure 1). Full-scale IQ testing for one patient (patient 5) demonstrated average IQ scores, although there was a decline in scores between follow-up time points. The remainder of patients demonstrated IQ scores that were between the ranges of low average to extremely low. Of the five patients with serial IQ testing, three patients demonstrated a decline in full-scale IQ scores over the follow-up period.
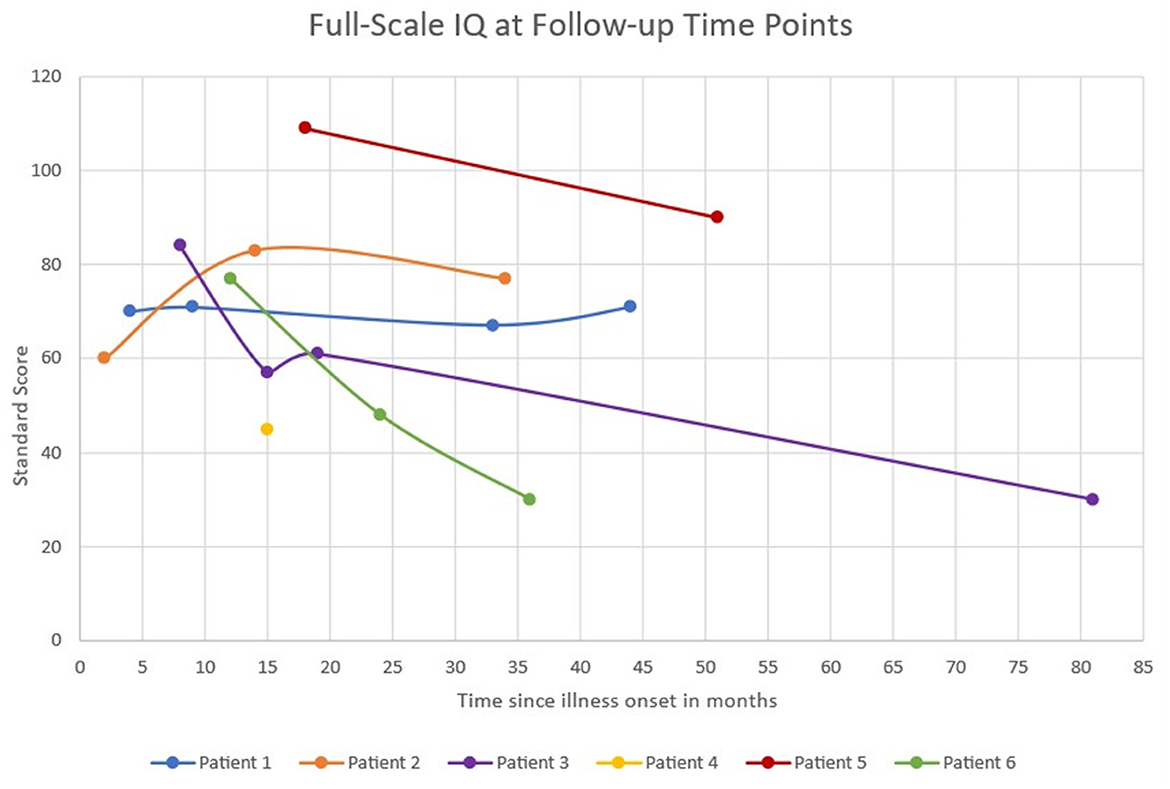
Figure 1. Full-scale IQ scores at serial follow-up time points. Full-scale IQ score categories: Average 90–109, Low average 80–89, Borderline 70–79, Extremely low <69.
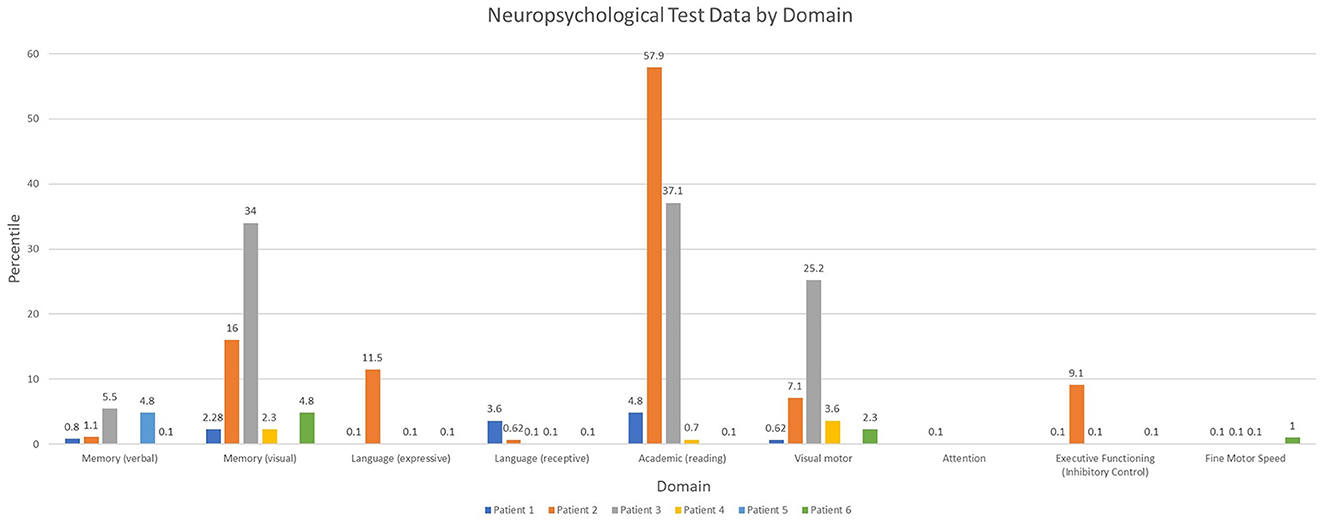
Overall, patients demonstrated a diffuse pattern of deficits across domains at the last follow-up time point (Figure 2). Deficits included verbal and non-verbal reasoning and visual spatial abilities, as well as memory deficits. Language weaknesses encompassed both receptive and expressive language impairment. Additionally, visual motor integration, fine motor speed and dexterity were each below age expectations, and weaknesses occurred across varying aspects of attention and executive functioning. Reading skills ranged from extremely low to average. Longitudinal testing demonstrated preservation of these skills over time, without evidence for decline or loss of skills. While longitudinal data was not available for other areas, there was a slight trend for lower math calculation skills when compared to reading. Spelling was more variable.
Parent, and in some cases, teacher report questionnaires (ABAS-3, BASC-3, and Vineland-3) were suggestive of concerns for symptoms of executive functioning deficits, psychosocial difficulties and overall adaptive functioning weaknesses in at least two patients. Parent or teacher BASC-3 questionnaires were completed in three children and suggestive of clinically significant hyperactivity. In two children, there was also indication of internalizing symptoms, including increased anxiety, somatization, or depression. At last follow-up, all patients continued to present with neuropsychological impairments and required special education services/interventions and/or accommodations for academic learning (Table 2).
Discussion
In this series of pediatric patients with FIRES treated acutely with anakinra, neuropsychological testing does not suggest a pattern of specific neurocognitive deficits, but rather a global decline in functioning. There is evidence that some patients continue to have further neurocognitive decline over time, although some patients demonstrate stable neuropsychological scores or improvement. All patients required educational support and interventions during the long-term follow-up period.
These findings are concordant with data supporting that super-refractory status epilepticus causes worse neurological outcomes and lower rates of return to baseline cognitive function than non-super-refractory status epilepticus (18). One study reported that 58% of patients with NORSE had neurocognitive deficits, of which 30% were severe (19). This is consistent with the current case series in which all patients demonstrate neurocognitive deficits including two patients classified as severe based on PCPC category. All patients in this series had return of speech and independent ambulation although no patients returned to their neurocognitive baseline, and all had some degree of disability at follow-up.
In general, patients had full-scale IQs below normal, and three patients showed a decline in full-scale IQ during period of long-term follow-up. This aligns with a previous study in which all patients with devasting epileptic encephalopathy in school-aged children (DESC) with prolonged status epilepticus had IQs below normal at follow-up, and serial follow-up neurocognitive testing showed progressive decline of intellectual functioning (19). At follow-up in another series of children with FIRES only 18% of survivors returned to normal function at last follow-up, although this classification also included the presence of learning disabilities (4). Poor cognitive outcomes were associated with younger age at FIRES onset and longer duration of induced burst suppression coma (4). The current case series reflects this with the youngest patients at FIRES onset exhibiting the second and third largest declines in IQ over the follow-up period with one of these patients also spending the longest time in burst suppression. However, this case series is not powered to statistically make comparisons between exposures. Three patients demonstrated ongoing decline in serial full-scale IQ over time. This decline may be multifactorial and related not only to initial FIRES presentation with refractory status epilepticus but also partly due to ongoing seizure activity in the chronic phase, medication effect, and factors related to the underlying etiology and pathophysiology of FIRES that need further exploration. However, it is also important to note the variability in serial full-scale IQ testing in this case series with one patient improving over time. This suggests that recovery of neurocognitive function can occur for some patients and highlights the importance of rehabilitation programs to maximize this potential.
When further evaluating domains of neuropsychological function this case series identified concern in all domains including verbal and non-verbal memory and visual spatial skills, as well as language weaknesses with very low receptive and expressive language scores and reduced fine motor speed and dexterity. Concordant with the present findings, neuropsychological testing in children with convulsive status epilepticus show decreased memory scores compared to a healthy control group (20). Though this study did not compare FIRES patients to a control group, all patients in this study had a verbal and visual memory score at last follow-up that fell in the bottom two quartiles. Additionally, there are prior reports of FIRES patients presenting with impairments in word finding, fluency, knowledge of words, and semantic comprehension (4, 19, 21).
Attention and executive functioning are also greatly impaired in patients with a diagnosis of FIRES. Executive function deficits are also seen in patients with drug-resistant frontal and temporal epilepsy and reduced impulse-inhibition in patients with temporal lobe epilepsy (22). These findings and those in the present case series may be in part due to ongoing drug-resistant epilepsy, localization of seizure onset, and cerebral dysfunction in the chronic phase of FIRES with multiple daily antiseizure medications.
Academic achievement was among the best performing domains for patients in this case series. This aligns with reports of higher scores for specific academic skills (such as arithmetic, spelling, reading recognition, and reading comprehension) relative to academic performance in patients with traumatic brain injury. Cognitive and behavioral deficits that impact performance may mediate this discrepancy (23).
Social and emotional functioning results were variable on parent report suggesting concerns for internalizing (attention, anxiety, depression) or externalizing (hyperactivity, aggression, conduct problems) for some patients although not all. In a prior study of children with DESC, all patients presented with emotional and behavioral disorders that included fits of anger, aggressiveness, agitation, apathy, and withdrawal behaviors (19). Another study of patients with convulsive status epilepticus found that approximately one-third of patients scored above the clinical cut-off on at least one behavioral scale (24). It is therefore reasonable to consider that these behavioral impairments may influence reports of academic performance in relation to academic skills. It is important to evaluate and address behavior and social-emotional functioning in the clinical care of patient with FIRES.
Most patients in this case series returned to some form of education but all had special education needs. This is reflected in the literature with prior reports of FIRES needing special education classes (19) and half of children with convulsive status epilepticus having special educational needs (21).
Overall, the described changes in cognitive function have multiple proposed explanations including the location of seizures, initial refractory status epilepticus, ongoing drug-resistant epilepsy, as well as antiseizure medication exposure in the acute and chronic phase of FIRES treatment. Additional causative considerations include the impact of the underlying pathophysiology of FIRES and status epilepticus. Seizures in children with FIRES often have a focal onset in both temporal-perisylvian areas of the brain, then spread to the frontal lobes (9, 25). PET scans show hypometabolism in the temporoparietal and orbitofrontal cortices (25). Damage in these areas is consistent with cognitive deficits found in FIRES, most commonly language, memory, behavior, and frontal lobe function. Follow-up brain imaging in this case series demonstrates diffuse atrophy and mesial temporal sclerosis or hippocampal volume consistent with prior reports in the chronic phase (26). These findings may additively contribute to cognitive impairments and future research evaluating serial magnetic resonance imaging (MRI) of the brain with long-term neurocognitive outcomes is warranted.
Antiseizure medication themselves can also contribute to cognitive and behavioral deficits. During the acute and chronic phases of FIRES patients receive treatment with multiple antiseizure medications as shown in this cohort with all patients receiving treatment with at least two antiseizure medications at the time of their last follow-up testing. Specifically high dose phenobarbital has been proposed in the acute treatment of FIRES (14) and in our cohort five patients received this treatment acutely with two patients remaining on phenobarbital at the last follow-up neuropsychological testing visit. Phenobarbital has been associated with an IQ that is on average 8.4 points lower than placebo in children with febrile seizures, as well as reduced performance on IQ when compared to valproic acid (27–29). There are additional antiseizure medications that can also be associated with cognitive and behavioral changes, such as topiramate or levetiracetam (29, 30). Overall, the cognitive and behavioral side effects of antiseizure medications may explain some of the cognitive deficits seen in this study population, although antiseizure medications are unlikely to account for the full extent of decline compared to baseline function prior to onset of FIRES.
One limitation of this study is small sample size, as FIRES is a rare pediatric condition, and only patients treated with anakinra were included. Additionally, because this was a multicenter, retrospective study, neuropsychological testing could not be standardized across all sites and patients, so patients received various neuropsychological tests at various timepoints. In some cases, alternative testing procedures were utilized as a result of the patient's cognitive limitations (e.g., use of WNV instead of WISC-V or WASI-II in a patient that presented with severe language deficits). While there was often no direct comparison of scores between or within patients, percentiles could be compared within the same category of neuropsychological tests. This allowed for an adequate understanding of patients' neurocognitive functioning at last follow-up compared to their peers. However, standardized serial monitoring of neuropsychological outcomes would be beneficial as part of routine follow-up care for patients with FIRES.
Future prospective studies of patients with FIRES in which standardized neuropsychological assessments are completed at set follow-up points would allow for a more direct analysis of change in neurocognitive function over time. This should be correlated with detailed neuroimaging and electrographic data to better understand these outcomes over time. It would also be helpful to include a comparative control group of patients not treated with anakinra to better determine any potential benefits of anakinra on neuropsychological outcomes. Ongoing multicenter collaboration and family engagement are encouraged to meet these objectives.
Conclusions
In summary, long-term follow-up of FIRES patients treated with anakinra demonstrates significant neurocognitive impairment across all neuropsychological testing domains with variable stability of scores over time. This reflects and builds upon neuropsychological data available in the literature. Future research needs to better understand the predictors of long-term neurocognitive outcomes and influence of acute treatment interventions. There is a need for standardized long-term serial neuropsychological assessments for all patients with FIRES and NORSE. It is recommended that this be incorporated into a multidisciplinary approach including not only neurologists and epilepsy specialists, as well as rehabilitation, neuropsychology, and mental health team members to support maximal neurocognitive recovery and support.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Colorado Multiple Institutional Review Board (COMIRB). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
EW, GB-S, EM, and KE contributed to the conception and design of the study. EW and KE developed the database. CS, KB, CV, SN, EW, and KE completed data collection. AS, EW, GB-S, and KE performed data review and analysis. EW and AS wrote the initial drafts of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
Funding for the submission and open-access publication fees were made possible by the family and friends of Finn Mussetter. The REDCap data tools were supported institutionally by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. The contents of the manuscript are the authors' sole responsibility and do not necessarily represent official NIH views.
Acknowledgments
Thank you also to Jan Martin and Craig Press who also cared for the patients from Children's Hospital Colorado included in the case series. Thank you also to H. Allison Bender ABPP-CN and Jessica Spat-Lemus who assisted with the care and neuropsychological testing at Weill Cornell Medicine/New York Presbyterian Hospital.
Conflict of interest
EM serves on the advisory board for Sobi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1100551/full#supplementary-material
References
1. Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. (2018) 59:739–44. doi: 10.1111/epi.14016
2. Pichler M, Hocker S, Hocker S, Wijdicks EFM, Kramer AH. Management of status epilepticus. Handb Clin Neurol. (2017) 3:140. doi: 10.1016/B978-0-444-63600-3.00009-X
3. Farias-Moeller R, Bartolini L, Staso K, Schreiber JM, Carpenter JL. Early ictal and interictal patterns in FIRES: The sparks before the blaze. Epilepsia. (2017) 58:1340–8. doi: 10.1111/epi.13801
4. Kramer U, Chi YS, Lin ZL, Specchio X, Sahin M, Olson H, et al. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome a multicenter study on 77 children. Epilepsia. (2011) 52:1956–65. doi: 10.1111/j.1528-1167.2011.03250.x
5. Lai YC, Muscal E, Wells E, Shukla N, Eschbach K, Lee KH, et al. Anakinra usage in febrile infection related epilepsy syndrome: an international cohort. Ann Clin Transl Neurol. (2020) 7:12. doi: 10.1002/acn3.51229
6. Clarkson BDS, Lafrance-Corey RG, Kahoud RJ, Farias-Moeller R, Payne ET, Howe CL. Functional deficiency in endogenous interleukin-1 receptor antagonist in patients with febrile infection-related epilepsy syndrome. Ann Neurol. (2019). 85:526–37. Available online at: https://onlinelibrary.wiley.com/doi/10.1002/ana.25439
7. Sakuma H, Tanuma N, Kuki I, Takahashi Y, Shiomi M, Hayashi M. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection-related refractory status epilepticus. J Neurol Neurosurg Psychiatry. (2015) 86:820–2. doi: 10.1136/jnnp-2014-309388
8. Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS. The cortical innate immune response increases local neuronal excitability leading to seizures. J Neurol. (2009) 2478–86. doi: 10.1093/brain/awp177
9. Nabbout R, Vezzani A, Dulac O, Chiron C. Personal view acute encephalopathy with infl ammation-mediated status epilepticus. Lancet Neurol. (2011) 10:99–108. doi: 10.1016/S1474-4422(10)70214-3
10. van Baalen A, Vezzani A, Häusler M, Kluger G. febrile infection-related epilepsy syndrome: clinical review and hypotheses of epileptogenesis. Neuropediatrics. (2017) 48:5–18. doi: 10.1055/s-0036-1597271
11. Gaspard N, Hirsch LJ. Sculier | Claudine, Loddenkemper T, van Baalen A, Lancrenon J, et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): State of the art and perspectives. Epilepsia. (2018) 59:745–52. doi: 10.1111/epi.14022
12. Varvel NH, Neher JJ, Bosch A, Wang W, Ransohoff RM, Miller RJ, et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. PNAS. (2016) E5665–74. doi: 10.1073/pnas.1604263113
13. Kenney-Jung DL, Vezzani A, Kahoud RJ, Lafrance-Corey RG, Ho ML, Muskardin TW, et al. Febrile infection-related epilepsy syndrome treated with anakinra. Annals Neurol. (2016) 80:939–45.
14. Koh S, Wirrell E, Vezzani A, Nabbout R, Muscal E, Kaliakatsos M, et al. Proposal to optimize evaluation and treatment of Febrile infection-related epilepsy syndrome (FIRES): a Report from FIRES workshop. (2020) 3:12447. doi: 10.1002/epi4.12447
15. Wickstrom R, Taraschenko O. Dilena | Robertino, Payne ET, Specchio N, Nabbout R, et al. International consensus recommendations for management of new onset refractory status epilepticus including febrile infection-related epilepsy syndrome: statements and supporting evidence. Epilepsia. (2022) 01:1–25.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
17. Stredny CM, Case S, Sansevere AJ, Son M, Henderson L, Gorman MP. Interleukin-6 blockade with tocilizumab in anakinra-refractory febrile infection-related epilepsy syndrome. FIRES. (2020) 7:2329048X2097925. doi: 10.1177/2329048X20979253
18. Vasquez A, Farias-Moeller R, Sánchez-Fernández I, Abend NS, Amengual-Gual M, Anderson A, et al. Super-refractory status epilepticus in children: s retrospective cohort study. Pediatric Critical Care Medicine. (2021) 22:E613–25. doi: 10.1097/PCC.0000000000002786
19. Mikaeloff Y, Jambaqué I, Hertz-Pannier L, Zamfirescu A, Adamsbaum C, Plouin P, et al. Devastating epileptic encephalopathy in school-aged children (DESC): s pseudo encephalitis. Epilepsy Res. (2006) 69:67–79. doi: 10.1016/j.eplepsyres.2006.01.002
20. Martinos MM, Pujar S, O'Reilly H, de Haan M, Neville BGR, Scott RC, et al. Intelligence and memory outcomes within 10 years of childhood convulsive status epilepticus. Epilepsy and Behavior. (2019) 95:18–25. doi: 10.1016/j.yebeh.2019.03.039
21. Pujar S, Scott RC. Long-term outcomes after childhood convulsive status epilepticus. Curr Opin Pediatr. (2019) 31:763–8. doi: 10.1097/MOP.0000000000000825
22. Esteso Orduña B. Fournier del Castillo M de la C, Cámara Barrio S, García Fernández M, Andrés Esteban EM, Álvarez-Linera Prado J, et al. Cognitive and behavioral profiles of pediatric surgical candidates with frontal and temporal lobe epilepsy. Epilepsy Behav. (2021) 117:107808. doi: 10.1016/j.yebeh.2021.107808
23. Ewing-Cobbs L, Barnes M, Fletcher JM, Levin HS, Swank PR, Song J. Modeling of longitudinal academic achievement scores after pediatric traumatic brain injury. Dev Neuropsychol. (2004) 25:107–33. doi: 10.1207/s15326942dn2501&2_7
24. Martinos MM, Pujar S, Gillberg C, Cortina-Borja M, Neville BGR, de Haan M, et al. Long-term behavioural outcomes after paediatric convulsive status epilepticus: a population-based cohort study. Dev Med Child Neurol. (2018) 60:409–16. doi: 10.1111/dmcn.13636
25. Mazzuca M, Jambaque I, Hertz-Pannier L, Bouilleret V, Archambaud F, Caviness V, et al. 8 F-FDG PET reveals frontotemporal dysfunction in children with fever-induced refractory epileptic encephalopathy. J Nucl Med. (2011) 52:40–7. doi: 10.2967/jnumed.110.077214
26. Culleton S, Talenti G, Kaliakatsos M, Pujar S, D'Arco F. The spectrum of neuroimaging findings in febrile infection-related epilepsy syndrome (FIRES): a literature review. Epilepsia. (2019) 60:585–92. doi: 10.1111/epi.14684
27. Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures—Effects on intelligence and seizure recurrence. N Engl J Med. (1990) 322:364–9. doi: 10.1056/NEJM199002083220604
28. Bourgeois BFD. Determining the effects of antiepileptic drugs on cognitive function in pediatric patients with epilepsy. J Child Neurol. (2004) 19:S15–24. doi: 10.1177/088307380401900103
29. Brandt C, Lahr D, May TW. Cognitive adverse events of topiramate in patients with epilepsy and intellectual disability. Epilepsy and Behavior. (2015) 45:261–4. doi: 10.1016/j.yebeh.2014.12.043
Keywords: new-onset refractory status epilepticus, NORSE, febrile infection-related epilepsy syndrome, FIRES, neuropsychological outcomes, cognitive outcomes, anakinra
Citation: Shrestha A, Wood EL, Berrios-Siervo G, Stredny CM, Boyer K, Vega C, Nangia S, Muscal E and Eschbach K (2023) Long-term neuropsychological outcomes in children with febrile infection-related epilepsy syndrome (FIRES) treated with anakinra. Front. Neurol. 14:1100551. doi: 10.3389/fneur.2023.1100551
Received: 16 November 2022; Accepted: 13 February 2023;
Published: 08 March 2023.
Edited by:
Nicolas Gaspard, Université Libre de Bruxelles, BelgiumReviewed by:
Sara Matricardi, University of Chieti, ItalyMarissa Kellogg, Oregon Health and Science University, United States
Copyright © 2023 Shrestha, Wood, Berrios-Siervo, Stredny, Boyer, Vega, Nangia, Muscal and Eschbach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krista Eschbach, krista.eschbach@childrenscolorado.org
†These authors share first authorship
 Anima Shrestha
Anima Shrestha E. Lynne Wood
E. Lynne Wood Gretchen Berrios-Siervo2,3
Gretchen Berrios-Siervo2,3 Coral M. Stredny
Coral M. Stredny Eyal Muscal
Eyal Muscal Krista Eschbach
Krista Eschbach