- 1Center of Cerebrovascular Diseases, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Rehabilitation Medicine Center, West China Hospital, Sichuan University, Chengdu, China
- 3The Second Department of Neurology, Shanxi Provincial People's Hospital, Xi'an, China
Background: Neutrophils and albumin are associated with outcomes in patients with acute ischemic stroke (AIS). We aimed to explore the association between the neutrophil percentage-to-albumin ratio (NPAR), a novel marker of inflammation and oxidative stress, and the 3-month functional outcome in AIS patients with reperfusion therapy.
Methods: This single-center, retrospective cohort study consecutively enrolled AIS patients with reperfusion therapy. Neutrophils and albumin were collected on admission. The primary outcome was a poor functional outcome, which was defined as a modified Rankin scale score of 3–6 at 3 months.
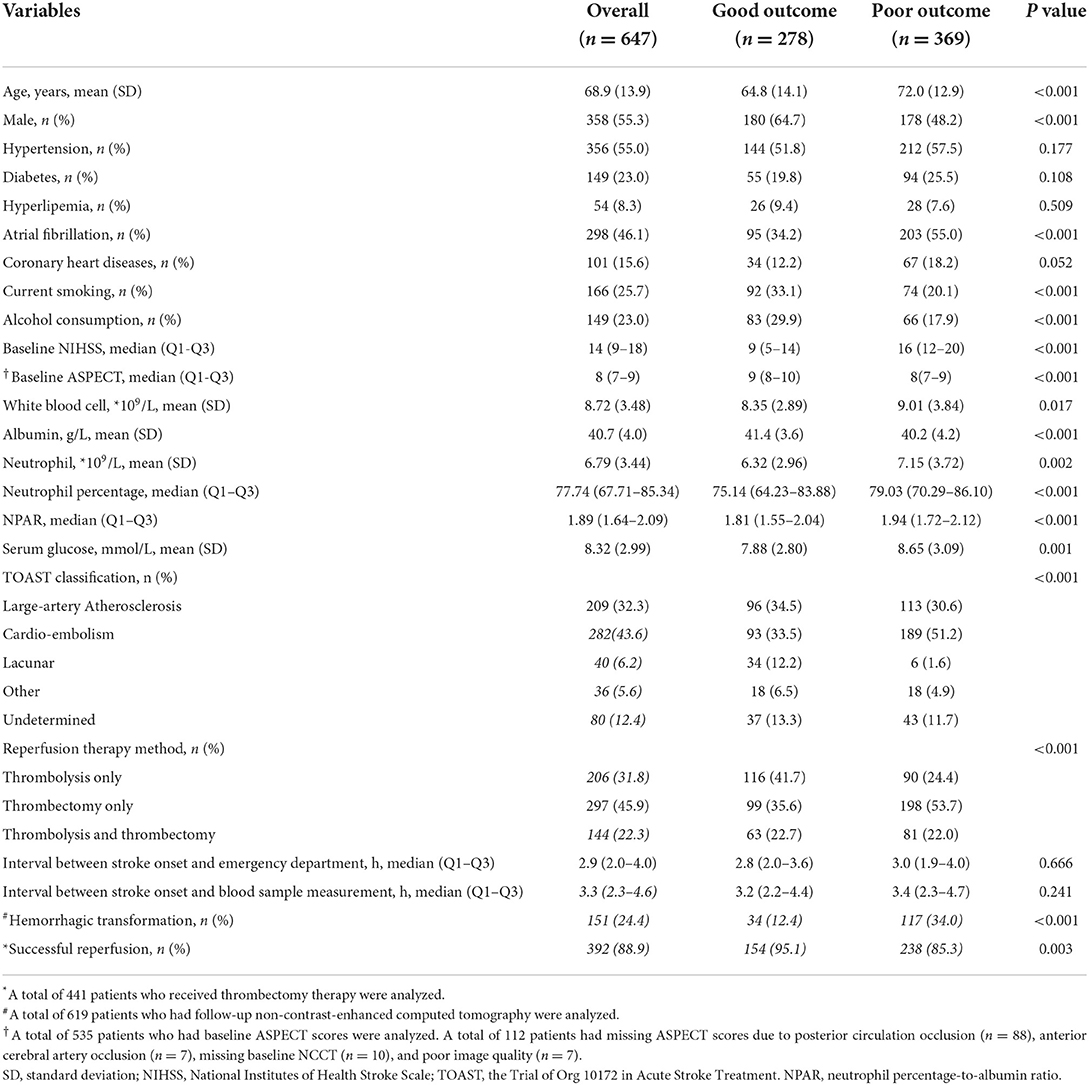
Results: A total of 647 patients with AIS who received reperfusion therapy were analyzed. The mean age was 68.9 ± 13.9 years, and 358 (55.3%) of the patients were men. The median NPAR was 1.89 (interquartile range [IQR] 1.64–2.09). The percentage of patients with a 3-month poor functional outcome was 57.0% (369/647). NPAR was positively associated with a poor functional outcome (odds ratio [OR] 2.76, 95% CI: 1.52–5.03, p = 0.001). When patients were classified into tertiles, patients in the upper tertile (2.03–7.59) had a higher risk of poor outcome than patients in the lower tertile after adjusting for potential confounders (0.78–1.73) (OR 2.10, 95% CI: 1.28–3.42, p = 0.003). The risk of poor outcome increased with NPAR tertiles (p-trend = 0.003). The optimal cut-off value of the NPAR for predicting a poor outcome was 1.72, with a sensitivity of 0.75, and a specificity of 0.43.
Conclusion: Neutrophil percentage-to-albumin ratio was significantly associated with 3-month poor functional outcomes in patients with AIS who received reperfusion therapy.
Introduction
Reperfusion therapy has become the standard of care for patients with acute ischemic stroke (AIS) (1). However, approximately half of the patients with AIS suffer poor clinical outcomes after reperfusion therapy (2, 3). Inflammation and oxidative stress are two critical variables influencing the prognosis of patients with AIS after reperfusion therapy (4).
During the acute phase of AIS, neutrophils are the earliest inflammatory cells that are abundantly present in cerebral microvessels, and their subsequent release of reactive oxygen species (ROS) is thought to be the main cause of reperfusion injury after AIS (5–9). Some studies found that serum albumin played a key role in scavenging ROS (10, 11) and might exert an anti-inflammatory effect by inhibiting neutrophil spreading (12, 13). Neutrophils and albumin are associated with outcomes in patients with AIS (14–18).
The neutrophil percentage-to-albumin ratio (NPAR) is an emerging marker of inflammation and oxidative stress. The NPAR has been reported to have prognostic significance in patients with cancer, spinal cord injury, acute kidney injury, acute myocardial infarction, and cardiogenic shock (19–27). Recently, a retrospective study explored the association between NPAR and infection in patients with AIS (28). However, there is uncertainty regarding the association between NPAR and 3-month functional outcomes in AIS patients with reperfusion therapy. We hypothesized that NPAR may reflect the severity of inflammation and ROS damage in the acute phase of AIS. We sought to assess the association between NPAR and patient outcomes after reperfusion therapy.
Materials and methods
Patients
This retrospective cohort study consecutively enrolled patients with AIS admitted to West China Hospital between 1 January 2018 and 31 December 2020. Patients who received reperfusion therapies, such as intravenous thrombolysis and/or mechanical or thrombus aspiration thrombectomy (or both), were included. The exclusion criteria were as follows: (1) without measuring neutrophil or albumin within 24 h of stroke onset. (2) Patients with severe liver damage (alanine aminotransferase [ALT] ≥150 IU/L and/or aspartate aminotransferase [AST] ≥120 IU/L for men; alanine aminotransferase [ALT] ≥120 IU/L and/or aspartate aminotransferase [AST] ≥105 IU/L for women). (3) Patients with severe kidney damage (estimated glomerular filtration rate [eGFR] ≤15 ml/min/1.73 m2) (29). (4) Patients who failed to follow-up. This study was approved by the Scientific Research Department of West China Hospital. We obtained oral informed consent from each patient or their relative.
Data collection
The variables collected included demographics (age and gender), medical histories (hypertension, atrial fibrillation, diabetes, hyperlipidemia, and coronary heart diseases), the National Institute of Health Stroke Scale (NIHSS) score, the Alberta Stroke Program Early CT Score (ASPECTS), hemorrhagic transformation (30), reperfusion therapy method, and the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification (31). Successful recanalization after endovascular therapy was defined as a modified Thrombolysis in Cerebral Infarction grading system score of 2b or 3 (32). Blood samples were collected for laboratory measurements in the emergency department. White blood cell (WBC) counts, including absolute numbers of neutrophils were measured using an automated hematology analyzer (Sysmex, Kobe, Japan). Serum albumin levels were determined with an Olympus AU-5400 automatic analyzer (Olympus, Tokyo, Japan).
Outcome
Follow-up for all included patients was conducted by telephone at 3 months to evaluate their functional outcome. A 3-month modified Rankin scale score of ≥3 was regarded as a poor functional outcome (33).
Statistical analysis
Means ± standard deviations (SDs) or medians (interquartile range [IQR]) were used to describe the quantitative variables as appropriate. Qualitative variables were reported as numbers and percentages. Student's t-test or the Mann–Whitney U-test for continuous variables and the χ2 test or Fisher's exact test for categorical data were used to conduct descriptive analyses of baseline characteristics and 3-month functional outcomes as appropriate. Variables within p < 0.10 in univariable analysis were defined as potential confounders. Multivariable logistic regression analysis was performed to explore the association between the NPAR and poor outcomes. Trends for the odds ratios (ORs) of poor outcome across NPAR tertiles (p-trend) were tested by entering the median value of NPAR in each tertile as a continuous variable (34). A receiver operating characteristic (ROC) curve was calculated to assess the diagnostic value of the NPAR in predicting outcome. The discrimination of NPAR was evaluated by the area under the ROC curve (AUC). The optimum NPAR cutoff point was determined by the Youden index. In addition, we compared the AUC of the NPAR with that of albumin, neutrophil percentage, and neutrophil-to-lymphocyte ratio in predicting a 3-month poor functional outcome (the Delong method) (35).
All analyses were performed using IBM SPSS Statistics (25.0; IBM, Armonk, NY, USA), R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria), and MedCalc 15.2.2 (MedCalc Software bvba, Ostend, West Flanders, Belgium). A two-sided p value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
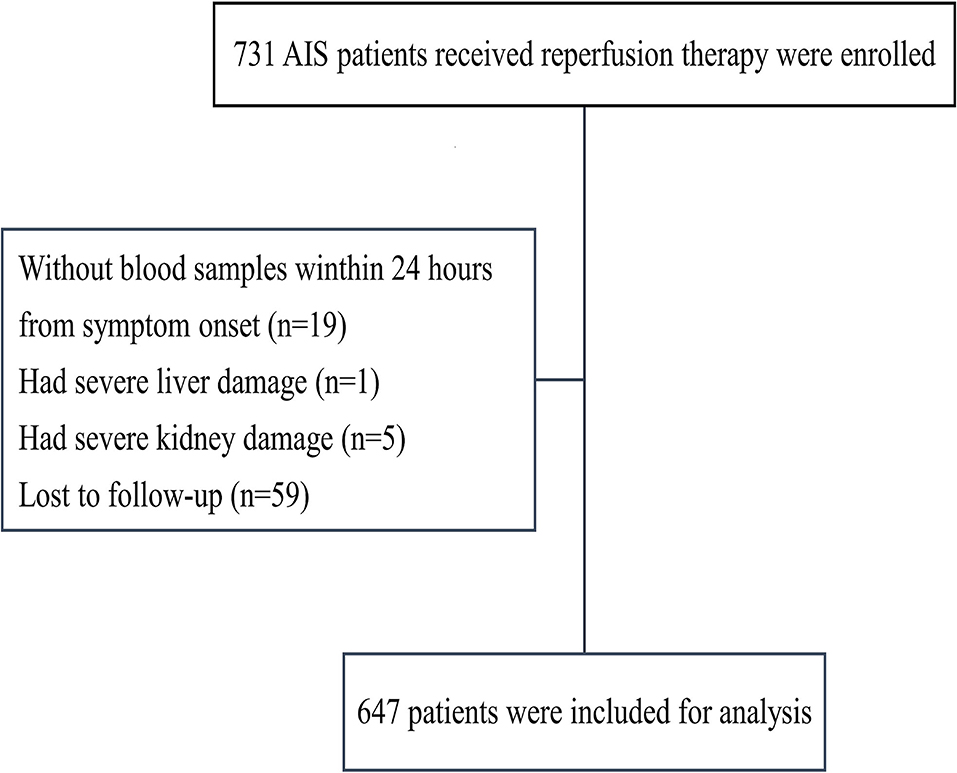
We enrolled 731 patients with reperfusion therapy; 19 patients were excluded because they did not have blood samples within 24 h of symptom onset; 6 patients had severe liver or kidney damage; and 59 patients were lost to follow-up (Figure 1). In total, 647 patients were included in this study.
As shown in Table 1, the mean age was 68.9 ± 13.9 years and 358 (55.3%) patients were men. The median NPAR was 1.89 (IQR 1.64–2.09). The median interval between stroke onset and blood sample measurement was 3.3 h (IQR 2.3–4.6 h). Approximately 32.0% (206/647) of patients received alteplase, 46% (297/647) of patients received endovascular therapy, and 22% (144/647) of patients received alteplase combined with endovascular therapy. The percentage of patients with a 3-month poor functional outcome was 57.0% (369/647). The baseline characteristics of patients stratified by the reperfusion therapy method are shown in Supplementary Table S1.
Association between NPAR and poor outcome
The univariable analysis showed that age, gender, baseline NIHSS score, atrial fibrillation, diabetes, coronary heart diseases, current smoking, drinking consumption, TOAST classification, serum glucose, reperfusion therapy method, and interval between stroke onset and blood sample measurement were significant confounders (P < 0.10, Table 2).
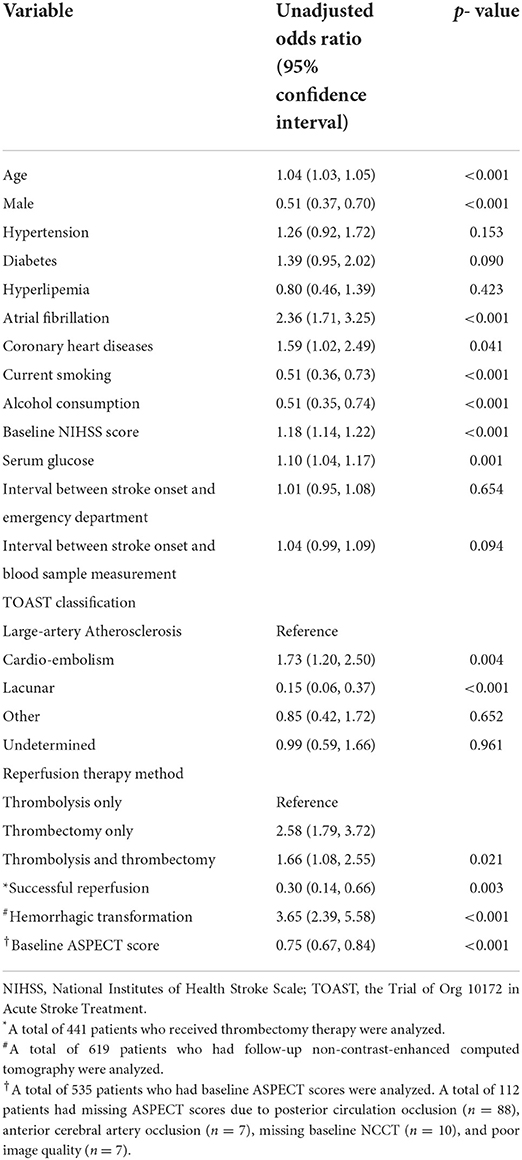
Table 2. Univariable logistic regression analysis of variables associated with poor 3-month outcome.
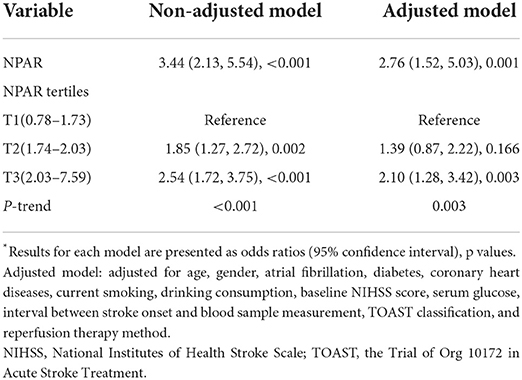
In univariable analysis, the NPAR was significantly associated with a 3-month poor functional outcome when it was treated as a continuous variable (OR 3.44, 95% CI: 2.13–5.54, p < 0.001, Table 3). After multivariable adjustment, the association between NPAR and poor outcome remained significant (OR 2.76, 95% CI: 1.52–5.03, p = 0.001).
When the NPAR was classified into tertiles, the OR of poor outcome was 1.85 for T2 and 2.54 for T3, when compared with T1 without adjustment. After multivariable adjustment, T3 still had a significant higher risk of poor outcome than T1 (OR 2.10, 95% CI: 1.28–3.42, p = 0.003), while T2 was no longer significantly different from T1 (OR 1.39, 95% CI: 0.87–2.22, p = 0.166). The risk of poor outcome significantly increased stepwise across NPAR tertiles (p-trend = 0.003).
The other four models were constructed to further explore the association between NPAR and outcome. In models 2, 3, and 4, after further adjustment for the ASPECT score, hemorrhagic transformation, and reperfusion status, respectively, the association between NPAR and outcome remained significant. In model 5, in addition to the variables in model 1, we further adjusted for the ASPECT score, hemorrhagic transformation, and reperfusion status together, and the association remained significant (OR 2.72, 95% CI: 1.19–6.26, p = 0.018, Supplementary Table S2).
Predictive value of NPAR
The ROC analysis suggested that the optimal cut-off value of the NPAR for predicting the 3-month poor functional outcome was 1.72, with a sensitivity of 0.75 and a specificity of 0.43 (Figure 2). Comparing the predictive value with other biomarkers, NPAR showed the highest AUC value [0.614 (0.575–0.652)] compared with albumin [0.578 (0.539–0.617)], neutrophil percentage [0.588 (0.549–0.626)], and neutrophil-to-lymphocyte ratio [0.588 (0.549–0.626)], but there was no significant difference between NPAR and albumin (p = 0.144).
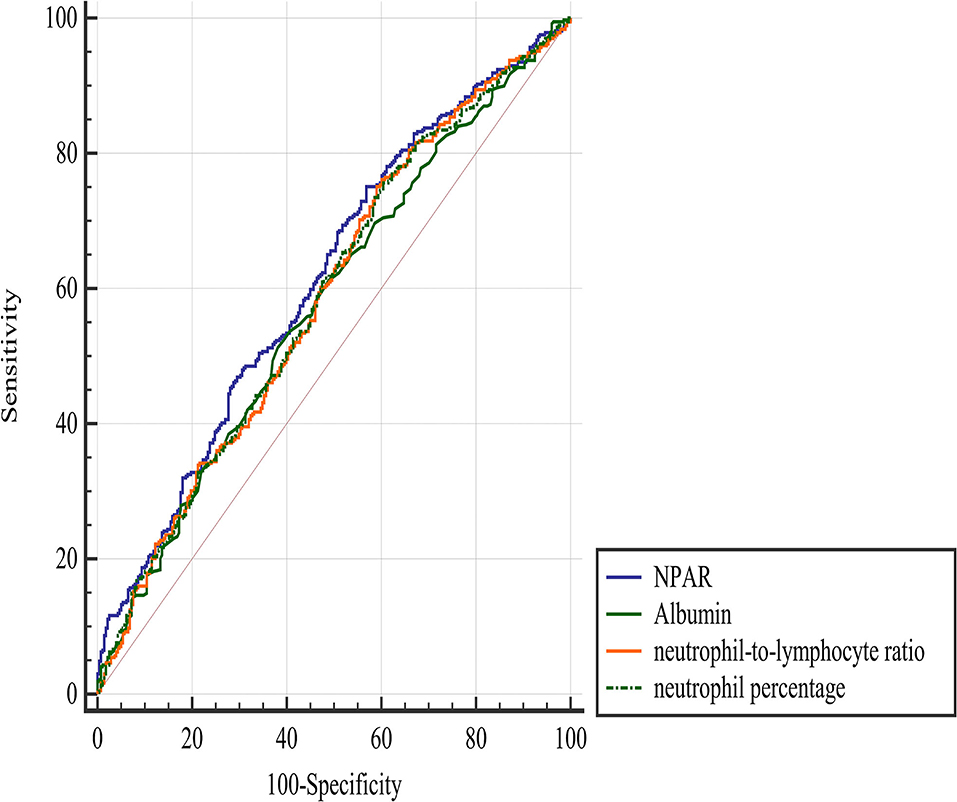
Figure 2. Comparison of predictive value between NPAR and other parameters in the prediction of a 3-month poor functional outcome. NPAR, neutrophil percentage-to-albumin ratio.
Discussion
In this retrospective observational study, we investigated the association between NPAR and 3-month functional outcome in patients with AIS who were treated with reperfusion therapies. We found that NPAR was positively associated with poor outcomes. The optimal cutoff value of the NPAR for predicting a poor outcome was 1.72 with a sensitivity of 0.75 and a specificity of 0.43. The predictive value of NPAR was significantly higher than that of neutrophil percentage and neutrophil-to-lymphocyte ratio but not albumin.
Post-ischemic inflammation and oxidative stress play essential roles in the response to brain ischemia-reperfusion injury in patients with AIS after reperfusion therapy (36). As markers of inflammation and oxidative stress response, neutrophils and albumin have been found to be associated with outcomes in AIS patients with reperfusion therapy (14–18). NPAR, which combines neutrophils with albumin, is accessible in daily clinical practice. Monitoring NPAR may help clinicians detect patients at high risk of poor outcome. However, the association between NPAR and outcome in AIS patients with reperfusion therapies remains unclear. Recently, a retrospective study found that NPAR could predict the occurrence of stroke-associated infection, but it did not focus on patients with reperfusion therapy and did not have data on the short-term outcome of patients with AIS (28). In the current study, we found that NPAR was positively associated with the poor functional outcome, and that the predictive value of NPAR for outcome was significantly higher than that of other conventional biomarkers, for example, the neutrophil-to-lymphocyte ratio, in these patients.
The underlying mechanism of the association between NPAR and outcome remains unclear and could be explained as follows: on one hand, during the acute phase of AIS, numerous pro-inflammatory cytokines and damage-associated molecular patterns (DAMPs) are released that promote neutrophil recruitment and activation, which in turn promote the release of ROS and ultimately result in poor functional outcome (5–9). On the other hand, it has been found that albumin may help limit the production of ROS and further scavenge ROS (10, 12). In addition, some studies found that albumin can also exert an anti-inflammatory effect by inhibiting neutrophil spreading (12, 13).
Our study has some limitations. First, this was a single-center retrospective study, which could lead to selection bias. Second, the interval between symptom onset and admission measurement of blood samples might lead to bias. However, we adjusted for this variable in multivariable analysis, and the result was significant. Third, regarding AUC, the cut-off value of its sensitivity and specificity was fairly low, and the clinical significance of NPAR may be limited. Fourth, although we adjusted for potential confounders using multivariate models, neutrophils may be influenced by infectious diseases, hematological or rheumatic disorders, and a history of long-term immunosuppressant drug use. Due to the retrospective study design, we could not adjust for these variables in our study. Fifth, we could not explore the association between NPAR and early neurological deterioration in this study due to limited data. Finally, previous studies have found that GFAP and S100B correlated with stroke severity and outcome (37, 38). However, due to the retrospective study design, we could not provide data on these prognostic biomarkers in our center, therefore, we could not compare the advantages and disadvantages between NPAR and these biological markers.
Conclusions
Neutrophil percentage-to-albumin ratio was independently associated with a 3-month poor functional outcome in AIS patients with reperfusion therapy.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by the Scientific Research Department of West China Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
BW conceived and designed the study. TC, CW, QZ, AW, XZ, SL, YY, and WS acquired the data, which TC analyzed. TC and CW aided in data interpretation and wrote the manuscript. All authors were involved in revising the article and approved the final version.
Funding
This work was supported by the National Natural Science Foundation of China (82071320 and 81870937), and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD18009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.898226/full#supplementary-material
References
1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
2. Kaesmacher J, Dobrocky T, Heldner MR, Bellwald S, Mosimann PJ, Mordasini P, et al. Systematic review and meta-analysis on outcome differences among patients with TICI2b versus TICI3 reperfusions: success revisited. J Neurol Neurosurg Psychiatry. (2018) 89:910–7. doi: 10.1136/jnnp-2017-317602
3. Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (London, England). (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
4. Carbone F, Bonaventura A, Montecucco F. Neutrophil-Related Oxidants Drive Heart and Brain Remodeling After Ischemia/Reperfusion Injury. Front Physiol. (2019) 10:1587. doi: 10.3389/fphys.2019.01587
5. Tang C, Wang C, Zhang Y, Xue L, Li Y, Ju C, et al. Recognition, Intervention, and Monitoring of Neutrophils in Acute Ischemic Stroke. Nano Lett. (2019) 19:4470–7. doi: 10.1021/acs.nanolett.9b01282
6. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. (2009) 7:97. doi: 10.1186/1479-5876-7-97
7. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cerebral Blood Flow Metabol. (2015) 35:888–901. doi: 10.1038/jcbfm.2015.45
8. Dhanesha N, Patel RB, Doddapattar P, Ghatge M, Flora GD, Jain M, et al. PKM2 promotes neutrophil activation and cerebral thrombo-inflammation: Therapeutic implications for ischemic stroke. Blood. (2021). doi: 10.1161/atvb.41.suppl_1.107
9. Wang Y, Jin H, Wang W, Wang F, Zhao H. Myosin1f-mediated neutrophil migration contributes to acute neuroinflammation and brain injury after stroke in mice. J Neuroinflammation. (2019) 16:77. doi: 10.1186/s12974-019-1465-9
10. Bourdon E, Blache D. The importance of proteins in defense against oxidation. Antioxid Redox Signal. (2001) 3:293–311. doi: 10.1089/152308601300185241
11. Halliwell B. Albumin–an important extracellular antioxidant? Biochem Pharmacol. (1988) 37:569–71. doi: 10.1016/0006-2952(88)90126-8
12. Nathan C, Xie QW, Halbwachs-Mecarelli L, Jin WW. Albumin inhibits neutrophil spreading and hydrogen peroxide release by blocking the shedding of CD43 (sialophorin, leukosialin). J Cell Biol. (1993) 122:243–56. doi: 10.1083/jcb.122.1.243
13. Parkkinen J, Ojala P, Niiranen J, Jolkkonen J. Molecular mechanisms underlying neuroprotective effects of albumin after ischemic stroke. Stroke. (2007) 38:255. doi: 10.1161/01.STR.0000254506.06583.2d
14. Kim J, Song T-J, Park JH, Lee HS, Nam CM, Nam HS, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. (2012) 222:464–7. doi: 10.1016/j.atherosclerosis.2012.02.042
15. Buck BH, Liebeskind DS, Saver JL, Bang OY, Yun SW, Starkman S, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. (2008) 39:355–60. doi: 10.1161/STROKEAHA.107.490128
16. Kumar AD, Boehme AK, Siegler JE, Gillette M, Albright KC, Martin-Schild S. Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J Stroke Cerebrov Dis. (2013) 22:e111–e7. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.008
17. Semerano A, Laredo C, Zhao Y, Rudilosso S, Renú A, Llull L, et al. Leukocytes, collateral circulation, and reperfusion in ischemic stroke patients treated with mechanical thrombectomy. Stroke. (2019) 50:3456–64. doi: 10.1161/STROKEAHA.119.026743
18. Gao J, Zhao Y, Du M, Guo H, Wan T, Wu M, et al. Serum albumin levels and clinical outcomes among ischemic stroke patients treated with endovascular thrombectomy. Neuropsychiatr Dis Treat. (2021) 17:401–11. doi: 10.2147/NDT.S293771
19. He HM, Zhang SC, He C, You ZB, Luo MQ, Lin MQ, et al. Association between neutrophil percentage-to-albumin ratio and contrast-associated acute kidney injury in patients without chronic kidney disease undergoing percutaneous coronary intervention. J Cardiol. (2022) 79:257–64. doi: 10.1016/j.jjcc.2021.09.004
20. Wang X, Wang J, Wu S, Ni Q, Chen P. Association Between the Neutrophil Percentage-to-Albumin Ratio and Outcomes in Cardiac Intensive Care Unit Patients. Int J Gen Med. (2021) 14:4933–43. doi: 10.2147/IJGM.S328882
21. Ferro M, Babă DF, de Cobelli O, Musi G, Lucarelli G, Terracciano D, et al. Neutrophil percentage-to-albumin ratio predicts mortality in bladder cancer patients treated with neoadjuvant chemotherapy followed by radical cystectomy. Future Sci OA. (2021) 7:Fso709. doi: 10.2144/fsoa-2021-0008
22. Dai K, Li Z, Luo Y, Xiong Q, Xiong Y, Song Z, et al. Neutrophil percentage-to-albumin ratio and monocyte-to-lymphocyte ratio as predictors of free-wall rupture in patients with acute myocardial infarction. J Clin Lab Anal. (2021) 36:e24136. doi: 10.1002/jcla.24136
23. Yu Y, Liu Y, Ling X, Huang R, Wang S, Min J, et al. The Neutrophil Percentage-to-Albumin Ratio as a New Predictor of All-Cause Mortality in Patients with Cardiogenic Shock. Biomed Res Int. (2020) 2020:7458451. doi: 10.1155/2020/7458451
24. Wang B, Li D, Cheng B, Ying B, Gong Y. The Neutrophil Percentage-to-Albumin Ratio Is Associated with All-Cause Mortality in Critically Ill Patients with Acute Kidney Injury. Biomed Res Int. (2020) 2020:5687672. doi: 10.1155/2020/5687672
25. Sun T, Shen H, Guo Q, Yang J, Zhai G, Zhang J, et al. Association between Neutrophil Percentage-to-Albumin Ratio and All-Cause Mortality in Critically Ill Patients with Coronary Artery Disease. Biomed Res Int. (2020) 2020:8137576. doi: 10.1155/2020/8137576
26. Gong Y, Li D, Cheng B, Ying B, Wang B. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol Infect. (2020) 148:e87. doi: 10.1017/S0950268820000771
27. Cui H, Ding X, Li W, Chen H, Li H. The Neutrophil Percentage to Albumin Ratio as a New Predictor of In-Hospital Mortality in Patients with ST-Segment Elevation Myocardial Infarction. Med Sci Monit. (2019) 25:7845–52. doi: 10.12659/MSM.917987
28. Zhang H, Wu T, Tian X, Lyu P, Wang J, Cao Y. High Neutrophil Percentage-To-Albumin Ratio Can Predict Occurrence of Stroke-Associated Infection. Front Neurol. (2021) 12:705790. doi: 10.3389/fneur.2021.705790
29. Ma Y-C, Zuo L, Chen J-H, Luo Q, Yu X-Q, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. (2006) 17:2937–44. doi: 10.1681/ASN.2006040368
30. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
31. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
32. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. (2013) 44:2650–63. doi: 10.1161/STROKEAHA.113.001972
33. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
34. Park S-Y, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann Intern Med. (2017) 167:228–35. doi: 10.7326/M16-2472
35. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
36. Sun M-S, Jin H, Sun X, Huang S, Zhang F-L, Guo Z-N, et al. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. (2018) 2018:3804979. doi: 10.1155/2018/3804979
37. Eriksson H, Löwhagen Hendén P, Rentzos A, Pujol-Calderón F, Karlsson J-E, Höglund K, et al. Acute symptomatic seizures and epilepsy after mechanical thrombectomy. Epilepsy & Behavior: E&B. (2020) 104:106520. doi: 10.1016/j.yebeh.2019.106520
Keywords: acute ischemic stroke, reperfusion therapy, neutrophil, albumin, outcome
Citation: Cui T, Wang C, Zhu Q, Li S, Yang Y, Wang A, Zhang X, Shang W and Wu B (2022) Association between neutrophil percentage-to-albumin ratio and 3-month functional outcome in acute ischemic stroke patients with reperfusion therapy. Front. Neurol. 13:898226. doi: 10.3389/fneur.2022.898226
Received: 08 April 2022; Accepted: 12 August 2022;
Published: 13 September 2022.
Edited by:
Yang-Kun Chen, Southern Medical University, ChinaReviewed by:
YongLin Liu, Southern Medical University, ChinaHaobo Chen, Guangzhou First People's Hospital, China
Li'An Huang, First Affiliated Hospital of Jinan University, China
Copyright © 2022 Cui, Wang, Zhu, Li, Yang, Wang, Zhang, Shang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wu, dr.bowu@hotmail.com
†These authors have contributed equally to this work
 Ting Cui
Ting Cui Changyi Wang
Changyi Wang Qiange Zhu3
Qiange Zhu3 Yuan Yang
Yuan Yang Xuening Zhang
Xuening Zhang Bo Wu
Bo Wu