- 1The Traditional Chinese Medicine Department, The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, China
- 2The Third Clinical Medical College, Zhejiang University of Traditional Chinese Medicine, Hangzhou, China
- 3Breast Medical Oncology Department, The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, China
Introduction: Chemotherapy-induced peripheral neuropathy (CIPN) is a common complication in patients with cancer during chemotherapy. It mainly leads to severe numbness of the hands and feet and causes great pain in patients. Electroacupuncture (EA) is considered to be beneficial in improving peripheral neuropathy and relieving numbness of the hands and feet. This trial aims to evaluate the therapeutic effect of different frequencies of EA on CIPN in patients with cancer.
Methods and analysis: This study is a randomized controlled trial. In total, 160 eligible CIPN patients are randomly assigned to the 2 Hz EA group, 100 Hz EA group, 2/100 Hz EA group, and control group in the ratio of 1:1:1:1. All patients in the EA treatment groups receive treatment with EA three times a week for 4 weeks and following up for 4 weeks. The patients in the control group are given Mecobalamin (MeCbl) tablets orally, one tablet at a time, three times a day, for 4 weeks, and following up for 4 weeks. The primary outcome measures are the participant neurotoxicity questionnaire (PNQ) and the peripheral neurotoxicity assessment rating (NCI CTCAE V5.0). Secondary outcomes are the quality of life scale (EORTC QLQ-C30) and the measurement of peripheral nerve conduction velocity (NCV). The results are evaluated at baseline, post-treatment phase, and following up for 4 weeks. All major analyses are based on the intention to treat principle.
Ethics/dissemination: This protocol was approved by the Medical Ethics Committee of the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) on 7 December 2021. The license number is IRB-2021-458. This study provides clinical efficacy data of different frequencies of EA in the treatment of CIPN. The results help to prove whether EA is an effective therapy for CIPN and optimize the frequency of EA for CIPN. The results of this study are shared with health care professionals, the public, and relevant organizations through the publication of manuscripts and conference reports.
Trial registration number: ChiCTR2100054458.
Background
Cancer is a common disease endangering human health. Chemotherapy is still one of the main treatment methods, but chemotherapy drugs often cause different side effects while killing cancer cells. Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of many chemotherapeutic drugs, especially platinum compounds, vinblastine, taxanes, and other chemotherapeutic drugs. For example, oxaliplatin is the third generation of broad-spectrum platinum compounds anticancer drugs after cisplatin and carboplatin. It is widely used in the treatment of gastrointestinal malignancies (1). However, the primary adverse reaction of oxaliplatin is CIPN, and the incidence of acute neurotoxicity can be as high as 85−95% (2). In contrast, its chronic neurotoxicity is closely related to the cumulative dose of drugs. It was reported that when the drug dose reaches 1,170 mg/m2, the incidence of neurotoxicity above grade III can be as high as 50% (3). About 40% of clinical colorectal cancer patients reduce the dose or even stop oxaliplatin due to CIPN; thus, the expected therapeutic effect cannot be achieved (4).
The main symptoms of CIPN are peripheral nerve damage, limb numbness, loss of sensation or abnormal sensation (such as tingling, electric shock and sensation, foreign body sensation, etc.), decreased tendon reflex, and sensory ataxia, which can be triggered or aggravated in case of cold stimulation. Based on the severity of symptoms, it is often divided into four levels. However, its pathogenesis is not clear. It is mainly considered to be related to the toxic accumulation of oxaliplatin itself and the metabolite oxalate. The main target of oxaliplatin is the dorsal root ganglia (DRG) without blood-brain barrier protection. The accumulation rate of chemotherapeutic drugs in DRG neurons is much higher than the clearance rate. This accumulation inhibits the rRNA synthesis in neurons, impairs the protein synthesis, and leads to the damage and degeneration of neurons (5). In addition, the measurement of nerve conduction velocity (NCV) is an important index for diagnosing and evaluating peripheral neuropathy and an objective standard for evaluating the degree of peripheral nerve damage (6). For example, peripheral neuropathy caused by diabetes can make the peripheral NCV slow. By measuring NCV, the degree of peripheral nerve damage can be assessed (7). CIPN caused by chemotherapeutic drugs can also significantly reduce the conduction velocity of peripheral nerves (8–11). According to current guidelines, there is no curative therapy while duloxetine is the only medication proven to be effective in alleviating the symptoms of CIPN. Antiepileptic drugs, calcium magnesium mixture, antioxidants, neuroprotective agents, and nutrients are generally used to treat CIPN, but they often bring other toxic and side effects or unsatisfactory curative effects. CIPN caused by chemotherapy drugs affect the treatment process of cancer and easily cause emotional instability and seriously affect the quality of life. Therefore, there is an urgent need to find an effective and less toxic and side effects treatment for CIPN.
Acupuncture, an important part of traditional Chinese medicine, played a prominent role in treating cancer-related diseases, especially in preventing and treating complications caused by chemotherapeutic drugs (11, 12). In addition, some studies have shown that acupuncture can effectively treat kinds of peripheral neuropathy, improve peripheral NCV, and alleviate patients' sensory abnormalities or pain symptoms at the end of limbs (13). Electroacupuncture (EA) is a new technology of acupuncture. It was developed by combining traditional acupuncture technology with electrical stimulation. In recent years, it has been widely used in clinical treatment. Many animal experiments show that EA can effectively treat CIPN, and the analgesic effect of low-frequency EA is better. For example, in the CIPN animal model, low-frequency EA (2Hz) can reduce the mechanical pain and hyperalgesia of CIPN induced by paclitaxel in rats (14) and also has a pronounced therapeutic effect on CIPN caused by oxaliplatin in rats (15); low frequency EA (10 Hz) is more effective than high frequency EA (100 Hz) in alleviating paclitaxel induced CIPN symptoms (16). While, in clinical research, it was reported that high-frequency EA (50–100 Hz) could achieve a good curative effect in the treatment of CIPN caused by oxaliplatin (17); Many researchers have also used density waves 2/100 Hz (18) and 2/120 Hz (19) to treat peripheral neuropathy and achieved positive results. However, some studies have pointed out that the efficacy of EA on CIPN is not clear. It is not as good as simply using drugs (20) and even aggravates the peripheral nerve symptoms of patients (21). According to the above, we can evaluate the degree of CIPN damage and treatment recovery by measuring NCV, and the clinical efficacy of EA in the treatment of CIPN needs to be further verified; The clinical effectiveness of different frequencies of EA in the treatment of CIPN may be different. Therefore, we designed an RCT involving drug control to accurately evaluate the efficacy of EA on CIPN and optimize the selection of EA frequency.
Methods
Objective
To demonstrate whether EA is an effective therapy for CIPN, the curative effect of EA with different frequencies on CIPN and its effect on NCV is observed.
Hypotheses
1. EA can significantly improve CIPN symptoms compared with drug intervention, as subjective (participant neurotoxicity questionnaire, PNQ) and objective (peripheral nerve conduction velocity, NCV) measurements after treatment.
2. EA can comprehensively improve patients' quality of life while improving CIPN, as measured by the quality of life scale (EORTC QLQ-C30).
3. There are differences in the efficacy of different frequencies of EA on CIPN.
Trial design
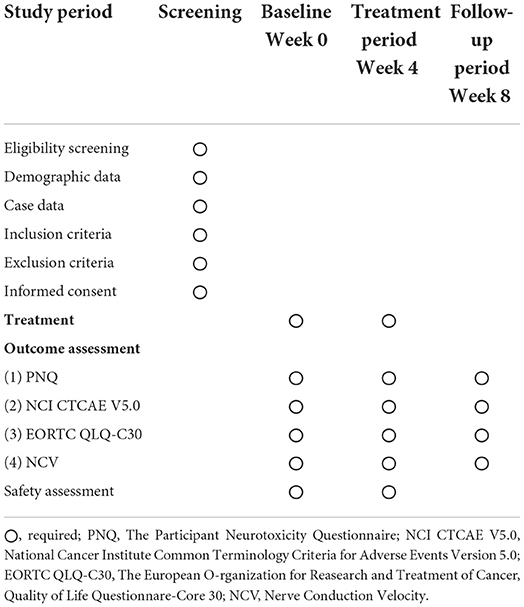
This trial was designed as a randomized, controlled, participant, researcher, and outcome evaluator blind experimental study to compare the therapeutic effects of three different frequencies of EA regimens on CIPN patients. In total, 160 participants with CIPN were randomly assigned to four treatment groups in the ratio of 1:1:1:1, namely the 2 Hz EA group, 100 Hz EA group, 2/100 Hz EA group, and control group. The flow chart of the study process is shown in Figure 1. Additionally, the trial schedule of enrolment, treatments, and assessments is shown in Table 1. The reporting of this protocol is based on the Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) (22) and the SPIRIT reporting guidelines (23).
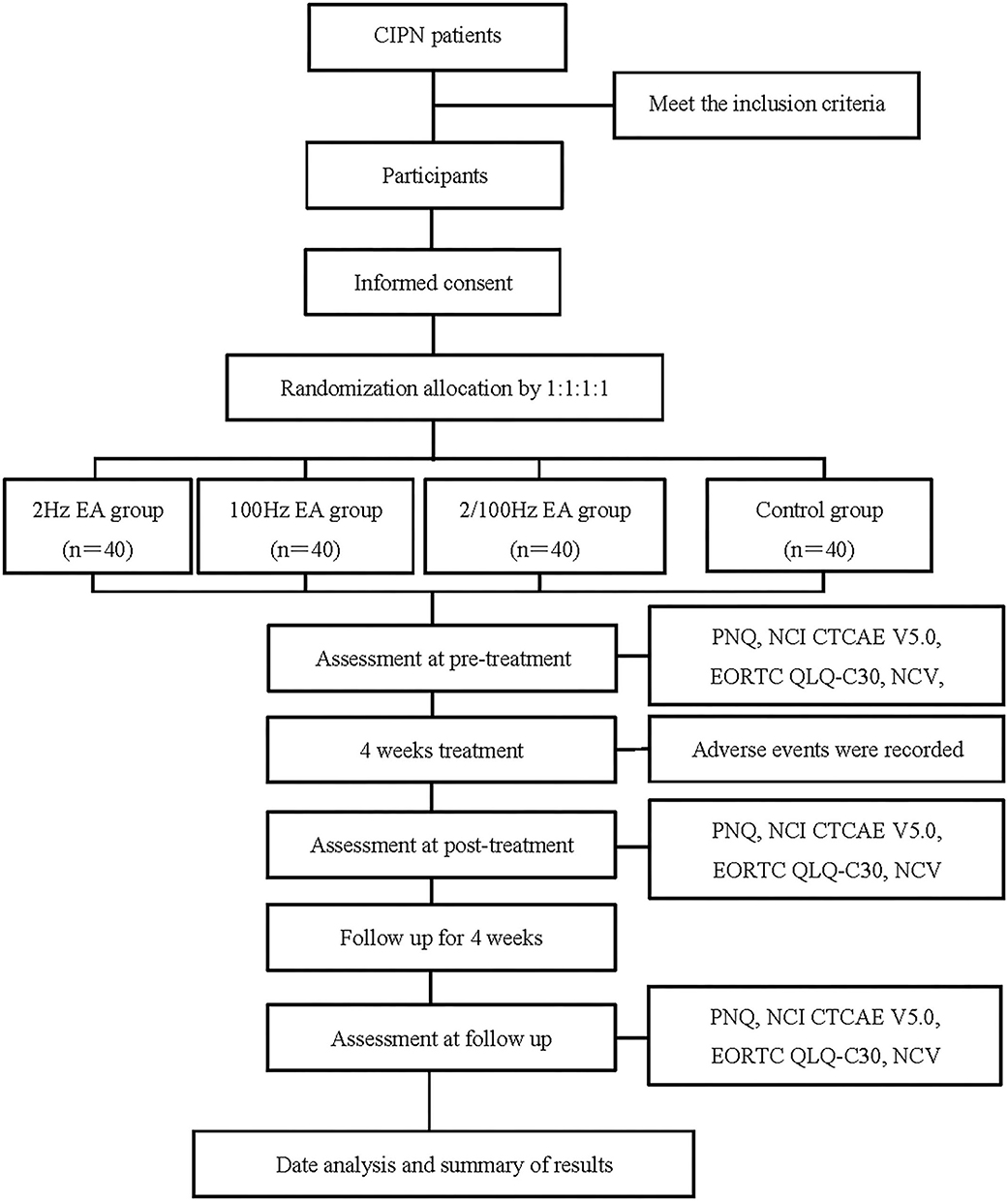
Figure 1. Flow chart of the study process. CIPN, Chemotherapy-induced peripheral neuropathy; EA, Electroacupuncture; PNQ, The Participant Neurotoxicity Questionnaire; NCI CTCAE V5.0, National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0; EORTC QLQ-C30, The European O-rganization for Reasearch and Treatment of Cancer, Quality of Life Questionnare-Core 30; NCV, Nerve Conduction Velocity.
Participants and recruitment
Participants with CIPN were recruited at the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital). Recruitment posters were placed in the outpatient hall to increase exposure, and the recruitment information for this trial was informed through online publicity.
Inclusion criteria
Participants who met all of the following requirements were considered for inclusion: (1) Over 18 and under 70 years old, and the expected survival time was ≥1 year; (2) Pathological diagnosis was a malignant tumor, and peripheral neurotoxicity above grade 1 occurred after chemotherapy with platinum compounds, paclitaxel, and vinblastine; (3) Have daily living ability, no hearing loss, and can cooperate independently to complete all examinations; (4) No severe heart, liver, brain, kidney and other diseases, Karnofsky functional status score (KPS) >60; (5) Clear consciousness without mental illness or cognitive impairment; (6) Signed the written informed consent form for the clinical trial.
Exclusion criteria
Patients were excluded if they were (1) diagnosed with relatively severe systemic diseases (cardiovascular disease, acute infectious disease, hematopathy, et al.); (2) diagnosed with severe mental disorders, like schizophrenia; (3) diagnosed with peripheral neuropathy caused by other causes, such as peripheral nerve injury or diabetic peripheral neuropathy; (4) pregnant and lactating women; (5) alcohol and/or other drug abuse or dependent; (6) participating in other clinical trials of acupuncture or drugs.
Randomization and allocation concealment
The randomization was performed by the Research Center of the Scientific Department, the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital). A randomization method was used to generate the random allocation sequence of four groups; four groups of random serial numbers were randomly placed in opaque sealed envelopes by other staff to ensure the concealment of distribution. The opaque sealed envelope was printed with the screening sequence of participants on the outside and randomly assigned groups on the inside, consecutively numbered and connected into a series. According to the subject's visit order, the envelope was selected with the corresponding serial number.
Blinding and informed consent
This is a participant-assessor-blinded trial in which participants are not aware of their group assignments. Participants were informed that they have a 25% chance of being allocated to receive either of the four treatments: 2 Hz EA group, 100 Hz EA group, 2/100 Hz EA group, and drugs in the control group. The follow-up assessors were also unaware of the patient's group allocation. Although acupuncturists were not blinded to the treatment assignments, they were not involved in the outcome assessments or data analyses.
To achieve blinding, the kind of disposable sterile steel needles, selection of acupoints, treatment times, and skin disinfection process were formed consistently and significantly. Meanwhile, the only variable was the frequency of EA. Participants were blinded to their group allocation.
Intervention
Intervention group
(1) 2 Hz EA group: acupuncture needle was routinely used. Table 2 shows the location of all selected acupoints. Bilateral Hegu (LI4), Houxi (SI3), Waiguan (SJ5), Quchi (LI11), and Baxie (EX-UE9) acupoints were taken in the upper limbs. Bilateral Zusanli (ST36), Yinlingquan (GB34), Yanglingquan (SP9), Sanyinjiao (SP6), Taichong (LR3), and Bafeng (EX-LE10) acupoints were taken in the lower limbs; Upper limb EA connects Waiguan (SJ5) and Hegu (LI4) points; Lower limb EA connects Zusanli (ST36) and Sanyinjiao(SP6) points. The frequency of 2 Hz was used in this group, and the stimulation intensity was based on the patient's tolerance. Each treatment time was 30 min, three times a week for 4 weeks. (2) 100 Hz EA group: the treatment method was the same as that of the 2 Hz EA group, while the EA frequency was 100 Hz. (3) 2/100 Hz EA group: the treatment method was the same as that of the 2 Hz EA group, and the EA frequency was 2/100 Hz. (Equipment: the disposable acupuncture needle adopts Huatuo brand filiform needle produced by Suzhou instrument factory, with models of 0.25 * 40 MM. The electro acupuncture adopts Hans-200a model).
Control group
The participants were given Mecobalamin (MeCbl) tablets orally, one tablet each time, three times a day for 4 weeks.
Outcome measures
The participant neurotoxicity questionnaire (PNQ) and peripheral neurotoxicity were assessed according to NCI CTCAE version 5.0, EORTC quality of life scale (QLQ-C30), and NCV measurement was the outcomes used to assess efficacy. Adverse events were recorded to assess safety.
Primary outcome
The participant neurotoxicity questionnaire
The participant neurotoxicity questionnaire is a questionnaire for diagnosis and quantification of CIPN (24, 25), which is validated and rated the highest overall assessment (26, 27). It includes two items (sensory nerve and motor nerve) and mainly assesses symptoms of paresthesia such as numbness, pain, tingling, and cold perception in the hands or feet. It was chosen as the primary assessment of neuropathy symptoms based on its capacity to detect both sensory nerve deficit and interference of neuropathy symptoms in activities of daily living (24, 28), as well as provide a robust patient-level assessment of neuropathy symptoms (26). The PNQ grades symptoms from grade 0 (no neuropathy) to grade 4 (very severe neuropathy).
Peripheral neurotoxicity assessment according to NCI CTCAE version 5.0
A total of five grades of peripheral neurotoxicity were identified according to NCI CTCAE version5.0 (29). Grade I, tendon reflex disappeared or sensory numbness (including acupuncture sensation), but does not affect function; Grade II, sensory loss or sensory numbness (including acupuncture sensation), which affects the function but does not affect the activities of daily life; grade III, sensory loss or sensory numbness (including acupuncture sensation), which affects the activities of daily life; grade IV, sensory motor neuropathy, which significantly interferes with the activities of daily life.
Secondary outcome
European Organization for research and treatment of Cancer quality of life scale (QLQ-C30)
The European Organization for research and treatment of Cancer (EORTC) QLQ-C30 is a quality-of-life instrument for use in international clinical trials in oncology (30). We used it to evaluate patients' quality of life before treatment, after treatment, and follow-up. QLQ-C30 scale includes five functional subscales (body, function, cognition, emotion, and Society), three symptom subscales (fatigue, pain, and nausea and vomiting), six single symptom measurement items (dyspnea, loss of appetite, insomnia, constipation, diarrhea, and economic difficulties), and one overall health subscale. The higher the score of the functional subscale, the better the quality of life. The higher the score of the symptom subscale, the worse the quality of life.
NCV measurement
Nerve conduction velocity detects the speed of nerve response by placing electrodes on the skin and stimulating nerves with electrical pulses. The room temperature was kept at 20–25°C during the examination. Before and after treatment, the motor conduction velocity (MCV) of the median nerve of the upper limb and the common peroneal nerve of the lower limb, and the sensory conduction velocity (SCV) of the median nerve and sural nerve were measured. The median nerve was stimulated at the wrist and elbow and recorded in the thenar and adductor pollicis muscles. The common peroneal nerve was stimulated at the ankle, under the fibular head and popliteal fossa, and the recording point was the extensor digitorum brevis. The sural nerve was stimulated at the middle and lower 1/3 junction of the lower leg, recorded at the lateral malleolus. The side with apparent feeling was selected for detection in both limbs. If both sides feel the same, one side was selected.
Other outcomes
All kinds of adverse reactions during treatment were recorded, including specific manifestations, occurrence time, treatment measures, results, and follow-up and indicated whether the case was continued to be included. In addition, all vital signs and adverse reactions were measured and recorded at each visit.
Setting
The trial was conducted in the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) between 1 January 2022 and 31 December 2024. This protocol was approved by the Medical Ethical Committee of the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) on 7 December 2021. The license number is IRB-2021-458.
The eligibility of prospective participants was determined by a researcher who was not involved in the assessment or treatment of the participants. On the first arrival for the screening meeting, patients were given more detailed information about the study procedures. Before the first treatment and after the last treatment, all patients were required to complete the PNQ, NCI CTCAE V5.0, EORTC QLQ-C30, and NCV measurement. After 4 weeks of treatment, follow-up was carried out, and the above indicators were measured again to evaluate the curative effect. The participants cannot take pain medications or unspecified CIPN-related therapeutic drugs during treatment. If the patient had to take pain medications or drugs that interfere with the results of the research due to changes in his condition, the patient would be considered to drop out. In order to promote enrollment and participant compliance, all treatment costs and all outcome measurements were free for participants.
Statistical methods
Sample size
This is a prospective clinical study. According to the randomized control scheme, three treatment groups and one control group are designed, with 40 patients in each group.
Statistical analysis
De-identified outcome data were analyzed by a statistician blinded to group allocations using the Statistical Package for the Social Sciences (SPSS) V 24.0 statistical software package. The data analysis was based on the intention-to-treat (ITT) population. All statistical analyses were two-sided tests except for the primary outcome. The level of significance was established at 0.05. Continuous data were represented by the mean, standard deviation, median, minimum value, and maximum value; categorical data were represented by percentages. To compare two independent samples, a t-test or nonparametric test was used for continuous data, and a chi-square test/the Fisher exact test/nonparametric tests were used for categorical data. For safety analysis, the incidence of adverse events was compared between the four groups using the chi-square test or the Fisher exact test.
Patient safety and quality control
All acupuncturists and evaluators were required to undergo special training before the trial to ensure consistent practices. The training program included CIPN diagnosis, participant inclusion, and exclusion criteria, location of the acupoints, EA techniques, and evaluation criteria of case report forms. Dropouts and withdrawals from the study were recorded through the intervention periods.
Any adverse events (described as unfavorable or unintended signs, symptoms, or diseases) related to acupuncture treatment were observed and reported by patients and practitioners during each patient visit. In addition, all vital signs and adverse events were measured and recorded at each visit. Throughout the trial, all study data were collected in the case report forms and entered into the electronic data capture system by independent researchers.
This trial is monitored by the scientific research department of the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital). They are responsible for monitoring the data and have the right to reveal blinded data and can verify the authenticity between the raw data and the recorded data to guarantee accuracy and quality throughout the study.
Discussion
Chemotherapy-induced peripheral neuropathy is one of the most frequent side effects caused by antineoplastic agents, with a prevalence from 19 to over 85% (31). Clinically, CIPN is a primarily sensory neuropathy that may be accompanied by motor and autonomic changes of varying intensity and duration (32). Due to its high prevalence, CIPN constitutes a significant clinical problem for patients with cancer and their health care providers, mainly because there is no single effective method to prevent CIPN. According to previously published data, we suggest that EA therapy may be effective in CIPN, while the dominant frequency of EA in the treatment of CIPN is not clear. Thus, we used EA technology as the primary treatment method and oral drugs as the control. Different frequencies of EA were used to treat CIPN for the first time. The curative effects of 2, 100, and 2/100 Hz EA were compared horizontally.
Selecting the appropriate acupuncture points to treat CIPN better and achieve the expected curative effect is one of the critical problems that need to be solved. We have screened the current high-frequency acupoints of acupuncture in the treatment of CIPN through published data (33, 34) and consulted experienced acupuncturists to determine the combination selection of acupoints.
Currently, there are no definite criteria or tools for evaluating CIPN (32, 35). Thus, selecting the suitable efficacy evaluation index to objectively evaluate the clinical efficacy of EA in the treatment of CIPN is also a critical problem that needs to be solved. In this study, the severity of CIPN was evaluated using the internationally recognized PNQ as the subjective indicator. Researchers graded the severity of CIPN according to the PNQ and clinical symptoms concerning NCI CTCAE version 5.0 and measured NCV as objective indicators to evaluate the degree of peripheral nerve injury and clinical treatment effect of CIPN patients. The quality of life of patients was comprehensively evaluated according to EORTC QLQ-C30, and adverse events during the study were recorded, which made the study results more convincing.
Mecobalamin is an active form of vitamin B12 that has been suggested to be beneficial in improving nerve conduction and neuropathic pain symptoms. As an auxiliary agent, it exerts neuronal protection by promoting regeneration of injured nerves and antagonizing glutamate-induced neurotoxicity and is already widely used to treat peripheral neuropathies (36, 37). Some clinical trials have shown that MeCbl can improve the numbness of hands and feet caused by peripheral neuropathy (38, 39). MeCbl is considered a safe, effective, and generally well-tolerated drug for treating peripheral neuropathies (36). Therefore, we used MeCbl as the treatment scheme for the control group to clarify the efficacy of EA.
By comparing the clinical efficacy of 2, 100, and 2/100Hz EA in the treatment of CIPN, this study can further clarify the effect of EA in the treatment of CIPN, optimize the selection of EA frequency, and provide technical guidance for clinical treatment. The results will further provide new clues, new ideas, and new methods for acupuncture in the treatment of nervous system diseases and cancer-related diseases. Finally, we hope the results of this study will effectively improve the CIPN symptoms of patients with tumors, reduce patients' pain, and improve their quality of life.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), on December 7, 2021. The license number is IRB-2021-458. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CL and RL contributed to the study's design, drafting, and editing of the manuscript. CL wrote the first manuscript for this trial and edited the final manuscript. RL revised the manuscript. DD participated in the design of the trial. GL evaluated participant information and collected CRF data. WB and SZ conducted the acupuncture operation. YW registered the study result data. All authors read and approved the final manuscript.
Funding
The work was supported by the Program of Science Research Foundation of Zhejiang Provincial TCM Administration (2022ZB058).
Acknowledgments
The authors appreciate the support from all participants who have been or will be included in this study, especially Professor Jianqiao Fang of the Zhejiang University of Traditional Chinese Medicine. His working experience gives us guidance on acupoint selection for EA technology treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Masanobu T, Tomoya T, Tadahumi T, Hirotaka S, Naohiro S, Kotaro S, et al. Pkc/mek inhibitors suppress oxaliplatin-induced neuropathy and potentiate the antitumor effects. Int J Cancer. (2015) 137:243–50. doi: 10.1002/ijc.29367
2. Carvalho LF, Amf S, Carvalho AA. The use of antioxidant agents for chemotherapy-induced peripheral neuropathy treatment in animal models. Clin Exp Pharmacol Physioly. (2017) 44:971–9. doi: 10.1111/1440-1681.12803
3. Lee JS, Kim YT, Jeon EK, Won HS, Cho YS, Ko YH. Effect of green tea extracts on oxaliplatin-induced peripheral neuropathy in rats. BMC Complement Altern Med. (2012) 12. doi: 10.1186/1472-6882-12-124
4. Park SB, Goldstein D, Lin CS, Krishnan AV, Friedlander ML, Kiernan MC. Acute abnormalities of sensory nerve function associated with oxaliplatin-induced neurotoxicity. J Clin Oncol. (2009) 27:1243–9. doi: 10.1200/JCO.2008.19.3425
5. Xiaobing C, Huifang L, Beibei C, Suxia L. Oxaliplatin induced neurotoxicity: mechanism and therapy. Chin Med Pharm. (2012) 2:38–40.
6. Domkundwar S, Autkar G, Khadilkar SV, Virarkar M. Ultrasound and emg-ncv study (electromyography and nerve conduction velocity) correlation in diagnosis of nerve pathologies. J Ultrasound. (2017) 20:111–22. doi: 10.1007/s40477-016-0232-3
7. Jiang DQ, Xu LC, Jiang LL, Li MX, Wang Y. Fasudil combined with methylcobalamin or lipoic acid can improve the nerve conduction velocity in patients with diabetic peripheral neuropathy: a meta-analysis. Medicine. (2018) 97:e11390. doi: 10.1097/MD.0000000000011390
8. Wozniak KM, Vornov JJ, Wu Y, Nomoto K, Littlefield BA, DesJardins C, et al. Sustained accumulation of microtubule-binding chemotherapy drugs in the peripheral nervous system: correlations with time course and neurotoxic severity. Cancer Res. (2016) 76:3332–9. doi: 10.1158/0008-5472.CAN-15-2525
9. Wozniak KM, Vornov JJ, Wu Y, Liu Y, Carozzi VA, Rodriguez-Menendez V, et al. Peripheral neuropathy induced by microtubule-targeted chemotherapies: Insights into acute injury and long-term recovery. Cancer Res. (2018) 78:817–29. doi: 10.1158/0008-5472.CAN-17-1467
10. Renn CL, Carozzi VA, Rhee P, Gallop D, Dorsey SG, Cavaletti G. Multimodal assessment of painful peripheral neuropathy induced by chronic oxaliplatin-based chemotherapy in mice. Mol Pain. (2011) 7:29. doi: 10.1186/1744-8069-7-29
11. Han X, Wang L, Shi H, Zheng G, He J, Wu W, et al. Acupuncture combined with methylcobalamin for the treatment of chemotherapy-induced peripheral neuropathy in patients with multiple myeloma. BMC Cancer. (2017) 17:40. doi: 10.1186/s12885-016-3037-z
12. Donald GK, Tobin I, Stringer J. Evaluation of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Acupunct Med. (2011) 29:230–3. doi: 10.1136/acupmed.2011.010025
13. Schröder S, Liepert J, Remppis A, Greten JH. Acupuncture treatment improves nerve conduction in peripheral neuropathy. Eur J Neurol. (2007) 14:276–81. doi: 10.1111/j.1468-1331.2006.01632.x
14. Yuanyuan L, Qinglin L, Chengyu Y, Yan T, Boyu L, Qimiao H, et al. Effect of low frequency electroacupuncture on the expression of trpv1 in dorsal root ganglia of paclitaxel induced cipn rats. J Zhejiang Chin Med Univ. (2019) 43:496–503+511. doi: 10.16466/j.issn1005-5509.2019.05.024
15. Guangwei C, Huaijin C, Hao C, Fan W, Tingting Z, Le K. Alleviating effect of electroacupuncture on oxaliplatin-induced peripheral neurotoxicity in rats. Chin J Tradit Chin Med Pharma. (2017) 32:2670–2.
16. Meng X, Zhang Y, Li A, Xin J, Lao L, Ren K, et al. The effects of opioid receptor antagonists on electroacupuncture-produced anti-allodynia/hyperalgesia in rats with paclitaxel-evoked peripheral neuropathy. Brain Res. (2011) 1414:58–65. doi: 10.1016/j.brainres.2011.08.004
17. Xianjun S, Shengli H, Hao C, Jie S, Weibin W, Luming L. Clinical study of electroacupuncture treatment for oxaliplatin neurotoxicity. Shanghai J Acupunct Moxibustion. (2012) 31:727–9.
18. Xiang Y, Xu N, Sui M. Clinical observation on electroacupuncture in the treatment of chemotherapy-induced pathologic neuropathyic pain. Chin J Integr Med. (2018) 16:1168–71.
19. Seunghoon L, Joo-Hee K, Kyung-Min S, Jung-Eun J, Tae-Hun K, Kyung-Won K, et al. Electroacupuncture to treat painful diabetic neuropathy: study protocol for a three-armed, randomized, controlled pilot trial. Trials. (2013) 14:225. doi: 10.1186/1745-6215-14-225
20. Al-Atiyyat N, Obaid A. Management of peripheral neuropathy induced by chemotherapy in adults with cancer: a review. Int J Palliat Nurs. (2017) 23:13–7. doi: 10.12968/ijpn.2017.23.1.13
21. Greenlee H, Crew KD, Capodice J, Awad D, Buono D, Shi Z, et al. Randomized sham-controlled pilot trial of weekly electro-acupuncture for the prevention of taxane-induced peripheral neuropathy in women with early stage breast cancer. Breast Cancer Res Treat. (2016) 156:453–64. doi: 10.1007/s10549-016-3759-2
22. MacPherson H, Altman DG, Hammerschlag R, Li Y, Wu T, White A, et al. Revised standards for reporting interventions in clinical trials of acupuncture (stricta): extending the consort statement. Acupunct Med. (2010) 28:83-93. doi: 10.1136/aim.2009.001370
23. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, KrleŽa-Jerić K, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583
24. Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, et al. Feasibility and validity of the patient neurotoxicity questionnaire during taxane chemotherapy in a phase iii randomized trial in patients with breast cancer: N-sas bc 02. Support Care Cancer. (2009) 17:1483–91. doi: 10.1007/s00520-009-0613-7
25. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. (2006) 33:15–49. doi: 10.1053/j.seminoncol.2005.12.010
26. McCrary JM, Goldstein D, Boyle F, Cox K, Grimison P, Kiernan MC, et al. Optimal clinical assessment strategies for chemotherapy-induced peripheral neuropathy (cipn): a systematic review and delphi survey. Support Care Cancer. (2017) 25:3485–93. doi: 10.1007/s00520-017-3772-y
27. Lu W, Giobbie-Hurder A, Freedman RA, Shin IH, Lin NU, Partridge AH, et al. Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer survivors: a randomized controlled pilot trial. Oncologist. (2020) 25:310–8. doi: 10.1634/theoncologist.2019-0489
28. Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, et al. Taxane-induced peripheral neuropathy and health-related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N-sas bc 02, a randomized clinical trial. Support Care Cancer. (2012) 20:3355–64. doi: 10.1007/s00520-012-1492-x
29. Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the national cancer institute's patient-reported outcomes version of the common terminology criteria for adverse events (pro-ctcae). J Natl Cancer Inst. (2014) 106:dju244. doi: 10.1093/jnci/dju244
30. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The european organization for research and treatment of cancer qlq-c30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
31. Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. (2019) 20:1451. doi: 10.3390/ijms20061451
32. Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. (2014) 155:2461–70. doi: 10.1016/j.pain.2014.09.020
33. Hwang MS, Lee HY, Choi TY, Lee JH, Ko YS, Jo DC, et al. A systematic review and meta-analysis of the efficacy of acupuncture and electroacupuncture against chemotherapy-induced peripheral neuropathy. Medicine. (2020) 99:e19837. doi: 10.1097/MD.0000000000019837
34. Franconi G, Manni L, Schröder S, Marchetti P, Robinson N. A systematic review of experimental and clinical acupuncture in chemotherapy-induced peripheral neuropathy. Evid Based Complement Alternat Med. (2013) 2013:1–7. doi: 10.1155/2013/516916
35. Cavaletti G, Frigeni B, Lanzani F, Piatti M, Rota S, Briani C, et al. The total neuropathy score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the national cancer institute-common toxicity scale. J Peripher Nerv Syst. (2007) 12:210–5. doi: 10.1111/j.1529-8027.2007.00141.x
36. Sawangjit R, Thongphui S, Chaichompu W, Phumart P. Efficacy and safety of mecobalamin on peripheral neuropathy: a systematic review and meta-analysis of randomized controlled trials. J Altern Complement Med. (2020) 26:1117–29. doi: 10.1089/acm.2020.0068
37. Zhang M, Han W, Hu S, Xu H. Methylcobalamin: a potential vitamin of pain killer. Neural Plast. (2013) 2013:424651. doi: 10.1155/2013/424651
38. Han Y, Wang M, Shen J, Zhang Z, Zhao M, Huang J, et al. Differential efficacy of methylcobalamin and alpha-lipoic acid treatment on symptoms of diabetic peripheral neuropathy. Minerva Endocrinol. (2018) 43:11–8. doi: 10.23736/S0391-1977.16.02505-0
Keywords: electroacupuncture, chemotherapy-induced peripheral neuropathy, randomized controlled trial, protocol, Mecobalamin
Citation: Lu C, Bao W, Deng D, Li R, Li G, Zou S and Wang Y (2022) Efficacy of electroacupuncture with different frequencies in the treatment of chemotherapy-induced peripheral neuropathy: A study protocol for a randomized controlled trial. Front. Neurol. 13:843886. doi: 10.3389/fneur.2022.843886
Received: 21 January 2022; Accepted: 07 July 2022;
Published: 28 July 2022.
Edited by:
Jeremy Micah Crook, Chris O'Brien Lifehouse, AustraliaReviewed by:
Sarah Prinsloo, University of Texas MD Anderson Cancer Center, United StatesManuel Morales, University of La Laguna, Spain
Copyright © 2022 Lu, Bao, Deng, Li, Li, Zou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Lu, 1069103617@qq.com
 Chao Lu
Chao Lu Wenlong Bao1
Wenlong Bao1 Rongrong Li
Rongrong Li