- 1School of Nursing, Naval Medical University, Shanghai, China
- 2Nursing School, Peking University, Beijing, China
Background: Chin tuck against resistance (CTAR) exercise was introduced to substitute for the commonly used Shaker exercise for dysphagia rehabilitation. The effects of CTAR exercise in stroke survivors needs to be validated.
Objective: To investigate the effects of Chin tuck against resistance (CTAR) exercise on the swallowing function and psychological condition in stroke survivors compared to no exercise intervention and the Shaker exercise.
Materials and methods: The Cochrane Library, PubMed, Web of Science, EMBASE, CINAHL and four Chinese databases were searched for randomized controlled trails (RCTs) and quasi-RCTs from inception to February 2022.
Results: After screened and assessed the methodological quality of the studies, nine studies with 548 stroke survivors were included in the systematic review. 8 studies were included in the meta-analysis using RevMan 5.4 software. The mean difference (MD) or standardized mean difference (SMD) with 95% confidence intervals (CIs) were calculated. The results revealed that CTAR exercise is effective in improving swallowing safety (MD, −1.43; 95% CI, −1.81 to −1.06; P < 0.0001) and oral intake ability (SMD, −1.82; 95% CI, −3.28 to −0.35; P = 0.01) compared with no exercise intervention, CTAR exercise is superior to Shaker exercise in improving swallowing safety (MD, −0.49; 95% CI, −0.83 to −0.16; P = 0.004). The psychological condition in CTAR group is significant better than the control group (MD, −5.72; 95% CI, −7.39 to −4.05; P < 0.00001) and Shaker group (MD, −2.20; 95% CI, −3.77 to −0.64; P = 0.006).
Conclusions: Our findings support CTAR exercise as a superior therapeutic exercise for post-stroke dysphagia rehabilitation than Shaker exercise. More high-qualities RCTs from larger multicenter are needed to analysis the effects of CTAR exercise in patients with different type and phase of stroke and explore the optimal training dose.
Introduction
Dysphagia is any difficulty during bolus transport from the oral cavity to the stomach in the swallowing process (1). It is one of the most common complications that affecting 37–78% stroke survivors (2, 3) and is strongly associated with a high risk of aspiration pneumonia, malnutrition, and increased mortality (4–6). For many patients, dysphagia resolves spontaneously within 14 days, but 50.9% of dysphagia persist at discharge, and 15% of patients still have dysphagia at 1 month of the onset of stroke, 11–50% still have dysphagia at 6 months (6–8). The residual functional deficits not only seriously affect the quality of life of stroke survivors, but also is a major cause of post-stroke depression and social isolation (9). Thus, exploring effective dysphagia rehabilitation methods is an essential concern of post-stroke care.
Therapeutic exercises that stimulating and strengthening the swallow-related muscles are strongly recommended for dysphagia rehabilitation (10). The suprahyoid muscle complex (SHM) is critical during the pharyngeal phase of swallowing as it controls the movement of the larynx, hyoid bone, and epiglottis to protect the airway, and the opening of upper esophageal sphincter to allow bolus transfer into the esophageal (11). For stroke survivors, based on the neuroplasticity principle, regular and repetitive resistance training can lead to the strength of swallowing muscles and may be effective on the recovery of sensorimotor control system of swallowing (12). Thus, SHM strengthening exercise has been a focus of research and practice in post-stroke dysphagia rehabilitation. The head-lift exercise (HLE), also called Shaker exercise, is the most commonly used SHM strengthening exercise that has been demonstrated to be effective in strengthen the SHM, reduce pyfiform sinus residue and increase upper esophageal sphincter opening in dysphagia (13, 14). It requires patients to lift their heads against gravity to look at their toes in a supine position (15). But Shaker exercise has some drawbacks. When patients raise their heads, the sternocleidomastoid muscle are inevitably activated, causing unnecessary muscle fatigue and physical effort (16). For elderly patients who are physically frail, repeated lifting of and holding their heads up is challenging. Several studies reported that participants showed a low compliance and felt frustrated (16–18). Therefore, chin tuck against resistance (CTAR) exercise was introduced as a new rehabilitative exercise that could substitute for Shaker exercise by Yoon et al. (19). For CTAR exercise, the patient is instructed by speech and language therapists to tuck their chin toward their manubrium sterni to squeeze an inflatable rubber ball that placed between their chin and chest while seated. Similar to Shaker exercise, CTAR exercise includes isometric and isokinetic tasks. The isokinetic task is the squeezing of the ball as hard as possible for successive repetitions, while the isometric task is the squeezing of the ball and sustaining the squeeze for a period of time (19). People can choose the appropriate resistance according to their physical condition. Several studies have been conducted to validate the biomechanics effects of CTAR exercise and compared with Shaker exercise using surface electromyography (sEMG), which demonstrate CTAR is effective in stimulating the SHM but there are inconsistent conclusions in the comparison of CTAR and Shaker exercise (20, 21). To our knowledge, a previous systematic review (22) summarized the applications of CTAR exercise, in which both healthy participants and patients with dysphagia were included. Due to the high heterogeneity and limit number of studies, they only performed a descriptive qualitative analysis that CTAR is more selective in the activation of the SHM than Shaker exercise. But whether the strength of SHM can elicit the improvement of swallowing function still needs to be verified, as post-stroke dysphagia is functional dysphagia caused by hemisphere damage rather than organic disorder. Additionally, we noticed that a series of RCTs that explore the effects of CTAR exercise in stroke survivors were reported recently. Therefore, the objective of this study was to included the newly published studies and performed a meta-analysis of the results on the effects of CTAR exercise in stroke survivors to provide a reliable evidence for the policy and practice development of post-stroke dysphagia rehabilitation.
Materials and methods
This systematic review and meta-analysis was conducted based on the Cochrane Handbook for Systematic Reviews of Interventions (https://training.cochrane.org/handbook), and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) (23, 24). This review was previously registered on PROSPERO (CRD42021265975).
Search strategy
We systematically searched the following electronic databases from inception to February 2022: Cochrane Library Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, Web of Science (WOS), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) for studies published in English, China Biology Medicine disc (CBM), China National Knowledge Infrastructure (CNKI), WanFang, and VIP database for studies published in Chinese. The following search terms were used: “chin tuck OR chin down OR CTAR” AND “stroke OR apoplexy OR cerebrovascular accident OR CVA OR brain vascular accident OR brain infarction OR cerebral infarction OR ischemic stroke OR hemorrhagic stroke” AND “dysphagia OR deglutition disorder OR swallowing disorder OR swallowing dysfunction OR impaired swallowing.” We also searched the reference lists of the included studies and Google Scholar to identify relevant studies.
Eligibility criteria
Studies were included if they met the following criteria: (1) study design: a randomized controlled trial (RCT) or a quasi-RCT; (2) participants: adults diagnosed with post-stroke dysphagia that confirmed by a videofluoroscopy swallowing study (VFSS) or fiberoptic endoscopic evaluation of swallowing (FESS) or standardized dysphagia assessment instrument; (3) intervention and comparison: a comparison of CTAR exercise with no exercise intervention or with Shaker exercise; and (4) outcome measures: the primary outcome are the swallowing safety and oral intake ability as measured by standardized dysphagia assessment scale; the second outcome is psychological condition as measured by Self-Rating Depression Scale (SDS). There was no restriction on the language of publication.
Studies were excluded if they (1) were reviews, case reports, conference abstracts, expert opinion articles or peer-review publications or if (2) their full texts were not available or valid outcome data could not be extracted.
Study selection and quality assessment
First, all searched studies were imported to NoteExpress 3.2.0 to delete duplicates. Then, two reviewers (L.J. and W.Q.Y.) independently completed the title and abstract screening to exclude irrelevant studies, followed by full-text screening according to the eligibility criteria. All reviewers were familiar with stroke rehabilitation and had taken an evidence-based training course.
The methodological quality of the included studies was assessed by two reviews (T.J. and Z.W. Q) independently. The Cochrane risk of bias tool for randomized controlled trials was used for 8 RCTs, in which 5 domains were examined: (a) selection bias, (b) performance bias, (c) detection bias, (d) attrition bias, and (e) reporting bias (25, 26). The risk of bias for each domain was reported as low, high, or unclear. The Joanna Briggs Institute (JBI) critical appraisal checklist was used for 1 quasi-experimental study (27). Discrepancies were resolved by discussion.
Data extraction
Two reviewers (L.J. and W.Q.Y.) independently extracted the data using a predefined form, and the following data were collected: first author's name, publication year, sample size, participants' characteristics (age, type and phase of stroke), protocols for the intervention and control groups (device, frequency, repetition, and duration), outcome measures and results. The authors were contacted via email if incomplete data were provided for analysis.
Statistical analysis
According to our objective, two comparisons were performed: CTAR exercise vs. no exercise intervention and CTAR exercise vs. Shaker exercise. Based on the Cochrane Handbook for Systematic Reviews of Interventions (version 6.3, 2022), meta-analysis consists of two stage. First, we calculated the mean change and standard difference from baseline to post-intervention in each group. The formulas were used if the standard difference was not presented (28):
To ensure that different scales represented the same effect direction for outcome measurement, we chose the most commonly used scale to determine the effect direction, and the mean change in scale scores with different directions was multiplied by −1. Then, the meta-analysis was conducted using Review Manager software (RevMan, version 5.4). Mean difference (MD) (when all studies measure the outcome using the same scale) or standardized mean difference (SMD) (when studies measure the outcome using different scales) was calculated for each study and synthesized into a pooled effect size with 95% confidence interval (CI).
The heterogeneity across the studies was analyzed by statistical testing with I2. I2 values <40, 40–75%, and >75% were considered low, moderate, and high heterogeneity, respectively. Random effects models were used to perform meta-analyses. In this study, a P-value < 0.05 was considered statistically significant.
Results
Study selection
A total of 154 articles were identified from databases, and 58 duplicates were removed using the duplicate finder tool in NoteExpress 3.4.0. Seventy-two studies were excluded after title and abstract screening, one study was excluded because full text was not available. Another 17 studies were excluded after reading the full text. One study from USA (29), one from Singapore (20), one from Turkey (30), and one from Netherlands (31) were excluded due to the wrong population. One study (32) from Greece was excluded because its intervention consisted of CTAR exercise and thermal-tactile stimulation so we could not evaluate the effects of CTAR exercise separately. Two ongoing RCTs (33, 34) from the UK and India were excluded. Finally, 9 studies were included in this systematic review, including 8 RCTs and one quasi-RCT. Five studies (35–39) came from China, three studies (40–42) came from South Korea, and one study (43) came from India. 8 studies was included in the meta-analysis as Lai's study (38) used the dysphagia screening tool rather than standardized assessment scale as outcome measures, which may limit the accuracy of the results. The PRISMA flow diagram shows the study selection process (see Figure 1).
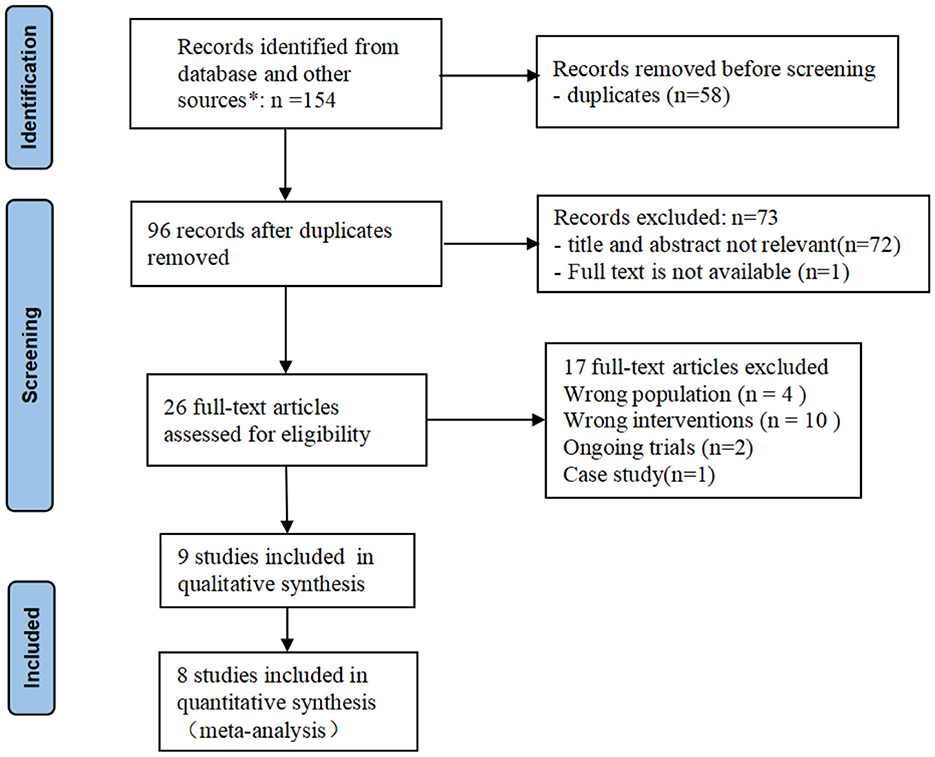
Figure 1. PRISMA flow diagram of study selection. *The number of records identified from each database searched: Cochrane Library: n = 11; Pubmed: n = 11; Web of science: n = 37; EMBASE: n = 27; CINAHL: n = 12; CBM: n = 2; CNKI: n = 35; WanFang: n = 13; VIP: n = 6.
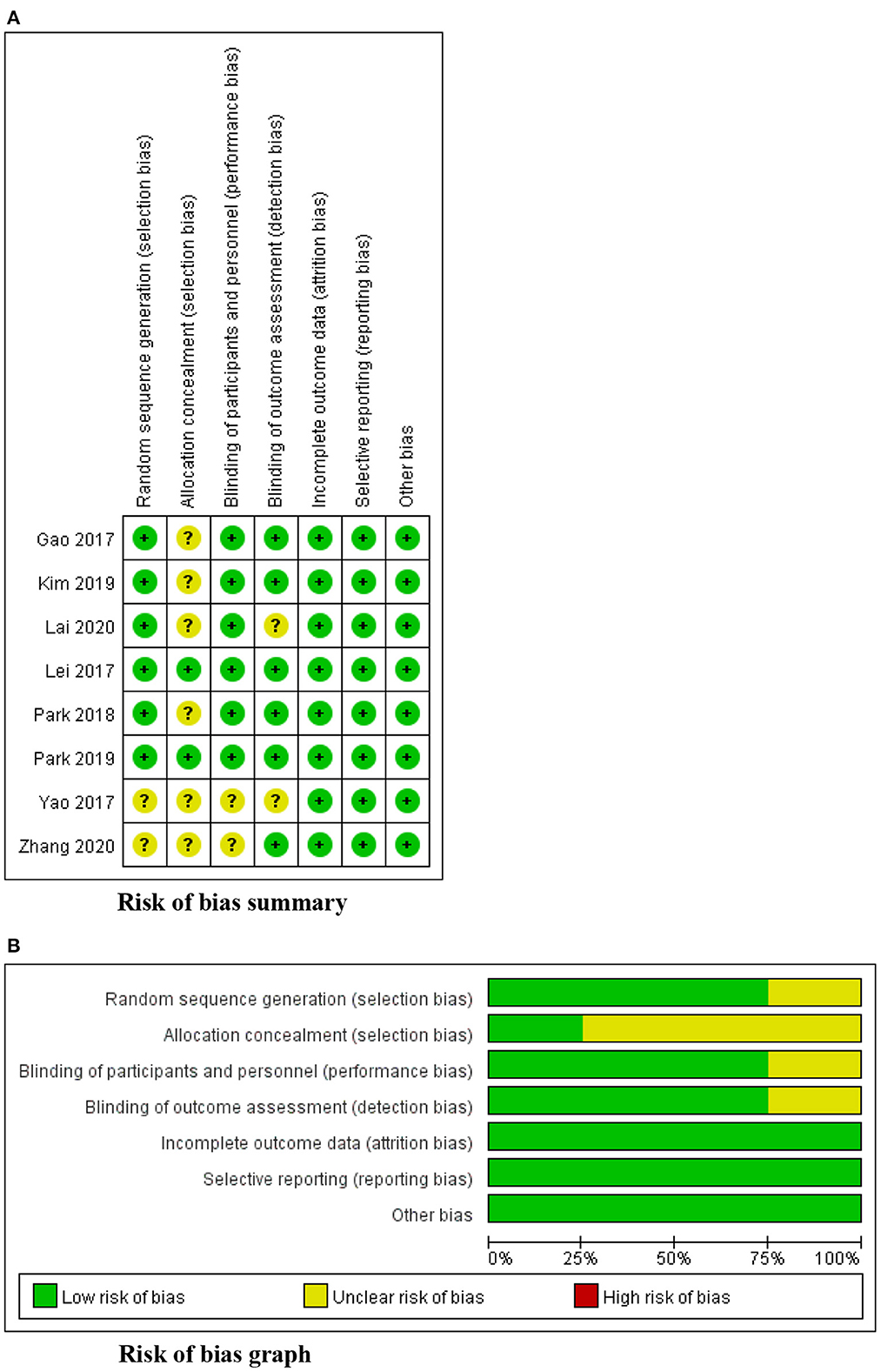
The risk of bias
Figure 2 displays a summary of the risk of bias assessment for the 8 RCTs. Two studies provided insufficient details about their methods of randomization (35, 37). Only two studies reported the process of allocation concealment (36, 41). None of the 8 RCTs reported the blinding of participants and outcome assessments, but considering that participant blinding was impossible in exercise-based intervention, six studies were determined to have a low risk of performance bias (36–42), and the other two studies were considered to have a high risk of between-group sample contamination because of their unclear randomization and allocation (35, 37). For detection bias, six studies measured swallowing function using a standard evaluation scale based on VFSS (35, 36, 39–42); thus, they were considered to have a low risk of detection bias. No selective reporting and other bias were identified. The overall appraisal of one quasi-experimental study was “include.”
Characteristics of the included studies
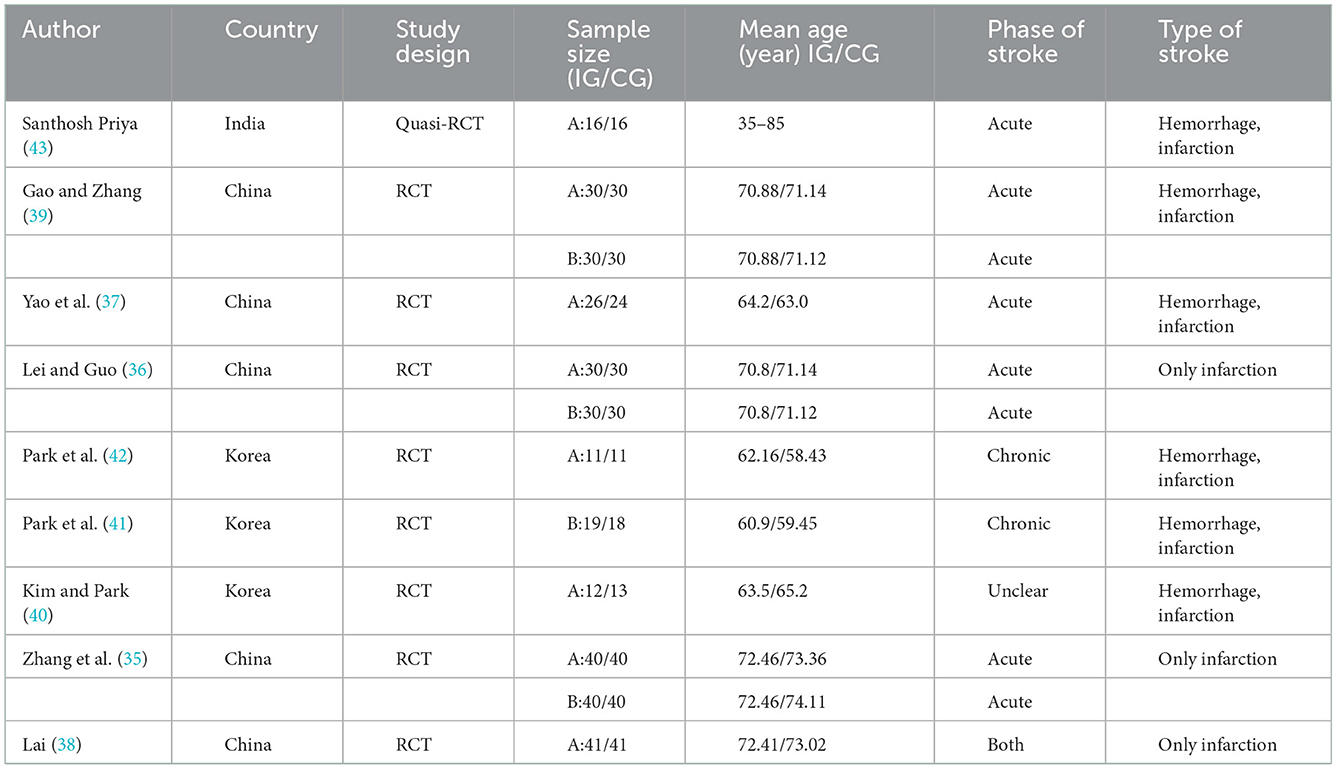
The characteristics of participants in the 9 included studies are shown in Table 1. A total of 548 participants were included, with sample sizes of each study ranging from 22 to 120. Five studies included both hemorrhagic and ischemic stroke patients (37, 40–43), and four studies included only ischemic stroke patients (35, 36, 38, 39). The post-stroke time varied from 4 days to 63 weeks.
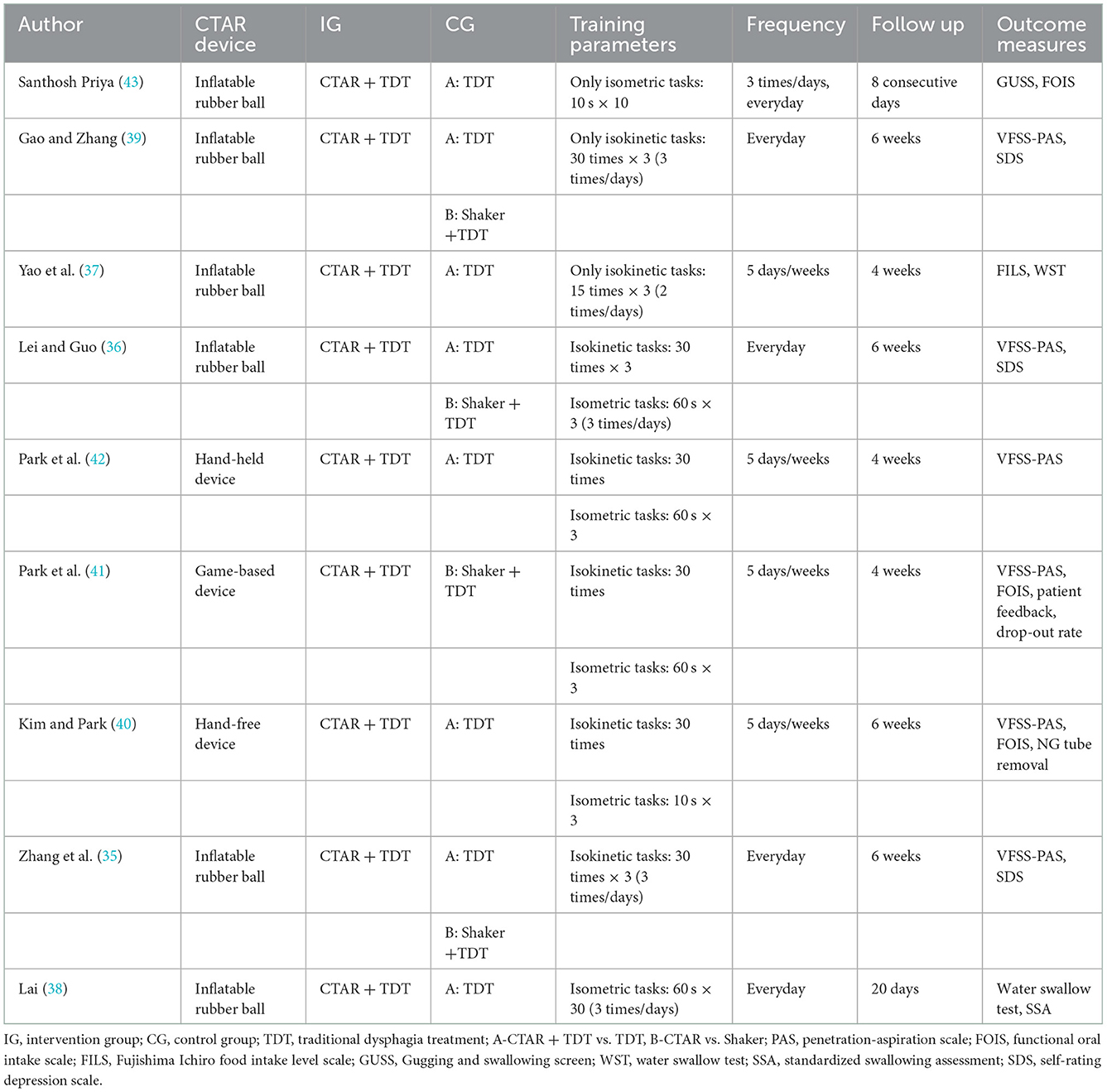
The characteristics of the intervention are shown in Table 2. Of the included 9 studies, 3 studies were three-arm trials (35, 39, 42). Thus, a total of 12 datasets were analyzed. Eight of the datasets (411 patients) compared CTAR exercise combined with traditional dysphagia treatments (TDT) vs. only TDT (e.g., oral facial massage, thermal-tactile stimulation, and transcranial direct current stimulation). The other four datasets (137 patients) compared the effects of CTAR exercise vs. Shaker exercise.
Regarding CTAR intervention, six studies used an inflatable rubber ball placed between the chin and the sternum (35–39, 43), 1 study used a hand-held flexible resistance bar (42), 1 study used a modified hand-free resistance bar secured to a desk surface (40), and 1 study used a hand-held resistance bar connected to a game-based PC tablet screen (41). Only the game-based device could adjust the intensity of training resistance. For the training protocols, 3 studies involved only isokinetic tasks (35, 37, 39), 2 studies involved only isometric tasks (38, 43), and the other 4 studies involved both (36, 40–42). The isokinetic task was 1 set of 30 consecutive squeezes, while the isometric task was the holding of the squeeze from 10 to 60 s for 3 to 10 repetitions. The training frequency varied from 1×/day for 5 days/weeks to 3×/day for 7 days/weeks. The duration of treatment varied from 8 days to 6 weeks.
For outcome measures, swallowing function and psychological condition were primary outcomes. A total of 11 different scales were used to measure swallowing function: (1) Swallowing safety: Six studies used the Penetration-Aspiration Scale (PAS) based on VFSS (35, 36, 39–42). The PAS is a widely used standard assessment scale to evaluate swallowing safety, which includes 8 points to reflect the depth of bolus penetration into the airway and the airway response to invasion. Higher scores indicate higher levels of airway aspiration and greater aspiration severity (44). (2) Oral intake ability: Three studies used the Functional Oral Intake Scale (FOIS) (40, 41, 43), and 1 study used the Fujishima Ichiro Food Intake Level Scale (FILS) (37). The FOIS is a 7-point scale that describes the feeding performance of oral intake (45), while the FILS is a 10-point scale. For both scales, a score of 1 indicates total nasogastric (NG) feeding, and higher scores represent better oral intake ability. (3) dysphagia screening tool: Two studies used the Water Swallow Test (WST) (37, 38), 1 study used the Gugging Swallowing Screen (GUSS) (43), and 1 study used the Standardized Swallowing Assessment (SSA) (38). The WST, GUSS and SSA are all simple bedside tools for dysphagia screening that are commonly used to identify high-risk populations with dysphagia and estimate the severity of dysphagia (46). Regarding psychological condition, 3 studies used the Self-depression scale (35, 36, 39).
Other outcomes included the compliance of patients (41), the score on a self-reporting questionnaire of enjoyment and physical fatigue (41), the NG tube removal rate (40, 41) and the physiological changes during the detailed phases of the swallowing process (41, 42).
Data synthesis
CTAR exercise vs. no exercise intervention
Swallowing safety as measured by PAS scores
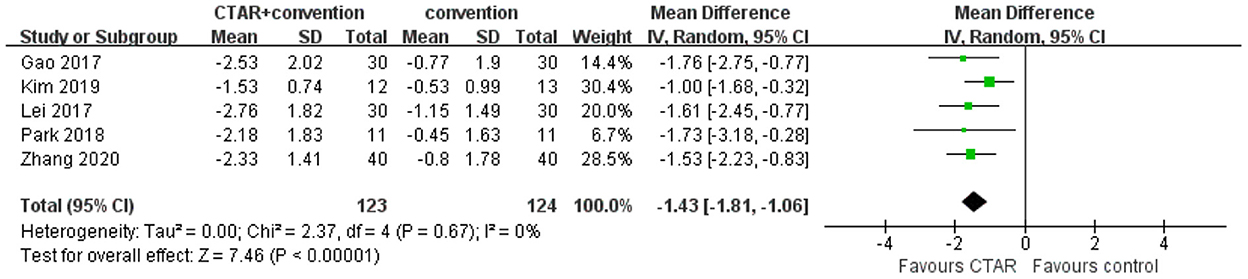
The aggregated results of 5 studies (123 patients in CTAR group and 124 in the control group) showed that CTAR group had a significantly lower PAS score than the no exercise intervention group (MD, −1.43; 95% CI, −1.81 to −1.06; P < 0.00001; I2, 0%) (see Figure 3), which suggested that patients in CTAR group had better swallowing safety and a lower risk of aspiration. The studies were homogenous.
Oral intake ability as measured by FOIS or FILS scores
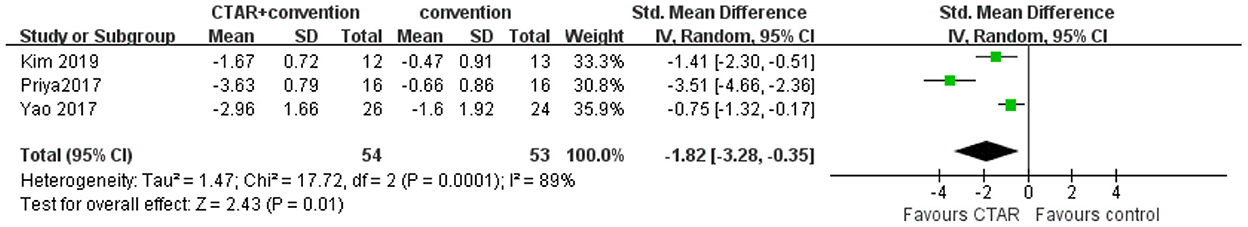
The aggregated results of 3 studies (54 patients in CTAR group and 53 in the control group) showed a greater intervention-induced effect of oral intake ability in the CTAR group than that of the control group (SMD, −1.82; 95% CI, −3.28 to −0.35; P = 0.01; I2, 89%) (see Figure 4). The heterogeneity between studies was high.
Psychological condition as measured by SDS scores
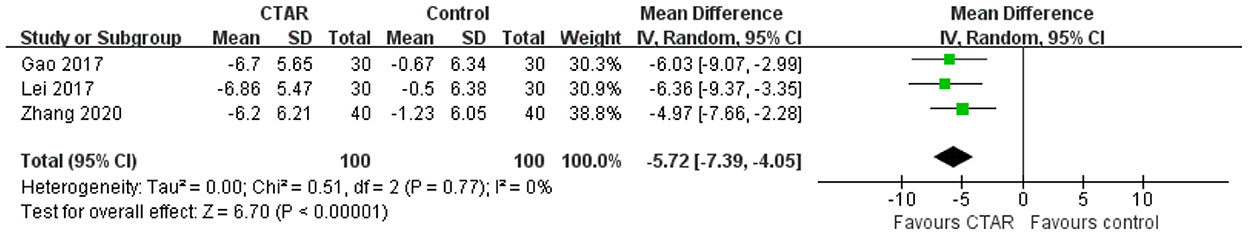
Three studies measured psychological condition using the SDS. The meta-analysis showed that the SDS scores in CTAR group were significantly lower than those in the control group (MD, −5.72; 95% CI, −7.39 to −4.05; P < 0.00001; I2 = 0%) (see Figure 5), which supported that the psychological condition of patients in CTAR group was significantly better than that in the control group. The studies were considered homogenous.
Other outcomes
Park et al. (42) evaluated the detailed phases of the complete swallowing process during a VFSS and found that CTAR group showed significantly better scores in the oral cavity and laryngeal elevation/epiglottic closure and less residue in the valleculae and pyriform sinuses. Kim et al. (40) reported that the rates of NG tube removal in CTAR and control groups were 25 and 15%, respectively.
CTAR exercise vs. Shaker exercise
Swallowing safety as measured by PAS scores
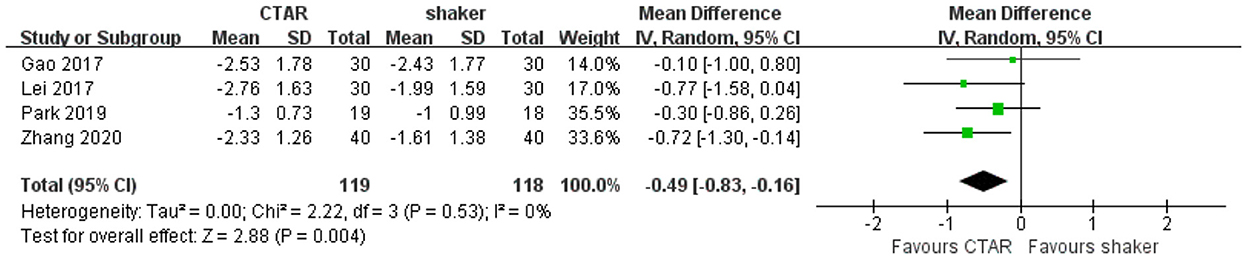
Four studies (119 patients in CTAR group and 118 in Shaker group) used the PAS to assess swallowing safety and compared the effects of CTAR and shaker exercises. The aggregated results showed that the change scores of CTAR group was significantly greater than that of Shaker group (MD, −0.49; 95% CI, −0.83 to −0.16; P = 0.004; I2, 0%) (see Figure 6), which suggested that CTAR exercise was more effective in improving swallowing safety than Shaker exercise. The studies were considered homogenous.
Oral intake ability
One study measured oral intake ability using the FOIS, and the results showed no significant difference in FOIS scores between CTAR and shaker group.
Psychological condition as measured by SDS scores
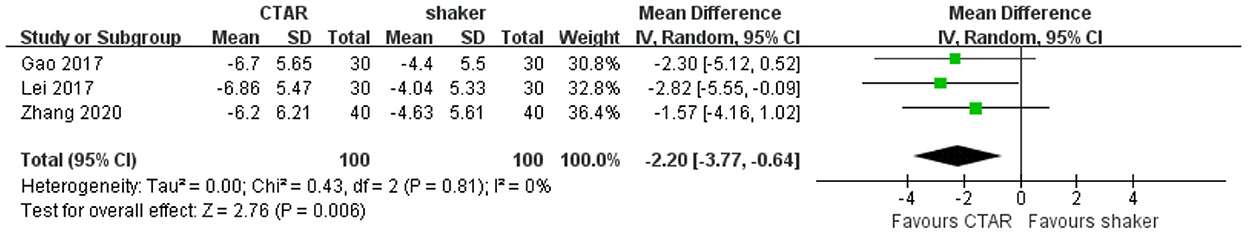
Three studies compared the SDS scores of CTAR and shaker groups. The aggregated results showed that the psychological condition of patients in CTAR group was significantly better than that of patients in Shaker group (MD, −2.20; 95% CI, −3.77 to −0.64 P = 0.006; I2 = 0%) (see Figure 7). The studies were considered homogenous.
Other outcomes
Park et al. (41) reported that CTAR group showed a significantly lower drop-out rate, better feedback in terms of motivation and interest/enjoyment, and lower physical fatigue than Shaker group.
Discussion
The purpose of this systematic review was to investigate the effects of CTAR exercise on swallowing function and psychological condition of stroke survivors. Overall, the results showed a positive effect of CTAR exercise on improving swallowing safety, oral intake ability, and psychological condition compared with no exercise intervention. Compared with Shaker exercise, the results of the meta-analyses suggested that CTAR exercise was more effective in improving swallowing safety and psychological condition.
To our knowledge, this is the first systematic review and meta-analysis to examine the clinical effects of CTAR exercise in stroke survivors and confirm that CTAR exercise has superior effects than Shaker exercise in post-stroke dysphagia rehabilitation. The main results are consistent with a previous systematic review, which compared the effects of CTAR exercise in improving swallowing safety compared with no exercise intervention (47). But they did not compare oral intake ability and the effect of CTAR with Shaker exercise. Our findings demonstrated that strengthening exercise of SHM is not only fit for dysphagia that resulting from upper esophageal sphincter dysfunction, but also effective for rehabilitation of post-stroke dysphagia due to hemisphere damage. Previous studies that evaluated the biochemical changes of CTAR exercise reported that for instant muscle performance, CTAR exercise could exhibit significantly higher instant mean and max muscle fatigue of the SHM, with less stimulation of the sternocleidomastoid muscle than performing Shaker exercise (20). And after 8-week training, a significant greater anterior tongue pressure, and maximum mouth opening were observed in participants performing CTAR exercise compared to Shaker exercise (30, 31). Our findings, focus on the clinical effects of CTAR exercise in stroke survivors, supported that performing CTAR exercise to repetitive stimulating the SHM could translate into the biomechanics changes during the swallowing process and subsequently lead to increased swallowing safety and better recovery of oral intake ability, which is meaningful to avoid the risk of penetration-aspiration and improve the quality of life of stroke survivors. Additionally, another strength of our study is that we verify patients performing CTAR exercise have better psychological condition compared with no exercise intervention and Shaker exercise, which can be explained by two aspects. First, studies have shown that post-stroke depression is strongly associated with the severity of functional impairment, so better improvements in swallowing function can induce a positive effect on the patients' psychological condition (48). Second, in contrast to a considerable number of patients feeling frustrated with their failure to perform Shaker exercise, patients provided positive subjective feedback about CTAR exercise, as they could complete the exercise with suitable training intensity based on their physical condition, and CTAR exercise was more interesting and motivating, especially when the exercise was combined with computer games (19, 41). Therefore, we recommend CTAR exercise as the first choice rehabilitative exercise for stroke survivors with dysphagia. For patients who cannot sit, Shaker exercise can be used as an alternative.
However, we also found that the training protocol varied greatly in previous CTAR studies. Exercised-based therapy utilizes the neuroplasticity principle that repetition, intensity, frequency and duration are especially important to achieve muscular hypertrophy (12, 49). Incorrect dose prescription may cause insufficient or negative effects. Blair et al. (50) considered it is unlikely to establish an optimal dosing for a training exercise, the dosing should be determined by the patient's age, primary dysphagia etiology, comorbidities, and physical fitness level. But several studies have demonstrated that it takes at least 4 weeks of resistance training to induce physiological changes in stroke patients (13, 51, 52). Most studies included in this review conducted a 4- or 6-week intervention (35–37, 39–42), and the other 2 studies conducted interventions for only 8 and 20 days (38, 43). For the repetitions, 30 consecutive repetitions for the isokinetic exercise and 3 sustained 60-s squeezes for the isometric exercise were adopted by most studies, which was consistent with the recommended dose for Shaker exercise (49). However, 2 studies performed only isometric tasks (38, 43), while the other 3 studies performed only isokinetic tasks (35, 37, 39). The training frequency also varied from 1×/day for 5 days/weeks to 3×/day for 7 days/weeks. Therefore, future research should continue to explore the relationship between the training dose and the training efficacy and establish universal standard parameters for CTAR exercise to maximize patient benefits.
This study has several limitations. First, there were heterogeneity between the included studies in terms of the patient conditions, type of stroke, and time post-stroke. The meta-analysis of oral intake ability and the overall severity of dysphagia showed substantial statistical heterogeneity, which may be due to the different measurement scales used in the studies. As Egger's publication bias test is known to have limit efficiency for meta-analysis that involving <10 studies, we did not conduct the test. Second, some studies had a high risk of bias in terms of randomization and allocation, which may limit the quality of the evidence. Third, most studies included in this review were from South Korea and China, which may limit the generalization of our findings. But this does not mean that no CTAR studies were performed in other countries. As CTAR exercise was proposed in 2014, most studies were conducted in healthy adults. We excluded these studies during study selection. Two CTAR trials from the UK and India were registered in the International Clinical Trials Registry Platform of the World Health Organization (WHO) recently. Thus, research on the effects of CTAR exercise on post-stroke dysphagia is continuously increasing, and more high-quality RCTs from different countries are warranted to enrich our findings.
Conclusion
In conclusion, the findings of this study suggest that CTAR exercise is an effective therapeutic method for post-stroke dysphagia rehabilitation and is superior to Shaker exercise in improving swallowing safety with positive effect to patients' psychological condition. Larger multicenter RCTs are needed to verify the effects of CTAR exercise in patients with different conditions, types of stroke, and times post stroke. It could also be worth to explore the optimal training dose of CTAR exercise to establish effective treatment protocols.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JL and QW: screened the title, abstract, full-text of the identified studies, and drafted the manuscript. JT: performed the data extraction. WaZ and YiG: evaluated the risk bias of included studies. XC, WeZ, and YaG: performed the data verification. LZ: conceptualization, supervision, and funding acquisition. All authors reviewed and approved the final manuscript.
Funding
This work was supported by the National Social Science Fund of China (Grant number: 21&ZD188). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We would like to thank AJE [https://www.aje.cn/#] for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CTAR, chin tuck against resistance; HLE, head lift exercise; SHM, suprahyoid muscle complex; VFSS, video fluoroscopic swallowing study; TDT, traditional dysphagia treatment; PAS, penetration-aspiration scale; FOIS, functional oral intake scale; FILS, Fujishima Ichiro food intake level scale; GUSS, Gugging Swallowing Screen; WST, water swallow test; SSA, standardized swallowing assessment; SDS, self-rating depression scale.
References
1. Umay E, Eyigor S, Ertekin C, Unlu Z, Selcuk B, Bahat G, et al. Best practice recommendations for stroke patients with dysphagia: a delphi-based consensus study of experts in Turkey-Part II: rehabilitation. Dysphagia. (2021) 36:800–20. doi: 10.1007/s00455-020-10218-8
2. Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. (2010) 9:105–18. doi: 10.1016/S1474-4422(09)70266-2
3. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. (2005) 36:2756–63. doi: 10.1161/01.STR.0000190056.76543.eb
4. Smithard DG, O'Neill PA, Morris J, Wyatt R, England R, Martin DF. Complications and outcome after acute stroke: does dysphagia matter? Stroke. (1996) 27:1200–4.
5. Cohen DL, Roffe C, Beavan J, Blackett B, Fairfield CA, Hamdy S, et al. Post-stroke dysphagia: a review and design considerations for future trials. Int J Stroke. (2016) 11:399–411. doi: 10.1177/1747493016639057
6. Arnold M, Liesirova K, Broeg-Morvay A, Meisterernst J, Schlager M, Mono ML, et al. Dysphagia in acute stroke: incidence, burden and impact on clinical outcome. PLoS ONE. (2016) 11:e148424. doi: 10.1371/journal.pone.0148424
7. Smithard DG, O'Neill PA, England RE, Park CL, Wyatt R, Martin DF, et al. The natural history of dysphagia following a stroke. Dysphagia. (1997) 12:188–93.
8. Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis. (2000) 10:380–6. doi: 10.1159/000016094
9. Towfighi A, Ovbiagele B, Husseini NE, Hackett ML, Jorge RE, Kissela BM, et al. Poststroke depression: a scientific statement for healthcare professionals from the American heart association/American stroke association. Stroke. (2017) 48:e30. doi: 10.1161/STR.0000000000000113
10. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke J Cerebral Circul. (2016) 2016:e98. doi: 10.1161/STR.0000000000000098
11. Lang IM. Brain stem control of the phases of swallowing. Dysphagia. (2009) 24:333–48. doi: 10.1007/s00455-009-9211-6
12. Robbins J, Butler SG, Daniels SK, Gross RD, Rosenbek J. Swallowing and Dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear R. (2008) 51:S276–300. doi: 10.1044/1092-4388(2008/021)
13. Park JS, Hwang NK, Oh DH, Chang MY. Effect of head lift exercise on kinematic motion of the hyolaryngeal complex and aspiration in patients with dysphagic stroke. J Oral Rehabil. (2017) 44:385–91. doi: 10.1111/joor.12492
14. Choi JB, Shim SH, Yang JE, Kim HD, Lee DH, Park JS. Effects of Shaker exercise in stroke survivors with oropharyngeal dysphagia. NeuroRehabilitation. (2017) 41:753–7. doi: 10.3233/NRE-172145
15. Shaker R, Easterling C, Kern M, Nitschke T, Massey B, Daniels S, et al. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. (2002) 122:1314–21. doi: 10.1053/gast.2002.32999
16. White KT, Easterling C, Roberts N, Wertsch J, Shaker R. Fatigue analysis before and after shaker exercise: physiologic tool for exercise design. Dysphagia. (2008) 23:385–91. doi: 10.1007/s00455-008-9155-2
17. Yoshida M, Groher ME, Crary MA, Mann GC, Akagawa Y. Comparison of surface electromyographic (sEMG) activity of submental muscles between the head lift and tongue press exercises as a therapeutic exercise for pharyngeal dysphagia. Gerodontology. (2007) 24:111–6. doi: 10.1111/j.1741-2358.2007.00164.x
18. Easterling C, Grande B, Kern M, Sears K, Shaker R. Attaining and maintaining isometric and isokinetic goals of the Shaker exercise. Dysphagia. (2005) 20:133–8. doi: 10.1007/s00455-005-0004-2
19. Yoon WL, Khoo JK, Rickard LS. Chin tuck against resistance (CTAR): new method for enhancing suprahyoid muscle activity using a Shaker-type exercise. Dysphagia. (2014) 29:243–8. doi: 10.1007/s00455-013-9502-9
20. Sze WP, Yoon WL, Escoffier N, Rickard LS. Evaluating the training effects of two swallowing rehabilitation therapies using surface electromyography: chin tuck against resistance (CTAR) exercise and the shaker exercise. Dysphagia. (2016) 31:195–205. doi: 10.1007/s00455-015-9678-2
21. Chang MC, Park S, Cho JY, Lee BJ, Hwang JM, Kim K, et al. Comparison of three different types of exercises for selective contractions of supra- and infrahyoid muscles. Sci Rep. (2021) 11:7131. doi: 10.1038/s41598-021-86502-w
22. Park JS, Hwang NK. Chin tuck against resistance exercise for dysphagia rehabilitation: a systematic review. J Oral Rehabil. (2021) 48:968–77. doi: 10.1111/joor.13181
23. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372: n160. doi: 10.1136/bmj.n160
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 10: n71. doi: 10.1136/bmj.n71
25. Higgins J, Savović J, Page M, Elbers R, Sterne J. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Alberta: Cochrane (2022).
26. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 10:l4898. doi: 10.1136/bmj.l4898
27. Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. (2020). doi: 10.46658/JBIMES-20-04
28. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Alberta: Cochrane (2022).
29. Hughes T, Watts CR. Effects of 2 resistive exercises on electrophysiological measures of submandibular muscle activity. Arch Phys Med Rehabil. (2016) 97:1552–7. doi: 10.1016/j.apmr.2015.11.004
30. Kilinç HE, Arslan SS, Demir N, Karaduman A. The effects of different exercise trainings on suprahyoid muscle activation, tongue pressure force and dysphagia limit in healthy subjects. Dysphagia. (2020) 35:717–24. doi: 10.1007/s00455-019-10079-w
31. Kraaijenga SA, van der Molen L, Stuiver MM, Teertstra HJ, Hilgers FJ, van den Brekel MW. Effects of strengthening exercises on swallowing musculature and function in senior healthy subjects: a prospective effectiveness and feasibility study. Dysphagia. (2015) 30:392–403. doi: 10.1007/s00455-015-9611-8
32. Ghemulet Politi C, Hamdy S, Papathanasiou I, Michou E. Effects of a sensorimotor short-term rehabilitation programme on dysphagia after cerebral infraction. A comparative study. Eur Stroke J. (2019) 4:59.
33. Kumari M, Sugumaran R. Effect of CTAR Exercise in Improving Swallowing Capacity Among Stroke Patients with Swallowing Difficulty. Geneva: World Health Organization (2021).
34. Simpson V. Chin Tuck Against Resistance with Feedback: Swallowing Rehabilitation in Frail Older People Admitted to Hospital with Pneumonia. A Feasibility Randomised Controlled Study of Two Types of Rehabilitation Exercise Using Chin Tuck Against Resistance to Improve Swallowing, Eating and Drinking. Geneva: World Health Organization (2022).
35. Zhang J, You L, Zhang SA, Song CH, Zhang Q. Effect of chin tuck against resistance and Shaker training on dysphagia and mental state after cerebral infarction. J Clin Pathol Res. (2020) 40:2636–41. doi: 10.3978/j.issn.2095-6959.2020.10.022
36. Lei YY, Guo XX. Effects of chin tuck against resistance exercise on patients with dysphagia after cerebral infarction. Chin J Gerontol. (2017) 37:2443–5. doi: 10.3969/j.issn.1005-9202.2017.10.041
37. Yao YH, Wu H, Shen J. Effect of resistance training combined with swallowing therapy on dysphagia after stroke. J Jiaxing Univ. (2017) 29:137–40.
38. Lai HM. Effects of electrical stimulation combined with resistance training in dysphagia after cerebral infarction. Chronic Pathematol J. (2020) 21:465–7. doi: 10.16440/j.cnki.1674-8166.2020.03.055
39. Gao J, Zhang HJ. Effects of chin tuck against resistance exercise vs. Shaker exercise on dysphagia and psychological state after cerebral infarction. Eur J Phys Rehabil Med. (2017) 53:426–32. doi: 10.23736/S1973-9087.16.04346-X
40. Kim HH, Park JS. Efficacy of modified chin tuck against resistance exercise using hand-free device for dysphagia in stroke survivors: a randomised controlled trial. J Oral Rehabil. (2019) 46:1042–6. doi: 10.1111/joor.12837
41. Park JS, Lee G, Jung YJ. Effects of game-based chin tuck against resistance exercise vs. head-lift exercise in patients with dysphagia after stroke: an assessor-blind, randomized controlled trial. J Rehabil Med. (2019) 51:749–54. doi: 10.2340/16501977-2603
42. Park JS, An DH, Oh DH, Chang MY. Effect of chin tuck against resistance exercise on patients with dysphagia following stroke: a randomized pilot study. NeuroRehabilitation. (2018) 42:191–7. doi: 10.3233/NRE-172250
43. Santhosh Priya N. A Study to Assess the Effectiveness of Chin Tuck Against Resistance (CTAR) Exercise in Improving Swallowing Ability Among Cerebrovascular Accident Patients with Dysphagia at Selected Hospital, Coimbatore. Coimbatore: PSG College of Nursing (2017).
44. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. (1996) 11:93–8.
45. Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. (2005) 86:1516–20. doi: 10.1016/j.apmr.2004.11.049
46. Park YH, Bang HL, Han HR, Chang HK. Dysphagia screening measures for use in nursing homes: a systematic review. J Korean Acad Nurs. (2015) 45:1–13. doi: 10.4040/jkan.2015.45.1.1
47. Speyer R, Cordier R, Sutt A, Remijn L, Heijnen BJ, Balaguer M, et al. Behavioural interventions in people with oropharyngeal dysphagia: a systematic review and meta-analysis of randomised clinical trials. J Clin Med. (2022) 11:685. doi: 10.3390/jcm11030685
48. Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke. (2014) 9:1026–36. doi: 10.1111/ijs.12356
49. Krekeler BN, Rowe LM, Connor NP. Dose in exercise-based dysphagia therapies: a scoping review. Dysphagia. (2021) 36:1–32. doi: 10.1007/s00455-020-10104-3
50. Blair SN, LaMonte MJ, Nichaman MZ. The evolution of physical activity recommendations: how much is enough? Am J Clin Nutr. (2004) 79:913S−20S. doi: 10.1093/ajcn/79.5.913S
51. Robbins JP, Kays SAM, Gangnon REP, Hind JAM, Hewitt ALM, Gentry LRM, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehab. (2007) 88:150–8. doi: 10.1016/j.apmr.2006.11.002
Keywords: chin tuck against resistance exercise, stroke, rehabilitation, dysphagia, systematic review
Citation: Liu J, Wang Q, Tian J, Zhou W, Gao Y, Chen X, Zhang W, Gao Y and Zhou L (2023) Effects of chin tuck against resistance exercise on post-stroke dysphagia rehabilitation: A systematic review and meta-analysis. Front. Neurol. 13:1109140. doi: 10.3389/fneur.2022.1109140
Received: 27 November 2022; Accepted: 20 December 2022;
Published: 09 January 2023.
Edited by:
Chunping Ni, Fourth Military Medical University, ChinaCopyright © 2023 Liu, Wang, Tian, Zhou, Gao, Chen, Zhang, Gao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanshu Zhou,  zhoulanshu@hotmail.com
zhoulanshu@hotmail.com
†These authors have contributed equally to this work
 Jing Liu
Jing Liu Qiuyi Wang1†
Qiuyi Wang1† Wei Zhang
Wei Zhang Yajing Gao
Yajing Gao Lanshu Zhou
Lanshu Zhou