- 1Department of Neurology, St John's Hospital, Vienna, Austria
- 2Department of Neurology, St Vincent's University Hospital, Dublin, Ireland
- 3Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
- 4Medical Faculty, Sigmund Freud University Vienna, Vienna, Austria
Management of stroke with minor symptoms may represent a therapeutical dilemma as the hemorrhage risk of acute thrombolytic therapy may eventually outweigh the stroke severity. However, around 30% of patients presenting with minor stroke symptoms are ultimately left with disability. The objective of this review is to evaluate the current literature and evidence regarding the management of minor stroke, with a particular emphasis on the role of IV thrombolysis. Definition of minor stroke, pre-hospital recognition of minor stroke and stroke of unknown onset are discussed together with neuroimaging aspects and existing evidence for IV thrombolysis in minor strokes. Though current guidelines advise against the use of thrombolysis in those without clearly disabling symptoms due to a paucity of evidence, advanced imaging techniques may be able to identify those likely to benefit. Further research on this topic is ongoing.
Introduction
A scenario known to every neurologist: a patient with acute onset mild stroke symptoms is admitted to the hospital. Imaging excludes an intracranial hemorrhage. Should intravenous thrombolysis be given? What are the risks and what are the benefits? It is frequently assumed that for those with mild stroke symptoms, risks of thrombolysis outweigh potential benefits. However, despite having “minor” symptoms, one-third of stroke patients were not functionally independent at 90 days when considered too mild to treat for intravenous thrombolysis (1–4). The purpose of this review is to provide an update on the acute treatment of patients with minor stroke with a special focus on intravenous thrombolysis.
Definition of Stroke With Mild Symptoms
The definition of a stroke with mild symptoms or minor stroke (MS) is not standardized. Definitions are often based on the National Institutes of Health Stroke Scale (NIHSS) requiring a score ≤ 1 on every item (5) or utilize certain limits, mostly NIHSS ≤ 6 (6). Other definitions include whether symptoms are disabling or non-disabling, e.g., isolated aphasia or a severe distal paresis of the arm will give a low NIHSS score but are very disabling symptoms.
Further questions arise in differentiating minor stroke from a transient ischemic attack (TIA). In the acute phase, it is not possible to tell whether symptoms will persist or resolve spontaneously. The definition of a TIA from the American Heart and the American Stroke Association from 2002, “a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction” (7) implies the use of an advanced imaging method to differentiate between TIA and minor stroke. This definition will build the basis for the 11th International Classification of Diseases (8). A majority of TIAs are of short duration, and once neurological deficits persist longer than 60 min they resolve in <15% within 24 h (9). Furthermore, only 2% of patients that received placebo in the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study were free of symptoms 24 h later (10).
Those with rapidly improving symptoms are usually excluded from receiving thrombolysis therapy. Rapidly improving symptoms are those which improve spontaneously after presentation, but the definition is ambiguous. However, their outcomes are not predictable, with 30% of those with minor stroke or rapidly improving symptoms not fully functionally independent at hospital discharge (1).
Disabling or Non-Disabling Symptoms?
Determining whether symptoms are “disabling” or not is an important factor in the management of acute MS. A pooled metanalysis of nine trials could show that thrombolysis treatment resulted in a nearly 10% better chance of an excellent functional outcome after 3 months in patients with clearly disabling deficits such as aphasia or hemiparesis (11, 12).
For those with non-disabling symptoms, however, less evidence exists. Only one of these nine trials–the Third International Stroke Trial (IST-3) (13)–did not exclude patients with non-disabling symptoms.
IST-3 found evidence of benefit for thrombolysis for those presenting within 6 h of symptoms of stroke, however the benefit increased with increasing NIHSS and was less beneficial for those with minor stroke symptoms. Out of the 106 patients randomized with NIHSS ≤ 5, 60% showed a favorable outcome after 3 months.
Non-disabling symptoms include transient, fluctuating or persistent symptoms without unilateral motor weakness or language/speech disturbance (e.g., hemi-body sensory symptoms, monocular vision loss, binocular diplopia, hemifield vision loss, dysarthria, dysphagia, or ataxia). The PRISMS trial, a randomized controlled trial (RCT), showed that among patients with a low NIHSS and no disabling deficit, rtPA may not provide a benefit and might increase the risk of symptomatic intracranial hemorrhage (6). A clearly disabling deficit was operationally defined as a deficit that, if unchanged, would prevent the patient from performing basic activities of daily living (i.e., bathing, ambulating, toileting, hygiene, and eating) or returning to work. Judging how disabling a deficit will be in the future is challenging in the hyperacute stroke setting.
A further obstacle to thrombolysis treatment in minor stroke is that patients with minor stroke symptoms do not receive the priority of emergency medical services and in-hospital triage pathways leading to relevant time delays in onset-to-door and door-to-imaging times (14).
Prehospital Recognition of Minor Stroke
The presentation of those with mild symptoms is frequently delayed compared to major stroke as it may not be recognized in the acute phase, leading to undertreatment. Public knowledge of stroke symptoms according to the FAST campaign is only about 70%, with the highest rate found in females and in the older and white population (15). Additionally, the mode of arrival at the hospital plays an important role. Patterns of emergency medical services pre-notification vary across countries. Data from a cohort study in New York showed that patients with minor stroke have longer door to needle times if the mode of arrival was without pre-notification (16).
The clinical significance of posterior circulation symptoms is often not recognized and, therefore, mostly remain undertreated in the acute phase. As in the NIHSS symptoms of the posterior circulation are underrepresented (e.g., vertigo, imbalance of gait), strokes in this territory are more likely to be defined as “minor” if a cut-off NIHSS score is used.
Wake up Stroke and Stroke of Unknown Onset
Those who wake up with stroke were traditionally excluded from revascularization therapies, due to unknown time of onset. Due to circadian rhythms there is diurnal variation in stroke onset, with a higher number occurring in the morning, which may be related to a surge in blood pressure (17). This suggests that the stroke may have occurred shortly before awakening, though the true time of onset is unknown. Modern imaging technologies, such as MRI DWI and FLAIR mismatch and or perfusion imaging, can help identify those who may benefit from thrombolysis or thrombectomy (18). The WAKE-UP trial showed that those with strokes evident from sleep with favorable MRI findings (DWI and FLAIR mismatch) who were treated with IV alteplase had significantly better functional outcomes, though more intracranial hemorrhages, than placebo at 90 days (19). The WAKE-UP trial included patients with all types of stroke severity, but the median NIHSS was of mild to moderate severity (median NIHSS 6, interquartile range 4–9). Analysis of patients with minor stroke has not been reported so far. Penumbral pattern identified using perfusion imaging is another recent radiological paradigm to identify those to benefit from reperfusion in the absence of onset time knowledge. Trials including ECASS4, EPITHET and EXTEND proved positively this concept for wake-up strokes and extended time window (4.5–9 h) thrombolysis (20).
Thrombolysis for wake-up stroke with minor symptoms has not been specifically studied. As mentioned previously, many stroke centers do not perform advanced imaging in those with NIHSS ≤ 6, and may be missing those with mismatch deficits or large vessel occlusions who could potentially benefit from thrombolysis. See also illustrative patient case in Figure 2.
Current Evidence of use of Thrombolytic Agents in Patients With Minor Stroke
Current guidelines and recommendations state that for patients with acute minor disabling ischemic stroke of <4.5 h duration, intravenous thrombolysis with recombinant alteplase is recommended/ may be reasonable (21, 22). RCTs and observational studies addressing this topic so far showed promising results with a good functional outcome and a low complication rate (Table 1).
For patients with acute minor non-disabling ischemic stroke of <4.5 h duration, no intravenous thrombolysis is recommended. One exception may be patients with non-disabling symptoms and a large vessel occlusion. However, many acute stroke centers do not perform angiography for those with NIHSS <6 as part of their internal protocol, and many centers do not have access to advanced imaging such as CT perfusion. Therefore, an unknown proportion of stroke with minor symptoms who have large vessel occlusions amenable to intervention are being missed. TEMPO 1, a case series of 50 patients with mild symptoms and intracranial vessel occlusion, which showed that administration of tenecteplase-tissue-type plasminogen activator in minor stroke with intracranial occlusion is feasible and safe (24). Wang et al. found that intravenous thrombolysis benefits though with mild stroke symptoms (NIHSS ≤ 5) and large artery atherosclerosis, though not those who had a tandem proximal intracranial occlusion and cervical internal artery lesion (complete occlusion or severe stenosis ≥ 90%) (30). They found that LAA-type patients (as defined by TOAST criteria) had significantly favorable outcomes after treatment with thrombolysis compared to untreated patients, however no such benefits were observed in other stroke subtypes, such as cardioembolic, small vessel occlusion and undetermined. This suggests that CT or MR angiography might be helpful to choose patients for thrombolysis that present with stroke with minor symptoms.
Alteplase or Tenecteplase in Patients With Minor Stroke
In recent years, the recombinant plasminogen activator tenecteplase is increasingly competing with the gold standard alteplase. The first publication of the EXTEND IA TNK study showed that higher perfusion rates and better clinical results can be achieved with tenecteplase in the 0.25 mg/kg dose than with alteplase in patients with an acute ischemic stroke (31). Tenecteplase was used as so-called bridging thrombolysis in the 4.5 h time window until the mechanical thrombectomy was performed. In addition, tenecteplase has advantages in handling, as it can be administered as single intravenous bolus and does not require a continuous infusion over 1 h, as alteplase does. The results of the EXTEND TNK study prompted the authors of the US guideline and the European Stroke Organization (ESO) to include tenecteplase in their recommendation as an alternative fibrinolytic (AHA/ASA Class IIb recommendation), although the AHA/ASA recommendation can also be considered to the 0.4 mg/kg dose for patients with less severe neurological impairments and if there are no large vessel occlusions (Level of Evidence: IIb) (22).
The second part of the EXTEND TNK study was recently published (32) which evaluated different doses of tenecteplase. The higher dose of tenecteplase (0.4 mg/kg) did not have any disadvantages in terms of safety: there were 16 and 22 death in the high and low dose groups, respectively. Symptomatic intracerebral hemorrhages 36 h after thrombolysis were numerically more frequent in the high dose group (7 vs. 2 patients), but four bleeding events in this group were associated with wire perforations during the endovascular procedure and were therefore not attributable to thrombolysis directly. The authors of the study report that the latter results are in contrast to an earlier study with the 0.40 mg/kg dose that was terminated prematurely for safety reasons, as some patients developed symptomatic intracranial hemorrhage. As a limitation, Campbell and colleagues point out that the study may not have been powered to reveal differences in efficacy. There was no restriction on clinical severity using NIHSS scores in these trials, but showed that probably a higher perfusion rate can be achieved with tenecteplase in patients with vessel occlusions. TEMPO 2 is an ongoing multicentre prospective randomized open label blinded-endpoint (PROBE) controlled trial of thrombolysis with low dose TEnecteplase vs. standard of care in Minor ischemic stroke with Proven acute symptomatic Occlusion (33). The hypothesis is that patients with mild (NIHSS < = 5) or even non-disabling symptoms due to identifiable vessel occlusion will benefit from IVT as compared to standard antiplatelet therapy. Results are expected in 2024. In summary, currently no evidence exists that tenecteplase should be preferred to alteplase in acute treatment of minor stroke patients, though further research is ongoing.
Time Trends of Using Intravenous Thrombolysis
In Austria, rates of rtPA treatment in patients with very mild symptoms (NIHSS 0-1) raised from 0.8% in 2006 to 3.5% in 2018 and for patients with a NIHSS 2–3 from 2.2% in 2006 to 17.2% in 2018 (34). Another large registry from 66 hospitals in Puerto Rico and Florida reported a substantial increase in thrombolysis rates of patients with minor stroke presenting within 4 h of stroke onset from 10% in 2010 to 25% in 2015 (14). The Get With the Guidelines Stroke database, which collects information from 1,783 hospitals across the United States, showed that the use of thrombolysis has increased from 45% in 2003 to 2005 to 82% in 2010 to 2011 (35). These substantial increases rtPA use in ischemic stroke patients with mild symptoms in different parts of the world document an increasing confidence in using this treatment according to the guidelines, which are regularly updated with regard to the minor stroke patient group.
Data from a prospective stroke thrombolysis registry in France (36) showed that a high rate (77%) of excellent outcome (3 month-modified Rankin Scale score ≤ 1) was observed in 1,035 minor stroke patients receiving thrombolysis. No symptomatic intracerebral hemorrhage occurred and the rate of any hemorrhagic transformation was 5%.
Patients With Very Mild Symptoms
A recent analysis from a large nationwide stroke registry in Austria shows that in patients with very mild symptoms (NIHSS 0–1), treatment with intravenous thrombolysis did not increase the likelihood of an excellent outcome as compared with those managed conservatively. On the contrary, those receiving IVT were more likely to suffer early neurological deterioration (adjusted OR 8.84, CI 6.61–11.83), symptomatic intracranial hemorrhage (adjusted OR 9.32, CI 4.53–19.15) and lower rate of excellent outcome (mRS 0–1) at 3 months (adjusted OR 0.67, CI 0.5–0.9). Proposed explanations for this phenomenon may include large vessel occlusion, thrombus migration, reperfusion injury, or re-embolization (26). Indeed, up to a third of patients of patients with initially mild symptoms may harbor a large vessel occlusion which may not respond well to IVT alone and may lead to secondary deterioration (37).
Dual Antiplatelet Therapy
If thrombolysis treatment is contraindicated or clinical assessment does preclude its use, current guidelines recommend dual antiplatelet therapy with Aspirin and Clopidogrel or Aspirin and Ticagrelor for a short time in patients with a minor stroke (NIHSS score ≤ 3) or high-risk TIA (ABCD2 score ≥ 4) (38). This treatment does not aim at vessel recanalization or rapid improvement of stroke symptoms, but rather to reduce early stroke recurrence.
The CHANCE study used a 300-mg clopidogrel (then 75 mg daily) and an aspirin loading dose of 75 to 300 mg followed by 75 mg daily within the first 24 h after TIA or minor stroke for a duration of 21 days (39). In the POINT trial 600-mg clopidogrel loading dose (then 75 mg daily) and an aspirin regimen of 50 to 325 mg daily started within in the first 12 h after TIA or minor stroke for up to 90 days was used (40). Additional analysis of the POINT trial could show that the effect of avoiding a recurrent stroke is primarily seen in the first 21 days, so that the recommendation is to treat these patients with a DAPT with loading doses immediately after TIA or minor stroke for no longer than 21 days. In both the CHANCE and POINT trials the benefits clearly outweighed the risk of bleeding.
In the THALES trial the use of ticagrelor (180-mg loading dose, then 90 mg twice daily) plus aspirin (300- to 325- mg loading does, then 75–100 mg daily) for 30 days was shown to be slightly superior to aspirin alone in preventing recurrent stroke with a significant increase in bleeding (41). In this study the preventive effect of the DAPT outweighed the risk of bleeding.
RCT's regarding the comparison of thrombolysis vs. DAPT in acute minor stroke patients are lacking. There is only one exploratory comparative analysis (42) showing a weak trend among intravenous thrombolysis, DAPT and Aspirin but not a significant difference in 90 day functional outcome in patients with minor stroke.
Discussion
Traditionally those presenting acutely with stroke with minor symptoms have been excluded from thrombolysis due to concerns that risks of hemorrhage would outweigh the benefits. However, as we have discussed above those presenting lower NIHSS scores may still experience long term disability. Currently it is challenging to judge what patients are likely to benefit most from thrombolysis (with or without thrombectomy) treatment with the lowest risk of bleeding complications.
Patients with vessel occlusions (like patients in Figures 1, 2) are likely to benefit from recanalizing treatments rather than (intensified) secondary prophylaxis with antiplatelets. Yet, in a majority of centers acute stroke treatment protocols do exclude patients with a low NIHSS from advanced imaging like MR/CT-angiography and perfusion. Therefore, the decision to initiate and organize these imaging modalities leads to time delays making thrombolysis less safe and efficient. Indeed, a recent study showed significant increased detection of LVO and increased frequencies of performed MTs after an in-house protocol change excluding the NIHSS criterion (43). Therefore, we would advocate that all patients presenting with stroke symptoms, including minor stroke symptoms, have angiography (typically CT) as part of their acute work up.
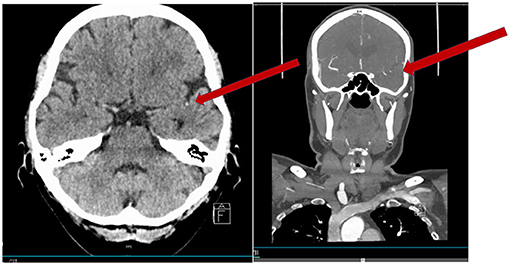
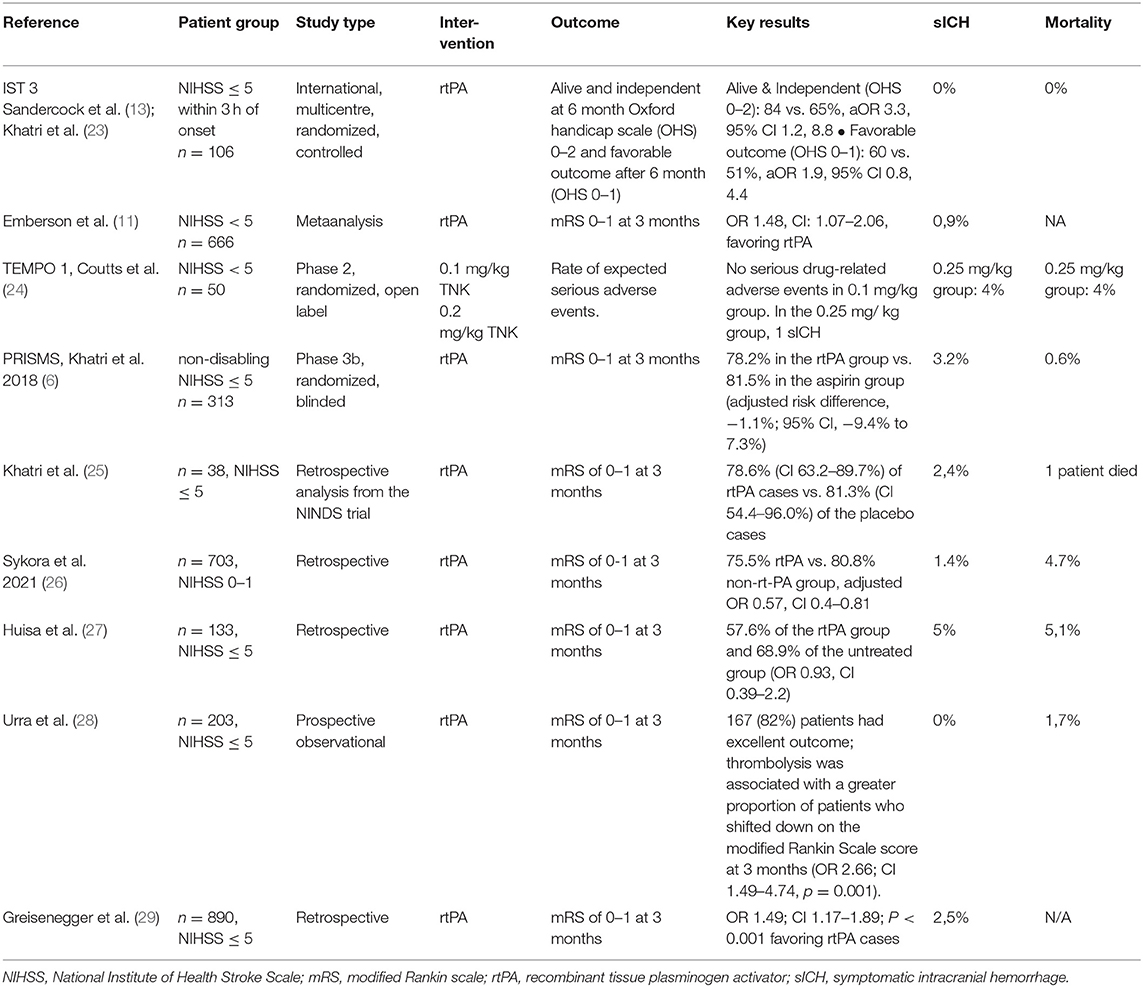
Figure 1. Fifty-nine-year-old woman with an acute onset of aphasia (NIHSS 1 disabling). CT scan showing a hyperdense vessel sign in M2 (arrow). CTA showing the occlusion in M2 (short in length).
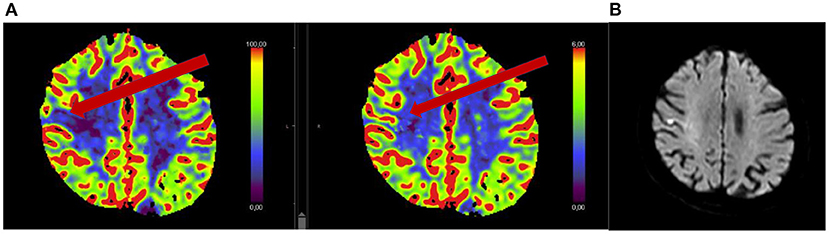
Figure 2. Seventy-five year old woman with acute onset of mild left hemi-body weakness and sensory loss (NIHSS 3). Patient received intravenous thrombolysis and showed clinical improvement. Acute CT perfusion showed a mismatch between normal cerebral blood volume (B) and reduced cerebral blood flow (A). Follow-up MRI performed 24 h after thrombolysis showed a small cortical ischemia.
There is some data which suggests that minor strokes of certain etiologies–e.g., like strokes due to large artery atherosclerosis (without large vessel occlusion) (30)–may benefit more from thrombolytic treatment.
Finally, the relevance of a neurological deficit can be extremely hard to judge in the acute stroke setting. Symptoms such as neglect, extinction, and cognitive deficits can be frequently under recognized in the emergency room. In particular, posterior circulation strokes tend to be misclassified as “minor stroke” (illustrative patient example Figure 3).
Figure 3. Fifty-five year old man with acute onset of mild dysathria and ataxia of the trunk, unable to walk (NIHSS 1). MRI showed an acute infarction [DWI positive (A), ADC negative (B), FLAIR negative (C)] in the territory of the occluded right posterior inferior cerebellar artery. The patient was treated with rtPA 3 h and 46 min after stroke onset.
The NIHSS has limitations with respect to its use when comparing the neurologic severity of a posterior circulation stroke and anterior circulation stroke (44). A patient with an acute ischemic stroke in the posterior circulation might have a comparably low score like patients with an acute ischemic stroke in the anterior circulation but be bedridden due to severe ataxia and/or vertigo, underlining that patients with a posterior circulation stroke need the same diagnostic and therapeutic measures (e.g., iv thrombolysis) like patients with an anterior circulation stroke (45).
Conclusion
Patients with a minor stroke are by no means to be classified as benign and may result in lasting significant neurological deficits. Even though the use of IV thrombolysis in this setting has substantially increased world-wild, current guidelines still recommend the administration of thrombolysis only to minor stroke patients with clear disabling symptoms, due to lack of convincing data from large randomized-controlled trials. Advanced imaging might help to better estimate the risk-benefit ratio of thrombolysis treatment in acute ischemic stroke with minor symptoms. Further results of ongoing trials on this topic are expected shortly.
Author Contributions
All authors contributed to the writing of the manuscript, literature search and provided patients cases. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smith EE, Fonarow GC, Reeves MJ, Cox M, Olson DM, Hernandez AF, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from Get with the guidelines-stroke. Stroke. (2011) 42:3110–5. doi: 10.1161/STROKEAHA.111.613208
2. Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? an analysis of patient eligibility. Neurology. (2001) 56:1015–20. doi: 10.1212/WNL.56.8.1015
3. Nedeltchev K, Schwegler B, Haefeli T, Brekenfeld C, Gralla J, Fischer U, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke. (2007) 38:2531–5. doi: 10.1161/STROKEAHA.107.482554
4. Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke. (2005) 36:2497–9. doi: 10.1161/01.STR.0000185798.78817.f3
5. Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, De Marchis GM, et al. What is a minor stroke? Stroke. (2010) 41:661–6. doi: 10.1161/STROKEAHA.109.572883
6. Khatri P, Kleindorfer DO, Devlin T, Sawyer RN, Starr M, Mejilla J, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the prisms randomized clinical trial. JAMA. (2018) 320:156–66. doi: 10.1001/jama.2018.8496
7. Albers GW, Caplan LR, Easton JD, Fayad PB, Mohr JP, Saver JL, et al. Transient ischemic attack–proposal for a new definition. N Engl J Med. (2002) 347:1713–6. doi: 10.1056/NEJMsb020987
8. Feigin V, Norrving B, Sudlow CLM, Sacco RL. Updated criteria for population-based stroke and transient ischemic attack incidence studies for the 21st century. Stroke. (2018) 49:2248–55. doi: 10.1161/STROKEAHA.118.022161
9. Levy DE. How transient are transient ischemic attacks? Neurology. (1988) 38:674–7. doi: 10.1212/WNL.38.5.674
10. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
11. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
12. Lees KR, Emberson J, Blackwell L, Bluhmki E, Davis SM, Donnan GA, et al. Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke. (2016) 47:2373–9. doi: 10.1161/STROKEAHA.116.013644
13. Sandercock P, IST-3 collaborative group, Wardlaw JM, Lindley RI, Dennis M, Cohen G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. (2012) 379:2352–63. doi: 10.1016/S0140-6736(12)60768-5
14. Asdaghi N, Wang K, Ciliberti-Vargas MA, Gutierrez CM, Koch S, Gardener H, et al. Predictors of thrombolysis administration in mild stroke: florida-puerto rico collaboration to reduce stroke disparities. Stroke. (2018) 49:638–45. doi: 10.1161/STROKEAHA.117.019341
15. Robinson TG, Reid A, Haunton VJ, Wilson A, Naylor AR. The face arm speech test: does it encourage rapid recognition of important stroke warning symptoms? Emerg Med J. (2013) 30:467–71. doi: 10.1136/emermed-2012-201471
16. Rostanski SK, Shahn Z, Elkind MSV, Liberman AL, Marshall RS, Stillman JI, et al. Door-to-needle delays in minor stroke: a causal inference approach. Stroke. (2017) 48:1980–2. doi: 10.1161/STROKEAHA.117.017386
17. Wouters A, Lemmens R, Dupont P, Thijs V. Wake-up stroke and stroke of unknown onset: a critical review. Front Neurol. (2014) 5:153. doi: 10.3389/fneur.2014.00153
18. Huang X, Alakbarzade V, Khandanpour N, Pereira AC. Management of a wake-up stroke. Pract Neurol. (2019) 19:326. doi: 10.1136/practneurol-2018-002179
19. Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Eng J Med. (2018) 379:611–22. doi: 10.1056/NEJMoa1804355
20. Campbell BCV, Ma H, Ringleb PA, Parsons MW, Churilov L, Bendszus M, et al. Extending thrombolysis to 4·5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. (2019) 394:139–47. doi: 10.1016/S0140-6736(19)31053-0
21. Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, et al. European stroke organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. (2021) 6:I–lxii. doi: 10.1177/2396987321989865
22. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
23. Khatri P, Tayama D, Cohen G, Lindley RI, Wardlaw JM, Yeatts SD, et al. Effect of intravenous recombinant tissue-type plasminogen activator in patients with mild stroke in the third international stroke trial-3: post hoc analysis. Stroke. (2015) 46:2325–7. doi: 10.1161/STROKEAHA.115.009951
24. Coutts SB, Dubuc V, Mandzia J, Kenney C, Demchuk AM, Smith EE, et al. Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke. (2015) 46:769–74. doi: 10.1161/STROKEAHA.114.008504
25. Khatri P, Kleindorfer DO, Yeatts SD, Saver JL, Levine SR, Lyden PD, et al. Strokes with minor symptoms: an exploratory analysis of the national institute of neurological disorders and stroke recombinant tissue plasminogen activator trials. Stroke. (2010) 41:2581–6. doi: 10.1161/STROKEAHA.110.593632
26. Sykora M, Krebs S, Simader F, Gattringer T, Greisenegger S, Ferrari J, et al. Intravenous thrombolysis in stroke with admission NIHSS score 0 or 1. Int J Stroke. (2021) 1747493021991969. doi: 10.1177/1747493021991969
27. Huisa BN, Raman R, Neil W, Ernstrom K, Hemmen TM. Intravenous tissue plasminogen activator for patients with minor ischemic stroke. J Stroke Cerebrovasc Dis. (2012) 21:732–6. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.009
28. Urra X, Ariño H, Llull L, Amaro S, Obach V, Cervera Á, et al. The outcome of patients with mild stroke improves after treatment with systemic thrombolysis. PLoS ONE. (2013) 8:e59420. doi: 10.1371/journal.pone.0059420
29. Greisenegger S, Seyfang L, Kiechl S, Lang W, Ferrari J. Thrombolysis in patients with mild stroke: results from the Austrian stroke unit registry. Stroke. (2014) 45:765–9. doi: 10.1161/STROKEAHA.113.003827
30. Wang D, Zhang L, Hu X, Zhu J, Tang X, Ding D, et al. Intravenous thrombolysis benefits mild stroke patients with large-artery atherosclerosis but no tandem steno-occlusion. Front Neurol. (2020) 11:340. doi: 10.3389/fneur.2020.00340
31. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Eng J Med. (2018) 378:1573–82. doi: 10.1056/NEJMoa1716405
32. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: The EXTEND-IA TNK Part 2 randomized clinical trial. JAMA. (2020) 323:1257–65. doi: 10.1001/jama.2020.1511
33. A Randomized Controlled Trial of TNK-tPA Versus Standard of Care for Minor Ischemic Stroke With Proven Occlusion. (2021). Available online at: https://ClinicalTrials.gov/show/NCT02398656
34. Marko M, Posekany A, Szabo S, Scharer S, Kiechl S, Knoflach M, et al. Trends of r-tPA (Recombinant Tissue-Type Plasminogen Activator) treatment and treatment-influencing factors in acute ischemic stroke. Stroke. (2020) 51:1240–7. doi: 10.1161/STROKEAHA.119.027921
35. Messé SR, Khatri P, Reeves MJ, Smith EE, Saver JL, Bhatt DL, et al. Why are acute ischemic stroke patients not receiving IV tPA? results from a national registry. Neurology. (2016) 87:1565–74. doi: 10.1212/WNL.0000000000003198
36. Laurencin C, Philippeau F, Blanc-Lasserre K, Vallet AE, Cakmak S, Mechtouff L, et al. Thrombolysis for acute minor stroke: outcome and barriers to management. results from the RESUVAL stroke network. Cerebrovasc Dis. (2015) 40:3–9. doi: 10.1159/000381866
37. Kim JT, Heo SH, Yoon W, Choi KH, Park MS, Saver JL, et al. Clinical outcomes of patients with acute minor stroke receiving rescue IA therapy following early neurological deterioration. J Neurointerv Surg. (2016) 8:461–5. doi: 10.1136/neurintsurg-2015-011690
38. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
39. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. (2013) 369:11–9. doi: 10.1056/NEJMoa1215340
40. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. (2018) 379:215–25. doi: 10.1056/NEJMoa1800410
41. Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. (2020) 383:207–17. doi: 10.1056/NEJMoa1916870
42. Wang P, Zhou M, Pan Y, Meng X, Zhao X, Liu L, et al. Comparison of outcome of patients with acute minor ischaemic stroke treated with intravenous t-PA, DAPT or aspirin. Stroke Vasc Neurol. (2021) 6:187–93. doi: 10.1136/svn-2019-000319
43. Mayer SA, Viarasilpa T, Panyavachiraporn N, Brady M, Scozzari D, Van Harn M, et al. CTA-for-All: Impact of emergency computed tomographic angiography for all patients with stroke presenting within 24 hours of onset. Stroke. (2020) 51:331–4. doi: 10.1161/STROKEAHA.119.027356
44. Sato S, Toyoda K, Uehara T, Toratani N, Yokota C, Moriwaki H, et al. Baseline NIH stroke scale score predicting outcome in anterior and posterior circulation strokes. Neurology. (2008) 70:2371–7. doi: 10.1212/01.wnl.0000304346.14354.0b
Keywords: minor stroke, thrombolysis/thrombolytic agents, DAPT (dual antiplatelet therapy), very mild severity, rapidly improving stroke symptoms
Citation: Ferrari J, Reynolds A, Knoflach M and Sykora M (2021) Acute Ischemic Stroke With Mild Symptoms–To Thrombolyse or Not to Thrombolyse? Front. Neurol. 12:760813. doi: 10.3389/fneur.2021.760813
Received: 18 August 2021; Accepted: 15 October 2021;
Published: 18 November 2021.
Edited by:
Mirjam R. Heldner, University Hospital Bern, SwitzerlandReviewed by:
Lauri Sakari Soinne, Helsinki University Central Hospital, FinlandSvetlana Lorenzano, Sapienza University of Rome, Italy
Copyright © 2021 Ferrari, Reynolds, Knoflach and Sykora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia Ferrari, julia.ferrari@bbwien.at
 Julia Ferrari
Julia Ferrari Audrey Reynolds
Audrey Reynolds Michael Knoflach3
Michael Knoflach3