- 1Department of Neuroradiology, Freiburg University Medical Center, University of Freiburg, Freiburg, Germany
- 2Faculty of Medicine, University of Freiburg, Freiburg, Germany
- 3Department of Neurology and Neuroscience, Freiburg University Medical Center, Freiburg, Germany
Introduction: Organizing regional stroke care considering thrombolysis as well as mechanical thrombectomy (MTE) remains challenging in light of a wide range of regional population distribution. To compare outcomes of patients in a stroke network covering vast rural areas in southwestern Germany who underwent MTE via direct admission to a single comprehensive stroke center [CSC; mothership (MS)] with those of patients transferred from primary stroke centers [PSCs; drip-and-ship (DS)], we undertook this analysis of consecutive stroke patients with MTE.
Materials and Methods: Patients who underwent MTE at the CSC between January 2013 and December 2016 were included in the analysis. The primary outcome measure was 90-day functional independence [modified Rankin score (mRS) 0–2]. Secondary outcome measures included time from stroke onset to recanalization/end of MTE, angiographic outcomes, and mortality rates.
Results: Three hundred and thirty-two consecutive patients were included (MS 222 and DS 110). Median age was 74 in both arms of the study, and there was no significant difference in baseline National Institutes of Health Stroke Scale scores (median MS 15 vs. 16 DS). Intravenous (IV) thrombolysis (IVT) rates differed significantly (55% MS vs. 70% DS, p = 0.008). Time from stroke onset to recanalization/end of MTE was 112 min shorter in the MS group (median 230 vs. 342 min, p < 0.001). Successful recanalization [thrombolysis in cerebral infarction (TICI) 2b-3] was achieved in 72% of patients in the MS group and 73% in the DS group. There was a significant difference in 90-day functional independence (37% MS vs. 24% DS, p = 0.017), whereas no significant differences were observed for mortality rates at 90 days (MS 22% vs. DS 17%, p = 0.306).
Discussion: Our data suggest that patients who had an acute ischemic stroke admitted directly to a CSC may have better 90-day outcomes than those transferred secondarily for thrombectomy from a PSC.
Introduction
According to the HERMES meta-analysis that combined the data of five randomized controlled trials (RCT), mechanical thrombectomy (MTE) is of benefit to most patients with acute ischemic stroke caused by occlusion of the proximal anterior circulation [large vessel occlusion (LVO)], irrespective of patient characteristics or geographical location (1). Organizing regional stroke care in light of this new evidence has been and still is a major challenge. The current version of the American Heart Association (AHA) guidelines for the “Early Management of Patients With Acute Ischemic Stroke” clearly states that regional systems of stroke care should be developed (2). They should include (a) health care facilities that provide initial emergency care, including administration of intravenous (IV) thrombolysis (IVT) and (b) centers performing endovascular stroke treatment with comprehensive periprocedural care to which rapid transport can be arranged when appropriate. This statement has both the highest level of evidence (Level A) and the highest class of recommendation (Class I). According to the same guideline, when several IVT-capable hospital options exist within a defined geographic region, the benefit of bypassing the closest to bring the patient to one that offers a higher level of stroke care, including MTE, is uncertain (2). These statements can be read as a recommendation for the drip-and-ship (DS) model—patients are transported to the nearest primary stroke center (PSC) to have rapid diagnostic imaging and administration of IVT followed by transport to the nearest PSC for MTE, as opposed to the mothership (MS) model, where patients are directly brought to the comprehensive stroke center (CSC) to minimize time to MTE. A recent RCT which compared MTE with or without IVT in patients with acute ischemic stroke due to LVO concluded that MTE alone was non-inferior with regard to functional outcome [modified Rankin score (mRS) ≤ 2 at 90 days] within a 20% margin of confidence to MTE preceded by IVT administered within 4.5 h after symptom onset (3). Nevertheless, data on non-inferiority of MTE alone are inconclusive; thus, the question of whether patients with LVO eligible for MTE should be transported to CSC directly is still unanswered.
To address this question, we analyzed functional outcomes of patients treated for acute ischemic strokes within our regional stroke network, comparing patients treated with a DS approach to patients sent directly to our CSC for MTE.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
We conducted an investigator-initiated, retrospective, open-label trial involving patients with acute ischemic stroke who either were directly sent to our CSC or were secondarily transferred from a PSC to undergo MTE at the CSC between January 1, 2013, and December 31, 2016. The study protocol was approved by our Ethics Committee (Faculty of Medicine, University of Freiburg, 077/09). Members of the trial steering committee and the local investigators designed the study, collected and analyzed the data, wrote the manuscript, and made the decision to submit the manuscript for publication.
Organization of Stroke Care
At the time of patient inclusion, our Interdisciplinary NeuroVascular Network South-West (INVAS) stroke network comprised 12 PSCs and one CSC at the University Hospital Freiburg. The catchment area of the INVAS stroke network covers a surface area of 9,357 km2 and a population of 2.3 million. It includes the rather densely populated Rhine valley as well as remote rural and mountainous regions of the southern black forest.1 Depending on the location of the patient presenting with symptoms of an acute ischemic stroke, transfer occurred either to one of the PSC or directly to the CSC. The decision of transportation destination was performed by an emergency medical team. Patients from the PSC were discussed via a telestroke platform with the attending stroke neurologist at the CSC. Patients from the PSC deemed eligible for MTE were secondarily transferred to the CSC. For definitions of PSC and CSC, please refer to the recent publication of Ernst et al. (4).
Patients
Patients from both PSC and CSC were eligible for MTE if they were >18 years of age, had a score of 6 or higher on the National Institutes of Health Stroke Scale (NIHSS) or a relevant functional deficit, a suspected (dense vessel sign) or proven [computed tomography angiography (CTA)/magnetic resonance angiography (MRA)] LVO of the anterior circulation, or a small core infarct with an Alberta Stroke Program Early CT Score (ASPECTS) >4. Initiation of MTE had to be possible within 6 h after stroke onset. Patients from PSC had repeated magnetic resonance imaging (MRI) or CT imaging if time from initial imaging to arrival at the CSC exceeded 2 h. Patients from both the PSC and CSC who did not present contraindication to systemic lysis received a weight-adapted dose of rtPA within 4.5 h after onset of acute ischemic stroke.
Endovascular Treatment
The majority of patients were treated under general anesthesia. The standard procedure included a 9F femoral access and 8F or 9F balloon guide catheters for the carotid artery. Thrombus retrieval stent retrievers were used for the large majority of cases (Table 1). Medical management and acute poststroke care after the procedure were consistent with the European Stroke Organization (ESO)/European Society for Minimally Invasive Neurological Therapy (ESMINT) and AHA guidelines (2, 5). A CT scan was performed within 24 h after the revascularization procedure to detect the presence of intracerebral hemorrhage (ICH).
Clinical and Radiological Assessments
All patients underwent clinical examination at baseline and discharge. Baseline data collected from the patients' medical chart included gender, age, stroke onset time, baseline NIHSS, time of initial CT/MRI, IVT given, time of IVT, time of CT/MRI at CSC for patients transferred from PSC, time of first angiographic run, time of recanalization, 24-h NIHSS, and NIHSS at discharge.
In addition, an independent rater who did not participate in the endovascular stroke treatment of included patients, with >10 years of experience in neuroimaging (R.H.), qualitatively evaluated pre-therapeutic MR, CT, and digital subtraction angiography (DSA) images as well as final angiograms after MTE and follow-up CT at 24 h. The extent of the initial core infarct was determined on pre-therapeutic CT or diffusion-weighted imaging (DWI) using ASPECTS (6). Revascularization was assessed by applying the modified thrombolysis in cerebral infarction (TICI) classification (7). Intracranial hemorrhage was defined on follow-up CT at 24 h as symptomatic in case of parenchymal hematoma with a clinical worsening of at least four points on the NIHSS scale according to the European Cooperative Acute Stroke Study (ECASS) definition (8). In addition, subarachnoid hemorrhages were reported.
Study Endpoints
The primary study outcome measure was disability at 90 days, as assessed by applying the mRS. Secondary clinical efficacy outcomes were NIHSS at discharge, mortality at 90 days, and the rate of symptomatic ICH (sICH), asymptomatic lobar hematoma, and subarachnoid hemorrhages. The last recorded NIHSS score was used as discharge NIHSS in patients who had deceased during hospitalization. The technical efficacy outcomes were successful reperfusion defined as angiographic demonstration of TICI scores of 2b (50–99% reperfusion) or 3 (complete reperfusion). In addition, we analyzed different time intervals including onset to first imaging, onset to IVT, onset to first angiographic run, and onset to recanalization/end of MTE for MS and DS patients. In DS patients, we additionally determined the transfer time defined as the time interval between first imaging at PSC and patient arrival at the CSC.
Ethics
The studies involving human participants were reviewed and approved by the Ethics Committee of the Faculty of Medicine, University of Freiburg, Freiburg, Germany.
Statistical Analysis
The data are expressed with standard descriptive statistics, including median, interquartile range (IQR) or range for ordinal and numerical variables, and absolute and relative frequencies for nominal variables as appropriate. Relative frequencies were rounded to whole numbers according to the common rules.
Comparisons among groups on baselines variables, peri-procedural time intervals, and outcome variables were made using Pearson's chi-square test for nominal variables (age, gender, IVT, target vessels, stent retrievers and catheters, mRS 0–2, TICI 2b/3, mortality, and hemorrhage data) and Mann–Whitney U-test for ordinal and numerical variables (age, NIHSS, ASPECTS, number of passes, and time intervals). Statistical significance was set at p < 0.05 for all contrasts of hypothesis. For small sample sizes with n < 30 (sICH and asymptomatic lobar hemorrhage), Fisher's exact test was used. Primary endpoint analysis was amended by logistic regression analysis.
The analyses were performed with IBM SPSS Statistics version 24. Outcome analyses of cerebral hemorrhages were conducted with R Studio software version 1.3.959. The figure was drawn with the use of Microsoft Excel 2013. No missing values were imputed. Statistical consulting was provided by the Institute of Medical Biometry and Statistics of the University of Freiburg.
Results
Baseline Results
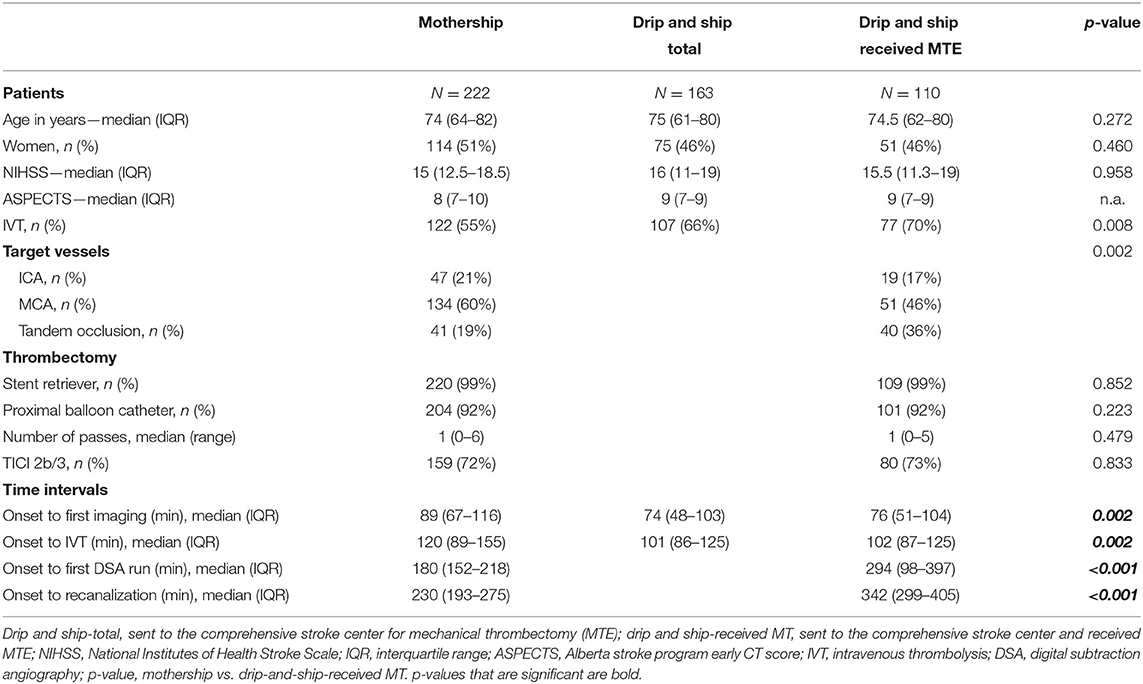
From January 1, 2013, to December 31, 2016, 385 patients were included in the analysis population. Two-hundred and twenty-two patients were directly admitted to the CSC with acute ischemic stroke and were treated with MTE (MS). One-hundred and sixty-three patients were admitted at PSC and transferred after telestroke consultation for MTE to the CSC (DS). Of 163 patients from the DS group (DS-total), 110 patients actually were treated by MTE (DS-received MTE) at the CSC. Patient demographics, NIHSS at presentation, initial ASPECTS score, and target vessel for MTE are summarized in Table 1. Supplementary Table 1 outlines the different reasons for not performing MTE in 53 patients from the DS-total group.
Mean age was 74 years (IQR 64–82) in the MS group compared to 75 years (IQR 62–80) in the DS-total group. In the MS group, 51% (114/222) of patients treated were female, whereas 46% (75/163) of the DS-total group were women. The median NIHSS did not differ significantly between groups and was 15 (IQR 12.5–18.5) for the MS group and 16 (IQR 11–19) for the DS-total group. With median ASPECTS scores of 8 (IQR 7–10), these were slightly lower in the MS group when compared to the DS-total group with 9 (IQR 7–9). Distribution of target vessels was slightly different between groups with 60% (134/222) middle cerebral artery (MCA) occlusions in the MS arm of the trial compared to 46% (51/110) MCA occlusion in the DS-received MTE group. The rate of patients with tandem occlusions (combined occlusion of the cervical ICA and MCA) was higher in the DS-received MTE group [36% (40/110)] than in the MS group [19% (41/222)].
Primary Endpoint Results
There was a significantly better primary outcome of functional independence (mRS 0–2) at 90 days in patients from the MS group compared to patients from the DS group [MS: 37% vs. DS-total: 26% (p = 0.024)/DS-received MTE: 24% (p = 0.017)]. Regression analysis showed a 1.7-fold increase in the good clinical outcome in MS-group patients (unadjusted OR 1,698; 95% KI 1,069–2,697; p = 0.025).
Secondary Endpoint Results
Time Intervals
Time intervals between onset and first imaging were significantly shorter in DS patients than in the MS group [MS 89 min (IQR 67–116) vs. DS-total: 74 min (IQR 48–103)/DS-received MTE: 76 min (IQR 51–104)]. In patients who received IVT, rtPA was administered significantly earlier in the DS group than in MS patients [MS 120 min (IQR 89–155) vs. DS-total: 101 min (IQR 86–125)/DS-received MTE: 102 min (IQR 87–125)]. The time intervals changed in favor of MS patients with respect to the MTE. MS patients had their first angiographic run of the MTE procedure 114 min earlier than did patients from the DS-received MTE group [MS 180 min (IQR 152–218) vs. DS-received MTE: 294 min (IQR 98–397)]. Successful recanalization again occurred 112 min earlier in MS patients than in patients from the DS-received MTE group [MS 230 min (IQR 193–275) vs. DS-received MTE: 342 min (IQR 299–405)]. Relevant time intervals are listed in Table 1.
Transfer Times
Distances between the PSC and the CSC range from 17.1 to 96.4 km, determined by Google Maps. Individual patient transfer times were based on the emergency transport reports. Ninety-one (56%) patients from the DS group were transported via helicopter, 46 (28%) patients were transported by ambulance, and 26 patients were missing information concerning the mode of transport. Transfer times defined as time from first imaging at the PSC to arrival at the CSC ranged from 1 h 55 min to 3 h 36 min. Distances and mean transport times for the different PSCs are detailed in Figure 1.
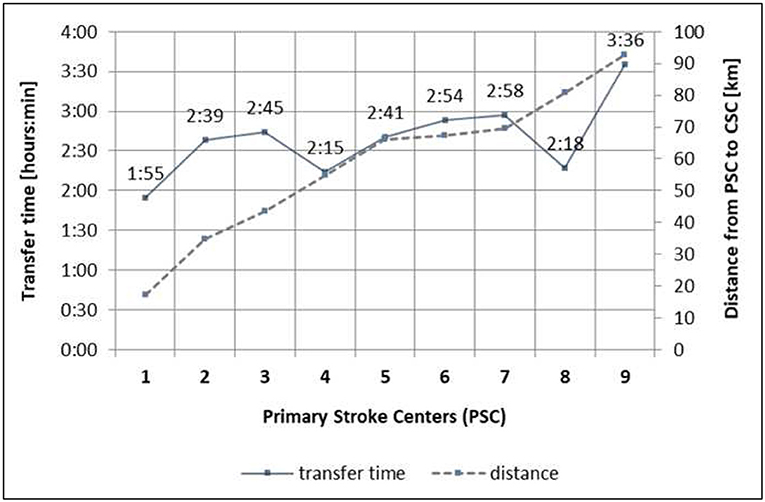
Figure 1. Neurovascular network with distances and transfer times from PSC to CSC. PSC, primary stroke centers; CSC, comprehensive stroke center (Freiburg); 1. PSC 1 with local stroke care, 2. PSC 2 with local stroke care, 3. PSC 3 with local stroke care, 4. PSC 4 with regional stroke care, 5. PSC 5 with regional stroke care, 6. PSC 6 with regional stroke care, 7. PSC 7 with regional stroke care, 8. PSC 8 with local stroke care, 9. PSC 9 with local stroke care.
Technical Efficacy Outcomes
In both groups, 99% of patients were treated with stent retrievers. The use of proximal balloon catheters did not differ (92% in both arms). There was no difference in the median number of stent retriever passes (one in both arms). Successful recanalization with angiographic demonstration of TICI scores of 2b (50–99% reperfusion) or 3 (complete reperfusion) was obtained in 159/220 (72%) of patients in the MS group and 80/110 (73%) of patients in the DS-received MTE group. Procedure-related information is summarized in Table 1.
Clinical Efficacy Outcomes
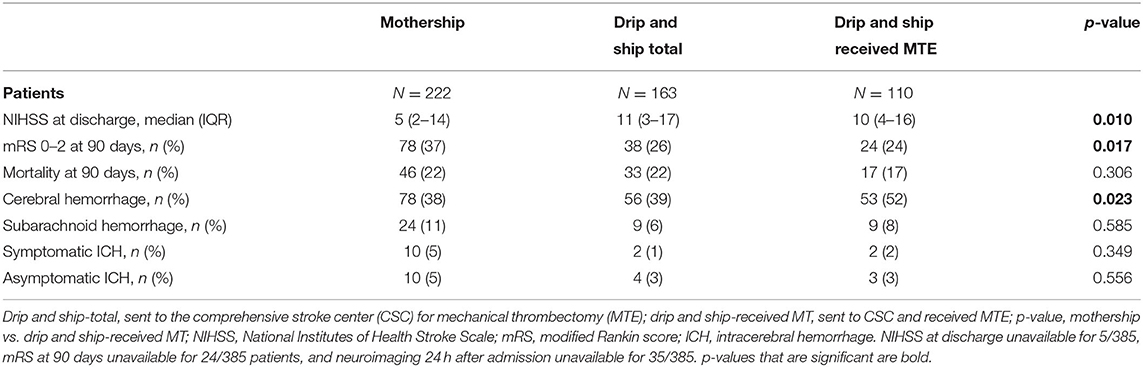
NIHSS at discharge differed significantly between the two arms of the study with a difference of five points on the NIHSS scale in favor of patients from the MS group [MS: 5 (range 2–14) vs. DS-received MTE: 10 (range 4–16)]. The mortality rates at 90 days did not differ significantly between groups (MS: 22% vs. DS-total: 22%/DS-received MTE: 17%).
A subgroup analysis of DS patients did not show a difference of good clinical outcome (mRS 0–2 vs. 3–6) when comparing patients with and without rtPA at PSC (p = 0.739). Good clinical outcome was not influenced by the different PSCs with differing transfer times in the DS group. In regression analysis, time to recanalization did show significant influence on good clinical outcome (p = 0.012). Forty-nine patients were recanalized outside the 6-h time window. Modified Rankin score from patients within 6-h time window was significantly better compared with that from patients outside the 6-h outcome (p = 0.042).
Symptomatic ICHs occurred more often in patients from the MS group [MS: 5% (10/220) vs. DS-received MTE: 2% (2/110)], such as asymptomatic lobar hematomas [MS: 5% (10/220) vs. DS-received MTE: 3% (3/110)] and subarachnoid hemorrhages [MS: 11% (24/220) vs. DS-received MTE: 8% (9/110)]. Those differences were statistically not significant. Clinical outcomes are summarized in Table 2.
Discussion
In this study, functional independence (mRS ≤ 2) at 90 days was significantly less likely in DS patients than in the control group of patients treated under an MS paradigm (MS 37% vs. DS-total 26%, DS-received MTE 24%; p = 0.017).
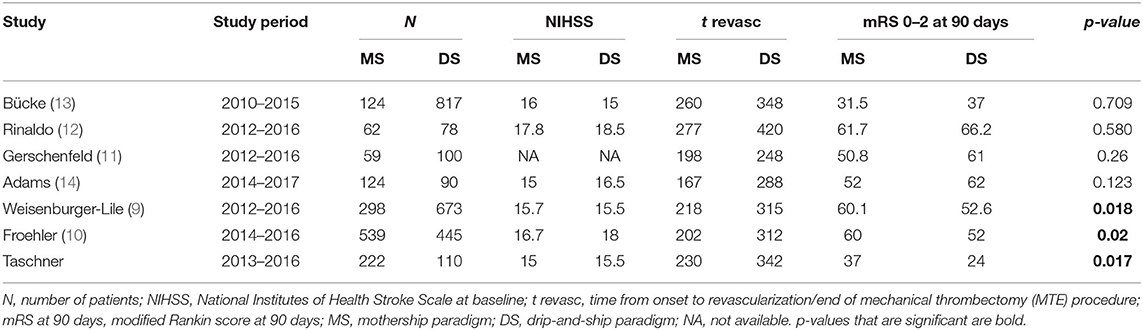
A review on the recent literature comparing MS and DS paradigms shows contradictory findings. Six different trials including a total of 3,741 patients (MS: 1,428 and DS: 2,313) unanimously reported significantly shorter onset to recanalization times for the MS groups when compared to DS patients. Only in two of these trials was the reported clinical outcome [functional independence (mRS ≤ 2)] at 90 days was significantly better in MS patients than in DS patients (9, 10). In the remaining four trials (11–14), patients in the DS group fared better than MS patients without these differences reaching statistical significance (Table 3).
A recent meta-analysis by Ismael et al. came to the conclusion that patients who had an acute ischemic stroke admitted directly to a CSC may have better 90-day outcomes than those receiving DS treatment. Their analysis was based on eight studies including 2,068 patients who were selected. Patients who underwent MTE in an MS setting had better functional independence than those undergoing DS, whereas no differences were found between the treatment pathways in successful reperfusion, symptomatic intracranial hemorrhage, and 90-day mortality (15).
The contradictory results of the different trials might in part be explained by the fact that clinical outcomes in acute stroke patients depend on a large number of different factors including topography of a stroke network, local infrastructure, onset time to first medical response, traffic, weather, local door-to-needle times at PSCs, experience with thrombolysis at the PSC, different thresholds for MTE patient selection in LVO, distances and mode of transportation between PSC and CSC, door-to-groin times at the CSC, and experience of the operator at the CSC.
The findings of our trial are corroborating the results from Froehler and Weisenburger-Lile as well as the meta-analysis by Ismael, and they do not correlate with current guidelines, which recommend rapid transport of patients with a stroke to the nearest primary stroke capable of providing IVT therapy, even if they are being considered for MTE (AHA and ESO/ESMINT guidelines). The recently published RCT comparing endovascular thrombectomy with or without IVT in patients with LVO showed that MTE without prior IVT turned out to be non-inferior with regard to functional outcome, raising concerns that patients with LVO eligible for MTE are not adequately treated in a DS setting (3). There are even indications that IVT before MTE for LVO is associated with distal embolization, which in turn may reduce the chance that MTE can be attempted and recanalization can be achieved (16). But data on direct transfer of patients to CSC are still inconclusive as there are also trials which failed to prove non-inferiority of the MS model and proved its benefit prior ivtPA (17). Also, detection of stroke patients with and without LVO especially in atypical presentation remains challenging especially in the MS model; besides, sending all patients with suspected LVO directly to the CSC would substantially increase the patient volume in these centers (18).
Novel approaches to identify LVO patients in the pre-hospital phase are under scrutiny. Among these are stroke assessment tools applied by first-aid providers. Yet these tests already seem to have difficulties in reliably identifying ischemic stroke patients, let alone recognizing ischemic stroke patients with LVO. The current guidelines conclude that better stroke identification tools are needed in the pre-hospital setting (2). Corresponding research projects are currently getting under way.2 A different approach consists in piloting specialized stroke ambulances (mobile stroke units) that are supposed to speed up diagnosis, triage, and emergency treatment of patients with acute ischemic stroke symptoms (19, 20).
Our findings suggest that MTE for acute stroke treatment organized in a decentralized system could be of benefit for patients with LVO. Brekenfeld et al. have shown the benefits of shipping the neurointerventionalists to two PSCs, located at driving distances of 53 and 63 km from the CSC at the University Hospital Hamburg Eppendorf. This approach allows for paralleling necessary steps such as transportation, reevaluation, and angiography instead of a more time-consuming sequential organization of stroke patient care (21). This is especially crucial since our data in accordance with others show that time to recanalization has a significant influence on good clinical outcome. Thus, speeding up timelines and eliminating unnecessary and time-consuming steps of acute stroke care organization especially in patients with LVO at PSC are some of the challenging needs to be worked on. In CSCs with greater catchment areas, physicians could alternatively be trained at the PSC to perform MTE locally. Rinaldo et al. reasoned that it may be beneficial to expand the availability of endovascular revascularization services to lower-volume hospitals to minimize the morbidity associated with transfer to larger endovascular centers. In a study that included 8,533 patients from 118 institutions, they were able to demonstrate significantly lower mortality rates and mortality indices in high-volume centers (>132 MTE/year) compared to medium- and low-volume centers (22). This effect might not only be explained by more experienced neurointerventionalists in high-volume centers but also reflects the quality of the intensive care management elements of peri-interventional stroke management based on the current evidence, pathophysiologic considerations, and personal experience in a larger CSC (23).
Study Limitations
Data for this monocentric trial were collected in a non-randomized fashion. The retrospective analysis of the prospectively maintained database included self-adjudicated primary imaging and clinical outcomes. We were confronted with the methodological problem on how to deal with patients diagnosed with LVO at the PSC secondarily sent to the CSC for MTE and who finally did not receive an MTE for different reasons (e.g., improvement under IV Alteplase and extended core infarct on secondary imaging at the CSC). Patients with LVO who improved prior to transfer from PSC to CSC were not included, leading to a potential time-reset effect (24). The findings of our trial reflect the specifics of our stroke network covering vast rural areas, and the generalizability of these results may thereby be limited.
Conclusion
Our data suggest that patients who had an acute ischemic stroke admitted directly to a CSC may have better 90-day outcomes than those transferred secondarily for thrombectomy from a PSC. Also, in accordance with others, time to recanalization has an especially significant influence on good clinical outcome with the necessity of eliminating influenceable time-consuming steps in acute stroke organization. As a consequence, on the one hand, we have come up with a study of an acute LVO detected by LVO scoring in the field with helicopter-based direct transfer to the CSC2 as well as a reorganization of acute stroke care in DS patients bypassing secondary imaging at CSC with direct transfer to the angio-suite.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee, Faculty of Medicine, University Freiburg. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CT, AT, and W-DN: substantial contributions to the conception or design of the work. All authors: acquisition, analysis, or interpretation of data for the work, drafting the work or revising it critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.743151/full#supplementary-material
Footnotes
1. ^https://en.wikipedia.org/wiki/Freiburg_(region) (accessed December 7, 2020).
2. ^LESTOR trial: https://www.drks.de/drks_web/setLocale_EN.do.
References
1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
2. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
3. Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. (2020) 382:1981–93. doi: 10.1056/NEJMoa2001123
4. Ernst M, Schlemm E, Holodinsky JK, Kamal N, Thomalla G, Fiehler J, et al. Modeling the optimal transportation for acute stroke treatment: the impact of the drip-and-drive paradigm. Stroke. (2020) 51:275–81. doi: 10.1161/STROKEAHA.119.027493
5. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke Organisation (ESO)- European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. (2019) 11:535–8. doi: 10.1136/neurintsurg-2018-014568
6. Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, et al. Use of the alberta stroke program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. (2001) 22:1534–42.
7. Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. (2003). 34:e109–37 [Erratum in: Stroke. (2003) 34:2774. doi: 10.1161/01.STR.0000082721.62796.09
8. Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. (1995) 274:1017–25. doi: 10.1001/jama.274.13.1017
9. Weisenburger-Lile D, Blanc R, Kyheng M, Desilles JP, Labreuche J, Piotin M, et al. Direct admission versus secondary transfer for acute stroke patients treated with intravenous thrombolysis and thrombectomy: insights from the endovascular treatment in ischemic stroke registry. Cerebrovasc Dis. (2019) 47:112–20. doi: 10.1159/000499112
10. Froehler MT, Saver JL, Zaidat OO, Jahan R, Aziz-Sultan MA, Klucznik RP, et al. Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke). Circulation. (2017) 136:2311–21. doi: 10.1161/CIRCULATIONAHA.117.028920
11. Gerschenfeld G, Muresan IP, Blanc R, Obadia M, Abrivard M, Piotin M, et al. Two paradigms for endovascular thrombectomy after intravenous thrombolysis for acute ischemic stroke. JAMA Neurol. (2017) 74:549–56. doi: 10.1001/jamaneurol.2016.5823
12. Rinaldo L, Brinjikji W, McCutcheon BA, Bydon M, Cloft H, Kallmes DF, et al. Hospital transfer associated with increased mortality after endovascular revascularization for acute ischemic stroke. J Neurointerv Surg. (2017) 9:1166–72. doi: 10.1136/neurintsurg-2016-012824
13. Bücke P, Pérez MA, Schmid E, Nolte CH, Bäzner H, Henkes H. Endovascular thrombectomy in acute ischemic stroke: outcome in referred versus directly admitted patients. Clin Neuroradiol. (2018) 28:235–44. doi: 10.1007/s00062-017-0558-z
14. Adams KM, Burns PA, Hunter A, Rennie I, Flynn PA, Smyth G, et al. Outcomes after thrombectomy in belfast: mothership and drip-and-ship in the real world. Cerebrovasc Dis. (2019) 47:231–7. doi: 10.1159/000500849
15. Ismail M, Armoiry X, Tau N, Zhu F, Sadeh-Gonik U, Piotin M, et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: a systematic review and meta-analysis. J Neurointerv Surg. (2019) 11:14–9. doi: 10.1136/neurintsurg-2018-014249
16. Flint AC, Avins AL, Eaton A, Uong S, Cullen SP, Hsu DP, et al. Risk of distal embolization from tPA (tissue-type plasminogen activator) administration prior to endovascular stroke treatment. Stroke. (2020) 51:2697–704. doi: 10.1161/STROKEAHA.120.029025
17. Suzuki K, Matsumaru Y, Takeuchi M, Morimoto M, Kanazawa R, Takayama Y, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. (2021) 325:244–53. doi: 10.1001/jama.2020.23522
18. Holodinsky JK, Williamson TS, Demchuk AM, Zhao H, Zhu L, Francis MJ, et al. Modeling stroke patient transport for all patients with suspected large-vessel occlusion. JAMA Neurol. (2018) 75:1477–86. doi: 10.1001/jamaneurol.2018.2424
19. Tsivgoulis G, Geisler F, Katsanos AH, Kõrv J, Kunz A, Mikulik R, et al. Ultraearly intravenous thrombolysis for acute ischemic stroke in mobile stroke unit and hospital settings. Stroke. (2018) 49:1996–9. doi: 10.1161/STROKEAHA.118.021536
20. Langhorne P, Audebert HJ, Cadilhac DA, Kim J, Lindsay P. Stroke systems of care in high-income countries: what is optimal? Lancet. (2020) 396:1433–42. doi: 10.1016/S0140-6736(20)31363-5
21. Brekenfeld C, Goebell E, Schmidt H, Henningsen H, Kraemer C, Tebben J, et al. 'Drip-and-drive': shipping the neurointerventionalist to provide mechanical thrombectomy in primary stroke centers. J Neurointerv Surg. (2018) 10:932–6. doi: 10.1136/neurintsurg-2017-013634
22. Rinaldo L, Brinjikji W, Rabinstein AA. Transfer to high-volume centers associated with reduced mortality after endovascular treatment of acute stroke. Stroke. (2017). 48:1316–21. doi: 10.1161/STROKEAHA.116.016360
23. Bösel J. Intensive care management of the endovascular stroke patient. Semin Neurol. (2016) 36:520–30. doi: 10.1055/s-0036-1592360
Keywords: stroke, mechanical thrombectomy, stroke outcome, drip-and-ship, mothership
Citation: Taschner CA, Trinks A, Bardutzky J, Brich J, Hartmann R, Urbach H and Niesen W-D (2021) Drip-and-Ship for Thrombectomy Treatment in Patients With Acute Ischemic Stroke Leads to Inferior Clinical Outcomes in a Stroke Network Covering Vast Rural Areas Compared to Direct Admission to a Comprehensive Stroke Center. Front. Neurol. 12:743151. doi: 10.3389/fneur.2021.743151
Received: 17 July 2021; Accepted: 27 September 2021;
Published: 01 November 2021.
Edited by:
Yong-Won Kim, Kyungpook National University Hospital, South KoreaReviewed by:
Hyungjong Park, Keimyung University, South KoreaDong-Seok Gwak, Kyungpook National University Hospital, South Korea
Copyright © 2021 Taschner, Trinks, Bardutzky, Brich, Hartmann, Urbach and Niesen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wolf-Dirk Niesen, wolf-dirk.niesen@uniklinik-freiburg.de
 Christian A. Taschner1,2
Christian A. Taschner1,2 Jürgen Bardutzky
Jürgen Bardutzky Jochen Brich
Jochen Brich Horst Urbach
Horst Urbach Wolf-Dirk Niesen
Wolf-Dirk Niesen