- Department of Neurology and Neuroscience Center, The First Hospital of Jilin University, Changchun, China
Progressive encephalomyelitis with rigidity and myoclonus (PERM) is part of the variant type of the Stiff Person Syndrome (SPS) and is a rare neurological disease. We report here a patient with PERM who had thymoma and was positive for anti-glutamic acid decarboxylase (anti-GAD) antibodies. Her symptoms improved after treatment with hormones and gamma globulin. We also summarized the literature review of patients with PERM accompanied by tumors reported.
Introduction
Stiff person syndrome is a rare neurological disease, mainly manifested by axial and limb muscle stiffness, and muscle painful spasms especially after tactile noise, and emotional stress stimulation. Moersch and Wohman (1) first reported in 1956 under the name “Still-man Syndrome.” Progressive encephalomyelitis with rigidity and myoclonus is a variant of stiff person syndrome. Its main clinical symptoms are in addition to the typical SPS symptoms, as well as sensory, brainstem symptoms (ataxia, vertigo), spinal cord symptoms, and autonomic symptoms, most patients have anti-glutamic acid decarboxylase (anti-GAD) antibodies, some patients found anti-glycine (GlyR) antibodies. In addition, PERM can be accompanied by tumors, such as thymoma (2–5), Hodgkin's lymphoma (6–8), small cell lung cancer (9, 10), breast cancer (11), etc. Here we report a case of GAD antibody- positive PERM associated with thymoma.
Case Presentation
A 60-year-old female patient was admitted to the hospital on May 21, 2019 due to “unstable walking and stiff legs for more than 10 days.” The patient had instability during walking more than 10 days before admission. Stiff bilateral lower limbs appeared at the time of walking, showing muscle tension in the lower limbs, affecting walking, and falling in severe cases, which gradually eased after about 1 min, and fell 5 times during the course of the disease. Accompanied by itching of the right lower extremity, mainly hip. The above symptoms aggravate after tension, emotional excitement, and touch. The patient denied trauma, infection, poisoning, drugs, psychiatric disease, and family genetic history.
On admission, she was alert and well-oriented. Admission examination of the nervous system: small horizontal nystagmus in both eyes; increased muscle tension in both lower extremities; marked rigidity of her lower limbs were also noticedactive tendon reflexes; hyperreflexia was observed in the extremities, and ankle cramps in both legs. There were no sensory disturbances.
Laboratory tests: blood routine, urine routine, liver and kidney function, blood lipids, ions, surgical comprehensive, and tumor markers were normal. Thyroid function: T3, T4 normal, TSH: 6.67 uIU/ml, antithyroid peroxidase antibody: 96.51 uIU/mL (<35), antithyroglobulin antibody: 272.5 uIU/mL (<115); IG antibody 18 U/L (0–12); anti-SSA-60 +, granular type 1: 100, anti-SSA-52/Ro52 +, anti-mitochondrial M2 antibody +, high-sensitivity C-reactive protein 6.03 (0–3.5 mg/L). Cerebrospinal fluid analysis showed no abnormality. The serum anti-GAD antibody IgG was positive and anti-GAD antibody in cerebrospinal fluid was not detected. Anti-islet cell antibody also was positive. Antibodies to Amhiphysin, Yo, Hu, Ri, CV2, Ma2, PCA-2, NMDA receptors, and VGKC were negative in serum. Myasthenia gravis antibodies also were negative. Unfortunately, We were not able to measure anti-glycine antibody titers in our patient due to limited conditions. Neostigmine test: negative.
Head MRI only showed right lacunar infarction. Spinal cord MRI was normal. Electromyographic examination: continuous motor unit activity (CMUA) was seen (Figure 1A). After the injection of 10 mg of diazepam, the CMUA gradually weakened and disappeared (Figure 1B). CT of the thorax revealed an anterior superior mediastinal mass, suggestive of a thymoma.
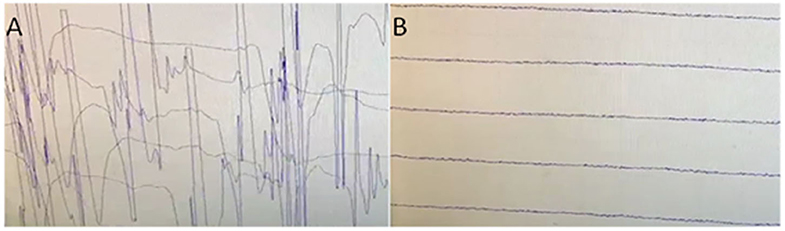
Figure 1. (A) Electromyographic examination: continuous motor unit activity (CMUA)was seen. (B) After the injection of 10 mg of diazepam, the CMUA gradually weakened and disappeared.
After admission, pregabalin (75 mg, 2/day) and baclofen (10 mg, 3/day) were given orally, but the symptoms did not improve. And the patient started difficulty water, dysphagia 10 days after admission. So much that a feeding tube was inserted 15 days after admission, binocular dyskinesia suddenly appeared, and eyes paralyzed to the right. Muscle stiffness and myoclonus in both lower extremities gradually increased, and itching in the right lower extremity was unbearable. To some extent, the patient also presented emotional irritability and anxiety. On examination, she was anxious with no evidence of cognitive deterioration. According to the medical history and auxiliary examination results, she was diagnosed with PERM supported by the clinical diagnosis criteria for SPS proposed by Dalakas et al. (12).
After taking the diagnosis of PERM into consideration, the patient was treated with intravenous methylprednisolone therapy (80 mg/day for 15 days) followed by maintenance therapy with oral methylprednisolone (60 mg/day). She also received intravenous immune globulin (20g/day) for 3 days. Clonazepam taken orally before bedtime. The patient's eye muscle paralysis disappeared after a few days, there was obvious relief of stiffness of lower limbs, paroxysmal myoclonus, difficulty swallowing, and itching and pain symptoms. Discharged neurological examination (2019-06-26): small horizontal nystagmus in both eyes, gastric tube removal, mild stiffness, and cramps in the lower limbs. Hyperreflexia was still observed in the extremities, and ankle cramps in right leg. The patient's thymoma had surgery indications. In view of the obvious improvement of symptoms and economic reasons, the patient and her family decided to postpone the operation.
After discharge, the methylprednisolone was gradually reduced orally, and clonazepam 1 mg was orally maintained before going to bed. On 2020-1-7 telephone follow-up, the patient experienced another episode of dysphagia and had to keep the gastric tube in the past 20 days. Later, the symptoms gradually improved with the increase of clonazepam dose. Recently, on 2020-7-15 telephone follow-up the patient had difficulty in swallowing again, and her lower limbs were so stiff that she dared not walk independently. As far as the patient's current situation is concerned, we recommend again that the removal of the thymoma and immunotherapy if possible.
Discussion
In 1976, Whiteley et al. (13) first reported two cases of encephalomyelitis associated with stiff-man syndrome (SPS) and subacute myoclonic spinal neuronitis, which had been classified as stiff-man superposition syndrome, and later confirmed to be progressive encephalomyelitis with rigidity and myoclonus (PERM). With people's understanding of the disease, PERM is found to be a severely life-threatening autoimmune disease characterized by rigidity, muscle pain and spasm, deep and shallow sensory disturbances and symptoms of the brainstem spinal cord, autonomic function, dyspnea, and stimulus-induced myoclonus. The patient here we reported had stiff muscles of the lower extremities, with paroxysmal myoclonus, itching, and pain; the course of the disease gradually develops, difficulty swallowing so severe that need nasal feeding, and paralysis of both eyes to the right; emotional changes and touch stimulation can induce or exacerbate the above symptom. Electromyographic examination: continuous motor unit activity can be seen, found in rectus abdominis, thoracolumbar paravertebral muscle, and proximal lower limb. After the injection of 10 mg diazepam, the CMUA gradually weakened and disappeared. These clinical symptoms and positive results for anti-GAD antibodies were consistent with PERM. Though there is still no clear diagnostic criteria for PERM. The patient was also accompanied by thymoma. The patient's symptoms improved after treatment with hormones, immunoglobulins, clonazepam, etc. Unfortunately, the patient did not perform thymectomy due to economic reasons and improved symptoms, and the patient experienced relapse of dysphagia, which may be related to the persistent cause of thymoma not removed.
Several neurologic disorders with similar clinical presentations should be considered, including neuromyotonia (NMT), tetanus, and psychogenic dyskinesia. Neuromyotonia is a clinical syndrome characterized by muscle peristalsis, fasciculation, muscle spasm, rigidity, and hyperhidrosis caused by a variety of reasons. Its muscle rigidity is characterized by no relief during sleep, The limbs are more common and rarely involve rectus abdominis. Typical EMG: continuous motor unit activity, slowed movement and sensory conduction, and no response to diazepam, all of which are obviously different from PERM. Tetanus usually has a history of trauma, with masseter muscle spasm as the first symptom. The typical symptom is opisthotonus, and strong muscle spasm caused by external stimuli, ranging from a few seconds to a few minutes each time. It can be distinguished from PERM by the history of trauma, typical symptoms, and the ineffectiveness of tranquilizers, stiff muscles during sleep, and the EMG CMUA can't be completely suppressed by tranquilizers. Psychogenic dyskinesia is mainly manifested as attacks began and ended abrutly, complex and variable manifestations, there are distraction of attention and entrainment effects and co-activation of the affected limb. There are many psychological factors, suggesting or placebo treatment may be effective. Anti-GAD antibodies and EMG are helpful for identification.
The cause of PERM is not clear and may be related to immunity. In recent years, it has been reported that it may be related to anti-glycine receptor (GlyR) antibody. Glycine receptor is a pentameric ligand-gated chloride channel. In the adult nervous system, they are mainly found in the spinal cord and brain stem, which mediate rapid inhibition (14). Antibodies targeted at Glycine receptor may lead to a persistent startle response. Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter. Glutamate decarboxylase(GAD) can catalyze the conversion of the excitatory neurotransmitter glutamic acid to GABA. Antibodies against GAD may affect functioning of GABA-ergic neurons (15). All of antibodies target proteins of GABAergic and glycinergic inhibitory synapses, except for dipeptidyl-peptidase-like protein-6 (DPPX)-antibodies (16). DPPX antibodies are a regulatory subunit of the Kv4.2 potassium channels on the surface of neurons, which are involved in somatodendritic signal integration and attenuation of back-propagation of action potentials. Patients with DPPX antibodies tend to have hyperekplexia, prominent cerebellar ataxia with marked eye movement disorder, trunk stiffness, sensory disturbance, and gastrointestinal symptoms. Our patient had anti-GAD antibodies which can explain the excessive neuronal activities resulting in the typical clinical symptoms. However, Our case simultaneously had many other antibodies but not meet the disease diagnosis criteria, such as antithyroid peroxidase antibody, anti-islet cell antibody, antithyroglobulin antibody, anticardiolipin IgG antibody, anti-SSA-60, granular type, anti-SSA-52/Ro52, anti-mitochondrial M2 antibody in her serum, which indicated the involvement of immune dysfunction. SPS and PERM are associated with other autoimmune conditions including diabetes mellitus, thyroiditis, autoimmune thyroid disorders, and pernicious anemia. Kraemer et al. reported a case of PERM that hypothyroidism was diagnosed 3 months before first motor symptoms and that both, the Hashimoto's thyroiditis and the diabetes mellitus were of recent onset (17). Therefore, it is still necessary for our patient to surveillance the development of potentially autoimmune diseases, such as thyroiditis and diabetes. The mechanisms of interaction between these antibodies need further investigation.
We summarized the cases of PERM reported in recent years (Tables 1, 2) and found that there are more men than women, and the reason for this difference is unknown. The age of onset varies, the age range at presentation is 14 months−81 years with a mean of 50 years. Patients with tumors are generally older. Patients have a variety of onset symptoms. Approximately 35% of patients have onset of muscle stiffness and spasms, and 25% of patients have onset of brainstem symptoms. However, the clinical manifestations are mainly stiff spasms of the trunk and limb muscles, accompanied by brainstem spinal cord symptoms, including eye movement paralysis, gaze paralysis, nystagmus and drooping eyelids, difficulty swallowing, and difficulty articulating. In general, patients will have obvious autonomic symptoms such as sweating, tachycardia, and urinary retention and even autonomic failure, which may include respiratory failure, and must be managed in intensive care. Twenty-five percentage of the patients require mechanical ventilation and mortality up to 40% (42). In most patients, there is no abnormality of the head and spinal cord magnetic resonance. MRI of one patient showed increased signal intensity throughout the length of the cervical cord and lower brainstem on the T2 weighted scan and reduced signal intensity in this region on the Tl weighted scan (20). MRI with diffusion-weighted and fluid-attenuated inversion recovery (FLAIR) weighted sequences (WS) of one patient showed left temporal and insular cortical hyperintensities without gadolinium enhancement (39). MRI of the head in 2 patients showed cerebellar atrophy (23, 33). MRI can differentially diagnose other diseases. EMG has diagnostic significance, and the emergence of CMUA is one of the important diagnostic criteria.
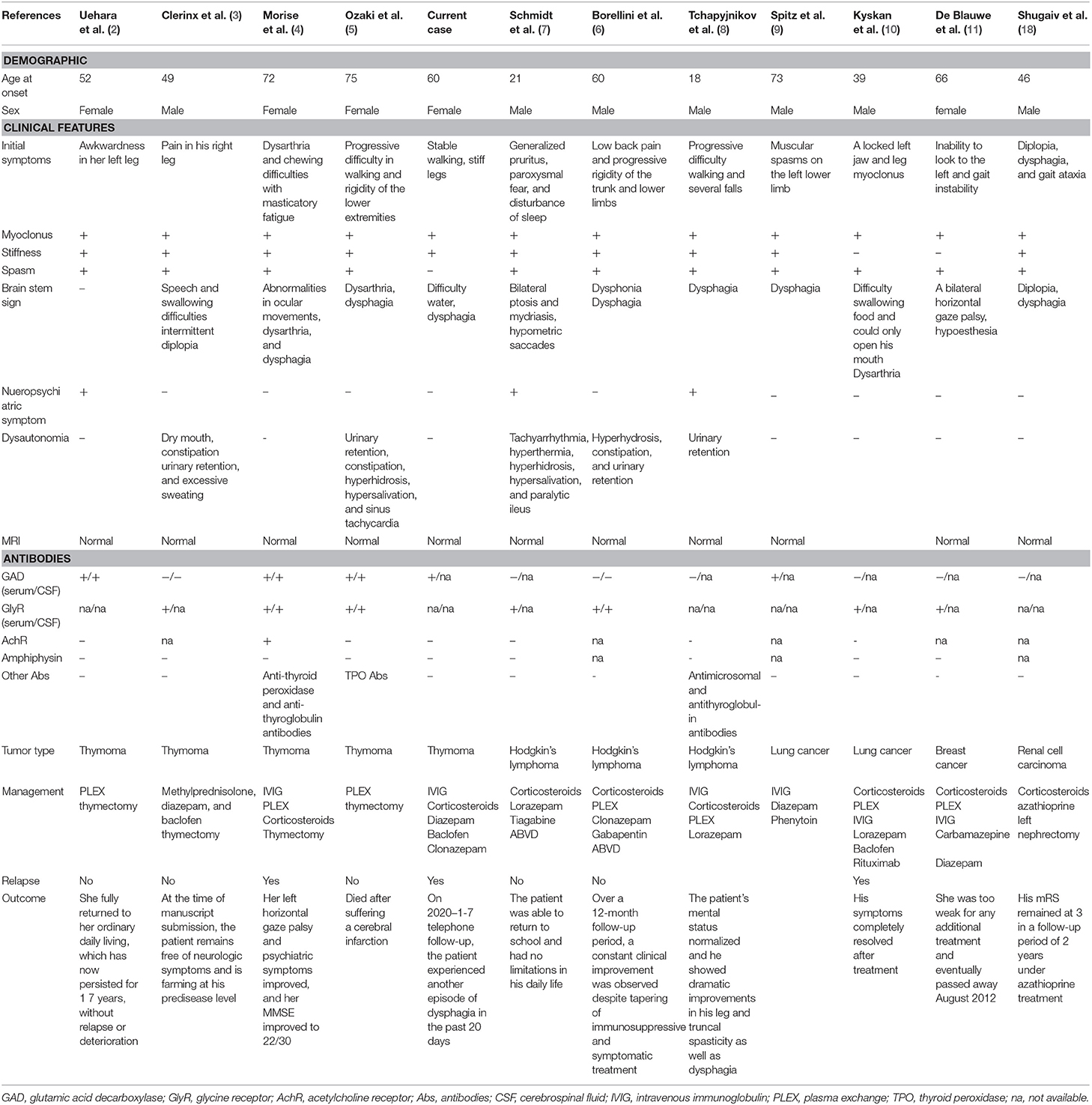
Table 1. Eleven reported cases of PERM associated with tumors; demographics, clinical features, investigation results, management, and outcome.
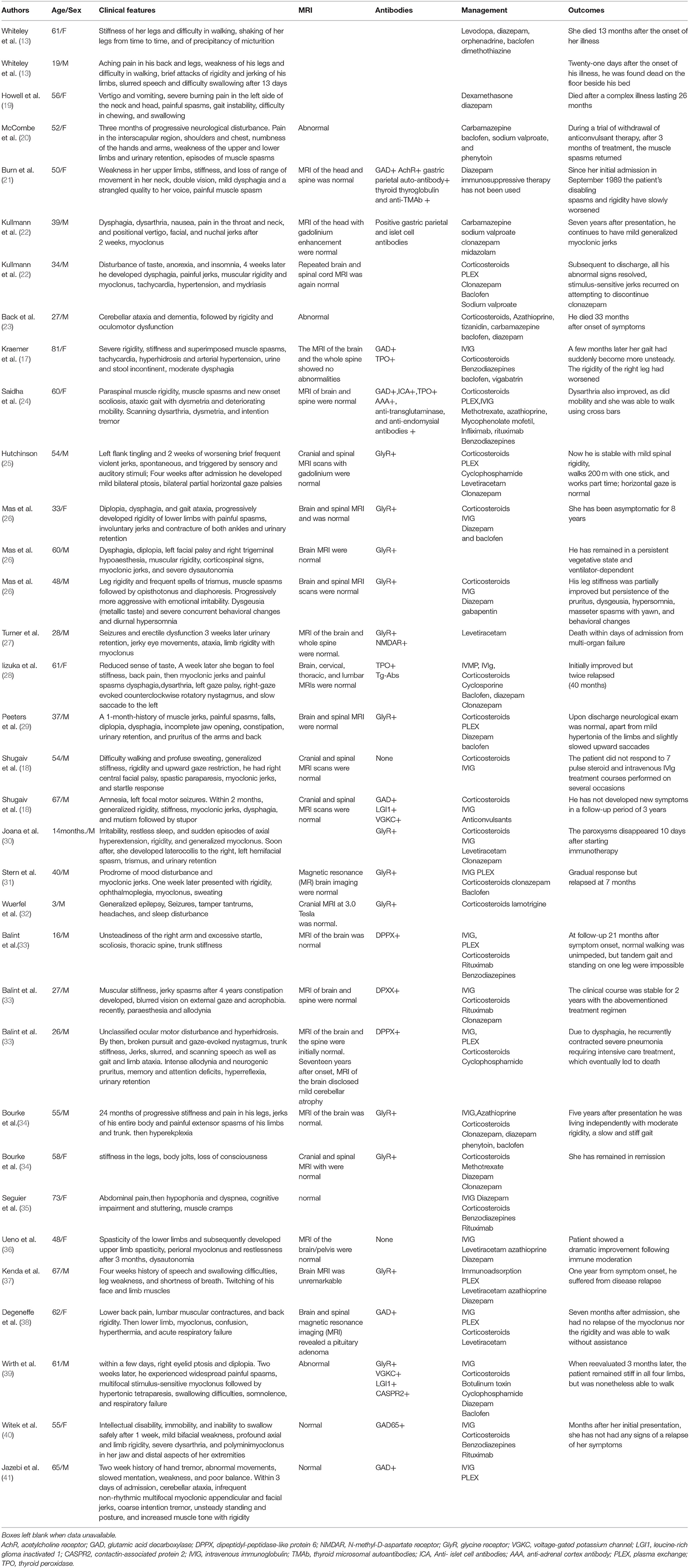
Table 2. Other reported cases of PERM without tumors; demographics, clinical features, investigation results, management and outcome.
The incidence rate of tumor in our statistics are about 20%, which is consistent with the previous literature report (43). We summarized these 12 cases in Table 1. There were 5 cases with thymoma (2–5), 3 cases with Hodgkin's lymphoma (6–8), 2 cases with lung cancer (9, 10), 1 case with breast cancer (11), 1 case with kidney cancer (18). All 7 patients who tested for GlyR antibodies were positive for GlyR antibodies. Almost all patients tested for GAD antibodies, but less than half of the patients were positive for GAD antibodies. Among the 12 patients with tumors, patients with glycine antibody positive were more likely to have dysautonomia.
A retrospective analysis recently found GlyR antibodies in patients of PERM, some of these also had GAD antibodies, DPPX antibodies (33), NMDAR antibodies (27). Patients with GlyR antibodies often have prominent brainstem involvement, and often sensory and autonomic symptoms. GAD antibodies associate with palatal myoclonus, epilepsy, sporadic progressive ataxia. Patients with DPPX antibodies tend to have hyperekplexia, prominent cerebellar ataxia with marked eye movement disorder, trunk stiffness, sensory disturbance, and gastrointestinal symptoms. Multiple antibodies found in some patients of PERM. Therefore, it is difficult to predict the specificity of these antibodies in PERM based on clinical evidence. Further research is needed to clarify each of their roles in PERM. When we consider that a patient may be diagnosed with PERM, we should screen for related antibodies as much as possible.
The treatment of PERM mainly includes symptomatic treatment and immunotherapy. There were 5 patients with thymoma among the patients with tumor, 4 of whom responded well to thymectomy. There were 3 patients with Hodgkin's lymphoma among the patients with tumor, 2 of whom responded well to ABVD chemotherapy. Patients with lung cancer, breast cancer, and kidney cancer have a poor prognosis. The stabilization and recovery may occur if the underlying tumor is detected and treated early. Therefore, in the early stage of diagnosis, tumors should be screened comprehensively, irrespective of the serology in all patients. Most cases of PERM without tumors showed substantial and sustained improvement with immunotherapies, usually with combinations of corticosteroids, IVIg, and plasma exchange, and Rituximab has been reported to have a significant effect.
We reported a patient with PERM who had thymoma and was positive for anti-GAD antibodies, and also summarized the current literature on clinical characteristics, investigation results, management, and outcome of patients of PERM. At the same time, this case also has some limitations, such that we can't measure the anti-glycine antibody titers due to limited lab conditions. In addition, the patient did not perform thymectomy due to economic reasons that maybe result in the patient experienced relapse of dysphagia after being discharged. When encountering similar clinical manifestations, it will be better to test related antibodies as comprehensively as possible and actively advise patients to perform surgical treatment to remove the original lesion to prevent recurrence. We hope that this report will provide a basis for further understanding of PERM and mention clinical doctors better identify and treat these similar presentations so as not to delay diagnosis and treatment.
PERM is a complex autoimmune disease that requires a clinician to diagnose it in combination with the patient's symptoms and signs and auxiliary examinations. With the in-depth study of PERM and the further development of detection technologies such as GAD antibodies and anti-glycine antibodies, people have gradually deepened their understanding of PERM disease, but the diagnosis still lacks specific antibodies. Further investigation is needed to uncover the nature of disease.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YS and LC participated in writing the manuscript. MZ and YL participated in collecting the information of the paper and analysis or interpretation of data. YZ participated in revising the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Natural Science Foundation of China (No. 81974194) (to YZ), by Jilin Province Health and Family Planning Commission (No. 2018J045) (to YZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Moersch FP, Woltman HW. Progressive fluctuating muscular rigidity and spasm (“stiff-man” syndrome); report of a case and some observations in 13 other cases. Proc Staff Meet Mayo Clin. (1956) 31:421–7.
2. Uehara T, Murai H, Yamasaki R, Kikuchi H, Shigeto H, Ohyagi Y, et al. Thymoma-associated progressive encephalomyelitis with rigidity and myoclonus successfully treated with thymectomy and intravenous immunoglobulin. Eur Neurol. (2011) 66:328–30. doi: 10.1159/000332033
3. Clerinx K, Breban T, Schrooten M, Leite MI, Vincent A, Verschakelen J, et al. Progressive encephalomyelitis with rigidity and myoclonus: resolution after thymectomy. Neurology. (2011) 76:303–4. doi: 10.1212/WNL.0b013e318207b008
4. Morise S, Nakamura M, Morita JI, Miyake K, Kunieda T, Kaneko S, et al. Thymoma-associated Progressive Encephalomyelitis with Rigidity and Myoclonus (PERM) with myasthenia gravis. Intern Med. (2017) 56:1733–7. doi: 10.2169/internalmedicine.56.7979
5. Ozaki K, Ohkubo T, Yamada T, Yoshioka K, Ichijo M, Majima T, et al. Progressive encephalomyelitis with rigidity and myoclonus resolving after thymectomy with subsequent anasarca: an autopsy case. Intern Med. (2018) 57:3451–8. doi: 10.2169/internalmedicine.1238-18
6. Borellini L, Lanfranconi S, Bonato S, Trezzi I, Franco G, Torretta L, et al. Progressive encephalomyelitis with rigidity and myoclonus associated with Anti-GlyR antibodies and hodgkin's lymphoma: a case report. Front Neurol. (2017) 8:401. doi: 10.3389/fneur.2017.00401
7. Schmidt C, Freilinger T, Lieb M, Rémi J, Klein M, Straube A, et al. Progressive encephalomyelitis with rigidity and myoclonus preceding otherwise asymptomatic Hodgkin's lymphoma. J Neurol Sci. (2010) 291:118–20. doi: 10.1016/j.jns.2009.12.025
8. Tchapyjnikov D, Borst AJ. Immune-related neurological symptoms in an adolescent patient receiving the checkpoint inhibitor nivolumab. J Immunother. (2017) 40:286–8. doi: 10.1097/CJI.0000000000000177
9. Spitz M, Ferraz HB, Barsottini OG, Gabbai AA. Progressive encephalomyelitis with rigidity: a paraneoplastic presentation of oat cell carcinoma of the lung. case report. Arq Neuropsiquiatria. (2004) 62:547–9. doi: 10.1590/S0004-282X2004000300033
10. Kyskan R, Chapman K, Mattman A, Sin D. Antiglycine receptor antibody and encephalomyelitis with rigidity and myoclonus (PERM) related to small cell lung cancer. BMJ Case Rep. (2013) 2013:bcr2013010027. doi: 10.1136/bcr-2013-010027
11. De Blauwe SN, Santens P, Vanopdenbosch LJ. Anti-glycine receptor antibody mediated progressive encephalomyelitis with rigidity and myoclonus associated with breast cancer. Case Rep Neurol Med. (2013) 2013:589154. doi: 10.1155/2013/589154
12. Dalakas MC. Stiff person syndrome: advances in pathogenesis and therapeutic interventions. Curr Treat Options Neurol. (2009) 11:102–10. doi: 10.1007/s11940-009-0013-9
13. Whiteley AM, Swash M, Urich H. Progressive encephalomyelitis with rigidity. Brain. (1976) 99:27–42. doi: 10.1093/brain/99.1.27
14. Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. (2009) 56:303–9. doi: 10.1016/j.neuropharm.2008.07.034
15. Gresa-Arribas N, Ariño H, Martínez-Hernández E, Petit-Pedrol M, Sabater L, Saiz A, et al. Antibodies to inhibitory synaptic proteins in neurological syndromes associated with glutamic acid decarboxylase autoimmunity. PLoS ONE. (2015) 10:e121364. doi: 10.1371/journal.pone.0121364
16. Balint B, Bhatia KP. Stiff person syndrome and other immune-mediated movement disorders - new insights. Curr Opin Neurol. (2016) 29:496–506. doi: 10.1097/WCO.0000000000000351
17. Kraemer M, Berlit P. Progressive encephalomyelitis with rigidity and myoclonus in an 81-year-old patient. Clin Neurol Neurosurg. (2008) 110:279–81. doi: 10.1016/j.clineuro.2007.10.004
18. Shugaiv E, Leite MI, Sehitoglu E, Woodhall M, Çavuş F, Waters P, et al. Progressive encephalomyelitis with rigidity and myoclonus: a syndrome with diverse clinical features and antibody responses. Eur Neurol. (2013) 69:257–62. doi: 10.1159/000342237
19. Howell DA, Lees AJ, Toghill PJ. Spinal internuncial neurones in progressive encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry. (1979) 42:773–85. doi: 10.1136/jnnp.42.9.773
20. McCombe PA, Chalk JB, Searle JW, Tannenberg AE, Smith JJ, Pender MP. Progressive encephalomyelitis with rigidity: a case report with magnetic resonance imaging findings. J Neurol Neurosurg Psychiatry. (1989) 52:1429–31. doi: 10.1136/jnnp.52.12.1429
21. Joanna Ball Andrew J. David J Burn. MATTERS ARISING: a case of progressive encephalomyelitis with rigidity and positive antiglutamic acid dehydrogenase antibodies. J Neurol Neurosurg Psychiatry. (1991) 54:1032. doi: 10.1136/jnnp.54.11.1032-e
22. Kullmann DM, Howard RS, Miller DH, Hirsch NP, Brown P, Marsden CD. Brainstem encephalopathy with stimulus-sensitive myoclonus leading to respiratory arrest, but with recovery: a description of two cases and review of the literature. Mov Disord. (1996) 11:715–8. doi: 10.1002/mds.870110618
23. Back T, Stoltenburg-Didinger G, Ploner CJ, Meisel H, Zschenderlein R. A new variant of progressive encephalomyelitis with rigidity associated with cerebellar ataxia and dementia: correlation of MRI and histopathological changes. a case report. Neurol Res. (1997) 19:187–91. doi: 10.1080/01616412.1997.11740794
24. Saidha S, Elamin M, Mullins G, Chaila E, Tormey VJ, Hennessy MJ. Treatment of progressive encephalomyelitis with rigidity and myoclonic jerks with rituximab: a case report. Eur J Neurol. (2008) 15:e33. doi: 10.1111/j.1468-1331.2008.02089.x
25. Hutchinson M, Waters P, McHugh J, Gorman G, O'Riordan S, Connolly S, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. (2008) 71:1291–2. doi: 10.1212/01.wnl.0000327606.50322.f0
26. Mas N, Saiz A, Leite MI, Waters P, Baron M, Castaño D, et al. Antiglycine-receptor encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry. (2011) 82:1399–401. doi: 10.1136/jnnp.2010.229104
27. Turner MR, Irani SR, Leite MI, Nithi K, Vincent A, Ansorge O. Progressive encephalomyelitis with rigidity and myoclonus: glycine and NMDA receptor antibodies. Neurology. (2011) 77:439–43. doi: 10.1212/WNL.0b013e318227b176
28. Iizuka T, Leite MI, Lang B, Waters P, Urano Y, Miyakawa S, et al. Glycine receptor antibodies are detected in progressive encephalomyelitis with rigidity and myoclonus (PERM) but not in saccadic oscillations. J Neurol. (2012) 32:544–9.
29. Peeters E, Vanacker P, Woodhall M, Vincent A, Schrooten M, Vandenberghe W. Supranuclear gaze palsy in glycine receptor antibody-positive progressive encephalomyelitis with rigidity and myoclonus. Mov Disord. (2012) 27:1830–2. doi: 10.1002/mds.25239
30. Damásio J, Leite MI, Coutinho E, Waters P, Woodhall M, Santos MA, et al. Progressive encephalomyelitis with rigidity and myoclonus: the first pediatric case with glycine receptor antibodies. JAMA Neurol. (2013) 70:498–501. doi: 10.1001/jamaneurol.2013.1872
31. Stern WM, Howard R, Chalmers RM, Woodhall MR, Waters P, Vincent A, et al. Glycine receptor antibody mediated Progressive Encephalomyelitis with Rigidity and Myoclonus (PERM): a rare but treatable neurological syndrome. Pract Neurol. (2014) 14:123–7. doi: 10.1136/practneurol-2013-000511
32. Wuerfel E, Bien CG, Vincent A, Woodhall M, Brockmann K. Glycine receptor antibodies in a boy with focal epilepsy and episodic behavioral disorder. J Neurol Sci. (2014) 343:180–2. doi: 10.1016/j.jns.2014.05.014
33. Balint B, Jarius S, Nagel S, Haberkorn U, Probst C, Blöcker IM, et al. Progressive encephalomyelitis with rigidity and myoclonus: a new variant with DPPX antibodies. Neurology. (2014) 82:1521–8. doi: 10.1212/WNL.0000000000000372
34. Bourke D, Roxburgh R, Vincent A, Cleland J, Jeffery O, Dugan N, et al. Hypoventilation in glycine-receptor antibody related progressive encephalomyelitis, rigidity and myoclonus. J Clin Neurosci. (2014) 21:876–8. doi: 10.1016/j.jocn.2013.07.014
35. Seguier J, Serratrice J, Lachaud A, Belenotti P, Benyamine A, Verschueren A, et al. [Abdominal pain in progressive encephalomyelitis with rigidity]. Rev Med Interne. (2015) 36:283–6. doi: 10.1016/j.revmed.2013.12.019
36. Ueno S, Miyamoto N, Shimura H, Ueno Y, Watanabe M, Hayashi A, et al. Successful immune moderation treatment for progressive encephalomyelitis with rigidity and myoclonus. Intern Med. (2015) 54:219–21. doi: 10.2169/internalmedicine.54.3760
37. Kenda J, Švigelj V, Rodi Z, Koritnik B, Graus F, Kojović M. Glycine receptor antibodies and progressive encephalomyelitis with rigidity and myoclonus with predominant motor neuron degeneration–expanding the clinical spectrum. J Neurol Sci. (2015) 353:177–8. doi: 10.1016/j.jns.2015.03.049
38. Degeneffe A, Dagonnier M, D'hondt A, Elosegi JA. A case report of rigidity and recurrent lower limb myoclonus: progressive encephalomyelitis rigidity and myoclonus syndrome, a chameleon. BMC Neurol. (2018) 18:173. doi: 10.1186/s12883-018-1176-3
39. Wirth T, Kaeuffer C, Chanson JB, Echaniz-Laguna A, Renaud M, Anheim M, et al. Progressive encephalomyelitis with rigidity and myoclonus, a diagnostic challenge. Rev Neurol. (2018) 174:343–6. doi: 10.1016/j.neurol.2017.09.012
40. Witek N, Hebert C, Gera A, Comella C. Progressive Encephalomyelitis With Rigidity and Myoclonus Syndrome Presenting as Catatonia. Psychosomatics. (2019) 60:83–7. doi: 10.1016/j.psym.2018.05.005
41. Jazebi N, Rodrigo S, Gogia B, Shawagfeh A. Anti-glutamic acid decarboxylase (GAD) positive cerebellar Ataxia with transitioning to progressive encephalomyelitis with rigidity and myoclonus (PERM), responsive to immunotherapy: a case report and review of literature. J Neuroimmunol. (2019) 332:135–7. doi: 10.1016/j.jneuroim.2019.04.003
42. Duddy ME, Baker MR. Stiff person syndrome. Front Neurol Neurosci. (2009) 26:147–65. doi: 10.1159/000212375
Keywords: progressive encephalomyelitis with rigidity and myoclonus, thymoma, anti-glycine receptor antibody, anti-glutamic acid decarboxylase antibodies, tumors
Citation: Su Y, Cui L, Zhu M, Liang Y and Zhang Y (2020) Progressive Encephalomyelitis With Rigidity and Myoclonus With Thymoma: A Case Report and Literature Review. Front. Neurol. 11:1017. doi: 10.3389/fneur.2020.01017
Received: 24 June 2020; Accepted: 03 August 2020;
Published: 18 September 2020.
Edited by:
Long-Jun Wu, Mayo Clinic, United StatesReviewed by:
Daishi Tian, Huazhong University of Science and Technology, ChinaWei Shihui, Chinese PLA General Hospital, China
Copyright © 2020 Su, Cui, Zhu, Liang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhang, zhang_ying99@jlu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Yana Su
Yana Su Li Cui
Li Cui Mingqin Zhu
Mingqin Zhu Yixuan Liang
Yixuan Liang Ying Zhang
Ying Zhang