- 1Department of Neurology, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 2Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 3China National Clinical Research Center for Neurological Diseases, Beijing, China
Objective: To explore cerebrospinal fluid (CSF) levels of soluble triggering receptor expressed on myeloid cells 2 (sTREM2) and neurofilament light proteins (NFL) in patients with neurosyphilis (NS).
Methods: We enrolled 71 NS patients (41 early-NS and 30 late-NS patients) and 20 syphilis but non-NS patients whose CSF samples were collected. The CSF levels of the microglial activation biomarker sTREM2 and neuronal injury biomarker NFL were measured using ELISA.
Results: CSF sTREM2 levels were significantly higher in NS patients compared to those in syphilis/non-NS patients (p < 0.001). In a subgroup analysis, the CSF sTREM2 levels elevated significantly in late-NS patients than those in early-NS patients (p < 0.001). The CSF sTREM2 levels in early-NS group were also significantly higher than those in syphilis/non-NS group (p = 0.024). Like CSF sTREM2, similar differences between groups were also found in CSF NFL. There was a moderate correlation between CSF sTREM2 and CSF NFL (r = 0.406, p < 0.001) in NS group.
Conclusions: CSF sTREM2 levels elevated in NS and peaked at the late stage, suggesting that CSF sTREM2 may be a useful marker to quantify microglia activation in NS and may play a role in the progression of NS. The positive correlation between CSF sTREM2 and CSF NFL indicates a linkage between microglial activation and neuronal injury in NS.
Introduction
NS is a chronic infectious disease caused by Treponema pallidum invading the central nervous system (CNS), which mainly damages the meninges, blood vessels, and brain, and spinal cord parenchyma (1, 2). A large number of activated microglia were found in the brain autopsies of NS, demonstrating that the microglial activation was an important pathological feature of NS (3, 4), which was rarely studied in CSF samples of patients with NS.
The triggering receptor expressed on myeloid cells-2 (TREM2) is a cell surface receptor protein, which is mainly expressed in myelocytes (5). Most relevant for the brain is the microglial expression of TREM2, which promotes phagocytosis, suppresses Toll-like receptor-induced inflammatory cytokine production, and enhances anti-inflammatory cytokine transcription in vitro (6–8). Increased soluble TREM2 (sTREM2) levels in CSF have been found in HIV infection (8), multiple sclerosis (MS) (9, 10), and Alzheimer's disease (AD) (11, 12). However, over activation of microglia may result in neuronal loss and neuropil damage (3, 4). Neurofilament light proteins (NFL) are the most widely distributed and important component of neurofilament proteins (NFs) (13). The levels of CSF NFL can reflect the degree of neuronal injury of the CNS and severity of the disease to a certain extent. A correlation between microglial activation and neuronal injury was found in HIV infection (8). NS patients, especially in the late stage, have mental abnormalities, cognitive changes, and brain atrophy (1, 2), indicating that NS patients have a degree of neuronal loss and injury. We speculate that the microglial dysfunction is probably involved in the pathogenesis of mental and neurocognitive disorders in NS.
The aim of this study was to determine the levels of CSF sTREM2 in different stages of NS patients and to explore the relationship between CSF sTREM2 and CSF NFL, so as to better understand the neuropathogenesis and progression of NS.
Materials and Methods
Patients
Between May 2018 and June 2019, 71 NS patients and 20 syphilis but non-NS controls hospitalized in the neurology department of Beijing Ditan Hospital and Beijing Tiantan Hospital were analyzed in this study. We recorded the medical history, neurological symptoms and signs, and serum and CSF laboratory testing results. According to the guidelines of NS in the USA, Europe and related literatures (14–18), the criteria for the diagnosis of NS in our study included positive syphilis serologies and one or more of the followings: (a) positive CSF rapid plasma regain (RPR); (b) positive CSF Treponema pallidum particle agglutination (TPPA) and fluorescent treponemal antibody absorption (FTA-ABS), with increased CSF protein (>45 mg/dl) or white blood cells (WBC) (> 5/μl) in absence of other known causes of these abnormalities. All of the enrolled patients were HIV negative. The exclusion criteria were as follows: treatment with antibiotics within the last 1-month, other infectious diseases (e.g., HIV), neurodegenerative disease (e.g., AD) and autoimmune diseases (e.g., MS). The patients enrolled in the control group were a serofast status without neurological symptoms and signs and underwent lumber puncture to rule out neurosyphilis.
This study was approved by the Ethics Committee of Beijing Ditan Hospital Affiliated to Capital Medical University, Beijing, People's Republic of China and written informed consent was obtained from all participants.
The enrolled NS patients were divided into early-NS that occurred during the primary stage or secondary stage, including asymptomatic, meningeal and meningovascular NS and late-NS that occurred years to decades after the primary infection, including general paresis and tabes dorsalis (1, 4).
Biomarker Measurement
CSF samples were immediately centrifuged and the supernatants were collected and stored at −80°C until the time of the biomarker assays. ELISA, to quantify CSF sTREM2 (ab224881, abcam) and NFL (CSB-E16094h, CUSA-BIO, Wuhan, China), was performed blinded to clinical information. All testing was performed according to the manufacturer's protocols and by the same technique.
Statistical Analysis
Data were analyzed by SPSS software (IBM SPSS 25.0 version) or Prism (GraphPad software 7.0 version). CSF levels of sTREM2 and NFL were log10 transformed where appropriate to reduce skewness. Continuous data were compared with the non-parametric Mann-Whitney U-test when the data were non-parametric or independent two-sample t-test when the data were parametric. Gender differences were assessed by χ2-test. Pearson' test (for parametric data) and Spearman's test (for non-parametric data) were used to ascertain the associations for analysis of correlations. The significance level was established at a two-sided p < 0.05.
Results
Patients Characteristics
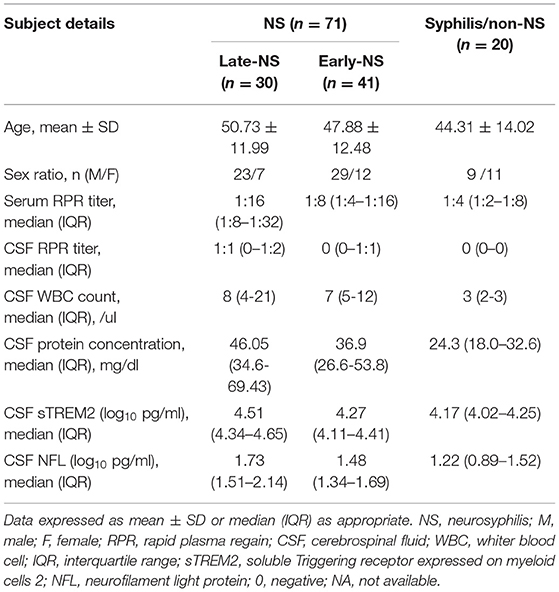
The clinical characteristics and concentrations of different CSF biomarkers were summarized in Table 1. A total of 71 NS patients (52 male and 19 female) and 20 syphilis but non-NS (9 male and 11 female) were studied. Forty-one patients with early-NS were enrolled, including asymptomatic NS (n = 34), meningeal NS (n = 1) and meningovascular NS (n = 6). In addition, 30 patients with late-NS were enrolled, including general paresis (n = 26) and tabes dorsalis (n = 4). The vast majority of patients received anti-syphilis treatment, and only 2 patients with paralytic dementia had no treatment history. The 30 cases of late-NS showed a variety of neurological symptoms and signs, including mental and behavior disorders (24/30), cognitive changes (23/30), sensory impairment (deduced vibration sense, Romberg sign, ataxia, painful polyradiculopathy, lighting pains) (8/30), parkinsonism (4/30), headache (3/30) and seizures (3/30). The symptoms of 41 cases of early-NS were asymptomatic (34/41), headache (1/41), stroke (6/41) and seizures (1/41).
There was no significant difference in age and gender between the NS group and the syphilis/non-NS group (p = 0.65 and p = 0.29). The serum RRP titer was significantly higher in the NS group than in the syphilis/non-NS group (p < 0.001). As for CSF laboratory findings between the late-NS group and early-NS group, we found that the incidence of positive CSF RPR rate was significantly higher in the late-NS group than in the early-NS group (p < 0.001), but no significant difference of serum RPR titer, CSF protein concentration and CSF WBC was found between the two groups (p = 0.081, p = 0.115, and p = 0.670, respectively).
CSF sTREM2 Levels Are Higher in NS Patients
The median levels of CSF sTREM2 were 21793.98 (16273.05-35218.54) pg/ml in NS group and 14783.12 (10567.25-17894.95) pg/ml in non-NS group. CSF sTREM2 (log10) values in the NS group were significantly higher than those in the syphilis/non-NS group (p < 0.001; Figure 1A). In the NS subgroup analysis, the higher CSF sTREM2 levels were found in the late-NS group [32700.63 (21979.94-44562.75) pg/ml] than those in the early-NS group [18641.82 (12839.14-25737.66) pg/ml]. There was a statistically significant difference of CSF sTREM2 (log10) values between the two NS groups (p < 0.001; Figure 1B). The CSF sTREM2 (log10) values in the early-NS group were also significantly higher than those in the syphilis/non-NS group (p = 0.024; Figure 1B).
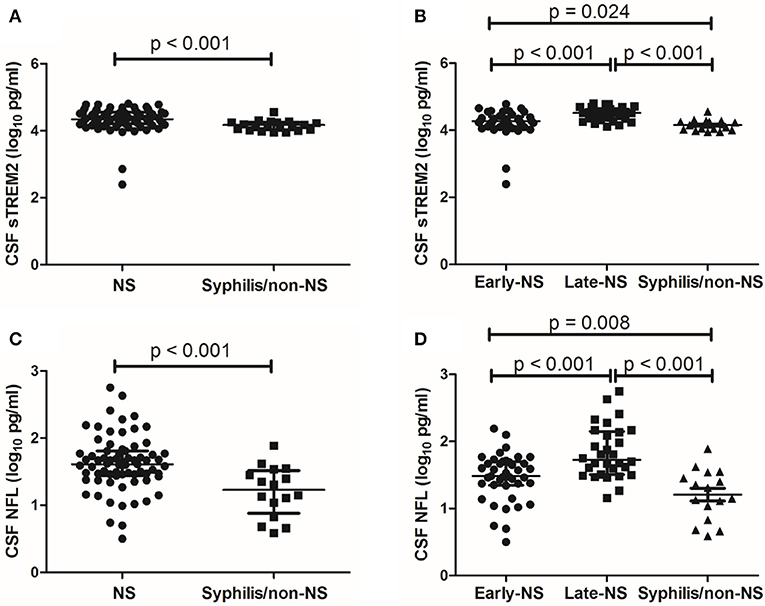
Figure 1. CSF levels of sTREM2 and NFL in NS and syphilis/non-NS patients. (A) Patients with NS had significantly higher levels of CSF sTREM2 (log10 pg/ml) than those with syphilis/non-NS. (B) The CSF sTREM2 (log10 pg/ml) levels in late-NS were significantly higher than in early-NS and syphilis/non-NS. The CSF sTREM2 (log10 pg/ml) levels in early-NS were also significantly higher than in syphilis/non-NS. (C) Patients with NS had significantly higher levels of CSF NFL (log10 pg/ml) than those with syphilis/non-NS. (D) The CSF NFL (log10 pg/ml) levels in late-NS were significantly higher than in early-NS and syphilis/non-NS. The CSF NFL (log10 pg/ml) levels in early-NS were also significantly higher than in syphilis/non-NS. NS, neurosyphilis; sTREM2, soluble triggering receptor expressed on myeloid cells 2; NFL, neurofilament light protein.
CSF NFL Levels Are Higher in NS Patients
Like CSF sTREM2, we found similar differences between groups in CSF NFL. The statistical results for CSF NFL of group comparisons were shown in Figures 1C,D.
Correlations of CSF sTREM2 Levels With CSF NFL and Age
There was a moderate correlation between CSF sTREM2 (log10) values and CSF NFL (log10) (r = 0.406, p < 0.001; Figure 2A) in NS group, while no significant correlation was found between CSF sTREM2 (log10) and CSF NFL (log10) in the syphilis/non-NS group (r = 0.02, p = 0.95; Figure 2B).
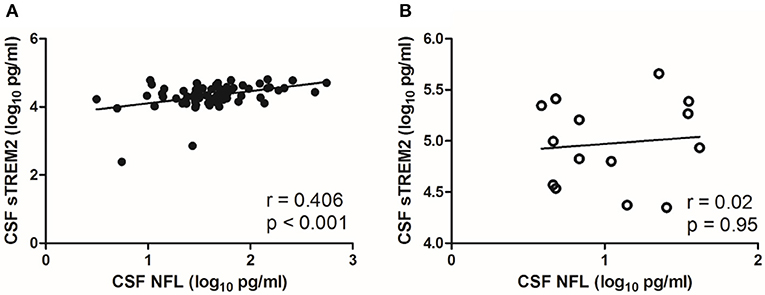
Figure 2. Correlations between CSF sTREM2 and CSF NFL in NS group and syphilis/non-NS group. (A) CSF sTREM2 (log10 pg/ml) levels were moderate correlated with CSF NFL (log10 pg/ml) in NS group. (B) No significant correlation was found between CSF sTREM2 (log10 pg/ml) and CSF NFL (log10 pg/ml) in syphilis/non-NS group. NS, neurosyphilis; CSF, cerebrospinal fluid; sTREM2, soluble triggering receptor expressed on myeloid cells 2; NFL, neurofilament light protein.
CSF sTREM2 (log10) levels were weak correlated with age in the NS group (r = 0.365, p = 0.002; Figure 3A), however, no significant correlation was found between CSF NFL (log10) and age in the NS group (r=0.06, p = 0.617; Figure 3B). The levels of CSF sTREM2 (log10) and CSF NFL (log10) were better correlated with age in syphilis/non-NS group (r = 0.556, p = 0.011 and p = 0.499, p = 0.049; Figures 3C,D).
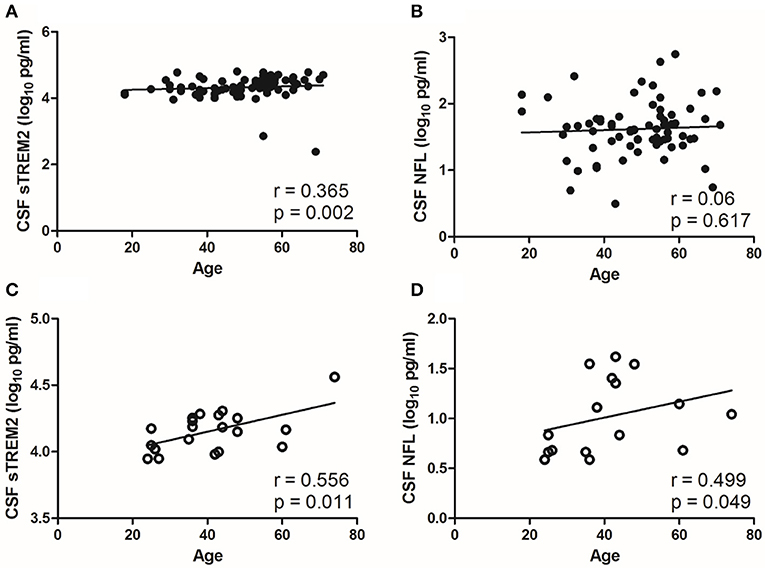
Figure 3. Correlations between CSF biomarkers and age in NS group and syphilis/non-NS group. (A) CSF sTREM2 (log10 pg/ml) levels were weak correlated with age in NS group. (B) No significant correlation was found between CSF NFL (log10 pg/ml) and age in NS group. (C) CSF sTREM2 (log10 pg/ml) levels were moderate correlated with age in syphilis/non-NS group. (D) CSF NFL (log10 pg/ml) levels were moderate correlated with age in syphilis/non-NS group. NS, neurosyphilis; CSF, cerebrospinal fluid; sTREM2, soluble triggering receptor expressed on myeloid cells 2; NFL, neurofilament light protein.
CSF sTREM2 Levels Are Higher in CSF RPR Positive Patients
To further evaluate the association between CSF sTREM2 and neuroinflammation, we divided the NS patients into CSF RPR positive group and CSF RPR negative group and compared the CSF sTREM2 levels between the two groups. Here, 43.7% (31/71) of our NS patients had CSF RPR positive, and the higher CSF sTREM2 levels were found in CSF RPR positive group. There were significant differences between CSF sTREM2 (log10) in the two groups (p = 0.035; Figure 4A). Like CSF sTREM2, CSF NFL levels in the CSF RPR positive group were significantly higher than those in the CSF RPR negative group (p=0.019; Figure 4B).
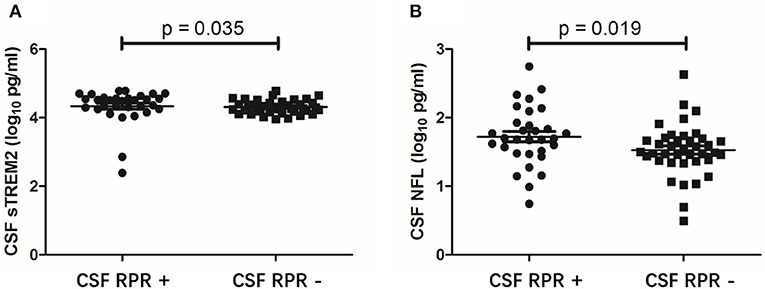
Figure 4. CSF levels of sTREM2 and NFL in NS patients with CSF RPR positive and CSF RPR negative. (A) The CSF sTREM2 (log10 pg/ml) levels in CSF RPR positive patients were significantly higher than in CSF RPR negative patients. (B) The CSF NFL (log10 pg/ml) levels in CSF RPR positive patients were significantly higher than in CSF RPR negative patients. CSF, cerebrospinal fluid; sTREM2, soluble triggering receptor expressed on myeloid cells 2; NFL, neurofilament light protein; RPR, rapid plasma regain.
Discussion
Microglia are important immunosurveillance cells in the CNS, and are important target cells for CNS infectious disease (19). Microglia activation may mediate neuroinflammation and cause dysfunction of neurons and neuropil in the disease (3, 4, 6, 7, 20, 21). TREM2 is a transmembrane protein selectively expressed by microglia in the brain, and its soluble variant (sTREM2) can be detected in CSF (9). In the present study, we investigated the changes of CSF sTREM2 levels at different stages of NS and explored the relationship between this microglial activation marker and neuronal injury marker NFL.
We found that the CSF sTREM2 levels elevated significantly in patients with early and late stages of NS compared to syphilis/non-NS patients, and peaked at late stage of NS. Researchers have found the pathophysiological function of TREM2 in animal models of different CNS diseases. In experimental autoimmune encephalitis, the expression levels of TREM2 on the surface of microglia increased significantly under inflammation. After blocking TREM2 expression, the inflammation and demyelination of the animals were aggravated (22). In AD mice, TREM2 silencing resulted in the increase expression of inflammatory factors, suggesting that TREM2 has an anti-inflammatory effect (20). Compared with non-inflammatory neurological diseases, the CSF sTREM2 levels increased in patients with MS and other inflammatory diseases, corroborating that sTREM2 reflects the neuroinflammatory process of the CNS (9). CSF RPR had an high diagnostic value in NS and was positively correlated with the inflammatory activity of the disease (15, 17, 18, 23). More patients in the late-NS group had positive CSF RPR, indicating persistent infection of treponema pallidum in the brain, which could cause and maintain neuroinflammation and tissue damage (24). The levels of CSF sTREM2 were higher in the NS group than those in the syphilis/non-NS group, as well as being higher in the CSF RPR positive group than those in the RPR negative group, suggesting that the expression of CSF sTREM2 was up-regulated under syphilis persistent infection to achieve an anti-inflammatory effect. One study of AD patients found that the expression of CSF sTREM2 increased, which was correlated with biomarkers of T-tau and P-tau, suggesting that the increase of inflammatory response was related to neurodegeneration (12). In our enrolled patients, 86.7% of late neurosyphilis were general paresis (26/30). The clinical manifestations of general paresis were very similar to those of AD. In addition, some studies found that general paresis was also associated with decreased CSF Aβ42 and elevated CSF t-tau and p-tau181 (25–28). CSF sTREM2 levels were higher in late-NS group, suggesting that sTREM2 may play an important role in the neurodegeneration and progression in the course of NS. Such a marker may be helpful to explore the pathogenesis of NS and provide a clinical usage for determining prognosis.
Another main finding of our study was that CSF NFL levels in the NS group were higher than those in the syphilis/non-NS group, reflecting ongoing neuronal injury. Compared with early-NS, late-NS mainly damages the parenchyma with more severe clinical symptoms and worse prognosis. The late-NS had the highest level of CSF NFL and CSF sTREM2 in this study, suggesting that the levels of these two indicators may reflect the progress of NS and be related to the disability or severity of the disease. A positive correlation was found between CSF sTREM2 and CSF NFL, suggesting that microglial activation was associated with neuronal injury in Treponema pallidum infection in CNS. A previous study also found a positive correlation between CSF sTREM2 and CSF NFL in patients with HIV infection (8).
We found a moderate correlation between the levels of CSF sTREM2 and CSF NFL with age in syphilis/non-NS patients. The brain displays an increasing microglia activation and neuronal injury with normal aging. Aging can result in CSF sTREM2 and NFL release (29, 30). Gisslén et al. found that CSF sTREM2 and CSF NFL levels were strong correlated with age in HIV patients and normal controls (8). Suárez-Calvet M found that sTREM2 and CSF NFL levels were significant correlated with age in preclinical AD, AD dementia and the control group (11). The correlations between CSF sTREM2 and CSF NFL levels with age in syphilis/non-NS group were better than those in NS group, suggesting that microglial activation and neuronal injury in NS patients may obscure the effect of age on the CSF sTREM2 and CSF NFL levels (31).
This study has several limitations. In theory, the disease duration should be considered in this study, but it was difficult for the patients in this study especially those with late neurosyphilis, to give the exact time of syphilis infection. Our study was also limited by the relatively small sample size. A retrospective cross-sectional study was used in this study. A longitudinal study of CSF sTREM2 and CSF NFL in patients with NS could further evaluate their dynamic changes before and after penicillin treatment, and perhaps correlate their clinical significance of using sTREM2 or NFL as a prognostic or a therapeutic marker. Further studies are expected to address these issues.
Conclusions
The study found that CSF concentrations of sTREM2 were higher in patients with NS compared to syphilis/non-NS patients, and peaked at late stage of NS, suggesting that CSF sTREM2 may be a useful marker of microglia activation in NS and play a role in the progression of NS. A positive correlation between the levels of CSF sTREM2 and CSF NFL indicates a certain linkage between microglial activation and neuronal injury in NS.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics committee of Beijing Ditan Hospital, Capital Medical University, Beijing, People's Republic of China (NO. 2019-026-001). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WL, HC, WW, LY and XZ conceived and designed the study. WL, MJ, JG, and YX carried out the laboratory analyses. LY, WL, DX, and YH collected and organized the data and drafted the manuscript. WL, HC, and XZ performed the statistical analysis. All authors read, reviewed, and approved the final manuscript.
Funding
This study was support by the Research Fund of Beijing Ditan Hospital (DTYM201806).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. Friedrich F, Geusau A, Greisenegger S, Ossege M, Aigner M. Manifest psychosis in neurosyphilis. Gen Hosp Psychiatry. (2009) 31:379–81. doi: 10.1016/j.genhosppsych.2008.09.010
3. Mao C, Gao J, Jin L, Peng B, Guo Y. Postmortem histopathologic analysis of neurosyphilis: a report of 3 cases with clinicopathologic correlations. J Neuropathol Exp Neurol. (2018) 77:296–301. doi: 10.1093/jnen/nly004
4. Sakai K, Fukuda T, Iwadate K, Maruyama-Maebashi K, Asakura K, Ozawa M, et al. A fatal fall associated with undiagnosed parenchymatous neurosyphilis. Am J Forensic Med Pathol. (2014) 35:4–7. doi: 10.1097/PAF.0000000000000068
5. Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. (2003) 3:445–53. doi: 10.1038/nri1106
6. Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. (2007) 184:92–9. doi: 10.1016/j.jneuroim.2006.11.032
7. Paradowska-Gorycka A, Jurkowska M. Structure, expression pattern and biological activity of molecular complex TREM-2/DAP12. Hum Immunol. (2013) 74:730–7. doi: 10.1016/j.humimm.2013.02.003
8. Gisslén M, Heslegrave A, Veleva E, Yilmaz A, Andersson LM, Hagberg L, et al. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol Neuroimmunol Neuroinflamm. (2018) 6:e512. doi: 10.1212/NXI.0000000000000512
9. Piccio L, Buonsanti C, Cella M, Tassi I, Schmidt RE, Fenoglio C, et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain. (2008) 131:3081–91. doi: 10.1093/brain/awn217
10. Öhrfelt A, Axelsson M, Malmeström C, Novakova L, Heslegrave A, Blennow K, et al. Soluble TREM-2 in cerebrospinal fluid from patients with multiple sclerosis treated with natalizumab or mitoxantrone. Mult Scler J. (2016) 22:1587–95. doi: 10.1177/1352458515624558
11. Suárez-Calvet M, Kleinberger G, Araque Caballero MÁ, Brendel M, Rominger A, Alcolea D, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer's disease and associate with neuronal injury markers. EMBO Mol Med. (2016) 8:466–76. doi: 10.15252/emmm.201506123
12. Heslegrave A, Heywood W, Paterson R, Magdalinou N, Svensson J, Johansson P, et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer's disease. Mol Neurodegener. (2016) 11:3. doi: 10.1186/s13024-016-0071-x
13. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. (2019) 90:870–81. doi: 10.1136/jnnp-2018-320106
14. Zhu L, Gu X, Peng RR, Wang C, Gao Z, Zhou P, et al. Comparison of the cerebrospinal fluid (CSF) toluidine red unheated serum test and the CSF rapid plasma reagin test with the CSF venereal disease research laboratory test for diagnosis of neurosyphilis among HIV-negative syphilis patients in China. J Clin Microbiol. (2014) 52:736–40. doi: 10.1128/JCM.02522-13
15. French P, Gomberg M, Janier M, Schmidt B, van Voorst Vader P, et al. IUSTI:2008 European guidelines on the management of syphilis. Int J STD AIDS. (2009) 20:300–9. doi: 10.1258/ijsa.2008.008510
16. Tuddenham S, Ghanem KG. Neurosyphilis: knowledge gaps and controversies. Sex Transm Dis. (2018) 45:147–51. doi: 10.1097/OLQ.0000000000000723
17. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. (2015) 64:1–137.
18. Li W, Jiang M, Xu D, Kou C, Zhang L, Gao J, et al. clinical and laboratory characteristics of symptomatic and asymptomatic neurosyphilis in hiv-negative patients: a retrospective study of 264 cases. Biomed Res Int. (2019) 2019:2426313. eCollection 2019. doi: 10.1155/2019/2426313
19. Garden GA, Moller T. Microglia biology in health and disease. Neuroimmune Pharmacol. (2006) 1:127–37. doi: 10.1007/s11481-006-9015-5
20. Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. (2005) 201:647–57. doi: 10.1084/jem.20041611
21. Pimenova AA, Marcora E, Goate AM. A tale of two genes: microglial apoe and Trem2. Immunity. (2017) 47:398–400. doi: 10.1016/j.immuni.2017.08.015
22. Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, et al. Blockade of TREM2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. (2007) 37:1290–01. doi: 10.1002/eji.200636837
23. Levchik N, Ponomareva M, Surganova V, Zilberberg N, Kungurov N. Criteria for the diagnosis of neurosyphilis in cerebrospinal fluid: relationships with intrathecal immunoglobulin synthesis and blood-cerebrospinal fluid barrier dysfunction. Sex Transm Dis. (2013) 40: 917–22. doi: 10.1097/OLQ.0000000000000049
24. Drago F, Javor S, Parodi A. Neurosyphilis: from infection to autoinflammation? Int J STD AIDS. (2016) 27:327–8. doi: 10.1177/0956462415590710
25. Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer's disease-role of spirochetes. J Alzheimer's Dis. (2008) 13:381–91. doi: 10.3233/JAD-2008-13404
26. Miklossy J. Historic evidence to support a causal relationship between spirochetal infections and Alzheimer's disease. Front Aging Neurosci. (2015) 7:46. doi: 10.3389/fnagi.2015.00046
27. Luo X, Shi H, Hou L, Zhong X, Chen X, Zhang Y, et al. Different cerebrospinal fluid levels of Alzheimer-type biomarker Aβ42 between general paresis and asymptomatic neurosyphilis. Eur J Neurol. (2015) 22:853–8. doi: 10.1111/ene.12680
28. Paraskevas GP, Kapaki E, Kararizou E, Mitsonis C, Sfagos C, Vassilopoulos D. Cerebrospinal fluid tau protein is increased in neurosyphilis: a discrimination from syphilis without nervous system involvement? Sex Transm Dis. (2007) 34:220–3. doi: 10.1097/01.olq.0000233738.23278.4e
29. Mecca C, Giambanco I, Donato R, Arcuri C. Microglia and aging: the role of the TREM2–DAP12 and CX3CL1-CX3CR1 axes. Int J Mol Sci. (2018) 19:318. doi: 10.3390/ijms19010318
30. Koellhoffer E, McCullough L, Ritzel R. Old maids: aging and its impact on microglia function. Int J Mol Sci. (2017) 18:769. doi: 10.3390/ijms18040769
Keywords: neurosyphilis, microglia, cerebrospinal fluid, soluble triggering receptor expressed on myeloid cells 2, neurofilament light proteins
Citation: Li W, Chang H, Wu W, Xu D, Jiang M, Gao J, Huang Y, Xu Y, Yin L and Zhang X (2020) Increased CSF Soluble TREM2 Concentration in Patients With Neurosyphilis. Front. Neurol. 11:62. doi: 10.3389/fneur.2020.00062
Received: 02 September 2019; Accepted: 16 January 2020;
Published: 05 February 2020.
Edited by:
Avindra Nath, National Institute of Neurological Disorders and Stroke (NINDS), United StatesReviewed by:
Lynn Pulliam, University of California, San Francisco, United StatesJayantee Kalita, Sanjay Gandhi Post Graduate Institute of Medical Sciences, India
Copyright © 2020 Li, Chang, Wu, Xu, Jiang, Gao, Huang, Xu, Yin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linlin Yin, yinlinlin@bjtth.org; Xinghu Zhang, xhzhtiantan@hotmail.com
 Wurong Li
Wurong Li Haoxiao Chang
Haoxiao Chang Wenqing Wu
Wenqing Wu Dongmei Xu1
Dongmei Xu1 Meijuan Jiang
Meijuan Jiang Junhua Gao
Junhua Gao Yun Xu
Yun Xu Linlin Yin
Linlin Yin Xinghu Zhang
Xinghu Zhang