- 1Department of Neurology, Japan Community Health Care Organization Kyushu Hospital, Fukuoka, Japan
- 2Department of Neurology, Neurological Institute, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- 3Department of Pathology, Japan Community Health Care Organization Kyushu Hospital, Fukuoka, Japan
- 4Division of Nephrology, Department of Internal Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan
Background: Several case reports have described the concurrence of chronic inflammatory demyelinating polyneuropathy (CIDP) and membranous nephropathy (MN). The presence of autoantibodies against podocyte antigens phospholipase A2 receptor (PLA2R) and thrombospondin type 1 domain containing 7A (THSD7A) in MN suggests an autoimmune mechanism. Some CIDP patients also harbor autoantibodies against paranodal proteins such as neurofascin 155 (NF155) and contactin-1 (CNTN1). We investigated the relationship between CIDP and MN by assaying autoantibodies against paranodal and podocyte antigens in a CIDP patient with MN, and by a literature survey on the clinical features of CIDP with MN.
Methods: Anti-CNTN1 and NF155 antibodies were measured by flow cytometry using HEK293 cell lines stably expressing human CNTN1 or NF155. Binding capacity of antibodies was validated by immunostaining mouse teased sciatic nerve fibers. Anti-PLA2R antibodies were measured by enzyme-linked sorbent assay and anti-THSD7A antibodies by indirect immunofluorescence assay. Clinical features between 14 CIDP with MN cases including two with anti-CNTN1 antibodies and 20 anti-CNTN1 antibody-positive CIDP cases were compared.
Results: A patient whose ages was in the late 70 s complained of progressive weakness and superficial and deep sensory impairment in four extremities over 6 months. Nerve conduction studies showed prominent demyelination patterns. The patient presented with nephrotic syndrome. Renal biopsy disclosed basement membrane thickening with local subepithelial projections and glomerular deposits of IgG4, compatible with MN. Autoantibody assays revealed the presence of IgG4 and IgG1 anti-CNTN1 antibodies, but an absence of anti-NF155, anti-PLA2R, and anti-THSD7A antibodies. The patient's serum stained paranodes of teased sciatic nerves. CIDP with MN and anti-CNTN1 antibody-positive CIDP commonly showed male preponderance, relatively higher age of onset, acute to subacute onset in 35–50% of cases, distal dominant sensorimotor neuropathy, proprioceptive impairment leading to sensory ataxia, and very high cerebrospinal fluid protein levels. However, 11 of 13 CIDP patients with MN had a favorable response to mono- or combined immunotherapies whereas anti-CNTN1 antibody-positive CIDP was frequently refractory to corticosteroids and intravenous immunoglobulin administration.
Conclusion: CIDP with MN and anti-CNTN1 antibody-positive CIDP show considerable overlap but are not identical. CIDP with MN is probably heterogeneous and some cases harbor anti-CNTN1 antibodies.
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an acquired immune-mediated disorder affecting the peripheral nerves, classically characterized by symmetrical weakness and impaired sensation. Although it is assumed that cell-mediated and humoral immunity play roles in this disease, the cause of CIDP remains to be elucidated. Recently, autoantibodies against paranodal proteins, such as neurofascin 155 (NF155) (1, 2), contactin-1 (CNTN1) (3–5), and contactin-associated protein 1 (CASPR1) (6), were reported to be present among subsets of CIDP patients.
Several case reports have described the concurrence of CIDP and membranous nephropathy (MN) (7–16), which is a major cause of nephrotic syndrome in adults. The autoimmune nature of idiopathic MN was clearly delineated by the identification of autoantibodies against podocyte antigens, namely phospholipase A2 receptor (PLA2R) and thrombospondin type 1 domain containing 7A (THSD7A) (17). Only one CIDP case with MN has been examined for paranodal protein antibodies and was found to be positive for anti-CNTN1 antibody (5). CNTN1 belongs to the immunoglobulin superfamily and is an adhesion molecule. Axonal CNTN1 and CASPR1 form septate-like junctions together with glial NF155 to maintain ion channel clustering at nodes of Ranvier (18). Because the loss of CNTN1 in genetically modified mice leads to decreased nerve conduction velocity (19), CNTN1 is regarded as fundamental for maintaining saltatory conduction. However, the roles of anti-CNTN1 antibodies in concomitant MN are unknown. In a previous report of anti-CNTN1 antibody-positive CIDP with MN, neither anti-PLA2R nor anti-THSD7A antibodies were studied (5). Therefore, a common pathway leading to both CIDP and MN remains to be elucidated. In the present study, we aimed to clarify the relationship between CIDP with MN and anti-CNTN1 antibody-positive CIDP by searching for autoantibodies to paranodal antigens and podocyte antigens in a patient with CIDP and MN, and by comparing the clinical features of CIDP with MN and anti-CNTN1 antibody-positive CIDP.
Materials and Methods
Case Presentation
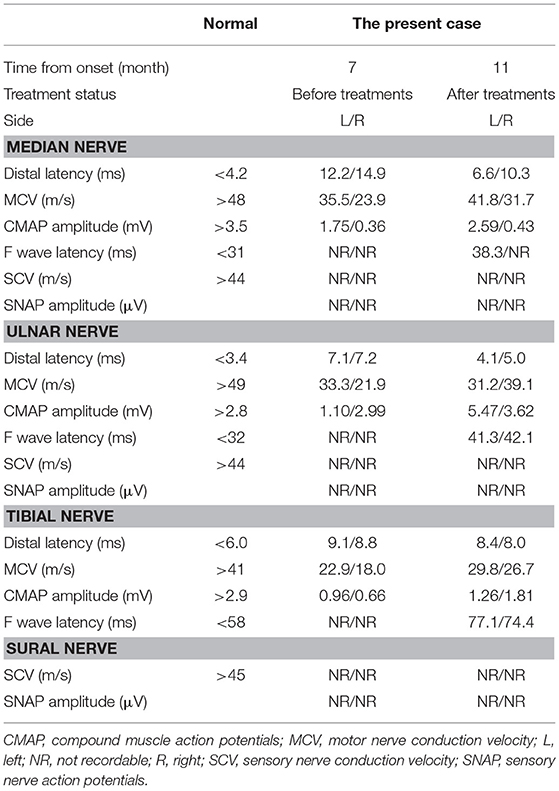
A patient whose age was in the late 70 s was referred to our hospital because of progressive weakness and paresthesia of the extremities over 6 months without any prodromal episode. The patient had no medical history for any neurological, renal, or collagen diseases. Physical examination revealed oral dryness, dry skin, bilateral leg edema, and wheezing in the right lower lung field. Neurologically, the patient showed symmetrical weakness that was more marked in the legs than in the arms, generalized areflexia, and severe superficial and deep sensory impairment in the distal parts of the four extremities. As a result, the patient could not walk without help. Mild orthostatic hypotension was observed. The patient showed no tremor or cranial nerve involvement. Nerve conduction studies showed prominent demyelinating patterns in all nerves examined, such as low-amplitude compound muscle action potentials with temporal dispersion, prolonged distal latencies, and reduced motor conduction velocities (Table 1), which met the European Federation of Neurological Societies/Peripheral Nerve Society electrophysiological criteria for definite CIDP. F waves and sensory nerve action potentials were not evoked. Magnetic resonance imaging showed thickening and gadolinium enhancement of the cauda equine (Figure 1). However, no demyelinating lesions of central nervous system tissues were detected.
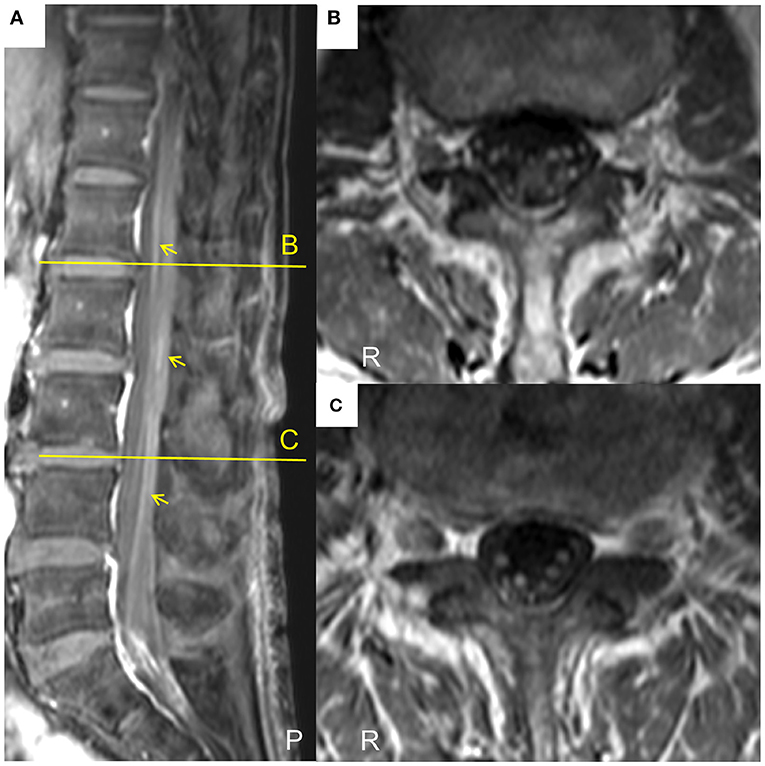
Figure 1. Lumbar magnetic resonance images of the current case of a CIDP patient with MN. (A)The sagittal T1-weighted spectral adiabatic inversion-recovery (SPAIR) magnetic resonance image shows gadolinium enhancement of cauda equine (arrows). (B,C) Axial T1-weighted SPAIR magnetic resonance images at the level of the indicated lines in (A). Thickening and gadolinium enhancement of the cauda equine are visible. P, posterior; R, right.
Regarding the cause of bilateral leg edema, the patient had severe hypoalbuminemia (0.7 g/dl), hypercholesterolemia (346 IU/l), and proteinuria (4.1 g in 24 h), which met the diagnostic criteria for nephrotic syndrome. Renal biopsy revealed stage 2 idiopathic membranous nephropathy (MN), as shown in the Results section. Other blood tests revealed positive anti-SSA/Ro antibodies (1:16), low vitamin B12 (57 pg/ml), and hypothyroidism (TSH 5.30 μIU/ml, FT4 0.83 ng/dl). Serological tests for infectious diseases (hepatitis B and C viruses, human immunodeficiency virus, and syphilis), anti-SSB/La antibodies, rheumatoid factor, anti-double-stranded DNA antibodies, anti-neutrophil cytoplasmic antibodies, M-protein, and cryoglobulins were negative. The patient's cerebrospinal fluid (CSF) protein level was 61 mg/dl and white blood cell count was 1/μl. There was no evidence of malignant tumor in the upper and lower gastrointestinal tracts by endoscopies and computed tomography. The patient was also diagnosed with Sjögren's syndrome based on increased anti-SSA/Ro antibodies and relevant clinical findings, such as xerostomia, xerophthalmia, lymphocytic interstitial pneumonia, atrophic gastritis, and subclinical hypothyroidism.
Generation of Transformed Cell Lines Stably Expressing Human CNTN1
We generated a HEK293 cell line stably expressing human CNTN1-turbo green fluorescent protein (GFP) fusion proteins using a plasmid containing a full-length cDNA encoding human CNTN1 (OriGene Technologies Inc., Rockville, MD). The plasmids were transfected into naive HEK 293 cells using FuGENE6 (Promega Corporation, Madison, WI) according to the manufacturer's recommendations. The transfected cells were cultured in selection medium containing G418 (Thermo Fisher Scientific, Waltham, MA). When single colonies of adequate size for clonal expansion had formed, individual colonies were picked up using a sterile cloning cylinder and scaled up to larger volumes.
Flow Cytometric and Conventional Cell-Based Assay for Anti-NF155 or Anti-CNTN1 Antibodies
Anti-CNTN1 antibodies were measured by the same methodology used for measuring anti-NF155 antibodies, as described previously (2). In brief, CNTN1-turbo GFP-transfected and naive HEK293 cells were evenly mixed and resuspended in Dulbecco's modified Eagle's medium containing 1% fetal bovine serum and 1 mM ethylenediaminetetraacetic acid (EDTA) (FCM buffer) at a concentration of 1.0 × 106 cells/ml, and rotated at 4°C for 60 min. Serum samples (2.5 μl) were mixed with 47.5 μl of cell-containing solution (1:20 dilution). After incubation at 4°C for 60 min, cells were washed and bound IgG was detected with Alexa Fluor 647-labeled anti-human IgG antibodies (Thermo Fisher Scientific) diluted 1:500 with FCM buffer. After incubation at 4°C for 60 min, cells were washed, resuspended in 100 μl phosphate-buffered saline (PBS) containing 5 mM EDTA and analyzed by MACSQuant Analyzer (Miltenyi Biotec, Bergisch Gladbach, Germany). The mean fluorescence intensity (MFI) of cell-associated turbo GFP and Alexa 647 was measured for each sample. The MFI of cell-associated Alexa 647 was measured to detect human IgG bound to CNTN1, using cells without CNTN1 expression as a negative control. For each serum sample, the MFI ratio was calculated by dividing Alexa 647 MFI of CNTN1-transfected cells by Alexa 647 MFI of CNTN1-untransfected cells, and the ΔMFI was calculated by subtracting Alexa-647 MFI of CNTN1-untransfected cells from Alexa 647 MFI of CNTN1-transfected cells. As a conventional cell-based assay, cell-containing solutions were placed on glass slides and viewed under a confocal microscope (A1; Nikon, Tokyo, Japan). In an anti-CNTN1 antibody-positive patient, IgG subclass profiles were examined using phycoerythrin-conjugated mouse anti-human IgG1, IgG2, IgG3, and IgG4 antibodies (Beckman Coulter Inc., Brea, CA) at 1:500 dilution.
Immunostaining of Mouse Teased Sciatic Nerve Fibers
Sciatic nerves were obtained from C57BL/6 mice, dissected, and fixed for 10 min in freshly prepared PBS containing 4% paraformaldehyde. After washing with PBS, fixed nerves were teased and transferred onto glass slides, permeabilized with 2% Triton X-100 in PBS for 30 min, blocked in 10% goat serum and 1% Triton X-100 in PBS for 60 min, and then incubated at 4°C in blocking solution containing rabbit anti-CASPR1 antibodies (diluted 1:500) (Abcam, Cambridge, UK) and sera from anti-CNTN1 antibody-positive CIDP patients or healthy controls (HCs) (diluted 1:100). After 24 h, teased nerve fibers were washed three times in PBS for 30 min, and incubated for 60 min with Alexa 488-labeled anti-human IgG and Alexa 647-labeled anti-rabbit IgG (Thermo Fisher Scientific), diluted 1:500. Finally, teased nerve fibers were washed three times in PBS for 30 min, mounted with PermaFluor (Thermo Fisher Scientific), and examined by confocal microscopy (A1; Nikon), using 488- and 638-nm lasers for excitation.
Assay for Anti-PLA2R or Anti-THSD7A Antibodies
Anti-PLA2R antibodies were measured by a commercial ELISA kit (Euroimmun AG, Lübeck, Germany) (20) and anti-THSD7A antibodies were assayed by a commercial indirect immunofluorescence assay kit (Euroimmun AG) (21).
Pathological and Immunofluorescence Studies for Renal Biopsy Specimens
Renal biopsy was performed to investigate the cause of the nephrotic syndrome. Tissues for light microscopy and immunofluorescence were routinely fixed in formalin and embedded in paraffin. The formalin-fixed paraffin-embedded sections were cut at 2 μm and stained with hematoxylin and eosin, periodic acid-Schiff, periodic acid methenamine silver, Masson's trichrome, and Congo red. Immunofluorescence staining was performed using formalin-fixed paraffin-embedded sections that were prepared by protease K (Dako, Copenhagen, Denmark) for 60 min. Fluorescein isothiocyanate-labeled rabbit anti-human IgG, IgA, IgM, C1q, C3, fibrinogen, kappa- and lambda-light chains (Dako) were used. Additionally, mouse monoclonal anti-human IgG1, IgG2, IgG4 (Chemicon, Temecula, CA, USA), and IgG3 (Thermo Fisher Scientific) antibodies, and rabbit polyclonal anti-PLA2R antibodies (Abcam) were used followed by Alexa 546 goat anti-mouse IgG (H+L) and Alexa 488 goat anti-rabbit IgG (H+L) (Thermo Fisher Scientific).
Literature Reviews
A PubMed search was performed to identify previous reports of CIDP with concurrent MN, using “CIDP” and “membranous nephropathy” or “membranous glomerulonephritis” as keywords. We reviewed all suitable cases by literature survey.
Results
Autoantibody Studies for Nodal and Podocyte Proteins
Autoantibodies in the patient's serum bound to the paranodal regions of mouse teased sciatic nerve fibers, with a staining pattern similar to anti-CASPR1 antibody (Figure 2A). Flow cytometry for anti-CNTN1 antibodies demonstrated the present case had a higher MFI ratio and ΔMFI values (the MFI ratio and ΔMFI were 14.2 and 106, respectively) than those of 30 HCs (mean MFI ratio and ΔMFI were 1.1 and 0.5, respectively; standard deviation of MFI ratio and ΔMFI were 0.16 and 1.43, respectively) (Figure 2B). A conventional cell-based assay visualized IgG binding to CNTN1-expressing HEK293 cells (Figure 2C). Subclass analysis of anti-CNTN1 antibodies revealed a predominant increase in IgG4 and IgG1 (Figure 2D). The MFI ratio and ΔMFI values of anti-NF155 antibodies were 1.04 and 1.02, respectively, and were similar to those of 10 healthy controls (mean MFI ratio and ΔMFI were 0.92 and −1.6, respectively; standard deviation of MFI ratio and ΔMFI were 0.14 and 4.0, respectively). However, for podocyte antigens, anti-PLA2R antibodies and anti-THSD7A antibodies were not positive in the serum. Finally, the patient was diagnosed with anti-CNTN1 antibody-positive CIDP with concurrent MN.
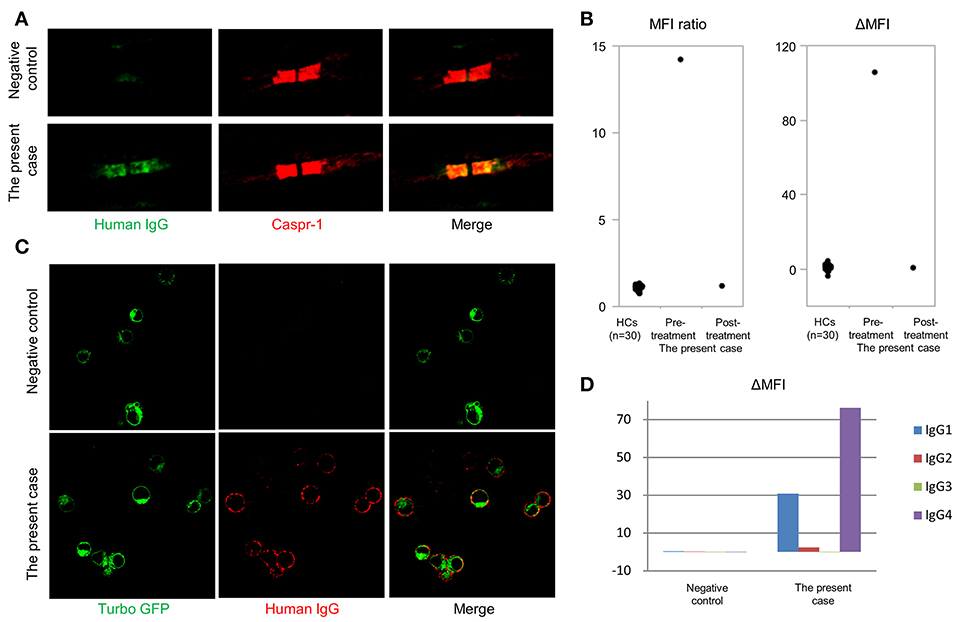
Figure 2. Detection of anti-contactin-1 antibodies. (A) Double immunostaining of mouse teased sciatic nerve fibers with anti-contactin-associated protein 1 (CASPR1) antibodies and sera from the anti-CNTN1 antibody-positive CIDP case or an anti-CNTN1 antibody-negative HC. A similar paranodal staining pattern is observed for anti-CASPR1 antibodies and the patient's serum. Scale bar = 5 μm. (B) Mean fluorescence intensity (MFI) ratio and ΔMFI for anti-contactin-1 (CNTN1) antibodies. Serum from the present case at admission (6th months from the onset) shows significantly higher MFI ratio and ΔMFI values (14.2 and 106, respectively) than those of 30 HCs (1.1 and 0.47, respectively). Standard deviation of MFI ratio and ΔMFI are 0.16 and 1.43, respectively. Eleven months after starting the treatment (18th months from the onset), anti-CNTN1 antibodies in the patient's serum disappeared concurrent with clinical and electrophysiological improvement. (C) Conventional cell-based assay shows that IgG in the patient's serum binds to HEK293 cells stably expressing human CNTN1-turbo green fluorescent protein (GFP). Scale bar = 20 μm. (D) Subclass analysis of anti-CNTN1 antibodies from the present case reveal the predominance of IgG1 and IgG4 subclasses.
Histological and Immunofluorescence Studies of Biopsied Renal Tissue
The glomeruli showed diffuse spikes of the glomerular basement membrane by periodic acid methenamine silver staining (Figure 3A). Immunofluorescence microscopy demonstrated granular deposits of IgG along the glomerular basement membrane (Figure 3B). These findings were consistent with stage 2 idiopathic MN. In an IgG subclass study, only IgG4 was detectable (Figures 3C–F). Deposition of complement C3 was not conspicuous. Staining for PLA2R was weakly positive by immunofluorescence (Figures 3G,H).
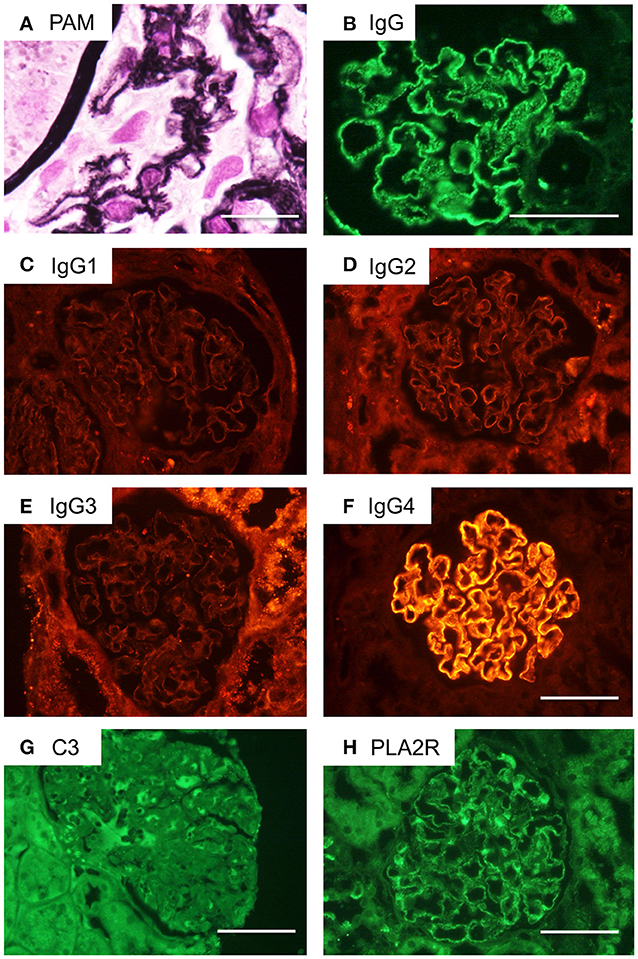
Figure 3. Renal pathological findings in the current case of a CIDP patient with MN. (A) Periodic acid methenamine silver (PAM) staining of the patient's glomeruli reveals a spiked appearance of the glomerular basement membrane. Scale bar = 50 μm. (B) Immunofluorescence microscopy demonstrates granular deposits of IgG along the glomerular basement membrane. Scale bar = 100 μm. (C–F) IgG subclass staining indicated only IgG4 deposits were detectable. Scale bar = 100 μm. (G) C3 deposition is not obvious by immunohistochemistry. Scale bar = 100 μm. (H) Immunostaining for phospholipase A2 receptor (PLA2R) is weakly positive. Scale bar = 100 μm.
Correlation of Anti-CNTN1 Antibody Levels With Clinical Course
Seven months after disease onset, the patient was treated with methylprednisolone pulse therapy (1000 mg/day for 3 days) followed by oral prednisolone (1 mg/kg/day with gradual taper). However, the patient had little improvement in muscle weakness and sensory disturbance, even though NCS findings tended to improve and the serum albumin level was increased (2.5 g/dl). Two months after initial treatment, intravenous immunoglobulin (IVIg) (400 mg/kg/day for 5 days) was administered. Thereafter, the patient's sensorimotor disturbance was ameliorated. Two months after the additional treatment, the patient was able to walk without help and NCS findings continued to improve (Table 1). Eleven months after starting immunotherapies (18th months from disease onset), the patient's anti-CNTN1 antibodies were undetectable (MFI ratio and ΔMFI were 1.40 and 0.97, respectively).
Characteristic Features of CIDP With Concurrent MN and Comparison With Anti-CNTN1 Antibody-Positive CIDP
By literature survey, we found 13 previously reported cases of CIDP and MN. The summary of 14 cases including the current case is shown in Table 2. Ten cases preceded CIDP while four showed concurrent onset of CIDP and MN. The mean age at the onset of CIDP was 47.6 ± 21.6 years (range 9–81 years old; 36% >60 years). The male-to-female ratio was 10:4 (2.5:1). Chronic onset of CIDP was seen in nine cases while four cases had acute onset (≤ 1 month) and one showed subacute onset (6 weeks). All patients were regarded as typical CIDP. Eleven cases (79%) showed distal dominant symmetrical sensory impairment while proprioception impairment or sensory ataxia was described in at least eight cases (57%). All but two cases had very high CSF levels (mean 291 ± 330 mg/dl; range 61–1,320 mg/dl; 12 cases (86%) >100 mg/dl).
Regarding the efficacy of immunotherapies on CIDP, corticosteroids were effective in 5/7 (71.4%) cases with monotherapy. Plasma exchange (PE) was effective in 3/3 (100%) cases with monotherapy. IVIg was effective in 2/2 (100%) cases with monotherapy. Combined immunotherapies were performed in four cases (one each of PE + methotrexate, PE + CS + azathioprine, PE + CS + IVIg, PE + CS + IVIg + cyclosporine), and were effective only in one case (PE + methotrexate). These observations suggest that most cases (11, 79%) responded favorably to immunotherapies such as CS, PE, and IVIg initially; however, three cases (21%) were refractory even to combined treatments including three or more immunotherapies.
Regarding the efficacy of immunotherapies on MN, favorable responses were described in 2/5 (40%) cases by CS monotherapy, in 0/1 (0%) by PE monotherapy, in 0/1 (0%) by IVIg monotherapy, and in 0/1 (0%) by immunosuppressants. Combined immunotherapies, including CS + immunosuppressant, CS + PE, PE + immunosuppressant, PE + CS + IVIg, and PE + CS + IVIg + immunosuppressant, were effective in 4/6 (67%) cases. Overall, only two cases responded favorably to monotherapy and five required combined immunotherapies to achieve improvement. There were at least five cases refractory to mono- or combined immunotherapies.
By literature survey, three case series reported 20 anti-CNTN1 antibody-positive CIDP patients (3, 4, 5) (Table 3). Ages at onset were 63.0 ± 13.5 (mean ± SD) years (range 33–81 years; 80% >60 years). The male-to-female ratio was 14:6 (2.3:1). Ten had a chronic progressive course while seven showed an acute or rapidly progressive course and three had subacute onset. Common clinical features were symmetrical manifestation, distal dominant involvement (90%) especially in sensory impairment, predominant sensory ataxia, an aggressive disease course, and poor response to CS and IVIg. Their CSF protein level was 253 ± 143 (mean ± SD) mg/dl (range: 79–693 mg/dl; 95% >100 mg/dl). Thus, common features between CIDP with MN and anti-CNTN1 antibody-positive CIDP are as follows (Table 3): male preponderance (about 2.5-fold higher than female), occasional acute to subacute onset (35.7 and 50%), relatively higher age of onset, distal dominant sensorimotor neuropathy with frequent proprioceptive sensory impairment leading to sensory ataxia, and high CSF protein levels. However, CIDP with MN was occasionally seen in younger people (< 30 years) and had a more favorable response to immunotherapies compared with anti-CNTN1 antibody-positive CIDP.
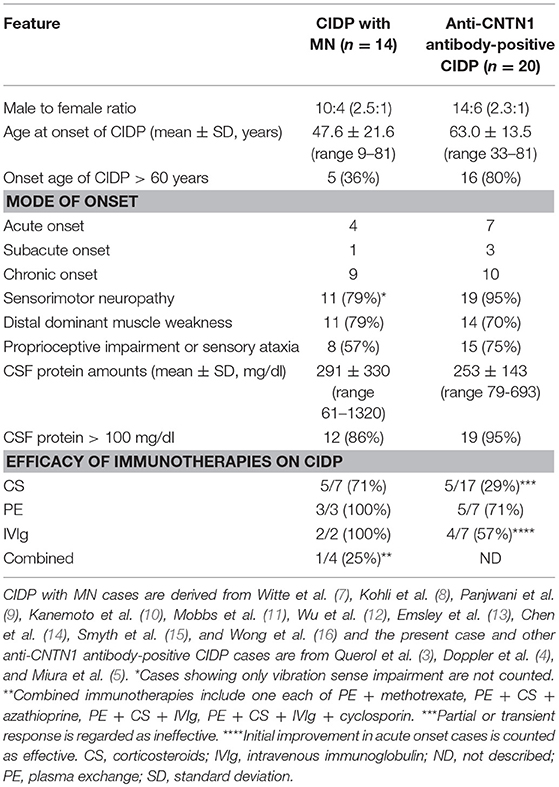
Table 3. Comparison of clinical features between CIDP with MN and anti-CNTN1 antibody-positive CIDP.
Discussion
The patient in this study is the second reported case of anti-CNTN1 antibody-positive CIDP with MN. Interestingly, the patient was negative for anti-PLA2R and anti-THSD7A antibodies, although IgG4 was deposited on the glomerular basement membrane in the kidney. The patient was concurrently diagnosed with Sjögren's syndrome. However, this usually manifests as chronic sensorimotor axonal polyradiculoneuropathy but not demyelinating neuropathy, and rarely accompanies MN (22, 23). Therefore, we consider anti-CNTN1 antibodies but not Sjögren's syndrome contributory to the present illness of our patient. In MN, the frequency of anti-PLA2R and anti-THSD7A antibodies are 50–80%, and 5–10%, respectively (17). Thus, target antigens in MN are still undetermined in 10–20% of cases. Our patients clearly had IgG4 deposition in the glomerular basement membrane, suggesting immune-mediated podocyte damage. However, anti-PLA2R and anti-THSD7A antibodies were double seronegative in this study patient. We could not totally exclude the involvement of low-titer anti-PLA2R antibodies because PLA2R glomerular deposits, one of the features of anti-PLA2R antibody-positive MN (24), were observed in this patient. It is worth searching for undetermined renal target antigens for CIDP with MN in the future, including CNTN1 whose mRNA is also weakly expressed in the kidney (25).
The decrease of anti-CNTN1 antibody levels along with improved CIDP by immunotherapy and staining of the paranodes by the patient's sera suggest a pathogenic role of anti-CNTN1 antibodies. Anti-CNTN1 antibodies in our patient were predominantly IgG4 and IgG1. In CIDP with anti-CNTN1 antibodies, it was reported that IgG1 and IgG3 subclasses were prominent in the acute stage of acute onset cases whereas IgG4 predominated at the chronic stage of acute onset cases as well as chronic onset cases (4, 5). IgG1 or IgG3 anti-CNTN1 antibodies may cause acute inflammation via complement activation at the acute inflammatory stage. By contrast, IgG4 does not bind to C1q or activate the classical complement pathway. Thus, blocking interactions between the CNTN1/CASPR1 complex and NF155 might be responsible for the pathogenic function of IgG4 anti-CNTN1 antibodies. Indeed, in human biopsied sural nerves, the detachment of Schwann cell terminal loops from axonal membranes at paranodes without inflammatory cell infiltration was observed by electron microscopy (26). Furthermore, IgG4 but not IgG1 can pass the paranodal barrier and disrupt the paranodal structure upon the passive transfer of anti-CNTN1 antibodies to rats (27). All these observations suggest that IgG4 anti-CNTN1 antibodies play pathogenic roles, especially in the chronic phase, by disrupting axo-glial interactions at the paranodes.
Among 14 cases of CIDP and MN found by literature survey (7–16), only two cases (including the current case) were examined for autoantibodies to paranodal antigens and both cases were positive for anti-CNTN1 antibodies (5). A comparison of the clinical features between CIDP with MN and anti-CNTN1 antibody-positive CIDP revealed male preponderance, relatively higher age of onset, occasional acute/subacute onset, distal dominant sensorimotor neuropathy suggestive of typical CIDP, frequent proprioceptive sensory impairment leading to sensory ataxia, and very high CSF protein levels as common features, although in some CIDP patients with MN including our case, CSF protein levels were not very high, possibly reflecting hypoalbuminemia caused by nephrotic syndrome. There were some differences between both conditions. None of the anti-CNTN1 antibody-positive CIDP had < 30 years of onset while three of 14 CIDP cases with MN (21.4%) were younger than 30 years at onset. Eleven of 13 treated CIDP patients with MN had a favorable response to mono- or combined immunotherapies such as CS, PE, and IVIg whereas anti-CNTN1 antibody-positive CIDP was frequently refractory to IVIg and CS. These observations suggest that CIDP with MN and anti-CNTN1 antibody-positive CIDP show some overlap but are not identical. Probably CIDP with MN is heterogeneous and some cases might be anti-CNTN1 antibody-positive CIDP. Thus, anti-CNTN1 antibodies should be surveyed in patients presenting with CIDP and MN.
Concluding Remarks
We describe the results of a detailed autoantibody study in a patient with anti-CNTN1 antibody-positive CIDP and MN, together with a comparison of the clinical features between CIDP with MN and anti-CNTN1 antibody-positive CIDP. We believe that the present study expands our understanding of the disease area related to anti-CNTN1 antibodies and that anti-CNTN1 antibodies are worth studying in cases with CIDP and MN.
Ethics Statement
The study was approved by the Kyushu University Hospital ethical standards committee.
Author Contributions
YH, HO, RY, TS, SK, KY, and JK made the study plan, conducted the research, and wrote the paper. HO, ZX, SA, and SM studied the autoantibodies. TM, TT, and AY collected patient samples, clinical data and supervised the analyses.
Funding
This work was partly supported by AMED under Grant Number JP17ek0109115h003 and JP18ek0109376, and by Grants-in-Aid for Scientific Research for Early-Career Scientists (No. 18K15454).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Abbreviations
CASPR1, contactin-associated protein 1; CIDP, chronic inflammatory demyelinating polyneuropathy; CNTN1, contactin-1; EDTA, ethylenediaminetetraacetic acid; GFP, green fluorescent protein; IVIg, intravenous immunoglobulin; MFI, mean fluorescence intensity; MN, Membranous nephropathy; PBS, phosphate-buffered saline; PLA2R, phospholipase A2 receptor; THSD7A, thrombospondin type 1 domain containing 7A.
References
1. Querol L, Nogales-Gadea G, Rojas-Garcia R, Diaz-Manera J, Pardo J, Ortega-Moreno A, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology (2014) 82:879–86. doi: 10.1212/WNL.0000000000000205
2. Ogata H, Yamasaki R, Hiwatashi A, Oka N, Kawamura N, Matsuse D, et al. Characterization of IgG4 anti-neurofascin 155 antibody-positive polyneuropathy. Ann Clin Transl Neurol. (2015) 2:960–71. doi: 10.1002/acn3.248
3. Querol L, Nogales-Gadea G, Rojas-Garcia R, Martinez-Hernandez E, Diaz-Manera J, Suárez-Calvet X, et al. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. (2013) 73:370–80. doi: 10.1002/ana.23794
4. Miura Y, Devaux JJ, Fukami Y, Manso C, Belghazi M, Wong AHY, et al. Contactin 1 IgG4 associates to chronic inflammatory demyelinating polyneuropathy with sensory ataxia. Brain (2015) 138:1484–91. doi: 10.1093/brain/awv054
5. Doppler K, Appeltshauser L, Wilhelmi K, Villmann C, Dib-Hajj SD, Waxman SG, et al. Destruction of paranodal architecture in inflammatory neuropathy with anti-contactin-1 autoantibodies. J Neurol Neurosurg Psychiatry (2015) 86:720–8. doi: 10.1136/jnnp-2014-309916
6. Doppler K, Appeltshauser L, Villmann C, Martin C, Peles E, Krämer HH, et al. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain (2016) 139:2617–30. doi: 10.1093/brain/aww189
7. Witte AS, Burke JF. Membranous glomerulonephritis associated with chronic progressive demyelinating neuropathy. Neurology (1987) 37:342–5.
8. Kohli A, Tandon P, Kher V. Chronic inflammatory demyelinating polyradiculoneuropathy with membranous glomerulonephritis: report of one case. Clin Neurol Neurosurg. (1992) 94:31–3.
9. Panjwani M, Truong LD, Eknoyan G. Membranous glomerulonephritis associated with inflammatory demyelinating peripheral neuropathies. Am J Kidney Dis. (1996) 27:279–83.
10. Kanemoto K, Nakahara C, Saitoh H, Fukushima T, Kashiwagi R, Takahashi M, et al. Renal glucosuria and membranous glomerulonephritis in chronic inflammatory demyelinating polyradiculoneuropathy: CIDP. Nihon Jinzo Gakkai Shi (1999) 41:511–6.
11. Mobbs RJ, Tuck RR, Hurley B. Chronic inflammatory demyelinating polyneuropathy associated with membranous glomerulonephritis: case report. J Clin Neurosci. (2000) 7:454–5. doi: 10.1054/jocn.1999.0235
12. Wu AD, Russell JA, Bouthout BA. Chronic inflammatory demyelinating polyneuropathy and membranous glomerulonephropathy: report of two cases. J Clin Neuromuscul Dis. (2001) 3:70–4. doi: 10.1097/00131402-200112000-00004
13. Emsley HCA, Molloy J. Inflammatory demyelinating polyradiculoneuropathy associated with membranous glomerulonephritis and thrombocytopaenia. Clin Neurol Neurosurg. (2002) 105:23–6. doi: 10.1016/S0303-8467(02)00087-2
14. Chen K-H, Chang C-T, Hung C-C. Glomerulonephritis associated with chronic inflammatory demyelinating polyneuropathy. Ren Fail (2006) 28:255–9. doi: 10.1080/08860220600580415
15. Smyth S, Menkes DL. Coincident membranous glomerulonephritis and chronic inflammatory demyelinating polyradiculoneuropathy: questioning the autoimmunity hypothesis. Muscle and Nerve (2008) 37:130–5. doi: 10.1002/mus.20841
16. Wong AHY, Kokubun N, Fukami Y, Miyaji K, Yuki N. Chronic inflammatory demyelinating polyneuropathy with membranous nephropathy. J Peripher Nerv Syst. (2015) 20:63–6. doi: 10.1111/jns.12113
17. Francis JM, Beck LH, Salant DJ. Membranous Nephropathy: a journey from bench to bedside. Am J Kidney Dis. (2016) 68:138–47. doi: 10.1053/j.ajkd.2016.01.030
18. Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court F a, et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron (2005) 48:737–42. doi: 10.1016/j.neuron.2005.10.019
19. Boyle MET, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron (2001) 30:385–97. doi: 10.1016/S0896-6273(01)00296-3
20. Timmermans SAMEG, Damoiseaux JGMC, Heerings-Rewinkel PTJ, Ayalon R, Beck LH, Schlumberger W, et al. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am J Clin Pathol. (2014) 142:29–34. doi: 10.1309/AJCP8QMOY5GLRSFP
21. Hoxha E, Beck LH, Wiech T, Tomas NM, Probst C, Mindorf S, et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7a-specific antibodies in membranous nephropathy. J Am Soc Nephrol. (2017) 28:520–31. doi: 10.1681/ASN.2016010050
22. Mori K, Iijima M, Koike H, Hattori N, Tanaka F, Watanabe H, et al. The wide spectrum of clinical manifestations in Sjögren's syndrome-associated neuropathy. Brain (2005) 128:2518–34. doi: 10.1093/brain/awh605
23. Ramos-Casals M, Brito-Zerón P, Seror R, Bootsma H, Bowman SJ, Dörner T, et al. Characterization of systemic disease in primary Sjögren's syndrome: EULAR-SS task force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatol (2015) 54:2230–8. doi: 10.1093/rheumatology/kev200
24. Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. (2011) 364:689–90. doi: 10.1056/NEJMc1011678
25. Reid RA, Bronson DD, Young KM, Hemperly JJ. Identification and characterization of the human cell adhesion molecule contactin. Brain Res Mol Brain Res. (1994) 21:1–8. doi: 10.1016/0169-328X(94)90372-7
26. Koike H, Kadoya M, Kaida K, Ikeda S, Kawagashira Y, Iijima M, et al. Paranodal dissection in chronic inflammatory demyelinating polyneuropathy with anti-neurofascin-155 and anti-contactin-1 antibodies. J Neurol Neurosurg Psychiatry (2017) 88:465–73. doi: 10.1136/jnnp-2016-314895
Keywords: contactin-1, autoantibody, chronic inflammatory demyelinating polyneuropathy, membranous nephropathy, nephrotic syndrome
Citation: Hashimoto Y, Ogata H, Yamasaki R, Sasaguri T, Ko S, Yamashita K, Xu Z, Matsushita T, Tateishi T, Akiyama S, Maruyama S, Yamamoto A and Kira J (2018) Chronic Inflammatory Demyelinating Polyneuropathy With Concurrent Membranous Nephropathy: An Anti-paranode and Podocyte Protein Antibody Study and Literature Survey. Front. Neurol. 9:997. doi: 10.3389/fneur.2018.00997
Received: 27 April 2018; Accepted: 05 November 2018;
Published: 27 November 2018.
Edited by:
Massimiliano Filosto, Asst degli Spedali Civili di Brescia, ItalyReviewed by:
Claudia Sommer, Universitätsklinikum Frankfurt, GermanyHugh J. Willison, University of Glasgow, United Kingdom
Eduardo Nobile-Orazio, Università degli Studi di Milano, Italy
Copyright © 2018 Hashimoto, Ogata, Yamasaki, Sasaguri, Ko, Yamashita, Xu, Matsushita, Tateishi, Akiyama, Maruyama, Yamamoto and Kira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-ichi Kira, kira@neuro.med.kyushu-u.ac.jp
†These authors have contributed equally to this work
 Yu Hashimoto
Yu Hashimoto Hidenori Ogata
Hidenori Ogata Ryo Yamasaki
Ryo Yamasaki Takakazu Sasaguri3
Takakazu Sasaguri3 Takuya Matsushita
Takuya Matsushita Shin'ichi Akiyama
Shin'ichi Akiyama Shoichi Maruyama
Shoichi Maruyama Jun-ichi Kira
Jun-ichi Kira