- 1Department of Rehabilitation Medicine, Guangdong Engineering and Technology Research Center for Rehabilitation Medicine and Translation, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2Department of Acupuncture and Moxibustion, The Secondary Medical College, Guangzhou University of Traditional Chinese Medicine, Guangzhou, China
- 3Xinhua College of Sun Yat-sen University, Guangzhou, China
Background: Stroke survivors may lack the cognitive ability to anticipate the required control for palmar grasp execution. The cortical mechanisms involved in motor anticipation of palmar grasp movement and its association with post-stroke hand function remains unknown.
Aims: To investigate the cognitive anticipation process during a palmar grasp task in subacute stroke survivors and to compare with healthy individuals. The association between cortical excitability and hand function was also explored.
Methods: Twenty-five participants with hemiparesis within 1–6 months after first unilateral stroke were recruited. Twenty-five matched healthy individuals were recruited as control. Contingent negative variation (CNV) was measured using electroencephalography recordings (EEG). Event related potentials were elicited by cue triggered hand movement paradigm. CNV onset time and amplitude between pre-cue and before movement execution were recorded.
Results: The differences in CNV onset time and peak amplitude were statistically significant between the subacute stroke and control groups, with patients showing earlier onset time with increased amplitudes. However, there was no statistically significant difference in CNV onset time and peak amplitude between lesioned and non-lesioned hemisphere in the subacute stroke group. Low to moderate linear associations were observed between cortical excitability and hand function.
Conclusions: The earlier CNV onset time and higher peak amplitude observed in the subacute stroke group suggest increased brain computational demand during palmar grasp task. The lack of difference in CNV amplitude between the lesioned and non-lesioned hemisphere within the subacute stroke group may suggest that the non-lesioned hemisphere plays a role in the motor anticipatory process. The moderate correlations suggested that hand function may be associated with cortical processing of motor anticipation.
Introduction
Cognitive Process of Movement Anticipation in Stroke Patients
Stroke is among the leading causes of long-term disability worldwide (1). It is one of the most severe issues encountered by the aging population (2). Seventy-five percent of stroke survivors have motor dysfunction that affects body coordination and motor skill (3). Motion prediction is a key component of cognitive function and is a high-level function that affects motor control (4). Motor function recovery is often measured in terms of motor execution, with little consideration given to the high level cognitive processes that feeds into the actual motor response (5). To execute activities of daily living such as reach and grasp, the upper extremity must apply the correct force, move the precise range and accurately coordinate multiple limb segments (6–10). The cognitive ability to anticipate the required movement control is therefore fundamental to hand motor performance. Published literature indicates that stroke patients lack the anticipatory ability of upper limb movement that is associated with palmar grasp (11–13). The lack of ability to anticipate was evidenced in the suboptimal application of force by producing markedly increased grip forces during lifting, holding and moving a hand-held object in patients with acute stroke (14). Patients with chronic stroke demonstrated a slower response to adapt to the perturbing force and exhibited smaller aftereffects when the perturbing force was unexpectedly removed than healthy controls (11).
Contingent Negative Variation
The electrophysiological processes associated with movement anticipation and the relationship between the electrophysiological changes and clinical impairment of hand function in stroke patients remain poorly understood (15). Contingent negative variation (CNV) is one of the electrophysiological substrates of motor anticipation. CNV was first reported by Walter et al. (16) as a slow-going, negative event-related potential (ERP) that reflects the cognitive processing (5). It refers to the sustained negativity that develops after the pre-cue (S1) and before the imperative stimulus (S2) that requires a response. If the interval between S1 and S2 is >2 s, CNV can be distinguished into initial CNV and late CNV (17). The late CNV that occurs before S2 is assumed to be the composition of a readiness potential and stimulus preceding negativity (18). The amplitude of late CNV is also modulated by the amount of advanced information provided by the pre-cue (19–21), which usually shows up as an increase in late CNV. Late CNV tends to increase when people perform intention-based action with advanced information provided by pre-cue (21–23). This is because pre-cue information enables people to have sufficient anticipation for the upcoming action (24). Source analysis suggests that there are multiple sources of the generation of late CNV. These include the cortical and subcortical generators of the (1) anterior cingulate cortex, (2) supplementary motor area, and (3) primary motor area, in particular the inferior parietal cortex (19, 25, 26). CNV is suggested to be an index of anticipation because it reflects the action preparation process (21, 27). Therefore, the CNV amplitude and latency represent cognitive processes of anticipation and movement preparation (28–30). Early literature reported that the time between the onset of brain potential recorded by electroencephalographic (EEG) and movement onset recorded with electromyography (EMG) were indicative of the preparation time for the required action (31). Studies of healthy individuals also indicated that non-dominant hand movement has an earlier onset of cortical potential when compared with the dominant hand movement. Thus, there is evidence to indicate that the longer anticipatory phase reflects the increase in computational demand when executing learned movement (32). In people with chronic stroke, CNV is significantly enhanced at the midline region with markedly slower response time during affected hand preparation (5). The increased amplitude of the midline region CNV correlates with a greater response-priming effect. Enlarged CNV amplitudes during intention-based actions are expected in people with stroke as they need to have increased cognitive preparation for the anticipation process when compared with healthy individuals. Similar findings were also reported in chronic stroke patients who had no residual paretic hand movements (33). Simultaneous recording of EEG and EMG indicated that early onset of cortical potential precede affected rather than unaffected hand movement.
To date, the cognitive process involved in movement anticipation during a palmar grasp task with advance pre-cuing in the subacute stroke population has not been studied using the CNV approach. Understanding the cortical regions that are involved during grasping preparation in the early stages of stroke can provide new insight into the neural plasticity mechanism for grasp motion recovery. The aim of the present study was to investigate the difference in the cognitive anticipation process between the lesioned and non-lesioned hemisphere in subacute stroke survivors and to compare the difference in the anticipation process with healthy individuals. This study hypothesized that subacute stroke patients demonstrated a statistically significant difference in CNV amplitude during intention-based action between the lesioned and non-lesioned side and that this differed significantly from healthy individuals. The study also investigated if the difference in the electrophysiological process was associated with a functional level as measured by the Action Research Arm Test (ARAT).
Methods
Participants
Twenty-five participants (thirteen males and 12 females; aged 55.2 ± 8.4 years) with subacute stroke were recruited. Twenty-five age and gender matched healthy individuals (thirteen males and 12 females; aged 53.8 ± 7.9 years) were recruited as controls. The functional level of the participants with subacute stroke was assessed by the ARAT (34), which consists of a total of 19 tests of arm motor function including grasp, hold, pinch and gross motor movement. Each test is allocated an ordinal value of 0, 1, 2, or 3, with higher values indicating better arm motor status. The total ARAT score is the sum of the 19 tests, and the maximum score of the scale is 57 (35). All recruited participants were right hand dominant as assessed by the Edinburgh handedness inventory (36). Table 1 gives a summary of the demographic data of the sample population.
The inclusion criteria for the subacute stroke cohort were as follows: (1) hemiparesis resulting from a unilateral subcortical lesion of the first occurrence of a stroke; (2) within 1–6 months of stroke occurrence; (3) magnetic resonance imaging (MRI) or computed tomography (CT) confirmed stroke; (4) age between 40 and 80 years old; (5) moderate to severe motor deficit of the affected upper limbs, having at least 10° of finger flexion and extension; (6) be able to sit at least 30 min without assistance; and (7) no severe cognitive impairment (Mini Mental State Examination >21) (37). None of the participants had any prior brain computer interface (BCI) experience. The exclusion criteria were as follows: (1) lacunar infarction; (2) massive cerebral infarction; (3) cerebellum or brainstem lesion; (4) open hand wound or hand deformity; and (5) visual field deficits. All of the healthy individuals had no history of psychiatric/neurological disease or musculoskeletal disorder.
Ethics Considerations
The study was approved by the Ethical Committee of The First Affiliated Hospital of Sun Yat-sen University. Data collection took place in the Department of Rehabilitation Medicine, The First Affiliated Hospital of Sun Yat-sen University. All of the participants were provided with a comprehensive explanation of the experimental protocol. They were given an information sheet about the study and encouraged to ask questions. Written informed consent was obtained from all participants.
Experimental Protocol
Data recording took place in an electrically shielded brain function laboratory. All participants were seated in front of a table. They were asked to place their shoulders at between 0 and 10° flexion, their forearms rested on the table with elbows flexed to 130°, and their wrists were oriented in a neutral position so that opening and closing of the hand occurred in the horizontal plane. Participants were asked to fix their gaze in the middle of the screen straight ahead, avoid eye movement and focus attention on task performance. All participants had one practice session before the ERP recording began.
Experimental Task and Procedure
All the participants first undertook 5 to 10 min of training to familiarize themselves with the entire procedure before the ERP recording began. At the beginning of the experiment, the introduction was displayed on the screen. The instructions given were as follows:
“Welcome to this experiment. During the experiment, you will see a fixated white cross appear on the screen to remind you to pay attention to the task. Then, you will see a picture of either a left hand grasp or a right hand grasp displayed on the screen accompanied by a sound to indicate a left or right hand grasp. At this stage, please keep both hands still. The screen will then turn to a grey window. During the grey window, please do a self-paced voluntary palmar grasp according to the previous cue. Throughout the experiment, please refrain from blinking and body movements, particularly during the opening and closing of the hands.”
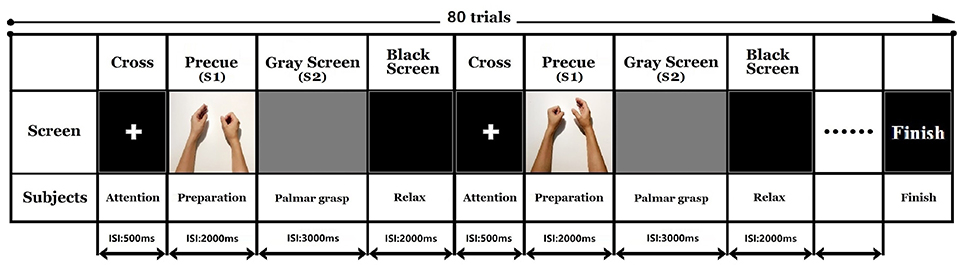
After understanding the displayed instructions, participants pressed the space bar to start the experiment. Participants first performed a modified audio-visual task that resembled a palmar grasp movement. The fixated white cross appeared on the screen for 500 ms to remind participants to pay attention to the task. Simultaneous visual and auditory imperative cues (S1) were given for 2,000 ms. A picture was displayed on the screen accompanied by a sound to indicate a right or left side palmar grasp. During S1, the participant was required to acquire the visual and auditory cues and judge the palmar grasp task. The screen then turned to a gray reaction window (S2) for 3,000 ms. The participants performed a self-paced voluntary palmar grasp during the S2 window. The experiment consisted of 40 trials for each hand, giving a total of 80 trials. The order of the trials was randomized in each experiment. The inter trial resting interval was marked by a dark screen that was displayed for 2,000 ms. The sequence of events in the trials is illustrated in Figure 1. All the participants were asked to avoid blinking and making compensatory/additional movements during the opening and closing of the hand.
EEG Signal Acquisition
The EEG data of each participant was recorded by a 32-channel QuickAmp amplifier and Ag/AgCl scalp electrodes (BrainProducts, Germany). The electrodes were positioned in accordance with the international 10–20 system (FP1, FP2, F3, Fz, F4, FC5, FC3, FC1, FCz, FC2, FC4, FC6, C5, C3, C1, Cz, C2, C4, C6, CP5, CP3, CP1, CPz, CP2, CP4, CP6, P3, POz, P4, POz, O1, O2; reference: FCz, ground: AFz). Data were recorded in DC mode with a sampling rate of 1,000 Hz. Electrodes were filled properly with conductive gel to maintain the impedance below 5 kΩ to ensure good quality recording.
For CNV amplitude analysis, topographic mapping was performed using six electrodes of interest. These electrodes were (i) F3 and C3 (left hemisphere), (ii) F4 and C4 (right hemisphere), and (iii) Fz and Cz (midline region). Figure 2 illustrates the topographic maps of participants when executing left or right hand movement tasks.
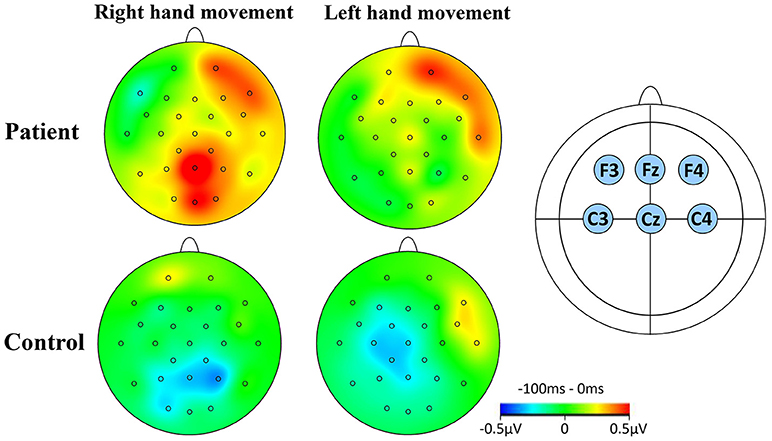
Figure 2. CNV topographical distributions. (A) The window for the mapping was −100 to 0 ms relative to S1. Red contour lines indicate positive activity, blue contour lines indicate negative activity. The greyscale fill between the contour lines indicates amount of activation (positive or negative). The small circles on the topographies indicate the electrodes sites. The rows indicate the two groups studied during RHM and LHM. (B) The location of the six electrodes of interest.
EEG Signal Processing
EEG signal analysis was performed using the Brain Vision Analyzer signal processing software 2.0 (BrainProducts, Germany). During the EEG pre-processing, the average potential of the bilateral mastoid was used as a new reference. Eye movement artifacts were removed through the Ocular Correction Independent Component Analysis (ICA) (38). The removal of eye movement artifacts is a standard operating procedure when analyzing EEG signals. The number of trials or the quality of data were not affected. Inspection of the raw data was conducted with a 50 Hz notch filter and a 0.1–30 Hz bandpass filter. The EEG data were segmented into epochs of 500 ms pre to 3,000 ms post-aligned to the cue (S1) to acquire stimulus-locked ERPs, and the baseline was corrected to the first 200 ms of the epochs, which was the 200 ms time window before S1 onset. For each epoch, the late CNV onset time was calculated as the last time the signal crossed the zero line before the rise of the CNV (17) (see Figure 4), and the peak amplitude was calculated from 1,000 to 2,000 ms since this time window contained the maximal variation of the CNV potential. CNV onset time and peak amplitude features were extracted.
In this study, the left and right hemispheres of subacute stroke participants were labeled as “lesioned” and “non-lesioned.” The left and right sided lateralised scalp sites were flipped in the participants with the right hemispheric lesion, (e.g., C3 and F3 for the right lesions and C4 and F4 for the left lesion) (33) to enable statistical comparisons between the lesioned and non-lesioned hemisphere. Thus, the “left” hemisphere was always the lesioned hemisphere in the subacute stroke group.
Statistical Analysis
All statistical analyses were performed using SPSS Statistic 21. Descriptive statistics were conducted to describe the sample population. CNV onset time and peak amplitudes were analyzed with a mixed model ANOVA analysis that incorporated the within-subjects factors TASK (Left hand movement and Right hand movement) and the LATERALITY (midline, contralateral, and ipsilateral regions). The between-subject factor of GROUP was used to compare the subacute stroke group to the matched healthy control group (stroke vs. control) during right-hand movement (RHM) and left-hand movement (LHM) and to compare the lesioned and non-lesioned hemisphere within the subacute stroke group during RHM and LHM. The Greenhouse-Geisser method were used to adjust the p-value and degrees of freedom when the assumption of sphericity was not met. Separate ANOVAs were calculated for each level of LATERALITY, using the same mixed model ANOVA format. To further investigate the differences between these groups, subsequent independent sample t-tests were performed. The significance level for all statistical analysis was set at 0.05. Bonferroni-adjusted significance tests were performed to correct the p-values of electrodes for multiple comparisons. Thus, the corrected significance level for LATERALITY was α = 0.05 ÷ 6 = 0.008. Spearman's rank correlation analysis was performed to investigate the association between the ARAT and electrophysiological measures. The interpretations of Spearman's rank correlation coefficient were as follow: “slight” (0.0–0.2); “low” (0.2–0.4); “moderate” (0.5–0.7); “high” (0.8–0.9) (39).
Results
Onset Time
The mixed model ANOVA analysis indicated a significant main effect of TASK (RHM, LHM) on the CNV onset time [F(1, 48) = 2.869, p = 0.028]. CNV onset time for patients with a subacute stroke was earlier than that of the control group both during RHM (p = 0.015) and LHM (p = 0.002). In addition, the difference was not statistically significant in CNV onset time between RHM (p = 0.323) and LHM (p = 0.202) within the subacute stroke group. Figure 3 illustrates the onset time for patients compared with controls.
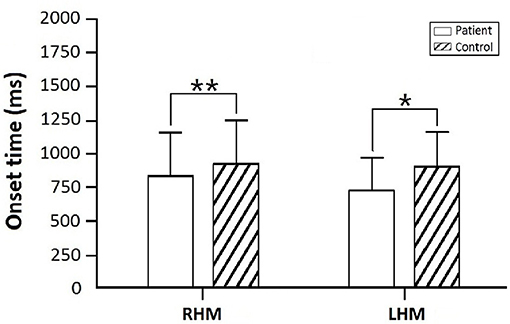
Figure 3. Onset time for the subacute stroke group in comparison to control group. Asterisks indicate significant differences within RHM and LHM (**p < 0.01; *p < 0.05).
Peak Amplitude
Analysis Between Subacute Stroke Group and Control Group
The mixed model ANOVA analysis on the CNV peak amplitude between the subacute stroke group and control group during RHM and LHM revealed a significant TASK × GROUP × LATERALITY interaction [F(5, 240) = 5.635, p < 0.001]. Furthermore, comparisons between the subacute stroke group and their match control group indicated a significant main effect of TASK for CNV peak amplitude [F(2.619, 125.713) = 3.390, p = 0.025]. Thus, separate ANOVAs were calculated for each level of TASK.
Comparison between subacute stroke group and control group within RHM condition
Analysis between the two groups during RHM indicated no significant LATERALITY × GROUP interaction [F(2.980, 71.520) = 1.605, p = 0.196]. Therefore, LATERALITY and GROUP were tested for individual effects. Significant GROUP effect [F(1, 24) = 9.379, p = 0.002] and LATERALITY effect [F(2.738, 262.837) = 4.364, p = 0.001] were observed. Then, subsequent independent sample t-tests revealed significantly larger CNV amplitudes in the subacute stroke group at midline (Fz, Cz), ipsilateral (F4, C4), and contralateral (C3, F3) electrodes than the control group when conducting right hand movement. Figure 4 gives the graphical representations of the CNV amplitudes during RHM. Table 2 illustrates the comparison of CNV peak amplitude between the two groups within RHM condition.
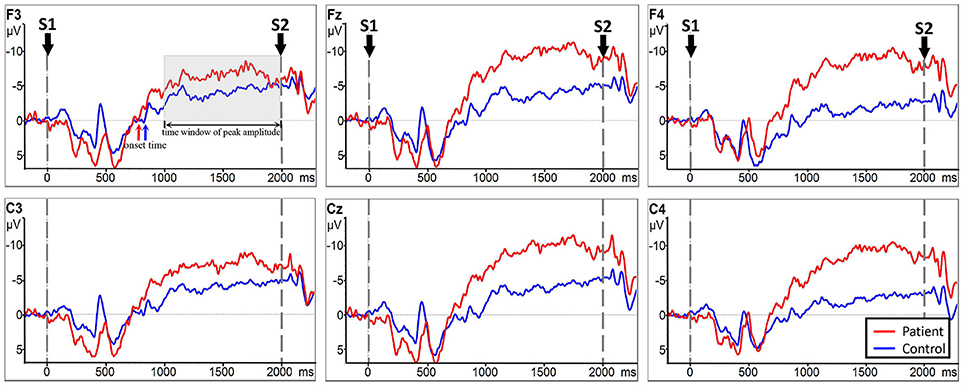
Figure 4. CNV amplitudes for the subacute stroke group (red line) and control group (blue line) during RHM. The Y-axis represents CNV waveform (μV) and the X-axis represents time (ms). Negative is plotted upwards. Baseline is the first 200 ms of the epoch, i.e., 200 ms before S1 onset. The 0 ms point is S1 onset. Red/blue arrows represent the onset of the late CNV in patient group/control group. The gray time window indicated the time window to detect the peak amplitude.
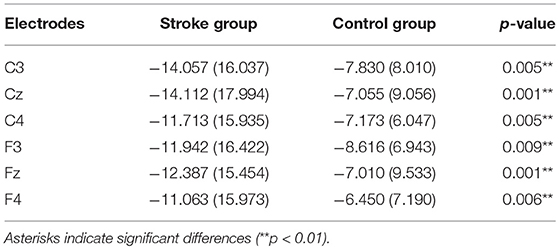
Table 2. Comparison of CNV peak amplitude between subacute stroke group and control group within RHM condition.
Comparison between subacute stroke group and control group within LHM condition
No significant LATERALITY × GROUP interaction [F(2.078, 49.883) = 0.905, p = 0.415] was observed in CNV amplitude between the subacute stroke group and the control group. Therefore, LATERALITY and GROUP were tested for individual effects. Analysis showed significant GROUP effect [F(1, 24) = 14.47, p = 0.001] and LATERALITY effect [F(2.920, 70.073) = 7.185, p < 0.001]. Further investigation of these effects revealed that there was a significantly larger CNV amplitude in the subacute stroke group at midline (Fz, Cz), contralateral (F4, C4), and ipsilateral (F3, C3) regions than the control group when conducting right hand movement. Figure 5 gives the graphical representations of CNV amplitudes for the patient and control groups during LHM. Table 3 shows the comparison of CNV peak amplitudes between subacute stroke group and control group within LHM condition.
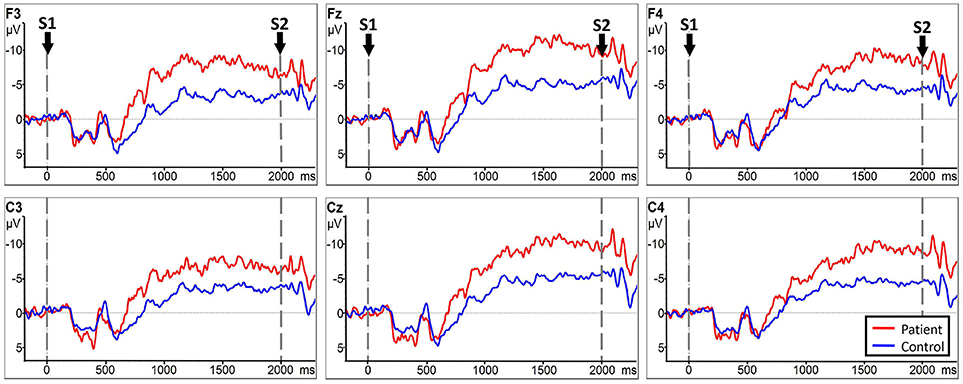
Figure 5. CNV amplitudes for the subject stroke group (red line) and control group (blue line) during LHM. The Y-axis represents CNV waveform (μV) and the X-axis represents time (ms). Negative is plotted upwards. Baseline is the first 200 ms of the epoch, i.e., 200 ms before S1 onset. The 0 ms point is S1 onset.
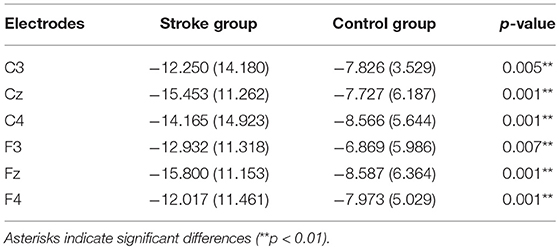
Table 3. Comparison of CNV peak amplitude between subacute stroke and control group within LHM condition.
Comparison Between RHM and LHM Within the Subacute Stroke Group
Analysis indicated no significant LATERALITY × GROUP interaction [F(2.153, 51.669) = 0.856, p = 0.438]. Further analysis showed no statistically significant main effect of GROUP [F(1, 24) = 0.100, p = 0.755] or LATERALITY [F(2.372, 56.938) = 1.209, p = 0.051] on CNV amplitude between RHM and LHM within the subacute stroke group. Thus, there was no statistically significant difference in CNV amplitude between RHM and LHM in the subacute stroke group. Figure 6 shows the EEG traces recorded at each electrode during LHM and RHM in the subacute stroke group.
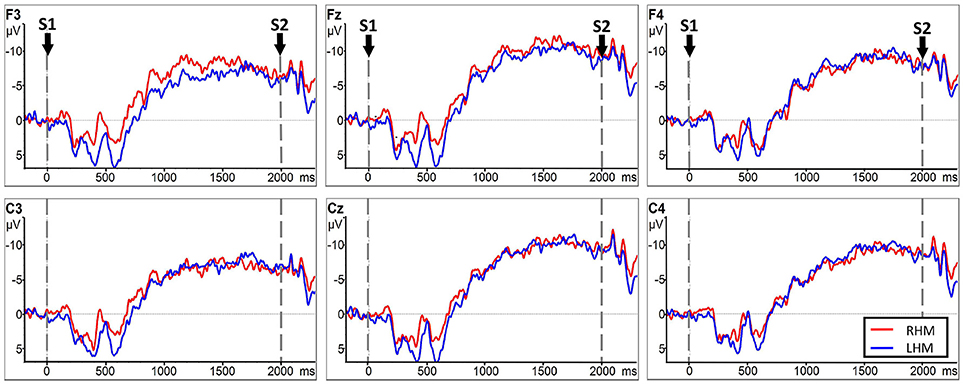
Figure 6. CNV amplitudes for the subacute stroke group group during RHM (red line) and LHM (blue line). The Y-axis represents CNV waveform (μV) and the X-axis represents time (ms). Negative is plotted upwards. Baseline is the first 200 ms of the epoch, i.e., 200 ms before S1 onset. The 0 ms point is S1 onset.
When comparing laterality factors (contralateral, midline, ipsilateral) within the TASK movement condition (RHM and LHM), there was no statistically significant hemisphere LATERALITY effect on CNV amplitude during RHM [F(5, 65.715) = 2.015, p = 0.126].
During LHM, a significantly main effect of LATERALITY on CNV amplitude was observed [F(1.944, 46.663) = 3.820, p = 0.030]. Further investigation of the hemisphere LATERALITY effect revealed that midline electrodes (Cz) had a higher CNV amplitude than contralateral (C4) electrodes (p = 0.007).
Correlations Between CNV Peak Amplitude With ARAT in the Subacute Stroke Group
Figure 7 shows the results of Spearman's rank correlation coefficient analysis between the CNV peak amplitude observed at different regions and the ARAT within the subacute stroke group. During RHM, significant correlations were observed in the front-ipsilateral side (r = 0.510, p = 0.009), midline (r = 0.428, p = 0.033), and ipsilateral side (r = 0.442, p = 0.027). During the LHM condition, a significant correlation was observed in the front-contralesional side (r = 0.496, p = 0.012), midline (r = 0.446, p = 0.026), and contralesional side (r = 0.496, p = 0.012).
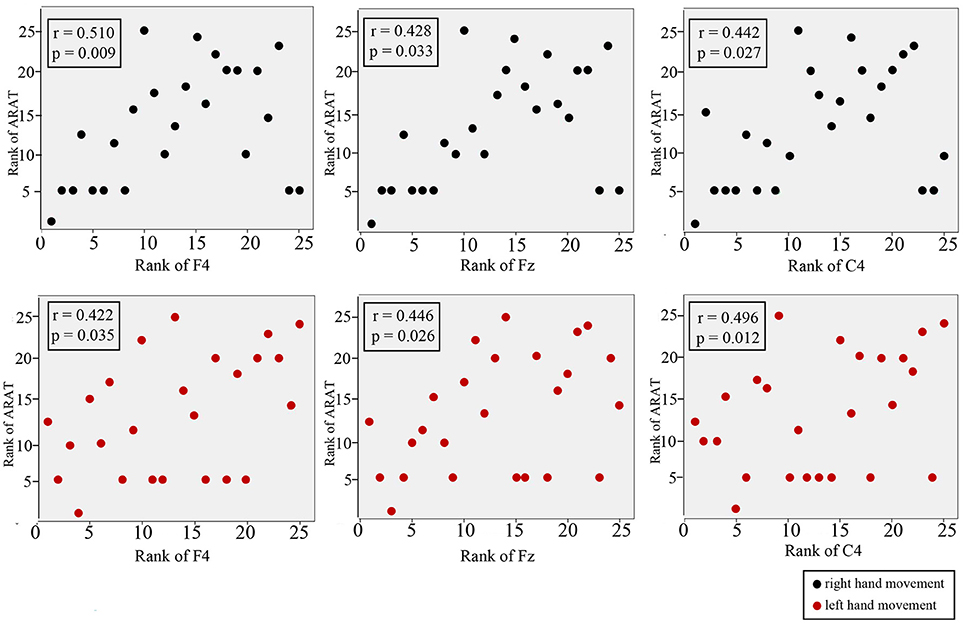
Figure 7. Correlation between CNV amplitude with ARAT in stroke participants. The Y-axis represents the rank of ARAT and the X-axis represents the rank of CNV. CNV recorded at midline, front-contralesional/front-ipsilateral side, and contralesional/ipsilateral side shown for RHM (black circles) and LHM (red circles).
Discussion
The primary aim of this study was to investigate the difference in the cognitive anticipation process between the lesioned and non-lesioned hemisphere in subacute stroke survivors and to compared those with healthy individuals. The association between the electrophysiological process and the functional level as measured by the ARAT in the stroke group was also investigated.
Onset of CNV
Results of this present study are consistent with existing literature reporting extended anticipation time. We found that this occurred in the subacute stroke population when compared with the control group, as evidenced by the statistically significant early CNV onset time during left hand and right hand movement. This finding is consistent with a previous investigation that reported a significantly earlier onset time in the paretic hand movement than in the non-paretic hand movement in people with chronic stroke using movement-related slow cortical potentials (SCP) (33). This supports the theory that extended planning time is needed for the brain to start exciting the motor network that leads to movement execution of the paretic arm. The results of this present study are consistent with existing literature suggesting that extended anticipation (planning) time is present within the subacute stroke population when compared with a control group. This study however did not observe any difference in CNV onset time between the lesioned and non-lesioned side during LHM and RHM. This is contrary to previous studies that reported a significant difference in CNV onset time between paretic and non-paretic hand movement (5, 33). The different findings between the two studies are likely related to the difference in experimental design. In the study by Deecke et al. (40), slow cortical potential measurement incorporated both anticipation of an upcoming movement as well as the motor potential occurring at the time of movement execution. Thus, SCP did not only reflect the cognitive processing behavior but also the additional time required for motor network excitation. The study by Dean et al. (5), recorded CNV onset from S2 (response cue) until feedback (task execution), whereas this study measured from S1 (pre-cue) until S2 without task execution. Thus, the potentials reported by Dean et al., did not only reflect the anticipation but also the potentials during movement. The findings in this study demonstrated for the first time that the motion anticipation phase alone is earlier in subacute stroke participants than healthy participants. Furthermore, advanced motor anticipation occurred not only in the paretic hand but also in the non-paretic hand, as evidenced by the lack of difference in CNV between the lesioned and non-lesioned hemisphere.
CNV Amplitude Related to Motor Expectancy
This study evaluated motor anticipation with CNV elicited by a stimulus-locked open/close hand movement paradigm. People with motor impairment are expected to have a larger peak amplitude that reflects the increase in the brain's computational demand to execute a movement (41). The study observed a significantly larger peak amplitude of CNV during RHM (affected hand) in the stroke group over the contralateral (C3), midline (Fz and Cz), and ipsilateral (C4) regions than that in the matched control group. This finding is consistent with published literature and supports the notion of an increase in psychological anticipation during the affected hand movement. This study also observed an increase in the activity pattern at contralateral (C4), midline (Fz and Cz), and ipsilateral (C3) locations during the LHM (unaffected hand) in the stroke group when compared with the control group. This suggests that increased psychological anticipation activity also occurs during unaffected hand movement. This higher activity level may be related to the compensatory over activation in the non-lesioned hemisphere and the lack of cross hemispheric inhibition from the lesioned to non-lesioned hemisphere.
Within the stroke group, extensive anticipation activation was observed at the frontal, contralateral, midline, and ipsilateral regions during the affected hand movement. No difference was found between the contralateral (C3) and ipsilateral (C4) side, with a significantly larger peak amplitude observed at the midline (Cz) than ipsilateral (C4) side. This suggests that there is no definitive hemisphere laterality and that most of the anticipation activity originates from the midline region during affected palmar movement. This is consistent with studies that reported increased effort in response to the inability to control the process in the hemisphere (42–44). These results did not demonstrate the effect a pattern of larger CNV amplitude over the contralateral region, as reported in previous research in the chronic stroke population (5, 19, 45, 46). The difference in findings may be related to the chronicity of the sample population. Cortical map expansion has been repeatedly reported in animal (47) and human experiments (48) after cortical damage. The expansion happens during the early stages of a stroke due to neuroplasticity and up to 8 weeks after training had stopped (49). Once the sequence of the motor task is learned, the size of the mapping representation returns to its original size (50). The lack of a significant difference in CNV amplitude between the left and right hemisphere may suggest that the subacute stroke patients are still going through the learning process to regain the palmar grasp movement. Thus, cortical expansion is occurring during this stage due to the neuroplasticity process, and a significantly larger CNV amplitude is generated along with bilateral changes in the adaptive compensation function of the brain (41). This theory is given further support from early literature that has repeatedly shown that movement of the affected hand is associated with increased bilateral activation of sensorimotor cortex (51–54). The lack of difference in the peak CNV amplitude may also be related to the incongruous over activation that occurs in the unaffected hemisphere or to an imbalance in inter hemispheric inhibition (55–59).
Correlation Between Electrophysiological Measures and Clinical Scale
A higher ARAT score is indicative of higher motor function of the paretic hand (60). Though it appeared that a better ARAT performance correlated with a smaller CNV peak amplitude, it is not possible to draw a firm conclusion because there is insufficient data available from the high and low CNV amplitude comparison. This study observed the strongest significant positive correlation at the front-ipsilateral side, which suggests that motor function may be represented by neural pathway modulation that is responsible for motor function. The significant moderate correlations suggested that hand function may be associated with cortical excitability. The correlation level observed in this study may be affected by the spread of the data. A known limitation of the correlation coefficient is that it is affected by the spread of the data, i.e., the bigger the data range, the higher the chance of a high correlation (61).
Limitations
There are some important points regarding the limitations of this study. One limitation was that we recruited a relatively small sample size of subacute stroke participants and matched healthy controls (both groups n = 25). Future research will be needed both to enroll chronic upper limb hemiparesis stroke subjects and enlarge the sample size to explore the effects of neurophysiologic mechanisms identified in the present study. Second, this study focused on participants with moderate to severe motor deficits of the affected upper limbs. Further research is suggested to investigate whether stroke patients with mild motor deficits of the affected upper limbs can also elicit electrophysiological changes of motor anticipation. Third, the ERP has the characteristic of temporal resolution of the millisecond, which can accurately capture extremely weak signals from the brain and observe the cortical excitability of motor anticipation in real time (62). To explore further insight into the strict frequency locked cortical excitability of motor anticipation, event related synchronization technology is recommended for further research. The flipping of scalp sites may be considered a limitation. However, it is not uncommon in published literature that involves ERP or fMRI to flip the scalp site in an attempt to increase statistical power. In addition, the preliminary analysis of the present results indicated there was no statistically significant difference in CNV onset time or amplitude between patients with a left and right hemispheric lesion. Thus, the inverted electrodes in right-hemisphere lesioned patients were unlikely to alter the current findings.
Conclusions
This study investigated the electrophysiological changes of motor anticipation during a palmar grasp task in subacute stroke survivors. The earlier CNV onset time and the higher peak amplitude are indicative of increased brain computational demand during palmar grasp task post-stroke. These are indicative of an increase in brain computational demand during the palmar grasp task. The lack of difference in CNV amplitude between the lesioned and non-lesioned hemisphere in the subacute stroke group may suggest that the non-lesioned hemisphere may play a role in the anticipatory process. Further investigation is required to understand the role of the non-lesioned side in movement recovery and the impact of intervention on electrophysiological changes.
Author Contributions
All authors have read and approved the final manuscript. All authors meet the four primary ICMJE criteria for authorship. In addition, all authors have been actively involved in the study in different capacities: LC designed the study and conducted all stages of the study including data collection, analysis, interpretation, and drafting of the manuscript. YM participated in the design of research protocol and interpreted the results. YL participated in the participants recruitment. MD participated in the recruitment and data analysis. LL revised the manuscript interpreted the data. JZ and ZX assessed the motor deficit of the affected upper limbs with Research Arm Test (ARAT). DH and WL managed the trial. WL analyzed and interpreted the data and revised the manuscript.
Funding
This study was supported by four research grants from Sun Yat-sen University Clinical Research 5010 Program (2014001), Provincial Science and technology project of Guangdong Province (2015B020233006, 2016A020220009), and Guangzhou science and technology project (201604020108).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE). 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (2016) 388:1603–58. doi: 10.1016/S0140-6736(16)31460-X
2. Vorkas PA, Shalhoub J, Lewis MR, Spagou K, Want EJ, Nicholson JK, et al. Metabolic phenotypes of carotid atherosclerotic plaques relate to stroke risk: an exploratory study. Eur J Vasc Endovasc Surg. (2016) 52:5–10. doi: 10.1016/j.ejvs.2016.01.022
3. Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke (2001) 32:1279–84. doi: 10.1161/01.STR.32.6.1279
4. Varghese JP, Merino DM, Beyer KB, McIlroy WE. Cortical control of anticipatory postural adjustments prior to stepping. Neuroscience (2016) 313:99–109. doi: 10.1016/j.neuroscience.2015.11.032
5. Dean PJA, Seiss E, Sterr A. Motor planning in chronic upper-limb hemiparesis: evidence from movement-related potentials. PLoS ONE (2012) 7:e44558. doi: 10.1371/journal.pone.0044558
6. Hakkennes S, Keating JL. Constraint-induced movement therapy following stroke: a systematic review of randomised controlled trials. Aust J Physiother. (2005) 51:221–31. doi: 10.1016/S0004-9514(05)70003-9
7. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics−2014 Update: a report from the american heart association. Circulation (2014) 129:e28–292. doi: 10.1161/01.cir.0000441139.02102.80
8. Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia (2009) 47:2953–66. doi: 10.1016/j.neuropsychologia.2009.06.025
9. Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil. (2005) 86:776–81. doi: 10.1016/j.apmr.2004.08.009
10. Kalaska JF. From intention to action: motor cortex and the control of reaching movements. Adv Exp Med Biol. (2009) 629:139–78. doi: 10.1007/978-0-387-77064-2_8
11. Takahashi CD, Reinkensmeyer DJ. Hemiparetic stroke impairs anticipatory control of arm movement. Exp Brain Res. (2003) 149:131–40. doi: 10.1007/s00221-002-1340-1
12. Hermsdörfer J, Hagl E, Nowak DA. Deficits of anticipatory grip force control after damage to peripheral and central sensorimotor systems. Hum Mov Sci. (2004) 23:643–62. doi: 10.1016/j.humov.2004.10.005
13. Tan C, Tretriluxana J, Pitsch E, Runnarong N, Winstein CJ. Anticipatory planning of functional reach-to-grasp: a pilot study. Neurorehabil Neural Repair (2012) 26:957–67. doi: 10.1177/1545968312437938
14. Nowak DA, Hermsd Rfer J, Topka H. Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol. (2003) 250:850–60. doi: 10.1007/s00415-003-1095-z
15. Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. (2015) CD006876. doi: 10.1002/14651858.CD006876.pub4
16. Walter WG, Cooper R, Aldridge VJ, Mccallum WC, Winter AL. Contingent negative veriation: an electric sign of sensorimotor association and expectancy in the human brain. Nature (1964) 203:380–4. doi: 10.1038/203380a0
17. Golob EJ, Ovasapyan V, Starr A. Event-related potentials accompanying motor preparation and stimulus expectancy in the young, young-old and oldest-old. Neurobiol Aging (2005) 26:531–42. doi: 10.1016/j.neurobiolaging.2004.04.002
18. van Boxtel GJ, Brunia CH. Motor and non-motor aspects of slow brain potentials. Biol Psychol. (1994) 38:37–51. doi: 10.1016/0301-0511(94)90048-5
19. Leuthold H, Jentzsch I. Neural correlates of advance movement preparation: a dipole source analysis approach. Brain Res Cogn Brain Res. (2001) 12:207–24. doi: 10.1016/S0926-6410(01)00052-0
20. Sterr A, Dean P. Neural correlates of movement preparation in healthy ageing. Eur J Neurosci. (2008) 27:254–60. doi: 10.1111/j.1460-9568.2007.05975.x
21. Leuthold H, Sommer W, Ulrich R. Preparing for action: inferences from CNV and LRP. J Psychophysiol. (2004) 18:77–88. doi: 10.1027/0269-8803.18.23.77
22. Vanhoutte S, Van Borsel J, Cosyns M, Batens K, van Mierlo P, Hemelsoet D, et al. CNV amplitude as a neural correlate for stuttering frequency: a case report of acquired stuttering. Neuropsychologia (2014) 64:349–59. doi: 10.1016/j.neuropsychologia.2014.09.036
23. Smith AL, Staines WR. Externally cued inphase bimanual training enhances preparatory premotor activity. Clin Neurophysiol. (2012) 123:1846–57. doi: 10.1016/j.clinph.2012.02.060
24. Falkenstein M, Hoormann J, Hohnsbein J, Kleinsorge T. Short-term mobilization of processing resources is revealed in the event-related potential. Psychophysiology (2003) 40:914–23. doi: 10.1111/1469-8986.00109
25. Gomez CM, Marco J, Grau C. Preparatory visuo-motor cortical network of the contingent negative variation estimated by current density. Neuroimage (2003) 20:216–24. doi: 10.1016/S1053-8119(03)00295-7
26. Praamstra P, Stegeman DF, Horstink MW, Cools AR. Dipole source analysis suggests selective modulation of the supplementary motor area contribution to the readiness potential. Electroencephalogr Clin Neurophysiol. (1996) 98:468–77. doi: 10.1016/0013-4694(96)95643-6
27. Brunia CHM, Boxtel GJMV, Böcker KBE. Negative slow waves as indices of anticipation: the Bereitschaftspotential, the contingent negative variation, and the stimulus-preceding negativity. In: The Oxford Handbook of Event-Related Potential Components. Oxford: Oxford University Press (2011). p. 189–207.
28. Bares M, Nestrasil I, Rektor I. The effect of response type (motor output versus mental counting) on the intracerebral distribution of the slow cortical potentials in an externally cued (CNV) paradigm. Brain Res Bull. (2007) 71:428–35. doi: 10.1016/j.brainresbull.2006.10.012
29. Bender S, Resch F, Weisbrod M, Oelkers-Ax R. Specific task anticipation versus unspecific orienting reaction during early contingent negative variation. Clin Neurophysiol. (2004) 115:1836–45. doi: 10.1016/j.clinph.2004.03.023
30. Brunia CH, van Boxtel GJ. Wait and see. Int J Psychophysiol. (2001) 43:59–75. doi: 10.1016/S0167-8760(01)00179-9
31. Lang W, Beisteiner R, Lindinger G, Deecke L. Changes of cortical activity when executing learned motor sequences. Exp Brain Res. (1992) 89:435–40. doi: 10.1007/BF00228259
32. Lang W, Obrig H, Lindinger G, Cheyne D, Deecke L. Supplementary motor area activation while tapping bimanually different rhythms in musicians. Exp Brain Res. (1990) 79:504–14. doi: 10.1007/BF00229320
33. Yilmaz O, Birbaumer N, Ramos-Murguialday A. Movement related slow cortical potentials in severely paralyzed chronic stroke patients. Front Hum Neurosci. (2015) 8:1033. doi: 10.3389/fnhum.2014.01033
34. Carroll, D. A quantitative test of upper extremity function. J Chronic Dis. (1965) 18:479–91. doi: 10.1016/0021-9681(65)90030-5
35. Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. (1981) 4:483–92. doi: 10.1097/00004356-198112000-00001
36. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia (1971) 9:97–113. doi: 10.1016/0028-3932(71)90067-4
37. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
38. Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology (2000) 37:163–78. doi: 10.1111/1469-8986.3720163
39. Krishef C. Fundamental Statistics for Human Services and Social Work. Boston, MA: PWS Publishers (1987).
40. Deecke L, Scheid P, Kornhuber HH. Distribution of readiness potential, pre-motion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Exp Brain Res. (1969) 7:158–68. doi: 10.1007/BF00235441
41. Jankelowitz SK, Colebatch JG. Movement related potentials in acutely induced weakness and stroke. Exp Brain Res. (2005) 161:104–13. doi: 10.1007/s00221-004-2051-6
42. Verleger R, Binkofski F, Friedrich M, Sedlmeier P, Kompf D, Anarchic-hand syndrome: ERP reflections of lost control over the right hemisphere. Brain Cogn. (2011) 77:138–50. doi: 10.1016/j.bandc.2011.05.004
43. Riecker A, Groschel K, Ackermann H, Schnaudigel S, Kassubek J, Kastrup A. The role of the unaffected hemisphere in motor recovery after stroke. Hum Brain Mapp. (2010) 31:1017–29. doi: 10.1002/hbm.20914
44. Slobounov S, Hallett M, Newell KM. Perceived effort in force production as reflected in motor-related cortical potentials. Clin Neurophysiol. (2004) 115:2391–402. doi: 10.1016/j.clinph.2004.05.021
45. Mathews S, Ainsley DP, Sterr A. EEG dipole analysis of motor-priming foreperiod activity reveals separate sources for motor and spatial attention components. Clin Neurophysiol. (2006) 117:2675–83. doi: 10.1016/j.clinph.2006.08.001
46. Leuthold H, Jentzsch I. Planning of rapid aiming movements and the contingent negative variation: are movement duration and extent specified independently? Psychophysiology (2009) 46:539–50. doi: 10.1111/j.1469-8986.2009.00799.x
47. Sanes JN, Suner S, Lando JF, Donoghue JP. Rapid reorganization of adult rat motor cortex somatic representation patterns after motor nerve injury. Proc Natl Acad Sci USA. (1988) 85:2003–7. doi: 10.1073/pnas.85.6.2003
48. Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science (1994) 263:1287–9. doi: 10.1126/science.8122113
49. Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA. (1998) 95:861–8. doi: 10.1073/pnas.95.3.861
50. Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve (2001) 24:1000–19. doi: 10.1002/mus.1104
51. Nelles G, Spiekermann G, Jueptner M, Leonhardt G, Muller S, Gerhard H, et al. Reorganization of sensory and motor systems in hemiplegic stroke patients. A positron emission tomography study. Stroke (1999) 30:1510–6. doi: 10.1161/01.STR.30.8.1510
52. Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. (1992) 31:463–72. doi: 10.1002/ana.410310502
53. Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. (1993) 33:181–9. doi: 10.1002/ana.410330208
54. Verleger R, Adam S, Rose M, Vollmer C, Wauschkuhn B, Kompf D. Control of hand movements after striatocapsular stroke: high-resolution temporal analysis of the function of ipsilateral activation. Clin Neurophysiol. (2003) 114:1468–76. doi: 10.1016/S1388-2457(03)00125-1
55. Battaglia F, Quartarone A, Ghilardi MF, Dattola R, Bagnato S, Rizzo V, et al. Unilateral cerebellar stroke disrupts movement preparation and motor imagery. Clin Neurophysiol. (2006) 117:1009–16. doi: 10.1016/j.clinph.2006.01.008
56. Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage (2005) 28:940–6. doi: 10.1016/j.neuroimage.2005.06.033
57. Hummel FC, Steven B, Hoppe J, Heise K, Thomalla G, Cohen LG, et al. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology (2009) 72:1766–72. doi: 10.1212/WNL.0b013e3181a609c5
58. Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair (2009) 23:64–56. doi: 10.1177/1545968309336661
59. Xu GQ, Lan Y, Zhang Q, Liu DX, He XF, Lin T. 1-Hz repetitive transcranial magnetic stimulation over the posterior parietal cortex modulates spatial attention. Front Hum Neurosci. (2016) 10:38. doi: 10.3389/fnhum.2016.00038
60. Sun R, Wong WW, Wang J, Tong RK. Changes in electroencephalography complexity using a brain computer interface-motor observation training in chronic stroke patients: a fuzzy approximate entropy analysis. Front Hum Neurosci. (2017) 11:444. doi: 10.3389/fnhum.2017.00444
61. Lee J, Koh D, Ong CN. Statistical evaluation of agreement between two methods for measuring a quantitative variable. Comput Biol Med. (1989) 19:61–70. doi: 10.1016/0010-4825(89)90036-X
Keywords: stroke, subacute, motor anticipatory, cortical excitability, movement-related potential
Citation: Chen L, Mao Y, Ding M, Li L, Leng Y, Zhao J, Xu Z, Huang DF and Lo WLA (2018) Assessing the Relationship Between Motor Anticipation and Cortical Excitability in Subacute Stroke Patients With Movement-Related Potentials. Front. Neurol. 9:881. doi: 10.3389/fneur.2018.00881
Received: 30 November 2017; Accepted: 28 September 2018;
Published: 17 October 2018.
Edited by:
Pavel Lindberg, INSERM U894 Centre de Psychiatrie et Neurosciences, FranceReviewed by:
Jun Yao, Northwestern University, United StatesHenry Ma, Monash University, Australia
Simon Morand-Beaulieu, Université de Montréal, Canada
Copyright © 2018 Chen, Mao, Ding, Li, Leng, Zhao, Xu, Huang and Lo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Feng Huang, huangdf@mail.sysu.edu.cn
Wai Leung Ambrose Lo, ambroselo0726@outlook.com
 Ling Chen
Ling Chen Yurong Mao
Yurong Mao Minghui Ding1
Minghui Ding1 Le Li
Le Li Jiangli Zhao
Jiangli Zhao Dong Feng Huang
Dong Feng Huang Wai Leung Ambrose Lo
Wai Leung Ambrose Lo