The effectiveness and safety of repetitive transcranial magnetic stimulation on spasticity after upper motor neuron injury: A systematic review and meta-analysis
- 1School of Health Preservation and Rehabilitation, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Mental Health Center, West China Hospital, West China School of Medicine, Sichuan University, Chengdu, China
- 3Department of Rehabilitation Medicine, West China Second University Hospital, Sichuan University, Chengdu, China
- 4Department of Rehabilitation Medicine, The Affiliated Meishan Hospital of Chengdu University of Traditional Chinese Medicine, Meishan, China
To systematically evaluate the effectiveness and safety of repetitive transcranial magnetic stimulation (rTMS) on spasticity after upper motor neuron (UMN) injury. Eight electronic databases were searched from inception to August 6, 2022. Randomized controlled trials (RCTs) investigating the effectiveness and safety of rTMS on spasticity after UMN injury were retrieved. Two reviewers independently screened studies, extracted data, and assessed the risk of bias. Review Manager 5.3 and Stata 14.0 software were used to synthesize data. The certainty of the evidence was appraised with the Grade of Recommendation, Assessment, Development and Evaluation tool. Forty-two studies with a total of 2,108 patients were included. The results of meta-analysis revealed that, compared with control group, rTMS could significantly decrease scores of the Modified Ashworth Scale (MAS) in patients with UMN injury. The subgroup analysis discovered that rTMS effectively decreased the MAS scores in patients with stroke. Meanwhile, rTMS treatment > 10 sessions has better effect and rTMS could decrease the MAS scores of upper limb. Thirty-three patients complained of twitching facial muscles, headache and dizziness, etc. In summary, rTMS could be recommended as an effective and safe therapy to relieve spasticity in patients with UMN injury. However, due to high heterogeneity and limited RCTs, this conclusion should be treated with caution.
Introduction
The spasticity refers to abnormal increase of muscle tone, which is associated with upper motor neuron (UMN) injury occurring in stroke, spinal cord injury (SCI), cerebral palsy (CP), multiple sclerosis (MS), and others (Dietz and Sinkjaer, 2012; Posteraro et al., 2018). After UMN injury, owing to loss of supraspinal inhibition, bulbospinal pathways become hyperexcitable, the presynaptic inhibition of muscle spindle afferents reduce and muscular tone increase (Li S. et al., 2021). Spasticity is characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerk (Feldman et al., 1980). Roughly, the prevalence of spasticity reaches to 42.6% (Harb and Kishner, 2022) in stroke patients, and 73.5% of patients with SCI may accompany spasticity (Strom et al., 2022). Moreover, approximately 80% of patients with MS (Arroyo González, 2018) and 69.8% children with CP (Pulgar et al., 2019) experience spasticity.
Spasticity could restrict joint movement, cause low dexterity of movement, abnormal limb postures, and pain (Naro et al., 2017). The spasticity reduces patients’ ability to undertake activities of daily living, such as walking, eating, and bathing (Ward, 2012). Patients with long-term spasticity usually accompany with depression, anxiety, bipolar disorder, and other mood disorders (Chen et al., 2013; Kes et al., 2013). The common pharmacological treatments for spasticity are oral muscle relaxants (Yelnik et al., 2009; Sommerfeld et al., 2012), intrathecal baclofen (Ertzgaard et al., 2017; Creamer et al., 2018), and botulinum neurotoxin injections (Chen et al., 2020; Harriss et al., 2021). However, the efficacy of antispastic drugs is limited, and long-term medication may cause undesirable side effects, such as drowsiness, cognitive impairment, and muscle weakness (Langhorne et al., 2011; Turner-Stokes et al., 2015). Consequently, it is necessary to find an effective and safe therapy to alleviate spasticity.
Repetitive transcranial magnetic stimulation (rTMS) is a method that delivers TMS pulses in trains with a constant frequency and intensity to induce changes in brain activity (Nardone et al., 2020). During rTMS treatment, a coil is placed on head, when a current is passing through a coil, a magnetic field can be generated (Roth et al., 1991). Magnetic field evokes a current which has impact on cortical excitability. Modulation of cortical excitability could induce cortical plastic changes (Nowak et al., 2009). Neuroplasticity refers to the ability of the nervous system to adjust activity after injury (Puderbaugh and Emmady, 2022). rTMS has been reported to be able to trigger neuroplasticity and potentiate synaptic transmission (Iglesias, 2020; Cantone et al., 2021). It is inferred that the anti-spastic effect of rTMS may be associated with the neuroplasticity modulation. Gottlieb et al. found that rTMS could reduce MAS scores in stroke patients and regulate neuronal plasticity (Gottlieb et al., 2021). Another study revealed that rTMS reduced spasticity in incomplete SCI patients by increasing synaptic transmission (de Araujo et al., 2017). Therefore, rTMS is a promising therapy to promote neuroplasticity and ameliorate spasticity.
Transcranial magnetic stimulation has been widely used to treat spasticity after UMN injury including stroke (Rastgoo et al., 2016), SCI (Kumru et al., 2013), CP (Rajak et al., 2019), and MS (San et al., 2019). Previous systematic reviews and meta-analyses (Gao et al., 2018; McIntyre et al., 2018; Xu P. et al., 2021; Wang et al., 2022) have been conducted to evaluate the effect of rTMS in patients with stroke and SCI. Gao et al. found that rTMS could improve the spasticity in patients with incomplete SCI (Gao et al., 2018). Wang et al. concluded that rTMS had a significant effect to relieve spasticity in patients with stroke (Wang et al., 2022). While the other two systematic reviews and meta-analyses (McIntyre et al., 2018; Xu P. et al., 2021) reported that rTMS was not effective to improve spasticity after stroke. Furthermore, the optimal protocols of rTMS (e.g., intensity, frequency, pulses, treatment site, number of sessions etc.) for spasticity remains to be investigated. Recently, several clinical trials of rTMS on spasticity after UMN injury have been conducted. We intended to conduct the systematic review and meta-analysis to update the current evidence of rTMS for spasticity after UMN injury and to explore optimal protocols of rTMS.
Methods
We conducted this systematic review and meta-analysis strictly following the A Measurement Tool to Assess Systematic Reviews (AMSTAR 2.0) (Shea et al., 2017) and reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis 2020 (PRISMA 2020) statement guidelines (Page et al., 2021). The protocol of this study has been registered in the international prospective register of systematic reviews (PROSPERO, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020213173). The registration number is CRD42020213173. The completed PRISMA 2020 checklist is shown in Supplementary Appendix 1.
Inclusion criteria
Type of studies
Randomized controlled trials (RCTs) and cross-over RCTs (Hui et al., 2015) that investigated the effect of rTMS for spasticity after UMN injury were included. The language was limited to Chinese or English.
Type of participants
Participants with spasticity after UMN injury (stroke, CP, MS, SCI, etc.) were included (Posteraro et al., 2018). The spasticity was defined that Modified Ashworth Scale (MAS) was greater than 0 (Balci, 2018), the Brunnstrom stage was greater than I or author reported spasticity. There were no restrictions on age, gender, race, or nation.
Type of interventions
The interventions included rTMS or rTMS combined with conventional rehabilitation (CR) training (physiotherapy, occupational therapy, orthotics, etc.).
Type of comparators
The comparators involved sham rTMS, CR or sham rTMS plus CR.
Outcome measurements
The primary outcome was MAS scores. The secondary outcomes included Hmax/Mmax ratio, F-wave latency, Fugl-Meyer-Assessment (FMA) and Barthel Index (BI), Hamilton anxiety scale (HAMA), Hamilton depression scale (HAMD). In addition, rTMS related adverse events (headache, seizures, hearing impairment, etc.) were assessed as safety measurements.
Exclusion criteria
Studies were excluded if they met one of the following criteria: (1) factorial RCTs (Montgomery et al., 2003), N of 1 RCT (Ulbrich-Zurni et al., 2018) or cluster RCTs (Ribeiro et al., 2018); (2) full text were unavailable through various approaches; (3) duplications; (4) the data cannot be extracted; (5) other patterns of TMS, such as deep TMS, paired associative stimulation; (6) other non-invasive brain stimulation techniques, such as transcranial direct current stimulation (tDCS) and electrical stimulation alone (Brihmat et al., 2022).
Search strategy
We systematically searched China National Knowledge Infrastructure, the Chinese Science and Technology Periodical Database, Wanfang database, China Biology Medicine, PubMed, Embase, the Cochrane Library, and Web of Science from their inception to August 6, 2022. The medical subject headings (MeSH) and free terms were combined using Boolean logic operators. The full search strategies which were tailored according to the characteristic of the above databases are listed in Supplementary Appendix 2. We manually searched gray literature, reference lists of identified studies for possible relevant literatures. Additionally, the Chinese Clinical Trial Registry and ClinicalTrials.gov were searched and the experts were consulted for eligible RCTs.
Studies selection
All the retrieved records were imported into Endnote (X9), then the duplicated records were removed. After that, two reviewers (Jin Fan and Hui Fu) independently screened titles and abstracts. Then, the rest records in full text were thoroughly reviewed according to eligible criteria. Any discrepancy was resolved by discussion or consultation with a third independent reviewer (Juan Li).
Data extraction
A standardized data extraction form was designed in advance. We piloted data extraction with three eligible studies, and evaluated the intraclass correlation coefficient (ICC) to achieve reliability in extraction. Two researchers (Yuxi Li and Xiaobo Liu) independently extracted the following data: (i) study information: the first author, year of publication, type of study; (ii) participant characteristics: sample size, gender, age, types of UMN injury, course of disease; (iii) intervention details: intervention, coil type, pulse, frequency, intensity, site, sessions of treatment; (iv) study outcomes: indicators of spasticity (MAS, Hmax/Mmax ration, F-wave latency, etc.) and other relevant outcomes; (v) information related to risk of bias. The original authors were contacted for missing data if necessary. For multi-arm RCTs, the comparison with inferior effect size was pooled to obtain more conservative results. After extraction, cross-check was performed to ensure no mistakes. Disagreements were arbitrated by a third reviewer (Rongjiang Jin).
Assessment of risk of bias
The revised Cochrane risk of bias tool for individually randomized, parallel group trials (ROB 2.0) tool was used to assess the risk of bias (Yang et al., 2017). Two independent reviewers (Xiaolong Xie and Huiling Zhang) studied the ROB 2.0, then the trained reviewers pre-assessed three eligible studies and calculated the ICC. After achieving good reliability in the risk of bias assessments, we performed formal evaluation.
Data analysis
The ICC was used to determine the level of reliability between reviewers. The classification of ICCs is: excellent reliability (ICC > 0.90), good reliability (ICC = 0.76–0.90), moderate reliability (ICC = 0.50–0.75), and poor reliability (ICC < 0.50) (Grgic et al., 2022). SPSS (version 25.0) was used to calculate ICC. For the cross-over RCTs, we extracted and analyzed the data at the first intervention phase. The change of MAS was used to estimate the effect size. The mean difference (MD) was used to analyze continuous outcomes with the same unit, otherwise standardized MD (SMD) was calculated. Heterogeneity of included studies was assessed using the Cochrane Q test and was quantified by the estimated I2 statistic. A fixed-effect model was applied if heterogeneity was acceptable (I2 ≤ 50%, P ≥ 0.1). Otherwise, a random-effect model was chosen. If outcomes could not be quantitatively analyzed, we narratively described these results. For all outcome variables, two-tailed P-values < 0.05 were considered statistically significant. Meta-analysis was conducted with the Review Manager (RevMan, version 5.3.5) and Stata (version 14.0) software.
Subgroup analysis
We conducted subgroup analysis based on the types of UMN injury (stroke, CP, SCI, MS), the frequency of rTMS (low frequency, high frequency), the intensity of rTMS [≤ 90% Motor threshold (MT), > 90% MT], the total sessions of rTMS (≤10, >10), the assessment position of the MAS (upper limb, lower limb).
Sensitivity analysis
The sensitivity analysis was conducted by deleting each study one by one to verify the robustness of the results.
Publication bias
The funnel plot was used to describe possible publication bias when ≥ 10 studies included in the analysis. In addition, the Begg’s test and Egger’s test were also used.
The certainty of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt et al., 2011) to appraise the certainty of evidence. The GRADE comprises five items: risk of bias, inconsistency, indirectness, imprecision, and publication bias (Atkins et al., 2004). To ensure a reliability in evaluation of GRADE, we pre-assessed three samples and calculated the ICC as well. The certainty of evidence of each outcome was considered as high, moderate, low, or very low by two independent reviewers (Yuxi Li and Dongling Zhong). GRADEpro (Version 3.6) software was adopted to summarize the findings.
Result
Selection of eligible studies
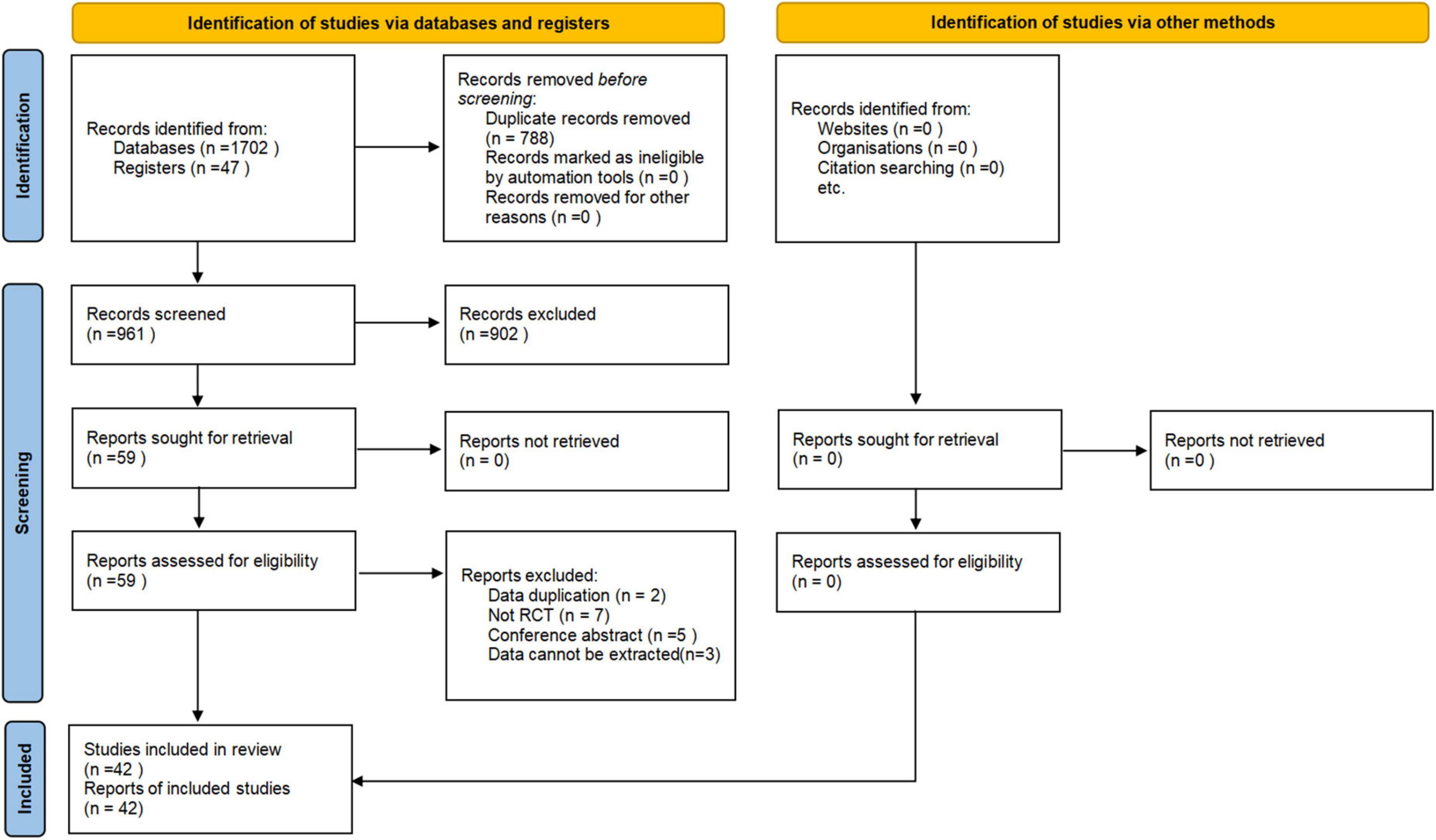
A total of 1,749 records were retrieved through electronic search. After removing duplicates, the title and abstract of the rest records were screened. Then, 59 articles were remained for scrutinization with the full texts. Seventeen studies were excluded, and the reasons for exclusion are listed in Supplementary Appendix 3. Eventually, 42 eligible RCTs with a total of 2,108 patients (Valle et al., 2007; Kumru et al., 2010, 2016; Benito et al., 2012; Nardone et al., 2014; Liao et al., 2015; Yan, 2015; Bao and Liu, 2016; Askin et al., 2017; Li, 2017; Ozkeskin et al., 2017; Sun et al., 2017; Wu, 2017; Chervyakov et al., 2018; Kong, 2018; Liu et al., 2018, 2019; Qin et al., 2018a,b; Tao and Wei, 2018; Watanabe et al., 2018; Xiao, 2018; Zhang, 2018; Dos Santos et al., 2019; Korzhova et al., 2019; Liu, 2019; Luo, 2020; Qi, 2020; Yuan, 2020; Zhang et al., 2020; Chen et al., 2021; Gottlieb et al., 2021; Liang et al., 2021; Mendonca et al., 2021; Xu R. et al., 2021; Yang, 2021; Zhao, 2021; Cheng et al., 2022; Xia et al., 2022a,b; Yang and Yang, 2022; Zang et al., 2022) were included. The PRISMA flow diagram is shown in Figure 1.
The characteristics of included studies
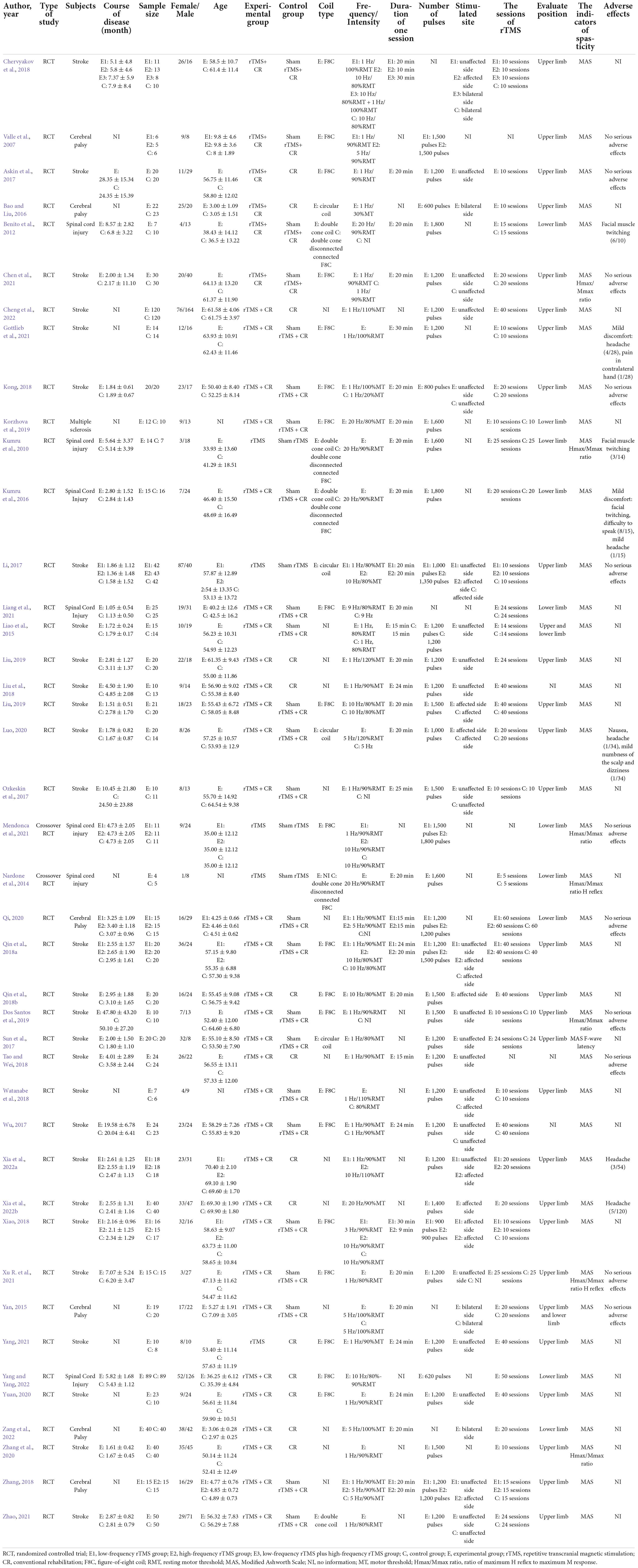
The included studies were published from 2007 to 2022. The age of patients with CP, SCI, stroke severally ranged from 1.54 to 14.4 years old, 20.33 to 65.18 years old, and 35.51 to 77.33 years old. The sample size of included trials varied from 9 to 240. Thirteen articles (Valle et al., 2007; Kumru et al., 2010, 2016; Benito et al., 2012; Nardone et al., 2014; Askin et al., 2017; Ozkeskin et al., 2017; Chervyakov et al., 2018; Watanabe et al., 2018; Dos Santos et al., 2019; Korzhova et al., 2019; Gottlieb et al., 2021; Mendonca et al., 2021) were published in English and twenty-nine articles (Liao et al., 2015; Yan, 2015; Bao and Liu, 2016; Li, 2017; Sun et al., 2017; Wu, 2017; Kong, 2018; Liu et al., 2018, 2019; Qin et al., 2018a,b; Tao and Wei, 2018; Xiao, 2018; Zhang, 2018; Liu, 2019; Luo, 2020; Qi, 2020; Yuan, 2020; Zhang et al., 2020; Chen et al., 2021; Liang et al., 2021; Xu R. et al., 2021; Yang, 2021; Zhao, 2021; Cheng et al., 2022; Xia et al., 2022a,b; Yang and Yang, 2022; Zang et al., 2022) in Chinese. Twenty-eight studies (Liao et al., 2015; Askin et al., 2017; Li, 2017; Ozkeskin et al., 2017; Sun et al., 2017; Wu, 2017; Chervyakov et al., 2018; Kong, 2018; Liu et al., 2018, 2019; Qin et al., 2018a,b; Tao and Wei, 2018; Watanabe et al., 2018; Xiao, 2018; Dos Santos et al., 2019; Liu, 2019; Luo, 2020; Yuan, 2020; Zhang et al., 2020; Chen et al., 2021; Gottlieb et al., 2021; Xu R. et al., 2021; Yang, 2021; Zhao, 2021; Cheng et al., 2022; Xia et al., 2022a,b) involved stroke, seven studies (Kumru et al., 2010, 2016; Benito et al., 2012; Nardone et al., 2014; Liang et al., 2021; Mendonca et al., 2021; Yang and Yang, 2022) related to SCI, six studies (Valle et al., 2007; Yan, 2015; Bao and Liu, 2016; Zhang, 2018; Qi, 2020; Zang et al., 2022) focused on CP, and one (Korzhova et al., 2019) about MS. The frequency of rTMS varied from 1 to 20 Hz. Seventeen studies (Kumru et al., 2010, 2016; Nardone et al., 2014; Li, 2017; Chervyakov et al., 2018; Qin et al., 2018b; Xiao, 2018; Zhang, 2018; Korzhova et al., 2019; Luo, 2020; Qi, 2020; Liang et al., 2021; Mendonca et al., 2021; Xia et al., 2022a,b; Yang and Yang, 2022; Zang et al., 2022) adopted high-frequency stimulation, and the remaining studies used low-frequency stimulation. The intensity of rTMS was from 20%MT to 120%MT. Twenty-three studies (Liao et al., 2015; Askin et al., 2017; Li, 2017; Ozkeskin et al., 2017; Sun et al., 2017; Wu, 2017; Chervyakov et al., 2018; Kong, 2018; Liu et al., 2018; Qin et al., 2018b; Tao and Wei, 2018; Watanabe et al., 2018; Zhang, 2018; Dos Santos et al., 2019; Korzhova et al., 2019; Liu, 2019; Yuan, 2020; Chen et al., 2021; Xu R. et al., 2021; Yang, 2021; Zhao, 2021; Cheng et al., 2022; Xia et al., 2022a) stimulated unaffected hemisphere, nine studies (Li, 2017; Chervyakov et al., 2018; Qin et al., 2018a,b; Xiao, 2018; Liu et al., 2019; Luo, 2020; Xia et al., 2022a,b) treated affected hemisphere, four studies (Yan, 2015; Bao and Liu, 2016; Chervyakov et al., 2018; Zang et al., 2022) involved bilateral rTMS, while eleven studies (Valle et al., 2007; Kumru et al., 2010, 2016; Benito et al., 2012; Nardone et al., 2014; Qi, 2020; Zhang et al., 2020; Gottlieb et al., 2021; Liang et al., 2021; Mendonca et al., 2021; Yang and Yang, 2022) did not specify the stimulation side. Among the included studies, there were comparisons of rTMS plus CR versus sham rTMS plus CR, rTMS plus CR versus CR, rTMS versus sham rTMS, and rTMS versus CR. The characteristics of the included studies are shown in Table 1.
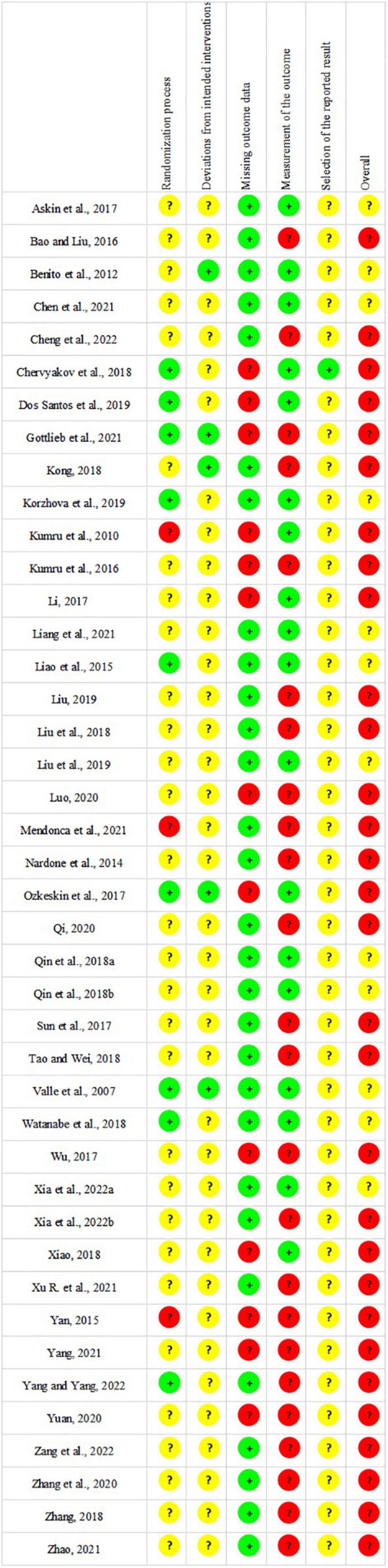
Risk of bias assessment
The ICC of each domain varied from 0.77 to 0.83, which indicated good reliability within risk of bias assessment. The results of risk of bias assessment are shown in Figure 2. Twenty-three RCTs (Valle et al., 2007; Liao et al., 2015; Yan, 2015; Bao and Liu, 2016; Askin et al., 2017; Li, 2017; Wu, 2017; Liu et al., 2018; Qin et al., 2018a,b; Tao and Wei, 2018; Xiao, 2018; Zhang, 2018; Liu et al., 2019; Qi, 2020; Chen et al., 2021; Gottlieb et al., 2021; Liang et al., 2021; Mendonca et al., 2021; Zhao, 2021; Xia et al., 2022b; Yang and Yang, 2022; Zang et al., 2022) adequately described methods of random sequences generation. Allocation concealment was performed in six studies (Ozkeskin et al., 2017; Chervyakov et al., 2018; Watanabe et al., 2018; Dos Santos et al., 2019; Korzhova et al., 2019; Mendonca et al., 2021), whereas the remaining studies did not report allocation concealment. Twelve studies (Valle et al., 2007; Kumru et al., 2010, 2016; Benito et al., 2012; Nardone et al., 2014; Liao et al., 2015; Li, 2017; Ozkeskin et al., 2017; Kong, 2018; Dos Santos et al., 2019; Korzhova et al., 2019; Mendonca et al., 2021) specified the blinding of patients and outcome assessors, and seventeen studies (Valle et al., 2007; Liao et al., 2015; Askin et al., 2017; Li, 2017; Ozkeskin et al., 2017; Chervyakov et al., 2018; Liu et al., 2018; Qin et al., 2018a,b; Watanabe et al., 2018; Xiao, 2018; Dos Santos et al., 2019; Korzhova et al., 2019; Liu et al., 2019; Chen et al., 2021; Liang et al., 2021; Xia et al., 2022a) mentioned the blinding of outcome assessors, while the rest of studies did not address whether blinding was used. In summary, the overall risk of bias of thirty studies (Kumru et al., 2010, 2016; Nardone et al., 2014; Yan, 2015; Bao and Liu, 2016; Li, 2017; Ozkeskin et al., 2017; Sun et al., 2017; Wu, 2017; Chervyakov et al., 2018; Kong, 2018; Liu et al., 2018; Tao and Wei, 2018; Xiao, 2018; Zhang, 2018; Dos Santos et al., 2019; Liu, 2019; Luo, 2020; Qi, 2020; Yuan, 2020; Zhang et al., 2020; Gottlieb et al., 2021; Mendonca et al., 2021; Xu R. et al., 2021; Yang, 2021; Zhao, 2021; Cheng et al., 2022; Xia et al., 2022b; Yang and Yang, 2022; Zang et al., 2022) was rated as “high risk of bias” and twelve studies (Valle et al., 2007; Benito et al., 2012; Liao et al., 2015; Askin et al., 2017; Qin et al., 2018a,b; Watanabe et al., 2018; Korzhova et al., 2019; Liu et al., 2019; Chen et al., 2021; Liang et al., 2021; Xia et al., 2022a) were considered as “some concerns.”
Primary outcome-the modified Ashworth scale
A total of 42 (Valle et al., 2007; Kumru et al., 2010, 2016; Benito et al., 2012; Nardone et al., 2014; Liao et al., 2015; Yan, 2015; Bao and Liu, 2016; Askin et al., 2017; Li, 2017; Ozkeskin et al., 2017; Sun et al., 2017; Wu, 2017; Chervyakov et al., 2018; Kong, 2018; Liu et al., 2018; Qin et al., 2018a,b; Tao and Wei, 2018; Watanabe et al., 2018; Xiao, 2018; Zhang, 2018; Dos Santos et al., 2019; Korzhova et al., 2019; Liu, 2019; Liu et al., 2019; Luo, 2020; Qi, 2020; Yuan, 2020; Zhang et al., 2020; Chen et al., 2021; Gottlieb et al., 2021; Liang et al., 2021; Mendonca et al., 2021; Xu R. et al., 2021; Yang, 2021; Zhao, 2021; Cheng et al., 2022; Xia et al., 2022a,b; Yang and Yang, 2022; Zang et al., 2022) studies reported the scores of MAS. However, the results of the MAS in five studies could not be extracted (Benito et al., 2012; Watanabe et al., 2018; Dos Santos et al., 2019; Mendonca et al., 2021; Zang et al., 2022), and one study did not provide the results of the MAS in control group (Chervyakov et al., 2018).
Repetitive transcranial magnetic stimulation plus conventional rehabilitation versus sham repetitive transcranial magnetic stimulation plus conventional rehabilitation
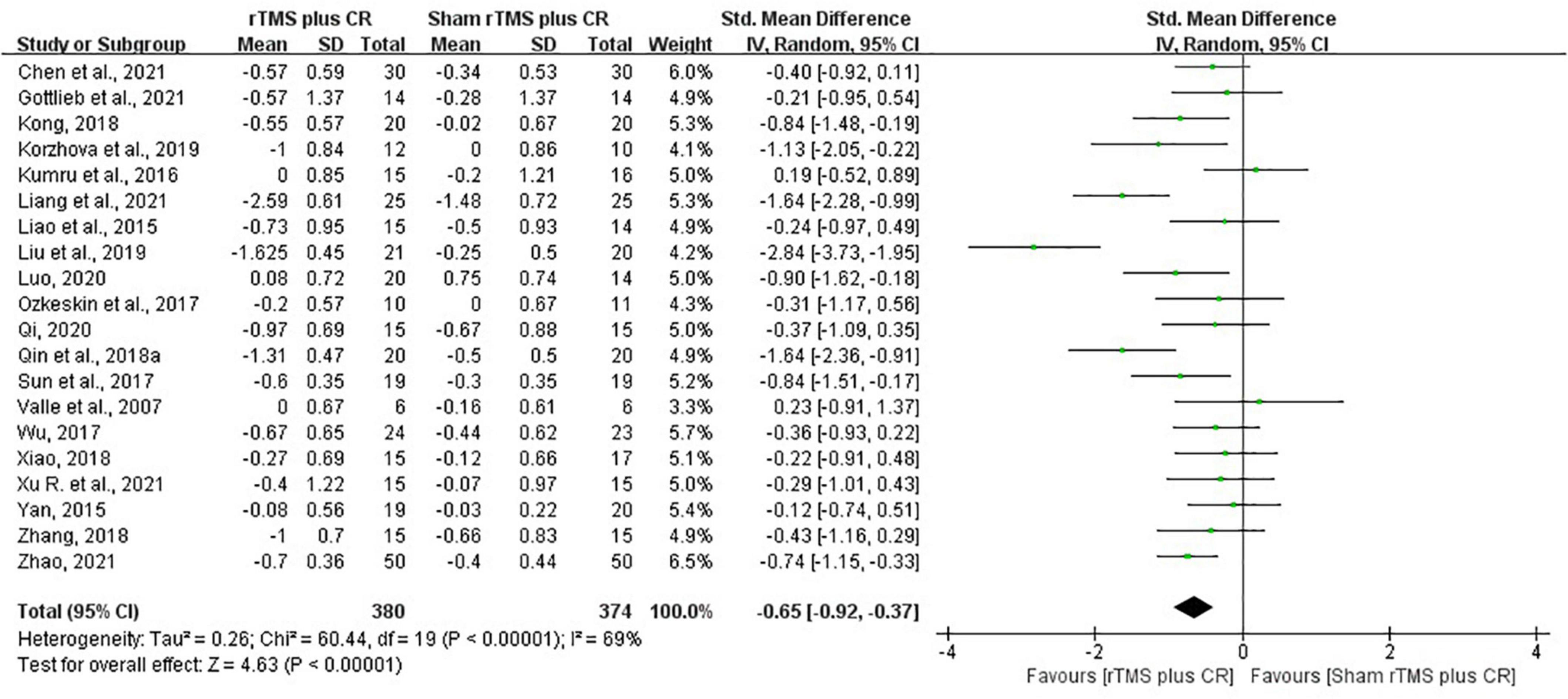
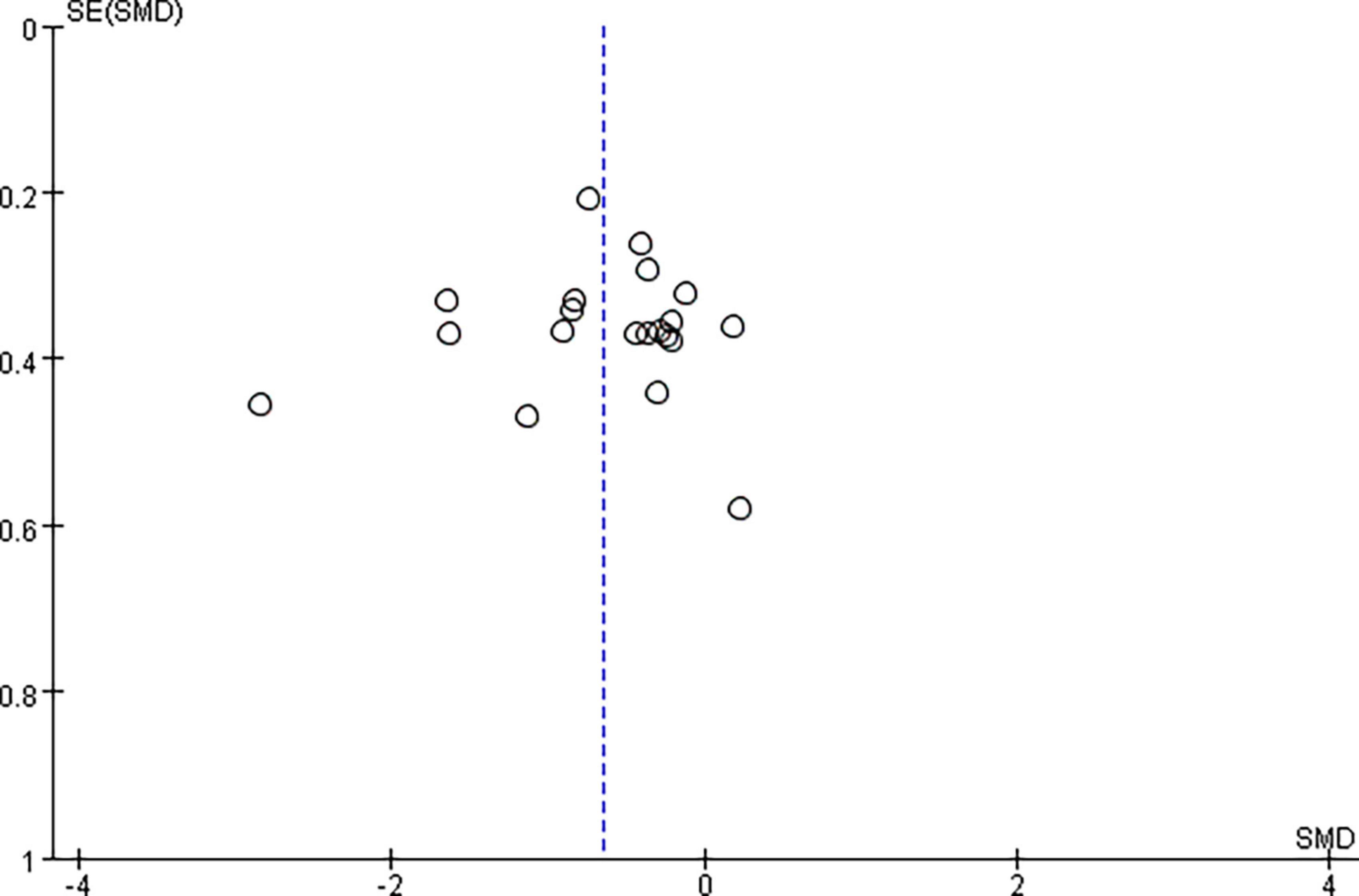
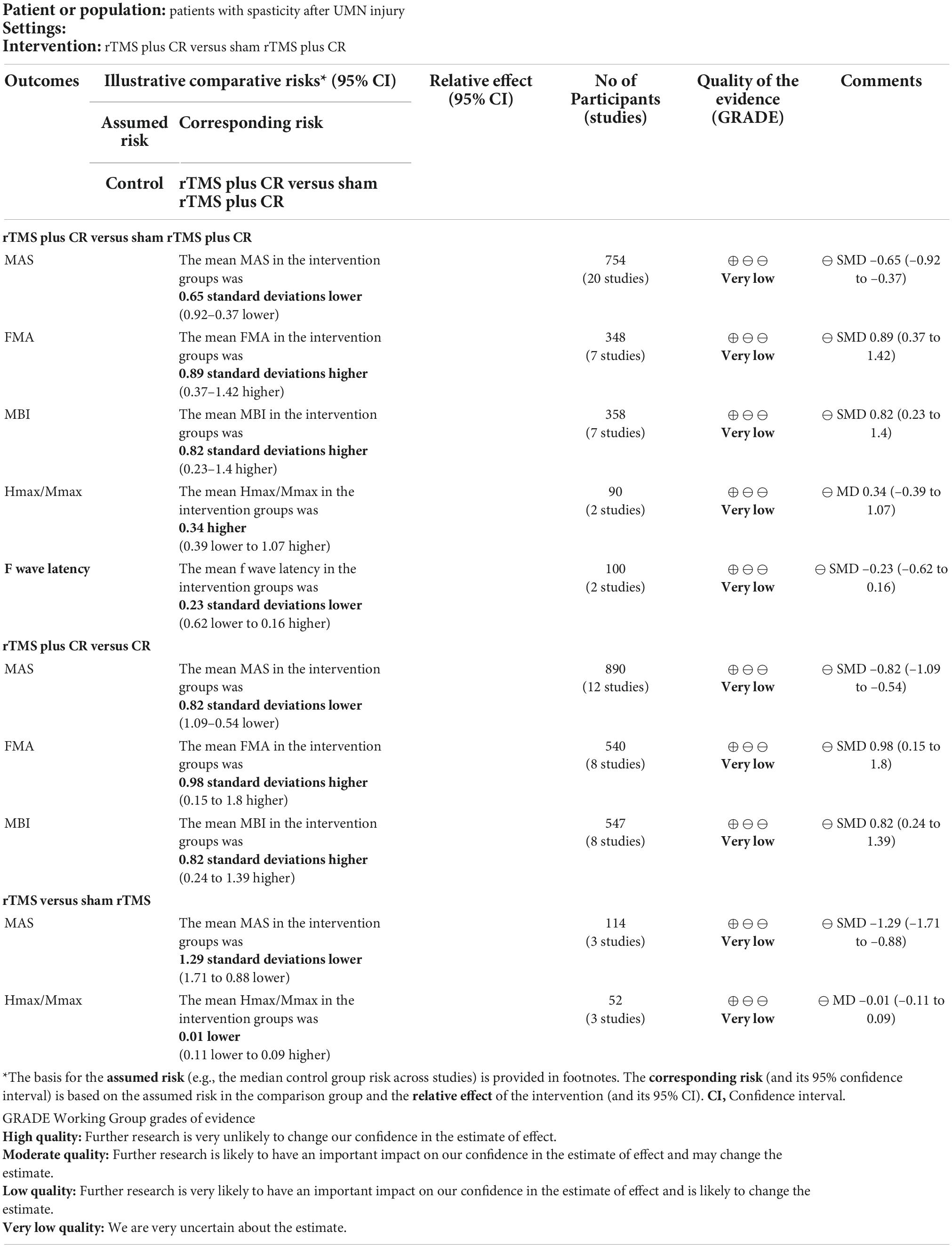
Pooled data from the twenty RCTs (Valle et al., 2007; Liao et al., 2015; Yan, 2015; Kumru et al., 2016; Ozkeskin et al., 2017; Sun et al., 2017; Wu, 2017; Kong, 2018; Qin et al., 2018b; Xiao, 2018; Zhang, 2018; Korzhova et al., 2019; Liu et al., 2019; Luo, 2020; Qi, 2020; Chen et al., 2021; Gottlieb et al., 2021; Liang et al., 2021; Xu R. et al., 2021; Zhao, 2021) revealed that rTMS plus CR decreased more MAS scores than sham rTMS plus CR (SMD = –0.65, 95%CI = –0.92 to –0.37, I2 = 69%, P < 0.00001) (Figure 3). The funnel plot, Egger’s test (P = 0.764) and Begg’s test (P = 0.922), of the MAS scores indicated no publication bias (Figure 4).
Repetitive transcranial magnetic stimulation plus conventional rehabilitation versus conventional rehabilitation
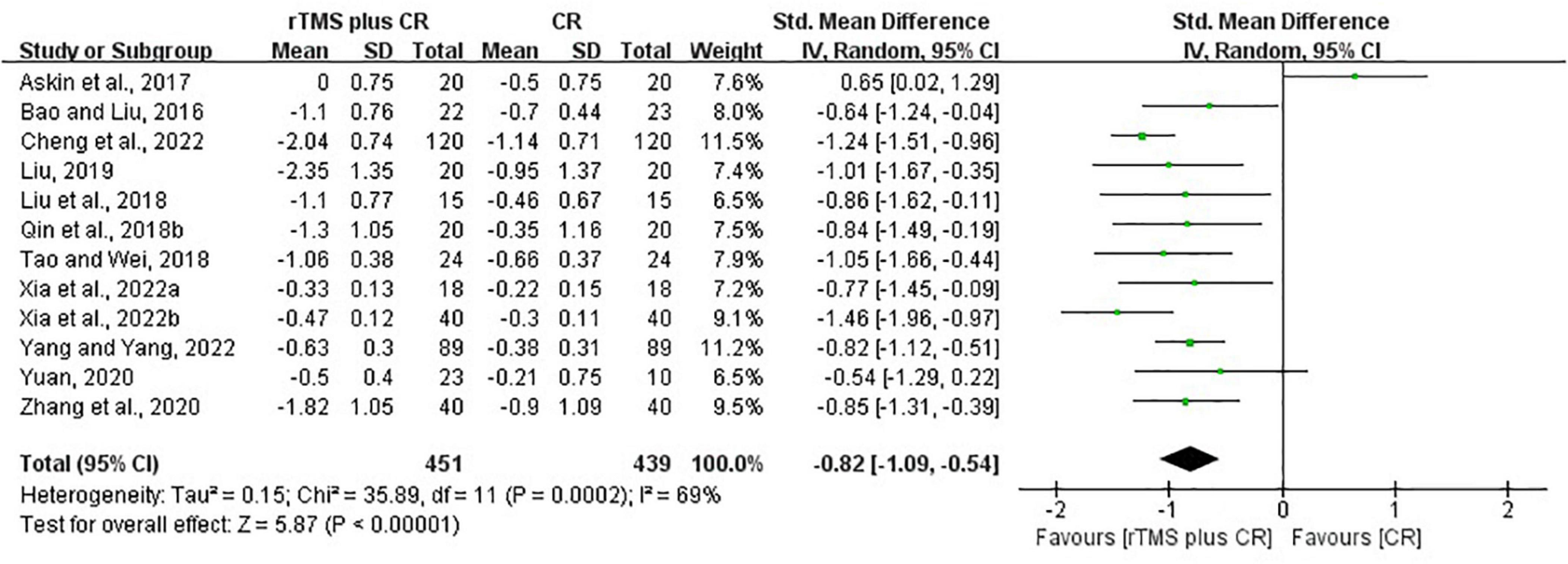
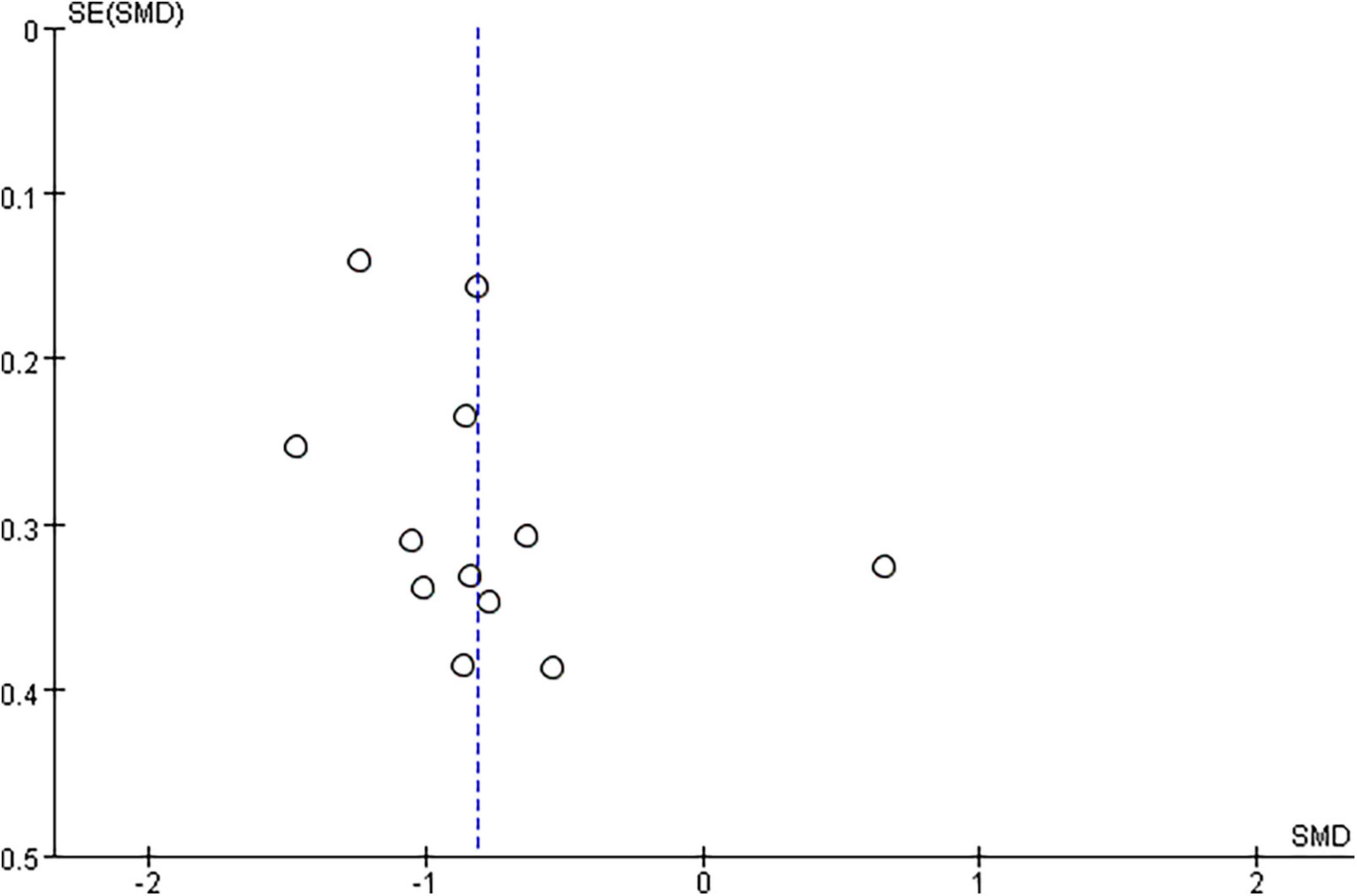
Based on the data of 12 RCTs (Bao and Liu, 2016; Askin et al., 2017; Liu et al., 2018; Qin et al., 2018a; Tao and Wei, 2018; Liu, 2019; Yuan, 2020; Zhang et al., 2020; Cheng et al., 2022; Xia et al., 2022a,b; Yang and Yang, 2022), we found that rTMS plus CR could reduce more MAS scores than CR (SMD = –0.82, 95%CI = –1.09 to –0.54, I2 = 69%, P < 0.00001) (Figure 5). The funnel plot, Egger’s test (P = 0.192) and Begg’s test (P = 0.304), demonstrated that there was no publication bias (Figure 6).
Repetitive transcranial magnetic stimulation versus sham repetitive transcranial magnetic stimulation
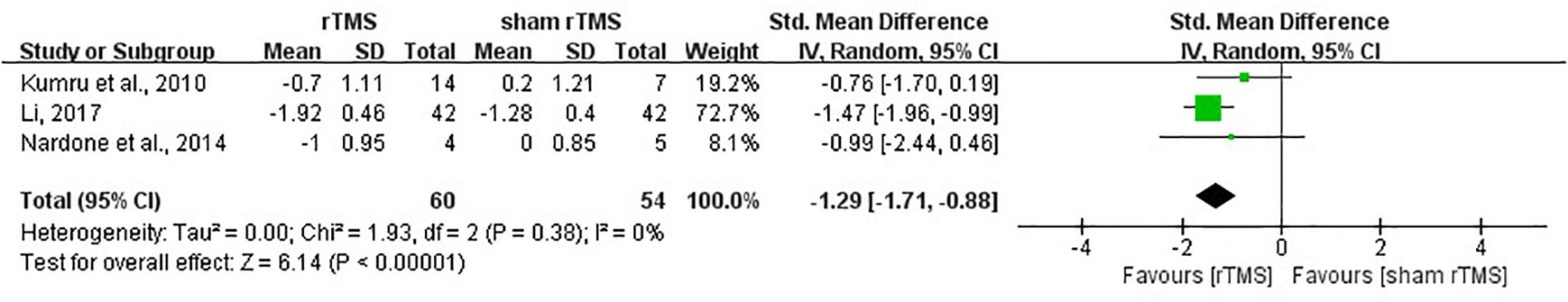
The results showed that rTMS was superior to sham rTMS in reduction of MAS scores according to the data from three studies (Kumru et al., 2010; Nardone et al., 2014; Li, 2017) (SMD = –1.29, 95%CI = –1.71 to –0.88, I2 = 0%, P < 0.00001) (Figure 7). No publication bias was detected based on the Egger’s test (P = 0.449) and Begg’s test (P = 1.000).
Repetitive transcranial magnetic stimulation versus conventional rehabilitation
Yang (2021) reported that rTMS effectively lowered MAS scores when compared with CR group.
Subgroup analysis of primary outcome
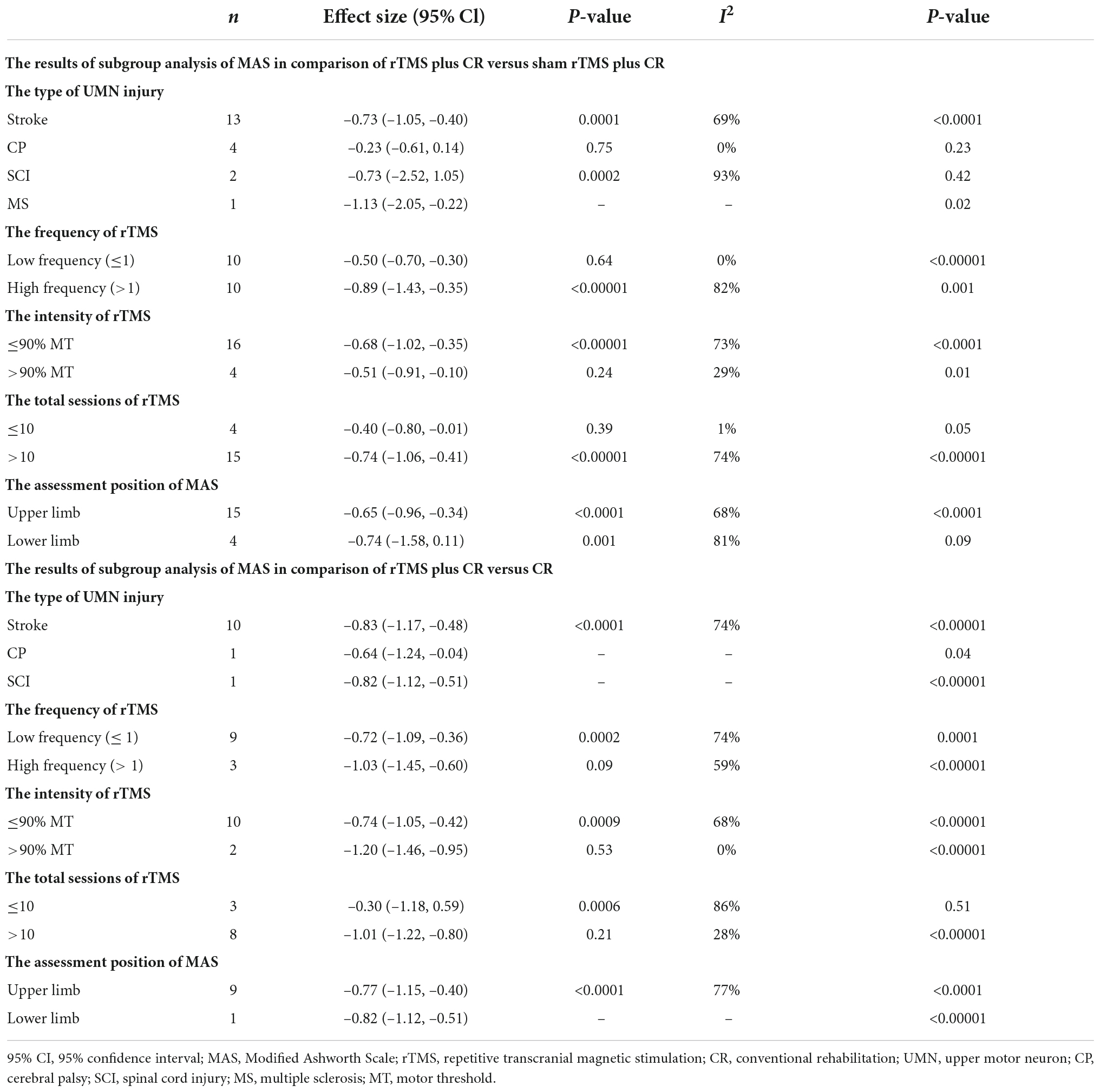
The results of subgroup analysis of MAS scores are presented in Table 2.
Repetitive transcranial magnetic stimulation plus conventional rehabilitation versus sham repetitive transcranial magnetic stimulation plus conventional rehabilitation
Compared with sham rTMS plus CR, rTMS plus CR was more effective in stroke and MS. Meanwhile, rTMS plus CR had better effect in upper limb. In the comparison of rTMS plus CR versus sham rTMS plus CR, rTMS with > 10 sessions decreased more MAS scores than rTMS ≤ 10 sessions.
Repetitive transcranial magnetic stimulation plus conventional rehabilitation versus conventional rehabilitation
Repetitive transcranial magnetic stimulation plus CR decreased more MAS scores than CR in spastic patients with stroke, SCI and CP. Moreover, rTMS with total sessions > 10 could decrease more MAS scores than rTMS with total sessions ≤ 10.
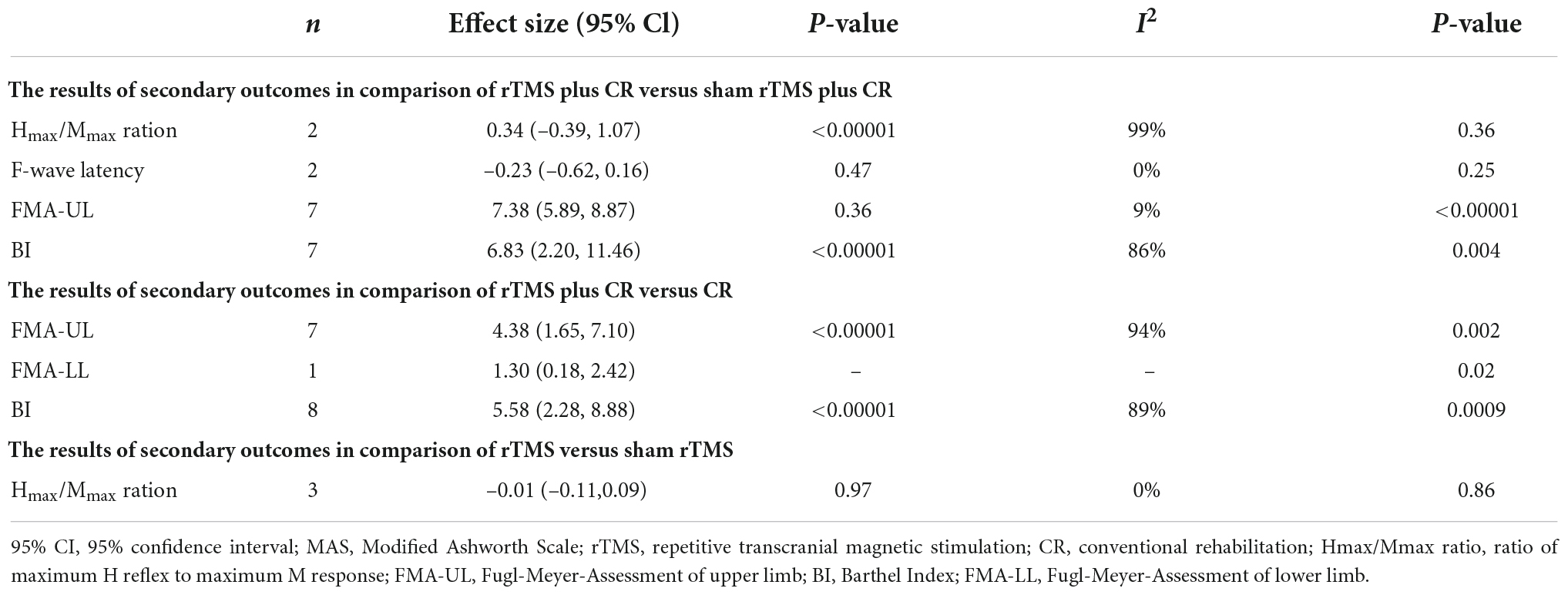
Secondary outcomes
As shown in Table 3, rTMS plus CR could increase more FMA scores and BI scores than control group. However, there was no difference between rTMS plus CR or rTMS group and control group in improving Hmax/Mmax ratio and F-wave latency.
There is only one study (Yang and Yang, 2022) reported that rTMS plus CR could effectively reduce HAMA and HAMD in contrast to the CR (P < 0.05).
Adverse events
Among forty-two included studies, eleven studies (Valle et al., 2007; Yan, 2015; Askin et al., 2017; Li, 2017; Kong, 2018; Tao and Wei, 2018; Dos Santos et al., 2019; Qi, 2020; Chen et al., 2021; Mendonca et al., 2021; Xu R. et al., 2021) reported that all patients could tolerate rTMS without complications, and no serious adverse effects were occurred. Seven studies (Kumru et al., 2010, 2016; Benito et al., 2012; Luo, 2020; Gottlieb et al., 2021; Xia et al., 2022a,b) described that 33 patients complained of twitching facial muscles, headache, pain in contralateral hand, dizziness, neck pain, and mild drowsiness after the rTMS treatment. The rest studies did not mention any adverse effects during rTMS treatment.
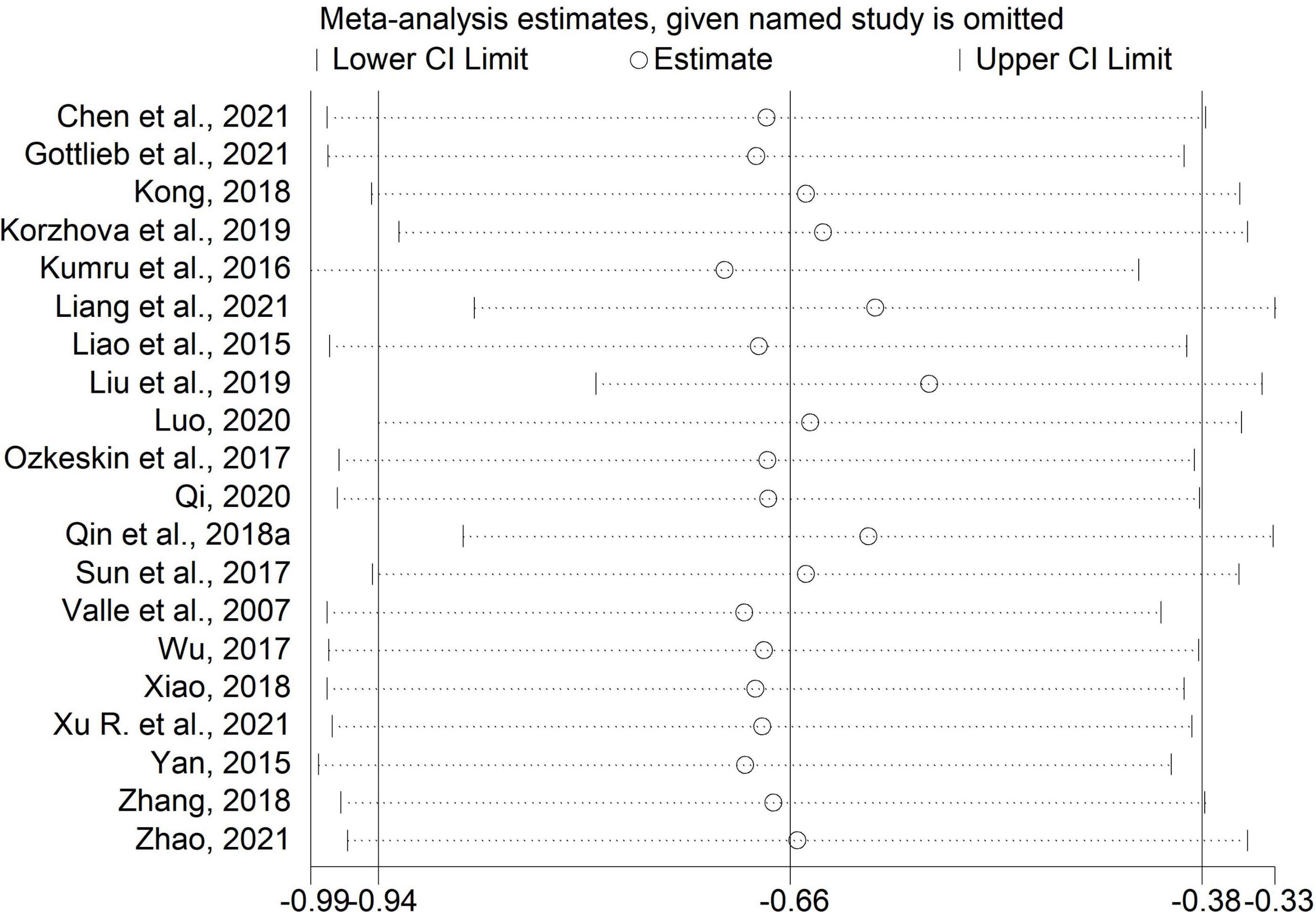
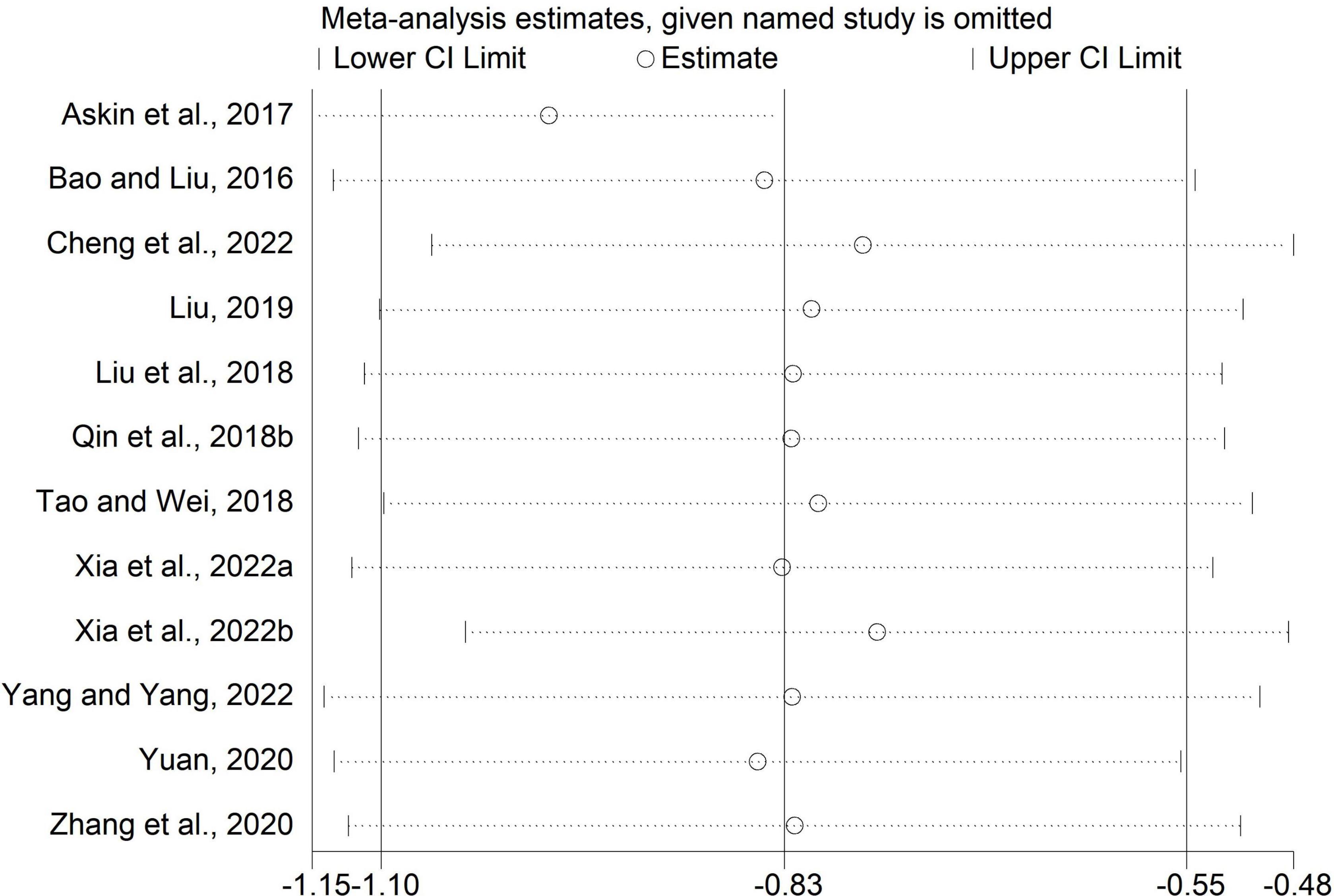
Sensitivity analysis
We performed sensitivity analysis by excluding one study each time. The results of the MAS scores in comparisons of rTMS plus CR versus sham rTMS plus CR and rTMS plus CR versus CR were unchanged (Figures 8, 9), which indicated these results were stable.
Certainty of evidence
The ICC for the independent assessments of each item in the GRADE ranged from 0.81 to 0.85, which indicated satisfactory reliability. The certainty of evidence of each outcome was considered very low. The downgraded certainty of evidence was mainly caused by high risk of bias, and inconsistency of results. The results are shown in Table 4.
Discussion
The effect of repetitive transcranial magnetic stimulation for spasticity
Repetitive transcranial magnetic stimulation combined with CR or rTMS alone could effectively decrease MAS scores in spastic patients after UMN injury. According to the results of subgroup analysis, rTMS plus CR was more effective than control group in patients with stroke, which was consistent with previous systematic reviews (Graef et al., 2016; McIntyre et al., 2018). The minimum clinically important difference (MCID) refers to the smallest clinical change which is significant to patients (Stratford et al., 1998). Chen et al. (2019) reported that the MCID of MAS in stroke patients, the MCID of MAS between 0.5 and 0.8 indicated moderate clinical effect, and the MCID greater than 0.8 meant high clinical effect. In our study, the SMD of MAS in stroke patients (rTMS plus CR versus sham rTMS plus CR) was 0.73, and the SMD of MAS (rTMS plus CR versus CR) was 0.83. These results demonstrated that rTMS plus CR had a moderate-to-high clinical effect to relieve spasticity in stroke patients.
With regard to other types of UMN injury, we found that the results of rTMS for SCI, CP were inconsistent in different comparisons. Moreover, there was only one study focusing on rTMS for MS. Due to limited studies and no MCID of MAS in patients with SCI, CP, MS, these results warranted further investigation.
Furthermore, it is reported that excitatory neurotransmitter and inhibitory neurotransmitter play an important role in the pathogenesis of spasticity (Liu et al., 2021). In mouse model of middle cerebral artery occlusion (MCAO), the concentration of excitatory neurotransmitter Glutamate (Glu) increased in the ischemic area of cerebral hippocampus (Qian et al., 2022). Sun et al. (2022) found that the expression of inhibitory neurotransmitter Gamma-aminobutyric acid (GABA) in mouse model of MCAO decreased in the brainstem. Currently, Poh et al. (2019) observed that the concentration of Glu in C57BL/6J mouse brain reduced after rTMS treatment. Peng et al. (2021) discovered that rTMS with low frequency was able to increase GABA level in the central nervous system. Therefore, we speculated that the anti-spastic effect of rTMS may be associated with the decrease of excitatory neurotransmitters and the increase of inhibitory neurotransmitters. However, the mechanism of rTMS for spasticity is still unclear and needs to be further studied.
The effect of repetitive transcranial magnetic stimulation for spasticity with different parameters
Different frequencies
The results of subgroup analysis demonstrated that rTMS with high or low frequency could alleviate spasticity after UMN injury. rTMS with high frequency (>1 HZ) can produce motor cortex excitation, whereas rTMS with low frequency (≤1 HZ) may induce motor cortex inhibition (Fitzgerald et al., 2006; Corti et al., 2012; Rossini et al., 2015). Fisicaro et al. reported that the affected hemisphere would produce a reduced inhibition on the unaffected hemisphere after stroke (Fisicaro et al., 2019). For stroke patients, rTMS with high-frequency stimulation on unaffected hemisphere or low-frequency stimulation on affected hemisphere may regulate the excitability of cerebral cortex, restore the inter-hemispheric excitation/inhibition balance, ameliorate spasticity, and enhance motor function (Takeuchi et al., 2005).
Three included studies reported that rTMS with high frequency was used to treat spastic patients with SCI, whereas the results were inconsistent. Quartarone et al. (2005) discovered that rTMS with high frequency could increase cortical excitability, while Mendonca et al. (2021) assumed that the increased cortical excitability induced by rTMS was not sufficient enough to influence the spasticity in SCI patients.
Three studies applied high frequency and two studies used low frequency to alleviate spasticity in patients with CP, while the results were contradictory. Furthermore, only one study investigated the effect of rTMS with high frequency for spasticity in MS patients. Therefore, more rigorous designed RCTs are needed to determine the effect of rTMS with different frequencies for spastic patients after UMN injury.
Different sessions
According to subgroup analysis, the rTMS > 10 sessions had better effect than ≤ 10 sessions in decreasing spasticity. Previous studies reported that rTMS with over 10 sessions could reduce more MAS scores in patients with SCI (Nardone et al., 2015), stroke, MS (Gunduz et al., 2014) and CP (Gupta et al., 2016). The spasticity was ameliorated with the increase sessions of rTMS. Whereas the dosage-effect relationship of rTMS stimulation for UMN injury remains to be explored.
Apart from the stimulation parameters mentioned above, the demographic factors (e.g., age, gender, disease duration) may have impact on the effect of rTMS. Todd et al. (2010) discovered that the effect of the 6 Hz rTMS was greater in young adults than in old individuals. Brihmat et al. (2022) concluded that young patients usually had greater potential for inducing plasticity changes in response to rTMS than elder participants. Hanlon and McCalley (2022) found that gender maybe a critical influencing factor on the effect of rTMS, and they inferred that the reason may be related with gender difference in gray matter density and gyrification, proximity of the brain to the scalp and cortical excitability. Furthermore, Fitzgerald et al. (2016) reported that the response to rTMS was greater in patients with shorter duration of illness. Future researches could focus on the influence of demographic factors on the effect of rTMS for spasticity in UMN injury.
The different assessment positions of the modified Ashworth scale
The present systematic review included 33 studies focusing on upper limb, and six studies on lower limb, the results demonstrated that compared with sham rTMS plus CR, rTMS plus CR was effective to alleviate spasticity of upper limb, while uneffective for lower limb. Lin et al. (2015) observed that most of studies investigated the effect of rTMS on motor dysfunction of upper extremity after stroke, but few studies paid attention to lower extremity. The reason maybe that the motor areas of lower limb is located in the deep inter-hemisphere fissure, and it is difficult for rTMS to deliver stimulation (Kakuda et al., 2013; Foerster et al., 2018).
The effect of repetitive transcranial magnetic stimulation for motor function and the activity of daily life
The results revealed that rTMS was effective to improve motor function and the activity of daily life. Li et al. (2022) reported that rTMS could dilate the cerebral blood vessels, increase the blood flow of brain tissue, and promote the regeneration of damaged axons, thus promoting the recovery of motor function (Sander et al., 1995; Wassermann and Lisanby, 2001). Previous studies also confirmed that rTMS could ameliorate muscle spasticity, improve motor function and the activity of daily life (Li D. et al., 2021; Kan et al., 2022).
The strength and limitations of this study
This is the latest systematic review and meta-analysis which focused on the effects of rTMS for UMN injury. Additionally, we conducted comprehensive search and assessed the risk of bias with ROB2.0. This systematic review and meta-analysis was conducted and reported strictly following the AMSTAR 2.0 and PRISMA 2020 statement guidelines. However, the present study has some limitations. First, MAS was used to evaluate spasticity among included studies, which is too subjective to accurately reflect the change of spasticity. Therefore, the objective indicators (e.g., Hmax/Mmax ratio, F-wave latency) of spasticity should be applied in future studies. Second, most of included studies did not comprehensively evaluate the effect of rTMS for spastic patients after UMN injury. Future studies should comprehensively assess the general health status, mood changes and quality of life of spastic patients after UMN injury. Third, owing to limited studies, we could not determine the optimal stimulation protocols of rTMS on spasticity after UMN injury (the optimal time of rTMS treatment, the optimal intensity, frequency, et al.). The optimal stimulation protocols of rTMS for spastic patients after UMN injury remain for further exploration. Last, there were comparisons of rTMS plus CR versus sham rTMS plus CR, rTMS plus CR versus CR, rTMS versus sham rTMS, and rTMS versus CR in this systematic review and meta-analysis, the researchers should pay attention to the effect of rTMS in contrast to other active interventions (tDCS, oral muscle relaxants, botulinum neurotoxin injections, et al.).
Conclusion
Repetitive transcranial magnetic stimulation could be recommended as an effective and safe therapy to relieve spasticity in patients with UMN injury. However, due to high heterogeneity and limited RCTs, this conclusion should be treated with caution. More rigorous designed RCTs are needed to determine the optimal protocol of rTMS for spastic patients after UMN injury.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JL, RJ, and ZZ conceived this study. All authors selected, extracted, assessed, and analyzed the data and revised the manuscript for intellectual content. JF, HF, and XX drafted the manuscript.
Funding
This study was supported by Sichuan Province Science and Technology Support Program (2014SZ0154), Sichuan Province Science and Technology Program (2019YFS0019), the Key Project of Sichuan Province Science and Technology (2020YFS0284), and National Natural Science Foundation of China (81873354).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncir.2022.973561/full#supplementary-material
References
Arroyo González, R. (2018). A review of the effects of baclofen and of THC:CBD oromucosal spray on spasticity-related walking impairment in multiple sclerosis. Expert Rev. Neurother. 18, 785–791. doi: 10.1080/14737175.2018.1510772
Askin, A., Tosun, A., and Demirdal, U. S. (2017). Effects of low-frequency repetitive transcranial magnetic stimulation on upper extremity motor recovery and functional outcomes in chronic stroke patients: A randomized controlled trial. Somatosens Mot. Res. 34, 102–107. doi: 10.1080/08990220.2017.1316254
Atkins, D., Best, D., Briss, P. A., Eccles, M., Falck-Ytter, Y., Flottorp, S., et al. (2004). Grading quality of evidence and strength of recommendations. BMJ 328:1490. doi: 10.1136/bmj.328.7454.1490
Balci, B. P. (2018). Spasticity measurement. Noro Psikiyatr Ars. 55(Suppl. 1), S49–S53. doi: 10.29399/npa.23339
Bao, N., and Liu, C. (2016). The effects of low frequency repetitive transcranial magnetic stimulation and occupational therapy on upper limbs function. Chin. Foreign Med. Res. 35, 5–7.
Benito, J., Kumru, H., Murillo, N., Costa, U., Medina, J., Tormos, J. M., et al. (2012). Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top. Spinal Cord Inj. Rehabil. 18, 106–112. doi: 10.1310/sci1802-106
Brihmat, N., Allexandre, D., Saleh, S., Zhong, J., Yue, G. H., and Forrest, G. F. (2022). Stimulation parameters used during repetitive transcranial magnetic stimulation for motor recovery and corticospinal excitability modulation in SCI: A scoping review. Front. Hum. Neurosci. 16:800349. doi: 10.3389/fnhum.2022.800349
Cantone, M., Lanza, G., Ranieri, F., Opie, G. M., and Terranova, C. (2021). Editorial: Non-invasive brain stimulation in the study and modulation of metaplasticity in neurological disorders. Front. Neurol. 12:721906. doi: 10.3389/fneur.2021.721906
Chen, C., Chen, C., Chen, H., Wu, C., Lin, K., Hsieh, Y. W., et al. (2019). Responsiveness and minimal clinically important difference of Modified Ashworth Scale in patients with stroke. Eur. J. Phys. Rehabil. Med. 55, 754–760. doi: 10.23736/S1973-9087.19.05545-X
Chen, C., Leys, D., and Esquenazi, A. (2013). The interaction between neuropsychological and motor deficits in patients after stroke. Neurology 80(3 Suppl. 2), S27–S34. doi: 10.1212/WNL.0b013e3182762569
Chen, Q., Huang, H., Chen, Z., and Ni, G. (2021). Effects of low- frequency repetitive transcranial magnetic stimulation combined with MOTOmed gracile on upper limb spasticity after stroke. Chin. J. Rehabil. Med. 36, 437–442.
Chen, Y., Zhang, C., Liu, Y., Magat, E., Verduzco-Gutierrez, M., Francisco, G. E., et al. (2020). The effects of botulinum toxin injections on spasticity and motor performance in chronic stroke with spastic hemiplegia. Toxins (Basel) 12:492. doi: 10.3390/toxins12080492
Cheng, R., Tang, H., Zhang, Y., and Chen, W. (2022). Effect of low frequency repetitive transcranial magnetic stimulation on patients with spastic dyskinesia after ischemic stroke. J. Beihua Univ. (Natural Science) 23, 79–83.
Chervyakov, A. V., Poydasheva, A. G., Lyukmanov, R. H., Suponeva, N. A., Chernikova, L. A., Piradov, M. A., et al. (2018). Effects of navigated repetitive transcranial magnetic stimulation after stroke. J. Clin. Neurophysiol. 35, 166–172. doi: 10.1097/WNP.0000000000000456
Corti, M., Patten, C., and Triggs, W. (2012). Repetitive transcranial magnetic stimulation of motor cortex after stroke: A focused review. Am. J. Phys. Med. Rehabil. 91, 254–270. doi: 10.1097/PHM.0b013e318228bf0c
Creamer, M., Cloud, G., Kossmehl, P., Yochelson, M., Francisco, G. E., Ward, A. B., et al. (2018). Effect of intrathecal baclofen on pain and quality of life in poststroke spasticity. Stroke 49, 2129–2137. doi: 10.1161/STROKEAHA.118.022255
de Araujo, A. V. L., Barbosa, V. R. N., Galdino, G. S., Fregni, F., Massetti, T., Fontes, S. L., et al. (2017). Effects of high-frequency transcranial magnetic stimulation on functional performance in individuals with incomplete spinal cord injury: study protocol for a randomized controlled trial. Trials 18:522. doi: 10.1186/s13063-017-2280-1
Dietz, V., and Sinkjaer, T. (2012). Spasticity. Handb. Clin. Neurol. 109, 197–211. doi: 10.1016/B978-0-444-52137-8.00012-7
Dos Santos, R. B. C., Galvao, S. C. B., Frederico, L. M. P., Amaral, N. S. L., Carneiro, M. I. S., de Moura, F. A. G., et al. (2019). Cortical and spinal excitability changes after repetitive transcranial magnetic stimulation combined to physiotherapy in stroke spastic patients. Neurol. Sci. 40, 1199–1207. doi: 10.1007/s10072-019-03765-y
Ertzgaard, P., Campo, C., and Calabrese, A. (2017). Efficacy and safety of oral baclofen in the management of spasticity: A rationale for intrathecal baclofen. J. Rehabil. Med. 49, 193–203. doi: 10.2340/16501977-2211
Feldman, R. G., Young, R. R., Koella, W. P., and Corporation, C. G. (1980). Spasticity: disordered motor control. Maryland Heights, MO: Year Book Medical Publishers.
Fisicaro, F., Lanza, G., Grasso, A. A., Pennisi, G., Bella, R., Paulus, W., et al. (2019). Repetitive transcranial magnetic stimulation in stroke rehabilitation: Review of the current evidence and pitfalls. Ther. Adv. Neurol. Disord. 12:1756286419878317. doi: 10.1177/1756286419878317
Fitzgerald, P. B., Fountain, S., and Daskalakis, Z. J. (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 117, 2584–2596. doi: 10.1016/j.clinph.2006.06.712
Fitzgerald, P. B., Hoy, K. E., Anderson, R. J., and Daskalakis, Z. J. (2016). A study of the pattern of response to rTMS treatment in depression. Depress Anxiety 33, 746–753. doi: 10.1002/da.22503
Foerster, A. S., Rezaee, Z., Paulus, W., Nitsche, M. A., and Dutta, A. (2018). Effects of cathode location and the size of anode on anodal transcranial direct current stimulation over the leg motor area in healthy humans. Front. Neurosci. 12:443. doi: 10.3389/fnins.2018.00443
Gao, Z., Niu, B., Gu, M., Li, Y., Liu, J., Wang, Y., et al. (2018). [Clinical effects of high frequency repeated transcranial magnetic stimulation therapy on dyskinesia in patients with incomplete spinal cord injury:a Meta-analysis]. Zhongguo Gu Shang 31, 47–55.
Gottlieb, A., Boltzmann, M., Schmidt, S. B., Gutenbrunner, C., Krauss, J. K., Stangel, M., et al. (2021). Treatment of upper limb spasticity with inhibitory repetitive transcranial magnetic stimulation: A randomized placebo-controlled trial. NeuroRehabilitation 49, 425–434. doi: 10.3233/nre-210088
Graef, P., Dadalt, M. L. R., Rodrigues, D., Stein, C., and Pagnussat, A. S. (2016). Transcranial magnetic stimulation combined with upper-limb training for improving function after stroke: A systematic review and meta-analysis. J. Neurol. Sci. 369, 149–158. doi: 10.1016/j.jns.2016.08.016
Grgic, J., Scapec, B., Mikulic, P., and Pedisic, Z. (2022). Test-retest reliability of isometric mid-thigh pull maximum strength assessment: a systematic review. Biol. Sport 39, 407–414. doi: 10.5114/biolsport.2022.106149
Gunduz, A., Kumru, H., and Pascual-Leone, A. (2014). Outcomes in spasticity after repetitive transcranial magnetic and transcranial direct current stimulations. Neural Regen. Res. 9, 712–718. doi: 10.4103/1673-5374.131574
Gupta, M., Lal, R. B., Bhatia, D., and Mukherjee, A. (2016). Effect of r-TMS over standard therapy in decreasing muscle tone of spastic cerebral palsy patients. J. Med. Eng. Technol. 40, 210–216. doi: 10.3109/03091902.2016.1161854
Guyatt, G. H., Oxman, A. D., Vist, G., Kunz, R., Brozek, J., Alonso-Coello, P., et al. (2011). GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J. Clin. Epidemiol. 64, 407–415. doi: 10.1016/j.jclinepi.2010.07.017
Hanlon, C. A., and McCalley, D. M. (2022). Sex/Gender as a factor that influences transcranial magnetic stimulation treatment outcome: three potential biological explanations. Front. Psychiatry 13:869070. doi: 10.3389/fpsyt.2022.869070
Harb, A., and Kishner, S. (2022). Modified ashworth scale. Treasure Island, FL: StatPearls Publishing.
Harriss, J., Roche, N., Cantu-Brito, C., Khatkova, S., Satero, P., Heitmann, S., et al. (2021). Spasticity in practice (SPACE): an international non-interventional study of botulinum neurotoxin type A in treatment-naive subjects with spasticity. Neurol. Neurochir. Pol. 55, 165–173. doi: 10.5603/PJNNS.a2021.0001
Hui, D., Zhukovsky, D. S., and Bruera, E. (2015). Which treatment is better? Ascertaining patient preferences with crossover randomized controlled trials. J. Pain Symptom Manage 49, 625–631. doi: 10.1016/j.jpainsymman.2014.11.294
Iglesias, A. H. (2020). Transcranial magnetic stimulation as treatment in multiple neurologic conditions. Curr. Neurol. Neurosci. Rep. 20:1. doi: 10.1007/s11910-020-1021-0
Kakuda, W., Abo, M., Nakayama, Y., Kiyama, A., and Yoshida, H. (2013). High-frequency rTMS using a double cone coil for gait disturbance. Acta Neurol. Scand. 128, 100–106. doi: 10.1111/ane.12085
Kan, R. L. D., Xu, G. X. J., Shu, K. T., Lai, F. H. Y., Kranz, G., and Kranz, G. S. (2022). Effects of non-invasive brain stimulation in multiple sclerosis: systematic review and meta-analysis. Ther. Adv. Chronic Dis. 13:20406223211069198. doi: 10.1177/20406223211069198
Kes, V. B., Cengic, L., Cesarik, M., Tomas, A. J., Zavoreo, I., Matovina, L. Z., et al. (2013). Quality of life in patients with multiple sclerosis. Acta Clin. Croat. 52, 107–111.
Kong, S. (2018). Clinical analysis of low frequency repetitive transcranial magnetic stimulation on treatment of upper extremity muscle spasm after ischemic stroke. Taichung: China Medical University.
Korzhova, J., Bakulin, I., Sinitsyn, D., Poydasheva, A., Suponeva, N., Zakharova, M., et al. (2019). High-frequency repetitive transcranial magnetic stimulation and intermittent theta-burst stimulation for spasticity management in secondary progressive multiple sclerosis. Eur. J. Neurol. 26, 680–e644. doi: 10.1111/ene.13877
Kumru, H., Benito, J., Murillo, N., Valls-Sole, J., Valles, M., Lopez-Blazquez, R., et al. (2013). Effects of high-frequency repetitive transcranial magnetic stimulation on motor and gait improvement in incomplete spinal cord injury patients. Neurorehabil. Neural Repair. 27, 421–429. doi: 10.1177/1545968312471901
Kumru, H., Benito-Penalva, J., Valls-Sole, J., Murillo, N., Tormos, J. M., Flores, C., et al. (2016). Placebo-controlled study of rTMS combined with Lokomat((R)) gait training for treatment in subjects with motor incomplete spinal cord injury. Exp. Brain Res. 234, 3447–3455. doi: 10.1007/s00221-016-4739-9
Kumru, H., Murillo, N., Samso, J. V., Valls-Sole, J., Edwards, D., Pelayo, R., et al. (2010). Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil. Neural Repair. 24, 435–441. doi: 10.1177/1545968309356095
Langhorne, P., Bermhardt, J., and Kwakkel, G. (2011). Stroke rehabilitation. Lancet 377, 1693–1702. doi: 10.1016/S0140-6736(11)60325-5
Li, J. (2017). Effectiveness of different frequencies of repetitive transcranial magnetic stimulation on the restoration of limbs motor dysfunction and degree of spasm in patients after cerebral infarction. Shandong: Shandong University.
Li, D., Cheng, A., Zhang, Z., Sun, Y., and Liu, Y. (2021). Effects of low-frequency repetitive transcranial magnetic stimulation combined with cerebellar continuous theta burst stimulation on spasticity and limb dyskinesia in patients with stroke. BMC Neurol. 21:369. doi: 10.1186/s12883-021-02406-2
Li, S., Francisco, G. E., and Rymer, W. Z. (2021). A new definition of poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabil. Neural Repair. 35, 601–610. doi: 10.1177/15459683211011214
Li, X., Lu, T., Yu, H., Shen, J., Chen, Z., Yang, X., et al. (2022). Repetitive transcranial magnetic stimulation for neuropathic pain and neuropsychiatric symptoms in traumatic brain injury: a systematic review and meta-analysis. Neural Plast. 2022:2036736. doi: 10.1155/2022/2036736
Liang, W., Wu, M., and Li, X. (2021). Effects of repetitive transcranial magnetic stimulation on muscle spasm and function in incomplete spinal cord injury. Chin. Manipulation Rehabil. Med. 12, 28–30.
Liao, C., Xin, W., Liu, R., and Liu, Z. (2015). “Effects of repetitive transcranial magnetic stimulation combined with rehabilitation exercises on hypermyotonia after cerebral apoplexy and the corresponding surface electromyography alterations,” in Proceedings of the the 10th beijing international rehabilitation forum (Beijin: China Rehabilitation Research Center), 55–60.
Lin, Y. N., Hu, C. J., Chi, J. Y., Lin, L. F., Yen, T. H., Lin, Y. K., et al. (2015). Effects of repetitive transcranial magnetic stimulation of the unaffected hemisphere leg motor area in patients with subacute stroke and substantial leg impairment: A pilot study. J. Rehabil. Med. 47, 305–310. doi: 10.2340/16501977-1943
Liu, J., Zhao, G., Niu, Y., Gan, T., Yan, Z., and Zhang, Y. (2021). Effect of electro-acupuncture therapy on limb spasm and excitability of motor neurons in stroke rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 50, 361–368. doi: 10.3724/zdxbyxb-2021-0007
Liu, S. (2019). Clinical research of low-frequency repetitive transcranial magnetic stimulation in elbow flexor spasticity after stroke. Zhengzhou: Zhengzhou University.
Liu, Y., Wang, X., Zhang, C., and Huang, D. (2018). Effects of low-frequency repetitive transcranial magnetic stimulation on upper limb spasticity after stroke: a task-state functional magnetic resonance study. Chin. J. Rehabil. Theory Pract. 7, 828–833.
Liu, Y., Zhang, C., and Qin, Y. (2019). Therapeutic effect of high frequency rTMS on upper limb spasticity after stroke. Chin. Foreign Med. Res. 11, 11–13.
Luo, L. (2020). Effects of high frequency rTMS on upper limb spasm and PAD in subacute stroke. Tianjin: Tianjin University of Sport.
McIntyre, A., Mirkowski, M., Thompson, S., Burhan, A. M., Miller, T., and Teasell, R. (2018). A systematic review and meta-analysis on the use of repetitive transcranial magnetic stimulation for spasticity poststroke. PM R 10, 293–302. doi: 10.1016/j.pmrj.2017.10.001
Mendonca, T., Brito, R., Luna, P., Campelo, M., Shirahige, L., Fontes, L., et al. (2021). Repetitive transcranial magnetic stimulation on the modulation of cortical and spinal cord excitability in individuals with spinal cord injury. Restor. Neurol. Neurosci. 39, 291–301. doi: 10.3233/RNN-211167
Montgomery, A. A., Peters, T. J., and Little, P. (2003). Design, analysis and presentation of factorial randomised controlled trials. BMC Med. Res. Methodol. 3:26. doi: 10.1186/1471-2288-3-26
Nardone, R., Holler, Y., Brigo, F., Orioli, A., Tezzon, F., Schwenker, K., et al. (2015). Descending motor pathways and cortical physiology after spinal cord injury assessed by transcranial magnetic stimulation: a systematic review. Brain Res. 1619, 139–154. doi: 10.1016/j.brainres.2014.09.036
Nardone, R., Höller, Y., Thomschewski, A., Brigo, F., Orioli, A., Höller, P., et al. (2014). rTMS modulates reciprocal inhibition in patients with traumatic spinal cord injury. Spinal Cord 52, 831–835. doi: 10.1038/sc.2014.136
Nardone, R., Sebastianelli, L., Versace, V., Brigo, F., Golaszewski, S., Pucks-Faes, E., et al. (2020). Effects of repetitive transcranial magnetic stimulation in subjects with sleep disorders. Sleep Med. 71, 113–121. doi: 10.1016/j.sleep.2020.01.028
Naro, A., Leo, A., Russo, M., Casella, C., Buda, A., Crespantini, A., et al. (2017). Breakthroughs in the spasticity management: Are non-pharmacological treatments the future? J. Clin. Neurosci. 39, 16–27. doi: 10.1016/j.jocn.2017.02.044
Nowak, D. A., Grefkes, C., Ameli, M., and Fink, G. R. (2009). Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil. Neural Repair. 23, 641–656. doi: 10.1177/1545968309336661
Ozkeskin, M., Ozturk, V., Çakmur, R., Kara, B., and Küçük, F. (2017). The effects of navigated repetitive transcranial magnetic simulation and brunnstrom movement therapy on upper extremity proprioceptive sense and spasticity in stroke patients: A double-blind randomized trial. J. Basic Clin. Health Sci. 2, 29–35.
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160. doi: 10.1136/bmj.n160
Peng, Y., Lin, Y., Yu, N., Liao, X., and Shi, L. (2021). [The clinical efficacy and possible mechanism of combination treatment of cerebral ischemic stroke with ginkgo biloba extract and low-frequency repetitive transcranial magnetic stimulation]. Sichuan Da Xue Xue Bao Yi Xue Ban 52, 883–889. doi: 10.12182/20210960202
Poh, E. Z., Hahne, D., Moretti, J., Harvey, A. R., Clarke, M. W., and Rodger, J. (2019). Simultaneous quantification of dopamine, serotonin, their metabolites and amino acids by LC-MS/MS in mouse brain following repetitive transcranial magnetic stimulation. Neurochem. Int. 131:104546. doi: 10.1016/j.neuint.2019.104546
Posteraro, F., Crea, S., Mazzoleni, S., Berteanu, M., Ciobanu, I., Vitiello, N., et al. (2018). Technologically-advanced assessment of upper-limb spasticity: a pilot study. Eur. J. Phys. Rehabil. Med. 54, 536–544. doi: 10.23736/S1973-9087.17.04815-8
Pulgar, S., Bains, S., Gooch, J., Chambers, H., Noritz, G. H., Wright, E., et al. (2019). Prevalence, patterns, and cost of care for children with cerebral palsy enrolled in medicaid managed care. J. Manag. Care Spec. Pharm. 25, 817–822. doi: 10.18553/jmcp.2019.25.7.817
Qi, S. (2020). Effects Of Repetitive Transcranial Magnetic Stimulation Combined With Exercise Therapy On Gross Motor Function In Children With Spastic Cerebral Palsy. Hebei: Hebei Normal University.
Qian, X., Ma, L., Mu, J., Zhang, Z., Sun, T., Yu, W., et al. (2022). [Study on the central mechanism of acupuncture for post-stroke spasticity based on the Na(+)/K(+)-ATPase-EAATs-Glu pathway]. Zhen Ci Yan Jiu 47, 283–289. doi: 10.13702/j.1000-0607.20210922
Qin, Y., Huang, D., Kang, G., and Liu, Y. (2018a). Effect observation on repetitive transcranial magnetic stimulation at scalp acupoints on spastic hemiplegia of upper extremity after stroke. Rehabil. Med. 6, 21–25.
Qin, Y., Liu, Y., Guo, X., and Zhang, C. (2018b). A comparative study of high and low frequency repetitive transcranial magnetic stimulation in treatment of upper limb spasticity after stroke. Chin. J. Stroke 6, 550–555.
Quartarone, A., Bagnato, S., Rizzo, V., Morgante, F., Sant’angelo, A., Battaglia, F., et al. (2005). Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp. Brain Res. 161, 114–124. doi: 10.1007/s00221-004-2052-5
Rajak, B. L., Gupta, M., Bhatia, D., and Mukherjee, A. (2019). Increasing number of therapy sessions of repetitive transcranial magnetic stimulation improves motor development by reducing muscle spasticity in cerebral palsy children. Ann. Indian Acad. Neurol. 22, 302–307. doi: 10.4103/aian.AIAN_102_18
Rastgoo, M., Naghdi, S., Nakhostin, A. N., Olyaei, G., Jalaei, S., Forogh, B., et al. (2016). Effects of repetitive transcranial magnetic stimulation on lower extremity spasticity and motor function in stroke patients. Disabil. Rehabil. 38, 1918–1926. doi: 10.3109/09638288.2015.1107780
Ribeiro, D. C., Milosavljevic, S., and Abbott, J. H. (2018). Sample size estimation for cluster randomized controlled trials. Musculoskelet Sci. Pract. 34, 108–111. doi: 10.1016/j.msksp.2017.10.002
Rossini, P. M., Burke, D., Chen, R., Cohen, L. G., Daskalakis, Z., Di Iorio, R., et al. (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 126, 1071–1107. doi: 10.1016/j.clinph.2015.02.001
Roth, B. J., Saypol, J. M., Hallett, M., and Cohen, L. G. (1991). A theoretical calculation of the electric field induced in the cortex during magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 81, 47–56. doi: 10.1016/0168-5597(91)90103-5
San, A. U., Yilmaz, B., and Kesikburun, S. (2019). The effect of repetitive transcranial magnetic stimulation on spasticity in patients with multiple sclerosis. J. Clin. Neurol. 15, 461–467. doi: 10.3988/jcn.2019.15.4.461
Sander, D., Meyer, B. U., Roricht, S., and Klingelhofer, J. (1995). Effect of hemisphere-selective repetitive magnetic brain stimulation on middle cerebral artery blood flow velocity. Electroencephalogr. Clin. Neurophysiol. 97, 43–48. doi: 10.1016/0924-980x(94)00247-5
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008. doi: 10.1136/bmj.j4008
Sommerfeld, D. K., Gripenstedt, U., and Welmer, A. K. (2012). Spasticity after stroke: an overview of prevalence, test instruments, and treatments. Am. J. Phys. Med. Rehabil. 91, 814–820. doi: 10.1097/PHM.0b013e31825f13a3
Stratford, P. W., Binkley, J. M., Riddle, D. L., and Guyatt, G. H. (1998). Sensitivity to change of the Roland-Morris back pain questionnaire: part 1. Phys. Ther. 78, 1186–1196. doi: 10.1093/ptj/78.11.1186
Strom, V., Manum, G., Arora, M., Joseph, C., Kyriakides, A., Le Fort, M., et al. (2022). Physical health conditions in persons with spinal cord injury across 21 countries worldwide. J. Rehabil. Med. 54:jrm00302. doi: 10.2340/jrm.v54.2040
Sun, T., Ma, L., Mu, J., Zhang, Z., Yu, W., Qian, X., et al. (2022). Acupuncture improves the structure of spastic muscle and decreases spasticity by enhancing GABA, KCC2, and GABAAgamma2 in the brainstem in rats after ischemic stroke. Neuroreport 33, 399–407. doi: 10.1097/WNR.0000000000001798
Sun, W., Zhao, C., Mou, X., and Liu, W. (2017). Clinical study of low frequency repetitive transcranial magnetic stimulation in the treatment of upper limb spasm in patients with stroke. Chin. J. Rehabil. 2, 102–105.
Takeuchi, N., Chuma, T., Matsuo, Y., Watanabe, I., and Ikoma, K. (2005). Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke 36, 2681–2686. doi: 10.1161/01.STR.0000189658.51972.34
Tao, J., and Wei, Y. (2018). Long term efficacy and safety of repetitive transcranial magnetic stimulation combined with repeated injection of botulinum toxin type A in the treatment of spasticity of lower limb muscles spasm after stroke. J. Brain Nervous Dis. 05, 272–276.
Todd, G., Kimber, T. E., Ridding, M. C., and Semmler, J. G. (2010). Reduced motor cortex plasticity following inhibitory rTMS in older adults. Clin. Neurophysiol. 121, 441–447. doi: 10.1016/j.clinph.2009.11.089
Turner-Stokes, L., Pick, A., Nair, A., Disler, P. B., and Wade, D. T. (2015). Multi-disciplinary rehabilitation for acquired brain injury in adults of working age. Cochrane Datab. Syst. Rev. 12:CD004170. doi: 10.1002/14651858.CD004170.pub3
Ulbrich-Zurni, S., Teut, M., Roll, S., and Mathie, R. T. (2018). The N-of-1 clinical trial: a timely research opportunity in homeopathy. Homeopathy 107, 10–18. doi: 10.1055/s-0037-1621731
Valle, A. C., Dionisio, K., Pitskel, N. B., Pascual-Leone, A., Orsati, F., Ferreira, M. J., et al. (2007). Low and high frequency repetitive transcranial magnetic stimulation for the treatment of spasticity. Dev. Med. Child Neurol. 49, 534–538. doi: 10.1111/j.1469-8749.2007.00534.x
Wang, X., Ge, L., Hu, H., Yan, L., and Li, L. (2022). Effects of non-invasive brain stimulation on post-stroke spasticity: A systematic review and meta-analysis of randomized controlled trials. Brain Sci. 12:836. doi: 10.3390/brainsci12070836
Ward, A. B. (2012). A literature review of the pathophysiology and onset of post-stroke spasticity. Eur. J. Neurol. 19, 21–27.
Wassermann, E. M., and Lisanby, S. H. (2001). Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin. Neurophysiol. 112, 1367–1377. doi: 10.1016/s1388-2457(01)00585-5
Watanabe, K., Kudo, Y., Sugawara, E., Nakamizo, T., Amari, K., Takahashi, K., et al. (2018). Comparative study of ipsilesional and contralesional repetitive transcranial magnetic stimulations for acute infarction. J. Neurol. Sci. 384, 10–14. doi: 10.1016/j.jns.2017.11.001
Wu, H. (2017). Clinical Observation and Longitudinal fMRI Study of Transcranial Magnetic Stimulation in the Treatment of Upper Limb Motor Dysfunction Caused by Spasticity After Stroke. Fujian: Fujian University of Traditional Chinese Medicine.
Xia, J., Chen, M., Lin, M., and Hao, Y. (2022a). Effect of high frequency and low frequency repetitive transcranial magnetic stimulation in the treatment of post-stroke spasticity: A comparative study. Clin. Focus 37, 427–430.
Xia, J., Hao, Y., Chen, M., and Shao, Y. (2022b). Clinical study of high frequency repetitive transcranial magnetic stimulation combined with peripheral magnetic stimulation in the treatment of post-stroke muscle spasm. Neural Injury Funct. Reconst. 17, 478–481. doi: 10.16780/j.cnki.sjssgncj.20210054
Xiao, C. (2018). Effectiveness of different high-frequency rTMS on upper-limbs motor function and ADL in patients with cerebral infarction. Guangzhou: Guangzhou Medical University.
Xu, P., Huang, Y., Wang, J., An, X., Zhang, T., Li, Y., et al. (2021). Repetitive transcranial magnetic stimulation as an alternative therapy for stroke with spasticity: a systematic review and meta-analysis. J. Neurol. 268, 4013–4022. doi: 10.1007/s00415-020-10058-4
Xu, R., Zhu, G., Wang, Y., Sun, T., and Xu, D. (2021). The effect of peripheral magnetic stimulation combined with transcranial magnetic stimulation on upper limb spasm after stroke. Chin. J. Rehabil. Med. 36, 943–948.
Yan, X. (2015). Effectiveness of repetitive transcranial magnetic stimulation on hand function and muscle recruitment in children with hemiplegic cerebral palsy: A randomized controlled trial. Guangzhou: Guangzhou Medical University.
Yang, X. (2021). Study on The Brain Network Mechanism of Low-frequency rTMS in The Treatment of Spastic Hemiplegia after Stroke Based on Resting State fMRI. FuJian: FuJian University of Traditional Chinese Medicine.
Yang, X., and Yang, W. (2022). Application value of repetitive transcranial magnetic stimulation in rehabilitation of incomplete spinal cord injury. Chin. J. Spine Spinal Cord 32.
Yang, Z. R., Sun, F., and Zhan, S. Y. (2017). [Risk on bias assessment: (2) Revised Cochrane risk of bias tool for individually randomized, parallel group trials (RoB2.0)]. Zhonghua Liu Xing Bing Xue Za Zhi 38, 1285–1291. doi: 10.3760/cma.j.issn.0254-6450.2017.09.028
Yelnik, A. P., Simon, O., Bensmail, D., Chaleat-Valayer, E., Decq, P., Dehail, P., et al. (2009). Drug treatments for spasticity. Ann. Phys. Rehabil. Med. 52, 746–756. doi: 10.1016/j.rehab.2009.09.005
Yuan, M. (2020). Multi-modal MRI study of low-frequency repetitive transcranial magnetic stimulation in the treatment of post-stroke spasticity. Fujian: Fujian University of Traditional Chinese Medicine.
Zang, Y., Kou, X., Li, B., and Yan, X. (2022). Effects of repetitive transcranial magnetic stimulation combined with knowledge transfer mode on gross motor function and ankle range of motion in children with cerebral palsy. Chin. J. Convalescent Med. 31, 620–622. doi: 10.13517/j.cnki.ccm.2022.06.018
Zhang, L., He, L., and Dan, L. (2020). Observation on the changes of cortex and spinal cord excitability in patients with upper extremity spastic paralysis of stroke after transcranial magnetic stimulation combined with physical therapy. Guizhou Med. J. 44, 91–92.
Zhang, Y. (2018). Effects of different frequency repetitive transcranial magnetic stimulation on motor function in children with spastic hemiplegia cerebral palsy. Jiamusi: Jiamusi University.
Keywords: upper motor neuron injury, repetitive transcranial magnetic stimulation, spasticity, systematic review, meta-analysis
Citation: Fan J, Fu H, Xie X, Zhong D, Li Y, Liu X, Zhang H, Zhang J, Huang J, Li J, Jin R and Zheng Z (2022) The effectiveness and safety of repetitive transcranial magnetic stimulation on spasticity after upper motor neuron injury: A systematic review and meta-analysis. Front. Neural Circuits 16:973561. doi: 10.3389/fncir.2022.973561
Received: 20 June 2022; Accepted: 17 October 2022;
Published: 08 November 2022.
Edited by:
Oscar Arias-Carrion, Hospital General Dr. Manuel Gea González, MexicoReviewed by:
Ying Shen, The First Affiliated Hospital of Nanjing Medical University, ChinaMariagiovanna Cantone, Gaspare Rodolico Hospital, Italy
Copyright © 2022 Fan, Fu, Xie, Zhong, Li, Liu, Zhang, Zhang, Huang, Li, Jin and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongjiang Jin, cdzyydxjrj@126.com; Juan Li, 785939016@qq.com; Zhong Zheng, 345244784@qq.com
†These authors have contributed equally to this work
 Jin Fan
Jin Fan Hui Fu3†
Hui Fu3†  Dongling Zhong
Dongling Zhong Yuxi Li
Yuxi Li Xiaobo Liu
Xiaobo Liu Jun Zhang
Jun Zhang Jiaxi Huang
Jiaxi Huang Juan Li
Juan Li Rongjiang Jin
Rongjiang Jin