Weighting of Celestial and Terrestrial Cues in the Monarch Butterfly Central Complex
- 1Biocenter, Zoology II, University of Wuerzburg, Würzburg, Germany
- 2Department of Biology and Center for Biological Clocks Research, Texas A&M University, College Station, TX, United States
- 3Department of Biology, Animal Physiology, Norwegian University of Science and Technology, Trondheim, Norway
Monarch butterflies rely on external cues for orientation during their annual long-distance migration from Northern US and Canada to Central Mexico. These external cues can be celestial cues, such as the sun or polarized light, which are processed in a brain region termed the central complex (CX). Previous research typically focused on how individual simulated celestial cues are encoded in the butterfly's CX. However, in nature, the butterflies perceive several celestial cues at the same time and need to integrate them to effectively use the compound of all cues for orientation. In addition, a recent behavioral study revealed that monarch butterflies can rely on terrestrial cues, such as the panoramic skyline, for orientation and use them in combination with the sun to maintain a directed flight course. How the CX encodes a combination of celestial and terrestrial cues and how they are weighted in the butterfly's CX is still unknown. Here, we examined how input neurons of the CX, termed TL neurons, combine celestial and terrestrial information. While recording intracellularly from the neurons, we presented a sun stimulus and polarized light to the butterflies as well as a simulated sun and a panoramic scene simultaneously. Our results show that celestial cues are integrated linearly in these cells, while the combination of the sun and a panoramic skyline did not always follow a linear integration of action potential rates. Interestingly, while the sun and polarized light were invariantly weighted between individual neurons, the sun stimulus and panoramic skyline were dynamically weighted when both stimuli were simultaneously presented. Taken together, this dynamic weighting between celestial and terrestrial cues may allow the butterflies to flexibly set their cue preference during navigation.
Introduction
Spatial orientation has been investigated behaviorally in many insects, ranging from desert ants (Wehner, 2003; Wehner and Müller, 2006), honeybees (Brines and Gould, 1979; Edrich et al., 1979; Rossel and Wehner, 1984), dung beetles (el Jundi et al., 2019; Dacke et al., 2021), and locusts (Homberg, 2015), to moths (Dreyer et al., 2018a,b). This also includes the monarch butterfly (Danaus plexippus), which covers a distance of about 4,000 kilometers on its annual migration to its overwintering spots in Central Mexico (Merlin et al., 2012; Merlin and Liedvogel, 2019). During this long-distance migration, the butterflies use the sun as their main orientation reference (Stalleicken et al., 2005). To successfully maintain their southerly direction over the course of a day, the butterflies integrate time information from the antennae (Merlin et al., 2009; Guerra et al., 2012) and the brain (Sauman et al., 2005) into their sun compass. In addition to the sun, monarch butterflies may also rely on the polarization pattern of the sky for orientation (Reppert et al., 2004). While the pattern of polarized light is perceived by a specialized dorsal region of the monarch butterfly eye, termed the dorsal rim area, the sun is detected by eye regions outside of the dorsal rim area (Sauman et al., 2005; Stalleicken et al., 2006). Celestial information is then transferred via the optic lobe and anterior optic tubercle to input neurons of the central complex (CX), termed tangential (TL) neurons (Heinze and Reppert, 2011; Nguyen et al., 2021). These neurons transfer celestial information from the bulb of the lateral complex to the central complex lower division in many insects (Held et al., 2016; el Jundi et al., 2018; Hensgen et al., 2020; Rother et al., 2021), including monarch butterflies (Figures 1A,B). As shown for other insects (Stone et al., 2017; Hardcastle et al., 2021), TL cells (in fruit flies termed ring neurons) synapse onto a network of heading-direction cells that flexibly encode the actual flight direction of an animal based on sensory-motor information (Seelig and Jayaraman, 2015; Green et al., 2017; Turner-Evans et al., 2017; Fisher et al., 2019; Kim et al., 2019; Okubo et al., 2020; Hulse et al., 2021). While previous research focused on how the CX processes single celestial stimuli in the monarch butterfly brain (Heinze and Reppert, 2011; Nguyen et al., 2021; Beetz et al., 2022), the controlled single cue conditions in the lab rarely reflect the compound cue conditions found in nature. Thus, to obtain a highly robust compass network, multiple visual cues, such as the sun and polarized light are integrated simultaneously in nature (el Jundi et al., 2014; Lebhardt and Ronacher, 2014). Moreover, experiments on tethered flying monarch butterflies suggest that the butterflies combine a sun stimulus and a panoramic skyline to keep a directed flight heading (Franzke et al., 2020), similar to what has been reported for Australian bull ants (Reid et al., 2011) and honeybees (Towne and Moscrip, 2008; Towne et al., 2017). But how visual sceneries composed of multiple stimuli, such as the sun and polarized light or the sun and a panoramic scene, are combined and how each of the cues is weighted neuronally has not been investigated in the monarch butterfly brain so far. To study this, we recorded intracellularly from TL cells in the monarch butterfly brain and analyzed how they respond to simultaneously presented stimuli, such as a simulated sun and polarized-light stimulus as well as a sun and panoramic-skyline stimulus.
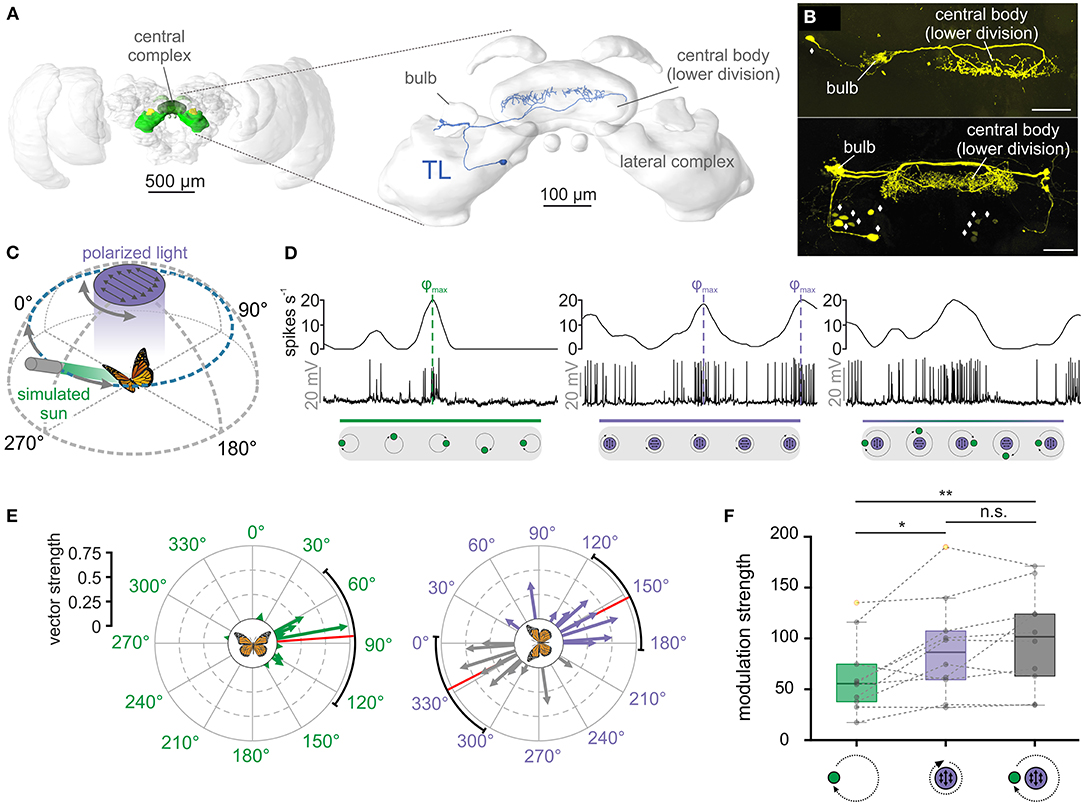
Figure 1. TL neurons of the central complex encode simulated celestial cues. (A) Left: Frontal view of the monarch butterfly brain. Highlighted in green are the neuropils of the central complex and lateral complex. Right: The central complex and lateral complex. A reconstructed tangential neuron (TL) is shown in blue. Modified from Heinze et al. (2013). (B) Two examples of TL neuron tracings (anterior views) during electrophysiological recordings (maximum intensity projection views). While single cell tracings of one TL cell was possible (top, synaptic input in the right bulb), in most experiments, several TL neurons from both hemispheres were labeled (bottom). Diamonds indicate the position of TL neuron somata. Scale bars: 50 μm. (C) Schematic illustration of the presented simulated celestial cues. The polarization stimulus was positioned dorsally to the butterfly and was rotated by 360°. The angle of polarization was aligned with the antero-posterior axis of the animal at the beginning of the rotation. The sun stimulus (elevation: 30°) was moved on a circular path around the animal. The angle of polarized light was oriented perpendicular to the direction of the sun stimulus, simulating their spatial relationship in nature. (D) Neural tuning of the same TL neuron to a moving sun stimulus (left), a rotating polarizer (middle) and when both stimuli were presented simultaneously (right). The upper curve of each plot shows the sliding window average of the action potential recordings (middle row). The lower gray boxes illustrate the position of the stimuli during a clockwise 360°-rotation. Preferred firing directions (φmax) to the sun (left) and polarized light (middle) are indicated by dashed vertical lines. (E) The preferred firing directions of the tested TL neurons (n = 10) in response to the sun stimulus (left) and the polarization stimulus (right). Each arrow represents a single neuron. Arrow length indicates the vector strength (directedness) of the neural tuning. The circular plots are labeled in relation to the animals' body axis (see schematic at the plot's center), with 0° being anterior, 90° being right, and 270° being left to the animal. The mean preferred firing directions (sun stimulus: 85.88° ± 54.94°; polarized light: 152.96° ± 34.16°) are indicated by the red solid lines and the confidence intervals (95%) by the black arcs. (F) Modulation strength of neural activity (n = 10) in response to the sun stimulus (left), polarized light (middle), and the combination of both celestial cues (right). The neural modulation to the sun stimulus was significantly weaker than to polarized light (pGREENvs.POL = 0.02, t = 2.99, n = 10; paired t-test) and the combination of the stimuli (pGREENvs.COMBO = 0.002, t = −4.38, n = 10; paired t-test), while the modulation strength to polarized light and the combined stimuli did not differ from each other (pPOLvs.COMBO = 0.23, t = −1.28; paired t-test). Gray circles show individual data points. Outliers are indicated in yellow. Dashed gray lines connect individual data points from the same TL neuron. Boxes indicate interquartile range. Whiskers extend to the 2.5th and 97.5th percentiles. Black horizontal lines show the median. n.s.: not significant, *p < 0.05, **p < 0.01.
Materials and Methods
Animals
Adult monarch butterflies (Danaus plexippus) of both sexes were kept in an incubator (I-30VL, Percival Scientific, Perry, IA, USA) with a 12:12 h light-dark cycle at 25°C in Würzburg (Germany). They were provided with 15% sugar solution ad libitum. Some animals were caught at College Station, TX, USA during their annual southward migration. These animals were kept in the incubator at an 11:13 h light-dark cycle at 23°C during light and 12°C during dark phases. They were fed with 20% honey solution every second day.
Preparation and Electrophysiology
After clipping off wings and legs, the butterflies were attached to a custom-built holder using dental wax (Omnident, Rodgau Nieder-Roden, Germany). The head capsule was opened frontally and muscle and fat tissue above the brain were removed. At least one of the antennae remained intact to avoid a disruption of circadian inputs to the compass network (Merlin et al., 2009; Guerra et al., 2012). To access the central brain with the electrode, the neural sheath was removed using fine tweezers. Throughout preparation and subsequent neuronal recording, the brain was immersed in monarch ringer (150 mM NaCl, 3 mM KCl, 10 mM TES, 25 mM sucrose, 3 mM CaCl2).
To record intracellularly from individual TL neurons, micropipettes were drawn from borosilicate glass capillaries (inner diameter: 0.75 mm and outer diameter: 1.5 mm, Hilgenberg, Malsfeld, Germany) using a Flaming/Brown horizontal puller (P-97, Sutter Instrument Company, Novato, CA, USA). After loading the micropipette with 4% Neurobiotin (Vector Laboratories, Burlingame, UK, dissolved in 1 M KCl), it was filled with a 1 M KCl solution. The micropipette was connected to an electrode holder with a chloridized silver wire, which was attached to a micromanipulator (Leica Microsystems, Wetzlar, Germany). Another chloridized silver wire served as reference electrode and was inserted into the opened head capsule close the butterfly's mouthparts. Detected signals were amplified 10x using a BA-03X bridge amplifier (npi Elelctronic GmbH, Tamm, Germany). The signal was digitized with sampling rates between 1-20 kHz using a digitizer (Power1401, Cambridge Electronic Design, Cambridge, UK). The neuronal activity was observed on a computer using the software Spike 2 (version 9.00, Cambridge Electronic Design). To obtain recordings from TL neurons, we targeted their output regions in the central complex. All recordings were thus likely obtained from the neurons' axons that enter the central body lower division anteriorly (Figure 1A).
Celestial Stimuli
To simulate celestial cues, the same stimulus was used as described in Nguyen et al. (2021). A rotation stage (DT-50, PI miCos GmbH, Karlsruhe, Germany) was dorsally positioned to the animals. For the polarized UV light stimulus, a polarizer was mounted on top of the rotation stage. Because monarch butterflies detect polarized light in the UV range (Sauman et al., 2005; Stalleicken et al., 2005) a UV-LED with an emission peak at 365 nm (LZ1-10UV00-0000, OSRAM Sylvania Inc., Wilmington, MA, US) and a quarter white diffuser (Nr. 251, LEE filters, Hampshire, UK) were placed behind a UV permeable linear polarizer (BVO UV, Bolder Vision Optik Inc., Boulder, CO, USA) in the center of the rotation stage. This allowed us to present equally illuminated polarized UV light to the butterflies. The sun stimulus was presented using an unpolarized green LED with an emission peak at 517 nm (LZ1-10G102-0000, OSRAM, Munich, Germany). This LED was mounted on one of four arms extending from the rotation stage. To control for the influence of wavelength information, a UV LED was attached to the arm opposite to the green LED. Both light spots were adjusted to an elevation of 30° relative to the animal's head and provided unpolarized light. The angle of the zenithal polarization filter was aligned perpendicular to the two LED arms, which allowed to present the celestial cues in the spatial relationship found in nature. All light stimuli (unpolarized green/UV light, polarized UV light) were adjusted to a photon flux of about 1.4 × 1014 photons/cm2/s, measured with a spectrometer (Maya2000 Pro, Ocean Optics) at the position where the animal faced the stimuli during recordings. Since the recordings were obtained via two setups with identical equipment, the angular positioning of the stimulus varied slightly. The polarization stimulus had an angular extent between 9.6°-10.4° at the butterfly's eye. The angular size of the unpolarized light spots was 1.3°-1.4°. The movements of the rotation stage were controlled via a custom-written script for the software MATLAB (Version R2019b, MathWorks, Natick, MA, USA). During the experiments, the rotation stage was turned by 360° in clock- and counterclockwise direction at a constant velocity of 60°/s while testing the response of the TL cells to a single stimulus (sun stimulus or polarized light) or a combination of the stimuli (sun stimulus and polarized light). As five of the TL neurons were obtained from migratory butterflies, we first tested if they differed in their general tuning characteristics from the recordings obtained in non-migratory butterflies. However, we did not find any differences in the relevant response characteristics tested here between both groups and decided to pool the data. As we often co-labeled several TL neurons from both brain hemispheres, we were unable to define for each recording from which brain hemisphere it was obtained. However, in six out of the ten celestial-cue experiments, TL neurons with synaptic input in the right bulb were either solely stained or showed a stronger staining (Figure 1B). In the remaining four experiments, TL neurons with inputs in the left bulb were either stained stronger (two experiments) or showed the same staining strength to TL neurons in the right brain hemisphere (two experiments).
Panoramic Skyline and Sun Stimuli
The panoramic skyline was simulated via an LED arena consisting of a circular array of 128x16 RGB-LEDs (M160256CA3SA1, iPixel LED Light Co., Ltd, Baoan Shenzhen, China). The arena covered a visual field of 360° along the horizontal and 43° along the vertical plane around the animal. The LEDs were controlled via a Raspberry Pi (Model 3B, Raspberry Pi Foundation, Cambridge, UK). We presented the same panoramic skyline to the butterflies that has been used in recent behavioral experiments on monarch butterflies (Franzke et al., 2020). Each LED above the horizon was adjusted to a photon flux of about 6.68 × 1010 photons/cm2/s in the blue range (emission peak: 458 nm). LEDs below the horizon were turned off. The panoramic scene was uploaded as an RGB image 8 bits/channel) to the Raspberry Pi and a custom-written program written in Go controlled the rotation movements of the stimulus. To avoid any dark adaption of the animals' eyes or history-dependent effects, a panorama with a flat horizon (flat panorama) was presented to the animals while searching for TL neurons. As soon as a TL neuron was successfully targeted, the panoramic scenery with the variable height profile (panoramic skyline) was used as a test stimulus. Light intensity differences between the panoramic scenery and the flat panorama were minimized by turning on a similar number of LEDs in both panoramas. To find TL neurons during our experiments, we first stimulated the animal with zenithal polarized light. Once a neuron responded to polarized light, the polarization stimulus was turned off and the panoramic skyline was presented and rotated by 360° around the animal in clock- and counterclockwise direction (at a constant velocity of 60°/s). To combine the panoramic scene with a sun stimulus, one LED above the horizon at an angular elevation of 18.9° was switched to a wavelength emission peak of about 516 nm and an intensity of about 6.14 × 1012 photons/cm2/s. We combined the sun stimulus either with the flat panorama or with the panoramic skyline. For the former, we moved the sun stimulus around the animal, while the flat panorama stayed stationary. For the latter, we rotated both stimuli around the animal.
Histology and Imaging
To evaluate anatomically the neuron type from which we recorded, Neurobiotin was iontophoretically injected into the cells (1-3.5 nA) for 3-5 min at the end of each experiment. After allowing the Neurobiotin to distribute for 20 min, the brains were dissected out of the head capsule and fixated for 18-24 h at 4°C in a sodium-phosphate buffer containing 4% paraformaldehyde, 0.2% picric acid, and 0.25% glutaraldehyde. They were then rinsed 4 × 15 min in 0.1 M phosphate buffered saline (PBS) and, afterwards, incubated with either Cy3-conjugated to streptavidin (Thermo Fisher Scientific, Waltham, MA USA, 1:1000) or Alexa568-conjugated to streptavidin (Molecular Probes, Eugene, OR, USA, 1:1000) diluted in PBS containing 0.3% Triton-X 100 (PBT) for 3 days at 4°C. The brains were then rinsed with PBT (3 × 20 min) and afterwards with PBS (2 × 20 min), before they were dehydrated through an ascending ethanol series (30, 50, 70, 90, 95, and 100%; 15 min each). Afterwards, the brains were immersed in a 1:1-mixture of ethanol and methyl salicylate (Fisher Scientific GmbH, Schwerte, Germany) for 20 min and then in 100% methyl salicylate for about 1 h at room temperature. The brains were then mounted in Permount (Fisher Scientific) between two cover slips with 10 reinforcement rings (Avery, Toronto, Canada) as spacers. Finally, they were imaged using a confocal microscope (Leica TCS SP8, Wetzlar, Germany) with a 10x air objective (HCX PL-Apo 10x/0.4 CS, Leica).
Data Analysis
To consider a neuron for analysis, the following criteria had to be fulfilled: i. stable baseline during stimulus presentation, ii. spike amplitudes clearly above noise level and iii. distinct immunolabeling of the recorded neuron. If a neuron passed these criteria, the recorded file was imported into MATLAB for further analysis via custom-written scripts that included the CircStat toolbox (Berens, 2009). Events during stimulation were detected based on a manually set threshold and were assigned to a particular polarization angle during the polarizer rotation or to a corresponding azimuthal angle during circling of a light spot. Neuronal spiking rates were estimated by low-pass filtering the instantaneous firing rate of the action potentials and illustrated as sliding window averages (Gaussian filtered, window size: 0.5 s) in the results. The preferred firing directions in response to the stimuli were determined as the mean vector of the bimodal (polarized light) or unimodal (sun stimulus) distribution of stimulus angles at the times of action potentials. The degree, to which the action potentials were clustered at this angular position, was defined as the vector strength. This value ranges from 0 to 1, with higher values indicating more directed responses
In addition to the aforementioned parameters, responses of each trial were binned into 18 bins and spike rate in each bin was calculated. This was used to obtain the modulation strength as described by Labhart (1996) using the following equation:
where n is the spiking rate (in spikes/s) and is the average spiking rate over the whole stimulation period. The higher the modulation strength, the stronger is the response of a neuron to a certain stimulus.
To predict the neuronal response to a combination of stimuli and to define the relevance of each cue on the neuronal coding, a weighted linear model was applied. This was based on the responses of the same neuron to the individual stimuli using the following equation:
where r1 and r2 describe the actual response of a neuron to an individual stimulus (e.g., sun stimulus and polarized light for the combined celestial stimuli condition), respectively and w indicates the weighting of them. If w < 0.5, the response of r2 is weighted higher, while w < 0.5 indicates that the neuronal response of r1 dominates the response. To identify the weighting that matches best the actual neuronal response to the combination of both stimuli, we calculated the correlation between the actual neuronal response and the modeled neuronal responses based on different linear weightings. The weighting that exhibited the highest correlation coefficient, was then considered for further analysis.
As we did not have any prediction of whether and how the TL neurons respond to the panoramic skyline, we used the inter-trial difference between single stimulus presentations as reference. Inter-trial differences were determined by treating clock- and counterclockwise responses separately. At first neuronal responses were grouped based on the stimulus rotation. Then, for both rotation directions, we calculated the mean neuronal response and correlated them with the response from each trial. Finally, the correlation coefficients were averaged within and then across rotation groups. The closer the correlation were to unity, the more similar were the neuronal responses across trials. To quantify the neuronal response to the panoramic skyline further, the averaged neuronal response for clock- and counterclockwise rotations was compared with the response to the flat panorama during a 6 s time frame. Both correlation values were averaged across rotation groups again. If the neurons encoded parts of the panoramic skyline, we expected that the neuronal response to the panoramic skyline and the response to the flat panorama correlate less with each other than the neuronal responses across trials.
To further characterize the response of the TL cells to the panoramic skyline, we applied an intensity-based model for different elevations. Circular, excitatory TL neurons' receptive fields were modeled by a Gaussian Kernel (11.5° width ± 2.9° standard deviation, corresponding to 5 pixels width and 2 pixels standard deviation of the arena) in MATLAB. This receptive field size is in a similar range as the excitatory component of measured locust TL neuron receptive fields (Takahashi et al., 2022). When the scene is rotated around the animal, this evokes changes in brightness in the modeled receptive field that are correlated with the silhouette of the panorama. Thus, if a bright sector of the panorama moves through the receptive field, it increases the spiking activity. In turn, if a dark sector of the panorama is moved through the receptive field, it will decrease the spiking activity. However, this change in spiking activity depends on the elevation of the receptive fields (Figure 3E), which may vary in monarch butterfly TL neurons, as shown for the homologous neurons in fruit flies (Seelig and Jayaraman, 2013). To reliably test if the TL neurons respond to the panoramic skyline, we defined each row of the LED arena between −16.1° and 16.1° as the center of a possible receptive field and modeled curves of the predicted neuronal modulation at different elevations when the panorama was rotated. To find the best match between the modeled response and the recorded neuronal response at different elevations, we calculated for the modeled responses at each elevation the cross correlation with the measured neuronal response. The modeled curve that exhibited the best match to the recorded neuronal response curve was included for further analysis.
Statistics
To identify whether action potentials in response to the simulated celestial cues are non-uniformly distributed, we applied the Rayleigh-test (significance level <0.05). To test whether the preferred firing directions are significantly clustered around the 0°-180° axis (polarized light) and around 90° (sun stimulus), we used the V-test (significance level <0.05). To test for normal distribution and similar variances of the modulation strengths, the Shapiro-Wilk test and the Levene-test were employed, respectively. If data were normally distributed and exhibited the same variance, parametric hypothesis tests were applied (unpaired t-test and paired t-test, respectively). Otherwise, non-parametric tests were used (Wilcoxon-rank-sum-test for unpaired and the Wilcoxon signed rank test for paired mean values). For partially paired data, like the observed weighting factors, a mixed linear model was used to test if the mean values differed significantly between the two test groups. Averaged parameters are shown as mean ± standard deviation if not mentioned otherwise.
Results
To understand how the monarch butterfly compass integrates multiple visual stimuli, we presented different visual cues in isolation and in combination to the animals while recording intracellularly from TL neurons of the central complex (Figures 1A,B). We successfully obtained recordings from 34 TL neurons. 15 TL neurons were tested with a combination of different celestial stimuli and 19 TL neurons with a combination of celestial and terrestrial wide field stimuli. Of the latter group, all TL cells were tested with the panoramic skyline, 15 of them were exposed long enough to the flat panorama to be included in the inter-trial response analyses and 13 of them were presented the combined sun stimulus and panoramic skyline.
Celestial Cue Integration in TL Neurons
We first tested the neuronal tuning to simulated celestial cues. Similar to previous experiments (Heinze and Reppert, 2011; Nguyen et al., 2021), a moving green light spot served as a sun stimulus while a rotating polarizer illuminated by UV light from the zenith was used to examine polarization sensitivity (Figure 1C). To simulate the natural spatial relationship between the sun and polarized light, we oriented the polarization angle perpendicular to the sun-stimulus direction (Figure 1C). As expected from previous experiments (Heinze and Reppert, 2011; Nguyen et al., 2021), TL neurons responded to both the sun stimulus and polarized light (Figure 1D, left and middle graph). Interestingly, the highest action potential rates (preferred firing directions, φmax) of the TL neuron to the sun stimulus and the polarization stimulus matched the 90°-relationship of the cues in nature. To investigate if this was true for all recorded TL cells, we analyzed the spatial distribution of the preferred firing directions (φmax) in response to the sun stimulus. The preferred firing directions were clustered around 90° in response to the sun stimulus (p = 0.008, v = 5.43; V-test; n = 10; Figure 1E, left) and along the 0° - 180° axis in response to polarized light (p = 0.03, v = 4.37; V-test; n = 10; V-test; Figure 1E, right). Taken together, the spatial relationship between the mean preferred firing directions of the recorded TL neurons when presenting sun and polarization stimulus in isolation matched the natural spatial relationship between both celestial cues.
When we presented both stimuli simultaneously, the neuronal tuning resembled a mixed response (Figure 1D, right graph), suggesting that TL neurons integrate both stimuli in a weighted manner. To quantify which of the two stimuli dominated the neuronal response, we compared the modulation strengths in response to the single stimuli (sun stimulus or polarized light) with the modulation strengths of the same neurons in response to the combined stimulus (sun stimulus and polarized light). The modulation strength in response to the sun stimulus (62.48 ± 37.05, n = 10) was significantly weaker than the modulation strength of the same neurons to the polarization stimulus (89.81 ± 48.64, n = 10; p = 0.02, t =2.99; paired t-test) and to the combination of the stimuli (98.90 ± 48.43, n = 10; p = 0.002, t =-4.38; paired t-test; Figure 1F). The modulation strength did not differ between the response to the polarizer and to the combination of the stimuli (p = 0.23, t =-1.28; paired t-test). This indicates that the polarization input is weighted stronger than the sun-stimulus input and that the response to the combined celestial cues seems to be mainly shaped by the polarization input.
Weighting of Celestial Cues in TL Neurons
To quantify whether polarized light truly dominates the response to the combined celestial cues and how they are weighted in TL neurons, we combined the responses to the isolated stimuli (Figure 2A, upper plot) in a weighted linear model and calculated a predicted response to the combined stimuli. By varying the weight between the neuronal response to the polarizer and sun stimulus, we modeled different expected neuronal responses to the combined stimuli and correlated these modeled responses with the actual response to the combined stimuli (Figure 2A, blue curve of lower plot). The modeled response with the highest similarity to the actual neuronal response (Figure 2A, red curve of lower plot) was selected to determine the neuron-specific cue weighting. A weighting factor of 0 indicated that the combined response was entirely characterized by the sun stimulus while a weighting of 1 represented a tuning that was purely described by the polarization input.
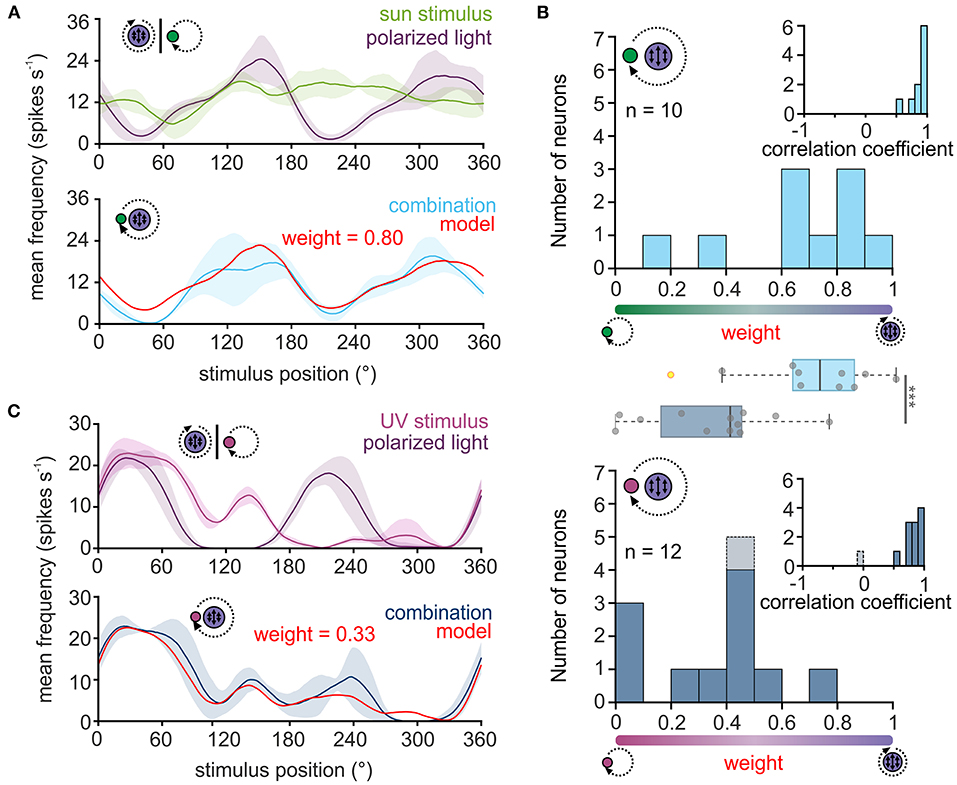
Figure 2. Weighting of celestial cues in TL neurons. (A,C) Upper plot: sliding window averages of the action potential rates of two TL neurons to the green sun stimulus [(A), green curve] or UV light spot [(C), magenta] as well as to the polarization stimulus [(A,C), violet curves] are shown. Lower plot: The response of the same neurons as shown in the corresponding upper plots when both stimuli were presented simultaneously [(A), light blue; (C) dark blue curve]. Based on the response to the single cues, a weighted linear model was fitted to the data (red curve) and a weighting of the single cues was calculated. A weight between 0 and 0.5 suggests that the sun stimulus (A) or the UV light spot (C) dominated the combined response while a weight between 0.5 and 1 indicates that the polarization input dominates the combined response. The shaded areas show the standard deviation. (B) Histograms of the weighting factors obtained for the experiments with the green sun stimulus and polarized light (top, n = 10) and the UV light spot and polarized light (bottom, n = 12). Insets show the correlation coefficients which describe how well the weighted linear model explains the measured neural response to the combined stimulus. The weighting factor that was obtained from a low correlation coefficient is shown in gray. Box plots in the middle: While the weighting is shifted to the polarization input when a green sun stimulus was combined with polarized light, the weighting is significantly shifted in favor of the light spot, when a UV light cue was combined with polarized light (p < 0.001, F = 113.31; linear mixed model ANOVA). Gray circles show individual data points (yellow circles indicate outliers). Boxes indicate interquartile range. Whiskers extend to the 2.5th and 97.5th percentiles. Black horizontal lines show the median. ***p < 0.001.
For all neurons, the correlation coefficients obtained through the comparison of the modeled and actual neuronal response were relatively high (Figure 2B, inset in upper histogram, 0.86 ± 0.12, n = 10), suggesting that the response of TL neurons to the combined celestial stimuli can be well described by the weighted linear model. When presenting sun stimulus and polarized light simultaneously, most TL cells responded stronger to the polarization information (Figure 2B, upper histogram), although the light intensity of the stimuli was set to the same photon flux. The bias toward the polarization stimulus may be induced by differences in the absolute sensitivity of the UV and green photoreceptors in the monarch butterfly eye. Thus, we assumed that the UV polarization stimulus may appear brighter to the butterflies than the green sun stimulus due to a higher sensitivity of the photoreceptors to UV light. To test whether this may explain the dominance of the polarization stimulus on the neuronal response to the combined stimuli, we repeated the experiments with a UV sun stimulus that had the same photon flux as the UV polarization stimulus (Figure 2C). Again, except for one neuron, the weighted linear model described the actual neuronal response well (Figure 2B, inset in lower histogram; 0.77 ± 0.28, n = 12), which further confirms that celestial information is linearly integrated in TL neurons. In contrast to the trials with the green sun stimulus and polarized light, the weighting to the combined UV stimuli shifted in favor of the UV sun stimulus (Figure 2B, lower histogram). The observed weightings differed significantly between the experiments with the green sun stimulus and polarized light (0.65 ± 0.28, n = 10) and the UV sun stimulus and polarized light (0.30 ± 0.23, n = 12; p < 0.001, F = 113.31; ANOVA, Figure 2B, boxplots). Taken together, our data show that TL neurons combine celestial cues linearly in monarch butterflies. However, the weighting between the sun and polarization input is highly affected by the spectral content and relative brightness of the presented stimuli.
TL Neurons Are Tuned to a Panoramic Skyline
In addition to celestial cues, recent experiments in Drosophila melanogaster suggest that central-complex neurons encode the entire visual scenery around the animal (Seelig and Jayaraman, 2015; Kim et al., 2019). One salient cue in a visual scene that can be used by many insects for orientation is the profile of a panoramic skyline (Graham and Cheng, 2009a,b; Reid et al., 2011; Legge et al., 2014; Franzke et al., 2020). In contrast to the sun and polarized skylight, coding a panoramic skyline neuronally is more complex as the neurons need to integrate information from different azimuths and elevations to precisely reproduce the silhouette of the panoramic skyline (Dewar et al., 2017). To grasp how the monarch butterfly central complex encodes a panoramic skyline, we placed the butterflies at the center of an LED arena and recorded the neuronal activity of TL neurons while the animals were exposed to a panoramic skyline that was presented at the inner surface of the LED arena. We used the same panoramic skyline that has recently been used to study the monarch butterfly orientation behavior (Franzke et al., 2020; Figure 3A). When we rotated the scene around the butterflies, we found that many TL neurons were modulated by the panoramic stimulus (Figure 3B). To exclude that these modulations occurred spontaneously, we analyzed the inter-trial variability of the neuronal activity and correlated the neuronal activity in response to each trial (Figure 3B, gray curves) with the averaged response (Figure 3B, orange curve). As a control, the neuronal responses of the same TL neurons to a flat panorama (Figure 3C) were correlated to the averaged response to the panoramic skyline. Neuronal responses to the panoramic skyline across trials were highly correlated with their averaged response (Figure 3D, upper plot) indicating a low inter-trial variability and that the neuronal modulations occurred in response to the rotating panoramic skyline. In contrast, the neuronal activity in the presence of the flat panorama was poorly correlated with the averaged response to the panoramic skyline (Figure 3D, lower plot). The correlation coefficients between inter-trial responses and the averaged response to the panoramic skyline were significantly higher (0.76 ± 0.07) than to the responses to the flat panorama and the averaged responses to the panoramic skyline (−0.02 ± 0.29; p < 0.001, sign rank = 120, Wilcoxon signed rank test, Figure 3D). This demonstrates that monarch butterfly TL neurons encode, in addition to celestial cues, panoramic skylines.
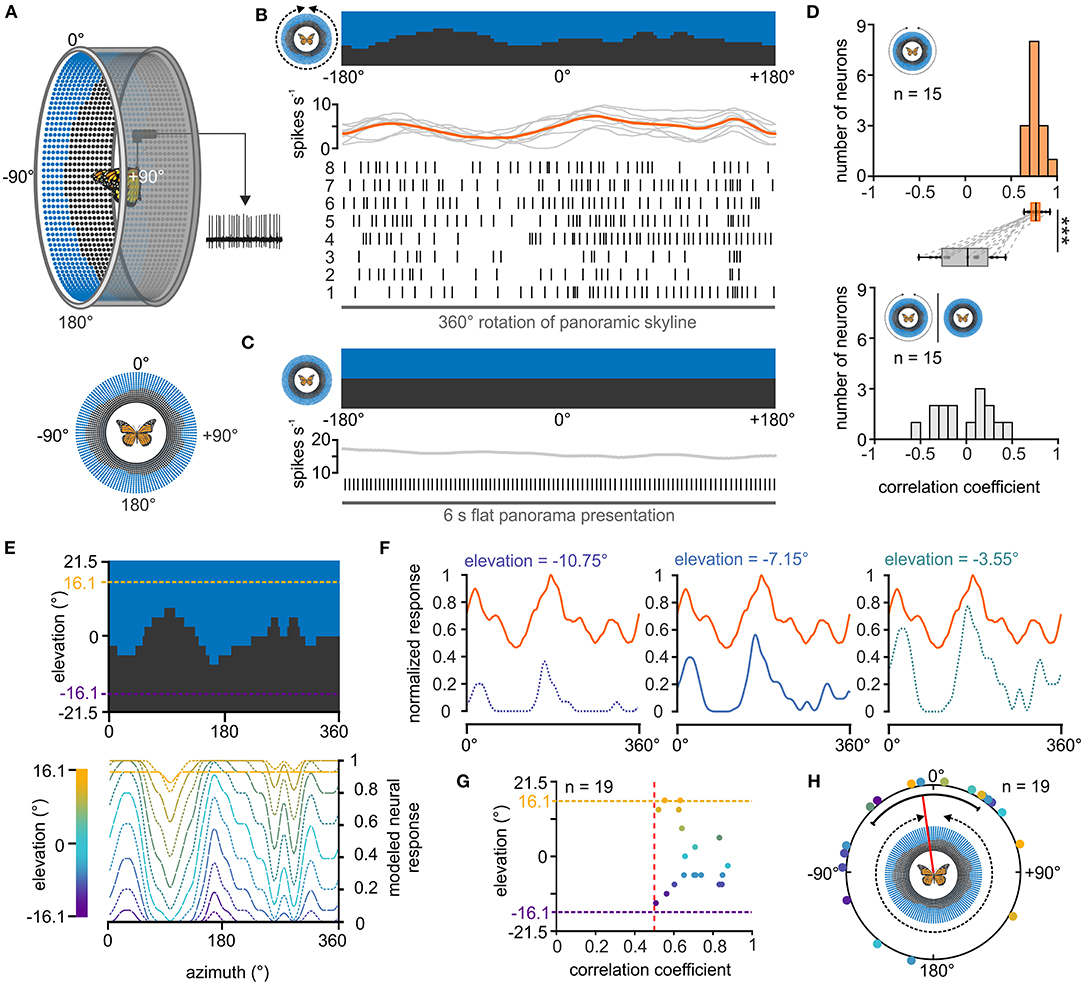
Figure 3. Response of central-complex TL neurons to a panoramic skyline. (A) Schematic drawing of the LED arena (top) that was used to test the coding of a panoramic scene in TL neurons. 0° was defined as the direction anterior to the butterfly, before the panoramic scene was rotated by 360° around the animal (bottom). (B) The response of a TL neuron to eight 360°-rotations of the panoramic scene is shown as raster plot (middle). Each vertical line represents an action potential. The diagram on top shows the sliding window average of the action potentials for the eight rotations (gray curves) as well as their mean (orange curve). The orientation of the panoramic scene prior to rotation is shown (top). (C) The TL neuron does not spontaneously modulate its action potential rate when a flat panorama is presented for 6s. (D) Distribution of correlation coefficients when comparing the neural response from each trial with the averaged response (orange, upper panel) and when comparing the neural response to the flat panorama with the averaged response to the panoramic skyline (gray, lower panel). Box plots: The neurons showed a higher inter-trial modulation to the panoramic scene compared to the modulation to the flat panorama (p < 0.001; sign rank = 120, Wilcoxon signed rank test). Paired data points across the tested groups are connected by dashed lines. Boxes indicate interquartile range. Whiskers extend to the 2.5th and 97.5th percentiles. Black horizontal lines show the median. ***p < 0.001. (E) Modeled neuronal response curves to the panoramic skyline of fictive TL neurons that have visual fields centered at different elevations (lower panel). The center of the receptive fields were set for elevations within the panoramic scene (between −16.1° and 16.1°, dashed lines, upper panel). (F) The measured neural response of one recorded TL neuron (orange curve) to the panoramic scene plotted against three modeled neural responses whose visual fields were centered at different elevations [see also lower panel in (E)]. The measured neural response showed the best match to the modeled modulation at an elevation of −7.15 (middle plot; correlation coefficient = 0.84). (G) Elevations of the highest match between the measured TL neuron response and the modeled response plotted against the corresponding correlation coefficients. Each point represents an individual TL neuron (n = 19). Vertical, red dashed line indicates a correlation coefficient of 0.5. The vertical dashed lines represent the elevation range for the modeled receptive fields (between −16.1° and 16.1°). (H) Azimuthal shifts leading to the maximum correlation between the measured and modeled neural response are plotted for each neuron (n = 19). Shifts were clustered in the frontal visual field (p = 0.045; Z = 3.06, n = 19, Rayleigh test). The mean is indicated by a red solid line and the confidence intervals (95%) by a black arc. The color code of the individual TL neurons in G and H corresponds to the color code of the heatmap in (E).
Although it is not trivial to predict the TL response to the presented panoramic skyline, we noticed that the modulation of the spiking activity seemed to correlate negatively with the troughs of the profile (Figure 3B), suggesting that they are tuned to changes in brightness during the stimulus rotation. To investigate this hypothesis, we modeled the neuronal responses for fictive TL cells whose neuronal tunings were based on changes in brightness during stimulus rotations. As the receptive fields of the TL neurons can cover patches of different elevations and azimuths (Seelig and Jayaraman, 2013; Takahashi et al., 2022), we varied both the azimuth and elevation (between −16.1° and +16.1°) of the center of the modeled neuron's receptive field (Figure 3E). For each of the modeled responses (Figure 3F, blue curves), we calculated its cross correlations with the measured neuronal TL response (Figure 3F, orange curves). If TL neurons encoded changes in brightness associated with rotations of the panoramic skyline, we expected that one of the modeled neuronal responses will align well with the measured neuronal response. Indeed, most measured neuronal responses correlated well with one of the modeled TL responses (Figure 3G). Not surprising, they showed the highest correlation at elevation values between −10.75° and +10.75°. In addition, cross correlations allowed us to calculate the azimuthal panorama position that gave the strongest neuronal response. The angular shifts between the measured and modeled TL response leading to the highest correlation coefficient clustered in the anterior field of the animals (p = 0.045; Z = 3.06, n = 19, Rayleigh test; Figure 3H).
Weighting of the Sun and Panoramic Scene in TL Neurons
We next wondered how TL neurons encode a visual scene that was composed of a simulated sun and the panoramic skyline. As demonstrated in the example TL neuron, the moving sun stimulus mainly dominated the neuronal response, irrespective of the absence/presence of the panoramic skyline (Figures 4A,B), but the modulation of the panoramic profile was additionally encoded in the neuronal response of the TL neuron (arrow in Figure 4B). To test whether the TL neurons combine both stimuli in a linear manner, as shown for the celestial cues, we also tested the same neurons' responses to the single stimuli. As expected, the TL neurons responded to the sun stimulus (Figure 4C, green curve) and the panoramic scene (Figure 4C, orange curve), when presented individually. Again, we used the neuronal tuning to the single stimuli to model the expected response of the TL neurons to a combined – sun and panorama – stimulus presentation. We then used the modeled neuronal response based on the weighted linear model (Figure 4C, red curve) and correlated it with the measured neuronal response to both stimuli (Figure 4C, blue curve). In contrast to the results for the combined celestial cues (Figure 2), the weighted linear model did not always result in high correlation values with the measured responses (Figure 4D, inset, 0.55 ± 0.29, n = 13). For five of the 13 TL neurons (correlation coefficient < 0.5; Figure 4D, inset), the neuronal response to the combined celestial and terrestrial cue, i.e., panoramic skyline, could not be explained with a linear model. The predicted weighting factors were highly variable. While responses of some TL neurons were dominated by the sun stimulus (weight < 0.5; Figure 4D), responses of other TL neurons were more dominated by the panoramic skyline (weight > 0.5; Figure 4D). Taken together, we found a high variance of neuronal coding in cue hierarchy between the sun and the panoramic skyline. This stands in contrast to the results observed with the sun stimulus and the polarized light. Although we were not able to define from which TL subtype we obtained our recordings (see discussion), the results indicate that the cue hierarchy between celestial and terrestrial cues shows a high inter-individual flexibility in monarch butterflies.
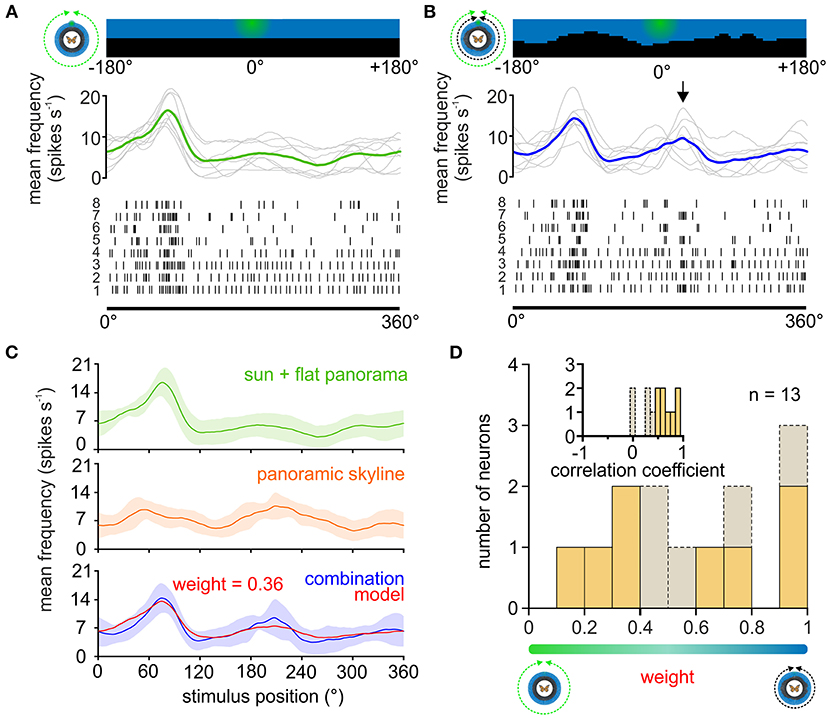
Figure 4. Weighting the sun and panoramic skyline in TL neurons. (A,B) The response of a TL neuron to eight 360°-rotations of the sun stimulus (A), and when the sun stimulus and the panoramic scene were rotated simultaneously around the animal (B). The stimulus position prior to rotation is shown (top). The action potentials are shown as raster plots (bottom). Above the raster plots, the sliding window averages of the rotations are shown (middle plot, gray curves). The sliding window averages of the mean spiking frequency are color coded [(A), moving sun stimulus in green; (B) moving sun stimulus and panorama in blue]. Arrow indicates an increased spiking activity that was not observed when presenting the sun stimulus alone and thus can be attributed to the panoramic skyline. (C) Sliding window averages of the same TL neuron as in (A,B) responding to a 360° rotation of different stimuli. From top to bottom: sun stimulus alone (green curve), panoramic skyline alone (orange curve), sun stimulus and panoramic skyline combined (blue curve). A weighted linear model (red curve, lower plot) was fitted to the observed neural activity to the combined stimulus. Shaded areas show the standard deviation. (D) The distribution of the weighting factors for experiments with the sun stimulus and panoramic skyline (n = 13). Low and high weighting values indicate that the sun stimulus and the panorama dominated the combined response, respectively. The correlation coefficient distribution in the inset indicates how well the weighted linear model described the measured neural response to the combined stimulus. Weighting factors obtained from a low correlation coefficient (<0.5), indicating that the neural response can be explained poorly with a linear model, are shown in gray.
Discussion
We show that the monarch butterfly CX integrates multiple visual cues, i.e., celestial and terrestrial panoramic skyline cues for orientation. While the sun stimulus and polarized light were integrated linearly, the coding of the sun stimulus and the panoramic skyline did not always match a linear summation of the neuronal response to the isolated stimuli. Moreover, while polarized light was usually weighted stronger than the green sun stimulus, the weighting of the sun versus the panorama stimulus was set in a variable manner across different TL neurons. This observation is in line with behavioral results on monarch butterflies tested within the same visual setting and might allow the butterflies to set the cue preference in a highly flexible manner between celestial and terrestrial cues (Franzke et al., 2020).
Celestial Coding in the Central Complex
Monarch butterfly TL neurons are sensitive to polarized light (Heinze and Reppert, 2011; Nguyen et al., 2021, this work), similar to what has been reported for TL cells of a wide range of other insects, including desert locusts (Vitzthum et al., 2002; Heinze et al., 2009; Bockhorst and Homberg, 2015; Pegel et al., 2018; Takahashi et al., 2022), field crickets (Sakura et al., 2008), dung beetles (el Jundi et al., 2015), sweat bees (Stone et al., 2017), and fruit flies (Hardcastle et al., 2021). In addition to polarized light, TL neurons in the present study were tuned to a green light spot – likely representing the sun. As we used the responsiveness to the polarization stimulus to physiologically identify the neurons, we might have missed TL neurons that were solely tuned to the sun stimulus. However, the here recorded TL neurons are suitable to combine information from the sun and the pattern of polarized light similar to what has been shown in TL neurons in desert locusts (Pegel et al., 2018; Takahashi et al., 2022) and dung beetles (el Jundi et al., 2015), as well as in previous experiments in monarch butterflies (Heinze and Reppert, 2011; Nguyen et al., 2021). Thus, the TL neuron sensitivity to celestial cues is highly conserved and may play a crucial role for the heading coding in a variety of insects. How the TL neurons' gain to visual cues is further affected by an animal's locomotor state, as shown for the corresponding neurons in fruit flies (Seelig and Jayaraman, 2013) and as suggested by a recent study on monarch butterflies (Beetz et al., 2022), awaits to be explored.
We found a fixed spatial relationship between the preferred firing direction to the sun stimulus and the polarization stimulus. The clustering of the preferred sun-stimulus directions to the butterflies' right side is likely a result of a bias in recordings from the right brain hemisphere (at least six out of ten recordings were obtained from right TL neurons). Thus, these cells receive likely visual input from the ipsilateral eye which is well in line with previous recordings from these compass neurons (Heinze and Reppert, 2011). Interestingly, we found that the preferred polarization directions of the same TL neurons were significantly aligned with the animals' longitudinal body axis which is at odds with a previous study (Heinze and Reppert, 2011). This resulted in an orthogonal relationship between the mean preferred sun and polarization directions, which parallels the 90°-relationship between the sun and polarization pattern in nature, an aspect that has also been reported in desert locust TL (Pegel et al., 2018) and optic lobe neurons (el Jundi et al., 2011). The bias in preferred polarization directions found in our TL neurons could thus be a consequence of a neuronal matched filter for celestial cues, allowing the butterflies to derive the same directional information from different celestial inputs.
Pegel et al. (2019) showed that the preferred firing directions to the sun stimulus differ between the three TL subtypes (TL2a, TL2b, TL3) in desert locusts. The monarch butterfly TL neurons can also be divided anatomically into three subtypes that innervate different layers in the lower division of the central body (Heinze et al., 2013). Unfortunately, we were not able to define from which subtype we performed our recordings as we often co-stained several TL subtypes in one experiment. As shown previously, monarch butterfly compass neurons show the same preferred firing direction, irrespective of the spectral information of the light stimulus (Heinze and Reppert, 2011; Nguyen et al., 2021). However, recordings from compass neurons in the desert locust suggest that the spectral influence on the preferred firing direction is strongly sensitive to the light intensity of the stimuli (Kinoshita et al., 2007; Pfeiffer and Homberg, 2007). It is therefore crucial to study the response characteristics of TL neurons to spectral cues at different light intensities in the future to shed light on how the monarch butterfly compass network may integrate different celestial cues into the central complex and how this represents the celestial cue hierarchy exhibited behaviorally.
Integration of the Panoramic Skyline in the Central Complex
In previous experiments, the sensitivity of TL cells has been studied with respect to vertical stripes (Bockhorst and Homberg, 2017; Omoto et al., 2017; Fisher et al., 2019), grating patterns (Rosner et al., 2019) or small light spots (Seelig and Jayaraman, 2013) in insects. We here found that TL neurons were sensitive to a simulated panoramic skyline by responding to changes in brightness while the panorama was rotated around the animal. As we only tested the response of the monarch TL neurons to one distinct panoramic scene, it has yet to be identified how modifying the frequency and amplitude of the panorama's profile will affect the tuning of the TL neurons. We chose this specific panoramic skyline as a recent behavioral study showed that monarch butterflies are able to use this setting to sustain a directed flight course (Franzke et al., 2020). However, as their orientation performance was indistinguishable from a flight stabilization strategy, it was unclear whether the butterflies can employ compass orientation with respect to a panoramic scene. Although our data do not exclude the possibility that TL neurons transfer motion information to the central complex, our data indicate that the central complex receives visual compass information of the panoramic scene. This suggests that monarch butterflies can use a panoramic skyline as a compass cue to compute a heading with respect to it, which parallels behavioral results from Australian desert ants that can use a panorama to calculate a heading direction (Graham and Cheng, 2009a,b). The structure and relative position of the receptive fields of the TL neurons studied with the panoramic scene are difficult to predict due to the complex nature of the stimulus. Exploring this requires to additionally map their receptive fields with respect to a small visual stimulus (Seelig and Jayaraman 2013), an aspect that was not feasible due to the short recording times of our intracellular recordings. Rather than encoding the current heading, the TL neurons seem to convey visual information into the insect compass, similar to the Drosophila ring neurons (Seelig and Jayaraman, 2013; Dewar et al., 2017). In both monarch butterflies and fruit flies, they synapse on a population of neurons termed CL1 neurons (Heinze et al., 2013), called EP-G cells in fruit flies, which likely represent a distinct heading direction within a visual scene based on multimodal information (Seelig and Jayaraman, 2015; Kim et al., 2019; Turner-Evans et al., 2020; Beetz et al., 2022). How monarch butterfly CL1 cells compute a heading based on terrestrial information from TL cells awaits to be answered through neuronal recordings during flight as the coding strongly depends on the animal's locomotory state (Beetz et al., 2022).
Flexible Weighting Between Celestial and Terrestrial Information
When we presented the sun and polarization stimulus simultaneously to the butterflies, the TL neurons combined these cues in a linear manner. These results differ from the dung beetle TL neurons (el Jundi et al., 2015) but are in line with desert locust columnar CX-neurons (Pegel et al., 2019). The polarization UV stimulus was consistently ranked higher than the green sun stimulus in TL neurons in monarch butterflies when presented with a similar relative light intensity. When we presented a UV light spot instead of a green one, the unpolarized light stimulus dominated the neuronal tuning. This switch in cue preference was likely not a result of a change in wavelength but rather a consequence of a change in relative intensity of light, which is in line with the stronger response of TL neurons to UV light than to green light (Nguyen et al., 2021). Thus, as the sun is several magnitudes brighter than the remaining sky in nature, it is likely the dominant cue being encoded in TL neurons under a real sky.
In general, the weighting between the simulated sun and polarized light was very similar across different TL neurons. This low variability in cue preference between TL neurons recorded in different monarch butterflies was similar to what has been found for TL neurons in dung beetles (el Jundi et al., 2015) and suggests that the weighting of celestial cues is determined at an early processing stage in the brain, such as at the level of the photoreceptors. In contrast, the simulated sun and the panoramic skyline were not always linearly integrated in the monarch butterfly central complex. Moreover, the cue preference was highly variable, which is well in line with the high inter-individual difference in the behavioral use of these cues for orientation in a flight simulator (Franzke et al., 2020). This high flexibility indicates that the weighting might not only be set based on the sensitivity of the inputs at the butterfly's eye but might additionally be adjusted at later stages in the brain network. This would allow a high inter-individual difference in weighting that is based on the animal's internal state, as well as its experience.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
TN, CM, KP, and BJ: study design. TN: conducting experiments and analysis of data. TN, MB, CM, KP, and BJ: interpretation of data. TN and BJ: drafting of the manuscript. MB, CM, and KP: critical review of the manuscript. BJ: acquired funding. All authors approved of the final version of the manuscript.
Funding
This work was supported by the Emmy Noether program of the Deutsche Forschungsgemeinschaft granted to BJ (GZ: EL784/1-1) and a DFG Grant to KP (PF714/5-1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. James Foster for fruitful comments on our analysis and Kolja Richter and Konrad Öchsner for their help in developing the LED arena. We thank Samantha Iiams, Aldrin Lugena, Guijun Wan and Ying Zhang for their help in capturing monarch butterflies and for checking them for Ophryocystis elektroscirrha. In addition, we would like to thank Sergio Siles and Marie Gerlinde Blaese (butterflyfarm.co.cr) for providing us with monarch butterfly pupae.
References
Beetz, M. J., Kraus, C., Franzke, M., Dreyer, D., Strube-Bloss, M. F., Roessler, W., et al. (2022). Flight-induced compass representation in the monarch butterfly heading network. Curr. Biol. 32, 338–349. doi: 10.1016/j.cub.2021.11.009
Berens, P. (2009). CircStat: A MATLAB toolbox for circular statistics. J. Stat. Softw. 31, 1–21. doi: 10.18637/jss.v031.i10
Bockhorst, T., and Homberg, U. (2015). Amplitude and dynamics of polarization-plane signaling in the central complex of the locust brain. J. Neurophysiol. 113, 3291–3311. doi: 10.1152/jn.00742.2014
Bockhorst, T., and Homberg, U. (2017). Interaction of compass sensing and object-motion detection in the locust central complex. J. Neurophysiol. 118, 496–506. doi: 10.1152/jn.00927.2016
Brines, M. L., and Gould, J. L. (1979). Bees have rules. Science 206, 571–573. doi: 10.1126/science.206.4418.571
Dacke, M., Baird, E., el Jundi, B., Warrant, E. J., and Byrne, M. (2021). How dung beetles steer straight. Ann. Rev. Entomol. 66, 243–256. doi: 10.1146/annurev-ento-042020-102149
Dewar, A. D. M., Wystrach, A., Phillippides, A., and Graham, P. (2017). Neural coding in the visual system of Drosophila melanogaster: how do small neural populations support visually guided behaviours? PLoS Comput. Biol. 13, e1005735. doi: 10.1371/journal.pcbi.1005735
Dreyer, D., el Jundi, B., Kishkinev, D., Suchentrunk, C., Campostrini, L., Frost, B. J., et al. (2018a). Evidence for a southward autumn migration of nocturnal noctuid moths in central Europe. J. Exp. Biol. 221, jeb179218. doi: 10.1242/jeb.179218
Dreyer, D., Frost, B., Mouritsen, H., Gunther, A., Green, K., Whitehouse, M., et al. (2018b). The Earth's magnetic field and visual landmarks steer migratory flight behavior in the nocturnal Australian bogong moth. Curr. Biol. 28, 2160–2166. doi: 10.1016/j.cub.2018.05.030
Edrich, W., Neumeyer, C., and von Heiversen, O. (1979). “Anti-sun orientation” of bees with regard to a field of ultraviolet light. J. Comp. Physiol. 134, 151–157. doi: 10.1007/BF00610473
el Jundi, B., Baird, E., Byrne, M. J., and Dacke, M. (2019). The brain behind straight-line orientation in dung beetles. J. Exp. Biol. 222, jeb192450. doi: 10.1242/jeb.192450
el Jundi, B., Pfeiffer, K., and Homberg, U. (2011). A distinct layer of the medulla integrates sky compass signals in the brain of an insect. PLoS One 6, e27855. doi: 10.1371/journal.pone.0027855
el Jundi, B., Smolka, J., Baird, E., Byrne, M. J., and Dacke, M. (2014). Diurnal dung beetles use the intensity gradient and the polarization pattern of the sky for orientation. J. Exp. Biol. 217, 2422–2429. doi: 10.1242/jeb.101154
el Jundi, B., Warrant, E. J., Byrne, M. J., Khaldy, L., Baird, E., Smolka, J., et al. (2015). Neural coding underlying the cue preference for celestial orientation. Proc. Natl. Acad. Sci. U. S. A. 112, 11395–11400. doi: 10.1073/pnas.1501272112
el Jundi, B., Warrant, E. J., Pfeiffer, K., and Dacke, M. (2018). Neuroarchitecture of the dung beetle central complex. J. Comp. Neurol. 526, 2612–2630. doi: 10.1002/cne.24520
Fisher, Y. E., Lu, J., D'Alessandro, I., and Wilson, R. I. (2019). Sensorimotor experience remaps visual input to a heading-direction network. Nature 576, 121–125. doi: 10.1038/s41586-019-1772-4
Franzke, M., Kraus, C., Dreyer, D., Pfeiffer, K., Beetz, M. J., Stöckl, A. L., et al. (2020). Spatial orientation based on multiple visual cues in non-migratory monarch butterflies. J. Exp. Biol. 223, 1–12. doi: 10.1242/jeb.223800
Graham, P., and Cheng, K. (2009a). Ants use the panoramic skyline as a visual cue during navigation. Curr. Biol. 19, R935–R937. doi: 10.1016/j.cub.2009.08.015
Graham, P., and Cheng, K. (2009b). Which portion of the natural panorama is used for view-based navigation in the Australian desert ant? J. Comp. Physiol. A 195, 681–689. doi: 10.1007/s00359-009-0443-6
Green, J., Adachi, A., Shah, K. K., Hirokawa, J. D., Magani, P. S., and Maimon, G. (2017). A neural circuit architecture for angular integration in Drosophila. Nature 546, 101–106. doi: 10.1038/nature22343
Guerra, P. A., Merlin, C., Gegear, R. J., and Reppert, S. M. (2012). Discordant timing between antennae disrupts sun compass orientation in migratory monarch butterflies. Nat. Comm. 3, 958. doi: 10.1038/ncomms1965
Hardcastle, B. J., Omoto, J. J., Kandimalla, P., Nguyen, B. M., Kele,ş, M. F., Boyd, N. K., et al. (2021). A visual pathway for skylight polarization processing in Drosophila. Elife 10, e63225. doi: 10.7554/eLife.63225
Heinze, S., Florman, J., Asokaraj, S., el Jundi, B., and Reppert, S. M. (2013). Anatomical basis of sun compass navigation II: the neuronal composition of the central complex of the monarch butterfly. J. Comp. Neurol. 521, 267–298. doi: 10.1002/cne.23214
Heinze, S., Gotthardt, S., and Homberg, U. (2009). Transformation of polarized light information in the central complex of the locust. J. Neurosci. 29, 11783–11793. doi: 10.1523/JNEUROSCI.1870-09.2009
Heinze, S., and Reppert, S. M. (2011). Sun compass integration of skylight cues in migratory monarch butterflies. Neuron 69, 345–358. doi: 10.1016/j.neuron.2010.12.025
Held, M., Berz, A., Hensgen, R., Muenz, T. S., Scholl, C., Roessler, W., et al. (2016). Microglomerular synaptic complexes in the sky-compass network of the honeybee connect parallel pathways from the anterior optic tubercle to the central complex. Front. Behav Neurosci. 10, 91–105. doi: 10.3389/fnbeh.2016.00186
Hensgen, R., England, L., Homberg, U., and Pfeiffer, K. (2020). Neuroarchitecture of the central complex in the brain of the honeybee: Neuronal cell types. J. Comp. Neurol. 20, 1–28. doi: 10.1002/cne.24941
Homberg, U. (2015). Sky compass orientation in desert locusts–Evidence from field and laboratory studies. Front. Behav. Neurosci. 9, 346. doi: 10.3389/fnbeh.2015.00346
Hulse, B. K., Haberkern, H., Franconville, R., Turner-Evans, D. B., Takemura, S.-,y., Wolff, T., et al. (2021). A connectome of the Drosophila central complex reveals network motifs suitable for flexible navigation and context-dependent action selection. Elife 10, e66039. doi: 10.7554/eLife.66039
Kim, S. S., Hermundstad, A. M., Romani, S., Abbott, L. F., and Jayaraman, V. (2019). Generation of stable heading representations in diverse visual scenes. Nature 576, 126–131. doi: 10.1038/s41586-019-1767-1
Kinoshita, M., Pfeiffer, K., and Homberg, U. (2007). Spectral properties of identified polarized-light sensitive interneurons in the brain of the desert locust Schistocerca gregaria. J. Exp. Biol. 210, 1350–1361. doi: 10.1242/jeb.02744
Labhart, T. (1996). How polarization-sensitive interneurones of crickets perform at low degrees of polarization. J. Exp. Biol. 199, 1467–1475. doi: 10.1242/jeb.199.7.1467
Lebhardt, F., and Ronacher, B. (2014). Interactions of the polarization and the sun compass in path integration of desert ants. J. Comp. Physiol. A 200, 711–720. doi: 10.1007/s00359-013-0871-1
Legge, E. L., Wystrach, A., Spetch, M. L., and Cheng, K. (2014). Combining sky and earth: desert ants (Melophorus bagoti) show weighted integration of celestial and terrestrial cues. J. Exp. Biol. 217, 4159–4166. doi: 10.1242/jeb.107862
Merlin, C., Gegear, R. J., and Reppert, S. M. (2009). Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 325, 1700–1704. doi: 10.1126/science.1176221
Merlin, C., and Liedvogel, M. (2019). The genetics and epigenetics of animal migration and orientation: birds, butterflies and beyond. J. Exp. Biol. 222, jeb191890. doi: 10.1242/jeb.191890
Merlin, C., Heinze, S., and Reppert, S. M. (2012). Unraveling navigational strategies in migratory insects. Curr. Opin. Neurobiol. 22, 353–361. doi: 10.1016/j.conb.2011.11.009
Nguyen, T. A. T., Beetz, M. J., Merlin, C., and el Jundi, B. (2021). Sun compass neurons are tuned to migratory orientation in monarch butterflies. Proc. Biol. Sci. 288, 20202988. doi: 10.1098/rspb.2020.2988
Okubo, T. S., Patella, P., D'Alessandro, I., and Wilson, R. I. (2020). A neural network for wind-guided compass navigation. Neuron 107, 924–940 e18. doi: 10.1016/j.neuron.2020.06.022
Omoto, J. J., Keleş, M. F., Nguyen, B. M., Bolanos, C., Lovick, J. K., and Frye, M. A. (2017). Visual input to the Drosophila central complex by developmentally and functionally distinct neuronal populations. Curr. Biol. 24, 1098–1110. doi: 10.1016/j.cub.2017.02.063
Pegel, U., Pfeiffer, K., and Homberg, U. (2018). Integration of celestial compass cues in the central complex of the locust brain. J. Exp. Biol. 221, jeb171207. doi: 10.1242/jeb.171207
Pegel, U., Pfeiffer, K., Zittrell, F., Scholtyssek, C., and Homberg, U. (2019). Two compasses in the central complex of the locust brain. J. Neurosci. 39, 3070–3080. doi: 10.1523/JNEUROSCI.0940-18.2019
Pfeiffer, K., and Homberg, U. (2007). Coding of azimuthal directions via time-compensated combination of celestial compass cues. Curr. Biol. 17, 960–965. doi: 10.1016/j.cub.2007.04.059
Reid, S. F., Narendra, A., Hemmi, J. M., and Zeil, J. (2011). Polarised skylight and the landmark panorama provide night-active bull ants with compass information during route following. J. Exp. Biol. 214, 363–370. doi: 10.1242/jeb.049338
Reppert, S. M., Zhu, H., and White, R. H. (2004). Polarized light helps monarch butterflies navigate. Curr. Biol. 14, 155–158. doi: 10.1016/j.cub.2003.12.034
Rosner, R., Pegel, U., and Homberg, U. (2019). Responses of compass neurons in the locust brain to visual motion and leg motor activity. J. Exp. Biol. 222, jeb196261. doi: 10.1242/jeb.196261
Rossel, S., and Wehner, R. (1984). Celestial orientation in bees: the use of spectral cues. J. Comp. Physiol. A 155, 605–613. doi: 10.1007/BF00610846
Rother, L., Kraft, N., Smith, D. B., el Jundi, B., Gill, R. J., and Pfeiffer, K. (2021). A micro-CT-based standard brain atlas of the bumblebee. Cell. Tissue Res. 386, 29–45. doi: 10.1007/s00441-021-03482-z
Sakura, M., Lambrinos, D., and Labhart, T. (2008). Polarized skylight navigation in insects: model and electrophysiology of e-vector coding by neurons in the central complex. J. Neurophysiol. 99, 667–682. doi: 10.1152/jn.00784.2007
Sauman, I., Briscoe, A. D., Zhu, H., Shi, D., Froy, O., Stalleicken, J., et al. (2005). Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron 46, 457–467. doi: 10.1016/j.neuron.2005.03.014
Seelig, J. D., and Jayaraman, V. (2013). Feature detection and orientation tuning in the Drosophila central complex. Nature 503, 262–266. doi: 10.1038/nature12601
Seelig, J. D., and Jayaraman, V. (2015). Neural dynamics for landmark orientation and angular path integration. Nature 521, 186–191. doi: 10.1038/nature14446
Stalleicken, J., Labhart, T., and Mouritsen, H. (2006). Physiological characterization of the compound eye in monarch butterflies with focus on the dorsal rim area. J. Comp. Physiol. A 192, 321–331. doi: 10.1007/s00359-005-0073-6
Stalleicken, J., Mukhida, M., Labhart, T., Wehner, R., Frost, B., and Mouritsen, H. (2005). Do monarch butterflies use polarized skylight for migratory orientation? J. Exp. Biol. 208, 2399–2408. doi: 10.1242/jeb.01613
Stone, T., Webb, B., Adden, A., Weddig, N. B., Honkanen, A., Templin, R., et al. (2017). An anatomically constrained model for path integration in the bee brain. Curr. Biol. 27, 3069–3085. doi: 10.1016/j.cub.2017.08.052
Takahashi, N., Zittrell, F., Hensgen, R., and Homberg, U. (2022). Receptive field structure for two celestial compass cues at the input stage of the central complex in the locust brain. J. Exp. Biol, jeb.243858. doi: 10.1242/jeb.243858
Towne, W. F., and Moscrip, H. (2008). The connection between landscapes and the solar ephemeris in honeybees. J. Exp. Biol. 211, 3729–3736. doi: 10.1242/jeb.022970
Towne, W. F., Ritrovato, A. E., Esposto, A., and Brown, D. F. (2017). Honeybees use the skyline in orientation. J. Exp. Biol. 220, 2476–2485. doi: 10.1242/jeb.160002
Turner-Evans, D., Wegener, S., Rouault, H., Franconville, R., Wolff, T., Seelig, J. D., et al. (2017). Angular velocity integration in a fly heading circuit. Elife 6, e23496. doi: 10.7554/eLife.23496
Turner-Evans, D. B., Jensen, K. T., Ali, S., Paterson, T., Sheridan, A., Ray, R. P., et al. (2020). The neuroanatomical ultrastructure and function of a biological ring attractor. Neuron 108, 145–163. doi: 10.1016/j.neuron.2020.08.006
Vitzthum, H., Müller, M., and Homberg, U. (2002). Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. J. Neurosci. 22, 1114. doi: 10.1523/JNEUROSCI.22-03-01114.2002
Wehner, R. (2003). Desert ant navigation: how miniature brains solve complex tasks. J. Comp. Physiol. A 189, 579–588. doi: 10.1007/s00359-003-0431-1
Keywords: insect, central complex, navigation, orientation, landmark, migration, panorama, lepidoptera
Citation: Nguyen TAT, Beetz MJ, Merlin C, Pfeiffer K and el Jundi B (2022) Weighting of Celestial and Terrestrial Cues in the Monarch Butterfly Central Complex. Front. Neural Circuits 16:862279. doi: 10.3389/fncir.2022.862279
Received: 25 January 2022; Accepted: 10 June 2022;
Published: 30 June 2022.
Edited by:
Wolfgang Stein, Illinois State University, United StatesReviewed by:
Hannah Haberkern, Janelia Research Campus, United StatesMark A. Frye, University of California, Los Angeles, United States
Copyright © 2022 Nguyen, Beetz, Merlin, Pfeiffer and el Jundi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Basil el Jundi, basil.el.jundi@ntnu.no
 Tu Anh Thi Nguyen1
Tu Anh Thi Nguyen1  M. Jerome Beetz
M. Jerome Beetz Christine Merlin
Christine Merlin Keram Pfeiffer
Keram Pfeiffer Basil el Jundi
Basil el Jundi