The Diversity of Cortical Inhibitory Synapses
- 1Division of Cerebral Circuitry, National Institute for Physiological Sciences, Okazaki, Japan
- 2Department of Physiological Sciences, The Graduate University for Advanced Studies (SOKENDAI), Okazaki, Japan
- 3Japan Science and Technology Agency, Core Research for Evolutional Science and Technology, Tokyo, Japan
- 4Laboratory of Neural Circuitry, Graduate School of Brain Science, Doshisha University, Kyoto, Japan
- 5Department of Mathematics, Kyoto University, Kyoto, Japan
The most typical and well known inhibitory action in the cortical microcircuit is a strong inhibition on the target neuron by axo-somatic synapses. However, it has become clear that synaptic inhibition in the cortex is much more diverse and complicated. Firstly, at least ten or more inhibitory non-pyramidal cell subtypes engage in diverse inhibitory functions to produce the elaborate activity characteristic of the different cortical states. Each distinct non-pyramidal cell subtype has its own independent inhibitory function. Secondly, the inhibitory synapses innervate different neuronal domains, such as axons, spines, dendrites and soma, and their inhibitory postsynaptic potential (IPSP) size is not uniform. Thus, cortical inhibition is highly complex, with a wide variety of anatomical and physiological modes. Moreover, the functional significance of the various inhibitory synapse innervation styles and their unique structural dynamic behaviors differ from those of excitatory synapses. In this review, we summarize our current understanding of the inhibitory mechanisms of the cortical microcircuit.
The microcircuit of the neocortex is a very complex, composed of excitatory neurons (including pyramidal cells and spiny stellate cells), inhibitory non-pyramidal interneurons (Jones, 1975; Kawaguchi and Kubota, 1997; Somogyi et al., 1998; Markram et al., 2004; DeFelipe et al., 2013), glutamatergic afferent axons arising from other cortical areas and from subcortical structures such as the thalamus (Jones, 2001; Kuramoto et al., 2009, 2015) as well as non-glutamatergic neuromodulatory afferents from many different brainstem nuclei (Gulledge and Stuart, 2005; Puig et al., 2014, 2015).
The GABAergic non-pyramidal cells, which function as cortical inhibitory interneurons, are located in all cortical layers, and send their axons to nearby areas or across layers or areas somewhat larger than their dendritic fields. At least ten or more GABAergic non-pyramidal cell subtypes had been identified, each with a unique form of axonal arborization (Kawaguchi and Kubota, 1997; Howard et al., 2005; Gonchar et al., 2007; Uematsu et al., 2008; Xu et al., 2010; Kubota et al., 2011; Kubota, 2014; Jiang et al., 2015; Markram et al., 2015) which innervate different domains: axon initial segment, soma, dendritic shaft and spine, of different subtypes of the pyramidal and non-pyramidal cells (Figures 1, 2; Somogyi, 1989; Kawaguchi and Kubota, 1998; Kubota et al., 2007, 2015; Jiang et al., 2013). So-called “basket” cells prefer somata as their synaptic target (Figure 3). They include parvalbumin (PV) positive fast spiking (FS) basket cells (Figures 3A,D–H), non-FS cholecystokinin (CCK) positive large basket cells (Figure 3C) which have a large perikaryon, and non-FS vasoactive intestinal polypeptide (VIP)/corticotrophin releasing factor (CRF)/CCK positive small basket cells with a small perikaryon and small dendritic and axonal fields which innervate inhibitory non-pyramidal and/or pyramidal cells (Peters, 1990; Kawaguchi and Kubota, 1996; Dalezios et al., 2002; Karube et al., 2004; Staiger et al., 2004; Uematsu et al., 2008; Kubota et al., 2011; Hioki et al., 2013; Pfeffer et al., 2013; Pi et al., 2013). In addition to the basket cells, alpha actinin 2 (AA2) positive late spiking (LS; Figure 3B) neurogliaform cells also prefer somata as their synaptic target (Price et al., 2005; Kubota et al., 2007, 2011; Uematsu et al., 2008). The other subtypes include PV positive FS chandelier cells which innervate almost exclusively axon initial segments of pyramidal cells (Kawaguchi and Kubota, 1998), non-FS somatostatin (SOM)/neuropeptide Y (NPY) positive Martinotti cells with ascending axons innervating apical (Silberberg and Markram, 2007) or apical tuft dendritic structures of the pyramidal cells (Wang et al., 2004; Jiang et al., 2013), non-FS VIP/calretinin (CR)/CRF/CCK positive double bouquet cells with descending axons innervating pyramidal cell and/or GABAergic non-pyramidal cells (Figure 1; Kawaguchi and Kubota, 1996, 1997; Karube et al., 2004; Uematsu et al., 2008; Kubota et al., 2011; Kubota, 2014). Other than the PV and somatostatin subgroups, all other subgroups appear to express 5-HT3A receptor (Lee et al., 2010; Rudy et al., 2011).
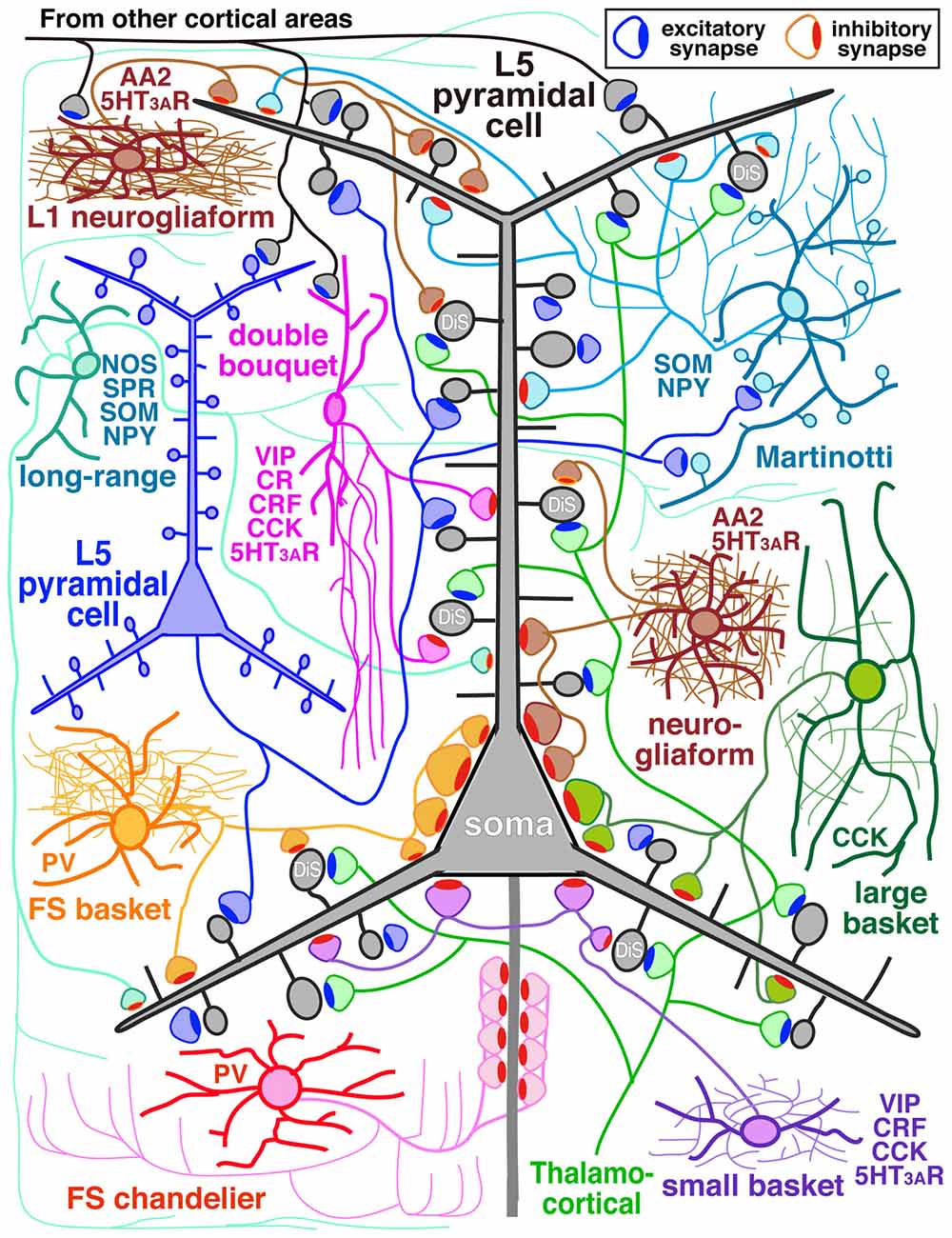
Figure 1. Diagram of cortical microcircuit showing the major subtypes of GABAergic inhibitory interneurons and their synaptic diversity. DiS, dually innervated spine; L5, layer V; AA2, alpha actinin 2; CCK, cholecystokinin; CR, calretinin; CRF, corticotrophin releasing factor; NOS, nitric oxide synthase; NPY, neuropeptide Y; PV, parvalbumin; SOM, somatostatin; SPR, substance P receptor; VIP, vasoactive intestinal polypeptide; 5HT3AR, 5-HT3A receptor.
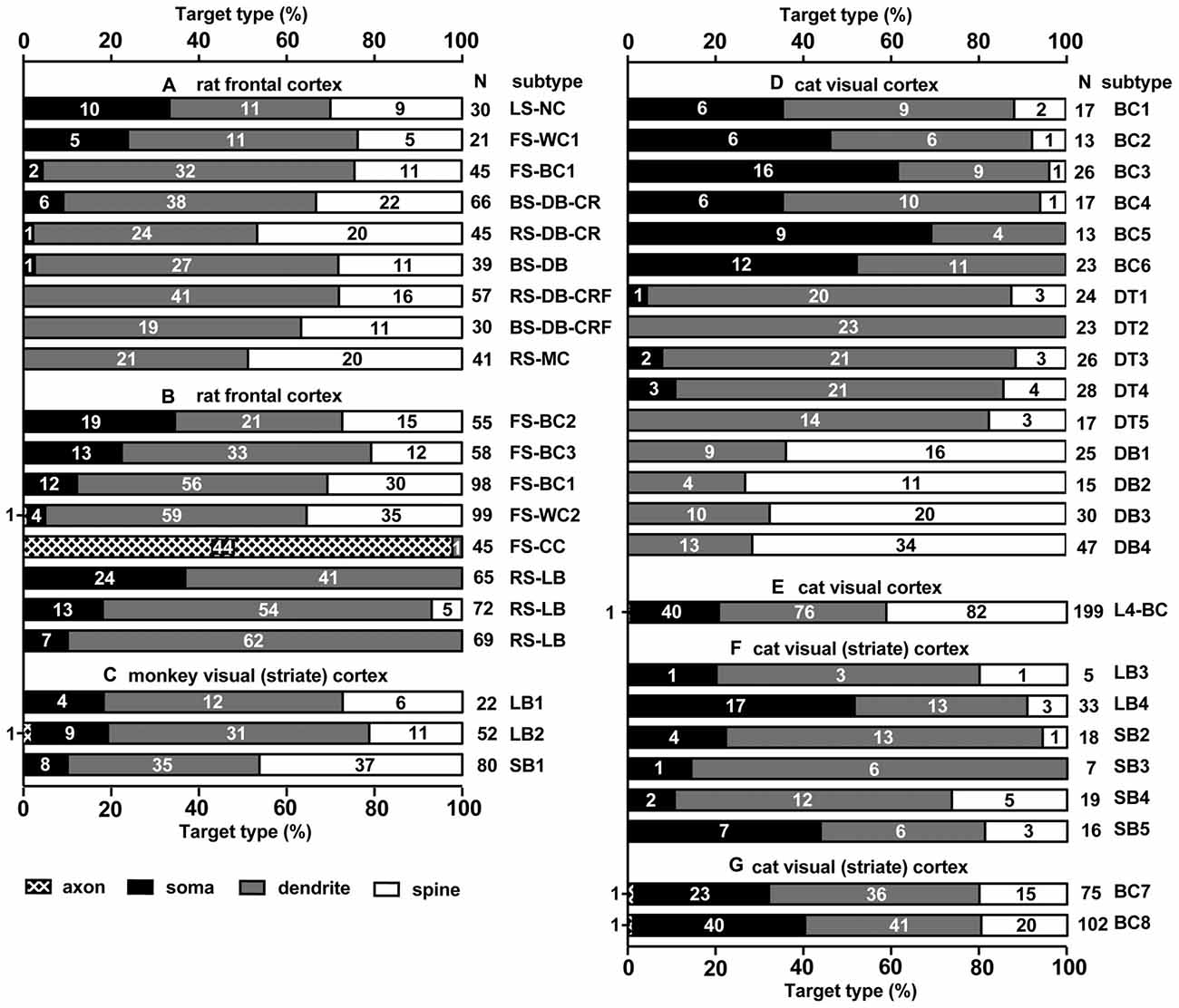
Figure 2. Summary of target structures innervated by different subtypes of cortical inhibitory non-pyramidal cells; in different cortical areas and different species. Each bar corresponds to a single analyzed neuron. Fast spiking (FS)-BC1 cell in (A,B) is the same cell, but analyzed at different occasions and methods. The numbers in the bar chart indicate the number of synaptic boutons analyzed on each type of postsynaptic structure. Total number of analyzed boutons are shown at the right of each bar chart (N). Most spines receiving an inhibitory synapse are dually innervated spines. LS, late spiking; FS, fast spiking; BS, burst spiking; RS, regular spiking; NC, neurogliaform cell; WC, wide arbor cell; BC, basket cell; DB, double bouquet cell; MC, Martinotti cell; CC, chandelier cell; LB, large basket cell; SB, small basket cell (including Clutch cell); DT, dendrite targeting cell; CR, calretinin; CRF, corticotropin releasing factor. (A) Rat frontal cortex is adapted from Kubota et al. (2007), (B) Rat frontal cortex (Kawaguchi and Kubota, 1998), (C) Monkey visual cortex (V1; Kisvárday et al., 1986), (D) Cat V1 (Tamás et al., 1997a), (E) Cat V1 (Kisvárday et al., 1987), (F) Cat V1 (Somogyi and Soltész, 1986), (G) Cat V1 (Somogyi et al., 1983).
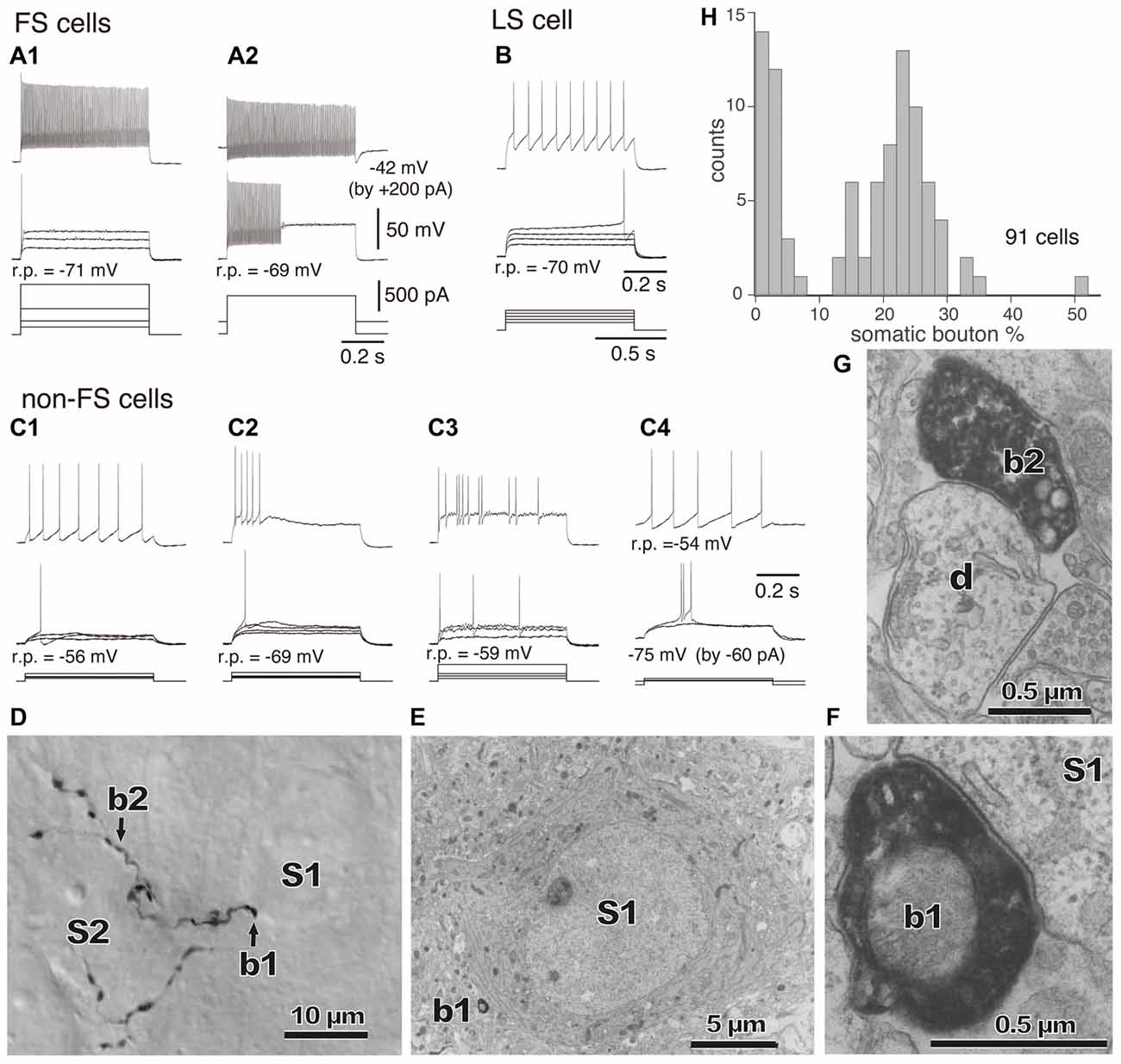
Figure 3. (A–C) Firing responses of nonpyramidal cells induced by depolarizing currents. r.p., Resting potential. DC injected into neuron to change membrane potential is indicated in parentheses. (A1,A2) FS cells. (B) A LS cell. (C1–C4) Non-FS cells. (D) Boutons of an FS basket cell (b1, b2) and their appositions on unstained somata (S1, S2) observed with differential interference contrast. (E) Electron micrograph of synaptic target unstained soma (S1) of the identified bouton (D, b1). (F) Synaptic contact of the bouton (b1) apposition on the soma (S1). (G) Electron micrographs of the bouton b2 and target dendrite (d). (H) Distribution of the somatic bouton percentage of nonpyramidal cells. Nonpyramidal cells were divided into cells with a low and high proportion of somatic boutons. Adapted from Karube et al. (2004).
In addition, some GABAergic cortical cells project to other areas of cortex in a “long-range” manner serving to coordinate large-scale network activity of the microcircuit with distant cortical areas (Figure 1; Tomioka et al., 2005; Caputi et al., 2013; Endo et al., 2016). They express substance P receptor (neurokinin 1 receptor)/neuronal nitric oxide synthase (nNOS)/SOM/NPY (Kubota et al., 1994, 2011; Endo et al., 2016) and have extensive axonal arborizations around the dendritic field with axonal branches extending a long distance vertically into all the layers and horizontally into other cortical areas. For instance the cells in the primary visual cortex (V1) extend their axon fibers into the secondary V1, retrosplenial cortex and across the subcortical white matter to the subiculum (Kubota et al., 1994, 2011; Endo et al., 2016). They lose dendritic spines during development (Kubota et al., 2011).
The different inhibitory non-pyramidal cell subtypes have distinct activity patterns during different cortical states and unique functional roles in the active cortical microcircuit (Klausberger and Somogyi, 2008; Isomura et al., 2009; Gentet et al., 2012; Lee et al., 2013; Pala and Petersen, 2015), although they comprise just 20% of the entire cortical neuron population. For more details, see Kawaguchi and Kubota (1997) and Kubota (2014).
The pyramidal cells are glutamatergic excitatory projection cells and are located in all cortical layers except layer I (Jones, 1984). Several pyramidal cell subtypes are found in each layer (van Aerde and Feldmeyer, 2015). They have different dendritic morphologies and electrophysiological properties, project to different target areas and receive inputs from different neurons (Thomson and Bannister, 2003). For instance, there are at least three pyramidal cell subtypes in the rodent layer 5 (L5), the crossed-corticostriatal (CCS) cells with slender-tufted apical dendrites, the corticopontine (CPn) cells with thick-tufted dendrites and the corticocortical non-striatal-projecting (CCnS) cells with slender-tufted dendrites (Wilson, 1987; Markram et al., 1997; Morishima and Kawaguchi, 2006; Brown and Hestrin, 2009; Morishima et al., 2011; Shepherd, 2013; Kim et al., 2015; Ramaswamy and Markram, 2015; van Aerde and Feldmeyer, 2015). The L5 CCS cells send their axons to ipsi-/contralateral striatum and to other ipsi- or contralateral cortical areas (Wilson, 1987), and receive inputs from layer II/III pyramidal cells, L5 CCS cells, L5 CPn cells and thalamus (Morishima et al., 2011; Hirai et al., 2012; Kim et al., 2015). On the other hand, the L5 CPn cell projects to ipsilateral striatum, thalamus, pons and spinal cord, and receives input from pyramidal cells in layer II/III, L5 CCS cells, CPn cells, basal forebrain and thalamus (Kaneko et al., 2000; Hirai et al., 2012; Ueta et al., 2013, 2014; Kim et al., 2015). The L5 CCnS cells show oval soma shape and significantly higher input resistance (263 or 249 MΩ) than the other subpopulations (CCS: 121 or 144 MΩ, CPn: 146 or 90 MΩ; Kim et al., 2015; van Aerde and Feldmeyer, 2015). This subtype in primary V1 innervates local cortex, but not striatum, and receives inputs from V1, thalamus and basal forebrain (Kim et al., 2015). Different pyramidal subtypes are likely to have different functional roles in the cortical microcircuit (Morita et al., 2012; Li et al., 2015; Lur et al., 2016).
The cortical microcircuit must be regulated and orchestrated exquisitely in each different cortical state, with a well coordinated ensemble of neuronal activities, among the diverse neuronal elements described above. Each of the inhibitory non-pyramidal cell subtypes has its own role in regulating the activity of the cortical microcircuit. For example, the coordination of network activity by gamma oscillations by PV or SOM cells (Cardin et al., 2009; Sohal et al., 2009; Kuki et al., 2015), the fine tuning of pyramidal cell activity with sensory inputs by the Martinotti/SOM cells (Murayama et al., 2009; Gentet et al., 2012), the disinhibition of pyramidal cell activity via excitatory inputs from the other cortical area by 5-HT3A receptor positive cells (Lee et al., 2013). The various inhibitory neurons also make very important contributions to cortical development, memory and synaptic plasticity associated with the critical period in the visual system (Hensch, 2005; Donato et al., 2013, 2015), as well as the acquisition and retention of new memories (Hensch, 2005; Donato et al., 2013, 2015). A deficit or abnormality in the cortical inhibitory systems may be one of the main causes of neuropsychiatric disorders such as epilepsy, autism, schizophrenia, or depression. Cortical non-pyramidal cell subtypes may be the key to the pathology associated with neurological disorders (Rubenstein and Merzenich, 2003; Lewis, 2011; Curley and Lewis, 2012; Lewis et al., 2012; Hunt et al., 2013; Sauer et al., 2015).
The domain specific selectivity of cortical inhibition is expressed as four different innervation styles: axo-somatic, axo-dendritic, axo-spinous or axo-axonic innervations, and the targets could include soma, axon, dendritic spines, proximal large dendrites and distal small dendrites on excitatory or inhibitory neurons (Figures 1, 2, 3, 4, 5; Somogyi, 1989; Somogyi et al., 1998; Thomson and Bannister, 2003; Kubota et al., 2007, 2015; Kubota, 2014). Each innervation style plays a specific role in the cortical microcircuit. This review surveys recent advances in our knowledge of cortical inhibitory mechanisms, especially inhibitory synapses on different domains of the target neuron: soma, dendritic shaft, dendritic spine and axon initial segments.
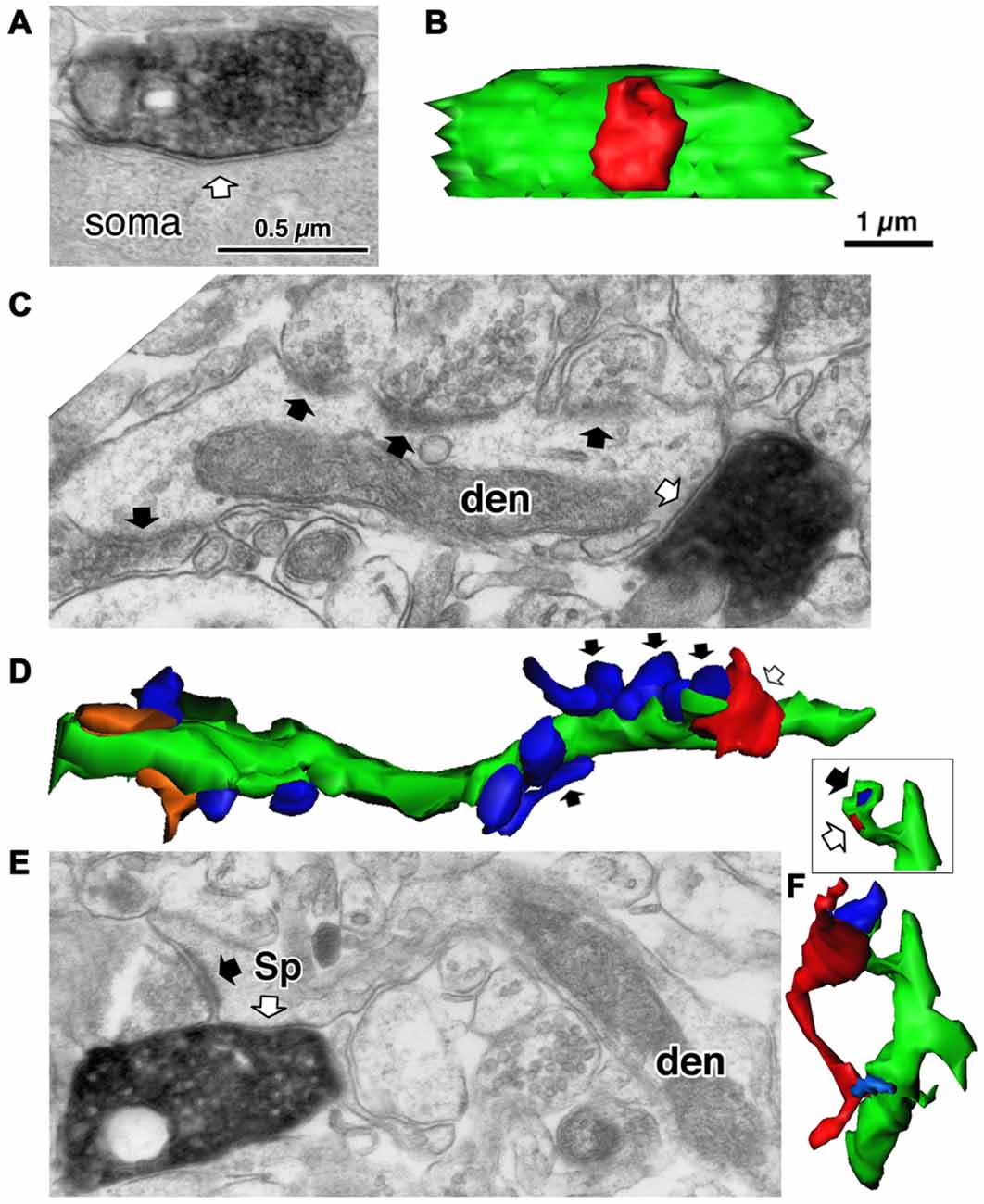
Figure 4. Three different target structures of the inhibitory synapses. (A) A symmetrical synapse (white arrow) of the LS neurogliaform (LS-NG) cell onto a postsynaptic soma. (B) 3D reconstructed image of the synaptic structure shown in (A). The axonal terminal of the LS NG cell (red) innervates the soma (green). (C) A symmetrical synapse (white arrow) from a corticotropin releasing factor (CRF) positive double bouquet cell onto a dendritic shaft (den) with frequent asymmetrical inputs (black arrows). (D) 3D reconstructed image (green) of the dendrite shown in (C). Arrows indicate the same axon terminal boutons shown in (C). Red is an axon terminal of the double bouquet cell. Blue structures are boutons forming asymmetrical synapses. Orange structures are boutons forming symmetrical synapses. (E) A symmetrical synapse (white arrow) from a CR positive double bouquet cell onto a spine head (Sp), which is also innervated by an asymmetrical synapse (black arrow). Scale in (A,C,E) shares the scale bar shown in (A). (F) 3D reconstructed image of synaptic structure shown in (E). An axon terminal of the double bouquet cell (red) innervates the spine head (green), which is also innervated by an asymmetrical synaptic terminal (blue). Inset shows the two synaptic junctions (red and dark blue) on the spine, without the presynaptic boutons. Scale in (B,D,F) share the scale bar shown in (B). Adapted from Kubota et al. (2007).
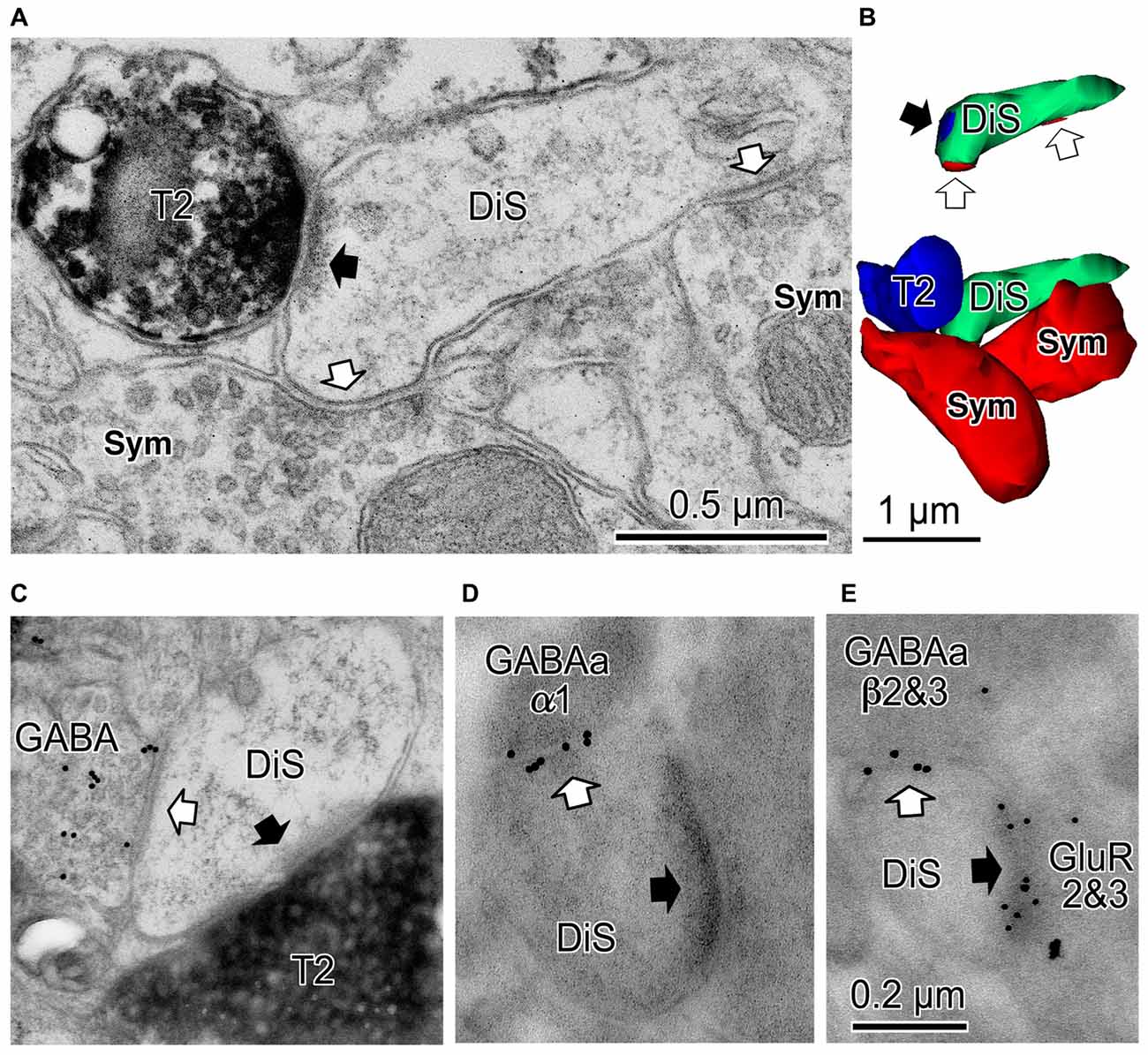
Figure 5. Cortical dually innervated spines. (A) A cortical spine (DiS) was co-innervated by a vesicular glutamate transporter 2 (VGLUT2)-positive (T2) asymmetrical synaptic terminal (black arrow) and two symmetrical synaptic terminals (Sym) (white arrows). (B) 3D reconstructed image of the spine and presynaptic terminals shown in (A). Bottom image is the VGLUT2-positive bouton (T2, blue) contacting the spine head (DiS, green) with a synaptic junction. This spine is also innervated by two symmetrical inputs (Sym, red). Upper image shows synaptic junctions (black arrow for VGLUT2 synapse (blue) and white arrows for symmetrical synapses (red)) on the spine head (DiS, green). (C) A spine head (DiS) innervated by both a VGLUT2-positive (T2) asymmetrical synaptic terminal (black arrow) and a GABA-positive (colloidal gold particle labeled) symmetrical synaptic terminal (white arrow). (D) A spine head (DiS) innervated by an asymmetrical synaptic terminal (black arrow) and also innervated by a symmetrical synaptic terminal (white arrow). Gold particles label GABAA α1 receptor subunits localized along the synaptic junction of the symmetrical synapse. (E) A spine head (DiS) innervated by an asymmetrical synaptic terminal (black arrow) and also innervated by a symmetrical synaptic terminal (white arrow). Larger gold particles (15 nm) labeled GABAA β2&3 receptor subunits localized along the synaptic junction of the symmetrical synaptic terminal and smaller particle (10 nm) labeled glutamate receptor GluR2&3 subunits of the associated asymmetrical synaptic junction. Scale in (C) shares the scale bar shown in (A), and the scale in (D) is the same as shown in (E). Adapted from Kubota et al. (2007).
Axo-Somatic Inhibition
The strong axo-somatic inhibition is the basis for the “classical” concept of inhibition in the cortical microcircuit (Somogyi, 1989; Kawaguchi and Kubota, 1996, 1998; Somogyi et al., 1998; Kubota et al., 2007, 2015; Kubota, 2014), however quantitatively axo-somatic contacts are just a small fraction of the total synaptic output of inhibitory non-pyramidal cells (for instances: 7% and 12% of the inhibitory synaptic contacts in the cat and monkey primary visual cortices, respectively; Figure 2; Beaulieu and Colonnier, 1985; Beaulieu et al., 1992). Even among the axon terminals of a basket cell, the axo-somatic terminals comprise only 10–37% in rat frontal cortex (Kawaguchi and Kubota, 1998), 10–70% in cat striate cortex (Somogyi et al., 1983; Kisvárday et al., 1985, 1987; Somogyi and Soltész, 1986; Tamás et al., 1997a), and 10–18% in monkey striate cortex (Kisvárday et al., 1986; Figure 2). Clearly the axo-somatic terminal represents only a fraction of the basket cell terminals. About half of the non-pyramidal cell subtypes innervate somata of target cells to various degrees. The FS basket cell, the small basket cell, the large basket cell, and the neurogliaform cell innervate somata frequently (Figures 1, 2; Karube et al., 2004; Kubota et al., 2007; Uematsu et al., 2008; Kubota, 2014). The FS basket cell, which expresses PV, and the large basket cell, which expresses CCK and the cannabinoid receptor (CB1R), respond differently to repetitive signals with a mechanism exquisitely regulated by cannabinoids (Hefft and Jonas, 2005; Glickfeld and Scanziani, 2006; Freund and Katona, 2007). The FS basket cells respond reliably and immediately to repetitive signals, whereas the CCK expressing large basket cells respond to repetitive signals with depression and delay in the hippocampus (Glickfeld and Scanziani, 2006). Interestingly CCK enhances the FS basket cell activity and induces endocannabinoid-mediated inhibition of GABA release from CCK/CB1R expressing basket cell terminals in the hippocampus (Foldy et al., 2007). During theta oscillations in vivo, the FS basket cells fire on the descending phase, whereas the CCK expressing large basket cells fire on the ascending phase (Klausberger et al., 2003, 2005). This is a typical example of how different non-pyramidal cell subtypes have different functional roles in the cortical microcircuit.
The “basket cell” was defined as a cell type making many axo-somatic boutons which form pericellular nests around the pyramidal cell somata, resembling a basket (Figures 3D–F; Jones and Hendry, 1984). The axo-somatic boutons are therefore called “basket terminals”. In rat frontal cortex, the basket cells are defined as having basket terminals for >12% and typically 25% or more among all its axonal boutons (Figure 3H; Karube et al., 2004). The basket terminals innervate pyramidal cell somata (Figures 1, 2, 3) and also somata of other basket cells (Jiang et al., 2015). Autaptic self-inhibition of a basket cell on its own soma, which usually induces a large inhibitory postsynaptic potential (IPSP), is also frequently found (Tamás et al., 1997b; Deleuze et al., 2014). The inhibitory somatic synapse junction area is larger than the other inhibitory synapses and its conductance is probably <2 nS (typically 0.4–1 nS; Kubota et al., 2015). We estimated the conductance using a unit value of electric charge/synaptic junction area (343.3 fC/μm2) measured by a paired recording study of the FS basket cell and the L5 CCS pyramidal cell combined with 3D reconstruction of the synaptic junction from serial electron micrographs and simulation analysis (Kubota et al., 2015). The multiple somatic synapses of a single presynaptic FS basket cell clustered on a single postsynaptic pyramidal cell soma are probably activated simultaneously, because of their large probability of release, as found in hippocampal glutamatergic synapses (Holderith et al., 2012). A typical FS cell makes, on average, 3.2 ± 2.0 (range 1–13 basket terminals per postsynaptic cell soma, analysis of 112 axon terminals of 7 presynaptic FS basket cells) contacts on the soma of a postsynaptic pyramidal cell (Figures 3D–H; Karube et al., 2004). One presynaptic FS basket cell may hyperpolarize the postsynaptic pyramidal cell soma by >1.33 mV with simultaneous activation of all its axo-somatic contacts, as shown by simulation analysis with holding membrane potential −65 mV (S1–S4 in Figures 6A,B; Kubota et al., 2015). Similar unitary IPSP amplitudes (0.4–3 mV) were observed in dual recording experiments in cortical or hippocampal slices (Buhl et al., 1994; Stuart, 1999; González-Burgos et al., 2005; Thomson and Lamy, 2007; Ma et al., 2012; Mercer et al., 2012) Thus a unitary IPSP would be sufficient to cancel an unitary excitatory postsynaptic potential (EPSP) induced by a single spike from a presynaptic pyramidal cell, because the EPSP amplitude is in a similar range (0.3–5.9 mV, Table 1 in Thomson et al., 2002; Feldmeyer et al., 2005; Frick et al., 2007, 2008; Thomson and Lamy, 2007). Multiple FS basket cells nearby may fire simultaneously due to frequent connection via gap junctions and mutual innervations (Amitai et al., 2002; Fukuda et al., 2006; Hu et al., 2011; Otsuka and Kawaguchi, 2013), so the postsynaptic pyramidal cell could be inhibited via much deeper hyperpolarization and/or via shunting inhibition mediated by synaptic high GABAA conductance under physiological conditions. This would counteract strong excitation and may prevent the pyramidal cell from firing (Kubota et al., 2015). In conclusion, the somatic GABAergic synapse has very strong inhibitory effect on target cell activity, although axo-somatic boutons comprise only a small fraction (a few to 10%) of all GABAergic synapses found on one postsynaptic neuron (Ahmed et al., 1997; Mátyás et al., 2004).
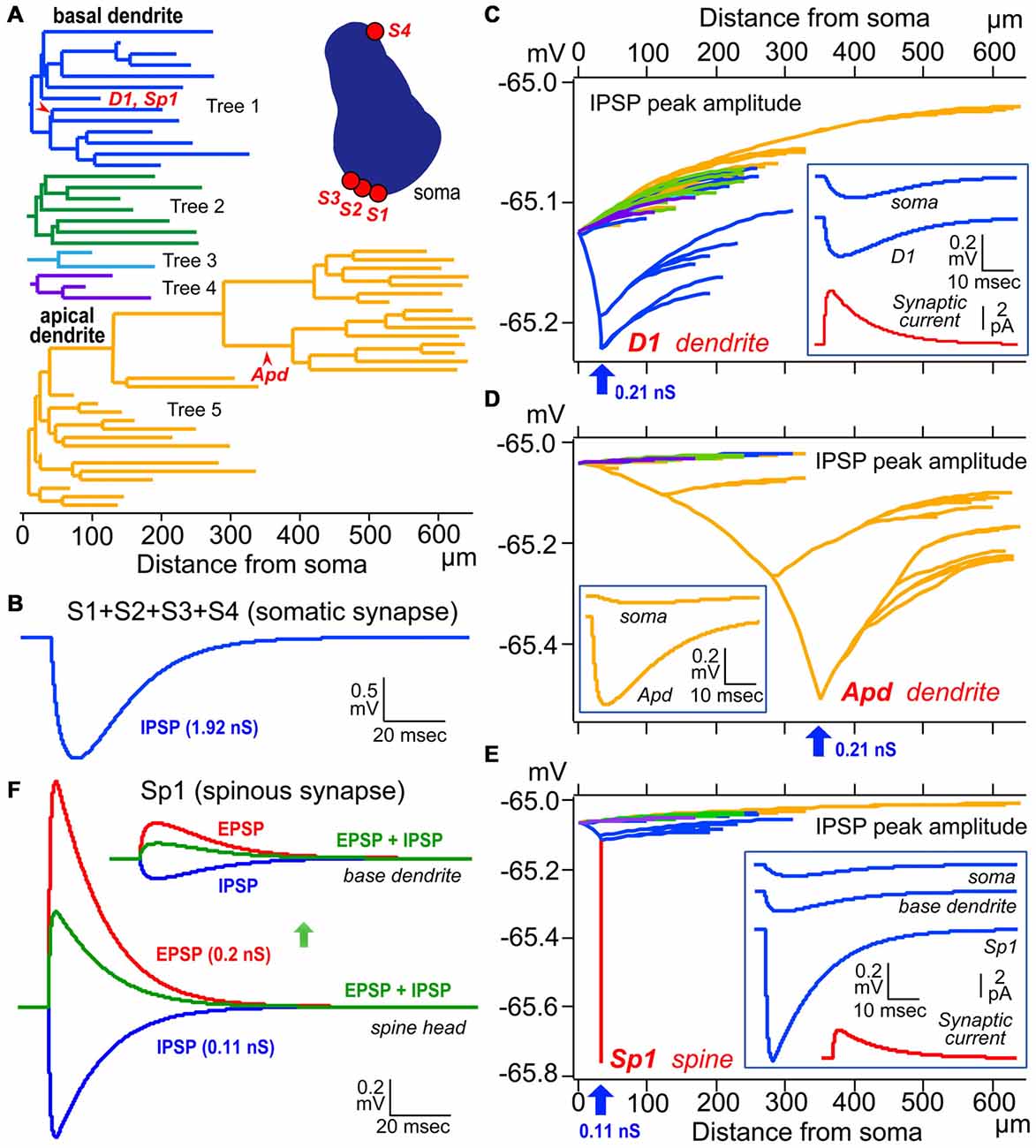
Figure 6. Simulation analysis of inhibitory postsynaptic potential (IPSP) on dendritic spine, shaft and somatic IPSCs. (A) Left, color-coded dendrogram with inhibitory synaptic current injection sites on the dendrites (red arrowheads). Right, a cross-section of the cell body of the model pyramidal cells with four somatic synaptic contacts. (B) The simulated IPSP wave-form at the soma induced by injection of a total of 1.92 nS inhibitory conductance summed across the somatic synapses S1–S4. (C) Peak dendro-somatic potential changes (color-coded as in A) induced by an inhibitory conductance of 0.21 nS (inset, red trace, simulated synaptic current) injected into the shaft of a basal dendritic branch (D1, blue arrow; corresponding to D1 in A). The simulated IPSP wave-forms at the injection site (D1) and soma are shown in the inset (blue traces). Resting potential is −65 mV. (D) Peak dendro-somatic potential changes induced by an inhibitory conductance of 0.21 nS (simulated IPSC is quite similar to the red trace shown in inset in C, red trace) injected into the shaft of an apical dendritic branch (Apd, blue arrow; corresponding to Apd in A). The simulated IPSP wave-forms at the injection site (Apd) and soma are shown in the inset (yellow traces). Resting potential is −65 mV. (E) Peak membrane potential changes over the somato-dendritic membrane induced by an inhibitory conductance of 0.11 nS injected at the spine head Sp1 (blue arrow, corresponding to Sp1 in A; simulated IPSC shown in red trace in inset). Peak inhibitory potential in the spine is indicated in red. The simulated IPSP wave-forms at the injection site (Sp1), base of dendrite and soma are shown in inset (blue traces). (F) Reduction (green) of the excitatory postsynaptic potential (EPSP) resulting from the injection of an EPSC waveform (with peak conductance of 0.2 nS; red) at the Sp1 spine head (lower superimposed traces) and base dendrite locus (upper superimposed traces)) by an IPSC (0.11 nS; blue) injected at the same site and time. Adapted from Kubota et al. (2015). The simulations were made with “NEURON” (Hines and Carnevale, 1997) and the CS56 pyramidal cell model neuron publicly available at ModelDB (Accession no: 183424; http://senselab.med.yale.edu/ModelDB/showModel.cshtml?model=183424. The datasets are also available at http://www.nips.ac.jp/circuit/).
Axo-Dendritic Inhibition
Except the chandelier/axo-axonic cells, all the inhibitory non-pyramidal cells in the rat frontal cortex innervate the dendritic shaft as their major target domain. Axo-dendritic boutons comprise 37–90% of the total synaptic contacts of the individual inhibitory non-pyramidal cell (Kawaguchi and Kubota, 1998; Kubota et al., 2007; Figures 1, 2, 3G, 4C,D). The inhibitory dendritic synapse junction area is smaller than the somatic inhibitory synapses and its estimated conductance is <1 nS (typically 0.1–0.8 nS; Kubota et al., 2015). The synaptic junction area size is well correlated with the target dendrite size, which probably provides an effective impedance matching (Kubota and Kawaguchi, 2000; Kubota et al., 2015). In our simulation analysis, inhibitory synaptic conductance (0.21 nS) injection into the basal dendritic shaft of a postsynaptic pyramidal cell held at a membrane potential of −65.0 mV (D1 in Figures 6A,C, 34 μm from soma) hyperpolarized the local dendrite by 0.23 mV. The hyperpolarization attenuated steeply in the proximal direction but relatively gently in the distal direction (Figure 6C), similar to the spread of an EPSP (Gidon and Segev, 2012). It caused a hyperpolarization of 0.13 mV at the soma (Figure 6C). The same inhibitory synaptic conductance (0.21 nS) injection on a further distal dendritic shaft located in the apical dendritic branch (Apd in Figure 6A, 350 μm from soma) hyperpolarized the local dendrite by 0.51 mV, but attenuated greatly at a distance (Figure 6D), hyperpolarizing the somatic membrane potential by only 0.04 mV (Figure 6D). These simulation analyses indicate that the inhibitory synapse on dendrites effectively hyperpolarizes the membrane potential only in the local dendritic domain. In addition, assuming more excitatory synaptic conductance in vivo, the inhibitory synaptic current would not spread any further. This assumes a only a simple inhibitory mechanism, although GABA shunting inhibition, voltage dependent conductance and so on might also be considered from a physiological point of view (Song et al., 2011; Doyon et al., 2016). In apical dendrites of pyramidal cells in layer 2/3 of the binocular region in the mouse primary V1, spine density is 4.42/10 μm, density of inhibitory synapses along the apical dendritic shaft is 1.68/10 μm and density of inhibitory synapses on spines is 0.71/10 μm. Densities are quite similar on basal dendrites (Chen et al., 2012; Villa et al., 2016). Usually pyramidal cell spine receives only one excitatory synapse, and almost all excitatory synapses on pyramidal cells are on spines (Chen et al., 2012), therefore the ratio of excitatory to inhibitory synapses on the dendrites can be about 2:1.1. In vivo, excitatory and inhibitory inputs are dynamically balanced in individual neurons from moment to moment (Xue et al., 2014). Stronger inhibition indicates simultaneously larger excitatory inputs. The excitatory conductance increase at dendrites would limit the spatial spread of increased inhibition thereby spatially confining the dendritic inhibitions (Doyon et al., 2016).
Axo-Spinous Inhibition of Dually Innervated Spines
Most inhibitory cells also innervate dendritic spines of pyramidal cells. The chandelier and CCK positive large basket cells are the only subtypes which do not target the spine (Figures 1, 2; Kawaguchi and Kubota, 1998). Inhibitory synapses on spines were discovered by electron microscopic observation of the cat cortex, and each recipient spine was found to receive also an asymmetrical (excitatory) synapse (Jones and Powell, 1969), so these spines were called dually innervated spines (DiS). Axo-spinous synapses originate from diverse non-pyramidal cell subtypes in different species: rodent, cat, monkey and human cerebral cortex. About 20–70% of the axon terminals of the cortical non-pyramidal cells target spines (Figures 1, 2, 4E,F, 5; Somogyi and Cowey, 1981; Somogyi et al., 1983; Kisvárday et al., 1985, 1987, 1990; Somogyi and Soltész, 1986; Tamás et al., 1997a; Kubota et al., 2007). The DiSs were found in the diverse cortical areas; frontal (Kubota et al., 2007, 2015), visual (Chen et al., 2012; van Versendaal et al., 2012; Villa et al., 2016) and somatosensory cortex (Knott et al., 2002; Isshiki et al., 2014), as well as in subcortical areas (Wilson et al., 1983; Ingham et al., 1998). This suggests that the inhibition targeting spines is a widespread motif in the microcircuitry of the brain.
The excitatory asymmetrical synaptic input on the cortical DiS originates from thalamocortical excitatory axon terminal that expresses vesicular glutamate transporter 2 (VGLUT2; (Figures 5A–C; Kubota et al., 2007). The excitatory afferents in the rodent frontal cortex are probably from the ventral anterior-ventral lateral (VA/VL) or ventral medial (VM) motor thalamic nuclei (Kuramoto et al., 2009, 2015; Shigematsu et al., 2015). The DiSs are large in volume and frequently receive a perforated synapse (Figure 5C). The inhibitory synapse of the DiS expresses GABAA alpha 1 and beta 2&3 receptors and the excitatory synapse has AMPA GluR2&3 receptors (Figures 5D,E; Kubota et al., 2007). These findings suggest that the DiS is a mature, stable spine (Matsuzaki et al., 2001; Holtmaat et al., 2006; Isshiki et al., 2014; Villa et al., 2016). The inhibitory synapse on the spine should efficiently veto the thalamic excitatory input and may reduce the probability of pyramidal cell firing (Sun et al., 2006; Gulledge et al., 2012; Chiu et al., 2013; Kubota et al., 2015). The largest proportion of spines with inhibitory synapses was found in layer 1 (16.3% of all VGLUT2 positive (thalamic) recipient spines). Towards the deeper layers, the fraction of VGLUT2-recipient spines which are dually innervated is gradually reduced (layer 2/3: 14.5%, layer 4: 8.7%, layer 5: 6.8% and layer 6: 4.4%; Kubota et al., 2007). The estimated fraction of DiS among all spines in each layer is <10% in layer 1, <3% in layer 2/3, <2% in layer 5 and <1% in layer 6.
The junction area of inhibitory spine synapse is similar or smaller than the dendritic inhibitory synapse, and its estimated conductance is <0.5 nS (typically 0.05–0.3 nS; Kubota et al., 2015). The synaptic junction area is well correlated with the target spine head volume as with the target dendrite (Kubota et al., 2015). Inhibitory synaptic conductance (0.11 nS) injected into the spine head (Sp1 in Figures 6A,E) resulted in a strong 0.78 mV hyperpolarization of the target spine head, but only 0.12 mV was conducted to the base dendritic region (Figure 6E). This indicates that the spine head is well isolated electrotonically by the high resistance of the spine neck (500 MΩ; Harnett et al., 2012). To analyze the effect of the IPSP on an EPSP at the DiS, 0.2 nS excitatory synaptic conductance in addition to the 0.11 nS inhibitory synaptic conductance were injected in the spine head. The excitatory current depolarized the local spine head by 1.3 mV and the inhibitory current reduced the EPSP by about a half (Figure 6F). Therefore the inhibitory spine synapse can effectively veto the local excitatory synaptic input, at least blocking NMDA receptor activation (Figure 6F; Gulledge et al., 2012; Chiu et al., 2013).
Strong Axo-Dendritic/Axo-Spinous Net Inhibition
Although the individual inhibitory axo-dendritic/axo-spinous synapses produce only a small local inhibitory conductance in comparison with the axo-somatic synapses, the local single-bouton IPSP in the dendrite is not any smaller than the local IPSP in the soma, and their integrated inhibition is powerful (Cossart et al., 2001; Gentet et al., 2012). Axo-dendritic inhibition is the major inhibition style among the cortical GABA synapses. About 90% or more of the cortical inhibitory synapses are axo-dendritic/axo-spinous (Ahmed et al., 1997; Mátyás et al., 2004). About 5–20 axonal boutons of one non-pyramidal cell usually contact different, distant dendrites of the target pyramidal cell, and the resulting hyperpolarizations may sum poorly (Kubota et al., 2015). Even so, the scattered inhibitory synapses on different dendritic branches can effectively reduce the integrated EPSP amplitude conducted to the soma if inhibitory cells fire at frequencies of 40–50 Hz (Isomura et al., 2009). Together with the GABA shunting effects (Gidon and Segev, 2012), axo-dendritic/axo-spinous inhibition can inhibit the spiking activity of the postsynaptic target pyramidal cell (Cossart et al., 2001; Gentet et al., 2012; Kubota et al., 2015). The inhibition of individual dendritic branches and/or spines may also have the advantage of inhibiting a functionally specific excitatory signal on the dendritic domain (Chen et al., 2013), which is in marked contrast to somatic inhibition, which inhibits all incoming excitation non-specifically.
Different Forms of GABA Mediated Inhibition
The cortical inhibition styles mentioned above mostly involve ionotropic GABAA receptors, which have a rapid synaptic event lasting only a few milliseconds and are mediated by Cl− ion flow through the synaptic channels. On the other hand, extrasynaptically located metabotropic GABAB receptors mediate very slow IPSCs lasting for tens of milliseconds or occasionally even longer (Kawaguchi, 1992; Tamás et al., 2003; Schwenk et al., 2010) and these slow IPSCs arise from non-synaptic volume transmission (Oláh et al., 2009) or high GABA release by burst firing of a presynaptic interneurons (Thomson and Destexhe, 1999). They also mediate inhibition by postsynaptic NMDA receptor mediated Ca2+ influx reduction acting via the downregulation of cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA), and by suppressing transmitter glutamate release by inhibition of presynaptic voltage-sensitive Ca2+ channels and/or by activation of K+ channels (Chalifoux and Carter, 2011; Lur and Higley, 2015; Urban-Ciecko et al., 2015). The apical tuft dendrites of the L5 pyramidal cell in layer 1 are inhibited by two different microcircuits using GABAA or GABAB-receptors (Palmer et al., 2012a). The first one uses exclusively GABAA-receptors and mediates feedback inhibition via the Martinotti cell after in vivo contralateral hindlimb stimulation. This circuit controls the sensitivity and dynamic range of the L5 pyramidal cell (Murayama et al., 2009). The first excited L5 pyramidal cell recruits the Martinotti cells to inhibit the tuft dendrites of the neighboring L5 cells as a surround inhibition (Palmer et al., 2012a). The second inhibitory circuit consists of the AA2 positive neurogliaform cells in layer I, excited by callosal excitatory axonal fibers activated by ipsilateral hindlimb stimulation. The AA2 positive neurogliaform cells inhibit the apical tuft dendrites of the L5 pyramidal cells with GABAB-mediated inhibition, and reduce their spiking activity by 25% in comparison to the control condition of the contralateral hindlimb stimulation only (Palmer et al., 2012b). These results illustrate how the different forms of inhibition in different cortical microcircuits are exquisitely used in regulating cortical activity in the living body.
Furthermore, a different form of inhibition, shunting inhibition, may suppress the excitatory signal more efficiently. Shunting inhibition attenuates the EPSP divisively, by reduction in input resistance of the postsynaptic membrane rather than by hyperpolarizing the postsynaptic membrane potential. This works effectively at neuronal domains where the membrane resting potential is similar to the inhibitory synaptic reversal potential (Gulledge and Stuart, 2003; Song et al., 2011), and the inhibitory synapse situated on the conduction pathway of EPSP to the action potential initiation site (Hao et al., 2009). The shunting is largely confined to the same branch and high for inhibitory synapses located in distal dendritic branches (Hao et al., 2009). A simulation analysis by Gidon and Segev (2012) suggested that shunting can spread beyond the anatomical domain demarcated by the inhibitory synapses, can effectively counteract the excitatory current generated in the nearby dendritic domain, even under higher excitation/inhibition ratios (>2; Megías et al., 2001; Merchán-Pérez et al., 2009; Gidon and Segev, 2012).
In Vivo Structural Dynamics of the Inhibitory Synapses
The cortical inhibitory synapse has its own structural dynamics which is different from that of the cortical excitatory synapse. Strong thalamic input during whisker stimulation for 24 h increases the number of DiS in somatosensory cortex (Knott et al., 2002). Monocular deprivation, a model of sensory input-dependent plasticity, induces loss of inhibitory dendritic shaft and spine synapses in the primary V1 (Chen et al., 2012; van Versendaal et al., 2012). The inhibitory synapses on DiS are more dynamic than the inhibitory synapses on the dendritic shaft or excitatory synapses on spines. They frequently exhibit recurrent dynamics, i.e., repetitive appearance and disappearance of inhibitory synapses on the DiS, under daily in vivo imaging, even in normal physiological circumstances. Under the same conditions, the excitatory synapses on the host spines remained stable (Villa et al., 2016). This characteristic structural dynamics of the inhibitory synapses provides a potential mechanism for reversible gating of specific excitatory connections, such as visual input from thalamic lateral geniculate nucleus (Villa et al., 2016).
Axo-Axonic GABA Response
Chandelier cells almost exclusively innervate the axon initial segments of pyramidal cells with vertically oriented axon-terminal bouton alignment and may target other chandelier cells as well (Figure 1; Somogyi, 1977; Jiang et al., 2015). Five to six chandelier cells may converge onto one axon initial segment of a pyramidal cell in layer 2/3 of cat striate cortex with about eight presynaptic axo-axonic terminal boutons per chandelier axonic bouton cartridge (Somogyi et al., 1982), and 3.8 ± 0.3 chandelier cells may participate in the innervation with 4.1 ± 0.2 presynaptic boutons in mouse somatosensory cortex (Inan et al., 2013). The chandelier cells do not innervate all the pyramidal cells evenly. Pyramidal cells in supragranular layers receive large number of axo-axonic synapses on the axon initial segment, 16–23 for callosal cells and 22–28 for ipsilateral corticocortical cells, whereas the corticothalamic pyramidal cells in the infragranular layers only receive 1–5 synapses in cat V1 (Fariñas and DeFelipe, 1991). The chandelier cells have been found in rodent, cat, monkey and human cortex (Somogyi, 1977; Somogyi et al., 1982; DeFelipe et al., 1989; Kawaguchi and Kubota, 1998; Szabadics et al., 2006; Inan et al., 2013; Jiang et al., 2015). Most chandelier cells express PV and are FS cells, while the others are CRF-positive (Lewis and Lund, 1990) and/or non-FS cell (Kawaguchi, 1995; Taniguchi et al., 2013). They probably suppress the target cell spiking activity by releasing the inhibitory transmitter GABA. This was shown by a local unitary field analysis of spike discharge of axo-axonic cells in the hippocampus, and by the population discharge activity timing of pyramidal and chandelier cells during theta rhythm oscillation (Klausberger and Somogyi, 2008; Glickfeld et al., 2009; Viney et al., 2013). However they may also excite the postsynaptic cell due to high Cl− concentration of the intracellular fluid of the target axon which is probably achieved by a low potassium chloride co-transporter 2 (KCC2) distribution in the axon initial segment of the target pyramidal cells (Szabadics et al., 2006; Woodruff et al., 2009; Báldi et al., 2010) and Na-K-2Cl co-transporter (NKCC1) existence on the neuronal membrane for Cl− uptake into intracellular space (Khirug et al., 2008). The equilibrium potential of GABA (EGABA) values obtained with local uncaging of GABA on axon initial segment, soma and dendrite of the dentate gyrus cells of hippocampus using gramicidin-perforated patch-clamp method were −59.4 ± 1.5 mV, −65.8 ± 1.2 mV, and −70.9 ± 1.5 mV, respectively, and the intracellular levels of Cl− [Cli] calculated on the basis of the EGABA values were 11, 7.9 and 6.0 mM, respectively (Khirug et al., 2008). Under a resting membrane potential of −72.7 ± 2.6 mV measured using the gramicidin-perforated patch-clamp method, a marked depolarizing driving force of about 13 mV can be mathematically predicted at the axon initial segment of the target cells, and GABA action may be associated with the efflux of chloride ions and the depolarization of the axon initial segment. With more excitatory synaptic conductances in vivo, the membrane potential is probably more depolarized and a weak hyperpolarizing driving force can be obtained. As a result, GABAA response could hyperpolarize the membrane potential at the axon initial segment by a small chloride ions influx. This is supported by in vivo studies or slice preparation (Klausberger and Somogyi, 2008; Glickfeld et al., 2009; Viney et al., 2013). Thus they could work in a diametrically opposite fashion depending on the circuit condition (Woodruff et al., 2011). This topic is still controversial and further studies should be done.
Excitatory/Inhibitory GABA Response
GABA responses at dendritic segments of cortical pyramidal cells could be excitatory under certain circumstances, whereas somatic GABA responses were inhibitory when coincident with excitatory synaptic potentials (Gulledge and Stuart, 2003). The ambient GABA could excite or inhibit adult hippocampal interneurons depending on the activated conductances, with its depolarizing reversal potential (Song et al., 2011). These suggest that the excitatory or inhibitory actions of GABA are dependent not only on the reversal potential, but also on the activated conductance. In the inflammatory chronic pain model mouse, the dendritic and somatic KCC2 expression is reduced in layer 2/3 pyramidal cells of somatosensory cortex, making the GABA equilibrium potential more positive than the control (Eto et al., 2012). In this condition, the GABA response could depolarize the pyramidal cells at the resting membrane potential, −70 ~ −80 mV (Gulledge and Stuart, 2003), but GABA actions still seem to be inhibitory on the basis of the GABAA receptor agonist/antagonist application studies in vivo (Eto et al., 2012). This suggests that GABAergic transmission could be inhibitory even in the case of a KCC2 reduction. To fully understand the GABAergic actions, we should know the dynamics of intracellular Cl− concentration, maybe dependent on the domain (somata, axons and dendritic shafts/spines) and the intracellular membrane potential at each domain, and how hyperpolarization and shunting inhibitions are used within individual domains (Song et al., 2011; Doyon et al., 2016).
Future Aspects
Some of the information on inhibitory styles in cortical microcircuits presented above has been obtained recently, using new techniques. However, we still have many unresolved questions concerning inhibition in the cortical microcircuit architecture and its functional implications. For instance, it would be interesting to know why one non-pyramidal cell innervates its target cell on different membrane domains, why inhibitory synapses on spines are recurrently dynamic, and how the diverse non-pyramidal cell subtypes exert coordinated inhibition to enable highly complex cortical activity patterns. Future studies are sure to make new and important findings on inhibitory structure and function in the cortical microcircuit.
Ethics Statement
Animal experimentation: All surgical and animal care methods was performed in strict accordance with the Guidelines for the Use of Animals of IBRO and our institutional Animal Care and Use committee (National Institute for Physiological Sciences) with reference number 15A091. All surgery was performed under ketamine and xylazine, or isoflurane anesthesia, and every effort was made to minimize suffering.
Author Contributions
YK: conception and design, acquisition of data or micrographs, analysis and interpretation of data, making figure panels, drafting or revising the article. FK: making figure panels, drafting or revising the article. MN: acquisition of simulation data. YK: conception and design, drafting or revising the article.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (A; 25250005), (B; 25290012), (15K14324) and on Innovative Areas “Adaptive circuit shift (No. 3603)” (26112006; 15H01456) and “Prediction and decision making (No. 4303)” (26120730) of The Ministry of Education, Culture, Sports, Science, and Technology, Japan, The NOVARTIS Foundation (Japan) for the Promotion of Science.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Sayuri Hatada for technical assistance, Drs. Steven R. Vincent and Ariel Agmon for valuable comments.
References
Ahmed, B., Anderson, J. C., Martin, K. A., and Nelson, J. C. (1997). Map of the synapses onto layer 4 basket cells of the primary visual cortex of the cat. J. Comp. Neurol. 380, 230–242. doi: 10.1002/(SICI)1096-9861(19970407)380:2<230::AID-CNE6>3.0.CO;2-4
Amitai, Y., Gibson, J. R., Beierlein, M., Patrick, S. L., Ho, A. M., Connors, B. W., et al. (2002). The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J. Neurosci. 22, 4142–4152.
Báldi, R., Varga, C., and Tamás, G. (2010). Differential distribution of KCC2 along the axo-somato-dendritic axis of hippocampal principal cells. Eur. J. Neurosci. 32, 1319–1325. doi: 10.1111/j.1460-9568.2010.07361.x
Beaulieu, C., and Colonnier, M. (1985). A laminar analysis of the number of round-asymmetrical and flat-symmetrical synapses on spines, dendritic trunks and cell bodies in area 17 of the cat. J. Comp. Neurol. 231, 180–189. doi: 10.1002/cne.902310206
Beaulieu, C., Kisvarday, Z., Somogyi, P., Cynader, M., and Cowey, A. (1992). Quantitative distribution of GABA-immunopositive and -immunonegative neurons and synapses in the monkey striate cortex (area 17). Cereb. Cortex 2, 295–309. doi: 10.1093/cercor/2.4.295
Brown, S. P., and Hestrin, S. (2009). Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature 457, 1133–1136. doi: 10.1038/nature07658
Buhl, E. H., Halasy, K., and Somogyi, P. (1994). Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368, 823–828. doi: 10.1038/368823a0
Caputi, A., Melzer, S., Michael, M., and Monyer, H. (2013). The long and short of GABAergic neurons. Curr. Opin. Neurobiol. 23, 179–186. doi: 10.1016/j.conb.2013.01.021
Cardin, J. A., Carlén, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K., et al. (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667. doi: 10.1038/nature08002
Chalifoux, J. R., and Carter, A. G. (2011). GABAB receptor modulation of synaptic function. Curr. Opin. Neurobiol. 21, 339–344. doi: 10.1016/j.conb.2011.02.004
Chen, J. L., Villa, K. L., Cha, J. W., So, P. T., Kubota, Y., and Nedivi, E. (2012). Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74, 361–373. doi: 10.1016/j.neuron.2012.02.030
Chen, T. W., Wardill, T. J., Sun, Y., Pulver, S. R., Renninger, S. L., Baohan, A., et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. doi: 10.1038/nature12354
Chiu, C. Q., Lur, G., Morse, T. M., Carnevale, N. T., Ellis-Davies, G. C., and Higley, M. J. (2013). Compartmentalization of GABAergic inhibition by dendritic spines. Science 340, 759–762. doi: 10.1126/science.1234274
Cossart, R., Dinocourt, C., Hirsch, J. C., Merchan-Perez, A., De Felipe, J., Ben-Ari, Y., et al. (2001). Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat. Neurosci. 4, 52–62. doi: 10.1038/82900
Curley, A. A., and Lewis, D. A. (2012). Cortical basket cell dysfunction in schizophrenia. J. Physiol. 590, 715–724. doi: 10.1113/jphysiol.2011.224659
Dalezios, Y., Luján, R., Shigemoto, R., Roberts, J. D., and Somogyi, P. (2002). Enrichment of mGluR7a in the presynaptic active zones of GABAergic and non-GABAergic terminals on interneurons in the rat somatosensory cortex. Cereb. Cortex 12, 961–974. doi: 10.1093/cercor/12.9.961
DeFelipe, J., Hendry, S. H., and Jones, E. G. (1989). Visualization of chandelier cell axons by parvalbumin immunoreactivity in monkey cerebral cortex. Proc. Natl. Acad. Sci. U S A 86, 2093–2097. doi: 10.1073/pnas.86.6.2093
DeFelipe, J., López-Cruz, P. L., Benavides-Piccione, R., Bielza, C., Larrañaga, P., Anderson, S., et al. (2013). New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 14, 202–216. doi: 10.1038/nrn3444
Deleuze, C., Pazienti, A., and Bacci, A. (2014). Autaptic self-inhibition of cortical GABAergic neurons: synaptic narcissism or useful introspection? Curr. Opin. Neurobiol. 26, 64–71. doi: 10.1016/j.conb.2013.12.009
Donato, F., Chowdhury, A., Lahr, M., and Caroni, P. (2015). Early- and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning. Neuron 85, 770–786. doi: 10.1016/j.neuron.2015.01.011
Donato, F., Rompani, S. B., and Caroni, P. (2013). Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276. doi: 10.1038/nature12866
Doyon, N., Vinay, L., Prescott, S. A., and De Koninck, Y. (2016). Chloride regulation: a dynamic equilibrium crucial for synaptic inhibition. Neuron 89, 1157–1172. doi: 10.1016/j.neuron.2016.02.030
Endo, T., Yanagawa, Y., and Komatsu, Y. (2016). Substance P activates Ca2+-permeable nonselective cation channels through a phosphatidylcholine-specific phospholipase C signaling pathway in nNOS-Expressing GABAergic Neurons in visual cortex. Cereb. Cortex 26, 669–682. doi: 10.1093/cercor/bhu233
Eto, K., Ishibashi, H., Yoshimura, T., Watanabe, M., Miyamoto, A., Ikenaka, K., et al. (2012). Enhanced GABAergic activity in the mouse primary somatosensory cortex is insufficient to alleviate chronic pain behavior with reduced expression of neuronal potassium-chloride cotransporter. J. Neurosci. 32, 16552–16559. doi: 10.1523/JNEUROSCI.2104-12.2012
Fariñas, I., and DeFelipe, J. (1991). Patterns of synaptic input on corticocortical and corticothalamic cells in the cat visual cortex. II. The axon initial segment. J. Comp. Neurol. 304, 70–77. doi: 10.1002/cne.903040106
Feldmeyer, D., Roth, A., and Sakmann, B. (2005). Monosynaptic connections between pairs of spiny stellate cells in layer 4 and pyramidal cells in layer 5A indicate that lemniscal and paralemniscal afferent pathways converge in the infragranular somatosensory cortex. J. Neurosci. 25, 3423–3431. doi: 10.1523/JNEUROSCI.5227-04.2005
Foldy, C., Lee, S. Y., Szabadics, J., Neu, A., and Soltesz, I. (2007). Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat. Neurosci. 10, 1128–1130. doi: 10.1038/nn1952
Freund, T. F., and Katona, I. (2007). Perisomatic inhibition. Neuron 56, 33–42. doi: 10.1016/j.neuron.2007.09.012
Frick, A., Feldmeyer, D., Helmstaedter, M., and Sakmann, B. (2008). Monosynaptic connections between pairs of L5A pyramidal neurons in columns of juvenile rat somatosensory cortex. Cereb. cortex 18, 397–406. doi: 10.1093/cercor/bhm074
Frick, A., Feldmeyer, D., and Sakmann, B. (2007). Postnatal development of synaptic transmission in local networks of L5A pyramidal neurons in rat somatosensory cortex. J. Physiol. 585, 103–116. doi: 10.1113/jphysiol.2007.141788
Fukuda, T., Kosaka, T., Singer, W., and Galuske, R. A. (2006). Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. J. Neurosci. 26, 3434–3443. doi: 10.1523/JNEUROSCI.4076-05.2006
Gentet, L. J., Kremer, Y., Taniguchi, H., Huang, Z. J., Staiger, J. F., and Petersen, C. C. (2012). Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci. 15, 607–612. doi: 10.1038/nn.3051
Gidon, A., and Segev, I. (2012). Principles governing the operation of synaptic inhibition in dendrites. Neuron 75, 330–341. doi: 10.1016/j.neuron.2012.05.015
Glickfeld, L. L., Roberts, J. D., Somogyi, P., and Scanziani, M. (2009). Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat. Neurosci. 12, 21–23. doi: 10.1038/nn.2230
Glickfeld, L. L., and Scanziani, M. (2006). Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nature Neurosci. 9, 807–815. doi: 10.1038/nn1688
Gonchar, Y., Wang, Q., and Burkhalter, A. (2007). Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front. Neuroanat. 1:3. doi: 10.3389/neuro.05.003.2007
González-Burgos, G., Krimer, L. S., Povysheva, N. V., Barrionuevo, G., and Lewis, D. A. (2005). Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J. Neurophysiol. 93, 942–953. doi: 10.1152/jn.00787.2004
Gulledge, A. T., Carnevale, N. T., and Stuart, G. J. (2012). Electrical advantages of dendritic spines. PLoS One 7:e36007. doi: 10.1371/journal.pone.0036007
Gulledge, A. T., and Stuart, G. J. (2003). Excitatory actions of GABA in the cortex. Neuron 37, 299–309. doi: 10.1016/s0896-6273(02)01146-7
Gulledge, A. T., and Stuart, G. J. (2005). Cholinergic inhibition of neocortical pyramidal neurons. J. Neurosci. 25, 10308–10320. doi: 10.1523/jneurosci.2697-05.2005
Hao, J., Wang, X. D., Dan, Y., Poo, M. M., and Zhang, X. H. (2009). An arithmetic rule for spatial summation of excitatory and inhibitory inputs in pyramidal neurons. Proc. Natl. Acad. Sci. U S A 106, 21906–21911. doi: 10.1073/pnas.0912022106
Harnett, M. T., Makara, J. K., Spruston, N., Kath, W. L., and Magee, J. C. (2012). Synaptic amplification by dendritic spines enhances input cooperativity. Nature 491, 599–602. doi: 10.1038/nature11554
Hefft, S., and Jonas, P. (2005). Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat. Neurosci. 8, 1319–1328. doi: 10.1038/nn1542
Hensch, T. K. (2005). Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888. doi: 10.1038/nrn1787
Hines, M. L., and Carnevale, N. T. (1997). The NEURON simulation environment. Neural Comput. 9, 1179–1209. doi: 10.1162/neco.1997.9.6.1179
Hioki, H., Okamoto, S., Konno, M., Kameda, H., Sohn, J., Kuramoto, E., et al. (2013). Cell type-specific inhibitory inputs to dendritic and somatic compartments of parvalbumin-expressing neocortical interneuron. J. Neurosci. 33, 544–555. doi: 10.1523/JNEUROSCI.2255-12.2013
Hirai, Y., Morishima, M., Karube, F., and Kawaguchi, Y. (2012). Specialized cortical subnetworks differentially connect frontal cortex to parahippocampal areas. J. Neurosci. 32, 1898–1913. doi: 10.1523/JNEUROSCI.2810-11.2012
Holderith, N., Lorincz, A., Katona, G., Rozsa, B., Kulik, A., Watanabe, M., et al. (2012). Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat. Neurosci. 15, 988–997. doi: 10.1038/nn.3137
Holtmaat, A., Wilbrecht, L., Knott, G. W., Welker, E., and Svoboda, K. (2006). Experience-dependent and cell-type-specific spine growth in the neocortex. Nature 441, 979–983. doi: 10.1038/nature04783
Howard, A., Tamas, G., and Soltesz, I. (2005). Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 28, 310–316. doi: 10.1016/j.tins.2005.04.004
Hu, H., Ma, Y., and Agmon, A. (2011). Submillisecond firing synchrony between different subtypes of cortical interneurons connected chemically but not electrically. J. Neurosci. 31, 3351–3361. doi: 10.1523/JNEUROSCI.4881-10.2011
Hunt, R. F., Girskis, K. M., Rubenstein, J. L., Alvarez-Buylla, A., and Baraban, S. C. (2013). GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci. 16, 692–697. doi: 10.1038/nn.3392
Inan, M., Blázquez-Llorca, L., Merchán-Pérez, A., Anderson, S. A., DeFelipe, J., and Yuste, R. (2013). Dense and overlapping innervation of pyramidal neurons by chandelier cells. J. Neurosci. 33, 1907–1914. doi: 10.1523/JNEUROSCI.4049-12.2013
Ingham, C. A., Hood, S. H., Taggart, P., and Arbuthnott, G. W. (1998). Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J. Neurosci. 18, 4732–4743.
Isomura, Y., Harukuni, R., Takekawa, T., Aizawa, H., and Fukai, T. (2009). Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat. Neurosci. 12, 1586–1593. doi: 10.1038/nn.2431
Isshiki, M., Tanaka, S., Kuriu, T., Tabuchi, K., Takumi, T., and Okabe, S. (2014). Enhanced synapse remodelling as a common phenotype in mouse models of autism. Nat. Commun. 5:4742. doi: 10.1038/ncomms5742
Jiang, X., Shen, S., Cadwell, C. R., Berens, P., Sinz, F., Ecker, A. S., et al. (2015). Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350:aac9462. doi: 10.1126/science.aac9462
Jiang, X., Wang, G., Lee, A. J., Stornetta, R. L., and Zhu, J. J. (2013). The organization of two new cortical interneuronal circuits. Nat. Neurosci. 16, 210–218. doi: 10.1038/nn.3305
Jones, E. G. (1975). Varieties and distribution of non-pyramidal cells in the somatic sensory cortex of the squirrel monkey. J. Comp. Neurol. 160, 205–267. doi: 10.1002/cne.901600204
Jones, E. G. (1984). “Laminar distribution of cortical efferent cells,” in Cerebral Cortex: Vol. 1, Cellular Components of the Cerebral Cortex, eds A. Peters and E. G. Jones (New York, NY: Plenum), 521–553.
Jones, E. G. (2001). The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 24, 595–601. doi: 10.1016/s0166-2236(00)01922-6
Jones, E. G., and Hendry, S. H. (1984). “Basket cells,” in Cerebral Cortex: Cellular Components of the Cerebral Cortex, ed. A. J. Peters (New York, NY: Plenum Press), 309–334.
Jones, E. G., and Powell, T. P. (1969). Morphological variations in the dendritic spines of the neocortex. J. Cell Sci. 5, 509–529.
Kaneko, T., Cho, R., Li, Y., Nomura, S., and Mizuno, N. (2000). Predominant information transfer from layer III pyramidal neurons to corticospinal neurons. J. Comp. Neurol. 423, 52–65. doi: 10.1002/1096-9861(20000717)423:1“52::aid-cne5”3.0.co;2-f
Karube, F., Kubota, Y., and Kawaguchi, Y. (2004). Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes. J. Neurosci. 24, 2853–2865. doi: 10.1523/JNEUROSCI.4814-03.2004
Kawaguchi, Y. (1992). Receptor subtypes involved in callosally-induced postsynaptic potentials in rat frontal agranular cortex in vitro. Exp. Brain Res. 88, 33–40. doi: 10.1007/bf02259126
Kawaguchi, Y. (1995). Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J. Neurosci. 15, 2638–2655.
Kawaguchi, Y., and Kubota, Y. (1996). Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J. Neurosci. 16, 2701–2715.
Kawaguchi, Y., and Kubota, Y. (1997). GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486. doi: 10.1093/cercor/7.6.476
Kawaguchi, Y., and Kubota, Y. (1998). Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience 85, 677–701. doi: 10.1016/s0306-4522(97)00685-4
Khirug, S., Yamada, J., Afzalov, R., Voipio, J., Khiroug, L., and Kaila, K. (2008). GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J. Neurosci. 28, 4635–4639. doi: 10.1523/JNEUROSCI.0908-08.2008
Kim, E. J., Juavinett, A. L., Kyubwa, E. M., Jacobs, M. W., and Callaway, E. M. (2015). Three types of cortical layer 5 neurons that differ in brain-wide connectivity and function. Neuron 88, 1253–1267. doi: 10.1016/j.neuron.2015.11.002
Kisvárday, Z. F., Cowey, A., and Somogyi, P. (1986). Synaptic relationships of a type of GABA-immunoreactive neuron (clutch cell), spiny stellate cells and lateral geniculate nucleus afferents in layer IVC of the monkey striate cortex. Neuroscience 19, 741–761. doi: 10.1016/0306-4522(86)90296-4
Kisvárday, Z. F., Gulyas, A., Beroukas, D., North, J. B., Chubb, I. W., and Somogyi, P. (1990). Synapses, axonal and dendritic patterns of GABA-immunoreactive neurons in human cerebral cortex. Brain 113, 793–812. doi: 10.1093/brain/113.3.793
Kisvárday, Z. F., Martin, K. A., Friedlander, M. J., and Somogyi, P. (1987). Evidence for interlaminar inhibitory circuits in the striate cortex of the cat. J. Comp. Neurol. 260, 1–19. doi: 10.1002/cne.902600102
Kisvárday, Z. F., Martin, K. A., Whitteridge, D., and Somogyi, P. (1985). Synaptic connections of intracellularly filled clutch cells: a type of small basket cell in the visual cortex of the cat. J. Comp. Neurol. 241, 111–137. doi: 10.1002/cne.902410202
Klausberger, T., Magill, P. J., Márton, L. F., Roberts, J. D., Cobden, P. M., Buzsáki, G., et al. (2003). Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421, 844–848. doi: 10.1038/nature01374
Klausberger, T., Marton, L. F., O’Neill, J., Huck, J. H., Dalezios, Y., Fuentealba, P., et al. (2005). Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J. Neurosci. 25, 9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005
Klausberger, T., and Somogyi, P. (2008). Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. doi: 10.1126/science.1149381
Knott, G. W., Quairiaux, C., Genoud, C., and Welker, E. (2002). Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34, 265–273. doi: 10.1016/s0896-6273(02)00663-3
Kubota, Y. (2014). Untangling GABAergic wiring in the cortical microcircuit. Curr. Opin. Neurobiol. 26, 7–14. doi: 10.1016/j.conb.2013.10.003
Kubota, Y., Hatada, S., Kondo, S., Karube, F., and Kawaguchi, Y. (2007). Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J. Neurosci. 27, 1139–1150. doi: 10.1523/jneurosci.3846-06.2007
Kubota, Y., Hattori, R., and Yui, Y. (1994). Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 649, 159–173. doi: 10.1016/0006-8993(94)91060-x
Kubota, Y., and Kawaguchi, Y. (2000). Dependence of GABAergic synaptic areas on the interneuron type and target size. J. Neurosci. 20, 375–386.
Kubota, Y., Kondo, S., Nomura, M., Hatada, S., Yamaguchi, N., Mohamed, A. A., et al. (2015). Functional effects of distinct innervation styles of pyramidal cells by fast spiking cortical interneurons. eLife 4:e07919. doi: 10.7554/eLife.07919
Kubota, Y., Shigematsu, N., Karube, F., Sekigawa, A., Kato, S., Yamaguchi, N., et al. (2011). Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb. Cortex 21, 1803–1817. doi: 10.1093/cercor/bhq252
Kuki, T., Fujihara, K., Miwa, H., Tamamaki, N., Yanagawa, Y., and Mushiake, H. (2015). Contribution of parvalbumin and somatostatin-expressing GABAergic neurons to slow oscillations and the balance in beta-gamma oscillations across cortical layers. Front. Neural Circuits 9:6. doi: 10.3389/fncir.2015.00006
Kuramoto, E., Furuta, T., Nakamura, K. C., Unzai, T., Hioki, H., and Kaneko, T. (2009). Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb. Cortex 19, 2065–2077. doi: 10.1093/cercor/bhn231
Kuramoto, E., Ohno, S., Furuta, T., Unzai, T., Tanaka, Y. R., Hioki, H., et al. (2015). Ventral medial nucleus neurons send thalamocortical afferents more widely and more preferentially to layer 1 than neurons of the ventral anterior-ventral lateral nuclear complex in the rat. Cereb. Cortex 25, 221–235. doi: 10.1093/cercor/bht216
Lee, S., Hjerling-Leffler, J., Zagha, E., Fishell, G., and Rudy, B. (2010). The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 30, 16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010
Lee, S., Kruglikov, I., Huang, Z. J., Fishell, G., and Rudy, B. (2013). A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 16, 1662–1670. doi: 10.1038/nn.3544
Lewis, D. A. (2011). The chandelier neuron in schizophrenia. Dev. Neurobiol. 71, 118–127. doi: 10.1002/dneu.20825
Lewis, D. A., Curley, A. A., Glausier, J. R., and Volk, D. W. (2012). Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35, 57–67. doi: 10.1016/j.tins.2011.10.004
Lewis, D. A., and Lund, J. S. (1990). Heterogeneity of chandelier neurons in monkey neocortex: corticotropin-releasing factor- and parvalbumin-immunoreactive populations. J. Comp. Neurol. 293, 599–615. doi: 10.1002/cne.902930406
Li, N., Chen, T. W., Guo, Z. V., Gerfen, C. R., and Svoboda, K. (2015). A motor cortex circuit for motor planning and movement. Nature 519, 51–56. doi: 10.1038/nature14178
Lur, G., and Higley, M. J. (2015). Glutamate receptor modulation is restricted to synaptic microdomains. Cell Rep. 12, 326–334. doi: 10.1016/j.celrep.2015.06.029
Lur, G., Vinck, M. A., Tang, L., Cardin, J. A., and Higley, M. J. (2016). Projection-specific visual feature encoding by layer 5 cortical subnetworks. Cell Rep. 14, 2538–2545. doi: 10.1101/028910
Ma, Y., Hu, H., and Agmon, A. (2012). Short-term plasticity of unitary inhibitory-to-inhibitory synapses depends on the presynaptic interneuron subtype. J. Neurosci. 32, 983–988. doi: 10.1523/JNEUROSCI.5007-11.2012
Markram, H., Lübke, J., Frotscher, M., Roth, A., and Sakmann, B. (1997). Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. 500, 409–440. doi: 10.1113/jphysiol.1997.sp022031
Markram, H., Muller, E., Ramaswamy, S., Reimann, M. W., Abdellah, M., Sanchez, C. A., et al. (2015). Reconstruction and simulation of neocortical microcircuitry. Cell 163, 456–492. doi: 10.1016/j.cell.2015.09.029
Markram, H., Toledo-Rodriguez, M., Wang, Y., Gupta, A., Silberberg, G., and Wu, C. (2004). Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807. doi: 10.1038/nrn1519
Matsuzaki, M., Ellis-Davies, G. C., Nemoto, T., Miyashita, Y., Iino, M., and Kasai, H. (2001). Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 4, 1086–1092. doi: 10.1038/nn736
Mátyás, F., Freund, T. F., and Gulyás, A. I. (2004). Convergence of excitatory and inhibitory inputs onto CCK-containing basket cells in the CA1 area of the rat hippocampus. Eur. J. Neurosci. 19, 1243–1256. doi: 10.1111/j.1460-9568.2004.03225.x
Megías, M., Emri, Z., Freund, T. F., and Gulyás, A. I. (2001). Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102, 527–540. doi: 10.1016/s0306-4522(00)00496-6
Mercer, A., Eastlake, K., Trigg, H. L., and Thomson, A. M. (2012). Local circuitry involving parvalbumin-positive basket cells in the CA2 region of the hippocampus. Hippocampus 22, 43–56. doi: 10.1002/hipo.20841
Merchán-Pérez, A., Rodriguez, J. R., Alonso-Nanclares, L., Schertel, A., and Defelipe, J. (2009). Counting synapses using FIB/SEM microscopy: a true revolution for ultrastructural volume reconstruction. Front. Neuroanat. 3:18. doi: 10.3389/neuro.05.018.2009
Morishima, M., and Kawaguchi, Y. (2006). Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J. Neurosci. 26, 4394–4405. doi: 10.1523/jneurosci.0252-06.2006
Morishima, M., Morita, K., Kubota, Y., and Kawaguchi, Y. (2011). Highly differentiated projection-specific cortical subnetworks. J. Neurosci. 31, 10380–10391. doi: 10.1523/JNEUROSCI.0772-11.2011
Morita, K., Morishima, M., Sakai, K., and Kawaguchi, Y. (2012). Reinforcement learning: computing the temporal difference of values via distinct corticostriatal pathways. Trends Neurosci. 35, 457–467. doi: 10.1016/j.tins.2012.04.009
Murayama, M., Pérez-Garci, E., Nevian, T., Bock, T., Senn, W., and Larkum, M. E. (2009). Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457, 1137–1141. doi: 10.1038/nature07663
Oláh, S., Füle, M., Komlósi, G., Varga, C., Báldi, R., Barzo, P., et al. (2009). Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461, 1278–1281. doi: 10.1038/nature08503
Otsuka, T., and Kawaguchi, Y. (2013). Common excitatory synaptic inputs to electrically connected cortical fast-spiking cell networks. J. Neurophysiol. 110, 795–806. doi: 10.1152/jn.00071.2013
Pala, A., and Petersen, C. C. (2015). In vivo measurement of cell-type-specific synaptic connectivity and synaptic transmission in layer 2/3 mouse barrel cortex. Neuron 85, 68–75. doi: 10.1016/j.neuron.2014.11.025
Palmer, L., Murayama, M., and Larkum, M. (2012a). Inhibitory regulation of dendritic activity in vivo. Front. Neural Circuits 6:26. doi: 10.3389/fncir.2012.00026
Palmer, L. M., Schulz, J. M., Murphy, S. C., Ledergerber, D., Murayama, M., and Larkum, M. E. (2012b). The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science 335, 989–993. doi: 10.1126/science.1217276
Peters, A. (1990). The axon terminals of vasoactive intestinal polypeptide (VIP)-containing bipolar cells in rat visual cortex. J. Neurocytol. 19, 672–685. doi: 10.1007/bf01188036
Pfeffer, C. K., Xue, M., He, M., Huang, Z. J., and Scanziani, M. (2013). Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076. doi: 10.1038/nn.3446
Pi, H. J., Hangya, B., Kvitsiani, D., Sanders, J. I., Huang, Z. J., and Kepecs, A. (2013). Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524. doi: 10.1038/nature12676
Price, C. J., Cauli, B., Kovacs, E. R., Kulik, A., Lambolez, B., Shigemoto, R., et al. (2005). Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J. Neurosci. 25, 6775–6786. doi: 10.1523/jneurosci.1135-05.2005
Puig, M. V., Gulledge, A. T., Lambe, E. K., and Gonzalez-Burgos, G. (2015). Editorial: neuromodulation of executive circuits. Front. Neural Circuits 9:58. doi: 10.3389/fncir.2015.00058
Puig, M. V., Rose, J., Schmidt, R., and Freund, N. (2014). Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents and birds. Front. Neural Circuits 8:93. doi: 10.3389/fncir.2014.00093
Ramaswamy, S., and Markram, H. (2015). Anatomy and physiology of the thick-tufted layer 5 pyramidal neuron. Front. Cell. Neurosci. 9:233. doi: 10.3389/fncel.2015.00233
Rubenstein, J. L., and Merzenich, M. M. (2003). Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267. doi: 10.1034/j.1601-183x.2003.00037.x
Rudy, B., Fishell, G., Lee, S., and Hjerling-Leffler, J. (2011). Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61. doi: 10.1002/dneu.20853
Sauer, J. F., Struber, M., and Bartos, M. (2015). Impaired fast-spiking interneuron function in a genetic mouse model of depression. eLife 4:e04979. doi: 10.7554/eLife.04979
Schwenk, J., Metz, M., Zolles, G., Turecek, R., Fritzius, T., Bildl, W., et al. (2010). Native GABA(B) receptors are heteromultimers with a family of auxiliary subunits. Nature 465, 231–235. doi: 10.1038/nature08964
Shepherd, G. M. (2013). Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 14, 278–291. doi: 10.1038/nrn3469
Shigematsu, N., Ueta, Y., Mohamed, A. A., Hatada, S., Fukuda, T., Kubota, Y., et al. (2015). Selective thalamic innervation of rat frontal cortical neurons. Cereb. Cortex doi: 10.1093/cercor/bhv124 [Epub ahead of print].
Silberberg, G., and Markram, H. (2007). Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53, 735–746. doi: 10.1016/j.neuron.2007.02.012
Sohal, V. S., Zhang, F., Yizhar, O., and Deisseroth, K. (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. doi: 10.1038/nature07991
Somogyi, P. (1977). A specific ‘axo-axonal’ interneuron in the visual cortex of the rat. Brain Res. 136, 345–350. doi: 10.1016/0006-8993(77)90808-3
Somogyi, P. (1989). “Synaptic organization of gabaergic neurons and GABAA receptors in the lateral geniculate nucleus and visual cortex,” in Neural Mechanisms of Visual Perception: Proceedings of the Second Retina Research Foundation Symposium, ed. D. K.-T. Lam and C. D. Gilbert (Texas, USA: Portfolio Pub Co), 35–62.
Somogyi, P., and Cowey, A. (1981). Combined Golgi and electron microscopic study on the synapses formed by double bouquet cells in the visual cortex of the cat and monkey. J. Comp. Neurol. 195, 547–566. doi: 10.1002/cne.901950402
Somogyi, P., Freund, T. F., and Cowey, A. (1982). The axo-axonic interneuron in the cerebral cortex of the rat, cat and monkey. Neuroscience 7, 2577–2607. doi: 10.1016/0306-4522(82)90086-0
Somogyi, P., Kisvarday, Z. F., Martin, K. A., and Whitteridge, D. (1983). Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience 10, 261–294. doi: 10.1016/0306-4522(83)90133-1
Somogyi, P., and Soltész, I. (1986). Immunogold demonstration of GABA in synaptic terminals of intracellularly recorded, horseradish peroxidase-filled basket cells and clutch cells in the cat’s visual cortex. Neuroscience 19, 1051–1065. doi: 10.1016/0306-4522(86)90122-3
Somogyi, P., Tamás, G., Lujan, R., and Buhl, E. H. (1998). Salient features of synaptic organisation in the cerebral cortex. Brain Res. Brain Res. Rev. 26, 113–135. doi: 10.1016/s0165-0173(97)00061-1
Song, I., Savtchenko, L., and Semyanov, A. (2011). Tonic excitation or inhibition is set by GABA(A) conductance in hippocampal interneurons. Nat. Commun. 2:376. doi: 10.1038/ncomms1377
Staiger, J. F., Masanneck, C., Schleicher, A., and Zuschratter, W. (2004). Calbindin-containing interneurons are a target for VIP-immunoreactive synapses in rat primary somatosensory cortex. J. Comp. Neurol. 468, 179–189. doi: 10.1002/cne.10953
Stuart, G. (1999). Voltage-activated sodium channels amplify inhibition in neocortical pyramidal neurons. Nat. Neurosci. 2, 144–150. doi: 10.1038/5698
Sun, Q. Q., Huguenard, J. R., and Prince, D. A. (2006). Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J. Neurosci. 26, 1219–1230. doi: 10.1523/jneurosci.4727-04.2006
Szabadics, J., Varga, C., Molnár, G., Oláh, S., Barzó, P., and Tamás, G. (2006). Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311, 233–235. doi: 10.1126/science.1121325
Tamás, G., Buhl, E. H., and Somogyi, P. (1997a). Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J. Physiol. 500, 715–738. doi: 10.1113/jphysiol.1997.sp022054
Tamás, G., Buhl, E. H., and Somogyi, P. (1997b). Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J. Neurosci. 17, 6352–6364.
Tamás, G., Lorincz, A., Simon, A., and Szabadics, J. (2003). Identified sources and targets of slow inhibition in the neocortex. Science 299, 1902–1905. doi: 10.1126/science.1082053
Taniguchi, H., Lu, J., and Huang, Z. J. (2013). The spatial and temporal origin of chandelier cells in mouse neocortex. Science 339, 70–74. doi: 10.1126/science.1227622
Thomson, A. M., and Bannister, A. P. (2003). Interlaminar connections in the neocortex. Cereb. Cortex 13, 5–14. doi: 10.1093/cercor/13.1.5
Thomson, A. M., and Destexhe, A. (1999). Dual intracellular recordings and computational models of slow inhibitory postsynaptic potentials in rat neocortical and hippocampal slices. Neuroscience 92, 1193–1215. doi: 10.1016/s0306-4522(99)00021-4
Thomson, A. M., and Lamy, C. (2007). Functional maps of neocortical local circuitry. Front. Neurosci. 1, 19–42. doi: 10.3389/neuro.01.1.1.002.2007
Thomson, A. M., West, D. C., Wang, Y., and Bannister, A. P. (2002). Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2-5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb. Cortex 12, 936–953. doi: 10.1093/cercor/12.9.936
Tomioka, R., Okamoto, K., Furuta, T., Fujiyama, F., Iwasato, T., Yanagawa, Y., et al. (2005). Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur. J. Neurosci. 21, 1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x
Uematsu, M., Hirai, Y., Karube, F., Ebihara, S., Kato, M., Abe, K., et al. (2008). Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb. Cortex 18, 315–330. doi: 10.1093/cercor/bhm056
Ueta, Y., Hirai, Y., Otsuka, T., and Kawaguchi, Y. (2013). Direction- and distance-dependent interareal connectivity of pyramidal cell subpopulations in the rat frontal cortex. Front. Neural Circuits 7:164. doi: 10.3389/fncir.2013.00164
Ueta, Y., Otsuka, T., Morishima, M., Ushimaru, M., and Kawaguchi, Y. (2014). Multiple layer 5 pyramidal cell subtypes relay cortical feedback from secondary to primary motor areas in rats. Cereb. Cortex 24, 2362–2376. doi: 10.1093/cercor/bht088
Urban-Ciecko, J., Fanselow, E. E., and Barth, A. L. (2015). Neocortical somatostatin neurons reversibly silence excitatory transmission via GABAb receptors. Curr. Biol. 25, 722–731. doi: 10.1016/j.cub.2015.01.035
van Aerde, K. I., and Feldmeyer, D. (2015). Morphological and physiological characterization of pyramidal neuron subtypes in rat medial prefrontal cortex. Cereb. Cortex 25, 788–805. doi: 10.1093/cercor/bht278
van Versendaal, D., Rajendran, R., Saiepour, M. H., Klooster, J., Smit-Rigter, L., Sommeijer, J. P., et al. (2012). Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74, 374–383. doi: 10.1016/j.neuron.2012.03.015
Villa, K. L., Berry, K. P., Subramanian, J., Cha, J. W., Oh, W. C., Kwon, H. B., et al. (2016). Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron 89, 756–769. doi: 10.1016/j.neuron.2016.01.010
Viney, T. J., Lasztoczi, B., Katona, L., Crump, M. G., Tukker, J. J., Klausberger, T., et al. (2013). Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat. Neurosci. 16, 1802–1811. doi: 10.1038/nn.3550
Wang, Y., Toledo-Rodriguez, M., Gupta, A., Wu, C., Silberberg, G., Luo, J., et al. (2004). Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 561, 65–90. doi: 10.1113/jphysiol.2004.073353
Wilson, C. J. (1987). Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J. Comp. Neurol. 263, 567–580. doi: 10.1002/cne.902630408
Wilson, C. J., Groves, P. M., Kitai, S. T., and Linder, J. C. (1983). Three-dimensional structure of dendritic spines in the rat neostriatum. J. Neurosci. 3, 383–388.
Woodruff, A. R., McGarry, L. M., Vogels, T. P., Inan, M., Anderson, S. A., and Yuste, R. (2011). State-dependent function of neocortical chandelier cells. J. Neurosci. 31, 17872–17886. doi: 10.1523/JNEUROSCI.3894-11.2011
Woodruff, A., Xu, Q., Anderson, S. A., and Yuste, R. (2009). Depolarizing effect of neocortical chandelier neurons. Front. Neural Circuits 3:15. doi: 10.3389/neuro.04.015.2009
Xu, X., Roby, K. D., and Callaway, E. M. (2010). Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 518, 389–404. doi: 10.1002/cne.22229
Keywords: inhibitory synapse, spine, pyramidal cell, dually innervated spine, veto, thalamocortical fiber, cortex
Citation: Kubota Y, Karube F, Nomura M and Kawaguchi Y (2016) The Diversity of Cortical Inhibitory Synapses. Front. Neural Circuits 10:27. doi: 10.3389/fncir.2016.00027
Received: 13 January 2016; Accepted: 29 March 2016;
Published: 25 April 2016.
Edited by:
Ariel Agmon, West Virginia University, USAReviewed by:
Michael Higley, Yale School of Medicine, USAAlex M. Thomson, UCL School of Pharmacy, UK
Copyright © 2016 Kubota, Karube, Nomura and Kawaguchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshiyuki Kubota, yoshiy@nips.ac.jp
†Present address: Masaki Nomura Center for iPS Cell Research and Application, Kyoto University, Kyoto, Japan
 Yoshiyuki Kubota
Yoshiyuki Kubota Fuyuki Karube
Fuyuki Karube Masaki Nomura
Masaki Nomura Yasuo Kawaguchi
Yasuo Kawaguchi