Specificity, Versatility, and Continual Development: The Power of Optogenetics for Epilepsy Research
- Graduate Program in Neuroscience and Department of Neuroscience, University of Minnesota, Minneapolis, MN, United States
Optogenetics is a powerful and rapidly expanding set of techniques that use genetically encoded light sensitive proteins such as opsins. Through the selective expression of these exogenous light-sensitive proteins, researchers gain the ability to modulate neuronal activity, intracellular signaling pathways, or gene expression with spatial, directional, temporal, and cell-type specificity. Optogenetics provides a versatile toolbox and has significantly advanced a variety of neuroscience fields. In this review, using recent epilepsy research as a focal point, we highlight how the specificity, versatility, and continual development of new optogenetic related tools advances our understanding of neuronal circuits and neurological disorders. We additionally provide a brief overview of some currently available optogenetic tools including for the selective expression of opsins.
Introduction
Optogenetics rests on the use of genetically encoded light sensitive proteins including opsins (Figure 1, Box 1; Deisseroth, 2011, 2015; Boyden, 2015). Through exogenous expression of these light sensitive proteins, researchers gain the ability to modulate neuronal activity, intracellular signaling pathways, or gene expression through the delivery of light (Figure 2). Spatially and temporally restricted delivery of light provides optogenetic approaches with both temporal and spatial specificity. The use of different light-sensitive proteins provides an additional element of experimental control, including the direction of modulation (e.g., excitation vs. inhibition of neuronal populations). Finally, the restricted expression of light-sensitive proteins allows cell-type specific modulation, that is, selectivity for which cell populations are directly affected when light is delivered (Figure 3, Box 2).
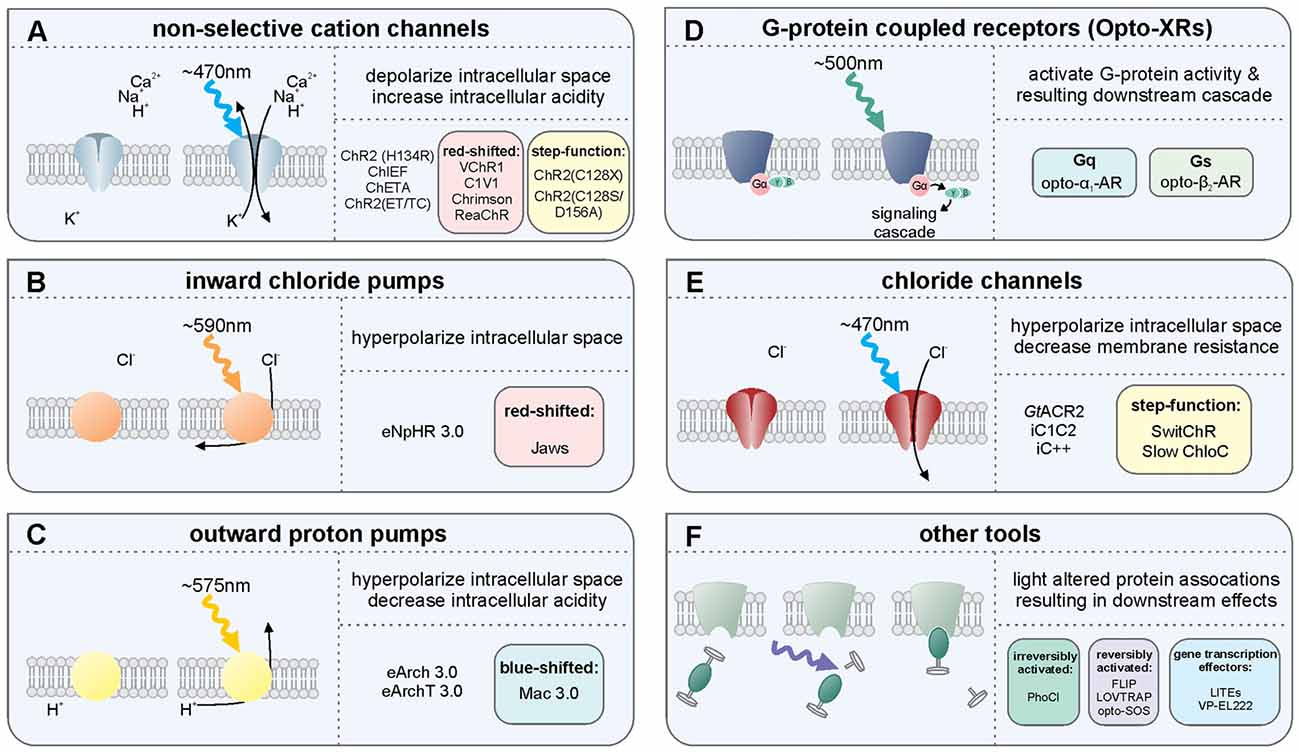
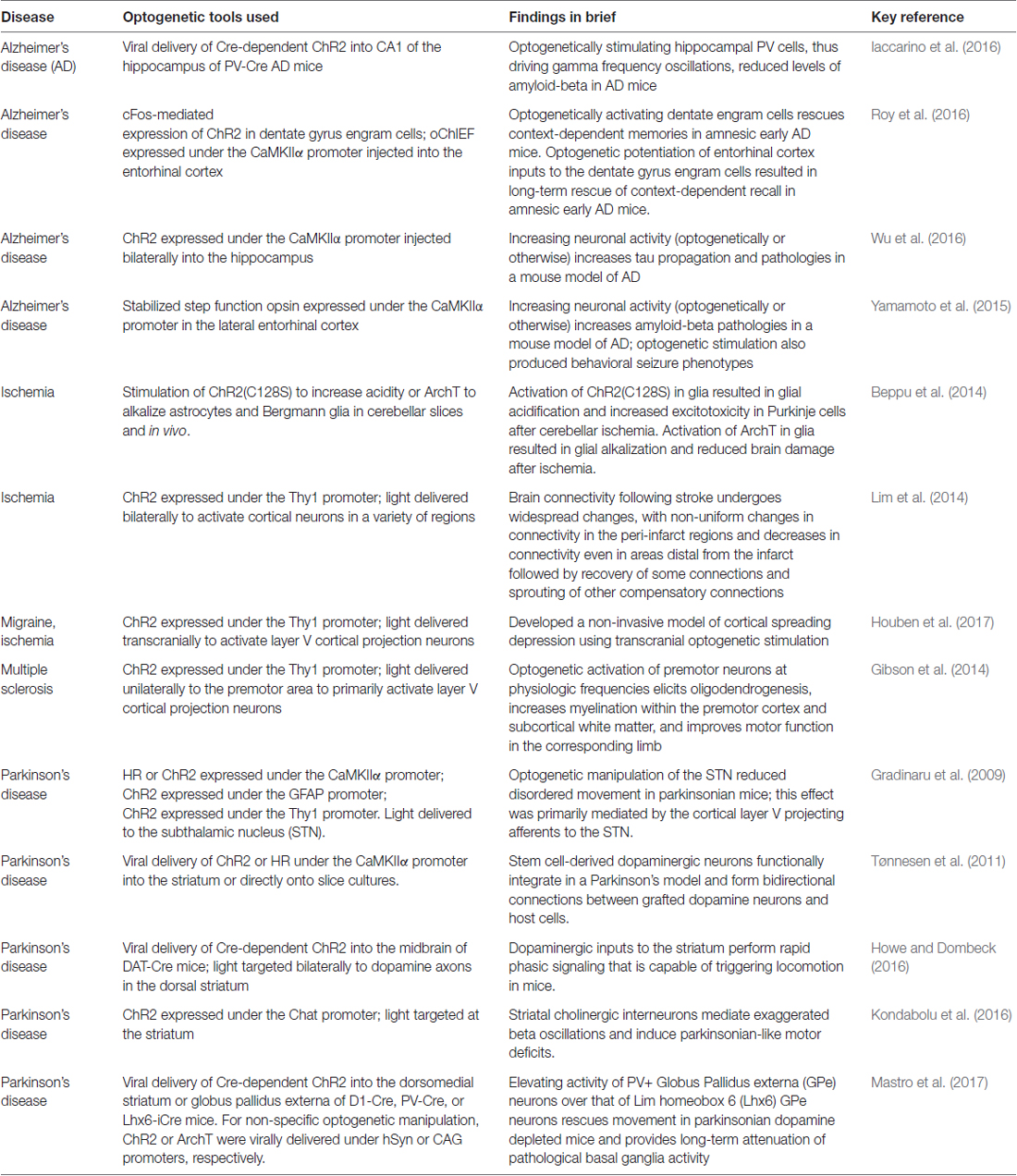
Figure 1. Overview of several light-sensitive proteins, their variants, and their effect on neurons. Throughout, refer to Box 1 for more information on the light-sensitive proteins listed. (A) The non-selective cation channels are perhaps the best-known family of excitatory opsins, with several variants stemming from the blue-light activated wild-type channelrhodopsin-2 found in Chlamydomonas reinhardtii. These non-selective cation channels allow for passive flow of protons and potassium, sodium, and calcium ions along their electrochemical gradient upon photostimulation with light at approximately 470 nm. In mammalian neurons, opening of these channels results in depolarization of the intracellular space, thus increasing the likelihood of action potential generation. The inward flow of protons has also been shown to affect intracellular pH. Several non-selective cation channel opsin variants are listed including a selection of the many blue-light activated opsin variants: ChR2(H134R), ChR2(C128X), ChiEF, ChETA and ChR2(ET/TC) variants; the red-shifted opsins: VChR1, C1V1, Chrimson and ReaChR; and the step function opsin (SFO) ChR2 (C128X), and stabilized SFO ChR2(C128S/D156A). (B) The inward chloride pumps are, to date, perhaps the most commonly used family of inhibitory opsins. Several of these inward chloride pumps, including the one listed (eNpHR3.0) are variants of the wild-type halorhodopsin (HR) found in Natronomonas pharaonis. These opsins are activated by 580 nm light and pump one chloride ion into the cell for each photon of light. The red-shifted opsin Jaws is a cruxhalorhodopsin derived from Haloarcula salinarum engineered to produce large inhibitory photocurrents induced by red light exposure. These inward chloride pumps hyperpolarize the intracellular space by increasing the intracellular chloride concentration, thus decreasing the likelihood of action potential generation. (C) There are several outward proton pumps used in mammalian neuron systems to silence neuronal activity as well. The outward proton pump opsins listed are eArch3.0, eArchT3.0 and eMac3.0, though there are several other versions not listed here. Each has enhanced membrane trafficking and slightly differing photoactivation/deactivation kinetics and resulting photocurrents. Peak photoactivation for eMac3.0 is stimulated by green wavelengths of light and is thus blue-shifted compared to eArch3.0 and eArchT3.0. Each of these outward proton pumps effectively inhibits neuronal activity by hyperpolarizing the intracellular space when protons are pumped out of the cell. Reducing the intracellular concentration of protons also reduces intracellular acidity. (D) G-protein coupled receptors indirectly affect neuronal activity by directly activating G proteins and their downstream targets, producing a signaling cascade. Opto-XRs are a family of G-protein coupled receptor opsins including the specific Gq-protein and Gs-protein coupled variants. (E) Chloride channel opsins allow passive flow of chloride ions down their electrochemical gradient upon photostimulation. In healthy adult mammalian neurons, photoactivation of these chloride channel opsins typically hyperpolarizes the intracellular space, in addition to decreasing membrane resistance, and thus reduce the likelihood of action potential generation. (F) There are several other optogenetic systems available that allow for photo-sensitive alteration of intracellular protein activity. Illustrated here is the irreversibly activated photocleavable protein, PhoCl. Other optogenetic protein alteration systems allow reversible alteration of protein-protein interactions, including FLIP, LOVTRAP and opto-SOS. Additional tools allow for light-altered gene expression, including LITEs and VP-EL222.
Box 1. A brief overview of key light-sensitive proteins, including opsins.
The non-selective cation channels (Figure 1A) are perhaps the best-known family of excitatory opsins. Non-selective cation channels allow passage of Na+, K+, H+ and Ca2+ along their concentration gradients, depolarizing the cell. In large part, the non-selective cation channel opsins stem from the naturally occurring channelrhodopsin-2 (ChR2) found in Chlamydomonas reinhardtii. To optimize ChR2 for use in manipulating mammalian neurons, several variants were made using point mutations. For instance, ChR2(H134R; Nagel et al., 2003), which has an H to R mutation at site 134, is widely used for neuronal manipulation as it displays larger photocurrents than the naturally occurring ChR2 due to an increase in light sensitivity and slower channel closing (Nagel et al., 2003). While the slower channel kinetics increase photocurrent amplitude, they also make ChR2(H134R) less temporally precise than wild-type ChR2. ChR2(H134R) is the most commonly used variant and is referred to simply as ChR2 throughout this review. Several other variants have also been created including: ChIEF (has increased steady-state responses and is able to generate action potentials with high fidelity with high-frequency light stimulation (Lin et al., 2009)); ChETA (has faster kinetics, but reduced photocurrent amplitudes (Gunaydin et al., 2010)); ChR2(ET/TC; has fast activation and deactivation kinetics and is able to generate action potentials with high fidelity at high stimulation frequencies across a wide range of light intensities (Berndt et al., 2011)); and the ChR2(C128X) mutations ChR2(C128A), ChR2(C128T) and ChR2(C128S; Berndt et al., 2009). These ChR2(C128X) mutations show delayed channel closure. Of these mutations, ChR2(C128S) showed photocurrents most similar to the wild-type ChR2 and had the most delayed channel closure. The ChR2(C128S) variant was further combined with a ChR2(D126A; Bamann et al., 2010) variant to produce an even more long-lasting bi-stable step-function opsin (more commonly known as the Stabilized Step Function Opsin (SSFO; Yizhar et al., 2011a). Neurons expressing SSFO display extended depolarization upon activation of the SSFO with a pulse of blue light. A second pulse of light, at a different wavelength (590 nm), is then used to deactivate the SSFO and return the expressing neurons to baseline levels of activity. Most non-selective cation channels related to the naturally occurring ChR2 are maximally activated by blue light at wavelengths of approximately 470 nm. One limitation of these blue-light activated opsins is that blue light has a relatively short wavelength and does not travel far through tissue. High power light delivery can heat tissue, having unintended consequences on physiology, including causing tissue damage at higher levels. In order to overcome this issue, several red-shifted channelrhodopsin variants have been developed/discovered including VChR1 (Zhang et al., 2008), C1V1 (Yizhar et al., 2011b), ReaChR (Lin et al., 2013) and Chrimson (Klapoetke et al., 2014). These red-shifted opsins can be activated with light delivered at lower powers with negligible heating effects from distances further from the target site. Indeed, both ReaChR and Chrimson have been shown to be capable of being activated transcranially in deep brain structures of rodents (Lin et al., 2013; Klapoetke et al., 2014). Recently, an additional transcranial optogenetic method was published, which was based on an alternate method of light delivery, rather than the development of a new opsin; near-infrared light was delivered transcranially and was then absorbed by previously injected upconversion nanoparticles in deep brain structures, that then locally converted the near-infrared photons into blue wavelengths, activating ChR2 (Chen et al., 2018). This tool (lanthanide-doped upconversion nanoparticles) has also been modified to emit green wavelengths (by codoping Er3+ and Yb3+ into the NaYF4 host lattice), capable of activating HR and Arch. These green-light emitting upconversion nanoparticles were used in vivo to inhibit acute, chemically induced, seizure activity (Table 2; Chen et al., 2018). Currently the nanoparticles need to be injected into the targeted brain region, preventing this technique from being used in a purely non-invasive manner. While the study examined up to a month past injection, the longer-term stability of the nanoparticles and effects of exposure to the upconversion nanoparticles remains to be studied (Chen et al., 2018). Additionally, if high levels of local blue light are achieved, a potential for tissue heating remains. Regardless of the light source, with the use of any non-selective cation channel opsin like ChR2, which also allow the passage of Ca2+, an additional aspect to consider is that the entry of Ca2+ into the cell may have unintended consequences on the cell’s physiology.
The inward chloride pumps (Figure 1B) are perhaps the best-known family of inhibitory opsins. As the name indicates, this family of opsins actively pumps chloride ions into the cell, thus increasing the intracellular chloride concentration, hyperpolarizing the intracellular space, and lowering the probability of action potential generation. As they actively pump chloride into the cell, chloride pumps can (temporarily) alter the intracellular chloride concentration (Alfonsa et al., 2015), an experimental caveat to be considered when using these opsins. On the flip side, as they are pumps, chloride pumps are not dependent on the reversal potential of chloride and can hyperpolarize and inhibit (at least acutely) even in situations where the chloride reversal potential may be altered (as can happen, for example, in epileptic tissue). Several of the inward chloride pump opsins stem from the naturally occurring halorhodopsin (HR) found in Natronomonas pharaonis (NpHR). As with ChR2, several variants of this naturally occurring inhibitory opsin were made in order to optimize their use for manipulating mammalian neurons (Gradinaru et al., 2008, 2010). Compared to the naturally occurring NpHR, eNpHR3.0 displays larger photocurrents due to better membrane trafficking, while preserving the resistance to inactivation seen in wild-type NpHR. As eNpHR3.0 is currently the primary inward chloride pump opsin used for research on mammalian neurons, we refer to it elsewhere in this review as simply HR. An additional inward chloride pump named Jaws, which was derived from Haloarcula salinarum, has a red-shifted activation spectrum that has also been shown to be effective in inhibiting neuronal activity through the production of large inhibitory photocurrents (Chuong et al., 2014).
The outward proton pumps (Figure 1C) are a distinct method for inhibiting neuronal activity. These opsins function through active pumping of protons out of the cell, thus decreasing the concentration of protons within the cell, hyperpolarizing (and alkalizing) the cell and decreasing the likelihood of action potential generation. These outward proton pump opsins stem from the naturally occurring archaerhodopsin-3 (Arch; Chow et al., 2010) found in Halorubrum sodomense. Several variants of this opsin have been developed to better allow sensitive manipulation of mammalian neurons, including ArchT (Han et al., 2011), eArch3.0 (Mattis et al., 2012) and eArchT3.0 (Mattis et al., 2012; enhanced versions have been modified to improve trafficking). Other outward proton pumps have also been discovered to work well in mammalian neurons when modified to improve trafficking including eMac3.0 (Chow et al., 2010; Mattis et al., 2012) and eBR (Gradinaru et al., 2010). Of these opsins, eArchT3.0 displays larger inhibitory photocurrents than either wild-type Arch, eNpHR3.0, or eMac3.0 (Mattis et al., 2012). Although eMac3.0 produces smaller inhibitory photocurrents relative to eArchT3.0 and eNpHR3.0, eMac3.0 is activated by green wavelengths of light (photoactivation spectrum not shown) and is sufficiently blue-shifted to allow for dual-optogenetic inhibition, as in Chow et al. (2010). An important experimental consideration in the use of proton pumps is the resulting alteration in pH. Note that extended light activation of proton pumps on presynaptic terminals can actually cause an increase in calcium influx (and thus downstream signaling) and neurotransmitter release via intracellular alkalization (Mahn et al., 2016).
The G-protein coupled receptor opsins (Figure 1D) offer methods for manipulating neural activity via intracellular signaling cascades. The family of G-protein coupled receptor opsins are referred to as OptoXRs (Airan et al., 2009), which includes opto-α1-AR (which triggers an α-adrenergic receptor-like response through activation of the Gq protein), and opto-β2-AR (which triggers a β-adrenergic receptor-like response through activation of the Gs protein). An important experimental consideration when using OptoXRs is the potential for expression of the exogenous proteins to alter native receptor expression, localization, or coupling to signaling pathways. Similarly, there is a lack of direct control over which signaling pathways (e.g., downstream of the G protein) the OptoXR expressed will preferentially couple.
The chloride channel opsins (Figure 1E), similar to inward chloride pumps and outward proton pumps, can be used for optogenetic inhibition of neural activity. The chloride channels allow for passive flow of chloride ions across the membrane, thus typically hyperpolarizing the cell, decreasing membrane resistance, and decreasing the likelihood of action potential generation. Unlike the inhibitory proton and chloride pumps, which only shuttle one ion per photon, chloride channel opsins allow for movement of hundreds of ions per photon, reducing the need for high intensity light delivery and reducing the likelihood of heating effects. Recently, two naturally occurring chloride channel opsins were discovered in the microbe Guillardia theta. These are Guillardia theta anion channelrhodopsin 1 and 2 (GtACR1 and GtACR2, respectively; Govorunova et al., 2015). GtACR2 has notably large inhibitory photocurrents and high light sensitivity and may therefore be especially useful in a variety of settings. Before the discovery of these naturally occurring inhibitory channel opsins, inhibitory chloride channels were engineered from the naturally occurring cation-selective channelrhodopsins. These include inhibitory C1C2 (iC1C2; Berndt et al., 2014), iC++ and a bi-stable variant SwiChR++ (Berndt et al., 2016), and a slow chloride-conducting channelrhodopsin (Slow ChloC; Wietek et al., 2014). While activation of chloride channel opsins can inhibit action potentials, when activated at synaptic terminals, rather than inhibiting neurotransmitter release, these opsins can actually trigger neurotransmitter release (Mahn et al., 2016). Regarding the importance of different chloride concentrations (even within the same neuron), and therefore the different impact that activation of chloride channel opsins in different locations on a neuron can have, the reader is further directed to a preprint, not yet peer-reviewed, reference that is particularly relevant: (Mahn et al., 2017). It should also be noted that these chloride channel opsins can both affect and be affected by the chloride ion concentration gradient. This is especially notable in the context of epilepsy research (Pathak et al., 2007; Moore et al., 2017; Wang et al., 2017).
There exist additional options for light-based control of chloride channels (and other receptors more broadly) through a system referred to as optogenetic pharmacology (Kramer et al., 2013; Lin et al., 2015; not illustrated). This method allows for receptor subtype-level control of neuronal activity. Experimental considerations when using an optogenetic pharmacology approach include the need to successfully conjugate the photoswitch prior to attempting neuromodulation; the potential for altering expression or localization of endogenous channels (directly or indirectly); the potential for other effects of the expressed channels, including prior to photoswitch conjugation (especially in contexts where the “photoswitch-ready” version of the subunit is expressed in addition to, rather than in place of, the native subunit); having a mix of light-sensitive and light-insensitive channels; and potential off-target effects or conjugation of the photoswitch itself.
Other tools include (Figure 1F) several optogenetic methods of altering protein activity. FLIP (Zhou et al., 2012) and LOVTRAP (Wang et al., 2016) are two methods by which one can conditionally activate proteins when light is delivered and then return the protein to an inactive/bound/caged state when light is terminated. These reversibly photo-inducible proteins are not ideal for long-term activation, as extended light delivery may cause negative phototoxic and heating effects, but these systems are well-suited for experiments that require on-demand or short-lived protein activation. The newly developed photocleavable tool PhoCl (Zhang et al., 2017) has been shown useful for long-term light-induced protein activation, including conditional, quick onset activation of Cre-recombinase (Zhang et al., 2017). Opto-SOS (Toettcher et al., 2013) can activate intracellular signaling proteins upon photo-stimulation. Light-inducible transcriptional effectors (LITES; Konermann et al., 2013) and VP-EL222 (Motta-Mena et al., 2014) are photo-sensitive tools for regulating gene transcription.
Additional notable optogenetic related tools not illustrated include eNPAC (Gradinaru et al., 2010), which is a hybrid of both NpHR and ChR2 and allows for excitation and inhibition of the same cell; BLINK1 (Cosentino et al., 2015), which is a blue-light gated potassium channel; and luminopsins (e.g., inhibitory luminopsins, or iLMO; Tung et al., 2015), which are optogenetic probes that genetically encode their own bioluminescent light source which can be activated in response to a chemical substrate.
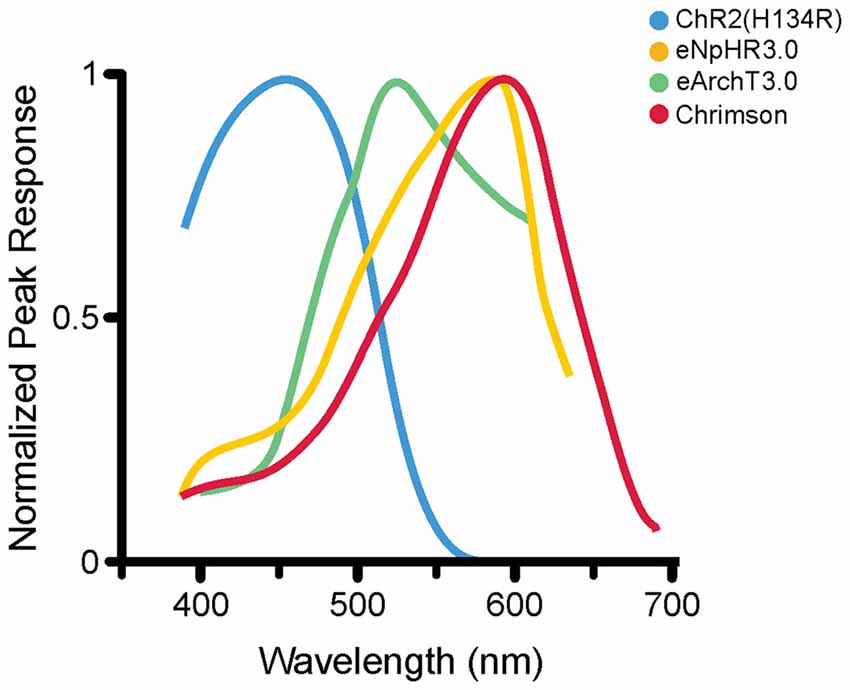
Figure 2. Photoactivation spectra. Action spectra from several opsins; each has a different peak excitation wavelength (shown here) as well as different photocurrent amplitudes and activation and deactivation kinetics (not shown here). ChR2(H134R; Nagel et al., 2005) is maximally excited by blue wavelengths of roughly 450 nm (illustrated activation spectrum for ChR2(H134R) modified from reference; Lin et al., 2009). eNpHR3.0 (Gradinaru et al., 2010) is a commonly used HR variant that is maximally excited at 590 nm (eNpHR3.0 activation spectrum modified from reference; Gradinaru et al., 2010). eArchT3.0, an outward proton pump, is maximally excited at roughly 520 nm (spectrum modified from reference; Mattis et al., 2012). Chrimson is a non-selective cation channel opsin, maximally activated at roughly 590 nm (spectrum modified from reference; Klapoetke et al., 2014). The diverse peak excitation wavelengths make it theoretically possible to do multi-color optogenetic experiments selectively targeting and manipulating different cell populations with reduced cross-talk (Gradinaru et al., 2010; Klapoetke et al., 2014). Note that different experimental conditions can produce different activation spectra; where possible, the illustrated activation spectra are taken from the first manuscript detailing the specific opsin variant illustrated. For an article directly comparing the induced photocurrents of various opsins, including some illustrated here, the reader is referred to reference Mattis et al. (2012).
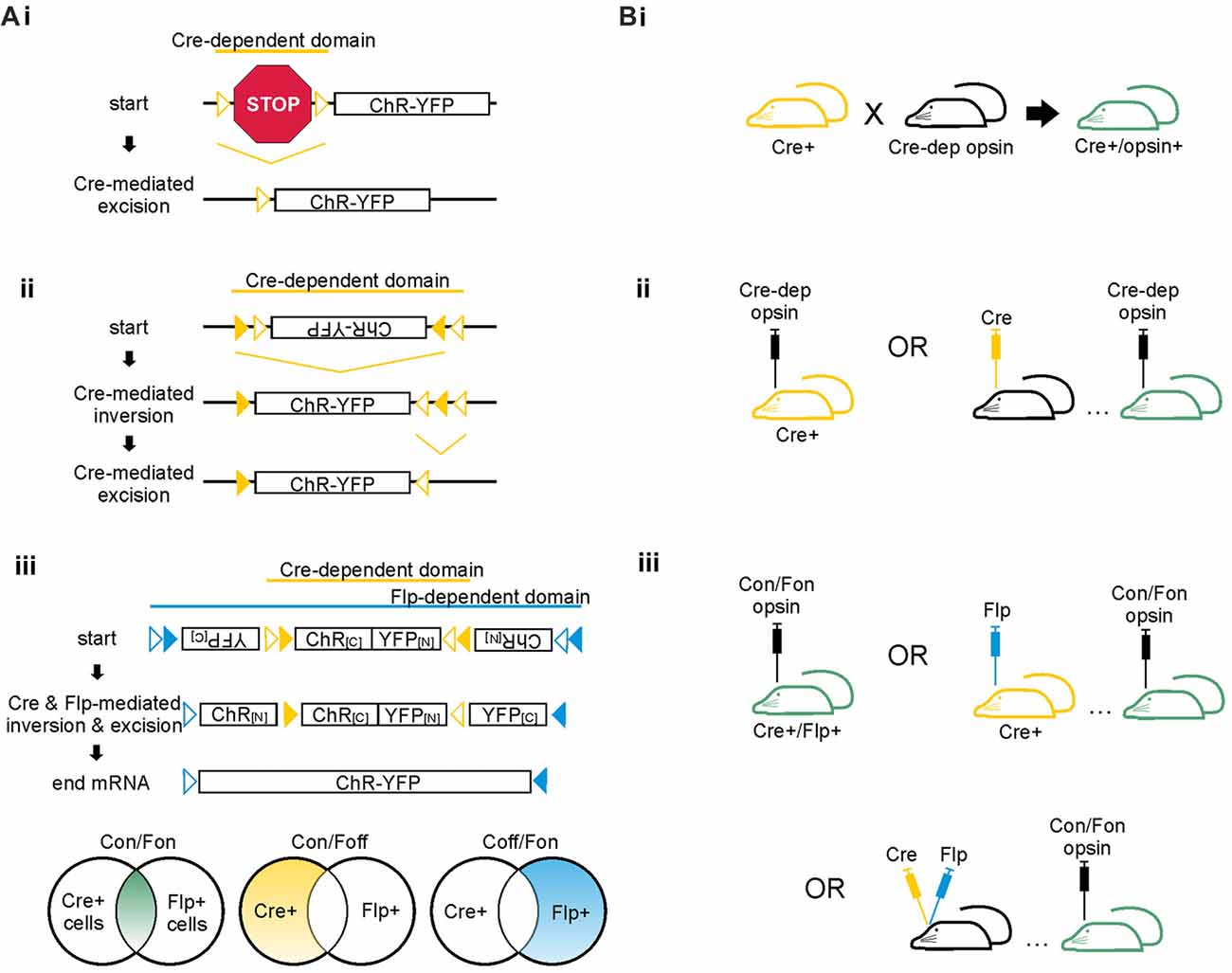
Figure 3. Cre-dependent strategies for achieving cell-type specific opsin expression. (A) Schematics of Cre-dependent expression strategies of the excitatory opsin channelrhodopsin (ChR) tagged with yellow fluorescent protein (YFP). (i) Two loxP sites (open triangles) flank a STOP codon that prevents expression of the ChR-YFP construct. Cre can mediate either inversion or excision of DNA located between two loxP sites. If the loxP sites are oriented in the same direction, Cre excises the DNA between the two sites; if the loxP sites are oriented in opposing directions, Cre inverts the DNA between the two sites. In the case shown here, Cre mediates excision of the floxed STOP codon, allowing expression of the opsin. (ii) The FLEX/DIO system utilizes one set of mutant loxP sites (closed triangles) and one set of typical loxP sites (open triangles). Each loxP site will only pair with its match. In this example, Cre mediates inversion of the opsin DNA between the mutant loxP sites. Cre then mediates excision of the mutant between the non-mutant loxP sites, preventing the DNA from flipping back into the anti-sense direction. (iii) The INTRSECT system utilizes both Cre- and Flp-mediated recombination to achieve opsin expression in cell populations defined by two markers. The Cre- and Flp-dependent viral vector illustrated is also known as a Cre-on/Flp-on system, or more simply: Con/Fon. Other options for INTRSECT approaches that allow selective opsin expression are diagramed below, including Cre-on/Flp-off (Con/Foff) and Cre-off/Flp-on (Coff/Fon). Con/Fon vector schematic modified with permission from Fenno et al. (2014). (B) Options for achieving cell-type specific expression using the floxed STOP system (i), the FLEX/DIO system (ii), or the INTRSECT system (iii) in mice. Transgenic mice expressing Cre are shown in yellow; mice with ultimate opsin expression through transgenic and/or viral methods are indicated in green. (i) To achieve Cre-dependent opsin expression using the floxed STOP system, one can use a transgenic approach in which a Cre-expressing mouse is crossed with a mouse expressing a Cre-dependent opsin, resulting in offspring that express the opsin Cre-dependently. Viral based methods for Cre expression are also possible, as shown for ii and iii. Viral vectors using the floxed STOP system for opsin expression are also theoretically possible but can result in unwanted/leaky expression of the opsin. Thus, the FLEX/DIO system for Cre-dependent opsin expression was created. (ii) In the FLEX/DIO system, a Cre-dependent opsin vector can be delivered into a transgenic Cre+ mouse or a mouse previously injected with a vector for expression of Cre. (iii) The Con/Fon INTRSECT viruses lead to opsin expression when injected into either Cre+/Flp+ transgenic mice, Cre+ mice injected with a vector encoding Flp-recombinase, Flp+ mice injected with a vector encoding Cre-recombinase (not shown), or mice previously injected with vectors encoding Cre and Flp-recombinases.
Box 2. Achieving opsin expression.
Opsins are derived from naturally occurring light-sensitive proteins found in various archaea and algal species. There is an ever-expanding toolbox of opsins and other light-sensitive proteins with distinct properties and unique downstream effects on the cells that express them (Box 1, Figure 1). This versatility is one of the features that make optogenetic tools so useful for neuroscience research. However, an early challenge to utilizing opsins’ power and versatility broadly in a neuroscience research setting was successfully achieving opsin expression in mammalian neurons. In 2005 the excitatory opsin, channelrhodopsin-2, was successfully expressed in cultured hippocampal neurons using a lentiviral gene delivery strategy (Boyden et al., 2005). This study demonstrated the viability of optogenetics for neuroscience and laid the foundation from which an entire field of study has grown (Boyden, 2015; Deisseroth, 2015).
When discussing the advantages of optogenetics over various other approaches, one must emphasize its specificity; optogenetics allows for bi-directional neuronal manipulation with incredible temporal, spatial, and cell-type specificity. Opsins are activated by light, allowing for potentially great temporal and spatial specificity, as light can be quickly delivered to a highly restricted and well-defined spatial location with relative ease. Opsins with rapid activation and deactivation kinetics have been engineered to allow fine-grained control over neuronal output (Box 1). However, opsins are not inherently cell-type specific. This is actually a strength and a trait that helps make opsins so versatile: with the proper gene delivery system, opsins can be expressed in virtually any cell population. For instance, opsins can be directly expressed under a general promoter, a pan-neuronal, or glial promoter. Importantly, more selective expression can also be achieved. Indeed, not only have new opsins and other genetically-encoded light-sensitive tools been developed over the past several years (Box 1), but methods for achieving specific expression have also improved greatly. The advancements to methods for achieving selective opsin expression (especially in mice) have given neuroscientists the ability to study specific cell populations’ activity in vitro and in vivo with relative speed and ease. We discuss in this box some current methods used to achieve selective opsin expression.
Opsins may be expressed directly under a promoter—as mentioned above, this promoter can be a broad or general promoter. If, however, more restricted expression is desired, a cell-type specific promoter can be used. There are a number of transgenic lines that utilize this approach. A cell-type specific promoter can also be used with a viral vector approach. The CaMKIIα promoter is one often used in this context (including, for example, several publications listed in Tables 1, 2). Using a cell-type specific promoter based approach has some major limitations, however. First, especially with a viral vector approach, leaky expression in other cell populations is sometimes reported. Second, if the promoter under which the opsin is to be expressed is long, it may not fit into commonly used vectors. And third, if the promoter of interest is a weak promoter, opsin expression will also be weak. To circumvent these limitations without sacrificing the ability to target opsin expression to a select population of cells, a Cre/loxP mediated approach is often taken (Figure 3). With such an approach, the opsin can be placed under a strong general promoter, but expression is made to be Cre-dependent.
There are several ways to achieve Cre-dependent opsin expression. A segment of DNA located between two loxP sites (i.e., a segment that is floxed) will be either irreversibly excised or reversibly inverted by Cre recombinase, depending on the relative orientation of the loxP sites. One method to achieve Cre-dependent opsin expression utilizing DNA excision is to insert a floxed STOP cassette upstream which prevents the opsin from being expressed until Cre-mediated excision of the STOP cassette (Figure 3Ai). This approach works well in transgenic mouse lines to restrict expression (Madisen et al., 2012). Because the excision of the STOP cassette is permanent, and the opsin itself is placed under a strong promoter, even weak levels of Cre-expression can be sufficient for robust opsin expression. This can circumvent issues arising due to cell-type specific promoters being weak or inconsistently expressed over time. It also can lead to difficulties, for example if a promoter is transiently used by a different cell population or during development. This is a caveat to consider for all recombination-based methods for selective expression.
Theoretically, a floxed STOP cassette could also be implemented in a viral vector, rather than solely in transgenic animals to achieve Cre-dependent opsin expression. This method was found to lead to leaky opsin expression, however. To overcome this limitation, another method for achieving Cre-dependent opsin expression in viral vectors is to place the opsin in the anti-sense direction between two loxP sites oriented such that Cre will invert the DNA between them, thus putting the opsin in the sense direction and allowing expression. Alone this method provides weak opsin expression, as the opsin can be re-inverted back to the anti-sense direction. However, when combined with a second set of mutated loxP sites, the inversion can be made irreversible (see Figure 3Aii). This method, referred to as a FLEX (flip-excision; Atasoy et al., 2008) or double-floxed inverse open reading frame (DOI; Zhang et al., 2010) is now commonly used to attain specific and strong opsin expression with viral-based gene delivery.
For all Cre-based approaches, Cre-expression can be achieved through a (second) transgenic line or through viral-based methods (Figure 3B). In some circumstances, viral vector-based methods can result in un-even or over-expression of proteins, evoke an immune response, or show diminished effectiveness due to an immune response. However, viral vector approaches provide an important compliment to purely transgenic approaches.
When vectors that produce retrograde labeling1 are used, viral based methods for Cre or opsin expression can also be used to gain selective expression in cell populations not defined by a neurochemical marker but instead by a projection target (Figure 6). There are a number of potential strategies here too, including the use of modified rabies (Wall et al., 2010; Sun et al., 2014; Chatterjee et al., 2018), canine adenovirus-2 (CAV; Junyent and Kremer, 2015; Namburi et al., 2015), herpes simplex virus (HSV; Fenno et al., 2014), engineered adeno-associated virus (AAV) AAV2-retro (Tervo et al., 2016), or Cre fused to wheat germ agglutinin (WGA-Cre; Gradinaru et al., 2010; Libbrecht et al., 2017). The use of WGA-Cre to achieve selective opsin expression recently allowed the examination of the role of mossy cells in temporal lobe seizure evolution (Bui et al., 2018; Table 2). Additionally, to overcome the limitation of cytotoxicity caused by previous rabies approaches, a class of relatively non-toxic rabies viral vectors have been developed (Chatterjee et al., 2018).
So far, all of the methods described above allow for expression of an opsin in a cell population as defined by one feature: for example, expression of a neurochemical marker (such as parvalbumin) or, in the case of target-based labeling, projection to a specific region. Cells are often defined by multiple characteristics, however, and distinct cell populations can share a given neurochemical marker or other partially defining feature. Recently, an approach to achieve opsin expression based on two features (rather than one) was developed. This intersectional approach extends the principles of the FLEX/DIO system described above and uses both the Cre/loxP and Flp/Frt recombination systems to limit expression (Figure 3Aiii). These cleverly designed viral vectors, known as INTRSECT, allow Boolean-logic based approaches to limit expression to cells that express both Cre and Flp (Con/Fon), or to cells that express Cre and not Flp (Con/Foff), or to cells that express Flp and not Cre (Coff/Fon). Intersectional optogenetic approaches allow for improved cell-type specificity and greatly improve the versatility and power of optogenetics for use in unraveling complex neuronal circuits.
The tools available to achieve selective opsin expression are, and hopefully will continue to be, rapidly expanding. Through selective opsin expression, selective neuromodulation is possible, and thereby we can gain greater insight into neurological circuits in health and disease.
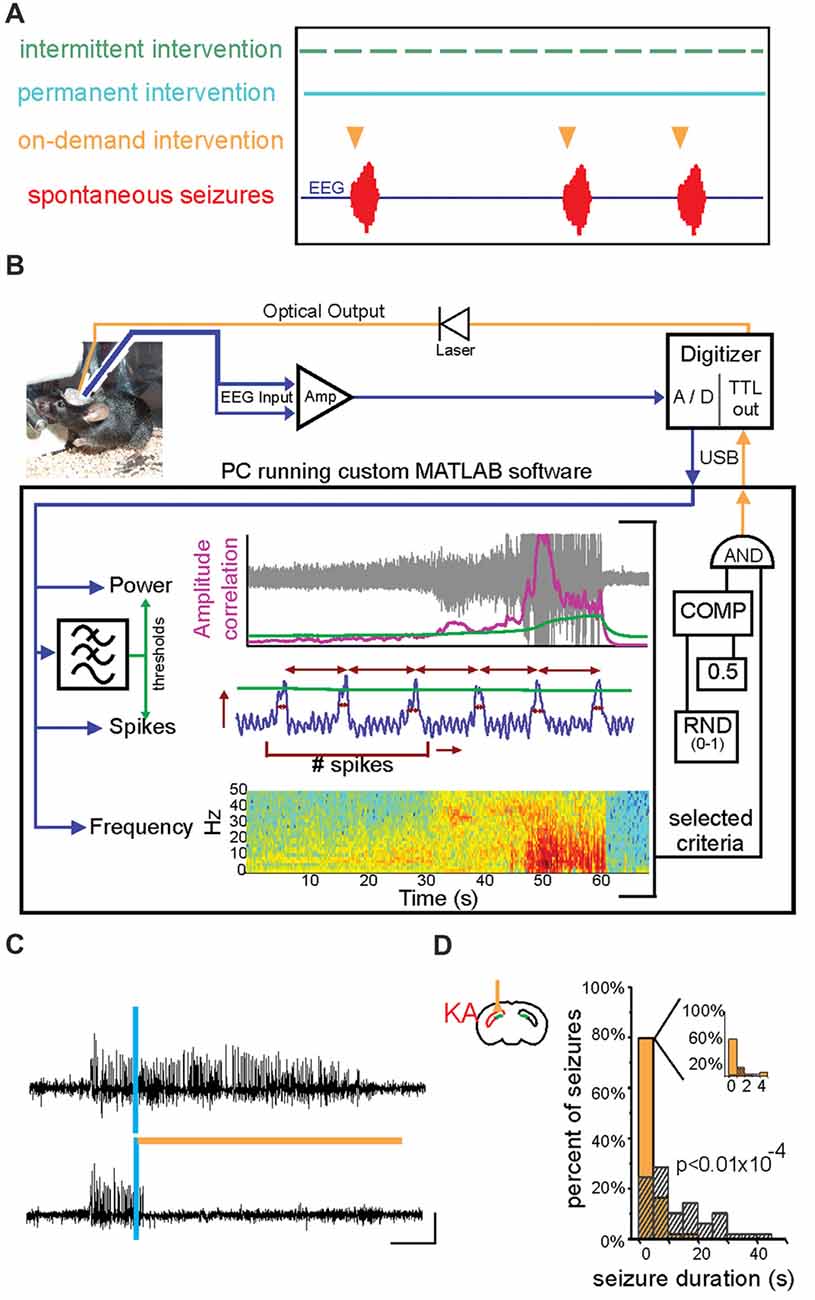
Figure 4. On-demand and closed-loop seizure intervention strategies. (A) Options for the timing of various seizure interventions illustrated relative to spontaneous seizure events (in red, bottom trace). Traditional seizure treatment options, including pharmacological and some neuromodulatory approaches, fall within the intermittent intervention strategy in which the therapy is delivered on a prescribed schedule irrespective of seizure timing (green). Other intervention strategies, such as surgical resection of the seizure focus, are clearly permanent and are also not sensitive to the timing or frequency of seizures (blue). In contrast, on-demand seizure interventions are applied only at the time of seizure prediction/detection and are therefore only used when necessary (orange). This on-demand method of seizure intervention may lead to fewer negative side effects and intervention-related complications. (B) An example of a closed-loop system for on-demand optogenetic seizure intervention in mice. EEG signals (blue) are amplified (Amp) and digitized (A/D) and dispatched into custom MATLAB software for real-time analysis of the signal power, frequency, and several spike characteristics (including spike number, width, amplitude, and rate). Threshold levels (green) for these characteristics are selected to achieve accurate seizure detection in each animal. A trigger signal is then sent to the digitizer and on to the LASER to begin a light protocol for a given percentage of detected seizures (in the illustrated system, 50% of detected seizures would be selected in a random fashion (RND) to trigger light delivery). COMP: digital comparator; USB: universal serial bus. (C) Representative spontaneous seizures recorded from the hippocampus of a mouse previously injected with kainate (KA) and which expresses HR in granule cells. Time of seizure detection by real-time analysis using custom MATLAB software (see also B) illustrated with vertical blue bar. The top trace shows a detected seizure that did not receive light intervention. In contrast, the bottom trace shows a seizure that triggered light delivery (orange horizontal bar). Note that the seizure in the bottom trace is quickly terminated after light delivery. (D) Summary data for one mouse showing a histogram of post-detection seizure durations with no light intervention (gray hashed bars) compared to those that did receive light intervention (orange solid bars). In these experiments (C,D), light (orange bar) was delivered ipsilateral to the site of previous KA injection (red in schematic of coronal section). Scale bar (D) = 0.2 mV, 5 s. (A) modified with permission after Figure 1A from Krook-Magnuson et al. (2015b) (B) from Krook-Magnuson et al. (2013). (C,D) modified with permission from Krook-Magnuson et al. (2015a).
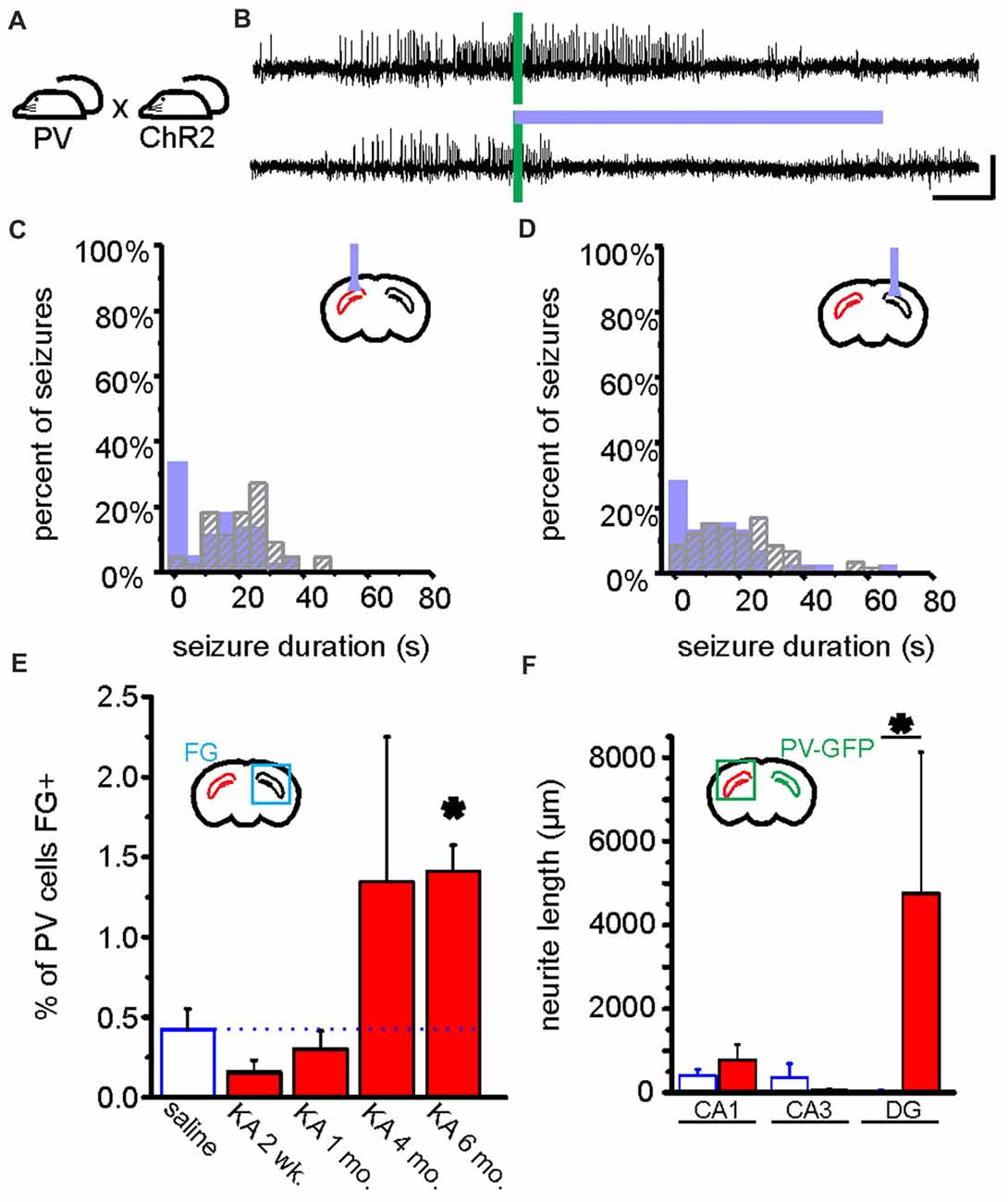
Figure 5. Optogenetic manipulation and circuit dissection of inhibitory PV cells in a mouse model of chronic temporal lobe epilepsy (TLE). (A) PV-Cre mice were crossed with mice expressing ChR2 in a Cre-dependent manner. Offspring of this cross expressed ChR2 selectively in PV cells. These mice received a unilateral intrahippocampal kainate (KA) injection to model chronic TLE. Blue light was targeted to the dorsal CA1 region of the hippocampus for optogenetic activation of hippocampal PV cells. Data from these mice are shown in (A–D). (B) An example seizure where no blue light was delivered (top trace), and where blue light was delivered (bottom trace). Green bars indicate time of seizure detection by the closed-loop algorithm (see Figure 4). Scale bars: 5 s, 100 μV. (C,D) Post-detection seizure duration of detected seizures where no blue light was delivered (gray hashed bars), and where blue light was delivered (filled bars). In (C,D), see inset schematics of light delivery (purple probe) relative to the hippocampus previously injected with KA (red). On-demand light delivery to activate PV cells increases the percentage of seizures stopping within 5 s of seizure detection, whether light was delivered ipsilateral (C) or contralateral (D) to the site previously injected with KA. (E) After an injection of the retrograde tracer Fluorogold (FG) into the left hippocampus (i.e., the hippocampus previously injected with KA or saline), the PV cells in the right hippocampus were analyzed for FG colocalization either 2 weeks, 1 month, 4 months, or 6 months following KA or saline. In the saline control mice (data collapsed across time points) approximately 0.5% of PV cells in the right hippocampus were colabeled with FG. Two weeks following KA, there was a slight, but non-significant decrease in the percentage of PV cells colabeled with FG followed by a progressive increase (asterisk indicates P value of less than 0.01; 6-month KA vs. saline controls). (F) Commissurally projecting PV cell axons were visualized and measured in a PV-Cre mouse line injected in the right hippocampus with a Cre-dependent GFP virus. Six months post-KA (red), there are significantly longer axons (more than 100x longer) in the dentate gyrus from commissurally projecting PV cells when compared to the 6-month post-saline controls. (A–D) modified with permission after Krook-Magnuson et al. (2013). (E,F) modified with permission after Christenson Wick et al. (2017).
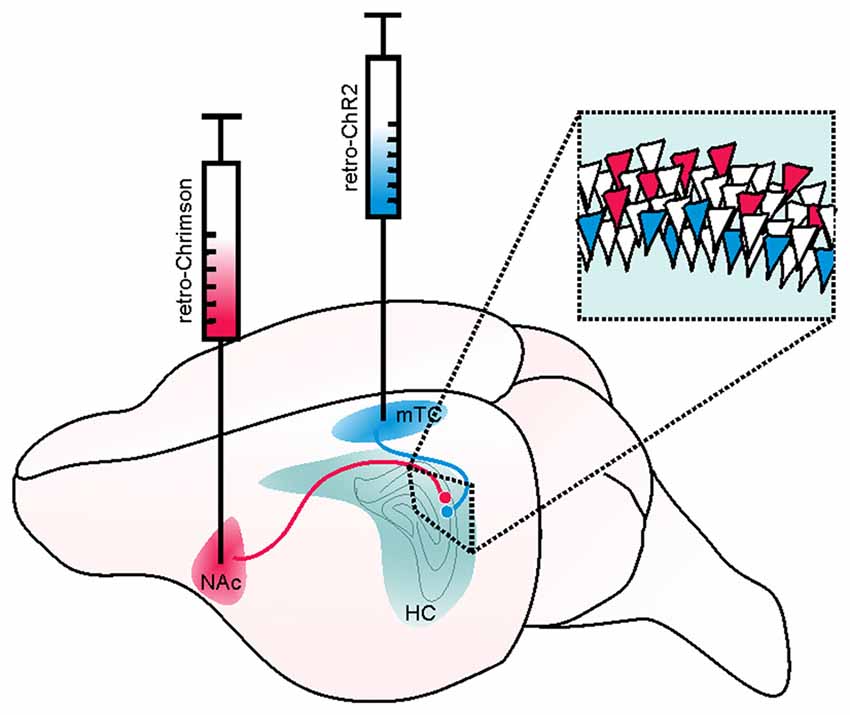
Figure 6. Example of a retrograde viral vector-based approach to selectively target deep and superficial pyramidal cells of the hippocampus (HC). Retrograde viral vector-based approaches to opsin delivery (such as WGA-Cre, AAV2-retro, or CAV) may be used to independently target and manipulate cells with distinct projection targets. In the illustrated hypothetical example, this method is used to separately target and manipulate deep and superficial pyramidal cells of the hippocampus in the same mouse. Such an approach could be used, for example, to test the hypothesis that deep and superficial pyramidal cells may contribute uniquely to hippocampal seizures or their spread. Superficial CA1 pyramidal cells (that is, those on the stratum radiatum side of the pyramidal cell layer) project to the medial temporal cortex (mTC) while deep pyramidal cells project to the nucleus accumbens (NAc). Therefore, by injecting a retrogradely transported viral vector carrying a blue-light activated opsin (e.g., ChR2, shown in blue) into the mTC, one could selectively target and manipulate superficial (and not deep) pyramidal cells. Likewise, by injecting a retrogradely transported viral vector carrying a red-shifted opsin (e.g., Chrimson, shown in red) into the NAc, one could selectively target and manipulate deep pyramidal cells. As these opsins have sufficiently distinct activation spectra, the two populations of neurons could be independently manipulated in the same animal. Inset illustrates a portion of stratum pyramidale in CA1 of the hippocampus, with schematic expression of ChR2 (blue) in superficial and Chrimson (red) in deep pyramidal cells.
Optogenetics thereby gives researchers an unprecedented ability to manipulate neuronal populations with cell type, spatial, directional and temporal specificity. This specificity makes optogenetics a powerful tool. Adding to its success, optogenetic techniques can be relatively easy to implement, in large part due to active decisions by tool developers to make tools widely accessible. Importantly, optogenetics is also a flexible platform, which can be adapted to the experimenter’s research needs. The continual creation and application of new optogenetic related tools further expands the usefulness of optogenetics in neuroscience research. Given these strengths (specificity, versatility, and continual development) of optogenetic approaches, it is not surprising that optogenetics is being rapidly embraced by a variety of neuroscience fields (Deisseroth, 2015; Montgomery et al., 2016).
Optogenetics has proven to be a highly powerful and useful tool for studying healthy and pathological brain activity from a cellular and systems/circuit level perspective (Boyden, 2015; Deisseroth, 2015). Indeed, optogenetics has become so ubiquitous a tool for studying the brain that it is not feasible to describe all of its uses and contributions in a single review. To illustrate the broad versatility and power of optogenetics, Table 1 highlights a few key examples of optogenetics being used to study neurological disorders that the World Health Organization has named among the most burdensome. One such neurological condition is epilepsy (Table 2; Krook-Magnuson and Soltesz, 2015).
Epilepsy is the fourth most common neurological disorder, affecting roughly 65 million people worldwide (England et al., 2012). Unfortunately, in large part due to a critical lack of understanding of many fundamental aspects of epilepsy, current treatment options fall short. It is estimated that one third or more of epilepsy patients do not achieve adequate seizure control with current treatment options, and current treatment options can have major negative side effects (de Tisi et al., 2011; Perucca and Gilliam, 2012; Duchowny and Bhatia, 2014; Laxer et al., 2014). Optogenetics, by providing researchers with a powerful and flexible tool, is allowing significant advances in our understanding of epilepsy (for additional reviews related to the general topic of epilepsy and optogenetics, see also Krook-Magnuson et al., 2014a; Krook-Magnuson and Soltesz, 2015; Tung et al., 2016; Choy et al., 2017; Forcelli, 2017; Tønnesen and Kokaia, 2017). Here, we discuss this work in epilepsy research highlighting how well-suited an optogenetic approach is to epilepsy research in particular and how the benefits of an optogenetic approach can be leveraged in future research. Optogenetic techniques have been uniquely valuable in identifying and testing brain circuit elements that may be capable of seizure control, testing hypotheses related to epilepsy, and determining the roles of specific neuronal populations in epilepsy and seizures.
While optogenetics is a powerful tool in neuroscience research, there are a number of practical hurdles which must be overcome before optogenetics can be used in a clinical setting for epilepsy or other neurological disorders. As discussed in more depth elsewhere (Kullmann et al., 2014; Krook-Magnuson and Soltesz, 2015), these challenges include demonstrating safety of opsin expression, determining the optimal methods to achieve opsin expression in human tissue, and devices for appropriate light delivery. To date, optogenetic techniques have had the most success in smaller animals, including rodents (see e.g., Tables 1, 2), zebrafish (e.g., Motta-Mena et al., 2014; Cosentino et al., 2015; Kainrath et al., 2017), and Drosophila (e.g., Zemelman et al., 2002; Dawydow et al., 2014; Liu et al., 2017; Mohammad et al., 2017). The “scaling up” of optogenetic techniques to larger animals is an additional potential issue which needs to be addressed; however, recent advancements in this arena have been made (e.g., Yazdan-Shahmorad et al., 2018). Despite these challenges, optogenetics may one day be used not only as a research tool, but also as a clinical tool. Optogenetic based approaches are currently entering early clinical trials for treating retinitis pigmentosa through companies including GenSight Biologics (Paris, France) and RetroSense Therapeutics (Ann Arbor, MI, USA). If these trials demonstrate that exogenous opsins can be safely expressed in humans, further investigation of the clinical potential of optogenetics can be expected. Epilepsy is likely to be one of the subsequent early targets for the clinical application of optogenetics. This is due to: (i) the success of some early preclinical optogenetic work in the epilepsy field discussed in part below; (ii) the opportunity for optogenetics to fill a clinical need for new treatment strategies for epilepsy that are more efficacious with fewer negative side effects; and (iii) practical reasons, as one current treatment strategy for epilepsy is surgical removal of brain tissue. Thus, an optogenetic approach could be tried prior to surgical resection, with opsin expression attempted in the brain area that would otherwise be removed; if the optogenetic approach failed, one could then proceed with tissue removal. A caveat to this, however, is if opsin expression were to spread outside of the would-be-transected region, for example through retrograde transport of the viral vector (Kaemmerer, 2017). Importantly, even if optogenetics itself does not reach the clinic, the insight gained through research using optogenetic methods is certain to have a beneficial impact on patient care (Rajasethupathy et al., 2016).
Using epilepsy research as a focal point, we discuss in turn examples of how the specificity of optogenetics can be harnessed; the diverse ways in which optogenetics can be used in research; and finally, how newly developed and future tools can aid in addressing outstanding questions in the field.
Specificity
One of the major benefits of an optogenetic approach is the level of specificity of intervention one can achieve. Therapeutically, an intervention strategy which is highly restricted may be able to avoid unnecessary negative side effects and intervention-related complications. Therefore, one of the first epilepsy research questions tested using this tool was whether optogenetics—a highly restricted intervention strategy—could also be an effective intervention strategy. Epilepsy is often referred to as a network or circuit disorder (Bernhardt et al., 2015; Jin et al., 2015; Vaughan et al., 2016) to emphasize the importance of neuronal interactions in seizures. In part because of this “network” view, it was unclear if a highly restricted intervention strategy (targeting one cell type in one region) could effectively inhibit seizures. This fundamental question was tested through a variety of experimental approaches, and optogenetics was ultimately shown to be effective at inhibiting: (i) epileptiform activity in vitro (Tønnesen et al., 2009; Berglind et al., 2014; Sessolo et al., 2015); (ii) induced, acute seizures in non-epileptic animals (Sukhotinsky et al., 2013; Berglind et al., 2014; Chiang et al., 2014; Ladas et al., 2015; Lu et al., 2016; Soper et al., 2016); and (iii) spontaneous seizures in various rodent models of different forms of epilepsy (Wykes et al., 2012; Krook-Magnuson et al., 2013; Krook-Magnuson et al., 2014b; Krook-Magnuson et al., 2015a; Paz et al., 2013; Kros et al., 2015; Sorokin et al., 2017; Bui et al., 2018). Table 2 provides an overview of studies using in vivo optogenetics in the field of epilepsy research. Importantly, a number of studies have harnessed not only the spatial specificity and cell-type specificity of optogenetics, but also the tool’s temporal specificity by implementing a closed-loop approach (Figure 4; Armstrong et al., 2013; Grosenick et al., 2015; Krook-Magnuson et al., 2015b; Nagaraj et al., 2015). In these studies, on-line seizure detection allowed light delivery to be restricted to times of spontaneous seizures (referred to as on-demand optogenetics), providing an additional element of specificity to the interventions (Krook-Magnuson et al., 2013; Krook-Magnuson et al., 2014b; Krook-Magnuson et al., 2015a; Paz et al., 2013; Sorokin et al., 2017; Bui et al., 2018).
Optogenetic Neuromodulation Allows More Direct Testing of Hypotheses Through Selective Perturbation
The specificity of intervention possible through on-demand optogenetic techniques also provides an important research tool to test additional hypotheses related to epilepsy. For example, in the field of temporal lobe epilepsy (TLE) research there is a long-standing hypothesis called “the dentate gate hypothesis” (Heinemann et al., 1992; Lothman et al., 1992). In healthy control tissue, the dentate gyrus is thought to act as a gate; due to intrinsic and circuit level properties, dentate gyrus granule cells only show very sparse activation in response to entorhinal cortex input. The dentate gate hypothesis posits that, in TLE, there is a breakdown of this gating function, granule cells become relatively over-active, and too much excitation flows through the hippocampal circuit. Whether the excitatory drive pushing seizures arrives from the entorhinal cortex, some other outside source, or from the dentate itself is currently under consideration (Meyer et al., 2016; Krook-Magnuson, 2017). Regardless of the source of the excitatory drive, fundamental to the dentate gate hypothesis is that granule cells show heightened activity levels. Supporting the dentate gate hypothesis, there are a number of changes in epileptic tissue that would reduce inhibition and increase excitation of dentate gyrus granule cells (Nadler et al., 1980; Coulter, 1999; Bouilleret et al., 2000; Zhang et al., 2012).
While there is abundant correlative data showing an increase in dentate excitability in TLE, until recently, strong evidence indicating causality (rather than merely correlation) to support the dentate gate hypothesis was lacking; prior to the advent of optogenetic techniques, it was difficult to directly test the dentate gate hypothesis. With on-demand optogenetic techniques however, researchers were able to test causality rather than just correlation by artificially causing a breakdown of the dentate gate (by optogenetically exciting granule cells) and by “restoring” the dentate gate (by optogenetically inhibiting granule cells) at the time of spontaneous seizures in epileptic animals (Krook-Magnuson et al., 2015a; Table 2). In doing so, researchers took advantage of: (i) the cell type specificity (targeting just granule cells); (ii) the temporal specificity (restoring the gate selectively at the time of seizures); and (iii) the control over the direction of modulation (excitation vs. inhibition) achievable with optogenetic approaches. Additionally, the authors examined whether the location of inhibition or excitation of granule cells mattered by delivering light either ipsilateral or contralateral to where the “break-down of the gate” was thought to occur.
Supporting the dentate gate hypothesis, the authors found that on-demand optogenetic excitation of granule cells (“flooding the gate”) worsened seizures in epileptic animals and even induced seizures in non-epileptic animals (Krook-Magnuson et al., 2015a). These findings also highlight that optogenetics can be used not only to inhibit seizures, but also to induce seizures. Optogenetic induction of seizures is not unique to activation of granule cells, and several studies have used optogenetic approaches to induce seizures (Osawa et al., 2013; Weitz et al., 2015; Khoshkhoo et al., 2017; Table 2). However, activation of the dentate may still be fairly distinct in regard to generating seizures: it was recently reported that optogenetic activation of neurons to produce cortical seizures requires increasingly stronger activations, with subsequent light deliveries, in order to continue to produce seizures (Khoshkhoo et al., 2017; Table 2). In contrast, optogenetic excitation of dentate granule cells in control animals displayed a rapid kindling effect, such that each subsequent light delivery produced a larger, more severe, seizure (Krook-Magnuson et al., 2015a). Providing further support for the dentate gate hypothesis, using non-optogenetic methods to increase the excitability of dentate granule cells is also sufficient to induce epilepsy (Pun et al., 2012).
Perhaps the greatest optogenetic support for the dentate gate hypothesis, however, comes from the experiments which have not induced seizures, but rather inhibited seizures in chronically epileptic mice. Experimenters used optogenetics to restore the dentate gate by selectively inhibiting granule cells at the time of seizures. When this optogenetic restoration of the dentate gate occurred ipsilateral to the presumed seizure focus (i.e., where the breakdown of the gate would be expected to occur), focal seizures were immediately inhibited (Figures 4C,D). Interestingly, and in-line with the hypothesis, inhibition of granule cells in the contralateral dentate gyrus (where the native gate should still be functioning) had no effect on the seizures.
Together, the findings provide strong support for the dentate gate hypothesis and illustrate the power of using the cell-type selective and temporally precise neuromodulation achievable with on-demand optogenetic methods. It also demonstrates the importance of direct control of the direction of modulation (excitation vs. inhibition), which can sometimes be difficult with electrical stimulation methods. Finally, the seizure inhibition achieved by selectively inhibiting granule cells was comparable to the level of seizure inhibition achieved previously by broadly inhibiting excitatory cells in the hippocampal formation (Krook-Magnuson et al., 2013; Table 2). Therefore, these findings further illustrate that identifying key components of the network (in this case, dentate gyrus granule cells) can allow refinement of an intervention without sacrificing efficacy.
An additional example of using the specificity of in vivo on-demand optogenetics to test hypotheses in epilepsy research is provided by work conducted by Paz and colleagues (Table 2). Paz et al. (2013) found that after an experimentally induced cortical stroke, rats developed epilepsy. The researchers hypothesized that cortical stroke was inducing thalamocortical epilepsy (i.e., that the thalamus was critically engaged during these seizures). To test this hypothesis, Paz et al. (2013) used a viral vector with a CaMKIIα promoter to express the inhibitory chloride pump halorhodopsin (HR) in the ventrobasal thalamus ipsilateral to the cortical stroke. Light was then delivered in an on-demand fashion to inhibit thalamic neurons. Seizures were truncated by this approach, supporting the importance of the thalamus in cortical stroke-induced epilepsy.
More recently, Paz and colleagues have used optogenetic methods to further demonstrate that the firing pattern of thalamocortical cells is critically important for seizure control (Sorokin et al., 2017; Table 2). Rather than briefly inhibiting thalamocortical neurons in an on-demand manner at the time of seizures as done previously, the researchers examined the effect of using an excitatory stabilized step function opsin (SSFO, see Figure 1A and Box 1) in genetic models of absence epilepsy. Light delivery placed the thalamocortical neurons in a tonic mode of firing, and (just as with brief inhibition in stroke induced seizures) inhibited seizures.
In the same study, Paz and colleagues also examined the effect of inducing burst firing interictally (that is, when the animal was not actively seizing) in animals genetically prone to absence seizures (Sorokin et al., 2017). Using pulses of light to briefly and repeatedly inhibit thalamocortical cells (which have strong T-type calcium channel-mediated currents), researchers were able to induce rebound firing in thalamocortical cells, entrain their firing, and mimic a burst firing mode. Such pulsed light delivery was able to initiate absence seizures in these animals. Together, the work by Paz and colleagues indicates that in epileptic animals, manipulation directed at the thalamus is able to induce thalamocortical absence seizures, and that disruption of on-going activity of thalamocortical cells during seizures (either by gentle excitation to induce tonic firing or by brief direct inhibition) can be used to truncate thalamocortical seizures, underscoring the importance of the thalamus in this seizure network.
The Broader Seizure Network?
The examples discussed above demonstrate the utility of on-demand optogenetics in more directly testing the importance of circuit elements hypothesized to be critically engaged in seizure networks. That is, by directly manipulating the thalamus, Paz and colleagues were able to provide strong support for the causal role the thalamus can play in thalamocortical absence epilepsy (Paz et al., 2013; Sorokin et al., 2017). Likewise, the importance of the dentate gyrus in TLE was demonstrated through direct manipulation of dentate gyrus granule cells (Krook-Magnuson et al., 2015a). Optogenetic methods are also useful in examining circuit elements that are not typically considered to be part of the seizure network but may still be able to provide seizure control. In this context, we will briefly discuss work using optogenetic methods to examine the utility of targeting the cerebellum or deep layers of the superior colliculus for a variety of epilepsies.
The cerebellum is not typically associated with epilepsy, but in large part due to recent work, including optogenetic work, that viewpoint is changing. Specifically, recent studies (Table 2) have shown that on-demand optogenetic manipulation of the cerebellum can inhibit seizures in rodent models of both thalamocortical absence epilepsy (Kros et al., 2015) and TLE (Krook-Magnuson et al., 2014b). Surprisingly, on-demand optogenetic excitation or inhibition of the cerebellar cortex was able to inhibit temporal lobe seizures (Krook-Magnuson et al., 2014b), suggesting that simply disrupting cerebellar activity was able to terminate hippocampal seizures. This is perhaps somewhat akin to results examining the effects of manipulation of thalamic cells during thalamocortical seizures discussed above (Paz et al., 2013; Sorokin et al., 2017; Table 2). More recently, Kros et al. (2015) examined the effect of optogenetic activation of the cerebellum (in particular, deep cerebellar nucleus neurons) during absence seizures, and found that on-demand stimulation was able to also abort thalamocortical seizures. Taken together, these results are very exciting, and suggest a potentially broad applicability of targeting the cerebellum in various epilepsies. Such a target with broad applicability would be valuable in treating patients whose seizures have multiple, diffuse, uncertain, or progressing seizure foci—these patient populations are currently very difficult to treat through directed neuromodulation methods.
Interestingly, when Kros et al. (2015) took a pharmacological, rather than optogenetic, approach to manipulate the deep cerebellar nuclei in absence epilepsy, inhibition (through local application of the GABAA receptor agonist muscimol) increased the likelihood of absence seizures (Kros et al., 2015). In contrast, and paralleling their optogenetic work, excitation (through application of the GABAA antagonist gabazine) increased the firing frequency and regularity of deep cerebellar neurons and inhibited seizures. These findings, and previous work showing mixed results using open-loop electrical stimulation of the cerebellum in epilepsy (e.g., Cooper et al., 1973; Šramka et al., 1976; Van Buren et al., 1978; Levy and Auchterlonie, 1979; Wright et al., 1984; Davis and Emmonds, 1992; Chkhenkeli et al., 2004; Velasco et al., 2005), raise an important question: is the timing of intervention a critical factor? That is, if Kros et al. (2015) had used on-demand optogenetics to briefly and acutely inhibit deep cerebellar nucleus neurons, would they have worsened seizures (paralleling their pharmacological experiments; Kros et al., 2015), or instead inhibited seizures (paralleling the on-demand optogenetics work targeting the cerebellar cortex for TLE (Krook-Magnuson et al., 2014b), as well as the on-demand optogenetics work targeting the thalamus for thalamocortical epilepsy; Paz et al., 2013; Sorokin et al., 2017)? Fortunately, the temporal specificity achievable with optogenetic methods allows future examination of this important topic.
In these studies, the researchers not only used optogenetics to manipulate neuronal activity, they also used more traditional methods to record neuronal activity. Paralleling previous findings in a variety of seizure models (e.g., Fernandez-Guardiola et al., 1962; Julien and Laxer, 1974; Heath et al., 1980; Kandel and Buzsáki, 1993) as well as in some human patients (Niedermeyer and Uematsu, 1974), both groups found that activity in the cerebellum was modulated by seizures; both hippocampal temporal lobe seizures (Krook-Magnuson et al., 2014b) and thalamocortical absence seizures (Kros et al., 2015) were mirrored in the cerebellum. The ability to record spontaneous seizure activity in the cerebellum suggests that scientists and clinicians may need to reject the adage that the cerebellum “does not seize.” It may also warrant reconsideration of which brain regions are considered to be part of the core hippocampal and thalamocortical seizure networks. When targeting the cerebellum, are we targeting a region distinct from the seizure network which is merely able to inhibit seizures as an outsider? Or, is the cerebellum a core part of the seizure network? Optogenetics, in combination with other brain mapping tools, can help address these questions.
The cerebellum is certainly not the only brain region which has been examined as a potential site for inhibiting a variety of seizure types (Gale, 1992; Dybdal and Gale, 2000; Fisher et al., 2010; Connor et al., 2012; Valentín et al., 2013). One such brain region, which was recently examined using an optogenetic approach, is the superior colliculus—in particular, the deep/intermediate layers of the superior colliculus (Soper et al., 2016; Table 2). Optogenetic activation of neurons in the superior colliculus was able to inhibit seizures induced by: (i) systemic administration of pentylenetetrazole; (ii) focal bicuculline application to Area Tempestas in the piriform cortex; (iii) systemic administration of gamma butyrolactone; or (iv) acoustic stimulation in animals genetically prone to audiogenic seizures (Soper et al., 2016). These results support the ability of intervention targeted at the deep layers of the superior colliculus to be effective in a wide variety of seizure types. Notably, the superior colliculus is a downstream target of the cerebellum and in turn projects to areas that are also directly targeted by the cerebellum, suggesting that the two structures may be part of a unified seizure-controlling network (Yu and Krook-Magnuson, 2015; Zeidler and Krook-Magnuson, 2016). Optogenetics provides an important toolbox also for testing this possibility in future work.
Optogenetic Cell-Type Selectivity for Local Circuit Dissection
The examples discussed above utilized the specificity achievable with on-demand optogenetic techniques to gain insight into the circuitry of epilepsy and seizures at a fairly macroscopic level—the importance of the dentate gyrus or thalamocortical interactions, and the relevance of other regions not classically associated with specific seizure networks. However, the cell-type specificity achievable with optogenetic techniques also allows probing of local circuit elements and the roles of different interneuron populations in healthy physiology and in neurological disorders such as epilepsy. In epilepsy research to date, these optogenetic tools have been most extensively used to investigate parvalbumin-expressing (PV) inhibitory neurons.
PV interneurons are a heterogeneous group of fast spiking inhibitory neurons, which together make up less than 5% of hippocampal neurons (Woodson et al., 1989; Freund and Buzsáki, 1996; Bezaire and Soltesz, 2013). While relatively few in number, PV cells are known to have a powerful impact in healthy circuits (Cobb et al., 1995; Freund and Buzsáki, 1996; Hajos and Paulsen, 2009; Sohal et al., 2009). Their role in seizures, however, has become somewhat controversial (Cammarota et al., 2013; Ledri et al., 2014; Maguire, 2015; Yi et al., 2015; Jiang et al., 2016). Using on-demand optogenetics with light delivery to the hippocampus and a model of chronic TLE, it was demonstrated that activation of PV cells selectively at the time of seizures, and early during seizures (before they progressed to having overtly behavioral manifestations), was able to significantly reduce the duration of electrographic seizures and reduce the likelihood that a seizure would progress to becoming a behavioral seizure (Krook-Magnuson et al., 2013). Seizures were significantly inhibited when light was delivered to the hippocampus ipsilateral to the presumed seizure focus or, somewhat surprisingly, to the contralateral hippocampus (Figure 5). This suggests that activation of PV cells can be an effective approach to inhibiting seizures. However, work done with evoked seizures in healthy tissue suggests that the role of PV cells may be more complicated than simply always providing seizure control. The effect of activating PV cells may be dependent on the timing of their activation (Ellender et al., 2014; Assaf and Schiller, 2016), the relative location of PV cell activation compared to the seizure focus (Sessolo et al., 2015), the type of seizure (Shiri et al., 2015, 2016; Lu et al., 2016) and/or the brain region targeted and expression levels of NKCC1/KCC2 (Wang et al., 2017). More work examining the role of PV neurons during spontaneous seizures in vivo in epileptic animals, as well as work parsing the role of different types of PV interneurons (as discussed more below) would help clarify these issues.
Optogenetics is a powerful tool for providing new insight into the role of populations of cells in neurological disorders. However, in the case of determining the role of PV interneurons during seizures, and to some extent interneurons more broadly, optogenetics has so far produced more questions than answers (Chiang et al., 2014; Ledri et al., 2014; Ladas et al., 2015; Yekhlef et al., 2015; Shiri et al., 2016; Khoshkhoo et al., 2017). Luckily, optogenetics also provides the opportunity to address these questions more thoroughly moving forward.
Versatility
The optogenetic toolbox is filled with a wide variety of tools (Box 1; Figure 1) which can be used for different purposes to distinct experimental ends. Moreover, optogenetic techniques are typically relatively easy to implement in conjunction with more traditional methods, further increasing the versatility of use of optogenetic techniques. As discussed above, in the context of epilepsy research, optogenetic methods have been used to study networks in epilepsy through both seizure inhibition and seizure induction. To date, this has relied primarily on the widely used opsins HR and ChR2 (an overview of optogenetic methods currently used in in vivo epilepsy research is included in Table 2), and a full harnessing of the varied tools (Box 1) available is still to come, as discussed more in the “Further Development and Application” section below. Importantly, optogenetics has also been used in epilepsy research to monitor changes in networks in epilepsy and with interventions. To illustrate the versatility of optogenetics, we discuss here some of the ways optogenetics has been paired with other techniques to gain these insights. While our discussion focuses on epilepsy research, it is important to note that these methods are more broadly applicable (Table 1); for example, optogenetics has not only been used to track changes in neuronal circuits in epileptic tissue, but also following stroke (Lim et al., 2014; listed in Table 1).
Axonal sprouting is a key example of network reorganization that can occur in epilepsy. A classic example of this in TLE is axonal sprouting of dentate granule cells (i.e., mossy fiber sprouting; Laurberg and Zimmer, 1981; Tauck and Nadler, 1985; Sutula et al., 1989; Buckmaster and Dudek, 1997; Althaus et al., 2016). Inhibitory axonal sprouting can also occur (Mathern et al., 1995; Zhang et al., 2009; Peng et al., 2013; Soussi et al., 2015; Christenson Wick et al., 2017), and this is one area in which optogenetic techniques have been paired with more traditional methods of studying anatomical reorganization. Recently, Peng et al. (2013) selectively expressed the excitatory opsin ChR2 in SOM cells of CA1 by injecting a Cre-dependent virus (Figure 3Aii) into the dorsal hippocampus of healthy and epileptic SOM-Cre mice. Because the expression of the virus was limited to SOM cells in the stratum oriens of CA1 and because the opsin was fluorescently tagged, the authors were able to clearly view the axonal arbors of SOM cells in healthy and epileptic mice. Upon visualizing the fluorescently labeled axonal arbors, Peng et al. (2013) found that SOM cells displayed axonal sprouting in epileptic mice. In healthy mice, the axonal arbors of labeled SOM cells in CA1 stopped rigidly before the hippocampal fissure—not crossing into the dentate. However, in epileptic mice, labeled SOM cells in CA1 had axonal arbors that crossed the hippocampal fissure, invading the dentate gyrus. Using optogenetics to activate labeled cells, and whole cell patch clamp methods to record from dentate granule cells in hippocampal slices, Peng et al. (2013) confirmed that these sprouted axons made functional connections onto dentate granule cells in the epileptic mice. More recently, work by others has shown that some CA1 SOM cells have axons that cross into the dentate in non-epileptic tissue (Katona et al., 2017; Szabo et al., 2017), suggesting the possibility that the specific population of CA1 SOM cells targeted may be a critical factor. An additional experimental caveat to consider in using optogenetic approaches in epilepsy research is that seizures can alter the traditional expression profile of markers including somatostatin, which can be transiently expressed in principal cells following seizures (Drexel et al., 2012). As discussed in a later section, intersectional approaches (Figure 3iii) may help alleviate both of these experimental considerations in future work. Importantly, in the study by Peng et al. (2013), an optogenetic approach, in combination with more traditional methods, allowed the demonstration of a novel form of plasticity in chronically epileptic tissue. Additionally, invasion of inhibitory axons from CA1 SOM neurons into the dentate gyrus, including in epileptic tissue, highlights that not only may local inhibitory neurons be harnessed to reinforce the dentate “gate,” but also that inhibitory input from other regions invading the dentate may also be able to help support the gate. In this context, recent work from our laboratory looking at axonal sprouting of PV cells is of interest.
As noted above, it was previously demonstrated that optogenetic activation of PV cells contralateral to the presumed seizure focus significantly inhibited temporal lobe seizures (Krook-Magnuson et al., 2013). This was a somewhat surprising finding, as directly inhibiting granule cells in the contralateral hippocampus had no effect on the seizures (Krook-Magnuson et al., 2015a). There are several possibilities for why activating contralateral PV cells might produce seizure inhibition when direct inhibition of contralateral granule cells does not. One such potential explanation was that PV cells, despite being typically thought of as local interneurons, might project to the contralateral hippocampus (Goodman and Sloviter, 1992; Zappone and Sloviter, 2001), and provide “outsider” inhibition (somewhat akin to SOM cells invading the dentate from CA1). To test this, we injected the retrograde tracer Fluorogold into the hippocampus of control (non-epileptic) and kainate-injected (to induce TLE) mice (Christenson Wick et al., 2017). In control animals, we found only very sparse Fluorogold labeling in contralateral PV cells (~0.5% of contralateral PV cells displayed Fluorogold labeling). In animals injected with kainate 6 months earlier, however, we found a significant increase in Fluorogold-labeled contralateral PV neurons (Figure 5E). To further examine this issue, we used viral injection to achieve Cre-dependent expression of enhanced yellow fluorescent protein (eYFP) in PV cells, allowing visualization of their contralaterally projecting fibers (Christenson Wick et al., 2017). We found long-range sprouting of this commissural inhibitory connection 6 months post-kainate injection (Figure 5F). Specifically, dramatic increases (greater than 100× increase) in labeled fiber lengths were found in the dentate gyrus, further supporting the relevance of this area (and inhibition to it) in TLE. As these studies illustrate, the versatility and flexibility of optogenetic tools to work alongside currently existing well-established techniques greatly increases the power of optogenetics to allow relatively easy expansion on previous findings, including in the domain of circuit changes in epilepsy.
Circuit reorganization can also occur following treatment approaches for epilepsy, including cell transplantation. Here, too, optogenetics can be useful. Specifically, it has been shown that transplantation of interneurons or of progenitors of GABAergic neurons into adult epileptic mice can reduce seizures (Hunt et al., 2013; Cunningham et al., 2014; Henderson et al., 2014; Hunt and Baraban, 2015). When such transplants express opsins, and standard slice electrophysiology techniques are combined with optogenetics, it is possible to demonstrate that the transplanted cells integrate into the circuit and provide synaptic inhibition to endogenous excitatory cells (Avaliani et al., 2014; Cunningham et al., 2014; Henderson et al., 2014; Hsieh and Baraban, 2017). Again, it is worth noting that such research is certainly not limited to the field of epilepsy. For example, optogenetics to study the integration of grafted cells has been used to examine integration of stem cell-derived dopaminergic neurons in a model of Parkinson’s disease (Tønnesen et al., 2011; Table 1). Further illustrating the relative ease at which optogenetics can be combined with other approaches, opsin expression in transplanted neurons was recently used alongside functional magnetic resonance imaging (fMRI; when paired with optogenetics, referred to as ofMRI) to examine larger-scale integration of transplants (Weitz and Lee, 2016).
The technique of ofMRI has also been used in epilepsy research to examine seizure dynamics across brain areas (Duffy et al., 2015; Choy et al., 2017). While ofMRI research so far has focused on using optogenetics to induce seizures in the scanner, future research could use ofMRI to examine seizure networks with spontaneous (rather than evoked) seizures in epileptic animals and use optogenetics to inhibit those seizures to examine seizure suppressive networks. This would be useful, for example, in gaining a better understanding of the networks engaged by interventions targeting the superior colliculus or the cerebellum. Indeed, ofMRI has recently been applied to cerebellar optogenetic stimulation in healthy animals (Choe et al., 2018). As a side note, ofMRI work also provides an excellent example of the need to always have appropriate experimental controls, and specifically the possibility of off-target effects of light; a recent report suggests that light itself can alter blood flow (Rungta et al., 2017).
With appropriate consideration of activation spectra and other methodological considerations, optogenetics can also be combined with cellular level imaging techniques such as calcium imaging. Khoshkhoo et al. (2017) paired optogenetically induced cortical seizures with fiber photometry in order to monitor the activity of different populations of neurons contralateral to the hemisphere receiving the optogenetic activation. In particular, they examined PV, SOM, vasoactive intestinal peptide-expressing (VIP) neurons and excitatory neurons. They found that evoked seizures produced significant increases in calcium signals for all cell types studied, with inhibitory populations showing a calcium signal increase coinciding with electrographic seizure onset, and excitatory cells showing increases after several seconds of lag. For these optogenetically induced seizures, PV and SOM calcium signals remained high for the duration of the seizure events. In addition to optogenetically inducing seizures, Khoshkhoo et al. (2017) used optogenetics to then investigate the effect of inhibiting populations of neurons on evoked seizures and found mixed effects. An additional method to track the activity of neuronal populations during seizures, or potentially across the development of epilepsy itself, is “opto-tagging.” For this, optogenetics is paired with unit recordings, with responsiveness to light being used to determine the cell-type, supplementing putative identification based on waveforms or firing patterns. This use has led to the development of optetrodes, capable of delivering light while performing multichannel recordings (Anikeeva et al., 2011). Relevant to epilepsy research, opto-tagging allows in vivo monitoring of the activity of specific cell types with higher temporal resolution than, for instance, calcium imaging. In addition, recordings with optetrodes provide details on how the optogenetic manipulation is affecting the cells’ activity (i.e., is the cell entraining to the stimulus; is the cell rebounding after photostimulation; has the chloride potential shifted such that chloride channel opsins are depolarizing rather than hyperpolarizing, etc.).
The examples discussed above demonstrate how optogenetics can be used in flexible ways and in combination with different technologies to illustrate its versatility. However, given the large diversity of optogenetic tools currently available (Box 1), and the continued development of new tools, the use of optogenetics in epilepsy research to date represents only a small fraction of what is possible. Continued development, alongside the use of the more varied tools, will allow epilepsy research to fully harness the versatility of optogenetic approaches. These issues are considered below.
Further Development and Application
There are several areas in which optogenetic techniques are being further developed. These include: (1) the light-sensitive proteins themselves and what they couple to; (2) methods for delivering light; and (3) methods for improving selectivity of expression (see also Box 2). Box 1 discusses classical opsins as well as more recently developed tools. Methods for delivering light, beyond traditional optical fibers, include μLEDs (Kim et al., 2013), transparent microprobe arrays (Lee et al., 2015), and wireless devices (for reviews, see Warden et al., 2014; Gutruf and Rogers, 2018). Light delivery approaches also include methods which take a fundamentally different approach, such as luminopsins (Tung et al., 2015) or upconversion nanoparticles (Chen et al., 2018; both briefly discussed in Box 1). A combination of fully harnessing available tools and development of new tools will allow optogenetics to make a significant impact on epilepsy research moving forward. Here, we discuss some ways in which newly developed, and not-yet developed, tools could be applied to epilepsy research to further the field, particularly focusing on improved cell-type specific targeting.
Increased cell-type specific targeting is certain to have a major impact on epilepsy research. For example, relatively little work has been done examining SOM-expressing inhibitory neurons in epileptic tissue (the work by Peng et al., 2013 discussed above is a notable exception). One major reason for this dearth of research is that GABAergic SOM neurons are difficult to selectively label and manipulate in animal models of epilepsy that display transient SOM expression in pyramidal cells (Hashimoto and Obata, 1991; Drexel et al., 2012). Use of Cre-based systems (Figure 3), in which opsin expression is Cre-dependent and Cre-expression is placed under the SOM promoter is problematic in these circumstances, as even transient expression of SOM (and thus Cre) in pyramidal cells is sufficient to irreversibly induce expression of the opsin in these excitatory cells. Therefore, maintenance of selective expression in inhibitory SOM neurons is compromised.
However, the increasing availability of Cre- and Flp-recombinase expressing mouse lines and systems (Taniguchi et al., 2011; Madisen et al., 2012, 2015; Tang et al., 2015; He et al., 2016) and recent advances in intersectional approaches for the restricted expression of opsins (Figure 3iii; Fenno et al., 2014) finally allow for more selective targeting and manipulation of cell populations. Importantly, these intersectional tools can aid in maintaining consistent selective expression in epileptic tissue. For instance, by crossing SOM-Cre mice with Dlx5/6-Flp mice, one can produce mice in which inhibitory SOM neurons express both Cre- and Flp-recombinase. Then, to selectively target and manipulate these neurons in a given brain region, one could inject an INTRSECT virus expressing the opsin of choice in a manner dependent on both Cre- and Flp-mediated recombination (Con/Fon; Figure 3iii). In this situation, transient expression of SOM in non-GABAergic populations would not compromise specificity of expression, as non-GABAergic populations would not express Flp and therefore not express the opsin. Therefore, through application of already available tools, the epilepsy field can harness optogenetic techniques to study populations of cells previously difficult to selectively target.
While the example above focuses on SOM cells in epileptic tissue, such intersectional approaches can also be used to examine other GABAergic populations that have been traditionally difficult to study, such as cholecystokinin-expressing (CCK) or neuronal nitric oxide synthase-expressing (nNOS) interneurons. While CCK and nNOS are both used as markers of certain populations of interneurons (Freund and Buzsáki, 1996; Armstrong et al., 2012), both are also natively expressed in excitatory principal cells (Burette et al., 2002; Lee and Soltesz, 2011). Therefore, targeting these cell populations with a traditional Cre-based system is insufficient. This has been a major problem for the field, but one which is now relatively straightforward to overcome; all the necessary tools are available to optogenetically excite populations of interneurons defined by their expression of molecular markers such as CCK or nNOS.
However, an additional hurdle for the field, alluded to earlier, is that neuronal markers such as SOM, PV, CCK or nNOS are fairly broad markers, with several distinct cell-types encompassed by each marker. For example, PV is expressed in hippocampal fast-spiking perisomatic targeting basket cells, in some dendritically targeting bistratified and oriens-lacunosum moleculare (O-LM) cells, and in axon-initial-segment targeting chandelier (axo-axonic) cells (Freund and Buzsáki, 1996; Bezaire and Soltesz, 2013). Each of these neuronal populations target different segments of the principal cell and may even have diametrically opposing effects on the postsynaptic cell. For instance, axo-axonic cells may actually increase the firing of the postsynaptic cell (Szabadics et al., 2006). Moving forward, it will be critically important for the field to gain better selectivity—to stop targeting “PV cells,” and start targeting more specifically defined cell populations. This may also help clarify the different outcomes seen when targeting broadly defined PV cells in epilepsy research (discussed above). Here too, intersectional approaches can be of great value. For example, in addition to expressing PV, bistratified and O-LM cells can express SOM (Freund and Buzsáki, 1996). Therefore, an intersectional approach, using PV-Flp or SOM-Flp mice in combination with SOM-Cre or PV-Cre mice, respectively, and INTRSECT viruses (Con/Fon, Coff/Fon, Con/Foff, Coff/Foff) can limit expression to subpopulations of PV cells (Fenno et al., 2014). Despite the progress this represents, selective targeting of just PV basket cells, for example, is not yet achievable, and will require the continued development of new tools. The need for continued development of new tools includes tools and insight not directly associated with optogenetics. For example, new mouse lines based on cell-type specific expression profiles gleamed through single-cell transcriptomics can provide important new avenues to specific opsin expression: a recent study identified neuron-derived neurotropic factor (Ndnf) expression in cortical neurogliaform cells, and then produced a Cre-line to allow targeting of this neuronal population (Tasic et al., 2016). While not directly an optogenetic development, this insight and new tool (the Ndnf-Cre line) allows the expansion of optogenetic methods. The same study which identified Ndnf-expression in cortical neurogliaform cells suggested that PV cells expressing Cpne5 (copine 5, a calcium-dependent protein) may correspond to axo-axonic cells (Tasic et al., 2016), which could provide a genetic handle for separating different classes of PV interneurons in the future.
In addition to gene-expression profiles, cell populations can also be segregated by projection targets. While the discussion above has focused on the use of intersectional approaches to improve cell-type specificity through the use of Flp and Cre mouse lines, intersectional approaches can be used in combination with other methods, including to selectively express opsins in cell populations defined by their projection targets. This development increases optogenetics’ power for exploring circuit-level interactions between brain regions in health and disease. For example, Cre and/or Flp expression can be achieved through retrograde expression systems, such as CAV2 (Junyent and Kremer, 2015), modified rabies (Wall et al., 2010; Chatterjee et al., 2018), or AAV2-retro (Tervo et al., 2016; Box 2, Figure 6). In this manner, dopaminergic neurons in the VTA that project to the nucleus accumbens (NAc) were selectively targeted in the original INTRSECT publication (Fenno et al., 2014). One potential application for an optogenetic examination of projection-defined neuronal populations in epilepsy is to explore the distinct contributions of deep vs. superficial CA1 pyramidal cells to epileptic neural activity, including seizure spread or generalization of spikes (Sheybani et al., 2018). Deep vs. superficial CA1 pyramidal cells project to distinct areas (Slomianka et al., 2011), interact with distinct local circuit elements (Krook-Magnuson et al., 2012; Lee et al., 2014), and display different neuromodulatory pathways, including via cannabinoid type-1 receptors (Maroso et al., 2016). For a review on CA1 pyramidal cell diversity, see reference (Soltesz and Losonczy, 2018). As deep and superficial pyramidal cells have distinct projection targets, it is possible to use a retrograde expression system to selectively label and manipulate just one population of pyramidal cells. This would not necessarily require an intersectional approach, but by combining retrograde-expression with an additional restriction (e.g., promoter-based Cre expression), opsin expression could be further limited. Such an approach would be useful, for example, in studying GABAergic projection neurons in situations where the target region is also targeted by excitatory principal neurons, such as entorhinal cortex inputs to the hippocampus (Melzer et al., 2012; Basu et al., 2016).
In addition to increased opsin delivery tools, increases in the availability of diverse light-sensitive proteins make it possible to combine two distinct optogenetic approaches within the same animal. For instance, the increasing availability of good red-shifted and blue-light activated opsins (Figure 2, Box 1) allow for simultaneous/dual-opto experiments in which two cell populations can be independently targeted and then independently manipulated. Building on the discussion of different targeting approaches, this dual-opto approach could be applied to epilepsy research in several ways. For instance, one could retrogradely target, and then manipulate, deep and superficial CA1 pyramidal cells independently, in the same animal. To do this, one could inject a CAV (or other retrograde) virus carrying a blue-light activated opsin (e.g., Chronos) into the NAc (to target deep pyramidal cells; McGeorge and Faull, 1989) and inject a CAV virus carrying a red-shifted opsin (such as Chrimson) into the medial temporal cortex (mTC; to target superficial pyramidal cells; Slomianka et al., 2011; Figure 6). Because the activation spectra of these two opsins are sufficiently distinct, one could activate one opsin without activating the other by using different wavelengths of light. With such an approach, one could examine if a given postsynaptic cell within CA1 was targeted by both populations of pyramidal cells, or selectively by one population or the other, and how synaptic reorganization associated with epilepsy might disrupt deep and superficial pyramidal cell wiring. To date INTRSECT has only been published for fluorescent proteins and ChR2 (Fenno et al., 2014). With further development of INTRSECT vectors to include additional opsins, INTRSECT approaches could be applied in dual-opto situations or in cases benefiting from inhibitory opsins.
The examples provided here are merely examples—the opportunities for combinatorial approaches that current and developing optogenetic tools provide are nearly endless. Clearly, optogenetic approaches are experimentally powerful, and will continue to be so moving forward.
While we have focused on the possibility for expansion of cell-type specific targeting of optogenetic methods in epilepsy research, and while the majority of optogenetic-aided epilepsy research (Table 2), and to some extent optogenetic-aided study of neurological disorders more broadly (Table 1), has been focused at the circuit level, use of optogenetics for molecular-level research is becoming increasingly possible, and deserves a brief discussion here. Indeed, several optogenetic tools are already available that would be very useful for studying neurological disorders, including epilepsy, from a molecular vantage (Box 1). These tools include light-activated G-protein coupled receptors (Opto-XRs; Airan et al., 2009), as well as a more diverse and ever-expanding tool box of specialized light-sensitive proteins.
One line of optogenetic tools which may be particularly useful for investigations at a more molecular level are systems in which light induces association or dissociation of proteins (Figure 1F, Box 1). There are a variety of these, including OptoSOS and the Phy-PIF system (Toettcher et al., 2013), LITEs and the CRY2-CIB1 system (Konermann et al., 2013), mutated Dronpa-based caging (Zhou et al., 2012), VP-EL222 and light-oxygen-voltage (LOV) domains (Motta-Mena et al., 2014), LOVTRAP (Wang et al., 2016), and photoactivatable Cre (Kawano et al., 2016). A recently developed system, PhoCl, is somewhat similar but rests on light-induced cleavage of a protein, making for a direct irreversible alteration by light (Zhang et al., 2017). The availability and advances to tools using light-induced alterations in protein-protein associations (or photocleavage of proteins) increases the possibilities for how optogenetics can be used to study the nervous system more broadly, by directly influencing the activity of intracellular proteins. As with other optogenetic approaches, these approaches can be used with the temporal and spatial specificity afforded through the use of light, and the cell-type specificity achievable through genetic encoding. Another use for systems in which light alters protein-protein interactions is to directly alter gene expression (e.g., LITEs (Konermann et al., 2013) and VP-EL222 (Motta-Mena et al., 2014)) or DNA itself (e.g., through a photoactivatable Cre system (Kawano et al., 2016)). Such light-based approaches may be able to achieve greater temporal specificity and reduced complications compared to more traditional methods like the Tetracycline/tamoxifen systems (Saunders, 2011). These tools are currently available, but not yet adopted in epilepsy research.
Additionally, the continued development and sharing of optogenetic tools, including optogenetic pharmacological tools, increases the potential for optogenetics as a strategy in cases where pharmacological tools may be lacking. In optogenetic pharmacology, a receptor (or subunit of a receptor) of interest is modified (e.g., through a single cysteine substitution) to allow a chemical photoswitch to (selectively and irreversibly) tether, thus combining genetic and chemical approaches. The photoswitches can be engineered to act as either an agonist or antagonist of the receptor in the absence or presence of a given wavelength of light. Previously, optogenetic pharmacology was available for various potassium channels (e.g., “SPARK” (Banghart et al., 2004)), glutamate receptors (e.g., “LiGluR” (Volgraf et al., 2006)) and acetylcholine receptors (e.g., “MAACh” (Tochitsky et al., 2012)). An optogenetic pharmacology approach was also recently developed for GABAA receptors (Lin et al., 2015). While the use of optogenetic pharmacology methods are arguably less straightforward than some other approaches, it offers the unique possibility of manipulating a select type of receptor in a particular cell type, in a particular brain region, at a particular time, and could be useful in the study of subunit specific contributions to epilepsy.
In addition to these optogenetic pharmacological tools, there have also been developments in other light-sensitive tools that may be useful where traditional pharmacological tools are currently lacking. Recently, Zhang et al. (2017) developed a photoactivatable version of the large-pore channel pannexin-1, which they termed Opto-Panx1. This tool could be directly relevant to research investigating pannexins or ATP release (as Opto-Panx1 activation induces ATP release) in health or disease states. Relevant to epilepsy research, ATP release is known to propagate glial calcium waves (Cotrina et al., 1998), which have been suggested to play a role in seizures (Gómez-Gonzalo et al., 2010). Also of note, glial calcium waves are blocked by some conventional anti-epileptic drugs (Tian et al., 2005). The potential impact of photocleavable proteins extends beyond pannexins and ATP, however. For an example, consider connexins. Connexins and the role of gap junctions in epilepsy is notoriously difficult to study owing to the lack of drugs that specifically and cleanly target connexins. While no Opto-connexin yet exists, one can easily imagine the potential uses for such a tool, and this is therefore an example of how continued tool development can aid future epilepsy research.
In addition to tools specifically designed to manipulate cellular processes at a molecular level, the currently widely available tools typically used for circuit-level investigations have been cleverly used in some instances for the study of more molecular phenomena. For example, in a stroke study by Beppu et al. (2014; listed in Table 1), the authors used ChR2(C128S) to induce inward flow of H+, and the inhibitory opsin ArchT to pump H+ out of the cell, in order to manipulate pH in glial cells. By optogenetically manipulating the acidity of glia in this manner, the authors demonstrated an impact on neurodegeneration at the site of ischemia. This is additionally an example of how considering how optogenetics has been used outside of the field of epilepsy (Table 1) could inspire future uses of optogenetics in epilepsy research.
The optogenetic tools available, both in terms of the opsins themselves and methods to achieve selective opsin expression, are rapidly expanding. As with any quickly growing field (or arguably any method), and discussed more in Box 1, optogenetics has limitations and considerations that must be acknowledged and controlled for in experimental design, including controlling for non-specific effects of light (including heat or sensory stimulation); expression of Cre, Flp, fluorescent proteins, or opsins themselves; potential effects of viral-based delivery; and “off-target” effects, including changes in ionic concentrations. Moreover, development of new tools is only one step in the process; the application of the tools to epilepsy research will depend in large part on the continued broad availability of the tools, and thus relies on tool developers making the conscious decision to make their tools widely available. With the specificity achievable with optogenetics, the versatility of optogenetic approaches, and the continued development (and sharing) of new tools, optogenetics will have a lasting impact on epilepsy research.
Conclusion
Optogenetics is a quickly growing field that provides researchers with the ability to manipulate neurons with unprecedented specificity. It is also a versatile tool, easily used in combination with other techniques to answer diverse experimental questions. Continued development and sharing of new methods, including for selective opsin expression, ensure the further expansion of optogenetics’ utility in epilepsy research.
Author Contributions
ZCW and EK-M drafted the manuscript.
FUNDING
Work in the Krook-Magnuson lab has been supported by the National Institute of Health through grants R01-NS104071, R03-NS098015 and R00-NS087110, by the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative, by the International Essential Tremor Foundation, the University of Minnesota’s Institute for Engineering in Medicine, the Winston and Maxine Wallin Neuroscience Discovery Fund and a McKnight Land-Grant Professorship (to EKM). ZCW was supported in part by National Institutes of Health grant T32-GM008471. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
- ^ “Vectors that produce retrograde labeling” should not be confused with the term “retrovirus,” which instead refers to a family of single-stranded RNA viruses, including HIV and lentiviruses.
References
Airan, R. D., Thompson, K. R., Fenno, L. E., Bernstein, H., and Deisseroth, K. (2009). Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029. doi: 10.1038/nature07926
Alfonsa, H., Merricks, E. M., Codadu, N. K., Cunningham, M. O., Deisseroth, K., Racca, C., et al. (2015). The contribution of raised intraneuronal chloride to epileptic network activity. J. Neurosci. 35, 7715–7726. doi: 10.1523/JNEUROSCI.4105-14.2015
Althaus, A. L., Zhang, H., and Parent, J. M. (2016). Axonal plasticity of age-defined dentate granule cells in a rat model of mesial temporal lobe epilepsy. Neurobiol. Dis. 86, 187–196. doi: 10.1016/j.nbd.2015.11.024
Anikeeva, P., Andalman, A. S., Witten, I., Warden, M., Goshen, I., Grosenick, L., et al. (2011). Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170. doi: 10.1038/nn.2992
Armstrong, C., Krook-Magnuson, E., Oijala, M., and Soltesz, I. (2013). Closed-loop optogenetic intervention in mice. Nat. Protoc. 8, 1475–1493. doi: 10.1038/nprot.2013.080
Armstrong, C., Krook-Magnuson, E., and Soltesz, I. (2012). Neurogliaform and Ivy cells: a major family of nNOS expressing GABAergic neurons. Front. Neural Circuits 6:23. doi: 10.3389/fncir.2012.00023
Assaf, F., and Schiller, Y. (2016). The antiepileptic and ictogenic effects of optogenetic neurostimulation of PV-expressing interneurons. J. Neurophysiol. 116, 1694–1704. doi: 10.1152/jn.00744.2015
Atasoy, D., Aponte, Y., Su, H. H., and Sternson, S. M. (2008). A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008
Avaliani, N., Sorensen, A. T., Ledri, M., Bengzon, J., Koch, P., Brüstle, O., et al. (2014). Optogenetics reveal delayed afferent synaptogenesis on grafted human-induced pluripotent stem cell-derived neural progenitors. Stem Cells 32, 3088–3098. doi: 10.1002/stem.1823
Bamann, C., Gueta, R., Kleinlogel, S., Nagel, G., and Bamberg, E. (2010). Structural guidance of the photocycle of channelrhodopsin-2 by an interhelical hydrogen bond. Biochemistry 49, 267–278. doi: 10.1021/bi901634p
Banghart, M., Borges, K., Isacoff, E., Trauner, D., and Kramer, R. H. (2004). Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386. doi: 10.1038/nn1356
Basu, J., Zaremba, J. D., Cheung, S. K., Hitti, F. L., Zemelman, B. V., Losonczy, A., et al. (2016). Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science 351:aaa5694. doi: 10.1126/science.aaa5694
Beppu, K., Sasaki, T., Tanaka, K. F., Yamanaka, A., Fukazawa, Y., Shigemoto, R., et al. (2014). Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 81, 314–320. doi: 10.1016/j.neuron.2013.11.011
Berglind, F., Ledri, M., Sørensen, A. T., Nikitidou, L., Melis, M., Bielefeld, P., et al. (2014). Optogenetic inhibition of chemically induced hypersynchronized bursting in mice. Neurobiol. Dis. 65, 133–141. doi: 10.1016/j.nbd.2014.01.015
Berndt, A., Lee, S. Y., Ramakrishnan, C., and Deisseroth, K. (2014). Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science 344, 420–424. doi: 10.1126/science.1252367
Berndt, A., Lee, S. Y., Wietek, J., Ramakrishnan, C., Steinberg, E. E., Rashid, A. J., et al. (2016). Structural foundations of optogenetics: determinants of channelrhodopsin ion selectivity. Proc. Natl. Acad. Sci. U S A 113, 822–829. doi: 10.1073/pnas.1523341113
Berndt, A., Schoenenberger, P., Mattis, J., Tye, K. M., Deisseroth, K., Hegemann, P., et al. (2011). High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl. Acad. Sci. U S A 108, 7595–7600. doi: 10.1073/pnas.1017210108
Berndt, A., Yizhar, O., Gunaydin, L. A., Hegemann, P., and Deisseroth, K. (2009). Bi-stable neural state switches. Nat. Neurosci. 12, 229–234. doi: 10.1038/nn.2247
Bernhardt, B. C., Bonilha, L., and Gross, D. W. (2015). Network analysis for a network disorder: the emerging role of graph theory in the study of epilepsy. Epilepsy Behav. 50, 162–170. doi: 10.1016/j.yebeh.2015.06.005
Bezaire, M. J., and Soltesz, I. (2013). Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus 23, 751–785. doi: 10.1002/hipo.22141
Bouilleret, V., Loup, F., Kiener, T., Marescaux, C., and Fritschy, J. M. (2000). Early loss of interneurons and delayed subunit-specific changes in GABAA-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus 10, 305–324. doi: 10.1002/1098-1063(2000)10:3<305::aid-hipo11>3.3.co;2-9
Boyden, E. S. (2015). Optogenetics and the future of neuroscience. Nat. Neurosci. 18, 1200–1201. doi: 10.1038/nn1215-1862b
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., and Deisseroth, K. (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268. doi: 10.1038/nn1525
Buckmaster, P. S., and Dudek, F. E. (1997). Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J. Comp. Neurol. 385, 385–404. doi: 10.1002/(sici)1096-9861(19970901)385:3<385::aid-cne4>3.3.co;2-y
Bui, A. D., Nguyen, T. M., Limouse, C., Kim, H. K., Szabo, G. G., Felong, S., et al. (2018). Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science 359, 787–790. doi: 10.1126/science.aan4074
Burette, A., Zabel, U., Weinberg, R. J., Schmidt, H. H., and Valtschanoff, J. G. (2002). Synaptic localization of nitric oxide synthase and soluble guanylyl cyclase in the hippocampus. J. Neurosci. 22, 8961–8970. doi: 10.1523/JNEUROSCI.22-20-08961.2002
Cammarota, M., Losi, G., Chiavegato, A., Zonta, M., and Carmignoto, G. (2013). Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J. Physiol. 591, 807–822. doi: 10.1113/jphysiol.2012.238154
Chang, M., Dian, J. A., Dufour, S., Wang, L., Moradi Chameh, H., Ramani, M., et al. (2018). Brief activation of GABAergic interneurons initiates the transition to ictal events through post-inhibitory rebound excitation. Neurobiol. Dis. 109, 102–116. doi: 10.1016/j.nbd.2017.10.007
Chatterjee, S., Sullivan, H. A., MacLennan, B. J., Xu, R., Hou, Y., Lavin, T. K., et al. (2018). Nontoxic, double-deletion-mutant rabies viral vectors for retrograde targeting of projection neurons. Nat. Neurosci. 21, 638–646. doi: 10.1038/s41593-018-0091-7
Chen, S., Weitemier, A. Z., Zeng, X., He, L., Wang, X., Tao, Y., et al. (2018). Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 359, 679–684. doi: 10.1126/science.aaq1144
Chiang, C. C., Ladas, T. P., Gonzalez-Reyes, L. E., and Durand, D. M. (2014). Seizure suppression by high frequency optogenetic stimulation using in vitro and in vivo animal models of epilepsy. Brain Stimul. 7, 890–899. doi: 10.1016/j.brs.2014.07.034
Chkhenkeli, S. A., Sramka, M., Lortkipanidze, G. S., Rakviashvili, T. N., Bregvadze, E., Magalashvili, G. E., et al. (2004). Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin. Neurol. Neurosurg. 106, 318–329. doi: 10.1016/j.clineuro.2004.01.009
Choe, K., Sanchez, C., Harris, N., Otis, T., and Mathews, P. (2018). Optogenetic fMRI and electrophysiological identification of region-specific connectivity between the cerebellar cortex and forebrain. Neuroimage 173, 370–383. doi: 10.1016/j.neuroimage.2018.02.047
Chow, B. Y., Han, X., Dobry, A. S., Qian, X., Chuong, A. S., Li, M., et al. (2010). High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102. doi: 10.1038/nature08652
Choy, M., Duffy, B. A., and Lee, J. H. (2017). Optogenetic study of networks in epilepsy. J. Neurosci. Res. 95, 2325–2335. doi: 10.1002/jnr.23767
Christenson Wick, Z., Leintz, C. H., Xamonthiene, C., Huang, B. H., and Krook-Magnuson, E. (2017). Axonal sprouting in commissurally projecting parvalbumin-expressing interneurons. J. Neurosci. Res. 95, 2336–2344. doi: 10.1002/jnr.24011
Chuong, A. S., Miri, M. L., Busskamp, V., Matthews, G. A., Acker, L. C., Sorensen, A. T., et al. (2014). Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129. doi: 10.1038/nn.3752
Cobb, S. R., Buhl, E. H., Halasy, K., Paulsen, O., and Somogyi, P. (1995). Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378, 75–78. doi: 10.1038/378075a0
Connor, D. E. Jr., Nixon, M., Nanda, A., and Guthikonda, B. (2012). Vagal nerve stimulation for the treatment of medically refractory epilepsy: a review of the current literature. Neurosurg. Focus 32:E12. doi: 10.3171/2011.12.FOCUS11328
Cooper, I. S., Amin, I., and Gilman, S. (1973). The effect of chronic cerebellar stimulation upon epilepsy in man. Trans. Am. Neurol. Assoc. 98, 192–196.
Cosentino, C., Alberio, L., Gazzarrini, S., Aquila, M., Romano, E., Cermenati, S., et al. (2015). Optogenetics. Engineering of a light-gated potassium channel. Science 348, 707–710. doi: 10.1126/science.aaa2787
Cotrina, M. L., Lin, J. H., Alves-Rodrigues, A., Liu, S., Li, J., Azmi-Ghadimi, H., et al. (1998). Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. U S A 95, 15735–15740. doi: 10.1073/pnas.95.26.15735
Coulter, D. A. (1999). Chronic epileptogenic cellular alterations in the limbic system after status epilepticus. Epilepsia 40, S23–33; discussion S40–S21. doi: 10.1111/j.1528-1157.1999.tb00875.x
Cunningham, M., Cho, J. H., Leung, A., Savvidis, G., Ahn, S., Moon, M., et al. (2014). hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 15, 559–573. doi: 10.1016/j.stem.2014.10.006
Davis, R., and Emmonds, S. E. (1992). Cerebellar stimulation for seizure control: 17-year study. Stereotact. Funct. Neurosurg. 58, 200–208. doi: 10.1159/000098996
Dawydow, A., Gueta, R., Ljaschenko, D., Ullrich, S., Hermann, M., Ehmann, N., et al. (2014). Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. U S A 111, 13972–13977. doi: 10.1073/pnas.1408269111
de Tisi, J., Bell, G. S., Peacock, J. L., McEvoy, A. W., Harkness, W. F., Sander, J. W., et al. (2011). The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378, 1388–1395. doi: 10.1016/S0140-6736(11)60890-8
Deisseroth, K. (2015). Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225. doi: 10.1038/nn.4091
Drexel, M., Kirchmair, E., Wieselthaler-Hölzl, A., Preidt, A. P., and Sperk, G. (2012). Somatostatin and neuropeptide Y neurons undergo different plasticity in parahippocampal regions in kainic acid-induced epilepsy. J. Neuropathol. Exp. Neurol. 71, 312–329. doi: 10.1097/NEN.0b013e31824d9882
Duchowny, M., and Bhatia, S. (2014). Epilepsy: preserving memory in temporal lobectomy-are networks the key? Nat. Rev. Neurol. 10, 245–246. doi: 10.1038/nrneurol.2014.67
Duffy, B. A., Choy, M., Chuapoco, M. R., Madsen, M., and Lee, J. H. (2015). MRI compatible optrodes for simultaneous LFP and optogenetic fMRI investigation of seizure-like afterdischarges. Neuroimage 123, 173–184. doi: 10.1016/j.neuroimage.2015.07.038
Dybdal, D., and Gale, K. (2000). Postural and anticonvulsant effects of inhibition of the rat subthalamic nucleus. J. Neurosci. 20, 6728–6733. doi: 10.1523/JNEUROSCI.20-17-06728.2000
Ellender, T. J., Raimondo, J. V., Irkle, A., Lamsa, K. P., and Akerman, C. J. (2014). Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous afterdischarges. J. Neurosci. 34, 15208–15222. doi: 10.1523/JNEUROSCI.1747-14.2014
England, M. J., Liverman, C. T., Schultz, A. M., and Strawbridge, L. M. (2012). Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav. 25, 266–276. doi: 10.1016/j.yebeh.2012.06.016
Fenno, L. E., Mattis, J., Ramakrishnan, C., Hyun, M., Lee, S. Y., He, M., et al. (2014). Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772. doi: 10.1038/nmeth.2996
Fernandez-Guardiola, A., Manni, E., Wilson, J. H., and Dow, R. S. (1962). Microelectrode recording of cerebellar and cerebral unit activity during convulsive afterdischarge. Exp. Neurol. 6, 48–69. doi: 10.1016/0014-4886(62)90014-6
Fisher, R., Salanova, V., Witt, T., Worth, R., Henry, T., Gross, R., et al. (2010). Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51, 899–908. doi: 10.1111/j.1528-1167.2010.02536.x
Forcelli, P. A. (2017). Applications of optogenetic and chemogenetic methods to seizure circuits: where to go next? J. Neurosci. Res. 95, 2345–2356. doi: 10.1002/jnr.24135
Freund, T. F., and Buzsáki, G. (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. doi: 10.1002/(sici)1098-1063(1996)6:4<347::aid-hipo1>3.0.co;2-i
Gale, K. (1992). Subcortical structures and pathways involved in convulsive seizure generation. J. Clin. Neurophysiol. 9, 264–277. doi: 10.1097/00004691-199204010-00007
Gibson, E. M., Purger, D., Mount, C. W., Goldstein, A. K., Lin, G. L., Wood, L. S., et al. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304. doi: 10.1126/science.1252304
Gómez-Gonzalo, M., Losi, G., Chiavegato, A., Zonta, M., Cammarota, M., Brondi, M., et al. (2010). An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 8:e1000352. doi: 10.1371/journal.pbio.1000352
Goodman, J. H., and Sloviter, R. S. (1992). Evidence for commissurally projecting parvalbumin-immunoreactive basket cells in the dentate gyrus of the rat. Hippocampus 2, 13–21. doi: 10.1002/hipo.450020103
Govorunova, E. G., Sineshchekov, O. A., Janz, R., Liu, X., and Spudich, J. L. (2015). NEUROSCIENCE. Natural light-gated anion channels: a family of microbial rhodopsins for advanced optogenetics. Science 349, 647–650. doi: 10.1126/science.aaa7484
Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M., and Deisseroth, K. (2009). Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359. doi: 10.1126/science.1167093
Gradinaru, V., Thompson, K. R., and Deisseroth, K. (2008). eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 36, 129–139. doi: 10.1007/s11068-008-9027-6
Gradinaru, V., Zhang, F., Ramakrishnan, C., Mattis, J., Prakash, R., Diester, I., et al. (2010). Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165. doi: 10.1016/j.cell.2010.02.037
Grosenick, L., Marshel, J. H., and Deisseroth, K. (2015). Closed-loop and activity-guided optogenetic control. Neuron 86, 106–139. doi: 10.1016/j.neuron.2015.03.034
Gunaydin, L. A., Yizhar, O., Berndt, A., Sohal, V. S., Deisseroth, K., and Hegemann, P. (2010). Ultrafast optogenetic control. Nat. Neurosci. 13, 387–392. doi: 10.1038/nn.2495
Gutruf, P., and Rogers, J. A. (2018). Implantable, wireless device platforms for neuroscience research. Curr. Opin. Neurobiol. 50, 42–49. doi: 10.1016/j.conb.2017.12.007
Hajos, N., and Paulsen, O. (2009). Network mechanisms of γ oscillations in the CA3 region of the hippocampus. Neural Netw. 22, 1113–1119. doi: 10.1016/j.neunet.2009.07.024
Han, X., Chow, B. Y., Zhou, H., Klapoetke, N. C., Chuong, A., Rajimehr, R., et al. (2011). A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front. Syst. Neurosci. 5:18. doi: 10.3389/fnsys.2011.00018
Hashimoto, T., and Obata, K. (1991). Induction of somatostatin by kainic acid in pyramidal and granule cells of the rat hippocampus. Neurosci. Res. 12, 514–527. doi: 10.1016/s0168-0102(09)80004-7
He, M., Tucciarone, J., Lee, S., Nigro, M. J., Kim, Y., Levine, J. M., et al. (2016). Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron 92:555. doi: 10.1016/j.neuron.2016.10.009
Heath, R. G., Dempesy, C. W., Fontana, C. J., and Fitzjarrell, A. T. (1980). Feedback loop between cerebellum and septal-hippocampal sites: its role in emotion and epilepsy. Biol. Psychiatry 15, 541–556.
Heinemann, U., Beck, H., Dreier, J. P., Ficker, E., Stabel, J., and Zhang, C. L. (1992). The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. Suppl. 7, 273–280.
Henderson, K. W., Gupta, J., Tagliatela, S., Litvina, E., Zheng, X., Van Zandt, M. A., et al. (2014). Long-term seizure suppression and optogenetic analyses of synaptic connectivity in epileptic mice with hippocampal grafts of GABAergic interneurons. J. Neurosci. 34, 13492–13504. doi: 10.1523/JNEUROSCI.0005-14.2014
Houben, T., Loonen, I. C., Baca, S. M., Schenke, M., Meijer, J. H., Ferrari, M. D., et al. (2017). Optogenetic induction of cortical spreading depression in anesthetized and freely behaving mice. J. Cereb. Blood Flow Metab. 37, 1641–1655. doi: 10.1177/0271678x16645113
Howe, M. W., and Dombeck, D. A. (2016). Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510. doi: 10.1038/nature18942
Hsieh, J. Y., and Baraban, S. C. (2017). Medial ganglionic eminence progenitors transplanted into hippocampus integrate in a functional and subtype-appropriate manner. eNeuro 4:ENEURO.0359-16.2017. doi: 10.1523/ENEURO.0359-16.2017
Hunt, R. F., and Baraban, S. C. (2015). Interneuron transplantation as a treatment for epilepsy. Cold Spring Harb. Perspect. Med. 5:a022376. doi: 10.1101/cshperspect.a022376
Hunt, R. F., Girskis, K. M., Rubenstein, J. L., Alvarez-Buylla, A., and Baraban, S. C. (2013). GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci. 16, 692–697. doi: 10.1038/nn.3392
Iaccarino, H. F., Singer, A. C., Martorell, A. J., Rudenko, A., Gao, F., Gillingham, T. Z., et al. (2016). γ frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235. doi: 10.1038/nature20587
Jiang, X., Lachance, M., and Rossignol, E. (2016). Involvement of cortical fast-spiking parvalbumin-positive basket cells in epilepsy. Prog. Brain Res. 226, 81–126. doi: 10.1016/bs.pbr.2016.04.012
Jin, S. H., Jeong, W., and Chung, C. K. (2015). Mesial temporal lobe epilepsy with hippocampal sclerosis is a network disorder with altered cortical hubs. Epilepsia 56, 772–779. doi: 10.1111/epi.12966
Julien, R. M., and Laxer, K. D. (1974). Cerebellar responses to penicillin-induced cerebral cortical epileptiform discharge. Electroencephalogr. Clin. Neurophysiol. 37, 123–132. doi: 10.1016/0013-4694(74)90002-9
Junyent, F., and Kremer, E. J. (2015). CAV-2—why a canine virus is a neurobiologist’s best friend. Curr. Opin. Pharmacol. 24, 86–93. doi: 10.1016/j.coph.2015.08.004
Kaemmerer, W. F. (2017). “Optogenetics for neurological disorders: what is the path to the clinic?,” in Optogenetics: From Neuronal Function to Mapping and Disease Biology, ed. K. Appasani (Cambridge: Cambridge University Press), 169–180.
Kainrath, S., Stadler, M., Reichhart, E., Distel, M., and Janovjak, H. (2017). Green-light-induced inactivation of receptor signaling using cobalamin-binding domains. Angew. Chem. Int. Ed Engl. 56, 4608–4611. doi: 10.1002/anie.201611998
Kandel, A., and Buzsáki, G. (1993). Cerebellar neuronal activity correlates with spike and wave EEG patterns in the rat. Epilepsy Res. 16, 1–9. doi: 10.1016/0920-1211(93)90033-4
Katona, L., Micklem, B., Borhegyi, Z., Swiejkowski, D. A., Valenti, O., Viney, T. J., et al. (2017). Behavior-dependent activity patterns of GABAergic long-range projecting neurons in the rat hippocampus. Hippocampus 27, 359–377. doi: 10.1002/hipo.22696
Kawano, F., Okazaki, R., Yazawa, M., and Sato, M. (2016). A photoactivatable Cre-loxP recombination system for optogenetic genome engineering. Nat. Chem. Biol. 12, 1059–1064. doi: 10.1038/nchembio.2205
Khoshkhoo, S., Vogt, D., and Sohal, V. S. (2017). Dynamic, cell-type-specific roles for GABAergic interneurons in a mouse model of optogenetically inducible seizures. Neuron 93, 291–298. doi: 10.1016/j.neuron.2016.11.043
Kim, T. I., McCall, J. G., Jung, Y. H., Huang, X., Siuda, E. R., Li, Y., et al. (2013). Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216. doi: 10.1126/science.1232437
Klapoetke, N. C., Murata, Y., Kim, S. S., Pulver, S. R., Birdsey-Benson, A., Cho, Y. K., et al. (2014). Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346. doi: 10.1038/nmeth.2836
Kondabolu, K., Roberts, E. A., Bucklin, M., McCarthy, M. M., Kopell, N., and Han, X. (2016). Striatal cholinergic interneurons generate β and γ oscillations in the corticostriatal circuit and produce motor deficits. Proc. Natl. Acad. Sci. U S A 113, E3159–E3168. doi: 10.1073/pnas.1605658113
Konermann, S., Brigham, M. D., Trevino, A. E., Hsu, P. D., Heidenreich, M., Cong, L., et al. (2013). Optical control of mammalian endogenous transcription and epigenetic states. Nature 500, 472–476. doi: 10.1038/nature12466
Kramer, R. H., Mourot, A., and Adesnik, H. (2013). Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 16, 816–823. doi: 10.1038/nn.3424
Krook-Magnuson, E. (2017). The gate and the source? The dentate gyrus takes central stage in temporal lobe epilepsy. Epilepsy Curr. 17, 48–49. doi: 10.5698/1535-7511-17.1.48
Krook-Magnuson, E., Armstrong, C., Bui, A., Lew, S., Oijala, M., and Soltesz, I. (2015a). In vivo evaluation of the dentate gate theory in epilepsy. J. Physiol. 593, 2379–2388. doi: 10.1113/JP270056
Krook-Magnuson, E., Gelinas, J. N., Soltesz, I., and Buzsáki, G. (2015b). Neuroelectronics and biooptics: closed-loop technologies in neurological disorders. JAMA Neurol. 72, 823–829. doi: 10.1001/jamaneurol.2015.0608
Krook-Magnuson, E., Armstrong, C., Oijala, M., and Soltesz, I. (2013). On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 4:1376. doi: 10.1038/ncomms2376
Krook-Magnuson, E., Ledri, M., Soltesz, I., and Kokaia, M. (2014a). How might novel technologies such as optogenetics lead to better treatments in epilepsy? Adv. Exp. Med. Biol. 813, 319–336. doi: 10.1007/978-94-017-8914-1_26
Krook-Magnuson, E., Szabo, G. G., Armstrong, C., Oijala, M., and Soltesz, I. (2014b). Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro 1:e2014. doi: 10.1523/eneuro.0005-14.2014
Krook-Magnuson, E., and Soltesz, I. (2015). Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nat. Neurosci. 18, 331–338. doi: 10.1038/nn.3943
Krook-Magnuson, E., Varga, C., Lee, S. H., and Soltesz, I. (2012). New dimensions of interneuronal specialization unmasked by principal cell heterogeneity. Trends Neurosci. 35, 175–184. doi: 10.1016/j.tins.2011.10.005
Kros, L., Eelkman Rooda, O. H., Spanke, J. K., Alva, P., van Dongen, M. N., Karapatis, A., et al. (2015). Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann. Neurol. 77, 1027–1049. doi: 10.1002/ana.24399
Kullmann, D. M., Schorge, S., Walker, M. C., and Wykes, R. C. (2014). Gene therapy in epilepsy-is it time for clinical trials? Nat. Rev. Neurol. 10, 300–304. doi: 10.1038/nrneurol.2014.43
Ladas, T. P., Chiang, C. C., Gonzalez-Reyes, L. E., Nowak, T., and Durand, D. M. (2015). Seizure reduction through interneuron-mediated entrainment using low frequency optical stimulation. Exp. Neurol. 269, 120–132. doi: 10.1016/j.expneurol.2015.04.001
Laurberg, S., and Zimmer, J. (1981). Lesion-induced sprouting of hippocampal mossy fiber collaterals to the fascia dentata in developing and adult rats. J. Comp. Neurol. 200, 433–459. doi: 10.1002/cne.902000310
Laxer, K. D., Trinka, E., Hirsch, L. J., Cendes, F., Langfitt, J., Delanty, N., et al. (2014). The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 37C, 59–70. doi: 10.1016/j.yebeh.2014.05.031
Ledri, M., Madsen, M. G., Nikitidou, L., Kirik, D., and Kokaia, M. (2014). Global optogenetic activation of inhibitory interneurons during epileptiform activity. J. Neurosci. 34, 3364–3377. doi: 10.1523/JNEUROSCI.2734-13.2014
Lee, J., Ozden, I., Song, Y. K., and Nurmikko, A. V. (2015). Transparent intracortical microprobe array for simultaneous spatiotemporal optical stimulation and multichannel electrical recording. Nat. Methods 12, 1157–1162. doi: 10.1038/nmeth.3620
Lee, S. H., Marchionni, I., Bezaire, M., Varga, C., Danielson, N., Lovett-Barron, M., et al. (2014). Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron 82, 1129–1144. doi: 10.1016/j.neuron.2014.03.034
Lee, S. Y., and Soltesz, I. (2011). Cholecystokinin: a multi-functional molecular switch of neuronal circuits. Dev. Neurobiol. 71, 83–91. doi: 10.1002/dneu.20815
Levy, L. F., and Auchterlonie, W. C. (1979). Chronic cerebellar stimulation in the treatment of epilepsy. Epilepsia 20, 235–245. doi: 10.1111/j.1528-1157.1979.tb04800.x
Libbrecht, S., Van den Haute, C., Malinouskaya, L., Gijsbers, R., and Baekelandt, V. (2017). Evaluation of WGA-Cre-dependent topological transgene expression in the rodent brain. Brain Struct. Funct. 222, 717–733. doi: 10.1007/s00429-016-1241-x
Lim, D. H., LeDue, J. M., Mohajerani, M. H., and Murphy, T. H. (2014). Optogenetic mapping after stroke reveals network-wide scaling of functional connections and heterogeneous recovery of the peri-infarct. J. Neurosci. 34, 16455–16466. doi: 10.1523/JNEUROSCI.3384-14.2014
Lin, J. Y., Knutsen, P. M., Muller, A., Kleinfeld, D., and Tsien, R. Y. (2013). ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508. doi: 10.1038/nn.3502
Lin, J. Y., Lin, M. Z., Steinbach, P., and Tsien, R. Y. (2009). Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814. doi: 10.1016/j.bpj.2008.11.034
Lin, W. C., Tsai, M. C., Davenport, C. M., Smith, C. M., Veit, J., Wilson, N. M., et al. (2015). A comprehensive optogenetic pharmacology toolkit for in vivo control of GABAA receptors and synaptic inhibition. Neuron 88, 879–891. doi: 10.1016/j.neuron.2015.10.026
Liu, Q., Gan, L., Ni, J., Chen, Y., Chen, Y., Huang, Z., et al. (2017). Dcf1 improves behavior deficit in Drosophila and mice caused by optogenetic suppression. J. Cell. Biochem. 118, 4210–4215. doi: 10.1002/jcb.26048
Lothman, E. W., Stringer, J. L., and Bertram, E. H. (1992). The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res. Suppl. 7, 301–313.
Lu, Y., Zhong, C., Wang, L., Wei, P., He, W., Huang, K., et al. (2016). Optogenetic dissection of ictal propagation in the hippocampal-entorhinal cortex structures. Nat. Commun. 7:10962. doi: 10.1038/ncomms12019
Madisen, L., Garner, A. R., Shimaoka, D., Chuong, A. S., Klapoetke, N. C., Li, L., et al. (2015). Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958. doi: 10.1016/j.neuron.2015.02.022
Madisen, L., Mao, T., Koch, H., Zhuo, J. M., Berenyi, A., Fujisawa, S., et al. (2012). A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802. doi: 10.1038/nn.3078
Maguire, J. L. (2015). Implicating interneurons: optogenetic studies suggest that interneurons are guilty of contributing to epileptiform activity. Epilepsy Curr. 15, 213–216. doi: 10.5698/1535-7511-15.4.213
Mahn, M., Gibor, L., Cohen-Kashi Malina, K., Patil, P., Printz, Y., Oring, S., et al. (2017). High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins. bioRxiv. doi: 10.1101/225847 [Epub ahead of Print].
Mahn, M., Prigge, M., Ron, S., Levy, R., and Yizhar, O. (2016). Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci. 19, 554–556. doi: 10.1038/nn.4266
Maroso, M., Szabo, G. G., Kim, H. K., Alexander, A., Bui, A. D., Lee, S. H., et al. (2016). Cannabinoid control of learning and memory through HCN channels. Neuron 89, 1059–1073. doi: 10.1016/j.neuron.2016.01.023
Mastro, K. J., Zitelli, K. T., Willard, A. M., Leblanc, K. H., Kravitz, A. V., and Gittis, A. H. (2017). Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat. Neurosci. 20, 815–823. doi: 10.1038/nn.4559
Mathern, G. W., Babb, T. L., Pretorius, J. K., and Leite, J. P. (1995). Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J. Neurosci. 15, 3990–4004. doi: 10.1523/jneurosci.15-05-03990.1995
Mattis, J., Tye, K. M., Ferenczi, E. A., Ramakrishnan, C., O’Shea, D. J., Prakash, R., et al. (2012). Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172. doi: 10.1038/nmeth.1808
McGeorge, A. J., and Faull, R. L. (1989). The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29, 503–537. doi: 10.1016/0306-4522(89)90128-0
Melzer, S., Michael, M., Caputi, A., Eliava, M., Fuchs, E. C., Whittington, M. A., et al. (2012). Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science 335, 1506–1510. doi: 10.1126/science.1217139
Meyer, M., Kienzler-Norwood, F., Bauer, S., Rosenow, F., and Norwood, B. A. (2016). Removing entorhinal cortex input to the dentate gyrus does not impede low frequency oscillations, an EEG-biomarker of hippocampal epileptogenesis. Sci. Rep. 6:25660. doi: 10.1038/srep25660
Mohammad, F., Stewart, J. C., Ott, S., Chlebikova, K., Chua, J. Y., Koh, T. W., et al. (2017). Optogenetic inhibition of behavior with anion channelrhodopsins. Nat. Methods 14, 271–274. doi: 10.1038/nmeth.4148
Montgomery, K. L., Iyer, S. M., Christensen, A. J., Deisseroth, K., and Delp, S. L. (2016). Beyond the brain: optogenetic control in the spinal cord and peripheral nervous system. Sci. Transl. Med. 8:337rv5. doi: 10.1126/scitranslmed.aad7577
Moore, Y. E., Kelley, M. R., Brandon, N. J., Deeb, T. Z., and Moss, S. J. (2017). Seizing control of KCC2: a new therapeutic target for epilepsy. Trends Neurosci. 40, 555–571. doi: 10.1016/j.tins.2017.06.008
Motta-Mena, L. B., Reade, A., Mallory, M. J., Glantz, S., Weiner, O. D., Lynch, K. W., et al. (2014). An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat. Chem. Biol. 10, 196–202. doi: 10.1038/nchembio.1430
Nadler, J. V., Perry, B. W., Gentry, C., and Cotman, C. W. (1980). Loss and reacquisition of hippocampal synapses after selective destruction of CA3-CA4 afferents with kainic acid. Brain Res. 191, 387–403. doi: 10.1016/0006-8993(80)91289-5
Nagaraj, V., Lee, S. T., Krook-Magnuson, E., Soltesz, I., Benquet, P., Irazoqui, P. P., et al. (2015). Future of seizure prediction and intervention: closing the loop. J. Clin. Neurophysiol. 32, 194–206. doi: 10.1097/wnp.0000000000000139
Nagel, G., Brauner, M., Liewald, J. F., Adeishvili, N., Bamberg, E., and Gottschalk, A. (2005). Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284. doi: 10.1016/j.cub.2005.11.032
Nagel, G., Szellas, T., Huhn, W., Kateriya, S., Adeishvili, N., Berthold, P., et al. (2003). Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U S A 100, 13940–13945. doi: 10.1073/pnas.1936192100
Namburi, P., Beyeler, A., Yorozu, S., Calhoon, G. G., Halbert, S. A., Wichmann, R., et al. (2015). A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678. doi: 10.1038/nature14366
Niedermeyer, E., and Uematsu, S. (1974). Electroencephalographic recordings from deep cerebellar structures in patients with uncontrolled epileptic seizures. Electroencephalogr. Clin. Neurophysiol. 37, 355–365. doi: 10.1016/0013-4694(74)90111-4
Osawa, S., Iwasaki, M., Hosaka, R., Matsuzaka, Y., Tomita, H., Ishizuka, T., et al. (2013). Optogenetically induced seizure and the longitudinal hippocampal network dynamics. PLoS One 8:e60928. doi: 10.1371/journal.pone.0060928
Pathak, H. R., Weissinger, F., Terunuma, M., Carlson, G. C., Hsu, F. C., Moss, S. J., et al. (2007). Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J. Neurosci. 27, 14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007
Paz, J. T., Davidson, T. J., Frechette, E. S., Delord, B., Parada, I., Peng, K., et al. (2013). Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 16, 64–70. doi: 10.1038/nn.3269
Peng, Z., Zhang, N., Wei, W., Huang, C. S., Cetina, Y., Otis, T. S., et al. (2013). A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J. Neurosci. 33, 14392–14405. doi: 10.1523/jneurosci.2045-13.2013
Perucca, P., and Gilliam, F. G. (2012). Adverse effects of antiepileptic drugs. Lancet Neurol. 11, 792–802. doi: 10.1016/S1474-4422(12)70153-9
Pun, R. Y., Rolle, I. J., Lasarge, C. L., Hosford, B. E., Rosen, J. M., Uhl, J. D., et al. (2012). Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron 75, 1022–1034. doi: 10.1016/j.neuron.2012.08.002
Rajasethupathy, P., Ferenczi, E., and Deisseroth, K. (2016). Targeting neural circuits. Cell 165, 524–534. doi: 10.1016/j.cell.2016.03.047
Roy, D. S., Arons, A., Mitchell, T. I., Pignatelli, M., Ryan, T. J., and Tonegawa, S. (2016). Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531, 508–512. doi: 10.1038/nature17172
Rungta, R. L., Osmanski, B. F., Boido, D., Tanter, M., and Charpak, S. (2017). Light controls cerebral blood flow in naive animals. Nat. Commun. 8:14191. doi: 10.1038/ncomms14191
Saunders, T. L. (2011). Inducible transgenic mouse models. Methods Mol Biol 693, 103–115. doi: 10.1007/978-1-60761-974-1_7
Sessolo, M., Marcon, I., Bovetti, S., Losi, G., Cammarota, M., Ratto, G. M., et al. (2015). Parvalbumin-positive inhibitory interneurons oppose propagation but favor generation of focal epileptiform activity. J. Neurosci. 35, 9544–9557. doi: 10.1523/jneurosci.5117-14.2015
Sheybani, L., Birot, G., Contestabile, A., Seeck, M., Zoltan Kiss, J., Schaller, K., et al. (2018). Electrophysiological evidence for the development of a self-sustained large-scale epileptic network in the kainate mouse-model of temporal lobe epilepsy. J. Neurosci. 38, 3776’3791. doi: 10.1523/JNEUROSCI.2193-17.2018
Shiri, Z., Manseau, F., Lévesque, M., Williams, S., and Avoli, M. (2015). Interneuron activity leads to initiation of low-voltage fast-onset seizures. Ann. Neurol. 77, 541–546. doi: 10.1002/ana.24342
Shiri, Z., Manseau, F., Lévesque, M., Williams, S., and Avoli, M. (2016). Activation of specific neuronal networks leads to different seizure onset types. Ann. Neurol. 79, 354–365. doi: 10.1002/ana.24570
Slomianka, L., Amrein, I., Knuesel, I., Sørensen, J. C., and Wolfer, D. P. (2011). Hippocampal pyramidal cells: the reemergence of cortical lamination. Brain Struct. Funct. 216, 301–317. doi: 10.1007/s00429-011-0322-0
Sohal, V. S., Zhang, F., Yizhar, O., and Deisseroth, K. (2009). Parvalbumin neurons and γ rhythms enhance cortical circuit performance. Nature 459, 698–702. doi: 10.1038/nature07991
Soltesz, I., and Losonczy, A. (2018). CA1 pyramidal cell diversity enabling parallel information processing in the hippocampus. Nat. Neurosci. 21, 484–493. doi: 10.1038/s41593-018-0118-0
Soper, C., Wicker, E., Kulick, C. V., N’Gouemo, P., and Forcelli, P. A. (2016). Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol. Dis. 87, 102–115. doi: 10.1016/j.nbd.2015.12.012
Sorokin, J. M., Davidson, T. J., Frechette, E., Abramian, A. M., Deisseroth, K., Huguenard, J. R., et al. (2017). Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron 93, 194–210. doi: 10.1016/j.neuron.2016.11.026
Soussi, R., Boulland, J. L., Bassot, E., Bras, H., Coulon, P., Chaudhry, F. A., et al. (2015). Reorganization of supramammillary-hippocampal pathways in the rat pilocarpine model of temporal lobe epilepsy: evidence for axon terminal sprouting. Brain Struct. Funct. 220, 2449–2468. doi: 10.1007/s00429-014-0800-2
Šramka, M., Fritz, G., Galanda, M., and Nádvorník, P. (1976). Some observations in treatment stimulation of epilepsy. Acta Neurochir. 23, 257–262. doi: 10.1007/978-3-7091-8444-8_41
Sukhotinsky, I., Chan, A. M., Ahmed, O. J., Rao, V. R., Gradinaru, V., Ramakrishnan, C., et al. (2013). Optogenetic delay of status epilepticus onset in an in vivo rodent epilepsy model. PLoS One 8:e62013. doi: 10.1371/journal.pone.0062013
Sun, Y., Nguyen, A. Q., Nguyen, J. P., Le, L., Saur, D., Choi, J., et al. (2014). Cell-type-specific circuit connectivity of hippocampal CA1 revealed through Cre-dependent rabies tracing. Cell Rep. 7, 269–280. doi: 10.1016/j.celrep.2014.02.030
Sutula, T., Cascino, G., Cavazos, J., Parada, I., and Ramirez, L. (1989). Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann. Neurol. 26, 321–330. doi: 10.1002/ana.410260303
Szabadics, J., Varga, C., Molnár, G., Oláh, S., Barzó, P., and Tamás, G. (2006). Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311, 233–235. doi: 10.1126/science.1121325
Szabo, G. G., Du, X., Oijala, M., Varga, C., Parent, J. M., and Soltesz, I. (2017). Extended interneuronal network of the dentate gyrus. Cell Rep. 20, 1262–1268. doi: 10.1016/j.celrep.2017.07.042
Tang, J. C., Rudolph, S., Dhande, O. S., Abraira, V. E., Choi, S., Lapan, S. W., et al. (2015). Cell type-specific manipulation with GFP-dependent Cre recombinase. Nat. Neurosci. 18, 1334–1341. doi: 10.1038/nn.4081
Taniguchi, H., He, M., Wu, P., Kim, S., Paik, R., Sugino, K., et al. (2011). A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013. doi: 10.1016/j.neuron.2011.07.026
Tasic, B., Menon, V., Nguyen, T. N., Kim, T. K., Jarsky, T., Yao, Z., et al. (2016). Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 19, 335–346. doi: 10.1038/nn.4216
Tauck, D. L., and Nadler, J. V. (1985). Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J. Neurosci. 5, 1016–1022. doi: 10.1523/jneurosci.05-04-01016.1985
Tervo, D. G., Hwang, B. Y., Viswanathan, S., Gaj, T., Lavzin, M., Ritola, K. D., et al. (2016). A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382. doi: 10.1016/j.neuron.2016.09.021
Tian, G. F., Azmi, H., Takano, T., Xu, Q., Peng, W., Lin, J., et al. (2005). An astrocytic basis of epilepsy. Nat. Med. 11, 973–981. doi: 10.1038/nm1277
Tochitsky, I., Banghart, M. R., Mourot, A., Yao, J. Z., Gaub, B., Kramer, R. H., et al. (2012). Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors. Nat. Chem. 4, 105–111. doi: 10.1038/nchem.1234
Toettcher, J. E., Weiner, O. D., and Lim, W. A. (2013). Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434. doi: 10.1016/j.cell.2013.11.004
Tønnesen, J., and Kokaia, M. (2017). Epilepsy and optogenetics: can seizures be controlled by light? Clin. Sci. 131, 1605–1616. doi: 10.1042/cs20160492
Tønnesen, J., Parish, C. L., Sørensen, A. T., Andersson, A., Lundberg, C., Deisseroth, K., et al. (2011). Functional integration of grafted neural stem cell-derived dopaminergic neurons monitored by optogenetics in an in vitro Parkinson model. PLoS One 6:e17560. doi: 10.1371/journal.pone.0017560
Tønnesen, J., Sorensen, A. T., Deisseroth, K., Lundberg, C., and Kokaia, M. (2009). Optogenetic control of epileptiform activity. Proc. Natl. Acad. Sci. U S A 106, 12162–12167. doi: 10.1073/pnas.0901915106
Tung, J. K., Berglund, K., and Gross, R. E. (2016). Optogenetic approaches for controlling seizure activity. Brain Stimul. 9, 801–810. doi: 10.1016/j.brs.2016.06.055
Tung, J. K., Gutekunst, C. A., and Gross, R. E. (2015). Inhibitory luminopsins: genetically-encoded bioluminescent opsins for versatile, scalable, and hardware-independent optogenetic inhibition. Sci. Rep. 5:14366. doi: 10.1038/srep14366
Tung, J. K., Shiu, F. H., Ding, K., and Gross, R. E. (2018). Chemically activated luminopsins allow optogenetic inhibition of distributed nodes in an epileptic network for non-invasive and multi-site suppression of seizure activity. Neurobiol. Dis. 109, 1–10. doi: 10.1016/j.nbd.2017.09.007
Valentín, A., García Navarrete, E., Chelvarajah, R., Torres, C., Navas, M., Vico, L., et al. (2013). Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 54, 1823–1833. doi: 10.1111/epi.12352
Van Buren, J. M., Wood, J. H., Oakley, J., and Hambrecht, F. (1978). Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J. Neurosurg. 48, 407–416. doi: 10.3171/jns.1978.48.3.0407
Vaughan, D. N., Rayner, G., Tailby, C., and Jackson, G. D. (2016). MRI-negative temporal lobe epilepsy: a network disorder of neocortical connectivity. Neurology 87, 1934–1942. doi: 10.1212/wnl.0000000000003289
Velasco, F., Carrillo-Ruiz, J. D., Brito, F., Velasco, M., Velasco, A. L., Marquez, I., et al. (2005). Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia 46, 1071–1081. doi: 10.1111/j.1528-1167.2005.70504.x
Volgraf, M., Gorostiza, P., Numano, R., Kramer, R. H., Isacoff, E. Y., and Trauner, D. (2006). Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2, 47–52. doi: 10.1038/nchembio756
Wall, N. R., Wickersham, I. R., Cetin, A., De La Parra, M., and Callaway, E. M. (2010). Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl. Acad. Sci. U S A 107, 21848–21853. doi: 10.1073/pnas.1011756107
Wang, H., Vilela, M., Winkler, A., Tarnawski, M., Schlichting, I., Yumerefendi, H., et al. (2016). LOVTRAP: an optogenetic system for photoinduced protein dissociation. Nat. Methods 13, 755–758. doi: 10.1038/nmeth.3926
Wang, Y., Xu, C., Xu, Z., Ji, C., Liang, J., Wang, Y., et al. (2017). Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron 95:1221. doi: 10.3410/f.727748591.793543895
Warden, M. R., Cardin, J. A., and Deisseroth, K. (2014). Optical neural interfaces. Annu. Rev. Biomed. Eng. 16, 103–129. doi: 10.1146/annurev-bioeng-071813-104733
Weitz, A. J., Fang, Z., Lee, H. J., Fisher, R. S., Smith, W. C., Choy, M., et al. (2015). Optogenetic fMRI reveals distinct, frequency-dependent networks recruited by dorsal and intermediate hippocampus stimulations. Neuroimage 107, 229–241. doi: 10.1016/j.neuroimage.2014.10.039
Weitz, A. J., and Lee, J. H. (2016). Probing neural transplant networks in vivo with optogenetics and optogenetic fMRI. Stem Cells Int. 2016:8612751. doi: 10.1155/2016/8612751
Wietek, J., Wiegert, J. S., Adeishvili, N., Schneider, F., Watanabe, H., Tsunoda, S. P., et al. (2014). Conversion of channelrhodopsin into a light-gated chloride channel. Science 344, 409–412. doi: 10.1126/science.1249375
Woodson, W., Nitecka, L., and Ben-Ari, Y. (1989). Organization of the GABAergic system in the rat hippocampal formation: a quantitative immunocytochemical study. J. Comp. Neurol. 280, 254–271. doi: 10.1002/cne.902800207
Wright, G. D., McLellan, D. L., and Brice, J. G. (1984). A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J. Neurol. Neurosurg. Psychiatry 47, 769–774. doi: 10.1136/jnnp.47.8.769
Wu, J. W., Hussaini, S. A., Bastille, I. M., Rodriguez, G. A., Mrejeru, A., Rilett, K., et al. (2016). Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 19, 1085–1092. doi: 10.1038/nn.4328
Wykes, R. C., Heeroma, J. H., Mantoan, L., Zheng, K., MacDonald, D. C., Deisseroth, K., et al. (2012). Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci. Transl. Med. 4:161ra152. doi: 10.1126/scitranslmed.3004190
Yamamoto, K., Tanei, Z., Hashimoto, T., Wakabayashi, T., Okuno, H., Naka, Y., et al. (2015). Chronic optogenetic activation augments aβ pathology in a mouse model of Alzheimer disease. Cell Rep. 11, 859–865. doi: 10.1016/j.celrep.2015.04.017
Yazdan-Shahmorad, A., Tian, N., Kharazia, V., Samaranch, L., Kells, A., Bringas, J., et al. (2018). Widespread optogenetic expression in macaque cortex obtained with MR-guided, convection enhanced delivery (CED) of AAV vector to the thalamus. J. Neurosci. Methods 293, 347–358. doi: 10.1016/j.jneumeth.2017.10.009
Yekhlef, L., Breschi, G. L., Lagostena, L., Russo, G., and Taverna, S. (2015). Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J. Neurophysiol. 113, 1616–1630. doi: 10.1152/jn.00841.2014
Yi, F., DeCan, E., Stoll, K., Marceau, E., Deisseroth, K., and Lawrence, J. J. (2015). Muscarinic excitation of parvalbumin-positive interneurons contributes to the severity of pilocarpine-induced seizures. Epilepsia 56, 297–309. doi: 10.1111/epi.12883
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M., and Deisseroth, K. (2011a). Optogenetics in neural systems. Neuron 71, 9–34. doi: 10.1016/j.neuron.2011.06.004
Yizhar, O., Fenno, L. E., Prigge, M., Schneider, F., Davidson, T. J., O’Shea, D. J., et al. (2011b). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178. doi: 10.1038/nature10360
Yu, W., and Krook-Magnuson, E. (2015). Cognitive collaborations: bidirectional functional connectivity between the cerebellum and the hippocampus. Front. Syst. Neurosci. 9:177. doi: 10.3389/fnsys.2015.00177
Zappone, C. A., and Sloviter, R. S. (2001). Commissurally projecting inhibitory interneurons of the rat hippocampal dentate gyrus: a colocalization study of neuronal markers and the retrograde tracer Fluoro-gold. J. Comp. Neurol. 441, 324–344. doi: 10.1002/cne.1415
Zeidler, Z., and Krook-Magnuson, E. (2016). One site to rule them all: toward a master regulator of ictal activity. Epilepsy Curr. 16, 170–171. doi: 10.5698/1535-7511-16.3.170
Zemelman, B. V., Lee, G. A., Ng, M., and Miesenböck, G. (2002). Selective photostimulation of genetically chARGed neurons. Neuron 33, 15–22. doi: 10.1016/s0896-6273(01)00574-8
Zhang, F., Gradinaru, V., Adamantidis, A. R., Durand, R., Airan, R. D., de Lecea, L., et al. (2010). Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439–456. doi: 10.1038/nprot.2009.226
Zhang, W., Huguenard, J. R., and Buckmaster, P. S. (2012). Increased excitatory synaptic input to granule cells from hilar and CA3 regions in a rat model of temporal lobe epilepsy. J. Neurosci. 32, 1183–1196. doi: 10.1523/jneurosci.5342-11.2012
Zhang, W., Lohman, A. W., Zhuravlova, Y., Lu, X., Wiens, M. D., Hoi, H., et al. (2017). Optogenetic control with a photocleavable protein, PhoCl. Nat. Methods 14, 391–394. doi: 10.1038/nmeth.4222
Zhang, F., Prigge, M., Beyrière, F., Tsunoda, S., Mattis, J., Yizhar, O., et al. (2008). Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat. Neurosci. 11, 631–633. doi: 10.1038/nn.2120
Zhang, W., Yamawaki, R., Wen, X., Uhl, J., Diaz, J., Prince, D. A., et al. (2009). Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J. Neurosci. 29, 14247–14256. doi: 10.1523/jneurosci.3842-09.2009
Zhang, H., Zhao, H., Zeng, C., Van Dort, C., Faingold, C. L., Taylor, N. E., et al. (2018). Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiol. Dis. 110, 47–58. doi: 10.1016/j.nbd.2017.11.003
Keywords: seizures, cell type specificity, neuronal circuitry, channelrhodopsin, halorhodopsin, archaerhodopsin, parvalbumin, intersectional genetics
Citation: Christenson Wick Z and Krook-Magnuson E (2018) Specificity, Versatility, and Continual Development: The Power of Optogenetics for Epilepsy Research. Front. Cell. Neurosci. 12:151. doi: 10.3389/fncel.2018.00151
Received: 03 March 2018; Accepted: 15 May 2018;
Published: 14 June 2018.
Edited by:
Jan Tønnesen, Achucarro Basque Center for Neuroscience, SpainReviewed by:
Ming-Chi Tsai, University of California, Berkeley, United StatesMy Sofia Andersson, Lund University, Sweden
Copyright © 2018 Christenson Wick and Krook-Magnuson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zoé Christenson Wick, chri3433@umn.edu
 Zoé Christenson Wick
Zoé Christenson Wick Esther Krook-Magnuson
Esther Krook-Magnuson