Brain-wide neuronal activation and functional connectivity are modulated by prior exposure to repetitive learning episodes
- Department of Cell Biology and Anatomy, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Memory storage and retrieval are shaped by past experiences. Prior learning and memory episodes have numerous impacts on brain structure from micro to macroscale. Previous experience with specific forms of learning increases the efficiency of future learning. It is less clear whether such practice effects on one type of memory might also have transferable effects to other forms of memory. Different forms of learning and memory rely on different brain-wide networks but there are many points of overlap in these networks. Enhanced structural or functional connectivity caused by one type of learning may be transferable to another type of learning due to overlap in underlying memory networks. Here, we investigated the impact of prior chronic spatial training on the task-specific functional connectivity related to subsequent contextual fear memory recall in mice. Our results show that mice exposed to prior spatial training exhibited decreased brain-wide activation compared to control mice during the retrieval of a context fear memory. With respect to functional connectivity, we observed changes in several network measures, notably an increase in global efficiency. Interestingly, we also observed an increase in network resilience based on simulated targeted node deletion. Overall, this study suggests that chronic learning has transferable effects on the functional connectivity networks of other types of learning and memory. The generalized enhancements in network efficiency and resilience suggest that learning itself may protect brain networks against deterioration.
Introduction
It has been well established that prior learning experiences alter the canvas against which new learning occurs. Learning results in numerous structural changes in the brain ranging from cellular and synaptic changes (Lendvai et al., 2000; Nyberg et al., 2003; Holtmaat et al., 2005; De Paola et al., 2006; Epp et al., 2013) to altered macroscale measurements of regional size and shape (Maguire et al., 2003; Draganski et al., 2004; Bermudez et al., 2008; Hyde et al., 2009; Scholz et al., 2009). A classic example of learning-induced structural changes is the change in hippocampal volume that occurs as a result of intense practice with spatial navigation in London taxi drivers (Maguire et al., 2000). Similar increases in hippocampal volume have also been observed in mice that were trained on a spatial learning task (Lerch et al., 2011).
To the extent that there are relationships between brain function and underlying structure, it should be predicted that learning-induced structural changes should also induce functional changes. Training-induced increases in hippocampal volume for example are also associated with enhanced memory performance (Bohbot et al., 2007).
In addition to structural changes, learning has been shown in some studies to change the organization of memories in the brain. In rats, previous studies have indicated that prior training with a memory task can prevent lesion-induced deficits in both similar and slightly distinct memory tasks (Clark and Delay, 1991; Ocampo et al., 2018). This suggested that prior learning experiences fundamentally change how and where future memories are encoded (Owen et al., 2010; Nouchi et al., 2012; West et al., 2017). Experiments such as these suggest some form of reorganization but do not give a complete picture as to how this reorganization has occurred.
Functional imaging experiments in humans have provided evidence that cognitive stimulation, or memory training, alters brain functional connectivity (Martínez et al., 2013; Dresler et al., 2017; Bagarinao et al., 2019; Miró-Padilla et al., 2019; Finc et al., 2020). These findings are of significant importance because reorganization of functional networks could increase the efficiency of learning and memory and could even increase the resilience of cognitive processes to damage or deterioration. However, investigating the influence of prior learning on altered functional connectivity in humans is complicated by the diverse cognitive, genetic and lifestyle differences in different individuals.
In the present study, to further elucidate the impact of prior learning on memory related functional connectivity, we have developed a mouse model in which mice are trained in a repeated acquisition spatial learning and memory task for several months. Using mice, we are able to control for environmental and genetic factors, and we can also control for prior learning experiences. Our aim was to investigate whether learning a spatial memory task would increase the efficiency of the functional networks underlying a different form of memory (contextual fear memory). Although spatial and contextual memories are independent of each other, the circuits involved in both of these forms of memory likely overlap in numerous places including, most notably, the hippocampus and connected structures. The repeated activation of regions that are mutually involved in circuits across multiple forms of learning and memory is likely a key factor in determining the breadth of tasks that would be influenced by prior learning. To examine the extent to which spatial memory training influences contextual memory circuits, we adopted a brain-wide functional connectivity approach using immediate early gene imaging that has been recently described (Wheeler et al., 2013; Vetere et al., 2017; Scott et al., 2020).
In this study, we show that chronic cognitive stimulation, in the form of spatial learning is sufficient to induce generalized changes in the organization of functional connectivity networks underlying a test of contextual fear memory.
Materials and methods
Mice
8-week-old male C57BL/6J mice purchased from The Jackson Laboratory (Bar Harbor, ME, United States) were used for all experiments. Upon arrival, mice were group housed, 3-4 mice per cage, under a 12-h light/12-h dark cycle with ad libitum access to food and water. Testing and handling was performed during the light phase of the cycle. Behavioral tests and network analyses were conducted using groups of n = 10. Mice from the Morris Water Maze training group and the cage control group were co-housed, and all mice received equal handling throughout the study. All procedures were conducted in accordance with protocols approved by the University of Calgary, Health Sciences Animal Care Committee, following the guidelines of the Canadian Council for Animal Care.
Morris water maze training
In order to provide mice with chronic cognitive stimulation, we trained half of the mice on a repeated acquisition and performance testing variant of the Morris Water Maze (MWM; see Figure 1A for an outline of the testing schedule and Figure 1B for the maze itself) (Spanswick et al., 2007). This task was chosen because it provides considerable flexibility in design, whereby a hidden platform can be moved to many different locations within the maze to encourage continuous learning. Furthermore, the spatial learning which occurs during MWM training is supported by many of the same neuroanatomical regions as contextual conditioning (Jo et al., 2007; Miller et al., 2014; Giustino and Maren, 2015; Kwapis et al., 2015; Milczarek et al., 2018). In this version of the task, the hidden escape platform is moved every second day which requires mice to repeatedly acquire new spatial memories throughout a 10-week training period. We used 10 different platform locations, with each platform location occurring on 2 separate occasions. Mice were trained 4 days per week (2 platform locations). During each daily session, mice were given four trials. Each trial lasted a maximum of 60 s and was initiated by placing the mouse gently into the pool, facing the wall. The start location was from a different cardinal compass position around the pool for each trial and the order of start locations was randomized each day. Trials were terminated once the mouse located the hidden platform. If the platform was not found after 60 s, mice were gently guided to the platform by the experimenter. Once on the platform, mice were given 15 s to remain on the platform before being returned to their cage. Trials were interleaved, whereby each mouse performed their first trial before the first mouse performed its second of four daily trials. This resulted in an intertrial interval of approximately 10 min. The circular pool had a diameter of 150 cm and a depth of 50 cm. The pool was filled so that the water level was 2 cm above the surface of a circular escape platform that had a diameter of 11 cm. The water was made opaque using white non-toxic tempera paint. The water was kept at a constant temperature of 22°C and stirred and cleaned of debris before each trial. Automated tracking software (ANY-Maze, Stoelting, Wood Dale, IL, United States) was used to record and analyze swim behaviors in the pool, primarily the distance traveled prior to locating the hidden platform. When analyzing these results, linear regression was applied to the mean distance traveled by each mouse across each training session to assess the influence of the memory of previous platform locations on the ability to learn new platform locations. The extent to which mice learned platform position within blocks of consistent locations was assessed by examining the mean slope of the regression lines between distance traveled at all first-day trials and between all second day trials.
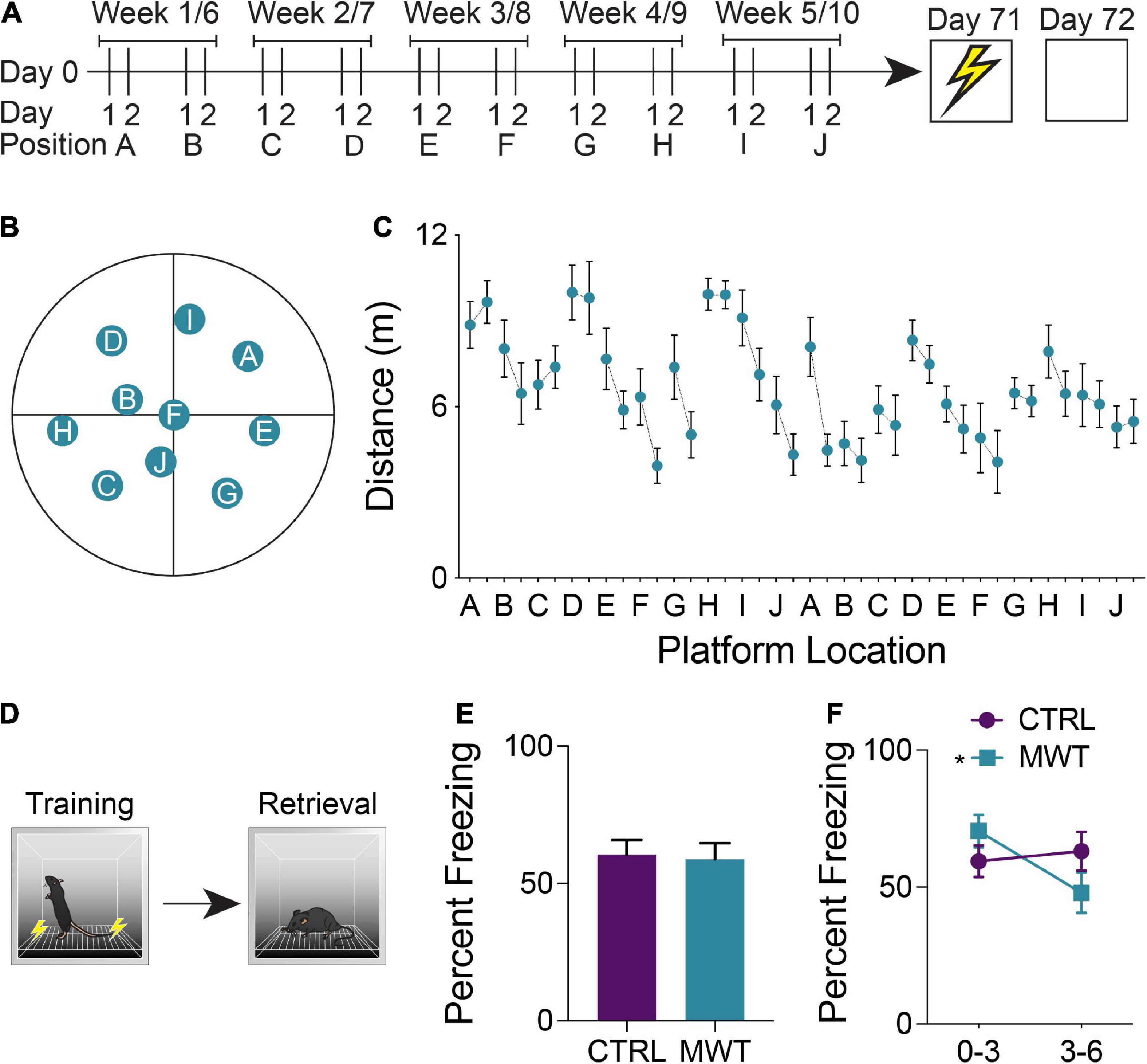
Figure 1. (A) Mice were trained on a repeated acquisition and performance testing variation of the MWM (n = 10) or kept in conventional housing conditions (n = 10) for 10 weeks. Afterward all mice underwent contextual fear conditioning and a retention test 24 h later. (B) During water maze training, the escape platform was moved every second day between 10 locations. After the 10th position, the platform was returned to the 1st position and the cycle was restarted. (C) Mean distance traveled in the water maze across each of the 20 locations. (D) During contextual fear memory retrieval, (E) there was no overall difference in freezing rates between the control group and the group which had underwent Morris Water Maze training (Paired two-tailed t test; P > 0.05). (F) Mice who had underwent Morris Water Maze training froze significantly more during the first half (minutes 0-3) of the test than they did during the second half of the test (minutes 3-6; Two-Way Repeated Measures ANOVA; Time × Treatment interaction: F1,18 = 7.763, p = 0.0122; MWM 0-3 – 3–6: p < 0.0066), while control mice showed no difference in task performance across these two halves of the test. Data shown are mean ± SEM when applicable.
Contextual fear conditioning
After the conclusion of the Morris Water Maze training protocol, all mice were trained in contextual fear conditioning. Training was conducted in sound-attenuated chambers with grated floors through which shocks (0.5 mA; 2 s) were delivered (Ugo Basile, Gemonio, Italy). Mice were first allowed to acclimate to the chamber for 2 min prior to the presentation of a series of 3 shocks, each separated by an interval of 90 s. 24 h after the training session, mice were returned to the conditioning chambers for a 6-min retention test. During this test, no shocks were administered, and behavior was monitored via an overhead infrared camera in conjunction with an automated tracking software (ANY-Maze, Stoelting, Wood Dale, IL, United States). The chamber was cleaned using 70% ethanol and allowed to dry before and after each trial. When analyzing these results, freezing criteria was defined as bouts of a minimum of two seconds without ambulation. The percentage of the trial spent exhibiting freezing behavior was compared between groups.
Perfusions and histology
Mice were transcardially perfused with 0.1 M phosphate buffered saline (PBS) followed by 4% formaldehyde 90 min after retention testing. Brains were then extracted and post-fixed in 4% formaldehyde for 24 h. Fixed brains were cryoprotected in 30% W/V sucrose solution at 4°C until no longer buoyant. From cryoprotected brains, serial coronal sections with a thickness of 40 μm were cut on a cryostat (Leica Biosystems, Concord, ON, Canada) and stored in 12 series at −20°C in antifreeze solution.
Immunohistochemistry
When conducting labeling for c-Fos expression, all tissue was processed concurrently. Tissue sections were washed 3 times (10 min per wash) in 0.1M PBS before being incubated in a primary antibody solution of 1:2000 rabbit anti-c-Fos primary antibody (226 003, Synaptic Systems, Göttingen, Germany), 3% normal donkey serum, and 0.03% Triton-X100 for 48 h at room temperature on a tissue shaker. Tissue sections were washed 3 × 10 min in 0.1M PBS before secondary antibody incubation. The secondary antibody solution was composed of 1:500 donkey anti-rabbit Alexa Fluor 488 (111-545-003, Cedar Lane Labs, Burlington, ON, Canada) in PBS for 24 h at room temperature. Sections were then transferred to 1:2000 DAPI solution for 15 min before being washed 3 × 10 minutes in 0.1M PBS. Labeled sections were mounted to glass slides and coverslipped with PVA-DABCO mounting medium.
Brain-wide c-Fos quantification
Quantification of fluorescent c-Fos labeled cells was conducted using a custom semi-automated segmentation and registration pipeline (Figure 2A). All slides were imaged as a single batch using an Olympus VS120-L100-W slide scanning microscope (Richmond Hill, ON, Canada). Images were collected using a 10x objective with a numerical aperture of 0.40 and a Hamamatsu ORCA-Flash4.0 camera. Labeled c-Fos was imaged using a FITC filter cube and a 9.00 V lamp at an intensity of 100% and an exposure time of 140 ms. DAPI staining was imaged under the same conditions, but with a DAPI filter cube and an exposure time of 65 ms. Cells expressing a c-Fos label were segmented using the machine learning-based pixel and object classification program, Ilastik (Berg et al., 2019). To further prepare Ilastik output images and DAPI channel photomicrographs for regional registration, a custom plug-in was written for ImageJ. The pixel intensity threshold of the Ilastik outputs was adjusted so as to only contain objects which the program determined to be within the correct range of pixel intensities and shapes. To compensate for inadequate regional area measurements at an image-by-image level in the atlas registration software, a mask of evenly spaced binary points was generated from the DAPI channel image. The pixel intensity thresholds of these images were adjusted to create a binary mask in the shape of the tissue section. Grid lines were then overlaid to create a mask of binary points arranged in a square grid in the shape of the tissue section. Adjacent binary points were spaced by 22 μm, therefore, each point in the mask accounted for an area of 484 μm2.
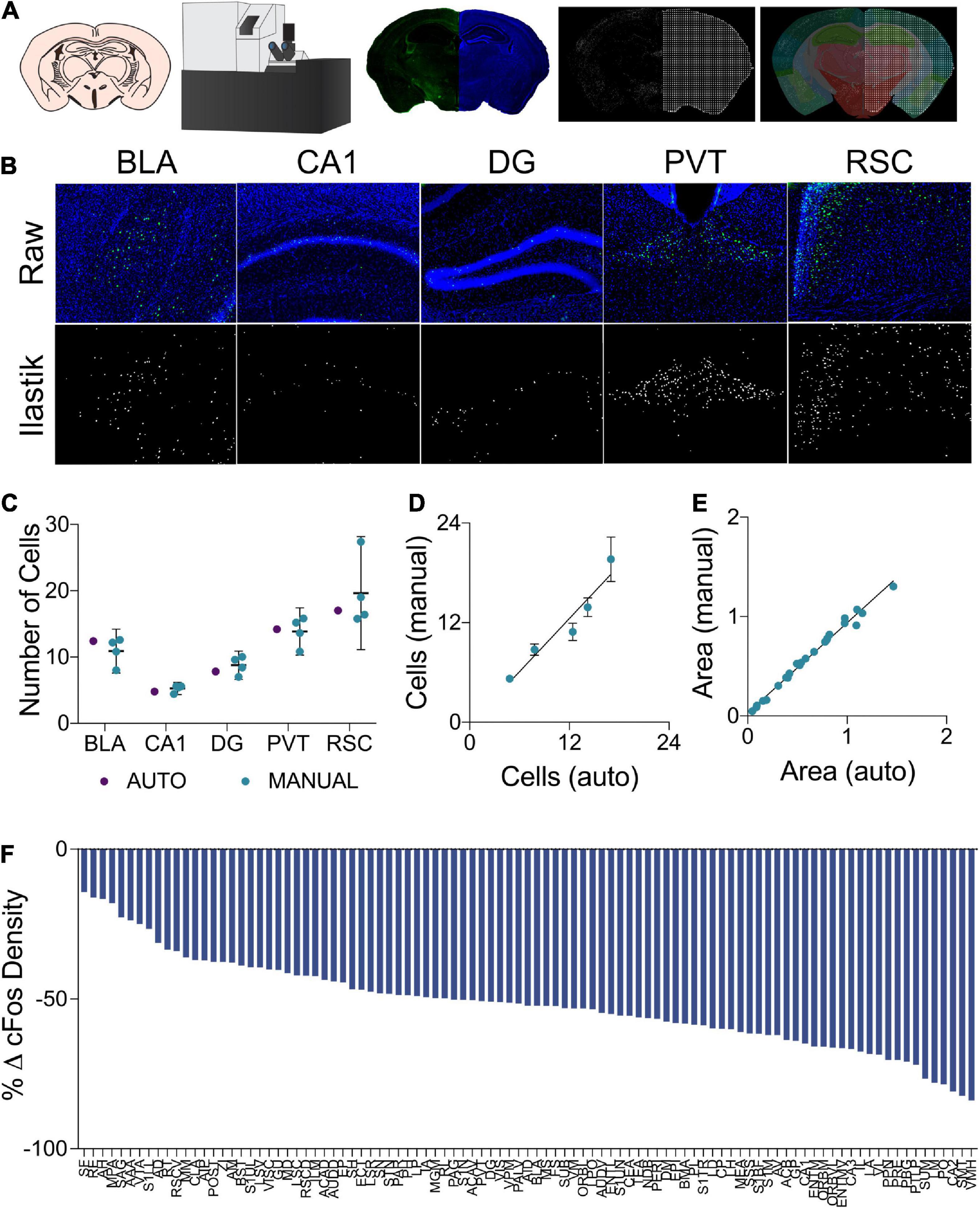
Figure 2. (A) In the cell segmentation and tissue registration pipeline, tissue was sectioned, immuno-labeled, and mounted on slides prior to being imaged on a fluorescent slide scanning microscope. Images of labeled c-Fos and DAPI staining were then processed using Ilastik and ImageJ to generate binary c-Fos labels and a mask of evenly spaced grid points in the shape of the tissue sections. These binary images were then applied to plates from the Allen Mouse Brain Atlas which had been morphed to align with the tissue sections using Whole Brain, yielding regional c-Fos densities. (B) Examples of raw c-Fos+ cells in the BLA, CA1, DG, PVT, and RSC (top, L-R) and cells segmented using Ilastik (bottom). (C) A set of ROIs was quantified for validation. Automated Ilastik segmentation yielded cell counts within the 95% confidence intervals of counts acquired from trained independent experimenters in each of the aforementioned regions (Two-Way ANOVA; segmentation method factor: F1,15 = 0.09515, p = 0.7620). Data presented as mean 95% ± confidence interval. (D) Cell counts collected using automated Ilastik processing were found to correlate highly with mean counts gathered through manual counting (Pearson r = 0.9610, p < 0.01). Data presented as mean 95% ± confidence interval. (E) Area approximations generated using the pipeline were correlated with areas acquired by tracing regions manually in ImageJ (Pearson r = 0.9941, p = < 0.0001). (F) c-Fos quantification across the 97 brain regions of interest. Relative to the control group, c-Fos expression in the mice that had previously received Morris Water Maze training was decreased to a variable extent in all brain regions.
Next, tissue sections were registered to plates of the Allen Mouse Brain Atlas using the R-based Whole Brain software (Fürth et al., 2018). Using this software, DAPI channel images were used as references to which the atlas plates were aligned. The number of segmented c-Fos labeled cells per neuroanatomical region was quantified in Whole Brain. Similarly, the binary point masks were processed to count the number of points in each region. Regional areas were then approximated using a Cavalieri-based point counting approach, whereby the number of mask points in each region was multiplied by the area accounted for by each point. This allowed for the c-Fos labeled cells to be normalized by area and presented as regional cell densities.
Validation of c-Fos quantification and regional area approximation
A separate cohort of mice was used for the validation of c-Fos labeled cell segmentation and regional area approximation. c-Fos immunostaining and imaging was identical to the methods described previously. To generate ground truth cell counts as a gold standard for our automated counting procedure, ten 500 μm x 500 μm regions of interest (ROI) were randomly generated from each of several regions including the basolateral amygdala, CA1, dentate gyrus, paraventricular nucleus, and the retrosplenial cortex. These ROIs were processed through our Ilastik label segmentation pipeline (representative raw and processed images in Figure 2B). In addition, the same ROIs were hand counted independently by 4 experimenters blind to the automated cell count results. The total numbers of cells counted across all counting boxes were then compared to assess whether or not Ilastik could segment fluorescent c-Fos labels within natural and acceptable inter-rater variability.
To assess the accuracy of regional area approximations, areas generated using the Cavalieri-based point counting approach were compared to the areas of these same regions which were manually traced in ImageJ. During this analysis, five photomicrographs of each of the following regions were examined: basolateral amygdala, CA1, dentate gyrus, paraventricular nucleus, and the retrosplenial cortex.
Functional connectivity network generation
We focused on a selection of 97 regions based on our ability to discriminate these regions using a DAPI stained image as reference (see Supplementary Table 1 for list of regions and abbreviations). From this list of regions, c-Fos label densities were cross correlated within each group to generate pairwise correlation matrices. Correlations were filtered by statistical significance and a false discovery rate of 5% (Benjamini and Hochberg, 1995; Bassett et al., 2009). For network analyses, correlation matrices were binarized to adjacency matrices based on Pearson’s correlation coefficient and statistical significance (r > 0.9; α = 0.005). This threshold allowed for sufficient network density to study global brain dynamics, while still limiting the analyses to only the strongest and most biologically plausible connections (Schneidman et al., 2006). To ensure that the thresholding parameters did not bias network analyses, additional adjacency matrices were generated using either more (r > 0.95; α = 0.0005) or less (r > 0.8; α = 0.05) conservative thresholds. To analyze adjacency matrices as network graphs, the 97 neuroanatomical regions were plotted as nodes. Connections were drawn between nodes whereby correlations surpassed correlation matrix thresholding parameters.
Functional connectivity network analysis
Graph theoretical analyses were applied to network graphs to examine global and local properties of the network. These analyses were guided by the use of the Brain Connectivity Toolkit (Rubinov and Sporns, 2010), the SBEToolbox (Konganti et al., 2013), and other custom analyses. Network properties examined include node degree, network density, global efficiency, betweenness centrality, Katz centrality, and network resiliency. In the following definitions, N is the array of nodes in the network represented by adjacency matrix A. The number of nodes in the network is represented by n and the number of connections between nodes is l. The variable aij is the index into the adjacency matrix which indicates the connection status of nodes i and j. The presence of a connection is represented by aij≠ 0 (Rubinov and Sporns, 2010). Node degree is the number of connections that link a node to the rest of the network (Rubinov and Sporns, 2010).
Network density is a metric of network dispersion. It is expressed as a proportion of the number of connections in a given network over the number of connections which would be required to saturate a network of the same size (Rubinov and Sporns, 2010).
Global efficiency is defined as the inverse of the average shortest path of connections between all possible pairs of nodes (Latora and Marchiori, 2001; Achard and Bullmore, 2007; Rubinov and Sporns, 2010).
Betweenness centrality and Katz centrality are measures which can be used to assess the importance of a node in the effective communication of a network. Betweenness centrality quantifies the number of shortest paths between nodes that pass-through a given node (Freeman, 1978; Brandes, 2001; Rubinov and Sporns, 2010).
Katz centrality applies an eigenvector approach to this metric by weighting the connections involving more highly connected nodes more heavily than those from lesser connected nodes when considering the makeup of the shortest paths which pass through a given node (Katz, 1953; Hubbell, 1965). The attenuation factor, α, used for this analysis was 0.1 (Zhan et al., 2017).
Network resiliency was assessed through targeted node deletion and an assessment of the size of the largest community of connected nodes and global network efficiency with each deletion. Nodes were targeted for deletion in decreasing order, from nodes with the highest degree to those with the lowest. Degree was recalculated after each deletion and the list was reordered accordingly.
Network metrics were both compared across conditions as well as used to assess small world-like network properties compared to random control network topology. Small world network distribution can be described as being efficient at both local and global scales (Watts and Strogatz, 1998). Random null control networks were generated for both the cognitive training and control groups and were matched for network size, overall degree, and degree distribution. Local efficiency was assessed by comparing mean clustering coefficients, while global efficiency was assessed by comparing bootstrapped global efficiency values with one hundred replacements. Networks were considered to display small world-like properties if they had displayed both the high global efficiency characteristic of a random network and increased local efficiency relative to random networks (Wheeler et al., 2013).
Statistical analyses
Behavioral data from all tasks was recorded and analyzed using ANY-Maze (Stoelting, Wood Dale, IL, United States). All t-tests and Two-Way ANOVA for comparing behavioral data, regional c-Fos expression, segmentation and regional area approximation validation, and functional connectivity networks were conducted using GraphPad Prism (GraphPad Software, San Diego, CA, United States). GraphPad Prism was also used to conduct the linear regression used to assess inter-position memory and repeated new learning in the Morris Water Task. The analysis of functional connectivity networks was conducted using MATLAB. Figures were generated using MATLAB, Cytoscape, and GraphPad Prism.
Results
Morris water maze training alters memory performance in unrelated tasks
A repeated acquisition and performance testing variant of the Morris Water Maze was used to provide chronic cognitive stimulation (Figures 1A–C). Simple linear regression was applied to assess inter-position memory and the presence of repeated new learning. The slope of the best fit line of the linear regression applied to the mean distance traveled by each mouse across each training session was −0.067. When examining the mean distances traveled across all first days at a given platform position, the line of best fit had a slope of −0.068. Across all second days at a given platform position, this slope was determined to be −0.069. Within each platform location, the line of best fit of the linear regression yielded a mean slope of −0.97 which differed significantly from zero and was indicative of improved performance over time within the same platform location (one sample t test; p = 0.0010).
To assess the generalization of improved cognitive performance following long-term spatial learning, mice were trained and tested in a contextual fear conditioning paradigm (Figure 1D). The percentage of time that mice exhibited freezing behavior was compared between groups and across the training and retention test sessions. Across both sessions, there were no significant differences in freezing behavior (Figure 1E). However, when the retention was divided into a first half and a second half, mice who had received cognitive training displayed increased freezing behavior during the first half of the test and then decreased freezing during the second half of the test (Figure 1F).
Validation of c-Fos segmentation and neuroanatomical atlas registration
To assess the reliability of the semi-automated c-Fos segmentation and mouse brain atlas registration pipeline used in this study (Figure 2A), we compared c-Fos counts obtained using this pipeline to those gathered manually (see representative images Figure 2B). We found that the number of c-Fos labeled cells quantified using Ilastik processing fell within the range of values counted manually by four different experimenters across a subset of regions with varying levels of background autofluorescence (Figure 2C). The inter-rater reliability was determined to be 15% across the datasets as a whole and the semi-automated cell counts were within 3.75% of the average of the manual cell counts. Manual and automated cell counts were highly correlated across the sampled brain regions (Figure 2D). WholeBrain region registration also produced regional area approximations that correlated highly with hand-traced regional area values (Figure 2E).
Prior spatial learning alters c-Fos expression associated with context memory recall
Changes in regional c-Fos expression density are depicted in Figure 2F as the percent change from the regional c-Fos expression density from the control condition to the group which had underwent long-term spatial learning. We observed decreased c-Fos expression density in all brain regions that were analyzed in mice that had received prior spatial training. With respect to brain-wide activity, prior spatial training resulted in an overall significant decrease in c-Fos expression (Supplementary Figure S1. Unpaired t test; p = 0.0003).
Morris water maze training alters functional connectivity network topology
Analyses of cross-correlated regional c-Fos expression density revealed differences in global functional connectivity network topology. On a global scale, we observed a reorganization of connections throughout the brain (Figures 3A–F). Relative to control conditions, mice that underwent prior cognitive training exhibited an increase in the overall density of functional connections during subsequent contextual fear memory retrieval (Figure 3G). Furthermore, the organization of these networks after prior cognitive training resulted in increased global efficiency relative to the control condition (Figure 3H). These increases were also present in the networks constructed with both more or less conservative thresholds, indicating that this effect was not an artifact of the thresholding level (Supplementary Figure S2).
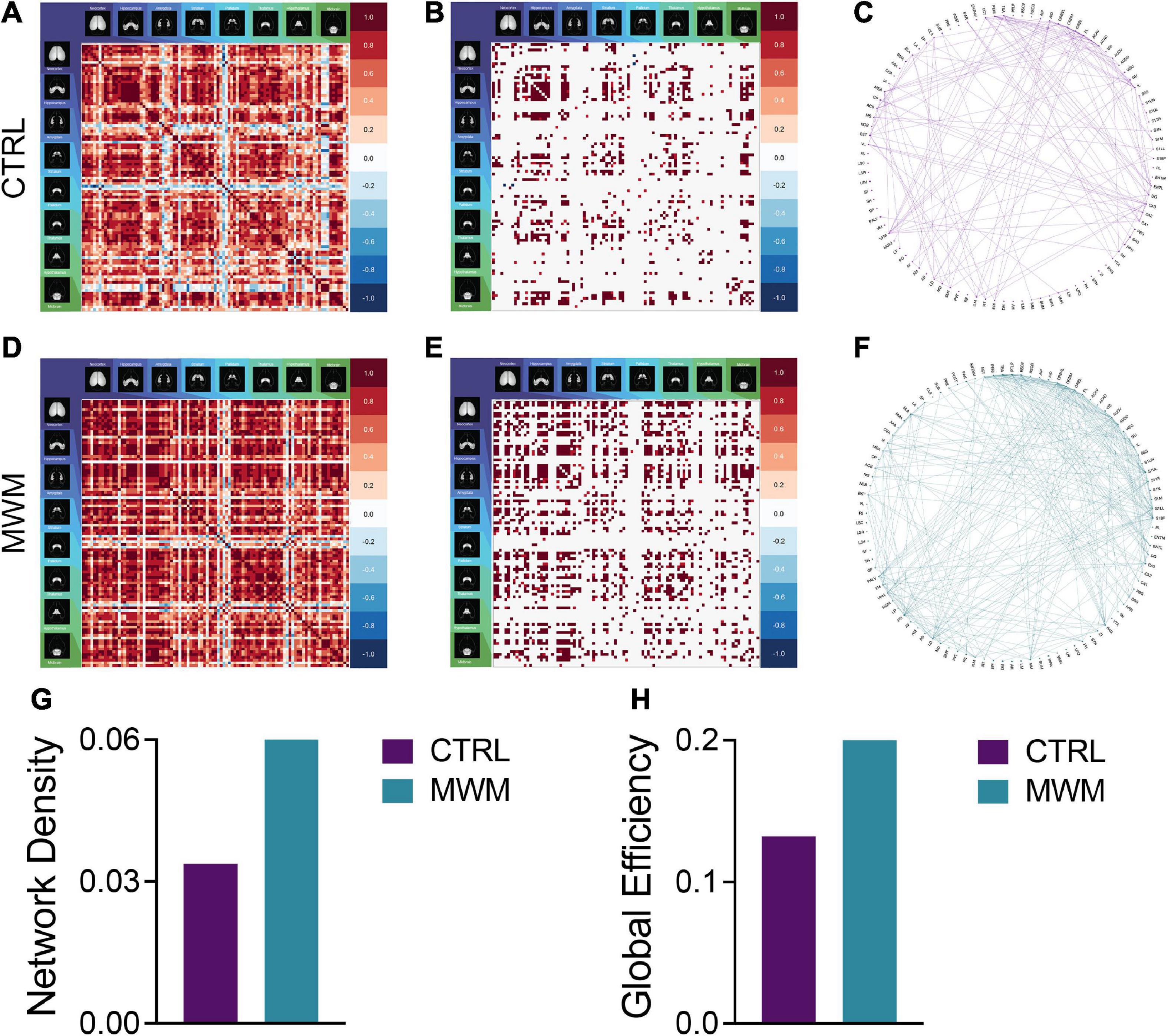
Figure 3. Pairwise correlation matrices (A,D), binarized adjacency matrices (B,E) and circle plots (C,F) showing significant correlations between regions for control (A-C) and Morris Water Maze trained (D-F) groups. See Supplementary Table 1 for full list of regions. MWM training increased (G) network density and (H) global network efficiency.
Control and morris water maze training networks exhibit small-world qualities
Networks from both control and spatial learning groups exhibited heavy-tailed degree distribution characteristic of a small-world network, with the majority of nodes making very few connections and a lesser number of nodes carrying disproportionate importance to the overall connectivity of the network (Figure 4A) (Bassett et al., 2006; Bullmore and Sporns, 2009). Comparisons to random null networks also highlighted that both the control and spatial learning networks maintained the high global efficiency characteristic of random networks (Figure 4B) while displaying increased clustering (Figure 4C). Together, these analyses indicate that the functional connectivity networks engaged during memory recall in both the control and spatial learning conditions exhibit properties that are consistent with small-world topology.
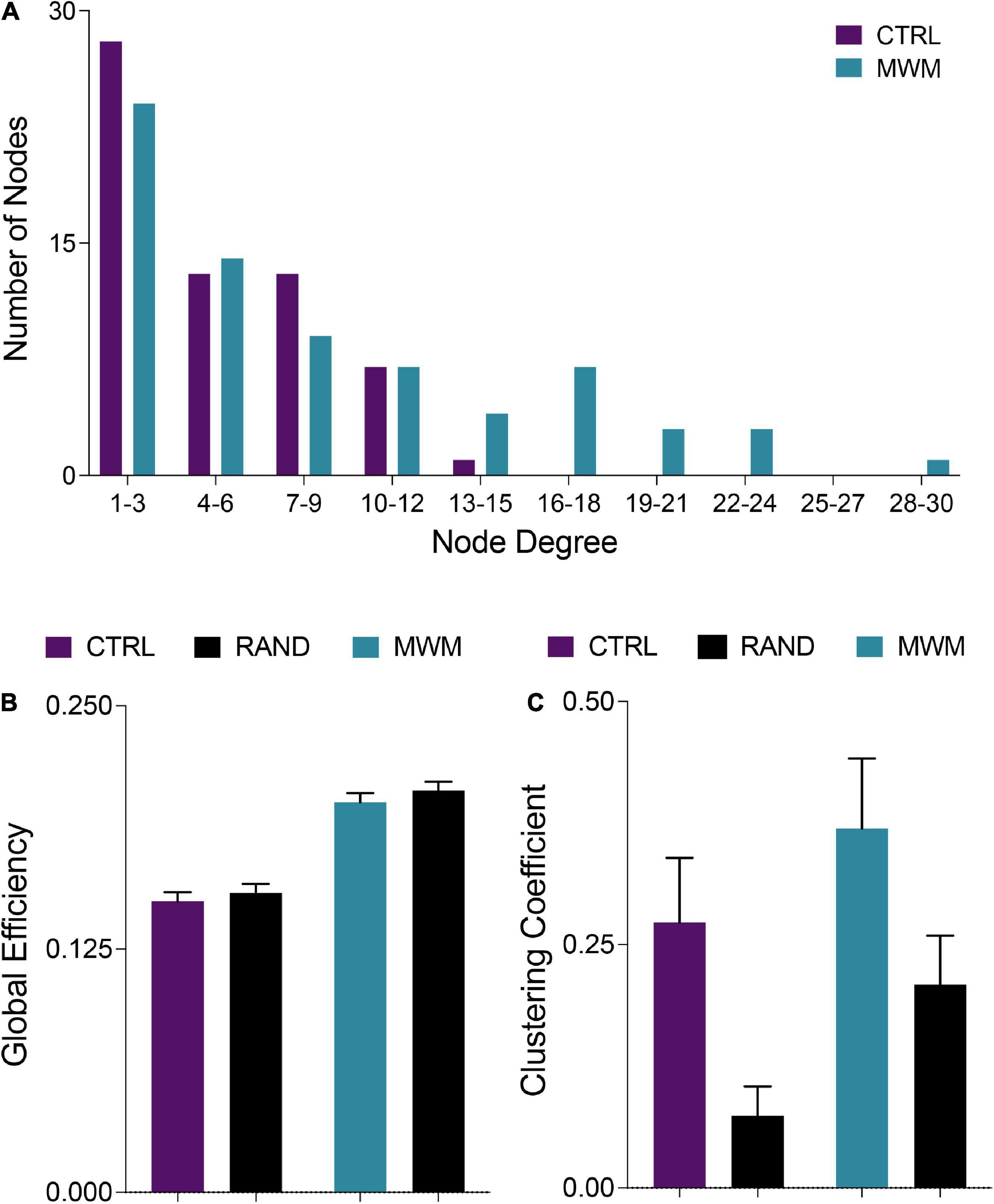
Figure 4. (A) Consistent with definitions of small world organization, in both control and MWM trained networks the majority of nodes were of a low degree. However, water maze training shifted the degree distribution and increased the number of highly connected nodes. (B) Also consistent with small world organization, both control and MWM trained networks showed equivalent global efficiency to random networks matched for degree distribution. (C) A third requirement for the classification of a small world organization is a higher clustering coefficient than a random network. Compared to random networks matched for degree distribution, both control and MWM trained networks showed heightened clustering. Data shown are mean ± 95% confidence intervals.
Morris water maze training alters cluster organization and connectivity
Changes in network topology were also observed at the local level. The organization of local communities within global networks changes with long-term spatial learning. While the size of the giant component (GC) (Figures 5A–C) underwent very little change with MWM training, differences arose in the connectivity patterns within this component. Within the GC, MWM training increases the mean number of connections per node (Figure 5D). The changes in connectivity coincided with changes in network resiliency. When faced targeted deletion of nodes, with deletions occurring in the order of decreasing degree, long-term spatial learning increased the ability of the network to preserve its giant component size (Figure 5E) and global efficiency (Figure 5F).
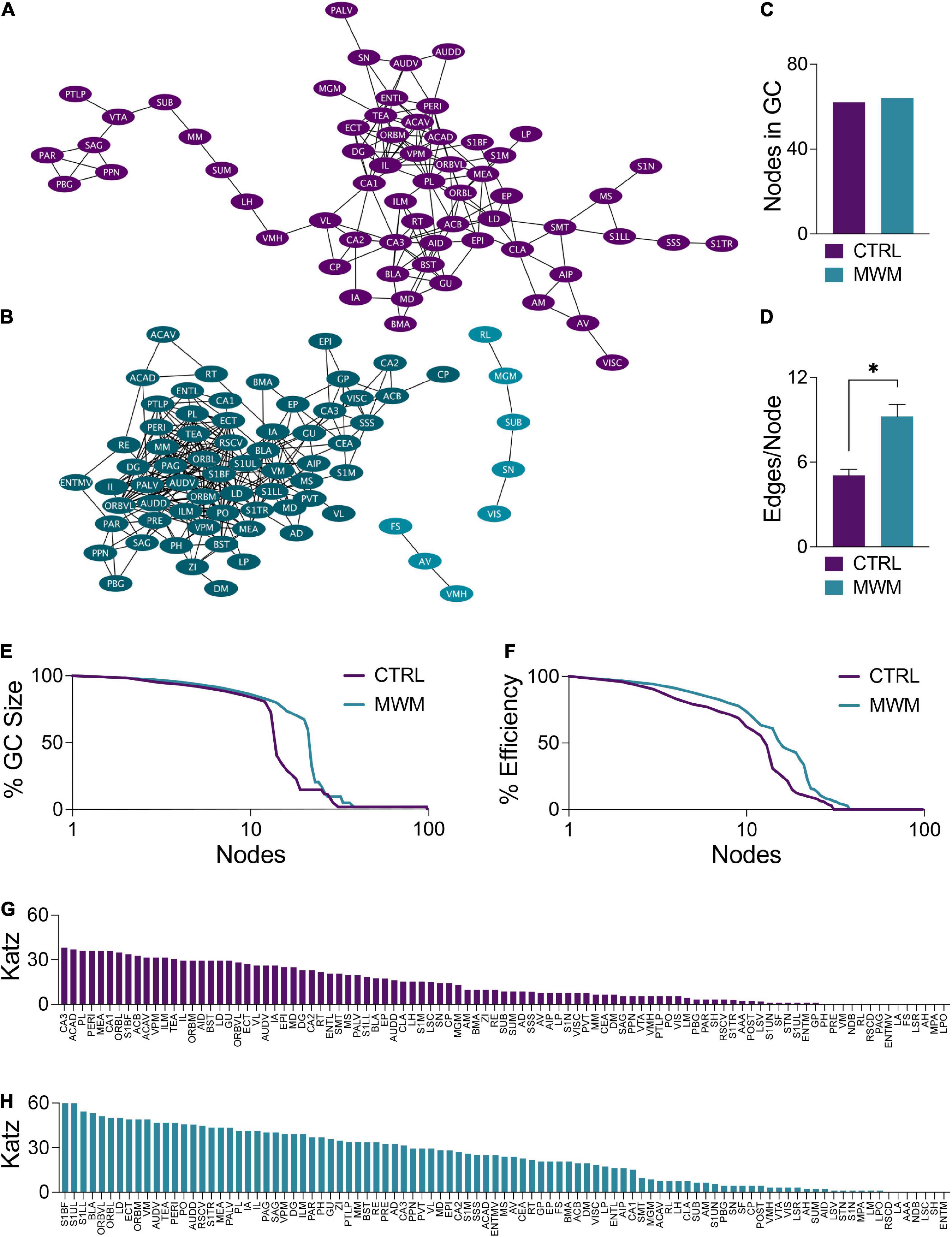
Figure 5. Network plots of (A) control and (B) MWM trained networks with the giant component (GC) highlighted in each in the darker shade. (C) In these networks, there is very little difference in the size of the GC. (D) Within the GC, there was an increase in the mean number of edges per node with MWM training (Two-tailed t test, p < 0.0001). MWM training also made the (E) integrity of the GC and the (F) global efficiency of the network more resilient to targeted node deletion. Relative to the control condition (G), MWM training (H) also increased Katz centrality of a subset of regions within the network. Data shown are mean ± SEM when applicable.
Coinciding with these changes in network resiliency were changes in Katz centrality. Katz centrality is a measure of centrality which differentially weighs connections based on the degrees of the nodes involved (Katz, 1953). This measure has previously been shown to correlate highly with neuronal activity compared to other measures of centrality (Fletcher and Wennekers, 2018). While most nodes in the control network had similar Katz centrality vectors (Figure 5G), MWM training increased the centrality of a subset of regions (Figure 5H). There was considerable overlap between these regions with increased Katz centrality and the regions in the most densely connected region of the GC. This was further corroborated by analysis of regional degree distribution (Supplementary Figure S3) and change in Katz centrality (Supplementary Figure S4) which further highlighted an increase in connectivity and of importance of numerous amygdala subregions following spatial learning.
Discussion
In the current study we employed a brain-wide activity mapping approach to examine the impact of a prolonged period of repeated spatial learning on brain-wide patterns of activation and functional connectivity. We posited that repeated activation of the circuits underlying spatial learning and memory might alter the networks that represent other forms of hippocampus dependent memory in the future. During the spatial learning manipulation, we saw that in changing the hidden platform location in the Morris Water Maze every second day, mice were encouraged to continuously learn. This continuous learning was evident by improved performance within platform positions between the first and second days, supported by a significantly negative slope in the regression analysis performed on the mean distances travelled between these days. Furthermore, there was minimal change in performance across all trials with new platform locations, as was evident by the minimal slope of the regression line when analyzing mean swimming distance across all trials, all first days only, and all second days only. These findings suggest that mice were continuously learning new platform positions and that this new learning was only minimally altered by prior learning of conflicting platform locations. Our results also clearly indicate that this prior spatial learning manipulation caused significant changes to the task-related functional connectivity associated with retrieval of a contextual memory. In the current study, prior exposure to repeated spatial learning episodes had minimal effects on the overall retrieval of a subsequently acquired contextual fear memory. Interestingly, when subdividing the retrieval period, we noticed that the nature of the memory was different in mice that had prior spatial training. Specifically, the retention was stronger in the first half of the test and decreased in the second half, whereas control mice showed stable retention throughout the test. This could be indicative of an increase in behavioral flexibility and/or increased rate of extinction in the absence of additional foot shocks. That we did not observe major differences in memory retrieval was not surprising given that the subjects were normal mice without memory deficits, the memory in control mice was already very strong and the retention interval was short (24 h). This similarity in behavioral performance between conditions allowed us to assess patterns of neuronal activation without the confound of differential memory ability. When we examined neuronal activation and network organization underlying the memory in these two groups, we observed a number of differences that could enhance retention/retrieval in the face of cognitive decline.
We were most interested in investigating whether such a manipulation, which might be viewed as memory practice or training, would enhance measures of efficiency when examining the storage and retrieval of future memories. The efficiency of brain activity underlying cognitive function is vulnerable to aging and disease. Decreased efficiency of cognitive processing has been reported in several neurological conditions, including major depressive disorder (Zhang et al., 2020), schizophrenia (Sheffield et al., 2016), and Alzheimer’s disease (Srivishagan et al., 2020). Even in healthy adults, patterns of brain activation become less efficient with age (Ajilore et al., 2014; Chong et al., 2019). These decreases in efficiency also coincide with decreased cognitive performance, thereby indicating that an intervention which can improve the efficiency of the functional connectome may preserve cognitive function in these conditions (van den Heuvel et al., 2009). We show here that the efficiency of brain-wide activation, as measured by c-Fos expression, is greatly increased in the mice that had prior spatial training. Efficiency can be defined as equal or greater memory performance with the expenditure of fewer resources (i.e., a decrease in activation) (McQuail et al., 2020). Our results show that contextual memory retrieval following spatial learning was associated with a decrease in the total c-Fos expressing cells throughout the brain compared to mice that had not previously experienced any spatial training. Previously, it has been reported that c-Fos expression density is increased in several neuroanatomical regions following water maze training (Guzowski et al., 2001; Teather et al., 2005). When interpreting the results of the current study, it is imperative that we highlight the differences in experimental designs which could underlie this difference. In these studies, c-Fos expression was tagged directly to water maze performance, while in the present study this expression was tagged to the recall of a contextually conditioned memory. Furthermore, the duration of the spatial learning period in these previous studies was much shorter than that used in the current design. Therefore, our results showing that all brain regions exhibited reduced activity are not contradictory with existing literature. Taken together, this pattern of activity perhaps indicates that the behavioral expression of the memory retrieval is more efficient on the level of neuronal activation.
At a network level, global efficiency can be estimated as the inverse of the average shortest path lengths between all network nodes. This measure represents the relative ease or difficulty of integrating information between nodes in a network. Using this measure, we found that mice which had received prior spatial training exhibited enhanced global efficiency and higher clustering compared to controls during subsequent contextual memory retrieval. Both of these findings are consistent with the effects of memory training observed using human functional neuroimaging (Langer et al., 2013). This analysis corroborates the interpretations of increased efficiency based on overall brain activation. Further corroborating these interpretations are changes in the Katz centrality within these networks. Centrality measures can be used as proxies of the relative importance of a node in the maintenance of effective communication across a network and as indicators of the functional segregation of the network as a whole. Spatial training increased the centrality of a subset of nodes. This pattern of distribution suggests a higher degree of network segregation and that this subset of regions is relatively more important to the behavioral expression of the context memory. Comparatively, based on the variability of regional Katz centrality, all regions in the network generated from untrained mice are of similar importance in the expression of this same behavior. These findings coincide with increased centrality in resting state memory networks following working memory training in human neuroimaging studies (Takeuchi et al., 2017). Together these metrics illustrate that the redistribution of neuronal activation induced by prior spatial learning is not only more efficient from the perspective of energetic resources, but also proves to be more efficient with respect to global flow of information throughout the brain.
Functional connectivity networks in the brain are considered to be complex networks. Many complex networks exhibit small world organization. Small-world networks balance global efficiency with local clustering by having a small proportion of nodes to contribute disproportionately to the overall connectivity of the network (Watts and Strogatz, 1998; Bassett et al., 2006; Sporns and Honey, 2006). This type of organization facilitates specialized processing in dense, local clusters while maintaining efficient information transfer between clusters. Regardless of prior spatial training exposure, the networks engaged by contextual memory retrieval displayed characteristic small-world properties. Compared to random networks, we observed that the memory networks had a small number of highly connected regions, an increase in clustering coefficient, and equivalent global efficiency. Small world organization facilitates specialized processing in densely connected local communities while also allowing for efficient transfer of information between local communities. We used Markov chain clustering to detect the structure of these communities within the networks. In both networks there was a large interconnected central component, referred to as the giant component, which did not change in size as a result of prior spatial training. However, there was a significant increase in the density of connections within the GC of the group of mice who had received prior spatial learning. The densely connected giant component at the core of the network underlying context memory expression in mice with prior spatial training contained many redundant connections. Therefore, we hypothesized that more successive deletions would be required to break apart communities in a way which would be consequential to the effective communication of the network. This hypothesis was supported by the results of targeted node deletion. By sequentially deleting nodes in the descending order of their degree, we noted that mice which had prior spatial training were able to retain a higher percentage of their basal global efficiency and giant component size. In being more resistant to targeted attack, this network can be said to be more resilient than the network obtained from control mice (Achard et al., 2006).
Increased resiliency to targeted node deletion presents interesting possibilities from the perspective of neurodegenerative disease. The pathology of many neurodegenerative conditions does not arise uniformly throughout the brain and rather targets the most highly involved regions of a network reviewed in Crossley et al. (2014). In addition to suffering from targeted attacks, the functional connectomes characteristic of many neurodegenerative conditions networks also display decreased redundancy in their connectivity patterns, rendering networks more vulnerable to these attack (Langella et al., 2021). An efficient network which is more resilient to attack has the potential to delay or reduce cognitive decline during early neurodegenerative disease progression (Rittman et al., 2019). The present study found that cognitive stimulation through repetitive learning experiences was able to increase network efficiency and resilience. Therefore, the potential exists for prior exposure to repetitive learning experiences to increase resiliency to deterioration. Future studies of this phenomenon might build upon this by examining whether prolonged cognitive stimulation and the resulting alterations in functional connectivity are sufficient for reducing cognitive deficits observed in early stages of neurodegeneration. From the current results it is not known whether the observed change in functional connectivity and activity would be observed in tasks other than contextual fear conditioning and as such this should also be investigated in future studies.
In the present study, networks were generated based on correlated expression of c-Fos across the brain. While this method has been demonstrated at various levels of regional organization in previous publications (Wheeler et al., 2013; Vetere et al., 2017; Silva et al., 2019; Scott et al., 2020), it is worth acknowledging the limitations of this approach. While exhibiting excellent spatial resolution at the single cell level, c-Fos expression is limited in its temporal resolution. It is important to consider the delay that occurs between cellular activity and c-Fos expression. A delay of 90 minutes between cellular activity and peak c-Fos expression allows us to use c-Fos to examine brain-wide activity tagged to behavioral paradigms which are incompatible with head-fixed neuroimaging techniques. However, it is impossible under the current design to establish patterns of c-Fos expression during distinct bouts of freezing or movement during conditioned context reintroduction. In an experiment such as this in which freezing behaviors were consistent between groups, it is possible that this limitation has less of an impact on the ability to interpret the results than in an experiment in which the behaviors corresponding with the tagged neuronal activity vary greatly between groups. In such scenarios, follow-up experiments using an in vivo measure of regional activation would be advised to assess the specificity functional connections to distinct behavioral outputs.
When analyzing networks based on brain-wide correlated c-Fos expression density the entire group is treated as a single network. This limits the inferential statistics that can be applied when comparing network metrics between groups. However, it can be easy to overlook that underlying each cell in a correlation matrix is a p value. In thresholding these correlation matrices to generate the binary adjacency matrices that form the bases of the presented network analyses, the statistical significance of each correlation is heavily weighted. The thresholds examined in the present study (α = 0.05, α = 0.005, and α = 0.0005) were implemented so as to only consider the most statistically significant correlations in the network analysis, thereby ensuring than any descriptive comparisons between networks were based on the organization of highly significant patterns of co-activation.
Additionally, while we attribute the changes in network topology to spatial learning, there are other possible factors present during these episodes which may also contribute to network reorganization. These heavily intertwined factors include, exercise, motor activity, sensory exposure, and repeated acute stress. Among these factors, there is considerable debate surrounding stress in the Morris water maze (Sandi et al., 1997; Engelmann et al., 2006; Harrison et al., 2009). Repeated exposure to this mild stressor could result in changes in the HPA axis, which could then impact behavior and the organization of functional connectivity networks underlying a contextually conditioned fear memory (Sandi et al., 2003; Rodríguez Manzanares et al., 2005). Mice given pool exposure matched to the duration of the water maze training group without an escape platform location to learn, commonly referred to as a yoked control group, may superficially control for exposure to the water maze. However, other issues arise with yoked control group as there may be differences between escapable and inescapable stressors. These points considered, it was decided that measures controlling for exercise, such as the use of a yoked control group might induce further variability. However, the important conclusion of the current study is that repeated learning and memory episodes induce widescale changes in brain activity and functional connectivity during encoding and/or retrieval of subsequent unrelated memory tasks. Future studies will be required to assess the relative influence of specific factors occurring during task exposure.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/dterstege/PublicationRepo/tree/main/Terstege2022A.
Ethics statement
The animal study was reviewed and approved by University of Calgary Health Sciences Animal Care Committee in accordance with the guidelines of the Canadian Council on Animal Care.
Author contributions
DJT and JRE conceived and designed the experiment and conducted the analyses and wrote the manuscript. DJT and IMD conducted the experiments. All authors contributed to the article and approved the submitted version.
Funding
Funding for this study was provided by The Brain Canada Foundation/The Azrieli Foundation Early Career Capacity Building Grant (4709) and an NSERC Discovery Grant (RGPIN-2018-05135) to JRE.
Acknowledgments
We acknowledge the Hotchkiss Brain Institute Advanced Microscopy Platform and the Cumming School of Medicine for support and use of the Olympus VS120-L100-W slide scanning microscope.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2022.907707/full#supplementary-material
References
Achard, S., and Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 3:e17. doi: 10.1371/journal.pcbi.0030017
Achard, S., Salvador, R., Whitcher, B., Suckling, J., and Bullmore, E. (2006). A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 26, 63–72. doi: 10.1523/JNEUROSCI.3874-05.2006
Ajilore, O., Lamar, M., and Kumar, A. (2014). Association of brain network efficiency with aging, depression, and cognition. Am. J. Geriatr. Psychiatry 22, 102–110. doi: 10.1016/j.jagp.2013.10.004
Bagarinao, E., Watanabe, H., Maesawa, S., Mori, D., Hara, K., Kawabata, K., et al. (2019). Reorganization of brain networks and its association with general cognitive performance over the adult lifespan. Sci. Rep. 9:11352. doi: 10.1038/s41598-019-47922-x
Bassett, D. S., Bullmore, E. T., Meyer-Lindenberg, A., Apud, J. A., Weinberger, D. R., and Coppola, R. (2009). Cognitive fitness of cost-efficient brain functional networks. Proc. Natl. Acad. Sci. U.S.A. 106, 11747–11752. doi: 10.1073/pnas.0903641106
Bassett, D. S., Meyer-Lindenberg, A., Achard, S., Duke, T., and Bullmore, E. (2006). Adaptive reconfiguration of fractal small-world human brain functional networks. Proc. Natl. Acad. Sci. U.S.A. 103, 19518–19523. doi: 10.1073/pnas.0606005103
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Berg, S., Kutra, D., Kroeger, T., Straehle, C. N., Kausler, B. X., Haubold, C., et al. (2019). ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232. doi: 10.1038/s41592-019-0582-9
Bermudez, P., Lerch, J. P., Evans, A. C., and Zatorre, R. J. (2008). Neuroanatomical Correlates of Musicianship as Revealed by Cortical Thickness and Voxel-Based Morphometry. Cereb. Cortex 19, 1583–1596. doi: 10.1093/cercor/bhn196
Bohbot, V. D., Lerch, J., Thorndycraft, B., Iaria, G., and Zijdenbos, A. P. (2007). Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J. Neurosci. 27, 10078–10083. doi: 10.1523/JNEUROSCI.1763-07.2007
Brandes, U. (2001). A faster algorithm for betweenness centrality. J. Math. Soc. 25, 163–177. doi: 10.1080/0022250X.2001.9990249
Bullmore, E., and Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. doi: 10.1038/nrn2575
Chong, J. S. X., Ng, K. K., Tandi, J., Wang, C., Poh, J.-H., Lo, J. C., et al. (2019). Longitudinal Changes in the Cerebral Cortex Functional Organization of Healthy Elderly. J. Neurosci. 39, 5534–5550. doi: 10.1523/JNEUROSCI.1451-18.2019
Clark, R. E., and Delay, E. R. (1991). Reduction of lesion-induced deficits in visual reversal learning following cross-modal training. Restor. Neurol. Neurosci. 3, 247–255. doi: 10.3233/RNN-1991-3503
Crossley, N. A., Mechelli, A., Scott, J., Carletti, F., Fox, P. T., McGuire, P., et al. (2014). The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137, 2382–2395. doi: 10.1093/brain/awu132
De Paola, V., Holtmaat, A., Knott, G., Song, S., Wilbrecht, L., Caroni, P., et al. (2006). Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49, 861–875. doi: 10.1016/j.neuron.2006.02.017
Draganski, B., Gaser, C., Busch, V., Schuierer, G., Bogdahn, U., and May, A. (2004). Changes in grey matter induced by training. Nature 427, 311–312. doi: 10.1038/427311a
Dresler, M., Shirer, W. R., Konrad, B. N., Müller, N. C. J., Wagner, I. C., Fernández, G., et al. (2017). Mnemonic Training Reshapes Brain Networks to Support Superior Memory. Neuron 93, 1227–1235.e6. doi: 10.1016/j.neuron.2017.02.003
Engelmann, M., Ebner, K., Landgraf, R., and Wotjak, C. T. (2006). Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm. Behav. 50, 496–501. doi: 10.1016/j.yhbeh.2006.04.009
Epp, J. R., Chow, C., and Galea, L. A. M. (2013). Hippocampus-dependent learning influences hippocampal neurogenesis. Front. Neurosci. 7:57. doi: 10.3389/fnins.2013.00057
Finc, K., Bonna, K., He, X., Lydon-Staley, D. M., Kühn, S., Duch, W., et al. (2020). Dynamic reconfiguration of functional brain networks during working memory training. Nat. Commun. 11:2435. doi: 10.1038/s41467-020-15631-z
Fletcher, J. M., and Wennekers, T. (2018). From Structure to Activity: Using Centrality Measures to Predict Neuronal Activity. Int. J. Neural. Syst. 28:1750013. doi: 10.1142/S0129065717500137
Freeman, L. C. (1978). Centrality in social networks conceptual clarification. Soc. Netw. 1, 215–239. doi: 10.1016/0378-8733(78)90021-7
Fürth, D., Vaissière, T., Tzortzi, O., Xuan, Y., Märtin, A., Lazaridis, I., et al. (2018). An interactive framework for whole-brain maps at cellular resolution. Nat. Neurosci. 21, 139–149. doi: 10.1038/s41593-017-0027-7
Giustino, T. F., and Maren, S. (2015). The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front. Behav. Neurosci. 9:298. doi: 10.3389/fnbeh.2015.00298
Guzowski, J. F., Setlow, B., Wagner, E. K., and McGaugh, J. L. (2001). Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-fos, and zif268. J. Neurosci. 21, 5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001
Harrison, F. E., Hosseini, A. H., and McDonald, M. P. (2009). Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav. Brain Res. 198, 247–251. doi: 10.1016/j.bbr.2008.10.015
Holtmaat, A. J. G. D., Trachtenberg, J. T., Wilbrecht, L., Shepherd, G. M., Zhang, X., Knott, G. W., et al. (2005). Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45, 279–291. doi: 10.1016/j.neuron.2005.01.003
Hubbell, C. H. (1965). An Input-Output Approach to Clique Identification. Sociometry 28, 377–399. doi: 10.2307/2785990
Hyde, K. L., Lerch, J., Norton, A., Forgeard, M., Winner, E., Evans, A. C., et al. (2009). Musical training shapes structural brain development. J. Neurosci. 29, 3019–3025. doi: 10.1523/JNEUROSCI.5118-08.2009
Jo, Y. S., Park, E. H., Kim, I. H., Park, S. K., Kim, H., Kim, H. T., et al. (2007). The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J. Neurosci. 27, 13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007
Katz, L. (1953). A New Status Index Derived From Sociometric Analysis. Psychometrika 18, 39–43. doi: 10.1007/BF02289026
Konganti, K., Wang, G., Yang, E., and Cai, J. J. (2013). SBEToolbox: A Matlab toolbox for biological network analysis. Evol. Bioinform. Online 9, 355–362. doi: 10.4137/EBO.S12012
Kwapis, J. L., Jarome, T. J., Lee, J. L., and Helmstetter, F. J. (2015). The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiol. Learn. Mem. 123, 110–116. doi: 10.1016/j.nlm.2015.06.007
Langella, S., Sadiq, M. U., Mucha, P. J., Giovanello, K. S., Dayan, E., Alzheimer’s Disease, et al. (2021). Lower functional hippocampal redundancy in mild cognitive impairment. Transl. Psychiatry 11:61. doi: 10.1038/s41398-020-01166-w
Langer, N., von Bastian, C. C., Wirz, H., Oberauer, K., and Jäncke, L. (2013). The effects of working memory training on functional brain network efficiency. Cortex 49, 2424–2438. doi: 10.1016/j.cortex.2013.01.008
Latora, V., and Marchiori, M. (2001). Efficient behavior of small-world networks. Phys. Rev. Lett. 87:198701. doi: 10.1103/PhysRevLett.87.198701
Lendvai, B., Stern, E. A., Chen, B., and Svoboda, K. (2000). Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 241404, 876–881. doi: 10.1038/35009107
Lerch, J. P., Yiu, A. P., Martinez-Canabal, A., Pekar, T., Bohbot, V. D., Frankland, P. W., et al. (2011). Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage 54, 2086–2095. doi: 10.1016/j.neuroimage.2010.09.086
Maguire, E. A., Gadian, D. G., Johnsrude, I. S., Good, C. D., Ashburner, J., Frackowiak, R. S., et al. (2000). Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. U.S.A. 97, 4398–4403. doi: 10.1073/pnas.070039597
Maguire, E. A., Spiers, H. J., Good, C. D., Hartley, T., Frackowiak, R. S. J., and Burgess, N. (2003). Navigation expertise and the human hippocampus: A structural brain imaging analysis. Hippocampus 13, 250–259. doi: 10.1002/hipo.10087
Martínez, K., Solana, A. B., Burgaleta, M., Hernández-Tamames, J. A., Alvarez-Linera, J., Román, F. J., et al. (2013). Changes in resting-state functionally connected parietofrontal networks after videogame practice: Videogame Practice and Functional Connectivity. Hum. Brain Mapp. 34, 3143–3157. doi: 10.1002/hbm.22129
McQuail, J. A., Dunn, A. R., Stern, Y., Barnes, C. A., Kempermann, G., Rapp, P. R., et al. (2020). Cognitive Reserve in Model Systems for Mechanistic Discovery: The Importance of Longitudinal Studies. Front. Aging Neurosci. 12:607685. doi: 10.3389/fnagi.2020.607685
Milczarek, M. M., Vann, S. D., and Sengpiel, F. (2018). Spatial memory engram in the mouse retrosplenial cortex. Curr. Biol. 28, 1975–1980.e6. doi: 10.1016/j.cub.2018.05.002
Miller, A. M. P., Vedder, L. C., Law, L. M., and Smith, D. M. (2014). Cues, context, and long-term memory: The role of the retrosplenial cortex in spatial cognition. Front. Hum. Neurosci. 8:586. doi: 10.3389/fnhum.2014.00586
Miró-Padilla, A., Bueichekú, E., Ventura-Campos, N., Flores-Compañ, M.-J., Parcet, M. A., and Ávila, C. (2019). Long-term brain effects of N-back training: An fMRI study. Brain Imaging Behav. 13, 1115–1127. doi: 10.1007/s11682-018-9925-x
Nouchi, R., Taki, Y., Takeuchi, H., Hashizume, H., Akitsuki, Y., Shigemune, Y., et al. (2012). Brain training game improves executive functions and processing speed in the elderly: A randomized controlled trial. PLoS One 7:e29676. doi: 10.1371/journal.pone.0029676
Nyberg, L., Sandblom, J., Jones, S., Neely, A. S., Petersson, K. M., Ingvar, M., et al. (2003). Neural correlates of training-related memory improvement in adulthood and aging. Proc. Natl. Acad. Sci. U.S.A. 100, 13728–13733. doi: 10.1073/pnas.1735487100
Ocampo, A. C., Squire, L. R., and Clark, R. E. (2018). The beneficial effect of prior experience on the acquisition of spatial memory in rats with CA1, but not large hippocampal lesions: A possible role for schema formation. Learn. Mem. 25, 115–121. doi: 10.1101/lm.046482.117
Owen, A. M., Hampshire, A., Grahn, J. A., Stenton, R., Dajani, S., Burns, A. S., et al. (2010). Putting brain training to the test. Nature 465, 775–778. doi: 10.1038/nature09042
Rittman, T., Borchert, R., Jones, S., van Swieten, J., Borroni, B., Galimberti, D., et al. (2019). Functional network resilience to pathology in presymptomatic genetic frontotemporal dementia. Neurobiol. Aging 77, 169–177. doi: 10.1016/j.neurobiolaging.2018.12.009
Rodríguez Manzanares, P. A., Isoardi, N. A., Carrer, H. F., and Molina, V. A. (2005). Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J. Neurosci. 25, 8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Sandi, C., Davies, H. A., Cordero, M. I., Rodriguez, J. J., Popov, V. I., and Stewart, M. G. (2003). Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur. J. Neurosci. 17, 2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x
Sandi, C., Loscertales, M., and Guaza, C. (1997). Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur. J. Neurosci. 9, 637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x
Schneidman, E., Berry, M. J. II, Segev, R., and Bialek, W. (2006). Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 440, 1007–1012. doi: 10.1038/nature04701
Scholz, J., Klein, M. C., Behrens, T. E. J., and Johansen-Berg, H. (2009). Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371. doi: 10.1038/nn.2412
Scott, G. A., Terstege, D. J., Vu, A. P., Law, S., Evans, A., and Epp, J. R. (2020). Disrupted Neurogenesis in Germ-Free Mice: Effects of Age and Sex. Front. Cell Dev. Biol. 8:407. doi: 10.3389/fcell.2020.00407
Sheffield, J. M., Repovs, G., Harms, M. P., Carter, C. S., Gold, J. M., MacDonald, A. W. III, et al. (2016). Evidence for Accelerated Decline of Functional Brain Network Efficiency in Schizophrenia. Schizophr. Bull. 42, 753–761. doi: 10.1093/schbul/sbv148
Silva, B. A., Burns, A. M., and Gräff, J. (2019). A cFos activation map of remote fear memory attenuation. Psychopharmacology 236, 369–381. doi: 10.1007/s00213-018-5000-y
Spanswick, S. C., Epp, J. R., Keith, J. R., and Sutherland, R. J. (2007). Adrenalectomy-induced granule cell degeneration in the hippocampus causes spatial memory deficits that are not reversed by chronic treatment with corticosterone or fluoxetine. Hippocampus 17, 137–146. doi: 10.1002/hipo.20252
Sporns, O., and Honey, C. J. (2006). Small worlds inside big brains. Proc. Natl. Acad. Sci. U.S.A. 103, 19219–19220. doi: 10.1073/pnas.0609523103
Srivishagan, S., Perera, A. A. I., Hojjat, A., and Ratnarajah, N. (2020). Brain Network Measures for Groups of Nodes: Application to Normal Aging and Alzheimer’s Disease. Brain Connect 10, 316–327. doi: 10.1089/brain.2020.0747
Takeuchi, H., Taki, Y., Nouchi, R., Sekiguchi, A., Kotozaki, Y., Nakagawa, S., et al. (2017). Neural plasticity in amplitude of low frequency fluctuation, cortical hub construction, regional homogeneity resulting from working memory training. Sci. Rep. 7:1470. doi: 10.1038/s41598-017-01460-6
Teather, L. A., Packard, M. G., Smith, D. E., Ellis-Behnke, R. G., and Bazan, N. G. (2005). Differential induction of c-Jun and Fos-like proteins in rat hippocampus and dorsal striatum after training in two water maze tasks. Neurobiol. Learn. Mem. 84, 75–84. doi: 10.1016/j.nlm.2005.03.006
van den Heuvel, M. P., Stam, C. J., Kahn, R. S., and Hulshoff Pol, H. E. (2009). Efficiency of functional brain networks and intellectual performance. J. Neurosci. 29, 7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009
Vetere, G., Kenney, J. W., Tran, L. M., Xia, F., Steadman, P. E., Parkinson, J., et al. (2017). Chemogenetic Interrogation of a Brain-wide Fear Memory Network in Mice. Neuron 94, 363–374.e4. doi: 10.1016/j.neuron.2017.03.037
Watts, D. J., and Strogatz, S. H. (1998). Collective dynamics of “small-world” networks. Nature 393, 440–442. doi: 10.1038/30918
West, G. L., Zendel, B. R., Konishi, K., Benady-Chorney, J., Bohbot, V. D., Peretz, I., et al. (2017). Playing Super Mario 64 increases hippocampal grey matter in older adults. PLoS One 12:e0187779. doi: 10.1371/journal.pone.0187779
Wheeler, A. L., Teixeira, C. M., Wang, A. H., Xiong, X., Kovacevic, N., Lerch, J. P., et al. (2013). Identification of a functional connectome for long-term fear memory in mice. PLoS Comput. Biol. 9:e1002853. doi: 10.1371/journal.pcbi.1002853
Zhan, J., Gurung, S., and Parsa, S. P. K. (2017). Identification of top-K nodes in large networks using Katz centrality. J. Big Data 4:16 doi: 10.1186/s40537-017-0076-5
Keywords: cognitive stimulation, functional connectivity, context memory, immediate early genes, mouse model
Citation: Terstege DJ, Durante IM and Epp JR (2022) Brain-wide neuronal activation and functional connectivity are modulated by prior exposure to repetitive learning episodes. Front. Behav. Neurosci. 16:907707. doi: 10.3389/fnbeh.2022.907707
Received: 30 March 2022; Accepted: 23 August 2022;
Published: 09 September 2022.
Edited by:
João J. Cerqueira, University of Minho, PortugalReviewed by:
Szu-Han Wang, University of Edinburgh, United KingdomDavid F. Werner, Binghamton University, United States
Copyright © 2022 Terstege, Durante and Epp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan R. Epp, Jonathan.epp1@ucalgary.ca
 Dylan J. Terstege
Dylan J. Terstege Isabella M. Durante
Isabella M. Durante  Jonathan R. Epp
Jonathan R. Epp