Nxf7 deficiency impairs social exploration and spatio-cognitive abilities as well as hippocampal synaptic plasticity in mice
- 1Laboratory of Biological Psychology, University of Leuven, KU Leuven, Leuven, Belgium
- 2Human Genome Laboratory, University of Leuven and VIB Center for the Biology of Disease, Leuven, Belgium
Nuclear RNA export factors (NXF) are conserved in all metazoans and are deemed essential for shuttling RNA across the nuclear envelope and other post-transcriptional processes (such as mRNA metabolism, storage and stability). Disruption of human NXF5 has been implicated in intellectual and psychosocial disabilities. In the present report, we use recently described Nxf7 knockout (KO) mice as an experimental model to analyze in detail the behavioral consequences of clinical NXF5 deficiency. We examined male Nxf7 KO mice using an extended cognitive and behavioral test battery, and recorded extracellular field potentials in the hippocampal CA1 region. We observed various cognitive and behavioral changes including alterations in social exploration, impaired spatial learning and spatio-cognitive abilities. We also defined a new experimental paradigm to discriminate search strategies in Morris water maze and showed significant differences between Nxf7 KO and control animals. Furthermore, while we observed no difference in a nose poke suppression in an conditioned emotional response (CER) protocol, Nxf7 KO mice were impaired in discriminating between differentially reinforced cues in an auditory fear conditioning protocol. This distinct neurocognitive phenotype was accompanied by impaired hippocampal Long-term potentiation (LTP), while long-term depression (LTD) was not affected by Nxf7 deficiency. Our data demonstrate that disruption of murine Nxf7 leads to behavioral phenotypes that may relate to the intellectual and social deficits in patients with NXF5 deficiency.
Introduction
Post-transcriptional processes governing transcription, transport and metabolism of neuronal mRNAs are key regulators of dendritic translation, and consequently of synaptic plasticity and cognitive functions. These processes involve a complex system of RNA binding proteins (RBPs) and non-coding RNAs ensuring proper translation activation or suppression with spatial and temporal specificity and precision (Thomas et al., 2014). Mutations in RBPs have been linked to a number of pathologies with associated cognitive dysfunction, such as fragile X mental retardation (e.g., FMRP) and frontotemporal dementia (e.g., TDP-43; Lukong et al., 2008; Tolino et al., 2012).
Nuclear RNA Export Factor (NXF) proteins interact with RBPs (Braun et al., 1999; Kang et al., 1999; Bachi et al., 2000), and hence play an essential role in post-transcriptional processes such as shuttling mRNA across the nuclear envelope (Izaurralde, 2002a,b, 2004), cytoplasmic mRNA trafficking (Takano et al., 2007), and mRNA stabilization (Zhang et al., 2007; Katahira et al., 2008). In the human genome, four functional NXF family members have been described (NXF1, NXF2, NXF3 and NXF5) of which NXF2, NXF3 and NXF5 are clustered on Xq22.1 (Herold et al., 2000; Jun et al., 2001). NXF3 and NXF5 proteins are present in the cytoplasm (Herold et al., 2000; Jun et al., 2001), but NXF5 appears to be the only one involved in brain development (Jun et al., 2001). NXF5 was shown to bind non-specifically to RNA, but failed to display RNA nuclear export activity like other NXF proteins (Bachi et al., 2000; Yang et al., 2001).
Several studies in intellectually impaired individuals implicated NXF5 deficiency, but actual causative information is lacking so far (Jun et al., 2001; Froyen et al., 2007; Grillo et al., 2010). Jun et al. (2001) described a middle-aged man with probable NXF5 deficiency-related intellectual disability, who displayed relatively unspecific, but severe cognitive impairment, complete lack of speech as well as very limited social and communicative abilities. Grillo et al. (2010) described a female patient with 1.1 Mb deletion of chromosome Xq22.1 containing part of the NXF cluster displaying severe mental retardation, autism, micro-brachycephaly and muscle and bone dystrophies. To investigate the importance of NXF5 in brain functioning more directly, we created a mouse model for this disorder. Based on detailed morphological, histological, and molecular analysis, we recently argued that Nxf7 is the functional analog of NXF5, and that Nxf7 knockout (KO) mice may therefore represent the most valid model for human NXF5 deficiency (Vanmarsenille et al., 2013).
In the present study, we investigated various aspects of behavioral and cognitive performance in Nxf7-deficient mice as well as hippocampal synaptic plasticity as the cellular correlate of complex learning abilities. We included these particular tests in reference to the intellectual and social defects in NXF5-deficient individuals. We assessed emotionality and social exploration, and spatio-cognitive abilities in these mice. Long-term potentiation (LTP) and long-term depression (LTD) of synaptic transmission were analyzed in the hippocampal CA1 region. These synaptic phenomena are widely regarded as cellular correlates of complex information storage in the brain (Huber et al., 2002; Denayer et al., 2008; Zhang et al., 2009; Balschun et al., 2010; Callaerts-Vegh et al., 2012; Lo et al., 2014).
Materials and Methods
Animal Colony and Behavioral Test Schedule
Nxf7-deficient mice (KO) were generated via targeted deletion of Nxf7 exon 3 as described in Vanmarsenille et al. (2013). Briefly, homologous recombination of exon 3 in 129Sv embryonic stem cells (ES) cells, and subsequent crosses of chimeric offspring with C57Bl/6 mouse produced male knock-in animals. Deletion of exon 3 was then achieved by mating a knock-in male with PGK-Cre-expressing females. For genotyping, PCR analysis of tail biopsies was performed as described before Vanmarsenille et al. (2013). Standard breeding procedures included crossing heterozygous (HTZ) Nxf7 females with C57Bl/6 wildtype (WT) males, which resulted in male NXF7 KO offspring. Animals used in behavior had been backcrossed to C57Bl/6 for over 20 generations.
Nxf7 KO males and their WT littermates were compared using an expanded test battery for motor, emotional, and cognitive performance (Table 1). Behavioral testing started at 8–10 weeks of age. Animals were kept in standard animal cages under conventional laboratory conditions (12 h/12 h light-dark cycle, 22°C) with ad libitum access to food and water (unless stated otherwise). All experiments were conducted during the light phase of their activity cycle. All protocols have been reviewed and approved by the animal experiments committee of the University of Leuven (Belgium), and were carried out in accordance with the European Community Council Directive (86/609/EEC).
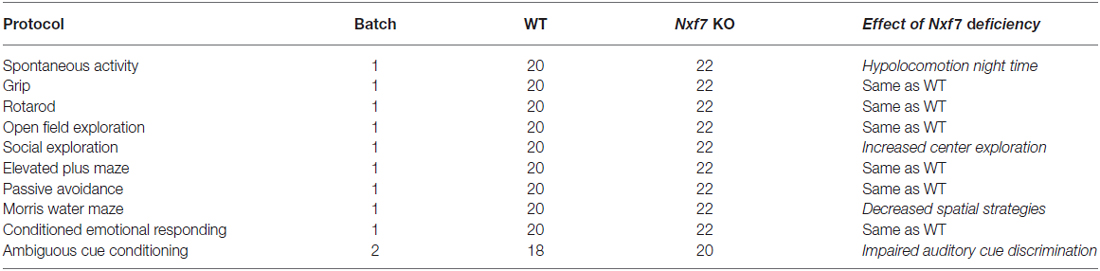
Table 1. Overview of behavioral tests, number of animals tested and summary of behavioral observations.
General Neuromotor Assessment
Cage activity and neuromotor coordination were assessed by various tests that we described and used previously: 23-h spontaneous cage activity, motor coordination/balance on the accelerating rotarod, and grip strength measurement (D’Hooge et al., 2005). Briefly, 23-h cage activity was recorded using a lab-built activity logger connected to three IR photo beams. Mice were placed individually in 20 × 30 cm2 transparent cages located between the photo beams. Over a period of 23 h, activity was recorded as number of beam crossings. To examine motor coordination, mice were first trained on a rotating rod at constant speed (4 rpm, 2 min), before starting four test trials (inter-trial interval, 10 min). During these test trials, the animals had to balance on a rotating rod that accelerated from 4 to 40 rpm in 5 min, and time until they dropped from the rod was recorded (up to the 5-min cut-off). Grip strength was measured using a device consisting of a T-shaped bar connected to a digital dynamometer (Ugo Basile, Comerio, Italy). Mice were placed before the bar, which they usually grabbed spontaneously, and gently pulled backwards until they released the bar (maximal readouts were recorded). Ten such measurements were obtained for each animal.
Exploration and Anxiety Assessment
Three tests were included that were based on spontaneous exploration. The first two, open field and social exploration, used a 50 × 50 cm2 square arena (D’Hooge et al., 2005; Callaerts-Vegh et al., 2006). Animals were placed in the dark for 30 min, and then placed individually in the arena for 11 min (1 min habituation and 10 min recording). Exploration was recorded using EthoVision video tracking equipment and software (Noldus, Wageningen, Netherlands). Total path length and corner crossings were included as measures of locomotor/exploratory activity. Entries into the center of the field were recorded as measures of conflict resolution or anxiolysis. For social exploration assessment, the arena contained a 15 cm round cage with two unfamiliar female mice. Again, exploratory activity was tracked for 11 min (1 min habituation and 10 min recording) using EthoVision software using predefined area templates. Finally, an elevated plus maze arena was used to assess anxiety-related exploration (Callaerts-Vegh et al., 2006). The arena consisted of a plus-shaped arena with two arms (5 cm wide) closed by side walls, and two arms without walls. Mice were placed at the center of the cross, and were allowed to explore freely for 11 min (1 min habituation and 10 min recording). Exploratory activity in this arena was recorded by five IR beams (four for arm entries, and one for open arm dwell) connected to a computerized activity logger.
Spatial Learning and Memory, and Spatio-Cognitive Performance
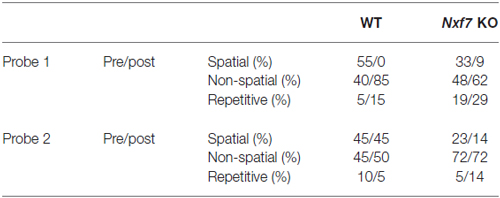
Spatial learning and memory were examined in a standard hidden-platform Morris water maze using acquisition and retention (i.e., long-term spatial memory) protocols. A 150 cm circular pool was filled with water, opacified with non-toxic white paint, and kept at 26°C as previously described (D’Hooge et al., 2005; Callaerts-Vegh et al., 2006). A 15 cm round platform was hidden 1 cm beneath the surface of the water at a fixed position. Mice were trained for 10 days (5 days followed by a 2-day break) with each daily session consisting of four swimming trials (15–30 min intertrial interval) that started randomly from four starting positions. Mice that failed to find the platform within 2 min were guided to the platform, where they remained for 15 s before being returned to their cages. Probe trials were conducted after five and 10 sessions (after the 2-day breaks and before continuation of acquisition training). During such probe trials, the platform was removed from the pool, and the search pattern of the mice was recorded for 100 s. Swimming paths were recorded using EthoVision video tracking equipment and software (Noldus, Wageningen, Netherlands). Search strategies during probe trails were analyzed with binary support-vector-machine (SVM) classifiers (Cortes and Vapnik, 1995). Each probe trial was divided into a time episode before the mouse first reached the virtual platform position, and the episode thereafter. Typically, animals that successfully learned the position of the platform, swim directly towards it and use spatial memory-dependent strategies, whereas less proficient mice employ search strategies that are not as cognitively advanced (i.e., non-spatial or repetitive strategies). We classified the strategy of each mouse during the probe trial periods using a 3-class scoring method (Brody and Holtzman, 2006; Callaerts-Vegh et al., 2012; Lo et al., 2013a).
Appetitive and Aversive Conditioning
Contextual fear conditioning was assessed with a passive avoidance protocol in a two- compartment box with a shock grid (D’Hooge et al., 2005). The box consisted of an illuminated and a dark compartment, separated by a guillotine door. The dark adapted mouse was placed in the illuminated box and latency to enter the dark compartment was measured. If the subject failed to enter the dark compartment within 5 min trial duration, it was gently probed to enter the dark part. Upon entry into the dark compartment, the door was closed and a 2 s foot shock (0.2 mA) was applied. The mouse was then removed from the box and placed in its home cage. Twenty-four hours later the dark-adapted mouse was again placed in the illuminated box and latency to enter the dark compartment was measured as retention of contextual memory.
Fear conditioning to ambiguous tones was studied using a modified protocol described by Tsetsenis et al. (2007). Testing was conducted in a freezing system (Panlab, Barcelona, Spain), containing a test chamber (26 cm wide × 26 cm long × 27 cm high) with a grid floor placed on top of a force transducer platform to record movements. Test chamber and startle platform were placed inside a ventilated and sound-attenuated cubicle (60 cm wide × 45 cm long × 48 cm high) with a speaker mounted on top of the test chamber. During fear acquisition, animals were presented two auditory stimuli (3 kHz and 8 kHz, 70 dB, 15 s). One stimulus always co-terminated with a mild foot shock (2 s, 0.3 mA; perfect cue, PER: 100% reinforced), while the other tone was either presented alone (no reinforcement) or in compound with the perfect cue and the shock (ambiguous cue, AMB: 50% reinforced; see Figure 4A, schematics of protocol). Each animal received 10 blocks of stimulus presentations (one block consists of (AMB-PER-shock) followed by 1 min inter-stimulus interval followed by AMB) with 1 min intervals between blocks. 48 h later, mice were placed in a novel context (grid floor covered by plastic sheet, changed odors and lighting). Animal movements were detected and recorded for 16 min by the force transducer with a sampling rate of 50 Hz and stored as raw data on a computer. During the first 3 min no stimulus was delivered (baseline freezing in new context), followed by a 5 min presentation of AMB, a 3 min inter-stimulus interval, and a 5 min presentation of PER. 14 days after conditioning, long-term retention was assessed by measuring fear potentiated startle using the same setup. Fear potentiated startle responses resulting from presentation of either the startle stimulus alone (5 kHz, 100–110 dB, 20 ms), or the startle stimulus preceded by the AMB or PER cue were recorded.
Scheduled appetitive conditioning, and acquisition and extinction of fear-induced response suppression were tested in a conditioned emotional response (CER) procedure using automated operant chambers (Coulbourn Instruments, Allentown, PA, USA), essentially as described previously (Callaerts-Vegh et al., 2006). Prior to the training, the animals were placed on a food restriction schedule to keep their body weights at 80–90% of their free-feeding weights. Under increasing reinforcement schedule (FR1-VI30), the animals were gradually shaped to nosepoke for food pellets (Noyes precision pellets, Research Diets, New Brunswick, NJ, USA) in 30 min daily sessions. After obtaining a stable response rate, eight fear acquisition trials with tone-shock presentations were superimposed on the VI-30 s schedule(Callaerts-Vegh et al., 2006), followed by extinction trials. Nosepoke rate during the auditory cues was compared to that during the interstimulus intervals (ISI) by calculating a suppression ratio (SR) as SR = RRCUE/(RRCUE + RRISI); with RRCUE and RRISI representing mean response rates in the presence and absence of the auditory cues, respectively. Thus, a SR of 0.5 indicates complete lack of suppression (equal response rates in the presence and in the absence of the cues), whereas a SR of 0 indicates complete suppression (complete lack of responding in the presence of the cues). Null responders were alloted SR of 0.
Field Potential Recordings on Hippocampal Slices
The preparation and methods applied were as detailed before Denayer et al. (2008). In brief, experimentally naïve mice of 3–5 months old were killed by cervical dislocation and the right hippocampus was immediately dissected into cold (4°C) artificial cerebrospinal fluid (ACSF) that was saturated with carbogen (95% O2 5% CO2). ACSF consisted of (in mM): 124 NaCl, 4.9 KCl, 25.6 NaHCO3, 1.2 KH2PO4, 2.0 CaCl2, 2.0 MgCl2, 10.0 D-glucose and was adjusted to pH 7.4. Transverse slices (400 μm thick) were prepared from the dorsal area of the hippocampus and transferred into a submerged-type slice chamber where they were maintained at 32°C and continuously perfused with ACSF at a flow-rate of 2.2 ml/min. After 90 min incubation, one slice was arbitrarily selected and a tungsten electrode was placed in CA1 stratum radiatum. For recording of field excitatory postsynaptic potentials (fEPSPs), a glass electrode (filled with ACSF, 3–7 MΩ) was placed in the stratum radiatum. The time course of the fEPSP was measured from their descending slope. After a further hour of incubation, input/output curves were determined, and the stimulation strength was adjusted to maintain a fEPSP slope of 35% of the initial maximum. Paired-pulse ratio (PPR) was investigated by applying two pulses in rapid succession (ISI of 10, 20, 50, 100, 200 and 500 ms) with 120 s between the different measurements. During baseline recording, three single stimuli (0.1 ms pulse width; 10 s interval) were measured every 5 min and averaged.
Synaptic plasticity in form of LTP and LTD were evaluated using previously described protocols (Denayer et al., 2008). Immediately after stimulation, evoked responses were monitored at 1, 4, 7 and 10 min, and then every further 5 min. The magnitude of non-NMDAR mediated post-tetanic potentiation (PTP) in both genotypes was investigated by bath application of the selective N-methyl-D-aspartate receptor (NMDAR) antagonist D-AP5, (D(-)-2-amino-5-phosphopentanoic acid; 50 μM). The drug was applied 10 min prior to delivering a single tetanus (100 Hz-1 s) and 10 min after. All drugs were obtained from Ascent Scientific Ltd. (Bristol, UK). In all cases, experiments on age matched WT and KO mice were interleaved with each other.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). To analyze behavior readouts, differences between mean values were determined using t-test or two-way repeated measures analysis of variance (RM-ANOVA) procedures with Tukey tests for post hoc comparison. We used Fisher’s exact test t compare ratios of search strategies between genotypes during the probe trials.
For field potential recordings, group differences between mean values were determined by repeated measures analysis of variance (RM-ANOVA) procedures with Holm-Sidak for post hoc comparisons. Intragroup comparisons were performed with Wilcoxon matched-pairs signed-rank test. Student t-tests were used for comparisons of PP Rs. All statistical tests were performed at the α level of significance at 0.05.
Results
Nxf7 KO Mice are Hypoactive
Overall spontaneous cage activity over 23 h was not different between Nxf7 KO and WT mice (Figure 1A). However, during the dark period, Nxf7 KO mice displayed a significantly decreased cage activity compared to WT (factor genotype: F1,983 = 5.07, P < 0.05 and interaction of factor genotype × time F23,983 = 1.567, P < 0.05, Figure 1B). Balancing on the rotating rod and grip strength was similar in Nxf7 KO and WT mice (Figures 1C,D), indicating a lack of major motor impairment.
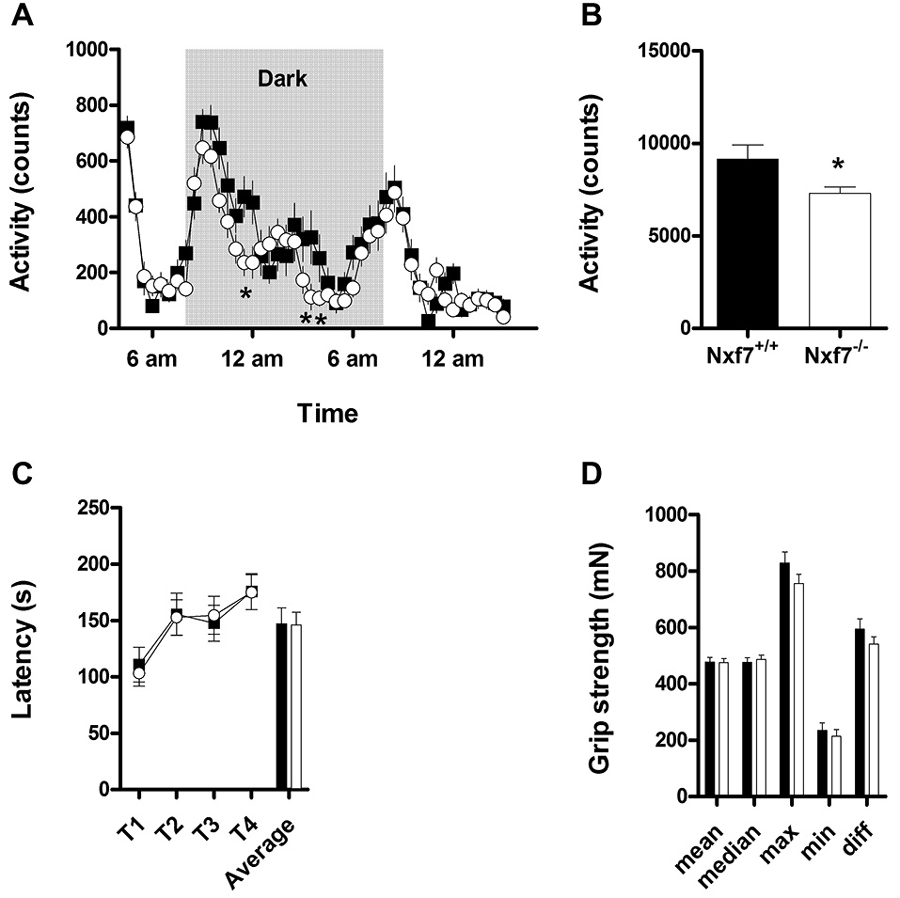
Figure 1. Activity and neuromotor assessment in wildtype (WT) and Nxf7 knockout (KO) mice. Overall 23 h activity was not different between the two genotypes (A), but during the dark period, Nxf7 KO (open symbols, N = 21) were significantly less active than WT mice (black symbols, N = 20) (B). Balance on the rotarod (C) and grip strength (D) was not different between the two genotypes. Data are expressed as means ± SEM. *p < 0.05 Nxf7 KO vs. WT (RM-ANOVA, post hoc comparison).
Nxf7 KO Mice Show Increased Social, but Normal Emotional Exploration
In open field exploration, no genotype differences were found in path length (Figure 2A), emotionality-related exploration patterns such as path length in the center (11.0 ± 1.0 m and 9.8 ± 1.0 m, for WT and Nxf7 KO, respectively), thigmotaxis (396 ± 15 s and 416 ± 19 s, for WT and Nxf7 KO, respectively), or time spent in center (Figures 2B,C).
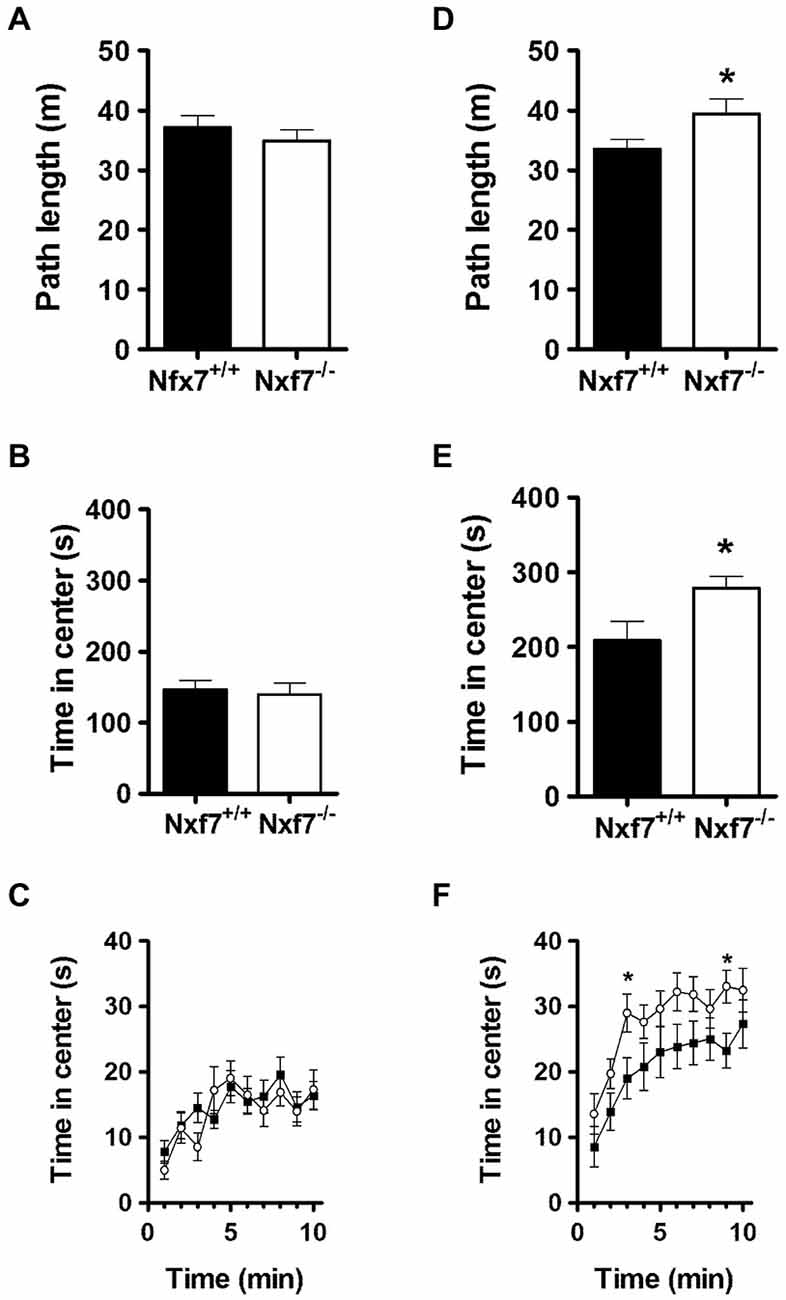
Figure 2. Exploratory activity in the open field and during social exploration. In the open field, Nxf7 KO (open symbols) covered the same distance (A), and spent the same amount of time in the center (B,C), as their WT littermates (black symbols). When two female mice were placed in the center of the open field, male Nxf7 KO (open symbols) were more active (D), and spent more time in the center (E), and approached the two female mice sooner (F) than their WT littermates. Data are presented as means ± SEMs. *p < 0.05 Nxf7 KO vs. WT.
In social exploration, however, total path length was significantly increased in Nxf7 KO mice (t38 = 2.33, P < 0.05; Figure 2D). Similarly, run speed was also increased in Nxf7 KO mice (5.58 ± 0.3 cm/s and 6.57 ± 0.4 cm/s for WT and KO mice, respectively; t38 = 2.03, P < 0.05). This increase in activity was directed towards the two unknown female mice placed in the center of the social exploration field, since Nxf7 KO spent significantly more time in the center area than WT littermates (t38 = 2.03, P < 0.05; Figures 2E,F). The presence of two female unknown mice induced social approach behavior (increased visit to the center when compared to open field, Figure 2C) and it was sooner and more pronounced in Nxf7 KO mice compared to WT (time F9,342 = 12.91, P < 0.001, genotype: F1,38 = 5.43, P < 0.03; Figure 2F).
In elevated plus maze, no differences were observed in general (total arm crosses 123 ± 8 and 126 ± 4, for WT and Nxf7 KO, respectively), or anxiety-related exploration (open arm dwell: 18.0 ± 1.4 and 21.0 ± 1.2% or open arm crosses: 21.0 ± 1.3 and 23.0 ± 1.6% for WT and Nxf7 KO, respectively).
Nxf7 KO Mice are Impaired in Spatio-Cognitive Performance
We have previously shown that spatial learning in the Morris water maze was significantly impaired in Nxf7 KO mice (Vanmarsenille et al., 2013). Further analysis indicated that this lower performance in the mutant group was not caused by differences in path length (Figure 3A, F1,409 = 2.72, P > 0.1), nor in swimming speed (F1,409 = 1.19, P > 0.1, data not shown), but apparently due to lower accuracy in their search for the hidden platform. The cumulative distance between the mouse and the target (platform center), a measure of spatial accuracy, indicated that Nxf7 KO were less accurate (longer target-mouse distance) than WT mice. RM-ANOVA of distance to the target was significantly different between genotypes (F1,39 = 8.33, P < 0.01), although both groups learned and reduced the distance significantly over trials (effect of trial F9,351 = 17.43, P < 0.001; Figure 3B). In addition, the interaction of genotype × trial was significant (F9,351 = 2.99, P < 0.005), consistent with the different learning curves. This difference was also evident during the two probe trials, where the average distance to the target center remained unchanged in Nxf7 KO mice compared to WT mice (Figure 3C). RM-ANOVA indicated a significant effect for genotype (F1,39 = 4.58, P > 0.05), while the contrasts between probe trials (F1,39 = 2.44) or the interaction (F1,39 = 3.15) were not significant. We used a previously described algorithm to categorize the search strategies employed during the probe trial (Callaerts-Vegh et al., 2012; Lo et al., 2013b, 2014). Mice use several strategies to find a hidden platform. Spatial strategies are the most efficient and rely predominantly on cognitive abilities (Brody and Holtzman, 2006; Lo et al., 2013a). However, when the platform is not found, the mouse either continues searching in the same spot (i.e., using spatial strategies), or it switches to non-spatial strategies. To test what strategies are being used, we divided each probe trial in two episodes: the first episode (pre) is defined by the path the animal swims until it crosses the platform position, followed by the period after this event (post). The different episodes were then scored based on the most prevalent search strategy (i.e., spatial, non-spatial and repetitive solutions). The first episode in a probe trial (swim to the platform position) is likely similar to the last acquisition trials. The second (after the first platform crossing), however, could yield additional information about robustness of cognitive searching and search strategy employed. During the first probe trial, we observed that the animals used spatial strategies in the pre episode (see Table 2), but after the crossing (post) non-spatial strategies were mostly employed (Figure 3D). No difference was observed between the genotypes. In contrast, during the second probe trial, we observed that both genotypes employed to a similar extent spatial strategies during the pre-episode (45 and 23% for WT and Nxf7 KO, respectively; Fisher’s exact test not significant). However, for the post episode, WT animals preferred spatial strategies, while Nxf7 KO mice relied mostly on non-spatial strategies. Fisher’s exact test comparing spatial vs. other strategies between WT and Nxf7 KO groups indicated a significant association between strategy and genotype (P < 0.05, Figure 3D).
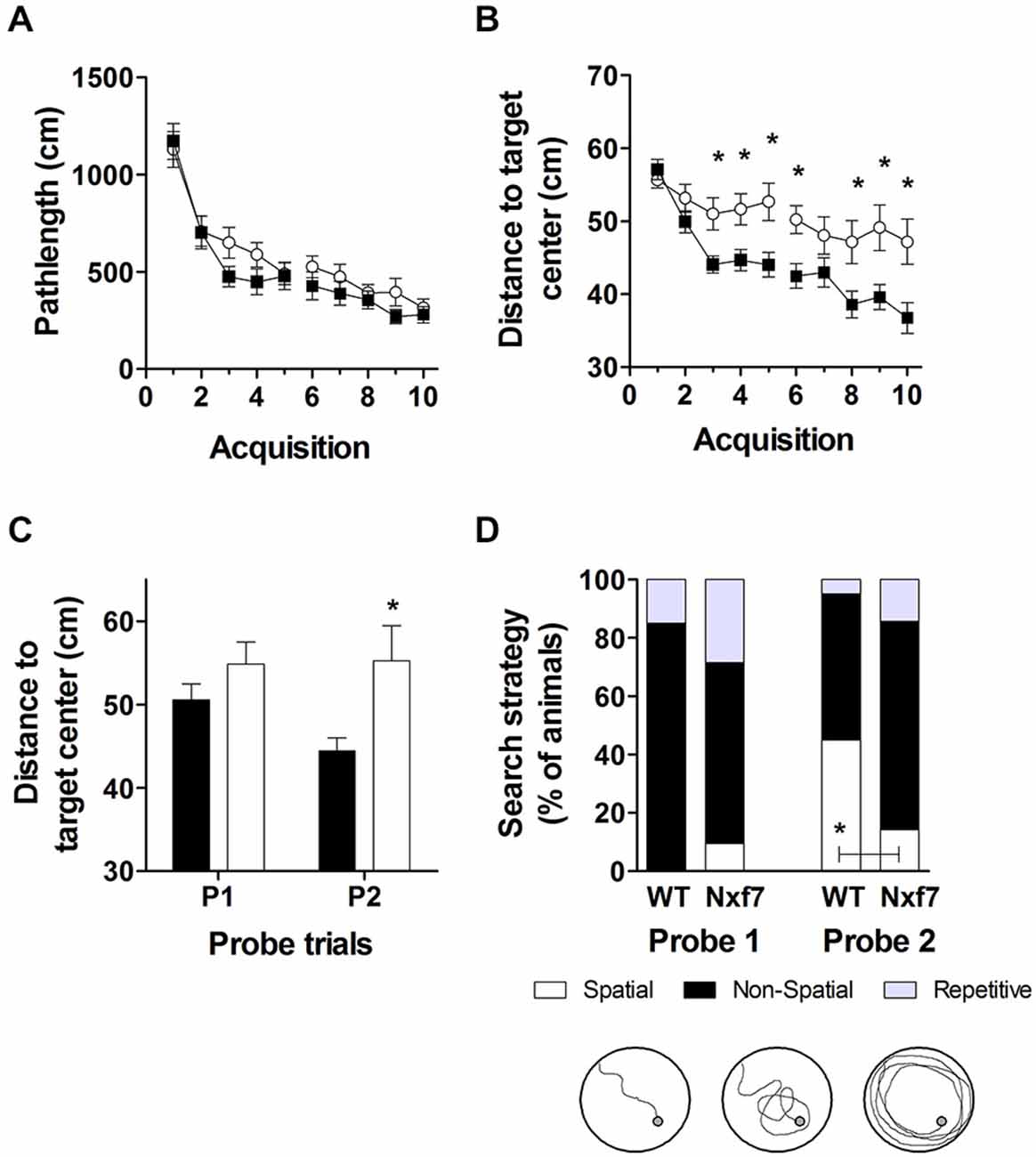
Figure 3. Spatial learning in the Morris water maze was impaired in Nxf7 KO (white symbols, N = 21) mice compared to WT (black symbols, N = 20) mice. While no difference was found in total path length (A), Nxf7 KO were less efficient in finding the platform and average distance to the hidden platform was significantly reduced during acquisition (B) and during interspersed probe trials (C). Results are presented as means ± SEMs. Search strategy analysis of the probe trials (D) indicated that 45% of WT animals employed spatial search strategies in the period after the first virtual platform crossing during the second probe trial In contrast, most Nxf7 KO mice (71%) relied on non-spatial strategies to search for the platform in the post-period. Tracks are depicting schematic representation of search strategies (for detailed information see Lo et al., 2013a, 2014). Data are presented as ratio. *p < 0.05 comparing to WT. See text for details.
Nxf7 KO Mice are Impaired in Cue Discrimination, but not in Appetitive or Aversive Conditioning
Passive avoidance was acquired in a single session and was tested 24 h later. No differences during training or testing were observed. Latency to enter the dark compartment during training was 14.4 ± 2 and 12.2 ± 1.3 s for WT and Nxf7 KO, respectively. Twenty-four hours later both groups hesitated to enter (latency to enter 221 ± 28 and 204 ± 25 s for WT and Nxf7 KO, respectively), indicating that 24 h after conditioning both groups learned to avoid the dark compartment.
Fear conditioning to ambiguous cues indicated that initial fear acquisition was similar in both genotypes (Figure 4B), but that long-term memory accuracy to distinguish between PER and AMB after 48 h (Figure 4C) and 14 days (Figure 4D) was impaired in Nxf7 KO mice. Two way ANOVA with factors genotype and phase (BSL, AMB, ISI, PER) for memory retention at 48 h revealed a significant effect of genotype (F1,151 = 6.706, P < 0.02), and phase (F3,151 = 63.43, P < 0.001). Post hoc analysis confirmed that 48 h after conditioning with two cues, WT animals displayed significantly more freezing to the perfect cue (PER) than the ambiguous cue (AMB; t = 2.41, P < 0.02), indicating that they distinguished between PER and AMB during conditioning. In contrast, Nxf7 KO mice showed robust cue-induced freezing but showed generalization to both cues (t = 1.3; Figure 4C). The robustness of fear memory was demonstrated by measuring fear-potentiated startle to PER or AMB 14 days after conditioning. WT showed a robust startle response to PER, not to AMB, while Nxf7 KO mice showed no differential response to the two cues (Figure 4D). Two-way ANOVA indicated a significant effect for factor CS (F1,55 = 4.13, P < 0.05), genotype (F3,55 = 4.55, P < 0.05), without significant interaction. Pairwise comparisons indicated that WT mice had a significantly higher startle response during PER than Nxf7 KO mice (t = 2.39, P < 0.05), but not during AMB (P > 0.5), and the difference between PER and AMB was significant in the WT group (t = 2.17, P < 0.05), but not in the Nxf7 KO group (P > 0.5). This effect was not due to differences in startle reactivity to the startle stimuli alone (19.9 ± 4.3 and 20.8 ± 3.8 arbitrary units, P > 0.5, for WT and Nxf7 KO mice, respectively).
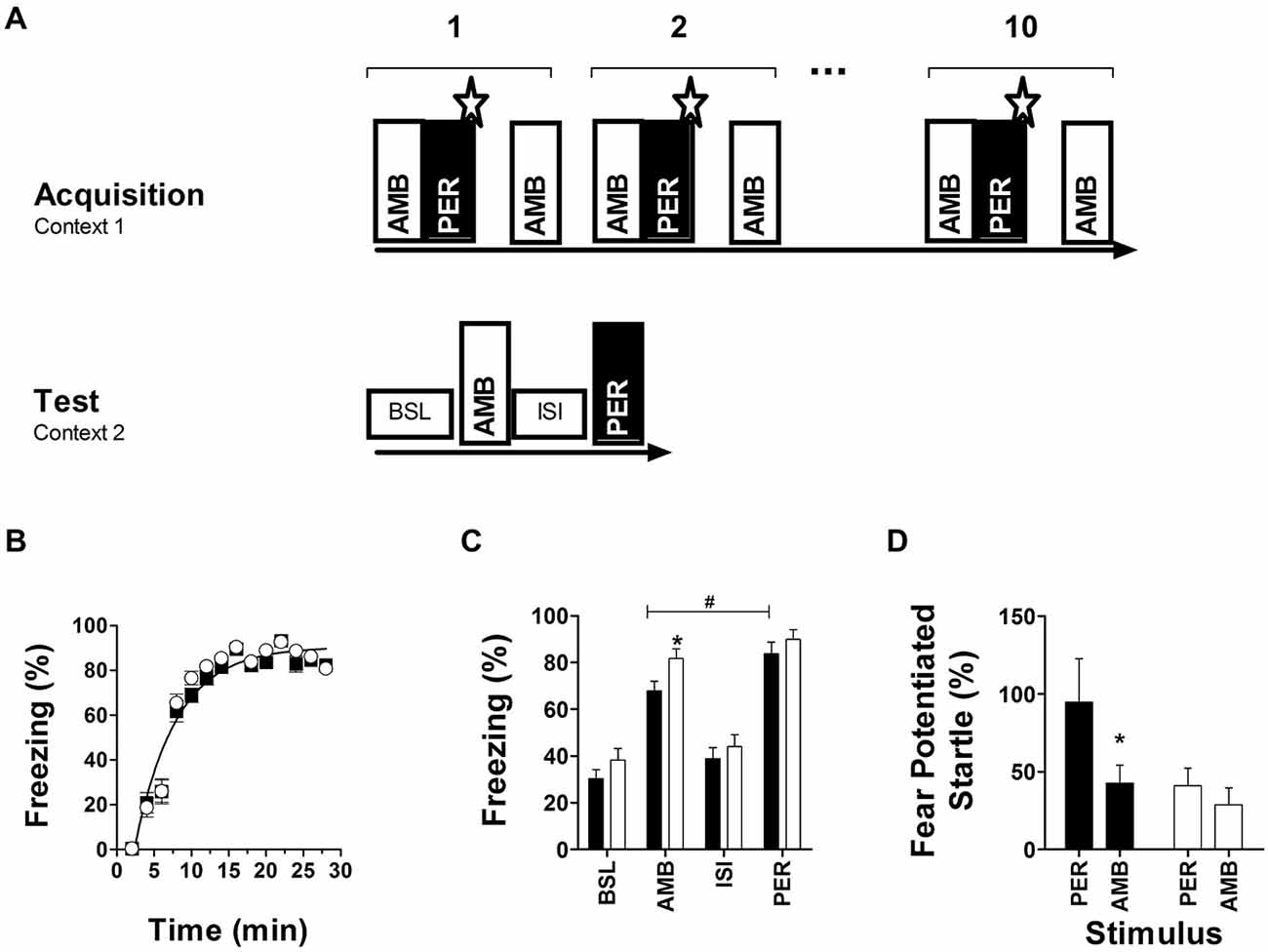
Figure 4. Fear conditioning to ambiguous auditory cues. Two auditory cues were presented during acquisition, and tested 48 h later (A). During conditioning, both genotypes developed robust freezing responses to negatively reinforced cues (WT, black symbols, n = 18; Nxf7 KO, white symbols, n = 20) (B). Forty-eight hours later memory strength was tested using freezing as a readout during baseline (novel context, no cues), and presentation of either ambiguous (AMB) or perfect (PER) cue. Presentation of the cue induced robust freezing in both genotypes, but Nxf7 KO mice were generalizing between PER and AMB. (*p < 0.05 Nxf7 KO vs. WT, #p < 0.05 AMB vs. PER) (C). Fourteen days later presentation of PER induced a significant increase in startle response compared to AMB in WT mice. In contrast, Nxf7 KO displayed a non-differentiating startle response to either cue (D) (*p < 0.05, AMB vs. PER). Data are presented as means ± SEM.
During appetitive conditioning, both genotypes increased nose poking in response to increasing reinforcement schedules, reaching a stable response rate of ~500 nosepokes /30 min (Figure 5A). During superimposed fear acquisition trials, both genotypes show a rapid and stable response suppression (Figure 5B), indicating no difference in fear learning between the two genotypes. Similarly, no difference between genotypes was observed during extinction learning (Figure 5C).
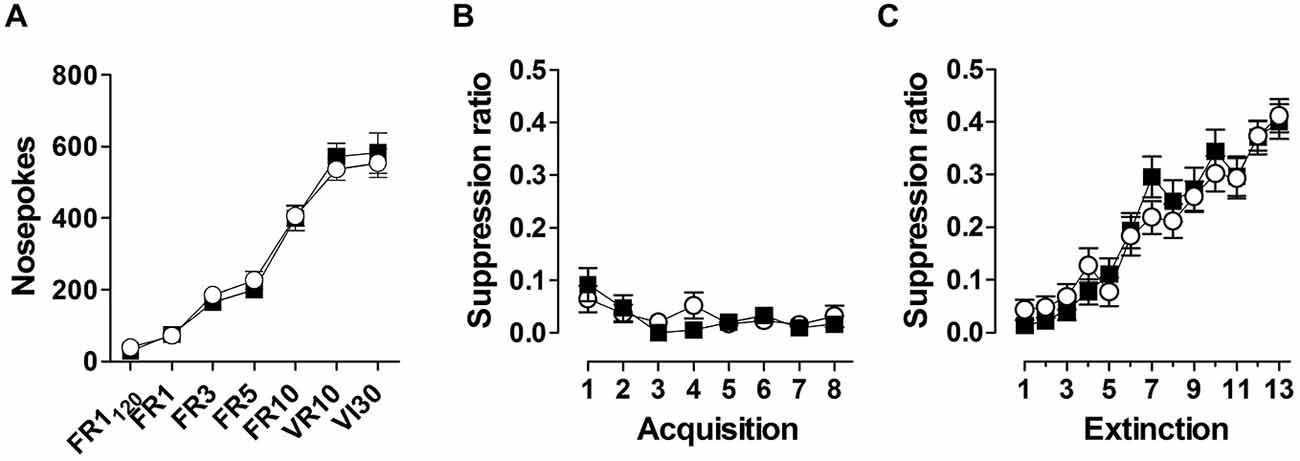
Figure 5. Animals were trained under increasing reinforcement schedules to obtain food pellets through nose poking (A). Reinforced schedules included fixed ratio (FR), variable ratio (VR) and variable interval (VI). Superimposed fear conditioning suppressed instrumental responding robustly (B), which recovered during subsequent extinction trials (C). Data are expressed as means ± SEM. No difference in performance between WT (black symbols) and Nxf7 KO (white symbols) were observed.
Long-Term Potentiation but not Long-Term Depression is Impaired in Nxf7 KO Mice
Synaptic plasticity in the form of LTP and LTD are considered cellular correlates of memory processes. While basal excitatory synaptic transmission (input/output curves, Figure 6A) was similar in WT and Nxf7 KO mice, paired-pulse facilitation, a form of presynaptic short-term plasticity, was reduced in Nxf7 KO mice at an interpulse interval of 50 ms (t36 = 2.711, P < 0.02; Student’s t-test, Figure 6B). When LTP in area-CA1 was examined, potentiation was readily evoked in Nxf7 KO and WT mice (1 min after TBS: WT 163 ± 17%, n = 6; KO 166 ± 8%, n = 8, Figure 6C). LTP was maintained for at least 2 h in WT (120 min: 123 ± 15%, n = 6), but decayed rapidly in Nxf7 KO, becoming indistinguishable from baseline already 25 min after tetanization (Wilcoxon matched-pairs signed-rank test). Statistical comparison by RM-ANOVA identified a significant group difference after tetanization (factor genotype F1,363 = 6.38, p < 0.05) and pairwise multiple comparisons (Holm-Sidak) revealed significant differences between Nxf7 KO and WT mice from 45 min onwards (Figure 6C). To exclude differences in NMDAR-independent PTP, we repeated the same tetanization protocol in the presence of 50 μM D-AP5. In both genotypes, we observed PTP of a similar magnitude as the potentiation without D-AP5 (WT, 159 ± 33%, n = 3; KO 166 ± 32%, n = 3, data not shown). Low frequency stimulation (LFS) of 900 pulses at 1 Hz resulted in similar stable LTD in both genotypes (1 min after LFS: WT 72 ± 5%, n = 8; KO 75 ± 4%, n = 12, Figure 6D). To summarize, we observed impaired hippocampal LTP, but intact LTD in Nxf7 KO, which may be related to their cognitive defects.
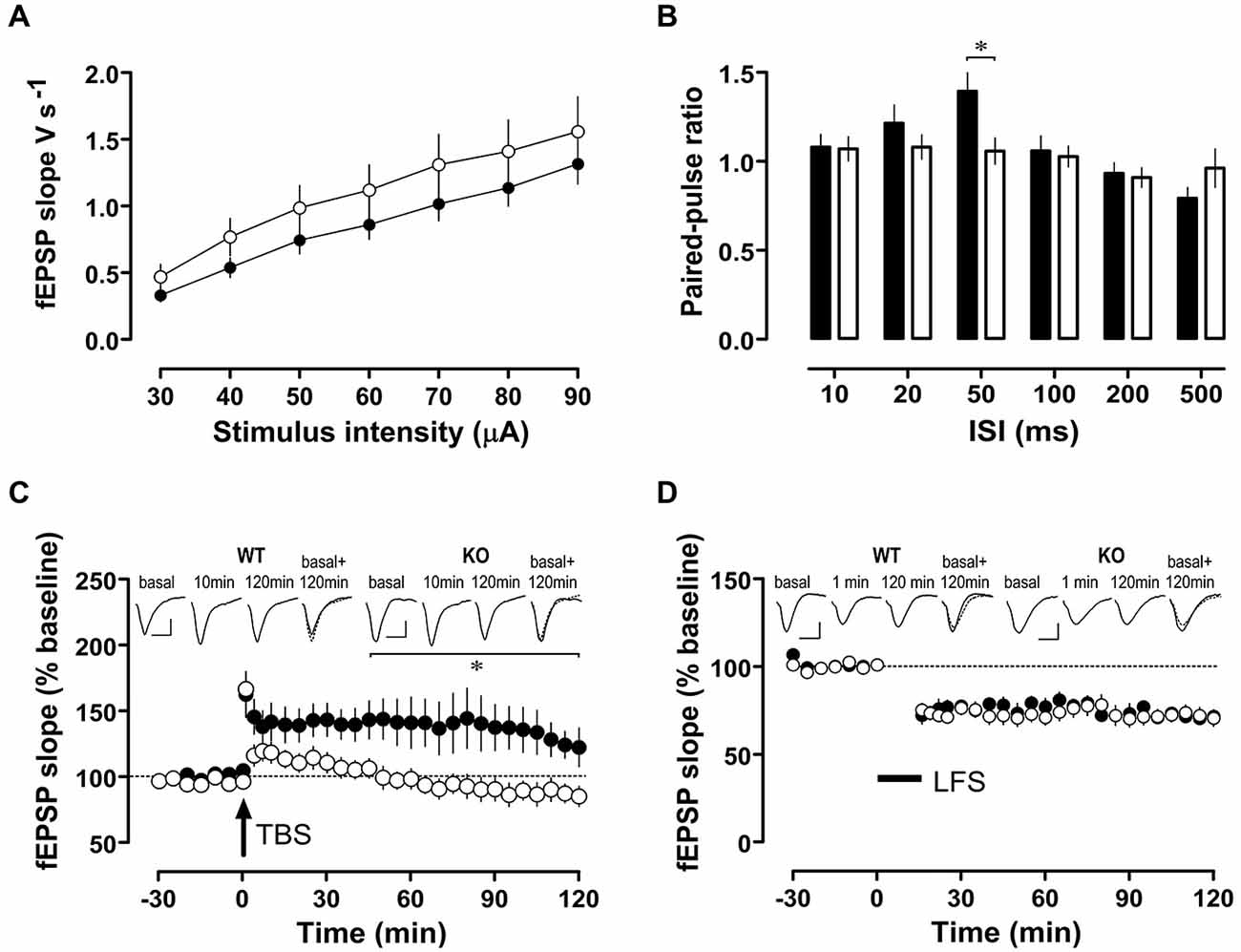
Figure 6. Synaptic plasticity in area-CA1 of hippocampus from Nxf7 KO (open symbols) and WT littermates (filled symbols). (A) Fast synaptic transmission in Nxf7 KO animals (n = 15) was similar to WT (n = 17) animals. (B) Paired-pulse facilitation was reduced in Nxf7 KO (n = 21) mice at 50 ms ISI compared to WT (n = 17; *P < 0.05, Students t-test). (C,D) Synaptic plasticity in area-CA1 of hippocampus from Nxf7 KO and WT littermates. (C) Long term potentiation induced by theta burst stimulation (TBS) was impaired in Nxf7 KO mice (n = 8) compared to WT animals (n = 6). Insets show representative analog traces collected during baseline recording, 10 min after tetanization (to exclude any confounding influence by post-tetanic potentiation) and 120 after tetanization. Traces on the right side display overlaid traces from baseline recording (full line) and 120 min after tetanization (broken line). Calibration bars indicate 0.5 mV and 10 ms, respectively. (D) LTD induced by low-frequency stimulation of 900 pulses at 1 Hz was similar in Nxf7 KO animals (n = 12) and in WT (n = 8). *p < 0.05, RM-ANOVA. See (C) for explanation of insets. Note that for LTD a 1-min-value is shown.
Discussion
In the present study, we modeled the functional consequences of NXF5 deficiency (Jun et al., 2001; Frints et al., 2003; Grillo et al., 2010), using mice deficient for Nxf7, the likely murine homolog of human NXF5 (Vanmarsenille et al., 2013). We examined the effect of genetic deletion of the nuclear export factor Nfx7 on a variety of behavioral tests and related the observed phenotype to changes in hippocampal synaptic transmission. Nxf7 KO mice were impaired in employing spatial strategies during acquisition and reference memory tasks in the water maze. We also observed altered social behavior in these mice, whereas motor performance and exploration were similar to WT mice. These behavioral changes coincided with reduced hippocampal LTP, whereas LTD remained unaffected.
Relating phenotypes of patients with loss-of-function mutations to those of disease gene-specific KO mice is often difficult, but Nxf7 KO mice did present social behavior and learning and long-term memory retention impairments. These deficits are reminiscent of the limited social capabilities and the intellectual disability observed in patients. We previously showed that Nxf7 male KO mice are healthy, fertile and do not show any obvious constitutional or progressive abnormalities. Furthermore, histological examination of KO brains did not show any alterations (Vanmarsenille et al., 2013). Nxf7 KO and WT littermates were compared in different functional assays using an extended battery of behavioral tests and synaptic plasticity recordings. No differences were observed in rotarod or grip strength assays indicating that (neuro)motor functions are largely unaffected. Furthermore, Nxf7 KO were undistinguishable from WT littermates in open field exploration and elevated plus maze, two tests that measure anxiety-related behavior and conflict resolution (Bailey and Crawley, 2009). However, Nxf7 KO mice showed behavioral alterations in the social exploration protocol. The changes we observed in Nxf7 mice could be related to loss in social inhibition or to increased sexually motivated approach. Notwithstanding obvious differences in social repertoire between mice and humans, patients with non-functional NXF5 also displayed a wide spectrum of personality disorders, including changes in social interaction (Jun et al., 2001; Frints et al., 2003; Grillo et al., 2010). Changes in social behavior have also been seen in other murine models of human disorder with altered social functions. For example, Pagani et al showed that activation of vasopressin 1b receptors (Avp1b) in hippocampal CA2 region affected social inhibition without changing anxiety-related behaviors (Pagani et al., 2015), whereas polymorphisms in patients were linked to disinhibition (i.e., ADHD-like behavior observed by Zai et al. (2012) and autism spectrum symptoms by Chakrabarti et al. (2009).
In addition to social behavior, we also tested Nxf7 KO mice in a variety of hippocampus-dependent avoidance and spatial learning tests, as a possible correlate of the cognitive difficulties observed in NXF5 patients. We observed no influence of Nxf7 deficiency on single-trial passive avoidance learning, a test that relies on simple contextual association (Bartus et al., 1980; Gower and Lamberty, 1993; Webster et al., 2014). Similar to WT littermates, NXf7 mice were able to increase performance in an appetitive conditioning shaping procedure. However, we observed defects in spatial learning and memory performance in the Morris water maze, which has become a prototypical test of hippocampus-dependent learning and memory (Brandeis et al., 1989; D’Hooge and De Deyn, 2001). In particular, the classification of search strategies in cognitive and non-cognitive approaches indicated a preference for non-spatial approaches in Nxf7-deficient mice. Finally, while we observed no difference in fear conditioning, Nxf7 KO mice were generalizing between two presented cues, a process shown to rely quite selectively on the hippocampus (Tsetsenis et al., 2007; Enkel et al., 2010). The observed defects therefore suggest selective hippocampal dysfunction in Nxf7-deficient mice.
Given these behavioral indications of hippocampal dysfunction in Nxf7-deficient mice, we investigated hippocampal electrophysiology on brain slices of Nxf7 KO mice. The electrophysiological recordings revealed changes specifically related to the processes of synaptic plasticity, because basal synaptic transmission was unaltered. Reduced paired-pulse facilitation in Nxf7 KO mice is furthermore indicative of defects in pre-synaptic processes of short-term plasticity (Zucker and Regehr, 2002). The impairment in hippocampal LTP contrasts with the induction of LTD, which was robust and similar to WT. The role of other NXF-family members in synaptic plasticity (LTP and LTD) has not been investigated, but important functions in synaptic plasticity have been reported for RNA binding/transport proteins such as Staufen and the FMR family (Kao et al., 2010). Down-regulation of Stau1 impaired chemically induced LTP in the CA1 region of organotypic cultures, whereas electrically induced LTP, and LTD induced by group I mGluR activation, were unaffected (Lebeau et al., 2008). Transcriptional silencing of the FMR1 gene and the concomitant loss of fragile-X mental retardation protein (FMRP) has been shown to have detrimental consequences causing fragile-X syndrome. Fmr1 KO mice have been characterized extensively, but show quite a different behavioral phenotype from the presently examined Nxf7 KO mice (Kooy, 2003). In Fmr1 KO animals, spatial learning and memory were normal, whereas reversal learning was impaired in different tasks. Such mice have unchanged LTP in hippocampal CA1 (Godfraind et al., 1996; Zhang et al., 2009), but group I mGluR-dependent LTD was enhanced (Huber et al., 2002; Zhang et al., 2009). Notably, mice lacking Fmr2, a gene associated with mild cognitive impairment in humans, did show an increase in NMDA receptor-dependent and -independent types of CA1-LTP (Gu et al., 2002). Similarly, downregulation of Cyfip1, a direct FMRP interacting protein, had no effect on hippocampal LTP, but increased DHPG-induced LTD (Bozdagi et al., 2012).
We propose that the different behavioral and physiological phenotypes observed upon disruption of Nxf7 and Fmrp could be due to Nxf7 and Fmrp representing distinct subsets of RBPs that regulate different sets of cellular mRNAs. Supportive evidence for this hypothesis comes from a number of different studies. Given the exclusive localization of Nxf7 in cytoplasmic granules (Tan et al., 2005), later defined as translating ribosomes, stress granules and P-bodies (Katahira et al., 2008), and its association with hnRNP A3 and Staufen1, Nxf7 was suggested to play a role in cytoplasmic mRNA transport, translation, degradation and/or storage (Tretyakova et al., 2005; Katahira et al., 2008). Another NXF family member, Nxf2, also controls mRNA dynamics (Takano et al., 2007), but interacts with the neuronal mRNA regulating protein Frmp (Lai et al., 2006; Zhang et al., 2007). Even though Fmrp and Stau1 have been demonstrated in the same RNP granules in Drosophila (Barbee et al., 2006; Bolduc et al., 2008) and mouse (Ferrari et al., 2007), they have been observed in separate particles as well (Barbee et al., 2006). Both proteins have been shown to interact with a shared set of RNA transcripts including Fmr1 and αCamKII (Ferrari et al., 2007). Still, 85% of Fmrp-interacting cellular mRNAs were not detected in Stau1-associated transcripts (Furic et al., 2008). Furthermore, Stau1 KO mice show impaired LTP comparable to our Nxf7 deficient mice, whereas behavioral and electrophysiological recordings of the Fmr1 KO mice were very different. Finally two other RBP models did not display cognitive differences in standard hippocampus dependent tests (Stein et al., 2006; Vessey et al., 2008), it would be interesting to expose these two models to complex tasks as described in our paper and determine if similar cognitive deficits could be observed.
In conclusion, we presently describe altered social behavior, impaired spatial learning and memory, and defective hippocampal LTP in Nxf7-deficient mice, which relate to behavioral and cognitive defects observed in NXF5-deficient humans. Nxf7-deficient mice appear to be a valid model of human NXF5 deficiency. Our data indeed support the hypothesis that mouse Nxf7 is the functional homolog of NXF5, and open the door for detailed molecular and behavioral analyses of the mechanistic basis of Nxf7/NXF5-dependent cognitive dysfunction.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants of the Flemish Fund for Scientific Research (FWO) to ZC-V, DB, GF and RD (G.0886.11, G.0746.13 and G.0587.14) and a GOA/12/008 project from the KU Leuven research fund. BV was supported by a postdoctoral fellowship from the Research Council Leuven and is currently a postdoctoral fellow of FWO-Flanders.
References
Bachi, A., Braun, I. C., Rodrigues, J. P., Panté, N., Ribbeck, K., von Kobbe, C., et al. (2000). The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6, 136–158. doi: 10.1017/s1355838200991994
Bailey, K. R., and Crawley, J. N. (2009). “Anxiety-related behaviors in mice,” in Methods of Behavior Analysis in Neuroscience, ed. J. J. Buccafusco (Boca Raton, FL: CRC Press), 77–101.
Balschun, D., Moechars, D., Callaerts-Vegh, Z., Vermaercke, B., Van Acker, N., Andries, L., et al. (2010). Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cereb. Cortex 20, 684–693. doi: 10.1093/cercor/bhp133
Barbee, S. A., Estes, P. S., Cziko, A. M., Hillebrand, J., Luedeman, R. A., Coller, J. M., et al. (2006). Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron 52, 997–1009. doi: 10.1016/j.neuron.2006.10.028
Bartus, R. T., Dean, R. L., Goas, J. A., and Lippa, A. S. (1980). Age-related changes in passive avoidance retention: modulation with dietary choline. Science 209, 301–303. doi: 10.1126/science.7384805
Bolduc, F. V., Bell, K., Cox, H., Broadie, K. S., and Tully, T. (2008). Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat. Neurosci. 11, 1143–1145. doi: 10.1038/nn.2175
Bozdagi, O., Sakurai, T., Dorr, N., Pilorge, M., Takahashi, N., and Buxbaum, J. D. (2012). Haploinsufficiency of Cyfip1 produces fragile X-like phenotypes in mice. PLoS One 7:e42422. doi: 10.1371/journal.pone.0042422
Brandeis, R., Brandys, Y., and Yehuda, S. (1989). The use of the morris water maze in the study of memory and learning. Int. J. Neurosci. 48, 29–69. doi: 10.3109/00207458909002151
Braun, I. C., Rohrbach, E., Schmitt, C., and Izaurralde, E. (1999). TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J. 18, 1953–1965. doi: 10.1093/emboj/18.7.1953
Brody, D. L., and Holtzman, D. M. (2006). Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp. Neurol. 197, 330–340. doi: 10.1016/j.expneurol.2005.10.020
Callaerts-Vegh, Z., Beckers, T., Ball, S. M., Baeyens, F., Callaerts, P. F., Cryan, J. F., et al. (2006). Concomitant deficits in working memory and fear extinction are functionally dissociated from reduced anxiety in metabotropic glutamate receptor 7-deficient mice. J. Neurosci. 26, 6573–6582. doi: 10.1523/jneurosci.1497-06.2006
Callaerts-Vegh, Z., Leo, S., Vermaercke, B., Meert, T., and D’Hooge, R. (2012). LPA(5) receptor plays a role in pain sensitivity, emotional exploration and reversal learning. Genes Brain Behav. 11, 1009–1019. doi: 10.1111/j.1601-183x.2012.00840.x
Chakrabarti, B., Dudbridge, F., Kent, L., Wheelwright, S., Hill-Cawthorne, G., Allison, C., et al. (2009). Genes related to sex steroids, neural growth and social-emotional behavior are associated with autistic traits, empathy and Asperger syndrome. Autism Res. 2, 157–177. doi: 10.1002/aur.80
Denayer, E., Ahmed, T., Brems, H., Van Woerden, G., Borgesius, N. Z., Callaerts-Vegh, Z., et al. (2008). Spred1 is required for synaptic plasticity and hippocampus-dependent learning. J. Neurosci. 28, 14443–14449. doi: 10.1523/JNEUROSCI.4698-08.2008
D’Hooge, R., and De Deyn, P. P. (2001). Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 36, 60–90. doi: 10.1016/s0165-0173(01)00067-4
D’Hooge, R., Lüllmann-Rauch, R., Beckers, T., Balschun, D., Schwake, M., Reiss, K., et al. (2005). Neurocognitive and psychotiform behavioral alterations and enhanced hippocampal long-term potentiation in transgenic mice displaying neuropathological features of human alpha-mannosidosis. J. Neurosci. 25, 6539–6549. doi: 10.1523/jneurosci.0283-05.2005
Enkel, T., Gholizadeh, D., von Bohlen Und Halbach, O., Sanchis-Segura, C., Hurlemann, R., Spanagel, R., et al. (2010). Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology 35, 1008–1015. doi: 10.1038/npp.2009.204
Ferrari, F., Mercaldo, V., Piccoli, G., Sala, C., Cannata, S., Achsel, T., et al. (2007). The fragile X mental retardation protein-RNP granules show an mGluR-dependent localization in the post-synaptic spines. Mol. Cell. Neurosci. 34, 343–354. doi: 10.1016/j.mcn.2006.11.015
Frints, S. G., Jun, L., Fryns, J. P., Devriendt, K., Teulingkx, R., Van den Berghe, L., et al. (2003). Inv(X)(p21.1;q22.1) in a man with mental retardation, short stature, general muscle wasting and facial dysmorphism: clinical study and mutation analysis of the NXF5 gene. Am. J. Med. Genet. A 119, 367–374. doi: 10.1002/ajmg.a.20195
Froyen, G., Van Esch, H., Bauters, M., Hollanders, K., Frints, S. G., Vermeesch, J. R., et al. (2007). Detection of genomic copy number changes in patients with idiopathic mental retardation by high-resolution X-array-CGH: important role for increased gene dosage of XLMR genes. Hum. Mutat. 28, 1034–1042. doi: 10.1002/humu.20564
Furic, L., Maher-Laporte, M., and DesGroseillers, L. (2008). A genome-wide approach identifies distinct but overlapping subsets of cellular mRNAs associated with Staufen1- and Staufen2-containing ribonucleoprotein complexes. RNA 14, 324–335. doi: 10.1261/rna.720308
Godfraind, J. M., Reyniers, E., De Boulle, K., D’Hooge, R., De Deyn, P. P., Bakker, C. E., et al. (1996). Long-term potentiation in the hippocampus of fragile X knockout mice. Am. J. Med. Genet. 64, 246–251. doi: 10.1002/(sici)1096-8628(19960809)64:2<246::aid-ajmg2>3.3.co;2-h
Gower, A. J., and Lamberty, Y. (1993). The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice. Behav. Brain Res. 57, 163–173. doi: 10.1016/0166-4328(93)90132-a
Grillo, L., Reitano, S., Belfiore, G., Spalletta, A., Amata, S., Bottitta, M., et al. (2010). Familial 1.1 Mb deletion in chromosome Xq22.1 associated with mental retardation and behavioural disorders in female patients. Eur. J. Med. Genet. 53, 113–116. doi: 10.1016/j.ejmg.2010.01.001
Gu, Y., McIlwain, K. L., Weeber, E. J., Yamagata, T., Xu, B., Antalffy, B. A., et al. (2002). Impaired conditioned fear and enhanced long-term potentiation in Fmr2 knock-out mice. J. Neurosci. 22, 2753–2763.
Herold, A., Suyama, M., Rodrigues, J. P., Braun, I. C., Kutay, U., Carmo-Fonseca, M., et al. (2000). TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture. Mol. Cell. Biol. 20, 8996–9008. doi: 10.1128/mcb.20.23.8996-9008.2000
Huber, K. M., Gallagher, S. M., Warren, S. T., and Bear, M. F. (2002). Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. U S A 99, 7746–7750. doi: 10.1073/pnas.122205699
Izaurralde, E. (2002a). A novel family of nuclear transport receptors mediates the export of messenger RNA to the cytoplasm. Eur. J. Cell Biol. 81, 577–584. doi: 10.1078/0171-9335-00273
Izaurralde, E. (2002b). Nuclear export of messenger RNA. Results Probl. Cell Differ. 35, 133–150. doi: 10.1007/978-3-540-44603-3_7
Izaurralde, E. (2004). Directing mRNA export. Nat. Struct. Mol. Biol. 11, 210–212. doi: 10.1038/nsmb0304-210
Jun, L., Frints, S., Duhamel, H., Herold, A., Abad-Rodrigues, J., Dotti, C., et al. (2001). NXF5, a novel member of the nuclear RNA export factor family, is lost in a male patient with a syndromic form of mental retardation. Curr. Biol. 11, 1381–1391. doi: 10.1016/s0960-9822(01)00419-5
Kang, Y., Bogerd, H. P., Yang, J., and Cullen, B. R. (1999). Analysis of the RNA binding specificity of the human tap protein, a constitutive transport element-specific nuclear RNA export factor. Virology 262, 200–209. doi: 10.1006/viro.1999.9906
Kao, D. I., Aldridge, G. M., Weiler, I. J., and Greenough, W. T. (2010). Altered mRNA transport, docking and protein translation in neurons lacking fragile X mental retardation protein. Proc. Natl. Acad. Sci. U S A 107, 15601–15606. doi: 10.1073/pnas.1010564107
Katahira, J., Miki, T., Takano, K., Maruhashi, M., Uchikawa, M., Tachibana, T., et al. (2008). Nuclear RNA export factor 7 is localized in processing bodies and neuronal RNA granules through interactions with shuttling hnRNPs. Nucleic Acids Res. 36, 616–628. doi: 10.1093/nar/gkm556
Kooy, R. F. (2003). Of mice and the fragile X syndrome. Trends Genet. 19, 148–154. doi: 10.1016/s0168-9525(03)00017-9
Lai, D., Sakkas, D., and Huang, Y. (2006). The fragile X mental retardation protein interacts with a distinct mRNA nuclear export factor NXF2. RNA 12, 1446–1449. doi: 10.1261/rna.94306
Lebeau, G., Maher-Laporte, M., Topolnik, L., Laurent, C. E., Sossin, W., Desgroseillers, L., et al. (2008). Staufen1 regulation of protein synthesis-dependent long-term potentiation and synaptic function in hippocampal pyramidal cells. Mol. Cell. Biol. 28, 2896–2907. doi: 10.1128/mcb.01844-07
Lo, A. C., Callaerts-Vegh, Z., Nunes, A. F., Rodrigues, C. M., and D’Hooge, R. (2013a). Tauroursodeoxycholic acid (TUDCA) supplementation prevents cognitive impairment and amyloid deposition in APP/PS1 mice. Neurobiol. Dis. 50, 21–29. doi: 10.1016/j.nbd.2012.09.003
Lo, A. C., De Maeyer, J. H., Vermaercke, B., Callaerts-Vegh, Z., Schuurkes, J. A., and D’Hooge, R. (2014). SSP-002392, a new 5-HT receptor agonist, dose-dependently reverses scopolamine-induced learning and memory impairments in C57Bl/6 mice. Neuropharmacology 85, 178–189. doi: 10.1016/j.neuropharm.2014.05.013
Lo, A. C., Tesseur, I., Scopes, D. I., Nerou, E., Callaerts-Vegh, Z., Vermaercke, B., et al. (2013b). Dose-dependent improvements in learning and memory deficits in APPPS1–21 transgenic mice treated with the orally active Abeta toxicity inhibitor SEN1500. Neuropharmacology 75, 458–466. doi: 10.1016/j.neuropharm.2013.08.030
Lukong, K. E., Chang, K. W., Khandjian, E. W., and Richard, S. (2008). RNA-binding proteins in human genetic disease. Trends Genet. 24, 416–425. doi: 10.1016/j.tig.2008.05.004
Pagani, J. H., Zhao, M., Cui, Z., Williams Avram, S. K., Caruana, D. A., Dudek, S. M., et al. (2015). Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatry 20, 490–499. doi: 10.1038/mp.2014.47
Stein, J. M., Bergman, W., Fang, Y., Davison, L., Brensinger, C., Robinson, M. B., et al. (2006). Behavioral and neurochemical alterations in mice lacking the RNA-binding protein translin. J. Neurosci. 26, 2184–2196. doi: 10.1523/jneurosci.4437-05.2006
Takano, K., Miki, T., Katahira, J., and Yoneda, Y. (2007). NXF2 is involved in cytoplasmic mRNA dynamics through interactions with motor proteins. Nucleic Acids Res. 35, 2513–2521. doi: 10.1093/nar/gkm125
Tan, W., Zolotukhin, A. S., Tretyakova, I., Bear, J., Lindtner, S., Smulevitch, S. V., et al. (2005). Identification and characterization of the mouse nuclear export factor (Nxf) family members. Nucleic Acids Res. 33, 3855–3865. doi: 10.1093/nar/gki706
Thomas, M. G., Pascual, M. L., Maschi, D., Luchelli, L., and Boccaccio, G. L. (2014). Synaptic control of local translation: the plot thickens with new characters. Cell. Mol. Life Sci. 71, 2219–2239. doi: 10.1007/s00018-013-1506-y
Tolino, M., Köhrmann, M., and Kiebler, M. A. (2012). RNA-binding proteins involved in RNA localization and their implications in neuronal diseases. Eur. J. Neurosci. 35, 1818–1836. doi: 10.1111/j.1460-9568.2012.08160.x
Tretyakova, I., Zolotukhin, A. S., Tan, W., Bear, J., Propst, F., Ruthel, G., et al. (2005). Nuclear export factor family protein participates in cytoplasmic mRNA trafficking. J. Biol. Chem. 280, 31981–31990. doi: 10.1074/jbc.m502736200
Tsetsenis, T., Ma, X. H., Lo Iacono, L., Beck, S. G., and Gross, C. (2007). Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat. Neurosci. 10, 896–902. doi: 10.1038/nn1919
Vanmarsenille, L., Verbeeck, J., Belet, S., Roebroek, A. J., Van de Putte, T., Nevelsteen, J., et al. (2013). Generation and characterization of an Nxf7 knockout mouse to study nxf5 deficiency in a patient with intellectual disability. PLoS One 8:e64144. doi: 10.1371/journal.pone.0064144
Vessey, J. P., Macchi, P., Stein, J. M., Mikl, M., Hawker, K. N., Vogelsang, P., et al. (2008). A loss of function allele for murine Staufen1 leads to impairment of dendritic Staufen1-RNP delivery and dendritic spine morphogenesis. Proc. Natl. Acad. Sci. U S A 105, 16374–16379. doi: 10.1073/pnas.0804583105
Webster, S. J., Bachstetter, A. D., Nelson, P. T., Schmitt, F. A., and Van Eldik, L. J. (2014). Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet. 5:88. doi: 10.3389/fgene.2014.00088
Yang, J., Bogerd, H. P., Wang, P. J., Page, D. C., and Cullen, B. R. (2001). Two closely related human nuclear export factors utilize entirely distinct export pathways. Mol. Cell 8, 397–406. doi: 10.1016/s1097-2765(01)00303-3
Zai, C. C., Muir, K. E., Nowrouzi, B., Shaikh, S. A., Choi, E., Berall, L., et al. (2012). Possible genetic association between vasopressin receptor 1B and child aggression. Psychiatry Res. 200, 784–788. doi: 10.1016/j.psychres.2012.07.031
Zhang, J., Hou, L., Klann, E., and Nelson, D. L. (2009). Altered hippocampal synaptic plasticity in the FMR1 gene family knockout mouse models. J. Neurophysiol. 101, 2572–2580. doi: 10.1152/jn.90558.2008
Zhang, M., Wang, Q., and Huang, Y. (2007). Fragile X mental retardation protein FMRP and the RNA export factor NXF2 associate with and destabilize Nxf1 mRNA in neuronal cells. Proc. Natl. Acad. Sci. U S A 104, 10057–10062. doi: 10.1073/pnas.0700169104
Keywords: hippocampal synaptic plasticity, nuclear RNA export factor, mouse model, social exploration, spatial learning and memory
Citation: Callaerts-Vegh Z, Ahmed T, Vermaercke B, Marynen P, Balschun D, Froyen G and D’Hooge R (2015) Nxf7 deficiency impairs social exploration and spatio-cognitive abilities as well as hippocampal synaptic plasticity in mice. Front. Behav. Neurosci. 9:179. doi: 10.3389/fnbeh.2015.00179
Received: 11 March 2015; Accepted: 25 June 2015;
Published: 10 July 2015.
Edited by:
Denise Manahan-Vaughan, Ruhr University Bochum, GermanyReviewed by:
Ayla Aksoy-Aksel, Philipps-Universität Marburg, GermanySajikumar Sreedharan, National University of Singapore, Singapore
Copyright © 2015 Callaerts-Vegh, Ahmed, Vermaercke, Marynen, Balschun, Froyen and D’Hooge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zsuzsanna Callaerts-Vegh, Laboratory of Biological Psychology, University of Leuven, KU Leuven, Tiensestraat 102, B-3000 Leuven, VB, Belgium, zsuzsanna.vegh@ppw.kuleuven.be
 Zsuzsanna Callaerts-Vegh
Zsuzsanna Callaerts-Vegh Tariq Ahmed
Tariq Ahmed Ben Vermaercke
Ben Vermaercke Peter Marynen
Peter Marynen Detlef Balschun
Detlef Balschun Guy Froyen
Guy Froyen Rudi D’Hooge
Rudi D’Hooge