2D SnS2 Nanostructure-Derived Photocatalytic Degradation of Organic Pollutants Under Visible Light
- 1Department of Physics, Institute of Science, Banaras Hindu University, Varanasi, India
- 2Center of Materials Science, University of Allahabad, Prayagraj, India
- 3Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi, India
- 4Department of Physics, Indian Institute of Science, Bangalore, India
Wastewater produced by the textile industry contains various dyes and organic compounds that directly or indirectly affect surface water or groundwater pollution. Visible-light-driven semiconductor photocatalysis is the leading pathway for the degradation of environmental pollutants. Herein we report the bottom-up hydrothermal growth of 2D tin disulfide nanostructures (SnS2 NSs) for the efficient photodegradation of organic pollutants such as Rhodamine B (Rh.B) and Methyl Violet (M.V) in an aqueous medium under visible light (λ > 400 nm) irradiation. The as-synthesized SnS2 NSs were characterized by various structural, morphological, and optical techniques such as XRD, RAMAN, TEM, UV–Vis, Brunauer–Emmett–Teller, etc. Furthermore, the low bandgap (∼1.6 eV), the high surface area (56 m2/g), and the anionic nature of SnS2 NSs attribute to it as an efficient photocatalyst for photocatalytic applications. The photocatalytic properties of SnS2 NSs showed good degradation efficiency of 94 and 99.6% for Rh. B and M.V, respectively, in 25 min. The kinetic rate constant of these dyes was estimated by using the Langmuir–Hinshelwood model. Here we also performed the recyclability test of the photocatalyst and discussed the plausible mechanism for the photocatalytic degradation of organic pollutants. The XPS spectra of SnS2 NSs were studied before and after the photodegradation of Rh.B and M.V, indicating the high stability of the photocatalyst. Moreover, in vitro cytotoxicity was also evaluated against human cervical cancer cell lines (HeLa cells) with different concentrations (0–1,000 μg/ml) of as-synthesized SnS2 NSs. This intended work provides a possible treatment for the degradation of organic pollutants under visible light to balance the aquatic ecosystems.
Introduction
The efficient disposal of environmental pollutants is one of the major problems, of which water pollution is the prime concern. Due to the rapid growth of industrial activity, chemical fertilizers and raw sewage have a direct or indirect impact on surface water or groundwater pollution (Schwarzenbach et al., 2010; Li et al., 2020a; Li et al., 2021). Commercially, over 100,000 dyes are available, and 700,000 tonnes are produced each year (Robinson et al., 2001). Among other pollutants, organic pollutants are becoming a major source of environmental contamination. Organic dyes are widely used in various industrial fields such as the leather industry, food, plastics, cosmetics, coloring agent in textile, and many more (Xie et al., 2000; Brillas and Martínez-Huitle, 2015). Approximately 10–20% of organic dyes that originate as wastewater from industries affect the balance of aquatic ecosystems. Organic dyes are toxic, mutagenic, carcinogenic, have disobedient molecules, and have low biodegradability (Xie et al.). To reduce the organic contaminants from wastewater, various methods have been adopted, such as ozonization, carbon adsorption, flocculation, and activated sludge process which is very tedious, unable to remove organic pollutants, requires expansive tools, and can also lead to secondary pollution (Patll and Shinde, 1988; Stock et al., 2000; Kim et al., 2016). However, to overcome these limitations, the photocatalytic degradation process is an effective and authentic technique that can be used to remove organic pollutants in the aqueous medium. In this process, the photocatalyst converts toxic organic dyes into nontoxic intermediates under ultraviolet or visible light irradiation (Zhong et al., 2009; Li et al., 2019).
In the last few years, semiconductor-based photocatalyst has been extensively used as an alternative to the degradation and decolorization of organic dyes. The semiconductor-based photocatalysts show tremendous properties such as high surface area, high charge transfer, active abundant site, a tunable optical band gap, and many more (Talebian and Nilforoushan, 2010; Opoku et al., 2017; Li et al., 2020b). In this context, various semiconductor-based photocatalysts such as SnO2, ZnO, TiO2, etc., have gained wide attention among the scientific community due to their excellent catalytic properties which require only the inexhaustible solar spectrum as the driving force for conducting the catalytic response (Cao et al., 2015; Sharma et al., 2017; Singh et al., 2017). However, the major disadvantage of these photocatalysts are high bandgap, less active sites, less low availability (3–5%) of UV light, and negotiated quantum efficiency (Štengl et al., 2013). Therefore, to overcome these limitations, the development of a novel photocatalyst with high absorption capacity and which can reduce bandgap is highly needed.
In the last few years, the semiconducting metal sulfide photocatalysts have attracted much attention among researchers due to their tremendous properties, such as light-absorbing ability in visible and near-field regions, high surface area, and abundant active surface sites (Guo et al., 2010; Xie et al., 2010; Zhang et al., 2011; Tian et al., 2017). Recently, in the IV–VI group semiconductors, tin disulfides (SnS2) have engrossed much consideration due to their strong anisotropic optical property in various potential applications, such as in electrical switching, solar cell, and catalytic reactions (Zhang et al., 2011a; Du et al., 2013; Song et al., 2013). SnS2 is an n-type semiconducting material with the general formula MX2 (where M stands for metal atom Sn, Mo, and W, and X stands for chalcogen atom S, Se, and Te) which belongs to the two-dimensional-layered metal dichalcogenides family. Structurally, SnS2 has a hexagonal CdI2-type crystal structure in which one layer of Sn atom has been sandwiched between two layers of S atom via in-plane covalent bonding and has weak van der Waals force between the adjacent layers. SnS2 has been widely used in various technological applications, such as sensors, energy storage, photodetectors, solar cell, and many more (Tan et al., 2011; Umar et al., 2013; Su et al., 2015; Srivastava et al., 2019). Moreover, tin disulfide is earth-abundant, cost-effective, has high carrier mobility, has a large surface active site, less toxic, and chemically stable in both acidic and neutral conditions, which make them a promising material for the degradation of organic pollutants (Li et al., 2012a; Umar et al., 2013; Lorenz et al., 2014). To date, various 2D SnS2-based nanostructures have been reported for photocatalytic application (Li et al., 2012b; Umar et al., 2013; Balu et al., 2018). However, all these methods have low degradation efficiency and were covered with inorganic materials or any organic surfactant molecules which restrict any interaction on their material surface and corresponds to a reduced catalytic activity. Therefore, it is of utmost importance to develop a novel photocatalyst which has abundant active sites, is biocompatible, has lower toxicity, and has a wider spectral response for the degradation of organic pollutants.
Therefore, in the present investigation, we report the synthesis of SnS2 NSs using a facile one-pot bottom-up ecofriendly hydrothermal method without a subsequent surface treatment. The as-synthesized SnS2 NSs show a low bandgap, a high surface area, and an anionic character. Furthermore, it has been observed that the degradation efficiency of organic dyes such as Rh.B and M.V is ∼94 and ∼99.6%, respectively, using SnS2 NSs as a photocatalyst under visible light in 25 min. The recyclability test and underneath detailed degradation mechanism were also discussed in the present work. Here we also estimated the in vitro cytotoxicity against human cervical cancer cell line (HeLa cells) with various concentrations of as-synthesized SnS2 NSs.
Experimental Techniques
Materials
Tin (IV) chloride pentahydrate (SnCl4·5H2O) and methyl violet were purchased from Molychem, India. Thioacetamide (C2H5NS) was purchased from OTTO Chemical, India. Rhodamine B and methyl violet were purchased from HIMEDIA, India. The human cervical cancer cell line (HeLa cells) was procured from the National Centre for Cell Sciences, Pune, India. The culture medium (RPMI-1640), fetal bovine serum, antibiotics, trypsin, and EDTA solutions were procured from Sigma Aldrich. The dimethyl sulphoxide (DMSO) was purchased from SRL Chemicals. Distilled water was used in the present work. All chemicals were used without any decontamination and were of analytical grade.
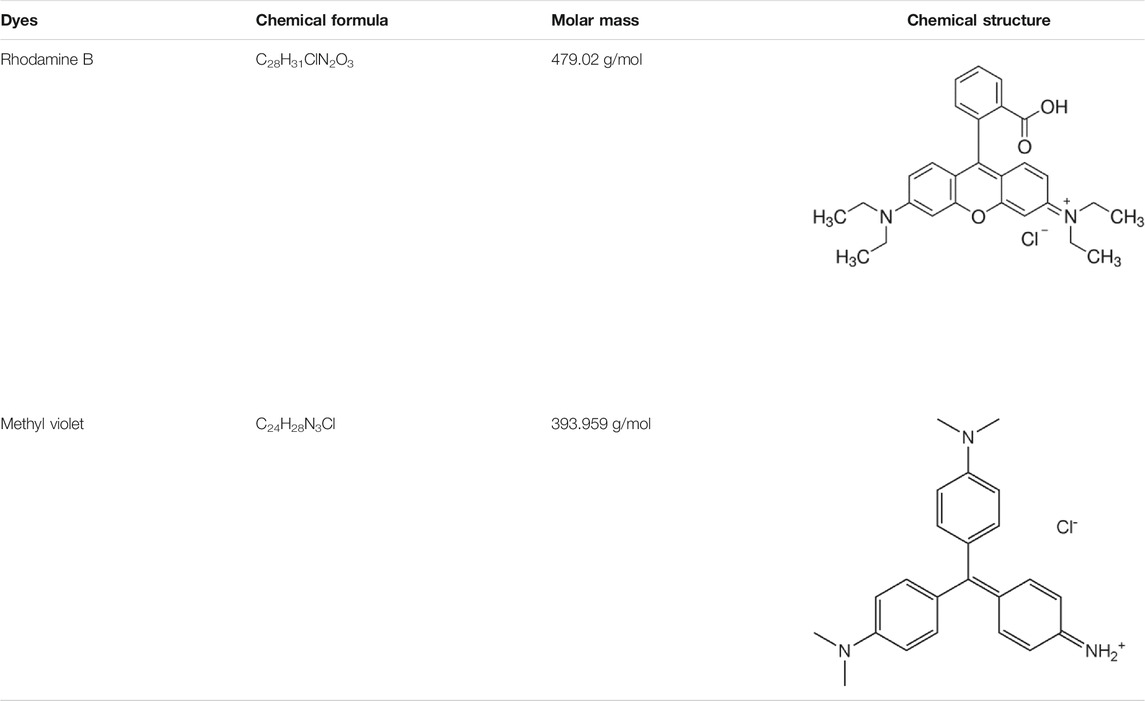
The molecular weight and chemical structure of organic dyes (such as Rhodamine B and Methyl Violet) used in the present work is shown above.
Hydrothermal Synthesis of SnS2 Nanostructures
Scheme 1 shows the schematic of the one-pot bottom-up hydrothermal synthesis of SnS2 NSs. SnCl4·5H2O and C2H5NS were used as the starting precursor of Tin (Sn+4) and sulfur (S−2) sources, respectively, for the growth of SnS2 NSs. In the typical reaction process, SnCl4·5H2O (∼1.8 g) and C2H5NS (∼1.6 g) were completely dissolved in 60 ml of distilled water and vigorously stirred for 120 min at room temperature. Afterward, the complete solution was transferred in a Teflon-lined autoclave at 180°C for 12 h. After the proposed reaction time, the autoclave is allowed to cool down at room temperature. A yellowish color solution was obtained, which was further washed several times with distilled water, and the sample was finally dried in vacuum at 80°C for 6 h.
Synthesis Reaction
During the hydrothermal synthesis, the hydrolysis of thioacetaamide produced ammonium acetate (CH3CO2NH4) and hydrogen sulfide (H2S) as products. The reaction between Sn+4 and H2S produced a yellow color perception which is responsible for SnS2 NSs. The possible reactions for the formation of SnS2 NSs are shown by Eqs. 1, 2 as follows (Zhang et al., 2011a):
The obtained powder was then further characterized in detail and used as an efficient photocatalyst for the photocatalytic degradation of Rh.B and M.V.
Instruments Used for Characterization
The morphology and the structure of as-synthesized SnS2 NSs were examined using a transmission electron microscope (TEM, FEI-Technai G2 F20, United States) with an accelerating voltage of 200 kV. The crystal phase and the crystallinity were examined by an X-ray diffractometer (PANalytical, United Kingdom) using Cu–Kα radiation (α = 1.54178 Å) at a scanning rate of 1°/s, ranging from 10° to 80°. Raman spectrophotometer (Renishaw, United Kingdom) was used to analyze the structural and the phase purities of as-synthesized SnS2 NSs. Fourier transform infrared (FTIR) spectrometer (Frontier, Perkin Elmer, United States) was used to determine the functional group. Total organic carbon (TOC) was measured using Lotix TOC Analyzer. The specific surface area was measured by Gemini VII 2390 surface area analyzer (Micromeritics, United States). All the photocatalytic activity of dyes was performed by using a UV–Vis spectrometer (UV-1800 SHIMADZU, Japan). X-ray photoelectron spectroscopy (XPS) was analyzed using PHI Versa Probe III.
Cell Culture and Cell Viability
Human cervical cancer cell line (HeLa cells) was cultured and maintained under a controlled atmospheric temperature at 37°C under 5% CO2 in RPMI-1640 medium, supplemented with 10% fetal bovine serum, penicillin (100 U ml−1), and streptomycin (100 mg ml−1). The 3-dimethylthiazol-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate cell viability after 24 h of cell seeding in the presence or absence of SnS2 NSs. In brief, HeLa cells were seeded in a 96-well polystyrene plate (TCP), with a density of 1 × 104 cells per well, and incubated under controlled conditions (5% CO2, 37°C) for 24 h. After that, the cells were treated with different concentrations (1.562, 3.125, 6.255, 12.5, 25, 50, 100, 200, 400, 800, and 1,000 μg/ml) of SnS2 NSs and incubated for 24 h at 37°C. After that, the cells were incubated with MTT solution (0.5 mg/ml in culture medium) at 37°C for 4 h and allowed formazan crystal formation. After incubation, the supernatant was removed, and 200 μl of DMSO was added and incubated for 30 min at 37°C. After the complete dissolution of formazan crystals, the optical density of the solution was measured at 540 nm by a microplate reader (PerkinElmer Victor 4 microplate reader). The wells without SnS2 NSs were used as controls. The values were expressed as mean ± standard deviation (n = 3). Cell viability was calculated using the following equation:
Sample Preparation for the Photocatalytic Activity of Dyes Using SnS2 NSs
The photocatalytic activities of the synthesized SnS2 NSs were evaluated by investigating the photodegradation of Rh.B and M.V under visible light radiation. The stock solution of 100 ml, with the concentration of 10 mg/L (10 ppm) of Rh.B and M.V, was prepared and stored in a dark room. A fluorescent lamp of 40 W (λ >420 nm), with a radiation intensity of 0.79 W/m2, was used for the light source. The volume of the initial solution (10 ml) of Rh.B and M.V was taken out for further photocatalytic activity. For the photocatalytic experiments, 5 mg of SnS2 NSs was added to 10 ppm solution of M.V and Rh.B and stirred for 5 min from the initial phase of the solution. The decomposed dyes of the different solutions were measured by using a UV spectrometer after regular time intervals. The concentration of aqueous M.V and Rh.B was determined by measuring its absorbance value, ranging from 400 to 800 nm, using a UV spectrophotometer. Furthermore, the detailed photodegradation study of M.V and Rh.B is also discussed in the present work.
Result and Discussions
Structural and Morphological Characterizations of SnS2 NSs
The as-synthesized SnS2 NSs are well characterized in terms of their structural, morphological, and optical properties. Powder X-Ray diffraction has been performed to determine the crystallinity and the crystal phase of as-synthesized SnS2 NSs, as shown in Figure 1A. The characteristic diffraction peaks observed at 15.0°, 28.1°, 32.1°, 46.1°, 50.1°, 52.4°, and 70.3° correspond to planes (001), (100), (101), (003), (110), (111), and (113), respectively.
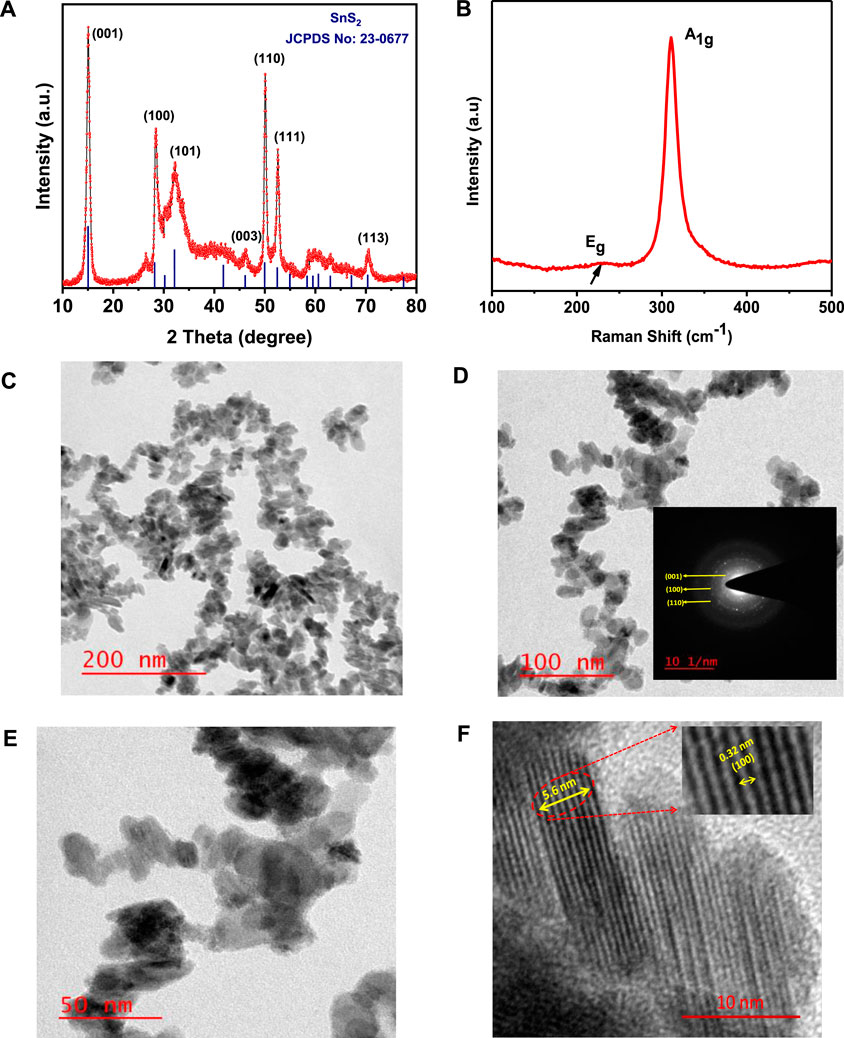
FIGURE 1. (A) X-ray diffraction pattern of SnS2 NSs. (B) RAMAN spectra of SnS2 NSs. (C–F) Transmission electron microscope and high-resolution transmission electron microscope images of as-synthesized SnS2 NSs with different magnification levels.
The observed XRD pattern of SnS2 NSs is well suited with JCPDPS card no. 22-0677, which suggests the hexagonal phase of SnS2 with lattice constant a = 3.649 Å and c = 5.899 Å and having space group: P-3m1 (164). The average crystallite size of as-synthesized materials was calculated by the Debye–Scherer formula which is given below:
where K represents the shape factor,
RAMAN is a versatile and non-destructive technique to evaluate the structural and vibrational properties of nanomaterials. The RAMAN spectra of as-synthesized SnS2 NSs were recorded with a laser excitation source of 532 nm, as shown in Figure 1B. There are two characteristic peaks located at 224 and 310 cm−1, which correspond to Eg (in-plane vibrational mode) and A1g (out-of-plane vibrational mode) modes, respectively (Deepika Bharatula et al., 2016).
The size and the morphology of as-synthesized SnS2 NSs were determined by TEM, as shown in Figures 1C–F. A typical high-resolution transmission electron microscope image of SnS2 is shown in Figure 1F. The morphology of as-synthesized SnS2 was found to be nanostructures in nature. The interlayer spacing of as-synthesized SnS2 NSs is 0.32 nm, which corresponds to the (100) crystalline plane, as shown in the inset of Figure 1F (Umar et al.).
Photophysical Characterization and Specific Surface Area Measurements of SnS2 NSs
The photophysical property of as-synthesized SnS2 NSs was estimated by UV–Vis diffuse reflectance spectra and FTIR. The absorption spectrum of SnS2 NSs was recorded in the wavelength range of 200–800 nm, as shown in Figure 2A. The broad absorption spectrum in the visible region indicates that the as-synthesized SnS2 NSs are capable of harvesting visible light and act as an efficient photocatalyst for photocatalytic reactions under a visible regime. The optical energy bandgap of as-synthesized SnS2 NSs was determined by the band edge absorption relation as follows:
where Eg is the optical energy bandgap, B is the optical-transition-dependent constant,
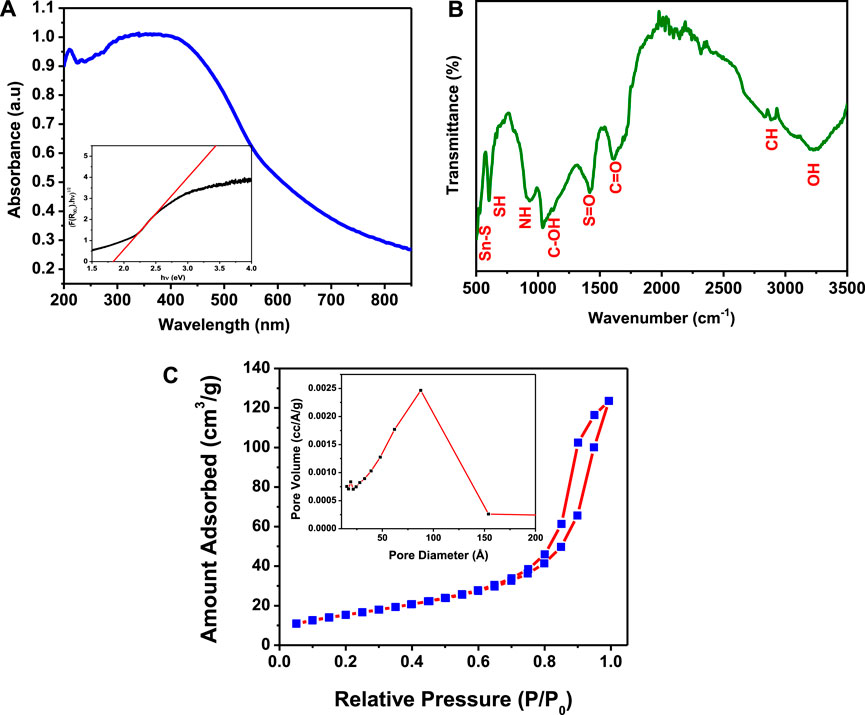
FIGURE 2. (A) Absorbance spectra. (B) Fourier transform infrared spectra. (C) Surface area measurements of SnS2 using the N2 adsorption–desorption isotherms and Brunauer–Emmett–Teller analysis.
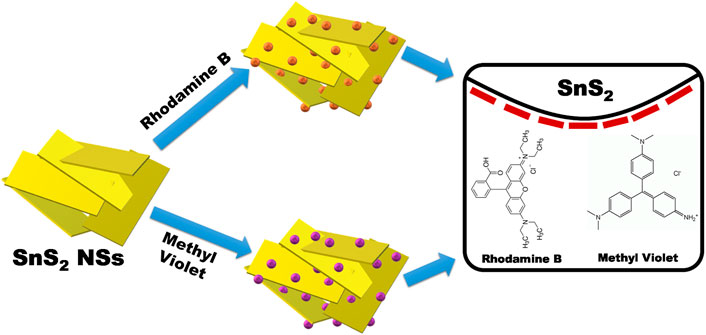
FTIR was performed to determine the function group over the surface of as-synthesized SnS2 NSs. In Figure 2B, the FTIR spectrum of SnS2 NSs was recorded, ranging from 400 to 4,000 cm−1. The peaks located at 3,226, 2,907, 1,610, 1,414, 1,194, 934, 612, and 545 cm−1 correspond to –OH, –CH, C=O, –S=O, –C–OH, N–H, C–H, and Sn–S, respectively. The presence of functional groups such as –OH, –COOH, and S=O may be responsible for making the SnS2 NSs anionic or negatively charged. This could lead to SnS2 as a promising material for the removal of cationic organic contaminants in an aqueous solution. Furthermore, electrostatic adsorption might be the prime factor for the interaction of selectively positive charged dyes such as Rh.B and M.V with negatively charged SnS2 (Han et al., 2017). Hence, SnS2 could be the superior adsorbent for industrial dyes, especially cationic dyes such as Rh.B and M.V, as shown in Scheme 2.
For photocatalytic applications, the designing of materials with a high surface area plays a crucial role in providing abundant active sites. This leads to the diffusion of dye molecules and enhancing the adsorption capability during the dye degradation process (Zhu et al., 2013; Han et al., 2017). In the present work, N2 adsorption–desorption isotherms and the corresponding Barratt–Joyner–Halenda (BJH) adsorption curve have been performed over SnS2 NSs, as shown in Figure 2C. The pore size of as-synthesized SnS2 NSs has been estimated by using the BJH method. This suggests that the pore size of SnS2 NSs varies from 5.5 to 16 nm, as shown in the inset of Figure 2C. The Brunauer–Emmett–Teller analysis suggests that the surface area of as-synthesized SnS2 NSs is 56.6 m2/g, with pore volume of 0.184 cc/g and average pore width of 10 nm. This measurement reveals that the as-synthesized SnS2 NSs possess a high surface area which provides an ample amount of surface adsorption sites, and a relatively large pore size might suggest the diffusion of organic dyes.
Cell Viability Performance
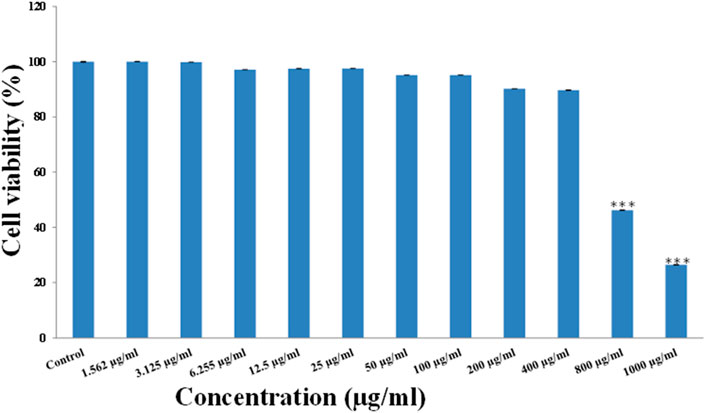
The biocompatibility of the SnS2 NSs was evaluated using MTT assay. In this assay, the metabolic activity of the cells was measured and correlated with the number of viable cells. The results of cell viability are shown in Figure 3, which suggests that SnS2 NSs do not exert obvious toxicity even at a very high concentration of 400 μg/ml (cell viability ∼90%), but at higher concentrations (800 and 1,000 μg/ml (46.22 and 26.45%)) significant toxicity was observed. Wang et al. (2020) reported that a hydrothermally synthesized SnS2 nanostructures shows good biocompatibility and use for photothermal therapy. Another study conducted by Feng et al. (2020) has developed a Ce-doped SnO2/SnS2-based photoelectrochemical cytosensor for recognition of tumor-associated macrophages. These studies significantly confirmed that SnS2-based materials have low toxicity and excellent biocompatibility for aquatic ecosystems.
Photocatalytic Activity
The photocatalytic activity of as-synthesized SnS2 NSs showed remarkable properties, such as adsorption of targeted organic pollutants, photoabsorption within a given light energy region, and the separation and transporting rate of the photogenerated electrons and holes in the photocatalyst.
The absorbance spectra of M.V and Rh.B are shown in Figure 4. The sequential change of the UV–Vis absorption spectra of M.V and Rh.B under visible light irradiation in regular time intervals are shown in Figures 4A,B. The photocatalytic degradation was recorded at wavelengths of 584 and 554 nm for M.V and Rh.B, respectively. Remarkably, it was found that, with increasing visible light time exposure, the absorption intensity continuously decreased in the presence of as-synthesized SnS2 NSs. The degradation efficiency or removal efficiency of organic dyes was estimated by the following equation:
where C and C0 are dye concentrations after and before exposure to visible light irradiation, respectively.
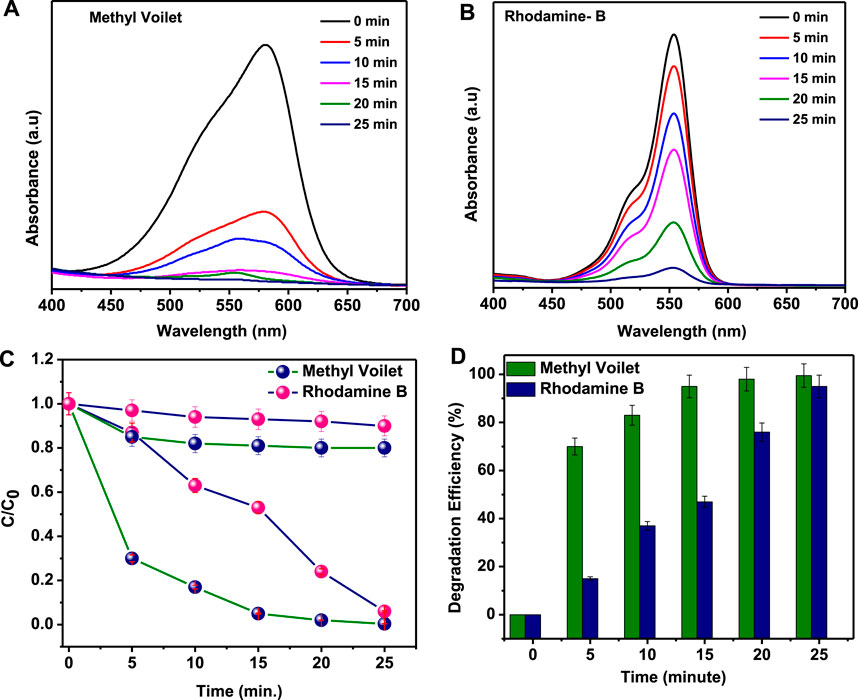
FIGURE 4. (A,B) Absorption spectra of the degradation of methyl violet and Rhodamine B, respectively, under visible light irradiation. (C) Relative concentration of decomposed dye. (D) Degradation efficiency (in %).
The relative concentration of organic dyes such as M.V and Rh.B has been analyzed in the absence and presence of visible light irradiation using SnS2 NSs, as shown in Figure 4C. The degradation histogram, as shown in Figure 4D, reveals that the organic dyes such as M.V and Rh.B are degraded up to 99.6 and 94.0%, respectively, in 25 min under visible light irradiation. However, in the absence of visible light irradiation, the degradation rate of Rh.B and M.V was almost negligible.
The behavior of photocatalytic activity is a tedious process that is driven by many parameters such as temperature, light intensity, catalyst loading, and concentration of organic pollutants. The photocatalytic kinetic behavior of SnS2 NSs has been obtained from the Langmuir–Henshelwood model. The catalytic decomposition was estimated by using a pseudo-first-order reaction kinetics equation which is given by:
where C0, C, k, and t are initial concentration, varying concentration, kinetic constant, and time, respectively. Figure 5A shows the kinetic rate constant (k) for the initial concentrations of M.V and Rh.B. The first-order reaction kinetic rate constant is 0.2236 and 0.0845 min−1 for M.V and Rh.B, respectively.
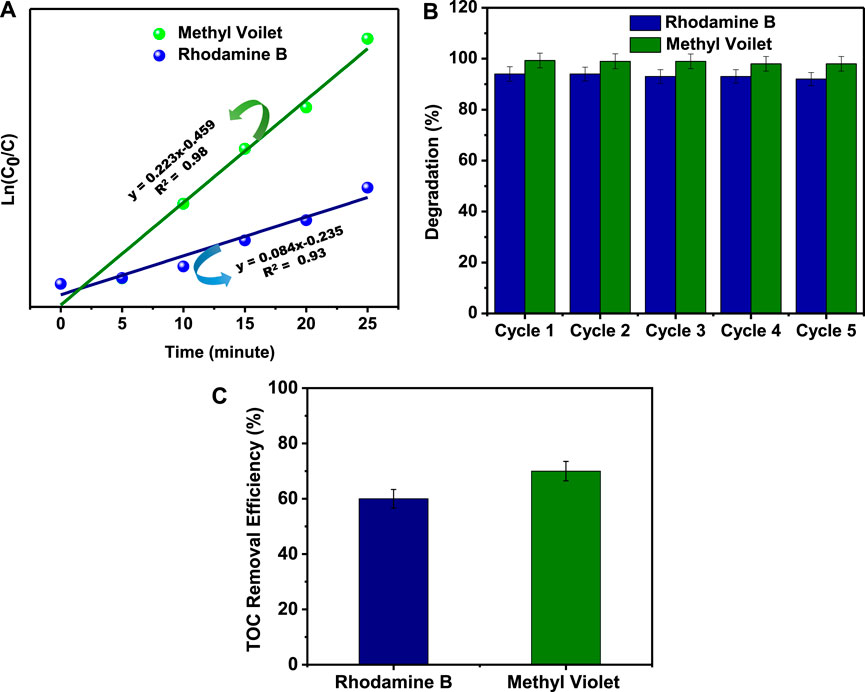
FIGURE 5. (A) First-order kinetic fit plots. (B) Recyclability performance of Rh.B and M.V. (C) Total organic carbon removal ratio of Rh.B and M.V using SnS2 NSs under visible light irradiation.
Stability and reusability play a vital role in the valuation of photocatalyst life cycle because photoanodic corrosion restricts their stability. To identify the stability and the reusability property of SnS2 NSs, the degradation process has been performed up to five times. In every cycle, SnS2 NSs were reused for their photocatalytic activity by using the separation and centrifuge method. A histogram graph has been plotted between the degradation efficiency of dye removal and the number of cycles, as shown in Figure 5B. In the first cycle, it has been observed that the photodegradation of aqueous solution of M.V and Rh.B has been degraded up to ∼99.6 and ∼94.0%, respectively. After performing this experiment, it has been found that the degradation efficiency is ∼91 and ∼97%, respectively, for Rh.B and M.V at the fifth cycle, which reveals the excellent stability and recyclability properties of SnS2 NSs.
The mineralization capability of photocatalysts is also an important criterion to evaluate their photocatalytic efficiency. Thus, the TOC removal efficiency of Rh.B and M.V by SnS2 NSs was investigated using a TOC analyzer. In Figure 5C, the TOC removal ratio of Rh.B and M.V reached ∼60 and ∼70% using SnS2 NSs under visible light irradiation in 25 min, respectively. These findings demonstrate that SnS2 NSs can easily mineralize Rh.B and M.V during the photocatalytic process and could be effectively mineralized into residual organic molecules, indicating their high potential for practical applications.
XPS was performed to determine the distribution of elements and compositional states on the surface of the sample. The XPS spectra of as-synthesized SnS2 NSs were investigated before and after the photocatalytic degradation of Rhodamine B and methyl violet after five successive cycles, as shown in Figure 6. The XPS survey spectrum (0–1,000 eV) evidences the presence of C, O, Sn, and S elements, as shown in Figure 6A. In Figures 6B,C, the XPS pattern of Sn 3d in pristine SnS2 NSs shows two prominent peaks at 486.4 and 494.8 eV, which correspond to Sn 3d5/2 and Sn3d3/2, respectively. The XPS spectra of S 2p in bare SnS2 NSs reveal two peaks at 161.4 and 162.4 eV, which correspond to S 2p3/2 and S 2p1/2, respectively (Hu et al., 2013). After the photodegradation of Rhodamine B and methyl violet, the Sn 3d and S 2p peaks of SnS2 NSs are slightly shifted to a lower binding energy (∼0.2 eV) compared to the pristine SnS2 NSs. This might be attributed to the photoreduction of SnS2 NSs that occurred on the surface throughout the photodegradation process, confirming the high stability and sustainability of the photocatalyst (Shi et al., 2010; Zhai et al., 2017).
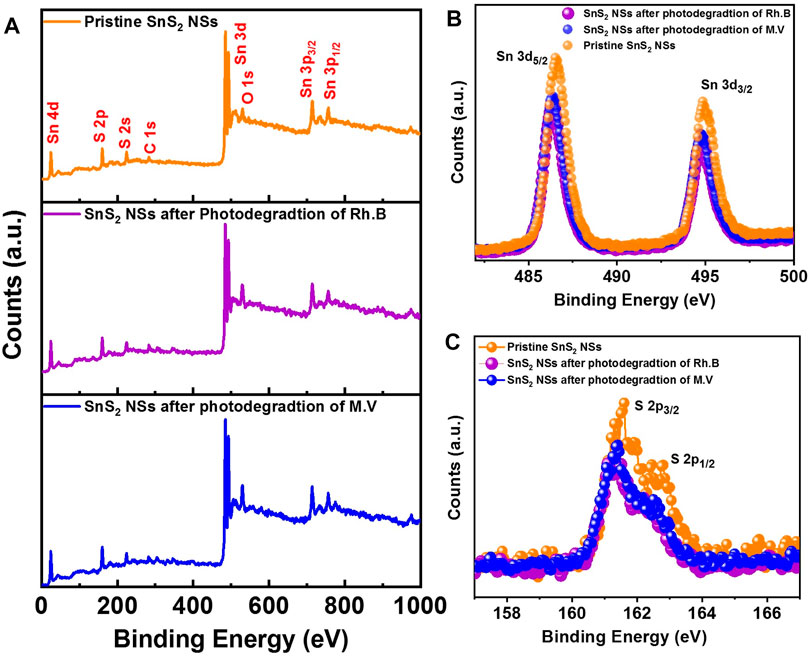
FIGURE 6. XPS spectrum of SnS2 NSs before and after photodegradation of Rh.B and M.V. (A) Survey spectra. (B) Sn 3d spectra. (C) S 2p spectra.
Among various 2D SnS2-based nanostructured materials, the as-synthesized SnS2 NSs revealed a highly efficient photocatalytic activity for organic pollutants such as Rh.B and M.V under visible-light-driven irradiation, as shown in Table 1.
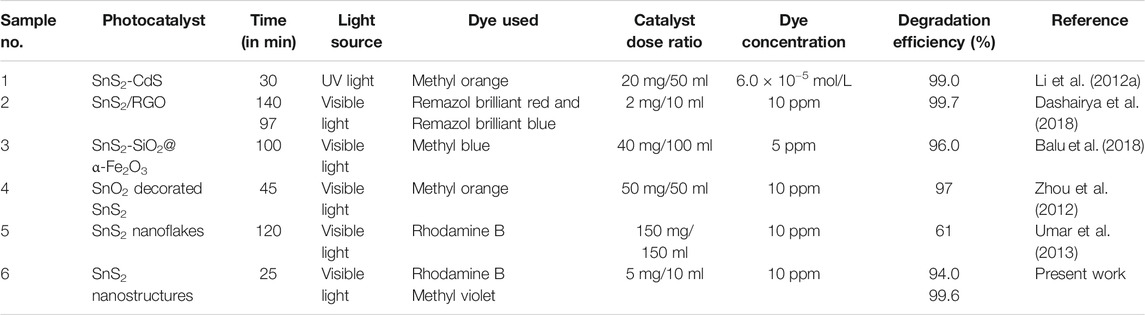
TABLE 1. Comparison between the photocatalytic activities of as-synthesized SnS2 NSs with other decorated/undecorated semiconducting SnS2-based photocatalysts.
Photocatalytic Mechanism
The main advantages of the photocatalytic activity of semiconductor catalysts are the photoabsorption ability within the given light regime and the separation and transfer of photogenerated electrons and holes in themselves. Under visible light irradiation, the degradation of organic dyes such as Rh. B and M.V over the surface of as-synthesized semiconducting SnS2 NSs has been demonstrated by a schematic, as shown in Scheme 3.
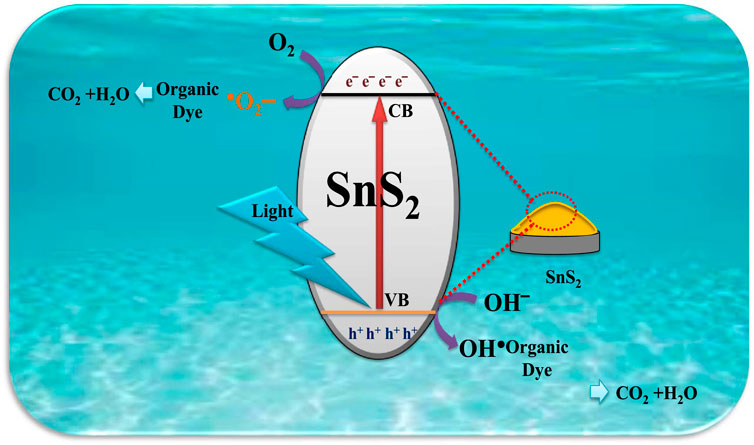
SCHEME 3. Schematic illustration of the degradation mechanism of organic dyes over the surface of SnS2 NSs.
A narrow bandgap semiconductor catalyst allows more photons to get absorbed, resulting in excellent catalytic activity under visible light irradiation. In the photocatalytic mechanism, a photocatalyst directly attacks the chromophore of organic pollutants due to the weak interaction between the photocatalyst and the dye (Saison et al., 2011). Due to the highly reactive free radicals in the photocatalytic degradation process, all contaminants break into inorganic substances or small molecules through the addition reaction, substitution reaction, and the electron transfer between free radicals and organic pollutants.
The generation of electron–hole pairs and the excitation of valence band electron will take place under visible light irradiation. The excited electron from the valence band will further transfer the electron to the conduction band, which results in the generation of e−/h+ pairs. The holes from the valence band of as-synthesized SnS2 NSs react with hydroxyl groups by breaking the water molecules and form highly reactive hydroxyl radicals (•OH), and the electron from the conduction band reacts with the oxygen molecules attached over the surface of SnS2 NSs and forms superoxide anion radicals (•O2−). Furthermore, these •O2− react with H+ and produce H2O2, and finally this H2O2 deteriorates into hydroxyl radicals (•OH) (Cao et al., 2017).
The plausible photocatalytic degradation mechanism for the catalytic behavior of as-synthesized semiconductor SnS2 NSs was suggested by the given equations as follows:
In general, highly reactive oxyradicals such as hydroxyl (OH•) and superoxide anion (O2•−) are responsible for the degradation of organic dyes such as Rh.B and M.V in the presence of visible light irradiation. However, at room temperature, the hydroxyl group (OH•) plays a vital role in the degradation of organic dyes with a simple magnetic stirring process. These highly reactive oxyradicals are formed over the surface of an as-synthesized SnS2 catalyst by the separation of electron–hole (e−/h+) pairs (Umar et al.). Organic dyes react with the hydroxyl groups (OH•) and superoxide anion (O2•−), break into smaller molecules, and generate intermediates, such as CO2, H2O, etc. Finally, under visible light irradiation, the transformation or oxidation of harmful organic dyes into less harmful chemicals takes place.
Conclusion
In summary, SnS2 NSs have been synthesized successfully using the facile single-step bottom-up hydrothermal method. The obtained SnS2 NSs have a low bandgap (∼1.6 eV), a high surface area (56 m2/g), and an anionic nature, resulting in an efficient photocatalyst for the degradation of organic dyes. The as-synthesized SnS2 NSs have been used for the removal of organic dyes such as Rh.B and M.V. SnS2 NSs exhibited good degradation efficiency for Rh.B (94%) and M.V (99.6%) in 25 min. The kinetic rate constant for Rh.B and M.V was calculated by using the first-order Langmuir–Hinshelwood model. The recyclability test suggests the excellent stability of the photocatalyst for up to five cycles. The XPS spectrum of SnS2 NSs was investigated before and after the photodegradation of Rhodamine B and Methyl Violet, suggesting the high stability of the photocatalyst. Herein we have also discussed the detailed degradation mechanism for the removal of dyes, which suggests the formation of a large number of oxyradicals and enhanced photocatalytic performance of as-synthesized SnS2 NSs. Moreover, in vitro cytotoxicity was also evaluated against human cervical cancer cell lines (HeLa cells) with different concentrations (0–1,000 μg/ml) of as-synthesized SnS2 NSs. In the present investigations, we believed that this highly responsive photocatalyst opened a new avenue for the degradation of environmental pollutants by the modification of the energy bandgap and charge nature on the photocatalyst surface.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
RS and AS conceived the idea of 2D SnS2 nanostructure-based photocatalytic degradation. RS carried out the whole experiments, analyzed the data, and wrote the draft of the manuscript. PK and SR helped to do photocatalytic experiments. SU discussed all experiment results. UY and PS also contributed to testing in-vitro cytotoxicity of 2D SnS2 nanostructures and to preparing a robotic model. SU given scientific suggestions and comments on the manuscript. AS supervised the research at all stages and led all groups. All the authors discussed the results and commented on the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
RS is thankful to UGC for providing fellowship. AS is thankful to DST India (DST-PURSE Scheme 5050), DST-SERB (EMR/2016/007720), and research grant for faculty (IoE Scheme) under Development Scheme No.: 6031 for providing financial assistance. AS is also thankful to the Department of Physics, BHU, for providing financial assistance under CAS scheme. The authors are also thankful to the University Science Instrumentation Center (USIC Level II) and the Department of Botany, Institute of Science, Banaras Hindu University, Varanasi, India, for UV–vis and TOC measurements, respectively.
References
Balu, S., Uma, K., Pan, G.-T., Yang, T., and Ramaraj, S. (2018). Degradation of Methylene Blue Dye in the Presence of Visible Light Using SiO2@α-Fe2O3 Nanocomposites Deposited on SnS2 Flowers. Materials 11, 1030. doi:10.3390/ma11061030
Brillas, E., and Martínez-Huitle, C. A. (2015). Decontamination of Wastewaters Containing Synthetic Organic Dyes by Electrochemical Methods. An Updated Review. Appl. Catal. B: Environ. 166–167, 603–643. doi:10.1016/j.apcatb.2014.11.016
Cao, S., Low, J., Yu, J., and Jaroniec, M. (2015). Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 27, 2150–2176. doi:10.1002/adma.201500033
Cao, Y., Li, Q., and Wang, W. 2017, Undefined Construction of a Crossed-Layer-Structure MoS2/gC3 N4 Heterojunction with Enhanced Photocatalytic Performance. Available at: https://pubs.rsc.org/--/content/articlehtml/2017/ra/c6ra26925g (Accessed May 12, 2021).
Dashairya, L., Sharma, M., Basu, S., and Saha, P. (2019). SnS2/RGO Based Nanocomposite for Efficient Photocatalytic Degradation of Toxic Industrial Dyes under Visible-Light Irradiation. J. Alloys Comp. 774, 625–636. doi:10.1016/j.jallcom.2018.10.008
Bharatula, L. D., Erande, M. B., Mulla, I. S., Rout, C. S., and Late, D. J. (2016). SnS2nanoflakes for Efficient Humidity and Alcohol Sensing at Room Temperature. RSC Adv. 6, 105421–105427. doi:10.1039/c6ra21252b
Du, Y., Yin, Z., Rui, X., Zeng, Z., Wu, X., Liu, J., et al. (2013). A Facile, Relative green, and Inexpensive Synthetic Approach Toward Large-Scale Production of SnS 2 Nanoplates for High-Performance Lithium-Ion Batteries. Nanoscale 5 (4), 1456–1459. doi:10.1039/c2nr33458e
Feng, R., Tian, K., Zhang, Y., Liu, W., Fang, J., Khan, M. S., et al. (2020). Recognition of M2 Type Tumor-Associated Macrophages with Ultrasensitive and Biocompatible Photoelectrochemical Cytosensor Based on Ce Doped SnO2/SnS2 Nano Heterostructure. Biosens. Bioelectron. 165, 112367. doi:10.1016/j.bios.2020.112367
Guo, Y., Wang, L., Yang, L., Zhang, J., Jiang, L., and Ma, X. (2011). Optical and Photocatalytic Properties of Arginine-Stabilized Cadmium Sulfide Quantum Dots. Mater. Lett. 65, 486–489. doi:10.1016/j.matlet.2010.10.057
Han, S., Liu, K., Hu, L., Teng, F., Yu, P., and Zhu, Y. (2017). Superior Adsorption and Regenerable Dye Adsorbent Based on Flower-Like Molybdenum Disulfide Nanostructure. Sci. Rep. 7, 1–11. doi:10.1038/srep43599
Hu, X., Song, G., Li, W., Peng, Y., Jiang, L., Xue, Y., et al. (2013). Phase-Controlled Synthesis and Photocatalytic Properties of SnS, SnS2 and SnS/SnS2 Heterostructure Nanocrystals. Mater. Res. Bull. 48, 2325–2332. doi:10.1016/j.materresbull.2013.02.082
Kim, S., Choi, M., and Bulletin, H. C.-M. R. (2016). Undefined Photocatalytic Activity of SnO2 Nanoparticles in Methylene Blue Degradation. Available at: https://www.sciencedirect.com/science/article/pii/S0025540815301586 (Accessed May 12, 2021).
Li, S., Chen, J., Hu, S., Wang, H., Jiang, W., and Chen, X. (2020a). Facile Construction of Novel Bi2WO6/Ta3N5 Z-Scheme Heterojunction Nanofibers for Efficient Degradation of Harmful Pharmaceutical Pollutants. Chem. Eng. J. 402, 126165. doi:10.1016/j.cej.2020.126165
Li, S., Hu, S., Jiang, W., Zhang, J., Xu, K., and Wang, Z. (2019). In Situ construction of WO3 Nanoparticles Decorated Bi2MoO6 Microspheres for Boosting Photocatalytic Degradation of Refractory Pollutants. J. Colloid Interf. Sci. 556, 335–344. doi:10.1016/j.jcis.2019.08.077
Li, S., Wang, C., Liu, Y., Xue, B., Jiang, W., Liu, Y., et al. (2021). Photocatalytic Degradation of Antibiotics Using a Novel Ag/Ag2S/Bi2MoO6 Plasmonic p-n Heterojunction Photocatalyst: Mineralization Activity, Degradation Pathways and Boosted Charge Separation Mechanism. Chem. Eng. J. 415, 128991. doi:10.1016/j.cej.2021.128991
Li, S., Xue, B., Chen, J., Jiang, W., and Liu, Y. (2020b). Nanoparticles as Highly Efficient Photocatalyst for the Treatment of Toxic Wastewater. Catalysts 1–15.
Li, X., Zhu, J., and Li, H. (2012b). Comparative Study on the Mechanism in Photocatalytic Degradation of Different-type Organic Dyes on SnS2 and CdS. Appl. Catal. B: Environ. 123–124, 174–181. doi:10.1016/j.apcatb.2012.04.009
Li, X., Zhu, J., and Li, H. (2012a). Undefined Comparative Study on the Mechanism in Photocatalytic Degradation of Different-Type Organic Dyes on SnS2 and CdS. Appl. Catal. B: Environ. 123–124, 174–181. doi:10.1016/j.apcatb.2012.04.009
Lorenz, T., Joswig, J., and Seifert, G. (2014). Unefined Combined SnS@ SnS2 Double Layers: Charge Transfer and Electronic Structure. Available at: https://iopscience.iop.org/article/10.1088/0268-1242/29/6/064006/meta (Accessed May 12, 2021).
Opoku, F., Govender, K. K., van Sittert, C. G. C. E., Govender, P. P., and Govender, P. P. (2017). Recent Progress in the Development of Semiconductor-Based Photocatalyst Materials for Applications in Photocatalytic Water Splitting and Degradation of Pollutants. Adv. Sust. Syst. 1, 1700006. doi:10.1002/adsu.201700006
Patil, S. S., and Shinde, V. M. (1988). Biodegradation Studies of Aniline and Nitrobenzene in Aniline Plant Wastewater by Gas Chromatography. Environ. Sci. Technol. 22, 1160–1165. doi:10.1021/es00175a005
Robinson, T., Mcmullan, G., Marchant, R., and Nigam, P. (2001). Remediation of Dyes in Textile Effluent: a Critical Review on Current Treatment Technologies with a Proposed Alternative. Bioresour. Tech. 77, 247–255. doi:10.1016/s0960-8524(00)00080-8
Saison, T., Chemin, N., Chanéac, C., Durupthy, O., Ruaux, V., Mariey, L., et al. (2011). Bi2O3, BiVO4, and Bi2WO6: Impact of Surface Properties on Photocatalytic Activity under Visible Light. J. Phys. Chem. C 115, 5657–5666. doi:10.1021/jp109134z
Schwarzenbach, R. P., Egli, T., Hofstetter, T. B., Von Gunten, U., and Wehrli, B. (2010). Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 35, 109–136. doi:10.1146/annurev-environ-100809-125342
Sharma, R., Khanuja, M., Sharma, S., Narayan, S., and Prakash, O. (2017). Undefined Reduced Band Gap & Charge Recombination Rate in Se Doped α-Bi2O3 Leads to Enhanced Photoelectrochemical and Photocatalytic Performance: Theoretical & Experimental Insight. Int. J. Hydrogen Energ. 42, 20638–20648. doi:10.1016/j.ijhydene.2017.07.011
Shi, R., Lin, J., Wang, Y., Xu, J., and Zhu, Y. (2010). Visible-Light Photocatalytic Degradation of BiTaO4 Photocatalyst and Mechanism of Photocorrosion Suppression. J. Phys. Chem. C 114, 6472–6477. doi:10.1021/jp9101866
Singh, S., Pendurthi, R., Khanuja, M., Islam, S. S., Rajput, S., and Shivaprasad, S. M. (2017). Copper-doped Modified ZnO Nanorods to Tailor its Light Assisted Charge Transfer Reactions Exploited for Photo-Electrochemical and Photo-Catalytic Application in Environmental Remediation. Appl. Phys. A. 123, 184. doi:10.1007/s00339-017-0806-8
Song, H. S., Li, S. L., Gao, L., Xu, Y., Ueno, K., Tang, J., et al. (2013). High-Performance Top-Gated Monolayer SnS2 Field-Effect Transistors and Their Integrated Logic Circuits. Nanoscale 5, 9666. doi:10.1039/c3nr01899g
Srivastava, R., Mishra, H., Singh, V., Vijay, K., Vikram, K. S., and Rajesh Kumar, S. (2019). Undefined pH Dependent Luminescence Switching of Tin Disulfide Quantum Dots. Available at: https://www.sciencedirect.com/science/article/pii/S0022231318322038?casa_token=veV8EeAPRBwAAAAA:Lc6w61aIaLsL1Y6Vi4FkA8NxqkP_CLUgE9Z3jv22mh5g9t1M-k5q2mnrPRd2JQ0jP0K4CNhFjA (Accessed May 12, 2021).
Štengl, V., Bakardjieva, S., Grygar, T. M., Bludská, J., and Kormunda, M. (2013). TiO2-graphene Oxide Nanocomposite as Advanced Photocatalytic Materials. Chem. Cent. J. 7. doi:10.1186/1752-153X-7-41
Stock, N. L., Peller, J., Vinodgopal, K., and Kamat, P. V. (2000). Combinative Sonolysis and Photocatalysis for Textile Dye Degradation. Environ. Sci. Technol. 34, 1747–1750. doi:10.1021/es991231c
Su, G., Hadjiev, V. G., Loya, P. E., Zhang, J., Lei, S., Maharjan, S., et al. (2015). Chemical Vapor Deposition of Thin Crystals of Layered Semiconductor Sns2 for Fast Photodetection Application. Nano Lett. 15, 506–513. doi:10.1021/nl503857r
Talebian, N., and Nilforoushan, M. R. (2010). Comparative Study of the Structural, Optical and Photocatalytic Properties of Semiconductor Metal Oxides toward Degradation of Methylene Blue. Thin Solid Films 518, 2210–2215. doi:10.1016/j.tsf.2009.07.135
Tan, F., Qu, S., Wu, J., Liu, K., Zhou, S., and Wang, Z. (2011). Preparation of Sns2 Colloidal Quantum Dots and Their Application in Organic/inorganic Hybrid Solar Cells. Nanoscale Res. Lett. 6, 298. doi:10.1186/1556-276X-6-298
Tian, H., Wan, C., Xue, X., Hu, X., and Wang, X. (2017). Effective Electron Transfer Pathway of the Ternary TiO2/RGO/Ag Nanocomposite with Enhanced Photocatalytic Activity under Visible Light. Catalysts 7, 156. doi:10.3390/catal7050156
Umar, A., Akhtar, M., Dar, G., Abaker, M., Hajry, A., Baskoutas, S., et al. (2013). Undefined Visible-Light-Driven Photocatalytic and Chemical Sensing Properties of SnS2 Nanoflakes. Available at: https://www.sciencedirect.com/science/article/pii/S0039914013001999?casa_token=vcL6-JhbVtYAAAAA:SahhMoWDddEeDRI7tELKMAdz-sYBCZlaQGHpzp8r-dIvB65ZFm1upZsf79ENsl0Ph6ESpP1cNw (Accessed May 12, 2021).
Wang, D., Tang, M., Jiang, H., Li, M., Jiang, S., Sun, L., et al. (2020). Helical Bowl-Like SnS2 with Structure-Induced Conversion Efficiency for Enhanced Photothermal Therapy. Chem. Eng. J. 400, 125814. doi:10.1016/j.cej.2020.125814
Xie, Y., Ali, G., Yoo, S. H., and Cho, S. O. (2010). Sonication-assisted Synthesis of CdS Quantum-Dot-Sensitized TiO2 Nanotube Arrays with Enhanced Photoelectrochemical and Photocatalytic Activity. ACS Appl. Mater. Inter. 2, 2910–2914. doi:10.1021/am100605a
Xie, Y., Chen, F., He, J., Zhao, J., and Hui, W. (2000). Undefined Photoassisted Degradation of Dyes in the Presence of Fe3+ and H2O2 Under Visible Irradiation. Available at: https://www.sciencedirect.com/science/article/pii/S1010603000003415?casa_token=z0HD7IXPPjIAAAAA:z-cYwzyN-KJTiJWIKql1Nxju7wmq1rTIcs-5WY6yFZVzf5glCqnsLKLvZ7-6RE5E27tTDiAUaw (Accessed May 12, 2021).
Zhai, H., Qi, J., Zhang, X., Li, H., Yang, L., Hu, C., et al. (2017). Preparation and Photocatalytic Performance of Hollow Structure LiNb3O8 Photocatalysts. Nanoscale Res. Lett. 12, 519. doi:10.1186/s11671-017-2291-6
Zhang, Y., Du, Z., Li, K., and Zhang, M. 2011, Undefined Size-Controlled Hydrothermal Synthesis of SnS2 Nanoparticles with High Performance in Visible Light-Driven Photocatalytic Degradation of Aqueous Methyl Orange. Sep. Purif. Tech. 81(1), 101–107. doi:10.1016/j.seppur.2011.07.016
Zhang, Y. C., Li, J., Zhang, M., and Dionysiou, D. D. (2011a). Size-Tunable Hydrothermal Synthesis of SnS2Nanocrystals with High Performance in Visible Light-Driven Photocatalytic Reduction of Aqueous Cr(VI). Environ. Sci. Technol. 45, 9324–9331. doi:10.1021/es202012b
Zhang, Y. C., Li, J., Zhang, M., and Dionysiou, D. D. (2011b). Size-Tunable Hydrothermal Synthesis of SnS2 Nanocrystals with High Performance in Visible Light-Driven Photocatalytic Reduction of Aqueous Cr(VI). Environ. Sci. Technol. 45, 9324–9331. doi:10.1021/es202012b
Zhong, H. E., Shaogui, Y., Yongming, J. U., and Cheng, S. (2009). Microwave Photocatalytic Degradation of Rhodamine B Using TiO2 Supported on Activated Carbon: Mechanism Implication. J. Environ. Sci. 21, 268–272. doi:10.1016/S1001-0742(08)62262-7
Zhou, X., Zhou, T., Hu, J., and Li, J. (2012). Controlled Strategy to Synthesize SnO2 Decorated SnS2 Nanosheets with Enhanced Visible Light Photocatalytic Activity. CrystEngComm 14, 5627. doi:10.1039/c2ce25309g
Keywords: semiconductor photocatalyst, 2D SnS2 NSs, hydrothermal growth, photocatalytic degradation, organic dyes
Citation: Srivastava RR, Kumar Vishwakarma P, Yadav U, Rai S, Umrao S, Giri R, Saxena PS and Srivastava A (2021) 2D SnS2 Nanostructure-Derived Photocatalytic Degradation of Organic Pollutants Under Visible Light. Front. Nanotechnol. 3:711368. doi: 10.3389/fnano.2021.711368
Received: 18 May 2021; Accepted: 29 July 2021;
Published: 23 August 2021.
Edited by:
Sibo Wang, Fuzhou University, ChinaCopyright © 2021 Srivastava, Kumar Vishwakarma, Yadav, Rai, Umrao, Giri, Saxena and Srivastava. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anchal Srivastava, anchalbhu@gmail.com
 Rohit Ranjan Srivastava1
Rohit Ranjan Srivastava1  Umakant Yadav
Umakant Yadav Suyash Rai
Suyash Rai Sima Umrao
Sima Umrao Anchal Srivastava
Anchal Srivastava