Clinical antidiabetic medication used in Alzheimer’s disease: From basic discovery to therapeutics development
- 1Department of Pharmacology and Chemical Biology, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Key Laboratory of Basic Pharmacology of Ministry of Education and Joint International Research Laboratory of Ethnomedicine of Ministry of Education, Zunyi Medical University, Zunyi, Guizhou, China
- 3School of Public Health, Zunyi Medical University, Zunyi, Guizhou, China
- 4The Third Affiliated Hospital of Zunyi Medical University (The First People’s Hospital of Zunyi), Zunyi, Guizhou, China
Alzheimer’s disease (AD) is a common neurodegenerative disease. Type 2 diabetes mellitus (T2DM) appears to increase and contributing to the risk of AD. Therefore, there is increasing concern about clinical antidiabetic medication used in AD. Most of them show some potential in basic research, but not in clinical research. So we reviewed the opportunities and challenges faced by some antidiabetic medication used in AD from basic to clinical research. Based on existing research progress, this is still the hope of some patients with special types of AD caused by rising blood glucose or/and insulin resistance.
Introduction
At present, Alzheimer’s disease (AD) lacks effective treatment methods and drugs. It is only delayed by some drugs that act on neurotransmitters (Marucci et al., 2021). In recent years, some progress has been made in anti-AD drugs, such as the Aducanumab approved by United States Federal Drug Administration (FDA), GV-971 approved by the National Medical Products Administration (NMPA), and Lecanemab, an initial decision on the drug’s approval by the FDA is expected by 2023, but they are all controversial (Karlawish and Grill, 2021; Xiao et al., 2021; The Lancet, 2022). The failure of a large number of drug studies on AD is largely related to the unknown pathogenesis of AD. Therefore, similar to the research of anti-tumor drugs, it is very promising to conduct more accurate subtype classifications for AD patients, and then conduct treatment drug research.
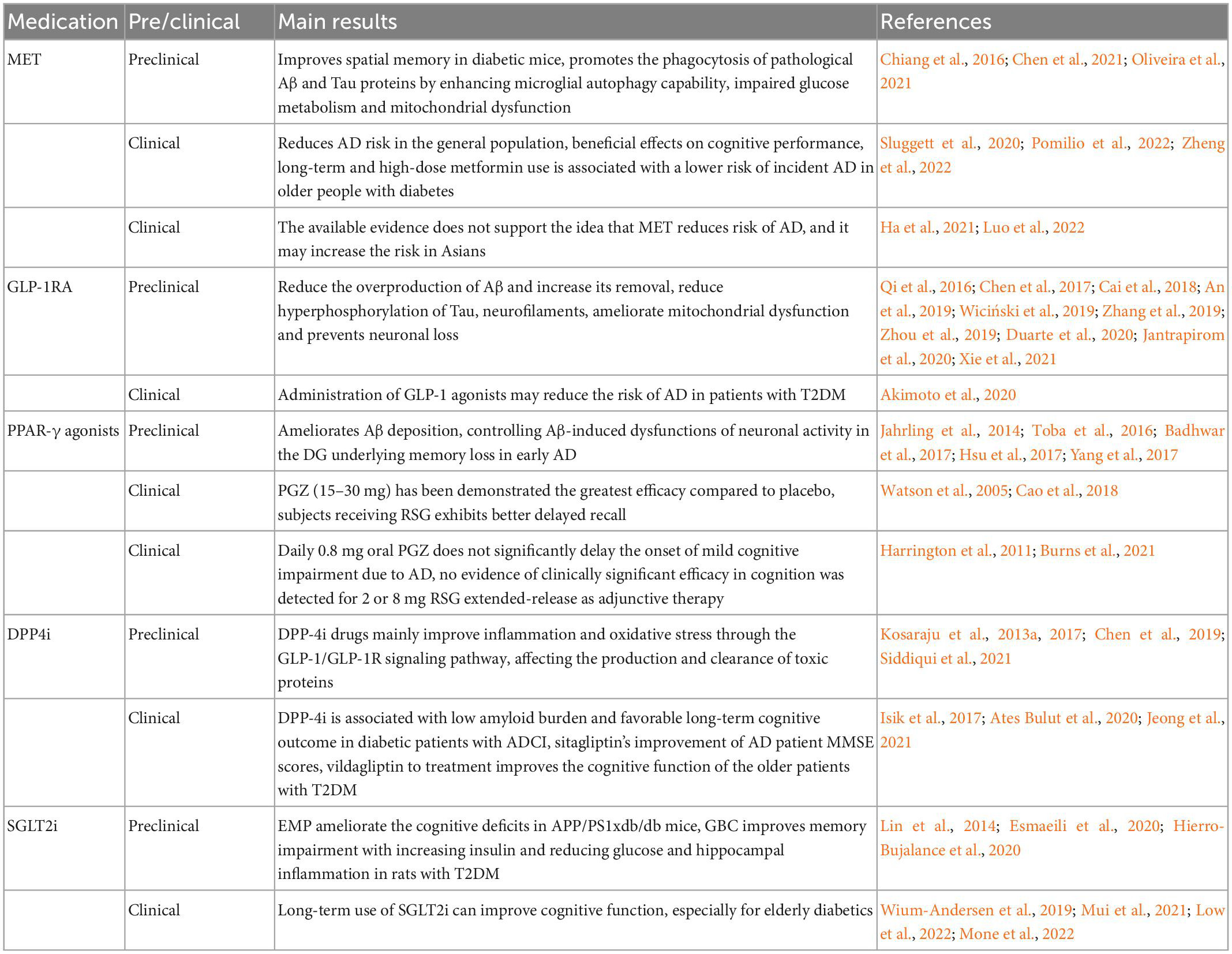
Epidemiological investigations of Type 2 diabetes mellitus (T2DM) and AD indicated that T2DM appears to increase and contributing to the risk of AD. Learning cognitive dysfunction, neuronal loss, etc., appear in T2DM patients (Noreen et al., 2018; Li et al., 2021). Further studies show they share lots of common link, including similar pathological features, etiology, targets, and involving same signaling pathways (Doherty et al., 2013; Chung et al., 2018; Zhang et al., 2018; Takeuchi et al., 2019; Gharibyan et al., 2020; Sun et al., 2020; Dekeryte et al., 2021; Liu et al., 2021; Yen et al., 2021). Thus, some researchers propose a theory that AD is Type 3 diabetes mellitus (T3DM) (Steen et al., 2005). Then, a growing number of studies have been carried out and showed that drugs for the treatment of T2DM also have certain improvement effect on AD (Akimoto et al., 2020). Most of them show some potential in basic research, but not in clinical research (Table 1). So we reviewed the opportunities and challenges faced by some antidiabetic medication used in AD from basic to clinical research. Based on existing research progress, this is still the hope of some patients with special types of AD caused by rising blood glucose or/and insulin resistance.
Metformin
Metformin (MET) is a common oral anti-diabetic drug, which can lower blood glucose in many ways. Among these mechanisms regulated by MET to lower blood glucose, the regulatory mechanism centered on AMP-activated protein kinase (AMPK) plays an important role not only in diabetes mellitus (DM) but also in AD (Markowicz-Piasecka et al., 2017; Ma et al., 2022). In streptozotocin (STZ)-induced Swiss Webster mice, MET improves spatial memory in diabetic mice, which can be associated with reducing p-Tau and β-amyloid (Aβ) plaque load and inhibition of neuronal death (Oliveira et al., 2021). And in APP/PS1 mice, MET increases the level of p-AMPK and insulin degrading enzyme (IDE) protein in mice, and significantly reduces the Aβ level in the brain. Although it does not affect the enzyme activity of Aβ-related secretion enzymes (Lu et al., 2020). Additionally, in the APP/PS1 mouse injected Tau, MET can promote the phagocytosis of pathological Aβ and Tau proteins by enhancing microglial autophagy capability (Chen et al., 2021). But this performance shows a certain gender difference. In AβPP mice aged 12–14 months, MET activates AMPK to show a protective effect in female mice, but it shows a damage effect in male mice (DiTacchio et al., 2015). The results of these pre-clinical studies show that MET has a certain potential treatment effect on AD.
Although in many preclinical studies, MET shows an exciting role, the results in clinical studies are indeed unsatisfactory. First of all, there are still certain controversy in whether MET reduces the risk of AD. Studies have shown that MET can be a reduced AD risk in the general population (Zheng et al., 2022). But analyzing among Asians, MET has the risk of increasing the prevalence of AD (Ha et al., 2021; Luo et al., 2022). We believe that this is not consistent with the selection and analysis goals of data. To a large extent, the risk of AD population based on DM-based diseases can be considered as useful. We speculate that this is related to some AD patients without blood glucose changes. The AMPK activator represented by MET may be defined as a significant role in AD patients with T3DM. And some clinical studies have also verified our speculation. The use of MET does not increase the risk of AD. And long-term and large doses of MET are related to the risk of lowering AD with elderly DM (Sluggett et al., 2020). Therefore, we believe that we should have targeted design clinical trials to screen patients with abnormal blood glucose or have DM themselves, or early intervention for patients with DM merged mild cognitive impairment. And when considering the selection of drugs, the stage, type, and gender of the disease itself should be comprehensively considered.
GLP-1 agonists
Glucagon-like peptide-1 (GLP-1) is one of the important targets for the treatment of diabetes. As an intestinal peptide, GLP-1 has glucose concentration dependent hypoglycemic effect via the potentiation of glucose-induced insulin secretion and the suppression of glucagon secretion (Keshava et al., 2017; Deacon, 2020). Moreover, numerous studies have demonstrated GLP-1 has potential neuroprotective and neurotrophic effects (Liu et al., 2021), so that GLP-1 based therapies may have favorable effects on AD. Such as liraglutide (LRGT), dulaglutide, lixisenatide, exenatide, and NLY01 have a significantly association with lowering risk of AD (Akimoto et al., 2020). The anti-AD effect of GLP-1 receptor (GLP-1R) agonist (GLP-1RA) has attracted the attention of researcher.
Liraglutide improves memory impairment in various AD models, decreasing AD-related insulin receptor (INSR), synaptic and Tau pathology in specific brain regions (Batista et al., 2018; Duarte et al., 2020). These effects involve multiple pathways. In Aβ, specifically, LRGT attenuates brain estradiol and GLP-1 and activates protein kinase A (PKA) levels, oxidative/nitrosative stress and inflammation in 11-month-old AD female mice, reduces their cortical Aβ1–42 levels (Duarte et al., 2020). LRGT can both reduce the overproduction of Aβ and increase its removal. One side, amyloid precursor protein (APP) is metabolized to Aβ by β-secretases and γ-secretases. LRGT decreases the formation of Aβ via inhibiting the activity of β-secretases and γ-secretases (Qi et al., 2016; Zhang et al., 2019; Jantrapirom et al., 2020). The other, binding to GLP-1R, LRGT activates the phosphoinositide-3 kinase/mitogen-activated protein kinase (PI3K/MAPK) dependent pathways, consequently following trafficking and clearing Aβ by increasing the presence of Aβ transporters in cerebrospinal fluid (Wiciński et al., 2019). In Tau, LRGT also reduces p-Tau, Aβ, via the protein kinase B/glycogen synthase kinase-3β (Akt/GSK-3β) pathways, reversing the p-INSR whose major downstream signaling molecules include insulin substrate 1, Akt and GSK-3β (Chen et al., 2017). At the same time, LRGT can reduce hyperphosphorylation of Tau, neurofilaments (NFs) and neuronal degeneration through restoring protein phosphatase-2A (PP2A) activity and altering in c-Jun N-terminal protein kainse (JNK) and extracellular regulated protein kinases (ERK) signaling apparently (Zhang et al., 2019; Jantrapirom et al., 2020). Additionally, LRGT ameliorates mitochondrial dysfunction and prevents neuronal loss with activation of the cAMP/PKA pathway in the brain of 5 × FAD mice. Activating the cAMP/PKA pathway, GLP-1 increases the p-DRP-1-s637 and mitigates mitochondrial fragmentation in Aβ-treated astrocytes. Then it further improves the Aβ-induced energy failure, mitochondrial reactive oxygen species (ROS) overproduction, mitochondrial membrane potential (MMP) collapse, and cell toxicity in astrocytes (Xie et al., 2021). In addition, Dulaglutide decreases the hyperphosphorylation of Tau and NFs proteins through improving the PI3K/Akt/GSK-3β signaling pathway (Zhou et al., 2019). Lixisenatide also plays an important role in memory formation, synaptic plasticity and cell proliferation of rats. It can reduce amyloid plaques, NFTs and neuroinflammation in the hippocampi of 12-month-old 3 × Tg female mice, which may be related to activating PKA-cAMP response element binding (CREB) signaling pathway and inhibiting p38-MAPK (Cai et al., 2018).
Generally speaking, these protection effects to a large extent rely on multiple pathways with regulatory regulation of insulin signal pathways as the core, thereby removing neurotoxic substances (Aβ and/or Tau). At the same time, it is difficult to define whether the control of inflammation and the protection of mitochondria is the cause or result. In addition, it is worth noting that a large number of preclinical studies on it come from China. And limited clinical research, it is difficult to prove the complex connection between correlation and clinical effectiveness. It is necessary to design more randomized controlled trial such as ELAD Study (Femminella et al., 2019). The clinical trials of it are worthy of attention. We look forward to these random dual-blind experiments that can have good results.
PPAR-γ agonists
The peroxisome proliferator-activated receptor γ (PPAR-γ) is a prototypical ligand-activated nuclear receptor that coordinates lipid, glucose and energy metabolism. The PPAR-γ agonists have emerged as potent insulin sensitizers used in the treatment of T2DM. Pioglitazone (PGZ) is a member of the thiazolidinedione (TZD) family. In a pre-clinical study, it improves cognitive deficits in AD animal models by reducing Aβ levels. And it normalizes the p35 protein and p-CRMP2 levels in the cerebellum, ameliorates impaired motor coordination ability and long-term depression (LTD) in APP/PS1 mice at the pre-Aβ accumulation stage (Toba et al., 2016). It also enhances peripheral and brain insulin sensitivity in diet-induced insulin resistance model rats, ameliorates Aβ1–42 deposition in the hippocampus by increasing IDE and PPARγ expression. Notably, activating the PI3K/Akt/GSK-3β pathway is also demonstrated to serve a role in PGZ-induced Aβ1–42 degradation, which is abrogated by the PPARγ antagonist GW9662 (Yang et al., 2017). Furthermore, PGZ treatment could inhibit Cdk5 activity by decreasing p35 protein level. More importantly, PGZ corrects long-term potentiation (LTP) deficit caused by Aβ exposure in cultured slices and rescues impaired LTP and spatial memory (Badhwar et al., 2017). Although clinical studies have shown that PGZ has the potential of AD for treatment, the results of clinical trials are indeed unsatisfactory. Daily 0.8 mg oral PGZ did not significantly delay the onset of mild cognitive impairment due to AD (Burns et al., 2021). Interestingly, PGZ 15–30 mg demonstrates the greatest efficacy compared to placebo in network meta-analysis (Cao et al., 2018).
The rosiglitazone (RSG) improves hippocampus-dependent cognitive deficits in some AD patients and ameliorates deficits in the Tg2576 mouse for AD amyloidosis (Jahrling et al., 2014). Then the research further verified RSG treatment rescues cognitive deficits and reduces aberrant activity of granule neurons in the dentate gyrus (DG) (Hsu et al., 2017). Clinical trials of RSG have shown some contradictions. Early studies showed some anti-AD potential of RSG (Watson et al., 2005), while subsequent clinical trials fail to achieve the desired results (Harrington et al., 2011). Therefore, in clinical trials on RSG, screening for multiple subgroups in the AD patient population and enrolling patients using predictive biomarkers has received attention (O’Bryant et al., 2021). We speculate that with the further development of AD typing and biomarkers, such studies may bring new hope.
DPP-4 inhibitors
Different from GLP-1 agonists, dipeptidyl peptidase 4 inhibitors (DPP4i) do not possess inherent glucose-lowering activity. It inhibits the activity of the enzyme DPP4, then it decreases blood glucose level through GLP1 to treat T2DM (Stoian et al., 2020). DPP-4i contains saxagliptin, vildagliptin, linagliptin, sitagliptin. They have beneficial effects on amyloid aggregation and longitudinal cognitive outcome in diabetic AD-related cognitive impairment (ADCI) (Jeong et al., 2021). However, the mechanism by which they work seems different.
Sitagliptin has been demonstrated to have antioxidative and antiapoptotic properties by modifying glutamate and glutathione levels within the region of hippocampus in mice (El-Sahar et al., 2015). Meanwhile, it increases the synaptic proteins and the O-Glycosylation (Chen et al., 2019). Moreover, sitagliptin improves the impaired cognitive by the potential mechanisms that regulating neuroinflammation, antioxidation, and antiapoptotic, and the level of GLP-1 and GLP-1R (Wiciński et al., 2018). Finally achieve the goal of protecting learning and memory. Interestingly, preliminary clinical results show that sitagliptin’s improvement of AD patient mini-mental state examination (MMSE) scores is better than MET (Isik et al., 2017). With a higher selectivity, saxagliptin has the same effect as sitagliptin that protect learning and memory through GLP-1/GLP-1R signaling pathway (Kosaraju et al., 2013a; Chen et al., 2019). Like sitagliptin, linagliptin treatment mitigates the cognitive deficits that attributed to the improvement of incretin levels and attenuate Aβ, p-Tau and neuroinflammation in the brain mice of 3 × Tg-AD and Aβ1–42 induced rat model of AD (Kosaraju et al., 2017; Siddiqui et al., 2021). Moreover, linagliptin can ameliorate cognitive deficits through insulin pathway (Siddiqui et al., 2021) and restore the impaired insulin signaling caused by Aβ in neuronal cells (Kornelius et al., 2015). Vildagliptin also demonstrates a unique mechanism for Aβ and Tau clearance and reverses the cognitive deficits and pathology observed in AD possibly via modulating Klotho protein together with Akt pathway (Kosaraju et al., 2013b; Yossef et al., 2020). The addition of vildagliptin to treatment improved the copying subdomain of cognitive function and metabolic control of the older patients with T2DM (Ates Bulut et al., 2020).
These results indicate that DPP-4i drugs mainly improve inflammation and oxidative stress through the GLP-1/GLP-1R signaling pathway, affecting the production and clearance of toxic proteins, thereby improving cognitive function. But most of the studies are basic research, although there are a small number of clinical studies on sitagliptin and vildagliptin in cognition, but they are all preliminary and short-term, and the sample size is small. Our suggestion would be best to carry out the anti-AD research of DPP-4i after a breakthrough in the anti-AD research of GLP-1/GLP-1R or the combination of DP-4i and the first approved effective anti-AD drug.
SGLT2 inhibitors
Sodium glucose cotransporter 2 inhibitors (SGLT2i) can reduce blood glucose by inhibiting its reabsorption in proximal tubules and by promoting urinary glucose excretion. A growing numbers evidence indicates that SGLT2i such as empagliflozin (EMP), canagliflozin, dapagliflozin, ertugliflozin, and sotagliflozin have neuroprotective potential in a murine mixed model of T2DM and AD (Lin et al., 2014; Rizvi et al., 2014; Shaikh et al., 2016; Hierro-Bujalance et al., 2020).
Empagliflozin help to limit cortical thinning and reduce neuronal loss, hemorrhage, microglia burdens and SPs burden, also improves cerebral microvascular eventually ameliorate the cognitive deficits in APP/PS1xdb/db mice (Lin et al., 2014; Hierro-Bujalance et al., 2020). Dapagliflozin and invokana might act as potent dual inhibitors of SGLT2 and AchE, which contributes to cognitive improvement, as well as ertugliflozin and sotagliflozin (Rizvi et al., 2014; Shaikh et al., 2016). Glibenclamide (GBC) treatment improves memory impairment with increasing insulin and reducing glucose and hippocampal inflammation in rats with T2DM and sporadic AD (Esmaeili et al., 2020). And SGLT2i exert anti-inflammatory and antioxidant effects at the cellular level mainly via regulation of the molecular target of rapamycin (mTOR) pathway, which could ameliorate the progression of AD (Esterline et al., 2020; Katsenos et al., 2022). And in nested case control study evaluating diagnoses of dementia in patients with T2DM, SGLT2i use showed a 42% reduction in dementia risk (Wium-Andersen et al., 2019). Interestingly, in the population-based cohort study of T2DM patients treated with SGLT2i and DPP4i, the use of SGLT2i is associated with lower risks of dementia, compared with DPP4i (Mui et al., 2021). And a prospective study shows significant beneficial effects of the EMP on cognitive in frail older adults with diabetes (Mone et al., 2022). In addition, SGLT2I’s ≥ 3 years use is related to the improvement of cognitive scores (Low et al., 2022). According to the existing evidence, long-term use of SGLT2i can improve cognitive function, especially for elderly diabetics. However, the role of AD patients still needs further study.
Conclusion
Based on the facts that T2DM and AD share common features, drugs used to treat T2DM are being investigated for efficacy in AD. Consequently, studies on drugs used for T2DM in AD found these treatments may represent a promising approach to fight AD, which include MET, GLP-1RA, PPAR-γ agonists, DPP-4i and SGLT2i (Cao et al., 2018). However, there are differences in their effects in basic and clinical research on anti-AD (Table 1). At the same time, the anti-AD effect of insulin is also controversial, but there are too many studies involved, so this review will not discuss it for the time being. We believe that the difference between the results of clinical antidiabetic medication in anti-AD treatment clinical trials and basic experiments is mainly related to the following: (1) We speculate that they are not effective for all types of AD, but may be a special type: AD patients who also suffer from diabetes. They may even be useful only for cognitive dysfunction caused by insulin resistance. (2) These effects interact with the improvement of insulin resistance, so perhaps early intervention may have a better effect. (3) Complex and interactive-oxidation, anti-neuroinflammation, and improve energy metabolism play an important role in it, so the combination of drugs to treat AD may have more potential. Such drugs are not a very good solution under the existing evidence conditions. Looking forward to more refined pathological research on AD classification, it may rekindle hope for the clinical research of such drugs.
But screening subjects based on more subtypes, or recruiting patients using predictive biomarkers, would severely narrow the pool of subjects who ultimately meet inclusion criteria and would substantially increase the cost of clinical trials. Unless a reasonable combination of predictive biomarkers can be found, or there is a well-defined classification of AD subtypes. Otherwise, it will still be a bottomless pit to rush to carry out relevant and more refined clinical trials, and it is not worth investing too much energy. Moreover, hypoglycemia, the side effect of such drugs, is still not negligible. In the elderly, falls caused by hypoglycemia often cause serious consequences. Therefore, we have to consider the scope of application of this type of drug and the direction that needs to be considered in the design of such drugs. It is best to regulate the insulin pathway and have little effect on blood glucose (or be able to control blood glucose stably within a reasonable range). Weighing the pros and cons is an unavoidable multiple-choice question in drug development. In addition to genes, diabetes is often closely related to eating habits, and intestinal flora also play a key role in it. Whether these drugs affect the intestinal flora and thus affect AD is also an aspect worthy of attention. It is also worth noting that in the absence of strong evidence-based medical evidence, the use of hypoglycemic drugs for the prevention and treatment of AD will face many risks.
In the current situation, we should not be pessimistic. While looking forward to the progress of basic research on AD, we should more actively conduct group statistics or subtype analysis on existing failed clinical trials, especially large-sample clinical trials. Not only may there be unexpected surprises, but it will also play a guiding role in the development of future clinical trials.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the Funds of National Natural Science Foundation of China (Nos. 82060728 and U18243) and Shijingshan’s Tutor Studio of Pharmacology (No. GZS-2016-07).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akimoto, H., Negishi, A., Oshima, S., Wakiyama, H., Okita, M., Horii, N., et al. (2020). Antidiabetic drugs for the risk of Alzheimer disease in patients with type 2 DM using FAERS. Am. J. Alzheimers Dis. Other Demen. 35:1533317519899546. doi: 10.1177/1533317519899546
An, J., Zhou, Y., Zhang, M., Xie, Y., Ke, S., Liu, L., et al. (2019). Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5×FAD mouse model of Alzheimer’s disease. Behav. Brain Res. 370:111932. doi: 10.1016/j.bbr.2019.111932
Ates Bulut, E., Sahin Alak, Z. Y., Dokuzlar, O., Kocyigit, S. E., Soysal, P., Smith, L., et al. (2020). Cognitive and metabolic outcomes of vildagliptin addition to the therapy in patients with type 2 diabetes mellitus: 26 week follow-up study. Arch. Gerontol. Geriatr. 88:104013. doi: 10.1016/j.archger.2020.104013
Badhwar, A., Brown, R., Stanimirovic, D. B., Haqqani, A. S., and Hamel, E. (2017). Proteomic differences in brain vessels of Alzheimer’s disease mice: normalization by PPARγ agonist pioglitazone. J. Cereb. Blood Flow Metab. 37, 1120–1136. doi: 10.1177/0271678x16655172
Batista, A. F., Forny-Germano, L., Clarke, J. R., Lyra, E. S. N. M., Brito-Moreira, J., Boehnke, S. E., et al. (2018). The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J. Pathol. 245, 85–100. doi: 10.1002/path.5056
Burns, D. K., Alexander, R. C., Welsh-Bohmer, K. A., Culp, M., Chiang, C., O’Neil, J., et al. (2021). Safety and efficacy of pioglitazone for the delay of cognitive impairment in people at risk of Alzheimer’s disease (TOMMORROW): a prognostic biomarker study and a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 20, 537–547. doi: 10.1016/s1474-4422(21)00043-0
Cai, H. Y., Yang, J. T., Wang, Z. J., Zhang, J., Yang, W., Wu, M. N., et al. (2018). Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 495, 1034–1040. doi: 10.1016/j.bbrc.2017.11.114
Cao, B., Rosenblat, J. D., Brietzke, E., Park, C., Lee, Y., Musial, N., et al. (2018). Comparative efficacy and acceptability of antidiabetic agents for Alzheimer’s disease and mild cognitive impairment: a systematic review and network meta-analysis. Diabetes Obes. Metab. 20, 2467–2471. doi: 10.1111/dom.13373
Chen, S., Sun, J., Zhao, G., Guo, A., Chen, Y., Fu, R., et al. (2017). Liraglutide improves water maze learning and memory performance while reduces hyperphosphorylation of tau and neurofilaments in APP/PS1/tau triple transgenic mice. Neurochem. Res. 42, 2326–2335. doi: 10.1007/s11064-017-2250-8
Chen, S., Zhou, M., Sun, J., Guo, A., Fernando, R. L., Chen, Y., et al. (2019). DPP-4 inhibitor improves learning and memory deficits and AD-like neurodegeneration by modulating the GLP-1 signaling. Neuropharmacology 157:107668. doi: 10.1016/j.neuropharm.2019.107668
Chen, Y., Zhao, S., Fan, Z., Li, Z., Zhu, Y., Shen, T., et al. (2021). Metformin attenuates plaque-associated tau pathology and reduces amyloid-β burden in APP/PS1 mice. Alzheimers Res. Ther. 13:40. doi: 10.1186/s13195-020-00761-9
Chiang, M. C., Cheng, Y. C., Chen, S. J., Yen, C. H., and Huang, R. N. (2016). Metformin activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against Amyloid-beta-induced mitochondrial dysfunction. Exp. Cell Res. 347, 322–331. doi: 10.1016/j.yexcr.2016.08.013
Chung, J. Y., Kim, H. S., and Song, J. (2018). Iron metabolism in diabetes-induced Alzheimer’s disease: a focus on insulin resistance in the brain. Biometals 31, 705–714. doi: 10.1007/s10534-018-0134-2
Deacon, C. F. (2020). Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 16, 642–653. doi: 10.1038/s41574-020-0399-8
Dekeryte, R., Franklin, Z., Hull, C., Croce, L., Kamli-Salino, S., Helk, O., et al. (2021). The BACE1 inhibitor LY2886721 improves diabetic phenotypes of BACE1 knock-in mice. Biochim. Biophys. Acta Mol. Basis of Dis. 1867:166149. doi: 10.1016/j.bbadis.2021.166149
DiTacchio, K. A., Heinemann, S. F., and Dziewczapolski, G. (2015). Metformin treatment alters memory function in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 44, 43–48. doi: 10.3233/jad-141332
Doherty, G. H., Beccano-Kelly, D., Yan, S. D., Gunn-Moore, F. J., and Harvey, J. (2013). Leptin prevents hippocampal synaptic disruption and neuronal cell death induced by amyloid β. Neurobiol. Aging 34, 226–237. doi: 10.1016/j.neurobiolaging.2012.08.003
Duarte, A. I., Candeias, E., Alves, I. N., Mena, D., Silva, D. F., Machado, N. J., et al. (2020). Liraglutide protects against brain amyloid-β(1-42) accumulation in female mice with early Alzheimer’s disease-like pathology by partially rescuing oxidative/nitrosative stress and inflammation. Int. J. Mol. Sci. 21:1746. doi: 10.3390/ijms21051746
El-Sahar, A. E., Safar, M. M., Zaki, H. F., Attia, A. S., and Ain-Shoka, A. A. (2015). Sitagliptin attenuates transient cerebral ischemia/reperfusion injury in diabetic rats: implication of the oxidative-inflammatory-apoptotic pathway. Life Sci. 126, 81–86. doi: 10.1016/j.lfs.2015.01.030
Esmaeili, M. H., Enayati, M., Khabbaz Abkenar, F., Ebrahimian, F., and Salari, A.-A. (2020). Glibenclamide mitigates cognitive impairment and hippocampal neuroinflammation in rats with type 2 diabetes and sporadic Alzheimer-like disease. Behav. Brain Res. 379:112359. doi: 10.1016/j.bbr.2019.112359
Esterline, R., Oscarsson, J., and Burns, J. (2020). A role for sodium glucose cotransporter 2 inhibitors (SGLT2is) in the treatment of Alzheimer’s disease? Int. Rev. Neurobiol. 155, 113–140. doi: 10.1016/bs.irn.2020.03.018
Femminella, G. D., Frangou, E., Love, S. B., Busza, G., Holmes, C., Ritchie, C., et al. (2019). Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomised controlled trial (ELAD study). Trials 20:191. doi: 10.1186/s13063-019-3259-x
Gharibyan, A. L., Islam, T., Pettersson, N., Golchin, S. A., Lundgren, J., Johansson, G., et al. (2020). Apolipoprotein E Interferes with IAPP Aggregation and Protects Pericytes from IAPP-Induced Toxicity. Biomolecules 10:134. doi: 10.3390/biom10010134
Ha, J., Choi, D. W., Kim, K. J., Cho, S. Y., Kim, H., Kim, K. Y., et al. (2021). Association of metformin use with Alzheimer’s disease in patients with newly diagnosed type 2 diabetes: a population-based nested case-control study. Sci. Rep. 11:24069. doi: 10.1038/s41598-021-03406-5
Harrington, C., Sawchak, S., Chiang, C., Davies, J., Donovan, C., Saunders, A. M., et al. (2011). Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr. Alzheimer Res. 8, 592–606. doi: 10.2174/156720511796391935
Hierro-Bujalance, C., Infante-Garcia, C., Del Marco, A., Herrera, M., Carranza-Naval, M. J., Suarez, J., et al. (2020). Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimers Res. Ther. 12:40. doi: 10.1186/s13195-020-00607-4
Hsu, W. J., Wildburger, N. C., Haidacher, S. J., Nenov, M. N., Folorunso, O., Singh, A. K., et al. (2017). PPARgamma agonists rescue increased phosphorylation of FGF14 at S226 in the Tg2576 mouse model of Alzheimer’s disease. Exp. Neurol. 295, 1–17. doi: 10.1016/j.expneurol.2017.05.005
Isik, A. T., Soysal, P., Yay, A., and Usarel, C. (2017). The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s disease. Diabetes Res. Clin. Pract. 123, 192–198. doi: 10.1016/j.diabres.2016.12.010
Jahrling, J. B., Hernandez, C. M., Denner, L., and Dineley, K. T. (2014). PPARγ recruitment to active ERK during memory consolidation is required for Alzheimer’s disease-related cognitive enhancement. J. Neurosci. 34, 4054–4063. doi: 10.1523/jneurosci.4024-13.2014
Jantrapirom, S., Nimlamool, W., Chattipakorn, N., Chattipakorn, S., Temviriyanukul, P., Inthachat, W., et al. (2020). Liraglutide suppresses tau hyperphosphorylation, amyloid beta accumulation through regulating neuronal insulin signaling and BACE-1 activity. Int. J. Mol. Sci. 21:1725. doi: 10.3390/ijms21051725
Jeong, S. H., Kim, H. R., Kim, J., Kim, H., Hong, N., Jung, J. H., et al. (2021). Association of dipeptidyl peptidase-4 inhibitor use and amyloid burden in patients with diabetes and AD-related cognitive impairment. Neurology 97:e1110. doi: 10.1212/WNL.0000000000012534
Karlawish, J., and Grill, J. D. (2021). The approval of Aduhelm risks eroding public trust in Alzheimer research and the FDA. Nat. Rev. Neurol. 17, 523–524. doi: 10.1038/s41582-021-00540-6
Katsenos, A. P., Davri, A. S., Simos, Y. V., Nikas, I. P., Bekiari, C., Paschou, S. A., et al. (2022). New treatment approaches for Alzheimer’s disease: preclinical studies and clinical trials centered on antidiabetic drugs. Expert. Opin. Investig. Drugs 31, 105–123. doi: 10.1080/13543784.2022.2022122
Keshava, H. B., Mowla, A., Heinberg, L. J., Schauer, P. R., Brethauer, S. A., and Aminian, A. (2017). Bariatric surgery may reduce the risk of Alzheimer’s diseases through GLP-1 mediated neuroprotective effects. Med. Hypotheses 104, 4–9. doi: 10.1016/j.mehy.2017.05.002
Kornelius, E., Lin, C. L., Chang, H. H., Li, H. H., Huang, W. N., Yang, Y. S., et al. (2015). DPP-4 inhibitor linagliptin attenuates Aβ-induced cytotoxicity through activation of AMPK in neuronal cells. CNS Neurosci. Ther. 21, 549–557. doi: 10.1111/cns.12404
Kosaraju, J., Gali, C. C., Khatwal, R. B., Dubala, A., Chinni, S., Holsinger, R. M., et al. (2013a). Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology 72, 291–300. doi: 10.1016/j.neuropharm.2013.04.008
Kosaraju, J., Holsinger, R. M. D., Guo, L., and Tam, K. Y. (2017). Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer’s disease. Mol. Neurobiol. 54, 6074–6084. doi: 10.1007/s12035-016-0125-7
Kosaraju, J., Murthy, V., Khatwal, R. B., Dubala, A., Chinni, S., Muthureddy Nataraj, S. K., et al. (2013b). Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J. Pharm. Pharmacol. 65, 1773–1784. doi: 10.1111/jphp.12148
Li, M., Li, Y., Liu, Y., Huang, H., Leng, X., Chen, Y., et al. (2021). Altered hippocampal subfields volumes is associated with memory function in type 2 diabetes mellitus. Front. Neurol. 12:756500. doi: 10.3389/fneur.2021.756500
Lin, B., Koibuchi, N., Hasegawa, Y., Sueta, D., Toyama, K., Uekawa, K., et al. (2014). Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 13:148. doi: 10.1186/s12933-014-0148-1
Liu, X. Y., Zhang, N., Zhang, S. X., and Xu, P. (2021). Potential new therapeutic target for Alzheimer’s disease: glucagon-like peptide-1. Eur. J. Neurosci. 54, 7749–7769. doi: 10.1111/ejn.15502
Low, S., Goh, K. S., Ng, T. P., Moh, A., Ang, S. F., Wang, J., et al. (2022). Association between use of sodium-glucose co-transporter-2 (SGLT2) inhibitors and cognitive function in a longitudinal study of patients with type 2 diabetes. J. Alzheimers Dis. 87, 635–642. doi: 10.3233/jad-215678
Lu, X. Y., Huang, S., Chen, Q. B., Zhang, D., Li, W., Ao, R., et al. (2020). Metformin ameliorates aβ pathology by insulin-degrading enzyme in a transgenic mouse model of Alzheimer’s disease. Oxid. Med. Cell Longev. 2020:2315106. doi: 10.1155/2020/2315106
Luo, A., Ning, P., Lu, H., Huang, H., Shen, Q., Zhang, D., et al. (2022). Association between metformin and Alzheimer’s disease: a systematic review and meta-analysis of clinical observational studies. J. Alzheimers Dis. 88, 1311–1323. doi: 10.3233/jad-220180
Ma, T., Tian, X., Zhang, B., Li, M., Wang, Y., Yang, C., et al. (2022). Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 603, 159–165. doi: 10.1038/s41586-022-04431-8
Markowicz-Piasecka, M., Sikora, J., Szydłowska, A., Skupień, A., Mikiciuk-Olasik, E., and Huttunen, K. M. (2017). Metformin – a future therapy for neurodegenerative diseases. Pharm. Res. 34, 2614–2627. doi: 10.1007/s11095-017-2199-y
Marucci, G., Buccioni, M., Ben, D. D., Lambertucci, C., Volpini, R., and Amenta, F. (2021). Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 190:108352. doi: 10.1016/j.neuropharm.2020.108352
Mone, P., Lombardi, A., Gambardella, J., Pansini, A., Macina, G., Morgante, M., et al. (2022). Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care 45, 1247–1251. doi: 10.2337/dc21-2434
Mui, J. V., Zhou, J., Lee, S., Leung, K. S. K., Lee, T. T. L., Chou, O. H. I., et al. (2021). Sodium-glucose cotransporter 2 (SGLT2) inhibitors vs. dipeptidyl peptidase-4 (DPP4) inhibitors for new-onset dementia: a propensity score-matched population-based study with competing risk analysis. Front. Cardiovasc. Med. 8:747620. doi: 10.3389/fcvm.2021.747620
Noreen, Z., DeJesus, J., Bhatti, A., Loffredo, C. A., John, P., Khan, J. S., et al. (2018). Epidemiological investigation of type 2 diabetes and Alzheimer’s disease in a Pakistani population. Int. J. Environ. Res. Public Health 15:1582. doi: 10.3390/ijerph15081582
O’Bryant, S. E., Zhang, F., Petersen, M., Johnson, L., Hall, J., and Rissman, R. A. (2021). A precision medicine approach to treating Alzheimer’s disease using rosiglitazone therapy: a biomarker analysis of the REFLECT trials. J. Alzheimers Dis. 81, 557–568. doi: 10.3233/jad-201610
Oliveira, W. H., Braga, C. F., Lós, D. B., Araújo, S. M. R., França, M. R., Duarte-Silva, E., et al. (2021). Metformin prevents p-tau and amyloid plaque deposition and memory impairment in diabetic mice. Exp. Brain Res. 239, 2821–2839. doi: 10.1007/s00221-021-06176-8
Pomilio, C., Pérez, N. G., Calandri, I., Crivelli, L., Allegri, R., Sevlever, G., et al. (2022). Diabetic patients treated with metformin during early stages of Alzheimer’s disease show a better integral performance: data from ADNI study. Geroscience 44, 1791–1805. doi: 10.1007/s11357-022-00568-6
Qi, L., Ke, L., Liu, X., Liao, L., Ke, S., Liu, X., et al. (2016). Subcutaneous administration of Liraglutide ameliorates learning and memory impairment by modulating tau hyperphosphorylation via the glycogen synthase kinase-3β pathway in an amyloid β protein induced Alzheimer disease mouse model. Eur. J. Pharmacol. 783, 23–32. doi: 10.1016/j.ejphar.2016.04.052
Rizvi, S. M., Shakil, S., Biswas, D., Shakil, S., Shaikh, S., Bagga, P., et al. (2014). Invokana (Canagliflozin) as a dual inhibitor of acetylcholinesterase and sodium glucose co-transporter 2: advancement in Alzheimer’s disease- diabetes type 2 linkage via an enzoinformatics study. CNS Neurol. Disord. Drug Targets 13, 447–451. doi: 10.2174/18715273113126660160
Shaikh, S., Rizvi, S. M., Shakil, S., Riyaz, S., Biswas, D., and Jahan, R. (2016). Forxiga (dapagliflozin): plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol. Appl. Biochem. 63, 145–150. doi: 10.1002/bab.1319
Siddiqui, N., Ali, J., Parvez, S., Zameer, S., Najmi, A. K., and Akhtar, M. (2021). Linagliptin, a DPP-4 inhibitor, ameliorates Aβ (1-42) peptides induced neurodegeneration and brain insulin resistance (BIR) via insulin receptor substrate-1 (IRS-1) in rat model of Alzheimer’s disease. Neuropharmacology 195:108662. doi: 10.1016/j.neuropharm.2021.108662
Sluggett, J. K., Koponen, M., Bell, J. S., Taipale, H., Tanskanen, A., Tiihonen, J., et al. (2020). Metformin and risk of Alzheimer’s disease among community-dwelling people with diabetes: a national case-control study. J. Clin. Endocrinol. Metab. 105:dgz234. doi: 10.1210/clinem/dgz234
Steen, E., Terry, B. M., Rivera, E. J., Cannon, J. L., Neely, T. R., Tavares, R., et al. (2005). Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J. Alzheimers Dis. 7, 63–80. doi: 10.3233/jad-2005-7107
Stoian, A. P., Sachinidis, A., Stoica, R. A., Nikolic, D., Patti, A. M., and Rizvi, A. A. (2020). The efficacy and safety of dipeptidyl peptidase-4 inhibitors compared to other oral glucose-lowering medications in the treatment of type 2 diabetes. Metabolism 109:154295. doi: 10.1016/j.metabol.2020.154295
Sun, Y., Ma, C., Sun, H., Wang, H., Peng, W., Zhou, Z., et al. (2020). Metabolism: a novel shared link between diabetes mellitus and Alzheimer’s disease. J. Diabetes Res. 2020:4981814. doi: 10.1155/2020/4981814
Takeuchi, S., Ueda, N., Suzuki, K., Shimozawa, N., Yasutomi, Y., and Kimura, N. (2019). Elevated membrane cholesterol disrupts lysosomal degradation to induce β-amyloid accumulation: the potential mechanism underlying augmentation of β-amyloid pathology by type 2 diabetes mellitus. Am. J. Pathol. 189, 391–404. doi: 10.1016/j.ajpath.2018.10.011
The Lancet (2022). Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet 400:1899. doi: 10.1016/s0140-6736(22)02480-1
Toba, J., Nikkuni, M., Ishizeki, M., Yoshii, A., Watamura, N., Inoue, T., et al. (2016). PPARγ agonist pioglitazone improves cerebellar dysfunction at pre-Aβ deposition stage in APPswe/PS1dE9 Alzheimer’s disease model mice. Biochem. Biophys. Res. Commun. 473, 1039–1044. doi: 10.1016/j.bbrc.2016.04.012
Watson, G. S., Cholerton, B. A., Reger, M. A., Baker, L. D., Plymate, S. R., Asthana, S., et al. (2005). Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am. J. Geriatr. Psychiatry 13, 950–958. doi: 10.1176/appi.ajgp.13.11.950
Wiciński, M., Socha, M., Malinowski, B., Wódkiewicz, E., Walczak, M., Górski, K., et al. (2019). Liraglutide and its neuroprotective properties-focus on possible biochemical mechanisms in Alzheimer’s disease and cerebral ischemic events. Int. J. Mol. Sci. 20:1050. doi: 10.3390/ijms20051050
Wiciński, M., Wódkiewicz, E., Słupski, M., Walczak, M., Socha, M., Malinowski, B., et al. (2018). Neuroprotective activity of sitagliptin via reduction of neuroinflammation beyond the incretin effect: focus on Alzheimer’s disease. Biomed. Res. Int. 2018:6091014. doi: 10.1155/2018/6091014
Wium-Andersen, I. K., Osler, M., Jørgensen, M. B., Rungby, J., and Wium-Andersen, M. K. (2019). Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur. J. Endocrinol. 181, 499–507. doi: 10.1530/eje-19-0259
Xiao, S., Chan, P., Wang, T., Hong, Z., Wang, S., Kuang, W., et al. (2021). A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res. Ther. 13:62. doi: 10.1186/s13195-021-00795-7
Xie, Y., Zheng, J., Li, S., Li, H., Zhou, Y., Zheng, W., et al. (2021). GLP-1 improves the neuronal supportive ability of astrocytes in Alzheimer’s disease by regulating mitochondrial dysfunction via the cAMP/PKA pathway. Biochem. Pharmacol. 188:114578. doi: 10.1016/j.bcp.2021.114578
Yang, S., Chen, Z., Cao, M., Li, R., Wang, Z., and Zhang, M. (2017). Pioglitazone ameliorates Aβ42 deposition in rats with diet-induced insulin resistance associated with AKT/GSK3β activation. Mol. Med. Rep. 15, 2588–2594. doi: 10.3892/mmr.2017.6342
Yen, Y. C., Kammeyer, A. M., Tirlangi, J., Ghosh, A. K., and Mesecar, A. D. (2021). A structure-based discovery platform for BACE2 and the development of selective BACE inhibitors. ACS Chem. Neurosci. 12, 581–588. doi: 10.1021/acschemneuro.0c00629
Yossef, R. R., Al-Yamany, M. F., Saad, M. A., and El-Sahar, A. E. (2020). Neuroprotective effects of vildagliptin on drug induced Alzheimer’s disease in rats with metabolic syndrome: role of hippocampal klotho and AKT signaling pathways. Eur. J. Pharmacol. 889:173612. doi: 10.1016/j.ejphar.2020.173612
Zhang, Y., Huang, N. Q., Yan, F., Jin, H., Zhou, S. Y., Shi, J. S., et al. (2018). Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 339, 57–65. doi: 10.1016/j.bbr.2017.11.015
Zhang, Y., Xie, J. Z., Xu, X. Y., Hu, J., Xu, T., Jin, S., et al. (2019). Liraglutide ameliorates hyperhomocysteinemia-induced alzheimer-like pathology and memory deficits in rats via multi-molecular targeting. Neurosci. Bull. 35, 724–734. doi: 10.1007/s12264-018-00336-7
Zheng, J., Xu, M., Walker, V., Yuan, J., Korologou-Linden, R., Robinson, J., et al. (2022). Evaluating the efficacy and mechanism of metformin targets on reducing Alzheimer’s disease risk in the general population: a Mendelian randomisation study. Diabetologia 65, 1664–1675. doi: 10.1007/s00125-022-05743-0
Keywords: Alzheimer’s disease, type 3 diabetes mellitus (T3DM), antidiabetic medication, type 2 diabetes mellitus, clinical research
Citation: Huang J, Huang N, Cui D, Shi J and Qiu Y (2023) Clinical antidiabetic medication used in Alzheimer’s disease: From basic discovery to therapeutics development. Front. Aging Neurosci. 15:1122300. doi: 10.3389/fnagi.2023.1122300
Received: 12 December 2022; Accepted: 30 January 2023;
Published: 10 February 2023.
Edited by:
Ju Gao, The University of Arizona, United StatesReviewed by:
Hans-Ulrich Demuth, Fraunhofer Institute for Cell Therapy and Immunology (IZI), GermanyCopyright © 2023 Huang, Huang, Cui, Shi and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Qiu,  qiuy@shsmu.edu.cn; Jingshan Shi,
qiuy@shsmu.edu.cn; Jingshan Shi,  shijs@zmu.edu.cn
shijs@zmu.edu.cn
†These authors have contributed equally to this work
 Juan Huang
Juan Huang Nanqu Huang
Nanqu Huang Di Cui2
Di Cui2  Jingshan Shi
Jingshan Shi