Melatonin as a Potential Neuroprotectant: Mechanisms in Subarachnoid Hemorrhage-Induced Early Brain Injury
- 1Department of Neurosurgery, The Children’s Hospital Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 2Department of Outpatient, The Children’s Hospital Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 3Department of Pharmacy, The Children’s Hospital Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
Subarachnoid hemorrhage (SAH) is a common cerebrovascular disease with high mortality and disability rates. Despite progressive advances in drugs and surgical techniques, neurological dysfunction in surviving SAH patients have not improved significantly. Traditionally, vasospasm has been considered the main cause of death and disability following SAH, but anti-vasospasm therapy has not benefited clinical prognosis. Many studies have proposed that early brain injury (EBI) may be the primary factor influencing the prognosis of SAH. Melatonin is an indole hormone and is the main hormone secreted by the pineal gland, with low daytime secretion levels and high nighttime secretion levels. Melatonin produces a wide range of biological effects through the neuroimmune endocrine network, and participates in various physiological activities in the central nervous system, reproductive system, immune system, and digestive system. Numerous studies have reported that melatonin has extensive physiological and pharmacological effects such as anti-oxidative stress, anti-inflammation, maintaining circadian rhythm, and regulating cellular and humoral immunity. In recent years, more and more studies have been conducted to explore the molecular mechanism underlying melatonin-induced neuroprotection. The studies suggest beneficial effects in the recovery of intracerebral hemorrhage, cerebral ischemia-reperfusion injury, spinal cord injury, Alzheimer’s disease, Parkinson’s disease and meningitis through anti-inflammatory, antioxidant and anti-apoptotic mechanisms. This review summarizes the recent studies on the application and mechanism of melatonin in SAH.
Introduction
Subarachnoid hemorrhage (SAH) is one of the common cerebrovascular diseases, and its incidence varies in different countries and regions; the overall incidence is about 6/100,000 people per year (Etminan et al., 2019). The incidence of SAH gradually increases with age. Due to the advances in medical and surgical techniques, the mortality rate of SAH has decreased over the past few decades but still remains prevalent (Macdonald and Schweizer, 2017). In addition, 33% of SAH survivors experienced a high disability rate and required long-term care (Al-Khindi et al., 2010). Vasospasm has traditionally been considered a major cause of death and disability post-SAH as it can induce delayed cerebral ischemia. Therefore, most of the studies in the past decades have focused on reducing vasospasm with the aim of improving outcomes of SAH patients (Kassell et al., 1985; Macdonald and Weir, 1991; Cook, 1995; Crowley et al., 2008; Macdonald, 2016; Etminan and Macdonald, 2021). However, delayed cerebral ischemia still occurs in a considerable proportion of SAH patients, even if the cerebral vasospasm is reversed in the early stage. In addition, not all cerebral infarction after SAH is caused by cerebral vasospasm in clinical practice. Cerebral infarction may occur immediately after the occurrence of SAH in some patients, without any cerebral angiography findings (Naidech et al., 2006). Several recent large clinical trials have suggested that treating vasospasm does not significantly improve patient outcomes (Macdonald et al., 2008, 2011; Shen et al., 2013). It is suggested that there may be other mechanisms of injury affecting the prognosis of SAH patients. Recently, the concept of early brain injury (EBI) was proposed, which refers to brain injury occurring within 72 h after the occurrence of SAH (Cahill and Zhang, 2009). EBI is an event with complex pathophysiological changes, including increased intracranial pressure, decreased cerebral blood flow, and direct hematoma toxicity to the brain tissue. These subsequently lead to the destruction of the blood-brain barrier (BBB), oxidative stress injury, cellular death, inflammatory response, microcirculation dysfunction and mitochondrial disorder, causing neurologic injury and poor outcome after SAH (Cahill et al., 2006; Ostrowski et al., 2006; Sehba et al., 2012; Fujii et al., 2013; Ji and Chen, 2016). Therefore, exploring efficient therapeutic methods targeting EBI is essential in treating SAH.
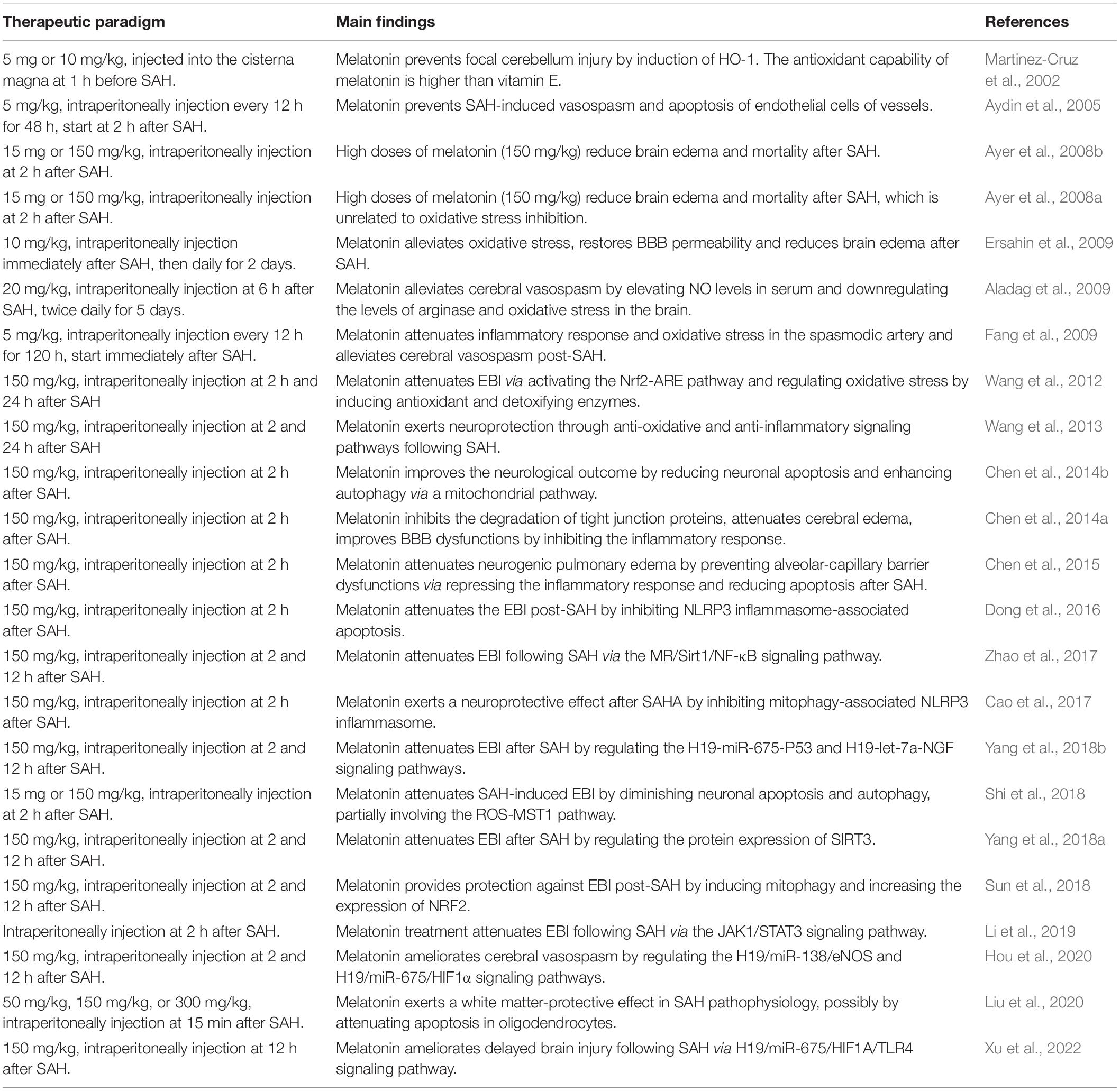
Melatonin is a type of neuroendocrine hormone produced by the pineal gland, with low daytime levels and high nighttime levels (Tordjman et al., 2017; Cardinali, 2021). It participates in regulating the immune, reproductive, endocrine and central nervous systems, and has attracted widespread attention due to its strong anti-inflammatory and antioxidant properties (Majidinia et al., 2018; He et al., 2021; Kvetnoy et al., 2022). Recent studies have shown that melatonin exerts a neuroprotective role in many neurological diseases such as stroke (Lee et al., 2007; Tai et al., 2010), trauma injury (Samantaray et al., 2009; Osier et al., 2018), neurodegenerative diseases (Alamdari et al., 2021; Roy et al., 2022), and spinal cord injury (Hong et al., 2010; Zhang et al., 2018). The mechanisms involved include anti-inflammation, anti-apoptosis, anti-oxidative stress, and BBB protection. In addition, numerous studies have been carried out on the role and mechanism of melatonin in SAH, and the results established that melatonin can improve brain injury after SAH through a variety of mechanisms, thus alleviating neurological impairment and improving prognosis (Martinez-Cruz et al., 2002). This study summarizes the current literature on melatonin treatment in EBI after SAH (Table 1). It explores the relevant mechanism of melatonin-induced neuroprotection, providing a theoretical basis for the experimental study and clinical application of melatonin treatment for SAH.
The Effect and Mechanism of Melatonin in Subarachnoid Hemorrhage
Melatonin and Vasospasm
Cerebral vasospasm is a common complication of SAH, which usually appears around 3 days after SAH, and reaches its peak within 10 days after SAH (Etminan et al., 2011; Gaspard, 2020). Delayed cerebral ischemia caused by cerebral vasospasm leads to cerebral infarction, cerebral hernia and other malignant complications (Kumar et al., 2016; Ikram et al., 2021). In the acute stage following SAH, the nitric oxide (NO)/NO synthase (NOS) system and vasoconstrictor factors may be involved in the occurrence of cerebral vasospasm. The NO/NOS system plays an important role regulating hemodynamics. NO regulates cerebral blood flow and blood pressure by dilating blood vessels, inhibiting platelet aggregation, and diminishing leukocyte adhesion to the intima (Toda et al., 2009; Attia et al., 2015; Vanhoutte, 2018). After SAH onset, decreased NO levels are observed, leading to CBF reduction, cerebral vasoconstriction, and platelet aggregation. In addition, as a form of free radical, NO enters the intima cells, causing damage to mitochondria and blood vessels (Sehba and Bederson, 2011; Crobeddu et al., 2016; Ehlert et al., 2016; Guo et al., 2016). Endothelin-1 (ET-1) is a potential vasoconstrictor mainly released by astrocytes and leukocytes during the early inflammatory response stage after SAH (Cosentino and Katusić, 1994; La and Reid, 1995; Vergouwen et al., 2012). Previous studies found that ET-1 levels in serum and plasma increased within a few minutes after SAH, and the expression of its receptor increased within 48 h (Lin et al., 2004, 2006). The declined levels of NO increases the expression of ET-1, causing continuous vasocontraction and degenerative morphological changes of the vessels. Notably, previous studies mainly focus on the spasms of large blood vessels, while ignoring the microvascular changes. The cerebral microvasculature has been recently identified as an important intervention target after SAH. Changes in the anatomical structure of cerebral microvessels, sufficient to cause functional deficits, are found in the early post-SAH period. After SAH occurrence, constriction of microvessels contributes to intima remodeling, basal lamina degradation, increased vascular permeability, and eventually leads to microcirculation disorders and brain injury (Nagai et al., 1976; Sehba and Friedrich, 2011).
To explore whether melatonin treatment reverses vasospasm in a SAH model, light microscopic measurements of the basilar arteries were performed to illustrate the arterial wall changes (Fang et al., 2009). Melatonin injection simultaneously with SAH or 2 h after SAH both were found to attenuate the constriction of vessels (Aydin et al., 2005). Additionally, melatonin-induced improvement of cerebral vasospasm is associated with increased serum NO levels, decreased arginase levels, and oxidative stress in the brain (Aladag et al., 2009). Furthermore, the potential underlying mechanism of melatonin treatment after SAH was studied. Hou et al. (2020) reported that melatonin ameliorates post-SAH vasospasm by regulating the expression of endothelial nitric oxide synthase (eNOS) and hypoxia-inducible factor-1 (HIF-1α) via the H19/miR-138/eNOS/NO and H19/miR-675/HIF-1α signaling pathways. However, the crosstalk of the pathway network is complex, and the exact mechanism behind the anti-vasospasm effect of melatonin needs further research. Recently, a clinical trial provided evidence for the delayed elevation of circulatory daytime melatonin after SAH and described the role of aneurysm location, resulting in high levels during the critical phase (Neumaier et al., 2021). However, the relationship between endogenous melatonin level changes and vasospasm were not discussed. Further clinical trials focusing on the role of endogenous changes and administration of melatonin in vasospasm after SAH may provide more clinical evidence for the clinical application of melatonin in SAH.
Melatonin and Inflammation
Previous studies have found that in the early SAH stage, erythrocyte degradation products accumulated in the subarachnoid space, activating an inflammatory response and participating in the acceleration of brain injury (Schallner et al., 2015; Zhang et al., 2016). Both experimental studies and clinical trials have demonstrated that the aseptic inflammatory response after SAH can aggravate tissue damage and is an independent predictor of mortality in SAH patients (Pradilla et al., 2010; Muroi et al., 2011; Luo et al., 2021; Devlin et al., 2022). Microglia are innate immune cells of the central nervous system and can be activated rapidly under inflammatory conditions, trauma, or other stimulating factors. Previous studies have confirmed that microglia can be activated within minutes of the occurrence of SAH and contribute to the process of inflammation (Coulibaly and Provencio, 2020; Heinz et al., 2021; Chen et al., 2022). When microglia are activated in a normal physiological state, they can remove harmful substances by phagocytosis. However, when microglia are overactivated, they exacerbate brain injury by releasing pro-inflammatory factors and oxidative metabolites, promoting the activation of neutrophils and macrophages, thus resulting in the destruction of BBB, inflammatory response and neuronal damage (Lucke-Wold et al., 2016; van Dijk et al., 2016; Schneider et al., 2018). Similar to microglia, astrocytes can also synthesize and secrete inflammatory factors (such as cytokines and chemokines) and participate in the inflammatory response of SAH (Gris et al., 2019; Zhang et al., 2021). In addition to inflammatory cells, inflammatory-related proteins such as C-reactive protein, intercellular adhesion molecule (ICAM)-1, high mobility group box 1 (HMGB1), and galectin-3 also play key roles in the inflammatory reaction following SAH (Lin et al., 2007; Sun et al., 2014; Nishikawa and Suzuki, 2018; Mota Telles et al., 2021). Damage-associated molecular patterns (DAMPs) are released by neurons, astrocytes, microglia and endothelial cells in the early stage of SAH, activating local and peripheral immune cells and releasing cytokines to promote an inflammatory response. This process leads to early brain injury (Chaudhry et al., 2018; Lu et al., 2018; Ahmed et al., 2021; Balança et al., 2021). Furthermore, pro-inflammatory cytokines, such as interleukin-1 β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor -α (TNF-α), can trigger an inflammatory cascade that ultimately leads to the destruction of the BBB, and cause secondary injury post-SAH (Duris et al., 2018; Savarraj et al., 2018; Okada and Suzuki, 2020). Therefore, therapies inhibiting the inflammatory response may alleviate the EBI after SAH, thereby improving the prognosis of patients.
Melatonin has shown anti-inflammatory properties in SAH. It can reduce neuroinflammation and improve axonal hypomyelination by modulating M1/M2 microglia polarization via the JAK2-STAT3-telomerase pathway (Zhou et al., 2021). Moreover, melatonin reduces the release of pro-inflammatory mediators (IL-1β, IL-6, and TNF-α, etc.), alleviates the inflammatory response, thus improving secondary brain damage and neurobehavioral dysfunction after SAH (Fang et al., 2009). In addition, inhibition of inflammation by melatonin effectively protects the integrity of the BBB structure and function, and reduces the degree of brain edema (Chen et al., 2014a,2015). Wang et al. (2013) found that melatonin markedly decreased the expressions of TLR4 pathway-related agents, such as HMGB1, toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), indicating the involvement of the TLR4 pathway in melatonin-induced neuroprotection. Among the inflammatory responses after SAH, NLRP3 inflammasome activation has recently been investigated. NLRP3 inflammasome activation promotes the maturation and secretion of pro-inflammatory cytokines and participates in cell death processes such as pyroptosis. Various studies have revealed that reduction of NLRP3 inflammasome activation exerts strong neuroprotective effects in the acute phase following SAH, which was associated with the downregulation of pro-inflammatory cytokines. Cao et al. (2017) proposed that melatonin is neuroprotective against EBI post-SAH via inhibiting mitophagy-associated NLRP3 inflammasome. In addition, Dong et al. (2016) demonstrated that melatonin treatment attenuates brain injury by inhibiting NLRP3 inflammasome-associated apoptosis following SAH. Notably, NLRP3 signal activation is performed by microglia, and reduced NLRP3 is related to decreased white matter injury after melatonin treatment in SAH (Liu et al., 2020).
Melatonin and Apoptosis
Apoptosis is one of the main pathophysiological processes of EBI, and its degree is closely related to the neurological function recovery after SAH (He et al., 2012; Hong et al., 2014). Previous experiments have shown that apoptosis of neurons begins within 10 min after the occurrence of SAH (Friedrich et al., 2012). The increased intracranial pressure, cerebral edema and oxidative stress induced by SAH can all lead to extensive cell apoptosis, including neurons, glial cells and vascular endothelial cells (Ostrowski et al., 2006; Hasegawa et al., 2011; Shao et al., 2020). Apoptosis after SAH involves many pathways, including the death receptor pathway, mitochondrial pathway, and dependent or independent caspase pathway (Cahill et al., 2006). The mitochondrial pathway is mediated by the Bcl-2 family and manifests as increased permeability of the mitochondrial membrane. Cytochrome c transfers from mitochondria to the cytoplasmic septum and participates in apoptosome assembly with apoptosis protease-activator factor 1 (Apaf-1), thus leading to the activation of caspase-9. Subsequently, caspase-3 is further activated to induce apoptosis (Ceccatelli et al., 2004; Bornstein et al., 2020). The caspase-independent pathway is mainly mediated by the mitochondrial release of apoptosis-inducing factor (AIF). AIF exists in the mitochondrial membrane gap in its normal state, and can be transferred to the nucleus after SAH to induce DNA destruction and cell apoptosis without caspase activation (Lorenzo et al., 1999; Lorenzo and Susin, 2007; Norberg et al., 2010). Death receptors participate in external apoptosis pathways. The expression of Fas and TNF ligands of death receptors are upregulated after SAH, binding to death receptors and activating the caspase cascade (Gorojod et al., 2017; Cha et al., 2019). These pathways interact with each other and participate in the occurrence and regulation of apoptosis after SAH. By intervening on the above pathways, apoptosis may be effectively alleviated, thus improving the neurological injury and promoting the recovery of neurologic function after SAH.
The neuroprotective role of melatonin by diminishing cellular apoptosis in EBI after SAH was investigated. A study published in 2005 first reported the anti-apoptosis effect of melatonin in a SAH rabbit model by reducing the apoptosis of endothelial cells (Aydin et al., 2005). Subsequently, many studies confirmed the role of melatonin in alleviating neuronal apoptosis after SAH, contributing to the amelioration of spatial learning and memory deficits and improvement of therapeutic outcomes (Wang et al., 2012, 2013; Dong et al., 2016; Sun et al., 2018). Moreover, melatonin can reduce oligodendrocyte apoptosis associated with the attenuation of white matter injury. The mechanism of the above anti-apoptosis effects is related to enhancing autophagy, which improves cell apoptosis via a mitochondrial pathway (Chen et al., 2014b). In addition, melatonin reduces cellular apoptosis partially via the regulation of the melatonin receptor/Sirt1/NF-κB signaling pathway (Zhao et al., 2017), ROS/mammalian sterile 20-like kinase 1 (MST1) pathway (Shi et al., 2018), ROS/SIRT3 pathway (Yang et al., 2018a), and JAK/STAT pathway (Li et al., 2019). Recently, many studies have shown that melatonin abolished apoptosis by regulating microRNAs. Xu et al. (2022) established that melatonin affects HIF-1α and ameliorates delayed brain injury following SAH via the H19/miR-675/HIF1A/TLR4 pathway. In contrast, Yang et al. (2018b) demonstrated that long non-coding RNA and microRNA-675/let-7a mediate the protective effect of melatonin against EBI after SAH via targeting TP53 and neural growth factor. Notably, melatonin not only reduces cell apoptosis in the brain but also prevents alveolar-capillary barrier dysfunctions via repressing cell apoptosis in the lung, thus attenuating neurogenic pulmonary edema after SAH (Chen et al., 2015).
Melatonin and Blood-Brain Barrier Disruption
BBB is mainly composed of capillary endothelial cells, pericytes, astrocytes, and vascular basilemma. Due to the tight connection between capillary endothelial cells, the cell gap is small. In the physiological state, most substances (such as plasma components, red blood cells, etc.) cannot pass the BBB except for a few lipid-soluble small molecules (Daneman and Barres, 2005; Zhao et al., 2015). In SAH, the expression of type IV collagen is significantly increased, which degrades the BBB basement membrane and is accompanied by the elevation of vascular endothelial growth factor, activation of endothelial cell apoptosis, resulting in the enhanced permeability of BBB (Yang and Rosenberg, 2011; Kanamaru and Suzuki, 2019; Li et al., 2020). Cerebral edema directly results from BBB dysfunction, which includes vasogenic and cytotoxic edema (Rosenberg, 1999; Sandoval and Witt, 2008). Vasogenic edema refers to blood flow from the vessels to brain tissue due to the apoptosis of endothelial cells and glial cells around blood vessels. The increase of intracranial pressure after SAH induces the decrease of CBF, which causes global cerebral ischemia, leading to the failure of the Na+ /K+ pump, and resulting in cytotoxic edema (Okada et al., 2020; Chen et al., 2021). Clinical studies have shown that about 8% of patients were found to have global cerebral edema after head CT examination upon admission, and another 12% of patients developed prominent cerebral edema within 6 days following SAH (Cahill et al., 2006). Severe cerebral edema often leads to increased intracranial pressure, acute cerebral ischemia, cerebral hernia, and death. Therefore, it is of great significance to protect the integrity of BBB and reduce the development of cerebral edema, aiming to improve the prognosis of patients (Michinaga and Koyama, 2015).
Pragmatic therapeutic strategies for brain edema such as acupuncture, osmotherapy, non-peptide vasopressin receptor antagonist, and calcium channel blockers are used in clinical practice (Rowland et al., 2019; Corry et al., 2020; Hinson et al., 2020; Guo et al., 2022). Although the above-mentioned treatment approaches have been well-studied and display partial protective effects in attenuating cerebral edema, a single therapy capable of inhibiting cerebral edema is yet to be found due to the complex mechanisms involved. Recently, experimental studies have shown that melatonin not only reduces cerebral edema but also protects the BBB by preventing the disruption of tight junction protein expression (ZO-1, occludin, and claudin-5), indicating that melatonin may be an effective alternative for alleviating brain edema after SAH (Ayer et al., 2008a,b; Chen et al., 2014a; Li et al., 2019; Liu et al., 2020). Additionally, melatonin can easily cross the BBB, while preserving BBB permeability and reducing brain edema (Ersahin et al., 2009).
Melatonin and Oxidative Stress
Superoxide dismutase (SOD), glutathione peroxidase, catalase and other important antioxidant enzymes in brain tissue are down-regulated after SAH, leading to a decrease in antioxidant capacity. Meanwhile, vasospasm and cerebral edema caused by SAH lead to cerebral ischemia, resulting in the production of a large number of oxygen ions (O2–) and hydrogen peroxide (H2O2) (Yang et al., 2017; Shao et al., 2020). The high concentration of Fe2+ and Fe3+ produced by erythrocyte degradation can combine with H2O2 and O2– by Fenton reaction to form hydroxyl radicals. Hydroxyl radicals are among the most toxic reactive oxygen species (ROS), which can directly damage neurovascular units and cause neurologic injury. In addition, free radicals-induced oxidative stress cause brain damage by promoting lipid peroxidation, protein degradation, and DNA destruction, resulting in neuronal apoptosis, endothelial cell damage, and BBB destruction. These changes result in severe brain injury and neurological deterioration after SAH (Lu et al., 2019; Wu et al., 2021). Therefore, the intervention of oxidative stress can inhibit the secondary cascade reaction of pathological changes and reduce subsequent brain damage (Zhang et al., 2014; Lin et al., 2021a).
Melatonin is a powerful antioxidant. Its antioxidant effects include direct scavenging of free radicals, stimulation of antioxidant enzyme activity and gene expression, stimulation of glutathione synthesis, reduction of electron leakage of mitochondrial electron transport chain, and reduction of cytokine production (Reiter et al., 2016; Galano and Reiter, 2018). Previous research has shown that lipopolysaccharides-induced hyperreactivity of vascular smooth muscle is mediated through enhanced release of ROS and prostanoids, and melatonin inhibits the vascular hyperreactivity via selective scavenging of ROS (Müller-Schweinitzer et al., 2004). Melatonin can cause a significant increase in brain glutathione (GSH) and superoxidase dismutase (SOD) content, as well as Na+-K+-ATPase activity and GSH/GSSG ratio, which is accompanied by significant decreases in ROS, malondialdehyde (MDA) levels, and myeloperoxidase (MPO) activity, thereby providing neuroprotection from EBI following SAH (Ersahin et al., 2009; Fang et al., 2009; Yang et al., 2018a). Besides, melatonin alleviates SAH-induced EBI by inhibiting the ROS-stimulated activation of the MST1 pathway (Shi et al., 2018), NLRP3 inflammasome (Cao et al., 2017), and SIRT3 pathway (Yang et al., 2018a). An in vivo and in vitro study demonstrated that H2O2 markedly upregulated the expression of H19, miR-675, and NGF, and downregulated let-7a and TP53 levels. These findings were reversed by melatonin treatment, revealing the potential antioxidant mechanisms of melatonin (Yang et al., 2018b). It is worth noting that Nrf2 is a global promoter of antioxidant response and has potential protective effects against post-SAH EBI. It has been shown that Nrf2-knockout animals have poorer outcomes in SAH. Melatonin can increase the effects of the antioxidant system by upregulating the expression of Nrf2 (Wang et al., 2012; Sun et al., 2018).
Conclusion and Prospects
Melatonin is a neuroendocrine hormone that protects the central nervous system mainly through anti-vasospasm, anti-oxidative stress, anti-inflammatory response, anti-apoptosis and BBB protection. At present, the study of melatonin in SAH is mostly limited to animal and cell models, and lack of clinical evidence. So far, four clinical studies with small cohort of patients have explored the association between melatonin and SAH patients. Melatonin could decrease fatigue, but has no significant impact on depression and apathy post-stroke (Gilard et al., 2016). In addition, melatonin administration has no effect on delayed cerebral ischemia, but may reduce mortality of SAH (Lin et al., 2021b). Another prospective and observational study enrolls 169 aneurysmal SAH patients, to ascertain the relationship between endogenous melatonin level and neurological outcome post-SAH. The results indicate that higher level of serum melatonin is associated with poor outcome after SAH (Zhan et al., 2021). As many factors can affects the concentrations of serum melatonin such as the severity of brain injury, and rhythm of melatonin secretion, the accurate influence and changes of melatonin after SAH need to be studied. Neumaier et al. (2021) reports that there is a delayed upregulation of circulatory daytime melatonin levels after SAH, and higher concentration of melatonin is related with patients with anterior communicating artery aneurysms or poor clinical outcome, indicating the potential role of hypothalamic dysfunction. Further large-scale clinical trials are needed to verify its neuroprotective effect in SAH patients. Additionally, the effects of melatonin in patients with different degrees of injury and ages should be explored. Moreover, the secretion of melatonin in the body follows the circadian rhythm. The time of administration likely plays an essential role in achieving the optimal therapeutic effect, and needs further study. Furthermore, the drug dosage and administration interval of melatonin used in current studies vary greatly. The optimal dose and administration frequency should be determined to effectively improve the therapeutic effect. Finally, melatonin regulates biological rhythms, and a large proportion of SAH patients have sleep disorders. Determining the therapeutic strategy of melatonin for this population is worth exploring.
Author Contributions
CX and ZH conceived the perspective of the work and searched the literature and drafted the manuscript. JL critically revised the article. All authors contributed to and approved to the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, H., Khan, M. A., Kahlert, U. D., Niemelä, M., Hänggi, D., Chaudhry, S. R., et al. (2021). Role of adaptor protein myeloid differentiation 88 (MyD88) in post-subarachnoid hemorrhage inflammation: a systematic review. Int. J. Mol. Sci. 22:4185. doi: 10.3390/ijms22084185
Aladag, M. A., Turkoz, Y., Parlakpinar, H., Ozen, H., Egri, M., and Unal, S. C. (2009). Melatonin ameliorates cerebral vasospasm after experimental subarachnoidal haemorrhage correcting imbalance of nitric oxide levels in rats. Neurochem. Res. 34, 1935–1944. doi: 10.1007/s11064-009-9979-7
Alamdari, A. F., Rahnemayan, S., Rajabi, H., Vahed, N., Kashani, H. R. K., Rezabakhsh, A., et al. (2021). Melatonin as a promising modulator of aging related neurodegenerative disorders: role of microRNAs. Pharmacol. Res. 173:105839. doi: 10.1016/j.phrs.2021.105839
Al-Khindi, T., Macdonald, R. L., and Schweizer, T. A. (2010). Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 41, e519–e536.
Attia, M. S., Lass, E., and Loch Macdonald, R. (2015). Nitric oxide synthases: three pieces to the puzzle? Acta Neurochir. Suppl. 120, 131–135. doi: 10.1007/978-3-319-04981-6_22
Aydin, M. V., Caner, H., Sen, O., Ozen, O., Atalay, B., Cekinmez, M., et al. (2005). Effect of melatonin on cerebral vasospasm following experimental subarachnoid hemorrhage. Neurol. Res. 27, 77–82. doi: 10.1179/016164105X18331
Ayer, R. E., Sugawara, T., Chen, W., Tong, W., and Zhang, J. H. (2008a). Melatonin decreases mortality following severe subarachnoid hemorrhage. J. Pineal Res. 44, 197–204. doi: 10.1111/j.1600-079X.2007.00508.x
Ayer, R. E., Sugawara, T., and Zhang, J. H. (2008b). Effects of melatonin in early brain injury following subarachnoid hemorrhage. Acta Neurochir. Suppl. 102, 327–330. doi: 10.1007/978-3-211-85578-2_62
Balança, B., Desmurs, L., Grelier, J., Perret-Liaudet, A., and Lukaszewicz, A. C. (2021). DAMPs and RAGE pathophysiology at the acute phase of brain injury: an overview. Int. J. Mol. Sci. 22:2439. doi: 10.3390/ijms22052439
Bornstein, R., Gonzalez, B., and Johnson, S. C. (2020). Mitochondrial pathways in human health and aging. Mitochondrion 54, 72–84. doi: 10.1016/j.mito.2020.07.007
Cahill, J., Calvert, J. W., and Zhang, J. H. (2006). Mechanisms of early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 26, 1341–1353.
Cahill, J., and Zhang, J. H. (2009). Subarachnoid hemorrhage: is it time for a new direction? Stroke 40, S86–S87. doi: 10.1161/STROKEAHA.108.533315
Cao, S., Shrestha, S., Li, J., Yu, X., Chen, J., Yan, F., et al. (2017). Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci. Rep. 7:2417. doi: 10.1038/s41598-017-02679-z
Cardinali, D. P. (2021). Melatonin and healthy aging. Vitam. Horm. 115, 67–88. doi: 10.1016/bs.vh.2020.12.004
Ceccatelli, S., Tamm, C., Sleeper, E., and Orrenius, S. (2004). Neural stem cells and cell death. Toxicol. Lett. 149, 59–66. doi: 10.1016/j.toxlet.2003.12.060
Cha, Z., Cheng, J., Xiang, H., Qin, J., He, Y., Peng, Z., et al. (2019). Celastrol enhances TRAIL-induced apoptosis in human glioblastoma via the death receptor pathway. Cancer Chemother. Pharmacol. 84, 719–728. doi: 10.1007/s00280-019-03900-8
Chaudhry, S. R., Hafez, A., Rezai Jahromi, B., Kinfe, T. M., Lamprecht, A., Niemelä, M., et al. (2018). Role of damage associated molecular pattern molecules (DAMPs) in aneurysmal subarachnoid hemorrhage (aSAH). Int. J. Mol. Sci. 19:2035. doi: 10.3390/ijms19072035
Chen, J., Chen, G., Li, J., Qian, C., Mo, H., Gu, C., et al. (2014a). Melatonin attenuates inflammatory response-induced brain edema in early brain injury following a subarachnoid hemorrhage: a possible role for the regulation of pro-inflammatory cytokines. J. Pineal Res. 57, 340–347. doi: 10.1111/jpi.12173
Chen, J., Wang, L., Wu, C., Hu, Q., Gu, C., Yan, F., et al. (2014b). Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J. Pineal Res. 56, 12–19. doi: 10.1111/jpi.12086
Chen, J., Qian, C., Duan, H., Cao, S., Yu, X., Li, J., et al. (2015). Melatonin attenuates neurogenic pulmonary edema via the regulation of inflammation and apoptosis after subarachnoid hemorrhage in rats. J. Pineal Res. 59, 469–477. doi: 10.1111/jpi.12278
Chen, J., Zheng, Z. V., Lu, G., Chan, W. Y., Zhang, Y., and Wong, G. K. C. (2022). Microglia activation, classification and microglia-mediated neuroinflammatory modulators in subarachnoid hemorrhage. Neural Regen. Res. 17, 1404–1411. doi: 10.4103/1673-5374.330589
Chen, S., Shao, L., and Ma, L. (2021). Cerebral edema formation after stroke: emphasis on blood-brain barrier and the lymphatic drainage system of the brain. Front. Cell Neurosci. 15:716825. doi: 10.3389/fncel.2021.716825
Cook, D. A. (1995). Mechanisms of cerebral vasospasm in subarachnoid haemorrhage. Pharmacol. Ther. 66, 259–284. doi: 10.1016/0163-7258(94)00080-m
Corry, J. J., Asaithambi, G., Shaik, A. M., Lassig, J. P., Marino, E. H., Ho, B. M., et al. (2020). Conivaptan for the reduction of cerebral edema in intracerebral hemorrhage: a safety and tolerability study. Clin. Drug Investig. 40, 503–509. doi: 10.1007/s40261-020-00911-9
Cosentino, F., and Katusić, Z. S. (1994). Does endothelin-1 play a role in the pathogenesis of cerebral vasospasm? Stroke 25, 904–908. doi: 10.1161/01.str.25.4.904
Coulibaly, A. P., and Provencio, J. J. (2020). Aneurysmal subarachnoid hemorrhage: an overview of inflammation-induced cellular changes. Neurotherapeutics 17, 436–445. doi: 10.1007/s13311-019-00829-x
Crobeddu, E., Pilloni, G., Tardivo, V., Fontanella, M. M., Panciani, P. P., Spena, G., et al. (2016). Role of nitric oxide and mechanisms involved in cerebral injury after subarachnoid hemorrhage: is nitric oxide a possible answer to cerebral vasospasm? J. Neurosurg. Sci. 60, 385–391.
Crowley, R. W., Medel, R., Kassell, N. F., and Dumont, A. S. (2008). New insights into the causes and therapy of cerebral vasospasm following subarachnoid hemorrhage. Drug Discov. Today 13, 254–260. doi: 10.1016/j.drudis.2007.11.010
Daneman, R., and Barres, B. A. (2005). The blood-brain barrier–lessons from moody flies. Cell 123, 9–12. doi: 10.1016/j.cell.2005.09.017
Devlin, P., Ishrat, T., and Stanfill, A. G. (2022). A systematic review of inflammatory cytokine changes following aneurysmal subarachnoid hemorrhage in animal models and humans. Transl. Stroke Res. doi: 10.1007/s12975-022-01001-y
Dong, Y., Fan, C., Hu, W., Jiang, S., Ma, Z., Yan, X., et al. (2016). Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J. Pineal Res. 60, 253–262. doi: 10.1111/jpi.12300
Duris, K., Lipkova, J., Splichal, Z., Madaraszova, T., and Jurajda, M. (2018). Early inflammatory response in the brain and anesthesia recovery time evaluation after experimental subarachnoid hemorrhage. Transl. Stroke Res. doi: 10.1007/s12975-018-0641-z
Ehlert, A., Manthei, G., Hesselmann, V., Mathias, K., Bein, B., and Pluta, R. (2016). A case of hyperacute onset of vasospasm after aneurysmal subarachnoid hemorrhage and refractory vasospasm treated with intravenous and intraventricular nitric oxide: a mini review. World Neurosurg. 91, .e611–.e618. doi: 10.1016/j.wneu.2016.04.047
Ersahin, M., Toklu, H. Z., Cetinel, S., Yüksel, M., Yeğen, B. C., and Sener, G. (2009). Melatonin reduces experimental subarachnoid hemorrhage-induced oxidative brain damage and neurological symptoms. J. Pineal Res. 46, 324–332. doi: 10.1111/j.1600-079X.2009.00664.x
Etminan, N., Chang, H. S., Hackenberg, K., De Rooij, N. K., Vergouwen, M. D. I., Rinkel, G. J. E., et al. (2019). Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 76, 588–597. doi: 10.1001/jamaneurol.2019.0006
Etminan, N., and Macdonald, R. L. (2021). Neurovascular disease, diagnosis, and therapy: subarachnoid hemorrhage and cerebral vasospasm. Handb. Clin. Neurol. 176, 135–169. doi: 10.1016/B978-0-444-64034-5.00009-2
Etminan, N., Vergouwen, M. D., Ilodigwe, D., and Macdonald, R. L. (2011). Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 31, 1443–1451. doi: 10.1038/jcbfm.2011.7
Fang, Q., Chen, G., Zhu, W., Dong, W., and Wang, Z. (2009). Influence of melatonin on cerebrovascular proinflammatory mediators expression and oxidative stress following subarachnoid hemorrhage in rabbits. Mediators Inflamm. 2009:426346. doi: 10.1155/2009/426346
Friedrich, V., Flores, R., and Sehba, F. A. (2012). Cell death starts early after subarachnoid hemorrhage. Neurosci. Lett. 512, 6–11. doi: 10.1016/j.neulet.2012.01.036
Fujii, M., Yan, J., Rolland, W. B., Soejima, Y., Caner, B., and Zhang, J. H. (2013). Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl. Stroke Res. 4, 432–446. doi: 10.1007/s12975-013-0257-2
Galano, A., and Reiter, R. J. (2018). Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J. Pineal Res. 65, e12514. doi: 10.1111/jpi.12514
Gaspard, N. (2020). How do i manage cerebral vasospasm and delayed cerebral ischemia? Minerva Anestesiol. 86, 1331–1339. doi: 10.23736/S0375-9393.20.14507-3
Gilard, V., Ferracci, F. X., Langlois, O., Derrey, S., Proust, F., and Curey, S. (2016). Effects of melatonin in the treatment of asthenia in aneurysmal subarachnoid hemorrhage. Neurochirurgie 62, 295–299. doi: 10.1016/j.neuchi.2016.06.010
Gorojod, R. M., Alaimo, A., Porte Alcon, S., Saravia, F., and Kotler, M. L. (2017). Interplay between lysosomal, mitochondrial and death receptor pathways during manganese-induced apoptosis in glial cells. Arch. Toxicol. 91, 3065–3078. doi: 10.1007/s00204-017-1936-7
Gris, T., Laplante, P., Thebault, P., Cayrol, R., Najjar, A., Joannette-Pilon, B., et al. (2019). Innate immunity activation in the early brain injury period following subarachnoid hemorrhage. J. Neuroinflammation 16:253. doi: 10.1186/s12974-019-1629-7
Guo, Z. N., Shao, A., Tong, L. S., Sun, W., Liu, J., and Yang, Y. (2016). The role of nitric oxide and sympathetic control in cerebral autoregulation in the setting of subarachnoid hemorrhage and traumatic brain injury. Mol. Neurobiol. 53, 3606–3615. doi: 10.1007/s12035-015-9308-x
Guo, Z. Q., Jiang, H., Huang, Y., Gu, H. M., Wang, W. B., and Chen, T. D. (2022). Early complementary acupuncture improves the clinical prognosis of traumatic brain edema: a randomized controlled trial. Medicine (Baltimore) 101:e28959. doi: 10.1097/MD.0000000000028959
Hasegawa, Y., Suzuki, H., Sozen, T., Altay, O., and Zhang, J. H. (2011). Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta Neurochir. Suppl. 110, 43–48. doi: 10.1007/978-3-7091-0353-1_8
He, F., Wu, X., Zhang, Q., Li, Y., Ye, Y., Li, P., et al. (2021). Bacteriostatic potential of melatonin: therapeutic standing and mechanistic insights. Front. Immunol. 12:683879. doi: 10.3389/fimmu.2021.683879
He, Z., Ostrowski, R. P., Sun, X., Ma, Q., Huang, B., Zhan, Y., et al. (2012). CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke 43, 484–490. doi: 10.1161/STROKEAHA.111.626432
Heinz, R., Brandenburg, S., Nieminen-Kelhä, M., Kremenetskaia, I., Boehm-Sturm, P., Vajkoczy, P., et al. (2021). Microglia as target for anti-inflammatory approaches to prevent secondary brain injury after subarachnoid hemorrhage (SAH). J. Neuroinflammation 18:36. doi: 10.1186/s12974-021-02085-3
Hinson, H. E., Sun, E., Molyneaux, B. J., Von Kummer, R., Demchuk, A., Romero, J., et al. (2020). Osmotherapy for malignant cerebral edema in a phase 2 prospective, double blind, randomized, placebo-controlled study of IV glibenclamide. J. Stroke Cerebrovasc. Dis. 29:104916. doi: 10.1016/j.jstrokecerebrovasdis.2020.104916
Hong, Y., Palaksha, K. J., Park, K., Park, S., Kim, H. D., Reiter, R. J., et al. (2010). Melatonin plus exercise-based neurorehabilitative therapy for spinal cord injury. J. Pineal Res. 49, 201–209. doi: 10.1111/j.1600-079X.2010.00786.x
Hong, Y., Shao, A., Wang, J., Chen, S., Wu, H., Mcbride, D. W., et al. (2014). Neuroprotective effect of hydrogen-rich saline against neurologic damage and apoptosis in early brain injury following subarachnoid hemorrhage: possible role of the Akt/GSK3β signaling pathway. PLoS One 9:e96212. doi: 10.1371/journal.pone.0096212
Hou, G., Chen, H., Yin, Y., Pan, Y., Zhang, X., and Jia, F. (2020). MEL ameliorates post-SAH cerebral vasospasm by affecting the expression of eNOS and HIF1α via H19/miR-138/eNOS/NO and H19/miR-675/HIF1α. Mol. Ther. Nucleic Acids 19, 523–532. doi: 10.1016/j.omtn.2019.12.002
Ikram, A., Javaid, M. A., Ortega-Gutierrez, S., Selim, M., Kelangi, S., Anwar, S. M. H., et al. (2021). Delayed cerebral ischemia after subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 30:106064.
Ji, C., and Chen, G. (2016). Signaling pathway in early brain injury after subarachnoid hemorrhage: news update. Acta Neurochir. Suppl. 121, 123–126. doi: 10.1007/978-3-319-18497-5_21
Kanamaru, H., and Suzuki, H. (2019). Potential therapeutic molecular targets for blood-brain barrier disruption after subarachnoid hemorrhage. Neural Regen. Res. 14, 1138–1143. doi: 10.4103/1673-5374.251190
Kassell, N. F., Sasaki, T., Colohan, A. R., and Nazar, G. (1985). Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 16, 562–572. doi: 10.1161/01.str.16.4.562
Kumar, G., Shahripour, R. B., and Harrigan, M. R. (2016). Vasospasm on transcranial doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J. Neurosurg. 124, 1257–1264. doi: 10.3171/2015.4.JNS15428
Kvetnoy, I., Ivanov, D., Mironova, E., Evsyukova, I., Nasyrov, R., Kvetnaia, T., et al. (2022). Melatonin as the cornerstone of neuroimmunoendocrinology. Int. J. Mol. Sci. 23:1835. doi: 10.3390/ijms23031835
La, M., and Reid, J. J. (1995). Endothelin-1 and the regulation of vascular tone. Clin. Exp. Pharmacol. Physiol. 22, 315–323. doi: 10.1111/j.1440-1681.1995.tb02008.x
Lee, M. Y., Kuan, Y. H., Chen, H. Y., Chen, T. Y., Chen, S. T., Huang, C. C., et al. (2007). Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res. 42, 297–309. doi: 10.1111/j.1600-079X.2007.00420.x
Li, S., Yang, S., Sun, B., and Hang, C. (2019). Melatonin attenuates early brain injury after subarachnoid hemorrhage by the JAK-STAT signaling pathway. Int. J. Clin. Exp. Pathol. 12, 909–915.
Li, Y., Wu, P., Bihl, J. C., and Shi, H. (2020). Underlying mechanisms and potential therapeutic molecular targets in blood-brain barrier disruption after subarachnoid hemorrhage. Curr. Neuropharmacol. 18, 1168–1179. doi: 10.2174/1570159X18666200106154203
Lin, C. L., Jeng, A. Y., Howng, S. L., and Kwan, A. L. (2004). Endothelin and subarachnoid hemorrhage-induced cerebral vasospasm: pathogenesis and treatment. Curr. Med. Chem. 11, 1779–1791. doi: 10.2174/0929867043364919
Lin, C. L., Kwan, A. L., Dumont, A. S., Su, Y. F., Kassell, N. F., Wang, C. J., et al. (2007). Attenuation of experimental subarachnoid hemorrhage-induced increases in circulating intercellular adhesion molecule-1 and cerebral vasospasm by the endothelin-converting enzyme inhibitor CGS 26303. J. Neurosurg. 106, 442–448. doi: 10.3171/jns.2007.106.3.442
Lin, C. L., Winardi, W., Jeng, A. Y., and Kwan, A. L. (2006). Endothelin-converting enzyme inhibitors for the treatment of subarachnoid hemorrhage-induced vasospasm. Neurol. Res. 28, 721–729. doi: 10.1179/016164106x152007
Lin, F., Li, R., Tu, W. J., Chen, Y., Wang, K., Chen, X., et al. (2021a). An update on antioxidative stress therapy research for early brain injury after subarachnoid hemorrhage. Front. Aging Neurosci. 13:772036. doi: 10.3389/fnagi.2021.772036
Lin, S. H., Galet, C., Zanaty, M., Bayman, E., Rogers, W. K., Hasan, D., et al. (2021b). Melatonin and risk of mortality in subjects with aneurysmal subarachnoid hemorrhage. Clin. Neurol. Neurosurg. 210:106990. doi: 10.1016/j.clineuro.2021.106990
Liu, D., Dong, Y., Li, G., Zou, Z., Hao, G., Feng, H., et al. (2020). Melatonin attenuates white matter injury via reducing oligodendrocyte apoptosis after subarachnoid hemorrhage in mice. Turk. Neurosurg. 30, 685–692. doi: 10.5137/1019-5149.JTN.27986-19.3
Lorenzo, H. K., and Susin, S. A. (2007). Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resist. Updat. 10, 235–255. doi: 10.1016/j.drup.2007.11.001
Lorenzo, H. K., Susin, S. A., Penninger, J., and Kroemer, G. (1999). Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 6, 516–524. doi: 10.1038/sj.cdd.4400527
Lu, Y., Zhang, X. S., Zhang, Z. H., Zhou, X. M., Gao, Y. Y., Liu, G. J., et al. (2018). Peroxiredoxin 2 activates microglia by interacting with toll-like receptor 4 after subarachnoid hemorrhage. J. Neuroinflammation 15:87. doi: 10.1186/s12974-018-1118-4
Lu, Y., Zhang, X. S., Zhou, X. M., Gao, Y. Y., Chen, C. L., Liu, J. P., et al. (2019). Peroxiredoxin 1/2 protects brain against H(2)O(2)-induced apoptosis after subarachnoid hemorrhage. FASEB J. 33, 3051–3062. doi: 10.1096/fj.201801150R
Lucke-Wold, B. P., Logsdon, A. F., Manoranjan, B., Turner, R. C., Mcconnell, E., Vates, G. E., et al. (2016). Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int. J. Mol. Sci. 17:497. doi: 10.3390/ijms17040497
Luo, F., Li, Y., Zhao, Y., Sun, M., He, Q., Wen, R., et al. (2021). Systemic immune-inflammation index predicts the outcome after aneurysmal subarachnoid hemorrhage. Neurosurg. Rev. 45, 1607–1615. doi: 10.1007/s10143-021-01681-4
Macdonald, R. L. (2016). Origins of the concept of vasospasm. Stroke 47, e11–15. doi: 10.1161/STROKEAHA.114.006498
Macdonald, R. L., Higashida, R. T., Keller, E., Mayer, S. A., Molyneux, A., Raabe, A., et al. (2011). Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 10, 618–625. doi: 10.1016/S1474-4422(11)70108-9
Macdonald, R. L., Kassell, N. F., Mayer, S., Ruefenacht, D., Schmiedek, P., Weidauer, S., et al. (2008). Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 39, 3015–3021. doi: 10.1161/STROKEAHA.108.519942
Macdonald, R. L., and Schweizer, T. A. (2017). Spontaneous subarachnoid haemorrhage. Lancet 389, 655–666.
Macdonald, R. L., and Weir, B. K. (1991). A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke 22, 971–982. doi: 10.1161/01.str.22.8.971
Majidinia, M., Reiter, R. J., Shakouri, S. K., Mohebbi, I., Rastegar, M., Kaviani, M., et al. (2018). The multiple functions of melatonin in regenerative medicine. Ageing Res. Rev. 45, 33–52. doi: 10.1016/j.arr.2018.04.003
Martinez-Cruz, F., Espinar, A., Pozo, D., Osuna, C., and Guerrero, J. M. (2002). Melatonin prevents focal rat cerebellum injury as assessed by induction of heat shock protein (HO-1) following subarachnoid injections of lysed blood. Neurosci. Lett. 331, 208–210. doi: 10.1016/s0304-3940(02)00884-4
Michinaga, S., and Koyama, Y. (2015). Pathogenesis of brain edema and investigation into anti-edema drugs. Int. J. Mol. Sci. 16, 9949–9975. doi: 10.3390/ijms16059949
Mota Telles, J. P., Rabelo, N. N., Junior, J. R., Teixeira, M. J., and Figueiredo, E. G. (2021). C-Reactive protein levels are higher in patients with fusiform intracranial aneurysms: a case-control study. World Neurosurg. 146, e896–e901. doi: 10.1016/j.wneu.2020.11.042
Müller-Schweinitzer, E., Gilles, H., Grapow, M., Kern, T., Reineke, D., and Zerkowski, H. R. (2004). Attenuation of lipopolysaccharide-induced hyperreactivity of human internal mammary arteries by melatonin. J. Pineal Res. 37, 92–97. doi: 10.1111/j.1600-079X.2004.00139.x
Muroi, C., Mink, S., Seule, M., Bellut, D., Fandino, J., and Keller, E. (2011). Monitoring of the inflammatory response after aneurysmal subarachnoid haemorrhage in the clinical setting: review of literature and report of preliminary clinical experience. Acta Neurochir. Suppl. 110, 191–196. doi: 10.1007/978-3-7091-0353-1_33
Nagai, H., Katsumata, T., Ohya, M., and Kageyama, N. (1976). Effect of subarachnoid haemorrhage on micro-circulation in hypothalamus and brain stem of dogs. Neurochirurgia (Stuttg) 19, 135–144. doi: 10.1055/s-0028-1090403
Naidech, A. M., Drescher, J., Tamul, P., Shaibani, A., Batjer, H. H., and Alberts, M. J. (2006). Acute physiological derangement is associated with early radiographic cerebral infarction after subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 77, 1340–1344. doi: 10.1136/jnnp.2006.089748
Neumaier, F., Weiss, M., Veldeman, M., Kotliar, K., Wiesmann, M., Schulze-Steinen, H., et al. (2021). Changes in endogenous daytime melatonin levels after aneurysmal subarachnoid hemorrhage - preliminary findings from an observational cohort study. Clin. Neurol. Neurosurg. 208:106870. doi: 10.1016/j.clineuro.2021.106870
Nishikawa, H., and Suzuki, H. (2018). Possible role of inflammation and galectin-3 in brain injury after subarachnoid hemorrhage. Brain Sci. 8:30. doi: 10.3390/brainsci8020030
Norberg, E., Orrenius, S., and Zhivotovsky, B. (2010). Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF). Biochem. Biophys. Res. Commun. 396, 95–100. doi: 10.1016/j.bbrc.2010.02.163
Okada, T., and Suzuki, H. (2020). Mechanisms of neuroinflammation and inflammatory mediators involved in brain injury following subarachnoid hemorrhage. Histol. Histopathol. 35, 623–636. doi: 10.14670/HH-18-208
Okada, T., Suzuki, H., Travis, Z. D., and Zhang, J. H. (2020). The stroke-induced blood-brain barrier disruption: current progress of inspection technique, mechanism, and therapeutic target. Curr. Neuropharmacol. 18, 1187–1212. doi: 10.2174/1570159X18666200528143301
Osier, N., Mcgreevy, E., Pham, L., Puccio, A., Ren, D., Conley, Y. P., et al. (2018). Melatonin as a therapy for traumatic brain injury: a review of published evidence. Int. J. Mol. Sci. 19:1539. doi: 10.3390/ijms19051539
Ostrowski, R. P., Colohan, A. R., and Zhang, J. H. (2006). Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol. Res. 28, 399–414. doi: 10.1179/016164106X115008
Pradilla, G., Chaichana, K. L., Hoang, S., Huang, J., and Tamargo, R. J. (2010). Inflammation and cerebral vasospasm after subarachnoid hemorrhage. Neurosurg. Clin. N. Am. 21, 365–379.
Reiter, R. J., Mayo, J. C., Tan, D. X., Sainz, R. M., Alatorre-Jimenez, M., and Qin, L. (2016). Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61, 253–278. doi: 10.1111/jpi.12360
Rowland, M. J., Ezra, M., Winkler, A., Garry, P., Lamb, C., Kelly, M., et al. (2019). Calcium channel blockade with nimodipine reverses MRI evidence of cerebral oedema following acute hypoxia. J. Cereb. Blood Flow Metab. 39, 285–301. doi: 10.1177/0271678X17726624
Roy, J., Wong, K. Y., Aquili, L., Uddin, M. S., Heng, B. C., Tipoe, G. L., et al. (2022). Role of melatonin in Alzheimer’s disease: from preclinical studies to novel melatonin-based therapies. Front. Neuroendocrinol. 65:100986. doi: 10.1016/j.yfrne.2022.100986
Samantaray, S., Das, A., Thakore, N. P., Matzelle, D. D., Reiter, R. J., Ray, S. K., et al. (2009). Therapeutic potential of melatonin in traumatic central nervous system injury. J. Pineal Res. 47, 134–142. doi: 10.1111/j.1600-079X.2009.00703.x
Sandoval, K. E., and Witt, K. A. (2008). Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 32, 200–219. doi: 10.1016/j.nbd.2008.08.005
Savarraj, J. P., Mcguire, M. F., Parsha, K., Hergenroeder, G., Bajgur, S., Ahn, S., et al. (2018). Disruption of thrombo-inflammatory response and activation of a distinct cytokine cluster after subarachnoid hemorrhage. Cytokine 111, 334–341. doi: 10.1016/j.cyto.2018.09.003
Schallner, N., Pandit, R., Leblanc, R. III, Thomas, A. J., Ogilvy, C. S., Zuckerbraun, B. S., et al. (2015). Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J. Clin. Invest. 125, 2609–2625. doi: 10.1172/JCI78443
Schneider, U. C., Xu, R., and Vajkoczy, P. (2018). Inflammatory events following subarachnoid hemorrhage (SAH). Curr. Neuropharmacol. 16, 1385–1395. doi: 10.2174/1570159X16666180412110919
Sehba, F. A., and Bederson, J. B. (2011). Nitric oxide in early brain injury after subarachnoid hemorrhage. Acta Neurochir. Suppl. 110, 99–103. doi: 10.1007/978-3-7091-0353-1_18
Sehba, F. A., and Friedrich, V. (2011). Early micro vascular changes after subarachnoid hemorrhage. Acta Neurochir. Suppl. 110, 49–55. doi: 10.1007/978-3-7091-0353-1_9
Sehba, F. A., Hou, J., Pluta, R. M., and Zhang, J. H. (2012). The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 97, 14–37. doi: 10.1016/j.pneurobio.2012.02.003
Shao, A., Lin, D., Wang, L., Tu, S., Lenahan, C., and Zhang, J. (2020). Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis. 11, 1537–1566. doi: 10.14336/AD.2020.0225
Shen, J., Pan, J. W., Fan, Z. X., Xiong, X. X., and Zhan, R. Y. (2013). Dissociation of vasospasm-related morbidity and outcomes in patients with aneurysmal subarachnoid hemorrhage treated with clazosentan: a meta-analysis of randomized controlled trials. J. Neurosurg. 119, 180–189. doi: 10.3171/2013.3.JNS121436
Shi, L., Liang, F., Zheng, J., Zhou, K., Chen, S., Yu, J., et al. (2018). Melatonin regulates apoptosis and autophagy Via ROS-MST1 pathway in subarachnoid hemorrhage. Front. Mol. Neurosci. 11:93. doi: 10.3389/fnmol.2018.00093
Sun, B., Yang, S., Li, S., and Hang, C. (2018). Melatonin upregulates nuclear factor erythroid-2 related factor 2 (Nrf2) and mediates mitophagy to protect against early brain injury after subarachnoid hemorrhage. Med. Sci. Monit. 24, 6422–6430. doi: 10.12659/MSM.909221
Sun, Q., Wu, W., Hu, Y. C., Li, H., Zhang, D., Li, S., et al. (2014). Early release of high-mobility group box 1 (HMGB1) from neurons in experimental subarachnoid hemorrhage in vivo and in vitro. J. Neuroinflammation 11:106. doi: 10.1186/1742-2094-11-106
Tai, S. H., Chen, H. Y., Lee, E. J., Chen, T. Y., Lin, H. W., Hung, Y. C., et al. (2010). Melatonin inhibits postischemic matrix metalloproteinase-9 (MMP-9) activation via dual modulation of plasminogen/plasmin system and endogenous MMP inhibitor in mice subjected to transient focal cerebral ischemia. J. Pineal Res. 49, 332–341. doi: 10.1111/j.1600-079X.2010.00797.x
Toda, N., Ayajiki, K., and Okamura, T. (2009). Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol. Rev. 61, 62–97. doi: 10.1124/pr.108.000547
Tordjman, S., Chokron, S., Delorme, R., Charrier, A., Bellissant, E., Jaafari, N., et al. (2017). Melatonin: pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 15, 434–443. doi: 10.2174/1570159X14666161228122115
van Dijk, B. J., Vergouwen, M. D., Kelfkens, M. M., Rinkel, G. J., and Hol, E. M. (2016). Glial cell response after aneurysmal subarachnoid hemorrhage - functional consequences and clinical implications. Biochim. Biophys. Acta 1862, 492–505. doi: 10.1016/j.bbadis.2015.10.013
Vanhoutte, P. M. (2018). Nitric oxide: from good to bad. Ann. Vasc. Dis. 11, 41–51. doi: 10.3400/avd.ra.17-00134
Vergouwen, M. D., Algra, A., and Rinkel, G. J. (2012). Endothelin receptor antagonists for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke 43, 2671–2676. doi: 10.1161/STROKEAHA.112.666693
Wang, Z., Ma, C., Meng, C. J., Zhu, G. Q., Sun, X. B., Huo, L., et al. (2012). Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J. Pineal Res. 53, 129–137. doi: 10.1111/j.1600-079X.2012.00978.x
Wang, Z., Wu, L., You, W., Ji, C., and Chen, G. (2013). Melatonin alleviates secondary brain damage and neurobehavioral dysfunction after experimental subarachnoid hemorrhage: possible involvement of TLR4-mediated inflammatory pathway. J. Pineal Res. 55, 399–408. doi: 10.1111/jpi.12087
Wu, F., Liu, Z., Li, G., Zhou, L., Huang, K., Wu, Z., et al. (2021). Inflammation and oxidative stress: potential targets for improving prognosis after subarachnoid hemorrhage. Front. Cell Neurosci. 15:739506. doi: 10.3389/fncel.2021.739506
Xu, Z., Zhang, F., Xu, H., Yang, F., Zhou, G., Tong, M., et al. (2022). Melatonin affects hypoxia-inducible factor 1α and ameliorates delayed brain injury following subarachnoid hemorrhage via H19/miR-675/HIF1A/TLR4. Bioengineered 13, 4235–4247. doi: 10.1080/21655979.2022.2027175
Yang, S., Chen, X., Li, S., Sun, B., and Hang, C. (2018a). Melatonin treatment regulates SIRT3 expression in early brain injury (EBI) due to reactive oxygen species (ROS) in a mouse model of subarachnoid hemorrhage (SAH). Med. Sci. Monit. 24, 3804–3814. doi: 10.12659/MSM.907734
Yang, S., Tang, W., He, Y., Wen, L., Sun, B., and Li, S. (2018b). Long non-coding RNA and microRNA-675/let-7a mediates the protective effect of melatonin against early brain injury after subarachnoid hemorrhage via targeting TP53 and neural growth factor. Cell Death Dis. 9:99. doi: 10.1038/s41419-017-0155-8
Yang, Y., Chen, S., and Zhang, J. M. (2017). The updated role of oxidative stress in subarachnoid hemorrhage. Curr. Drug Deliv. 14, 832–842. doi: 10.2174/1567201813666161025115531
Yang, Y., and Rosenberg, G. A. (2011). Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42, 3323–3328. doi: 10.1161/STROKEAHA.110.608257
Zhan, C. P., Zhuge, C. J., Yan, X. J., Dai, W. M., and Yu, G. F. (2021). Measuring serum melatonin concentrations to predict clinical outcome after aneurysmal subarachnoid hemorrhage. Clin. Chim. Acta 513, 1–5. doi: 10.1016/j.cca.2020.12.006
Zhang, L., Guo, K., Zhou, J., Zhang, X., Yin, S., Peng, J., et al. (2021). Ponesimod protects against neuronal death by suppressing the activation of A1 astrocytes in early brain injury after experimental subarachnoid hemorrhage. J. Neurochem. 158, 880–897. doi: 10.1111/jnc.15457
Zhang, X. S., Zhang, X., Zhou, M. L., Zhou, X. M., Li, N., Li, W., et al. (2014). Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. J. Neurosurg. 121, 42–54. doi: 10.3171/2014.2.JNS13730
Zhang, Y., Zhang, W. X., Zhang, Y. J., Liu, Y. D., Liu, Z. J., Wu, Q. C., et al. (2018). Melatonin for the treatment of spinal cord injury. Neural Regen. Res. 13, 1685–1692.
Zhang, Z. H., Han, Y. L., Wang, C. X., Zhou, C. H., Wu, L. Y., Zhang, H. S., et al. (2016). The effect of subarachnoid erythrocyte lysate on brain injury: a preliminary study. Biosci. Rep. 36:e00359. doi: 10.1042/BSR20160100
Zhao, L., Liu, H., Yue, L., Zhang, J., Li, X., Wang, B., et al. (2017). Melatonin attenuates early brain injury via the melatonin receptor/Sirt1/NF-κB signaling pathway following subarachnoid hemorrhage in mice. Mol. Neurobiol. 54, 1612–1621. doi: 10.1007/s12035-016-9776-7
Zhao, Z., Nelson, A. R., Betsholtz, C., and Zlokovic, B. V. (2015). Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078. doi: 10.1016/j.cell.2015.10.067
Zhou, Q., Lin, L., Li, H., Wang, H., Jiang, S., Huang, P., et al. (2021). Melatonin reduces neuroinflammation and improves axonal hypomyelination by modulating M1/M2 microglia polarization via JAK2-STAT3-telomerase pathway in postnatal rats exposed to lipopolysaccharide. Mol. Neurobiol. 58, 6552–6576. doi: 10.1007/s12035-021-02568-7
Keywords: subarachnoid hemorrhage, early brain injury, melatonin, mechanism, apoptosis, inflammation, vasospasm, oxidative stress
Citation: Xu C, He Z and Li J (2022) Melatonin as a Potential Neuroprotectant: Mechanisms in Subarachnoid Hemorrhage-Induced Early Brain Injury. Front. Aging Neurosci. 14:899678. doi: 10.3389/fnagi.2022.899678
Received: 19 March 2022; Accepted: 12 April 2022;
Published: 29 April 2022.
Edited by:
Anwen Shao, Zhejiang University, ChinaReviewed by:
Gongchang Yu, Shandong First Medical University, ChinaYuanhan Zhu, Jiaxing University Medical College, China
Copyright © 2022 Xu, He and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiabin Li, 6513077@zju.edu.cn
†These authors have contributed equally to this work
 Chengyan Xu
Chengyan Xu Zixia He2†
Zixia He2†  Jiabin Li
Jiabin Li