Astrocytes exhibit diverse Ca2+ changes at subcellular domains during brain aging
- 1Britton Chance Center for Biomedical Photonics, Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, Wuhan, China
- 2MoE Key Laboratory for Biomedical Photonics, School of Engineering Sciences, Huazhong University of Science and Technology, Wuhan, China
- 3Brain Research Center and State Key Laboratory of Trauma, Burns, and Combined Injury, Third Military Medical University, Chongqing, China
- 4Advanced Institute for Brain and Intelligence and School of Physical Science and Technology, Guangxi University, Nanning, China
- 5Brain Research Instrument Innovation Center, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, Suzhou, China
- 6Combinatorial NeuroImaging Core Facility, Leibniz Institute for Neurobiology, Magdeburg, Germany
- 7Guangyang Bay Laboratory, Chongqing Institute for Brain and Intelligence, Chongqing, China
- 8Center for Neurointelligence, School of Medicine, Chongqing University, Chongqing, China
Astrocytic Ca2+ transients are essential for astrocyte integration into neural circuits. These Ca2+ transients are primarily sequestered in subcellular domains, including primary branches, branchlets and leaflets, and endfeet. In previous studies, it suggests that aging causes functional defects in astrocytes. Until now, it was unclear whether and how aging affects astrocytic Ca2+ transients at subcellular domains. In this study, we combined a genetically encoded Ca2+ sensor (GCaMP6f) and in vivo two-photon Ca2+ imaging to determine changes in Ca2+ transients within astrocytic subcellular domains during brain aging. We showed that aging increased Ca2+ transients in astrocytic primary branches, higher-order branchlets, and terminal leaflets. However, Ca2+ transients decreased within astrocytic endfeet during brain aging, which could be caused by the decreased expressions of Aquaporin-4 (AQP4). In addition, aging-induced changes of Ca2+ transient types were heterogeneous within astrocytic subcellular domains. These results demonstrate that the astrocytic Ca2+ transients within subcellular domains are affected by aging differently. This finding contributes to a better understanding of the physiological role of astrocytes in aging-induced neural circuit degeneration.
Introduction
Astrocytes are distributed throughout the brain in a grid-like manner and form a dense network of interactions with neurons, other glial cells, and blood vessels (Kosaka and Hama, 1986; Khakh and Sofroniew, 2015; Liu et al., 2020; Descalzi, 2021). These electrically non-excitable astrocytes use Ca2+ signals as a substrate for their excitability to communicate with the surrounding milieu (Jianxiong Zhang and Chen, 2017; Semyanov, 2019; Semyanov and Verkhratsky, 2021). Astrocytic Ca2+ elevations (transients) can influence the activities of surrounding neurons by releasing gliotransmitters (Araque et al., 2014), regulating K+ uptake (Wang et al., 2012), and controlling local blood flow (Otsu et al., 2015). Therefore, spatially and temporally controlled Ca2+ signals represent a major component of ‘astrocytic languages’ which mediate learning and memory (Zhang et al., 2021; Wu and Gao, 2022). More importantly, astrocytic Ca2+ transients occur more frequently following CNS injuries and are abnormally increased in regions of amyloid deposition in murine models of Alzheimer’s disease (Shigetomi et al., 2019; Verkhratsky, 2019), indicating that astrocytic Ca2+ signaling is highly adaptable and changeable. Additional studies have demonstrated that multiple patterns of astrocytic Ca2+ transients determine multiple states of neuronal networks (Semyanov, 2019). As such, the pathological changes in astrocytic Ca2+ transients contribute to astrocytic pathology associated with deficient neuroprotection or failure in glial homeostatic support (Nanclares et al., 2021).
Astrocytes extend highly branched processes that are coupled to the rest of the cell by narrow cytoplasmic channels (Grosche et al., 1999). These astrocytic processes are categorized as primary branches emanating from the soma (Figure 1A, right), higher-order branchlets and terminal leaflets that occupy most of the astrocytic territory (Figure 2A, right), and endfeet that contact blood vessels (Figure 3A, left; Lim et al., 2021; Semyanov and Verkhratsky, 2021). In contrast to neurons, the somatic astrocyte region is not a central signaling hub. Instead, the local Ca2+ transients widely distributed in astrocytic processes are thought to trigger downstream signaling cascades that modify local neuronal signaling (Semyanov et al., 2020). These local Ca2+ events in astrocytic processes occupy approximately 75% of the astrocyte volume (Bindocci et al., 2017) and occur independently in the soma (Lim et al., 2021). Further investigations have demonstrated that these local astrocytic Ca2+ transients in processes are the sites for synapse-astrocyte communications and are important for the control of synaptic transmission and plasticity (Di Castro et al., 2011). In addition, at the vascular interface, astrocytic Ca2+ transients are mostly restricted to individual endfeet as potential regulators of neurovascular coupling during synaptic activity (Otsu et al., 2015).
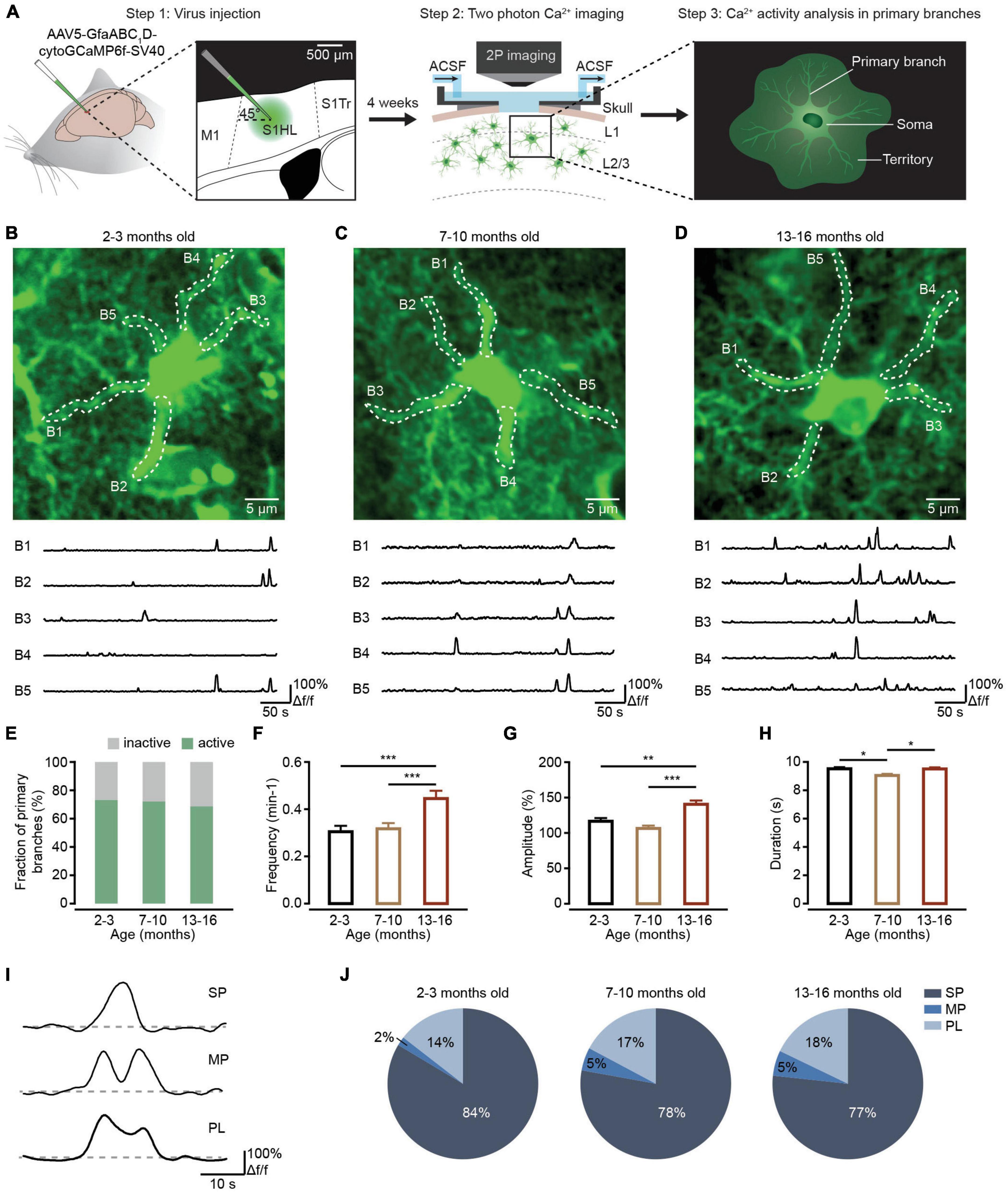
Figure 1. Ca2+ transient changes within primary branches of cortical astrocytes during brain aging. (A) Experimental flow diagram. Left: AAV5-GfaABC1D-cytoGCaMP6f-SV40 was injected into the S1 area. Middle: in vivo two-photon Ca2+ imaging was performed on the cortical astrocytes 4 weeks after viral injection. Right: in vivo Ca2+ transients were analyzed in primary astrocyte branches. (B–D) Top, representative two-photon images of GCaMP6f-labeled astrocytes in the 2–3-month-old (B; imaging depth, 110 μm), 7–10-month-old (C; imaging depth, 107 μm), and 13–16-month-old groups (D; imaging depth, 80 μm). Primary branch ROIs are outlined using white dashed lines. Below are example traces of spontaneous Ca2+ transients from each ROI. (E) The fraction of active primary branches within the 2–3-month-old (n = 183 ROIs from six mice), 7–10-month-old (n = 197 ROIs from nine mice), and 13–16-month-old groups (n = 169 ROIs from six mice); all groups: χ2 = 0.9739, P = 0.6145, χ2-test. (F) Bar graphs summarizing the frequencies of Ca2+ transients within all active branches of the 2–3 month-old (n = 129 ROIs from six mice), 7–10-month-old (n = 141 ROIs from nine mice), and 13–16-month-old (n = 110 ROIs from six mice) group. The 2–3-month-old group versus the 7–10 month-old group: P = 1.000; the 2–3-month-old group versus the 13–16-month-old group: P = 0.0003; the 7–10 month-old group versus the 13–16-month-old group: P = 0.0005; ***P < 0.001, Kruskal–Wallis test followed by Dunn’s multiple comparisons test. (G) Bar graphs summarizing the amplitudes of Ca2+ transients within active primary branches of the 2–3-month-old (n = 444 Ca2+ events from six mice), 7–10-month-old (n = 432 Ca2+ events from nine mice), and 13–16-month-old groups (n = 548 Ca2+ events from six mice). The 2–3-month-old group versus the 7–10-month-old group: P = 0.6555; the 2–3-month-old group versus the 13–16-month-old group: P = 0.0026; 7–10 months old group versus 13–16 months old group: P = 1.30 × 10– 5; ***P < 0.001,**P < 0.01, Kruskal–Wallis test followed by Dunn’s multiple comparisons test. (H) Bar graphs summarizing the durations of Ca2+ transients within active primary branches of the 2–3-month-old (n = 453 Ca2+ events from six mice), 7–10-month-old (n = 444 Ca2+ events from nine mice), and 13–16-month-old groups (n = 562 Ca2+ events from six mice). The 2–3 month-old group versus the 7–10-month-old group: P = 0.0173; the 2–3-month-old group versus 13–16-month-old group: P = 1.0000; the 7–10-month-old group versus the 13–16-month-old group: P = 0.0361; *P < 0.05, Kruskal–Wallis test followed by Dunn’s multiple comparisons test. (I) Example Ca2+ traces of the 3 peak types: singlepeaks, multipeaks, and plateaus. (J) Proportions of different peak types of Ca2+ transients within astrocytic branches of the 2–3-month-old (n = 469 Ca2+ events from six mice), 7–10-month-old (n = 465 Ca2+ events from nine mice) and 13–16-month-old groups (n = 588 Ca2+ events from six mice). All groups: χ2 = 12.1912, P = 0.0160; the 2–3-month-old group versus the 7–10-month-old group: SP: P = 0.0791, MP: P = 0.0330, PL: P = 0.7728; the 2–3 month-old group versus the 13–16-month-old group: SP: P = 0.0170, MP: P = 0.0096, PL: P = 0. 4278; the 7–10-month-old group versus the 13–16-month-old group: SP: P = 1.0000, MP: P = 1.0000, PL: P = 1.0000. χ2-test followed by Bonferroni correction. All data are shown as the mean ± SEM. SP, singlepeaks; MP, multipeaks; PL, plateaus; B, branch.
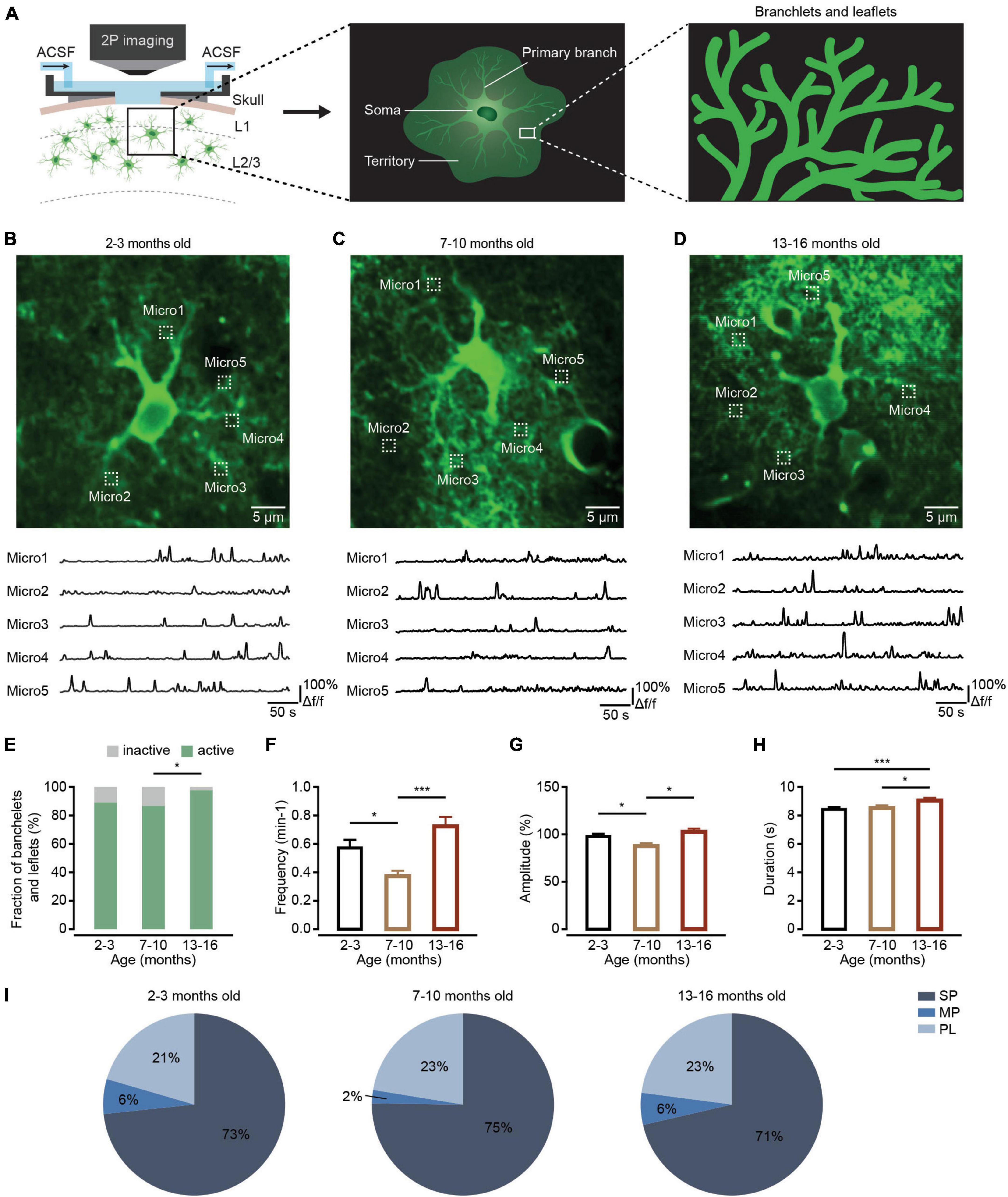
Figure 2. Ca2+ transient changes within branchlets and leaflets of cortical astrocytes during brain aging. (A) Schematic of two-photon Ca2+ imaging of astrocytic branchlets and leaflets expressing GCaMP6f. (B–D) Top, representative two-photon images of GCaMP6f-labeled ROIs of branchlets and leaflets in the 2–3-month-old (B; imaging depth, 172 μm), 7–10-month-old (C; imaging depth, 151 μm), and 13–16-month-old groups (D; imaging depth, 134 μm). Regions of branchlets and leaflets are outlined by white dashed squares. Below are example traces of spontaneous Ca2+ transients from each ROI. (E) The fraction of active branchlets and leaflets within the 2–3-month-old (n = 119 ROIs from six mice), 7–10-month-old (n = 112 ROIs from seven mice) and 13–16-month-old groups (n = 88 ROIs from six mice). All groups: χ2 = 7.6671, P = 0.0216; the 2-3-month-old group versus the 7–10-month-old group: P = 1.000; the 2–3-month-old group versus the 13–16-month-old group: P = 0.0529; the 7–10 month-old group versus the 13–16-month-old group: P = 0.0154, *P < 0.05, χ2-test followed by Bonferroni correction. (F) Bar graphs summarizing the frequencies of Ca2+ transients within active astrocytic branchlets and leaflets of the 2–3 month-old (n = 97 ROIs from six mice), 7–10-month-old (n = 96 ROIs from seven mice) and 13–16-month-old (n = 85 ROIs from six mice) groups. The 2–3 month-old group versus the 7–10-month-old group: P = 0.0133; the 2–3-month-old group versus the 13–16-month-old group: P = 0.0695; the 7–10 month-old group versus the 13–16-month-old group: P = 1.58 × 10– 6, *P < 0.05, ***P < 0.001, Kruskal–Wallis test followed by Dunn’s multiple comparisons test. (G) Bar graphs summarizing the amplitudes of Ca2+ transients within astrocytic branchlets and leaflets of the 2–3 month-old (n = 743 Ca2+ events from six mice), 7–10-month-old (n = 347 Ca2+ events from seven mice) and 13–16-month-old groups (n = 601 Ca2+ events from six mice). The 2–3-month-old group versus the 7–10-month-old group: P = 0.0241; the 2–3-month-old group versus 13–16-month-old group: P = 1.0000; the 7–10-month-old group versus the 13–16-month-old group: P = 0.0404, *P < 0.05, Kruskal–Wallis test followed by Dunn’s multiple comparisons test. (H) Bar graphs summarizing the durations of Ca2+ transients within astrocytic branchlets and leaflets of the 2–3-month-old (n = 749 Ca2+ events from 6 mice), 7–10-months old (n = 365 Ca2+ events from seven mice) and 13–16-month-old groups (n = 638 Ca2+ events from six mice). The 2–3-month-old group versus 7–10-month-old group: P = 1.0000; the 2–3-month-old group versus the 13–16-month-old group: P = 0.0002; the 7–10-month-old group versus the 13–16-month-old group: P = 0.0234, *P < 0.05, ***P < 0.001, Kruskal–Wallis test followed by Dunn’s multiple comparisons test. (I) Proportions of different peak types of Ca2+ transients within astrocytic branchlets and leaflets of the 2–3 month-old (n = 791 Ca2+ events from six mice), 7–10-month-old (n = 379 Ca2+ events from seven mice), and 13–16-month-old groups (n = 650 Ca2+ events from six mice), all groups: χ2 = 9.1670, P = 0.0571, χ2-test. All data are shown as the mean ± SEM. SP, singlepeaks; MP, multipeaks; PL, plateaus; Micro, micro-domain.
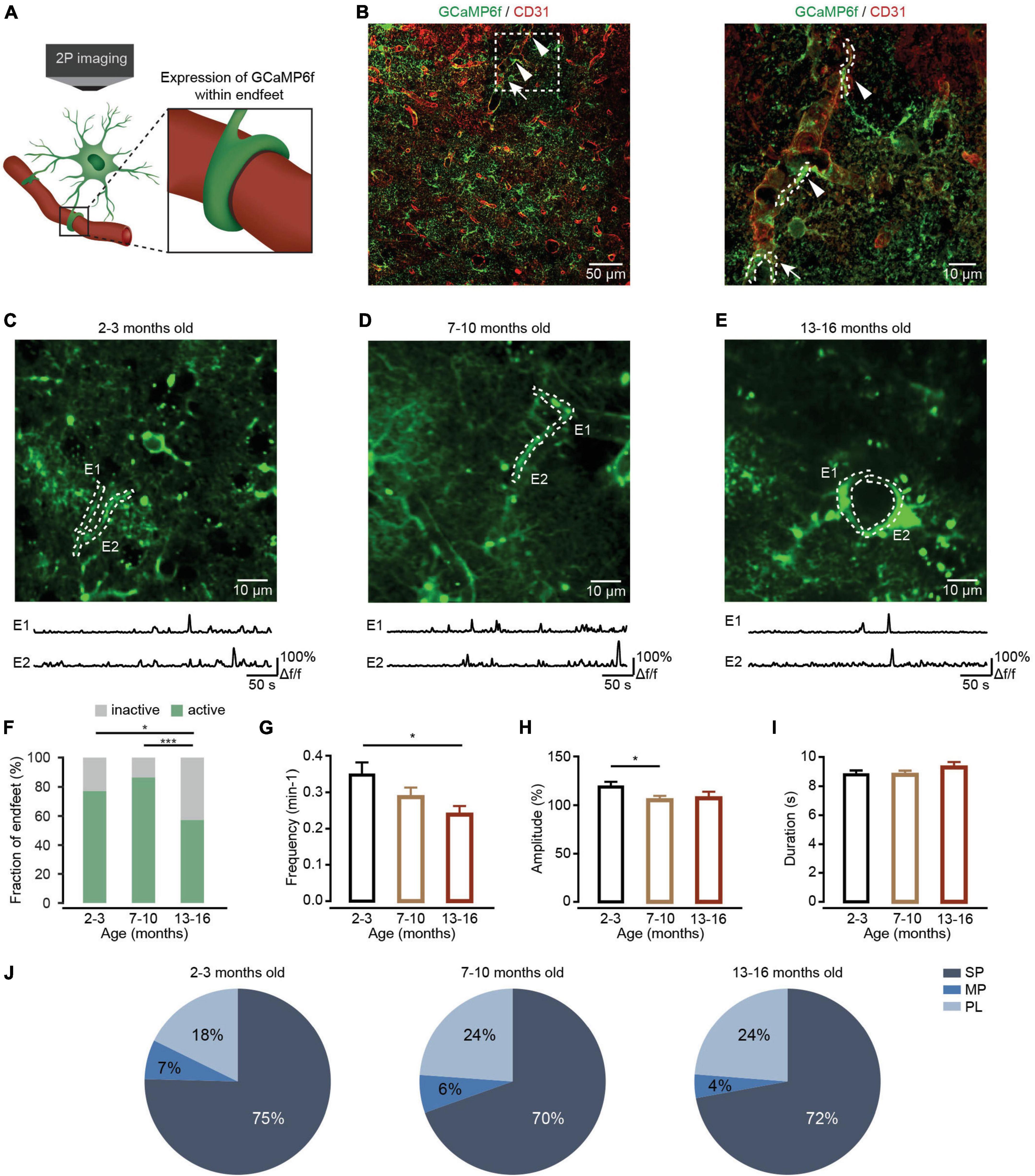
Figure 3. Ca2+ transient changes within endfeet of cortical astrocytes during brain aging. (A) Schematic of two-photon Ca2+ imaging of astrocytic endfeet expressing GCaMP6f. (B) Left, a representative image showing GCaMP6f (green)-labeled astrocytes and CD31 (red)-labeled blood vessels in the cortices of mice 4 weeks after AAV5-GfaABC1D-cytoGCaMP6f-SV40 injection. Right, a high-magnification image showing immunostaining of GCaMP6f (green) and CD31 (red) as indicated by the white-dashed box in the left panel. The arrowheads point to the sagittal sections of the endfeet. The arrow points to the coronal sections of the endfeet. (C–E) Top, representative two-photon images of GCaMP6f-labeled endfeet ROIs in the 2–3-month-old (C; imaging depth, 155 μm), 7–10-month-old (D; imaging depth, 107 μm), and 13–16-month-old groups (E; imaging depth, 113 μm). Endfeet are outlined by a white-dashed line. Below are example traces of spontaneous Ca2+ transients from each ROI. (F) The fraction of active endfeet within the 2–3-month-old (n = 79 ROIs from seven mice), 7–10-month-old (n = 89 ROIs from seven mice), and 13–16-month-old groups (n = 89 ROIs from seven mice). All groups: χ2 = 20.3088, P = 3.89 × 10– 5; the 2–3 month-old group versus the 7–10-month-old group: P = 0.3484; the 2–3 month-old group versus the 13–16-month-old group: P = 0.0189; the 7–10-month-old group versus the 13–16-month-old group: P = 4.35 × 10– 5, *P < 0.05, ***P < 0.001, χ2-test followed by Bonferroni correction. (G) Bar graphs summarizing the frequencies of Ca2+ transients within active astrocytic endfeet of the 2–3-month-old (n = 55 ROIs from seven mice), 7–10-month-old (n = 70 ROIs from seven mice), and 13–16-month-old (n = 48 ROIs from seven mice) groups. All groups: P = 0.0436; the 2–3 month-old-group versus 7–10-month-old group: P = 0.3749; the 2–3 months-old-group versus the 13–16-month-old group: P = 0.0389; the 7–10-month-old group versus the 13–16-month-old group: P = 0.7588, *P < 0.05, Kruskal–Wallis test followed by Dunn’s multiple comparisons test. (H) Bar graphs summarizing the amplitudes of Ca2+ transients within astrocytic endfeet of the 2–3-month-old (n = 234 Ca2+ events from seven mice), 7–10-month-old (n = 274 Ca2+ events from seven mice), and 13–16-month-old groups (n = 109 Ca2+ events from seven mice). The 2–3-month-old group versus the 7–10-month-old group: P = 0.0164; the 2–3 month-old group versus the 13–16-month-old group: P = 0.2502; the 7–10-month-old group versus the 13–16-month-old group: P = 1.0000, *P < 0.05, Kruskal–Wallis test followed by Dunn’s multiple comparisons test. (I) Bar graphs summarizing the durations of Ca2+ transients within astrocytic endfeet of the 2–3-month-old (n = 237 Ca2+ events from seven mice), 7–10-month-old (n = 279 Ca2+ events from seven mice), and 13–16-month-old groups (n = 120 Ca2+ events from seven mice). All groups: P = 0.1706, Kruskal–Wallis test. (J) Proportions of different peak types of Ca2+ transients within astrocytic endfeet of the 2–3-month-old (n = 248 Ca2+ events from seven mice), 7–10-month-old (n = 289 Ca2+ events from seven mice), and 13–16-month-old groups (n = 122 Ca2+ events from seven mice). All groups: χ2 = 4.408, P = 0.3540, χ2-test. All data are shown as the mean ± SEM. SP, singlepeaks; MP, multipeaks; PL, plateaus; E, endfoot.
Brain aging is associated with a progressive loss of function that causes deficits in learning and memory (Verkhratsky, 2019). This degenerative progression was found to produce numerous detrimental changes in astrocytes (Matias et al., 2019), including reduced glutamate uptake (Popov et al., 2021), decreased astroglial metabolic support (Verkhratsky et al., 2021), and impaired glymphatic clearance (Kress et al., 2014). However, the effects of aging on astrocytic Ca2+ transients are not currently well-characterized. Some in vitro data indicate age-dependent remodeling of ionotropic signaling (Lalo et al., 2011) and decreased Ca2+ transients with aging (Gómez-Gonzalo et al., 2017; Lalo et al., 2018) occurs in astrocytes. In contrast, in vivo data based on Ca2+ analysis restricted to the soma suggest that Ca2+ wave activity increases in astrocytes during aging (Mathiesen et al., 2013). Until now, little was known about whether and how local Ca2+ signals change in astrocytic subcellular domains like branches, branchlets, leaflets, and endfeet during brain aging. Clarifying the spatial-temporal changes of these local Ca2+ transients during aging is a crucial step in our understanding of the astrocyte role in brain function (Zhang et al., 2021) and could reveal astrocytes as novel therapeutic targets to treat neurodegenerative diseases (Zhang et al., 2016; Yang et al., 2022).
To determine the aging-induced in vivo changes of Ca2+ transients in astrocytic processes, including the primary branches, branchlets and leaflets, and endfeet, we combined in vivo two-photon Ca2+ imaging with genetically encoded Ca2+ indicators (GECIs) to monitor astrocytic Ca2+ transients in these subcellular domains during brain aging. We found that astrocytic Ca2+ transients diversely change during aging within the astrocytic subcellular domains. This finding provides us with a more precise understanding of how astrocytes contribute to brain function and dysfunction during the aging process.
Methods
Animals
Male C57BL/6J mice were obtained from the GemPharmatech Company in Nanjing. The mice were divided into three groups according to their ages: the 2–3-month-old, 7–10-month-old, and 13–16-month-old groups. 6–9 mice were used per group, and 23 mice were used in total. Mice were maintained in a 12-h light-dark cycle at 22–25°C and 50–60% relative humidity with freely available food and water. All animal experiments were performed according to the Institutional Animal Care and Use Committee of the Third Military Medical University, China.
Virus injection
The mice were anesthetized with 1–1.5% isoflurane in pure O2 and placed in stereotaxic frames. A small vertical incision was made in the skin. The part of the skull directly above the injection site was thinned using a small bone drill to allow penetration by a glass micropipette containing pAAV. To detect the characteristics of Ca2+ transients of astrocytes, the AAV construct (AAV5-GfaABC1D-cytoGCaMP6f-SV40, Cat# 52925-AAV5, Addgene) was injected with a glass micropipette at a 45° forward tilt into the somatosensory cortex [anteroposterior (AP): + 0.5 mm; mediolateral (ML): −1.56 mm, Figure 1A, left]. Two injection sites (200∼300 nl/site, without dilution: titer ≥ 7 × 1012 vg/mL) were applied at dorsoventral (DV): −0.7 and −0.5 mm. After the injection, the pipette was held in place for 15 min before being slowly retracted from the brain. The scalp incision was closed with tissue adhesive (Vetbond; 3M Animal Care Products); postinjection analgesics were administered to aid recovery.
In vivo two-photon Ca2+ imaging of cortical astrocytes
Approximately 4 weeks after virus injections, in vivo two-photon Ca2+ imaging was performed as previously described (Zhang et al., 2016, 2021). Briefly, mice were anesthetized by 1–1.5% isoflurane in pure O2. The skin was removed after local application of xylocaine. A custom-made recording chamber was then glued to the skull with cyanoacrylate glue (UHU). A craniotomy (approximately 3 mm diameter) centered on the previous virus injection site was made by a cranial drill. During the two-photon Ca2+ imaging, the concentration of isoflurane decreased to 0.5–1% (breathing rates of mice were kept at 100–120 times per minute). The recording chamber was perfused with warm artificial cerebrospinal fluid (ACSF), as previously described (Zhang et al., 2021), which maintains the cell survival environment (Figure 1A, middle).
In vivo two-photon Ca2+ imaging of cortical astrocytes was performed with a custom-built two-photon microscope system (Zhang et al., 2016, 2021). Full-frame images were acquired at 20 Hz by custom-written software based on LabVIEW (National Instruments) and resampled at 5 Hz. The wavelength of the excitation laser was set at 920 nm. The average power delivered to the brain was adjusted to 15–30 mW depending on image depth to avoid phototoxicity on astrocytes.
Immunohistochemistry
Mice were anesthetized with 1% pentobarbital (0.1 mL/g) and perfused with 4% paraformaldehyde (PFA). Free-floating coronal brain slices (40 μm thick) were obtained and stained as previously described (Qin et al., 2020; Zhang et al., 2021). In brief, brain slices were permeabilized, blocked, and incubated with primary antibodies overnight at 4°C (chicken anti-GFP, 1:500, Abcam, ab13970; rat anti-CD31,1;100, BD Biosciences, 550274; rabbit anti-S100β, 1:500, Abcam, ab41548; rabbit anti-NeuN, 1:500, Abcam, ab177487; goat anti GFAP, 1:500, Abcam, ab53554; rabbit anti AQP4, 1:500, Sigma, A5971). Sections were rinsed in PBS, followed by a 2 h incubation, in the dark at room temperature, with secondary antibodies directed against the immunoglobulins of the appropriate species coupled to AF594 and AF488 (1:500 dilution, Invitrogen). Sections were mounted with Fluorescence Mounting Medium (Fluoro-Gel II with Dapi, Electron Microscopy Sciences, Hatfield, United Kingdom). Images were acquired with a Leica TCS SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) equipped with standard filter sets and oil immersion objectives (60×/1.42 and 20×/0.85).
Data analysis and statistics
Data analysis of two-photon Ca2+ imaging was completed according to our previously reported methods (Zhang et al., 2021) using LabVIEW 2014 (National Instruments), MATLAB 2014a (MathWorks), or Igor Pro 5.0 (version 6.0.3.1; WaveMetrics) in conjunction with custom-written macros. Upon displaying the maximum fluorescence projections, ROIs were manually defined for the soma, primary branches and endfeet based on high resolution (600 × 600 pixel) anatomical images (Taheri et al., 2017; Stobart et al., 2018b). Microdomains (branchlets and leaflets) were chosen at about 12–20 μm from the soma perimeter (1 × 1 μm box) according to the previous report (Taheri et al., 2017) using our custom-written macros. Astrocytic Ca2+ transients were expressed as relative fluorescence changes (Δf/f), corresponding to the mean fluorescence from all pixels within specified regions of interest (ROIs), as reported by our previous studies (Zhang et al., 2016, 2021). The Ca2+ signal for each ROI was expressed as Δf/f = (f – f0)/f0, where the fluorescence f0 was estimated as the 25th percentile of the entire fluorescence recording (Zhang et al., 2021). Ca2+ signals were defined as ‘Ca2+ transients’ when the maxima of the Ca2+ signals were above 3 × SD of the baseline that was the average of the entire 10 min Ca2+ signals. ROIs were classified as active if ≥ 1 Ca2+ transients occurred during the 10 min recording (Gómez-Gonzalo et al., 2017; Fordsmann et al., 2019). Therefore, inactive ROIs include weak Ca2+ signals that were smaller than 3 × SD of the baseline traces. We classified the Ca2+ transients as singlepeak (SP), multipeak (MP), and plateau (PL) according to a previous study (Taheri et al., 2017; Stobart et al., 2018b). SP means a signal with one clear peak, without any subsequent major oscillations or bumps; MP means a signal that has more than one peak in succession; PL means a signal with one main peak and subsequent bump or oscillation (Figure 1I). All statistics were performed in GraphPad Prism (version 8.0) and SPSS (version 25.0). The outliers of Ca2+ imaging data were removed according to the rule of 1.5 × interquartile range (IQR) before statistics. Normality was tested by the D’Agostino–Pearson test since the data didn’t fit the normal distribution. As such, multiple group comparisons were performed using a Kruskal–Wallis test followed by Dunn’s multiple comparisons test. Fractions of active/inactive ROIs (primary branches, branchlets and leaflets, or endfeet) and types of Ca2+ transients were compared with χ2-test or Fisher’s exact test followed by Bonferroni correction. Data were expressed as the mean ± SEM. P < 0.05 was considered statistically significant.
Results
Ca2+ transients within astrocytic primary branches are increased during aging
The primary branches are the astrocytic processes emanating from the soma (Figure 1A, right; Semyanov and Verkhratsky, 2021). Local Ca2+ elevations in the peripheral districts of astrocytic processes like branchlets and leaflets could generate propagating Ca2+ signals in the primary branches through the opening of endoplasmic reticulum (ER) Ca2+ release channels (Semyanov and Verkhratsky, 2021). Therefore, Ca2+ transients in the primary branches integrate the local Ca2+ events in the surrounding branchlets and leaflets and are important for understanding the principles of Ca2+ events integration within single astrocytes. First, we sought to detect aging-dependent changes to Ca2+ transients within the primary branches. Based on previous in vivo physiological studies on astrocytes (Ghosh et al., 2013; Lind et al., 2013; Kanemaru et al., 2014; Zhang et al., 2016), we injected AAV5-GfaABC1D-cytoGCaMP6f-SV40 into the somatosensory cortex of mice and expressed cytosolic GCaMP6f, a genetically encoded Ca2+ sensor, within astrocytic processes in different age groups (Figure 1A, left). Consistent with the high efficiency and specificity of GCaMP6f labeling in our previous studies (Qin et al., 2020; Zhang et al., 2021), the microinjection of this virus resulted in reliable and specific labeling of GCaMP6f within astrocytes based on immunohistochemical analysis (Supplementary Figure 1). Four weeks later, we implanted a chronic cranial window and imaged the animals by two-photon microscopy while under isoflurane anesthesia (Figure 1A, middle). Finally, we analyzed the Ca2+ transients within the primary branches recorded during in vivo two-photon imaging separately in each group (Figure 1A, right).
Figures 1B–D illustrate experiments in which we monitored the Ca2+ activity within primary branches of different age groups. During our analysis, we selected the primary branches from visible structures in baseline images (Figures 1B–D). The results indicated that the fractions of active primary branches did not change during the aging process (Figure 1E). However, spontaneous Ca2+ transients within the primary branches displayed a greater frequency (Figure 1F), a larger mean amplitude (Figure 1G), and a longer duration (Figure 1H) within active primary branches in the 13–16-month-old group than in the 2–3-month-old or 7–10 month-old groups. Based on their shape, the peaks of these Ca2+ transients were divided into three different classes: SP, MP, and PL (Figure 1I), which were classified according to previous studies (Taheri et al., 2017; Stobart et al., 2018b). Statistical analysis indicated that the percentage of SP decreased, while the percentages of MP increased during aging within active primary branches (Figure 1J).
Ca2+ transients within astrocytic branchlets and leaflets first decreased and then increased during aging
Branchlets and leaflets are higher-order and terminal processes surrounding the primary branches. Most cannot be resolved with diffraction-limited optical imaging, appear as a spongiform cloud, and occupy most of the astrocyte territory (Figure 2A, middle and right) (Semyanov and Verkhratsky, 2021). Most of spontaneous Ca2+ activity occurs as localized transient elevations of [Ca2+]i are detected in branchlets and leaflets (Lim et al., 2021). These local Ca2+ transients are often triggered by neurotransmitters and modify local neuronal signaling (Semyanov et al., 2020).
We then detected aging-dependent changes to Ca2+ transients within astrocytic branchlets and leaflets. Using the above-described methods, astrocytic branchlets and leaflets were labeled by cytosolic GCaMP6f 4 weeks after microinjection of AAV5-GfaABC1D-cytoGCaMP6f-SV40 into the somatosensory cortex. The Ca2+ transients within astrocytic branchlets and leaflets were recorded using in vivo two-photon microscopy in each group (Figure 2A). According to the previous report (Taheri et al., 2017), Ca2+ activities in microdomains (1 × 1 μm box) at approximately 12–20 μm from the soma perimeter were applied to represent Ca2+ transients within the branchlets and leaflets (Figures 2B–D). We found that the fraction of active branchlets and leaflets increased during aging (Figure 2E). The frequency (Figure 2F) and amplitude (Figure 2G) of spontaneous Ca2+ transients within the active branchlets and leaflets decreased in the 7–10-month-old group and increased in 13–16-month-old group, while the duration of Ca2+ transients within the active branchlets and leaflets was unchanged in the 7–10-month-old group and increased in 13–16-month-old group (Figure 2H). In addition, consistent with the changes of the frequency and amplitude during aging, the percentage of MP type of Ca2+ transients decreased in the 7–10-month-old group and increased in 13-16-month-old group (all groups: P = 0.0571, χ2-test; Figure 2I).
Ca2+ transients within astrocytic endfeet decreased during aging
Endfeet are the astrocytic domains closest to blood vessels and envelop these vessels. Ca2+ transients within the astrocytic endfeet are important for regulating neurovascular coupling (Otsu et al., 2015). To determine whether aged astrocytes show differences in the characteristics of Ca2+ transients within endfeet, we expressed cytosolic GCaMP6f in astrocytic endfeet with injections of AAV5-GfaABC1D-cytoGCaMP6f-SV40. Four weeks after virus injection, Ca2+ transients within astrocytic endfeet were recorded by in vivo two-photon microscopy in anesthetized mice of different age groups (Figure 3A). Immunohistochemistry data shows that astrocytic endfeet expressing GCaMP6f envelop CD31-positive blood vessels (Figure 3B). During data analysis, endfeet regions were selected from visible structures in baseline images according to the previous study (Stobart et al., 2018a,b; Figures 3C–E). Statistical results demonstrated that aging decreased the fractions of active endfeet (Figure 3F). In addition, the frequency and amplitude of Ca2+ transients decreased during aging within active endfeet (Figures 3G,H). However, the duration of astrocytic Ca2+ transients (Figure 3I) and the percentages of Ca2+ transient types (all groups: P = 0.3540, χ2-test; Figure 3J) were not affected by aging within active endfeet.
Aquaporin-4 (AQP4) is the predominant water channel localized in the astrocytic endfeet and mediates water transport into the brain parenchyma (Vandebroek and Yasui, 2020). A previous study suggested that AQP4 is involved in regulating the Ca2+ transients within the endfeet (Thrane et al., 2011). We also detected the expressions of AQP4 in endfeet in different groups by immunohistochemistry (Figure 4A). Similar to our previous report (Yang et al., 2022), the current results indicated that AQP4 expression in endfeet decreased during brain aging (Figures 4B,C), which could decrease Ca2+ transients within endfeet (Thrane et al., 2011).
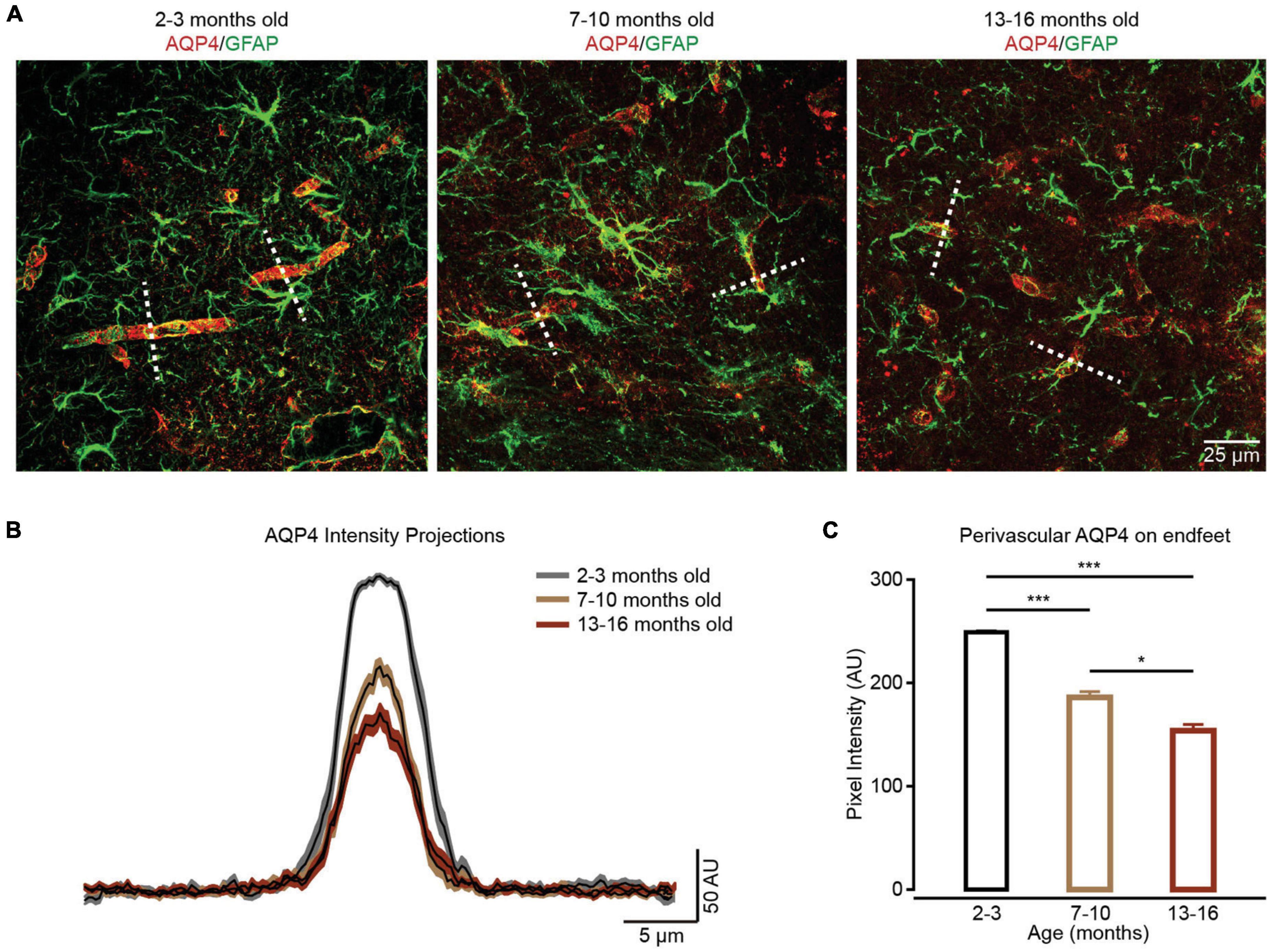
Figure 4. The localization of perivascular AQP4 on endfeet is lost during brain aging. (A) In contrast to the well-maintained localization of perivascular AQP4 (red) on endfeet (perivascular GFAP labeled green structure) in the 2–3-month-old group (left panel), perivascular AQP4 localization becomes more dispersed in the 7–10-month-old and 13–16-month-old groups (middle and right panel). (B) AQP4 immunofluorescence was evaluated in linear regions of interest (dashed lines, A) extending outward from vessels. (C) Bar graph summarizing measurement of perivascular AQP4 expression on endfeet. Compared with the 2–3-month-old group (n = 47 vessels from four mice), perivascular AQP4 expression on endfeet decreased in the 7–10-month-old group (n = 40 vessels from four mice) and 13–16-month-old group (n = 45 vessels from four mice); the 2–3-month-old group versus 7–10-month-old group: P = 6.67 × 10– 10; the 2–3-month-old group versus the 13–16-month-old group: P = 4.1959 × 10– 20; the 7–10-month-old group versus the 13–16-month-old group: P = 0.0359, *P < 0.05, ***P < 0.001, Kruskal–Wallis test followed by Dunn’s multiple comparisons test. All data are shown as the mean ± SEM.
In addition, changes in Ca2+ transients within the somata of astrocytes were assessed during brain aging. Data indicated that aging did not affect the characteristics (frequency, amplitude, and duration) and proportions of Ca2+ transient types within astrocytic somata (all groups: P = 0.7468, Fisher’s exact test; Supplementary Figure 2). This result suggests that astrocytic processes and endfeet are more sensitive to aging-induced chronic stress than somata.
Discussion
Arborization of astrocytes is classified into (i) primary branches emanating from soma; (ii) branchlets and leaflets that occupy most of the astrocyte territory also known as an anatomic domain; and (iii) endfeet that are specialized extensions of astrocytic branches contacting and plastering blood vessel (Semyanov and Verkhratsky, 2021). Most of spontaneous Ca2+ transients reside in the above astrocyte subcellular domains (Lim et al., 2021), heterogeneously occur in an individual astrocyte (Bindocci et al., 2017), and are independent of somatic Ca2+ signals (Lim et al., 2021). These local Ca2+ transients in peripheral processes and endfeet of astrocytes are important for their normal functions: in primary branches, Ca2+ transients, representing the signal communications between soma and processes, indicate the intrinsic principles of Ca2+ signal integration in an individual astrocyte (Semyanov et al., 2020); in branchlets and leaflets, Ca2+ transients modify local neuronal signaling and control synaptic transmission (Lim et al., 2021); in endfeet, Ca2+ transients contribute to the regulation of neurovascular coupling (Otsu et al., 2015). Therefore, deciphering the complex and heterogeneous “Ca2+ language” in these subcellular domains is essential for defining how astrocytes contribute to brain function and dysfunction.
Aging reduces the functional capacity of astrocytes, including reduced K+ buffering and glutamate clearance (Popov et al., 2021), decreased astroglial gap junctional coupling, and impaired astroglial metabolism (Jiang and Cadenas, 2014). In aging brains, astrocytes exhibit ionic excitability mediated by changes in the intracellular concentration of ions (Verkhratsky, 2019) and show peculiar reorganization of astrocytic Ca2+ signals (Popov et al., 2021). This aging-induced aberrant Ca2+ signaling impacts synaptic plasticity and affects long-term potentiation in hippocampal synapses (Popov et al., 2021). Importantly, it has been suggested that changes in Ca2+ homeostasis and Ca2+ signaling are a general mechanism of neural cell aging, which was formalized in a “calcium hypothesis of brain aging” (Khachaturian, 1987; Verkhratsky and Toescu, 1998). More detailed analyses revealed that aging seems to lead to enhanced sensitivity of the brain to changes in [Ca2+]i. Aged brain cells could be more vulnerable because of a decreased ability to down-regulate [Ca2+]i (Müller et al., 1996). Considering the functional importance of subcellular Ca2+ signaling in astrocytes, we studied whether and how these local Ca2+ transients change during aging to further illustrate the role of astrocytes in brain aging.
The present study found that aging alters characteristics of Ca2+ transients in all kinds of subcellular domains, including primary branches, branchlets and leaflets, and endfeet. However, these age-dependent changes differ between these subcellular domains, suggesting there are distinct aging-induced degenerative mechanisms occurring in separate local regions within an individual astrocyte.
In primary branches, Ca2+ transients are initiated by the release of Ca2+ from the ER through the inositol 1,4,5-trisphosphate receptor type 2 (IP3R2) in response to stimulation of metabotropic receptors (Shigetomi et al., 2013; Kanemaru et al., 2014; Lim et al., 2021). Previous studies have indicated that astrocytes show an age-dependent increase in oxidative metabolism and reactive oxygen species (ROS) (Jiang and Cadenas, 2014). In addition, ROS causes oxidation of the IP3R2 and sensitization of Ca2+ release to promote cytoplasmic Ca2+ oscillations (Bánsághi et al., 2014). ROS also activates ryanodine receptor Ca2+ release channels in ER and increases the number of Ca2+ transients (Prosser et al., 2013). Therefore, the enhanced oxidative stress during physiological aging could increase Ca2+ transients within primary branches of astrocytes. Furthermore, the surface-to-volume ratio (SVR) sets the threshold for the generation of spreading Ca2+ events and determines the probability of Ca2+ transient initiation (Rungta et al., 2016; Wu et al., 2019). The primary branches have higher SVR than the somata. As such, Ca2+ entering the primary branch will produce larger increases in Ca2+ concentration, which are more likely than increases in somatic Ca2+ concentration to reach the threshold for amplification by Ca2+-dependent Ca2+ release (Semyanov et al., 2020). This could be why aging caused Ca2+ transient changes in primary branches, but not in somata (Figure 1 and Supplementary Figure 2).
In branchlets and leaflets, Ca2+ transients are generated by Ca2+ release from ER and mitochondria in branchlets and Ca2+ entry through the plasma membrane via sodium/calcium exchanger (NCX), ionotropic receptors or channels in leaflets (Semyanov et al., 2020). In this study, we found that the Ca2+ transients within branchlets and leaflets first decreased in 7–10-months-old mice, and then increased in 13–16-months-old mice (Figure 2). The decreased Ca2+ transients in 7–10-months-old mice could be caused by the age-dependent remodeling of ionotropic signaling in astrocytes (Lalo et al., 2011). Certain kinds of ionotropic receptors peak in young adults (3-months-old) and decrease during aging (Lalo et al., 2011). The age-dependent increase in oxidative metabolism in astrocytes (Jiang and Cadenas, 2014) could induce the following increased Ca2+ transients in 13–16-month-old mice. It has been shown that increased ROS levels not only enhance Ca2+ release from ER (Prosser et al., 2013), but also promote Ca2+ efflux from mitochondria via the mitochondrial permeability pore (mPTP) (Agarwal et al., 2017). Additionally, the increased Ca2+ transients in branchlets and leaflets during aging could be caused by decreases in the partial pressure of oxygen (PO2) in the aged brain (Mathiesen et al., 2013). It has been reported that lowering PO2 in the aged brain resulted in increased cytosolic NADH that induced pronounced increase in Ca2+ signaling in astrocytes (Requardt et al., 2012).
Previous studies have found that Ca2+ transients within the endfeet are involved in the modulation of neurovascular coupling (Otsu et al., 2015). These Ca2+ transients are partly triggered by AQP4-mediated water influx, which promotes ATP release and activation of P2 purinergic receptors (Thrane et al., 2011). In a normal physiological state, the expression of AQP4 is localized to the astrocytic endfeet. However, a previous study (Valenza et al., 2019) and our data (Figure 4) both indicated that the localization of perivascular AQP4 on endfeet is lost during brain aging. Therefore, the decreased Ca2+ transients within the endfeet (Figure 3) could be caused by decreased expression of AQP4 on endfeet, which results in decreased water influx, the release of ATP, and activation of P2 purinergic receptors. At the same time, Ca2+ transients within the endfeet are also mediated by mitochondria (Göbel et al., 2020) and GABAA receptors (Lind et al., 2018). Therefore, the aging-induced impairment of mitochondrial Ca2+ uptake (Göbel et al., 2020) and decreases in ionotropic receptor expression (Lalo et al., 2011) can also contribute to decreases in Ca2+ transients observed within endfeet. In addition, according to previous studies, IP3R2-mediated Ca2+ transients are present within branches and branchlets (Shigetomi et al., 2013; Kanemaru et al., 2014), but absent within endfeet (Bonder and McCarthy, 2014), which could lead to the diverse impacts of aging on Ca2+ transients within these subcellular domains.
Apart from that, both the age-dependent morphological changes and astrocytic network alterations may induce the aberrant astrocytic Ca2+ transients at subcellular domains during brain aging. Previous studies indicated that aging reduces cellular surface area and morphological complexity of astrocytes (Popov et al., 2021; Yang et al., 2022). These age-dependent morphological changes are correlated to spatiotemporal reorganization and increased duration of Ca2+ transients in old astrocytes (Popov et al., 2021). In addition, astrocytic network alterations have been reported in neurodegenerative disorders, which induced the elevated resting Ca2+ and more frequent Ca2+ transients in astrocytic network (Kuchibhotla et al., 2009; Delekate et al., 2014).
There are three different Ca2+ transient types based on their shapes: singlepeaks, multipeaks, and plateaus (Figure 1I). Different Ca2+ transient types indicate diverse mechanisms of signaling (Stobart et al., 2018b). In this study, we found that the percentages of Ca2+ transient types changed with aging in primary branches (Figure 1J) and branchlets and leaflets (Figure 2I), but remained unchanged in endfeet (Figure 3J). This suggests that, aging differentially affects the mechanisms of Ca2+ signaling in astrocyte subcellular domains. Specifically, it suggested that the IP3-mediated release of ER calcium stores contributed to singlepeaks and multipeaks (Stobart et al., 2018b). As such, aging-changed singlepeak and multipeak proportions further indicated that ROS-mediated IP3R2 oxidation (Bánsághi et al., 2014) could be the main mechanism of Ca2+ transient changes during aging within primary branches (Figure 1J), branchlets and leaflets (Figure 2I).
Our study indicated that there was no change of Ca2+ transients within astrocytic somata during brain aging (Supplementary Figure 2), suggesting branches, branchlets and leaflets, or endfeet were more sensitive to aging-induced oxidative stress than somata. Furthermore, previous studies indicated that astrocytic Ca2+ transients remained stable (Gómez-Gonzalo et al., 2017) or declined (Lalo et al., 2018) during aging, which could be caused by the in vitro methods and chemical calcium indicators used in these experiments.
Altogether, our in vivo results demonstrate that aging alters the characteristics of Ca2+ transients within astrocyte subcellular domains. However, these changes in Ca2+ transients are heterogeneous in the subcellular domains, which indicates that the aging-induced degenerative mechanisms differ at the subcellular level in astrocytes. This finding provides us a better understanding of how astrocytes contribute to brain dysfunction during aging and other neural degenerative diseases, like Alzheimer’s disease and Parkinson’s disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of the Third Military Medical University.
Author contributions
LF and KZ designed the work. FD, SL, RL, ZY, YH, SY, QD, JZ, JLy, ZZ, MH, HW, JLi, CY, YW, MG, SC, and HJ performed the main experiments. FD, ZY, MH, and HW completed the virus injections. FD, RL, YH, and JZ performed the two-photon Ca2+ image. FD, ZY, JLi, CY, YW, and MG performed the immunohistochemistry. FD, SL, QD, JLy, ZZ, HJ, and XL performed the data analysis. XC, XL, LF, and KZ wrote the manuscript with input from all co-authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (Nos. 61890952 to LF and 81771175 to KZ) and the National Key R&D Program of China (No. 2018YFA0109600).
Acknowledgments
We thank Jia Lou for the technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.1029533/full#supplementary-material
Supplementary Figure 1 | Specific and efficient expression of GCaMP6f in cortical astrocytes 4 weeks after AAV5-GfaABC1D-cytoGCaMP6f-SV40 injection. (A) Representative image showing most of the GCaMP6f (green)-labeled astrocytes were S100β (red) positive cells. (B) High-magnification image showing immunostaining of GCaMP6f (green) and S100β (red) as indicated by the white-dashed box in the left panel. (C) Representative image showing none of the GCaMP6f-labeled astrocytes were stained with NeuN.
Supplementary Figure 2 | No changes to Ca2+ transients within the somata of cortical astrocytes during brain aging. (A) The fraction of active somata within the 2–3-month-old (n = 126 ROIs from seven mice), 7–10-month-old (n = 126 ROIs from seven mice), and 13–16-month-old groups (n = 116 ROIs from seven mice), all groups: χ2 = 1.2468, P = 0.5261, χ2-test. (B) Bar graphs summarizing the frequencies of Ca2+ transients within active astrocytic somata of the 2–3-month-old (n = 48 ROIs from seven mice), 7–10-month-old (n = 53 ROIs from seven mice), and 13–16-month-old (n = 42 ROIs from seven mice) groups. All groups: P = 0.3233, Kruskal–Wallis test. (C) Bar graphs summarizing the amplitudes of Ca2+ transients within astrocytic somata of the 2–3-month-old (n = 134 Ca2+ events from seven mice), 7–10-month-old (n = 124 Ca2+ events from seven mice), and 13–16-month-old (n = 83 Ca2+ events from seven mice) groups. All groups: P = 0.5258, Kruskal–Wallis test. (D) Bar graphs summarizing the durations of Ca2+ transients within astrocytic somata of the 2–3-month-old (n = 133 Ca2+ events from seven mice), 7–10-month-old (n = 117 Ca2+ events from seven mice), and 13–16-month-old (n = 80 Ca2+ events, seven mice) groups. All groups: P = 0.0920, Kruskal–Wallis test. (E) Proportions of different peak types of Ca2+ transients within somata of the 2–3-month-old (n = 138 Ca2+ events from seven mice), 7–10-month-old (n = 128 Ca2+ events from seven mice), and 13–16-month-old groups (n = 87 Ca2+ events from seven mice). All groups: P = 0.7468, Fisher’s exact test. All data are shown as the mean ± SEM. SP, singlepeaks; MP, multipeaks; PL, plateaus; S, soma.
References
Agarwal, A., Wu, P. H., Hughes, E. G., Fukaya, M., Tischfield, M. A., Langseth, A. J., et al. (2017). Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93, 587–605.e7. doi: 10.1016/j.neuron.2016.12.034
Araque, A., Carmignoto, G., Haydon, P. G., Oliet, S. H., Robitaille, R., and Volterra, A. (2014). Gliotransmitters travel in time and space. Neuron 81, 728–739. doi: 10.1016/j.neuron.2014.02.007
Bánsághi, S., Golenár, T., Madesh, M., Csordás, G., RamachandraRao, S., Sharma, K., et al. (2014). Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J. Biol. Chem. 289, 8170–8181. doi: 10.1074/jbc.M113.504159
Bindocci, E., Savtchouk, I., Liaudet, N., Becker, D., Carriero, G., and Volterra, A. (2017). Three-dimensional Ca(2+) imaging advances understanding of astrocyte biology. Science 356:eaai8185. doi: 10.1126/science.aai8185
Bonder, D. E., and McCarthy, K. D. (2014). Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J. Neurosci. 34, 13139–13150. doi: 10.1523/JNEUROSCI.2591-14.2014
Delekate, A., Füchtemeier, M., Schumacher, T., Ulbrich, C., Foddis, M., and Petzold, G. C. (2014). Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 5, 5422. doi: 10.1038/ncomms6422
Descalzi, G. (2021). Cortical astrocyte-neuronal metabolic coupling emerges as a critical modulator of stress-induced hopelessness. Neurosci. Bull. 37, 132–134. doi: 10.1007/s12264-020-00559-7
Di Castro, M. A., Chuquet, J., Liaudet, N., Bhaukaurally, K., Santello, M., Bouvier, D., et al. (2011). Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284.
Fordsmann, J. C., Murmu, R. P., Cai, C., Brazhe, A., Thomsen, K. J., Zambach, S. A., et al. (2019). Spontaneous astrocytic Ca(2+) activity abounds in electrically suppressed ischemic penumbra of aged mice. Glia 67, 37–52. doi: 10.1002/glia.23506
Ghosh, A., Wyss, M. T., and Weber, B. (2013). Somatotopic astrocytic activity in the somatosensory cortex. Glia 61, 601–610.
Gómez-Gonzalo, M., Martin-Fernandez, M., Martínez-Murillo, R., Mederos, S., Hernández-Vivanco, A., Jamison, S., et al. (2017). Neuron-astrocyte signaling is preserved in the aging brain. Glia 65, 569–580. doi: 10.1002/glia.23112
Grosche, J., Matyash, V., Möller, T., Verkhratsky, A., Reichenbach, A., and Kettenmann, H. (1999). Microdomains for neuron-glia interaction: Parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 2, 139–143. doi: 10.1038/5692
Göbel, J., Engelhardt, E., Pelzer, P., Sakthivelu, V., Jahn, H. M., Jevtic, M., et al. (2020). Mitochondria-endoplasmic reticulum contacts in reactive astrocytes promote vascular remodeling. Cell Metab. 31, 791–808.e8. doi: 10.1016/j.cmet.2020.03.005
Jiang, T., and Cadenas, E. (2014). Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13, 1059–1067.
Kanemaru, K., Sekiya, H., Xu, M., Satoh, K., Kitajima, N., Yoshida, K., et al. (2014). In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca(2+) indicator. Cell Rep. 8, 311–318. doi: 10.1016/j.celrep.2014.05.056
Khachaturian, Z. S. (1987). Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiol. Aging 8, 345–346.
Khakh, B. S., and Sofroniew, M. V. (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952.
Kosaka, T., and Hama, K. (1986). Three-dimensional structure of astrocytes in the rat dentate gyrus. J. Comp. Neurol. 249, 242–260.
Kress, B. T., Iliff, J. J., Xia, M., Wang, M., Wei, H. S., Zeppenfeld, D., et al. (2014). Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861.
Kuchibhotla, K. V., Lattarulo, C. R., Hyman, B. T., and Bacskai, B. J. (2009). Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323, 1211–1215. doi: 10.1126/science.1169096
Lalo, U., Bogdanov, A., and Pankratov, Y. (2018). Diversity of astroglial effects on aging- and experience-related cortical metaplasticity. Front. Mol. Neurosci. 11:239. doi: 10.3389/fnmol.2018.00239
Lalo, U., Palygin, O., North, R. A., Verkhratsky, A., and Pankratov, Y. (2011). Age-dependent remodelling of ionotropic signalling in cortical astroglia. Aging Cell 10, 392–402. doi: 10.1111/j.1474-9726.2011.00682.x
Lim, D., Semyanov, A., Genazzani, A., and Verkhratsky, A. (2021). Calcium signaling in neuroglia. Int. Rev. Cell Mol. Biol. 362, 1–53.
Lind, B. L., Brazhe, A. R., Jessen, S. B., Tan, F. C., and Lauritzen, M. J. (2013). Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc. Natl. Acad. Sci. U.S.A. 110, E4678–E4687. doi: 10.1073/pnas.1310065110
Lind, B. L., Jessen, S. B., Lønstrup, M., Joséphine, C., Bonvento, G., and Lauritzen, M. (2018). Fast Ca(2+) responses in astrocyte end-feet and neurovascular coupling in mice. Glia 66, 348–358.
Liu, J. H., Li, Z. L., Liu, Y. S., Chu, H. D., Hu, N. Y., Wu, D. Y., et al. (2020). Astrocytic GABA(B) receptors in mouse hippocampus control responses to behavioral challenges through Astrocytic BDNF. Neurosci. Bull. 36, 705–718. doi: 10.1007/s12264-020-00474-x
Mathiesen, C., Brazhe, A., Thomsen, K., and Lauritzen, M. (2013). Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. J. Cereb. Blood Flow Metab. 33, 161–169. doi: 10.1038/jcbfm.2012.175
Matias, I., Morgado, J., and Gomes, F. C. A. (2019). Astrocyte heterogeneity: Impact to brain aging and disease. Front. Aging Neurosci. 11:59. doi: 10.3389/fnagi.2019.00059
Müller, W. E., Eckert, A., Hartmann, H., Velbinger, K., and Förstl, H. (1996). [The calcium hypothesis of brain aging]. Nervenarzt 67, 15–24.
Nanclares, C., Baraibar, A. M., Araque, A., and Kofuji, P. (2021). Dysregulation of astrocyte-neuronal communication in Alzheimer’s disease. Int. J. Mol. Sci. 22:7887. doi: 10.3390/ijms22157887
Otsu, Y., Couchman, K., Lyons, D. G., Collot, M., Agarwal, A., Mallet, J. M., et al. (2015). Calcium dynamics in astrocyte processes during neurovascular coupling. Nat. Neurosci. 18, 210–218.
Popov, A., Brazhe, A., Denisov, P., Sutyagina, O., Li, L., Lazareva, N., et al. (2021). Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell 20:e13334. doi: 10.1111/acel.13334
Prosser, B. L., Khairallah, R. J., Ziman, A. P., Ward, C. W., and Lederer, W. J. (2013). X-ROS signaling in the heart and skeletal muscle: Stretch-dependent local ROS regulates [Ca2+]i. J. Mol. Cell. Cardiol. 58, 172–181. doi: 10.1016/j.yjmcc.2012.11.011
Qin, H., He, W., Yang, C., Li, J., Jian, T., Liang, S., et al. (2020). Monitoring Astrocytic Ca(2+) activity in freely behaving mice. Front. Cell. Neurosci. 14:603095. doi: 10.3389/fncel.2020.603095
Requardt, R. P., Hirrlinger, P. G., Wilhelm, F., Winkler, U., Besser, S., and Hirrlinger, J. (2012). Ca2+ signals of astrocytes are modulated by the NAD+/NADH redox state. J. Neurochem. 120, 1014–1025.
Rungta, R. L., Bernier, L. P., Dissing-Olesen, L., Groten, C. J., LeDue, J. M., Ko, R., et al. (2016). Ca(2+) transients in astrocyte fine processes occur via Ca(2+) influx in the adult mouse hippocampus. Glia 64, 2093–2103. doi: 10.1002/glia.23042
Semyanov, A. (2019). Spatiotemporal pattern of calcium activity in astrocytic network. Cell Calcium 78, 15–25.
Semyanov, A., Henneberger, C., and Agarwal, A. (2020). Making sense of astrocytic calcium signals - from acquisition to interpretation. Nat. Rev. Neurosci. 21, 551–564. doi: 10.1038/s41583-020-0361-8
Semyanov, A., and Verkhratsky, A. (2021). Astrocytic processes: From tripartite synapses to the active milieu. Trends Neurosci. 44, 781–792. doi: 10.1016/j.tins.2021.07.006
Shigetomi, E., Bushong, E. A., Haustein, M. D., Tong, X., Jackson-Weaver, O., Kracun, S., et al. (2013). Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J. Gen. Physiol. 141, 633–647. doi: 10.1085/jgp.201210949
Shigetomi, E., Saito, K., Sano, F., and Koizumi, S. (2019). Aberrant calcium signals in reactive astrocytes: A key process in neurological disorders. Int. J. Mol. Sci. 20:996. doi: 10.3390/ijms20040996
Stobart, J. L., Ferrari, K. D., Barrett, M. J. P., Stobart, M. J., Looser, Z. J., Saab, A. S., et al. (2018b). Long-term in vivo calcium imaging of astrocytes reveals distinct cellular compartment responses to sensory stimulation. Cereb. Cortex 28, 184–198. doi: 10.1093/cercor/bhw366
Stobart, J. L., Ferrari, K. D., Barrett, M. J. P., Glück, C., Stobart, M. J., Zuend, M., et al. (2018a). Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron 98, 726–735.e4. doi: 10.1016/j.neuron.2018.03.050
Taheri, M., Handy, G., Borisyuk, A., and White, J. A. (2017). Diversity of evoked astrocyte Ca(2+) dynamics quantified through experimental measurements and mathematical modeling. Front. Syst. Neurosci. 11:79. doi: 10.3389/fnsys.2017.00079
Thrane, A. S., Rappold, P. M., Fujita, T., Torres, A., Bekar, L. K., Takano, T., et al. (2011). Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. U.S.A. 108, 846–851. doi: 10.1073/pnas.1015217108
Valenza, M., Facchinetti, R., Steardo, L., and Scuderi, C. (2019). Altered waste disposal system in aging and Alzheimer’s disease: Focus on astrocytic aquaporin-4. Front. Pharmacol. 10:1656. doi: 10.3389/fphar.2019.01656
Vandebroek, A., and Yasui, M. (2020). Regulation of AQP4 in the central nervous system. Int. J. Mol. Sci. 21:1603.
Verkhratsky, A. (2019). Astroglial calcium signaling in aging and Alzheimer’s disease. Cold Spring Harb. Perspect. Biol. 11:a035188.
Verkhratsky, A., Augusto-Oliveira, M., Pivoriūnas, A., Popov, A., Brazhe, A., and Semyanov, A. (2021). Astroglial asthenia and loss of function, rather than reactivity, contribute to the ageing of the brain. Pflugers Arch. 473, 753–774. doi: 10.1007/s00424-020-02465-3
Wang, F., Smith, N. A., Xu, Q., Fujita, T., Baba, A., Matsuda, T., et al. (2012). Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci. Signal. 5:ra26. doi: 10.1126/scisignal.2002334
Wu, J. L., and Gao, T. M. (2022). Monitoring the activity of astrocytes in learning and memory. Neurosci. Bull. 38, 1117–1120.
Wu, Y. W., Gordleeva, S., Tang, X., Shih, P. Y., Dembitskaya, Y., and Semyanov, A. (2019). Morphological profile determines the frequency of spontaneous calcium events in astrocytic processes. Glia 67, 246–262. doi: 10.1002/glia.23537
Yang, Z., Gong, M., Jian, T., Li, J., Yang, C., Ma, Q., et al. (2022). Engrafted glial progenitor cells yield long-term integration and sensory improvement in aged mice. Stem Cell Res. Ther. 13:285. doi: 10.1186/s13287-022-02959-0
Zhang, K., Chen, C., Yang, Z., He, W., Liao, X., Ma, Q., et al. (2016). Sensory response of transplanted astrocytes in adult mammalian cortex in vivo. Cereb. Cortex 26, 3690–3704. doi: 10.1093/cercor/bhw213
Zhang, K., and Chen, X. (2017). Sensory response in host and engrafted astrocytes of adult brain in vivo. Glia 65, 1867–1884. doi: 10.1002/glia.23181
Keywords: astrocyte, Ca2+ transients, branches, branchlets and leaflets, endfeet, aging
Citation: Ding F, Liang S, Li R, Yang Z, He Y, Yang S, Duan Q, Zhang J, Lyu J, Zhou Z, Huang M, Wang H, Li J, Yang C, Wang Y, Gong M, Chen S, Jia H, Chen X, Liao X, Fu L and Zhang K (2022) Astrocytes exhibit diverse Ca2+ changes at subcellular domains during brain aging. Front. Aging Neurosci. 14:1029533. doi: 10.3389/fnagi.2022.1029533
Received: 27 August 2022; Accepted: 11 October 2022;
Published: 28 October 2022.
Edited by:
Alberto Javier Ramos, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Mazahir T. Hasan, Achucarro Basque Center for Neuroscience, SpainPotier Brigitte, Université Paris-Saclay, France
Copyright © 2022 Ding, Liang, Li, Yang, He, Yang, Duan, Zhang, Lyu, Zhou, Huang, Wang, Li, Yang, Wang, Gong, Chen, Jia, Chen, Liao, Fu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Liao, xiang.liao@cqu.edu.cn; Ling Fu, lfu@mail.hust.edu.cn; Kuan Zhang, zhangkuan@tmmu.edu.cn
†These authors have contributed equally to this work
 Fusheng Ding
Fusheng Ding Shanshan Liang
Shanshan Liang Ruijie Li
Ruijie Li Zhiqi Yang3
Zhiqi Yang3  Jianxiong Zhang
Jianxiong Zhang Haoyu Wang
Haoyu Wang Shangbin Chen
Shangbin Chen Hongbo Jia
Hongbo Jia Xiaowei Chen
Xiaowei Chen Xiang Liao
Xiang Liao Kuan Zhang
Kuan Zhang