Demyelination of the Optic Nerve: An Underlying Factor in Glaucoma?
- State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China
Neurodegenerative disorders are characterized by typical neuronal degeneration and axonal loss in the central nervous system (CNS). Demyelination occurs when myelin or oligodendrocytes experience damage. Pathological changes in demyelination contribute to neurodegenerative diseases and worsen clinical symptoms during disease progression. Glaucoma is a neurodegenerative disease characterized by progressive degeneration of retinal ganglion cells (RGCs) and the optic nerve. Since it is not yet well understood, we hypothesized that demyelination could play a significant role in glaucoma. Therefore, this study started with the morphological and functional manifestations of demyelination in the CNS. Then, we discussed the main mechanisms of demyelination in terms of oxidative stress, mitochondrial damage, and immuno-inflammatory responses. Finally, we summarized the existing research on the relationship between optic nerve demyelination and glaucoma, aiming to inspire effective treatment plans for glaucoma in the future.
Introduction
Neurodegenerative diseases are a group of heterogeneous diseases with progressive and selective loss of neurons (Lin and Beal, 2006). These diseases have some common pathological changes such as axonal dysfunction, demyelination, and irreversible neuronal death. Glaucoma is a neurodegenerative disease characterized by chronic loss of retinal ganglion cells (RGCs) and their axons, with typical clinical manifestations including optic nerve atrophy, visual field defects, and diminution of vision (Weinreb et al., 2014; Jones-Odeh and Hammond, 2015; He et al., 2018). The data from population-based surveys showed that 60 million people suffered from glaucoma worldwide, of which 8.4 million became bilaterally blind, and 112 million people are expected to be suffering from glaucoma by 2040 (Quigley, 2011; Cook and Foster, 2012; Jonas et al., 2017). Developing countries lack efficient and low-cost early screening methods, so the diagnosis of glaucoma is usually confirmed at an advanced stage at which point the optimal treatment period is already missed. Glaucoma disease management in many developing countries needs further improvement. Although it is the leading cause of irreversible blindness worldwide and has been extensively investigated, the pathogenesis of glaucoma is still not yet fully understood (Weinreb et al., 2014). Therefore, it is imperative to explore the pathogenesis and establish an early diagnostic method for glaucoma.
Myelin sheath, a vital part of the nerve, is a multilayered glial membrane surrounding the axons of the myelinated nerve fiber in vertebrates. In the central nervous system (CNS), oligodendrocytes extend the processes to form myelin sheaths (Luse, 1956; Nave and Werner, 2014). Under physiological conditions, myelin plays a critical role in providing survival support to the axons, by maintaining connections and enabling efficient transmission of action potentials. The deterioration of the myelin sheaths and oligodendrocytes, known as demyelination, can affect multiple nerve fibers and form multiple disseminated lesions, leading to serious consequences, including nervous system dysfunctions (Baumann and Pham-Dinh, 2001). As the earliest pathological changes, destruction of the myelin sheath may be the basis of neurodegenerative diseases, such as multiple sclerosis, a typical demyelinating disease with axonal injury (Kaminska et al., 2017), or Alzheimer’s disease (Cai and Xiao, 2016). Therefore, demyelination plays a decisive role in neurodegenerative diseases (Coleman, 2005; Mitew et al., 2014).
It has been proposed that demyelination occurs in neurodegenerative diseases even earlier than neuronal death and axonal dysfunction (You et al., 2019). Some researchers suggested that demyelination is a potential etiological factor of glaucoma. However, it has not been fully accepted due to the prevailing notion that demyelination occurs secondary to neuronal death and axonal dysfunction. Whether demyelination is an underlying factor in glaucoma and if the identification of demyelination could help in the early diagnosis of glaucoma remain controversial. We intend to review the possible mechanisms and manifestations of demyelination and discuss the relationships between demyelination and glaucomatous neurodegeneration that may help explore possible therapeutic directions for glaucoma in the future (Figure 1).
Figure 1. Vision impairment from glaucoma significantly influences daily life. In this article, we explored the relationship between demyelination and glaucoma, aiming to discover an early diagnosis for glaucoma in the future.
The Phenomenon of Demyelination
As a type of plasma membrane, the main components of the myelin sheath are lipids and proteins. Lipids, which account for 70–75% of the dry weight of myelin, mainly contain cholesterol, galactosylceramide, and ethanolamine plasmalogen (Schmitt et al., 2015). Proteins comprise 25–30% of myelin, and the major protein components are myelin basic proteins (MBP) and proteolipid proteins (PLP) (Aggarwal et al., 2013). There is a close relationship between myelin, oligodendrocytes and axons. Therefore, morphological and functional changes in oligodendrocytes, myelin, and axons can be observed when demyelination occurs.
Morphological Changes
Changes of Oligodendrocytes and Other Glial Cells
Demyelination affects the number of oligodendrocyte-lineage cells at various stages, such as the increased number of oligodendrocyte precursor cells (OPCs) after demyelination. Increased OPC can further differentiate into premyelinating oligodendrocytes and remyelinating oligodendrocytes after demyelination (Son et al., 2010; Jennings and Carroll, 2015). Other related cell types can also be affected by demyelination. For example, astrocytes are activated during the early stages of demyelination, characterized by changes in proteins such as glial fibrillary acidic protein and vimentin. During the late stages of demyelination, the number of microglial cells is increased to phagocytose the myelin sheath fragments (Rotshenker et al., 2008; Rotshenker, 2009; Son et al., 2010).
Changes in the Morphology of Myelin Sheaths and the Contents of Lipids and Proteins
Myelin has an unusual lipid composition — the lipid-to-protein ratio in the myelin membrane is the reverse of other cellular membranes. The molar ratio of cholesterol, phospholipids, galactolipids, and plasmalogens in myelin is approximately 2:2:1:1, respectively (Norton and Poduslo, 1973; Nave and Werner, 2014). Phospholipids are essential for the adhesive properties of myelin, and decreased phospholipid content results in phospholipid disarrangement. Based on the fact, the demyelination process can be monitored using Luxol Fast Blue (LFB), a Cu-Ti dye that interacts with phospholipids. When the optic nerve cross-sections are stained with LFB, the myelin sheaths appear as ring- or strip-shaped structures stained in blue, while other parts are transparent (Jennings and Carroll, 2015; Renner et al., 2017).
During demyelination, the contents of myelin proteins, such as MBP and myelin oligodendrocyte glycoprotein (MOG), are dramatically decreased in the myelin sheaths (Renner et al., 2017). These protein components play a critical role in the compaction of myelin sheaths. The interaction of MBP with the cytosolic membrane surfaces allows MBP to switch from an intrinsically unstructured polypeptide chain to a tightly packed protein phase, which could make a tight bond between cytoplasmic surfaces. The highly condensed cytoplasmic surfaces can be observed under EM as major dense lines (Stadelmann et al., 2019). MOG, located on the surface of myelin sheaths and oligodendrocyte processes, is crucial for the structural maintenance of the myelin sheath, especially compaction (Brunner et al., 1989). PLP is a transmembrane protein in myelin and a candidate for the tight apposition of membrane sheaths due to its hydrophilic extracellular domains (Slavin et al., 1997; Stadelmann et al., 2019). When demyelination occurs, the transcription and translation of these proteins such as MBP and MOG are affected, which could be detected by immunofluorescence and RT-qPCR (Renner et al., 2017).
Ultrastructural Changes in Myelin Sheaths and Axons
Morphological changes during demyelination can be evaluated using different approaches. Among them, the most intuitive and reliable method is to observe the ultrastructure and analyze the material composition of myelin sheaths by electron microscopy (EM) (Helvacioglu and Dagdeviren, 2019).
Under normal circumstances, it was observed that the myelin sheath is a stack of alternating electron-dense and electron-lucent layers, which are named the major dense line and intraperiod line, respectively (Stadelmann et al., 2019). When demyelination, vacuolation and splitting of the myelin sheath can be observed. It is widely believed that the G-ratio (ratio of the inner to the outer radius of the myelin sheath wrapped around the axon) is a parameter for assessing axonal myelination (Chomiak and Hu, 2009). An increase in the G-ratio usually means the myelin sheath thinning or the swelling of the axoplasm without affecting myelin thickness (Stikov et al., 2015). Normal axons have a normal G-ratio (0.6–0.75), while demyelinated axons lack myelin (G-ratio 1.0). Some demyelinated axons have undergone spontaneous remyelination, with thin sheaths and G-ratio greater than 0.75 (Chomiak and Hu, 2009; Berman et al., 2018; Duncan et al., 2018).
In addition, the change in axons is the breakdown of the cytoskeleton, including axoplasmic microtubules and neurofilaments, which are transformed into amorphous and granular materials. The morphological manifestations of axonal degeneration are as follows: (1) dark degeneration, where axons become dark, with dense axoplasm and miscellaneous organelles; (2) watery degeneration, where axons become pale, swollen, enlarged, and the axoplasm is either filled with an amorphous or granular material or is completely devoid of organelles (Saggu et al., 2010). Enlarged and swollen mitochondria are observed in axons, accompanied by fading cristae in the mitochondria (Coughlin et al., 2015; Thai et al., 2019). Mitochondrial dysfunction impairs mitochondrial transport along the axons and triggers necrosis of neurons (Chandrasekaran et al., 2019; Feng et al., 2021). Some RGCs have an intact cell membrane with swollen organelles; some RGCs are severely damaged with disrupted cytoplasmic organelles and electron-dense clumped nuclear remnants distributed in the cytoplasm (Narciso et al., 2001).
Functional and Imaging Changes
Demyelination could also disrupt the integrity of the visual pathway, as reflected by reduced nerve conduction velocity and axonal damage. The action potential could not be recorded by giving a stimulation in an experimentally demyelinated sciatic nerve induced by lysophosphatidylcholine (Waxman et al., 1994). The delays in conduction along the visual system could be measured with visual evoked potentials (VEPs) (Berman et al., 2020). In addition to the morphological examination, axonal damage could be reflected by functional and imaging examination, such as diffusion tensor imaging (DTI) (Ngamsombat et al., 2020).
Visual Evoked Potentials
As an electrophysiological examination, visual evoked potentials (VEPs) can detect the integrity of the entire visual pathway from the retina to the visual cortex (You et al., 2015). The latency of VEP reflects the speed of signal transduction along the visual pathway, where a prolonged VEP latency implies demyelination (Heidari et al., 2019). Furthermore, the decrease in VEP amplitude indicates axon loss, inflammation, and/or demyelination (You et al., 2012).
Diffusion Tensor Imaging
Diffusion tensor imaging is the development and deepening of diffusion-weighted imaging, the only non-invasive examination method that can effectively observe and track the bundles of white matter fibers in the brain (Li et al., 2019). The function change can be revealed by the parameters of DTI, such as radial diffusivity (λ⊥), axial diffusivity (λ ‖), and fractional anisotropy (FA). λ⊥, the mean value of λ2 and λ3, increases when myelin sheaths are damaged due to the weakened restriction of perpendicular water molecule diffusion by myelin sheaths during demyelination. λ ‖ reflects axonal integrity. FA decreases upon axonal degeneration and myelin sheath damage (Xu et al., 2013).
Visual evoked potential and diffusion tensor imaging have been used to screen for glaucoma in high-risk groups. Recently, several studies have investigated the utility of DTI in the diagnosis of glaucoma, and it has been suggested that the parameters of DTI in the optic nerve and brain may be sensitive and reliable biomarkers for glaucoma evaluation (Li et al., 2019; Schmidt et al., 2019; Nucci et al., 2020). However, compared to IOP screening or visual field examination, VEP and DTI are relatively expensive and inconvenient, especially when the relationship between demyelination and glaucoma is unknown.
Mechanisms of Demyelination
There has been much discussion about the mechanism of demyelination. Here, we highlight three key points: (1) Mitochondrial dysfunction in oligodendrocytes. Such dysfunction leads to impaired synthesis of lipid, a major myelin component (Schoenfeld et al., 2010). (2) Increased oxidative stress in oligodendrocytes. Reactive oxygen free radicals or reactive nitrogen free radicals may play a potential role in oligodendrocyte death (Roth and Núñez, 2016). (3) Immune and inflammatory injury (Figure 2). Interactions between T cells and glial cells could promote oligodendrocyte injury and myelin damage (Kunkl et al., 2020; Sidoryk-Węgrzynowicz and Strużyńska, 2021). Autoantibody production and complement system also play a role in demyelination (Stern and Keskin, 2008).
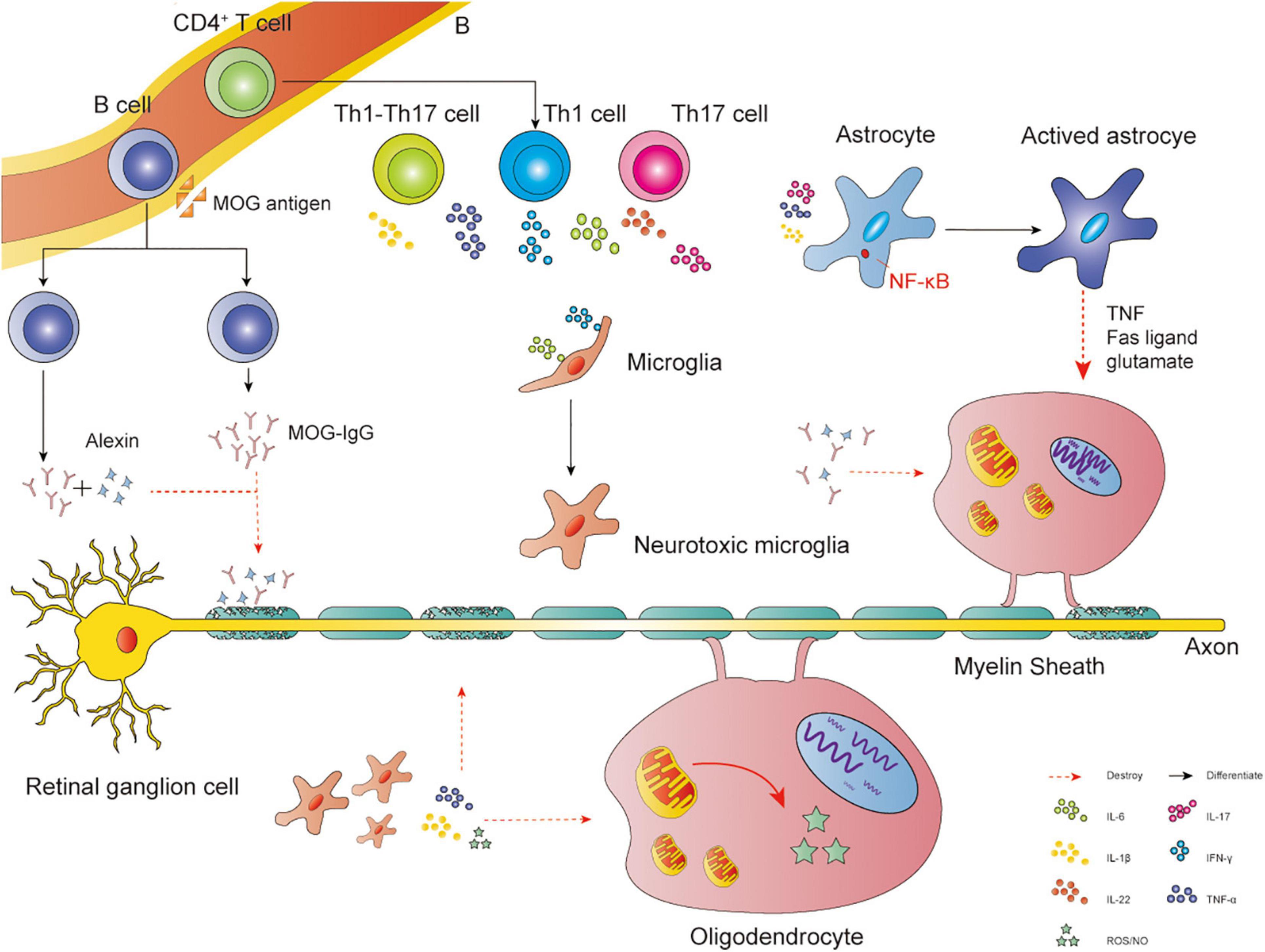
Figure 2. Immune and inflammatory injury. Interactions between glial cells and self-reactive lymphocytes in the CNS: CD4+ Th cells may differentiate into Th1, Th17, and Th1-Like Th17 cells. The main cytokines released by Th1 cells are IFN-γ and TNF-α, Th17 cells are IL-22 and IL-17, Th1-Like Th17 cells are IL-17 and IFN-γ. TNF-α, IL-1β, and IL-17 could activate astrocytes via NF-κB, leading to the apoptosis of oligodendrocytes via Fas ligand, TNF, and the release of NO or glutamate. IL-6 and IFN-γ could transfer microglia into neurotoxic microglia, producing NO, ROS, IL-1β, and TNF-α to attack oligodendrocytes. Pathogenic autoantibody and complement system could also play a role in demyelination. MOG antigen leaks into the peripheral blood, then transfers B cells into MOG-specific B cells, producing MOG-IgG. The antibody disrupts the interaction between MOG proteins and MBP proteins, causing demyelination. Meanwhile, MOG could directly activate the classical pathway of the complement cascade, exerting a cytotoxic effect.
Mitochondrial Dysfunction in Oligodendrocytes
Mitochondria are essential for oligodendrocyte survival and myelination. Mitochondria are semi-autonomous organelles that have their own genetic material, mtDNA. Functional defects and morphological changes in the mitochondria have been observed in neurodegenerative diseases (Lin and Beal, 2006). The nodes of Ranvier contain a large number of mitochondria, which are responsible for respiratory reactions and encode proteins involved in the oxidative phosphorylation system. In oligodendrocytes, the transcription and translation of mitochondrially expressed genes play an essential role in differentiation. Mitochondrial fatty acid oxidation transcripts are activated to synthesize cholesterol, which is required for myelination; the transcription of DBI gene, which transfers mitochondrial cholesterol to the cytoplasm, is also increased; transcription of other myelination genes such as myelin-associated oligodendrocytic basic protein and fyn proto-oncogene are also induced (Schoenfeld et al., 2010). The dysfunction of mtDNA reduce the transcription and translation of these genes, leading to demyelination. Mitochondrial DNA double-strand breaks are also a risk factor for oligodendrocyte cell death, which then cause secondary damage such as demyelination (Madsen et al., 2017). Other research groups have also demonstrated that mitochondria are crucial metabolic factors that affect oligodendrocyte myelination.
Increased Oxidative Stress in Oligodendrocytes
Oxidative stress can lead to demyelination of the nervous system (Ohl et al., 2016). Oligodendrocytes have relatively low levels of antioxidant ability, extensive elaborations of membranes and high levels of iron content. All features predispose oligodendrocytes to oxidative damage. Meanwhile, the myelin sheath is a preferential target of oxidative stress because of its composition and high lipid to protein ratio (French et al., 2009). Oxidative stress is caused by an imbalance between oxidants and antioxidants, or the accumulation of reactive oxygen free radicals or reactive nitrogen free radicals (Kehrer and Klotz, 2015; Kimura et al., 2017). Oxidative stress and mitochondrial damage can promote each other, forming a vicious cycle (Mahad et al., 2015). According to a previous study, oxygen free radicals can attack DNA in oligodendrocytes, and oxidative stress can also affect mitochondrial membrane phospholipids, enzymes, protein complexes, and mtDNA (Spaas et al., 2021). Oxidative stress attacks other components, in addition to mitochondrial damage. For example, matrix metalloproteinases (MMPs) are susceptible to redox state, oxygen free radicals facilitate the transcription and translation of MMP1/2/9. As an interstitial collagenase, MMPs could breakdown the basement membrane of the blood-brain barrier and degrade MBP, leading to the invasion of immune cells and demyelination (Offen et al., 2004; Ljubisavljevic, 2016).
Immune and Inflammatory Injury
Accumulated evidence indicates that demyelination is closely associated with immune dysfunctions. For example, some neurodegenerative diseases with demyelination, such as multiple sclerosis and Alzheimer’s disease, have been associated with immune system abnormalities (Heneka, 2017; Kunkl et al., 2020). Furthermore, T cells have been found to be involved in the degeneration of the optic nerve in glaucoma (Chen et al., 2018).
The effects of T cells on demyelination in neurodegenerative diseases have been widely recognized. CD4+ Th cells are activated by mature dendritic cells. Subsequently, dendritic cells and other costimulatory molecules promote CD4+ Th cell proliferation and differentiation. CD4+ Th cells may differentiate into Th1, Th17, and Th1-Like Th17 cells. These cells can invade the CNS and interact with microglia and astrocytes, resulting in inflammatory responses by cytokines and inhibiting the maturation of oligodendrocytes and myelin sheath (Uccelli et al., 2003; Constantinescu et al., 2011; Kunkl et al., 2020). For example, inflammatory stimuli such as TNF-α, interleukin (IL)-1β, and IL-17 could cause nuclear translocation of the nuclear factor κ-light-chain-enhancer (NF-κB). The activation of NF-κB is a central step in astrocyte activation, promoting apoptosis of oligodendrocytes via Fas ligand, TNF, and NO or glutamate release (Linnerbauer et al., 2020). Microglia could transfer into neurotoxic microglia, which presents MHCII and CD86 by stimulating IL-6 and IFN-γ. Then, they produce cytokines such as NO, ROS, IL-1β, and TNF-α to promote oligodendrocyte damage (Geladaris et al., 2021).
Pathogenic autoantibodies specific to CNS antigens produced by B cells and the activation of the complement pathway also cause demyelination. For example, inflammation may disrupt the blood-brain barrier, permitting entry of MOG antigen (Zamvil and Slavin, 2015). Then the antigen could activate CD4+ T cells and recruit MOG-specific B cells, which produce a large number of antibodies. The MOG-IgG mediates the damage of MBP, destroying the myelin sheath (Ambrosius et al., 2020). Meanwhile, MOG could directly activate the classical pathway of the complement cascade, leading to demyelination (Johns and Bernard, 1999).
Is Demyelination Associated With Glaucoma?
Thus far, it is unknown whether demyelination is an underlying factor in glaucoma. However, we do find a correlation between the two in both basic science studies and clinical patients. Clinically, in patients with primary open-angle glaucoma, researchers have observed the delay of VEP latency and an increase of λ⊥ in the optic radiation in DTI (You et al., 2019). This phenomenon indicates that demyelination occurs in patients with glaucoma. Such relevance is also proved indirectly in other demyelinating diseases. For example, more than fifty percent of patients suffer from high IOP or glaucoma in Charcot–Marie–Tooth disease type 1, whose characterized pathology is demyelination (Laššuthová et al., 2018). Furthermore, in basic research studies, researchers used laser photocoagulation to build a chronic glaucoma model in rhesus monkeys. Transmission electron microscopy analysis provided evidence of demyelination by observing myelin swelling in the lateral geniculate nucleus (Yan et al., 2017).
Thus far, only a few studies have focused on the relationship between demyelination and glaucoma. Available references are listed in Table 1.
Evidence That Supports an Association Between Demyelination and Glaucoma
Morphological Changes of Demyelination in Glaucoma
The morphology of myelin sheath is mainly observed by immunohistochemistry (LFB staining), immunofluorescence (MBP staining) and EM. Reinehr et al. (2016) established an experimental autoimmune glaucoma (EAG) model by injecting bovine optic nerve homogenate antigens. Data showed that the pathogenesis of this model is the activation of the complement system via the lectin pathway. Activation occurred at 7 days with significant RGC loss observed at 28 days; however, changes in the myelin sheath appeared early. A rapid increase in the MBP-positive area was observed at 3 days, MBP-positive area fell back to the control values after 7 days, and a decrease in the MBP-positive area occurred at 14 days (Reinehr et al., 2016). Based on the above results, we speculate that the early changes in the myelin sheath indicate glaucoma. Consistent with these results, researchers also revealed a change in MBP in a rabbit model of ocular hypertension. The authors suggest that MBP activity is a marker of stress under incipient degeneration, during which myelin is destroyed, and loosely compacted myelin sheaths can also be observed under scanning electron microscope (SEM). However, the underlying mechanisms have not yet been carefully investigated (Shen et al., 2017).
Another morphological change of demyelination reflects as the changes of oligodendrocytes. A significant change in oligodendrocytes was observed in a chronic ocular hypertensive model established by laser photocoagulation. The key point of pathogenesis is the increase in TNF-α levels. The level of TNF-α increased rapidly at 3 days after laser photocoagulation, while the increased protein levels were maintained for at least 14 days. The oligodendrocyte loss occurred at 1 week, and a loss of RGCs occurred 2 weeks after oligodendrocyte loss (Nakazawa et al., 2006).
Functional Changes of Demyelination in Glaucoma
In a recent study, 20 patients with bilateral chronic primary angle-closure glaucoma were examined using DTI for axonal and myelin damage of the optic nerves and optic radiations. Compared with the control group, the patients had significantly decreased FA and increased λ⊥, and increased λ⊥ correlated with mfVEP latency. Surprisingly, an increase in λ⊥ was observed not only in the optic radiation fibers projecting to the affected visual hemifield, but also in the unaffected visual hemifield. This phenomenon indicates that myelin damage precedes axonal degeneration (Garaci et al., 2009; Zhang et al., 2015).
Other Circumstantial Evidence
Some experiments shows that demyelination could be a target for predicting glaucoma. Increased serum anti-MBP antibody levels are a key indicator of brain damage or demyelination (Wa̧sik et al., 2020). South Korean scientists and German scientists proved that the serum levels of anti-MBP antibodies were higher in patients with primary open-angle glaucoma (POAG) and normal-tension glaucoma (NTG). Therefore, anti-MBP antibodies in serum produced by demyelination may be a marker to distinguish between control participants and patients with glaucoma (Joachim et al., 2008; Shin et al., 2020).
Some experiments have suggested that demyelination may play a role in the pathogenesis of glaucoma. Elevated peptidyl arginine deiminase 2 and decreased arginyl methylation, which mediate protein citrullination, were detected in POAG optic nerve and glaucomatous DBA/2J mice. MBP is a major citrullinated protein in the POAG optic nerve (Bhattacharya et al., 2006). Citrullinated MBP lost the ability to maintain the compaction of myelin sheaths and gave rise to large monolingual vesicles, leading to demyelination (Boggs et al., 1997). Therefore, they hypothesized that citrullination causes changes in the dynamics of myelin components, leading to the initiation of glaucomatous neuropathy (Bhattacharya et al., 2006).
Evidence That Contradicts an Association Between Demyelination and Glaucoma
Changes in the Morphology of Myelin Sheaths
Some researchers regard demyelination as a manifestation of axon damage rather than an underlying factor. Kuehn et al., verified that the main pathogenic factor in the EAG model established by S100B directly destroys the axons of the optic nerve. The first change was optic nerve degeneration at 3 days and the beginning of RGC loss at 14 days, while apparent demyelination was detected at 21 days by LFB staining (Kuehn et al., 2018). Researchers revealed that a decrease in PLP gene expression and oligodendrocyte loss must follow rather than precede axon loss in old DBA/2J mice (a secondary glaucoma model induced by genetic elements and accompanied by progressively increasing IOP). Next, they analyzed an acute glaucoma rat model; half of the axons were lost 10 days after the increase in IOP, while no loss of oligodendrocytes was observed at this time (Son et al., 2010). In this case, oligodendrocyte loss is an accompanying phenomenon. Consistent with these results, in the model animals injected with N-methyl-D-aspartate (NMDA), the RGC number was significantly decreased in the peripheral and middle regions at 3 days after injury, and damage to the optic nerves occurred at 14 days. Myelin sheaths were kept intact after injury by LFB staining (Kuehn et al., 2017).
Ultrastructural Changes in Myelin Sheaths and Axons
Scientists have discovered different phenomena in the same DBA/2J mouse optic nerves. A study showed that myelin sheath thickness outpaced axon diameter increase, leading to a decreased G-ratio. However, the researchers did not interpret the results as an indication of early demyelination and attributed this to the upregulation of genes controlling myelin sheath synthesis. They believed that hypermyelination in the DBA/2J optic nerves was protective (Smith et al., 2018). In conclusion, changes in myelin need to be investigated further.
Demyelination Could Be an Underlying Factor in Glaucoma
At present, most experiments focus on an unmyelinated region of the optic nerve with only few studies focusing on the myelin sheath. Even the study of glaucoma that mentions myelin sheath only describes the morphology of the myelin sheath. There are very few in-depth studies on the relationship between demyelination and glaucoma.
Based on the summary of the above literature, there is insufficient evidence from clinical studies and translational studies. Animal models are primarily used to explore whether demyelinating is the underlying factor of glaucoma, but the results are controversial. The possible reasons are summarized as follows: (1) The different pathogenesis of the models leads to significant differences in the results. Most of the current basic studies use a single animal model, which only reflects part of the pathogenesis of glaucoma (Reinehr et al., 2016; Kuehn et al., 2017; Schnichels et al., 2021). (2) The dynamic changes of the myelin sheath especially the precise time point of demyelinating have not been comprehensively studied. Most animal experiments only detect changes in myelin sheath at the late stage, while the early changes have been overlooked (Son et al., 2010). (3) The standardized and reliable quantitation methods have not been well established. The LFB and MBP staining are the most commonly used methods because they are relatively simple and feasible. However, LFB and MBP staining is a semi-quantitative technique that does not allow absolute quantitation of the myelin sheath, leading to differences in results (Khodanovich et al., 2018; Kuehn et al., 2018).
Conclusion and Perspectives
Vision loss from glaucoma causes significant inconvenience in daily life. The incomplete understanding of the pathogenesis of glaucoma limits our ability to alleviate injury to the optic nerve and visual field defects (Weinreb et al., 2014). Currently, reducing intraocular pressure is still considered the only efficient approach to treat glaucoma (Weinreb et al., 2014; Donegan and Lieberman, 2016). Disease progression in glaucoma patients is difficult to prevent even though the intraocular pressure can be reduced by medication or surgery (De Moraes et al., 2017). Therefore, exploration of new therapeutic directions for glaucoma is highly desirable. Prevention/repair of myelin damage has become an essential part of therapy for neurodegenerative diseases, which also shows the potential to become an underlying therapeutic approach for glaucoma.
After analyzing the existing research and issues, we found several directions worthy of discussion: (1) Whether or not demyelination happens in glaucoma patients, and where it occurs, if yes. We could use various animal models in basic experiments and establish standardized quantitative methods to explore this issue. Clinically, imaging technology needs to be further developed to examine the patients. (2) If demyelination exists, whether it is helpful for the early diagnosis of glaucoma. At present, early diagnosis relies on structural and functional evaluation, mainly through optical coherence tomography and perimetry. Further study on the changes in DTI and VEP, or related antibodies and elements in the serum such as anti-MBP antibodies, could help us screen early glaucoma patients more comprehensively and efficiently. (3) We could explore the mechanism of demyelination and screen for optic neuroprotective drugs. We would like to find the critical targets of demyelination, such as muscarinic acetylcholine receptors and sphingosine one phosphate receptors, which have been explored. We hope to screen out drugs with high specificity and minor side effects, laying the foundation for translational research and clinical application.
In future, the exploration of whether demyelination is an underlying factor in glaucoma will provide new directions for the treatment of glaucoma.
Author Contributions
JFX, YTZ, and YQL contributed to the central idea of the review and wrote the manuscript. ZL, JCL, and YJNL helped to revise the manuscript. YHZ and YQL designed and coordinated the study, and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Key Research and Development Project of China (Grant Number 2020YFA0112701), National Natural Science Foundation of China (Grant No. 81870657), Science and Technology Program of Guangzhou, China (202102010216), Natural Science Foundation of Guangdong Province, China (Grant No. 2018A030313049), Medical Scientific Research Foundation of Guangdong Province (A2018052), and Fundamental Research Funds for the Youth Scholars of Sun Yat-sen University (18ykpy32).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
References
Aggarwal, S., Snaidero, N., Pähler, G., Frey, S., Sánchez, P., Zweckstetter, M., et al. (2013). Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLoS Biol. 11:e1001577. doi: 10.1371/journal.pbio.1001577
Ambrosius, W., Michalak, S., Kozubski, W., and Kalinowska, A. (2020). Myelin oligodendrocyte glycoprotein antibody-associated disease: current insights into the disease pathophysiology, diagnosis and management. Int. J. Mol. Sci. 22:100. doi: 10.3390/ijms22010100
Baumann, N., and Pham-Dinh, D. (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 81, 871–927.
Berman, S., West, K. L., Does, M. D., Yeatman, J. D., and Mezer, A. A. (2018). Evaluating g-ratio weighted changes in the corpus callosum as a function of age and sex. Neuroimage 182, 304–313. doi: 10.1016/j.neuroimage.2017.06.076
Berman, S., Backner, Y., Krupnik, R., Paul, F., Petrou, P., Karussis, D., et al. (2020). Conduction delays in the visual pathways of progressive multiple sclerosis patients covary with brain structure. Neuroimage 221:117204. doi: 10.1016/j.neuroimage.2020.117204
Bhattacharya, S. K., Crabb, J. S., Bonilha, V. L., Gu, X., Takahara, H., and Crabb, J. W. (2006). Proteomics implicates peptidyl arginine deiminase 2 and optic nerve citrullination in glaucoma pathogenesis. Invest. Ophthalmol. Vis. Sci. 47, 2508–2514. doi: 10.1167/iovs.05-1499
Boggs, J. M., Yip, P. M., Rangaraj, G., and Jo, E. (1997). Effect of posttranslational modifications to myelin basic protein on its ability to aggregate acidic lipid vesicles. Biochemistry 36, 5065–5071. doi: 10.1021/bi962649f
Brunner, C., Lassmann, H., Waehneldt, T. V., Matthieu, J. M., and Linington, C. (1989). Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2’,3’-cyclic nucleotide 3’-phosphodiesterase in the CNS of adult rats. J. Neurochem. 52, 296–304. doi: 10.1111/j.1471-4159.1989.tb10930.x
Cai, Z., and Xiao, M. (2016). Oligodendrocytes and Alzheimer’s disease. Int. J. Neurosci. 126, 97–104. doi: 10.3109/00207454.2015.1025778
Chandrasekaran, K., Anjaneyulu, M., Choi, J., Kumar, P., Salimian, M., Ho, C. Y., et al. (2019). Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD (+)-dependent SIRT1-PGC-1α-TFAM pathway. Int. Rev. Neurobiol. 145, 177–209. doi: 10.1016/bs.irn.2019.04.002
Chen, H., Cho, K. S., Vu, T. H. K., Shen, C. H., Kaur, M., et al. (2018). Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat. Commun. 9:3209. doi: 10.1038/s41467-018-05681-5689
Chomiak, T., and Hu, B. (2009). What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? a theoretical approach. PLoS One 4:e7754. doi: 10.1371/journal.pone.0007754
Coleman, M. (2005). Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 6, 889–898. doi: 10.1038/nrn1788
Constantinescu, C. S., Farooqi, N., O’Brien, K., and Gran, B. (2011). Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 164, 1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x
Cook, C., and Foster, P. (2012). Epidemiology of glaucoma: what’s new? Can. J. Ophthalmol. 47, 223–226. doi: 10.1016/j.jcjo.2012.02.003
Coughlin, L., Morrison, R. S., Horner, P. J., and Inman, D. M. (2015). Mitochondrial morphology differences and mitophagy deficit in murine glaucomatous optic nerve. Invest. Ophthalmol. Vis. Sci. 56, 1437–1446. doi: 10.1167/iovs.14-16126
De Moraes, C. G., Liebmann, J. M., and Levin, L. A. (2017). Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog. Retin. Eye Res. 56, 107–147. doi: 10.1016/j.preteyeres.2016.10.001
Donegan, R. K., and Lieberman, R. L. (2016). Discovery of molecular therapeutics for glaucoma: challenges, successes, and promising directions. J. Med. Chem. 59, 788–809. doi: 10.1021/acs.jmedchem.5b00828
Duncan, I. D., Radcliff, A. B., Heidari, M., Kidd, G., August, B. K., and Wierenga, L. A. (2018). The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. U S A. 115, E11807–E11816. doi: 10.1073/pnas.1808064115
Feng, Y., Guo, M., Zhao, H., Han, S., Hao, Y., Yuan, Y., et al. (2021). Dl-3-n-Butylphthalide alleviates demyelination and improves cognitive function by promoting mitochondrial dynamics in white matter lesions. Front. Aging Neurosci. 13:632374. doi: 10.3389/fnagi.2021.632374
French, H. M., Reid, M., Mamontov, P., Simmons, R. A., and Grinspan, J. B. (2009). Oxidative stress disrupts oligodendrocyte maturation. J. Neurosci. Res. 87, 3076–3087. doi: 10.1002/jnr.22139
Garaci, F. G., Bolacchi, F., Cerulli, A., Melis, M., Spano, A., Cedrone, C., et al. (2009). Optic nerve and optic radiation neurodegeneration in patients with glaucoma: in vivo analysis with 3-T diffusion-tensor MR imaging. Radiology 252, 496–501. doi: 10.1148/radiol.2522081240
Geladaris, A., Häusler, D., and Weber, M. S. (2021). Microglia: the missing link to decipher and therapeutically control MS progression? Int. J. Mol. Sci. 22:3461. doi: 10.3390/ijms22073461
He, S., Stankowska, D. L., Ellis, D. Z., Krishnamoorthy, R. R., and Yorio, T. (2018). Targets of neuroprotection in glaucoma. J. Ocul. Pharmacol. Ther. 34, 85–106. doi: 10.1089/jop.2017.0041
Heidari, M., Radcliff, A. B., McLellan, G. J., Ver Hoeve, J. N., Chan, K., Kiland, J. A., et al. (2019). Evoked potentials as a biomarker of remyelination. Proc. Natl. Acad. Sci. U S A. 116, 27074–27083. doi: 10.1073/pnas.1906358116
Helvacioglu, F., and Dagdeviren, A. (2019). Myelin ultrastructure terminology in disease and recovery processes. Arch. Ital. Biol. 157, 76–88. doi: 10.12871/00039829201924
Heneka, M. T. (2017). Inflammasome activation and innate immunity in Alzheimer’s disease. Brain Pathol. 27, 220–222. doi: 10.1111/bpa.12483
Jennings, A. R., and Carroll, W. M. (2015). Oligodendrocyte lineage cells in chronic demyelination of multiple sclerosis optic nerve. Brain Pathol. 25, 517–530. doi: 10.1111/bpa.12193
Joachim, S. C., Reichelt, J., Berneiser, S., Pfeiffer, N., and Grus, F. H. (2008). Sera of glaucoma patients show autoantibodies against myelin basic protein and complex autoantibody profiles against human optic nerve antigens. Graefes Arch. Clin. Exp. Ophthalmol. 246, 573–580. doi: 10.1007/s00417-007-0737-738
Johns, T. G., and Bernard, C. C. (1999). The structure and function of myelin oligodendrocyte glycoprotein. J. Neurochem. 72, 1–9. doi: 10.1046/j.1471-4159.1999.0720001.x
Jonas, J. B., Aung, T., Bourne, R. R., Bron, A. M., Ritch, R., and Panda-Jonas, S. (2017). Glaucoma. Lancet 390, 2183–2193. doi: 10.1016/s0140-6736(17)31469-31461
Jones-Odeh, E., and Hammond, C. J. (2015). How strong is the relationship between glaucoma, the retinal nerve fibre layer, and neurodegenerative diseases such as Alzheimer’s disease and multiple sclerosis? Eye (Lond) 29, 1270–1284. doi: 10.1038/eye.2015.158
Kaminska, J., Koper, O. M., Piechal, K., and Kemona, H. (2017). Multiple sclerosis - etiology and diagnostic potential. Postepy Hig. Med. Dosw (Online) 71, 551–563. doi: 10.5604/01.3001.0010.3836
Kehrer, J. P., and Klotz, L. O. (2015). Free radicals and related reactive species as mediators of tissue injury and disease: implications for Health. Crit. Rev. Toxicol. 45, 765–798. doi: 10.3109/10408444.2015.1074159
Khodanovich, M. Y., Kisel, A. A., Akulov, A. E., Atochin, D. N., Kudabaeva, M. S., Glazacheva, V. Y., et al. (2018). Quantitative assessment of demyelination in ischemic stroke in vivo using macromolecular proton fraction mapping. J. Cereb. Blood Flow Metab. 38, 919–931. doi: 10.1177/0271678x18755203
Kimura, A., Namekata, K., Guo, X., Noro, T., Harada, C., and Harada, T. (2017). Targeting oxidative stress for treatment of glaucoma and optic neuritis. Oxid. Med. Cell Longev. 2017:2817252. doi: 10.1155/2017/2817252
Kuehn, S., Meissner, W., Grotegut, P., Theiss, C., Dick, H. B., and Joachim, S. C. (2018). Intravitreal S100B injection leads to progressive glaucoma like damage in retina and optic nerve. Front. Cell Neurosci. 12:312. doi: 10.3389/fncel.2018.00312
Kuehn, S., Rodust, C., Stute, G., Grotegut, P., Meissner, W., Reinehr, S., et al. (2017). Concentration-Dependent inner retina layer damage and optic nerve degeneration in a NMDA model. J. Mol. Neurosci. 63, 283–299. doi: 10.1007/s12031-017-0978-x
Kunkl, M., Frascolla, S., Amormino, C., Volpe, E., and Tuosto, L. (2020). T helper cells: the modulators of inflammation in multiple sclerosis. Cells 9:482. doi: 10.3390/cells9020482
Laššuthová, P., Vill, K., Erdem-Ozdamar, S., Schröder, J. M., Topaloglu, H., Horvath, R., et al. (2018). Novel SBF2 mutations and clinical spectrum of Charcot-Marie-Tooth neuropathy type 4B2. Clin. Genet. 94, 467–472. doi: 10.1111/cge.13417
Li, M., Ke, M., Song, Y., Mu, K., Zhang, H., and Chen, Z. (2019). Diagnostic utility of central damage determination in glaucoma by magnetic resonance imaging: an observational study. Exp. Ther. Med. 17, 1891–1895. doi: 10.3892/etm.2018.7134
Lin, M. T., and Beal, M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. doi: 10.1038/nature05292
Linnerbauer, M., Wheeler, M. A., and Quintana, F. J. (2020). Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622. doi: 10.1016/j.neuron.2020.08.012
Ljubisavljevic, S. (2016). Oxidative stress and neurobiology of demyelination. Mol. Neurobiol. 53, 744–758. doi: 10.1007/s12035-014-9041-x
Luse, S. A. (1956). Formation of myelin in the central nervous system of mice and rats, as studied with the electron microscope. J. Biophys. Biochem. Cytol. 2, 777–784.
Madsen, P. M., Pinto, M., Patel, S., McCarthy, S., Gao, H., Taherian, M., et al. (2017). Mitochondrial DNA double-strand breaks in oligodendrocytes cause demyelination, axonal injury, and CNS inflammation. J. Neurosci. 37, 10185–10199. doi: 10.1523/JNEUROSCI.1378-17.2017
Mahad, D. H., Trapp, B. D., and Lassmann, H. (2015). Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 14, 183–193. doi: 10.1016/S1474-4422(14)70256-X
Mitew, S., Hay, C. M., Peckham, H., Xiao, J., Koenning, M., and Emery, B. (2014). Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276, 29–47. doi: 10.1016/j.neuroscience.2013.11.029
Nakazawa, T., Nakazawa, C., Matsubara, A., Noda, K., Hisatomi, T., She, H., et al. (2006). Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J. Neurosci. 26, 12633–12641. doi: 10.1523/jneurosci.2801-06.2006
Narciso, M. S., Hokoç, J. N., and Martinez, A. M. (2001). Watery and dark axons in Wallerian degeneration of the opossum’s optic nerve: different patterns of cytoskeletal breakdown? An. Acad. Bras Cienc. 73, 231–243. doi: 10.1590/s0001-37652001000200008
Nave, K. A., and Werner, H. B. (2014). Myelination of the nervous system: mechanisms and functions. Annu. Rev. Cell Dev. Biol. 30, 503–533. doi: 10.1146/annurev-cellbio-100913-113101
Ngamsombat, C., Tian, Q., Fan, Q., Russo, A., Machado, N., Polackal, M., et al. (2020). Axonal damage in the optic radiation assessed by white matter tract integrity metrics is associated with retinal thinning in multiple sclerosis. Neuroimage Clin. 27:102293. doi: 10.1016/j.nicl.2020.102293
Norton, W. T., and Poduslo, S. E. (1973). Myelination in rat brain: changes in myelin composition during brain maturation. J. Neurochem. 21, 759–773. doi: 10.1111/j.1471-4159.1973.tb07520.x
Nucci, C., Garaci, F., Altobelli, S., Di Ciò, F., Martucci, A., Aiello, F., et al. (2020). Diffusional kurtosis imaging of white matter degeneration in glaucoma. J. Clin. Med. 9:3122. doi: 10.3390/jcm9103122
Offen, D., Gilgun-Sherki, Y., Barhum, Y., Benhar, M., Grinberg, L., Reich, R., et al. (2004). A low molecular weight copper chelator crosses the blood-brain barrier and attenuates experimental autoimmune encephalomyelitis. J. Neurochem. 89, 1241–1251. doi: 10.1111/j.1471-4159.2004.02428.x
Ohl, K., Tenbrock, K., and Kipp, M. (2016). Oxidative stress in multiple sclerosis: central and peripheral mode of action. Exp. Neurol. 277, 58–67. doi: 10.1016/j.expneurol.2015.11.010
Reinehr, S., Reinhard, J., Gandej, M., Kuehn, S., Noristani, R., Faissner, A., et al. (2016). Simultaneous complement response via lectin pathway in retina and optic nerve in an experimental autoimmune glaucoma model. Front. Cell Neurosci. 10:140. doi: 10.3389/fncel.2016.00140
Renner, M., Stute, G., Alzureiqi, M., Reinhard, J., Wiemann, S., Schmid, H., et al. (2017). Optic nerve degeneration after retinal ischemia/reperfusion in a rodent model. Front. Cell Neurosci. 11:254. doi: 10.3389/fncel.2017.00254
Roth, A. D., and Núñez, M. T. (2016). Oligodendrocytes: functioning in a delicate balance between high metabolic requirements and oxidative damage. Adv. Exp. Med. Biol. 949, 167–181. doi: 10.1007/978-3-319-40764-7_8
Rotshenker, S. (2009). The role of Galectin-3/MAC-2 in the activation of the innate-immune function of phagocytosis in microglia in injury and disease. J. Mol. Neurosci. 39, 99–103.
Rotshenker, S., Reichert, F., Gitik, M., Haklai, R., Elad-Sfadia, G., and Kloog, Y. (2008). Galectin-3/MAC-2, Ras and PI3K activate complement Receptor-3 and scavenger Receptor-AI/II mediated myelin phagocytosis in microglia. Glia 56, 1607–1613.
Saggu, S. K., Chotaliya, H. P., Blumbergs, P. C., and Casson, R. J. (2010). Wallerian-like axonal degeneration in the optic nerve after excitotoxic retinal insult: an ultrastructural study. BMC Neurosci. 11:97. doi: 10.1186/1471-2202-11-97
Schmidt, M. A., Engelhorn, T., Dörfler, A., and Michelson, G. (2019). [Impairment of the visual system in glaucoma]. Klin Monbl Augenheilkd 236, 134–141. doi: 10.1055/a-0762-0822
Schmitt, S., Castelvetri, L. C., and Simons, M. (2015). Metabolism and functions of lipids in myelin. Biochim. Biophys. Acta 1851, 999–1005. doi: 10.1016/j.bbalip.2014.12.016
Schnichels, S., Paquet-Durand, F., Löscher, M., Tsai, T., Hurst, J., Joachim, S. C., et al. (2021). Retina in a dish: Cell cultures, retinal explants and animal models for common diseases of the retina. Prog. Retin Eye Res. 81:100880. doi: 10.1016/j.preteyeres.2020.100880
Schoenfeld, R., Wong, A., Silva, J., Li, M., Itoh, A., Horiuchi, M., et al. (2010). Oligodendroglial differentiation induces mitochondrial genes and inhibition of mitochondrial function represses oligodendroglial differentiation. Mitochondrion 10, 143–150.
Shen, J., Yang, Q., Yu, D., Wu, J., Zhu, Y., and Guo, W. (2017). Vulnerability study of myelinated and unmyelinated nerve fibers in acute ocular hypertension in rabbit. Mol. Med. Rep. 16, 6794–6802. doi: 10.3892/mmr.2017.7474
Shin, Y. J., Kim, E., Han, B. K., and Yi, K. (2020). Serum biomarkers for the diagnosis of glaucoma. Diagnostics (Basel) 11:20. doi: 10.3390/diagnostics11010020
Sidoryk-Węgrzynowicz, M., and Strużyńska, L. (2021). Astroglial and microglial purinergic P2X7 receptor as a major contributor to neuroinflammation during the course of multiple sclerosis. Int. J. Mol. Sci. 22:8404. doi: 10.3390/ijms22168404
Slavin, A. J., Johns, T. G., Orian, J. M., and Bernard, C. C. (1997). Regulation of myelin oligodendrocyte glycoprotein in different species throughout development. Dev. Neurosci. 19, 69–78. doi: 10.1159/000111187
Smith, M. A., Plyler, E. S., Dengler-Crish, C. M., Meier, J., and Crish, S. D. (2018). Nodes of ranvier in glaucoma. Neuroscience 390, 104–118. doi: 10.1016/j.neuroscience.2018.08.016
Son, J. L., Soto, I., Oglesby, E., Lopez-Roca, T., Pease, M. E., Quigley, H. A., et al. (2010). Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia 58, 780–789. doi: 10.1002/glia.20962
Spaas, J., van Veggel, L., Schepers, M., Tiane, A., van Horssen, J., and Wilson, D. M. III, et al. (2021). Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell Mol. Life Sci. 78, 4615–4637. doi: 10.1007/s00018-021-03802-3800
Stadelmann, C., Timmler, S., Barrantes-Freer, A., and Simons, M. (2019). Myelin in the central nervous system: structure, function, and pathology. Physiol. Rev. 99, 1381–1431. doi: 10.1152/physrev.00031.2018
Stern, J. N., and Keskin, D. B. (2008). Strategies for the identification of loci responsible for the pathogenesis of multiple sclerosis. Cell Mol. Biol. Lett. 13, 656–666. doi: 10.2478/s11658-008-0030-39
Stikov, N., Campbell, J. S., Stroh, T., Lavelee, M., Frey, S., Novek, J., et al. (2015). In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage 118, 397–405. doi: 10.1016/j.neuroimage.2015.05.023
Thai, T. Q., Nguyen, H. B., Sui, Y., Ikenaka, K., Oda, T., and Ohno, N. (2019). Interactions between mitochondria and endoplasmic reticulum in demyelinated axons. Med. Mol. Morphol. 52, 135–146. doi: 10.1007/s00795-018-0212-210
Uccelli, A., Pedemonte, E., Narciso, E., and Mancardi, G. (2003). Biological markers of the inflammatory phase of multiple sclerosis. Neurol. Sci. 24, (Suppl. 5), S271–S274. doi: 10.1007/s10072-003-0172-175
Wa̧sik, N., Sokół, B., Hołysz, M., Mańko, W., Juszkat, R., Jagodziński, P. P., et al. (2020). Serum myelin basic protein as a marker of brain injury in aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 162, 545–552. doi: 10.1007/s00701-019-04185-4189
Waxman, S. G., Utzschneider, D. A., and Kocsis, J. D. (1994). Enhancement of action potential conduction following demyelination: experimental approaches to restoration of function in multiple sclerosis and spinal cord injury. Prog. Brain Res. 100, 233–243. doi: 10.1016/s0079-6123(08)60790-6
Weinreb, R. N., Aung, T., and Medeiros, F. A. (2014). The pathophysiology and treatment of glaucoma: a review. JAMA 311, 1901–1911. doi: 10.1001/jama.2014.3192
Xu, J., Shimony, J. S., Klawiter, E. C., Snyder, A. Z., Trinkaus, K., Naismith, R. T., et al. (2013). Improved in vivo diffusion tensor imaging of human cervical spinal cord. Neuroimage 67, 64–76. doi: 10.1016/j.neuroimage.2012.11.014
Yan, Z., Liao, H., Chen, H., Deng, S., Jia, Y., Deng, C., et al. (2017). Elevated intraocular pressure induces Amyloid-β deposition and tauopathy in the lateral geniculate nucleus in a monkey model of glaucoma. Invest. Ophthalmol. Vis. Sci. 58, 5434–5443. doi: 10.1167/iovs.17-22312
You, Y., Gupta, V. K., Chitranshi, N., Reedman, B., Klistorner, A., and Graham, S. L. (2015). Visual evoked potential recording in a rat model of experimental optic nerve demyelination. J. Vis. Exp. 101:e52934. doi: 10.3791/52934
You, Y., Joseph, C., Wang, C., Gupta, V., Liu, S., Yiannikas, C., et al. (2019). Demyelination precedes axonal loss in the transneuronal spread of human neurodegenerative disease. Brain 142, 426–442. doi: 10.1093/brain/awy338
You, Y., Klistorner, A., Thie, J., Gupta, V. K., and Graham, S. L. (2012). Axonal loss in a rat model of optic neuritis is closely correlated with visual evoked potential amplitudes using electroencephalogram-based scaling. Invest. Ophthalmol. Vis. Sci. 53:3662. doi: 10.1167/iovs.12-9843
Zamvil, S. S., and Slavin, A. J. (2015). Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol. Neuroimmunol. Neuroinflamm. 2:e62. doi: 10.1212/nxi.0000000000000062
Keywords: glaucoma, demyelination, therapy, degeneration, neuroprotection, retinal ganglion cell
Citation: Xue J, Zhu Y, Liu Z, Lin J, Li Y, Li Y and Zhuo Y (2021) Demyelination of the Optic Nerve: An Underlying Factor in Glaucoma? Front. Aging Neurosci. 13:701322. doi: 10.3389/fnagi.2021.701322
Received: 27 April 2021; Accepted: 13 October 2021;
Published: 02 November 2021.
Edited by:
Denis Gris, Université de Sherbrooke, CanadaReviewed by:
Vinicius Toledo Ribas, Federal University of Minas Gerais, BrazilRaghu R. Krishnamoorthy, University of North Texas Health Science Center, United States
Copyright © 2021 Xue, Zhu, Liu, Lin, Li, Li and Zhuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiqing Li, liyiqing3@mail.sysu.edu.cn; Yehong Zhuo, zhuoyh@mail.sysu.edu.cn
†These authors have contributed equally to this work
 Jingfei Xue
Jingfei Xue Yingting Zhu
Yingting Zhu Zhe Liu
Zhe Liu  Yiqing Li
Yiqing Li Yehong Zhuo
Yehong Zhuo