- Resource Institute for Chinese and Ethnic Materia Medica, Guizhou University of Traditional Chinese Medicine, Guiyang, China
Sweating is one of the most important primary processing methods of Chinese medicinal materials. Dipsacus asper is a typical representative of sweating treatment that is recommended by the Chinese Pharmacopoeia. The color change of the fracture surface of the root is the prominent feature of sweating treatment. However, few studies have focused on the mechanism of color change during sweating treatment. In this study, widely targeted metabolomics and ITS high-throughput sequencing technologies were applied to detect metabolites and microbial structure and diversity in the root of D. asper during sweating treatment. A total of 667 metabolites, including 36 downregulated and 78 upregulated metabolites, were identified in D. asper following sweating treatment. The significantly differential metabolites were divided into 12 classes, including terpenoids and phenolic acids. Moreover, all the differential terpenoids were upregulated and 20 phenolic acids showed a significant change after sweating treatment. In addition, microbial community diversity and richness increased following sweating treatment. The composition of microbial communities revealed that the relative abundances of Ascomycota and Basidiomycota significantly changed after sweating treatment. Correlation analysis revealed that Ascomycota (Fusarium sp., Macrophomina sp., Ilyonectria sp., Memnoniella sp., Penicillium sp., Cyphellophora sp., Neocosmospora sp., unclassified_f_Nectriaceae, and unclassified_o_Saccharomycetales) and Basidiomycota (Armillaria sp.) were associated with the content of terpenoids (6-deoxycatalpol and laciniatoside III) and phenolic acids (3-(4-hydroxyphenyl)-propionic acid, ethyl caffeate, 4-O-glucosyl-4-hydroxybenzoic acid, 2-acetyl-3-hydroxyphenyl−1-O-glucoside, 4-O-glucosyl-3,4-dihydroxybenzyl alcohol, 3-O-feruloylquinic acid, 3,4-O-dicaffeoylquinic acid methyl ester, O-anisic acid, and coniferyl alcohol). We speculate that the Ascomycota and Basidiomycota affect the content of terpenoids and phenolic acids, resulting in color change during sweating treatment in D. asper. This study provides a foundation for analyzing the mechanism involved in the processing of Chinese medicinal materials.
Introduction
Chinese medicinal herbs have played a vital role in clinical therapy for thousands of years, and most of them should carry out primary processing to form clinical medicinal materials. The primary processing of Chinese medicinal materials includes cleaning, steaming, boiling, sweating, and drying, and the different processing methods can affect the characteristics and quality of the materials (Wu et al., 2019). Sweating is a special processing method in which fresh herbs are harvested and semi-dried before being piled up and heated, resulting in the internal water of the fresh herbs evaporating outward and appearing on the surface of the herbs; this is known as sweating or “diaphoretic” processing in the Chinese Pharmacopoeia (version 2020; Tao et al., 2020). It has been reported that sweating can accelerate the drying rate of medicinal materials by redistributing the internal water, and increase their flavor or reduce their irritation (Duan et al., 2013). Furthermore, sweating affects the temperature and humidity inside the medicinal materials, which then changes the microbial communities and the activity of enzymes in biological tissues. These changes may provide conditions for the biological and chemical conversion of primary or secondary metabolites, directly influencing the quality of medicinal materials (Chen et al., 2018; Wu et al., 2019).
Five Chinese medicinal materials have been clearly proposed to require sweating, including Eucommia ulmoides Oliv., Magnolia officinalis Rehd. et Wils, and Dipsacus asper Wall. ex C.B. Clarke. Color change is an important characteristic during sweating treatment. Study of M. officinalis has suggested that sweating can affect the contents of the main active substances, and this change may be caused by the variation in the microbial community during the sweating process (Wu et al., 2019). Moreover, previous studies have shown that the change in the appearance and color of medicinal materials is due to a series of physicochemical reactions that cause the internal chemical composition change (Zhu et al., 2022). The color change during sweating may be related to the change in the metabolites or the variation in the microbial community. However, the mechanism between color change and sweating in Chinese medicine has not yet been explained.
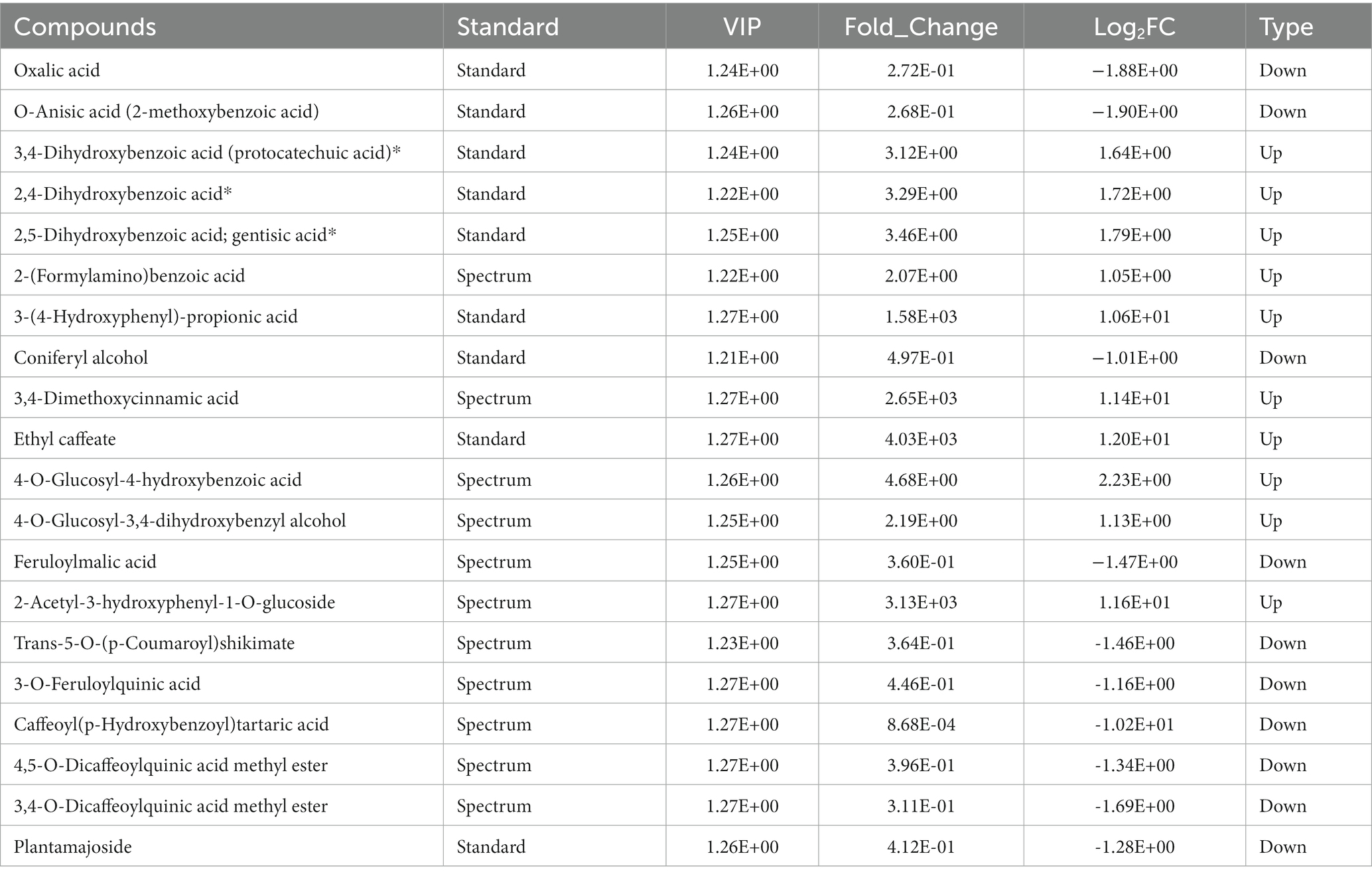
D. asper is an important traditional Chinese herb. The root is a Chinese traditional medicine that undergoes sweating treatment, and it is a well-known medicine used for the treatment of bone diseases, traumatic hematoma, and uterine bleeding (Niu et al., 2011; Yu et al., 2012a,b; Tao et al., 2020). Pharmacological research has revealed that D. asper plays an important role in the treatment of depression (Zhang et al., 2020). Previous studies have isolated approximately 100 components from D. asper, including triterpenoids, iridoids, phenolic acids, essential oils, alkaloids, lignin, and fatty acids, and triterpenoid saponins are the major bioactive compound (Jung et al., 2012). A study investigating the spectrum-effect relationship between sweated and crude D. asper in terms of cell proliferation and differentiation suggested that sweating can affect the efficacy of D. asper by changing the content of its main material basis, including asperosaponin VI, loganin, and caffeic acid (Yang et al., 2019). In addition, sweating can change the color of the fracture surface of D. asper medicine (Figure 1), which is regarded as one of the important indicators of quality in D. asper. However, it is not clear what causes the color change and how the color change relates to the variation of compounds and microorganisms in this valuable Chinese herbal medicine.
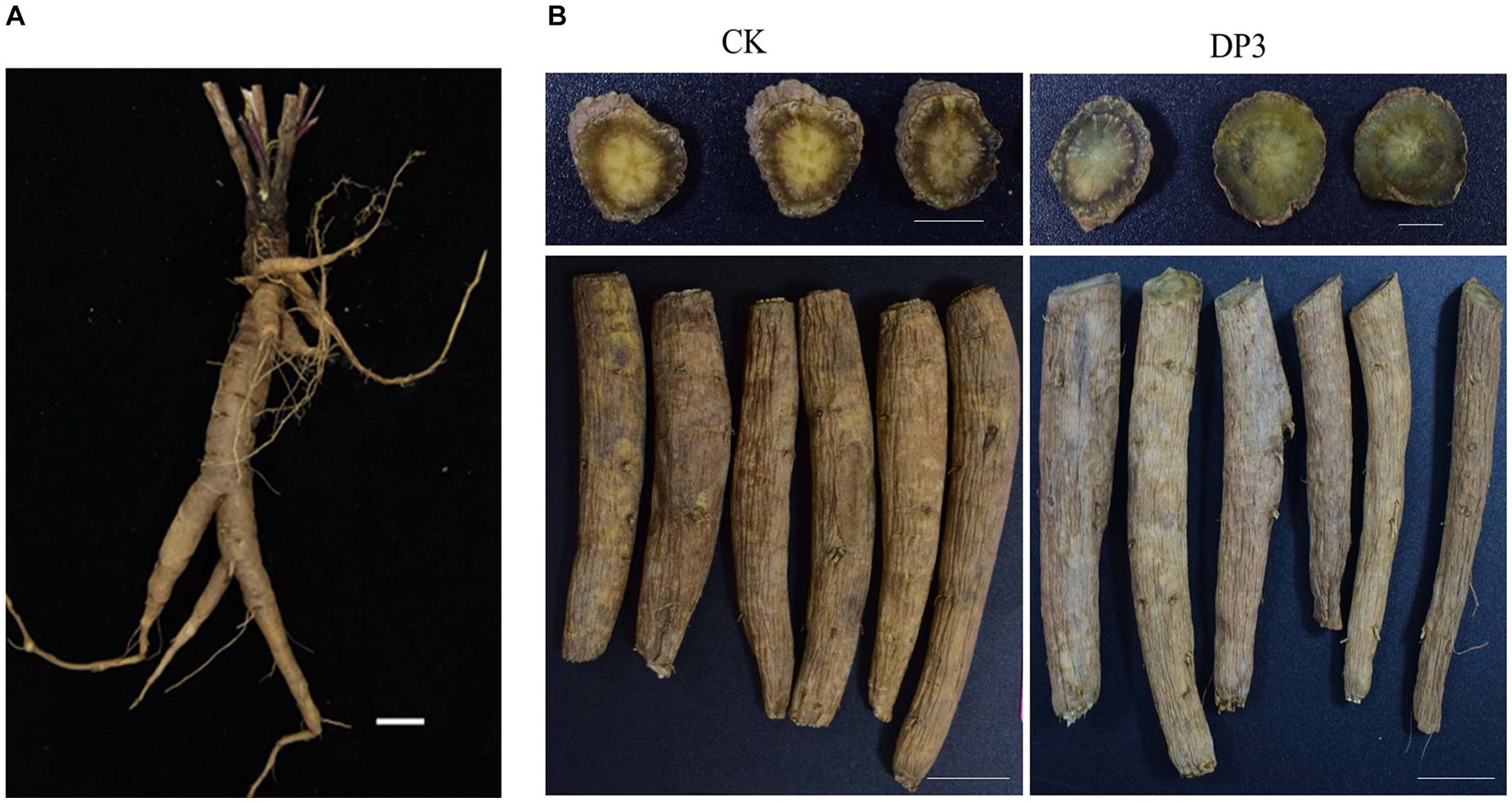
Figure 1. Phenotype of the root in Dipsacus asper. (A) The fresh root. (B) The root before (CK) and after (DP3) sweating. Scale bars = 2 cm.
Our previous study also showed that sweating can affect the content of total triterpene saponins and asperosaponin VI, which is the index component of D. asper (He et al., 2021), while the content of asperosaponin VI in D. asper showed no correlation with the color change of the cross-section in stable sweating conditions (Zhou et al., 2021). These results implied that color change during the sweating process may be related to other metabolites. Based on the above results, widely targeted metabolomics was used to comprehensively analyze metabolite composition and relative content in D. asper root between the control and the sweating group. Then, high-throughput sequencing technology was used to analyze fungal community variations between the two groups. Metabolic and microbiome association analysis was conducted to find the metabolites and fungi that led to the color change following sweating treatment of D. asper root. These results would provide insight into the very nature of sweating processing in Chinese medicine.
Materials and methods
Plant materials and sample preparation
D. asper plants were cultivated for more than 2 years at Guizhou University of Traditional Chinese Medicine, Guizhou Province, China. The fresh root of D. asper was collected and washed. Then, the roots were cut into approximately 10-cm-thick sections. All the samples were divided in half for sweating treatment.
Sweating treatment
Fresh D. asper was collected and processed according to the sweating method established in our previous study (Zhou et al., 2021). All the samples were dried in a dryer until the relative water content reached 40%, and these samples were used for sweating processing. One part of these samples was taken as the control, named CK in the subsequent analysis. The other samples were sweated for 3 days at 25°C in an airtight environment and were named DP3. These two samples were stored in a − 80°C freezer for widely targeted metabolomics and high-throughput sequencing, simultaneously. There were three biological replicates per treatment.
Metabolite extraction
The above samples with three biological replicates were subjected to widely targeted metabolomic analysis (Metware Biotechnology Co., Ltd. Wuhan, China). The samples were freeze-dried with a vacuum freeze-dryer (Scientz-100F) and then crushed using a mixer mill (MM 400, Retsch) with a zirconia bead for 1.5 min at 30 Hz. The lyophilized powder (100 mg) was dissolved in 1.2 mL of 70% methanol solution, vortexed for 30 s every 30 min (for a total of six times), and then stored at 4°C overnight. Following centrifugation at 12,000 rpm for 10 min, the extracts were filtrated (0.22 μm pore size) before ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) analysis.
UHPLC-ESI-Q TRAP-MS/MS and analysis
The sample extracts were analyzed using an UHPLC-ESI-MS/MS system (UHPLC, SHIMADZU Nexera X2, Shanghai, China; MS, Applied Biosystems 4,500 Q TRAP, Thermo Fisher) with an Agilent SB-C18 column (1.8 μm, 2.1 mm × 100 mm). The mobile phase consisted of solvent A (pure water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid). The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS.LIT and triple quadrupole (QQQ) scans were acquired on a mass spectrometer (Q TRAP; AB4500 Q TRAP UHPLC/MS/MS System) equipped with an ESI Turbo Ion-Spray interface, operating in positive and negative ion mode and controlled by Analyst 1.6.3 software (AB Sciex). Based on the self-built database MWDB V2.0 (Metware Biotechnology Co., Ltd. Wuhan, China), primary and secondary mass spectrometry data were subjected to qualitative analysis. Isotopic signals, repeating signals containing K+ ions, Na+ ions, NH4+ ions, and fragment ions that were other larger molecular weight compounds were removed from the analysis. Moreover, the accurate mass, MS/MS spectra, and retention time (RT) of the identified metabolites in the CK and DP3 samples were also compared with the compounds in the self-built database MWDB V2.0 to improve the accuracy of the identification. The comparison was performed with the following parameters: the mass error of the parent ion and fragment ion of the metabolites was set below 20 ppm, and the RT values were set with a variation of less than 0.2 min. Metabolite quantification was performed using triple quadrupole mass spectrometry in multiple reaction monitoring (MRM) (Fraga et al., 2010).
Unsupervised principal component analysis (PCA) was performed using R software (www.r-project.org/). The clustering heat map was analyzed with normalized data. Significantly different accumulated metabolites (DAMs) between groups were determined by variable importance in projection (VIP) of ≥1 and absolute log2 (fold change) of ≥1.
DNA extraction and its amplification
Microbial DNA was extracted from the two sample groups mentioned above (CK, DP3), each with three biological replicates, using an E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, United States) according to the manufacturer’s protocols. Total DNA concentration and quality were determined using a NanoDrop 2000 UV–vis spectrophotometer (Thermo Scientific, Wilmington, United States) and agarose gel electrophoresis. The ITS regions of the fungal 18S rRNA genes were amplified with primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′). The PCR reactions were conducted using the following protocol: 95°C for 3 min, followed by 36 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s, with a final extension at 72°C for 10 min. The PCRs (Gene Amp 9,700, ABI, United States) were performed in triplicate. Amplicons were extracted from 2% agarose gels, further purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States), and quantified using QuantiFluor™-ST (Promega, United States) according to the manufacturer’s protocol.
Illumina MiSeq sequencing
Purified amplicons were pooled in equimolar ratios and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, United States) according to the standard protocols described by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). After Illumina sequencing, the fungi raw sequences were deposited in the Sequence Read Archive database at the NCBI (the accession number: PRJNA1005339). Raw fastq files were demultiplexed, quality-filtered using Trimmomatic, and merged using FLASH with the following criteria: (i) the reads were truncated at any site receiving an average quality score of <20 over a 50 bp sliding window; (ii) primers were exactly matched, allowing two nucleotide mismatching, and reads containing ambiguous bases were removed; and (iii) sequences with overlaps longer than 10 bp were merged according to their overlap sequence.
Sequencing data analysis
Operational taxonomic units (OTUs) were clustered with a 97% similarity cutoff using UPARSE (version 7.0 http://drive5.com/uparse/), and chimeric sequences were identified and removed using USEARCH. The taxonomy of each 18S rRNA gene sequence was analyzed using the RDP Classifier algorithm (version 2.2 http://sourceforge.net/projects/rdp-classifier/) against the Silva (SSU123) database using a confidence threshold of 70%.
Alpha diversity indices were used to evaluate microbial abundance and diversity. Chao, ACE indexes, and principal coordinate analysis (PCoA) were analyzed using the online tool of the Majorbio Cloud Platform (Ren et al., 2022). Unweighted Pair-group Method with Arithmetic Mean (UPGMA) was used to construct a phylogenetic tree to investigate beta-diversity patterns.
Correlation analysis
Correlation analysis was performed on the differential fungi and metabolites detected in each group. The Spearman correlation coefficient of microorganisms and metabolites was calculated (Franzosa et al., 2019). The Spearman correlation value r is between −1 and + 1. Green indicates a positive correlation with r > 0, and red represented a negative correlation with r < 0. And p - value below 0.05 indicates a significant correlation. Metabolites with a correlation greater than 0.8 and a correlation significance test p - value <0.05 were used to generate the chord diagram and heatmap.
Results and discussion
Phenotype observation after sweating in Dipsacus asper
Sweating treatment is a traditional processing method in D. asper. According to the Chinese Pharmacopoeia and a previous study (Zhou et al., 2021), the roots of D. asper were used for sweating treatment (Figure 1A). After sweating treatment, the color of the cross-section in DP3 changed from yellow to green, which was consistent with the Chinese Pharmacopoeia description (version 2020) (Figure 1B). There was little difference in the root epidermis and root morphology, and color change was the key feature of sweating treatment. It has been shown that the contents of protodioscin, diosgenin, and narcissoside have a significantly positive correlation with color change during the processing of Polygonatum kingianum Rhizoma (Wang et al., 2022). Previous studies have indicated that carotenoids play an important role in the colors that mostly range from yellow to red, which affected the coloration in plants (Nisar et al., 2015; Zheng et al., 2019). The accumulation of anthocyanins in some mature fruits, including grapes, strawberries, and apples, contributed to fruits with rich colors (Zhang et al., 2008). Moreover, the content of tanshinone and salvianolic acid is involved in the root color of Salvia miltiorrhiza (Deng et al., 2020; Hao et al., 2020). In conclusion, metabolites such as carotenoids, anthocyanins, saponins, and phenolic acids were related to color changes. Hence, we speculated that metabolite changes led to color changes after sweating treatment in D. asper.
Metabolites in control and sweated Dipsacus asper
To investigate the relationship between metabolites and sweating treatment, widely targeted metabolomic analysis was performed with control (CK) and sweated (DP3) D. asper. A total of 667 metabolites were identified. The metabolites could be classified into 12 categories: including terpenoids, tannins, phenolic acids, organic acids, nucleotides and derivatives, lipids, lignans and coumarins, flavonoids, steroids, amino acid and derivatives, alkaloids, and other metabolites (Figure 2A). The detailed information of these metabolites is shown in Supplementary Table 1. Among them, the classifications with the highest number of metabolites were lipids (18.1%), phenolic acids (14.6%), and terpenoids (13.2%). These three types of metabolites were considered the main components in D. asper. Previous studies have shown that there are 52 common components in raw and sweated D. asper based on UPLC-Triple-TOF/MS analysis, including triterpenoid saponins, iridoids, and phenolic acids (Hong et al., 2020). We also identified a variety of metabolites in D. asper with sweating treatment, and there was a large number of alkaloids and lipids in addition to terpenoids and phenolic acids.
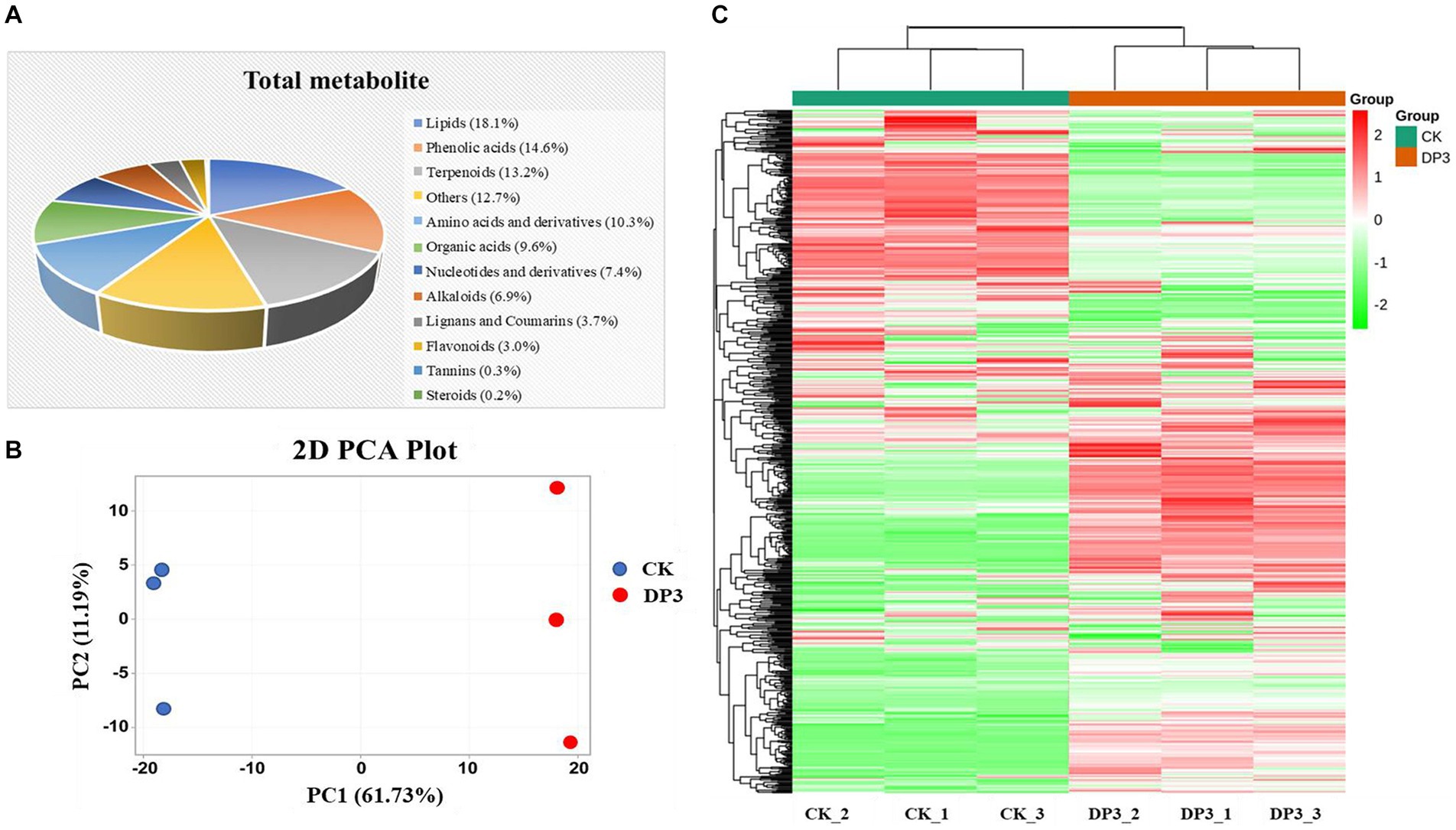
Figure 2. Metabolites detected in D. asper. (A) Classification and proportion of 667 metabolites detected in D. asper. (B) Principal component analysis (PCA) of D. asper samples. (C) Hierarchical clustering heatmap of metabolite accumulation in D. asper.
Moreover, PCA analysis showed that these two samples were clearly distinguishable, with PC1 and PC2 accounting for 61.73% and 11.19% of the variance, respectively (Figure 2B). There was a significant difference in PC1 between CK and DP3. The results indicated that the metabolite compositions of CK and DP3 were different. Moreover, the hierarchical clustering heatmap also showed that the metabolites were quite different following sweating treatment (Figure 2C). The results revealed that the differential metabolites may be responsible for color changes caused by sweating treatments in D. asper.
Differential metabolites in control and sweated Dipsacus asper
To better understand the effects of the sweating treatment on the changes of the metabolites, a comparison of metabolites between control and sweated D. asper was analyzed. As shown in the volcano plot, out of the 667 metabolites detected, there were 114 significantly different accumulated metabolites after sweating, including 36 downregulated and 78 upregulated metabolites (Figure 3A; Supplementary Figure S1). Furthermore, the heatmap showed that the metabolites from all the 12 classes significantly differed between control and sweated D. asper. Based on the number and proportion of the significantly different accumulated metabolites, sweating mainly affected the content of the metabolites from the classes of phenolic acids, organic acids, amino acids and derivatives, and terpenoids (Supplementary Figure S2).

Figure 3. Differential metabolites analysis. (A) Volcano plots of differential metabolites between CK and DP3. (B) Cluster analysis of differential metabolites between CK and DP3.
There were more phenolic acids and terpenoids among the significantly differential compounds (Supplementary Figure S3). All the terpenoids were upregulated and showed a significant change after sweating treatment, including two iridoids, laciniatoside III, and 6-deoxycatalpol (Figure 3B). Iridoids represent a large group of cyclopentano[c]pyran monoterpenoids, which are widely distributed in medicinal plants (Zhao and Shi, 2011). Owing to the unstable nature of their C1-OH group, iridoids often react with sugar to form glycosides, and they often decompose and transform during the processing of traditional Chinese medicines. Based on the structure and properties of iridoids, factors such as temperature, relative humidity, pH, and water content can affect the decomposition and transformation of iridoids during processing (Wang et al., 2020; Du et al., 2021). According to a previous study and Chinese Pharmacopoeia, Gentiana macrophylla, a Chinese medicinal herb, also needs sweating. It has been shown that the color change is related to secoiridoid glycoside content during the sweating processing (Wang et al., 2017). In our study, laciniatoside III is a bis-iridoid that was upregulated after the sweating treatment, and was first isolated from the aerial parts of D. laciniatus (Kocsis and Szabó, 1993). The iridoid glycoside 6-deoxycatalpol has a close relationship with catalpol, which also significantly correlates with the apparent color during the processing of Rehmanniae Radix (Chen et al., 2022). Hence, we think that the two iridoids, laciniatoside III and 6-deoxycatalpol, might play an important role in the color change of D. asper sweating.
In addition, 20 phenolic acids showed a significant content change compared with the control (Table 1). The phenolic acid metabolites, such as oxalic acid, O-anisic acid, and caffeoyl tartaric acid, were the downregulated metabolites with large fold changes after sweating treatment, whereas metabolites such as ethyl caffeate, 2-acetyl-3-hydroxyphenyl−1-O-glucoside, and 3,4-dimethoxycinnamic acid were the upregulated metabolites with large fold changes after sweating treatment (Figure 3B; Supplementary Figure S3).
Phenolic acids, metabolites that contain phenolic hydroxyl and carboxyl groups, exist in many medicine plants. In plant tissues, most of the phenolic acids are bound phenolics and occur in the form of esters and insoluble bound complexes. In addition to their important pharmacological activities, they are involved in the color change in Chinese medicinal herbs. Yang reported that the color change in Lycii Fructus was strongly correlated with a water-soluble pigment extract solution, which mainly contains phenolic acids and rutin (Yang et al., 2015). Additionally, phenols are involved in the color change in the primary processing of the roots of Rehmannia glutinosa with the action of the polyphenol oxidase (Duan et al., 2013). A study on the marked greening observed in some foods during food processing suggested that the reaction of chlorogenic acid or caffeic acid ester with a primary amino compound can form a green pigment compound (Yabuta et al., 2001). In our study, ethyl caffeate was upregulated after sweating, and amino acids were also present in the sweating samples; therefore, we speculated that ethyl caffeate may be involved in the green color formation in sweated D. asper.
Hence, all these results showed that terpenoids and phenolic acids play a pivotal role in the green color formation following sweating treatment. Factors leading to changes in metabolites that cause the cross-section color change during sweating would be the focus of our next study.
Given that sweating can affect the structure of microbial communities and the activity of enzymes in the tissues, and microbial communities can influence the transformation of the metabolites (Zhang et al., 2019), we speculated that the microbial community variation between the CK and DP3 groups could mediate the changes in differential metabolites.
Diversity and composition analysis of fungal communities in control and sweated Dipsacus asper
To find out the differential microbial communities in control and sweated D. asper, high-throughput sequencing of the above two samples was completed. A total of 314,688 quality-filtered 18S rRNA gene sequences were obtained with an average length of 255 bp. The coverage index values of these two samples exceeded 99%. This suggested that most of the fungal communities could be detected and that the results could be used for further analysis. In our study, 246 and 322 OTUs were obtained for the fungal communities in CK and DP3, respectively, based on 97% similarity (Figure 4A). A total of 163 OTUs were the same in CK and DP3 (Figure 4B).
Figure 4. OTU numbers and Venn diagram analysis. (A) Number of OTUs in the fungal community. (B) Venn diagram of the fungal community.
To investigate the microbial diversity and richness of the microbial communities in CK and DP3, alpha-diversity analysis was conducted. The Chao index based on ITS data showed that DP3 had a higher diversity and species richness than CK (Figure 5A). The Ace index showed similar results (Figure 5B). Hence, the results revealed that sweating treatment could elevate the richness and diversity in the root of D. asper. The alteration patterns of the microbial communities in CK and DP3 were investigated through beta diversity. Principal coordinate analysis (PCoA) showed that the microbial communities were separated between DP3 and CK by sweating treatment (Figure 5C), and the result of hierarchical clustering analysis was similar to that of PCoA (Figure 5D). The results revealed that the diversity of fungal community structure increased following sweating process.
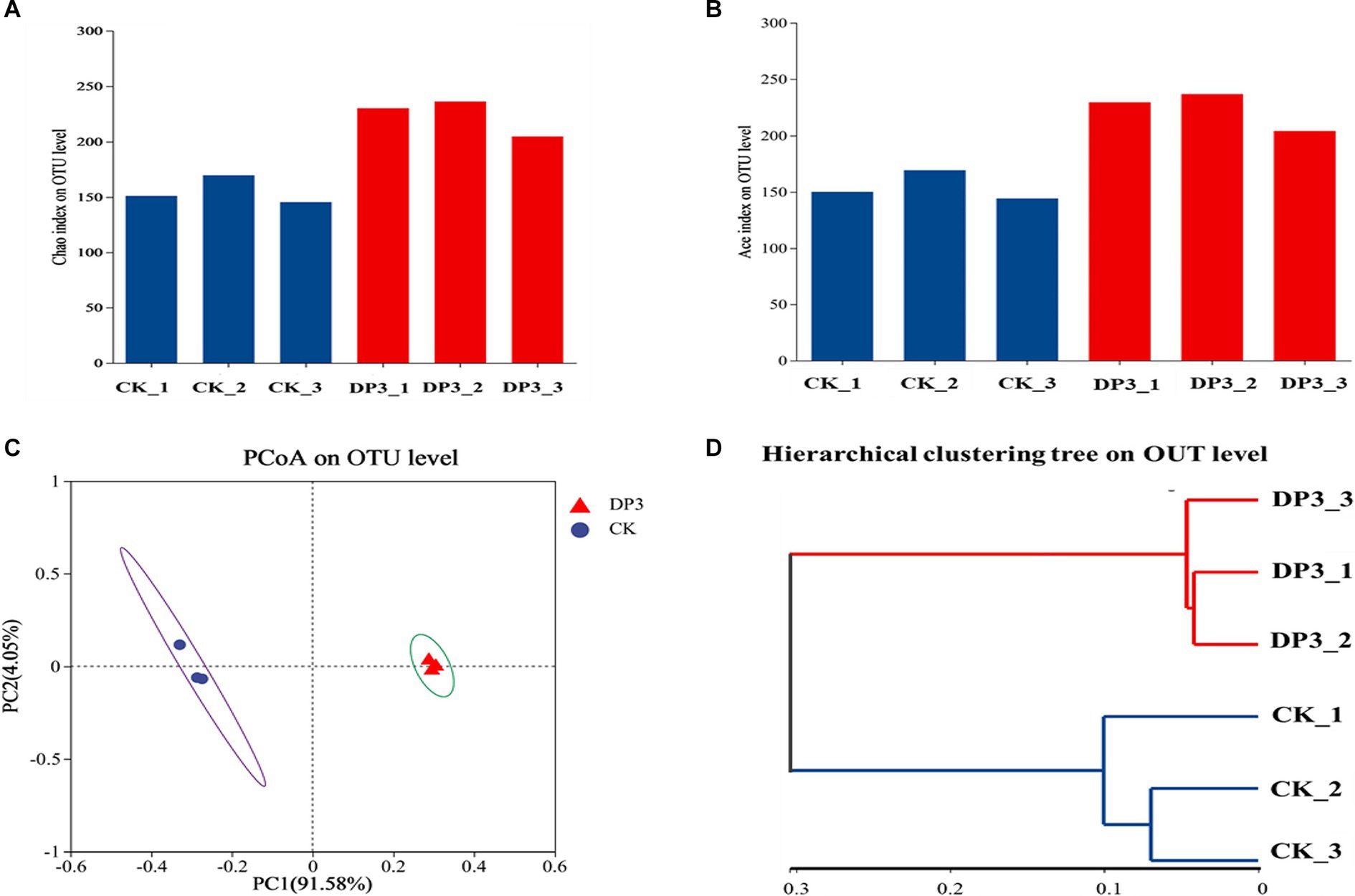
Figure 5. Fungal diversity analysis. The Chao indices (A) and ACE indices (B) of the fungal community. (C) PCoA analysis of the fungal community. (D) Hierarchical clustering analysis of the fungal community.
Furthermore, the composition of microbial communities in the above two samples was analyzed. The OTUs were divided into four main phyla: Ascomycota, Basidiomycota, Olpidiomycota, and others (Figure 6A). There was obvious variation at the phylum level between CK and DP3. The relative abundance of Ascomycota was significantly increased, whereas the relative abundance of Basidiomycota and Olpidiomycota was significantly decreased in DP3. Moreover, the relative abundance of community variation at the genus level had a similar variation to that at the phylum level (Figure 6B). The relative abundance of Ascomycota was increased in DP3, including Fusarium sp., Ilyonectria sp., Memnoniella sp., and Neocosmospora sp. Additionally, the relative abundance of Armillaria sp., which belong to the Basidiomycota, was decreased in DP3. The results showed that the balance between the Basidiomycota and Ascomycota was disrupted by the sweating process in D. asper. The fungi with significant relative abundance variation were associated with color change caused by sweating treatment in D. asper. Based on the analysis of the microbial communities in the process of sweating in M. officinalis, the relative abundance of Enterobacter, Klebsiella, Weissella, Bacillus, and Candida would be conducive to improving the quality of M. officinalis (Wu et al., 2019). Hence, we speculate that differential metabolites may be involved in the variation of microbial communities.
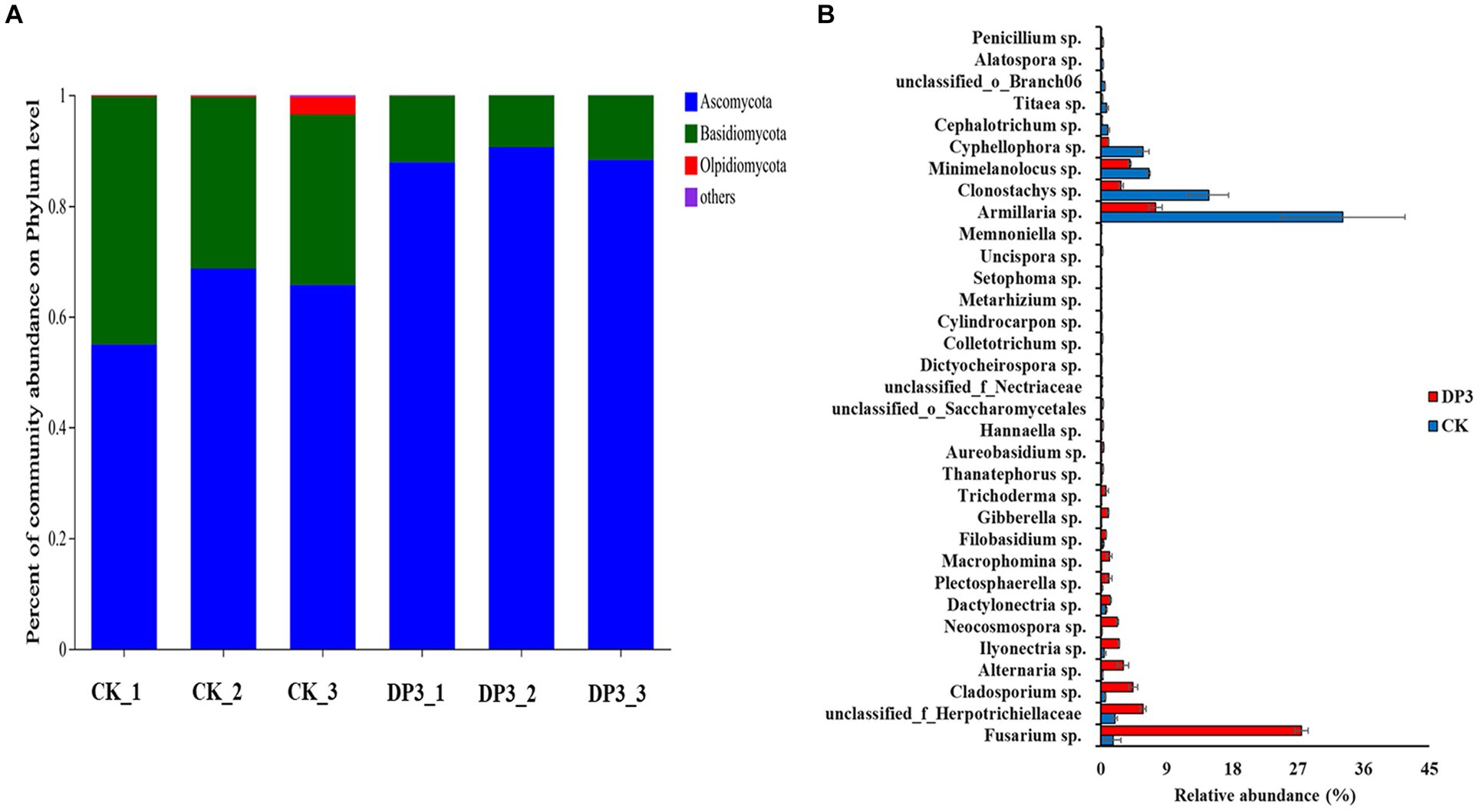
Figure 6. The composition of fungal communities in control and sweated D. asper. (A) Percentage of community abundance at the phylum level. (B) Fungi with significant relative abundance at the genus level.
Correlation analysis of differential metabolites and microbial communities
Based on the differential metabolite enrichment analysis, the differential phenolic acids and terpenoids may be the key compounds related to the cross-section color change caused by the sweating process. Hence, correlation analysis was conducted between the differential metabolites and the differential microbial communities in control and sweated D. asper. The results suggested that the differential fungi of 28 genera correlated with 25 differential metabolites of phenolic acids and terpenoids (Figure 7A). Some fungi that enriched significantly in DP3 showed a significant correlation with these metabolites (Figure 7B). Neocosmospora sp. had a significant positive correlation with two iridoid compounds, 6-deoxycatalpol and laciniatoside III, which were upregulated after sweating treatment. Moreover, Macrophomina sp. and Memnoniella sp. were positively correlated with phenolic acid compounds, including ethyl caffeate, 3-(4-hydroxyphenyl)-propionic acid, 2,4-dihydroxybenzoic acid, and 2-acetyl-3-hydroxyphenyl−1-O-glucoside. Additionally, the results showed that unclassified_o_Saccharomycetales and unclassified_f_Nectriaceae were positively correlated with 4-O-glucosyl-4-hydroxybenzoic acid and 4-O-glucosyl-3,4-dihydroxybenzyl alcohol. Both Fusarium sp. and Ilyonectria sp. had a negative correlation with two phenolic acid compounds, 3-O-feruloylquinic acid and 3,4-O-dicaffeoylquinic acid methyl ester, which were downregulated following sweating treatment. Furthermore, some fungi with decreased relative abundance in DP3 showed a significant correlation with metabolites that were downregulated in DP3. The results showed that Armillaria sp. and Cyphellophora sp. were significantly correlated with O-anisic acid, 3-O-feruloylquinic acid, and 3,4-O-dicaffeoylquinic acid methyl ester. Penicillium sp. showed a significant correlation with 3,4-O-dicaffeoylquinic acid methyl ester and coniferyl alcohol. These metabolites were phenolic acids and downregulated in DP3.
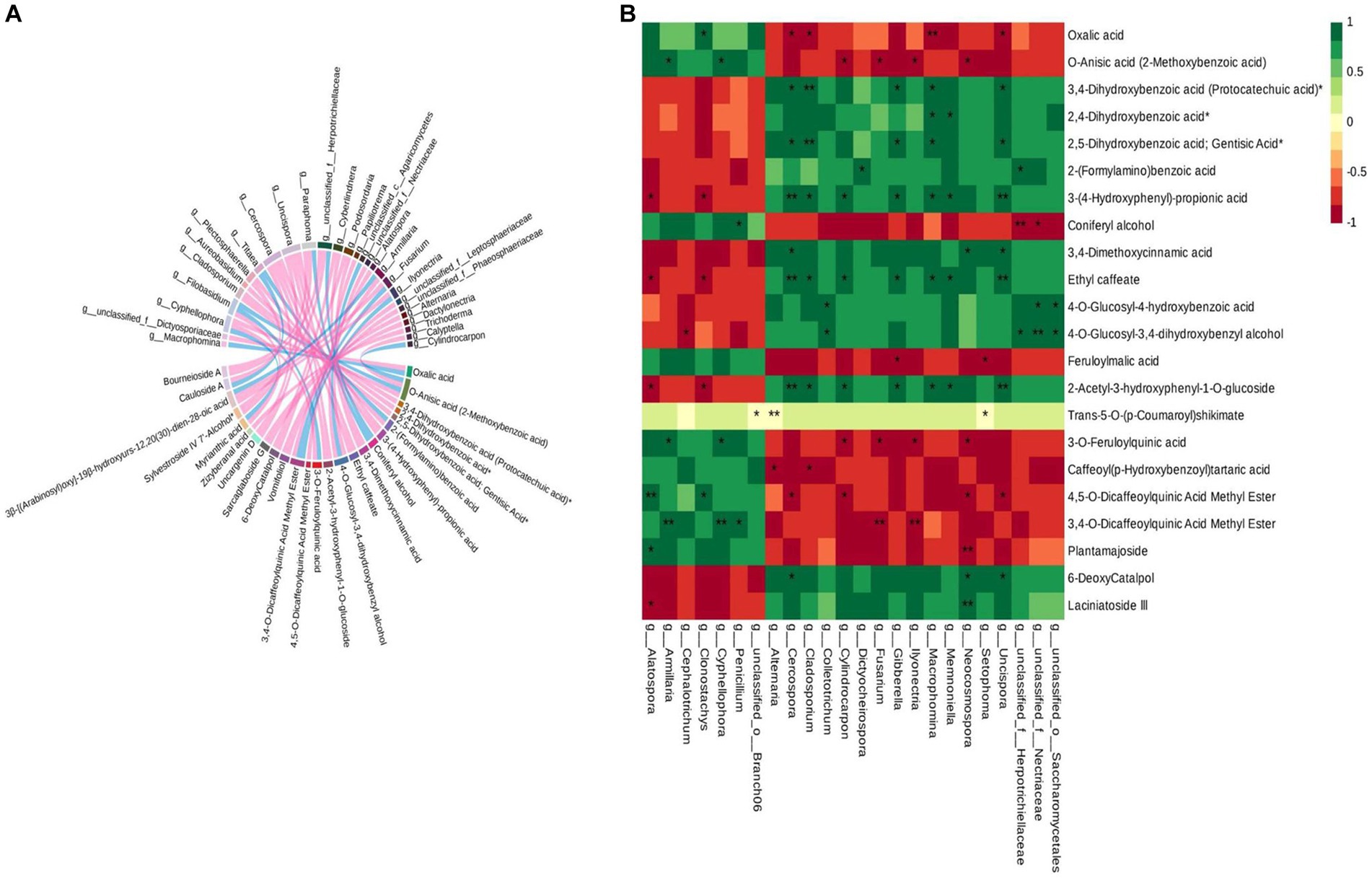
Figure 7. Correlation analysis of the differential metabolites and differential microorganisms in control and sweated D. asper. (A) Correlation analysis of differential metabolites and differential fungi. (B) Correlation analysis of phenolic acids, terpenoids and differential fungi.
It has been reported that Fusarium and Neocosmospora sp. could colonize pistachio wood and cause vascular discolorations. They were also capable of producing discoloration in stems of clonal rootstocks (Crespo et al., 2019). The discoloration of the stems was similar to the color change of the root that was caused by sweating treatment. Additionally, the study revealed that a gene cluster from Macrophomina phaseolina was responsible for the biosynthesis of novel DTAs, macrophasetins (Yu et al., 2022). It has been shown that M. phaseolina can produce phomeolic acid in a potato dextrose medium (Singh et al., 2022). Macrophomina sp. can produce secondary metabolites, including phenolic acids. In addition, an automatic reconstruction revealed 24 Penicillium sp., which have the potential to produce secondary metabolites such as terpenoids and phenylpropanoids (Prigent et al., 2018). Hence, Macrophomina sp. and Penicillium sp. may have the potential to influence the content of phenolic acids in D. asper root with sweating treatment. Therefore, we speculated that 24 fungi interact with 22 differential metabolites to regulate the cross-section color change caused by the sweating processing in D. asper.
Conclusion
In this study, color change was the key phenotype in the sweating process. A total of 667 metabolites in D. asper following sweating were identified by widely targeted metabolomics. The content of terpenoids and phenolic acids was involved in green color formation after sweating treatment. In addition, microbial community diversity and richness were increased after sweating treatment. Correlation analysis revealed that the fungi from the Basidiomycota and Ascomycota with the significant relative abundance variation were associated with the content of terpenoids and phenolic acids, which caused the color change following sweating treatment in D. asper. This study provides a foundation for analyzing the mechanism in the processing of Chinese medicinal materials.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/sra/,PRJNA1005339.
Author contributions
HH, JX, and TaoZ conceived and designed the study. HH wrote the manuscript. TmZ and HH performed the experiments. JX, CY, and LL contributed to the data analysis. YY, CX, and CZ revised the manuscript. All authors contributed to the article and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81860675 and 82160725), Guizhou Provincial Science and Technology Projects (Qian Ke He Ji Chu [2020]1Y370, [2019]1026), Research Platform Team Project of the Education Department of Guizhou Province [Qian Jiao Ji [2022]021], and High-Level Innovative Talents of Guizhou Province of China (Qian Ke He Ping Tai Ren Cai [2018] 5638–2).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1195088/full#supplementary-material
References
Chen, R., Chen, C., Yang, X., Yu, J., Zhao, R., and Gu, W. (2018). Modern research progress of Chinese materia medica diaphoretic processing method. Chin. Trad. Herb. Drugs 49, 489–493. doi: 10.7501/j.issn.0253-2670.2018.02.033
Chen, Q., Lei, X., Gao, Y., Xu, J., Wang, L., Yang, M., et al. (2022). Quality evaluation in processing of Rehmanniae Radix praeparata processed with Amomi Fructus and Citri Reticulatae pericarpium based on "color as paint,sweet as maltose". Chin. J. ETMF 28, 154–162. doi: 10.13422/j.cnki.syfjx.20211859
Crespo, M., Lawrence, D., Nouri, M., Doll, D., and Trouillas, F. (2019). Characterization of fusarium and Neocosmospora species associated with crown rot and stem canker of pistachio rootstocks in California. Plant Dis. 103, 1931–1939. doi: 10.1094/PDIS-11-18-2012-RE
Deng, C., Shi, M., Fu, R., Zhang, Y., Wang, Q., Zhou, Y., et al. (2020). ABA-responsive transcription factor bZIP1 is involved in modulating biosynthesis of phenolic acids and tanshinones in Salvia miltiorrhiza. J. Exp. Bot. 71, 5948–5962. doi: 10.1093/jxb/eraa295
Du, Y., Wang, M., Li, L., Zhou, K., Bai, Y., Li, Y., et al. (2021). Research progress on effect of processing on properties and efficacy of traditional Chinese medicine containing iridoid terpenoids based on stability of compounds. Chin. Trad. Herb. Drugs 52, 5039–5051. doi: 10.7501/j.issn.0253-2670.2021.16.029
Duan, J., Su, S., Yan, H., Guo, S., Liu, P., Qian, D., et al. (2013). “Sweating” of traditional Chinese medicinal materials during primary processing and its mechanisms of enzymatic reaction and chemical conversion. Chin. Trad. Herb. Drugs 44, 1219–1225. doi: 10.7501/j.issn.0253-2670.2013.10.001
Fraga, C., Clowers, B., Moore, R., and Zink, E. (2010). Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 82, 4165–4173. doi: 10.1021/ac1003568
Franzosa, E., Sirota-Madi, A., Avila-Pacheco, J., Fornelos, N., Haiser, H., Reinker, S., et al. (2019). Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 4, 293–305. doi: 10.1038/s41564-018-0306-4
Hao, X., Pu, Z., Cao, G., You, D., Zhou, Y., Deng, C., et al. (2020). Tanshinone and salvianolic acid biosynthesis are regulated by SmMYB98 in Salvia miltiorrhiza hairy roots. J. Adv. Res. 23, 1–12. doi: 10.1016/j.jare.2020.01.012
He, H., Xu, J., Zhou, T., Zhou, T., Guo, J., Jiang, W., et al. (2021). Content changes of triterpene saponins in crude and sweated Dipsacus asper: an iTRAQ-based analysis. China J. Chin. Mat. Med. 46, 4730–4735. doi: 10.19540/j.cnki.cjcmm.20210311.303
Hong, Z., Du, W., Yang, Y., Kang, X., Hong, H., Zhu, W., et al. (2020). Analysis of components of crude and sweated Dipsaci Radix by UPLC-triple-TOF/MS. Chin. Trad. Herb. Drugs 51, 1233–1241. doi: 10.7501/j.issn.0253-2670.2020.05.020
Jung, H., Jung, J., Son, K., Lee, D., Kang, T., Kim, Y., et al. (2012). Inhibitory effects of the root extract of Dipsacus asperoides C.Y. Cheng et al t.M.Ai on collagen-induced arthritis in mice. J. Ethnopharmacol. 139, 98–103. doi: 10.1016/j.jep.2011.10.020
Kocsis, K., and Szabó, L. (1993). New bis-iridoids from Dipsacus laciniatus. J. Nat. Prod. 56, 1486–1499. doi: 10.1021/np50099a007
Nisar, N., Li, L., Lu, S., Khin, N., and Pogson, B. (2015). Carotenoid metabolism in plants. Mol. Plant 8, 68–82. doi: 10.1016/j.molp.2014.12.007
Niu, Y., Li, Y., Huang, H., Kong, X., Zhang, R., Liu, L., et al. (2011). Asperosaponin VI, a Saponin component from Dipsacus asper wall, induces osteoblast differentiation through bone morphogenetic Protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway. Phytother. Res. 25, 1700–1706. doi: 10.1002/ptr.3414
Prigent, S., Nielsen, J., Frisvad, J., and Nielsen, J. (2018). Reconstruction of 24 Penicillium genome-scale metabolic models shows diversity based on their secondary metabolism. Biotechnol. Bioeng. 115, 2604–2612. doi: 10.1002/bit.26739
Ren, Y., Yu, G., Shi, C., Liu, L., Guo, Q., Han, C., et al. (2022). Majorbio cloud: a one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 1:e12. doi: 10.1002/imt2.12
Singh, G., Kumar, A., Verma, M., Gupta, P., and Katoch, M. (2022). Secondary metabolites produced by Macrophomina phaseolina, a fungal root endophyte of Brugmansia aurea, using classical and epigenetic manipulation approach. Folia Microbiol. (Praha) 67, 793–799. doi: 10.1007/s12223-022-00976-3
Tao, Y., Chen, L., and Yan, J. (2020). Traditional uses, processing methods, phytochemistry, pharmacology and quality control of Dipsacus asper wall. Ex C.B. Clarke: a review. J. Ethnopharmacol. 258:112912. doi: 10.1016/j.jep.2020.112912
Wang, C., Gong, X., Bo, A., Zhang, L., Zhang, M., Zang, E., et al. (2020). Iridoids: research advances in their Phytochemistry, biological activities, and pharmacokinetics. Molecules 25:287. doi: 10.3390/molecules25020287
Wang, X., Ling, X., Li, J., Chen, H., and Guo, H. (2017). Correlation between the content of active components and chromaticity in different processed Gentiana macrophylla. J. Zhejiang Agric. Sci. 58, 976–980.
Wang, S., Wang, L., Fang, J., Liu, K., Wang, Y., and Zhang, C. (2022). Correlation analysis between color and content changes of five components of wine-processed Polygonatum kingianum Rhizoma during processing. Chin. J. ETMF 28, 156–162. doi: 10.13422/j.cnki.syfjx.20220252
Wu, Q., Wei, D., Dong, L., Liu, Y., Ren, C., Liu, Q., et al. (2019). Variation in the microbial community contributes to the improvement of the main active compounds of Magnolia officinalis rehd.Et Wils in the process of sweating. Chin. Med. 14:45. doi: 10.1186/s13020-019-0267-4
Yabuta, G., Koizumi, Y., Namiki, K., Hida, M., and Namiki, M. (2001). Structure of green pigment formed by the reaction of caffeic acid esters (or chlorogenic acid) with a primary amino compound. Biosci. Biotechnol. Biochem. 65, 2121–2130. doi: 10.1271/bbb.65.2121
Yang, L., Chen, H., Li, X., Wang, F., Chen, L., and Liu, Y. (2015). Correlation between color and pigment components' contents of Lycii Fructus expressing different degree of color-changing. Chin. J. ETMF 21, 47–50. doi: 10.13422/j.cnki.syfjx.2015080047
Yang, Y., Kang, X., Du, W., Lai, P., and Ge, W. (2019). Spectral-effect relationship between HPLC fingerprint and cell proliferation and differentiation of crude and sweated Dipsaci Radix. Chin. Trad. Herb. Drugs 50, 3909–3916. doi: 10.7501/j.issn.0253-2670.2019.16.025
Yu, C., Chen, L., Gao, Y., Liu, J., Li, P., Zhang, M., et al. (2022). Discovery and biosynthesis of macrophasetins from the plant pathogen fungus Macrophomina phaseolina. Front. Virol. 13:1056392. doi: 10.3389/fmicb.2022.1056392
Yu, X., Wang, L., du, Q. M., Ma, L., Chen, L., You, R., et al. (2012a). Akebia Saponin D attenuates amyloid β-induced cognitive deficits and inflammatory response in rats: involvement of Akt/NF-κB pathway. Behav. Brain Res. 235, 200–209. doi: 10.1016/j.bbr.2012.07.045
Yu, X., Wang, L., Ma, L., You, R., Cui, R., Ji, D., et al. (2012b). Akebia saponin D attenuates ibotenic acid-induced cognitive deficits and pro-apoptotic response in rats: involvement of MAPK signal pathway. Pharmacol. Biochem. Behav. 101, 479–486. doi: 10.1016/j.pbb.2012.02.014
Zhang, Z., Gao, Y., Zang, P., Zhao, Y., He, Z., and Zhu, H. (2019). Research progress on active components of fungi transforming Chinese materia medica. Chin. Trad. Herb. Drugs 50, 2736–2742. doi: 10.7501/j.issn.0253-2670.2019.11.034
Zhang, W., Li, X., Zheng, J., Wang, G., Sun, C., Ferguson, I., et al. (2008). Bioactive components and antioxidant capacity of Chinese bayberry (Myrica rubra Sieb. And Zucc.) fruit in relation to fruit maturity and postharvest storage. Eur. Food Res. Technol. 227, 1091–1097. doi: 10.1007/s00217-008-0824-z
Zhang, J., Yi, S., Li, Y., Xiao, C., Liu, C., Jiang, W., et al. (2020). The antidepressant effects of asperosaponin VI are mediated by the suppression of microglial activation and reduction of TLR4/NF-κB-induced IDO expression. Psychopharmacology 237, 2531–2545. doi: 10.1007/s00213-020-05553-5
Zhao, Y., and Shi, Y. (2011). Phytochemicals and biological activities of Dipsacus species. Chem. Biodivers. 8, 414–430. doi: 10.1002/cbdv.201000022
Zheng, X., Zhu, K., Sun, Q., Zhang, W., Wang, X., Cao, H., et al. (2019). Natural variation in CCD4 promoter underpins species-specific evolution of red coloration in Citrus Peel. Mol. Plant 12, 1294–1307. doi: 10.1016/j.molp.2019.04.014
Zhou, T., Gong, A., He, H., Li, L., Xu, J., Jiang, W., et al. (2021). “Sweating” condition of Dipsaci Radix and correlation between content of Asperosaponin VI and chromaticity. Mod. Chin. Med. 23, 1250–1253. doi: 10.13313/j.issn.1673-4890.20201222003
Keywords: Dipsacus asper, metabolomics, microbiome, sweating, color changes
Citation: He H, Xu J, Zhou T, Yang Y, Yang C, Xiao C, Zhang C, Li L and Zhou T (2023) Metabolomic and microbiomic insights into color changes during the sweating process in Dipsacus asper. Front. Microbiol. 14:1195088. doi: 10.3389/fmicb.2023.1195088
Edited by:
Jesús Navas-Castillo, CSIC, SpainReviewed by:
Yi Tao, Zhejiang University of Technology, ChinaJieying Zhang, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, China
Copyright © 2023 He, Xu, Zhou, Yang, Yang, Xiao, Zhang, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Zhou, taozhou88@163.com
†These authors have contributed equally to this work
 Hua He
Hua He Jiao Xu
Jiao Xu Taimin Zhou
Taimin Zhou Chenghong Xiao
Chenghong Xiao Tao Zhou
Tao Zhou