- 1Breast Department, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, China
- 2Beijing University of Chinese Medicine, Beijing, China
- 3Department of Thyroid and Breast Surgery, Liaoning Provincial People's Hospital, People's Hospital of China Medical University, Shenyang, China
Introduction: Granulomatous mastitis (GM) is a chronic inflammatory breast disease. In recent years, the role of Corynebacterium in GM onset has received more and more attention. This study aims to detect the dominant bacterium in GM patients and analyze the association between clinical characteristics and infectious factors.
Methods: In this study, 88 samples from 44 GM patients, six acute lactation mastitis (ALM) patients, and 25 non-inflammatory breast disease (NIB) patients were divided into a GM pus group, a GM tissue group, an ALM pus group, and a NIB tissue group; then, 16S ribosomal DNA sequencing was used to explore their microbiota. The clinical data of all 44 GM patients were also retrospectively collected and analyzed to determine their relationship with infection.
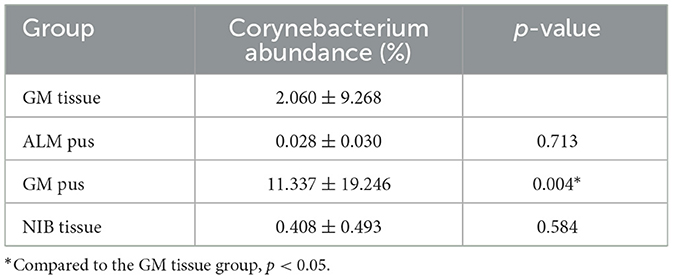
Results: The median age of the 44 GM patients was 33 years, and 88.6% of patients had primary-onset cases, while 11.4% were recurrences; additionally, 89.5% of patients were postpartum and 10.5% were nulliparous. The serum prolactin level was abnormal in nine patients (24.3%). Samples from 15 GM patients (34.1%) had a Corynebacterium abundance of >1% (1.08–80.08%), with eight (53.3%) displaying an abundance of >10%. Corynebacterium was the only genus with significant differences between the GM pus group and the other three groups (p < 0.05). Corynebacterium kroppenstedtii was the predominant Corynebacterium species. Among clinical characteristics, a statistical difference in breast abscess formation was observed according to Corynebacterium abundance in Corynebacterium-positive and- negative patients (p < 0.05).
Discussion: This study explored the relationship between Corynebacterium infection and GM, compared the clinical characteristics between Corynebacterium-positive and- negative patients, and provided support for the role of Corynebacterium species-in particular, C. kroppenstedtii-in the pathogenesis of GM. The detection of Corynebacterium can predict GM onset, especially in patients with high prolactin levels or a history of recent lactation.
Introduction
Granulomatous mastitis (GM), a type of non-lactation mastitis, is a benign chronic inflammatory breast disease that commonly affects women of reproductive age with a history of gestation and lactation (Fujii et al., 2018). In recent years, the incidence of GM has increased rapidly (Bacon et al., 2021; Bi et al., 2021). Clinical manifestations of GM and include breast lumps, abscesses, fistulae, and nipple retractions. Sometimes, GM mimics breast carcinoma (Dobinson et al., 2015). Some patients present with extramammary symptoms, such as erythematous nodosum and joint pain, especially in the lower limbs (Saydam et al., 2020). The golden diagnostic standard of GM is pathological examination (Johnstone et al., 2017). Typical morphology under the microscope includes lobulocentric granulomatous inflammation, non-caseating granulomas, and neutrophils (Yu et al., 2016; Johnstone et al., 2017; Bi et al., 2021).
Currently, the etiology of GM remains hypothetical; however, relevant factors reported to date include immune dysregulation, microbial infection, and hormonal disturbances (Fujii et al., 2018; Koksal et al., 2020; Yin et al., 2021). In recent years, infection-related factors have received increasing attention. Corynebacterium species, particularly Corynebacterium kroppenstedtii (Ck), have been linked to GM (Tauch et al., 2016).
Nowadays, with the advent of new technologies, such as 16S ribosomal RNA, 16S ribosomal DNA (16SrDNA) sequencing, and matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (Li et al., 2020), clinical microbiology laboratories can identify organisms down to both the genus and species levels more accurately and promptly (Saraiya and Corpuz, 2019). However, although emerging technologies offer excellent convenience for the detection of GM pathogens, there is still often an absence of clinical information about the bacterium, causing insufficient attention to be paid to infectious factors in the GM diagnosis and treatment process.
An agreement has not yet been reached regarding the treatment of GM (Yaghan et al., 2020). Conservative strategy involves immunosuppressants, steroids, and antibiotics, while surgery is a treatment choice available for patients showing an insufficient response to conservative therapy (Wolfrum et al., 2018; Chen et al., 2021). For Ck-related GM, choosing lipophilic antibiotics is very important. To avoid the development of drug resistance and obtain better efficacy, the course of antibiotics should not be short (Williams et al., 2021). Therefore, accurately identifying the bacteria species present will help the clinic tailor antibiotic use.
In the enrolled cases, we explored whether the identified bacteria were pathogens of GM, which bacterium was the primary pathogen of GM, the relationship between the patient's clinical manifestations and the pathogen, and the difference in bacterium abundance among different types of samples from the same patient. The microbiota in breast pus and tissue samples from GM patients were examined by 16SrDNA sequencing and compared to those of samples from patients with acute lactation mastitis (ALM) or non-inflammatory breast diseases (NIBs), including benign or malignant breast tumors. This is the first study to report bacterial differences in pus and tissue samples, and it is also the first to investigate the contrast between GM patients and ALM and NIB patients. This study aims to detect the dominant bacterium in GM patients and analyze the association between clinical characteristics and infectious factors to provide reliable evidence for early, precise treatment. Figure 1 contains a flowchart of the study selection process.
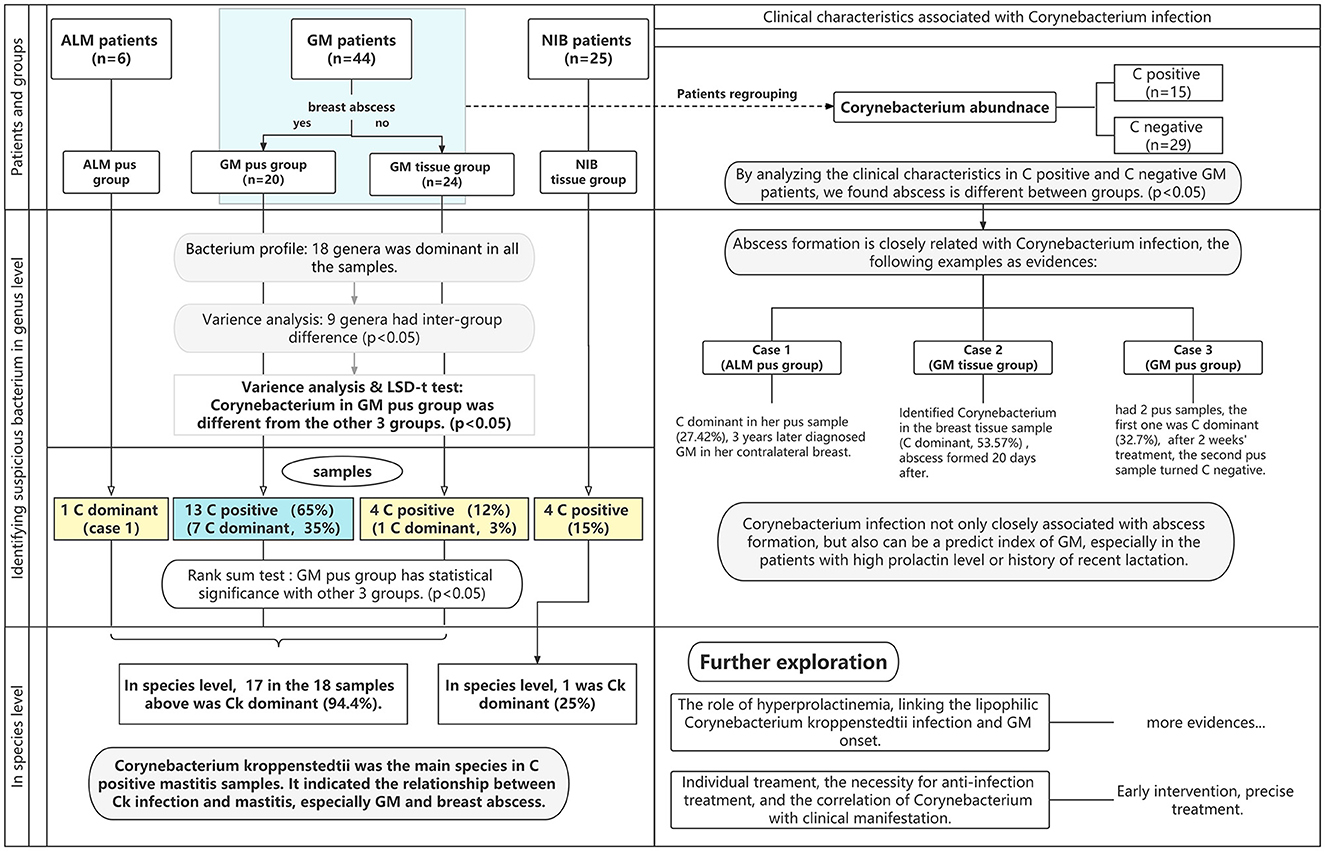
Figure 1. The flowchart of this study. The blue area represents the observation group, while the yellow areas represent the control group. Abbreviations: ALM, acute lactation mastitis; Ck, Corynebacterium kroppenstedtii; GM, granulomatous mastitis; NIB, non-inflammation breast disease. “C” is the abbreviation for Corynebacterium; C-dominant means the abundance of Corynebacterium is >10%, and C-positive means the abundance of Corynebacterium is >1%.
Materials and methods
Patients and specimens
From March 2019 to March 2022, 44 GM patients, 6 ALM patients, and 25 NIB patients were recruited for this study. GM diagnosis was proven by pathologic evidence. All enrolled patients were from the Breast department, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, and written informed consent was obtained from all participants.
Among the 44 GM patients, 20 GM patients had breast abscesses; pus samples of all of these patients were taken, and tissue samples of nine patients were also collected. A total of 24 GM patients did not have an abscess, so only tissue samples were obtained from these individuals. All six ALM patients had breast abscesses, and we collected six pus samples from them. None of the 25 NIB patients had an abscess, so only their tissue samples were obtained. As shown in Figure 1, this study divided the patients into two pus groups (GM pus group and ALM pus group) and two tissue groups (GM tissue group and NIB tissue group). We obtained 29 pus samples and 59 tissue samples (patients with multiple samples are noted in Figure 2). During microbiota analysis, the ALM pus and NIB tissue groups were mainly used as control groups for the two GM groups.
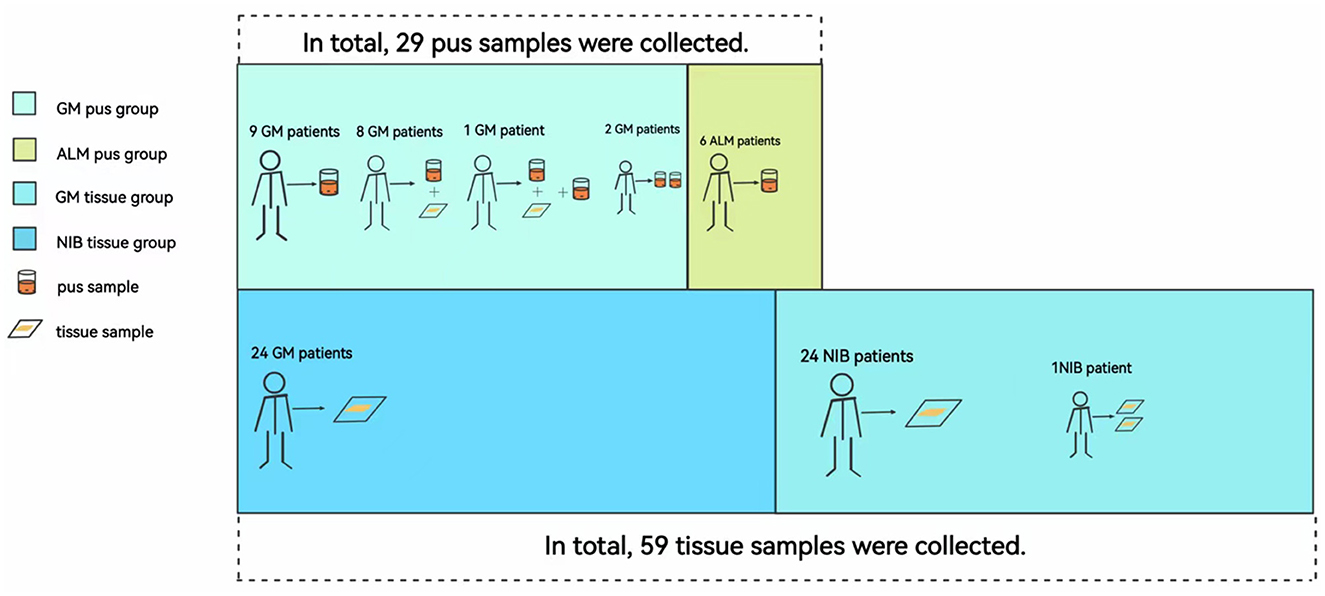
Figure 2. Correspondence between samples and patients in each group. Abbreviations: ALM, acute lactation mastitis; Ck, Corynebacterium kroppenstedtii; GM, granulomatous mastitis; NIB, non-inflammation breast disease. Patients had different samples due to their disease condition and the number of follow-up visits. In this figure, we can see that eight patients in the GM pus group had both pus and tissue samples; one patient had two pus samples taken at two different times and one tissue sample. In the NIB tissue group, one patient had two tissue samples collected because she had bilateral fibromas and underwent two surgeries at different times.
Pus samples were obtained by syringe negative-pressure aspiration, and tissue samples were obtained from puncture or surgery. All operations followed the aseptic principle, and samples were quickly transferred to sterile containers after sampling.
Clinical data were collected for the 44 GM patients, including age, newly diagnosed or relapsed status, laterality, maternity history, clinical manifestation, serum prolactin level, and disease duration.
16SrDNA sequencing and bioinformatics analysis
16SrDNA sequencing was completed by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). The extracted genomic DNA was examined by agarose electrophoresis to check the integrity and concentration of the genomic DNA. Genomic DNA was precisely quantified using the Qubit 2.0 DNA assay kit (Thermo Fisher Scientific, Waltham, MA, United States), to determine the amount of DNA incorporated into the polymerase chain reaction (PCR). The primers used for PCR were fused to universal primers for the MiSeq sequencing platform (Illumina, San Diego, CA, United States). The PCR products were subjected to agarose electrophoresis after PCR, and the DNA was purified by using VAHTS DNA Clean Beads (Vazyme Biotech, Nanjing, China). Qubit 2.0 was used to quantify the refined products, and all samples were mixed in a 1:1 ratio according to the measured DNA concentration and shaken evenly. This mixed sample was sequenced using the MiSeq platform.
Next, the raw image data files were converted into sequenced reads by base calling analysis (Schmieder and Edwards, 2011), labeled “raw data”, and stored in a fastq (.fq) file format, which contains sequence information for sequencing sequences (reads) and their corresponding sequencing quality information.
Down-machine sequencing obtained pair-ended (PE) sequence data, and the sequencing sequence contained the barcode sequence as well as primer and adapter sequences added during sequencing. The primer adapter sequence was removed, and then, according to the overlapping relationship between PE reads, the paired reads were spliced into a sequence. Next, samples were identified and differentiated according to the barcode label sequence to obtain each sample's data. Finally, the quality of each sample's data was quality-controlled and filtered to obtain effective data for that sample.
The data-optimization methods and parameters were as follows (Marcel, 2011; Edgar, 2013): (1) we used cutadapt to remove the Read13' terminal sequencing primer linker AGATCGGAAGAGACGTCTGAACTCCAGTCA and the Read23' terminal sequencing primer linker AGATCGGAAGAGCGTGGTGTAGGGAAAGAGT; (2) we used PEAR to merge pairs of reads into a sequence according to the overlapping relationship between PE reads; (3) we split the data of each sample from the spliced data according to the barcode sequence and primer sequence of each sample, then corrected the sequence direction; and (4) we used PRINSEQ to excise bases below 20 at the tail of reads and set a window of 10 bp. If the average mass value in the window was lower than 20, we cutoff the back-end bases from the window, filtered the N-containing sequences and short sequences after quality control, and finally filtered out the sequences with low complexity.
Subsequently, OTU clustering analysis was used for each sample-optimization sequence. To be precise to the species level, BLAST was used for comparison with the GTDB database, and the community composition of each sample was finally counted at each taxonomic level as follows: domain, phylum, class, order, family, genus, and species (Altschul et al., 1997).
Statistical analysis
SPSS version 25.0 (IBM Corporation, Armonk, NY, United States) was used for data analysis and measurement data were presented as mean ± standard deviation values while counting data were presented as numbers (percentages). STAMP version 2.1.3 was used for variance analysis to determine which variables among the many control variables had a significant impact on the observed variables. A one-way analysis of variance was performed on the top 10 flora of the four groups at the genus level, and the flora that contributed greatly to differences between groups was obtained; meanwhile, the main differentiating bacteria between the GM pus group, GM tissue group, and other groups were obtained by least significant difference multiple comparisons post-hoc testing. The Corynebacterium degree between the four groups was determined by non-parametric rank-sum testing. Finally, the clinical characteristics of GM patients between the Corynebacterium-positive and Corynebacterium-negative groups were tested by the chi-square test or exact probability method, and p < 0.05 was considered statistically significant.
Results
Characteristics of GM patients
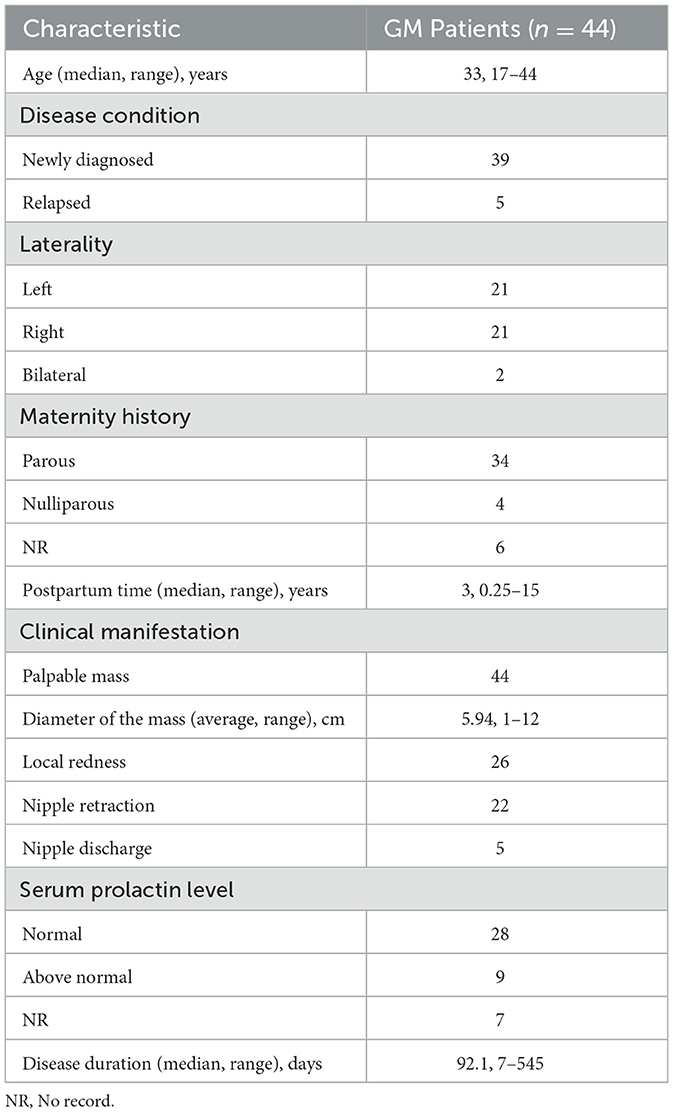
Table 1 summarizes the clinical characteristics of the 44 patients with GM enrolled in this study. Among them, one patient had accessory axillary GM. The median age of GM patients was 33 years (range, 17–44 years), and 39 patients (88.6%) had been recently diagnosed for the first time, while five patients (11.4%) had relapsed. The interval between incipience and recurrence ranged from 3 months to 3 years, and 42 cases (95.5%) were unilateral and two (4.5%) were bilateral. According to the recorded data, four patients (10.5%) were nulliparous, while 34 patients (89.5%) were postpartum, and the median postpartum time was 3 years. The most common clinical manifestation was a palpable breast mass with an average diameter of 5.94 cm. A total of 26 patients (59.1%) had local redness, 22 patients (50%) were found to have nipple retraction, and five patients (11.4%) had nipple discharge. Serum prolactin levels were available in 37 patients, including nine patients (24.3%) with levels above the normal range. The disease duration ranged from 7 days to 1.5 years.
Bacterial profiles in samples (genus level)
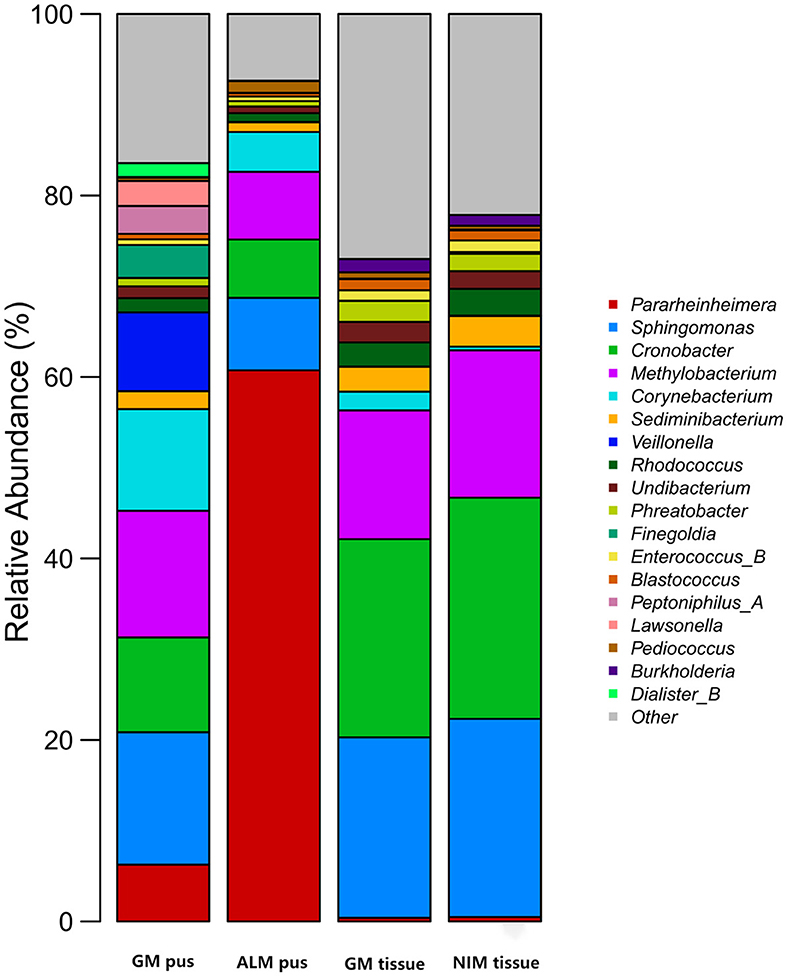
Figure 3 shows the top 10 genera in the four groups, respectively. A total of 18 genera were involved in the four groups. Figure 4 is the collinearity relationship plot, which reflects the proportion of the dominant genera composition for each group and the distribution proportion of each dominant genus between the different groups.

Figure 4. Collinearity relationship plot. The right semicircle indicates the genera abundance composition of the groups, while the left semicircle shows the proportion of genera in different groups. The number at the edge of the circle represents the genera abundance of the right semicircle and the proportion of genera on the left semicircle.
Contributing bacteria
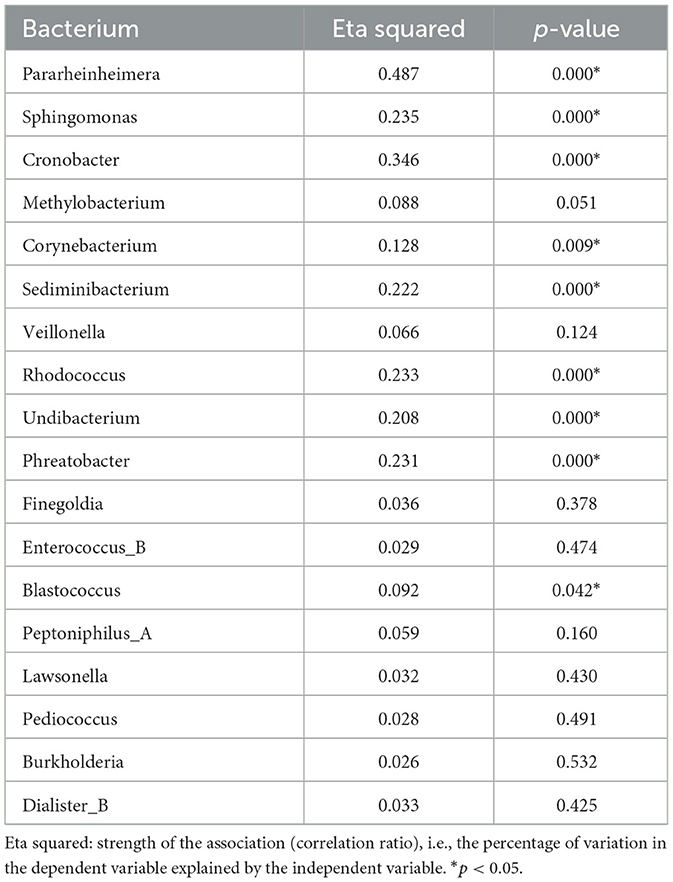
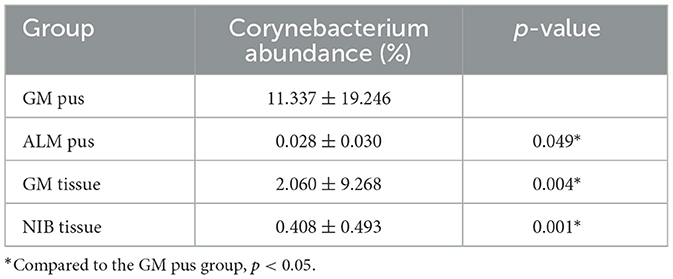
Table 2 shows the results of contributing bacteria in the four groups. All 18 genera we just mentioned were compared in these four groups, and nine genera had statistically significant differences. We further analyzed the nine genera with differences between groups, mainly focusing on the differences between the GM pus/tissue group and the other two groups. Eventually, we found that the genus Corynebacterium was the only bacterial genus with a significant difference between the GM pus group and the other three groups (Table 3). There was no significant difference between the GM tissue group and the two control groups (ALM pus group and NIB tissue group) (Table 4).
Corynebacterium in samples
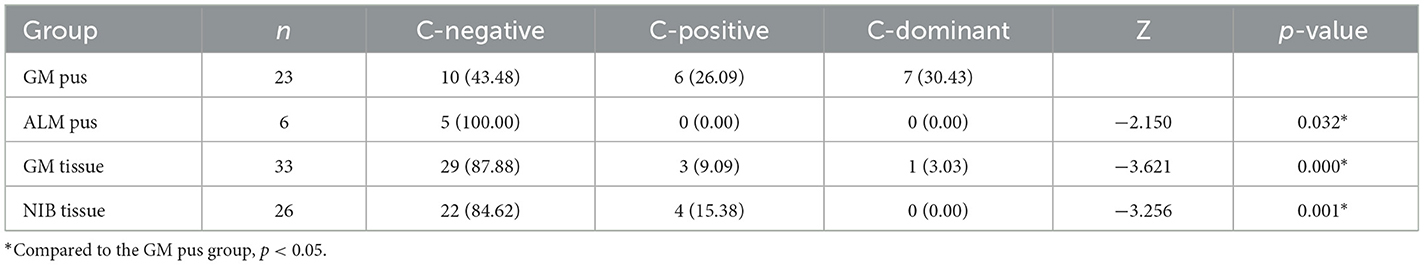
Since Corynebacterium was found to be the only contributing bacteria in the GM group, we further analyzed the abundance of Corynebacterium in all samples. We assigned three degrees according to the Corynebacterium abundance, where an abundance of 1–10% (including 1%) was “Corynebacterium-positive” (C-positive), an abundance of ≥10% was “Corynebacterium-dominant” (C-dominant), and an abundance of <1% was “Corynebacterium-negative” (C-negative), respectively. As Table 5 shows, six samples (26.09%) were C-positive in the GM pus group and seven samples (30.43%) were C-dominant. In total, Corynebacterium abundance ranged from 1.08 to 80.08%. In the ALM pus group, five samples were C-negative and another sample was excluded because the patient developed GM later (this will be discussed as case 1 in section 3.8). In the GM tissue group, three samples (9.09%) were C-positive and one sample (3.03%) was C-dominant, and the total Corynebacterium abundance ranged from 1.11 to 53.57% (the mentioned C-dominant sample will be discussed as case 2 in section 3.8.). In the NIB tissue group, four samples (15.38%) were C-positive, and the total Corynebacterium abundance ranged from 1.11 to 2.04%. As Table 5 shows, the Corynebacterium abundance degree in the GM pus group differed significantly from that in the other three groups.
Figure 5 shows that, in this study, nine GM patients had pus and tissue samples, and the pus samples of seven (77.78%) of these GM patients were C-positive or C-dominant. In contrast, their tissue samples were C-negative. Overall, 11.1% of patients had C-positive findings in both pus and tissue, while one patient's tissue sample was C-positive (the Corynebacterium abundance was 1.11%) but her pus sample was C-negative (the Corynebacterium abundance was 0.30%). After data tracing of this patient, we found that the tissue sample was collected before the pus sample; successive treatment may explain why the tissue result was C-positive but the pus result was C-negative.
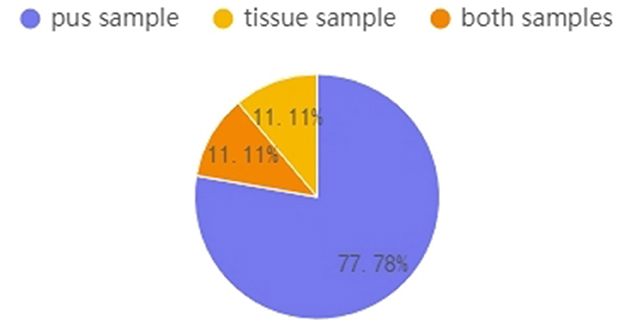
Figure 5. Patients who had multiple samples collected (n = 9). Percentages represent the proportions of C-positive and C-dominant samples from the nine GM patients.
Different Corynebacterium species in samples
A total of three Corynebacterium species were detected in this study, including Ck, Corynebacterium jeddahense, and Corynebacterium pseudogenitalium. Figure 6 shows the predominant species proportion in each group. In the GM pus group, Ck was predominant in 12 of the 13 C-positive samples (92.31%), while C. pseudogenitalium and C. jeddahense were more common in NIB tissue samples. Interestingly, one GM pus sample, in which the abundance of Corynebacterium was 80.08%—the highest—had a predominance of C. pseudogenitalium.
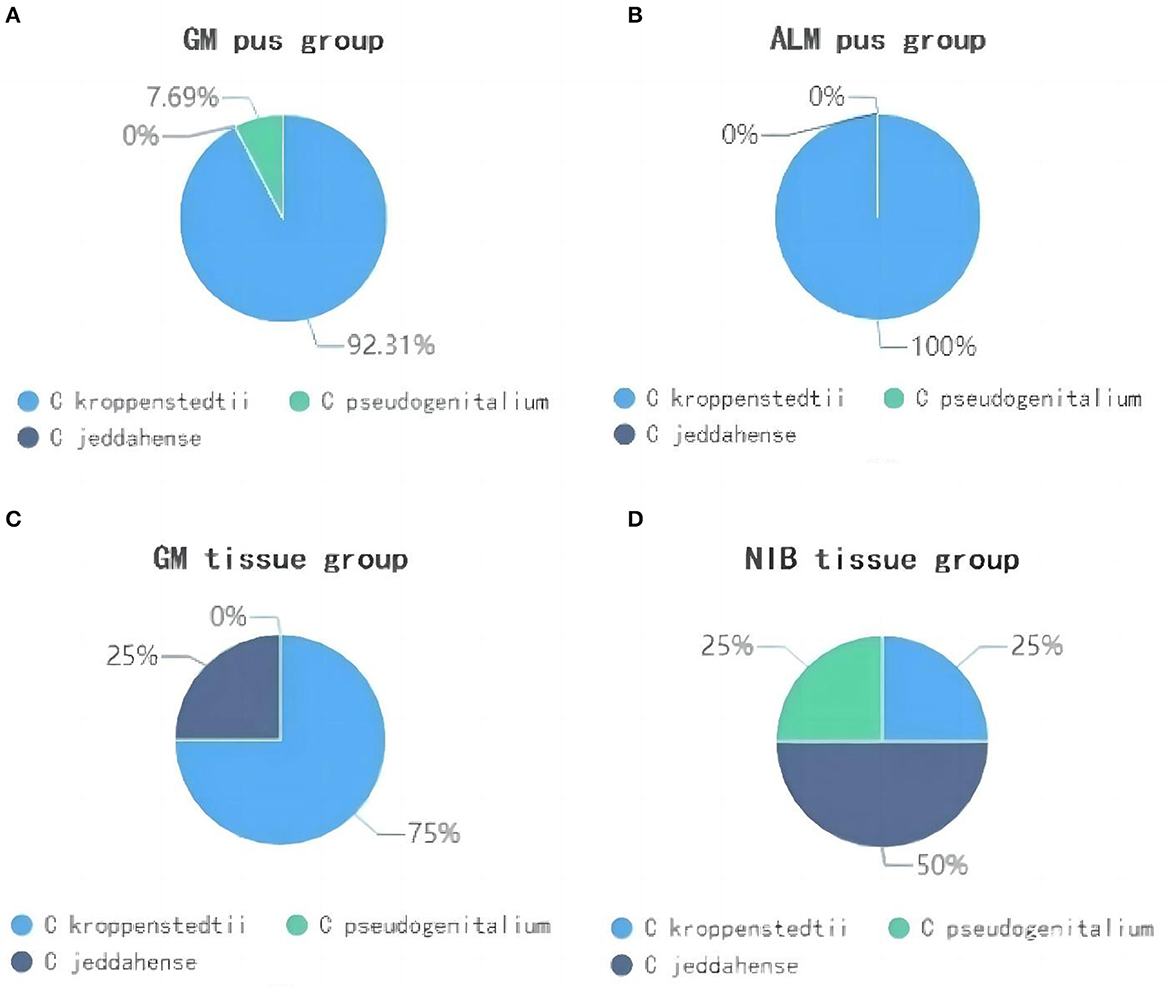
Figure 6. The proportion of Corynebacterium species in four groups. (A) shows the GM pus group; (B) shows the ALM pus group; (C) shows the GM tissue group; (D) shows the NIB tissue group.
Clinical characteristics associated with Corynebacterium infection
To explore the relationship between Corynebacterium infection and clinical characteristics, we divided the patients into two groups, Corynebacterium-positive (C-positive, where the abundance was ≥1%) and Corynebacterium-negative (C-negative, where the abundance was <1%). Under this delineation, 15 patients were C-positive and 29 patients were C-negative. We compared their clinical characteristics and found breast abscess was the only one that showed a significant difference between the two groups (p < 0.05) (Table 6).
Examples of typical cases
Case 1 was a patient in the ALM pus group. She visited our department 2 weeks after giving birth to her second child. Painful redness and a swollen lump had been present in her right breast for a week (Figure 7), and the diagnosis was ALM. Since a breast abscess had formed, we aspirated the pus by syringe to reduce the abscess tension. The pus sample was tested, and the result was C-dominance. Her symptoms improved after treatment, and the lump in her right breast eventually disappeared. However, 3 years later, a new mass had developed in the left breast, and a core needle biopsy confirmed it was GM.
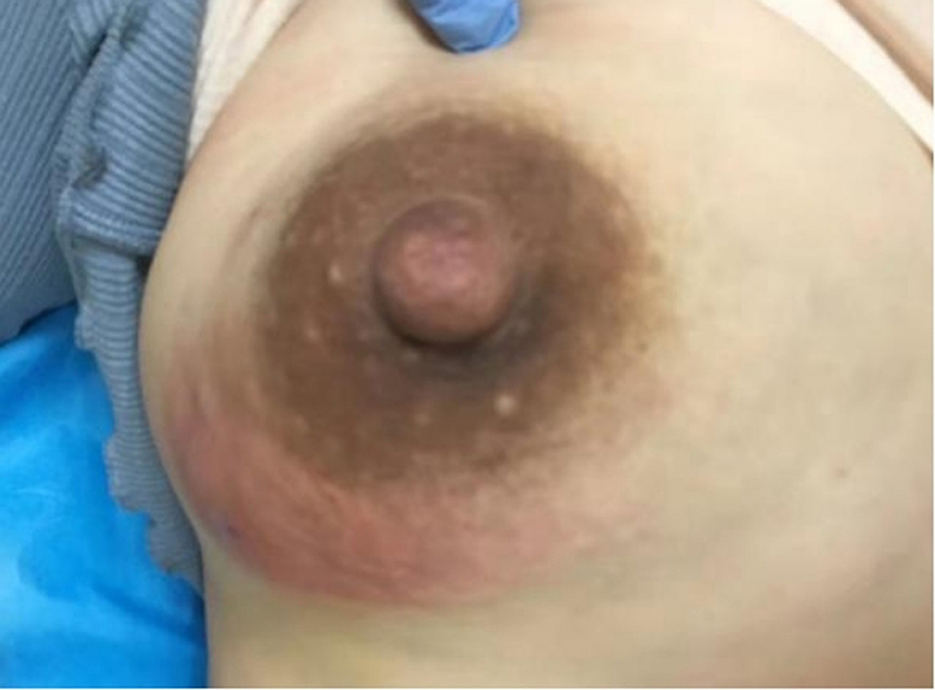
Figure 7. Case 1. A lesion of an ALM pus group patient is seen. The lower quadrant of the right breast was red and swollen.
Case 2 was a C-dominant patient in the GM tissue group. She visited our department in the acute stage with a 1-week disease history, complaining of mild red swelling and a mass in her left breast. We performed a core needle biopsy to diagnose, and the tissue sample showed C-dominance with an abundance of 53.57%. Since no anti-infection treatment was administered, after 20 days, GM developed, and an abscess formed.
Case 3 had a 1-month history of a painful right breast lump before her initial visit to our department (Figure 8). A physical examination indicated bilateral nipple reversal, left nipple discharge, and red swelling around the areola. The diameter of the lump was ~7 cm, and an abscess had formed. The pus was aspirated, and an anti-infection treatment was introduced to relieve her symptoms. After 2 weeks, her pain had decreased, and the mass was smaller in size than before. We repeated aspiration in the residue abscess and compared the pus samples obtained these two times. A considerable difference was found as follows: in the first pus sample, the abundance of Corynebacterium was 32.74%, while, in the second one, it was only 0.91%.
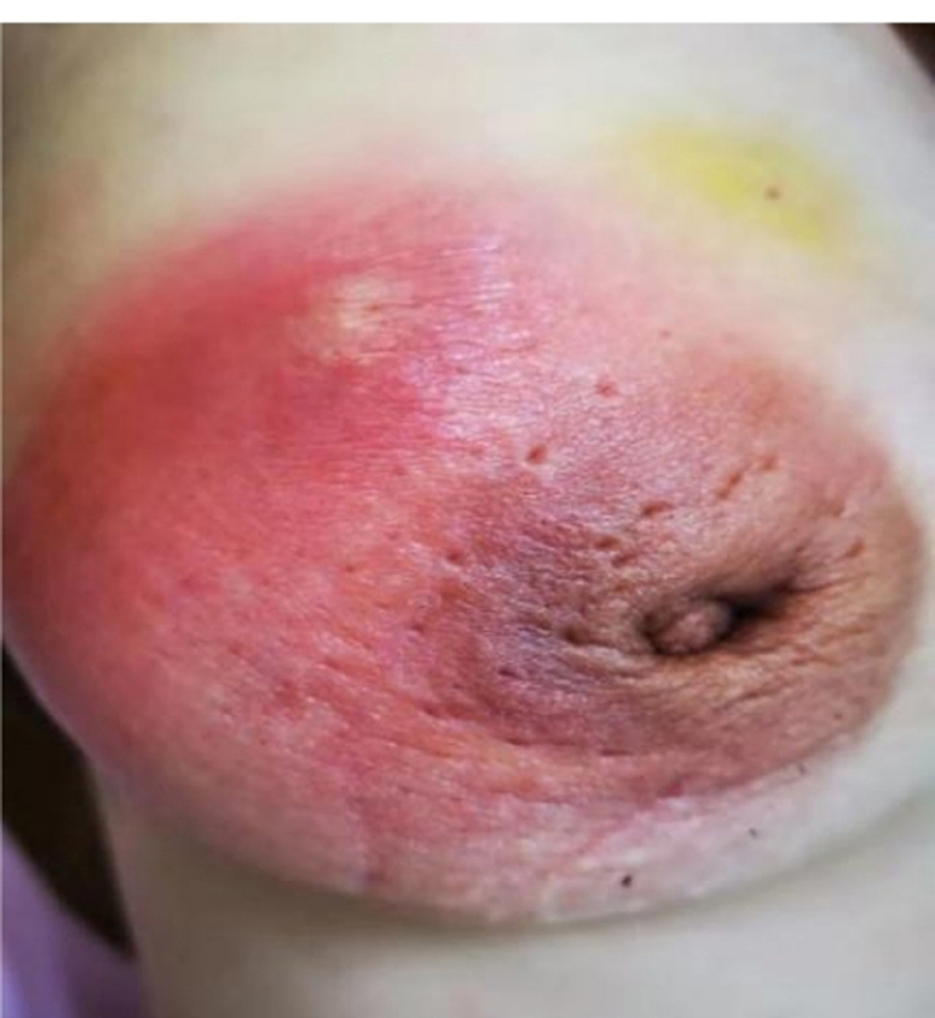
Figure 8. Case 2. A lesion of a GM pus group patient photographed during her initial visit is seen. The diameter of the lump was ~7 cm, and red swelling was present around the areola, with abscess formation.
Discussion
The theory that the “normal breast is a non-sterile environment” has already become a consensus (Saraiya and Corpuz, 2019), and diverse bacteria keep their balance in the breast microenvironment. In recent years, infectious factors have gained increasing attention in the pathogenesis of GM. Though there were some studies that focused on the relationship between infection and GM, especially Corynebacterium species (Dobinson et al., 2015; Wolfrum et al., 2018; Tan et al., 2020), whether this bacterium plays an essential role in the onset of GM and the relationship between bacterial infection and clinical manifestation is still not clear. Our study divided GM samples into two groups (pus and tissue groups) according to the sample type and set two control groups (an ALM pus group and a NIB tissue group) to compare the microbiota between the different groups. To the best of our knowledge, this is the first study to compare GM samples with both ALM pus samples and NIB tissue samples, rather than samples of the normal breast or another type of mastitis only, in order to identify the pathogen in GM. We also clarified the difference in pathogen abundance in GM tissue and pus, and the results offer great value for samples chosen in the microbe study of GM. The relationship between bacterial infection, clinical manifestation, and the prognosis was explored too.
Granulomatous mastitis (GM) usually occurs in women of reproductive age, ~2–6 years after delivery (Angelopoulou et al., 2018; Azizi et al., 2020; Wu and Turashvili, 2020). In this study, the average age of patients was 33 years, and the median postpartum time was 3 years. In total, 89.5% of patients were within 5 years of childbearing, and 24.3% had elevated serum prolactin levels. All these data show that GM is correlated with excessive duct secretion. Milk stasis can exist for several years after weaning, and elevated prolactin levels can stimulate ducts to oversecrete (Nikolaev et al., 2016; Bi et al., 2021; Yin et al., 2021). This can induce mammary duct dilation and cause chronic inflammation and local immune response. Moreover, the residual milk in the mammary duct provides an excellent growth environment for the lipophilic Corynebacterium organism.
In this study, we compared different genera in four groups and identified nine genera, such as Pararheinheimera, Sphingomonas, Cronobacter, Corynebacterium, Sediminibacterium, Rhodococcus, Undibacterium, Phreatobacter, and Blastococcus. We further performed pairwise comparisons between the four groups and found that Corynebacterium was the only bacterium distinguishable in the GM group; notably, its abundance in GM patients was revealed to be relatively higher by comparing with ALM and NIB patients.
Corynebacterium is also detectable in normal breast tissues. Due to this, whether Corynebacterium is a colonizer, contaminant, or pathogen has been debated for years (Wolfrum et al., 2018; Wong et al., 2018). In normal breast tissue, however, the Corynebacterium abundance is generally <0.5% (Li et al., 2020). Based on this premise, in this study, we chose an abundance of Corynebacterium of ≥1% to indicate its pathogenic nature. We divided the samples into three groups (C-negative, C-positive, and C-dominant) and used 1% and 10% as the cutoff points to delineate them. A total of 15 of 44 GM patients (34.1%) were C-positive or C-dominant, with the abundance ranging from 1.08 to 80.08%, which is significantly higher than that in the ALM and NIB groups.
At the species level, we found three Corynebacterium species, such as Ck, C. jeddahense, and C. pseudogenitalium. Among these, Ck was the predominant species in 88.2% of C-positive and C-dominant GM samples. Other studies have also identified Ck as the dominant species in samples. Yu et al. conducted a metagenomic analysis of 19 GM patients and found that Ck was predominant in 11 patients (Yu et al., 2016), while Bi et al. performed next-generation sequencing on 25 GM patients' samples and detected Ck in 40% (Bi et al., 2021).
Though the Corynebacterium abundance in the GM group was higher than that in the ALM pus and NIB tissue groups, not all GM samples were C-positive. To explore the clinical differences between samples, we also divided the GM patients into two groups, C-positive and C-negative, and compared their clinical characteristics. Laterality, local redness, nipple retraction, maternity history, and recurrence were compared, and we found that breast abscess displayed the only significant difference between the two groups. C-positive patients, especially C-dominant patients, are more likely to form abscesses, which has been proven by other research (Saraiya and Corpuz, 2019). GM recurrence related to Corynebacterium colonization has been reported in other studies (Le Flèche-Matéos et al., 2012; Tan et al., 2019, 2020). However, this study did not find a significant difference in recurrence between the two groups, maybe because the number of relapsed patients was too small.
Since Corynebacterium-related GM is more likely to result in abscess formation, it is easy to understand why this bacterium is always more abundant in pus samples than in tissue samples. Some studies tested pus samples together with tissue samples (Johnstone et al., 2017) or tested the tissue samples only (Fujii et al., 2018; Tariq et al., 2022) and rarely compared the difference between the two sample types. In this study, 56.5% of GM pus samples were C-positive, while only 8.3% of GM tissue samples were C-positive. To confirm this opinion that pus samples always have a greater Corynebacterium abundance than tissue samples, we analyzed nine patients with both pus and tissue samples available and found that eight patients' pus samples were C-positive, while their tissue samples generally had a lower Corynebacterium abundance. This result validated the view that Corynebacterium is prone to driving abscess formation, thus, it is more easily found in pus than in tissue.
The presence of Corynebacterium in pus can also be a predicting signal of GM onset. Case 1 in this study was a mother who, 2 weeks after delivery of her second child, presented with acute lactation mastitis with abscess formation in the right breast. Unlike the other lactating women whose pus samples were Staphylococcus-dominant, this patient's pus sample was C-dominant, and her left breast was diagnosed with GM later.
Since Corynebacterium is a pathogen of GM, controlling this pathogen is necessary for treatment success. If we can administer anti-infection therapy promptly, the therapeutic effect should be good and vice versa. Cases 2 and 3 are two opposing examples of GM treatment success. Case 2 had a 1-week history of a breast lump with red swelling and pain at the first visit to our department. The Corynebacterium abundance in her tissue sample was 53.57%, but no anti-infection treatment had been given, and a breast abscess formed 20 days later. Case 3 presented with a breast abscess and severe clinical manifestation on her first visit. Her first pus sample was C-dominant (32.47%). After 2 weeks of anti-infection treatment, her symptoms were much relieved, and the Corynebacterium abundance in the second sample was reduced to <1%. These two cases validate the idea that timely anti-infection treatment is effective and necessary for inhibiting Corynebacterium (Wolfrum et al., 2018; Yaghan et al., 2020). As Corynebacterium abundance decreases, the symptoms will also resolve (Johnstone et al., 2017; Zeng et al., 2022).
Our study has several limitations. First, as a retrospective study, the absence of some clinical data impacted the analysis, and bias was difficult to avoid. Second, the sample size was relatively small, especially that of the ALM pus control group. Third, due to the limited number of recurrences, the relationship between recurrence and Corynebacterium infection was not well defined. This will be further assessed in a future study.
Conclusion
This study supports the correlation of Corynebacterium species with GM pathogenesis and identified Ck as the main species in GM onset. We found that Corynebacterium abundance in the GM group was obviously greater than that in other groups, and the abundance of Corynebacterium in GM pus samples was more than that in GM tissue samples. We also found that abscess formation is closely related to Corynebacterium infection, and early detection of Corynebacterium in a tissue sample may be a sign of a higher risk of abscess formation. Corynebacterium infection can also be a predictor of GM, especially in patients with high prolactin levels or a history of recent lactation. Timely application of anti-infection therapy is necessary for disease remission.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number PRJNA947767.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DZ and QH contributed to the conception and design of the study. WC, YZ, JY, JW, and SL collected and analyzed the data from previous studies. WC wrote the first draft of the manuscript. DZ, JC, and KM revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the Beijing Ten Million Talent Plan Project (No. 2019A31), Research and Transformation of Clinical Diagnosis and Treatment Technology in Beijing (Z211100002921020), Gansu Provincial Science and Technology Program Subsidized Projects (21JRIRG303), and Young Doctor Scholar Project (2022).
Acknowledgments
We thank all authors who contributed valuable methods and data and made them public.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. doi: 10.1093/nar/25.17.3389
Angelopoulou, A., Field, D., Ryan, C. A., Stanton, C., Hill, C., Ross, R. P., et al. (2018). The microbiology and treatment of human mastitis. Med Microbiol Immunol. 207, 83–94. doi: 10.1007/s00430-017-0532-z
Azizi, A., Prasath, V., Canner, J., Gharib, M., Sadat Fattahi, A., Naser Forghani, M., et al. (2020). Idiopathic granulomatous mastitis: management and predictors of recurrence in 474 patients. Breast J. 26, 1358–1362. doi: 10.1111/tbj.13822
Bacon, D. R., Ngeve, S. M., and Jordan, S. G. (2021). Granulomatous mastitis: an underdiagnosed inflammatory disease afflicting minority women. Radiol. Case R. 16, 3990–3994. doi: 10.1016/j.radcr.2021.09.044
Bi, J., Li, Z., Lin, X., Li, F., Xu, H., Yu, X., et al. (2021). Etiology of granulomatous lobular mastitis based on metagenomic next-generation sequencing. Int. J. Infect. Dis. 113, 243–250. doi: 10.1016/j.ijid.2021.10.019
Chen, X., Zhang, W., Yuan, Q., Hu, X., Xia, T., Cao, T., et al. (2021). A novel therapy for granulomatous lobular mastitis: local heat therapy. Exp. Ther. Med. 22, 1156. doi: 10.3892/etm.2021.10590
Dobinson, H. C., Anderson, T. P., Chambers, S. T., Doogue, M. P., Seaward, L., Werno, A., et al. (2015). Antimicrobial treatment options for granulomatous mastitis caused by corynebacterium species. J Clin Microbiol. 53, 2895–2899. doi: 10.1128/JCM.00760-15
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Fujii, M., Mizutani, Y., Sakuma, T., Tagami, K., Okamoto, K., Kuno, Y., et al. (2018). Corynebacterium kroppenstedtii in granulomatous mastitis: analysis of formalin-fixed, paraffin-embedded biopsy specimens by immunostaining using low-specificity bacterial antisera and real-time polymerase chain reaction. Pathol. Int. 68, 409–418. doi: 10.1111/pin.12683
Johnstone, K. J., Robson, J., Cherian, S. G., Cheong, J. W. S., Kerr, K., Bligh, J. F., et al. (2017). Cystic neutrophilic granulomatous mastitis associated with corynebacterium including Corynebacterium kroppenstedtii. Pathol. 49, 405–412. doi: 10.1016/j.pathol.2017.01.006
Koksal, H., Vatansev, H., Artac, H., and Kadoglou, N. (2020). The clinical value of interleukins-8,−10, and−17 in idiopathic granulomatous mastitis. Clin. Rheumatol. 39, 1671–1677. doi: 10.1007/s10067-020-04925-8
Le Flèche-Matéos, A., Nicolas, B., Fabienne, L., Yolande, A. Le Guern Anne-Sophie, India, L., Maria, B. A., and Jean-Claude, M. (2012). Recurrent breast abscesses due to Corynebacterium kroppenstedtii, a human pathogen uncommon in Caucasian women. Case Rep. Infect. Dis. 2012, 120968. doi: 10.1155/2012/120968
Li, X. Q., Wu, H. L., Yuan, J. P., Liu, T. G., Sun, S. R., Chen, C., et al. (2020). Bacteria associated with granulomatous lobular mastitis and the potential for personalized therapy. J. Invest. Surg. 35, 164–170. doi: 10.1080/08941939.2020.1833262
Marcel, M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 2226–6089. doi: 10.14806/ej.17.1.200
Nikolaev, A., Blake, C. N., and Carlson, D. L. (2016). Association between Hyperprolactinemia and granulomatous mastitis. Breast J. 22, 224–231. doi: 10.1111/tbj.12552
Saraiya, N., and Corpuz, M. (2019). Corynebacterium kroppenstedtii: a challenging culprit in breast abscesses and granulomatous mastitis. Curr. Opin. Gynecol. Obstet. 31, 325–332. doi: 10.1097/GCO.0000000000000541
Saydam, M., Yilmaz, K. B., Sahin, M., Yanik, H., Akinci, M., Yilmaz, I., et al. (2020). New findings on autoimmune etiology of idiopathic granulomatous mastitis: serum IL-17, IL-22 and IL-23 levels of patients. J. Invest. Surg. 34, 993–997. doi: 10.1080/08941939.2020.1725190
Schmieder, R., and Edwards, R. (2011). Quality control and preprocessing of metagenomic datasets. Bioinform. 27, 863–864. doi: 10.1093/bioinformatics/btr026
Tan, C., Lu, F. I., Aftanas, P., Tsang, K., Mubareka, S., Chan, A., et al. (2020). Whole genome sequence of Corynebacterium kroppenstedtii isolated from a case of recurrent granulomatous mastitis. IDCases. 23, e01034. doi: 10.1016/j.idcr.2020.e01034
Tan, Q. T., Tay, S. P., Gudi, M. A., Nadkarni, N. V., Lim, S. H., Chuwa, E. W. L., et al. (2019). Granulomatous mastitis and factors associated with recurrence: an 11-year single-centre study of 113 patients in Singapore. World J. Surg. 43, 1737–1745. doi: 10.1007/s00268-019-05014-x
Tariq, H., Menon, P. D., Fan, H., Vadlamudi, K. V., Pandeswara, S. L., Nazarullah, A. N., et al. (2022). Detection of Corynebacterium kroppenstedtii in granulomatous lobular mastitis using real-time polymerase chain reaction and sanger sequencing on formalin-fixed, paraffin-embedded tissues. Arch Pathol Lab Med. 146, 749–754. doi: 10.5858/arpa.2021-0061-OA
Tauch, A., Fernández-Natal, I., and Soriano, F. (2016). A microbiological and clinical review on Corynebacterium kroppenstedtii. Int. J. Infect. Dis. 48, 33–39. doi: 10.1016/j.ijid.2016.04.023
Williams, M. S., McClintock, A. H., Bourassa, L., and Laya, M. B. (2021). Treatment of granulomatous mastitis: is there a role for antibiotics? Eur. J. Breast Health 17, 239–246. doi: 10.4274/ejbh.galenos.2021.2021-3-1
Wolfrum, A., Kümmel, S., Theuerkauf, I., Pelz, E., and Reinisch, M. (2018). Granulomatous mastitis: a therapeutic and diagnostic challenge. Breast Care. 13, 413–418. doi: 10.1159/000495146
Wong, S. C. Y., Poon, R. W. S., Foo, C. H., Ngan, A. H. Y., Tse, H., Lam, V. C. M., et al. (2018). Novel selective medium for the isolation of corynebacterium kroppenstedtii from heavily colonised clinical specimens. J. Clin. Pathol. 71, 781–786. doi: 10.1136/jclinpath-2017-204834
Wu, J. M., and Turashvili, G. (2020). Cystic neutrophilic granulomatous mastitis: an update. J. Clin. Pathol. 73, 445–453. doi: 10.1136/jclinpath-2019-206180
Yaghan, R. J., Ayoub, N. M., Hamouri, S., Al-Mohtaseb, A., Gharaibeh, M., Yaghan, L., et al. (2020). The role of establishing a multidisciplinary team for idiopathic granulomatous mastitis in improving patient outcomes and spreading awareness about recent disease trends. Int. J. Breast Cancer. 2020, 5243958. doi: 10.1155/2020/5243958
Yin, Y., Liu, X., Meng, Q., Han, X., Zhang, H., Lv, Y., et al. (2021). Idiopathic granulomatous mastitis: etiology, clinical manifestation, diagnosis and treatment. J. Invest. Surg. 35, 709–720. doi: 10.1080/08941939.2021.1894516
Yu, H., Deng, H., Ma, J., Huang, S., Yang, J., Huang, Y., et al. (2016). Clinical metagenomic analysis of bacterial communities in breast abscesses of granulomatous mastitis. Int. J. Infect. Dis. 53, 30–33. doi: 10.1016/j.ijid.2016.10.015
Keywords: granulomatous mastitis, clinical characteristic, microbiota analysis, corynebacterium, corynebacterium kroppenstedtii
Citation: Chen W, Zhang D, Zeng Y, Cui J, Yu J, Wang J, Li S, Huang Q and Mansoor KM (2023) Clinical characteristics and microbiota analysis of 44 patients with granulomatous mastitis. Front. Microbiol. 14:1175206. doi: 10.3389/fmicb.2023.1175206
Received: 27 February 2023; Accepted: 23 March 2023;
Published: 17 April 2023.
Edited by:
Michal Letek, Universidad de León, SpainReviewed by:
Syed Hoda, Weill Cornell Medicine, Cornell University, United StatesMuhammad Ahmad, Weill Cornell Medical Center, NewYork-Presbyterian New York City, United States, in collaboration with reviewer SH
Efe Sezgin, Izmir Institute of Technology, Türkiye
Copyright © 2023 Chen, Zhang, Zeng, Cui, Yu, Wang, Li, Huang and Mansoor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongxiao Zhang, morningdong@163.com
 Wen Chen
Wen Chen Dongxiao Zhang
Dongxiao Zhang Yifei Zeng1,2
Yifei Zeng1,2