- 1Department of Soil and Water Sciences, University of Florida, Gainesville, FL, United States
- 2Southwest Florida Research and Education Center, University of Florida, Immokalee, FL, United States
Biocrusts are communities of microorganisms within the top centimeter of soil, often dominated by phototrophic dinitrogen-fixing (N2-fixing) organisms. They are common globally in arid ecosystems and have recently been identified in agroecosystems. However, unlike natural ecosystem biocrusts, agroecosystem biocrusts receive regular fertilizer and irrigation inputs. These inputs could influence seasonal biocrust N2-fixation and their relationship with soil nutrients in perennial agroecosystems, which is of particular interest given crop management requirements. In this study, biocrust and adjacent bare soil N2-fixation activity was measured in the field during the summer, fall, spring, and winter seasons in a Florida citrus orchard and vineyard using both acetylene reduction assays and 15N2 incubations. Samples were analyzed for microbial and extractable carbon (MBC, EC), nitrogen (MBN, EN), and phosphorus (MBP, EP). In both agroecosystems, biocrusts had greater microbial biomass and extractable nutrients compared to bare soil. The citrus and grape biocrusts were both actively fixing N2, despite crop fertilization, with rates similar to those found in natural arid and mesic systems, from 0.1 to 142 nmol of C2H4 g–1 of biocrust dry weight h–1 (equivalent to 1–401 μmol m–2h–1). Lower soil temperatures and higher EC:EN ratios were associated with higher N2-fixation rates in citrus biocrusts, while higher soil moisture and higher EP were associated with higher N2-fixation rates in grape biocrusts. The N2-fixation activity of these agroecosystem biocrusts indicates the possibility of biocrusts to enhance N cycling in perennial agroecosystems, with potential benefits for crop production.
Introduction
Biological soil crusts (biocrusts) are communities of organisms on soil surfaces and often include diazotrophic organisms such as lichens (Kuske et al., 2012), cyanobacteria (Yeager et al., 2007), and heterotrophic bacteria (da Rocha et al., 2015), in addition to other non-diazotrophs such as archaea (Zhao et al., 2020), green algae, fungi (Bates et al., 2010), and bryophytes (Seitz et al., 2017). Biocrusts occur on all continents (Colesie et al., 2016), and are adapted to higher light exposure (Garcia-Pichel and Castenholz, 1991), lower water availability (Garcia-Pichel and Pringault, 2001), and cycles of water saturation and drying typical in deserts (Rajeev et al., 2013). However, biocrusts are not restricted to arid conditions and have also been identified in mesic ecosystems (Veluci et al., 2006; Seitz et al., 2017), where they experience temporary dry conditions, similar to their arid counterparts, which require adaptations to desiccation and high light exposure (Colesie et al., 2016). Recently, biocrusts have also been identified in managed mesic ecosystems, specifically perennial Florida agroecosystems (Nevins et al., 2020, 2022) where they occur in unshaded areas between trees (e.g., citrus orchards) and grape vines. However, unlike the natural ecosystems where biocrusts have been well studied, considerably less is known about the processes and functions of biocrusts in agroecosystems.
Biological nitrogen fixation (N2-fixation) is one of the key biocrust processes, and it is estimated that biocrusts contribute approximately half of the total N fixed in arid lands (Elbert et al., 2012), with rates between 0.08 and 10 kg N ha–1 year–1 (Malam Issa et al., 2001; Belnap, 2002; Billings et al., 2003; Russow et al., 2005; Housman et al., 2006; Holst et al., 2009). Similarly, mesic biocrusts fix 1.3 kg N ha–1 year–1 in a temperate savannah (Veluci et al., 2006), 5.2 kg N ha–1 year–1 in a seasonally flooded savannah (Williams et al., 2018), and 4 kg N ha–1 year–1 in the seasonally flooded Everglades (Liao and Inglett, 2014).
In agroecosystem biocrusts, the possibility of naturally occurring N2-fixation is of particular interest due to the fertilizer requirements for perennial crops. However, agroecosystem biocrust N2-fixation rates may differ from those of natural ecosystems due to the influence of N and phosphorus (P) fertilization applications. In particular, fertilizer N could inhibit biocrust N2-fixation, as shown both in field-collected biocrusts and multi-species laboratory cultures. For example, N additions mimicking atmospheric deposition significantly reduced N2-fixation rates in arid light and dark biocrusts (Belnap et al., 2008). In addition, 25 days of exposure to 55 lbs N acre–1 reduced N2-fixation of agroecosystem biocrust cell cultures by 80% (Peng and Bruns, 2019). Based on this N application rate with the assumption that 25 days of constant fertilizer exposure are equivalent to a yearly fertilization rate, it is common to split liquid fertilization in citrus into 26 biweekly doses. Such a fertilization rate would be within the lower range for a 1 to 3-year-old citrus in the field: 25–200 lbs acre–1 (Obreza and Morgan, 2020). Therefore, it is expected that agroecosystem biocrusts will have drastically reduced N2-fixation rates compared to their natural ecosystem counterparts.
In contrast to N, however, P addition could stimulate N2-fixation. Phosphorus addition to a P-limited environment enhanced N2-fixation activity and labile P concentration in non-biocrust soils of prairie and tropical rainforest (Reed et al., 2007a,b). Furthermore, the balance of P and N availability dictated N2-fixation rates of wetland biocrusts (Liao and Inglett, 2014). Biocrust N2-fixation in a P amended agroecosystem could therefore be higher than in natural ecosystem biocrusts.
While N2-fixation positively responded to increases in moisture in arid ecosystems (Zhao et al., 2010; Caputa et al., 2013) and seasonally flooded biocrusts of restored wetlands (Liao and Inglett, 2012, 2014), agroecosystem biocrust N2-fixation rates are not expected to vary as strongly seasons because of consistent moisture provided by irrigation. While higher temperature ranges are associated with higher N2-fixation rates in natural ecosystem biocrusts from regions with temperatures ranging from below 0 to 30°C (Zhao et al., 2010; Caputa et al., 2013), higher N2-fixation rates are not expected in agroecosystem biocrusts during the warmer seasons of fall (17–28°C), spring (16–26°C), and summer (25–29°C) than in the cooler winter (10–22°C) due to their narrow temperature range.1
Biocrust N2-fixation activities have not been examined in perennial agroecosystems, and the influence of consistent N and P fertilization and irrigation on agroecosystem biocrust N2-fixation activities is unknown. Therefore, we conducted a year-long field study of agroecosystem biocrusts and bare soil controls to quantify seasonal N2-fixation rates, compare biocrust and bare soil nutrient concentrations, and identify the relationships between biocrust N2-fixation activity, soil nutrients, and environmental variables. We chose two agroecosystems with biocrusts (a vineyard and a citrus orchard) that had similar climatic conditions but differed in fertilization and irrigation management. We hypothesized that due to limited seasonal temperature change and consistent water input through irrigation, N2-fixation rates of these agroecosystem biocrusts would not have a seasonal pattern. However, based on differences in crop management, we hypothesized that the availability of N and P fertilizer would regulate biocrust N2-fixation patterns and relationships with biocrust nutrient concentrations more strongly than soil temperature and soil moisture.
Materials and methods
Site and plot selection
This study was conducted in a subtropical climate receiving 813–929 mm total precipitation during the study period from August 2019 to May 2020 with mean air temperatures ranging from 21°C to 22°C.1 Agroecosystem biocrusts were assessed in two perennial crops: 2-month-old Vitis rotundifolia (muscadine grape) located at the University of Florida Plant Science Research and Education Unit in Citra, Florida (referred to as ‘Grape’, 29.407195, –82.139980) and a 2-year-old Citrus sinensis (orange) orchard located at the University of Florida Citrus Research and Education Center in Lake Alfred, Florida (referred to as ‘Citrus’, 28.115496, –81.713458). Soils of both sites were classified as excessively drained Entisols of the Candler series, sandy soil formed from eolian and loamy marine deposits with 6.0–6.5 pH as measured by Jalpa et al. (2020) and Nevins et al. (2020). Citrus was irrigated daily through micro-sprinklers, while Grape was irrigated daily through a drip system. Each Grapevine received at least 3.8 L of water per day from May to June, and then this amount was reduced by half during the other months. Each Citrus tree received approximately 34 L of water per day, and this amount was reduced by half during the winter. Grape had 11 N kg ha–1 10–10–10 NPK granulated fertilizer applied in June 2019, July 2019, and March 2020, while Citrus was fertigated (5–0–7 NPK or 7–2–7 NPK) weekly and received 29 N kg ha–1 12–4–8 NPK controlled release fertilizer in July 2019 (Supplementary Table 1).
Samples were collected at Citrus in September 2019 (Summer), November 2019 (Fall), January 2020 (Winter), and May 2020 (Spring); and at Grape in August 2019 (Summer), October 2019 (Fall), January 2020 (Winter), and May 2020 (Spring). Samples were collected from six plots at each location. Each plot was randomly located on either side of the crop trunk or vine and contained an intact biocrust and adjacent bare soil (no more than 10 cm away from the biocrust) located within the crop row (Supplementary Figure 1). There were minimal weeds in each plot due to herbicide control using glyphosate, and plots were located 122 cm away from the trunk or vine.
Biocrusts and bare soils were randomly sampled at each plot and ranged in area from 1045 cm2 up to 6427 cm2. Plots were at least 2 m apart from each other within a site. After each sampling point, the sample collection plot locations were shifted to the nearest intact biocrusts (no more than 2 m away from the original plot location) because not enough material remained for repeated sampling.
Qualitative biocrust characterization
Biocrusts were identified by field observations and referencing the visual development scale (Belnap et al., 2008). The bare soil for each plot had no visible surface roughness or darkening (Belnap et al., 2008). To further characterize biocrusts, two replicates of biocrusts from sampling times during which they exhibited the highest N2-fixation rates from both sites (Grape: August 2019; Citrus: May 2020) were examined for the presence of cyanobacteria and algae using an inverted microscope, Nikon Eclipse Ti2 (Nikon Instrument Inc., Japan).
Field environmental parameters
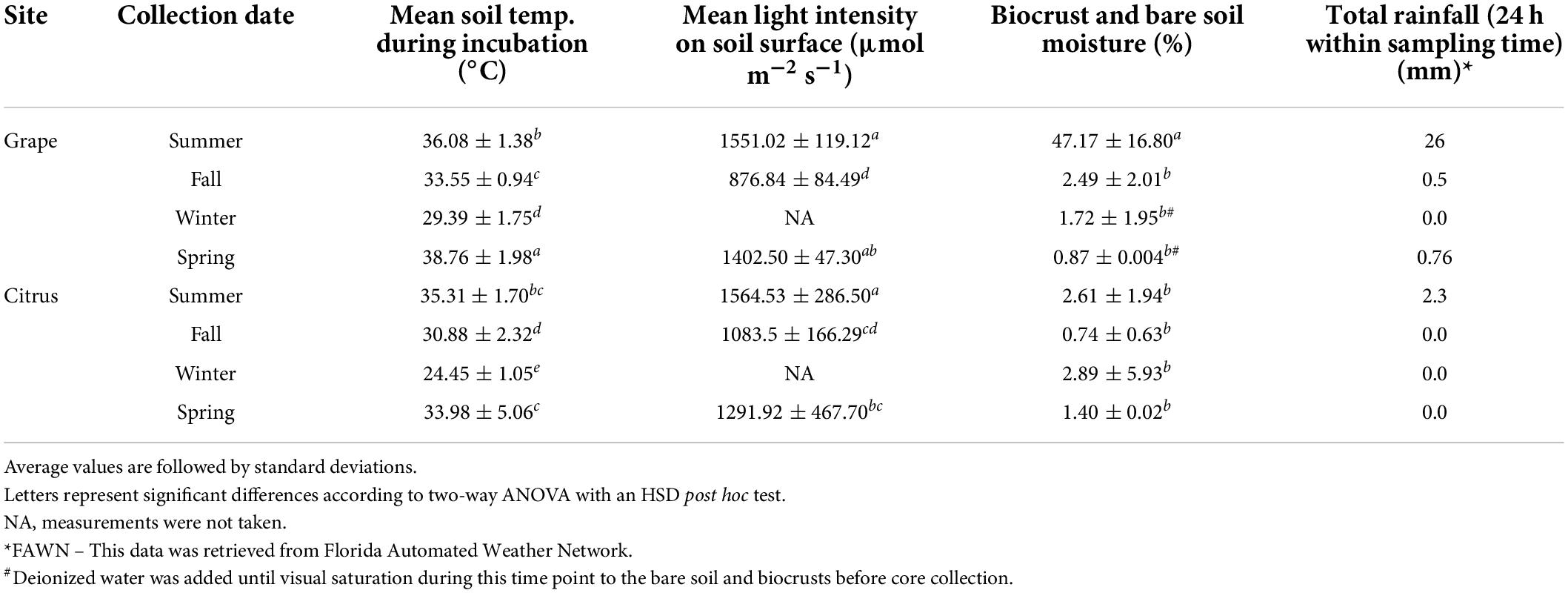
Soil surface temperature and light intensity were measured at each plot (n = 6) when samples were collected. Temperatures were measured at the surface without plant shading using a thermocouple attached to a DIGI-SENSE 20250-02 temperature meter (Cole Parmer, Vernon Hills, IL, United States). Light intensity measurements were recorded using a LI-250A light meter (LI-COR Biosciences, Lincoln, NE United States). Additional data about precipitation and solar irradiance were obtained from the Florida Automated Weather Network1 (Supplementary Table 1).
Sample collection
Three subreplicates each of biocrust and adjacent bare soil were collected intact from each plot to 0.5 cm depth using a 3 cm diameter corer (Supplementary Figure 1). Cores were placed in airtight jars for N2-fixation assays and subsequent measurements of soil moisture, microbial biomass, and extractable nutrients. Additional subreplicates for 15N enrichment incubations and analysis were collected during the summer season in Grape (biocrusts: n = 3, bare soils: n = 3) and during the fall season in Citrus (biocrusts: n = 2). Bare soil samples from Citrus were not collected for 15N2 enrichment incubations.
Both sites were irrigated daily in the morning, including the morning before sample collection. However, at the January and May 2020 sample collections, the Grape soil was very dry, therefore, on these dates, the soil was saturated with deionized water before soil core collection. No deionized water was added during other collection times.
Field N2-fixation rate measurements
Biocrust and bare soil N2-fixation was measured under field conditions immediately after collection using an adapted version of the acetylene reduction assay (ARA) (Stewart and Bergersen, 1980; Inglett, 2013). Measurements were made using a 2-h field incubation in airtight 138 mL glass jars with 10% acetylene headspace. Non-acetylated sample blanks were simultaneously incubated for biocrusts and bare soils. Intact soil and biocrust core samples were placed on the inside lid of inverted glass jars to allow for increased ambient light access to potentially N2-fixing phototrophic organisms (Supplementary Figure 2). The lids had a butyl rubber septum installed into a drilled hole for gas injections. To maintain field soil temperatures and prevent overheating, jars were incubated in a shallow water bath monitored with a thermocouple. After incubation, a 5 mL headspace gas sample was collected into a 3.5 mL exetainer after vigorously shaking each jar for 4 s. The jars were then opened and aerated in the field for at least 10 min before closing and storing them at 4°C for transport to the University of Florida Wetlands Biogeochemistry Laboratory in Gainesville, FL. Gas samples from ARA measurements were stored at 25°C for later ethylene analysis by gas chromatography.
Acetylene reduction assay calibration incubations
To identify the conversion ratio from acetylene reduction to N2 fixed, separate incubations with 15N2 gas were conducted simultaneously with ARA on two biocrust replicates from Citrus in the fall, three biocrust replicates from Grape in the summer, and three bare soil replicates from Grape in the summer season following the method of Inglett (2013). These incubations were identical to those of ARA, but without injection of acetylene. Briefly, 20 mL of 98% 15N2 was injected into the jar headspace of samples and into three empty jars. Gas samples were collected from these jars after a 2-h incubation to determine the headspace 15N2 concentration. The jars of 15N2 enriched samples were then opened and aerated for 10 min before being placed on ice to stop the incubation. These samples were used for N isotopic determination. Acetylated samples from the same plots where 15N2-incubated samples were collected served as unenriched controls. 15N2-enriched biocrust subreplicates from 15N2-enriched jars and non-enriched control biocrust subreplicates from acetylated jars were separated from loose soil particles with a 0.5 mm sieve. The three sieved enriched biocrust, non-enriched biocrusts, enriched bare soil, and unenriched bare soil subreplicates from each plot were pooled, homogenized, and dried at 70°C.
Laboratory analysis
Biocrust samples from acetylated jars were separated from loose soil particles with a 0.5 mm sieve. The three sieved biocrust and bare soil subreplicates from each plot were pooled, homogenized, and then subsampled for microbial biomass, extractable nutrients, and moisture determination (n = 6). The soil moisture content of biocrusts and bare soils was determined gravimetrically after drying in the laboratory oven for 72 h at 70°C to avoid additional mass loss due to organic matter volatilization and to allow for subsequent N analyses (Susha Lekshmi et al., 2014). Biocrust and bare soil moisture measurements from each plot were averaged together for soil moisture comparison between seasons across sites because no significant difference was detected between biocrust and bare soil moisture (n = 12). However, only biocrust soil moistures were averaged together for principal component analysis (PCA).
Microbial biomass C (MBC), N (MBN), and P (MBP) were measured using the fumigation extraction approach (Liao et al., 2014). Briefly, 1 g of sample was incubated for 24 h either in the presence of chloroform (fumigated) or without exposure to chloroform (non-fumigated) at room temperature and then extracted with 0.5 M potassium sulfate (MBC and MBN) or 0.5 M sodium bicarbonate (MBP). The C and N extracts were analyzed using a Shimadzu TOC-5050 (Japan, Tokyo) analyzer with an N module. The P extracts were digested with sulfuric acid and potassium persulfate, resuspended in double deionized water, and analyzed on the Shimadzu UV-160 spectrophotometer (Kyoto, Japan) using the molybdenum blue method (EPA Method 365.3).
Microbial biomass C, MBN, and MBP were determined by calculating the differences between fumigated and non-fumigated sample pairs with 0.37 adjustment factor (extraction efficiency) for MBC, 0.54 for MBN, and no adjustment factor for MBP following McLaughlin et al. (1986). Non-fumigated C and N fractions were quantified as extractable C (EC) and extractable N (EN), while unfumigated P extract was quantified as extractable P (EP) (Olsen et al., 1954). EC and EN are equivalent to KCl-extractable C and N, respectively, while EP is considered to contain available P in both organic and inorganic forms (Liao et al., 2014).
Gas samples from ARA measurements were stored at 25°C and analyzed for ethylene within 2 weeks of collection using a Shimadzu GC-8A gas chromatograph equipped with a flame ionization detector (FID) and HayeSep N column (2 m). The operating temperatures for the column and injection ports were 80 and 110°C, respectively. A 100 ppm standard C2H4 gas (Airgas, Radnor Township, PA, United States) was used for calibration, and results were reported as micromoles of C2H4 per square meter of soil core surface area per hour (μmol m–2h–1).
15N2-enriched biocrust subreplicates from 15N2-enriched jars and non-enriched control biocrust subreplicates from acetylated jars were analyzed for isotopic N, and total N. Atom% 15N was measured using a Thermo Finnigan Delta Plus XL isotope ratio mass spectrometer with a ConFlo III preparation system at the UF/IFAS Soil and Water Sciences Elemental Analysis Laboratory, Gainesville, FL, United States. Total N was simultaneously determined using a Costech ECS 4010 CHNS-O elemental analyzer. Atom% 15N of headspace N2 was calculated by subtracting 15N/14N atom% of air from 15N/14N atom% of gas blanks. Atom% excess biomass was divided by atom% excess in headspace to calculate the fraction of N2-fixation derived N according to the equation adapted from Inglett (2013).
Total N in the enriched biocrust samples was then used to calculate the N2-fixation rate in nmols of N-N2 g–1 DW h–1. The conversion factor from acetylene reduction to N2-fixation was calculated by dividing the average biocrust acetylene reduction rate by the average enriched biocrust N2-fixation rate. The conversion factor was obtained separately for the summer season Grape and the fall season Citrus samples. Analogous estimates were also made using the theoretical conversion factor of 3 (Howarth et al., 1988).
Data analysis
All data analysis was performed in R statistical software (R Core Team, 2019). First, biocrust measurements from acetylene reduction rates, microbial biomass, and nutrient measurements were compared across sites and seasons to determine the influence of interactions using general linear mixed model analysis with emmeans (Lenth, 2019) and nlme (Pinheiro et al., 2019) packages. Second, biocrusts measurements from acetylene reduction rates, microbial biomass, and nutrients were compared to bare soils within each site and season using general linear mixed model analysis with emmeans (Lenth, 2019) and nlme (Pinheiro et al., 2019) packages. A random effect for paired bare soil and biocrust samples was added to the statistical model. Biocrust and bare soil moisture, field measured light intensity, and field measured soil temperature were compared across seasons and sites using a Two-Way ANOVA with an HSD post hoc test. Biocrust microbial biomass, nutrients, and nutrient ratios were compared across seasons and sites using a Two-Way ANOVA with an HSD post hoc test. Normality was tested by the Shapiro–Wilk test for distribution and homogeneity of residuals. Non-normal data containing zeros were square root transformed, while non-normal data without zeros were log transformed. The results for general linear mixed-model analysis were reported as significant when p < 0.05 according to Tukey post hoc test. Plots were created using the ggplot2 package in R (Wickham, 2016).
To determine variables influencing N2-fixation rates of biocrusts, PCA was performed with the princomp function (stats 4.0.3). Each site was analyzed separately and only measurements from biocrusts were included. Prior to analysis, the data were preprocessed by filtering out zeros (Grape n = 21; Citrus n = 23), testing for multivariate normality using the mvn function from the MVN package (Korkmaz et al., 2014), and for Mardia’s multivariate skewness and kurtosis. Grape N2-fixation rates were log transformed, while other variables were left untransformed. For Citrus, all nutrient ratios were log transformed, moisture was root square transformed, and the rest of the variables were not transformed. Following transformations, the data were z-score standardized. The elbow plot and latent root criteria using base R were used to determine the number of principal components that best explained the data variation. Bootstrapped eigenvectors and loadings of at least 0.3 were used to determine the significance of loadings at the 0.01 significance level (Peres-Neto et al., 2003). PerMANOVA was performed to determine if samples were significantly separated by season using the adonis function from the vegan 2.5-7 package (Oksanen et al., 2013). Pairwise PerMANOVA was performed to determine which pairs of seasons were significantly separated from each other using wrapper function pairwise.adonis for multilevel pairwise comparison using adonis from package ‘vegan’ (Martinez Arbizu, 2020). PCA for each site was plotted with the ggbiplot function from the devtools package (currently in development by Vincent Q Vu) by only including variables with significant loadings as determined by bootstrapping. When vectors with significant loadings were not visible in the PCA plot, only one representative vector was shown.
Results
Qualitative biocrust characterization
Biocrust visual development scale, as qualitatively determined by surface coloration and roughness (Belnap et al., 2008) was at least 5 or 6 (Figures 1A,D). Microscopic inspections identified that Grape and Citrus biocrusts were both dominated by heterocystous and non-heterocystous cyanobacteria. Grape biocrusts also had filamentous algae, whereas Citrus biocrusts also contained mosses and single-celled algae (Figures 1D,E).

Figure 1. Images and micrographs of Grape and Citrus biocrusts: (A) Grape biocrust close-up, (B) filamentous algae bundles next to a heterocystous cyanobacterial filament in Grape, (C) non-heterocystous cyanobacterial bundles in Grape, (D) Citrus biocrust close-up with moss pointed out with a red bracket, (E) heterocystous filamentous cyanobacteria in Citrus, and (F) non-heterocystous cyanobacterial filament and unicellular algae in Citrus. Red arrows point to heterocystous cells, the blue arrow points to a group of unicellular algae, and the purple arrow points to a non-heterocystous cyanobacterial filament.
Field environmental conditions
Soils at both sites received the highest total precipitation 24 h before sampling during the summer season (Table 1), which exceeded other seasons by 2.3 mm (Citrus) and 26 mm (Grape). At the Grape site, biocrust and bare soil moisture ranged from 0.004 to 78%, with the highest average temperature in summer and the lowest average in spring, while at the Citrus site, it ranged from 0.002 to 22%, with the highest average in the winter and lowest average in fall (Table 1). At the Grape site, biocrust and bare soil moisture were significantly greater during the summer by at least 44% compared to fall, winter, and spring, even with the inclusion of added water during winter and spring collections (Table 1, p < 0.001). At the Grape site, biocrust and bare soil temperature ranged from 27.0 to 41.9°C, with the highest average temperature in spring and the lowest average in winter, while at the Citrus site, it ranged from 23.1 to 41.8°C, with the highest average in the summer and lowest average in winter (Table 1). At the Grape site, the light intensity on the soil surface ranged from 691 to 1741 μmol m–2 s–1 with the highest average intensity in summer and the lowest average in fall, while at the Citrus site it ranged from 504 to 1920 μmol m–2 s–1 with the highest average in summer and lowest average in fall (Table 1). Light intensity was not measured during winter at both sites.
N2-fixation rates
There were significant interactions between season, site, and sample type (biocrust or bare soil) for acetylene reduction rates (Supplementary Table 2). In Grape, average biocrust acetylene reduction rates were significantly greater in biocrusts (98%) than in bare soils during the summer, fall, and winter. In Citrus, average biocrust acetylene reduction rates were significantly greater in biocrusts (98%) during the fall, winter, and spring (Figure 2).
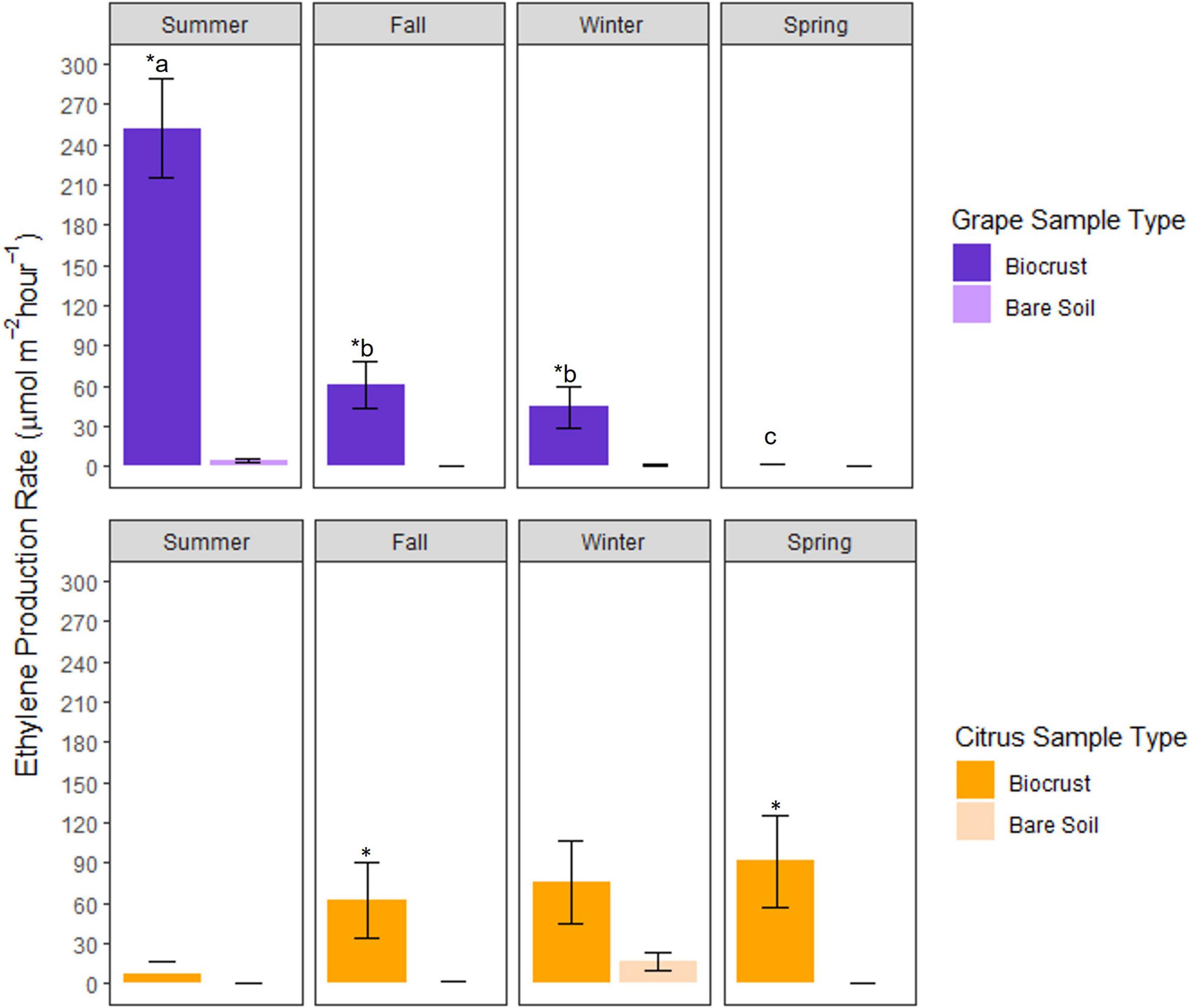
Figure 2. Seasonal rates of acetylene reduction (ethylene production) for biocrusts at the Grape and Citrus sites. The asterisk above the biocrust bar within a season indicates a significant difference between biocrusts and bare soils for a given sampling date, while letters indicate a significant difference between biocrusts of different seasons (*P < 0.05; n = 6, mean ± SE). One month prior to sampling in the summer at Citrus, controlled release fertilizer was applied at a rate of 29 kg N ha–1.
Grape biocrusts had higher average acetylene reduction rates than Citrus biocrusts. Grape biocrust acetylene reduction rates ranged from undetectable to 401 μmol m–2 h–1 with the highest average rate in summer (260 μmol m–2 h–1) and the lowest average rate in spring (4.19 μmol m–2 h–1) (Figure 2). Acetylene reduction rates of Citrus biocrusts ranged from undetectable to 326 μmol m–2 h–1 with the highest rate in winter (78 μmol m–2 h–1) and the lowest in summer (13 μmol m–2 h–1).
Average Grape biocrust acetylene reduction rates decreased from summer to spring, while average Citrus biocrust acetylene reduction rates increased from summer to spring. Grape biocrust acetylene reduction rates were significantly greater during the summer when compared to Grape biocrust acetylene reduction rates during fall, winter, and spring (Figure 2). While not significant, there was a trend of increasing acetylene reduction rates from summer to spring in Citrus (Figure 2).
The C2H4:N2 conversion factor was lower in Citrus (1.22 ± 0.23, standard deviation) than in Grape (2.69 ± 0.92), leading to higher average annual N input estimates from N2-fixation in Citrus. Estimates of biocrust N2-fixation rates using both the experimental and theoretical 3:1 ratios are shown in Table 2. Assuming a constant C2H4:N2 conversion factor and using experimental ratios, the N2-fixation rates in Grape ranged from 1.87 × 10–6 to 4.18 × 10–3 g N m–2 h–1, while in Citrus it ranged from 2.05 × 10–6 to 7.49 × 10–3 g N m–2 h–1. Based on the assumption that phototrophic diazotrophs were engaged in N2-fixation for 10 light hours, we estimated the daily N2-fixation rate of these agroecosystem biocrusts to be 6–32 mg of N per day. Averaging N2-fixation inputs separately for each season and site, and then adding for an annual estimate with the assumption that biocrust agroecosystems had 12.5% biocrust soil surface area coverage, Citrus biocrusts were estimated to provide 8.1 kg N ha–1 year–1, while Grape could provide 4.9 kg N ha–1 year–1.
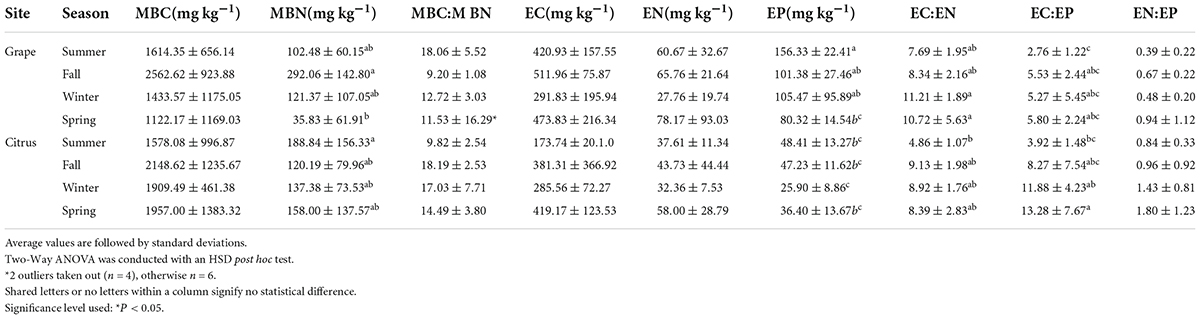
Microbial biomass nutrients and ratios
Microbial and extractable carbon was significantly greater in Grape biocrusts compared to bare soil only during the fall, while at the Citrus, MBC was significantly greater in biocrusts compared to bare soil during the fall, winter, and spring seasons (Supplementary Figure 3). Grape biocrust MBC ranged from 19 to 4036 mg kg–1 with the highest concentration in fall (2563 mg kg–1) and lowest in spring (1122 mg kg–1). Citrus biocrust MBC ranged from 232 to 3998 mg kg–1 with the highest concentration in fall (2149 mg kg–1) and lowest in summer (1578 mg kg–1) (Supplementary Figure 3).
Grape MBN was significantly greater in biocrusts compared to bare soil in the fall and winter, while MBN was not significantly different between Citrus biocrusts and bare soils in all seasons (Supplementary Figure 3). Grape biocrust MBN ranged from 2 to 520 mg kg–1 with the highest concentration in winter (121 mg kg–1) and lowest in spring (36 mg kg–1). Citrus biocrust MBN ranged from 16 to 480 mg kg–1 with the highest concentration in summer (189 mg kg–1) and lowest in fall (120 mg kg–1). There was also a significant interaction between site and season for biocrust MBN (Supplementary Table 1), where Grape biocrust MBN was significantly greater in the fall compared to the spring (Table 1).
Grape biocrust MBP ranged from undetectable to 83 mg kg–1 with the highest concentration in summer (29 mg kg–1) and lowest in winter (10 mg kg–1). Citrus biocrust MBP ranged from undetectable to 68 mg kg–1 with the highest concentration in spring (29.5 mg kg–1) and lowest in winter (3 mg kg–1) (Supplementary Figure 3). Due to the high variability and MBP being below the detection limit in 30% of samples, it was not analyzed for significant differences.
There were different seasonal patterns in MBC:MBN ratios in Grape and Citrus. Mass-based MBN:MBP and MBC:MBP ratios were excluded because 30% of MBP values were below the detection limit. Grape biocrust MBC:MBN ranged from 6 to 44, while Citrus biocrust MBC:MBN ranged from 7 to 30. The highest biocrust MBC:MBN in Grape was during the summer (18.1 ± 5.52), while in Citrus it was during the fall (18.2 ± 2.53). The lowest biocrust MBC:MBN in Grape was in fall (9.2 ± 1.08), while in Citrus it was during the summer (9.82 ± 2.54) (Table 1).
Extractable nutrient concentrations and ratios
In general, EC, EN, and EP were higher in biocrusts than in bare soils in Grape and Citrus. EC was significantly greater in biocrusts than in bare soils during the summer (Grape), fall (Grape and Citrus), and spring (Citrus) (Supplementary Figure 4). Grape biocrust EC ranged from 106 to 780 mg kg–1 with a high in fall (512 mg kg–1) and a low in spring (292 mg kg–1). Citrus biocrust EC ranged from 83 to 1107 mg kg–1 with a high in spring (419 mg kg–1) and a low in summer (174 mg kg–1).
EN was significantly greater in biocrusts than in bare soils during the summer (Citrus), fall (Grape), winter (Citrus), and spring (Citrus). Grape biocrust EN ranged from 7 to 255 mg kg–1 with a high in spring (78 mg kg–1) and a low in winter (28 mg kg–1). Citrus biocrust EN ranged from 9 to 132 mg kg–1 with a high in spring (58 mg kg–1) and a low in winter (32 mg kg–1).
There was a significant interaction between site and sample type for EP (Supplementary Table 2). EP tended to be higher in Grape biocrusts than in Citrus biocrusts. EP was significantly greater in biocrusts than in bare soils during the summer (Grape), fall (Citrus), and winter (Grape). EP in Grape biocrusts decreased from summer to spring, while in Citrus there was no significant seasonal trend (Table 1). Grape biocrust EP ranged from 36 to 268 mg kg–1 with a high in summer (156 mg kg–1) and a low in spring (80 mg kg–1). Citrus biocrust EP ranged from 12 to 67 mg kg–1 with a high in summer (48 mg kg–1) and a low in winter (26 mg kg–1).
No strong seasonal patterns or significant differences between sites, biocrusts and bare soils, or seasons were detected in extractable nutrient ratios. Due to undetected EN and EP, certain ratios were excluded from the following comparisons. Grape biocrust EC:EN ranged from 3 to 18, while Grape bare soil EC:EN ranged from 7 to 15. Citrus biocrust EC:EN ranged from 3 to 13, while Citrus bare soil EC:EN ranged from 1 to 10. Grape biocrust EN:EP ranged from 0.05 to 3, while Grape bare soil EN:EP ranged from 0.2 to 0.6. Citrus biocrust EN:EP ranged from 0.2 to 4, while Citrus bare soil EN:EP ranged from 0.2 to 2. Highest average Grape biocrust EC:EN was in winter (11.2 ± 1.89), while the lowest Grape biocrust EC:EN was in summer (4.9 ± 1.07). Highest average Citrus biocrust EC:EN was in fall (9.1 ± 1.98), while the lowest Citrus biocrust EC:EN was in summer (4.86 ± 1.07). Highest average Grape biocrust EN:EP was in spring (0.9 ± 1.12), while the lowest Grape biocrust EN:EP was in summer (0.4 ± 0.22). Highest average Citrus biocrust EN:EP was in spring (1.8 ± 1.23), while the lowest Citrus biocrust EN:EP was in summer (0.8 ± 0.33) (Table 1).
Relationship between N2-fixation activity, nutrients, and environmental conditions
Variation in actively N-fixing biocrusts in Grape and Citrus was explained by the variation in environmental conditions, nutrients, biomass, and acetylene reduction rates. Biotic variables (MBC, MBN, MBC:MBN), nutrient variables (EC, EN, EP, EC:EN, EN:EP, EC:EP), acetylene reduction rates, and environmental variables (soil moisture, soil temperature) measured in N2-fixing biocrusts were included in the PCA. At Grape, MBC:MBN, extractable nutrients, extractable nutrient ratios, and soil moisture were significant drivers of variation, as indicated by the significance of loadings (Supplementary Table 3). Based on significant loading, PC1 in Grape may represent nutrients, PC2 may represent N2-fixation activity, and PC3 may represent microbial biomass. At Citrus, microbial biomass, MBC:MBN, EC, EN, EC:EP, EN:EP EC:EN, and temperature were significant drivers of variation (Supplementary Table 2). Based on significant loading, Citrus PC1 may represent nutrients and microbial biomass carbon, PC2 may represent N2-fixation activity, and PC3 may represent microbial biomass nitrogen, nutrients, and environmental variables.
Actively N2-fixing biocrust samples in Grape (46%) and Citrus (29%) were significantly separated based on the season (Supplementary Table 4). N2-fixing biocrust samples in summer significantly differed from the fall and winter seasons in both Grape and Citrus (Supplementary Table 4). Grape summer samples were significantly different from fall and winter samples mainly because of higher EC, EN, MBC:MBN, EN:EP, and EC:EP but lower EC:EN (PC1); and higher acetylene reduction rates, soil moisture, and EP, but lower EC:EN (PC2, Supplementary Table 3). Citrus summer samples were significantly different from fall and winter samples mainly because of lower MBC, EC, EN, EN:EP, and EC:EP (PC1); and lower acetylene reduction rates, MBC:MBN, and EC:EN but higher soil temperatures (PC2, Supplementary Table 3).
Discussion
Agroecosystem biocrusts were identified as dark algal biocrusts in Grape and dark cyanobacterial biocrusts in Citrus. During select seasons at both sites, biocrust N2-fixation rates, MBC, MBN, EC, EN, and EP were significantly higher than in bare soils. The N2-fixation rates in Grape significantly decreased from summer to spring, while in Citrus an opposite but non-significant trend was measured. In addition, in Grape biocrust MBN increased from summer to fall, but then decreased from fall to spring. The N2-fixing biocrust acetylene reduction rates, nutrient concentrations, microbial biomass, soil moisture, and soil temperature significantly differed across seasons. Higher soil moisture and higher EP were associated with higher N2-fixation rates in Grape, while lower soil temperatures and higher EC:EN ratios were associated with higher N2-fixation rates in citrus biocrusts.
The Florida agroecosystem biocrusts in this study appeared to be dark algal and cyanobacterial biocrusts based on assessments using microscopy and the visual biocrust development scale (Belnap et al., 2008). In addition, despite large climatic differences, subtropical Florida agroecosystem biocrusts had average MBC values similar to natural cyanobacterial biocrusts of a semi-arid Mediterranean desert (Miralles et al., 2012). The lower range of MBN from these agroecosystem biocrusts was also similar to the MBN of natural lichen-dominated biocrusts of Mediterranean grassland (Castillo-Monroy et al., 2010).
Biocrusts in both citrus and grape agroecosystems fixed atmospheric N2, with rates within the ranges recorded in both arid and mesic natural ecosystem biocrusts (Table 2), including biocrusts in restored Florida wetlands (Liao and Inglett, 2014). To calculate rates of N2-fixation, we converted ethylene production rates into mg of N inputs by using an experimental ratio for moles of C2H4 to moles of N2. Grape biocrusts fixed between 0.6 and 2.7 mg N m–2 h–1, while Citrus biocrusts fixed between 1.7 and 3.1 mg N m–2 h–1 (Table 2). In citrus and grape agroecosystems, biocrust could potentially contribute between 0.6 and 3 mg of N m2 h–1 through N2-fixation based on experimental conversion ratios (Table 2). As these agroecosystem biocrusts appear to be similar to dark cyanobacterial biocrusts, cyanobacteria could be the dominant contributors to N2-fixation of these agroecosystem biocrusts. Based on the assumption that phototrophic diazotrophs were engaged in N2-fixation for 10 light hours, we estimated the daily N2-fixation rate of these agroecosystem biocrusts to be 6–32 mg of N per day, which constitutes less than 1% of fertigation for a single citrus tree from our site per application (4–5 g N, based on fertigation information specific to citrus) (Obreza and Morgan, 2020). It should be noted, however, that this is likely an underestimate as these rates are single time point measurements in a diel cycle, and therefore do not include potential dark or nighttime N2-fixation.
If it is assumed that biocrust agroecosystems had 100% coverage over a hectare, Citrus biocrusts could contribute 64 kg N ha–1 year–1, while Grape biocrusts could provide 39 kg N ha–1 year–1 per hectare of biocrust. However, while in natural ecosystems up to 70% of soil surface can be covered with biocrusts (Ferrenberg et al., 2015), agroecosystem biocrusts tend to grow in crop interspaces and only between crop rows, leaving at most 12.5% of the total field area available for biocrust growth (based on field observations). Assuming 12.5% biocrust soil surface area coverage, Citrus biocrusts are estimated to provide 8.1 kg N ha–1 year–1, while Grape could provide 4.9 kg N ha–1 year–1, which satisfies 7 and 14% of total yearly N input, respectively. Regardless of potential overestimation and underestimation due to lack of night and diurnal measurements, these estimated rates fit well within the expected N input contribution of natural ecosystem biocrusts (Malam Issa et al., 2001; Belnap, 2002; Billings et al., 2003; Russow et al., 2005; Housman et al., 2006; Holst et al., 2009).
Higher precipitation favors higher N2-fixation rates in natural ecosystem biocrusts, and if coupled with higher moisture, warmer temperatures can also lead to higher N2-fixation rates (Zhao et al., 2010; Caputa et al., 2013). Similar to dark and light cyanobacterial biocrusts from Southwestern U.S. deserts (Belnap, 2002), N2-fixation activity in Grape biocrusts peaked during the season of higher precipitation and after a high precipitation event (Figure 2 and Table 3). In the summer, 46% of Grape N2-fixation rates were significantly higher compared to fall and winter seasons potentially because of higher moisture. Biocrust moisture was also significantly higher in the summer and was one of the significant drivers of variation along PC2 of Grape N2-fixing biocrust samples (Figure 3 and Supplementary Tables 3, 4).
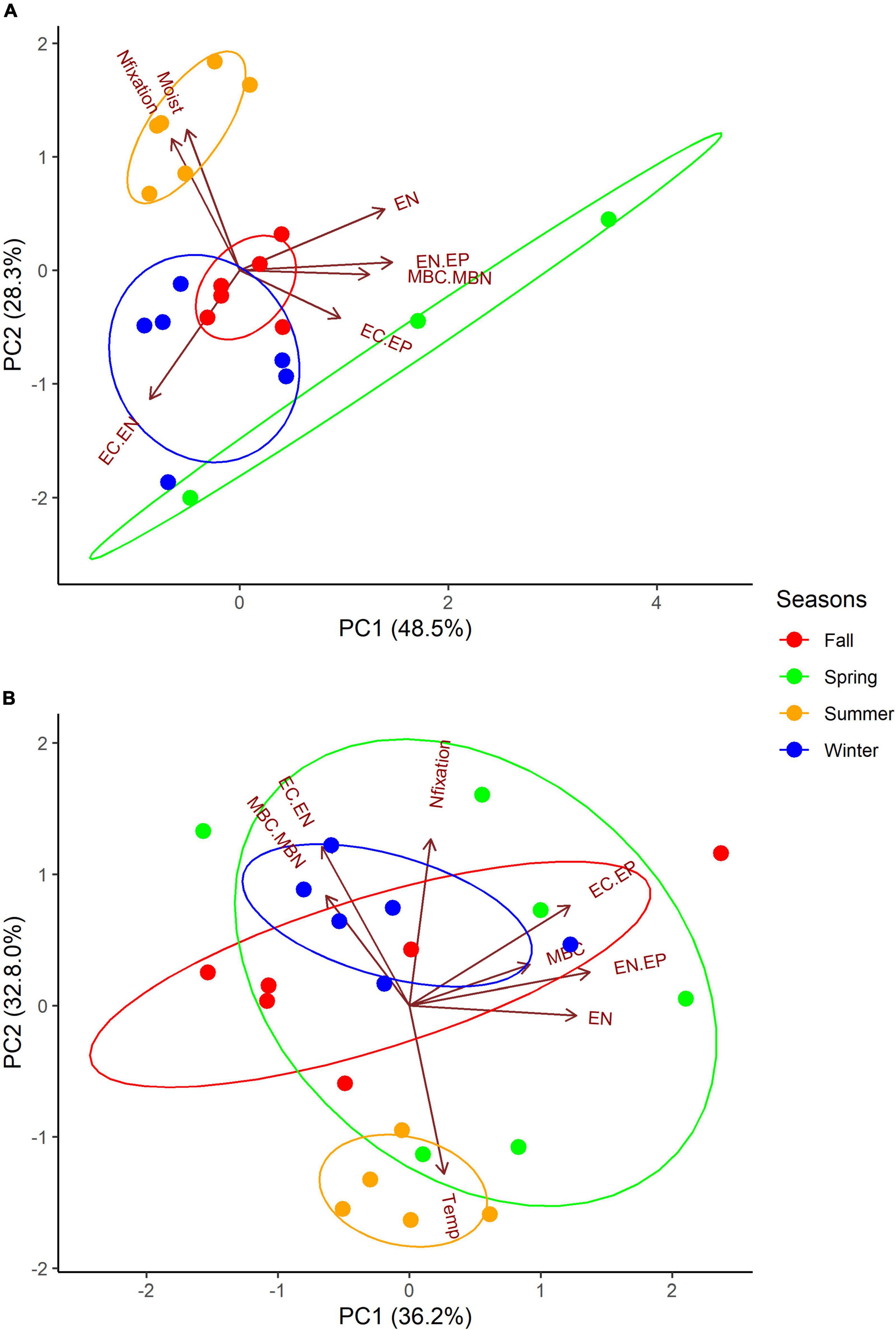
Figure 3. Principle component analysis (PCA) of seasonally N2-fixing biocrusts from (A) Grape (n = 21) and (B) Citrus (n = 23). Vectors represent variables with significant loadings used to generate Euclidean distance: N2-fixation (Nfixation), biocrust moisture (Moist), soil temperature (Temp), extractable nutrients (EC, EN, EP, EC.EN) and their ratios (EN.EP, EC.EP), and microbial nutrients (MBC, MBN) and their ratio (MBC.MBN). In Grape, the following vectors represent multiple variables: Nfixation (EP) and EN (EC). In Citrus, the following vector represents two variables: EN (EC). Ellipses are drawn around seasons at 98% probability. Vector loadings and their significance with all variables included are shown in Supplementary Table 3. PerMANOVA results of differences based on the season are shown in Supplementary Table 4.
Irrigation management may also be responsible for the seasonal pattern differences in microbial biomass and N2-fixation rates between Grape and Citrus biocrusts. For example, rainfall may have impacted N2-fixation rates more for the drip-irrigated Grape system (Table 3 and Figure 2), while Citrus was irrigated with a higher-intensity microjet system. Thus, there was likely less variability of surface soil moisture at Citrus, and a greater dependence on precipitation at Grape, potentially explaining the lower Grape biocrust N2-fixation rates in drier seasons of fall, winter, and spring (Figures 2, 3) and the weak relationship between soil moisture and N2-fixation rates (Figure 3) at Citrus.
Despite regular N fertilizer inputs, biocrusts in these perennial agroecosystems had detectable N2-fixation activity for most of the year (Figure 2). While the presence of available N can inhibit N2-fixation (Nordlund and Ludden, 2004; Masepohl and Forchhammer, 2007), the continued N2-fixation by these agroecosystem biocrusts indicates that these organisms may tolerate a threshold of N fertilizer in their environment. For example, below the threshold of 55 lbs acre–1, N2-fixation of lab-grown cyanobacteria sourced from agroecosystem biocrust continued to fix N2 at a steady rate (Peng and Bruns, 2019). In Florida citrus, this threshold of 55 lbs acre–1 (Peng and Bruns, 2019) would be equivalent to the lowest recommended fertilizer rate for a tree that has been in the orchard for 1–3 years (Obreza and Morgan, 2020), and the trees in our study were less than 3 years old during our sample collections. However, controlled release fertilizer application may have reduced N2-fixation rates of Citrus biocrusts in the summer (lowest N2-fixation rates and highest MBN) which occurred 1 month after application of a controlled release fertilizer containing ammonium and nitrate (Supplementary Table 1). This could mean that within a month of controlled release fertilizer application, the microbial community assimilated the fertilizer N and did not require biological N2-fixation to meet N demands. Two months later, however, Citrus had significantly higher N2-fixation rates and MBN dropped by approximately 60 mg kg–1 (Table 1), suggesting an ability and necessity to resume N2-fixation activity. While Citrus received weekly fertigation, the controlled release fertilizer form may have a stronger negative effect on biocrust N2-fixation than the liquid form due to its higher concentration of N (Supplementary Table 1). The exponential increase in released N (Sempeho et al., 2014) might have resulted in a longer period of higher N concentrations in proximity to biocrusts. In contrast, liquid fertilizer N, especially the nitrate form applied to sandy soils in this Citrus (Gaines and Gaines, 1994), may leach quickly (Kadyampakeni et al., 2018), reducing the concentration of N and allowing for higher N2-fixation activity.
Grape and citrus have different fertilizer requirements, which may have also resulted in different N2-fixation rates between the sites (Figure 3). For example, the vineyards in this study received a 1:1 ratio of N to phosphate, versus 7:2 for citrus (Supplementary Table 1). In the Grape biocrusts, as EP increased, N2-fixation also tended to increase, possibly suggesting that higher EP led to higher N2-fixation rates (Figure 3). Phosphorus addition is known to stimulate N2-fixation activity in highly weathered soils such as Oxisols and Ultisols (Reed et al., 2011), and increased P inputs can shift an ecosystem from P to N limitation, resulting in increased N2-fixation activity of cyanobacterial communities and biocrusts (Inglett et al., 2009; Liao and Inglett, 2012). Oxisols and Ultisols tend to be more common in tropical climates, and while our sites are located in the subtropics, they have Entisols that are not known to commonly be P limited. However, the biocrusts on a smaller scale could still be P limited due to having different properties than the soil below.
Extractable nutrients and their ratios explained 33–38% of the total variation in N2-fixing biocrusts (Figure 3), which is not surprising because nutrient status has been shown to govern N2-fixation activity (Inglett et al., 2004, 2009, 2011). The N:P of the Grape fertilizer was lower than at Citrus (Supplementary Table 1), so fertilization likely led to the N limitation in Grape. A higher EC:EN was also associated with higher N2-fixation rates in Citrus (Figure 3 and Supplementary Table 3), and higher EC and EC:EN ratios coincided with seasons of higher N2-fixation activity in Citrus (fall, winter, and spring) (Table 2). Therefore, Citrus biocrusts might be experiencing C and N colimitation that regulated N2-fixation. Despite the fertilizer differences between Grape and Citrus, EN:EP ratio of biocrusts at both sites did not exceed 4, which was also true for a desert biocrust N:P (Zhou et al., 2016). Such a ratio is well below the threshold for N limitation of grassland soil microorganisms (Griffiths et al., 2012), suggesting that the biocrusts might have been experiencing N limitation at both sites.
Differences in environmental conditions, planting history, and fertilization management likely result in organism level differences between Citrus and Grape biocrusts. Diazotrophic community composition may be responsible for the seasonal pattern differences in biomass and N2-fixation rates between Grape and Citrus biocrusts. Grape and citrus have different disturbance histories from planting, which could explain the differences in their biocrust morphologies. Using the qualitative indicators of potentially different organism compositions in biocrusts (Belnap et al., 2008), Grape biocrusts had a homogeneous dark green color after wetting, while Citrus biocrusts had heterogeneous dark green and dark brown coloration after wetting (Figure 1). Certain organisms were also not detected microscopically in both sites; for example, filamentous algae were specific to the Grape biocrusts, while single-celled algae and mosses were specific to Citrus biocrusts. The older tree age of Citrus (i.e., more time since major soil disturbance) compared to grape (2 years > 2 months) might have allowed Citrus biocrusts to become more homogeneous in coloration and to contain mosses. Also, despite similar climatic conditions, N2-fixation rates followed contrasting seasonal patterns in Grape and Citrus (Figure 2), indicating potentially different organism communities and growth/senescence cycles which may be supported by MBN seasonal patterns (Supplementary Figure 3). Finally, the conversion factor for moles of C2H4 to moles of N2 was not the same for the two sites (3 for Grape and 1 for Citrus), suggesting that different communities of organisms were involved in N2-fixation at each site. Due to the variety of diazotroph nitrogenases, the conversion factor can differ between not only phyla but also species of cyanobacteria, as it did between Anabaena culture (4–5) and Nostoc culture (0.1–0.5) (Liengen, 1999). Additionally, conversion factors lower than 2 can be the result of N2-fixers with alternative nitrogenases that use vanadium or iron cofactors, which is common for asymbiotic soil N2-fixers (Bellenger et al., 2014). Therefore, based on the experimentally derived conversion ratios, diazotrophs from Grape and Citrus could contain organisms that use different nitrogenases.
Conclusion
Despite regular N fertilizer inputs, biocrusts in Florida citrus and grape agroecosystems maintained N2-fixation activity within ranges of natural biocrusts. These agroecosystem biocrusts have the potential to supplement available N for the crops through N2-fixation activity. While biocrusts could contribute less than 1% of the daily fertigation requirement of 1–3-year-old citrus, their continued N2-fixation activity during favorable conditions could contribute 7-14% of yearly N requirements for perennial crops such as grape and citrus.
To arrive at more accurate N2-fixation rates and patterns for better estimates of N inputs from N2-fixation on a crop field yearly scale, studies across more time and spatial scales are necessary. Additional diel N2-fixation rate measurements would show if there are contributions from non-phototrophic diazotrophs (dark), or more N2-fixation activity during other times of the day that this study did not consider. This study used 3 cm diameter cores for N2-fixation rate measurements, but there is a need to scale up to the whole field area for more accurate N input estimates, which could be done with more and larger core collections, biocrust percent cover measurements, and potentially paired with remote sensing technology as was done for biocrusts in natural ecosystems (Havrilla et al., 2020).
As hypothesized, crop-specific fertilization and irrigation management appeared to impact N2-fixation rates as fertilization and soil temperatures were the main controls of N2-fixation in citrus systems, while P and soil moisture were the main controls in vineyards. The differences in crop management and patterns of microbial biomass and N2-fixation patterns point to the possibility that the microbial communities of these crops could be distinct. To further explore microbial community differences between grape and citrus biocrusts, biocrust DNA could be analyzed for community composition and diversity.
Detailed analysis of biocrust microbial communities could help identify agroecosystem biocrusts N2-fixing organisms and their N2-fixation strategies, which could be important for developing management strategies to encourage N2-fixation activity in biocrusts. To further examine the relationship between N and P fertilization and N2-fixation in agricultural biocrusts, experiments with controlled N and P addition would better establish the potential for nutrient thresholds for suppression (N) and stimulation (P) of biocrust N2-fixation rates. Ultimately, further agroecosystem biocrust studies should aid in determining whether biocrust N inputs make any substantial improvements to crop productivity, or at least help satisfy N crop requirements more sustainably.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
PI, SS, and KS designed the experiment. KS conducted the experiment, processed the samples, analyzed the data, and wrote the manuscript. SS and PI revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by the United States Department of Agriculture – National Institute of Food and Agriculture – Agriculture and Food Research Initiative grant (2018-67019-27707).
Acknowledgments
We thank Devin Leonard and Sophia Barbour of Wetland Biogeochemistry Laboratory in Gainesville, FL, United States, for training KS and helping conduct soil biogeochemical analyses. We thank DR. Kathryn Curtis of Stable Isotope Mass Spectrometry Laboratory in Gainesville, FL, United States, for conducting an isotopic analysis of soil samples. We also thank Dr. Conor MacDonnell of Wetland Biogeochemistry Laboratory in Gainesville, FL, United States, and Rachel Berner, Diderot Saintilma, DR. Antonio Castellano-Hinojosa, DR. Clayton Nevins, and Brittney Monus of the Soil Microbiology Laboratory at the UF Southwest Research and Education Center in Immokalee, FL, United States, for assisting with field N2-fixation measurements and sample collection. Caroline Tardivo from the UF Southwest Research and Education Center in Immokalee assisted with microscopy pictures of biocrusts. James Colee and Dr. Antonio Castellano-Hinojosa of the UF Institute of Food and Agricultural Sciences assisted with statistical analyses. Special thanks to the University of Florida Plant Science Research and Education Unit in Citra, FL, United States, and the University of Florida Citrus Research and Education Center in Lake Alfred, Florida, for allowing the use of an orchard and a vineyard as our study sites.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.892266/full#supplementary-material
Footnotes
References
Abed, R. M. M., Al Kharusi, S., Schramm, A., and Robinson, M. D. (2010). Bacterial diversity, pigments and nitrogen fixation of biological desert crusts from the Sultanate of Oman. FEMS Microbiol. Ecol. 72, 418–428. doi: 10.1111/j.1574-6941.2010.00854.x
Bates, S. T., Nash, T. H., Sweat, K. G., and Garcia-Pichel, F. (2010). Fungal communities of lichen-dominated biological soil crusts: Diversity, relative microbial biomass, and their relationship to disturbance and crust cover. J. Arid Environ. 74, 1192–1199. doi: 10.1016/j.jaridenv.2010.05.033
Bellenger, J. P., Xu, Y., Zhang, X., Morel, F. M. M., and Kraepiel, A. M. L. (2014). Possible contribution of alternative nitrogenases to nitrogen fixation by asymbiotic N2-fixing bacteria in soils. Soil Biol. Biochem. 69, 413–420. doi: 10.1016/j.soilbio.2013.11.015
Belnap, J. (2002). Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biol. Fertil. Soils 35, 128–135. doi: 10.1007/s00374-002-0452-x
Belnap, J., Phillips, S. L., Flint, S., Money, J., and Caldwell, M. (2008). Global change and biological soil crusts: Effects of ultraviolet augmentation under altered precipitation regimes and nitrogen additions. Glob. Change Biol. 14, 670–686. doi: 10.1111/j.1365-2486.2007.01509.x
Billings, S. A., Schaeffer, S. M., and Evans, R. D. (2003). Nitrogen fixation by biological soil crusts and heterotrophic bacteria in an intact Mojave Desert ecosystem with elevated CO2 and added soil carbon. Soil Biol. Biochem. 35, 643–649. doi: 10.1016/s0038-0717(03)00011-7
Caputa, K., Coxson, D., and Sanborn, P. (2013). Seasonal patterns of nitrogen fixation in biological soil crusts from British Columbia’s Chilcotin grasslands. Bot. Bot. 91, 631–641. doi: 10.1139/cjb-2013-0014
Castillo-Monroy, A. P., Maestre, F. T., Delgado-Baquerizo, M., and Gallardo, A. (2010). Biological soil crusts modulate nitrogen availability in semi-arid ecosystems: Insights from a Mediterranean grassland. Plant Soil 333, 21–34. doi: 10.1007/s11104-009-0276-7
Colesie, C., Felde, V. J. M. N. L., and Büdel, B. (2016). “Composition and Macrostructure of Biological Soil Crusts In,” in Biological Soil Crusts: An Organizing Principle in Drylands, eds B. Weber, B. Büdel, and J. Belnap (Berlin: Springer), 159–172. doi: 10.1007/978-3-319-30214-0_9
da Rocha, U. N., Cadillo-Quiroz, H., Karaoz, U., Rajeev, L., Klitgord, N., Dunn, S., et al. (2015). Isolation of a significant fraction of non-phototroph diversity from a desert Biological Soil Crust. Front. Microbiol. 6:14. doi: 10.3389/fmicb.2015.00277
Elbert, W., Weber, B., Burrows, S., Steinkamp, J., Budel, B., Andreae, M. O., et al. (2012). Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 5, 459–462. doi: 10.1038/ngeo1486
Ferrenberg, S., Reed, S. C., Belnap, J., and Schlesinger, W. H. (2015). Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc. Natl. Acad. Sci. U.S.A. 112, 12116–12121. doi: 10.1073/pnas.1509150112
Gaines, T. P., and Gaines, S. T. (1994). Soil texture effect on nitrate leaching in soil percolates. Commun. Soil Sci. Plant Anal. 25, 2561–2570. doi: 10.1080/00103629409369207
Garcia-Pichel, F., and Pringault, O. (2001). Microbiology: Cyanobacteria track water in desert soils. Nature 413, 380–381. doi: 10.1038/35096640
Garcia-Pichel, F., and Castenholz, R. W. (1991). Characterization And Biological Implications Of Scytonemin, A Cyanobacterial Sheath Pigment. J. Phycol. 27, 395–409. doi: 10.1111/j.0022-3646.1991.00395.x
Griffiths, B. S., Spilles, A., and Bonkowski, M. (2012). C:N:P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol. Process. 1:6. doi: 10.1186/2192-1709-1-6
Havrilla, C. A., Villarreal, M. L., DiBiase, J. L., Duniway, M. C., and Barger, N. N. (2020). Ultra-high-resolution mapping of biocrusts with Unmanned Aerial Systems. Remote Sens. Ecol. Conserv. 6, 441–456. doi: 10.1002/rse2.180
Holst, J., Butterbach-Bahl, K., Liu, C. Y., Zheng, X. H., Kaiser, A. J., Schnitzler, J. P., et al. (2009). Dinitrogen fixation by biological soil crusts in an Inner Mongolian steppe. Biol. Fertil. Soils 45, 679–690. doi: 10.1007/s00374-009-0378-7
Housman, D. C., Powers, H. H., Collins, A. D., and Belnap, J. (2006). Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. J. Arid Environ. 66, 620–634. doi: 10.1016/j.jaridenv.2005.11.014
Howarth, R. W., Marino, R., Lane, J., and Cole, J. J. (1988). Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 1. Rates and importance. Limnol. Oceanogr. 33, 669–687. doi: 10.4319/lo.1988.33.4part2.0669
Inglett, P. W., D’Angelo, E. M., Reddy, K. R., McCormick, P. V., and Hagerthey, S. E. (2009). Periphyton nitrogenase activity as an indicator of wetland eutrophication?: Spatial patterns and response to phosphorus dosing in a northern Everglades ecosystem. Wetl. Ecol. Manage. 17, 131–144. doi: 10.1007/s11273-008-9095-5
Inglett, P. W. (2013). “Biological dinitrogen fixation,” in Methods in biogeochemistry of wetlands, SSSA book series 10, eds R. D. DeLaune, K. R. Reddy, C. J. Richardson, and J. P. Megonigal (Madison, WI: Soil Science Society of America), 593–602.
Inglett, P. W., Reddy, K. R., and McCormick, P. V. (2004). Periphyton chemistry and nitrogenase activity in a northern Everglades ecosystem. Biogeochemistry 67, 213–233. doi: 10.1023/B:BIOG.0000015280.44760.9a
Inglett, P. W., Rivera-Monroy, V. H., and Wozniak, J. R. (2011). Biogeochemistry of nitrogen across the everglades landscape. Crit. Rev. Environ. Sci. Technol. 41, 187–216. doi: 10.1080/10643389.2010.530933
Jalpa, L., Mylavarapu, R. S., Hochmuth, G. J., Wright, A. L., and van Santen, E. (2020). Apparent recovery and efficiency of nitrogen fertilization in tomato grown on sandy soils. HortTechnology 30, 204–211. doi: 10.21273/HORTTECH04480-19
Kadyampakeni, D. M., Nkedi-Kizza, P., Leiva, J. A., Muwamba, A., Fletcher, E., and Morgan, K. T. (2018). Ammonium and nitrate transport during saturated and unsaturated water flow through sandy soils. J. Plant Nutr. Soil Sci. 181, 198–210. doi: 10.1002/jpln.201700405
Korkmaz, S., Goksuluk, D., and Zararsiz, G. (2014). MVN: An R package for assessing multivariate normality. R J. 6, 151–162. doi: 10.32614/rj-2014-031
Kuske, C. R., Yeager, C. M., Johnson, S., Ticknor, L. O., and Belnap, J. (2012). Response and resilience of soil biocrust bacterial communities to chronic physical disturbance in arid shrublands. ISME J. 6, 886–897. doi: 10.1038/ismej.2011.153
Lenth, R. (2019). Emmeans: Estimated Marginal Means, aka Least-Squares Means. Package Version 1.3.3.
Liao, X. L., and Inglett, P. W. (2012). Biological Nitrogen Fixation in Periphyton of Native and Restored Everglades Marl Prairies. Wetlands 32, 137–148. doi: 10.1007/s13157-011-0258-4
Liao, X. L., and Inglett, P. W. (2014). Dynamics of periphyton nitrogen fixation in short-hydroperiod wetlands revealed by high-resolution seasonal sampling. Hydrobiologia 722, 263–277. doi: 10.1007/s10750-013-1709-0
Liao, X. L., Inglett, P. W., and Inglett, K. S. (2014). Vegetation and microbial indicators of nutrient status: Testing their consistency and sufficiency in restored calcareous wetlands. Ecol. Indic. 46, 358–366. doi: 10.1016/j.ecolind.2014.07.001
Liengen, T. (1999). Conversion factor between acetylene reduction and nitrogen fixation in free-living cyanobacteria from high arctic habitats. Can. J. Microbiol. 45, 223–229. doi: 10.1139/cjm-45-3-223
Malam Issa, O., Stal, L. J., Defarge, C., Coute, A., and Trichet, J. (2001). Nitrogen fixation by microbial crusts from desiccated Sahelian soils (Niger). Soil Biol. Biochem. 33, 1425–1428. doi: 10.1016/s0038-0717(01)00046-3
Martinez Arbizu, P. (2020). Pairwiseadonis: Pairwise Multilevel Comparison using adonis. R Package Version 0.4.
Masepohl, B., and Forchhammer, K. (2007). “Regulatory Cascades to Express Nitrogenases,” in Biology of the Nitrogen Cycle, eds H. Bothe, S. Ferguson, and W. E. Newton (Amsterdam: Elsevier B.V), doi: 10.1016/B978-044452857-5.50010-2
McLaughlin, M. J., Alston, A. M., and Martin, J. K. (1986). Measurement Of Phosphorus In The Soil Microbial Biomass - A Modified Procedure For Field Soils. Soil Biol. Biochem. 18, 437–443. doi: 10.1016/0038-0717(86)90050-7
Miralles, I., Domingo, F., García-Campos, E., Trasar-Cepeda, C., Leirós, M. C., and Gil-Sotres, F. (2012). Biological and microbial activity in biological soil crusts from the Tabernas desert, a sub-arid zone in SE Spain. Soil Biol. Biochem. 55, 113–121. doi: 10.1016/j.soilbio.2012.06.017
Nevins, C. J., Inglett, P. W., Reardon, C. L., and Strauss, S. L. (2022). Seasonality drives microbiome composition and nitrogen cycling in soil below biocrusts. Soil Biol. Biochem. 166:108551. doi: 10.1016/j.soilbio.2022.108551
Nevins, C. J., Strauss, S. L., and Inglett, P. W. (2020). Biological soil crusts enhance moisture and nutrients in the upper rooting zone of sandy soil agroecosystems. Plant Nutr. Soil Sci. C 183, 615–626. doi: 10.1002/jpln.202000218
Nordlund, S., and Ludden, P. W. (2004). “Post-translational regulation of nitrogenase in photosynthetic bacteria,” in Genetics and regulation of nitrogen fixation in free-living bacteria, eds W. Klipp, B. Masepohl, J. R. Gallon, and W. E. Newton (Alphen aan den Rijn: Kluwer Academic Publishers). doi: 10.1007/1-4020-2179-8
Obreza, T. A., and Morgan, K. T. (2020). Nutrition of Florida Citrus Trees 3rd Edition. Gainesville: University of Florida IFAS Extension.
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., and O’Hara, R. B. (2013). Package vegan. Package “vegan”: community ecology package. R package version 2.0-7.
Olsen, S. R., Watanabe, F. S., Cosper, H. R., Larson, W. E., and Nelson, L. B. (1954). Residual phosphorus availability in long-time rotations on calcareous soils. Soil Sci. 78, 141–152. doi: 10.1097/00010694-195408000-00008
Peng, X., and Bruns, M. A. (2019). Development of a nitrogen-fixing cyanobacterial consortium for surface stabilization of agricultural soils. J. Appl. Phycol. 31, 1047–1056. doi: 10.1007/s10811-018-1597-9
Peres-Neto, P. R., Jackson, D. A., and Somers, K. M. (2003). Giving meaningful interpretation to ordination axes: Assessing loading significance in principal component analysis. Ecology 84, 2347–2363. doi: 10.1890/00-0634
Pinheiro, J., Bates, D., DebRoy, S., and Sarkar, D. R Core Team. (2019). Linear and Nonlinear Mixed Effects Models.
R Core Team. (2019). R: A Language and Environment for Statistical Computing. Vienna: R foundation for Statistical Computing.
Rajeev, L., Da Rocha, U. N., Klitgord, N., Luning, E. G., Fortney, J., Axen, S. D., et al. (2013). Dynamic cyanobacterial response to hydration and dehydration in a desert biological soil crust. ISME J. 7, 2178–2191. doi: 10.1038/ismej.2013.83
Reed, S. C., Cleveland, C. C., and Townsend, A. R. (2007a). Controls over leaf litter and soil nitrogen fixation in two lowland tropical rain forests. Biotropica 39, 585–592. doi: 10.1111/j.1744-7429.2007.00310.x
Reed, S. C., Cleveland, C. C., and Townsend, A. R. (2011). Functional Ecology of Free-Living Nitrogen Fixation: A Contemporary Perspective. Annu. Rev. Ecol. Evol. Syst. 42, 489–512. doi: 10.1146/annurev-ecolsys-102710-145034
Reed, S. C., Seastedt, T. R., Mann, C. M., Suding, K. N., Townsend, A. R., and Cherwin, K. L. (2007b). Phosphorus fertilization stimulates nitrogen fixation and increases inorganic nitrogen concentrations in a restored prairie. Appl. Soil Ecol. 36, 238–242. doi: 10.1016/j.apsoil.2007.02.002
Russow, R., Veste, M., and Bohme, F. (2005). A natural N-15 approach to determine the biological fixation of atmospheric nitrogen by biological soil crusts of the Negev Desert. Rapid Commun. Mass Spectrom. 19, 3451–3456. doi: 10.1002/rcm.2214
Seitz, S., Nebel, M., Goebes, P., Kappeler, K., Schmidt, K., Shi, X. Z., et al. (2017). Bryophyte-dominated biological soil crusts mitigate soil erosion in an early successional Chinese subtropical forest. Biogeosciences 14, 5775–5788. doi: 10.5194/bg-14-5775-2017
Sempeho, S. I., Kim, H. T., Mubofu, E., and Hilonga, A. (2014). Meticulous Overview on the Controlled Release Fertilizers. Adv. Chem. 2014, 1–16. doi: 10.1155/2014/363071
Stewart, W. D., and Bergersen, F. J. (1980). “Systems involving blue-green algae (Cyanobacteria),” in Methods for Evaluating Biological Nitrogen Fixation, ed. F. J. Bergersen (Chichester.: John Wiley & Sons Ltd).
Strauss, S. L., Day, T. A., and Garcia-Pichel, F. (2012). Nitrogen cycling in desert biological soil crusts across biogeographic regions in the Southwestern United States. Biogeochemistry 108, 171–182. doi: 10.1007/s10533-011-9587-x
Susha Lekshmi, S. U., Singh, D. N., and Shojaei Baghini, M. (2014). A critical review of soil moisture measurement. Measurement 54, 92–105. doi: 10.1016/j.measurement.2014.04.007
Torres-Cruz, T. J., Howell, A. J., Reibold, R. H., McHugh, T. A., Eickhoff, M. A., and Reed, S. C. (2018). Species-specific nitrogenase activity in lichen-dominated biological soil crusts from the Colorado Plateau, USA. Plant Soil 429, 113–125. doi: 10.1007/s11104-018-3580-2
Veluci, R. M., Neher, D. A., and Weicht, T. R. (2006). Nitrogen fixation and leaching of biological soil crust communities in mesic temperate soils. Microb. Ecol. 51, 189–196. doi: 10.1007/s00248-005-0121-3
Williams, W., Budel, B., and Williams, S. (2018). Wet season cyanobacterial N enrichment highly correlated with species richness and Nostoc in the northern Australian savannah. Biogeosciences 15, 2149–2159. doi: 10.5194/bg-15-2149-2018
Yeager, C. M., Kornosky, J. L., Morgan, R. E., Cain, E. C., Garcia-Pichel, F., Housman, D. C., et al. (2007). Three distinct clades of cultured heterocystous cyanobacteria constitute the dominant N2-fixing members of biological soil crusts of the Colorado Plateau, USA. FEMS Microbiol. Ecol. 60, 85–97. doi: 10.1111/j.1574-6941.2006.00265.x
Zhao, L., Liu, Y., Yuan, S., Li, Z., Sun, J., and Li, X. (2020). Development of archaeal communities in biological soil crusts along a revegetation chronosequence in the Tengger Desert, north central China. Soil Tillage Res. 196:104443. doi: 10.1016/j.still.2019.104443
Zhao, Y., Xu, M., and Belnap, J. (2010). Potential nitrogen fixation activity of different aged biological soil crusts from rehabilitated grasslands of the hilly Loess Plateau. China. J. Arid Environ. 74, 1186–1191. doi: 10.1016/j.jaridenv.2010.04.006
Zhou, X., Smith, H., Silva, A. G., Belnap, J., and Garcia-Pichel, F. (2016). Differential Responses of Dinitrogen Fixation, Diazotrophic Cyanobacteria and Ammonia Oxidation Reveal a Potential Warming-Induced Imbalance of the N-Cycle in Biological Soil Crusts. PLoS One 11:e0164932. doi: 10.1371/journal.pone.0164932
Keywords: nitrogenase (acetylene reducing) activity, nitrogen fixation, biocrust, vineyard, citrus orchard, agroecosystem
Citation: Sorochkina K, Strauss SL and Inglett PW (2022) Contrasting seasonal patterns and factors regulating biocrust N2-fixation in two Florida agroecosystems. Front. Microbiol. 13:892266. doi: 10.3389/fmicb.2022.892266
Received: 08 March 2022; Accepted: 11 July 2022;
Published: 03 August 2022.
Edited by:
Shubin Lan, Northeast Normal University, ChinaReviewed by:
Marina Gonzalez Polo, Instituto de Investigaciuones en Biodiversidad y Medioambiente (CONICET-UNCOMA), ArgentinaLindsey Christine Slaughter, Texas Tech University, United States
Copyright © 2022 Sorochkina, Strauss and Inglett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick W. Inglett, pinglett@ufl.edu
 Kira Sorochkina
Kira Sorochkina Sarah L. Strauss
Sarah L. Strauss Patrick W. Inglett
Patrick W. Inglett