- Department of Infectious Diseases and Immunology, College of Veterinary Medicine, University of Florida, Gainesville, FL, United States
Brucellosis is a disease of livestock that is commonly asymptomatic until an abortion occurs. Disease in humans results from contact of infected livestock or consumption of contaminated milk or meat. Brucella zoonosis is primarily caused by one of three species that infect livestock, Bacillus abortus in cattle, B. melitensis in goats and sheep, and B. suis in pigs. To aid in disease prophylaxis, livestock vaccines are available, but are only 70% effective; hence, improved vaccines are needed to mitigate disease, particularly in countries where disease remains pervasive. The absence of knowing which proteins confer complete protection limits development of subunit vaccines. Instead, efforts are focused on developing new and improved live, attenuated Brucella vaccines, since these mimic attributes of wild-type Brucella, and stimulate host immune, particularly T helper 1-type responses, required for protection. In considering their development, the new mutants must address Brucella’s defense mechanisms normally active to circumvent host immune detection. Vaccination approaches should also consider mode and route of delivery since disease transmission among livestock and humans is believed to occur via the naso-oropharyngeal tissues. By arming the host’s mucosal immune defenses with resident memory T cells (TRMs) and by expanding the sources of IFN-γ, brucellae dissemination from the site of infection to systemic tissues can be prevented. In this review, points of discussion focus on understanding the various immune mechanisms involved in disease progression and which immune players are important in fighting disease.
Introduction
Brucellosis remains a worldwide problem ranking third among eight neglected zoonotic diseases (Mableson et al., 2014), and is the most common zoonotic disease worldwide (Corbel, 1997; Godfroid et al., 2005). In humans, brucellosis is generally results from the consumption of unpasteurized dairy products or exposure to aerosols from infected livestock (Spink, 1956; Foley et al., 1970; Centers for Disease Control (CDC), 1983; Young, 1983; Chomel et al., 1994; Malik, 1997; Troy et al., 2005; de Figueiredo et al., 2015). In livestock, brucellosis was originally believed to be solely a sexually transmitted disease resulting in fetal abortion (Poester et al., 2013; Olsen and Palmer, 2014; de Figueiredo et al., 2015), but oropharyngeal infection is deemed to be the probable mode of transmission following exposure to an aborted fetus or birthing tissues (Olsen and Palmer, 2014; Cotterill et al., 2018). In fact, one study found that Brucella-infected bovine umbilicus contained 2 × 108 to 4 × 109 CFUs/g and bovine fetal cotyledons, 5 × 1011 to 1 × 1013 CFUs/g tissue (Alexander et al., 1981). Thus, brucellae can concentrate to high numbers, and following abortion, the placenta and fetus can expose herd members to high numbers of brucellae. On the human side, this Gram-negative species is highly infectious with less than 100 CFUs needed for blood borne exposure (Pappas et al., 2006) or less than 2,000 CFUs for pulmonary infection (Teske et al., 2011; Henning et al., 2012). Regardless of the route of infection, brucellae can disseminate systemically resulting in flu-like symptoms (Young, 1983; Malik, 1997; Troy et al., 2005; de Figueiredo et al., 2015). Since current vaccines are not completely protective in livestock, more efficacious brucellosis vaccines are needed to protect livestock and to prevent zoonosis. Vaccines for humans would also aid in protecting livestock producers.
This review considers alternate routes of vaccination, specifically, mucosal routes, specifically in the context of Brucella prophylaxis. Because natural infection generally occurs via crossing of the mucosal barrier, vaccination via this route should improve vaccine efficacy by mounting both local and distal responses of effector and memory B and T cells. While Brucella vaccination is often administered parentally in cattle and wildlife, mucosal vaccination via the conjunctival route is practiced in sheep as a strategy to reduce vaccine-induced abortions. While the T cell response is considered crucial to the Brucella vaccine-induced protective response, few studies have examined the mucosal T cell responses in ruminant species following conjunctival vaccination. Similarly, most vaccine studies in experimental animal models focus on the systemic response following parenteral vaccination. Thus, a void exists between the understanding host immunity and the routes of vaccination particularly in natural hosts. Past research in livestock species have focused on protection against abortion, serological responses, and peripheral blood T cell responses. More evaluations are needed in both natural hosts and laboratory animal models in determining additional parameters of protection against both abortion and infection. Infection prevention is particularly relevant when considering vaccine development for humans.
To meet this objective, the review first explores a historical perspective of brucellosis and its prevalence through the course of time followed by the etiology of this disease. We then describe current knowledge of host immunity to brucellosis, examining various cell-mediated immunity parameters that correlate with protection. A brief description of currently available vaccines is provided. With this background information, we move to studies examining mucosal routes of vaccination. We conclude describing the potential benefits of mucosal vaccination in both animal and human hosts.
Historical perspective
Brucellosis is believed to have been problematic for humans for at least several millennia or maybe longer dating to the domestication of goats and sheep. Some of the first suggestions of brucellosis infections in humans date back about 3,300 years, whereby a possible Brucella peptide signature was identified in cheese remnants found in Egyptian tombs (Greco et al., 2018). In addition, lesions in human vertebrae remains from Early Bronze Age and the Pompeii volcanic eruption in 79 AD are suggestive of Brucella infection (D'Anastasio et al., 2011). Not until 1887 was the etiological agent responsible for brucellosis discovered by the British surgeon, David Bruce, while serving in Malta. He first identified small coccobacilli causing “Malta Fever,” when isolated from the spleen of a victim (Bruce, 1889; Vassallo, 1992), and called it Micrococcus melitensis (Bruce, 1887). After its discovery in goats’ blood and later in their milk, consumption of goats’ milk was surmised to be the source of Malta Fever transmission. This suspicion was corroborated when the British military prohibited goat milk consumption by its Malta personnel, resulting in a dramatic decline in disease incidence (Vassallo, 1992). In 1895, the Danish veterinary pathologist, L. F. Benhard Bang, discovered the related, Bacillus abortus, responsible for abortion in cattle, and upon subsequent infection of an isolate, could induce abortion in cattle, sheep, and goats fulfilling Koch’s postulates (Bang, 1897; Bang, 1906). Based on the observation that both Bacillus abortus and Micrococcus melitensis induced abortion, presence in milk, and shared morphology and seroreactivity, Evans concluded these species were related (Evans, 1918). In 1920, in honor of Dr. Bruce, the three related species of M. melitensis, B. abortus, and B. suis were named in the new genus, Brucella (Vassallo, 1992).
Brucella etiology
Brucella, the pathogen
The Gram-negative Brucella species are highly homogeneous sharing more than 94% DNA homology (Wattam et al., 2009; Van der Henst et al., 2013; Whatmore and Foster, 2021), and 12 species have been identified as animal and human pathogens (Olsen and Palmer, 2014; Roop et al., 2021; Whatmore and Foster, 2021). Their genomes are composed of two circular chromosomes (Wattam et al., 2009; Roop et al., 2021). Brucella species are ubiquitous, infecting both land and marine animals (Guzmán-Verri et al., 2012; Roop et al., 2021; Whatmore and Foster, 2021). Three species are the primary cause of disease in livestock and can be problematic for humans due to incidental exposures. Brucella melitensis causes disease primarily in goats and sheep (Poester et al., 2013; Olsen and Palmer, 2014). Bacillus abortus is primarily a disease of cattle, but has been introduced into wildlife both in the United States and other countries (Poester et al., 2013; Olsen and Palmer, 2014). Brucella suis is primarily a disease of domestic and feral pigs (Corbel, 1997; Poester et al., 2013; Roop et al., 2021). Each of these is the primary cause of disease in humans, and the remaining 8 species are found in specific hosts (Wattam et al., 2009; Roop et al., 2021; Whatmore and Foster, 2021). However, opportunistic species have been reported in limited or rare human infections including, B. inopinata (Scholz et al., 2010; Roop et al., 2021), the amphibian B. inopinata-like (Rouzic et al., 2021), and B. canis (Roop et al., 2021). Recent genomic evidence suggests that a number of Ochrobactrum species as being related to Brucella, which would dramatically expand this genus (Hördt et al., 2020; Moreno et al., 2022).
Livestock disease
The four major Brucella pathogens responsible for livestock disease (Poester et al., 2013; Olsen and Palmer, 2014; Goodwin and Pascual, 2016) are B. abortus, B. melitensis, B. suis, and B. ovis, and each cause abortion (Bang, 1897; Godfroid et al., 2011; Poester et al., 2013; Byndloss and Tsolis, 2016). The absence of symptoms prior to abortion is problematic. Brucellosis is a chronic disease with possible swelling of lymph nodes near sites of infection. The most common clinical manifestation of animal brucellosis is reproductive loss resulting from abortion, birth of weak offspring, or infertility (Poester et al., 2013; Olsen and Palmer, 2014; Goodwin and Pascual, 2016; Rossetti et al., 2022). Brucella abortus localizes and replicates within the rough endoplasmic reticulum of trophoblastic epithelial cells in pregnant ruminants (Meador and Deyoe, 1989). However, placental infections frequently cause abortions after infection, particularly for the first offspring, and reduce fertility (Enright, 1990; Olsen and Palmer, 2014). Fetal pneumonia and necrotizing placentitis are implicated as the cause of abortion (Poester et al., 2013; Olsen and Palmer, 2014). Exposure to the aborted fetus and placenta is likely responsible for the persistence of B. abortus, B. melitensis, and B. suis in herds and populations. Though not zoonotic, B. ovis, is responsible for disease primarily in sheep, but is limited to mostly rams and transiently infect ewes (Ridler and West, 2011; Olsen and Palmer, 2014; Rossetti et al., 2022). Brucella ovis can cause epididymitis, which can result in infertility, and its presence in semen can be detected in rams with or without epididymitis (Ridler and West, 2011; Olsen and Palmer, 2014; Rossetti et al., 2022).
Human disease
Human brucellosis can be a debilitating disease, especially if untreated (Pappas et al., 2005, 2006; Franco et al., 2007; Cross et al., 2019). Brucellosis poses an occupational hazard common to abattoir workers, or by needle-stick by laboratory workers and veterinarians administering live brucellosis vaccines (Buswell et al., 2016; Pereira et al., 2020). Human brucellosis is more often acquired subsequent to consumption of unpasteurized dairy products (Chomel et al., 1994; Pappas et al., 2005; Baldi and Giambartolomei, 2013; Dadar et al., 2019; Adetunji et al., 2020). The American Academy of Pediatrics recommends against the consumption of unpasteurized milk by pregnant women and children (American Academy of Pediatrics, 2014). Brucellosis prevails along the Mediterranean rim, Middle East, Central Asia, South America, and the United States bordering Mexico (Chomel et al., 1994; Malik, 1997; Pappas et al., 2005; Corbel, 2006; Pappas et al., 2006). Its presence is attributed mostly to the inability to rid disease from livestock. The enormous cost of brucellosis to the livestock industry, as well as its impact on public health, has prompted many countries to adopt brucellosis control and eradication programs (Olsen and Stoffregen, 2005). In the United States, a brucellosis eradication program was established in 1954 aiding in the elimination of B. abortus infections from cattle. Vaccination of heifers using B. abortus strain 19 (S19), then subsequently with RB51, has been practiced to reduce the incidence of the disease and prevent B. abortus-induced abortions (Schurig et al., 2002). Ridding brucellosis from animal herds and pasteurization of dairy products reduce disease in humans.
The incidence of disease varies among the endemic regions (Pappas et al., 2006; Franco et al., 2007), but actual case numbers may be higher by as much as 26-fold due to misdiagnosis and underreporting (Franco et al., 2007; Hull and Schumaker, 2018). The high incidence of Brucella infections is attributed to the sustained prevalence of brucellosis in infected livestock (Young, 1983), that are the source of unpasteurized milk consumed in various dairy products (Pappas et al., 2005; Baldi and Giambartolomei, 2013; Dadar et al., 2019; Adetunji et al., 2020). In addition, a number of cases have been attributed to an aerosol exposure from Brucella-infected livestock (Corbel, 1997), laboratory acquired (Traxler et al., 2013), or an accidental biopharmaceutical release (Pappas, 2022). In the latter case, more than 10,000 individuals became infected with brucellosis (Pappas, 2022). However acquired, brucellosis is seldom (<0.5% of cases) life-threatening in humans (Ariza et al., 1995; Corbel, 1997), but human abortions occur, though rare [rev. in (Arenas-Gamboa et al., 2016)]. Acute disease manifests with flu-like symptoms such as fever, chills, malaise, headaches, with the presence of hepatomegaly and splenomegaly (Corbel, 1997; Pappas et al., 2005; Corbel, 2006; Franco et al., 2007). Despite rigorous antibiotic treatment, brucellosis can progress to a chronic disease exhibiting symptoms of relapsing undulant fever, protracted fatigue, and malaise (Young, 1983; Ariza et al., 1995; Franco et al., 2007; Baldi and Giambartolomei, 2013; Hull and Schumaker, 2018), and have positive Brucella blood cultures (Ariza et al., 1995; Corbel, 1997). Patients can further develop neurological complications, endocarditis, or arthritis (Rajapakse, 1995; Reguera et al., 2003; Shen, 2008; Adetunji et al., 2018; Lacey et al., 2018). Although Brucella is sensitive to antibiotics via a prolonged two-antibiotic regimen (Ariza et al., 1995; Corbel, 1997), sequelae can still remain in ~16% of the infected individuals (Ariza et al., 1995), of which 50% remain bacteremic (Ariza et al., 1995). Mucosal infection is the most likely means of brucellosis transmission, yet the absence of symptoms or pathology in the intestinal tract (Ariza et al., 1995; Ablin et al., 1997; Ron-Román et al., 2014) suggests that brucellae rapidly transverse the intestinal tract, local phagocytic cells become infected and leave this tissue, or that other mucosal sites are sensitive to infection. Oropharyngeal tissues are the most likely site of infection since pharyngitis is often observed (Carpenter and Boak, 1932; Poelma and Pickens, 1932; Suraud et al., 2007; Zachou et al., 2008; Ron-Román et al., 2014), and cervical lymph nodes are selectively infected (von Bargen et al., 2015; Rouzic et al., 2021). Treatment requires a combination therapy of antibiotics for 6 weeks generally involving doxycycline + streptomycin, doxycycline + gentamicin, or doxycycline + rifampin (Ariza et al., 2007; Bosilkovski et al., 2021).
Brucella immunology
Brucella, a stealth pathogen
As a means to sustain intracellular survival, Brucella has evolved a number of mechanisms to avoid host recognition and establish infection (Celli et al., 2019; Roop et al., 2021). Following bacteremia, macrophages are one of the principal cells targeted by Brucella to sustain infection (Gomes et al., 2012; Celli et al., 2019; Bhagyaraj et al., 2021). Once brucellae achieve intracellular infection, their elimination proves to be more difficult as these have a number of tools to evade the host immune system. As such, brucellae exist in Brucella-containing vacuoles (BCVs; Gomes et al., 2012; Celli et al., 2019; Roop et al., 2021), which traffic in the endocytic pathway incorporating endosomal membrane proteins, e.g., calreticulin and calnexin1, avoiding phagolysosome maturation and killing (Pizarro-Cerdá et al., 1998; Celli et al., 2003; Starr et al., 2008; Gomes et al., 2012; Celli et al., 2019; Bhagyaraj et al., 2021; Roop et al., 2021) in a VirB-dependent fashion (Celli et al., 2003; Starr et al., 2008; Gomes et al., 2012; Roop et al., 2021). To avoid TLR4 and other LPS-sensitive innate detection sensors, Brucella expresses a low endotoxic LPS (Forestier et al., 2000; Martirosyan et al., 2011; Conde-Álvarez et al., 2012; Byndloss and Tsolis, 2016). Brucella can also infect dendritic cells (Bhagyaraj et al., 2021), and suppress dendritic cell maturation (Salcedo et al., 2008). To interfere with TLR2 and TLR4 signaling, Brucella produces TcpB, an analog for mammalian Toll/interleukin 1 receptor (TIR) domain-containing adaptor protein (TIRAP), to suppress NF-κB activation and cytokine secretion (Alaidarous et al., 2014; Snyder et al., 2014). TcpB can also degrade caspases 1, 4, and 11, and ultimately suppress IL-1 production (Jakka et al., 2017). To minimize host adaptive immune responses, Brucella has the capacity to interrupt antigen presentation via inhibition of MHC class I (Barrionuevo et al., 2013; Velásquez et al., 2017; Barrionuevo and Giambartolomei, 2019) and class II molecules (Barrionuevo et al., 2008; Velásquez et al., 2017; Barrionuevo and Giambartolomei, 2019; Milillo et al., 2019).
Type 1 IFNs are often deemed important for anti-viral defense (McNab et al., 2015; Takaoka and Yamada, 2019), and few studies have considered the role of type 1 IFNs following infection with wild-type (wt) Brucella (de Almeida et al., 2011; Gorvel et al., 2014; Khan et al., 2016; Costa Franco et al., 2018; Guimarães et al., 2019). Brucella infection interferes with monocytic DC maturation (Gorvel et al., 2014). The induction of the type 1 IFN pathway was found to be sensing stimulator of interferon genes (STING)-dependent evidenced by the recognition of both Brucella’s DNA (de Almeida et al., 2011; Costa Franco et al., 2018) and c-di-GMP (Khan et al., 2016; Costa Franco et al., 2018; Guimarães et al., 2019). Wt Brucella showed enhanced splenic colonization in STING−/− mice (Costa Franco et al., 2018; Khan et al., 2020) supporting the notion that wt B. melitensis can suppress STING early in infection.
Th1 cell immunity and brucellosis
Cellular immunity is essential for protection against brucellosis (Table 1). An inflammatory or T helper (Th)1 cell response is required to eliminate brucellae in an IL-12- (Zhan and Cheers, 1995) and TNF-α-dependent manner (Zhan and Cheers, 1998) for the stimulation of IFN-γ. As a result, protection to Brucella is abated in IFN-γ−/− mice (Murphy et al., 2001; Skyberg et al., 2012) further supporting the importance of IFN-γ to protection. Yet, there may exist alternative or cooperative pathways of protection. One critical observation is that the degree of susceptibility in IFN-γ−/− mice varied between C57BL/6 (B6) and BALB/c backgrounds. B6 IFN-γ−/− mice are more highly susceptible to death sooner than BALB/c IFN-γ−/− mice (Murphy et al., 2001; Skyberg et al., 2012). Moreover, vaccination of BALB/c IFN-γ−/− mice with ΔznuA B. melitensis mutant did show reduced brucellae colonization from challenge suggesting other mechanisms that can contribute to immune protection (Clapp et al., 2011, 2016), and these vaccinated IFN-γ−/− mice did not show increased susceptibility to death. Both strains of IFN-γ−/− mice showed increased susceptibility to osteoarthritis (Skyberg et al., 2012; Lacey et al., 2016). In fact, Brucella-infected patients with reduced IFN-γ capacity showed increased sensitivity to osteoarticular complications (Rafiei et al., 2006; Hedayatizadeh-Omran et al., 2010).
Essential sources of IFN-γ include CD4+ (Murphy et al., 2001; Vitry et al., 2012; Yingst et al., 2013; Vitry et al., 2014; Yang et al., 2016), CD8+ T cells (Clapp et al., 2011; Durward-Diioia et al., 2015; Clapp et al., 2016; Yang et al., 2016; Wang et al., 2020), or both (Araya et al., 1989; Hanot Mambres et al., 2016; Goodwin et al., 2022). NK cells also provide IFN-γ to activate macrophages and DCs (Dornand et al., 2004; Bhagyaraj et al., 2021). The role of CD4+ and CD8+ T cells has been extensively studied to learn correlates of protection against brucellosis (Table 1). How IFN-γ-producing T cells are elicited is dependent upon the Brucella mutant or vaccine strain used, as well as, its mode of delivery for vaccination (Table 1). Clearly, CD4+ Th1 cells provide a primary source of IFN-γ to combat Brucella infection (Zhan et al., 1995; He et al., 2001; Vitry et al., 2012; Yingst et al., 2013; Vitry et al., 2014), and vaccination by the parenteral route with livestock vaccines elicited mostly CD4+ T cell-dependent responses (Table 1). In one study, protection was deemed CD4+ T cell-dependent since orally vaccinated CD8−/− mice with a purine auxotrophic B. melitensis mutant were not protected against virulent B. melitensis challenge (Yingst et al., 2013). In a separate study, vaccination of MHC class II−/− mice was accomplished using a low-dose parenteral infection with wt B. melitensis 16M followed by antibiotic treatment. When challenged with wt B. melitensis 16M, these mice showed elevated splenic brucellae burden and reduced IFN-γ-producing cells when compared to similarly vaccinated and challenged immunocompetent mice (Vitry et al., 2014). In a subsequent study (Hanot Mambres et al., 2016), a similar strategy was applied with a nasal wt B. melitensis 16M infection of MHC class II−/− and TAP1−/− mice followed by antibiotic treatment prior to nasal challenge with wt B. melitensis 16M. Both MHC class II−/− and TAP1−/− mice showed equivalent protection to similarly immunized and challenged immunocompetent mice. The investigators concluded that the source of IFN-γ-producing cells can be either CD4+ or CD8+ T cells for conferring protection. Such results corroborate findings from an earlier study where adoptive transfer of immune CD4+ or CD8+ T cells from B. abortus S19-vaccinated mice reduced splenic brucellae colonization following challenge of recipients with wt B. abortus (Araya et al., 1989). Thus, based upon these findings, dependence on CD4+ T cell immunity for protection against virulent Brucella challenge is influenced by the source of the immunizing Brucella strain used (Table 1).
CD8+ T cell immunity and brucellosis
The lack of memory CD8+ T cells has been suggested as a means for enabling brucellae persistence (Durward-Diioia et al., 2015). In contrast, others suggest that CD8+ T cell immunity is expandable for brucellosis (Vitry et al., 2012; Yingst et al., 2013; Vitry et al., 2014). Only a few studies have investigated the role of CD8+ T cells in immunity to Brucella infections (Table 1). One study focused on using mice deficient of their immunoproteasome (lacking β1i, β2i, and β5i subunits) to minimize MHC class I antigen presentation. Infection of these mice with wt B. abortus 2308 resulted in nearly complete loss of IFN-γ+ CD8+ T cells, as well as, a substantive reduction in IFN-γ+ CD4+ T cells (Guimarães et al., 2018). Such evidence points to CD8+ T cells contributing to IFN-γ generation. CD8+ T cell immunity may also be influenced by targeted mutations made in Brucella to attenuate its infection. One notable mutation was the deletion of znuA, a gene involved in zinc uptake, as a means to inactivate zinc-dependent enzymes in Brucella, particularly its superoxide dismutase. The ΔznuA B. abortus mutant exhibited diminished growth capacity in macrophages and rendered protective qualities, when administered parenterally, similar to conventional B. abortus S19 and RB51 vaccines (Yang et al., 2006).
The route of vaccination may also influence the T cell response elicited (Table 1). Adapting this same mutation in B. melitensis resulted in an attenuation signature similar to that of ΔznuA B. abortus. Nasal vaccination with the ΔznuA B. melitensis mutant showed a T cell bias noted by the stimulation of IFN-γ+ CD8+ T cells than by CD4+ T cells (Clapp et al., 2016). Such attribute posed an interesting question of whether the preferential stimulation of CD8+ T cells was due to the mutation or the mode of vaccination. To test this question, a second mutation was introduced into ΔznuA B. abortus. The norD gene, which encodes for nitric oxide reductase was selected since it showed a modest reduction in virulence as a single gene deletion in B. suis (Loisel-Meyer et al., 2006). The development of the double ΔznuA ΔnorD B. abortus mutant carrying the lacZ reporter gene (znBAZ) prompted testing via parenteral immunization resulting in the stimulation of both IFN-γ-producing and polyfunctional CD4+ and CD8+ T cells (Yang et al., 2016). Yet, the numbers of splenic IFN-γ+ and polyfunctional CD4+ T cells exceeded the counterparts for CD8+ T cells.
To learn how these mutations in Brucella may influence the types of T cell responses elicited following mucosal vaccination (Table 1), an oral prime, nasal boost vaccination regimen was devised for znBAZ (Wang et al., 2020). The notable attribute that distinguished znBAZ’s immunogenicity was the number of IFN-γ+ CD8+ and polyfunctional CD8+ T cells doubled those of IFN-γ+ CD4+ and polyfunctional CD4+ T cells present in the lungs. Similarly primed, boosted mice with RB51 showed no bias for IFN-γ+ CD8+ T cells, mostly being IFN-γ+ CD4+ T cells (Wang et al., 2020). Another key attribute found with orally primed, nasally boosted mice with znBAZ is the induction of resident memory T cells (TRMs; Table 1). TRMs are distinguished by their expression of memory markers, CD44high L-selectin− CD69+ and either CD103+ or CD103−, and reside in the mucosal epithelium (Turner et al., 2013; Mueller and Mackay, 2016; Szabo et al., 2019). The mucosal epithelium is where exposure to wt Brucella is most likely to occur; hence, the need to arm the epithelium with memory T cells is deemed essential to eradicate Brucella-infected cells. As a result, both CD4+ and CD8+ TRMs were elicited to greater levels subsequent to znBAZ vaccination than the level seen in those mice similarly vaccinated with RB51 (Wang et al., 2020). In a similar vein, the same genetic mutations were performed to generate the ΔznuA ΔnorD B. melitensis mutant carrying the mCherry reporter gene (znBM-mC; Goodwin et al., 2022). As accomplished with znBAZ vaccination, CD4+ and CD8+ TRMs were induced in the lungs of mice orally primed, nasally boosted with znBM-mC (Table 1), but TEMs and TCMs were also detected (Goodwin et al., 2022). This evidence suggests that the mode of vaccination can contribute to types of IFN-γ+ and polyfunctional T cells, as well as to the TRMs induced, but cannot diminish the influence of the Brucella’s mutations used to develop these strains.
Th17 cell immunity and brucellosis
As suggested above, alternative immune players may contribute to protection against Brucella infections. One such possibility examined is the role of a different proinflammatory cytokine, IL-17. IL-17, important for protection against various extracellular mucosal pathogens, is derived from innate and adaptive lymphocytes (Mills, 2022). IL-17 is involved in neutrophil recruitment and promotes IL-22 and antimicrobial peptide production (Mills, 2022). Although often overlooked, IL-17 has had varied impact on protection against brucellosis, but IL-17 may be more relevant upon extracellular brucellae release from killed host cells. For example, mice vaccinated with ΔznuA Brucella mutants and treated in vivo with an anti-IL-17 antibody (Ab) showed significant increases in colonization suggesting a role for IL-17 and protection (Clapp et al., 2011; Pasquevich et al., 2011; Clapp et al., 2016). Although IL-17 production is augmented in mucosally vaccinated IFN-γ−/− mice (Clapp et al., 2011, 2016), IL-17 production is not linked to the development of osteoarthritis in Brucella-infected IFN-γ−/− mice (Skyberg et al., 2012). In contrast, oral prime, nasal boost with znBAZ did elicit IL-17+ CD4+ and CD8+ TRMs, but in vivo neutralization of IL-17 during the post-challenge phase failed to reverse znBAZ’s protective qualities, i.e., the diminished lung and splenic colonization by wt B. abortus 2308 remained intact (Wang et al., 2020). However, the spleens from IL-17−/− mice that were orally primed, nasally boosted with znBM-mC showed increased brucellae colonization following pulmonary challenge with wt B. melitensis 16M suggesting that IL-17 may be important for maintaining systemic protection (Goodwin et al., 2022). Nasal infection with virulent B. melitensis 16M minimally impacted brucellae colonization of the lungs and spleen 4 weeks post-infection of IL-17RA−/− and IL-23p19−/− mice, but did enable greater colonization of the lungs at 5 and 12 days post-infection of IL-17RA−/− mice. This suggests IL-17’s role may be more relevant early in infection (Hanot Mambres et al., 2016), possibly tied to neutrophil recruitment. IL-17 derived from γδ T cells is also thought to be important early in B. abortus infection (Skyberg et al., 2011).
Th17 cells have been reported to produce GM-CSF and IL-22 following RORγt activation (Liang et al., 2006; Zheng et al., 2007; Codarri et al., 2011; El-Behi et al., 2011). Some consider IL-22-producing T cells as a separate entity, e.g., Th22 cells (Dudakov et al., 2015), since Runx1 has been recently found to coactivate RORγt (Sekimata et al., 2019). The stimulation of IL-22 is associated with protection of the mucosal epithelium by enhancing the epithelial barrier and increasing defensins production (Valeri and Raffatellu, 2016), and is responsible for stimulation of the heme scavenger, hemopexin, to sequester iron from bacteria (Sakamoto et al., 2017). Examination of IL-22’s role was pursued subsequent mucosal vaccination with ΔznuA B. melitensis, and IL-22 production was found to be augmented in both immunocompetent and IFN-γ−/− mice (Clapp et al., 2011, 2016). For nasally ΔznuA B. melitensis-vaccinated mice, IL-22 was mostly derived from CD8+ T cells (Clapp et al., 2016). Another consideration for IL-22’s relevance is the observed lack of intestinal infection by Brucella (Ariza et al., 1995; Ablin et al., 1997). IL-22 production was augmented following oral ΔznuA B. melitensis vaccination (Clapp et al., 2011). Oral infection remains to be tested in IL-22−/− mice to determine if increased intestinal pathology occurs because of a reduced barrier. However, IL-22−/− mice infected parenterally with virulent B. melitensis 16M showed only a minimal impact early in infection with a modest reduction in IFN-γ+ CD4+ T cells (Vitry et al., 2012).
γδ T cells and brucellosis
γδ T cells serve as sentinels in mucosal surfaces and respond to infectious agents via their variable TCRs and pathogen recognition receptors (Holderness et al., 2013). These cells are an important source of IL-17 (Agerholm and Bekiaris, 2021), but also responsible for the production of other proinflammatory cytokines including IFN-γ (Skyberg et al., 2011; Demars et al., 2019). γδ T cells contribute to early innate cell activation noted by the increased brucellae burden following infection of TCRδ-deficient mice with wt B. abortus 2308 (Skyberg et al., 2011) or wt B. melitensis 16M (Hanot Mambres et al., 2016). Consistent with the notion of early involvement, macrophages in the presence of γδ T cells benefitted by the enhanced clearance of infecting brucellae (Skyberg et al., 2011). However, γδ T cells’ impact is not relevant during the late phase of infection evidenced by the lack of differences in brucellae tissue burdens when compared to similarly infected wt mice (Skyberg et al., 2011; Hanot Mambres et al., 2016). Instead, γδ T cells’ contribution to brucellae clearance from tissues may be dependent upon route of exposure (Skyberg et al., 2011; Hanot Mambres et al., 2016; Demars et al., 2019). TCRδ-deficient mice given an intradermal infection with wt B. melitensis 16M showed no difference in brucellae tissue burdens from wt mice at early or late time points (Demars et al., 2019), suggesting that peripheral γδ T cells may be less impacted by Brucella infections than mucosal γδ T cells (Hanot Mambres et al., 2016).
Livestock T cell immunity
The above studies focused primarily on results obtained in mice to learn relevant correlates of protection. Such studies are often more difficult to conduct in the natural host due to limitations in readily available genetic models to test various hypotheses. Nonetheless, significant knowledge can still be extrapolated from rodent studies and in vitro studies using livestock lymphocytes. Surprisingly, only a few studies have delved into studying IFN-γ-producing T cell responses in livestock following infection with wt Brucella or subsequent vaccination. These studies also varied in their mode of Brucella delivery. Additionally, studies that did investigate host T cell responses mostly relied upon peripheral blood, not tissue-derived T cells. The limitation of peripheral blood T cell responses is that these lymphocytes are ever evolving, and timing is critical to capture the moment of optimal responsiveness. As in the case of one study, cows were conjunctivally infected with B. suis or B. abortus, and their peripheral blood CD4+ T cells were shown to produce IFN-γ following in vitro Ag restimulation using Brucellergene®, (a preparation of cytoplasmic proteins derived from a rough mutant of B. melitensis; Weynants et al., 1998), Little IFN-γ production was detected for CD8+ or γδ T cells (Weynants et al., 1998). However, in a separate study, bovine γδ T cells could aid in activating macrophages in vitro via IFN-γ, suggesting that γδ T cells may be more important early during the infection if sufficiently activated (Skyberg et al., 2011). Another study investigated IFN-γ production by peripheral blood T cells from intramuscular (IM) RB51-vaccinated heifers, and following Ag restimulation, the induced IFN-γ was mostly derived from CD4+ T cells (Boggiatto et al., 2020). However, one study did pursue an evaluation of memory T cells (up to one and one-half years) and various cytokine responses by peripheral blood T cells from subcutaneously (SC) RB51-vaccinated calves (Dorneles et al., 2015). These heifers were dosed twice with RB51 one year apart. While both CD4+ and CD8+ T cells showed increased cytokine production following in vitro Ag restimulation, the majority of the IFN-γ and IL-17 came from CD4+ T cells (Dorneles et al., 2015). Granzyme B+ and perforin+ CD8+ T cells were also augmented subsequent the RB51 boost.
An in-depth immune analysis was performed following conjunctival infection with wt B. melitensis H38S of sheep (Suraud et al., 2008). Brucellae colonization of various mucosal sites including nasal and eye secretions, eyelids and lacrimal glands, tonsils, various head and neck lymph nodes (HNLNs), and distal mesenteric LNs, precapsular LNs, and spleen were monitored up to 4 weeks post-infection. Increases in IFN-γ production following Brucella Ag-restimulation of regional and distal LN lymphocytes were observed (Suraud et al., 2008). A separate study examined T cell profiles following variable SC dosing of sheep with Rev. 1 vaccine, and found elevated CD4+ and CD8+ T cell levels, but did not discern whether these CD8+ T cells included γδ T cells (Curina et al., 2018). Total memory T cells were elevated, but did not distinguish between CD4+ and CD8+ T cells. Another study measured T cell responses to bp26 and Omp31 peptides in sheep SC vaccinated thrice with B. melitensis M5-90 vaccine, and found elevated IFN-γ responses mostly derived from CD4+ T cells (Wang et al., 2014). The investigators speculated that regulatory T cells (Tregs) were increased because of the increased CD25 expression, but did not confirm Foxp3 co-expression, regulatory cytokine production, nor functional measurements. While Tregs may express CD25, CD25 is also indicative of activated T cells, so whether the elevated levels measured were truly all Tregs or a mixture with activated T cells is unclear. One study evaluated CD4+ and γδ T cell responses following infection of pregnant goats (11 weeks of gestation) with wt B. melitensis 16M or B. melitensis Rev. 1 vaccine (Higgins et al., 2018). They did find a significant increase in the number of IFN-γ-producing γδ T cells 4 weeks post-infection from Rev. 1-infected goats compared to those infected with wt B. melitensis 16M.
B cell immunity and brucellosis
The role of B cells in brucellosis is less understood since induced Abs only modestly or do not protect against Brucella challenge (Vitry et al., 2012; de Figueiredo et al., 2015), and B cell-deficient (μMT) mice are found to be more resistant to Brucella infection (Goenka et al., 2011; Vitry et al., 2012; Dadelahi et al., 2020). Anti-Brucella polysaccharide Abs have less value (Vitry et al., 2012; Boggiatto et al., 2020) than cell-mediated immunity in protection to brucellosis (Godfroid et al., 2005; Goenka et al., 2012; Vitry et al., 2012, 2014; de Figueiredo et al., 2015; Yang et al., 2016; López-Santiago et al., 2019; Wang et al., 2020). Passive transfer of anti-Brucella serum was shown to diminish splenic B. abortus S19 colonization (Araya et al., 1989), as well as, the passive transfer of an O-polysaccharide-specific mAb for prevention of virulent B. abortus in infection (Winter et al., 1989); however, cell-mediated immunity is still required for protection (Jiang and Baldwin, 1993; Zhan and Cheers, 1995; Rodriguez-Zapata et al., 1996; Zhan and Cheers, 1998; Murphy et al., 2001; Ko et al., 2002; Skyberg et al., 2011; Bhagyaraj et al., 2021).
Interestingly, human and animal B cells can be directly infected by Brucella (Bratescu et al., 1981; Goenka et al., 2011, 2012; Pesce Viglietti et al., 2016; García-Gil et al., 2019). Consequently, B cells may serve as a reservoir since Brucella cannot replicate in B cells (Goenka et al., 2012; Pesce Viglietti et al., 2016), which in turn enables the development of immunosuppressive B cells (Atluri et al., 2011; Goenka et al., 2011, 2012; Spera et al., 2014). Hence, brucellae can persist and sequester in B cells. Further inquiry into B cells’ role in immunity has found that μMT mice were more resistant to B. abortus infection than immunocompetent mice (Goenka et al., 2011). Since B. abortus can also infect B cells, infection results in the production of B cell-derived TGF-β1, whose anti-inflammatory property may exacerbate chronic Brucella infection (Goenka et al., 2012). In fact, adoptive transfer of B and CD4+ T cells into Rag1−/− recipients dampened the protective effects of only transferring CD4+ T cells against wt B. melitensis 16M infection (Dadelahi et al., 2020), further suggesting that infection of B cells leads to suppression of host inflammatory responses (Atluri et al., 2011; Goenka et al., 2011, 2012; Spera et al., 2014).
Brucella vaccines
Live Brucella vaccines
As noted, stimulation of Th1-type immunity derived from CD4+ or CD8+ T cells is required for protection to eliminate brucellae from the intracellular compartment (Martirosyan et al., 2011; de Figueiredo et al., 2015; Pascual et al., 2018). Given the high DNA homology among Brucella species (Wattam et al., 2009; Whatmore and Foster, 2021), vaccination against one species can protect against infection from heterologous Brucella species. Current vaccines are limited to four live vaccines used for protecting livestock: B. abortus strain 19 (S19) for cattle; rough B. abortus RB51 for cattle; B. melitensis Rev. 1 for sheep and goats; and B. suis strain 2 (S2) for pigs (Olsen and Palmer, 2014; Byndloss and Tsolis, 2016; Goodwin and Pascual, 2016). No vaccines exist for humans, although S19 had been used to vaccinate livestock workers in the former Soviet Union to successfully diminish the incidence of brucellosis (Vershilova, 1961). When the same S19 isolate from P. A. Vershilova along with Rev. 1 were tested in United States volunteers, four of the S19 vaccinees exhibited undesirable sequelae with two recipients being hospitalized; 11 of 16 Rev. 1 vaccinees also showed undesirable sequelae with four of them requiring hospitalization (Spink et al., 1962). A subsequent study using a reduced dose of Rev. 1 still produced some symptoms in vaccinees (Pappagianis et al., 1966).
Bacillus abortus strain 19 vaccine
Derived from a spontaneously attenuated isolate, the B. abortus S19 has a 703 base pair deletion of the erythritol catabolic genes (Sangari et al., 1994). S19 was pursued as a cattle vaccine (Buck, 1930), and proved to be effective against brucellosis-induced abortion in cattle (Confer et al., 1985), though its efficacy is only 70% (Lubroth et al., 2007). S19 can also be used therapeutically to reduce incidence of new infections in existing Brucella-infected herds (Olsen and Stoffregen, 2005). For susceptible herds, S19 is given to female calves at 3–6 months of age as a single SC dose or to adults at reduced SC or conjunctival dose (Nicoletti, 1990; World Animal Health Organization, 2014). Since S19 still retains its LPS and produces a possible positive serology test, the vaccination regimen for heifers permits protection with a lessened chance of a persistent Ab-reactive response, and also reduces the likelihood of vaccine-induced abortion and vaccine excretion into milk (World Animal Health Organization, 2014).
Bacillus abortus RB51 vaccine
A spontaneous rough mutant was selected after repeated passages of wt B. abortus 2308 on rifampin- and penicillin-containing media, and led to the derivation of B. abortus RB51 strain (Schurig et al., 1991). RB51’s mutation is due to an interruption of the enzyme, wboA glycosyltransferase, that is involved in O-Ag biosynthesis (Vemulapalli et al., 1999). The advantage of RB51 as a vaccine is its lack of O-Ag (LPS), thus providing a method to distinguish vaccinated from naturally B. abortus-infected animals. Since RB51 fails to produce a positive serological reaction by conventional tests, its use allows ease for diagnosis of positive Brucella reactivity (Stevens et al., 1994).
Vaccination of cattle with RB51 proved to be safe and efficacious in precluding Brucella-induced abortion and fetal infection (Olsen, 2000). Efficacy with RB51 is age-dependent noted by the responsiveness to calf vaccination at >5–6 months of age showing no abortions by pregnant mature cows (2–3 years of age) subjected to B. abortus abortion challenge. In contrast, calves vaccinated too young at 3 months of age showed reduced efficacy when challenged as pregnant adults (Cheville et al., 1996; Olsen and Stoffregen, 2005). RB51’s efficacy was found to be similar to S19 (Olsen, 2000). The United states and many other countries have since replaced S19 with RB51 as part of their brucellosis eradication program.
Brucella melitensis Rev. 1 vaccine
Originating from an avirulent streptomycin-dependent strain, B. melitensis Rev. 1, was subsequently derived as an isolated revertant that became streptomycin-resistant (Herzberg and Elberg, 1955). Rev. 1 vaccine is administered either as a SC injection or via the conjunctiva to sheep and goats, and is protective against virulent B. melitensis abortion challenge (Olsen and Stoffregen, 2005). However, Rev. 1 can induce vaccine-induced abortion in pregnant animals, and small ruminants are generally not vaccinated when pregnant (Olsen and Stoffregen, 2005; Byndloss and Tsolis, 2016). Although effective against B. melitensis-induced abortion, Rev. 1 being a smooth vaccine makes it difficult to distinguish vaccinated from naturally infected animals. Rev. 1 is also effective in protecting against other Brucella species (Byndloss and Tsolis, 2016).
Brucella suis S2 vaccine
Brucella suis, responsible for abortion of fetal pigs, is not problematic for United States pork producers since its eradication from commercial herds in 2011, but it does threaten cattle where feral pigs are problematic (Olsen and Tatum, 2016; Franco-Paredes et al., 2017). Brucella suis infections of swine remain an international problem (Olsen and Tatum, 2016; Franco-Paredes et al., 2017). The B. suis strain 2 (S2) vaccine was developed in China from an isolate taken from an aborted B. suis-infected fetal pig, and then subsequently attenuated by repeated passages (Xin, 1986; Zhu et al., 2016). S2 is administered orally via drinking water, and has been successfully used in China since 1971 to reduce brucellosis incidence in swine (Xin, 1986; Zhu et al., 2016; Hou et al., 2019). S2 has also been shown to be effective against infection by other Brucella species (Xin, 1986; Bosseray and Plommet, 1990; Zhu et al., 2016; Hou et al., 2019).
Mucosal approaches to brucellosis vaccine delivery
Advantages of mucosal vaccinations
Brucellosis disseminates systemically regardless of the route of exposure (Ko and Splitter, 2003; Atluri et al., 2011; de Figueiredo et al., 2015). A striking advantage of mucosal vaccination is that it arms the mucosa near the sites of infection (Silva-Sanchez and Randall, 2019; Kiyono et al., 2021; Lavelle and Ward, 2022; Mettelman et al., 2022) resulting in the stimulation of memory T cells that prevent reinfection (Hirahara et al., 2021; Lange et al., 2022; Zheng and Wakim, 2022; Figure 1). In addition to stimulating mucosal immunity, mucosal vaccination results in systemic immunity (Silva-Sanchez and Randall, 2019; Kiyono et al., 2021; Lavelle and Ward, 2022; Mettelman et al., 2022). Thus, a key advantage of mucosal vaccination is its capacity to confer protective immunity in both mucosal and systemic tissues. In fact, infection with Brucella is the result of a mucosal exposure (Goodwin and Pascual, 2016; Pascual et al., 2018; López-Santiago et al., 2019).
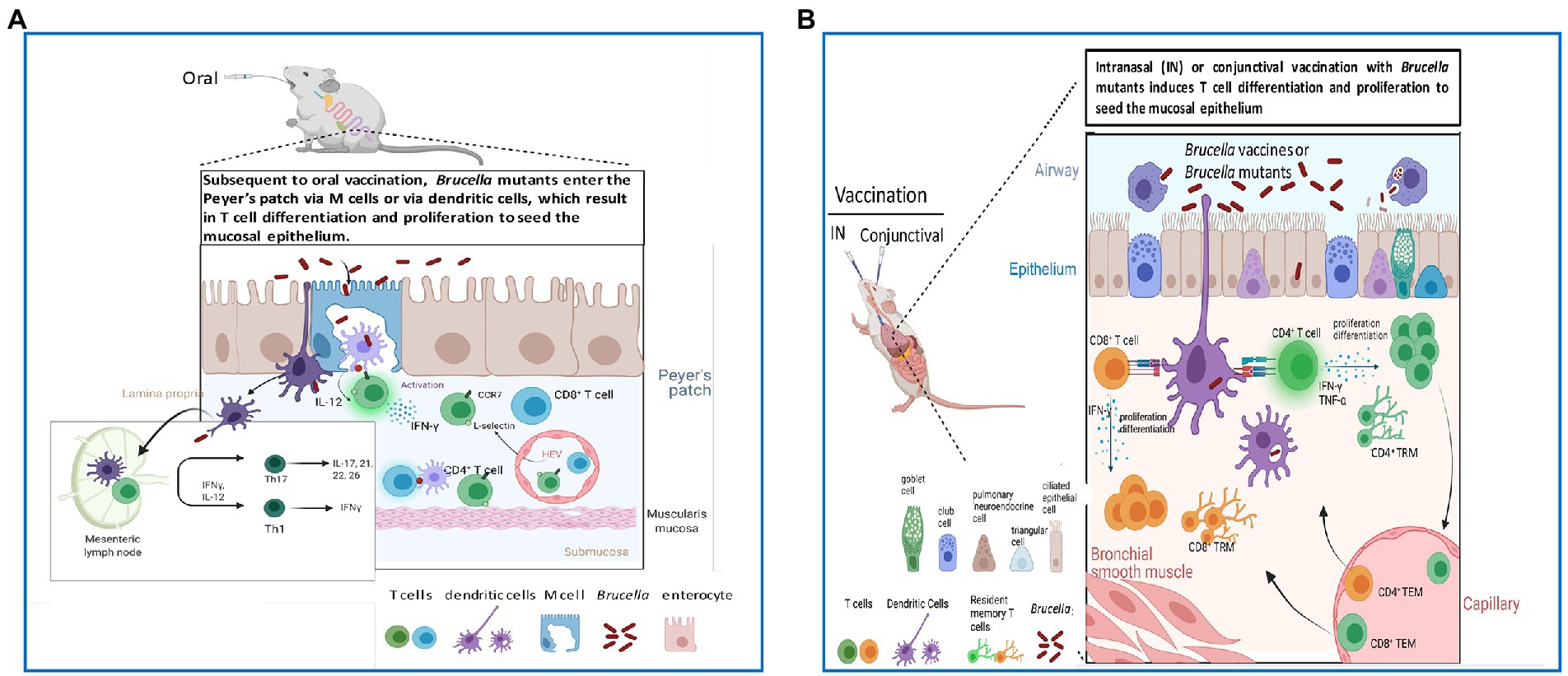
Figure 1. Mucosal routes of vaccination improve host capacity to target T cells to infiltrate the epithelium to combat Brucella pathogens. (A) Oral vaccination with Brucella vaccines or mutant strains enables stimulation of IFN-γ-producing T cells in the Peyer’s patch and eventual dissemination into regional lymph nodes (LNs) and mucosal epithelium. (B) Intranasal (IN) and conjunctival vaccination with Brucella vaccines or mutant strains enables induction of resident memory T cells (TRMs) to reside in the epithelium to combat reinfection with virulent Brucella.
Oral infection with Brucella is believed to be mediated via the prion protein expressed on intestinal microfold cells (Ackermann et al., 1988; Paixão et al., 2009; Nakato et al., 2012; Figure 1), but brucellae are unable to infect gut tissues (Ablin et al., 1997; Delpino et al., 2007). Rather, infections are believed to be more localized to the lymphoid tissues associated with the naso-oropharyngeal lymph nodes (Meador et al., 1988) resulting from animals sniffing or licking Brucella-infected aborted fetuses and/or infected placental tissues (Samartino and Enright, 1993; Schumaker, 2013), and possibly spread through grooming (Rhyan et al., 2019). Similar sites of sensitivity, e.g., tonsils, are also evident in infected humans (Carpenter and Boak, 1932; Poelma and Pickens, 1932; Suraud et al., 2007; Zachou et al., 2008; Ron-Román et al., 2014).
Vaccination via naso-oropharyngeal routes offers a potent means for inducing mucosal immunity both locally and at distal immune sites. The nasopharyngeal-associated lymphoid tissue (NALT) and various HNLNs support induction of this process (Csencsits et al., 2002). The HNLNs include the facial or parotid gland LNs (PrLNs); the submandibular gland LNs (SMLNs) also referred to as the superficial cervical LNs; and the deep cervical LNs (CLNs) dorsal to the brachial plexus deep within the musculature of the neck (Tilney, 1971). The SMLNs drain the nasal submucosa, while the CLNs drain the NALT (Tilney, 1971; Hameleers et al., 1990; Kuper et al., 1992). In contrast, the PRLNs are responsible for draining the skin of the head and neck as well as the conjunctiva (Collins, 1978; Freeman and Troutt, 1985; Cserr and Knopf, 1992; Wu et al., 1997; Cashion et al., 1999; Dickstein et al., 1999). It is believed that aerosolization of bacteria infects via the conjunctiva (Collins, 1978) since spread of Salmonella was prevented in goggled guinea pigs (Moore, 1957). Vaccination via the conjunctiva resembles nasal vaccine administration in that similar draining LNs, e.g., SMLNs and PrLNs, are stimulated (Blasco, 1997; Seo et al., 2010). Many of the same regional and distal compartments acquire immune B and T cells noted by the presence of secretory IgA in the tears, saliva, and nasal vaginal washes (Seo et al., 2010). Thus, nasal and conjunctival vaccine delivery impacts cellular immunity presence in the various effector tissues in the head and neck, e.g., naso-oropharyngeal tissues, salivary glands, lungs, and genitourinary tract (Hameleers et al., 1990; Wu et al., 1997; Csencsits et al., 2001, 2002; Seo et al., 2010; Kiyono et al., 2021; Lavelle and Ward, 2022; Mettelman et al., 2022). Given the shared homing signaling among these tissues, vaccination via the naso-oropharyngeal and conjunctival tissues can provide regional, systemic, and distal immunity.
Despite the fact that Brucella most commonly infects via the oral route for both animals and humans, many brucellosis vaccines are parenterally administered both for convenience, and experimentally to mimic systemic disease. Vaccination of a large number of livestock in a short time frame may prove cumbersome and subject the vaccinator to the risk of needle-stick injuries (Buswell et al., 2016; Pereira et al., 2020). The alternative is consideration of mucosal delivery methods. Mucosal vaccinations can circumvent needle-stick injuries since mucosal delivery is needle-free. However, there are limitations as well in mechanizing or implementing mucosal vaccinations for a large number of animals within a limited time period. Nasal vaccinations have the concern of possible draining or sneezing nasal fluids back onto the applicator. Oral vaccination may require gavaging, which may require animal restraint, to administer successfully. Alternatively, food may be mixed with the vaccine (Edmonds et al., 2001; Rhyan et al., 2019) or supplied in the drinking water as done for B. suis S2 vaccine, but the latter may not adequately deliver standard doses among the animals (Kiyono et al., 2021).
Oral vaccination has also been tested with S19 to ascertain its capacity for protection against Brucella-induced abortion (Nicoletti and Milward, 1983; Nicoletti, 1984). Pregnant heifers in their first trimester were orally vaccinated with 5 × 1011 CFUs B. abortus S19, and orally challenged with 3.4 × 109 CFUs wt B. abortus 2308 at midgestation (Nicoletti and Milward, 1983). Half of all unvaccinated, challenged heifers aborted or had premature delivery and two-thirds were culture-positive while the vaccinated heifers showed only 5% birthing prematurely and 20% being culture-positive (Nicoletti and Milward, 1983). Oral S19 vaccination was found to confer equivalent protection against B. abortus-induced abortion challenge to pregnant heifers vaccinated by conventional parenteral or conjunctival routes (Nicoletti, 1984). Oral RB51 vaccination also proved efficacious against B. abortus-induced challenge (Elzer et al., 1998). Unbred heifers were orally vaccinated with 5 × 1010–1 × 1011 CFUs B. abortus RB51 mixed with corn syrup on hay for oral consumption, and were bred 6 weeks post-vaccination. All vaccinated and unvaccinated pregnant heifers were challenged by the conjunctival route with 2 × 107 CFUs wt B. abortus 2,308. Of the challenged control animals, 80% were culture-positive and 70% aborted compared to RB51-vaccinated animals showing 20% culture-positive and 30% aborted (Elzer et al., 1998). Thus, oral S19 or RB51 conferred equivalent protection to those vaccinated by conventional means (Nicoletti and Milward, 1983; Nicoletti, 1984; Elzer et al., 1998). As shown with B. suis S2 vaccine (Xin, 1986), oral delivery of brucellosis vaccines proves to be an effective means for vaccination of livestock and possibly wildlife (Rhyan et al., 2019). To this end, microencapsulated S19 plus sheep liver fluke, Fasciola hepatica. Vitelline protein B (VpB), was used to orally vaccinate red deer (Arenas-Gamboa et al., 2009). The VpB protein was included to delay vaccine release from the microspheres. Improved efficacy was observed against conjunctival challenge with 109 CFUs of wt B. abortus 2308 compared to red deer vaccinated SC or orally without the VpB. The investigators measured tissue colonization of the spleen, liver, lungs, SMLNs, mammary gland LNs, and mesenteric LNs. These results demonstrate the potential of oral vaccination of ruminants.
Conjunctival vaccination of livestock
The conjunctiva, or the membrane lining the inner surface of the eyelids, is a surface often susceptible to infection as a result of aerosol dispersion or direct contact with an infectious agent, e.g., Staphylococcus aureus and Chlamydia trachomatis. The conjunctiva is vascularized and composed of epithelial cells and goblet cells, and has supportive draining lymphatics responsible for immune protection and lubrication for the eyes (Royer et al., 2019). The eyes are considered an immune privileged site (Royer et al., 2019), yet the conjunctiva is often used to vaccinate against brucellosis, especially for goats and sheep (Blasco, 1997; Olsen and Stoffregen, 2005; Olsen and Palmer, 2014). SC Rev. 1 vaccination of sheep and goats during pregnancy results in vaccine-induced abortion, even at a reduced dose (Blasco, 1997; Olsen and Stoffregen, 2005). Too low of a dose results in insufficient protective immunity; hence, conjunctival vaccination was tested and found to reduce the frequency of Rev. 1-induced abortion when using a reduced dose in sheep (Blasco, 1997), but not goats (Zundel et al., 1992). Despite the reduced frequency of vaccine-induced abortion, Rev. 1 administered via the conjunctival route can still cause abortion if given to pregnant animals. Reduced abortion frequency was also noted in sheep if vaccinated via the conjunctiva during the last month of pregnancy (Jiménez de Bagués et al., 1989). Conjunctival Rev. 1 vaccination also has the advantage of reducing vaccine expression in the milk (Blasco, 1997). Given these outcomes, Rev. 1 vaccination is widely used for vaccination of sheep (Blasco, 1997; Olsen and Stoffregen, 2005).
Conjunctival vaccination with Rev. 1 vaccination of rams limited vaccine infection to the HNLNs and spleen, unlike SC vaccination, which resulted in more generalized infection (Muñoz et al., 2008). Conjunctival vaccination of cattle is not commonly done, but has shown to be beneficial for vaccinating with S19 to elicit lesser enduring serum Ab responses, yet remained protective (Nicoletti, 1990; Chand et al., 2015).
Mucosal vaccination of experimental animals
Only a limited number of studies have examined the effectiveness of mucosal vaccine approaches, which can induce localized immunity and are more apt to prevent infection. First, to examine the impact of adopting oral vaccination method, one study using orally delivered RB51 proved modestly effective against intraperitoneal (IP; Stevens et al., 1996; Pasquevich et al., 2010) or oral challenge with virulent B. abortus 2308 (Stevens et al., 1996). Oral vaccination with irradiated RB51 or B. neotomae, a pathogen of wood rats and possibly humans (Suárez-Esquivel et al., 2017), decreased brucellae tissue colonization after IP or nasal challenge with virulent B. abortus (Dabral et al., 2014). Examination of various attenuated mutants revealed that oral vaccination with the ΔpurEK B. melitensis 16M (WR201) strain elicited robust protection against nasal B. melitensis 16M challenge as shown by abating colonization of the lungs and reducing systemic spread (Izadjoo et al., 2004; Yingst et al., 2013). In a similar vein, oral ΔznuA B. melitensis vaccination elicited robust protection against pulmonary wt B. melitensis 16M challenge, whereby 58 and 83% of the vaccinated mice showed no detectable brucellae in their lungs and spleens, respectively, in an IFN-γ-dependent fashion (Clapp et al., 2016). Interestingly, oral vaccination of IFN-γ−/− mice with the ΔznuA B. melitensis mutant elicited even some protection, e.g., reduced tissue colonization by virulent B. melitensis 16M, partially, in an IL-17-dependent fashion (Clapp et al., 2011).
Examination of studies adopting nasal delivery methods revealed that nasal immunization with RB51 or a modified RB51 carrying Brucella superoxide dismutase (SOD), proved ineffective against pulmonary wt B. abortus challenge (Surendran et al., 2011). Prime and boosting failed to augment protection with either RB51 or RB51-SOD. Using a WboA-modified RB51, which produces low amount of cytoplasmic O-polysaccharide, also failed to confer protection against nasal challenge with wt B. abortus 2308 (Surendran et al., 2011). However, when RB51WboA was later modified to include overexpression of the wbkF gene and referred to as RB51WboAKF strain, increased detectable O-polysaccharide production was capable of eliciting anti-LPS Abs, but still retained its rough phenotype (Dabral et al., 2019). When administered parenterally, it was highly effective in conferring potent protection of the spleen against wt B. abortus 2308, wt B. melitensis 16M, and wt B. suis 1330 challenges (Dabral et al., 2019). The inclusion of soluble TLR2 or TLR4 agonist upon nasal RB51 vaccination enhanced partial protection only in the lungs (Surendran et al., 2013). In contrast, a single, nasal dose of ΔznuA B. melitensis potently protected mice against pulmonary B. melitensis challenge, wherein more than half of the mice had no detectable brucellae in their lungs or spleens (Clapp et al., 2016). As with the oral vaccinates, protection was IFN-γ-dependent, and the reduced protection in nasally vaccinated IFN-γ−/− mice was abrogated upon IL-17 neutralization (Clapp et al., 2016).
Examination of the types of T cells elicited subsequent to nasal vaccination with ΔznuA B. melitensis revealed induction of effector memory CD8+ T cells in the lungs producing IFN-γ, TNF-α, and granzyme B with the majority of IFN-γ derived from CD8+ T cells (Clapp et al., 2016; Table 1). To ascertain the effectiveness of B. melitensis Rev. 1 vaccine and ΔznuA B. melitensis mutant to protection relative to contributions by CD4+ and CD8+ T cells, groups of B6, CD4−/−, and CD8−/− mice were nasally vaccinated once, and then nasally challenged 6 weeks later with wt B. melitensis 16M. Both B6 and CD4−/− mice showed complete protection in the spleen and lungs by Rev. 1 and ΔznuA B. melitensis showing reliance on CD8+ T cells (Clapp et al., 2016). Both Rev. 1 and ΔznuA B. melitensis exhibited reduced protection in CD8−/− mice. This T cell bias was preserved upon introduction of a second norD mutation into ΔznuA B. abortus strain. Oral prime, nasal boost of CD8−/− mice with znBAZ resulted in the loss of CD8+ T cell-mediated protection against pulmonary challenge with virulent B. abortus 2308 (Wang et al., 2020). In contrast, mice vaccinated with znBM-mC depended upon either CD4+ or CD8+ T cells for protection despite having elevated numbers of CD8+ T cells. CD4−/− and CD8−/− mice, orally primed, nasally vaccinated with znBM-mC, showed equivalent protection against virulent pulmonary challenge with wt B. melitensis 16M (Goodwin et al., 2022). Such finding indicated that CD4+ T cells can compensate for CD8 T cell deficiency.
Conclusion
Brucellosis is a disease of potential impact to 3.5 billion people (Rossetti et al., 2017), and is considered as a neglected disease (Mableson et al., 2014). Due to its debilitating effects in humans (Pappas et al., 2005, 2006; Franco et al., 2007; Cross et al., 2019), and abortions, reduced fertility, and reduced milk and meat production in livestock (McDermott et al., 2013), this disease poses significant economic hardship to livestock producers in affected countries (World Health Organization, 2005; Rubach et al., 2013; Welburn et al., 2015; Rossetti et al., 2017). Eradication programs used by the United States and some Western countries successfully eliminated and/or controlled livestock brucellosis (Schurig et al., 2002; Olsen and Stoffregen, 2005), but application of such process would prove costly in countries unable to compensate or replace the seropositive livestock (McDermott et al., 2013; Rossetti et al., 2017). Given that current vaccines are only 70% efficacious (Olsen, 2000; Olsen and Stoffregen, 2005; Lubroth et al., 2007), the development of novel vaccines is warranted to help reduce brucellosis incidence. Cheap and readily available vaccines could improve livestock production, improve the standard of living for livestock producers, and reduce incidence of brucellosis in humans.
Most work in brucellosis vaccine development relies on live attenuated mutants. The basis for their employment is their relative success by B. abortus S19 and RB51, B. melitensis Rev. 1, and B. suis S2 vaccines in reducing livestock disease (Olsen and Palmer, 2014; de Figueiredo et al., 2015; Byndloss and Tsolis, 2016; Goodwin and Pascual, 2016). Although these are not completely effective, they provide the basis for successfully using live vaccines to elicit the desired protective response. While not discussed in this article, a subunit vaccine approach could have promise; however, no single Brucella protein has been identified yet that is capable of conferring complete protection. Most of the protein candidates are not able to confer more than a two log reduction in tissue colonization (Pascual et al., 2018). More likely, some combination of epitopes is needed to be delivered in such a manner that potent Th1-type responses are elicited.
Consideration of mucosal vaccination delivery methods does need more attention because of their potential impact on immunizing diverse tissues, protecting sites of infection, improving vaccine efficacy, and conferring conventional and alternative mechanisms of protection. Implementing parenteral vaccinations lessens the opportunities for eliciting memory T cell responses at sites of infection that could potentially prevent or limit systemic spread. Several of the studies conducted with ruminants vaccinated or infected with wt Brucella did evaluate host T cell responses (Weynants et al., 1998; Suraud et al., 2008; Wang et al., 2014; Dorneles et al., 2015; Curina et al., 2018; Higgins et al., 2018; Boggiatto et al., 2020), but many of these studies limited evaluations to peripheral blood T cells, and not mucosal T cells. Peripheral blood T cells vary in their stage of activation, and are in transition in homing to their targeted tissues. Aside from looking at peripheral blood, T cell restimulation methods may also influence Ag-specific responses. Among these studies, the described restimulation methods varied, where often whole LPS-bearing brucellae were used. LPS can alter host T cell responses via activation of co-cultured Ag-presenting cells (APCs). Finally, mode of Ag restimulation may bias T cell responses where soluble Ags mostly stimulate via the MHC class II-dependent pathway activating CD4+ T cells, and in contrast, an infection process, e.g., live infection of APCs, followed by subsequent APC inactivation, promotes MHC class I-driven CD8+ T cell responses. Future studies are warranted to compare the influence of the route of delivery, e.g., SC, conjunctival, oral, and nasal, upon eliciting memory T cell responses, especially the naso-oropharyngeal tissues of animals and humans, which are sensitive to Brucella infection (Carpenter and Boak, 1932; Poelma and Pickens, 1932; Suraud et al., 2007, 2008; Zachou et al., 2008; Arenas-Gamboa et al., 2009; Ron-Román et al., 2014; von Bargen et al., 2015; Rouzic et al., 2021).
Proof of mucosal vaccination effectiveness has been shown experimentally in livestock, wildlife, and rodents. Oral vaccination with S19 (Nicoletti and Milward, 1983; Nicoletti, 1984) and RB51 (Elzer et al., 1998) proved effective in pregnant heifers against B. abortus-induced abortion. Oral vaccination of red deer with microencapsulated S19 effectively reduced tissue colonization (Arenas-Gamboa et al., 2009). Conjunctival Rev. 1 vaccination of sheep proved effective in protection against abortion and vaccine-induced abortion (Jiménez de Bagués et al., 1989; Blasco, 1997). Conjunctival brucellosis vaccination is commonly practiced (Blasco, 1997; Chand et al., 2015), but understanding why this route is effective in livestock is less understood. Given difficulties in conducting immune analyses in livestock, experimental animal systems can shed insight into mechanisms of protection not otherwise possible in livestock. Using the ΔznuA B. melitensis mutant (Clapp et al., 2011, 2016), znBAZ mutant (Wang et al., 2020), znBM-mC mutant (Goodwin et al., 2022), Rev. 1 vaccine (Clapp et al., 2016), and ΔpurEK B. melitensis (WR201) mutant (Izadjoo et al., 2004; Yingst et al., 2013), studies proved that potent protection can be achieved in the mucosal and systemic compartments. An interesting finding derived from the ΔznuA B. melitensis (Clapp et al., 2011, 2016), znBAZ (Wang et al., 2020), and znBM-mC (Goodwin et al., 2022) studies was the predilection for stimulation of IFN-γ+ CD8+ T cell responses, which is attributed to either the mutant or mode of delivery. IP vaccination with znBAZ stimulated both CD4+ and CD8+ T cells, but the number of splenic IFN-γ+ CD4+ T cells nearly doubled those induced by CD8+ T cells (Yang et al., 2016) suggesting that the route has an influence. This was particularly noted with nasal Rev. 1 vaccination resulting CD8+ T cell-dependent immunity as observed by the reduction in protection in CD8−/− mice (Clapp et al., 2016). However, oral vaccination with the WR201 mutant induced CD4+ T cell-dependent protection since protection against nasal challenge with wt B. melitensis 16M were equivalent in B6 and CD8−/− mice (Yingst et al., 2013). This finding disputes the idea that route of vaccination is the only factor, but the mutant is also an important consideration. These collective findings demonstrate that immune protection can be achieved with either CD4+ or CD8+ T cells, but eliciting memory responses proximal the site of infection, e.g., the stimulation of TRMs, are more apt to protect, possibly preventing or limiting brucellae dissemination as suggested by the ΔznuA B. melitensis (Clapp et al., 2016), znBAZ (Wang et al., 2020), and znBM-mC (Goodwin et al., 2022) studies. Mucosal vaccination with znBAZ or znBM-mC stimulated robust CD8+ and CD4+ TRM responses in the lungs to facilitate protection against pulmonary wt challenge, and may have prevented further brucellae dissemination (Wang et al., 2020; Goodwin et al., 2022). Thus, the stimulation of alternative immune pathways is beneficial, and may improve vaccine efficacy warranting further testing.
The study of different Brucella species may assist in learning Brucella’s pathogenesis and tenets of host protection. The discovery of B. microti in voles (Scholz et al., 2008) and later in other host species (Occhialini et al., 2022), extends the genus diversity, whereby B. microti shares metabolic traits, conserved VirB type IV secretion system, and early granuloma formation with other Brucella pathogens of livestock (Scholz et al., 2008; Hanna et al., 2011; Jiménez de Bagüés et al., 2011; Occhialini et al., 2022). Brucella microti offers another model to study brucellosis since it can be lethal in mice and actively proliferates in murine macrophages. Cell-mediated immunity is essential for protection against B. microti and NK cells are required (Jiménez de Bagüés et al., 2011) showing the relevance of experimental animal systems.
Finally, an improved vaccine for one species has the potential to cross-protect against other Brucella species. A number of studies have shown such cross-protection as evidenced in humans with S19 for protection against B. melitensis infection (Vershilova, 1961; van Straten et al., 2016); B. neotomae for protection against B. abortus (Dabral et al., 2014); S19, Rev. 1, and S2 for cross-protection against B. suis, B. melitensis, and B. abortus (Bosseray and Plommet, 1990); and RB51WboAKF for B. suis, B. melitensis, and B. abortus (Dabral et al., 2019). Herein lies the potential for a universal brucellosis vaccine.
Author contributions
DP conceived and designed the entire review and wrote the paper. ZG, EB, CH, and XY reviewed and edited the manuscript. ZG, EB and DP constructed the figures. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by U. S. Public Health Grants R01 AI123244 and R01 AI125546, and ZG was in part supported by AI123244-S1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ablin, J., Mevorach, D., and Eliakim, R. (1997). Brucellosis and the gastrointestinal tract. The odd couple. J. Clin. Gastroenterol. 24, 25–29. doi: 10.1097/00004836-199701000-00005
Ackermann, M. R., Cheville, N. F., and Deyoe, B. L. (1988). Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Vet. Pathol. 25, 28–35. doi: 10.1177/030098588802500104
Adetunji, S. A., Ramirez, G., Ficht, A. R., Perez, L., Foster, M. J., and Arenas-Gamboa, A. M. (2020). Building the evidence base for the prevention of raw milk-acquired brucellosis: a systematic review. Front. Public Health 8:76. doi: 10.3389/fpubh.2020.00076
Adetunji, S. A., Ramirez, G., Foster, M. J., and Arenas-Gamboa, A. M. (2018). A systematic review and meta-analysis of the prevalence of osteoarticular brucellosis. PLoS Negl. Trop. Dis. 13:e0007112. doi: 10.1371/journal.pntd.0007112
Agerholm, R., and Bekiaris, V. (2021). Evolved to protect, designed to destroy: IL-17-producing γδ T cells in infection, inflammation, and cancer. Eur. J. Immunol. 51, 2164–2177. doi: 10.1002/eji.202049119
Alaidarous, M., Ve, T., Casey, L. W., Valkov, E., Ericsson, D. J., Ullah, M. O., et al. (2014). Mechanism of bacterial interference with TLR4 signaling by Brucella toll/interleukin-1 receptor domain-containing protein Tcp B. J. Biol. Chem. 289, 654–668. doi: 10.1074/jbc.M113.523274
Alexander, B., Schnurrenberger, P. R., and Brown, R. R. (1981). Numbers of Brucella abortus in the placenta, umbilicus and fetal fluid of two naturally infected cows. Vet. Rec. 108:500. doi: 10.1136/vr.108.23.500
American Academy of Pediatrics (2014). Policy statement: consumption of raw or unpasteurized milk and milk products by pregnant women and children. Pediatrics 133, 175–179. doi: 10.1542/peds.2013-3502
Araya, L. N., Elzer, P. H., Rowe, G. E., Enright, F. M., and Winter, A. J. (1989). Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J. Immunol. 143, 3330–3337.
Arenas-Gamboa, A. M., Ficht, T. A., Davis, D. S., Elzer, P. H., Kahl-McDonagh, M., Wong-Gonzalez, A., et al. (2009). Oral vaccination with microencapsuled strain 19 vaccine confers enhanced protection against Brucella abortus strain 2308 challenge in red deer (Cervus elaphus elaphus). J. Wildl. Dis. 45, 1021–1029. doi: 10.7589/0090-3558-45.4.1021
Arenas-Gamboa, A. M., Rossetti, C. A., Chaki, S. P., Garcia-Gonzalez, D. G., Adams, L. G., and Ficht, T. A. (2016). Human brucellosis and adverse pregnancy outcomes. Curr. Trop. Med. Rep. 3, 164–172. doi: 10.1007/s40475-016-0092-0
Ariza, J., Bosilkovski, M., Cascio, A., Colmenero, J. D., Corbel, M. J., Falagas, M. E., et al. (2007). International Society of Chemotherapy; Institute of Continuing Medical Education of Ioannina. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 4:e317. doi: 10.1371/journal.pmed.0040317
Ariza, J., Corredoira, J., Pallares, R., Viladrich, P. F., Rufi, G., Pujol, M., et al. (1995). Characteristics of and risk factors for relapse of brucellosis in humans. Clin. Infect. Dis. 20, 1241–1249. doi: 10.1093/clinids/20.5.1241
Atluri, V. L., Xavier, M. N., de Jong, M. F., den Hartigh, A. B., and Tsolis, R. M. (2011). Interactions of the human pathogenic Brucella species with their hosts. Annu. Rev. Microbiol. 65, 523–541. doi: 10.1146/annurev-micro-090110-102905
Baldi, P. C., and Giambartolomei, G. H. (2013). Immunopathology of Brucella infection. Recent Pat. Antiinfect. Drug Discov. 8, 18–26. doi: 10.2174/1574891x11308010005
Barrionuevo, P., Cassataro, J., Delpino, M. V., Zwerdling, A., Pasquevich, K. A., García Samartino, C., et al. (2008). Brucella abortus inhibits major histocompatibility complex class II expression and antigen processing through interleukin-6 secretion via toll-like receptor 2. Infect. Immun. 76, 250–262. doi: 10.1128/IAI.00949-07
Barrionuevo, P., Delpino, M. V., Pozner, R. G., Velásquez, L. N., Cassataro, J., and Giambartolomei, G. H. (2013). Brucella abortus induces intracellular retention of MHC-I molecules in human macrophages down-modulating cytotoxic CD8+ T cell responses. Cell. Microbiol. 15, 487–502. doi: 10.1111/cmi.12058
Barrionuevo, P., and Giambartolomei, G. H. (2019). Inhibition of antigen presentation by Brucella: many more than many ways. Microbes Infect. 21, 136–142. doi: 10.1016/j.micinf.2018.12.004
Bhagyaraj, E., Wang, H., Yang, X., Hoffman, C., Akgul, A., Goodwin, Z. I., et al. (2021). Mucosal vaccination primes NK cell-dependent development of CD8+ T cells against pulmonary Brucella infection. Front. Immunol. 12:697953. doi: 10.3389/fimmu.2021.697953
Blasco, J. M. (1997). A review of the use of B. melitensis Rev 1 vaccine in adult sheep and goats. Prev. Vet. Med. 31, 275–283. doi: 10.1016/s0167-5877(96)01110-5
Boggiatto, P. M., Schaut, R. G., and Olsen, S. C. (2020). Enhancing the detection of Brucella-specific CD4+ T cell responses in cattle via in vitro antigenic expansion and restimulation. Front. Immunol. 11:1944. doi: 10.3389/fimmu.2020.01944
Bosilkovski, M., Keramat, F., and Arapovic, J. (2021). The current therapeutical strategies in human brucellosis. Infection 49, 823–832. doi: 10.1007/s15010-021-01586-w
Bosseray, N., and Plommet, M. (1990). Brucella suis S2, Brucella melitensis Rev. 1 and Brucella abortus S19 living vaccines: residual virulence and immunity induced against three Brucella species challenge strains in mice. Vaccine 8, 462–468. doi: 10.1016/0264-410x(90)90247-j
Bratescu, A., Mayer, E. P., and Teodorescu, M. (1981). Binding of bacteria from the genus Brucella to human lymphocytes-B. Infect. Immun. 31, 816–821. doi: 10.1128/iai.31.2.816-821
Bruce, D. (1887). Note on the discovery of a micro·organism in Malta fever. Practitioner 39, 161–170.
Bruce, D. (1889). Observations on Malta fever. Br. Med. J. 1, 1101–1105. doi: 10.1136/bmj.1.1481.1101
Buck, J. M. (1930). Studies of vaccination during calfhood to prevent bovine infectious abortion. J. Agric. Res. 41, 667–689.
Buswell, M. L., Hourigan, M., Nault, A. J., and Bender, J. B. (2016). Needlestick injuries in agriculture workers and prevention programs. J. Agromedicine 21, 82–90. doi: 10.1080/1059924X.2015.1106996
Byndloss, M. X., and Tsolis, R. M. (2016). Brucella spp. virulence factors and immunity. Annu. Rev. Anim. Biosci. 4, 111–127. doi: 10.1146/annurev-animal-021815-111326
Carpenter, C. M., and Boak, R. A. (1932). The isolation of Brucella abortus from tonsils. JAMA 99, 296–298. doi: 10.1001/jama.1932.02740560022007
Cashion, M. F., Banks, W. A., Bost, K. L., and Kastin, A. J. (1999). Transmission routes of HIV-1 gp 120 from brain to lymphoid tissues. Brain Res. 822, 26–33. doi: 10.1016/s0006-8993(99)01069-0
Celli, J. (2019). Chap. 7: the intracellular life cycle of Brucella spp. In Bacteria and Intracellularity. eds. Cossart, P., Roy, C. R., and Sansonetti, P. Washington, DC: American Society for Microbiology, 7.
Celli, J., de Chastellier, C., Franchini, D. M., Pizarro-Cerda, J., Moreno, E., and Gorvel, J. P. (2003). Brucella evades macrophage killing via Vir B-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198, 545–556. doi: 10.1084/jem.20030088
Centers for Disease Control (CDC) (1983). Epidemiologic notes and reports brucellosis -- Texas. MMWR Morb. Mortal. Wkly Rep. 32, 548–553.
Chand, P., Chhabra, R., and Nagra, J. (2015). Vaccination of adult animals with a reduced dose of Brucella abortus S19 vaccine to control brucellosis on dairy farms in endemic areas of India. Tropl. Anim. Health Prod. 47, 29–35. doi: 10.1007/s11250-014-0678-2
Cheville, N. F., Olsen, S. C., Jensen, A. E., Stevens, M. G., Palmer, M. V., and Florance, A. M. (1996). Effects of age at vaccination on efficacy of Brucella abortus strain RB51 to protect cattle against brucellosis. Am. J. Vet. Res. 57, 1153–1156.
Chomel, B. B., DeBess, E. E., Mangiamele, D. M., Reilly, K. F., Farver, T. B., Sun, R. K., et al. (1994). Changing trends in the epidemiology of human brucellosis in California from 1973 to 1992: a shift toward foodborne transmission. J Infect Dis 170, 1216–1223. doi: 10.1093/infdis/170.5.1216
Clapp, B., Skyberg, J. A., Yang, X., Thornburg, T., Walters, N., and Pascual, D. W. (2011). Protective live oral brucellosis vaccines stimulate Th1 and Th17 cell responses. Infect. Immun. 79, 4165–4174. doi: 10.1128/IAI.05080-11
Clapp, B., Yang, X., Thornburg, T., Walters, N., and Pascual, D. W. (2016). Nasal vaccination stimulates CD8+ T cells for potent protection against mucosal Brucella melitensis challenge. Immunol. Cell Biol. 94, 496–508. doi: 10.1038/icb.2016.5
Codarri, L., Gyülvészi, G., Tosevski, V., Hesske, L., Fontana, A., Magnenat, L., et al. (2011). RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567. doi: 10.1038/ni.2027
Collins, F. M. (1978). Cellular antimicrobial immunity. CRC Crit. Rev. Microbiol. 7, 27–91. doi: 10.3109/10408417909101177
Conde-Álvarez, R., Arce-Gorvel, V., Iriarte, M., Mancek-Keber, M., Barquero-Calvo, E., Palacios-Chaves, L., et al. (2012). The lipopolysaccharide core of Brucella abortus acts as a shield against innate immunity recognition. PLoS Pathog. 8:e1002675. doi: 10.1371/journal.ppat.1002675
Confer, A. W., Hall, S. M., Faulkner, C. B., Espe, B. H., Deyoe, B. L., Morton, R. J., et al. (1985). Effects of challenge dose on the clinical and immune responses of cattle vaccinated with reduced doses of Brucella abortus strain 19. Vet. Microbiol. 10, 561–575. doi: 10.1016/0378-1135(85)90065-3
Corbel, M. (1997). Brucellosis: an overview. Emerg. Infect. Dis. 3, 213–221. doi: 10.3201/eid0302.970219
Costa Franco, M. M., Marim, F., Guimarães, E. S., Assis, N. R. G., Cerqueira, D. M., Alves-Silva, J., et al. (2018). Brucella abortus triggers a cGAS-independent STING pathway to induce host protection that involves Guanylate-binding proteins and inflammasome activation. J. Immunol. 200, 607–622. doi: 10.4049/jimmunol.1700725
Cotterill, G. G., Cross, P. C., Cole, E. K., Fuda, R. K., Rogerson, J. D., Scurlock, B. M., et al. (2018). Winter feeding of elk in the greater Yellowstone ecosystem and its effects on disease dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373:20170093. doi: 10.1098/rstb.2017.0093
Cross, A. R., Baldwin, V. M., Roy, S., Essex-Lopresti, A. E., Prior, J. L., and Harmer, N. J. (2019). Zoonoses under our noses. Microbes Infect. 21, 10–19. doi: 10.1016/j.micinf.2018.06.001
Csencsits, K. L., Jutila, M. A., and Pascual, D. W. (2002). Mucosal addressin expression and binding-interactions with naive lymphocytes vary among the cranial, oral, and nasal-associated lymphoid tissues. Eur. J. Immunol. 32, 3029–3039. doi: 10.1002/1521-4141(200211)32:11<3029::AID-IMMU3029>3.0.CO;2-9
Csencsits, K. L., Walters, N., and Pascual, D. W. (2001). Cutting edge: dichotomy of homing receptor dependence by mucosal effector B cells: αE versus L-selectin. J. Immunol. 167, 2441–2445. doi: 10.4049/jimmunol.167.5.2441
Cserr, H. F., and Knopf, P. M. (1992). Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol. Today 13, 507–512. doi: 10.1016/0167-5699(92)90027-5
Curina, G., Nardini, R., Corneli, S., D'Avino, N., Tentellini, M., Montagnoli, C., et al. (2018). Evaluation of immune responses in mice and sheep inoculated with a live attenuated Brucella melitensis REV1 vaccine produced in bioreactor. Vet. Immunol. Immunopathol. 198, 44–53. doi: 10.1016/j.vetimm.2018.02.010
Dabral, N., Burcham, G. N., Jain-Gupta, N., Sriranganathan, N., and Vemulapalli, R. (2019). Overexpression of wbk F gene in Brucella abortus RB51WboA leads to increased O-polysaccharide expression and enhanced vaccine efficacy against B. abortus 2308, B. melitensis 16M, and B. suis 1330 in a murine brucellosis model. PLoS One 14:e0213587. doi: 10.1371/journal.pone.0213587
Dabral, N., Moreno-Lafont, M., Sriranganathan, N., and Vemulapalli, R. (2014). Oral immunization of mice with gamma-irradiated Brucella neotomae induces protection against intraperitoneal and intranasal challenge with virulent B. abortus 2308. PLoS One 9:e107180. doi: 10.1371/journal.pone.0107180
Dadar, M., Shahali, Y., and Whatmore, A. M. (2019). Human brucellosis caused by raw dairy products: a review on the occurrence, major risk factors and prevention. Int. J. Food Microbiol. 292, 39–47. doi: 10.1016/j.ijfoodmicro.2018.12.009
Dadelahi, A. S., Lacey, C. A., Chambers, C. A., Ponzilacqua-Silva, B., and Skyberg, J. A. (2020). B cells inhibit CD4+ T cell-mediated immunity to Brucella infection in a major histocompatibility complex class II-dependent manner. Infect. Immun. 88, e00075–e00020. doi: 10.1128/IAI.00075-20
D'Anastasio, R., Staniscia, T., Milia, M. L., Manzoli, L., and Capasso, L. (2011). Origin, evolution and paleoepidemiology of brucellosis. Epidemiol. Infect. 139, 149–156. doi: 10.1017/S095026881000097X
de Almeida, L. A., Carvalho, N. B., Oliveira, F. S., Lacerda, T. L., Vasconcelos, A. C., Nogueira, L., et al. (2011). MyD88 and STING signaling pathways are required for IRF3-mediated IFN-ß induction in response to Brucella abortus infection. PLoS One 6:e23135. doi: 10.1371/journal.pone.0023135
de Figueiredo, P., Ficht, T. A., Rice-Ficht, A., Rossetti, C. A., and Adams, L. G. (2015). Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am. J. Pathol. 185, 1505–1517. doi: 10.1016/j.ajpath.2015.03.003
Delpino, M. V., Marchesini, M. I., Estein, S. M., Comerci, D. J., Cassataro, J., Fossati, C. A., et al. (2007). A bile salt hydrolase of Brucella abortus contributes to the establishment of a successful infection through the oral route in mice. Infect. Immun. 75, 299–305. doi: 10.1128/IAI.00952-06
Demars, A., Lison, A., Machelart, A., Van Vyve, M., Potemberg, G., Vanderwinden, J. M., et al. (2019). Route of infection strongly impacts the host-pathogen relationship. Front. Immunol. 10:1589. doi: 10.3389/fimmu.2019.01589
Dickstein, J. B., Moldofsky, H., Lue, F. A., and Hay, J. B. (1999). Intracerebroventricular injection of TNF-a promotes sleep and is recovered in cervical lymph. Am. J. Physiol. 276. (Regulatory Integrative Comp Physiol. 45, R1018–R1022. doi: 10.1152/ajpregu.1999.276.4.R1018
Dornand, J., Lafont, V., Oliaro, J., Terraza, A., Castaneda-Roldan, E., and Liautard, J. P. (2004). Impairment of intramacrophagic Brucella suis multiplication by human natural killer cells through a contact-dependent mechanism. Infect. Immun. 72, 2303–2311. doi: 10.1128/IAI.72.4.2303-2311.2004
Dorneles, E. M., Lima, G. K., Teixeira-Carvalho, A., Araújo, M. S., Martins-Filho, O. A., Sriranganathan, N., et al. (2015). Immune response of calves vaccinated with Brucella abortus S19 or RB51 and revaccinated with RB51. PLoS One 10:e0136696. doi: 10.1371/journal.pone.0136696
Dudakov, J. A., Hanash, A. M., and van den Brink, M. R. (2015). Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785. doi: 10.1146/annurev-immunol-032414-112123
Durward-Diioia, M., Harms, J., Khan, M., Hall, C., Smith, J. A., and Splitter, G. A. (2015). CD8+ T cell exhaustion, suppressed gamma interferon production, and delayed memory response induced by chronic Brucella melitensis infection. Infect. Immun. 83, 4759–4771. doi: 10.1128/IAI.01184-15
Edmonds, M. D., Samartino, L. E., Hoyt, P. G., Hagius, S. D., Walker, J. V., Enright, F. M., et al. (2001). Oral vaccination of sexually mature pigs with Brucella abortus vaccine strain RB51. Am. J. Vet. Res. 62, 1328–1311. doi: 10.2460/ajvr.2001.62.1328
El-Behi, M., Ciric, B., Dai, H., Yan, Y., Cullimore, M., Safavi, F., et al. (2011). The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575. doi: 10.1038/ni.2031
Elzer, P. H., Enright, F. M., Colby, L., Hagius, S. D., Walker, J. V., Fatemi, M. B., et al. (1998). Protection against infection and abortion induced by virulent challenge exposure after oral vaccination of cattle with Brucella abortus strain RB51. Am. J. Vet. Res. 59, 1575–1578.
Enright, F. M. (1990). “The pathogenesis and pathobiology of Brucella infection in domestic animals” in Animal Brucellosis. ed. J. R. Duncan (Boca Raton, FL: CRC Press), 301–320.
Evans, A. C. (1918). Further studies on bacterium abortus and related bacteria: II. A comparison of bacterium abortus with bacterium bronchisepticus and with the organism which causes Malta fever. J Infect Dis 22, 580–593.
Foley, B. V., Clay, M. M., and O'Sullivan, D. J. (1970). A study of a brucellosis epidemic. Ir. J. Med. Sci. 3, 457–462. doi: 10.1007/BF02958986
Forestier, C., Deleuil, F., Lapaque, N., Moreno, E., and Gorvel, J. P. (2000). Brucella abortus lipopolysaccharide in murine peritoneal macrophages acts as a down-regulator of T cell activation. J. Immunol. 165, 5202–5210. doi: 10.4049/jimmunol.165.9.5202
Franco, M. P., Mulder, M., Gilman, R. H., and Smits, H. L. (2007). Human brucellosis. Lancet Infect. Dis. 7, 775–786. doi: 10.1016/S1473-3099(07)70286-4
Franco-Paredes, C., Chastain, D., Taylor, P., Stocking, S., and Sellers, B. (2017). Boar hunting and brucellosis caused by Brucella suis. Travel Med. Infect. Dis. 16, 18–22. doi: 10.1016/j.tmaid.2017.03.006
Freeman, L. E., and Troutt, H. F. (1985). Lymph drainage of the conjunctiva: topographic anatomic study in calves. Am. J. Vet. Res. 46, 1967–1970.
García-Gil, A., Lopez-Bailon, L. U., and Ortiz-Navarrete, V. (2019). Beyond the antibody: B cells as a target for bacterial infection. J. Leukoc. Biol. 105, 905–913. doi: 10.1002/JLB.MR0618-225R
Godfroid, J., Cloeckaert, A., Liautard, J. P., Kohler, S., Fretin, D., Walravens, K., et al. (2005). From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36, 313–326. doi: 10.1051/vetres:2005003
Godfroid, J., Scholz, H. C., Barbier, T., Nicolas, C., Wattiau, P., Fretin, D., et al. (2011). Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev. Vet. Med. 102, 118–131. doi: 10.1016/j.prevetmed.2011.04.007
Goenka, R., Guirnalda, P. D., Black, S. J., and Baldwin, C. L. (2012). B lymphocytes provide an infection niche for intracellular bacterium Brucella abortus. J Infect Dis 206, 91–98. doi: 10.1093/infdis/jis310
Goenka, R., Parent, M. A., Elzer, P. H., and Baldwin, C. L. (2011). B cell-deficient mice display markedly enhanced resistance to the intracellular bacterium Brucella abortus. J Infect Dis 203, 1136–1146. doi: 10.1093/infdis/jiq171
Gomes, M. T., Campos, P. C., de Almeida, L. A., Oliveira, F. S., Costa, M. M., Marim, F. M., et al. (2012). The role of innate immune signals in immunity to Brucella abortus. Front. Cell. Infect. Microbiol. 2:130. doi: 10.3389/fcimb.2012.00130
Goodwin, Z. I., and Pascual, D. W. (2016). Brucellosis vaccines for livestock. Vet. Immunol. Immunopathol. 181, 51–58. doi: 10.1016/j.vetimm.2016.03.011
Goodwin, Z. I., Yang, X., Hoffman, C., and Pascual, D. W. (2022). Live mucosal vaccination stimulates potent protection via varied CD4+ and CD8+ T cell subsets against wild-type Brucella melitensis 16M challenge. Front. Immunol. 13:995327. doi: 10.3389/fimmu.2022.995327
Gorvel, L., Textoris, J., Banchereau, R., Ben Amara, A., Tantibhedhyangkul, W., von Bargen, K., et al. (2014). Intracellular bacteria interfere with dendritic cell functions: role of the type I interferon pathway. PLoS One 9:e99420. doi: 10.1371/journal.pone.0099420
Greco, E., El-Aguizy, O., Ali, M. F., Foti, S., Cunsolo, V., Saletti, R., et al. (2018). Proteomic analyses on an ancient Egyptian cheese and biomolecular evidence of brucellosis. Anal. Chem. 90, 9673–9676. doi: 10.1021/acs.analchem.8b02535
Guimarães, E. S., Gomes, M. T. R., Campos, P. C., Mansur, D. S., Dos Santos, A. A., Harms, J., et al. (2019). Brucella abortus cyclic Dinucleotides trigger STING-dependent unfolded protein response that favors bacterial replication. J. Immunol. 202, 2671–2681. doi: 10.4049/jimmunol.1801233
Guimarães, G., Gomes, M. T. R., Campos, P. C., Marinho, F. V., de Assis, N. R. G., Silveira, T. N., et al. (2018). Immunoproteasome subunits are required for CD8+ T cell function and host resistance to Brucella abortus infection in mice. Infect. Immun. 86, e00615–e00617. doi: 10.1128/IAI.00615-17
Guzmán-Verri, C., González-Barrientos, R., Hernández-Mora, G., Morales, J. A., Baquero-Calvo, E., Chaves-Olarte, E., et al. (2012). Brucella ceti and brucellosis in cetaceans. Front. Cell. Infect. Microbiol. 2:3. doi: 10.3389/fcimb.2012.00003
Hameleers, D. M., van der Ven, I., Sminia, T., and Biewenga, J. (1990). Anti-TNP-forming cells in rats after different routes of priming with TNP-LPS followed by intranasal boosting with the same antigen. Res. Immunol. 141, 515–528. doi: 10.1016/0923-2494(90)90020-y
Hanna, N., Jiménez de Bagüés, M. P., Ouahrani-Bettache, S., El Yakhlifi, Z., Köhler, S., and Occhialini, A. (2011). The virB operon is essential for lethality of Brucella microti in the Balb/c murine model of infection. J Infect Dis 203, 1129–1135. doi: 10.1093/infdis/jiq163
Hanot Mambres, D., Machelart, A., Potemberg, G., De Trez, C., Ryffel, B., Letesson, J. J., et al. (2016). Identification of immune effectors essential to the control of primary and secondary intranasal infection with Brucella melitensis in mice. J. Immunol. 196, 3780–3793. doi: 10.4049/jimmunol.1502265
He, Y., Vemulapalli, R., Zeytun, A., and Schurig, G. G. (2001). Induction of specific cytotoxic lymphocytes in mice vaccinated with Brucella abortus RB51. Infect. Immun. 69, 5502–5508. doi: 10.1128/IAI.69.9.5502-5508.2001
Hedayatizadeh-Omran, A., Rafiei, A., Hajilooi, M., and Haghshenas, M. (2010). Interferon-gamma low producer genotype +5644 over presented in patients with focal brucellosis. Pak. J. Biol. Sci. 13, 1036–1041. doi: 10.3923/pjbs.2010.1036.1041
Henning, L. N., Miller, S. M., Pak, D. H., Lindsay, A., Fisher, D. A., Barnewall, R. E., et al. (2012). Pathophysiology of the rhesus macaque model for inhalational brucellosis. Infect. Immun. 80, 298–310. doi: 10.1128/IAI.05878-11
Herzberg, M., and Elberg, S. S. (1955). Immunization against Brucella infection. III. Response of mice and guinea pigs to injection of viable and nonviable suspensions of a streptomycin-dependent mutant of Brucella melitensis. J. Bacteriol. 69, 432–435. doi: 10.1128/jb.69.4.432-435.1955
Higgins, J. L., Bowen, R. A., and Gonzalez-Juarrero, M. (2018). Cell mediated immune response in goats after experimental challenge with the virulent Brucella melitensis strain 16M and the reduced virulence strain Rev. 1. Vet. Immunol. Immunopathol. 202, 74–84. doi: 10.1016/j.vetimm.2018.06.004
Hirahara, K., Kokubo, K., Aoki, A., Kiuchi, M., and Nakayama, T. (2021). The role of CD4+ resident memory T cells in local immunity in the mucosal tissue-protection versus pathology. Front. Immunol. 12:616309. doi: 10.3389/fimmu.2021.616309
Holderness, J., Hedges, J. F., Ramstead, A., and Jutila, M. A. (2013). Comparative biology of γδ T cell function in humans, mice, and domestic animals. Annu. Rev. Anim. Biosci. 1, 99–124. doi: 10.1146/annurev-animal-031412-103639
Hördt, A., López, M. G., Meier-Kolthoff, J. P., Schleuning, M., Weinhold, L. M., Tindall, B. J., et al. (2020). Analysis of 1, 000+ type-strain genomes substantially improves taxonomic classification of Alphaproteobacteria. Front. Microbiol. 11:468. doi: 10.3389/fmicb.2020.00468
Hou, H., Liu, X., and Peng, Q. (2019). The advances in brucellosis vaccines. Vaccine 37, 3981–3988. doi: 10.1016/j.vaccine.2019.05.084
Hull, N. C., and Schumaker, B. A. (2018). Comparisons of brucellosis between human and veterinary medicine. Infect Ecol. Epidemiol. 8:1500846. doi: 10.1080/20008686.2018.1500846
Izadjoo, M. J., Bhattacharjee, A. K., Paranavitana, C. M., Hadfield, T. L., and Hoover, D. L. (2004). Oral vaccination with Brucella melitensis WR201 protects mice against intranasal challenge with virulent Brucella melitensis 16M. Infect. Immun. 72, 4031–4039. doi: 10.1128/IAI.72.7.4031-4039.2004
Jakka, P., Namani, S., Murugan, S., Rai, N., and Radhakrishnan, G. (2017). The Brucella effector protein Tcp B induces degradation of inflammatory caspases and thereby subverts non-canonical inflammasome activation in macrophages. J. Biol. Chem. 292, 20613–20627. doi: 10.1074/jbc.M117.815878
Jiang, X., and Baldwin, C. L. (1993). Effects of cytokines on intracellular growth of Brucella abortus. Infect. Immun. 61, 124–134. doi: 10.1128/iai.61.1.124-134.1993
Jiménez de Bagüés, M. P., de Martino, A., Quintana, J. F., Alcaraz, A., and Pardo, J. (2011). Course of infection with the emergent pathogen Brucella microti in immunocompromised mice. Infect. Immun. 79, 3934–3939. doi: 10.1128/IAI.05542-11
Jiménez de Bagués, M. P., Marin, C. M., Barberán, M., and Blasco, J. M. (1989). Responses of ewes to B. melitensis Rev 1 vaccine administered by subcutaneous or conjunctival routes at different stages of pregnancy. Ann. Rech. Vet. 20, 205–213.
Khan, M., Harms, J. S., Liu, Y., Eickhoff, J., Tan, J. W., Hu, T., et al. (2020). Brucella suppress STING expression via mi R-24 to enhance infection. PLoS Pathog. 16:e1009020. doi: 10.1371/journal.ppat.1009020
Khan, M., Harms, J. S., Marim, F. M., Armon, L., Hall, C. L., Liu, Y. P., et al. (2016). The bacterial second messenger cyclic di-GMP regulates Brucella pathogenesis and leads to altered host immune response. Infect. Immun. 84, 3458–3470. doi: 10.1128/IAI.00531-16
Kiyono, H., Yuki, Y., Nakahashi-Ouchida, R., and Fujihashi, K. (2021). Mucosal vaccines: wisdom from now and then. Int. Immunol. 33, 767–774. doi: 10.1093/intimm/dxab056
Ko, J., Gendron-Fitzpatrick, A., and Splitter, G. A. (2002). Susceptibility of IFN regulatory factor-1 and IFN consensus sequence binding protein-deficient mice to brucellosis. J. Immunol. 168, 2433–2440. doi: 10.4049/jimmunol.168.5.2433
Ko, J., and Splitter, G. A. (2003). Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 16, 65–78. doi: 10.1128/CMR.16.1.65-78.2003
Kuper, C. F., Koornstra, P. J., Hameleers, D. M., Biewenga, J., Spit, B. J., Duijvestijn, A. M., et al. (1992). The role of nasopharyngeal lymphoid tissue. Immunol. Today 13, 219–224. doi: 10.1016/0167-5699(92)90158-4
Lacey, C. A., Keleher, L. L., Mitchell, W. J., Brown, C. R., and Skyberg, J. A. (2016). CXCR2 mediates Brucella-induced arthritis in interferon γ-deficient mice. J Infect Dis 214, 151–160. doi: 10.1093/infdis/jiw087
Lacey, C. A., Mitchell, W. J., Dadelahi, A. S., and Skyberg, J. A. (2018). Caspase-1 and Caspase-11 mediate Pyroptosis, inflammation, and control of Brucella joint infection. Infect. Immun. 86, e00361–e00318. doi: 10.1128/IAI.00361-18
Lange, J., Rivera-Ballesteros, O., and Buggert, M. (2022). Human mucosal tissue-resident memory T cells in health and disease. Mucosal Immunol. 15, 389–397. doi: 10.1038/s41385-021-00467-7
Lavelle, E. C., and Ward, R. W. (2022). Mucosal vaccines - fortifying the frontiers. Nat. Rev. Immunol. 22, 236–250. doi: 10.1038/s41577-021-00583-2
Liang, S. C., Tan, X. Y., Luxenberg, D. P., Karim, R., Dunussi-Joannopoulos, K., Collins, M., et al. (2006). Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279. doi: 10.1084/jem.20061308
Loisel-Meyer, S., Jiménez de Bagüés, M. P., Bassères, E., Dornand, J., Köhler, S., Liautard, J. P., et al. (2006). Requirement of nor D for Brucella suis virulence in a murine model of in vitro and in vivo infection. Infect. Immun. 74, 1973–1976. doi: 10.1128/IAI.74.3.1973-1976.2006
López-Santiago, R., Sánchez-Argáez, A. B., De Alba-Núñez, L. G., Baltierra-Uribe, S. L., and Moreno-Lafont, M. C. (2019). Immune Response to Mucosal Brucella Infection. Front. Immunol. 10:1759. doi: 10.3389/fimmu.2019.01759
Lubroth, J., Rweyemamu, M. M., Viljoen, G., Diallo, A., Dungu, B., and Amanfu, W. (2007). Veterinary vaccines and their use in developing countries. Rev. Sci. Tech. 26, 179–201. doi: 10.20506/rst.26.1.1737
Mableson, H. E., Okello, A., Picozzi, K., and Welburn, S. C. (2014). Neglected zoonotic diseases-the long and winding road to advocacy. PLoS Negl. Trop. Dis. 8:e 2800. doi: 10.1371/journal.pntd.0002800
Malik, G. M. (1997). A clinical study of brucellosis in adults in the Asir region of southern Saudi Arabia. Am. J. Trop. Med. Hyg. 56, 375–377. doi: 10.1056/NEJM199804093381520
Martirosyan, A., Moreno, E., and Gorvel, J. P. (2011). An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 240, 211–234. doi: 10.1111/j.1600-065X.2010.00982.x
McDermott, J., Grace, D., and Zinsstag, J. (2013). Economics of brucellosis impact and control in low-income countries. Rev. Sci. Tech. 32, 249–261. doi: 10.20506/rst.32.1.2197
McNab, F., Mayer-Barber, K., Sher, A., Wack, A., and O'Garra, A. (2015). Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103. doi: 10.1038/nri3787
Meador, V. P., and Deyoe, B. L. (1989). Intracellular localization of Brucella abortus in bovine placenta. Vet. Pathol. 26, 513–515. doi: 10.1177/030098588902600609
Meador, V. P., Warner, D. P., and Deyoe, B. L. (1988). Distribution of Brucella abortus organisms in calves after conjunctival exposure. Am. J. Vet. Res. 49, 2015–2017.
Mettelman, R. C., Allen, E. K., and Thomas, P. G. (2022). Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 55, 749–780. doi: 10.1016/j.immuni.2022.04.013
Milillo, M. A., Trotta, A., Serafino, A., Marin Franco, J. L., Marinho, F. V., Alcain, J., et al. (2019). Bacterial RNA contributes to the Down-modulation of MHC-II expression on monocytes/macrophages diminishing CD4+ T cell responses. Front. Immunol. 10:2181. doi: 10.3389/fimmu.2019.02181
Mills, K. H. G. (2022). IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 5, 1–17. doi: 10.1038/s41577-022-00746-9
Moore, B. (1957). Observations pointing to the conjunctiva as the portal of entry in salmonella infection of guinea-pigs. J. Hyg. 55, 414–433. doi: 10.1017/s0022172400037311
Moreno, E., Blasco, J. M., Letesson, J. J., Gorvel, J. P., and Moriyón, I. (2022). Pathogenicity and its implications in taxonomy: the Brucella and Ochrobactrum case. Pathogens 11:377. doi: 10.3390/pathogens11030377
Mueller, S. N., and Mackay, L. K. (2016). Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89. doi: 10.1038/nri.2015.3
Muñoz, P. M., de Miguel, M. J., Grilló, M. J., Marín, C. M., Barberán, M., and Blasco, J. M. (2008). Immunopathological responses and kinetics of Brucella melitensis Rev 1 infection after subcutaneous or conjunctival vaccination in rams. Vaccine 26, 2562–2569. doi: 10.1016/j.vaccine.2008.03.03
Murphy, E. A., Sathiyaseelan, J., Parent, M. A., Zou, B., and Baldwin, C. L. (2001). Interferon-γ is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103, 511–518. doi: 10.1046/j.1365-2567.2001.01258.x
Nakato, G., Hase, K., Suzuki, M., Kimura, M., Ato, M., Hanazato, M., et al. (2012). Cutting edge: Brucella abortus exploits a cellular prion protein on intestinal M cells as an invasive receptor. J. Immunol. 189, 1540–1544. doi: 10.4049/jimmunol.1103332
Nicoletti, P. (1984). Vaccination of cattle with Brucella abortus strain 19 administered by differing routes and doses. Vaccine 2, 133–135. doi: 10.1016/0264-410x(84)90004-5
Nicoletti, P., and Milward, F. W. (1983). Protection by oral administration of Brucella abortus strain 19 against an oral challenge exposure with a pathogenic strain of Brucella. Am. J. Vet. Res. 44, 1641–1643.
Occhialini, A., Hofreuter, D., Ufermann, C. M., Al Dahouk, S., and Köhler, S. (2022). The retrospective on atypical Brucella species leads to novel definitions. Microorganisms 10:813. doi: 10.3390/microorganisms10040813
Olsen, S. C. (2000). Immune responses and efficacy after administration of a commercial Brucella abortus strain RB51 vaccine to cattle. Vet. Ther. 1, 183–191.
Olsen, S. C., and Palmer, M. V. (2014). Advancement of knowledge of Brucella over the past 50 years. Vet. Pathol. 51, 1076–1089. doi: 10.1177/0300985814540545
Olsen, S. C., and Stoffregen, W. S. (2005). Essential role of vaccines in brucellosis control and eradication programs for livestock. Expert Rev. Vaccines 4, 915–928. doi: 10.1586/14760584.4.6.915
Olsen, S. C., and Tatum, F. M. (2016). Swine brucellosis: current perspectives. Vet. Med. 8, 1–12. doi: 10.2147/VMRR.S91360
Paixão, T. A., Roux, C. M., den Hartigh, A. B., Sankaran-Walters, S., Dandekar, S., Santos, R. L., et al. (2009). Establishment of systemic Brucella melitensis infection through the digestive tract requires urease, the type IV secretion system, and lipopolysaccharide O antigen. Infect. Immun. 77, 4197–4208. doi: 10.1128/IAI.00417-09
Pappagianis, D., Elberg, S. S., and Crouch, D. (1966). Immunization against Brucella infections. Effects of graded doses of viable attenuated Brucella melitensis in humans. Am. J. Epidemiol. 84, 21–31. doi: 10.1093/oxfordjournals.aje.a120624
Pappas, G. (2022). The Lanzhou Brucella leak: the largest laboratory accident in the history of infectious diseases? Clin. Infect. Dis. 75, 1845–1847. doi: 10.1093/cid/ciac463
Pappas, G., Akritidis, N., Bosilkovski, M., and Tsianos, E. (2005). Brucellosis. N. Engl. J. Med. 352, 2325–2336. doi: 10.1056/NEJMra050570
Pappas, G., Panagopoulou, P., Christou, L., and Akritidis, N. (2006). Brucella as a biological weapon. Cell. Mol. Life Sci. 63, 2229–2236. doi: 10.1007/s00018-006-6311-4
Pappas, G., Papadimitriou, P., Akritidis, N., Christou, L., and Tsianos, E. V. (2006). The new global map of human brucellosis. Lancet Infect. Dis. 6, 91–99. doi: 10.1016/S1473-3099(06)70382-6
Pascual, D. W., Yang, X., Wang, H., Goodwin, Z., Hoffman, C., and Clapp, B. (2018). Alternative strategies for vaccination to brucellosis. Microbes Infect. 20, 599–605. doi: 10.1016/j.micinf.2017.12.006
Pasquevich, K. A., García Samartino, C., Coria, L. M., Estein, S. M., Zwerdling, A., Ibañez, A. E., et al. (2010). The protein moiety of Brucella abortus outer membrane protein 16 is a new bacterial pathogen-associated molecular pattern that activates dendritic cells in vivo, induces a Th1 immune response, and is a promising self-adjuvanting vaccine against systemic and oral acquired brucellosis. J. Immunol. 184, 5200–5212. doi: 10.4049/jimmunol.0902209
Pasquevich, K. A., Ibañez, A. E., Coria, L. M., García Samartino, C., Estein, S. M., Zwerdling, A., et al. (2011). An oral vaccine based on U-Omp 19 induces protection against B. abortus mucosal challenge by inducing an adaptive IL-17 immune response in mice. PLoS One 6:e16203. doi: 10.1371/journal.pone.0016203
Pereira, C. R., de Almeida JVF, C., Cardoso de Oliveira, I. R., Faria de Oliveira, L., Pereira, L. J., Zangerônimo, M. G., et al. (2020). Occupational exposure to Brucella spp.: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 14:e0008164. doi: 10.1371/journal.pntd.0008164
Pesce Viglietti, A. I., Arriola Benitez, P. C., Giambartolomei, G. H., and Delpino, M. V. (2016). Brucella abortus-infected B cells induce osteoclastogenesis. Microbes Infect. 18, 529–535. doi: 10.1016/j.micinf.2016.04.001
Pizarro-Cerdá, J., Méresse, S., Parton, R. G., van der Goot, G., Sola-Landa, A., Lopez-Goñi, I., et al. (1998). Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66, 5711–5724. doi: 10.1128/IAI.66.12.5711-5724.1998
Poelma, L. J., and Pickens, E. M. (1932). Brucella abortus in human tonsils. J. Bacteriol. 23, 112–113.
Poester, F. P., Samartino, L. E., and Santos, R. L. (2013). Pathogenesis and pathobiology of brucellosis in livestock. Rev. Sci. Tech. 32, 105–115. doi: 10.20506/rst.32.1.2193
Rafiei, A., Ardestani, S. K., Kariminia, A., Keyhani, A., Mohraz, M., and Amirkhani, A. (2006). Dominant Th1 cytokine production in early onset of human brucellosis followed by switching towards Th2 along prolongation of disease. J. Infect. 53, 315–324. doi: 10.1016/j.jinf.2005.11.024
Rajapakse, C. N. (1995). Bacterial infections: osteoarticular brucellosis. Baillieres Clin. Rheumatol. 9, 161–177. doi: 10.1016/s0950-3579(05)80153-0
Reguera, J. M., Alarcón, A., Miralles, F., Pachón, J., Juárez, C., and Colmenero, J. D. (2003). Brucella endocarditis: clinical, diagnostic, and therapeutic approach. Eur. J. Clin. Microbiol. Infect. Dis. 22, 647–650. doi: 10.1007/s10096-003-1026-z
Rhyan, J., Nol, P., Wehtje, M., Bosco-Lauth, A., Marlenee, N., McCollum, M., et al. (2019). Partial protection in BALB/c house mice (Mus musculus) and Rocky Mountain elk (Cervus canadensis) after vaccination with a killed, mucosally delivered Brucella abortus vaccine. J. Wildl. Dis. 55, 794–803. doi: 10.7589/2018-08-190
Ridler, A. L., and West, D. M. (2011). Control of Brucella ovis infection in sheep. Vet. Clin. North Am. Food Anim. Pract. 27, 61–66. doi: 10.1016/j.cvfa.2010.10.013
Rodriguez-Zapata, M., Salmeron, I., Manzano, L., Salmeron, O. J., Prieto, A., and Alvarez-Mon, M. (1996). Defective interferon-gamma production by T-lymphocytes from patients with acute brucellosis. Eur. J. Clin. Invest. 26, 136–140. doi: 10.1046/j.1365-2362.1996.108250.x
Ron-Román, J., Ron-Garrido, L., Abatih, E., Celi-Erazo, M., Vizcaíno-Ordóñez, L., Calva-Pacheco, J., et al. (2014). Human brucellosis in Northwest Ecuador: typifying Brucella spp., seroprevalence, and associated risk factors. Vector Borne Zoonotic Dis. 14, 124–133. doi: 10.1089/vbz.2012.1191
Roop, R. M., Barton, I. S., Hopersberger, D., and Martin, D. W. (2021). Uncovering the hidden credentials of Brucella virulence. Microbiol. Mol. Biol. Rev. 85, e00021–e00019. doi: 10.1128/MMBR.00021-19
Rossetti, C. A., Arenas-Gamboa, A. M., and Maurizio, E. (2017). Caprine brucellosis: a historically neglected disease with significant impact on public health. PLoS Negl. Trop. Dis. 11:e0005692. doi: 10.1371/journal.pntd.0005692
Rossetti, C. A., Maurizio, E., and Rossi, U. A. (2022). Comparative review of brucellosis in small domestic ruminants. Front. Vet. Sci. 9:887671. doi: 10.3389/fvets.2022.887671
Rouzic, N., Desmier, L., Cariou, M. E., Gay, E., Foster, J. T., Williamson, C. H. D., et al. (2021). First case of brucellosis caused by an amphibian-type Brucella. Clin. Infect. Dis. 72, e404–e407. doi: 10.1093/cid/ciaa1082
Royer, D. J., Montegomery, M. L., and Carr, D. J. J. (2019). “Chap. 17 - Mucosal regulatory system for balanced ocular immunity” in Mucosal Vaccines: Innovation for Preventing Infectious Diseases. eds. H. Kiyono and D. W. Pascual. 2nd ed (London, United Kingdom: Academic Press), 299–312.
Rubach, M. P., Halliday, J. E., Cleaveland, S., and Crump, J. A. (2013). Brucellosis in low-income and middle-income countries. Curr. Opin. Infect. Dis. 26, 404–412. doi: 10.1097/QCO.0b013e3283638104
Sakamoto, K., Kim, Y. G., Hara, H., Kamada, N., Caballero-Flores, G., Tolosano, E., et al. (2017). IL-22 controls iron-dependent nutritional immunity against systemic bacterial infections. Sci. Immunol. 2:eaai 8371. doi: 10.1126/sciimmunol.aai8371
Salcedo, S. P., Marchesini, M. I., Lelouard, H., Fugier, E., Jolly, G., Balor, S., et al. (2008). Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp 1. PLoS Pathog. 4:e21. doi: 10.1371/journal.ppat.0040021
Samartino, L. E., and Enright, F. M. (1993). Pathogenesis of abortion of bovine brucellosis. Comp. Immunol. Microbiol. Infect. Dis. 16, 95–101. doi: 10.1016/0147-9571(93)90001-l
Sangari, F. J., García-Lobo, J. M., and Agüero, J. (1994). The Brucella abortus vaccine strain B19 carries a deletion in the erythritol catabolic genes. FEMS Microbiol. Lett. 121, 337–342. doi: 10.1111/j.1574-6968.1994.tb07123.x
Scholz, H. C., Hubalek, Z., Sedlácek, I., Vergnaud, G., Tomaso, H., Al Dahouk, S., et al. (2008). Brucella microti sp. nov., isolated from the common vole Microtus arvalis. Int. J. Syst. Evol. Microbiol. 58, 375–382. doi: 10.1099/ijs.0.65356-0
Scholz, H. C., Nöckler, K., Göllner, C., Bahn, P., Vergnaud, G., Tomaso, H., et al. (2010). Brucella inopinata sp. nov., isolated from a breast implant infection. Int. J. Syst. Evol. Microbiol. 60, 801–808. doi: 10.1099/ijs.0.011148-0
Schumaker, B. (2013). Risks of Brucella abortus spillover in the greater Yellowstone area. Rev. Sci. Tech. 32, 71–77. doi: 10.20506/rst.32.1.2185
Schurig, G. G., Roop, R. M. 2nd, Bagchi, T., Boyle, S., Buhrman, D., and Sriranganathan, N. (1991). Biological properties of RB51; a stable rough strain of Brucella abortus. Vet. Microbiol. 28, 171–188. doi: 10.1016/0378-1135(91)90091-s
Schurig, G. G., Sriranganathan, N., and Corbel, M. J. (2002). Brucellosis vaccines: past, present and future. Vet. Microbiol. 90, 479–496. doi: 10.1016/s0378-1135(02)00255-9
Sekimata, M., Yoshida, D., Araki, A., Asao, H., Iseki, K., and Murakami-Sekimata, A. (2019). Runx 1 and RORγt cooperate to Upregulate IL-22 expression in Th cells through its distal enhancer. J. Immunol. 202, 3198–3210. doi: 10.4049/jimmunol.1800672
Seo, K. Y., Han, S. J., Cha, H. R., Seo, S. U., Song, J. H., Chung, S. H., et al. (2010). Eye mucosa: an efficient vaccine delivery route for inducing protective immunity. J. Immunol. 185, 3610–3619. doi: 10.4049/jimmunol.1000680
Shen, M. W. (2008). Diagnostic and therapeutic challenges of childhood brucellosis in a nonendemic country. Pediatrics 121, e1178–e1183. doi: 10.1542/peds.2007-1874
Silva-Sanchez, A., and Randall, T. D. (2019). “Chap. 2 - Anatomical uniqueness of the mucosal immune system (GALT, NALT, iBALT) for the induction of mucosal immunity and tolerance” in Mucosal Vaccines: Innovation for Preventing Infectious Diseases. eds. H. Kiyono and D. W. Pascual. 2nd ed (London, United Kingdom: Academic Press), 21–54.
Skyberg, J. A., Thornburg, T., Kochetkova, I., Layton, W., Callis, G., Rollins, M. F., et al. (2012). IFN-γ-deficient mice develop IL-1-dependent cutaneous and musculoskeletal inflammation during experimental brucellosis. J. Leukoc. Biol. 92, 375–387. doi: 10.1189/jlb.1211626
Skyberg, J. A., Thornburg, T., Rollins, M., Huarte, E., Jutila, M. A., and Pascual, D. W. (2011). Murine and bovine γδ T cells enhance innate immunity against Brucella abortus infections. PLoS One 6:e21978. doi: 10.1371/journal.pone.0021978
Snyder, G. A., Deredge, D., Waldhuber, A., Fresquez, T., Wilkins, D. Z., Smith, P. T., et al. (2014). Crystal structures of the toll/Interleukin-1 receptor (TIR) domains from the Brucella protein Tcp B and host adaptor TIRAP reveal mechanisms of molecular mimicry. J. Biol. Chem. 289, 669–679. doi: 10.1074/jbc.M113.523407
Spera, J. M., Comerci, D. J., and Ugalde, J. E. (2014). Brucella alters the immune response in a prp A-dependent manner. Microb. Pathog. 67-68, 8–13. doi: 10.1016/j.micpath.2014.01.003
Spink, W. W., Hall, J. W. 3rd, Finstad, J., and Mallet, E. (1962). Immunization with viable Brucella organisms. Results of a safety test in humans. Bull. World Health Organ. 26, 409–419.
Starr, T., Ng, T. W., Wehrly, T. D., Knodler, L. A., and Celli, J. (2008). Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 9, 678–694. doi: 10.1111/j.1600-0854.2008.00718.x
Stevens, M. G., Hennager, S. G., Olsen, S. C., and Cheville, N. F. (1994). Serologic responses in diagnostic tests for brucellosis in cattle vaccinated with Brucella abortus 19 or RB51. J. Clin. Microbiol. 32, 1065–1066. doi: 10.1128/jcm.32.4.1065-1066.1994
Stevens, M. G., Olsen, S. C., Palmer, M. V., and Pugh, G. W. Jr. (1996). Immune responses and resistance to brucellosis in mice vaccinated orally with Brucella abortus RB51. Infect. Immun. 64, 4534–4541. doi: 10.1128/iai.64.11.4534-4541.1996
Suárez-Esquivel, M., Ruiz-Villalobos, N., Jiménez-Rojas, C., Barquero-Calvo, E., Chacón-Díaz, C., Víquez-Ruiz, E., et al. (2017). Brucella neotomae infection in humans, Costa Rica. Emerg. Infect. Dis. 23, 997–1000. doi: 10.3201/eid2306.162018
Suraud, V., Jacques, I., Olivier, M., and Guilloteau, L. A. (2008). Acute infection by conjunctival route with Brucella melitensis induces IgG+ cells and IFN-γ producing cells in peripheral and mucosal lymph nodes in sheep. Microbes Infect. 10, 1370–1378. doi: 10.1016/j.micinf.2008.08.00
Suraud, V., Olivier, M., Bodier, C. C., and Guilloteau, L. A. (2007). Differential expression of homing receptors and vascular addressins in tonsils and draining lymph nodes: effect of Brucella infection in sheep. Vet. Immunol. Immunopathol. 115, 239–250. doi: 10.1016/j.vetimm.2006.11.008
Surendran, N., Sriranganathan, N., Boyle, S. M., Hiltbold, E. M., Tenpenny, N., Walker, M., et al. (2013). Protection to respiratory challenge of Brucella abortus strain 2308 in the lung. Vaccine 31, 4103–4110. doi: 10.1016/j.vaccine.2013.06.078
Surendran, N., Sriranganathan, N., Lawler, H., Boyle, S. M., Hiltbold, E. M., Heid, B., et al. (2011). Efficacy of vaccination strategies against intranasal challenge with Brucella abortus in BALB/c mice. Vaccine 29, 2749–2755. doi: 10.1016/j.vetmic.2010.06.001
Szabo, P. A., Miron, M., and Farber, D. L. (2019). Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol. 4:eaas 9673. doi: 10.1126/sciimmunol.aas9673
Takaoka, A., and Yamada, T. (2019). Regulation of signaling mediated by nucleic acid sensors for innate interferon-mediated responses during viral infection. Int. Immunol. 31, 477–488. doi: 10.1093/intimm/dxz034
Teske, S. S., Huang, Y., Tamrakar, S. B., Bartrand, T. A., Weir, M. H., and Haas, C. N. (2011). Animal and human dose-response models for Brucella species. Risk Anal. 31, 1576–1596. doi: 10.1111/j.1539-6924.2011.01602.x
Tilney, N. L. (1971). Patterns of lymphocyte drainage in the adult laboratory rat. J. Anat. 109, 369–383.
Traxler, R. M., Lehman, M. W., Bosserman, E. A., Guerra, M. A., and Smith, T. L. (2013). A literature review of laboratory-acquired brucellosis. J. Clin. Microbiol. 51, 3055–3062. doi: 10.1128/JCM.00135-13
Troy, S. B., Rickman, L. S., and Davis, C. E. (2005). Brucellosis in San Diego: epidemiology and species-related differences in acute clinical presentations. Medicine 84, 174–187. doi: 10.1097/01.md.0000165659.20988.25
Turner, D. L., Bickham, K. L., Thome, J. J., Kim, C. Y., D’Ovidio, F., Wherry, E. J., et al. (2013). Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 7, 501–510. doi: 10.1038/mi.2013.67
Valeri, M., and Raffatellu, M. (2016). Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 74:ftw111. doi: 10.1093/femspd/ftw111
Van der Henst, C., de Barsy, M., Zorreguieta, A., Letesson, J. J., and De Bolle, X. (2013). The Brucella pathogens are polarized bacteria. Microbes Infect. 15, 998–1004. doi: 10.1016/j.micinf.2013.10.008
van Straten, M., Bardenstein, S., Keningswald, G., and Banai, M. (2016). Brucella abortus S19 vaccine protects dairy cattle against natural infection with Brucella melitensis. Vaccine 34, 5837–5839. doi: 10.1016/j.vaccine.2016.10.011
Vassallo, D. J. (1992). The corps disease: brucellosis and its historical association with the Royal Army Medical Corps. J. R. Army Med. Corps 138, 140–150. doi: 10.1136/jramc-138-03-09
Velásquez, L. N., Milillo, M. A., Delpino, M. V., Trotta, A., Fernández, P., Pozner, R. G., et al. (2017). Brucella abortus down-regulates MHC class II by the IL-6-dependent inhibition of CIITA through the downmodulation of IFN regulatory factor-1 (IRF-1). J. Leukoc. Biol. 101, 759–773. doi: 10.1189/jlb.4A0416-196R
Velásquez, L. N., Milillo, M. A., Delpino, M. V., Trotta, A., Mercogliano, M. F., Pozner, R. G., et al. (2017). Inhibition of MHC-I by Brucella abortus is an early event during infection and involves EGFR pathway. Immunol. Cell Biol. 95, 388–398. doi: 10.1038/icb.2016.111
Vemulapalli, R., McQuiston, J. R., Schurig, G. G., Sriranganathan, N., Halling, S. M., and Boyle, S. M. (1999). Identification of an IS711 element interrupting the wbo a gene of Brucella abortus vaccine strain RB51 and a PCR assay to distinguish strain RB51 from other Brucella species and strains. Clin. Diagn. Lab. Immunol. 6, 760–764. doi: 10.1128/CDLI.6.5.760-764.1999
Vershilova, P. A. (1961). The use of live vaccine for vaccination of human beings against brucellosis in the USSR. Bull. World Health Organ. 24, 85–89.
Vitry, M. A., De Trez, C., Goriely, S., Dumoutier, L., Akira, S., Ryffel, B., et al. (2012). Crucial role of IFN-γ-producing CD4+ Th1 cells but dispensable function of CD8+ T cell, B cell, Th2, and Th17 responses in the control of Brucella melitensis infection in mice. Infect. Immun. 80, 4271–4280. doi: 10.1128/IAI.00761-12
Vitry, M. A., Hanot Mambres, D., De Trez, C., Akira, S., Ryffel, B., Letesson, J. J., et al. (2014). Humoral immunity and CD4+ Th1 cells are both necessary for a fully protective immune response upon secondary infection with Brucella melitensis. J. Immunol. 192, 3740–3752. doi: 10.4049/jimmunol.1302561
von Bargen, K., Gagnaire, A., Arce-Gorvel, V., de Bovis, B., Baudimont, F., Chasson, L., et al. (2015). Cervical lymph nodes as a selective niche for Brucella during oral infections. PLoS One 10:e121790. doi: 10.1371/journal.pone.0121790
Wang, H., Hoffman, C., Yang, X., Clapp, B., and Pascual, D. W. (2020). Targeting resident memory T cell immunity culminates in pulmonary and systemic protection against Brucella infection. PLoS Pathog. 16:e1008176. doi: 10.1371/journal.ppat.1008176
Wang, W., Wu, J., Qiao, J., Weng, Y., Zhang, H., Liao, Q., et al. (2014). Evaluation of humoral and cellular immune responses to BP26 and OMP31 epitopes in the attenuated Brucella melitensis vaccinated sheep. Vaccine 32, 825–833. doi: 10.1016/j.vaccine.2013.12.028
Wattam, A. R., Williams, K. P., Snyder, E. E., Almeida, N. F. Jr., Shukla, M., Dickerman, A. W., et al. (2009). Analysis of ten Brucella genomes reveals evidence for horizontal gene transfer despite a preferred intracellular lifestyle. J. Bacteriol. 191, 3569–3579. doi: 10.1128/JB.01767-08
Welburn, S. C., Beange, I., Ducrotoy, M. J., and Okello, A. L. (2015). The neglected zoonoses - the case for integrated control and advocacy. Clin. Microbiol. Infect. 21, 433–443. doi: 10.1016/j.cmi.2015.04.011
Weynants, V., Walravens, K., Didembourg, C., Flanagan, P., Godfroid, J., and Letesson, J. J. (1998). Quantitative assessment by flow cytometry of T-lymphocytes producing antigen-specific γ-interferon in Brucella immune cattle. Vet. Immunol. Immunopathol. 66, 309–320. doi: 10.1016/s0165-2427(98)00205-0
Whatmore, A. M., and Foster, J. T. (2021). Emerging diversity and ongoing expansion of the genus Brucella. Infect. Genet. Evol. 92:104865. doi: 10.1016/j.meegid.2021.104865
Winter, A. J., Duncan, J. R., Santisteban, C. G., Douglas, J. T., and Adams, L. G. (1989). Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect. Immun. 57, 3438–3444. doi: 10.1128/iai.57.11.3438-3444.1989
World Animal Health Organization (2014). Manual of diagnostic tests and vaccines for terrestrial animals World Animal Health Organization.
World Health Organization (2005). The control of neglected zoonotic diseases: A route to poverty alleviation. Reports of a joint WHO/DIFD-animal health Programme meeting with the participation of FAO and OIE, Geneva, 20–21. Paris, France: Geneva.
Wu, H. Y., Nikolova, E. B., Beagley, K. W., Eldridge, J. H., and Russell, M. W. (1997). Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect. Immun. 65, 227–235. doi: 10.1128/iai.65.1.227-235.1997
Xin, X. (1986). Orally administrable brucellosis vaccine: Brucella suis strain 2 vaccine. Vaccine 4, 212–216. doi: 10.1016/0264-410x(86)90131-3
Yang, X., Becker, T., Walters, N., and Pascual, D. W. (2006). Deletion of znuA virulence factor attenuates Brucella abortus and confers protection against wild-type challenge. Infect. Immun. 74, 3874–3879. doi: 10.1128/IAI.01957-05
Yang, X., Clapp, B., Thornburg, T., Hoffman, C., and Pascual, D. W. (2016). Vaccination with a Δnor D Δznu a Brucella abortus mutant confers potent protection against virulent challenge. Vaccine 34, 5290–5297. doi: 10.1016/j.vaccine.2016.09.004
Yingst, S. L., Izadjoo, M., and Hoover, D. L. (2013). CD8 knockout mice are protected from challenge by vaccination with WR201, a live attenuated mutant of Brucella melitensis. Clin. Dev. Immunol. 2013:686919. doi: 10.1155/2013/686919
Zachou, K., Papamichalis, P. A., and Dalekos, G. N. (2008). Severe pharyngitis in stockbreeders: an unusual presentation of brucellosis. Occup. Med. 58, 305–307. doi: 10.1093/occmed/kqn020
Zhan, Y., and Cheers, C. (1995). Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infect. Immun. 63, 1387–1390. doi: 10.1128/iai.63.4.1387-1390.1995
Zhan, Y., and Cheers, C. (1998). Control of IL-12 and IFN-γ production in response to live or dead bacteria by TNF and other factors. J. Immunol. 161, 1447–1453.
Zhan, Y., Kelso, A., and Cheers, C. (1995). Differential activation of Brucella-reactive CD4+ T cells by Brucella infection or immunization with antigenic extracts. Infect. Immun. 63, 969–975. doi: 10.1128/iai.63.3.969-975.1995
Zheng, Y., Danilenko, D. M., Valdez, P., Kasman, I., Eastham-Anderson, J., Wu, J., et al. (2007). Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651. doi: 10.1038/nature05505
Zheng, M. Z. M., and Wakim, L. M. (2022). Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 15, 379–388. doi: 10.1038/s41385-021-00461-z
Zhu, L., Feng, Y., Zhang, G., Jiang, H., Zhang, Z., Wang, N., et al. (2016). Brucella suis strain 2 vaccine is safe and protective against heterologous Brucella spp. infections. Vaccine 34, 395–400. doi: 10.1016/j.vaccine.2015.09.116
Keywords: Brucella, mucosal immunity, vaccination, pathogenesis, cell-mediated immunity, CD8+ T cells, resident memory T cells
Citation: Pascual DW, Goodwin ZI, Bhagyaraj E, Hoffman C and Yang X (2022) Activation of mucosal immunity as a novel therapeutic strategy for combating brucellosis. Front. Microbiol. 13:1018165. doi: 10.3389/fmicb.2022.1018165
Edited by:
Som G. Nanjappa, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Menachem Banai, Kimron Veterinary Institute, IsraelEric Muraille, Université libre de Bruxelles, Belgium
Copyright © 2022 Pascual, Goodwin, Bhagyaraj, Hoffman and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David W. Pascual, pascuald@ufl.edu
 David W. Pascual
David W. Pascual Zakia I. Goodwin
Zakia I. Goodwin Ella Bhagyaraj
Ella Bhagyaraj