- Division of Basic Biomedical Sciences, The Sanford School of Medicine of the University of South Dakota, Vermillion, SD, USA
Viral infections of the upper respiratory tract are associated with a variety of invasive diseases caused by Streptococcus pyogenes, the group A streptococcus, including pneumonia, necrotizing fasciitis, toxic shock syndrome, and bacteremia. While these polymicrobial infections, or superinfections, are complex, progress has been made in understanding the molecular basis of disease. Areas of investigation have included the characterization of virus-induced changes in innate immunity, differences in bacterial adherence and internalization following viral infection, and the efficacy of vaccines in mitigating the morbidity and mortality of superinfections. Here, we briefly summarize viral-S. pyogenes superinfections with an emphasis on those affiliated with influenza viruses.
Introduction
The study of microbial pathogenesis typically examines a single pathogen in an experimental system. The reductionist approach has proven to be highly informative and forms the foundation of our understanding of pathogenesis. Nonetheless, humans are often exposed to an assortment of viral and bacterial pathogens and the factors determining the outcome of these host-pathogen interactions are less well characterized. Viral infections complicated by a concurrent, or subsequent, bacterial infection are known as coinfections or superinfections, respectively. Due to synergistic effects, the mortality of these infections is often greater than that of either the virus or the bacteria alone (Morens and Taubenberger, 2015).
Streptococcus pyogenes, the group A streptococcus (GAS), can colonize humans asymptomatically or cause infections of the skin and pharynx. In severe cases, the gram-positive pathogen can cause a variety of life threatening invasive diseases (iGAS diseases) including bacteremia, toxic shock syndrome, pneumonia, and necrotizing fasciitis (the so-called “flesh eating disease”). GAS causes approximately 700 million infections annually, including 1.8 million invasive infections. The mortality rate of these infections is ~20% (Carapetis et al., 2005), despite the availability of antibiotics that are effective ex vivo. Here we briefly summarize the epidemiological reports supporting the idea that upper respiratory tract viral infections contribute to the development of iGAS diseases and review studies aimed at identifying the molecular underpinnings of viral-GAS pathogenesis.
The Contribution of Varicella, Morbilla Virus, and Epstein–Barr Virus to IGAS Diseases
The association between chickenpox and GAS necrotizing fasciitis was described as early as the nineteenth century and varicella continues to be an important contributor to iGAS diseases among children, which is most evident in regions of the world that choose not to vaccinate against chickenpox or that lack the resources to do so (Wilson et al., 1995; Schreck et al., 1996). GAS is the most common bacterial cause of varicella coinfections (Aebi et al., 1996), which typically results in necrotizing fasciitis, bacteremia, and toxic shock syndrome. Usually these diseases occur as the result of infected skin lesions, which provide portals for bacterial entry into the blood. However, varicella-GAS superinfections can also occur, albeit rarely, when varicella skin lesions are not apparent suggesting that the viral infection causes systemic decreases in innate immunity (Laskey et al., 2000; Sivakumar and Latifi, 2008). Prior to the use of the vaccine, the incidence of varicella-GAS coinfections was estimated to be between 15 and 36% (Laupland et al., 2000; Zachariadou et al., 2014). Results of a study that spanned both the pre and post-varicella vaccination periods (1993–2001) showed that the incidence of these coinfections declined from 27% prior to 1995 to just 2% after the availability of the vaccine (Patel et al., 2004).
Both GAS and Epstein–Barr virus (EBV) are common causes of pharyngitis. Two studies focused on patients 25 years-of-age or younger and determined the incidence of EBV-GAS coinfection to be 18 and 29% (Henke et al., 1973; Rush and Simon, 2003). In contrast, < 3% of college students with EBV were also infected with GAS (Chretien and Esswein, 1976). While there are sporadic and infrequent reports of children with infectious mononucleosis developing iGAS diseases, including streptococcal toxic shock syndrome (Pontes and Antunes, 2004) and GAS bacteremia (DuBois and Baehner, 1979), a causal role for EBV in iGAS diseases remains unclear (Watanabe et al., 2012).
Measles virus (MV) infection can also enhance host susceptibility to bacterial infections (de Vries et al., 2012). While the incidence of MV-GAS coinfections is currently low, this has not always been the case. In 1917, an epidemic of severe GAS diseases coincided with a measles epidemic among U.S. Army personnel resulting in bronchopneumonia, empyema, and bacteremia, which were associated with significant mortality (Morens and Taubenberger, 2015). While GAS is currently a relatively uncommon cause of MV superinfections, sporadic cases still occur (Gremillion and Crawford, 1981; Duke and Mgone, 2003; Gahr et al., 2014).
The Contribution of Influenza to IGAS Diseases
The most thoroughly characterized bacterial superinfections are those involving the influenza virus (McCullers and Rehg, 2002; Peltola and McCullers, 2004). Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, and GAS are causes of “excess mortality” due to influenza coinfections and superinfections (Peltola and McCullers, 2004; Brundage, 2006), which were first described nearly a century ago (Laennec, 1923). An analysis of lung biopsies from the 1918 influenza pandemic revealed that S. pneumoniae and GAS were the most frequently observed bacteria and together contributed substantially to the deaths of approximately 50 million people (Morens and Fauci, 2007). The surprisingly high incidence of cases involving GAS was likely due to the circulation of particularly virulent GAS strains and high carriage rate during this time (Morens and Taubenberger, 2015). More recently during the 2009 H1N1 influenza pandemic, 27% of fatalities among people with laboratory confirmed IAV-bacterial coinfections were caused by GAS1. A report of 10 patients in California with laboratory confirmed H1N1-GAS infections during this pandemic revealed that despite treating at least 6 patients with both antivirals and antibiotics, 7 of the 10 patients died and the median age of the deceased was 37 (Jean et al., 2010).
In addition to these cases in the US, iGAS diseases associated with IAV occur Worldwide. In England, the incidence of all iGAS diseases increased by 26% between July 2009 and January 2011 with the highest increase reported in December 2010, which was attributed to an increase in influenza during the same time period (Zakikhany et al., 2011). A retrospective study conducted in France found that one third of children infected with GAS also had laboratory confirmed influenza infection (Parola et al., 2011). In Israel during the 2009–2010 influenza pandemic there was an increase in GAS bacteremia (Tasher et al., 2011). Similarly, the incidence of iGAS diseases in Sweden increased markedly in 2012 compared to previous years, which was attributed to an increase in respiratory viral infections, particularly influenza (Darenberg et al., 2013).
A study of military recruits in the U.S. provides a different perspective illustrating the contribution of IAV to GAS diseases. The recruits were less likely to experience any GAS disease if they were vaccinated against influenza compared to unvaccinated controls, and protection against GAS diseases ranged from 50 to 77% depending on the year of the study (Lee et al., 2008).
In addition to IAV, influenza B virus (IBV) is associated with iGAS diseases (Aebi et al., 2010). In a study conducted in Canada, 8% of patients with iGAS diseases occurring between 1996 and 2008 were also infected with IBV (Allard et al., 2012). In a study conducted in England, 14 of 19 cases of iGAS diseases including bacteremia, septic arthritis, endometritis, and pneumonia had evidence of a previous influenza-like infection and 26% had laboratory confirmed influenza infections, mostly IBV. Significantly, ten of the patients died even though 9 of those 10 were being treated with antibiotics that are effective against GAS ex vivo (Scaber et al., 2011).
Collectively, these clinical observations indicate that a substantial number of iGAS diseases are associated with influenza. Moreover, despite the availability of influenza vaccines, the practice of prophylactically treating severely ill influenza patients with antibiotics (Mandell et al., 2007), and the fact that all isolates of GAS are sensitive to penicillin ex vivo, the mortality rate of influenza-GAS superinfections is substantial.
Mechanisms of IAV-GAS Superinfection
The effect of influenza virus infection on host susceptibility to bacterial infections has been most thoroughly studied with S. pneumoniae and several excellent reviews on this topic are available (McCullers, 2006, 2014; Metzger and Sun, 2013; Rynda-Apple et al., 2015). Influenza infections increase host susceptibility to bacterial infections in a variety of ways including an increase in bacterial adherence after the removal of sialic acid from respiratory cells by viral neuraminidase (McCullers and Bartmess, 2003; McCullers, 2014); destruction of the mucocilliary escalator by viral induced lysis of respiratory epithelial cells (Ohashi et al., 1991; Pittet et al., 2010); decreased expression by alveolar macrophages of the scavenger receptor MARCO following influenza induction of IFN-γ (Sun and Metzger, 2008); depletion of resident alveolar macrophages following influenza infection (Ghoneim et al., 2013). As a result, macrophage-mediated clearance of bacteria is diminished.
Comparatively little is known about the host-pathogen interactions of IAV-GAS superinfection; however, the areas that have been investigated include the development of murine models of superinfection, characterization of viral-induced changes in bacterial adherence and internalization, and assessments of the efficacy of vaccines targeting either the virus or bacteria in decreasing the morbidity and mortality associated with superinfections.
Murine Models of IAV-GAS Superinfection
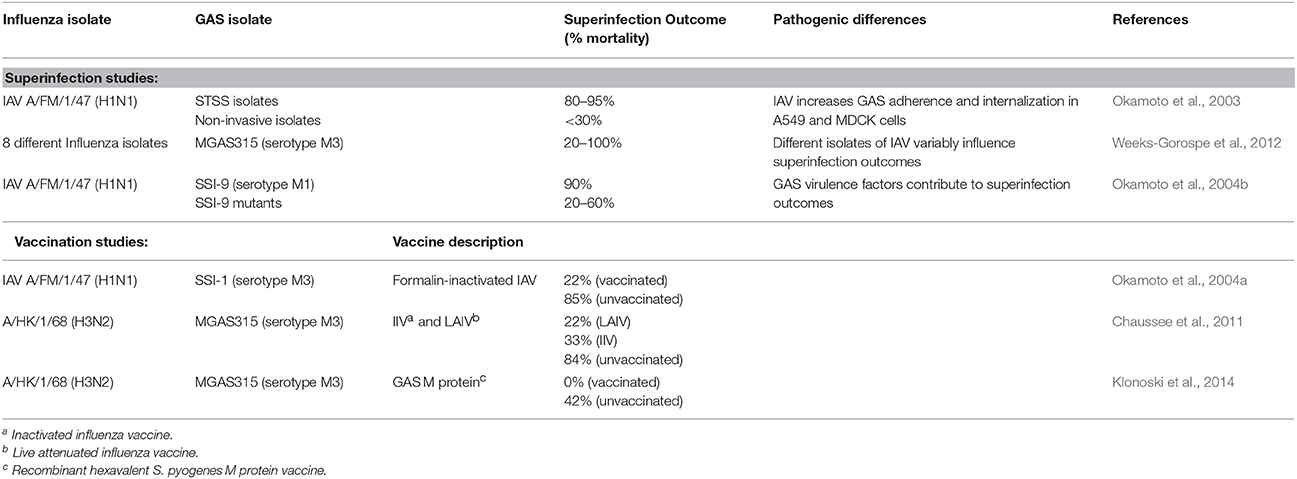
Murine models of IAV-GAS superinfection typically use sub-lethal intranasal inoculations of IAV and GAS, which are separated by 2–7 days. These studies are summarized in Table 1. As is likely the case with human disease, the outcome of murine IAV-GAS infections varies greatly depending on the specific viral and bacterial strains used in the study. In one case, the outcome of an IAV-GAS coinfection was compared between mice inoculated with isolates of GAS from patients with toxic shock syndrome and those from non-invasive infections using an interval of 2 days between viral and bacterial inoculations (Okamoto et al., 2003). Coinfection with isolates from cases of toxic shock syndrome resulted in the death of 80–95% of the mice while inoculation with the same dose of the isolates in the absence of antecedent viral infection resulted in no mortality. In contrast, when mice were coinfected with GAS isolates from non-invasive infections the mortality was < 30% (Okamoto et al., 2003). A subsequent study used a 5 day interval between IAV and GAS inoculations, which allows mice time to begin to recover from the viral infection prior to being infected with bacteria. Using this model, the contribution of eight different isolates of IAV to the outcome of superinfection was determined with a single isolate of GAS (Weeks-Gorospe et al., 2012). The mortality varied between 100 and 20% indicating that the outcome of IAV-GAS superinfections is also affected by the particular type of influenza virus (Weeks-Gorospe et al., 2012). Together, the results indicate that the severity of IAV-GAS superinfections is likely to vary considerably depending on the specific types of IAV and GAS circulating in the population.
The mortality of IAV-GAS superinfections in mice is due, in part, to increased persistence of the bacteria in the lungs of superinfected mice compared to mice infected with only GAS (Chaussee et al., 2011). Persistence is associated with subsequent bacteremia and multi-organ infection. As a result of dissemination, ~10% of superinfected mice develop necrotizing fasciitis (Okamoto et al., 2003).
Since the respiratory tract is altered during IAV infection, including changes in cell types, cytokine milieu, host protein composition and abundance, and endothelial and effector cell functions, it is of interest to know if GAS virulence factors important in a GAS monoinfection are similarly important in the context of a coinfection or superinfection. To address this issue, a serotype M1 strain of GAS and several mutant derivatives were analyzed with a murine H1N1 superinfection model (Okamoto et al., 2004b). Strains included those with mutations in the hasA gene (encoding the first enzyme involved in the synthesis of the hyaluronic acid capsule), slo (encoding the secreted thiol-activated cytolysin SLO), speB (encoding a secreted cysteine protease), sagA (encoding a secreted cytolysin SLS), and mga (encoding a global transcriptional regulatory protein; Okamoto et al., 2004b). Only 10% of mice coinfected with the parental GAS strain survived. In contrast, between 40 and 80% of mice coinfected with the mutant derivatives survived. The hasA gene had the most significant contribution to disease, which was attributed to decreased bacterial adherence due to the loss of the capsule (Okamoto et al., 2004b), which is an adhesion (Schrager et al., 1998). However, the capsule is also antiphagocytic, which likely contributed to the ability of the parental strain, but not the hasA mutant, to persist in the lungs of coinfected mice. In this respect, the hasA mutant had the fewest bacteria in the lungs of superinfected mice 24 h after inoculation compared to the wild-type strain and the other mutants. The coinfection studies were completed using an interval of 2 days between viral and bacterial inoculations (Okamoto et al., 2004b). The results indicate that virulence factors important in a monoinfection also contribute to coinfection and it will be of interest to determine if similar results are obtained using a superinfection model with a longer interval between viral and bacterial inoculations.
Adherence and Internalization
IAV infection increases the abundance of fibrinogen (Fg) in the respiratory tract beginning approximately 4 days after infection (Keller et al., 2006). The binding of GAS to Fg has been recognized for decades as being important for GAS adherence and internalization within host epithelial cells and for conferring resistance to phagocytosis (Cunningham, 2000). IAV infection enhanced the adherence of GAS to cultured Madin–Darby canine kidney (MDCK) cells in an Fg-dependent manner (Sanford et al., 1982). The finding was confirmed by a subsequent study showing that both bacterial adherence and internalization were enhanced in cells infected with IAV, and that the effect was also dependent on the GAS M protein, which binds to a variety of host proteins including Fg (Hafez et al., 2010). In addition to Fg, Streptococcus dysgalactiae binds directly to IAV hemagglutinin (HA) localized to the surface of infected cells. Binding was diminished by the addition of GAS, which indicated that GAS was also likely to bind directly to viral HA (Pan et al., 1979). In this regard, IAV infection of cultured cells (A549 and MDCK) increased GAS adherence and internalization compared to uninfected cells (Okamoto et al., 2003) and GAS bound directly to progeny virus budding from infected cells, presumably by binding to viral HA (Okamoto et al., 2003). Moreover, treating superinfected mice intravenously with antibodies against HA decreased the number of streptococci in the lungs as well as other organs and mortality (Okamoto et al., 2003). Similarly the adherence and internalization of a GAS serotype M6 isolate (JRS4) to MDCK cells was enhanced 4- and 20-fold, respectively, in viral infected cells compared to uninfected cells (Hafez et al., 2010). Both adherence and internalization were dependent on the M6 protein, and both were enhanced by host Fg and fibronectin (Fn) (Hafez et al., 2010).
The influenza neuraminidase cleaves sialic acid, which exposes host proteins involved in pneumococcal adherence (McCullers and Bartmess, 2003). Neuraminidase also stimulates the expression of bacterial receptors by activating TGF-β (Li et al., 2015), which also increases the production of Fn (Schultz-Cherry and Hinshaw, 1996). Similar to Fg, Fn is an important ligand for GAS adherence (Beachey and Courtney, 1989) and internalization (Chaussee et al., 2000; Wang et al., 2006). Using TGF-β knockout mice, Li et al. found that GAS colonization of the lungs 3 days after IAV infection was less than that observed with wild-type mice (Li et al., 2015). Thus several studies using a variety of different isolates of virus and bacteria indicate that IAV infection increases both GAS adherence and internalization by binding to viral HA on the infected cell surface and following increases in the abundance of, and access to, bacterial receptors and the GAS ligands Fg and Fn.
Vaccination
Vaccination of mice against IAV provides significant protection against mortality from GAS secondary infections (Okamoto et al., 2004a; Chaussee et al., 2011). In one study, mice were vaccinated against influenza and subsequently infected with an H1N1 virus 2 days prior to infection with GAS; only 15% of unvaccinated mice survived compared to 78% of vaccinated mice (Okamoto et al., 2004a). A subsequent study compared the efficacy of live attenuated and killed influenza vaccines in a superinfection model in which mice were inoculated intranasally with a normally sub-lethal dose of GAS 7 days after IAV infection (Chaussee et al., 2011). Morbidity (measured by weight loss) following IAV infection was undetectable among mice vaccinated with either formulation, consistent with protection against the virus (Chaussee et al., 2011). Nonetheless, 22 and 33% of mice receiving the attenuated or killed vaccine, respectively died as a result of GAS superinfection. Thus, the influenza vaccines prevented viral illness and decreased the mortality associated with IAV-GAS superinfections; however, they did not provide sterilizing immunity nor did they abrogate mortality. The results suggest that IAV infection or exposure, even among vaccinated people, may still contribute to the incidence of iGAS diseases.
A related study examined the effectiveness of vaccinating against GAS using a hexavalent polypeptide corresponding to surface localized M proteins (Dale, 1999). In contrast to vaccination against influenza and despite signs of illness after IAV infection, vaccinated mice were completely protected from mortality (Klonoski et al., 2014). One possible explanation for the difference in protection between influenza vaccines and the GAS vaccine is that vaccination against GAS may confer the host with the ability to clear the bacteria by an antibody-dependent mechanism that is not compromised by antecedent IAV infection. In contrast, following vaccination against IAV viral exposure may still impair host defenses sufficiently to promote bacterial superinfection, even in the absence of a clinically apparent viral infection. Interestingly, a recent report using a conjugate vaccine against S. pneumoniae showed that this vaccine did not result in 100% protection as obtained with the anti-M protein vaccine (Metzger et al., 2015). This indicates that there is still much to learn regarding the differences in the pathogenesis of IAV-bacterial superinfections and the contributions of vaccine-induced immunity in the context of polymicrobial infections. Future studies focused on defining the mechanisms of vaccine-induced protection will aid in the interpretation of these observations and guide the design of new therapeutic strategies to mitigate the morbidity and mortality of IAV superinfections.
Summary
GAS causes several life threatening invasive diseases including pneumonia, bacteremia, necrotizing fasciitis, and toxic shock syndrome. Decades of study with GAS in monoculture and with monoinfected animal models has yielded significant insights into the virulence factors and host responses that contribute to invasive diseases (Cunningham, 2000; Fieber and Kovarik, 2014; Walker et al., 2014). Similarly, genomic studies conducted during the past decade have led to insights into the genetic characteristics of GAS isolates from invasive infections (Musser and Shelburne, 2009). A less well understood contributor to iGAS diseases is antecedent, or concurrent, viral infection of the upper respiratory tract. Epidemiological studies and clinical reports support the idea that several viruses, and particularly influenza, increase the incidence of iGAS diseases. Although comparatively little is known about the molecular bases underlying the observations, virus-induced changes in bacterial adherence and effector cell-mediated clearance appear to be particularly important. Future studies will likely identify additional host and bacterial factors important in the development of viral associated iGAS diseases and polymicrobial diseases in general. Such knowledge is especially important in the design of new strategies to control the mortality of influenza-GAS superinfections, which is significant despite the availability of influenza vaccines, anti-viral treatments, and the ex vivo susceptibility of GAS to penicillin.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are supported by award 1R15AI094437-01A1 to MC and NIH grant 2 P20 RR016479 from the INBRE Program of the National Center for Research Resources.
Footnotes
1. ^Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. Morb. Mortal. Wkly. Rep. 58, 1071–1074.
References
Aebi, C., Ahmed, A., and Ramilo, O. (1996). Bacterial complications of primary varicella in children. Clin. Infect. Dis. 23, 698–705. doi: 10.1093/clinids/23.4.698
Aebi, T., Weisser, M., Bucher, E., Hirsch, H. H., Marsch, S., and Siegemund, M. (2010). Co-infection of Influenza B and Streptococci causing severe pneumonia and septic shock in healthy women. BMC Infect. Dis. 10:308. doi: 10.1186/1471-2334-10-308
Allard, R., Couillard, M., Pilon, P., Kafka, M., and Bedard, L. (2012). Invasive bacterial infections following influenza: a time-series analysis in Montreal, Canada, 1996-2008. Influenza Other Respi. Viruses 6, 268–275. doi: 10.1111/j.1750-2659.2011.00297.x
Beachey, E. H., and Courtney, H. S. (1989). Bacterial adherence of group A streptococci to mucosal surfaces. Respiration 55(Suppl. 1), 33–40. doi: 10.1159/000195749
Brundage, J. F. (2006). Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect. Dis. 6, 303–312. doi: 10.1016/S1473-3099(06)70466-2
Carapetis, J. R., Steer, A. C., Mulholland, E. K., and Weber, M. (2005). The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5, 685–694. doi: 10.1016/S1473-3099(05)70267-X
Chaussee, M. S., Cole, R. L., and van Putten, J. P. (2000). Streptococcal erythrogenic toxin B abrogates fibronectin-dependent internalization of Streptococcus pyogenes by cultured mammalian cells. Infect. Immun. 68, 3226–3232. doi: 10.1128/iai.68.6.3226-3232.2000
Chaussee, M. S., Sandbulte, H. R., Schuneman, M. J., Depaula, F. P., Addengast, L. A., Schlenker, E. H., et al. (2011). Inactivated and live, attenuated influenza vaccines protect mice against influenza: Streptococcus pyogenes super-infections. Vaccine 29, 3773–3781. doi: 10.1016/j.vaccine.2011.03.031
Chretien, J. H., and Esswein, J. G. (1976). How frequent is bacterial superinfection of the pharynx in infectious mononucleosis? Observations on incidence, recognition, and management with antibiotics. Clin. Pediatr. 15, 424–427. doi: 10.1177/000992287601500505
Cunningham, M. W. (2000). Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13, 470–511. doi: 10.1128/CMR.13.3.470-511.2000
Dale, J. B. (1999). Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine 17, 193–200. doi: 10.1016/S0264-410X(98)00150-9
Darenberg, J., Henriques-Normark, B., Lepp, T., Tegmark-Wisell, K., Tegnell, A., and Widgren, K. (2013). Increased incidence of invasive group A streptococcal infections in Sweden, January 2012-February 2013. Euro Surveill. 18, 20443. doi: 10.2807/1560-7917.ES2013.18.14.20443
de Vries, R. D., Mesman, A. W., Geijtenbeek, T. B., Duprex, W. P., and de Swart, R. L. (2012). The pathogenesis of measles. Curr. Opin. Virol. 2, 248–255. doi: 10.1016/j.coviro.2012.03.005
DuBois, D. R., and Baehner, R. L. (1979). Infectious mononucleosis associated with fatal beta hemolytic streptococcal infection. Clin. Pediatr. 18, 511–512. doi: 10.1177/000992287901800816
Duke, T., and Mgone, C. S. (2003). Measles: not just another viral exanthem. Lancet 361, 763–773. doi: 10.1016/S0140-6736(03)12661-X
Fieber, C., and Kovarik, P. (2014). Responses of innate immune cells to group A Streptococcus. Front. Cell. Infect. Microbiol. 4:140. doi: 10.3389/fcimb.2014.00140
Gahr, P., DeVries, A. S., Wallace, G., Miller, C., Kenyon, C., Sweet, K., et al. (2014). An outbreak of measles in an undervaccinated community. Pediatrics 134, e220–e228. doi: 10.1542/peds.2013-4260
Ghoneim, H. E., Thomas, P. G., and McCullers, J. A. (2013). Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J. Immunol. 191, 1250–1259. doi: 10.4049/jimmunol.1300014
Gremillion, D. H., and Crawford, G. E. (1981). Measles pneumonia in young adults. An analysis of 106 cases. Am. J. Med. 71, 539–542. doi: 10.1016/0002-9343(81)90203-5
Hafez, M. M., Abdel-Wahab, K. S., and El-Fouhil, D. F. (2010). Augmented adherence and internalization of group A Streptococcus pyogenes to influenza A virus infected MDCK cells. J. Basic Microbiol. 50(Suppl. 1), S46–S57. doi: 10.1002/jobm.200900427
Henke, C. E., Kurland, L. T., and Elveback, L. R. (1973). Infectious mononucleosis in Rochester, Minnesota, 1950 through 1969. Am. J. Epidemiol. 98, 483–490.
Jean, C., Louie, J. K., Glaser, C. A., Harriman, K., Hacker, J. K., Aranki, F., et al. (2010). Invasive group A streptococcal infection concurrent with 2009 H1N1 influenza. Clin. Infect. Dis. 50, e59–e62. doi: 10.1086/652291
Keller, T. T., van der Sluijs, K. F., de Kruif, M. D., Gerdes, V. E., Meijers, J. C., Florquin, S., et al. (2006). Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ. Res. 99, 1261–1269. doi: 10.1161/01.RES.0000250834.29108.1a
Klonoski, J. M., Hurtig, H. R., Juber, B. A., Schuneman, M. J., Bickett, T. E., Svendsen, J. M., et al. (2014). Vaccination against the M protein of Streptococcus pyogenes prevents death after influenza virus: S. pyogenes super-infection. Vaccine 32, 5241–5249. doi: 10.1016/j.vaccine.2014.06.093
Laennec, T. H. R. (1923). Translation of Selected Passages from De l'Ausculation Mediate. New York, NY: William Wood & Co.
Laskey, A. L., Johnson, T. R., Dagartzikas, M. I., and Tobias, J. D. (2000). Endocarditis attributable to group A beta-hemolytic streptococcus after uncomplicated varicella in a vaccinated child. Pediatrics 106:E40. doi: 10.1542/peds.106.3.e40
Laupland, K. B., Davies, H. D., Low, D. E., Schwartz, B., Green, K., and McGeer, A. (2000). Invasive group A streptococcal disease in children and association with varicella-zoster virus infection. Ontario group A streptococcal study group. Pediatrics 105:E60. doi: 10.1542/peds.105.5.e60
Lee, S. E., Eick, A., Bloom, M. S., and Brundage, J. F. (2008). Influenza immunization and subsequent diagnoses of group A streptococcus-illnesses among U.S. Army trainees, 2002-2006. Vaccine 26, 3383–3386. doi: 10.1016/j.vaccine.2008.04.041
Li, N., Ren, A., Wang, X., Fan, X., Zhao, Y., Gao, G. F., et al. (2015). Influenza viral neuraminidase primes bacterial coinfection through TGF-beta-mediated expression of host cell receptors. Proc. Natl. Acad. Sci. U.S.A. 112, 238–243. doi: 10.1073/pnas.1414422112
Mandell, L. A., Wunderink, R. G., Anzueto, A., Bartlett, J. G., Campbell, G. D., Dean, N. C., et al. (2007). Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl. 2), S27–S72. doi: 10.1086/511159
McCullers, J. A. (2006). Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 19, 571–582. doi: 10.1128/CMR.00058-05
McCullers, J. A. (2014). The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 12, 252–262. doi: 10.1038/nrmicro3231
McCullers, J. A., and Bartmess, K. C. (2003). Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 187, 1000–1009. doi: 10.1086/368163
McCullers, J. A., and Rehg, J. E. (2002). Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186, 341–350. doi: 10.1086/341462
Metzger, D. W., Furuya, Y., Salmon, S. L., Roberts, S., and Sun, K. (2015). Limited efficacy of antibacterial vaccination against secondary serotype 3 pneumococcal pneumonia following influenza infection. J. Infect. Dis. 212, 445–452. doi: 10.1093/infdis/jiv066
Metzger, D. W., and Sun, K. (2013). Immune dysfunction and bacterial coinfections following influenza. J. Immunol. 191, 2047–2052. doi: 10.4049/jimmunol.1301152
Morens, D. M., and Fauci, A. S. (2007). The 1918 influenza pandemic: insights for the 21st century. J. Infect. Dis. 195, 1018–1028. doi: 10.1086/511989
Morens, D. M., and Taubenberger, J. K. (2015). A forgotten epidemic that changed medicine: measles in the US Army, 1917-18. Lancet Infect. Dis. 15, 852–861. doi: 10.1016/S1473-3099(15)00109-7
Musser, J. M., and Shelburne, S. A. III. (2009). A decade of molecular pathogenomic analysis of group A Streptococcus. J. Clin. Invest. 119, 2455–2463. doi: 10.1172/JCI38095
Ohashi, Y., Nakai, Y., Esaki, Y., Ohno, Y., Sugiura, Y., and Okamoto, H. (1991). Influenza A virus-induced otitis media and mucociliary dysfunction in the guinea pig. Acta oto-laryngologica. Supplementum 486, 135–148. doi: 10.3109/00016489109134991
Okamoto, S., Kawabata, S., Fujitaka, H., Uehira, T., Okuno, Y., and Hamada, S. (2004a). Vaccination with formalin-inactivated influenza vaccine protects mice against lethal influenza Streptococcus pyogenes superinfection. Vaccine 22, 2887–2893. doi: 10.1016/j.vaccine.2003.12.024
Okamoto, S., Kawabata, S., Nakagawa, I., Okuno, Y., Goto, T., Sano, K., et al. (2003). Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J. Virol. 77, 4104–4112. doi: 10.1128/JVI.77.7.4104-4112.2003
Okamoto, S., Kawabata, S., Terao, Y., Fujitaka, H., Okuno, Y., and Hamada, S. (2004b). The Streptococcus pyogenes capsule is required for adhesion of bacteria to virus-infected alveolar epithelial cells and lethal bacterial-viral superinfection. Infect. Immun. 72, 6068–6075. doi: 10.1128/IAI.72.10.6068-6075.2004
Pan, Y. T., Schmitt, J. W., Sanford, B. A., and Elbein, A. D. (1979). Adherence of bacteria to mammalian cells: inhibition by tunicamycin and streptovirudin. J. Bacteriol. 139, 507–514.
Parola, P., Colson, P., Dubourg, G., Million, M., Charrel, R., Minodier, P., et al. (2011). Letter to the editor. Group A streptococcal infections during the seasonal influenza outbreak 2010/11 in South East England. Euro Surveill. 16:19816. Available online at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19816
Patel, R. A., Binns, H. J., and Shulman, S. T. (2004). Reduction in pediatric hospitalizations for varicella-related invasive group A streptococcal infections in the varicella vaccine era. J. Pediatr. 144, 68–74. doi: 10.1016/j.jpeds.2003.10.025
Peltola, V. T., and McCullers, J. A. (2004). Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr. Infect. Dis. J. 23, S87–S97. doi: 10.1097/01.inf.0000108197.81270.35
Pittet, L. A., Hall-Stoodley, L., Rutkowski, M. R., and Harmsen, A. G. (2010). Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am. J. Respir. Cell Mol. Biol. 42, 450–460. doi: 10.1165/rcmb.2007-0417OC
Pontes, T., and Antunes, H. (2004). [Group A beta-hemolytic streptococcal toxic shock]. Acta Med. Port. 17, 395–398.
Rush, M. C., and Simon, M. W. (2003). Occurrence of Epstein-Barr virus illness in children diagnosed with group A streptococcal pharyngitis. Clin. Pediatr. 42, 417–420. doi: 10.1177/000992280304200505
Rynda-Apple, A., Robinson, K. M., and Alcorn, J. F. (2015). Influenza and Bacterial Superinfection: Illuminating the Immunologic Mechanisms of Disease. Infect. Immun. 83, 3764–3770. doi: 10.1128/IAI.00298-15
Sanford, B. A., Davison, V. E., and Ramsay, M. A. (1982). Fibrinogen-mediated adherence of group A Streptococcus to influenza A virus-infected cell cultures. Infect. Immun. 38, 513–520.
Scaber, J., Saeed, S., Ihekweazu, C., Efstratiou, A., McCarthy, N., and O'Moore, É. (2011). Group A streptococcal infections during the seasonal influenza outbreak 2010/11 in South East England. Euro Surveill. 16:19780. Available online at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19780
Schrager, H. M., Alberti, S., Cywes, C., Dougherty, G. J., and Wessels, M. R. (1998). Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J. Clin. Invest. 101, 1708–1716. doi: 10.1172/JCI2121
Schreck, P., Bradley, J., and Chambers, H. (1996). Musculoskeletal complications of varicella. J. Bone Joint Surg. Am. 78, 1713–1719.
Schultz-Cherry, S., and Hinshaw, V. S. (1996). Influenza virus neuraminidase activates latent transforming growth factor beta. J. Virol. 70, 8624–8629.
Sivakumar, S., and Latifi, S. Q. (2008). Varicella with stridor: think group A streptococcal epiglottitis. J. Paediatr. Child Health 44, 149–151. doi: 10.1111/j.1440-1754.2007.01280.x
Sun, K., and Metzger, D. W. (2008). Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 14, 558–564. doi: 10.1038/nm1765
Tasher, D., Stein, M., Simoes, E. A., Shohat, T., Bromberg, M., and Somekh, E. (2011). Invasive bacterial infections in relation to influenza outbreaks, 2006-2010. Clin. Infect. Dis. 53, 1199–1207. doi: 10.1093/cid/cir726
Walker, M. J., Barnett, T. C., McArthur, J. D., Cole, J. N., Gillen, C. M., Henningham, A., et al. (2014). Disease manifestations and pathogenic mechanisms of group a Streptococcus. Clin. Microbiol. Rev. 27, 264–301. doi: 10.1128/CMR.00101-13
Wang, B., Li, S., Southern, P. J., and Cleary, P. P. (2006). Streptococcal modulation of cellular invasion via TGF-beta1 signaling. Proc. Natl. Acad. Sci. U.S.A. 103, 2380–2385. doi: 10.1073/pnas.0506668103
Watanabe, T., Sugawara, H., Tamura, H., Ishii, A., Matsubayashi, H., Kakei, M., et al. (2012). Co-infection with group A Streptococci and Epstein-Barr virus presenting with acute glomerulonephritis and acute left ventricular dysfunction. Intern. Med. 51, 2639–2643. doi: 10.2169/internalmedicine.51.7761
Weeks-Gorospe, J. N., Hurtig, H. R., Iverson, A. R., Schuneman, M. J., Webby, R. J., McCullers, J. A., et al. (2012). Naturally occurring swine influenza A virus PB1-F2 phenotypes that contribute to superinfection with Gram-positive respiratory pathogens. J. Virol. 86, 9035–9043. doi: 10.1128/jvi.00369-12
Wilson, G. J., Talkington, D. F., Gruber, W., Edwards, K., and Dermody, T. S. (1995). Group A streptococcal necrotizing fasciitis following varicella in children: case reports and review. Clin. Infect. Dis. 20, 1333–1338. doi: 10.1093/clinids/20.5.1333
Zachariadou, L., Stathi, A., Tassios, P. T., Pangalis, A., Legakis, N. J., Papaparaskevas, J., et al. (2014). Differences in the epidemiology between paediatric and adult invasive Streptococcus pyogenes infections. Epidemiol. Infect. 142, 512–519. doi: 10.1017/S0950268813001386
Zakikhany, K., Degail, M. A., Lamagni, T., Waight, P., Guy, R., Zhao, H., et al. (2011). Increase in invasive Streptococcus pyogenes and Streptococcus pneumoniae infections in England, December 2010 to January 2011. Euro Surveill. 16:19785. Available online at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19785
Keywords: Streptococcus pyogenes, group A streptococcus, influenza
Citation: Herrera AL, Huber VC and Chaussee MS (2016) The Association between Invasive Group A Streptococcal Diseases and Viral Respiratory Tract Infections. Front. Microbiol. 7:342. doi: 10.3389/fmicb.2016.00342
Received: 15 January 2016; Accepted: 03 March 2016;
Published: 21 March 2016.
Edited by:
Frederic Lamoth, Lausanne University Hospital, SwitzerlandReviewed by:
Mattias Collin, Lund University, SwedenAnnelies Sophie Zinkernagel, University Hospital Zurich, University of Zurich, Switzerland
Copyright © 2016 Herrera, Huber and Chaussee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael S. Chaussee, michael.chaussee@usd.edu
 Andrea L. Herrera
Andrea L. Herrera Victor C. Huber
Victor C. Huber Michael S. Chaussee
Michael S. Chaussee