Association between the β-blocker use and patients with sepsis: a cohort study
- Department of Critical Care, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Objective: This study aimed to assess whether β-blockers are associated with mortality in patients with sepsis.
Method: We conducted a retrospective cohort study of patients with sepsis using the Medical Information Market for Intensive Care (MIMIC)-IV and the emergency intensive care unit (eICU) databases. The primary outcome was the in-hospital mortality rate. The propensity score matching (PSM) method was adopted to reduce confounder bias. Subgroup and sensitivity analyses were performed to test the stability of the conclusions.
Results: We included a total of 61,751 patients with sepsis, with an overall in-hospital mortality rate of 15.3% in MIMIC-IV and 13.6% in eICU. The inverse probability-weighting model showed that in-hospital mortality was significantly lower in the β-blockers group than in the non-β-blockers group [HR = 0.71, 95% CI: 0.66–0.75, p < 0.001 in MIMIC-IV, and HR = 0.48, 95% CI: 0.45–0.52, p < 0.001 in eICU]. In subgroups grouped according to sex, age, heart rate, APSIII, septic shock, and admission years, the results did not change.
Conclusion: β-blocker use is associated with lower in-hospital mortality in patients with sepsis, further randomized trials are required to confirm this association.
Background
Sepsis, a dysregulated immune response caused by infection, is a common syndrome in the ICU (1). However, despite medical advances and the initiation of active treatment, as well as the treatment of underlying infections and support for failing organ function, sepsis still has a high mortality rate of 20%–30% (2).
Djillali et al. showed sympatho-adrenergic system activation and significantly elevated circulating catecholamine levels in patients with sepsis (3). In critically ill patients, transient activation of the sympathetic nervous system is beneficial; however, excessive activation is detrimental to the organism (4). In addition, tachycardia is an independent predictor of poor prognosis in patients with sepsis (5). β-blockers are common drugs for the treatment of cardiovascular diseases, including chronic heart failure (6, 7) and myocardial infarction (8, 9) occur by reducing sympathetic neural activity and controlling the heart rate. Therefore, we hypothesized that β-blockers could inhibit sympathetic activation, reduce catecholamine release, and control tachycardia in sepsis patients, thereby improving the prognosis of sepsis patients.
Several studies have shown that the application of β-blockers in sepsis reduces heart rate, morbidity, and mortality (10–13), but there is still a lack of studies with large samples. Moreover, the application of β-blockers in sepsis may also lead to decreased cardiac output, microcirculation, and tissue perfusion, resulting in adverse events (14). Therefore, we conducted a retrospective study to determine the association between the use of β-blockers and mortality in patients with sepsis to better guide clinical management.
Methods
Data sources and setting
The two patient cohorts used in this study were drawn from two large public databases, the Medical Information in Intensive Care database (MIMIC-IV) and the eICU Collaborative Research database v2.0. The MIMIC-IV is a large clinical database containing comprehensive high-quality data on ICU inpatients at the Beth Israel Deaconess Medical Center between 2008 and 2019, to which we obtained access (certification number: 36508141). The eICU database covers 200,859 patients admitted to the intensive care units of 208 hospitals in the United States between 2014 and 2015.
Study population
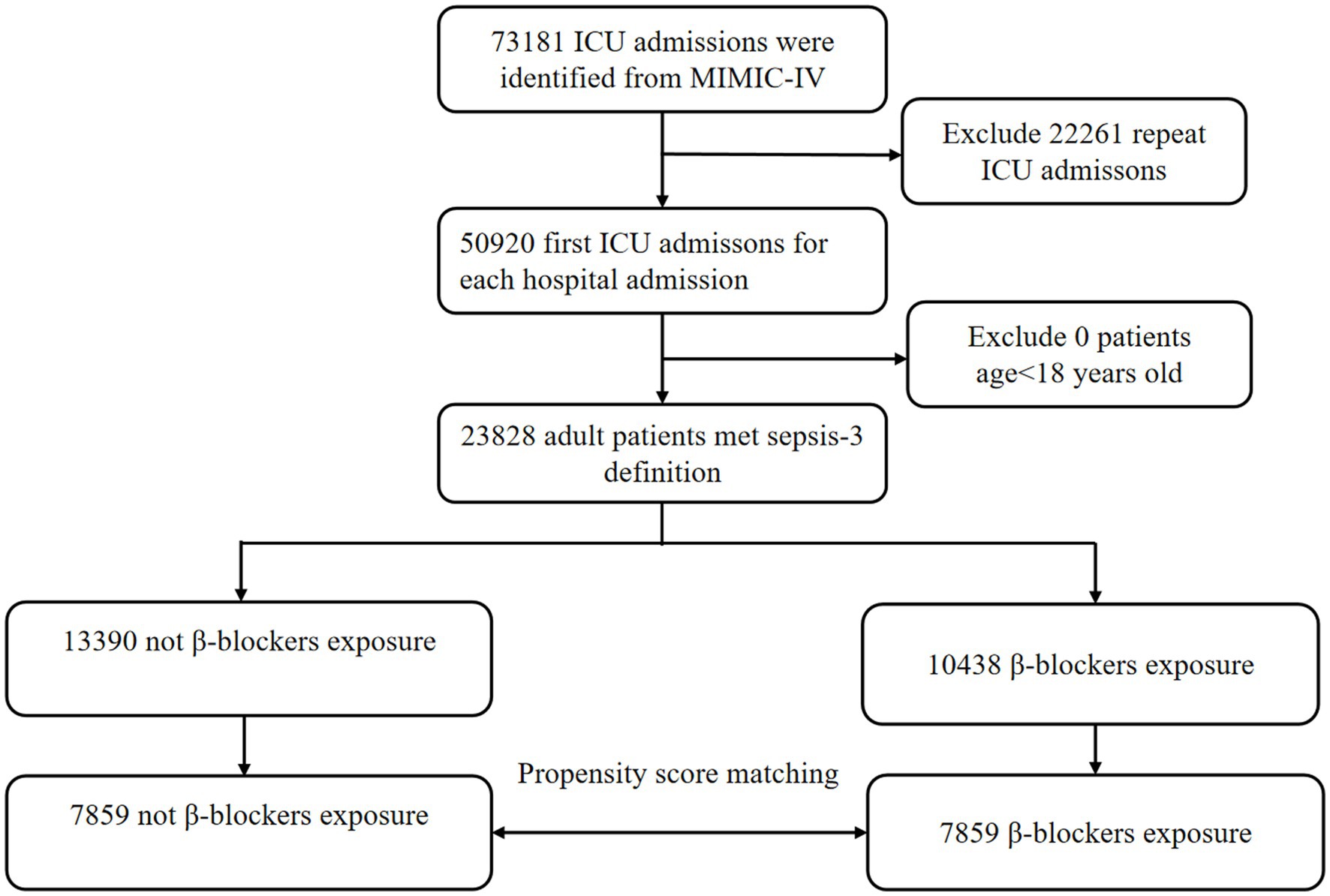
All patients in the MIMIC-IV and eICU databases were eligible for inclusion. Adults (older than or equal to 18 years) diagnosed with sepsis-3 were included in our study. The diagnostic criterion was an increase in the Sequential Organ Failure Assessment (SOFA) score of 2 points caused by a dysregulated host response to infection. For patients with repeated ICU admissions, only those admitted to the ICU for the first time were considered.
β-blockers exposure
Exposure to β-blockers was defined as a prescription containing a β-blocker within 3 days of admission to the ICU. The β-blockers included “Acebutolol,” “Atenolol,” “Esmolol,” “Betaxolol,” “Bisoprolol,” “Metoprolol,” “Nadolol,” and “Propranolol.” The routes of administration of β-blocker include intravenous push and oral administration.
Covariates
We included the following variables: heart rate (HR), mean arterial pressure (MAP), blood glucose, temperature, SpO2, hemoglobin, platelet, white blood cell (WBC), bicarbonate, blood urea nitrogen (BUN), chloride, creatinine, potassium, acute physiology score (APS) III, simplified acute physiology score (SAPS) II, sequential organ failure score (SOFA), Charlson Comorbidity Index, and comorbidities (including Peripheral vascular disease, rheumatic disease, peptic ulcer, diabetes, septic shock), use of β-blockers prior to ICU admission, use of statins during hospitalization and the use of ventilator and renal replacement therapy (RRT) on the first day of ICU admission. Basic information for hospital admission registration was obtained which included demographic characteristics (e.g., age and gender).
Outcome
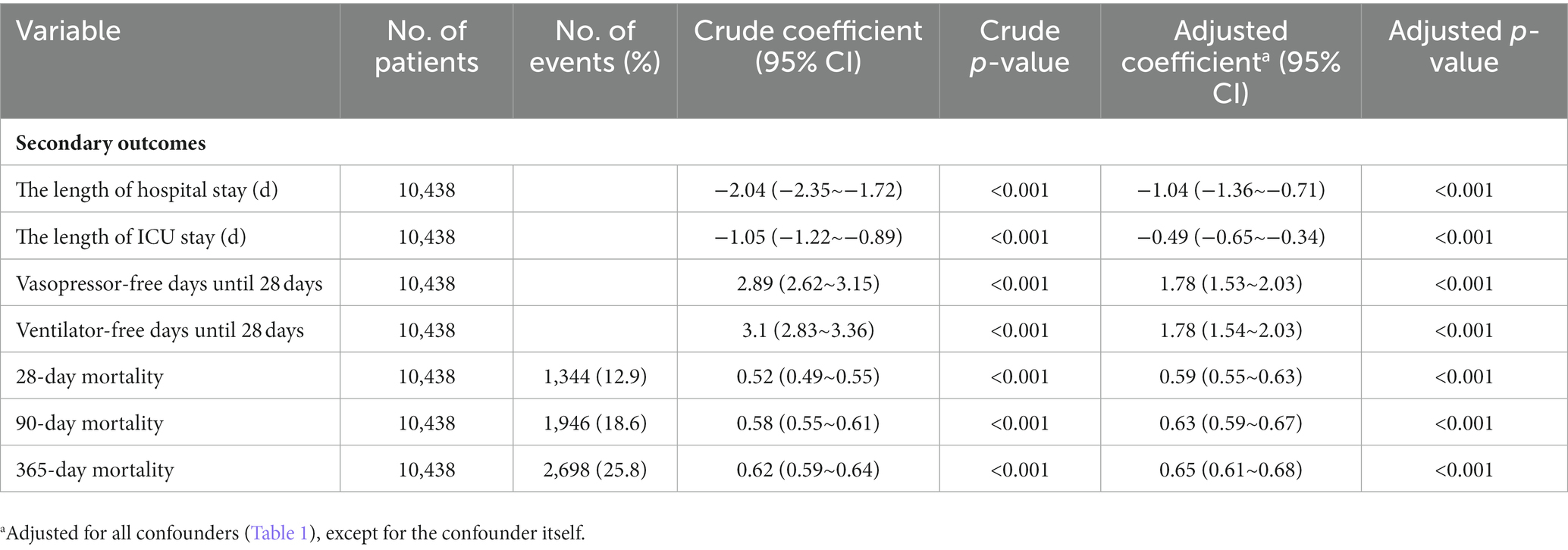
The primary outcome was in-hospital mortality. The secondary outcomes included the length of ICU stay, length of hospital stay, ventilator-free days until 28 days, vasopressor-free days until 28 days, 28-day mortality, 90-day mortality, and 365-day mortality.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation (SD) or median and interquartile range (IQR), and categorical variables were presented as frequency (percentage). For continuous variables, the Student’s t-test or Wilcoxon rank-sum test was used to determine whether they conformed to normal distributions. Pearson’s chi-squared test or Fisher’s exact test was used for categorical data, as appropriate.
Multivariable Cox regression analyses were used to assess the independent association between-blocker use and in-hospital mortality and to calculate hazard ratios (HRs) and their 95% confidence intervals (CIs). Survival curves were generated using Kaplan–Meier analysis and compared by log-rank analysis. To balance the baseline characteristics between the -blocker and β-blocker use groups, we used the PSM method, which was performed in our study by a 1:1 nearest neighbor matching algorithm with a caliper width of 0.2. The variables shown in Table 1 were all selected to generate the propensity score, and the standardized mean difference (SMD) was used to examine the degree of PSM. Less than 0.1 was considered an acceptable threshold. The primary outcome was further verified using inverse probability of treatment weighted (IPTW), which was created using the estimated propensity scores as weights. Subgroup analyses were stratified according to relevant covariates to reduce the impact of survival bias.
A two-tailed test was performed and p < 0.05 was considered statistically significant. All analyses were performed with R 3.3.2 (http://www.R-project.org, The R Foundation).
Results
Population
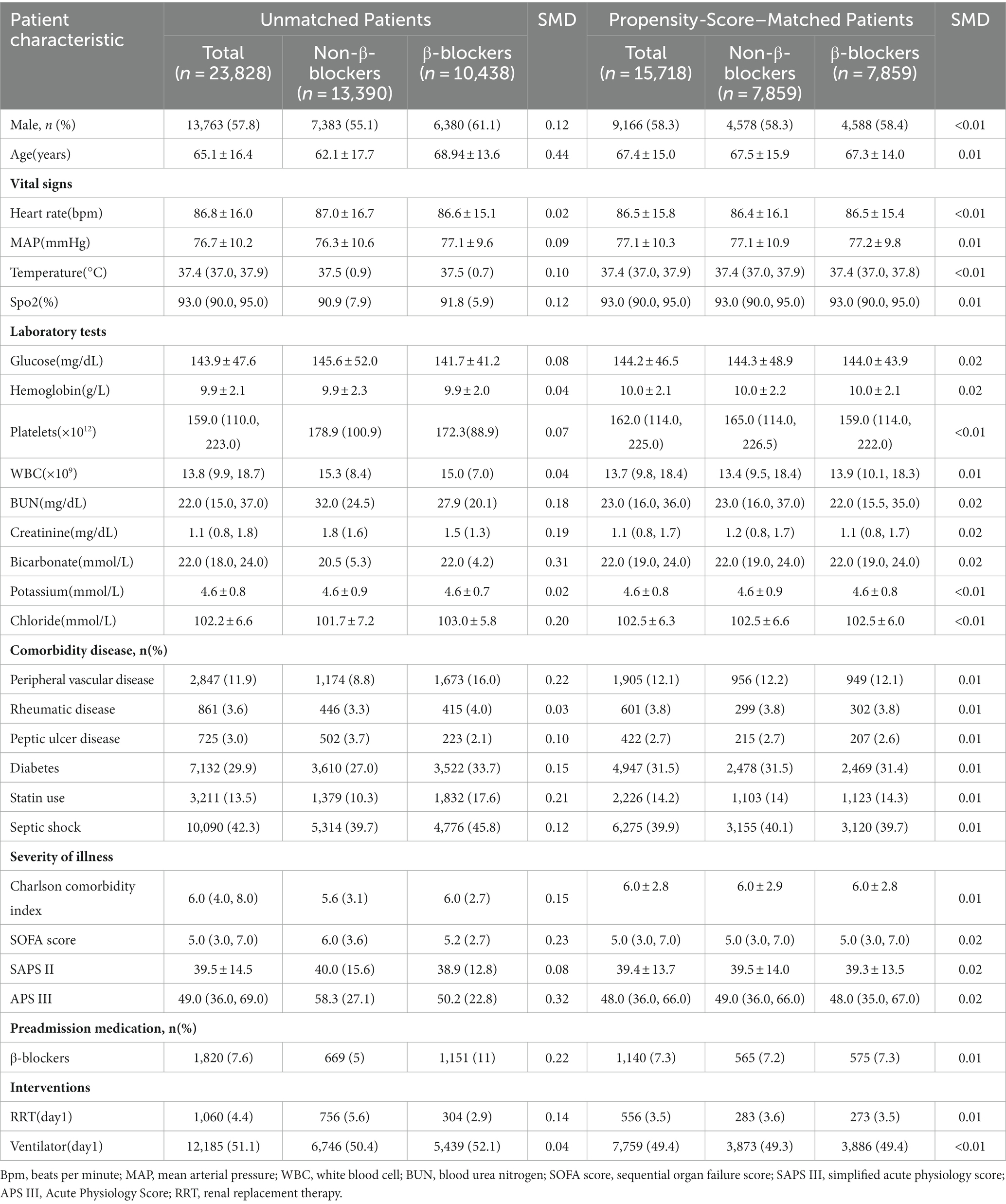
In the MIMIC-IV database, a total of 23,828 patients with sepsis were enrolled in our cohort, of whom 10,438 patients (43.8%) were exposed to β-blockers, and 13,390 (56.2%) were not (Figure 1). After PSM, 7,859 patients were successfully matched. In the eICU database, out of 200,859 unique patients, 37,923 patients were included in the study (Supplementary Figure 1).
Study cohort and patients’ characteristics
The baseline patients’ characteristics are listed in Table 1. The baseline characteristics of the two groups after PSM are almost balanced in Table 1. The mean age was 65.1 ± 16.4 years old, 7,383 (55.1%) were males. The baseline characteristics of the included patients in eICU database are shown in Supplementary Table 1.
Primary outcome
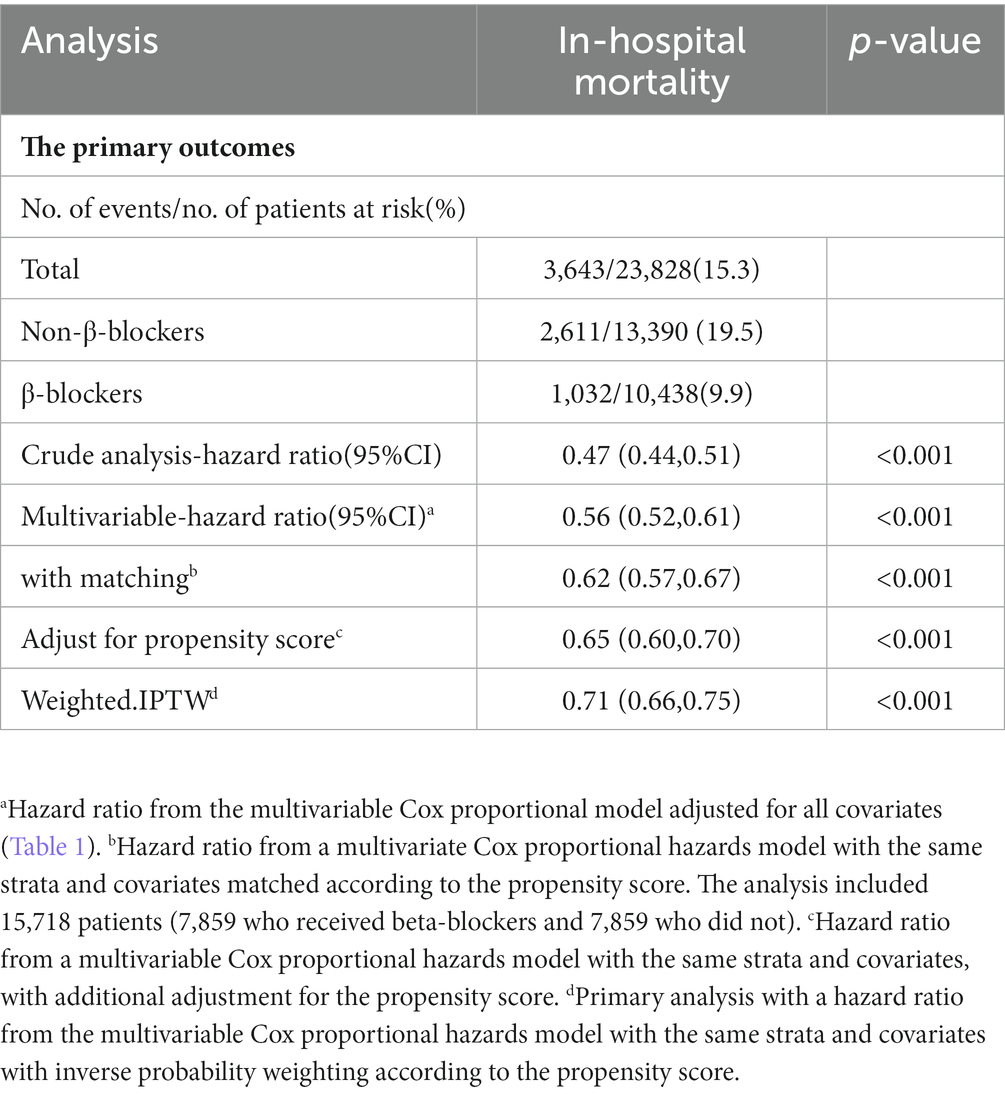
The overall in-hospital mortality rates were 15.3% in the MIMIC-IV group (Table 2) and 13.6% in the eICU (Supplementary Table 1). In the MIMIC-IV database, the in-hospital mortality for the β-blockers and non-β-blockers groups was 9.9%(1,032/10,438) and 19.5%(2,611/13,390), respectively. In univariable Cox proportional hazards regression, the HR was 0.47 (95% CI, 0.44–0.51, p < 0.001). In Multivariate Cox proportional hazards regression, the HR was 0.56 (95% CI, 0.52–0.61, p < 0.001). Similar to the results in the pre-matched cohort, both PSM (HR = 0.62, 95%CI, 0.57–0.67, p < 0.001) and IPTW (HR = 0.71,95% CI, 0.66–0.75, p < 0.001) indicated that β-blockers use was significantly associated with reduced in-hospital mortality. Before PSM, the in-hospital Kaplan–Meier survival curve also showed that β-blockers users had lower in-hospital mortality (Log-rank test: p < 0.0001; Figure 2). In the eICU database, we similarly observed that β-blockers were associated with reduced in-hospital mortality (adjusted HR 0.48, 95% CI, 0.45–0.52, p < 0.001; Supplementary Table 2).
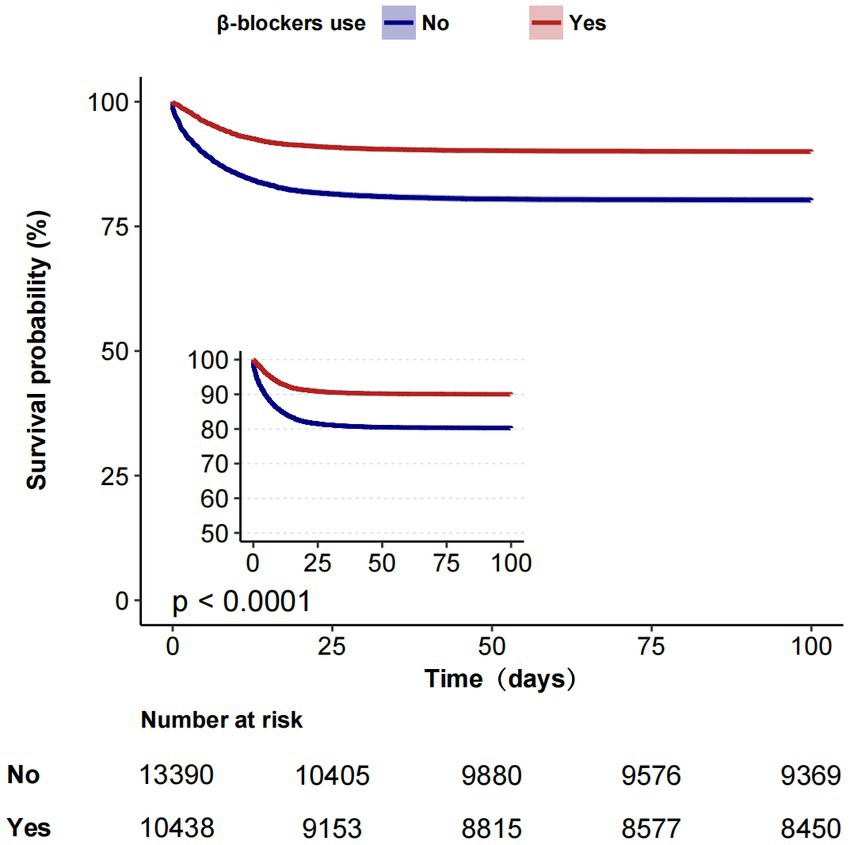
Figure 2. Kaplan–Meier estimates of cumulative probabilities of in-hospital survival for septic patients in β-blockers group and non-β-blockers group.
Subgroup analysis
Although subgroup analysis was performed according to some confounders, the in-hospital mortality survival analysis showed that patients in the β-blockers group had lower in-hospital mortality compared with the non-β-blockers in all the subgroups (Figure 3). However, some interactions were found between APS III, septic shock, and preadmission β-blockers use (p for interaction < 0.05).
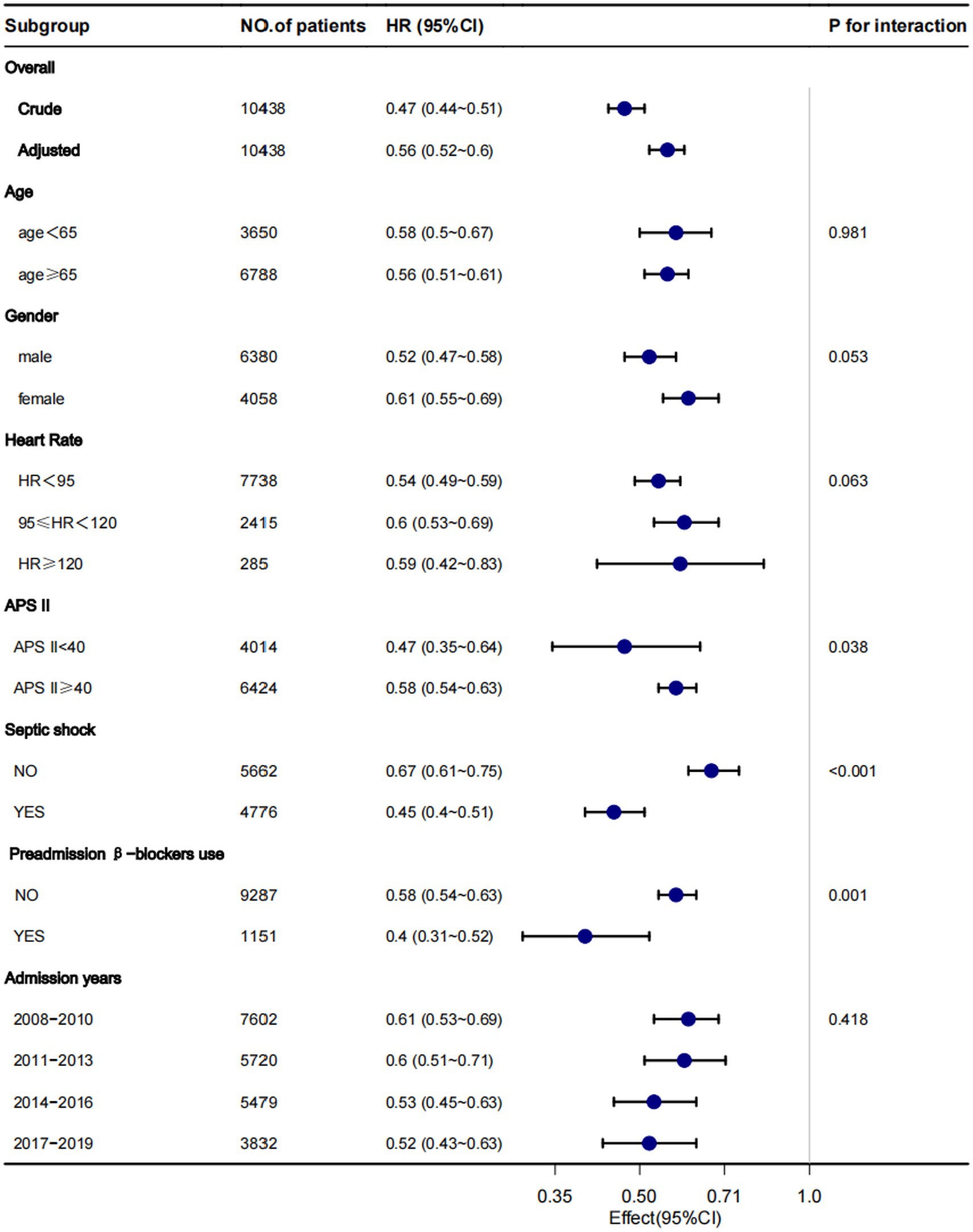
Figure 3. Subgroup analysis for the associations between β-blocker use and in-hospital mortality. Each stratification adjusted for all confounders (Table 1), except for the stratification factor itself.
Sensitivity analysis
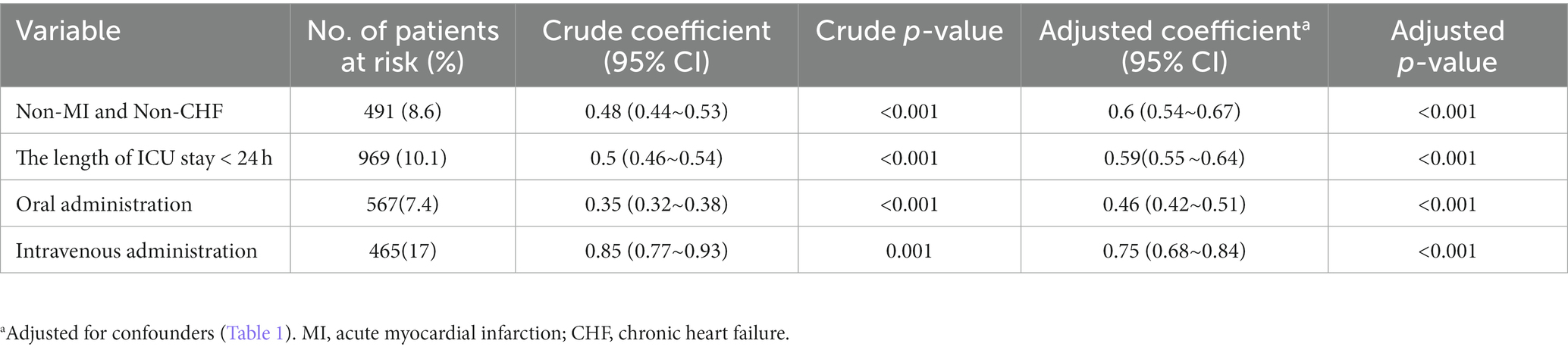
After we excluded patients with ICU stays of less than 24 h, acute myocardial infarction, and chronic heart failure, in-hospital mortality remained lower in the β-blocker patients than in the non-β-blocker patient group (all p < 0.001). Whether administered orally or intravenously, β-blockers were associated with reduced in-hospital mortality compared with the non-β-blockers group (Both p < 0.001; Table 3).
Secondary outcomes
Table 4 shows secondary efficacy outcomes. Patients receiving β-blockers showed improved outcomes in terms of length of ICU stay, length of hospital stay, ventilator-free days until 28 days, vasopressor-free days until 28 days, 28-day mortality, 90-day mortality, 365-day mortality compared to those not receiving β-blockers.
Discussion
In this large retrospective cohort study, we found that patients who received β-blockers after admission to the intensive care unit had a lower in-hospital mortality before and after removing the interference of possible confounding factors using PSM, compared with the non-β-blockers group. In the eICU database, we similarly observed that the β-blockers group was associated with reduced in-hospital mortality.
Morelli et al. conducted the first randomized controlled study to verify the efficacy and safety of esmolol in the treatment of septic shock. Akin to our findings, the results showed that the esmolol group had significantly lower 28-day mortality (49%) compared to 80.5% in the control group (adjusted hazard ratio, 0.39,95% CI,0.26 to 0.59, p < 0.001) (10). However, they only included patients with septic shock requiring vasopressors to maintain blood pressure, whereas we expanded the study population to include patients with sepsis (with and without vasopressors). Similarly, an observational study (n = 1,186) reported by Dmitri et al. showed that long-term β -blocker therapy (≥1 year) prior to hospital admission reduced 30-day all-cause mortality in patients with sepsis, but the study was conducted mainly in patients with sepsis on medicine wards, not in the ICU (11).
An RCT conducted by Kakihana et al. (n = 151) showed that landiolol significantly reduced heart rate and incidence of new-onset arrhythmia in patients with sepsis-related tachyarrhythmias. However, in their study, landiolol did not show a significant reduction in 28-day mortality in patients with sepsis (9% vs. 15%, p > 0.05), which is inconsistent with our findings, possibly because of the differences in sample size between the two studies and differences in patient demographics at baseline (e.g., patients in Kakihana’s study had higher SOFA scores, mean SOFA = 10.1) (15).
Interestingly, in our study, the mortality rate associated with sepsis was lower than that reported in previous studies (2), which may have been due to the use of different definitions of sepsis. However, in Rhee’s retrospective study of 173,690 septic patients with sepsis (according to the definition of sepsis-3) in the United States from 2009 to 2014, the in-hospital mortality rate of sepsis was 15.6%, which is comparable to our results (16), suggesting that our cohort was similar to those of previous studies.
One of the strengths of our study is that it included patients with sepsis from two large public databases (n = 62,676) and was the largest population included in any study that has simultaneously examined the relationship between β-blockers and sepsis.
In most studies, β-blockers are administered intravenously and rarely orally. In our study, the route of administration was both oral and intravenous, it was a real-world study combining the effects of β-blockers on sepsis mortality with oral as well as intravenous administration.
It has been labeled that β-blockers are protective in patients with cardiovascular disease (acute myocardial infarction, chronic heart failure), and in our study population, which included a subset of patients with comorbid cardiovascular disease (acute myocardial infarction, chronic heart failure), we were able to observe a survival benefit in patients receiving β-blockers even after excluding these patients.
However, the mechanism underlying the association between the use of β-blockers and lower mortality in patients with sepsis remains unclear. First, the protective effect of β-blockers in sepsis patients may be related to a lower heart rate and reduced sympathetic overexcitation in sepsis patients. In 1969, Berk et al. were the first to investigate the use to reduce sympathetic nerve excitability in sepsis, which is associated with improved survival (17). Second, it may be related to improved hemodynamics in patients with sepsis; Morelli et al. showed that esmolol can increase the stroke index, systemic vascular resistance, and left ventricular stroke work indices (10). Third, it may be related to the role of β-blockers in regulating inflammatory response. β-blockers can down-regulate inflammatory mediators (e.g., TNF-α, IL-6, HMGB-1) in animal models of sepsis to reduce the inflammatory response (13, 18, 19) as well as inhibit cardiomyocyte apoptosis (20, 21). In addition, β-blockers can activate the anti-inflammatory effects of cholecystokinin receptors and thus play a role in protecting the function of the intestinal barrier (18). It has also been shown that β-blockers (landiolol) may improve sepsis-induced acute lung injury through the pulmonary endothelin-1 system (22).
This study had several limitations. First, it was a retrospective analysis and hence suffered from potential selection and ascertainment bias; the baseline characteristics were different between the two groups. To cope with these imbalances, we applied a multivariable Cox proportional hazards regression model and propensity score matching to confirm the robustness of our findings; however, we are not confident that estimation bias of the effects can be completely excluded. Second, due to the retrospective nature of this cohort study, the indications for treatment and treatment intervention were not standardized. Therefore, it was difficult to investigate the impact of dosage on mortality in all participants. Third, owing to the lack of data, we were unable to obtain specific sites of infection as well as objective laboratory indicators of the severity of the inflammatory response to sepsis, such as lactate, PCT, and C-reactive protein levels. Therefore, the results should be interpreted with caution. Finally, a prospective randomized controlled double-blind clinical trial is urgently needed to evaluate the potential benefit and safety of β-blockers given in various doses and routes in septic patients based on sepsis criteria to further prove the results.
Conclusion
This cohort study suggests that β-blocker use is associated with lower in-hospital mortality in patients with sepsis. Further large-scale prospective studies are needed to verify these findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://mimic.mit.edu/.
Ethics statement
The requirement of ethical approval was waived by the Clinical Research Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University for the studies involving humans because the MIMIC-IV database has received ethical approval from the institutional review boards (IRBs) at Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the database does not contain protected health information.
Author contributions
QY: Data curation, Methodology, Software, Writing – review & editing. TK: Data curation, Methodology, Software, Writing – original draft. ZB: Data curation, Writing – original draft. SY: Data curation, Writing – original draft. XC: Software, Writing – original draft. JZ: Methodology, Writing – original draft. XX: Writing – review & editing. DW: Supervision, Writing – review & editing. ZZ: Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors gratefully acknowledge financial support by Guangzhou Municipal Science and Technology Project (202102010068) and The Natural Science Foundation of Guangdong Province (2022A1515010397 and 2023A1515011988).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1272871/full#supplementary-material
References
1. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Angus, DC, and van der Poll, T. Severe sepsis and septic shock. N Engl J Med. (2013) 369:840–51. doi: 10.1056/NEJMra1208623
3. Annane, D, Trabold, F, Sharshar, T, Jarrin, I, Blanc, AS, Raphael, JC, et al. Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med. (1999) 160:458–65. doi: 10.1164/ajrccm.160.2.9810073
4. Dünser, MW, and Hasibeder, WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. (2009) 24:293–316. doi: 10.1177/0885066609340519
5. Hayase, N, Yamamoto, M, Asada, T, Isshiki, R, Yahagi, N, and Doi, K. Association of Heart Rate with N-terminal pro-B-type natriuretic peptide in septic patients: a prospective observational cohort study. Shock. (2016) 46:642–8. doi: 10.1097/SHK.0000000000000673
6. Satwani, S, Dec, GW, and Narula, J. Beta-adrenergic blockers in heart failure: review of mechanisms of action and clinical outcomes. J Cardiovasc Pharmacol Ther. (2004) 9:243–55. doi: 10.1177/107424840400900404
7. Kubon, C, Mistry, NB, Grundvold, I, Halvorsen, S, Kjeldsen, SE, and Westheim, AS. The role of beta-blockers in the treatment of chronic heart failure. Trends Pharmacol Sci. (2011) 32:206–12. doi: 10.1016/j.tips.2011.01.006
8. Cucherat, M. Quantitative relationship between resting heart rate reduction and magnitude of clinical benefits in post-myocardial infarction: a meta-regression of randomized clinical trials. Eur Heart J. (2007) 28:3012–9. doi: 10.1093/eurheartj/ehm489
9. Kjekshus, JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term acute myocardial infarction intervention trials. Am J Cardiol. (1986) 57:F43–9. doi: 10.1016/0002-9149(86)90888-X
10. Morelli, A, Ertmer, C, Westphal, M, Rehberg, S, Kampmeier, T, Ligges, S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. (2013) 310:1683–91. doi: 10.1001/jama.2013.278477
11. Guz, D, Buchritz, S, Guz, A, Ikan, A, Babich, T, Daitch, V, et al. β-Blockers, tachycardia, and survival following sepsis: an observational cohort study. Clin Infect Dis. (2021) 73:e921–6. doi: 10.1093/cid/ciab034
12. Hasegawa, D, Sato, R, Prasitlumkum, N, Nishida, K, Takahashi, K, Yatabe, T, et al. Effect of ultrashort-acting β-blockers on mortality in patients with sepsis with persistent tachycardia despite initial resuscitation: a systematic review and meta-analysis of randomized controlled trials. Chest. (2021) 159:2289–300. doi: 10.1016/j.chest.2021.01.009
13. Zhang, J, Chen, C, Liu, Y, Yang, Y, Yang, X, and Yang, J. Benefits of esmolol in adults with sepsis and septic shock: an updated meta-analysis of randomized controlled trials. Medicine. (2022) 101:e29820. doi: 10.1097/MD.0000000000029820
14. Gore, DC, and Wolfe, RR. Hemodynamic and metabolic effects of selective beta1 adrenergic blockade during sepsis. Surgery. (2006) 139:686–94. doi: 10.1016/j.surg.2005.10.010
15. Kakihana, Y, Nishida, O, Taniguchi, T, Okajima, M, Morimatsu, H, Ogura, H, et al. Efficacy and safety of landiolol, an ultra-short-acting β1-selective antagonist, for treatment of sepsis-related tachyarrhythmia (J-land 3S): a multicentre, open-label, randomised controlled trial. Lancet Respir Med. (2020) 8:863–72. doi: 10.1016/S2213-2600(20)30037-0
16. Rhee, C, Dantes, R, Epstein, L, Murphy, DJ, Seymour, CW, Iwashyna, TJ, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. (2017) 318:1241–9. doi: 10.1001/jama.2017.13836
17. Berk, JL, Hagen, JF, Beyer, WH, Gerber, MJ, and Dochat, GR. The treatment of endotoxin shock by beta adrenergic blockade. Ann Surg. (1969) 169:74–81. doi: 10.1097/00000658-196901000-00007
18. Tan, S, Zhou, F, Zhang, Z, Wang, J, Xu, J, Zhuang, Q, et al. Beta-1 blocker reduces inflammation and preserves intestinal barrier function after open abdominal surgery. Surgery. (2021) 169:885–93. doi: 10.1016/j.surg.2020.11.004
19. Hagiwara, S, Iwasaka, H, Maeda, H, and Noguchi, T. Landiolol, an ultrashort-acting beta1-adrenoceptor antagonist, has protective effects in an LPS-induced systemic inflammation model. Shock. (2009) 31:515–20. doi: 10.1097/SHK.0b013e3181863689
20. Wang, Y, Wang, Y, Yang, D, Yu, X, Li, H, Lv, X, et al. β₁-adrenoceptor stimulation promotes LPS-induced cardiomyocyte apoptosis through activating PKA and enhancing CaMKII and IκBα phosphorylation. Crit Care. (2015) 19:76. doi: 10.1186/s13054-015-0820-1
21. Ackland, GL, Yao, ST, Rudiger, A, Dyson, A, Stidwill, R, Poputnikov, D, et al. Cardioprotection, attenuated systemic inflammation, and survival benefit of beta1-adrenoceptor blockade in severe sepsis in rats. Crit Care Med. (2010) 38:388–94. doi: 10.1097/CCM.0b013e3181c03dfa
Keywords: β-blocker, sepsis, in-hospital mortality, critically ill patients, septic shock
Citation: Yang Q, Kong T, Bao Z, Yang S, Chen X, Zheng J, Xiong X, Wen D and Zhang Z (2023) Association between the β-blocker use and patients with sepsis: a cohort study. Front. Med. 10:1272871. doi: 10.3389/fmed.2023.1272871
Edited by:
Ayaz Shahid, Western University of Health Sciences, United StatesReviewed by:
Sebastien Gibot, Centre Hospitalier Universitaire de Nancy, FranceTakehiko Oami, Chiba University, Japan
Copyright © 2023 Yang, Kong, Bao, Yang, Chen, Zheng, Xiong, Wen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhui Zhang, zhzhhicu@126.com; Deliang Wen, deliangw@163.com
†These authors have contributed equally to this work
‡ORCID: Qilin Yang https://orcid.org/0000-0002-9870-7017
Xuming Xiong https://orcid.org/0000-0003-2583-9411
Zhenhui Zhang https://orcid.org/0000-0002-2801-8865
 Qilin Yang
Qilin Yang Tianyu Kong
Tianyu Kong Ziping Bao†
Ziping Bao†  Jiezhao Zheng
Jiezhao Zheng Zhenhui Zhang
Zhenhui Zhang