Predictors and outcomes of recurrent retroperitoneal liposarcoma with multiple tumors
- 1Department of Gastrointestinal Surgery, Peking University First Hospital, Beijing, China
- 2Department of General Surgery, The First Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, China
Background: Retroperitoneal liposarcoma (RLS) is a rare but severe disease. Repeated postoperative recurrence with multiple tumors is a therapeutic dilemma. The clinical outcomes and survival predictors of recurrent RLS with multiple tumors remain to be explored.
Methods: Patients with recurrent RLS were retrospectively analyzed. Univariate and multivariate analysis was performed to find independent prognostic factors that were correlated with Overall survival (OS) or progression-free survival (PFS). Factors significant in univariate analysis were further included into multivariate Cox proportional hazards regression model. The nomogram model was built to predict the survival status of patients. Variables that were significant in multivariable analysis were added to the internally validated nomogram models. The analysis of OS and PFS was performed by Kaplan–Meier analysis and log-rank test.
Results: A total of 113 recurrent RLS patients with multiple tumors were enrolled in the study. The 1-, 3-, and 5-years OS (PFS) rates were 70.7% (76.1%), 35.9% (76.1%), and 30.9% (76.1%), respectively. Univariate and multivariate analyses showed that number of surgeries, resection methods, tumor size, status of pathological differentiation, pathological subtypes, and recurrence patterns were important prognostic factors for OS or PFS (each p < 0.05). Nomogram models were established to efficiently predict the prognostic status of patients. Patients with the local recurrence (LR) pattern had a poor prognosis and would derive no survival benefit from combined organ resection and R0/R1 resection (each p < 0.05).
Conclusion: RLS patients recurrence with multiple tumors had a poor prognosis. Those patients should be followed up more frequently after surgery. The strategies of aggressive resection may not improve the survival of patients with LR pattern in the retroperitoneum. Prognostic factors in the efficient nomogram models should be considered in the individualized clinical management of recurrent RLS with multiple tumors.
Introduction
Retroperitoneal liposarcoma (RLS) is the most common type of retroperitoneal sarcoma (1). According to official classifications, RLS can be further divided into the following four subtypes: well-differentiated liposarcoma (WDL), dedifferentiated liposarcoma (DDL), myxoid cell liposarcoma (MLS), and pleomorphic liposarcoma (PLS) (2, 3). Due to the tumor growth in the deep abdomen, RLS without typical symptoms, evading the early clinical detection. A large number of patients with RLS tend to have the possibility of recurrence. The prognosis of patients with recurrent RLS is not optimistic, although great improvements in treatment have been achieved in recent years. Postoperative recurrence with multiple tumors is a frequent outcome among patients with RLS. Usually, those patients with multiple tumors has a poor prognosis (4). Therefore, there is an urgent need to find high-efficient predictive factors for the prognosis of recurrent RLS with multiple tumors.
Numerous studies have investigated the clinicopathological features and outcomes of recurrent RLS. Some studies have demonstrated that radical resection of tumors will improve the survival status of patients with RLS (5). However, the multiple and large volume of tumors in recurrent RLS may restrict the ability of surgeon to achieve a true radical resection (6). The complicated anatomic structures of recurrent RLS also limit the potential of the complete surgical margin, which associated with a poor prognosis. Likewise, complete capsular resection and combined organ resection are difficlut to achieve during the surgical procedures of recurrent RLS (7). These factors are therapeutic obstacle and poor predictors for patients with recurrent RLS.
The pathological subtype is an important prognostic factor for the survival of patients with recurrent RLS (8). During clinical practice, we found that pathological subtype could alter as the tumor number increasing in some recurrent cases. The phenomenon indicates that tumor number may associated with the status of pathological differentiation. However, no related studies have clarified the potential mechanism of this correlation.
The prognosis of recurrent RLS also correlated with the relapsed tumor numbers. Previous studies have demonstrated that RLS patients usually growth with multiple tumors simultaneously (4). Although radical resection is currently the effective treatment for RLS, recurrent cases with multiple tumors has higher propensity of postoperative recurrence and poor prognosis (9). The tumor numbers have been proved as a poor prognostic factor for RLS. Nevertheless, research on the characteristics and prognostic outcomes of recurrent RLS with multiple tumors is currently limited. Clarification the effects of tumor numbers on the prognosis of recurrent RLS would be helpful for the better understanding of recurrence mechanism and providing clinical management strategies.
We analyzed the basic clinical and pathological features of recurrent RLS with multiple tumors to investigate the important prognostic predictors and outcomes in the subset of patients. This study may provide evidence for individualized clinical management strategies for recurrent RLS with multiple tumors.
Materials and methods
Patient selection
All patients with RLS experienced multiple recurrences and underwent at least two surgeries from February 2000 to August 2017 at the First Medical Center, Chinese People Liberation Army General Hospital. The pathological subtype was diagnosed and confirmed by experienced pathologists based on WHO (World Health Organization) pathologic criteria (10, 11). Patients with distant organ metastasis were excluded in the study. Patients with adjuvant radiotherapy or chemotherapy at any point of treatment were also excluded. Patients who experienced recurrence but without complete medical records or follow-up data were excluded. The recurrence confirmation of RLS combined preoperative radiological examinations, postoperative pathological examinations and surgical records. All patients signed the consent forms for the series of studies. This study was approved by the Protection of Human Subjects Committee of the Chinese People’s Liberation Army General Hospital.
Definitions
Multiple tumors were defined as the presence of two or more non-contiguous multifocal RLS concurrently. Local recurrence (LR) was defined as tumor relapse at the same anatomical compartment in the retroperitoneum. Non-LR was defined as tumor recurrence at another compartment but without distant organ metastasis (4). Tumor growth rate (TGR) was defined as tumor size (the maximum dimension of the largest mass recorded on final pathological records) divided by the time from last resection to this recurrence diagnosed (12). Overall survival (OS) referred to the time from surgical resection to the end of 5-years follow-up or death. Progression-free survival (PFS) was defined as the time from surgical resection to the initial data of documented tumor progression or death within 5-years (13).
Statistical analyses
Continuous variables are expressed as the median (Q1–Q3). The categorical data are expressed as frequencies (percentages), it compared using the chi-square or Fisher exact test. Univariate and multivariate analyses were applied for the exploration of independent prognostic factors that were associated with OS or PFS. Factors significant in univariate analysis were further included into multivariate Cox proportional hazards regression model. The Cox regression models were conducted by the survival coxph function of the R package. The nomogram models based on Cox regression were built to predict the survival status of recurrent RLS patients with multiple tumors. The Kaplan–Meier curves were applied to estimate the OS and PFS. Data were analyzed using IBM SPSS Statistics (Version 25.0). A two-sided p value <0.05 was considered statistically significant.
Results
Basic clinicopathological characteristics
Based on the above enrollment criteria, one hundred thirteen recurrent RLS patients with multiple tumors were finally enrolled (Figure 1). Sixty-two patients were male, fifty-one patients were female, and the median age of all patients was 53-years. The median tumor size was 18 cm, and the median TGR was 1.29 cm/month. Fifty-four percent of patients had a change of pathological differentiation, and almost half of patients experienced recurrence at the local sites in the retroperitoneum. The important clinical and pathological characteristics of patients with multiple tumors are shown in Table 1.
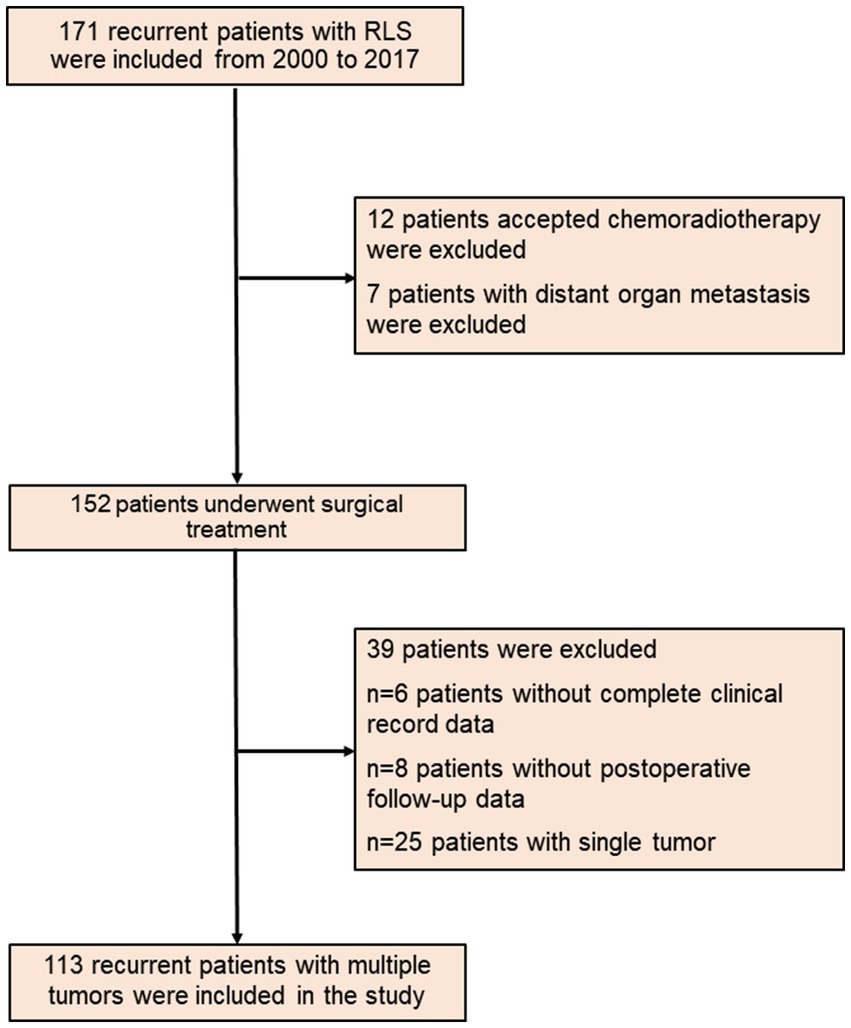
Figure 1. The flow diagram of recurrent RLS patients with multiple tumors. RLS, retroperitoneal liposarcoma.

Table 1. Demographic, clinical, and pathological characteristics of included patients with recurrent RLS.
Predictors for OS and PFS
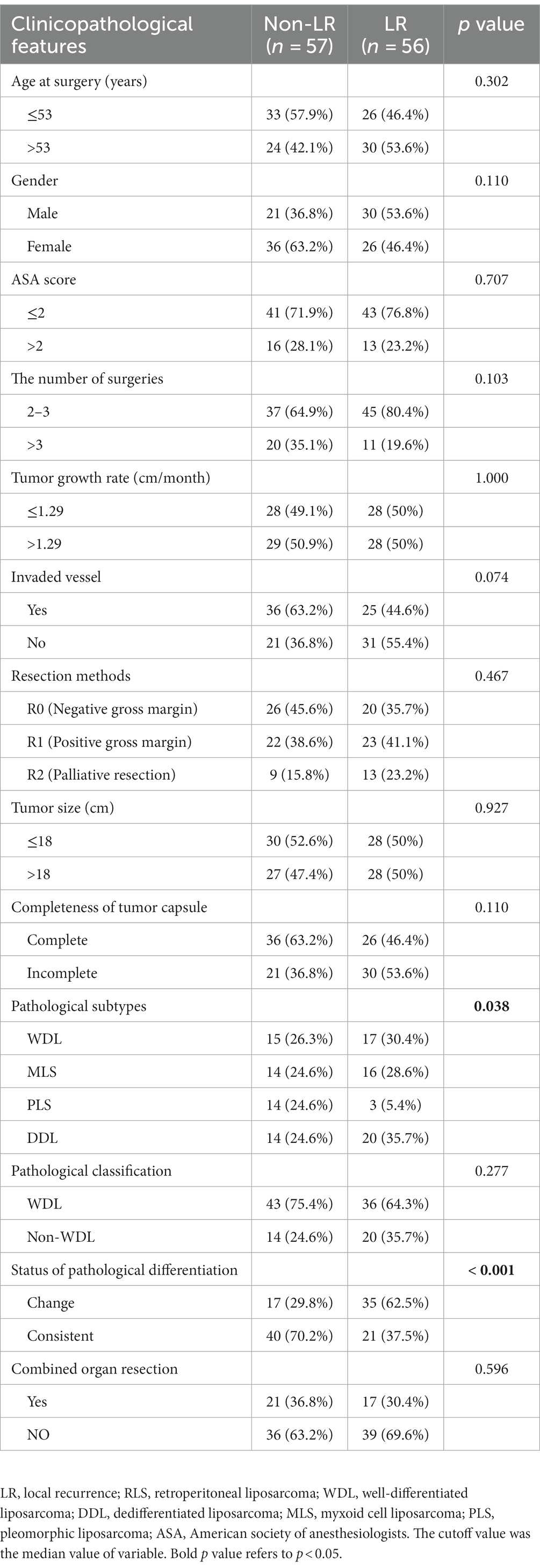
Univariate and multivariate analyses were performed to explore clinicopathologic variables associated with 5-years OS (Table 2). The results showed that resection method, tumor size, status of pathological differentiation, and recurrence pattern were independent risk factors for OS (each p < 0.05). Likewise, we analyzed the prognostic factors associated with 5-years PFS for all patients. The univariate and multivariate analyses showed that number of surgeries, resection method, recurrence pattern and pathological subtype were the significant factors associated with PFS (each p < 0.05) (Table 3). Considering the importance of the recurrence pattern of recurrent RLS, we further divided the cohort into two groups. The comparison of clinicopathological feature between LR and non-LR patterns showed that pathological subtypes and status of pathological differentiation were significantly different (each p < 0.05) (Table 4).
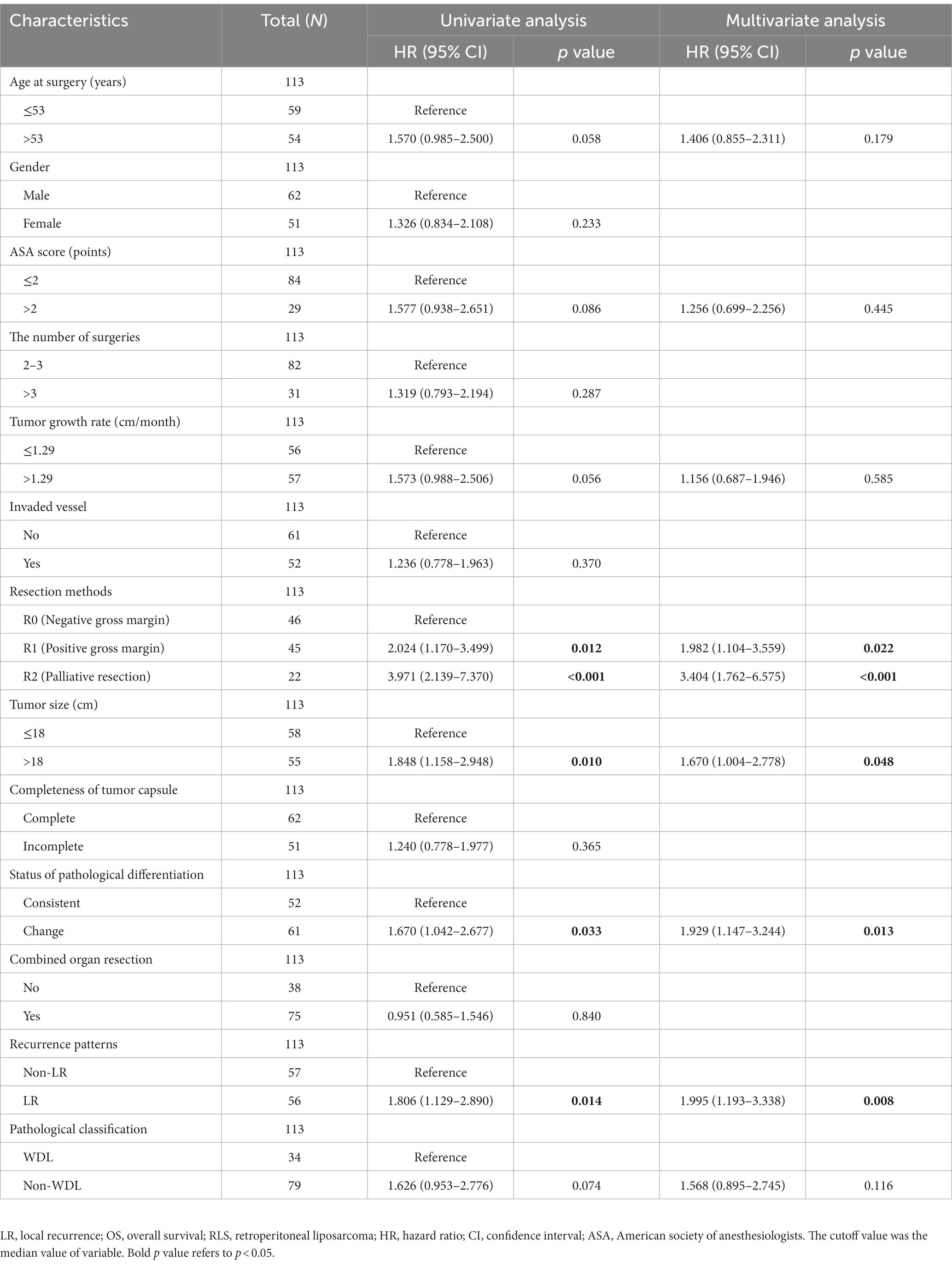
Table 2. Univariate and multivariate analysis of clinicopathologic variables associated with 5-years OS.
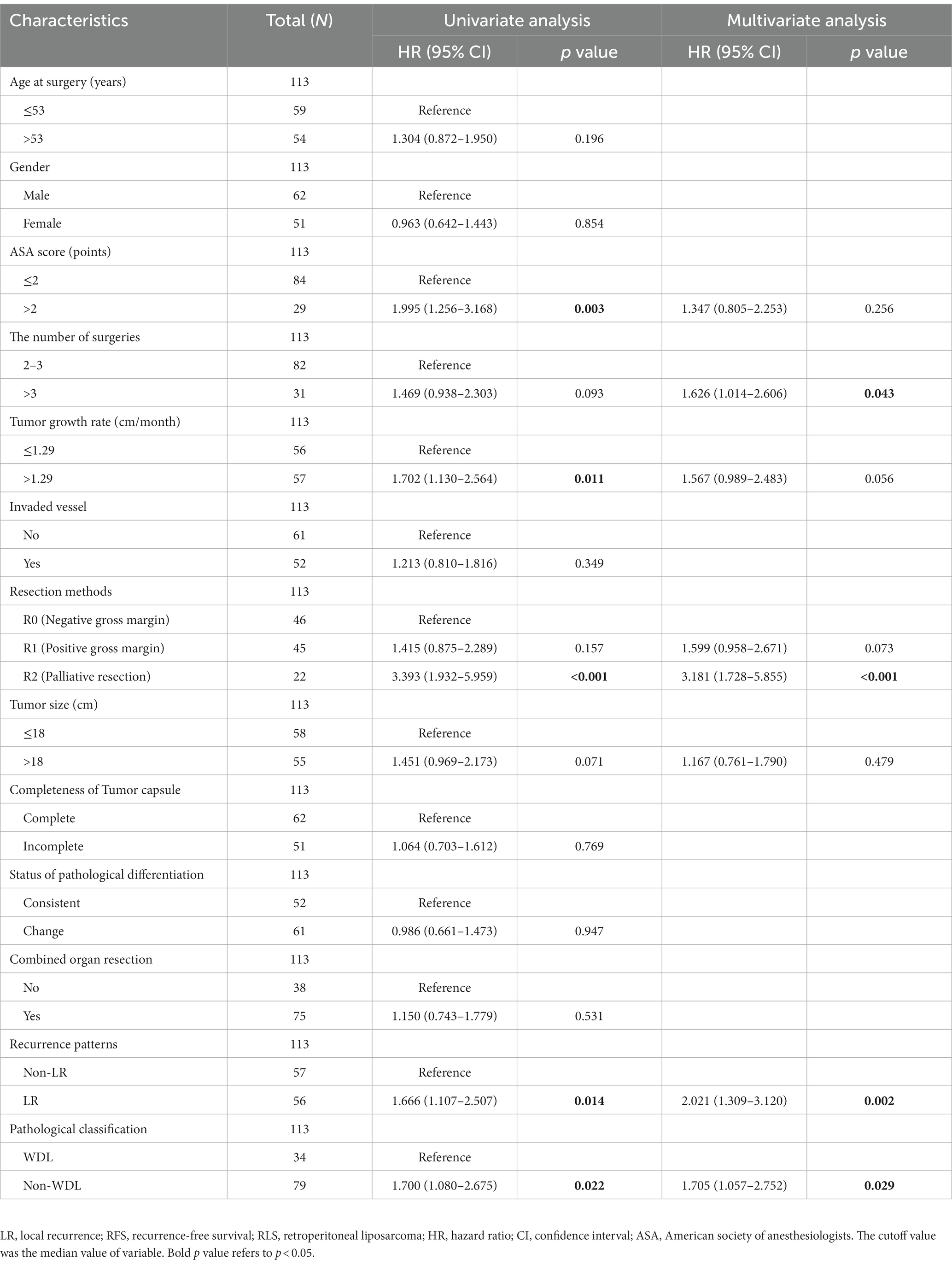
Table 3. Univariate and multivariate analysis of clinicopathologic variables associated with 5-years PFS.
Nomogram models to predict prognostic status
The predictors enrolled in nomogram models were significant factors in the multivariate Cox model. The models can be applied to accurately predict the OS rate of RLS patients who experienced recurrence with multiple tumors (Figure 2). Each predictor corresponds to a point on the top of the nomogram model. We added each score of the four risk factors to obtain a total score, which indicated the overall risk of survival in a patient. The three horizontal lines on the bottom of the nomogram corresponds to the predicted 1-, 2-, and 3-years OS. A vertical line that would intersect the total point axis and the 1-, 2-, and 3-years survival axis indicate the probability of 1-, 2-, and 3-years of OS predicted by the model (Figure 2A). We further built the internal validated models by dividing the cases into training (60%) and test sets (40%). The calibration curves showed an optimal agreement between the predicted and observed OS. The concordance index for the nomogram was 0.703 (95% CI 0.623–0.783) in the training set (Figure 2B) and 0.695 (95% CI 0.565–0.825) in the test set (Figure 2C).
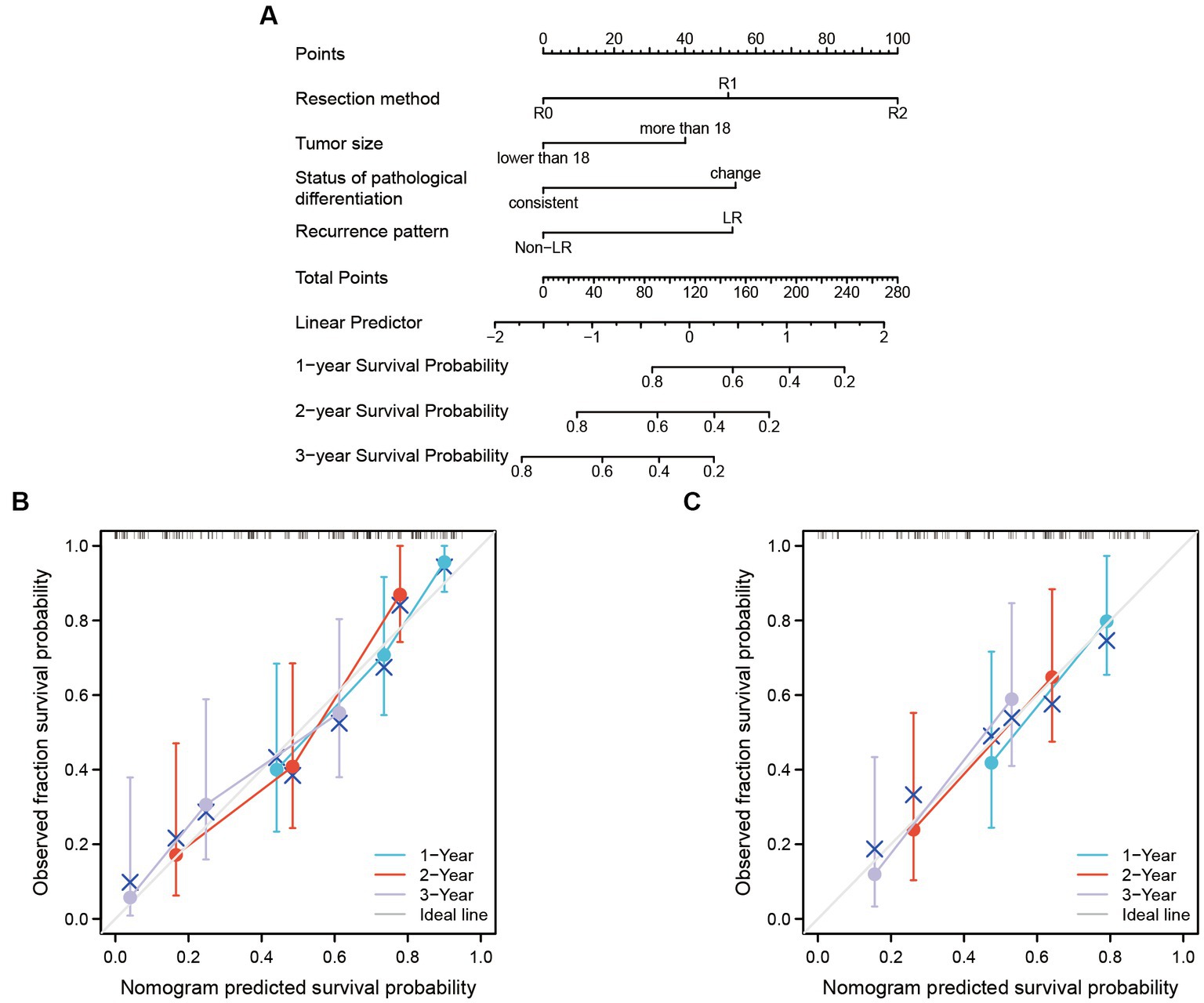
Figure 2. The nomogram model was built to predict the OS of recurrent RLS patients with multiple tumors. (A) Nomogram for 1-year, 2-years and 3-years overall survival in all enrolled patients. (B) Calibration plots of training set for 1-year, 2-years, and 3-years OS in all enrolled patients. (C) Calibration plots of validation set for 1-year, 2-years, and 3-years OS in all enrolled patients. The X-axis: bootstrap-predicted survival; the Y-axis: actual outcome. LR, local recurrence; RLS, retroperitoneal liposarcoma; OS, overall survival. The cutoff value of tumor size was the median value of the variable (the median value included in the lower side; the 5-years OS was too low to be displayed in the nomogram model).
Another nomogram model was established to predict 1-, 2-, and 3-years PFS (Figure 3A). The internal validation again demonstrated an optimal agreement between the predicted and observed PFS. The concordance index for the nomogram was 0.703 (95% CI 0.623–0.783) in the training set (Figure 3B) and 0.695 (95% CI 0.565–0.825) in the test set (Figure 3C). Based on the nomogram models, the cohort was divided into two groups according to the overall risk score. The Kaplan–Meier analyses showed a significant difference in 5-years survival status between the low-risk group and the high-risk group (Figure 4).
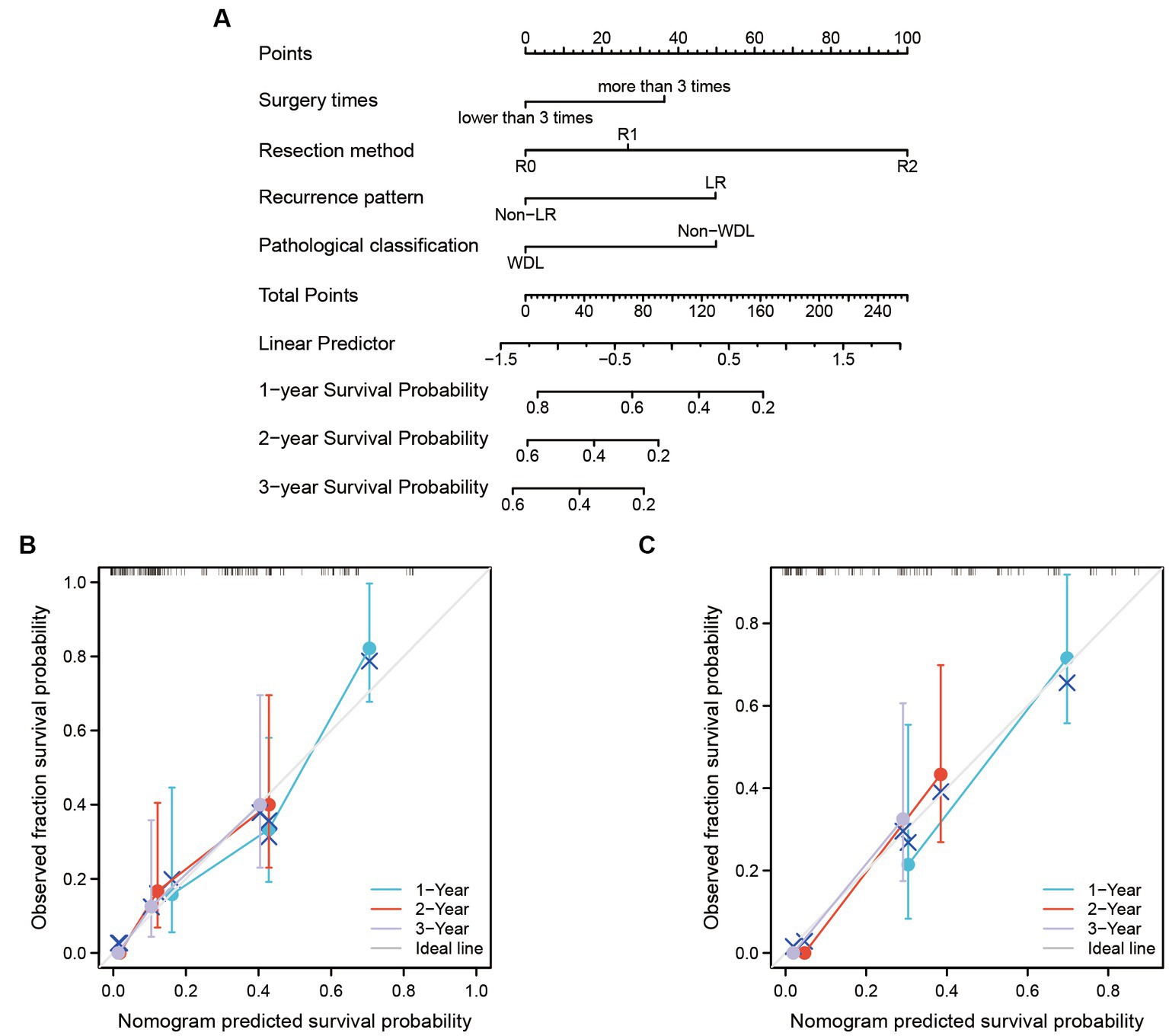
Figure 3. The nomogram model was built to predict the PFS of recurrent RLS patients with multiple tumors. (A) Nomogram for 1-year, 2-years, and 3-years PFS in all enrolled patients. (B) Calibration plots of training set for 1-year, 2-years, and 3-years PFS in all enrolled patients. (C) Calibration plots of validation set for 1-year, 2-years, and 3-years PFS in all enrolled patients. The X-axis: bootstrap-predicted survival; the Y-axis: actual outcome. LR, local recurrence; RLS, retroperitoneal liposarcoma; PFS, progression-free survival. The cutoff value of surgery times was the median value of the variable (the median value included in the lower side; the 5-years PFS was too low to be displayed in the nomogram model).

Figure 4. The prognostic analysis of recurrent RLS patients with multiple tumors based on risk scores predicted by nomogram models. (A) The OS analysis of enrolled patients in different risk grade. (B) The PFS analysis of enrolled patients in different risk grade. The cutoff value of risk score was the median value of the risk scores in all patients. RLS, retroperitoneal liposarcoma; LR, local recurrence; OS, overall survival.
Overall survival and progression-free survival
The Kaplan–Meier analysis showed that the median survival time of patients was approximately 23-months in this study. The 1-, 3-, and 5-years OS rates were 70.7, 35.9, and 30.9%, respectively (Figure 5A). The main causes of death in the study was showed in the Supplementary Table S1. The resection method had an important prognostic value for OS. Patients with R0 resection had a better prognosis in the entire cohort (Figure 5B). Tumor size lower than 18 cm was associated with a better OS (Figure 5C). The difference of survival outcome was not significant between the WDL and non-WDL pathological subtypes (Figure 5D). However, patients had a better prognosis if the pathological subtype was consistent with that of the last occurrence (Figure 5E). Recurrence pattern is an important predictor for prognosis. In this study, we found that patients who experienced recurrence with the LR pattern had poor OS (Figure 5F). For patients with the LR pattern, the strategy of combined organ resection seemed to have no effect on OS (Figure 5G). Similarly, patients with the LR pattern did not obtain a survival benefit from the R0 or R1 resection method (Figure 5H).
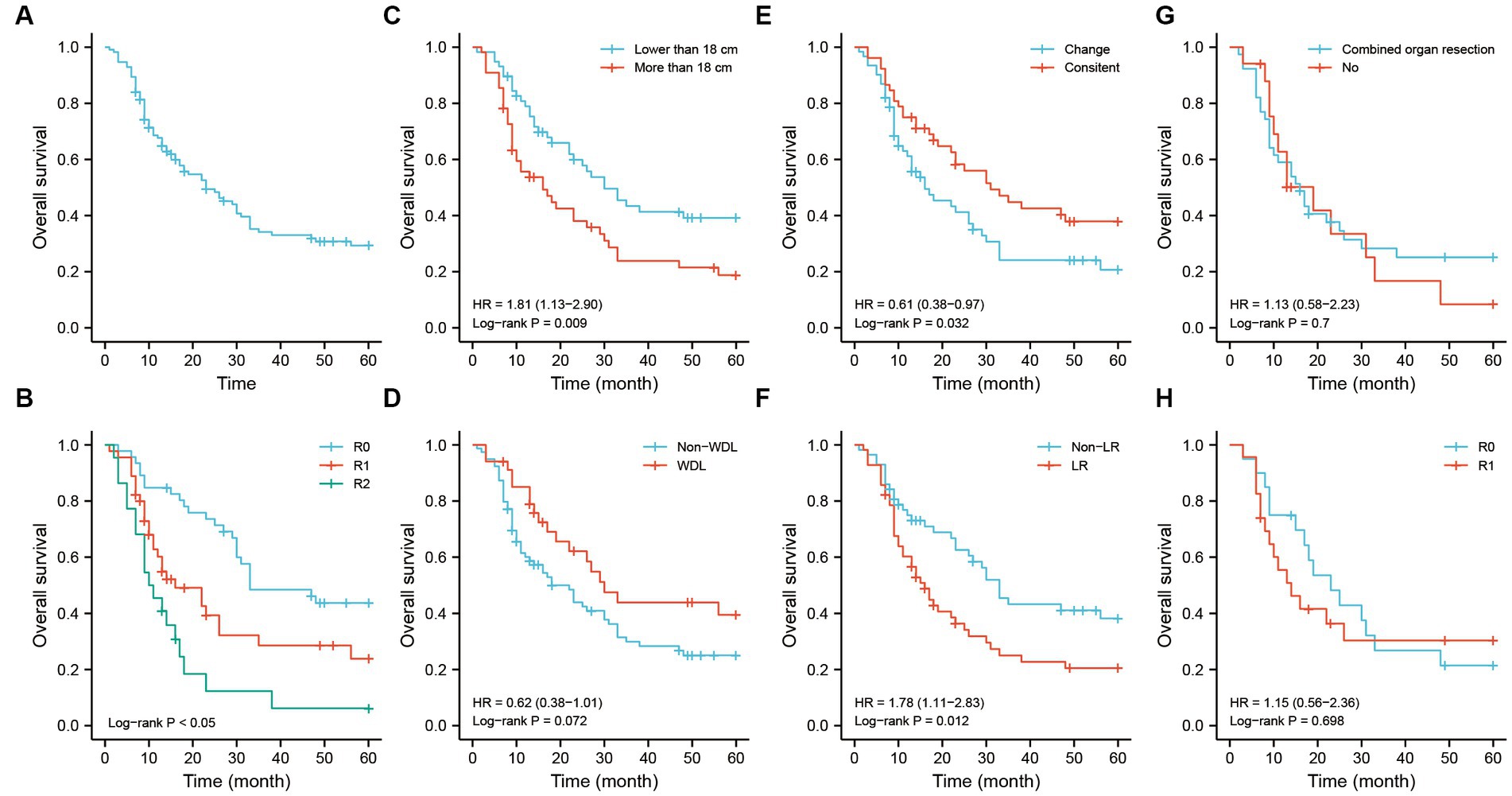
Figure 5. The OS analysis of recurrent RLS patients with multiple tumors. (A) The OS analysis of 113 recurrent RLS patients with multiple tumors. (B) The OS analysis of 113 enrolled patients with three resection methods. (C) The OS analysis of enrolled patients with two types of tumor size. (D) The OS analysis of 113 enrolled patients with two types of pathological classification. (E) The OS analysis of 113 enrolled patients with different status of pathological differentiation. (F) The OS analysis of 113 enrolled patients with different recurrence patterns. (G) The OS analysis of enrolled patients with LR pattern after combined organ resection. (H) The OS analysis of enrolled patients with LR pattern after R0/R1 resection. The cutoff value of tumor size was the median value of the variable (the median value included in the lower side). OS, overall survival; RLS, retroperitoneal liposarcoma; LR, local recurrence.
The Kaplan–Meier analysis illustrated that the median progression time of patients was approximately 23-months. The 1-, 3-, and 5-years PFS rates were 76.1, 50.8, and 34.4%, respectively (Figure 6A). Patients with R0 and R1 resection had better PFS than those with R2 resection (Figure 6B). The PFS was not significant among the tumor size-stratified groups in the study (Figure 6C). However, patients with the WDL subtype had better PFS regardless of the status of pathological differentiation (Figures 6D,E). As in the OS analysis, patients with the LR pattern exhibited poor PFS, and strategies of combined organ resection and R0/R1 resection did not contribute the survival to those patients (Figures 6F,H).
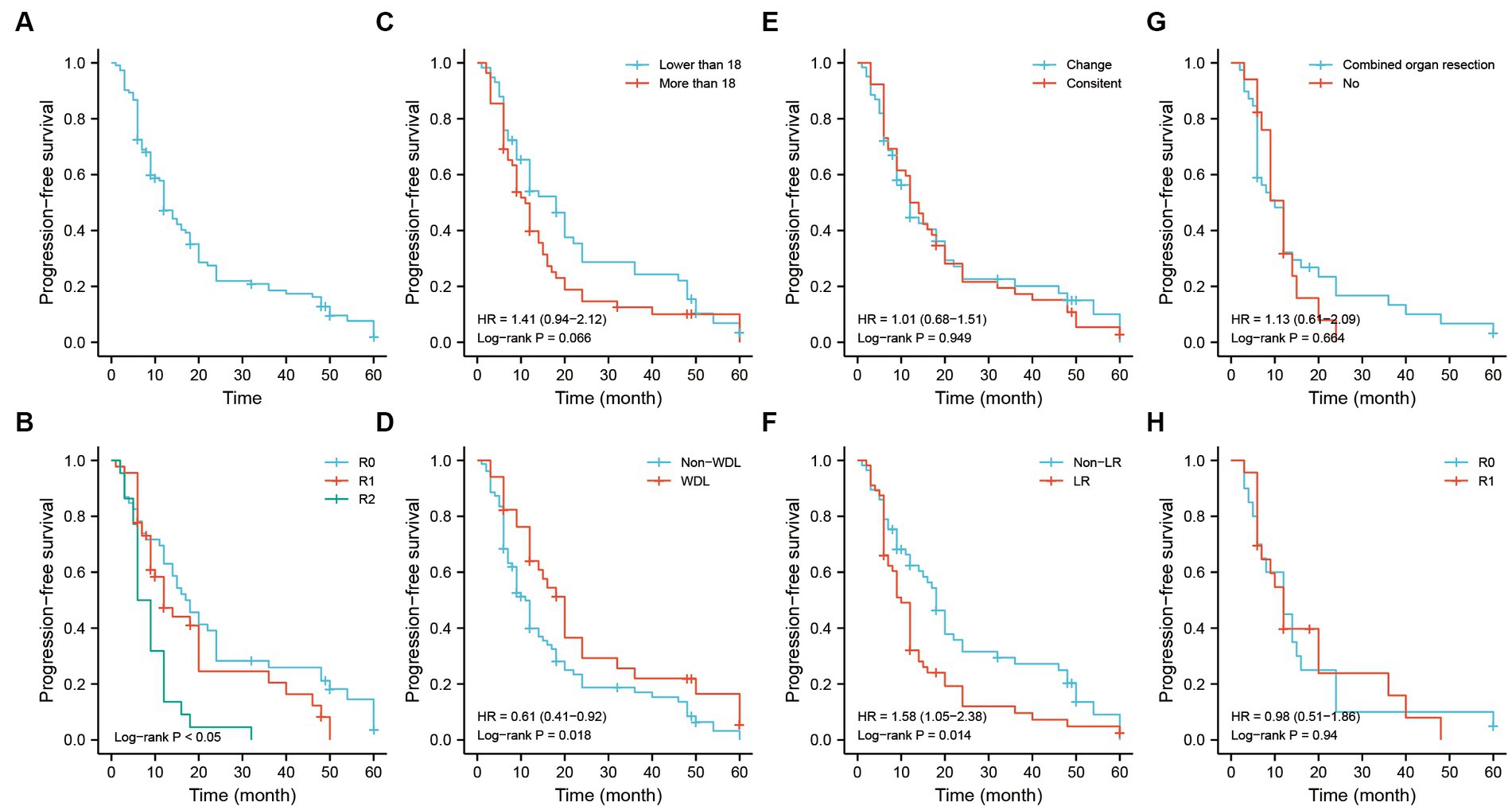
Figure 6. The PFS analysis of recurrent RLS patients with multiple tumors. (A) The PFS analysis of 113 recurrent RLS patients with multiple tumors. (B) The PFS analysis of 113 enrolled patients with three resection methods. (C) The PFS analysis of enrolled patients with two types of tumor size. (D) The PFS analysis of 113 enrolled patients with two types of pathological classification. (E) The PFS analysis of 113 enrolled patients with different status of pathological differentiation. (F) The PFS analysis of 113 enrolled patients with different recurrence patterns. (G) The PFS analysis of enrolled patients with LR pattern after combined organ resection. (H) The PFS analysis of enrolled patients with LR pattern after R0/R1 resection. The cutoff value of tumor size was the median value of the variable (the median value included in the lower side). PFS, progression-free survival; RLS, retroperitoneal liposarcoma; LR, local recurrence.
Discussion
RLS is a potentially severe disease, need a deeper understanding of the disease mechanisms. WDL and DDL are the most common RLS subtypes representing 40–45% of all RLSs (3). Patients with RLS are susceptible to relapses and even death, has a poor prognosis (14, 15). Recurrent RLS with multiple tumors is a more malignant and aggressive liposarcoma, has poor clinical outcomes (16). The main limiting factor for the long-term survival of RLS patients with multiple tumors is repeated recurrences regardless of the treatment method (17, 18). Hence, it is necessary to clarify factors related to recurrence with multiple tumors and explore the predictors of survival status.
Previous studies have explored the risk factors influencing the prognosis of RLS (19, 20). In this study, we investigated the clinicopathological features of recurrent RLS with multiple tumors and explored the surgical factors that correlated with OS or PFS. The univariate and multivariate analyses showed that number of surgeries, tumor size, pathological subtype, status of pathological differentiation, resection method and recurrence pattern were important prognostic factors of OS and PFS. Nomogram models based on multivariate analysis have been built to predict the survival status of RLS (2, 21). However, a highly efficient model that predicting the prognosis of recurrent RLS with multiple tumors is lacking. Gronchi et al. built a high-efficient model to predict the OS of patients with retroperitoneal soft tissue sarcoma. They enrolled the FNCLCC grade, histologic subtype, extent of resection, multifocality, and tumor size into the models. Interestingly, they found the patients with multifocal disease had better OS in their cohort, the new finding deserves praise and spread (22). Our internal validated models demonstrated an optimal agreement between the predicted and observed OS and PFS. In addition, the risk score–stratified survival analysis revealed the validity of the models. In previous studies, the recurrence pattern was rarely included in the nomogram models (2, 20, 23). We found that the recurrence with LR pattern were the poor prognostic factor in our nomogram models. Consequently, the above prognostic factors input into the nomogram models could be considered in the individualized clinical management of recurrent RLS with multiple tumors.
The number of surgeries and tumor size are important predictors for the prognosis of recurrent RLS. We found that the number of surgeries was an independent risk factor for PFS, and patients who underwent more than three surgeries had a poor prognosis (Table 3). Ishii et al. concluded that recurrence rate might increase as the frequency of recurrence and surgery increased in RLS (18). Tumor size has been seen as an important predictor of recurrent RLS (24). The giant tumor volume of RLS limits the ability of surgeon to achieve the radical resection of tumors. In this study, we found that patients with tumor sizes greater than 18 cm behaved poor OS, which is consistent with the previous study (12).
The pathological subtype of RLS plays an important role in the survival prediction. A number of studies have proved that patients with well differentiated RLS benefited better survival than those with lower differentiation (8, 25). However, this feature is a little different from recurrent patients with multiple tumors. Our survival analysis showed that patients with the WDL subtype had better PFS but without a significant difference in OS. This phenomenon may result from the change of pathological differentiation during recurrence. During clinical practice, we found that some cases repeated recurrence with multiple tumors. It induces that the recurrence cases could not maintain the original status of pathological differentiation. Surprisingly, a large number of recurrence cases grew with multiple tumors, which underwent a change of pathological differentiation (Table 1). In the study finished by Tseng et al. (4), the proportion of multifocal disease in recurrent RLS accounting for approximately 50%. The phenomenon mainly correlated with multiple potential tumor growth point in the retroperitoneum cavity. Some previous studies found that different pathological subtypes of RLS has specific typical gene aberrations and biological features, and the multiple gene aberrations can exist in a patient simultaneously (26–28). It is possible that the tumor growth point with a new pathological subtype conceals in the distant retroperitoneum cavity, exhibiting a change of pathological differentiation during recurrence. It inevitably impacts the survial status of those patients. The uncertainty of pathological differentiation during recurrence influences the long-term survival outcomes of patients. Therefore, patients with the WDL subtype could exhibit better PFS in once recurrence, but the long-term OS may not be benefited. The research conducted by Carolyn et al. also found that the changes of pathological differentiation could impact the prognosis of RLS (29). In our study, patients with the change of pathological differentiation behaved poor OS, whereas, the PFS was not influenced regardless of the status of pathological differentiation. We believe that patients of RLS recurrence with an uncertain pathology, which might influence the long-term OS. However, recurrent patients with consistent pathological differentiation benefited better long-term prognosis (Figure 5E). In the study, RLS patients recurrence with multifocal tumors were more likely to experience the change of pathological differentiation, which resulting the relatively poor prognosis.
The postoperative recurrence is a frequent event in RLS. Local recurrence influences the prognosis of patients with recurrent RLS. However, there is no reliable consensus for the definition of the range of local recurrence in RLS. Some studies defined all recurrences in the retroperitoneum or intra-abdominal regions as local recurrences (5, 17, 29). Distant recurrence has been deemed as tumor recurrence at another compartment of the retroperitoneum or distant organ metastases (4, 30). To explore the outcomes of local recurrence in recurrent RLS, we defined an explicit anatomical ranges of local recurrence in the retroperitoneum. The LR pattern of RLS was defined as the liposarcoma repeated recurrence in the same compartment of the retroperitoneum. The further survival analysis illustrated that patients with the LR pattern had poor OS and PFS. Tseng et al. reported that the differences of OS between patients with multifocal and unifocal disease were dependent on the disease status. They found the patients with multifocal disease or recurrent status had poor prognosis. It is the first literature that described the unusual pattern of multifocal recurrence in RLS (4).
In previous studies, complete resection and combined organ resection have been recommended as the most effective treatments for the recurrence of RLS (9, 31, 32). In our entire cohort, the patients with multiple tumors, who underwent the R0 resection had a better prognosis than those with R1/R2 resection. We further conducted a subgroup survival analysis on patients with the LR pattern. The results showed that those patients with the LR pattern behaved poor OS and PFS. The strategies of combined organ resection and R0/R1 resection would not contribute to the survival of patients, who experienced local recurrence with multiple tumors. These observations indicate that comprehensive strategies need to be considered when applying the aggressive resection to those patients. The follow up strategy should be re-consider for RLS patients. In the previous researches and protocols, the follow up interval was recommended every 6 or 12-months. However, patients with multiple tumors had shorter recurrence intervals. Combining research findings, we suggest shorter interval of follow up for RLS patients with multiple tumors.
Study limitations
Our study has several limitations. First, this study involved only a single institution, the number of cases was limited, and adjuvant therapies and distant metastases were unable to be further analyzed. Second, the long-term survival and disease-specific death for recurrent RLS should be evaluated. Third, our cases were retrotspective, mainly based on our institution’s medical records, and lacked prospective data. Fourth, the tumor necrosis and the mitotic count that used in the FNCLCC grading, were missed in this retrospective study, it induced the effective sarculator system could not used in the study.
Conclusion
Prognostic factors that significant in the nomogram models could be considered into the individualized clinical management of recurrent RLS. Those patients recurrence with multiple tumors had a poor prognosis, and should be followed up more frequently after surgery. The strategies of aggressive resection may not improve the survival of patients, who experienced local recurrence with multiple tumors in the retroperitoneum.
Data availability statement
The datasets presented in this article are not readily available because institution limitations. Requests to access the datasets should be directed to BW, weibo@vip.163.com.
Ethics statement
This study was approved by the Protection of Human Subjects Committee of the Chinese People’s Liberation Army General Hospital. All patients signed consent forms for the study.
Author contributions
HD: conceptualisation, data curation, investigation, project administration, writing-original draft, and writing and editing. JG and XX: data curation, investigation, methodology, and formal analysis. GL: built the calibration plots of the dynamic OS nomogram. LS: writing abstract and editing the writing-original draft. JH: data analysis and table editing. BW: conceptualisation, research investigation, project administration, and editing-original draft. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by grants from the National Natural Science Foundation of China (81773135) and National Key Research and Development Project of China (2019YFB1311505).
Acknowledgments
We would like to thank colleagues from Chinese PLA General Hospital for their valuable assistance in the statistical analysis and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1161494/full#supplementary-material
References
1. Chouairy, CJ, Abdul-Karim, FW, and MacLennan, GT. Retroperitoneal liposarcoma. J Urol. (2007) 177:1145. doi: 10.1016/j.juro.2006.12.006
2. Xue, G, Wang, Z, Li, C, Lv, A, Tian, X, Wu, J, et al. A novel nomogram for predicting local recurrence-free survival after surgical resection for retroperitoneal liposarcoma from a chinese tertiary cancer center. Int J Clin Oncol. (2021) 26:145–53. doi: 10.1007/s10147-020-01796-6
3. De Vita, A, Mercatali, L, Recine, F, Pieri, F, Riva, N, Bongiovanni, A, et al. Current classification, treatment options, and new perspectives in the management of adipocytic sarcomas. Onco Targets Ther. (2016) 9:6233–46. doi: 10.2147/OTT.S112580
4. Tseng, WW, Madewell, JE, Wei, W, Somaiah, N, Lazar, AJ, Ghadimi, MP, et al. Locoregional disease patterns in well-differentiated and dedifferentiated retroperitoneal liposarcoma: implications for the extent of resection? Ann Surg Oncol. (2014) 21:2136–43. doi: 10.1245/s10434-014-3643-4
5. Molina, G, Hull, MA, Chen, Y, DeLaney, TF, De Amorim, BK, Choy, E, et al. Preoperative radiation therapy combined with radical surgical resection is associated with a lower rate of local recurrence when treating unifocal, primary retroperitoneal liposarcoma. J Surg Oncol. (2016) 114:814–20. doi: 10.1002/jso.24427
6. Strauss, DC, Hayes, AJ, Thway, K, Moskovic, EC, Fisher, C, and Thomas, JM. Surgical management of primary retroperitoneal sarcoma. Br J Surg. (2010) 97:698–706. doi: 10.1002/bjs.6994
7. Lee, SY, Goh, BKP, Teo, MCC, Chew, MH, Chow, PKH, Wong, WK, et al. Retroperitoneal liposarcomas: the experience of a tertiary asian center. World J Surg Oncol. (2011) 9:12. doi: 10.1186/1477-7819-9-12
8. Tan, MCB, Brennan, MF, Kuk, D, Agaram, NP, Antonescu, CR, Qin, L, et al. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg. (2016) 263:593–600. doi: 10.1097/SLA.0000000000001149
9. Trans-Atlantic, RSWG . Management of metastatic retroperitoneal sarcoma: a consensus approach from the trans-Atlantic retroperitoneal sarcoma working group (tarpswg). Ann Oncol. (2018) 29:857. doi: 10.1093/annonc/mdy052
10. Jo, VY, and Fletcher, CDM. Who classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. (2014) 46:95–104. doi: 10.1097/PAT.0000000000000050
11. The WHO . Classification of tumours editorial board. WHO classification of tumour soft tissue and bone tumours. 5th ed. Lyon: IARC Press (2022).
12. Park, JO, Qin, L, Prete, FP, Antonescu, C, Brennan, MF, and Singer, S. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma. Ann Surg. (2009) 250:977–82. doi: 10.1097/SLA.0b013e3181b2468b
13. Jennifer, JG, Joyce, C, Erik, B, Jacquelyn, S, Suparna, BW, Harpreet, S, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US food and drug administration pooled analysis. Lancet Oncol. (2020) 21:250–60. doi: 10.1016/S1470-2045(19)30804-6
14. Adarsh, V, and Lakshmi, R. Retroperitoneal liposarcoma: a comprehensive review. Am J Clin Oncol. (2013) 12:5665–7. doi: 10.1097/COC.0b013e31829b5667
15. Casadei, L, de Faria, F, Lopez-Aguiar, A, Pollock, RE, and Grignol, V. Targetable pathways in the treatment of retroperitoneal liposarcoma. Cancers (Basel). (2022) 14:1362. doi: 10.3390/cancers14061362
16. Anaya, DA, Lahat, G, Liu, J, Xing, Y, Cormier, JN, Pisters, PW, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann Surg. (2009) 249:137–42. doi: 10.1097/SLA.0b013e3181928f2f
17. Bagaria, SP, Gabriel, E, and Mann, GN. Multiply recurrent retroperitoneal liposarcoma. J Surg Oncol. (2018) 117:62–8. doi: 10.1002/jso.24929
18. Ishii, K, Yokoyama, Y, Nishida, Y, Koike, H, Yamada, S, Kodera, Y, et al. Characteristics of primary and repeated recurrent retroperitoneal liposarcoma: outcomes after aggressive surgeries at a single institution. Jpn J Clin Oncol. (2020) 50:1412–8. doi: 10.1093/jjco/hyaa126
19. Keung, EZ, Hornick, JL, Bertagnolli, MM, Baldini, EH, and Raut, CP. Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery. J Am Coll Surg. (2014) 218:206–17. doi: 10.1016/j.jamcollsurg.2013.10.009
20. Gronchi, A, Strauss, DC, Miceli, R, Bonvalot, S, Swallow, CJ, Hohenberger, P, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (rps). Ann Surg. (2016) 263:1002–9. doi: 10.1097/SLA.0000000000001447
21. Zhuang, A, Wu, Q, Tong, H, Zhang, Y, and Lu, W. Development and validation of a nomogram for predicting recurrence-free survival of surgical resected retroperitoneal liposarcoma. Cancer Manag Res. (2021) 13:6633–9. doi: 10.2147/CMAR.S321324
22. Gronchi, A, Miceli, R, Shurell, E, Eilber, FC, Eilber, FR, Anaya, DA, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. (2013) 31:1649–55. doi: 10.1200/JCO.2012.44.3747
23. Li, Y, Wu, G, Zhang, Y, Yang, W, Wang, X, Duan, L, et al. Development and validation of a prognostic model to predict the prognosis of patients with retroperitoneal liposarcoma: a large international population-based cohort study. Front Oncol. (2022) 12:857827. doi: 10.3389/fonc.2022.857827
24. Yi-Xi, W, Jun-Yan, L, Jjia-Jia, L, Yan, P, Bo, T, You-Hong, C, et al. A retrospective, single-center cohort study on 65 patients with primary retroperitoneal liposarcoma. Oncol Lett. (2018) 15:1799–810. doi: 10.3892/ol.2017.7533
25. Gronchi, A, Collini, P, Miceli, R, Valeri, B, Renne, SL, Dagrada, G, et al. Myogenic differentiation and histologic grading are major prognostic determinants in retroperitoneal liposarcoma. Am J Surg Pathol. (2015) 39:383–93. doi: 10.1097/PAS.0000000000000366
26. Thway, K . Well-differentiated liposarcoma and dedifferentiated liposarcoma: an updated review. Semin Diagn Pathol. (2019) 36:112–21. doi: 10.1053/j.semdp.2019.02.006
27. Pal, S, Sengupta, S, Bose, K, and Jana, S. Mixed type retroperitoneal liposarcoma-combination of myxoid and well-differentiated type. J Cancer Res Ther. (2016) 12:424–6. doi: 10.4103/0973-1482.172590
28. Tyler, R, Wanigasooriya, K, Taniere, P, Almond, M, Ford, S, Desai, A, et al. A review of retroperitoneal liposarcoma genomics. Cancer Treat Rev. (2020) 86:102013. doi: 10.1016/j.ctrv.2020.102013
29. Nessim, C, Raut, CP, Callegaro, D, Barretta, F, Miceli, R, Fairweather, M, et al. Analysis of differentiation changes and outcomes at time of first recurrence of retroperitoneal liposarcoma by transatlantic australasian retroperitoneal sarcoma working group (tarpswg). Ann Surg Oncol. (2021) 28:7854–63. doi: 10.1245/s10434-021-10024-y
30. Singer, S, Antonescu, CR, Riedel, E, and Brennan, MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. (2003) 121:52–65. doi: 10.1097/01.sla.0000086542.11899.38
31. Raut, CP, and Swallow, CJ. Are radical compartmental resections for retroperitoneal sarcomas justified? Ann Surg Oncol. (2010) 17:1481–4. doi: 10.1245/s10434-010-1061-9
Keywords: retroperitoneal liposarcoma, recurrent, multiple tumors, pathological differentiation, local recurrence, predictor, nomogram model
Citation: Deng H, Xu X, Gao J, Huang J, Liu G, Song L and Wei B (2023) Predictors and outcomes of recurrent retroperitoneal liposarcoma with multiple tumors. Front. Med. 10:1161494. doi: 10.3389/fmed.2023.1161494
Edited by:
Alice Chen, Consultant, Potomac, MD, United StatesReviewed by:
Raphael Pollock, The Ohio State University, United StatesRahul Gupta, Synergy Institute of Medical Sciences, India
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy
Copyright © 2023 Deng, Xu, Gao, Huang, Liu, Song and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wei, weibo@vip.163.com
 Huan Deng1,2
Huan Deng1,2  Bo Wei
Bo Wei