Unmet needs and perspectives in rheumatoid arthritis-associated interstitial lung disease: A critical review
- 1Department of Biomedical Sciences, Humanitas University, Milan, Italy
- 2Division of Respiratory Medicine, IRCCS Humanitas Research Hospital, Milan, Italy
- 3IRCCS Humanitas Research Hospital, Milan, Italy
- 4Division of Rheumatology and Clinical Immunology, IRCCS Humanitas Research Hospital, Milan, Italy
- 5Department of Radiology, IRCCS Humanitas Research Hospital, Milan, Italy
- 6Division of Thoracic Surgery, IRCCS Humanitas Research Hospital, Milan, Italy
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by synovitis as the most common clinical manifestation, and interstitial lung disease (RA-ILD) represents one of the most common and potentially severe extra-articular features. Our current understanding of the mechanisms and predictors of RA-ILD is limited despite the demonstration that an early identification of progressive fibrosing forms is crucial to provide timely treatment with antifibrotic therapies. While high resolution computed tomography is the gold standard technique for the diagnosis and follow-up of RA-ILD, it has been hypothesized that serum biomarkers (including novel and rare autoantibodies), new imaging techniques such as ultrasound of the lung, or the application of innovative radiologic algorithms may help towards predicting and detecting early forms of diseases. Further, while new treatments are becoming available for idiopathic and connective tissue disease-associated forms of lung fibrosis, the treatment of RA-ILD remains anecdotal and largely unexplored. We are convinced that a better understanding of the mechanisms connecting RA with ILD in a subgroup of patients as well as the creation of adequate diagnostic pathways will be mandatory steps for a more effective management of this clinically challenging entity.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease in which an autoimmune mechanism causes chronic inflammation which predominantly involves the synovia at the peripheral joints (1). Although the disease etiology remains largely unknown, genetic predisposition, environmental triggers, and aberrant immune system activation are well established factors determining RA pathogenesis (2). A large proportion of patients report extra-articular manifestations, including cardiovascular, respiratory, and cutaneous involvement (3). The presence of extra-articular manifestations may in some cases predate the clinical onset of arthritis, may require specific management measures, and ultimately impact therapy (3, 4). Focusing on the respiratory manifestations of RA, it has been estimated that lung disease accounts for 10%–20% of mortality in subjects with RA, being inferior only to cardiovascular events (5). While the lung parenchyma, airways, pleura, and vasculature may all be affected, RA-associated interstitial lung disease (RA-ILD) is the most common and potentially severe manifestation, as it can present with a progressive fibrosing phenotype (6). Acute exacerbations of RA-ILD are defined as a rapidly progressing, potentially life-threatening respiratory decline characterized by new extensive alveolar abnormalities superimposed on underlying pulmonary fibrosis (7). Acute exacerbations are a rare but severe complication carrying a 12% to 64% mortality (8–11). To provide a better overview of RA-ILD, we will herein review the prevalence, risk factors, clinical characteristics, and therapeutic perspective of RA-ILD.
Prevalence, incidence, and mortality of RA-ILD
It has been estimated that RA-ILD explains about 8% of all cases of ILD (12). The prevalence of IL among patients with RA ranges between 1.8% and 67% and according to a recently published meta-analysis, the prevalence of clinically detected RA-ILD is also lower than radiologically detected cases (13). Chest high-resolution computed tomography (HRCT) is the most sensitive technique to screen for the presence of ILD in patients with RA and allows its characterization and quantification (14). The presence of symptoms and signs (i.e.: exercise dyspnea, cyanosis, inspiratory velcro-like crackles, digital clubbing) makes “clinically-driven” detection of RA-ILD ineffective and leads to delayed diagnosis at later stages (15). Thus, the use of different case finding methods explains, at least in part, the heterogeneity of RA-ILD prevalence that is reported in the literature.
Second, with the adoption of HRCT in clinical practice, an increase in RA-ILD prevalence has been observed over time (16). ILD has been detected in up to 7.5% subjects with early RA (17), while interstitial lung abnormalities (ILA, vide infra) may be more common (18). It has been estimated that 10% patients with established RA have clinically significant ILD (i.e., signs and symptoms, latent respiratory insufficiency, severe lung function impairment) (19). Moreover, ILD can precede RA clinical onset in a significant proportion of cases (20). Third and last, genetic susceptibility can be hypothesized to explain geographical differences (21).
While RA-associated general mortality has decreased over the last decades, mortality due to RA-ILD remained stable (15, 19) resulting in a 3–10 times higher risk of death in patients with RA-ILD compared to patients with RA without lung involvement (14, 20). RA-ILD not only increases the risk of all-cause and respiratory mortality, but also seems to be associated with elevated risk of cancer-related mortality (22) with pulmonary malignancy being the most common cancer-related cause of death in patients with RA, especially if ILD is present (22–24). The incidence of lung cancer is higher in patients <60 years with rheumatic disease-associated ILD (RD-ILD) than patients without rheumatic disease (25) and the incidence of lung cancer in RA-ILD is comparable to idiopathic pulmonary fibrosis (IPF) (26) but how these data apply to RA is unclear. Last, a trend towards a mortality reduction is associated with the early diagnosis of RA-ILD at HRCT and with the use of immunosuppressive therapy (27), while it has been demonstrated that diagnostic delay in RA-ILD diagnosis leads to increased mortality (28).
Risk factors and prognostic factors of RA-ILD
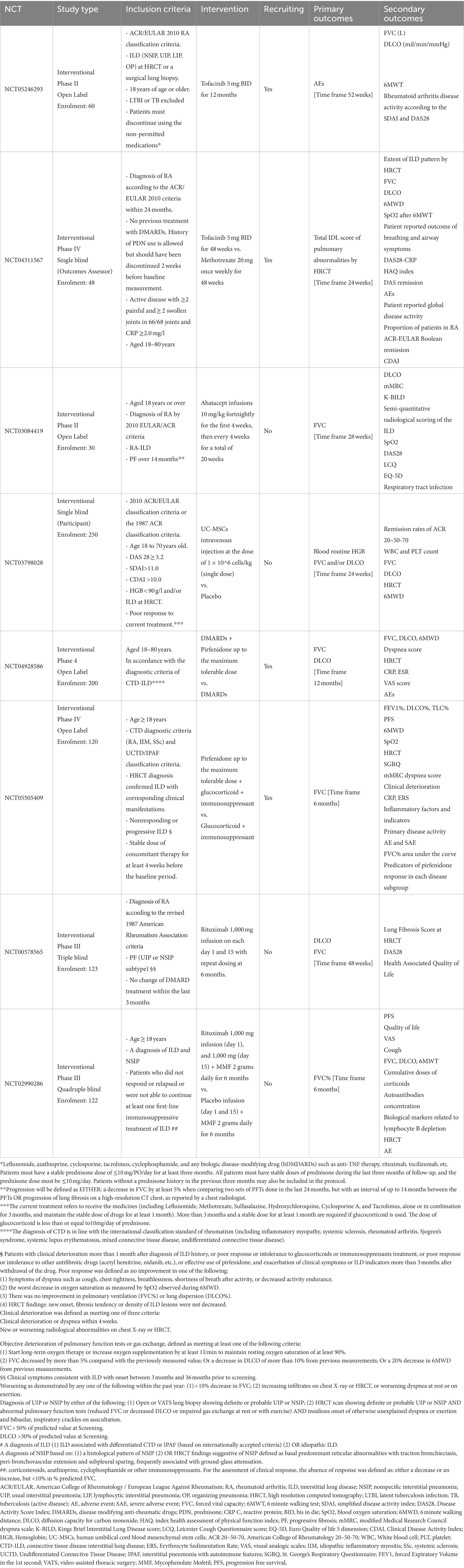
Established risk factors for RA-ILD are summarized in Table 1 and include demographics such as older age, male sex, obesity, and smoking history, along with the presence of respiratory comorbidities (22, 29). In addition, RA disease features, such as longer disease duration (13), high disease activity (22) and serum autoantibodies, in particular rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) significantly increase the risk of developing ILD. Furthermore, novel emerging biomarkers seem to play a prognostic role (27, 29) while genetic risk factors such as gain-of-function MUC5B promoter variant rs35705950 increase the risk of ILD in patients with RA and are associated with the usual interstitial pneumonia (UIP) pattern at HRCT (30) apart from being associated with IPF (31). Additionally, single-nucleotide polymorphisms (SNPs) in TERT, TOLLIP, and FAM13A loci have also been associated with pulmonary fibrosis in patients with RA (32).
While time-dependent decline in lung function correlates with mortality in RA-ILD (13, 33), other prognostic factors include older age, male sex, smoking habit, or the presence of comorbidities (13, 34) such as RA disease activity and the use of systemic glucocorticoids (13, 34). Additional poor prognostic features include pleural effusion, short time between RA diagnosis and ILD occurrence (35), as well as the radiologic pattern at HRCT with UIP pattern predicting mortality (13, 33, 36) and correlating with an increased risk of acute exacerbations and lung cancer (36).
Progressive pulmonary fibrosis and RA-ILD
According to the Fleischner Society glossary, fibrosing ILD is defined in the presence of reticular abnormalities, traction bronchiectasis and bronchiolectasis, architectural distortion, and/or honeycombing on HRCT scan (37–39). These radiologic changes reflect the exuberant deposition of extracellular matrix within the pulmonary interstitium, and may lead to the development of progressive fibrosing ILD in a subset of patients (39).
Progressive pulmonary fibrosis (PPF) has been defined as the progression of at least two domains among clinical and/or functional and/or radiological status, occurring within 1 year, without any alternative explanation, in a patient with an established diagnosis of fibrosing ILD other than IPF (40). One third of patients with RA-ILD develop PPF (12, 41, 42), particularly when a UIP pattern is described at HRCT, although a minority of subjects with these features do not progress (43). PPF is associated with increased mortality and unfavorable outcomes (44) and the antifibrotic drug nintedanib is now recommended in this subset of patients (40), thus making an early diagnosis a major clinical need.
Comorbidities in RA-ILD
Several comorbidities can impact the course of RA-ILD, affecting disease control and leading to impaired quality of life. According to Mena-Vázquez et al. ILD is independently associated with multimorbidity in patients with RA, with the most frequent comorbid conditions being traditional cardiovascular risk factors, depression, and osteoporosis (45).
Among respiratory comorbidities, RA-ILD can be associated with airway disease, including COPD, bronchiectasis and asthma (46). RA-ILD patients with COPD or emphysema have higher mortality risk in different cohorts (47–49). Interestingly, pre-existing COPD has been associated with a higher incidence of ILD in newly diagnosed RA patients (50). Bronchiectasis in RA are associated with increased risk of infections per se (46) and in patients treated with biologic disease modifying anti-rheumatic drugs (DMARDs) (51). RA-ILD is a risk factor for pneumonia (51, 52), in particular when associated to an organizing pneumonia pattern, and with daily doses of prednisone exceeding 10 mg. (52) A relevant concern in terms of infections is also represented by COVID-19 (53), since patients with RA are at increased risk of developing severe COVID-19, the risk appearing even higher in those with pre-existing ILD (54, 55).
Sleep disorders are frequently associated with RA. A recent meta-analysis has shown that the incidence of obstructive sleep apnea syndrome (OSAS) is 29.8% among RA cohorts, however with a significant heterogeneity, and high BMI is the principal risk factor (56). Although the epidemiology of sleep disorders is still blurred in RA-ILD, it is reasonable to consider OSAS as a significant complication in this subgroup of patients due to its relevance in other ILDs, including IPF, and can cause an extremely poor sleep quality that correlates with poor quality of life (57, 58). Thus, OSAS and prolonged oxygen desaturation during sleep have been associated with a worse prognosis in IPF, both in terms of mortality and clinical progression. (59)
Pulmonary hypertension associated to interstitial lung disease is an established clinical entity, owing to class III World Health Organization (WHO) classification (60). However, pulmonary arterial hypertension (PAH, i.e., class I WHO) can represent a rare complication patients suffering from RA (60, 61). Also, the prothrombotic effect associated to chronic inflammation increases the risk of venous thromboembolism (62), and chronic thromboembolic pulmonary hypertension should be taken into account when approaching to the differential diagnosis of pulmonary hypertension in patients with RA and RA-ILD. Interestingly, the dominating cause of pulmonary hypertension may change over time, making the diagnostic and therapeutic process more challenging (63).
Traditional and novel biomarkers in RA-ILD
Biomarkers are measurable indicators of biologic and pathologic processes (64), and include well-established serum autoantibodies used in routine clinical or research settings in addition to RF and ACPA and non-autoimmune markers of lung damage. Table 2 summarizes the main established and investigational biomarkers in RA-ILD.
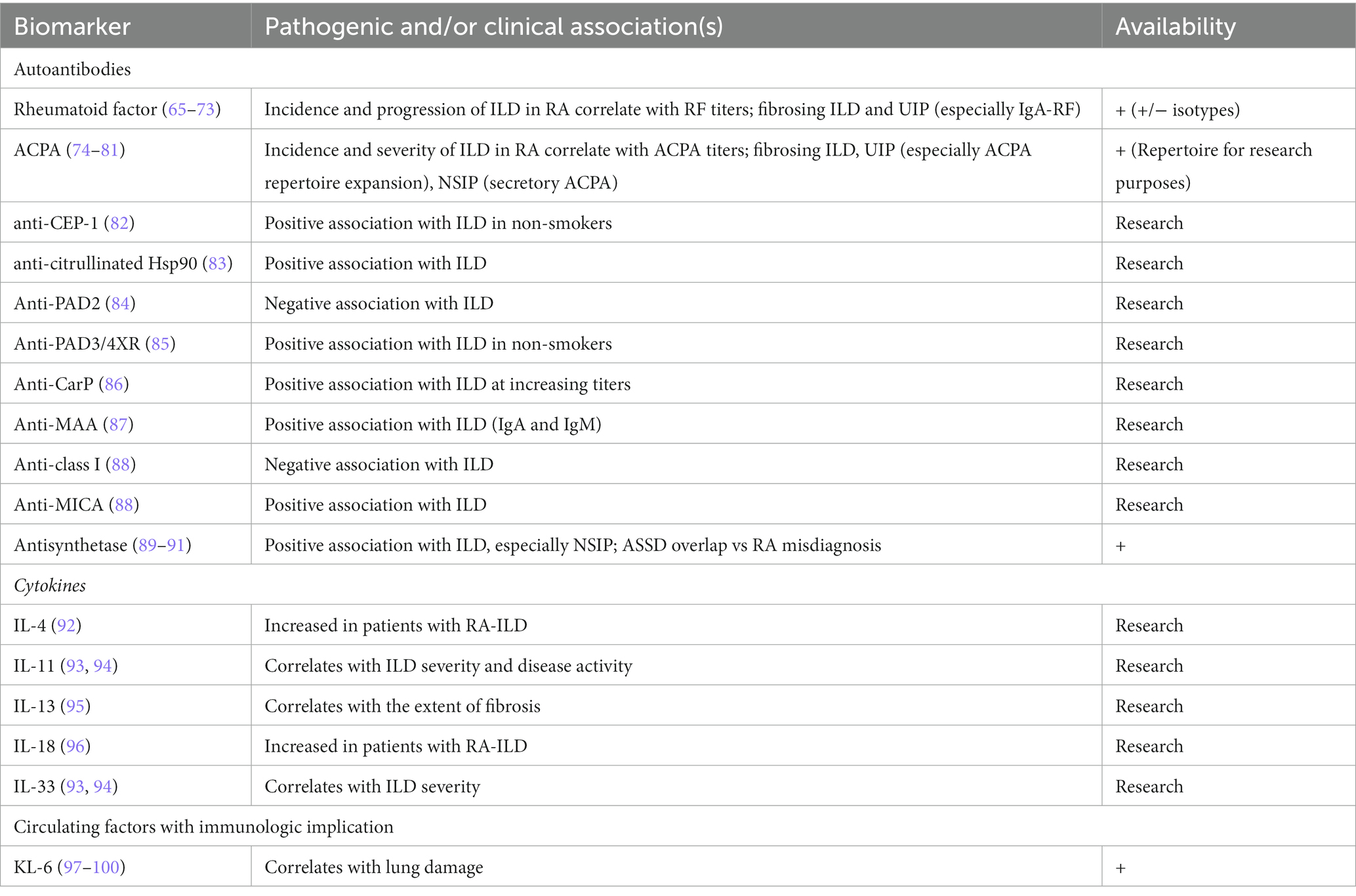
Table 2. Established and candidate biomarkers for RA-ILD; the proposed mechanistic links and setting of use are specified for all markers.
Rheumatoid factors (RF)
RF is represented by immunoglobulins, mainly IgM but also IgG and IgA, directed towards the constant (Fc) portion of another immunoglobulin (101); serum RF is positive in up to 80% of patients with RA, albeit with lower specificity (101) since non-rheumatic conditions (e.g., infective endocarditis, hepatitis B and C, primary biliary cholangitis, lymphoma) and rheumatic diseases other than RA (e.g., Sjogren’s syndrome, cryoglobulinemia) manifest different degrees of RF positivity (102). RF can be tested with different laboratory methods, including latex fixation test, Waaler-Rose reaction, and enzyme linked immunosorbent assay (ELISA) (103), with the latex fixation test and Waaler-Rose reaction capable of detecting only IgM-RF, while immunoassays can also identify IgG and IgA isotypes, possibly increasing the diagnostic sensitivity (104, 105).
RF positivity is an established risk factor for ILD development in patients with RA (65–67). It has been demonstrated that the prevalence and incidence of RA-ILD correlates with serum titers of RF (68) and high-titer RF is associated with an increased risk of progression and elevated mortality in patients with RA-ILD (69), along with a more aggressive form of RA with a higher risk of erosions (70). Moreover, signs of advanced fibrosis at HRCT (including honeycombing) have been associated with RF seropositivity in patients with RA-ILD (69).
In contrast to IgG and IgM RF isotypes, IgA-RF correlates with more severe articular disease, development of bone erosions, and increased prevalence of extra-articular manifestations including ILD (71, 72) with a higher prevalence of UIP pattern at HRCT (73). Despite this preliminary evidence, the clinical significance of different RF isotypes needs to be further explored in longitudinal studies.
Anti-citrullinated protein antibodies
Serum ACPA are associated with an increased risk of ILD in patients with RA (74) in a titer-dependent manner (75). Also, the prevalence of ILD at disease onset is higher among subjects with high ACPA titers (76). There is also a direct correlation between ACPA and disease severity in terms of clinical presentation (i.e., symptoms, signs, presence of respiratory insufficiency), worse lung function, and extent of ILD on HRCT (77). In a meta-analysis by Zhu and colleagues, ACPA status predicted ILD in RA and was significantly associated with an increased risk of fibrosing ILD (78) at degrees correlating with ACPA titers (77). Since signs of fibrosis at HRCT are an established risk factor for PPF (40), patients with RA testing positive for ACPA could be at higher risk for PPF development and may warrant a closer monitoring of lung changes.
ACPA formation against different citrullinated peptides and epitope spreading, which is the development of immunity against self-antigens release during autoimmune responses (79), are established mechanisms in the pathogenesis of RA (80). As such, as RA progresses, the ACPA repertoire (i.e., the number of different autoantigens and specific moieties recognized by ACPA) expands. This phenomenon has been associated with an increased risk of fibrosing ILD, lower lung volumes and DLCO, and higher prevalence of UIP pattern at HRCT (81). This is relevant considering that such functional and radiological features can predict PPF and suggests that analyzing the ACPA repertoire during the disease course may help individuating patients with RA-ILD at high risk of PPF.
Among autoantibody subtypes, patients testing positive for serum ACPA with secretory components have more frequently a nonspecific interstitial pneumonia (NSIP) pattern at HRCT, in contrast to what is commonly seen in RA-ILD (73).
Novel autoantibodies in RA-ILD
Among the non-classical autoantibodies putatively correlated with ILD in patients with RA, anti-citrullinated alpha-enolase peptide 1 (anti-CEP-1) have been identified as a subset of ACPA associated with erosive RA and ILD (82), particularly at high titers (106), and have been proposed for early detection of RA-ILD in at risk non-smoking patients (82). Anti-citrullinated heat shock protein 90 (Hsp90) antibodies are found in a subset of patients with RA-ILD, but not in patients with RA without ILD or in other forms of ILD (83). They are also present in the bronchoalveolar lavage fluid (BALF) (107) while autoreactive Th1 lymphocytes directed towards citrullinated Hsp90 have been detected in the peripheral blood of patients with RA-ILD (108). Peptidylarginine deaminase (PAD) is the most important enzyme causing citrullination (109), as demonstrated for the oral bacterium P. gingivalis (110). Autoantibodies against the human PAD isoforms PAD2, PAD3 and PAD4 have been detected in patients with RA (109) and may be useful in the risk stratification for lung disease. Anti-PAD2 antibodies have been described in a subset of patients with RA characterized by milder articular damage, as well as less frequent and less severe extra-articular manifestations, especially ILD (84), whereas anti-PAD4 antibodies correlate with a more aggressive disease (111). A subgroup of patients with RA possesses cross-reactive antibodies towards both PAD3 and PAD4, named anti-PAD3/4XR antibodies, that may predict ILD occurrence, especially in never-smoking patients (85), an association not found for anti-PAD3 or anti-PAD4 antibodies alone. Recent data demonstrate a possible association between double anti-PAD3 and PAD-4 positivity with both ILD and more erosive disease and the authors have hypothesized that such patients might have anti-PAD3/4XR positivity (112). Serum anti-carbamylated proteins antibodies (anti-CarP) have been reported at higher frequency in patients with RA-ILD compared to RA patients without ILD, independent of the smoking status (86). Malondialdehyde-acetaldehyde adducts (MAA) are highly expressed in the lung tissue from patients with RA-ILD, and antibodies against MAA (anti-MAA) have been associated with RA-ILD (87), especially high titers and the IgA or IgM isotypes (87). Furukawa and Colleagues have described the presence of autoantibodies to human leukocyte antigen (HLA) class I (anti-class I) and HLA class I related chain A (anti-MICA) in a cohort of patients with RA. Notably, higher levels of anti-MICA antibodies and higher values of the anti-MICA/anti-class I ratio were found in patients with RA-ILD, compared to patients without lung involvement (88). Antisynthetase antibodies are directed towards aminoacyl-tRNA-synthetase complex and are mainly found in a cluster of patients with inflammatory myositis, namely the antisynthetase syndrome (ASSD) (113) with NSIP as the most common ILD pattern observed at HRCT (114). In a cohort of patients with RA, the prevalence of serum antisynthetase antibodies was 6%, and ILD occurred more frequently (57%) in seropositive than seronegative (22%) patients. Specifically, anti-PL-7 was the most frequently reported among antisynthetase antibodies, whereas a low prevalence of anti-Jo-1 was observed; this contrasts with what is commonly seen in ASSD cohorts, where anti-Jo-1 is the most common antibody. Furthermore, opposite to RA-ILD with conventional antibodies like RF and ACPA, NSIP was the most frequent pattern at HRCT in antisynthetase antibody-positive subjects with RA (89). The association of antisynthetase antibodies and RA-ILD was confirmed in an independent cohort (90), and a case of anti-EJ and ACPA positive RA with ILD-only onset was also reported (115). Remarkably, ASSD is frequently misdiagnosed and treated as RA, especially when arthritis is the predominant manifestation (91).
Routine laboratory tests
Among tests usually performed in the clinical setting, an unsuspected role has been proposed for serum uric acid, for which higher levels were observed in RA-ILD with a prevailing UIP pattern at HRCT (116). Serum uric acid is already included in the DETECT algorithm for early detection of pulmonary arterial hypertension in patients with systemic sclerosis (SSc) (117). Higher neutrophil and monocyte counts are independent predictors of mortality in RA-ILD, particularly when both are elevated (118).
Other serum biomarkers
Among cytokines, serum titers of IL-4 (92) and IL-18 (96) are increased in patients with ILD, compared to the general RA population while serum IL-13 is increased in patients with RA-ILD and correlates with the extent of fibrosis at HRCT (95). However, such observations warrant further investigation since, as an example, IL-4 is a strict autocrine cytokine and serum levels may not differ even between subjects with IL-4-dependent diseases and healthy controls (119). Both arthritis and ILD severity correlate with the presence and serum concentrations of IL-11 and IL-33, independent of the RF and ACPA status, with the former being also associated with RA disease activity (93, 94). Within the IL23-IL17 axis, Zhang and colleagues reported that lung fibroblasts from patients with RA-ILD express significantly higher levels of the IL-17A receptor (IL-17RA) compared to patients without ILD or IPF (120) while IL-23 contributes to the epithelial-mesenchymal transition in the lung of RA-ILD (121). While the role of IL-17 and IL-23 remains elusive, these results support the use of monoclonal antibodies against IL-17 (e.g., secukinumab, ixekizumab) and IL-23 (e.g., guselkumab, risankizumab, and ustekinumab) which are currently used in spondyloarthritis, psoriasis and psoriatic arthritis, and inflammatory bowel disease for RA-ILD despite being proven ineffective on the articular manifestations of RA (122, 123). Krebs von den Lungen 6 (KL-6, a glycoprotein expressed by type II alveolar cells) serum levels correlate with lung damage in patients with ILD (97) with higher baseline values associated with mortality in RA-ILD, especially with a UIP pattern at HRCT (98). Changes in KL-6 values over time may predict acute exacerbations of RA-ILD (99) to make routine tests a putative screening method for ILD in patients with RA (100). In combination with KL-6, the oncological markers CA 19-9, CA 125, and CEA correlate with the presence and severity of ILD in patients with RA (124) while serum HE4, a biomarker for ovarian cancer, may identify RA cases at risk for subclinical ILD (125). It was demonstrated that serum onco-marker CA 15–3 is a valid alternative to KL-6, with comparable sensitivity and specificity in differentiating fibrosing and non-fibrosing ILD (126). Other proposed molecules involved at different levels in the immune, inflammatory, and fibrotic response characterizing RA-ILD include matrix metalloproteinase 7 (MMP-7), C-X-C motif chemokine ligand 10 (CXCL10) (127), Dickkopf 1 (DKK1) (128), and soluble programmed death 1 (sPD-1) (129). Circulating endothelial progenitor cells (EPCs) are associated with the repair of alveolar damage and are increased in RA-ILD compared to RA patients without lung involvement. However, their levels are lower in comparison to patients with IPF (130). While we acknowledge that observed differences refer to tests performed only for research purposes (67, 131), it should also be noted that non-coding RNAs (132, 133) and metabolomic profiling (134) have also been proposed with promising preliminary results.
Biomarkers in RA-ILD: Unmet needs and research questions
Except for traditional RA autoantibodies (i.e., RF and ACPA), no biomarker is currently used in clinical practice for the screening of RA-ILD, thus, further studies are required. First, it is of critical importance to individuate at baseline (or as early as possible) which patients with RA are at high risk of developing ILD. Second, once RA-ILD is established, there is a need to understand which subjects are likely to develop clinically significant disease or are going to require specific therapies (even in the presence of subclinical disease). Third, since fibrosis and PPF are major concerns in the management of RA-ILD, biomarkers are required for early identification of patients at risk of developing progressive fibrosing ILD. Fourth, there is an urgent need to understand whether antifibrotic therapy can be started only when PPF has established or, vice versa, whether there is any benefit from starting such therapy in patients ‘at high risk of PPF’. Fifth, since ‘ILD’ does not always mean ‘fibrosis’, biomarkers could help in discriminating different ‘treatable traits’ when clinical worsening occurs (e.g., progressive fibrosis versus inflammation versus superimposed infection, etc.). Sixth, predictive biomarkers that inform us of therapeutic effects are warranted.
Imaging in RA-ILD
To date, there are neither consensus statements or guidelines / recommendations on radiologic screening and follow-up of pulmonary involvement in RA, despite HRCT remaining the preferred tool for the identification of lung involvement in RA with a better sensitivity compared to chest X ray at early stages (135). Of importance, HRCT allows to discriminate between inflammatory and fibrotic lesions (136) with prognostic implications (33).
Preclinical thoracic findings
Lung involvement may predate the onset of RA, particularly with ancillary signs suggesting rheumatic involvement including RA-ILD, pleural effusion, pleuritis, bronchiectasis, rheumatoid nodules, pulmonary vascular diseases, and drug-associated lung complications (137).
ILAs, incidental findings involving at least 5% of lung parenchyma at HRCT in individuals in which ILD in not suspected (38) can be the first detectable sign both in patients with early and longstanding RA (138), with the latter having more frequent HRCT abnormalities (139). Gabbay et al. (140) detected ILAs in 44% of RA cases screened for lung involvement while others found HRCT abnormalities in nearly 50% of the patients with no respiratory symptoms. Factors significantly associated with HRCT abnormalities were age older than 40 years, positive tests for IgM-RF, hypoxia at rest, and lung function test evidence of distal airway disease (141). A lower incidence (22%) has been reported in a retrospective study of 293 patients with RA undergoing HRCT; 29% of these manifested progression over 4.4 years, particularly with subpleural distribution and higher baseline ILA extent. HRCT scans were performed for non-pulmonary indications in 46% patients, and ILAs were detected in a considerable proportion (44%) of these subjects. This supports the hypothesis that pulmonary involvement in RA is largely underdiagnosed (142). A 57% progression rate in ILAs has been described in a different study, largely related to past cigarette smoking (143).
ILD patterns at imaging
In patients with RA, UIP is the most common pattern at presentation, followed by NSIP while other types of ILD, i.e., organizing pneumonia (OP), desquamative interstitial pneumonia (DIP) and lymphocytic interstitial pneumonia (LIP), are found less frequently (144, 145). Typical UIP pattern is characterized by heterogenous honeycombing of the pulmonary bases and periphery, peripheral basilar predominant reticular abnormalities, and architectural distortions. However, the presence of anterior upper lobe honeycombing sign, where honeycombing is distributed both in the anterior upper lobes as well as in pulmonary bases, or the presence of exuberant honeycombing sign, where honeycombing is hypertrophic and distributed across multiple layers, are frequently associated with RD-ILDs, including RA-ILD (146, 147). The UIP pattern has been associated with an increased mortality in RA-ILD in different studies (137, 148–150) while Yunt et al. did not report any difference in survival between subjects with definite UIP versus those with possible UIP (137). A recent meta-analysis confirmed that UIP pattern at HRCT, presence of emphysema, and both the occurrence and number of acute exacerbations were associated with increased mortality in RA-ILD (151). Different from UIP, the NSIP pattern is characterized by ground-glass opacities (GGO), fine reticulation or traction bronchiectasis within GGO, and airspace consolidation while honeycombing is rarely present (152). Patients with NSIP develop pulmonary involvement at younger age and longer after RA diagnosis compared to the UIP pattern (153). However, they seem to respond better to immunosuppressive treatment (154, 155) and have a longer duration of articular manifestations and a lower risk of disease progression (155). In terms of natural history, RA-ILD may lead to progressive fibrosis, particularly with UIP (42, 156–159) or a widespread fibrosis (148, 160) with intercurrent acute exacerbations, with over 40% of patients fulfilling the criteria for PPF (161). It has been observed that the UIP pattern at HRCT (9, 10) is per se associated to an increased risk of acute exacerbation of pulmonary fibrosis, including fibrotic RA-ILD (162).
While there is no consensus or guidelines on the use and evaluation of HRCT to detect disease progression, visual evaluation is not an ideal tool to estimate the percentage of lung volume containing fibrotic features (40). Despite the absence of universal methods, the quantitative assessment (computer-based quantitative HRCT) of lung fibrosis and progression is a more objective and reproducible method (163, 164), as represented by the MeVis PULMO 3D system using the threshold value of -800HU correlating with both human observers and physiological impairment (165). A different automated quantification system includes the evaluation of lung fibrosis (as the sum of reticulation and traction bronchiectasis) and ILD (as the sum of lung fibrosis, honeycombing, and GGO) scores with a good performance in predicting prognosis in 144 patients with RA-ILD (166). Jacob et al. combined two visual staging systems in a cohort of RA-ILD patients, reaching good prognostic stratification, thus being able to identify a subpopulation of patients with progression characteristics similar to IPF (167).
Lung ultrasonography
Lung ultrasonography (LUS) is emerging as a novel diagnostic approach for ILD (168), with the main pathologic findings being alterations in the pleural line and appearance of vertical artifacts called “B lines.” The former lesion is defined by the pleural line becoming irregular and thickened and may appear blurred and fragmented while B lines are vertical hyperechoic laser beam-like artifacts that arise from the pleural line and extend to the end of the screen without fading, erasing A lines, and moving synchronously with the pleural sliding until defining the “interstitial syndrome” (169–171). Several protocols have been proposed for LUS but there is no consensus or guidelines about the ideal examination protocol for ILD. According to different studies, LUS are able to screen for ILD in RA patients with a good sensitivity and specificity (100, 172–176) and Cogliati et al. reported that LUS is a reliable screening tool not only if performed by a trained physician using a standard 72 lines protocol but also if performed by a short-trained physician using a pocket-size lung ultrasound device (173). The presence of B lines has a sensitivity and a specificity, respectively, of 92% and 56% for RA-ILD when LUS is compared to HRCT and this is only slightly reduced (89 and 50%) when an ultrasound pocket device is used and 8 rather than 72 zones are explored (173). Results from a meta-analysis on the use of LUS diagnostic studies on RD-ILD, including RA-ILD, reaffirms the high sensitivity and specificity of LUS. Moreover, of six examined scanning protocols, a simplified method scanning only 14 lung intercostal spaces showed very high sensitivity and specificity with a short scanning time (177).
The combination of LUS with serum KL-6 demonstrated to increase the correlation with HRCT and disease severity in 150 RA cases with serum KL-6 positively correlating with LUS score and HRCT. Cut-off values of KL-6 and LUS score were 277.5 U/ml and < 5.5, with sensitivity 86.7 and 100%, and specificity 88 and 100%, respectively (100), thus confirming data from a retrospective study on patients with ILD and rheumatic diseases, including RA (178). LUS may be helpful also in the longitudinal follow-up of patients on treatment, as suggested by one case report (179).
Lung function tests in RA-ILD
To date, there are no consensus statements nor guidelines/recommendations on functional screening and follow-up of RA-ILD. However, lung function tests are a reliable and easily accessible tool to detect lung involvement, staging disease severity, and monitor for disease progression.
Due to the systemic manifestations of RA, that can lead to musculoskeletal limitation and major exercise intolerance, lung function tests seem to be a better screening tool compared to clinical evaluation alone. Topcu et al. highlighted that symptom-related patient-reported outcome measures could be used to evaluate health-related quality of life in RA-ILD. However, they may not be very helpful in differentiating ‘ILD’ from ‘non-ILD’ causes in patients complaining respiratory symptoms, such as cough or dyspnea (180). On the other hand, concomitant comorbidities and complications due to the systemic disease involvement can represent confounding factors when assessing ILD severity (181).
RA-ILD is typically associated with a restrictive pattern with reduced carbon monoxide diffusing capacity (DLCO) on lung function tests. However, patients with RA can also develop obstructive lung disease, even in association with ILD (182).
Lung function tests can also help predicting the progression of RA-ILD. Lower forced vital capacity (FVC) and DLCO, as well as their decline over a 6-month period are associated to severe disease (159). Also, higher levels of DLCO have been associated with a better prognosis in an observational cohort (160), while DLCO ≤54% predicted has been identified as a cut-off with good sensitivity and specificity to individuate high risk of RA-ILD progression (183).
In another study enrolling 140 RA-ILD patients, most subjects experienced stable or slowly declining lung function. In 5% cases, however, rapid FVC (expressed as % predicted) deterioration was observed, especially in older adults (age > 70 years) with early diagnosis of RA. To note, the lung function trajectory did not go in parallel with RA disease activity (184).
Most RA patients are studied for lung involvement only when suggestive symptoms occur, and pulmonary disease has already evolved. However, since ILA and early ILD are present in asymptomatic patients, in our opinion it is reasonable to screen all subjects with a new diagnosis of RA with lung function tests and thoracic physical examination, looking for velcro-like crackles. Moreover, lung function monitoring and physical examination should be repeated at least once a year during follow-up; prompt radiological evaluation should be obtained in case of impaired baseline lung function tests, abnormal thoracic physical examination, or lung function decline according to recent guidelines on progressive fibrosing ILD (40).
The current clinical practice in the management of RA-ILD
There are significant gaps in the physician knowledge regarding RA-ILD and this is well represented by the underestimated prevalence of ILD in patients with RA (185). Despite the significant burden of RA-ILD, there are no established recommendations for the management of this condition. It is disconcerting that ILD is not mentioned in the latest 2022 European Alliance of Associations for Rheumatology (EULAR) recommendations for the management of RA (186) while the 2021 American College of Rheumatology (ACR) guidelines only advise to pay attention on the role of methotrexate (MTX) in patients with a previous diagnosis of lung or airway disease without addressing ILD (187). At a local level, ILD was included in the Taiwan Society of Rheumatology recommendations for the management of comorbidities and extra-articular manifestations of RA (188), and in the Spanish Societies of Rheumatology (SER), Pneumology and Thoracic Surgery (SEPAR) guidelines for the management of RA-ILD (189, 190). In the aforementioned documents, there is general accordance against the use of MTX and leflunomide (LEF), in favor of rituximab or abatacept (188, 189), despite a recent meta-analysis found that MTX is not associated with the risk of ILD in RA (191). Remarkably, both guidelines are characterized by low quality of evidence. No recommendations or guidelines from international respiratory societies have been specifically directed towards the management of RA-ILD.
The optimal treatment choices and timing for RA-ILD have not been established and limited evidence is currently available. No RCT has investigated the role of immunosuppressants in the treatment of RA-ILD. Despite the lack of evidence, glucocorticoids are often used, and seem to be effective especially in case of NSIP and OP patterns on HRCT (192, 193). Evidence supporting the use of immunosuppressive drugs is mainly derived from large studies investigating ILD associated to systemic sclerosis (194, 195); also, in contrast to IPF, immunosuppressive therapy is safe in patients with RA-ILD also when a UIP pattern is observed (196). Cyclophosphamide (197) and mycophenolate (198) have been used with varying success, despite information on RA-ILD has been often extrapolated from studies investigating heterogeneous populations of patients with ILD associated to different rheumatic diseases (199). In a retrospective study, treatment with either azathioprine, mycophenolate or rituximab was associated with improved pulmonary function at 12 months with no difference among the treatment regimens (200), while in another retrospective study rituximab has shown some efficacy in RA-ILD patients with progressive ILD despite treatment with glucocorticoids and conventional synthetic DMARDs or immunosuppressants (201) Further evidence is required to support the use of specific immunosuppressive drugs in RA-ILD, and a precision medicine approach is warranted to target specific disease pheno- and endotypes.
Concern has been raised towards the use of anti-TNF therapy in patients with RA-ILD, since cases of disease progression and safety issues have been reported but the clinical relevance and prevalence of these observations require further data-based confirmation (202). Biologic DMARDs with targets other than TNF-alpha appear to be associated with slower rate of progression of lung disease, whereas anti-TNF therapy does not correlate with a risk of ILD worsening (203). Despite the promising role of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in patients with ILD secondary to systemic sclerosis (204), further evidence is required concerning RA. Tocilizumab has demonstrated potential efficacy in maintaining lung function with a good safety profile, in a retrospective cohort of patients with RA-ILD (205). Evidence from a large retrospective registry might encourage the use of rituximab in patients with RA-ILD (206). The RECITAL trial has demonstrated efficacy and safety of rituximab in patients with connective tissue disease-associated ILD, but the study did not include RA-ILD (207). A possible role for Janus kinase (JAK)-inhibitors has been suggested from animal models of ILD associated with arthritis (208) as the JAK2 isoform specifically mediates TGF-beta signaling and the activation of myofibroblasts, and has been advocated in the molecular pathophysiology of RA-ILD (209, 210). However, due to the lack of solid evidence, the use of JAK-inhibitors cannot be encouraged for the treatment of RA-ILD (211, 212). Abatacept has shown promising results in different clinical studies (213–216); the results of the APRIL trial (NCT03084419) which is evaluating change in lung function at 24 weeks in RA-ILD patients treated with abatacept, are still expected. Iguratimod, a novel synthetic DMARD approved in Japan and China, prevents nuclear factor kappa B (NF-kB) migration into the cellular nucleus, thus impairing the transcription of proinflammatory genes and blocking the inflammatory response (217). Iguratimod has been evaluated in a study on 101 RA-ILD patients showing reduction of general inflammation, disease activity, and improvement in lung function (218).
With regard for antifibrotic molecules, these include nintedanib and pirfenidone but only the former is suggested as a therapeutic option in patients with RA ILD who meet the criteria for PPF according to ATS/ERS/JRS/ALAT Guidelines (40). In fact, the INBUILD trial demonstrated the efficacy and safety of nintedanib in patients with PPF other than IPF and significantly reduced the lung function decline at 52 weeks (219); a post hoc analysis found significant results in patients with autoimmune disease-related PPF without a specific analysis for RA-ILD (220).
On the other hand, pirfenidone did not achieve the same results and the TRAIL study (NCT02808871), a RCT enrolling patients with RA-ILD to compare pirfenidone to placebo, has been stopped early due to slow recruitment during the COVID-19 pandemic. However, preliminary results seem to suggest the efficacy of pirfenidone in slowing the rate of decline of FVC over time in patients with RA-ILD, although caution in interpreting results is necessary since the study was unpowered (221).
Regarding AE of RA-ILD, no consensus or management guidelines have been published, and, notably, diagnostic criteria are derived from AE in IPF (7). Few retrospective studies have analyzed AE in different RD-ILD (222) and RA-ILD alone (10, 11, 223, 224). In most cases, patients were treated with high doses steroids and best supportive care. One retrospective study failed to demonstrate benefits in term of survival from the use of cyclophosphamide in AE of RA-ILD (223). On the other hand, Ota et al. retrospectively found that the use of high doses of steroids and immunosuppressants (including cyclophosphamide, tacrolimus and cyclosporine) could improve the prognosis in AE of RA-ILD (224). Further studies are necessary to better understand pathologic mechanisms behind AEs in RA-ILD and improve management and prognosis of this severe complication.
Table 3 reports all ongoing RCTs on RA-ILD obtained from a systematic research of different registry trials (1ISRCTN registry and EU clinical trials register) on RA-ILD patients or trials on RA patients evaluating ILD among secondary outcomes. Unfortunately, these are based on variable encoded approaches for the diagnosis and management of RA-ILD and underlines the gaps in a uniform approach to this condition.
The management of RA-ILD patients should be not only based on the treatment of ILD itself, but should also address the clinical consequences and comorbidities of RA. With regard to obstructive lung disease, there are no specific guidelines targeting the management in RA patients, and the impact still remains largely unexplored (46). However, smoking cessation programs should be proposed to all patients to reduce risk of death and improve quality of life (225). Patients should be screened for obstructive lung disease and treated accordingly to the current guidelines. The use of conventional DMARDs has been explored both in asthma and in COPD cohorts, and methotrexate may exert a modest steroid-sparing effect (46). As both bronchiectasis (46) and ILD (51, 52) can be associated with an increased risk of lower tract respiratory infections, a multidisciplinary approach including pulmonologists and rheumatologists is warranted for all patients, in order to evaluate the best pharmacologic interventions and reduce the risk of infections (46, 51, 52). Moreover, microbiological sampling should be considered in case of infection, particularly pneumonia, and DMARDs should be suspended and recommenced only once the antibiotic therapy is completed and clinical symptoms have resolved (226). Pneumococcal and annual Influenza vaccinations should be offered to all patients with RA, regardless of the treatment (226, 227), along with SARS-CoV-2 immunization (228, 229). Finally, since treatment with anti-TNF therapy is associated with an increased risk of developing TB, screening and treatment for latent TB should be proposed to all RA patients (226).
Pulmonary hypertension secondary to ILD has been associated to reduced exercise capacity, increased need for supplemental oxygen, worse quality of life and prognosis (230–232). Screening for pulmonary hypertension should be performed in all RA-ILD patients although no recommendation on timing and frequency is available (233). Recently, the INCREASE trial (234) has shown significant improvements in exercise capacity in ILD patients with PH treated with inhaled treprostinil. Clinical worsening also occurred less frequently in the treprostinil group, compared with placebo. The trial also included RD-ILD patients, but subgroup analysis has not been performed, and targeted clinical trials are warranted to confirm these results in specific populations, as is the case of RA-ILD patients. Since subjects with RA-ILD are at higher risk of developing malignancy and in particular lung neoplasms (22, 24), cancer screening should be systematically performed; however, there is no clear indication regarding timing and frequency (24).
In addition to clinical comorbidities, several relevant treatable traits have been identified in ILD and should be addressed in RA-ILD (235), including dyspnea, exercise-induced hypoxemia, and exercise intolerance. In RA-ILD patients, these conditions can be worsened by musculoskeletal involvement due to RA itself. Referral to pulmonary rehabilitation should be considered as an important component of comprehensive patient care (236). On the other hand, in case of end stage disease, a palliative approach is preferable to reduce the burden of symptoms and improve the quality of life (237). Finally, lung transplantation could be considered in selected patients with RA-ILD; no significant differences have been described in terms of survival, acute and chronic rejection, or extrapulmonary organ dysfunction compared to IPF (238, 239). Thus, lung transplant could offer a chance to improve the quality of life in the appropriate patients’ subsets (239).
Conclusion
Available data on RA-ILD epidemiology remain unconclusive and heterogeneous for both clinical and research purposes and significant more research efforts are required to finely define incidence, prevalence, and mortality of ILD in the RA population. In particular, one priority is the harmonization of the detection methods since HRCT is the gold standard technique for the diagnosis of ILD. Second, it is essential to define which patients with RA are at increased risk of ILD and, thus, deserve early radiologic investigations as delayed diagnosis is associated with increased mortalityss. Third, the timing, frequency, and the potential role of alternative screening methods, such as lung function tests, serum biomarkers and LUS, also need to be determined (26), likely with the use of biomarkers, including both autoantibodies and non-autoimmune biomarkers. Fourth, the proportion of patients with radiologic ILD who will progress to clinically overt disease is unknown, as is the proportion of patients with radiologic ILD who might benefit from early treatment. Ultimately, efforts are required to imbricate clinical, biological, radiological, and functional risk factors to find reproducible prediction models to estimate the risk and prognosis of ILD in the RA population and to stratify patients with RA-ILD at risk of developing PPF.
Author contributions
AS, AT, and GB: review of relevant papers and manuscript preparation. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1.Smolen, JS, Aletaha, D, and McInnes, IB. Rheumatoid arthritis. Lancet. (2016) 388:2023–38. doi: 10.1016/S0140-6736(16)30173-8
2.McInnes, IB, and Schett, G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 365:2205–19. doi: 10.1056/NEJMra1004965
3.Marcucci, E, Bartoloni, E, Alunno, A, Leone, MC, Cafaro, G, Luccioli, F, et al. Extra-articular rheumatoid arthritis. Reumatismo. (2018) 70:212–24. doi: 10.4081/reumatismo.2018.1106
4.Figus, FA, Piga, M, Azzolin, I, McConnell, R, and Iagnocco, A. Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmun Rev. (2021) 20:102776. doi: 10.1016/j.autrev.2021.102776
5.Alunno, A, Gerli, R, Giacomelli, R, and Carubbi, F. Clinical, epidemiological, and Histopathological features of respiratory involvement in rheumatoid arthritis. Biomed Res Int. (2017) 2017:1–8. doi: 10.1155/2017/7915340
6.Esposito, AJ, Chu, SG, Madan, R, Doyle, TJ, and Dellaripa, PF. Thoracic manifestations of rheumatoid arthritis. Clin Chest Med. (2019) 40:545–60. doi: 10.1016/j.ccm.2019.05.003
7.Collard, HR, Ryerson, CJ, Corte, TJ, Jenkins, G, Kondoh, Y, Lederer, DJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med. (2016) 194:265–75. doi: 10.1164/rccm.201604-0801CI
8.Hozumi, H, Kono, M, Hasegawa, H, Kato, S, Inoue, Y, Suzuki, Y, et al. Acute exacerbation of rheumatoid arthritis-associated interstitial lung disease: mortality and its prediction model. Respir Res. (2022) 23:57. doi: 10.1186/s12931-022-01978-y
9.Izuka, S, Yamashita, H, Iba, A, Takahashi, Y, and Kaneko, H. Acute exacerbation of rheumatoid arthritis-associated interstitial lung disease: clinical features and prognosis. Rheumatology (Oxford). (2021) 60:2348–54. doi: 10.1093/rheumatology/keaa608
10.Bs, K, Hy, L, J, C, Ej, C, S, H, and Jw, S. Acute respiratory deterioration in rheumatoid arthritis-associated interstitial lung disease: a single-center study. Chest [Internet]. (2022) 162:136–44. doi: 10.1016/j.chest.2022.01.007
11.Hozumi, H, Nakamura, Y, Johkoh, T, Sumikawa, H, Colby, TV, Kono, M, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open. (2013) 3:e003132. doi: 10.1136/bmjopen-2013-003132
12.Nasser, M, Larrieu, S, Boussel, L, Si-Mohamed, S, Bazin, F, Marque, S, et al. Estimates of epidemiology, mortality and disease burden associated with progressive fibrosing interstitial lung disease in France (the PROGRESS study). Respir Res. (2021) 22:162. doi: 10.1186/s12931-021-01749-1
13.Fazeli, MS, Khaychuk, V, Wittstock, K, Han, X, Crocket, G, Lin, M, et al. Rheumatoid arthritis-associated interstitial lung disease: epidemiology, risk/prognostic factors, and treatment landscape. Clin Exp Rheumatol. (2021) 39:1108–18. doi: 10.55563/clinexprheumatol/h9tc57
14.Bongartz, T, Nannini, C, Medina-Velasquez, YF, Achenbach, SJ, Crowson, CS, Ryu, JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. (2010) 62:1583–91. doi: 10.1002/art.27405
15.Jeganathan, N, Nguyen, E, and Sathananthan, M. Rheumatoid arthritis and associated interstitial lung disease: mortality rates and trends. Annals ATS. (2021) 18:1970–7. doi: 10.1513/AnnalsATS.202102-115OC
16.Raimundo, K, Solomon, JJ, Olson, AL, Kong, AM, Cole, AL, Fischer, A, et al. Rheumatoid arthritis-interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J Rheumatol. (2019) 46:360–9. doi: 10.3899/jrheum.171315
17.Paulin, F, Secco, A, Benavidez, F, Rodríguez Moncalvo, JJ, Carballo, OG, Ingenito, F, et al. Lung involvement prevalence in patients with early rheumatoid arthritis without known pulmonary disease: a multicentric cross sectional study. Adv Rheumatol. (2021) 61:52. doi: 10.1186/s42358-021-00209-0
18.Dong, H, Julien, PJ, Demoruelle, MK, Deane, KD, and Weisman, MH. Interstitial lung abnormalities in patients with early rheumatoid arthritis: a pilot study evaluating prevalence and progression. Eur J Rheumatol. (2019) 6:193–8. doi: 10.5152/eurjrheum.2019.19044
19.Olson, AL, Swigris, JJ, Sprunger, DB, Fischer, A, Fernandez-Perez, ER, Solomon, J, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. (2011) 183:372–8. doi: 10.1164/rccm.201004-0622OC
20.Hyldgaard, C, Hilberg, O, Pedersen, AB, Ulrichsen, SP, Løkke, A, Bendstrup, E, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis. (2017) 76:1700–6. doi: 10.1136/annrheumdis-2017-211138
21.Wheeler, AM, Baker, JF, Poole, JA, Ascherman, DP, Yang, Y, Kerr, GS, et al. Genetic, social, and environmental risk factors in rheumatoid arthritis-associated interstitial lung disease. Semin Arthritis Rheum. (2022) 57:152098. doi: 10.1016/j.semarthrit.2022.152098
22.Sparks, JA, Jin, Y, Cho, SK, Vine, S, Desai, R, Doyle, TJ, et al. Prevalence, incidence and cause-specific mortality of rheumatoid arthritis-associated interstitial lung disease among older rheumatoid arthritis patients. Rheumatology (Oxford). (2021) 60:3689–98. doi: 10.1093/rheumatology/keaa836
23.Fu, Q, Wang, L, Li, L, Li, Y, Liu, R, and Zheng, Y. Risk factors for progression and prognosis of rheumatoid arthritis-associated interstitial lung disease: single center study with a large sample of Chinese population. Clin Rheumatol. (2019) 38:1109–16. doi: 10.1007/s10067-018-4382-x
24.Fragoulis, GE, and Chatzidionysiou, K. Lung cancer in rheumatoid arthritis. Is there a need for better risk assessment and screening? Clin Rheumatol. (2020) 39:957–61. doi: 10.1007/s10067-019-04882-x
25.Choi, WI, Lee, DY, Choi, HG, and Lee, CW. Lung cancer development and mortality in interstitial lung disease with and without connective tissue diseases: a five-year Nationwide population-based study. Respir Res. (2019) 20:117. doi: 10.1186/s12931-019-1094-y
26.Choi, WI, Park, SH, Park, BJ, and Lee, CW. Interstitial lung disease and lung cancer development: a 5-year Nationwide population-based study. Cancer Res Treat. (2018) 50:374–81. doi: 10.4143/crt.2017.119
27.Kelly, CA, Nisar, M, Arthanari, S, Carty, S, Woodhead, FA, Price-Forbes, A, et al. Rheumatoid arthritis related interstitial lung disease – improving outcomes over 25 years: a large multicentre UK study. Rheumatology. (2021) 60:1882–90. doi: 10.1093/rheumatology/keaa577
28.Cano-Jiménez, E, Vázquez Rodríguez, T, Martín-Robles, I, Castillo Villegas, D, Juan García, J, Bollo de Miguel, E, et al. Diagnostic delay of associated interstitial lung disease increases mortality in rheumatoid arthritis. Sci Rep. (2021) 11:9184. doi: 10.1038/s41598-021-88734-2
29.Kronzer, VL, Huang, W, Dellaripa, PF, Huang, S, Feathers, V, Lu, B, et al. Lifestyle and clinical risk factors for incident rheumatoid arthritis-associated interstitial lung disease. J Rheumatol. (2021) 48:656–63. doi: 10.3899/jrheum.200863
30.Juge, PA, Lee, JS, Ebstein, E, Furukawa, H, Dobrinskikh, E, Gazal, S, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. (2018) 379:2209–19. doi: 10.1056/NEJMoa1801562
31.Seibold, MA, Wise, AL, Speer, MC, Steele, MP, Brown, KK, Loyd, JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. (2011) 364:1503–12. doi: 10.1056/NEJMoa1013660
32.Jönsson, E, Ljung, L, Norrman, E, Freyhult, E, Ärlestig, L, Dahlqvist, J, et al. Pulmonary fibrosis in relation to genetic loci in an inception cohort of patients with early rheumatoid arthritis from northern Sweden. Rheumatology (Oxford). (2022) 61:943–52. doi: 10.1093/rheumatology/keab441
33.Solomon, JJ, Chung, JH, Cosgrove, GP, Demoruelle, MK, Fernandez-Perez, ER, Fischer, A, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. (2016) 47:588–96. doi: 10.1183/13993003.00357-2015
34.England, BR, Sayles, H, Michaud, K, Caplan, L, Davis, LA, Cannon, GW, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). (2016) 68:36–45. doi: 10.1002/acr.22642
35.Ekici, M, Baytar, Y, Kardas, RC, Sari, A, Akdogan, A, Durhan, G, et al. Predictors of mortality in rheumatoid arthritis-associated lung disease: a retrospective study on ten years. Joint Bone Spine. (2021) 88:105133. doi: 10.1016/j.jbspin.2021.105133
36.Kakutani, T, Hashimoto, A, Tominaga, A, Kodama, K, Nogi, S, Tsuno, H, et al. Related factors, increased mortality and causes of death in patients with rheumatoid arthritis-associated interstitial lung disease. Mod Rheumatol. (2020) 30:458–64. doi: 10.1080/14397595.2019.1621462
37.Hansell, DM, Bankier, AA, Mac Mahon, H, McLoud, TC, Müller, NL, and Remy, J. Fleischner society: glossary of terms for thoracic imaging. Radiology. (2008) 246:697–722. doi: 10.1148/radiol.2462070712
38.Hatabu, H, Hunninghake, GM, Richeldi, L, Brown, KK, Wells, AU, Remy-Jardin, M, et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner society. Lancet Respir Med. (2020) 8:726–37. doi: 10.1016/S2213-2600(20)30168-5
39.Spagnolo, P, Ryerson, CJ, Putman, R, Oldham, J, Salisbury, M, Sverzellati, N, et al. Early diagnosis of fibrotic interstitial lung disease: challenges and opportunities. Lancet Respir Med. (2021) 9:1065–76. doi: 10.1016/S2213-2600(21)00017-5
40.Raghu, G, Remy-Jardin, M, Richeldi, L, Thomson, CC, Inoue, Y, Johkoh, T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. (2022) 205:e18–47. doi: 10.1164/rccm.202202-0399ST
41.Wijsenbeek, M, and Cottin, V. Spectrum of fibrotic lung diseases. N Engl J Med. (2020) 383:958–68. doi: 10.1056/NEJMra2005230
42.Mena-Vázquez, N, Rojas-Gimenez, M, Romero-Barco, CM, Manrique-Arija, S, Francisco, E, Aguilar-Hurtado, MC, et al. Predictors of progression and mortality in patients with prevalent rheumatoid arthritis and interstitial lung disease: a prospective cohort study. J Clin Med. (2021) 10:874. doi: 10.3390/jcm10040874
43.Cottin, V, Hirani, NA, Hotchkin, DL, Nambiar, AM, Ogura, T, Otaola, M, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. (2018) 27:180076. doi: 10.1183/16000617.0076-2018
44.Fischer, A, and Distler, J. Progressive fibrosing interstitial lung disease associated with systemic autoimmune diseases. Clin Rheumatol. (2019) 38:2673–81. doi: 10.1007/s10067-019-04720-0
45.Mena-Vázquez, N, Rojas-Gimenez, M, Romero-Barco, CM, Gandía-Martínez, M, Perez-Gómez, N, Godoy-Navarrete, FJ, et al. Analysis of comorbidity in rheumatoid arthritis-associated interstitial lung disease: a nested case-cohort study. Biomed Pharmacother. (2023) 157:114049. doi: 10.1016/j.biopha.2022.114049
46.Matson, SM, Demoruelle, MK, and Castro, M. Airway disease in rheumatoid arthritis. Ann Am Thorac Soc. (2022) 19:343–52. doi: 10.1513/AnnalsATS.202107-876CME
47.Ng, KH, Chen, DY, Lin, CH, Chao, WC, and Chen, HH. Analysis of risk factors of mortality in rheumatoid arthritis patients with interstitial lung disease: a nationwide, population-based cohort study in Taiwan. RMD Open. (2022) 8:e002343. doi: 10.1136/rmdopen-2022-002343
48.Qiu, M, Jiang, J, Nian, X, Wang, Y, Yu, P, Song, J, et al. Factors associated with mortality in rheumatoid arthritis-associated interstitial lung disease: a systematic review and meta-analysis. Respir Res. (2021) 22:264. doi: 10.1186/s12931-021-01856-z
49.Nikiphorou, E, de Lusignan, S, Mallen, C, Roberts, J, Khavandi, K, Bedarida, G, et al. Prognostic value of comorbidity indices and lung diseases in early rheumatoid arthritis: a UK population-based study. Rheumatology (Oxford). (2020) 59:1296–305. doi: 10.1093/rheumatology/kez409
50.Zheng, B, Soares de Moura, C, Machado, M, Pineau, CA, Curtis, JR, Vinet, E, et al. Association between chronic obstructive pulmonary disease, smoking, and interstitial lung disease onset in rheumatoid arthritis. Clin Exp Rheumatol. (2022) 40:1280–4. doi: 10.55563/clinexprheumatol/i9au1r
51.Honne, K, Bando, M, Mieno, MN, Iwamoto, M, and Minota, S. Bronchiectasis is as crucial as interstitial lung disease in the severe pneumonia that occurs during treatment with biologic DMARDs in rheumatoid arthritis: a retrospective cohort study in a single facility. Rheumatol Int. (2022) 42:1341–6. doi: 10.1007/s00296-021-04934-z
52.Zamora-Legoff, JA, Krause, ML, Crowson, CS, Ryu, JH, and Matteson, EL. Risk of serious infection in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. (2016) 35:2585–9. doi: 10.1007/s10067-016-3357-z
53.Al-Adhoubi, NK, Ali, M, Wahshi, HA, Salmi, IA, Al-Balushi, F, Lawati, TA, et al. COVID-19 mortality in patients with rheumatic diseases: a real concern. Curr Rheumatol Rev. (2022) 18:234–42. doi: 10.2174/1573397118666220412114514
54.Kelly, C. Increased risk of severe COVID-19 outcomes in patients with rheumatoid arthritis and interstitial lung disease. Lancet Rheumatol. (2022) 4:e741–3. doi: 10.1016/S2665-9913(22)00256-9
55.Figueroa-Parra, G, Gilbert, EL, Valenzuela-Almada, MO, Vallejo, S, Neville, MR, Patel, NJ, et al. Risk of severe COVID-19 outcomes associated with rheumatoid arthritis and phenotypic subgroups: a retrospective, comparative, multicentre cohort study. Lancet Rheumatol. (2022) 4:e765–74. doi: 10.1016/S2665-9913(22)00227-2
56.Thakur, B, Pathak, M, Singh, P, and Padhan, P. Prevalence of obstructive sleep apnea among patients with rheumatoid arthritis and its association with age and body mass index: a systematic review and meta-analysis. Int J Rheum Dis. (2021) 24:1354–61. doi: 10.1111/1756-185X.14178
57.Raghu, G, Collard, HR, Egan, JJ, Martinez, FJ, Behr, J, Brown, KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. (2011) 183:788–824. doi: 10.1164/rccm.2009-040GL
58.Bosi, M, Milioli, G, Parrino, L, Fanfulla, F, Tomassetti, S, Melpignano, A, et al. Quality of life in idiopathic pulmonary fibrosis: the impact of sleep disordered breathing. Respir Med. (2019) 147:51–7. doi: 10.1016/j.rmed.2018.12.018
59.Bosi, M, Milioli, G, Fanfulla, F, Tomassetti, S, Ryu, JH, Parrino, L, et al. OSA and prolonged oxygen desaturation during sleep are strong predictors of poor outcome in IPF. Lung. (2017) 195:643–51. doi: 10.1007/s00408-017-0031-4
60.Panagiotidou, E, Sourla, E, Kotoulas, SX, Akritidou, S, Bikos, V, Bagalas, V, et al. Rheumatoid arthritis associated pulmonary hypertension: clinical challenges reflecting the diversity of pathophysiology. Respir Med Case Rep. (2017) 20:164–7. doi: 10.1016/j.rmcr.2017.02.006
61.Shahane, A. Pulmonary hypertension in rheumatic diseases: epidemiology and pathogenesis. Rheumatol Int. (2013) 33:1655–67. doi: 10.1007/s00296-012-2659-y
62.Chung, WS, Peng, CL, Lin, CL, Chang, YJ, Chen, YF, Chiang, JY, et al. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis. (2014) 73:1774–80. doi: 10.1136/annrheumdis-2013-203380
63.Szturmowicz, M, Franczuk, M, Jędrych, ME, Wyrostkiewicz, D, Oniszh, K, Darocha, S, et al. Dominating cause of pulmonary hypertension may change over time-diagnostic and therapeutic considerations in a patient with pulmonary hypertension due to rheumatoid arthritis with lung involvement. Diagnostics (Basel). (2021) 11:1931. doi: 10.3390/diagnostics11101931
64.Califf, RM. Biomarker definitions and their applications. Exp Biol Med (Maywood). (2018) 243:213–21. doi: 10.1177/1535370217750088
65.Kamiya, H, Panlaqui, OM, Izumi, S, and Sozu, T. Systematic review and meta-analysis of prognostic factors for idiopathic inflammatory myopathy-associated interstitial lung disease. BMJ Open. (2018) 8:e023998. doi: 10.1136/bmjopen-2018-023998
66.S, X, S, L, B, C, Q, Z, L, X, and F, L. Serum anti-citrullinated protein antibodies and rheumatoid factor increase the risk of rheumatoid arthritis-related interstitial lung disease: a meta-analysis. Clinical Rheumatol [Internet]. (2021) 40:4533–43. doi: 10.1007/s10067-021-05808-2
67.Doyle, TJ, Patel, AS, Hatabu, H, Nishino, M, Wu, G, Osorio, JC, et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med. (2015) 191:1403–12. doi: 10.1164/rccm.201411-1950OC
68.Natalini, JG, Baker, JF, Singh, N, Mahajan, TD, Roul, P, Thiele, GM, et al. Autoantibody Seropositivity and risk for interstitial lung disease in a prospective male-predominant rheumatoid arthritis cohort of U.S. Veterans Ann Am Thorac Soc. (2021) 18:598–605. doi: 10.1513/AnnalsATS.202006-590OC
69.Tyker, A, Ventura, IB, Lee, CT, Strykowski, R, Garcia, N, Guzy, R, et al. High-titer rheumatoid factor seropositivity predicts mediastinal lymphadenopathy and mortality in rheumatoid arthritis-related interstitial lung disease. Sci Rep. (2021) 11:22821. doi: 10.1038/s41598-021-02066-9
70.Smolen, JS, Landewé, RBM, Bijlsma, JWJ, Burmester, GR, Dougados, M, Kerschbaumer, A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. (2020) 79:685–99. doi: 10.1136/annrheumdis-2019-216655
71.Jónsson, T, and Valdimarsson, H. Is measurement of rheumatoid factor isotypes clinically useful? Ann Rheum Dis. (1993) 52:161–4. doi: 10.1136/ard.52.2.161
72.Jónsson, T, and Valdimarsson, H. What about IgA rheumatoid factor in rheumatoid arthritis? Ann Rheum Dis. (1998) 57:63–4. doi: 10.1136/ard.57.1.63
73.Oka, S, Higuchi, T, Furukawa, H, Shimada, K, Okamoto, A, Hashimoto, A, et al. Serum rheumatoid factor IgA, anti-citrullinated peptide antibodies with secretory components, and anti-carbamylated protein antibodies associate with interstitial lung disease in rheumatoid arthritis. BMC Musculoskelet Disord. (2022) 23:46. doi: 10.1186/s12891-021-04985-0
74.Kamiya, H, and Panlaqui, OM. Systematic review and meta-analysis of the risk of rheumatoid arthritis-associated interstitial lung disease related to anti-cyclic citrullinated peptide (CCP) antibody. BMJ Open. (2021) 11:e040465. doi: 10.1136/bmjopen-2020-040465
75.Correia, CS, Briones, MR, Guo, R, and Ostrowski, RA. Elevated anti-cyclic citrullinated peptide antibody titer is associated with increased risk for interstitial lung disease. Clin Rheumatol. (2019) 38:1201–6. doi: 10.1007/s10067-018-04421-0
76.Chen, RX, Zhao, LD, Xiao, XY, Song, L, Du, HY, Xu, ZJ, et al. Distinctive clinical characteristics and outcome of ILD-onset rheumatoid arthritis and ACPA-positive ILD: a longitudinal cohort of 282 cases. Clin Rev Allergy Immunol. (2021) 60:46–54. doi: 10.1007/s12016-020-08819-0
77.Rocha-Muñoz, AD, Ponce-Guarneros, M, Gamez-Nava, JI, Olivas-Flores, EM, Mejía, M, Juárez-Contreras, P, et al. Anti-cyclic Citrullinated peptide antibodies and severity of interstitial lung disease in women with rheumatoid arthritis. J Immunol Res. (2015) 2015:151626:1–10. doi: 10.1155/2015/151626
78.Zhu, J, Zhou, Y, Chen, X, and Li, J. A Metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum Anticitrullinated protein antibody positivity. J Rheumatol. (2014) 41:1282–9. doi: 10.3899/jrheum.131341
79.Vanderlugt, CJ, and Miller, SD. Epitope spreading. Curr Opin Immunol. (1996) 8:831–6. doi: 10.1016/S0952-7915(96)80012-4
80.Kongpachith, S, Lingampalli, N, Ju, CH, Blum, LK, Lu, DR, Elliott, SE, et al. Affinity maturation of the anti-Citrullinated protein antibody Paratope drives epitope spreading and polyreactivity in rheumatoid arthritis. Arthritis Rheumatol. (2019) 71:507–17. doi: 10.1002/art.40760
81.Giles, JT, Danoff, SK, Sokolove, J, Wagner, CA, Winchester, R, Pappas, DA, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis. (2014) 73:1487–94. doi: 10.1136/annrheumdis-2012-203160
82.Alunno, A, Bistoni, O, Pratesi, F, La Paglia, GMC, Puxeddu, I, Migliorini, P, et al. Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology (Oxford). (2018) 57:850–5. doi: 10.1093/rheumatology/kex520
83.Harlow, L, Rosas, IO, Gochuico, BR, Mikuls, TR, Dellaripa, PF, Oddis, CV, et al. Identification of citrullinated hsp 90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. (2013) 65:869–79. doi: 10.1002/art.37881
84.Darrah, E, Giles, JT, Davis, RL, Naik, P, Wang, H, Konig, MF, et al. Autoantibodies to peptidylarginine Deiminase 2 are associated with less severe disease in rheumatoid arthritis. Front Immunol. (2018) 9:2696. doi: 10.3389/fimmu.2018.02696
85.Giles, JT, Darrah, E, Danoff, S, Johnson, C, Andrade, F, Rosen, A, et al. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PLoS One. (2014) 9:e98794. doi: 10.1371/journal.pone.0098794
86.Castellanos-Moreira, R, Rodríguez-García, SC, Gomara, MJ, Ruiz-Esquide, V, Cuervo, A, Casafont-Solé, I, et al. Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: evidence of a new autoantibody linked to interstitial lung disease. Ann Rheum Dis. (2020) 79:587–94. doi: 10.1136/annrheumdis-2019-216709
87.England, BR, Duryee, MJ, Roul, P, Mahajan, TD, Singh, N, Poole, JA, et al. Malondialdehyde-acetaldehyde adducts and antibody responses in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. (2019) 71:1483–93. doi: 10.1002/art.40900
88.Furukawa, H, Oka, S, Shimada, K, Masuo, K, Nakajima, F, Funano, S, et al. Autoantibody profiles in collagen disease patients with interstitial lung disease (ILD): antibodies to major histocompatibility complex class I-related chain a (MICA) as markers of ILD. Biomark Insights. (2015) 10:63–73. doi: 10.4137/BMI.S28209
89.Matsushita, M, Tamura, N, Ogasawara, M, Tada, K, Yamaji, K, and Takasaki, Y. The association of anti-aminoacyl-transfer ribonucleic acid synthetase antibodies in patients with rheumatoid arthritis and interstitial lung disease. Arch Rheumatol. (2018) 33:26–32. doi: 10.5606/ArchRheumatol.2018.6401
90.Emad, Y, Ragab, Y, Hammam, N, El-Shaarawy, N, Ibrahim, O, Gamal, RM, et al. Autoantibodies to extractable nuclear antigens (ENAs) pattern in rheumatoid arthritis patients: relevance and clinical implications. Reumatol Clin [Internet]. (2021) 17:250–7. doi: 10.1016/j.reuma.2019.10.001
91.Kumar, RR, Jha, S, Dhooria, A, Naidu, GSRSNK, Minz, RW, Kumar, S, et al. Anti-Jo-1 syndrome often misdiagnosed as rheumatoid arthritis (for many years): a single-center experience. J Clin Rheumatol. (2021) 27:150–5. doi: 10.1097/RHU.0000000000001234
92.Shen, H, Xia, L, and Lu, J. Interleukin-4 in rheumatoid arthritis patients with interstitial lung disease: a pilot study. Indian J Med Res. (2013) 138:919–21.
93.Wang, X, Zhu, G, Ren, Q, Wu, J, Gu, B, Su, D, et al. Increased interleukin-11 associated with disease activity and development of interstitial lung disease in patients with rheumatoid arthritis. Clin Exp Rheumatol. (2022) 40:135–41. doi: 10.55563/clinexprheumatol/mccyj0
94.Xiangyang, Z, Lutian, Y, Lin, Z, Liping, X, Hui, S, and Jing, L. Increased levels of interleukin-33 associated with bone erosion and interstitial lung diseases in patients with rheumatoid arthritis. Cytokine. (2012) 58:6–9. doi: 10.1016/j.cyto.2011.12.010
95.Hussein, MS, El-Barbary, AM, Nada, DW, Gaber, RA, Elkolaly, RM, and Aboelhawa, MA. Identification of serum interleukin-13 and interleukin-13 receptor subunit expressions: rheumatoid arthritis–associated interstitial lung disease. Int J Rheum Dis. (2021) 24:591–8. doi: 10.1111/1756-185X.14084
96.Matsuo, T, Hashimoto, M, Ito, I, Kubo, T, Uozumi, R, Furu, M, et al. Interleukin-18 is associated with the presence of interstitial lung disease in rheumatoid arthritis: a cross-sectional study. Scand J Rheumatol. (2019) 48:87–94. doi: 10.1080/03009742.2018.1477989
97.Billi, PM, Castellví, I, Martinez, LM, Aparicio, F, Franquet, T, Vidal, OS, et al. Diagnostic value of serum KL-6 in interstitial lung disease: preliminary results from an European cohort. Eur Respir J. (2018) 52:4724–32. doi: 10.21037/jtd.2018.07.54
98.Kim, HC, Choi, KH, Jacob, J, and Song, JW. Prognostic role of blood KL-6 in rheumatoid arthritis–associated interstitial lung disease. PLoS One. (2020) 15:e0229997. doi: 10.1371/journal.pone.0229997
99.Tanaka, N, Nishimura, K, Waki, D, Kadoba, K, Murabe, H, and Yokota, T. Annual variation rate of KL-6 for predicting acute exacerbation in patients with rheumatoid arthritis-associated interstitial lung disease. Mod Rheumatol. (2021) 31:1100–6. doi: 10.1080/14397595.2021.1879346
100.Fotoh, DS, Helal, A, Rizk, MS, and Esaily, HA. Serum Krebs von den Lungen-6 and lung ultrasound B lines as potential diagnostic and prognostic factors for rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. (2021) 40:2689–97. doi: 10.1007/s10067-021-05585-y
101.Sutton, B, Corper, A, Bonagura, V, and Taussig, M. The structure and origin of rheumatoid factors. Immunol Today. (2000) 21:177–83. doi: 10.1016/S0167-5699(00)01589-9
102.Shmerling, RH, and Delbanco, TL. The rheumatoid factor: an analysis of clinical utility. Am J Med. (1991) 91:528–34. doi: 10.1016/0002-9343(91)90190-9
103.Klein, F, and Janssens, MB. Standardisation of serological tests for rheumatoid factor measurement. Ann Rheum Dis. (1987) 46:674–80. doi: 10.1136/ard.46.9.674
104.Ingegnoli, F, Castelli, R, and Gualtierotti, R. Rheumatoid factors: clinical applications. Dis Markers. (2013) 35:727–34. doi: 10.1155/2013/726598
105.Sieghart, D, Platzer, A, Studenic, P, Alasti, F, Grundhuber, M, Swiniarski, S, et al. Determination of autoantibody Isotypes increases the sensitivity of Serodiagnostics in rheumatoid arthritis. Front Immunol. (2018) 9:876. doi: 10.3389/fimmu.2018.00876
106.Liu, Y, Liu, C, Li, L, Zhang, F, Li, Y, and Zhang, S. High levels of antibodies to citrullinated α-enolase peptide-1 (CEP-1) identify erosions and interstitial lung disease (ILD) in a Chinese rheumatoid arthritis cohort. Clin Immunol. (2019) 200:10–5. doi: 10.1016/j.clim.2019.01.001
107.Harlow, L, Gochuico, BR, Rosas, IO, Doyle, TJ, Osorio, JC, Travers, TS, et al. Anti-citrullinated heat shock protein 90 antibodies identified in bronchoalveolar lavage fluid are a marker of lung-specific immune responses. Clin Immunol. (2014) 155:60–70. doi: 10.1016/j.clim.2014.08.004
108.Chen, J, Song, S, Liu, Y, Liu, D, Lin, Y, Ge, S, et al. Autoreactive T cells to citrullinated HSP90 are associated with interstitial lung disease in rheumatoid arthritis. Int J Rheum Dis. (2018) 21:1398–405. doi: 10.1111/1756-185X.13316
109.Curran, AM, Naik, P, Giles, JT, and Darrah, E. PAD enzymes in rheumatoid arthritis: pathogenic effectors and autoimmune targets. Nat Rev Rheumatol. (2020) 16:301–15. doi: 10.1038/s41584-020-0409-1
110.Montgomery, AB, Kopec, J, Shrestha, L, Thezenas, ML, Burgess-Brown, NA, Fischer, R, et al. Crystal structure of Porphyromonas gingivalis peptidylarginine deiminase: implications for autoimmunity in rheumatoid arthritis. Ann Rheum Dis. (2016) 75:1255–61. doi: 10.1136/annrheumdis-2015-207656
111.Kolarz, B, Ciesla, M, Rosenthal, AK, Dryglewska, M, and Majdan, M. The value of anti-car P and anti-PAD4 as markers of rheumatoid arthritis in ACPA/RF negative rheumatoid arthritis patients. Ther Adv Musculoskelet Dis. (2021) 13:1759720X2198986. doi: 10.1177/1759720X21989868
112.Palterer, B, Vitiello, G, Del Carria, M, D’Onofrio, B, Martinez-Prat, L, Mahler, M, et al. Anti-protein arginine deiminase antibodies are distinctly associated with joint and lung involvement in rheumatoid arthritis. Rheumatology (Oxford). (2022):keac 667. doi: 10.1093/rheumatology/keac667
113.Marco, JL, and Collins, BF. Clinical manifestations and treatment of antisynthetase syndrome. Best Pract Res Clin Rheumatol. (2020) 34:101503. doi: 10.1016/j.berh.2020.101503
114.Waseda, Y, Johkoh, T, Egashira, R, Sumikawa, H, Saeki, K, Watanabe, S, et al. Antisynthetase syndrome: pulmonary computed tomography findings of adult patients with antibodies to aminoacyl-tRNA synthetases. Eur J Radiol. (2016) 85:1421–6. doi: 10.1016/j.ejrad.2016.05.012
115.Tomioka, H, Kaneko, M, Kogata, Y, Katsuyama, E, Ishikawa, S, and Fujii, T. Case of interstitial lung disease with anti-EJ and anti-CCP antibodies preceding rheumatoid arthritis. Respir Investig. (2012) 50:66–9. doi: 10.1016/j.resinv.2012.04.003
116.Wang, Z, Wang, W, Xiang, T, Gong, B, and Xie, J. Serum uric acid as a diagnostic biomarker for rheumatoid arthritis-associated interstitial lung disease. Inflammation. (2022) 45:1800–14. doi: 10.1007/s10753-022-01661-w
117.Guillén-Del Castillo, A, Callejas-Moraga, EL, García, G, Rodríguez-Palomares, JF, Román, A, Berastegui, C, et al. High sensitivity and negative predictive value of the DETECT algorithm for an early diagnosis of pulmonary arterial hypertension in systemic sclerosis: application in a single center. Arthritis Res Ther. (2017) 19:135. doi: 10.1186/s13075-017-1327-8
118.Saku, A, Fujisawa, T, Nishimoto, K, Yoshimura, K, Hozumi, H, Karayama, M, et al. Prognostic significance of peripheral blood monocyte and neutrophil counts in rheumatoid arthritis-associated interstitial lung disease. Respir Med. (2021) 182:106420. doi: 10.1016/j.rmed.2021.106420
119.Vlaykov, AN, Tacheva, TT, Vlaykova, TI, and Stoyanov, VK. Serum and local IL-4, IL-5, IL-13 and immunoglobulin E in allergic rhinitis. Postepy Dermatol Alergol. (2020) 37:719–24. doi: 10.5114/ada.2020.100483
120.Zhang, J, Wang, D, Wang, L, Wang, S, Roden, AC, Zhao, H, et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. (2019) 316:L487–97. doi: 10.1152/ajplung.00301.2018
121.Zhang, C, Wang, S, Lau, J, Roden, AC, Matteson, EL, Sun, J, et al. IL-23 amplifies the epithelial-mesenchymal transition of mechanically conditioned alveolar epithelial cells in rheumatoid arthritis-associated interstitial lung disease through mTOR/S6 signaling. Am J Physiol Lung Cell Mol Physiol. (2021) 321:L1006–22. doi: 10.1152/ajplung.00292.2021
122.Blanco, FJ, Möricke, R, Dokoupilova, E, Codding, C, Neal, J, Andersson, M, et al. Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. (2017) 69:1144–53. doi: 10.1002/art.40070
123.Smolen, JS, Agarwal, SK, Ilivanova, E, Xu, XL, Miao, Y, Zhuang, Y, et al. A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann Rheum Dis. (2017) 76:831–9. doi: 10.1136/annrheumdis-2016-209831
124.Zheng, M, Lou, A, Zhang, H, Zhu, S, Yang, M, and Lai, W. Serum KL-6, CA19-9, CA125 and CEA are diagnostic biomarkers for rheumatoid arthritis-associated interstitial lung disease in the Chinese population. Rheumatol Ther. (2021) 8:517–27. doi: 10.1007/s40744-021-00288-x
125.Liang, L, Chen, J, Di, C, Zhan, M, Bao, H, Xia, C, et al. Serum human epididymis protein 4 as a novel biomarker in identifying patients with interstitial lung disease in rheumatoid arthritis. Front Med (Lausanne). (2021) 8:755268. doi: 10.3389/fmed.2021.755268
126.Kruit, A, Gerritsen, WBM, Pot, N, Grutters, JC, van den Bosch, JMM, and Ruven, HJT. CA 15-3 as an alternative marker for KL-6 in fibrotic lung diseases. Sarcoidosis Vasc Diffuse Lung Dis. (2010) 27:138–46.
127.Furukawa, H, Oka, S, Higuchi, T, Shimada, K, Hashimoto, A, Matsui, T, et al. Biomarkers for interstitial lung disease and acute-onset diffuse interstitial lung disease in rheumatoid arthritis. Ther Adv Musculoskelet Dis. (2021) 13:1759720X2110225. doi: 10.1177/1759720X211022506
128.Xue, J, Wang, YJ, Xia, HC, Liang, XY, Cui, JD, Yu, M, et al. Circulating Dickkof-1 as a potential biomarker associated with the prognosis of patients with rheumatoid arthritis-associated interstitial lung disease. Chin Med J. (2021) 134:1119–21. doi: 10.1097/CM9.0000000000001267
129.Xu, L, Jiang, L, Nie, L, Zhang, S, Liu, L, Du, Y, et al. Soluble programmed death molecule 1 (sPD-1) as a predictor of interstitial lung disease in rheumatoid arthritis. BMC Immunol. (2021) 22:69. doi: 10.1186/s12865-021-00460-6
130.Pulito-Cueto, V, Remuzgo-Martínez, S, Genre, F, Mora-Cuesta, VM, Iturbe-Fernández, D, Fernández-Rozas, S, et al. Endothelial progenitor cells as a potential biomarker in interstitial lung disease associated with rheumatoid arthritis. J Clin Med. (2020) 9:E 4098. doi: 10.3390/jcm9124098
131.Kass, DJ, Nouraie, M, Glassberg, MK, Ramreddy, N, Fernandez, K, Harlow, L, et al. Comparative profiling of serum protein biomarkers in rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis. Arthritis Rheumatol. (2020) 72:409–19. doi: 10.1002/art.41123
132.Zhou, W, Zheng, J, Yuan, M, Yuan, L, Jia, X, and Liu, H. Differentially expressed lnc RNAs in peripheral blood mononuclear cells from middle-aged female patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. (2020) 39:2281–9. doi: 10.1007/s10067-020-04977-w
133.Oka, S, Furukawa, H, Shimada, K, Hashimoto, A, Komiya, A, Fukui, N, et al. Plasma mi RNA expression profiles in rheumatoid arthritis associated interstitial lung disease. BMC Musculoskelet Disord. (2017) 18:21. doi: 10.1186/s12891-017-1389-4
134.Furukawa, H, Oka, S, Shimada, K, Okamoto, A, Hashimoto, A, Komiya, A, et al. Serum Metabolomic profiling in rheumatoid arthritis patients with interstitial lung disease: a case-control study. Front Med (Lausanne). (2020) 7:599794. doi: 10.3389/fmed.2020.599794
135.Dawson, JK. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. (2001) 56:622–7. doi: 10.1136/thx.56.8.622
136.Paulin, F, Babini, A, Mamani, M, Mercado, J, and Caro, F. Practical approach to the evaluation and Management of Rheumatoid Arthritis-Interstitial Lung Disease Based on its proven and hypothetical mechanisms. Rev Investig Clin. (2017) 69:235–42. doi: 10.24875/RIC.17002162
137.Yunt, ZX, and Solomon, JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin N Am. (2015) 41:225–36. doi: 10.1016/j.rdc.2014.12.004
138.Mori, S, Cho, I, Koga, Y, and Sugimoto, M. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol. (2008) 35:1513–21.
139.Lucchino, B, Di Paolo, M, Gioia, C, Vomero, M, Diacinti, D, Mollica, C, et al. Identification of subclinical lung involvement in ACPA-positive subjects through functional assessment and serum biomarkers. Int J Mol Sci. (2020) 21:E 5162. doi: 10.3390/ijms21145162
140.Gabbay, E, Tarala, R, Will, R, Carroll, G, Adler, B, Cameron, D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. (1997) 156:528–35. doi: 10.1164/ajrccm.156.2.9609016
141.Zrour, SH, Touzi, M, Bejia, I, Golli, M, Rouatbi, N, Sakly, N, et al. Correlations between high-resolution computed tomography of the chest and clinical function in patients with rheumatoid arthritis. Prospective study in 75 patients. Joint Bone Spine. (2005) 72:41–7. doi: 10.1016/j.jbspin.2004.02.001
142.Kawano-Dourado, L, Doyle, TJ, Bonfiglioli, K, Sawamura, MVY, Nakagawa, RH, Arimura, FE, et al. Baseline characteristics and progression of a Spectrum of interstitial lung abnormalities and disease in rheumatoid arthritis. Chest. (2020) 158:1546–54. doi: 10.1016/j.chest.2020.04.061
143.Gochuico, BR, Avila, NA, Chow, CK, Novero, LJ, Wu, HP, Ren, P, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. (2008) 168:159–66. doi: 10.1001/archinternmed.2007.59
144.Solomon, JJ, Ryu, JH, Tazelaar, HD, Myers, JL, Tuder, R, Cool, CD, et al. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir Med. (2013) 107:1247–52. doi: 10.1016/j.rmed.2013.05.002
145.Ascherman, DP. Interstitial lung disease in rheumatoid arthritis. Curr Rheumatol Rep. (2010) 12:363–9. doi: 10.1007/s11926-010-0116-z
146.Yamakawa, H, Ogura, T, Sato, S, Nishizawa, T, Kawabe, R, Oba, T, et al. The potential utility of anterior upper lobe honeycomb-like lesion in interstitial lung disease associated with connective tissue disease. Respir Med. (2020) 172:106125. doi: 10.1016/j.rmed.2020.106125
147.Palmucci, S, Galioto, F, Fazio, G, Ferlito, A, Cancemi, G, Di Mari, A, et al. Clinical and radiological features of lung disorders related to connective-tissue diseases: a pictorial essay. Insights Imaging. (2022) 13:108. doi: 10.1186/s13244-022-01243-2
148.Kelly, CA, Saravanan, V, Nisar, M, Arthanari, S, Woodhead, FA, Price-Forbes, AN, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics--a large multicentre UK study. Rheumatology (Oxford). (2014) 53:1676–82. doi: 10.1093/rheumatology/keu165
149.Nieto, MA, Rodriguez-Nieto, MJ, Sanchez-Pernaute, O, Romero-Bueno, F, Leon, L, Vadillo, C, et al. Mortality rate in rheumatoid arthritis-related interstitial lung disease: the role of radiographic patterns. BMC Pulm Med. (2021) 21:205. doi: 10.1186/s12890-021-01569-5
150.Kim, EJ, Collard, HR, and King, TE. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. (2009) 136:1397–405. doi: 10.1378/chest.09-0444
151.Qiu, M, Chen, Y, and Ye, Q. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Clin Respir J. (2018) 12:1084–92. doi: 10.1111/crj.12631
152.Yoshinouchi, T, Ohtsuki, Y, Fujita, J, Yamadori, I, Bandoh, S, Ishida, T, et al. Nonspecific interstitial pneumonia pattern as pulmonary involvement of rheumatoid arthritis. Rheumatol Int. (2005) 26:121–5. doi: 10.1007/s00296-004-0527-0
153.Mohning, MP, Amigues, I, Demoruelle, MK, Fernández Pérez, ER, Huie, TJ, Keith, RK, et al. Duration of rheumatoid arthritis and the risk of developing interstitial lung disease. ERJ Open Res. (2021) 7:00633–2020. doi: 10.1183/23120541.00633-2020
154.Duarte, AC, Porter, JC, and Leandro, MJ. The lung in a cohort of rheumatoid arthritis patients-an overview of different types of involvement and treatment. Rheumatology (Oxford). (2019) 58:2031–8. doi: 10.1093/rheumatology/kez177
155.Kadura, S, and Raghu, G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev. (2021) 30:210011. doi: 10.1183/16000617.0011-2021
156.Mena-Vázquez, N, Rojas-Gimenez, M, Romero-Barco, CM, Manrique-Arija, S, Hidalgo Conde, A, Díez, A, et al. Characteristics and predictors of progression interstitial lung disease in rheumatoid arthritis compared with other autoimmune disease: a retrospective cohort study. Diagnostics (Basel). (2021) 11:1794. doi: 10.3390/diagnostics11101794
157.Chen, N, Diao, CY, Gao, J, and Zhao, DB. Risk factors for the progression of rheumatoid arthritis-related interstitial lung disease: clinical features, biomarkers, and treatment options. Semin Arthritis Rheum. (2022) 55:152004. doi: 10.1016/j.semarthrit.2022.152004
158.Liu, L, Fang, C, Sun, B, Bao, R, and Zhang, H. Predictors of progression in rheumatoid arthritis-associated interstitial lung disease: a single-center retrospective study from China. Int J Rheum Dis. (2022) 25:795–802. doi: 10.1111/1756-185X.14351
159.Zamora-Legoff, JA, Krause, ML, Crowson, CS, Ryu, JH, and Matteson, EL. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. (2017) 69:542–9. doi: 10.1002/art.39971
160.Kim, EJ, Elicker, BM, Maldonado, F, Webb, WR, Ryu, JH, Van Uden, JH, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. (2010) 35:1322–8. doi: 10.1183/09031936.00092309
161.Molina-Molina, M, Castellví, I, Valenzuela, C, Ramirez, J, Rodríguez Portal, JA, Franquet, T, et al. Management of progressive pulmonary fibrosis associated with connective tissue disease. Expert Rev Respir Med. (2022) 16:765–74. doi: 10.1080/17476348.2022.2107508
162.Cereser, L, Passarotti, E, De Pellegrin, A, Patruno, V, Poi, ED, Marchesini, F, et al. Chest high-resolution computed tomography in patients with connective tissue disease: pulmonary conditions beyond “the usual suspects.”. Curr Probl Diagn Radiol. (2022) 51:759–67. doi: 10.1067/j.cpradiol.2021.07.007
163.Hansell, DM, Goldin, JG, King, TE, Lynch, DA, Richeldi, L, and Wells, AU. CT staging and monitoring of fibrotic interstitial lung diseases in clinical practice and treatment trials: a position paper from the Fleischner society. Lancet Respir Med. (2015) 3:483–96. doi: 10.1016/S2213-2600(15)00096-X
164.Chen, A, Karwoski, RA, Gierada, DS, Bartholmai, BJ, and Koo, CW. Quantitative CT analysis of diffuse lung disease. Radiographics. (2020) 40:28–43. doi: 10.1148/rg.2020190099
165.Marten, K, Dicken, V, Kneitz, C, Hoehmann, M, Kenn, W, Hahn, D, et al. Computer-assisted quantification of interstitial lung disease associated with rheumatoid arthritis: preliminary technical validation. Eur J Radiol. (2009) 72:278–83. doi: 10.1016/j.ejrad.2008.07.008
166.Oh, JH, Kim, GHJ, Cross, G, Barnett, J, Jacob, J, Hong, S, et al. Automated quantification system predicts survival in rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford). (2022) 61:4702–10. doi: 10.1093/rheumatology/keac184
167.Jacob, J, Hirani, N, van Moorsel, CHM, Rajagopalan, S, Murchison, JT, van Es, HW, et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J. (2019) 53:1800869. doi: 10.1183/13993003.00869-2018
168.Laursen, CB, Clive, A, Hallifax, R, Pietersen, PI, Asciak, R, Davidsen, JR, et al. European Respiratory Society statement on thoracic ultrasound. Eur Respir J. (2021) 57:2001519. doi: 10.1183/13993003.01519-2020
169.Volpicelli, G, Elbarbary, M, Blaivas, M, Lichtenstein, DA, Mathis, G, Kirkpatrick, AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. (2012) 38:577–91. doi: 10.1007/s00134-012-2513-4
170.Gargani, L, and Volpicelli, G. How I do it: lung ultrasound. Cardiovasc Ultrasound. (2014) 12:1–10. doi: 10.1186/1476-7120-12-25
171.Soldati, G, Smargiassi, A, Demi, L, and Inchingolo, R. Artifactual lung ultrasonography: it is a matter of traps, order, and disorder. Appl Sci. (2020) 10:1570. doi: 10.3390/app10051570
172.Mena-Vázquez, N, Jimenez-Núñez, FG, Godoy-Navarrete, FJ, Manrique-Arija, S, Aguilar-Hurtado, MC, Romero-Barco, CM, et al. Utility of pulmonary ultrasound to identify interstitial lung disease in patients with rheumatoid arthritis. Clin Rheumatol. (2021) 40:2377–85. doi: 10.1007/s10067-021-05655-1
173.Cogliati, C, Antivalle, M, Torzillo, D, Birocchi, S, Norsa, A, Bianco, R, et al. Standard and pocket-size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology (Oxford). (2014) 53:1497–503. doi: 10.1093/rheumatology/keu033
174.Moazedi-Fuerst, FC, Kielhauser, S, Brickmann, K, Tripolt, N, Meilinger, M, Lufti, A, et al. Sonographic assessment of interstitial lung disease in patients with rheumatoid arthritis, systemic sclerosis and systemic lupus erythematosus. Clin Exp Rheumatol. (2015) 33:S87–91.
175.Gutierrez, M, Ruta, S, Clavijo-Cornejo, D, Fuentes-Moreno, G, Reyes-Long, S, and Bertolazzi, C. The emerging role of ultrasound in detecting interstitial lung disease in patients with rheumatoid arthritis. Joint Bone Spine. (2022) 89:105407. doi: 10.1016/j.jbspin.2022.105407
176.Esposito, AJ, Sparks, JA, Gill, RR, Hatabu, H, Schmidlin, EJ, Hota, PV, et al. Screening for preclinical parenchymal lung disease in rheumatoid arthritis. Rheumatology (Oxford). (2022) 61:3234–45. doi: 10.1093/rheumatology/keab891
177.Xie, HQ, Zhang, WW, Sun, DS, Chen, XM, Yuan, SF, Gong, ZH, et al. A simplified lung ultrasound for the diagnosis of interstitial lung disease in connective tissue disease: a meta-analysis. Arthritis Res Ther. (2019) 21:93. doi: 10.1186/s13075-019-1888-9
178.Wang, Y, Chen, S, Lin, Z, Du, G, Lin, J, Lin, Q, et al. Imaging and serum biomarkers in connective tissue disease-associated interstitial lung diseases: correlation between lung ultrasound B-lines and KL-6 levels. Ann Rheum Dis. (2019) 78:573–5. doi: 10.1136/annrheumdis-2018-214098
179.Laria, A, Lurati, A, and Scarpellini, M. Ultrasound in rheumatologic interstitial lung disease: a case report of nonspecific interstitial pneumonia in rheumatoid arthritis. Case Rep Rheumatol. (2015) 2015:107275:1–4. doi: 10.1155/2015/107275
180.Topcu, A, Mursaloglu, HH, Yalcinkaya, Y, Karakurt, S, Yagiz, B, Alaca, Z, et al. Evaluation of rheumatoid arthritis and connective tissue disease-related interstitial lung disease with pulmonary physiologic test, HRCT, and patient-based measures of dyspnea and functional disability. Clin Rheumatol. (2021) 40:3797–805. doi: 10.1007/s10067-021-05693-9
181.Wells, A, Devaraj, A, Renzoni, EA, and Denton, CP. Multidisciplinary evaluation in patients with lung disease associated with connective tissue disease. Semin Respir Crit Care Med. (2019) 40:184–93. doi: 10.1055/s-0039-1684020
182.Nannini, C, Medina-Velasquez, YF, Achenbach, SJ, Crowson, CS, Ryu, JH, Vassallo, R, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken). (2013) 65:1243–50. doi: 10.1002/acr.21986
183.Dawson, JK, Fewins, HE, Desmond, J, Lynch, MP, and Graham, DR. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann Rheum Dis. (2002) 61:517–21. doi: 10.1136/ard.61.6.517
184.Chang, SH, Lee, JS, Ha, YJ, Kim, MU, Park, CH, Lee, JS, et al. Lung function trajectory of rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford). (2023):kead 027. doi: 10.1093/rheumatology/kead027
185.Jj, S, Jj, S, M, K, M, P, K, A, Am, HV, et al. The attitudes and practices of physicians caring for patients with rheumatoid arthritis-associated interstitial lung disease: an international survey. Rheumatology. (2022) 61:1459–67. doi: 10.1093/rheumatology/keab552
186.Smolen, JS, Landewé, RBM, Bergstra, SA, Kerschbaumer, A, Sepriano, A, Aletaha, D, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheumatic Diseases [Internet]. (2022) 82:3–18. doi: 10.1136/ard-2022-223356
187.Fraenkel, L, Bathon, JM, England, BR, St Clair, EW, Arayssi, T, Carandang, K, et al. 2021 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. (2021) 73:1108–23. doi: 10.1002/art.41752
188.Yu, KH, Chen, HH, Cheng, TT, Jan, YJ, Weng, MY, Lin, YJ, et al. Consensus recommendations on managing the selected comorbidities including cardiovascular disease, osteoporosis, and interstitial lung disease in rheumatoid arthritis. Medicine (Baltimore). (2022) 101:e28501. doi: 10.1097/MD.0000000000028501
189.Narváez, J, Díaz Del Campo Fontecha, P, Brito García, N, Bonilla, G, Aburto, M, Castellví, I, et al. SER-SEPAR recommendations for the management of rheumatoid arthritis-related interstitial lung disease. Part 2: treatment. Reumatol Clin (Engl Ed). (2022) 18:501–12. doi: 10.1016/j.reuma.2022.03.005
190.Rodríguez Portal, JA, Brito García, N, Díaz Del Campo Fontecha, P, Valenzuela, C, Ortiz, AM, Nieto, MA, et al. SER-SEPAR recommendations for the management of rheumatoid arthritis-related interstitial lung disease. Part 1: epidemiology, risk factors and prognosis. Reumatol Clin (Engl Ed). (2022) 18:443–52. doi: 10.1016/j.reuma.2022.02.009
191.Juge, PA, Lee, JS, Lau, J, Kawano-Dourado, L, Rojas Serrano, J, Sebastiani, M, et al. Methotrexate and rheumatoid arthritis associated interstitial lung disease. Eur Respir J. (2021) 57:2000337. doi: 10.1183/13993003.00337-2020
192.Song, JW, Lee, HK, Lee, CK, Chae, EJ, Jang, SJ, Colby, TV, et al. Clinical course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. (2013) 30:103–12.
193.Hallowell, RW, and Horton, MR. Interstitial lung disease in patients with rheumatoid arthritis: spontaneous and drug induced. Drugs. (2014) 74:443–50. doi: 10.1007/s40265-014-0190-z
194.Tashkin, DP, Elashoff, R, Clements, PJ, Goldin, J, Roth, MD, Furst, DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. (2006) 354:2655–66. doi: 10.1056/NEJMoa055120
195.Tashkin, DP, Roth, MD, Clements, PJ, Furst, DE, Khanna, D, Kleerup, EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. (2016) 4:708–19. doi: 10.1016/S2213-2600(16)30152-7
196.Idiopathic Pulmonary Fibrosis Clinical Research NetworkMartinez, FJ, de Andrade, JA, Anstrom, KJ, King, TE, and Raghu, G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. (2014) 370:2093–101. doi: 10.1056/NEJMoa1401739
197.Kelly, C, Palmer, E, Gordon, J, Woodhead, F, Nisar, M, Arthanari, S, et al. OP0037 pulsed cyclophosphamide in the treatment of rheumatoid arthritis-related interstitial lung disease (RA-ILD). Ann Rheum Dis. (2014) 73. doi: 10.1136/annrheumdis-2014-eular.2342
198.Fischer, A, Brown, KK, Du Bois, RM, Frankel, SK, Cosgrove, GP, Fernandez-Perez, ER, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. (2013) 40:640–6. doi: 10.3899/jrheum.121043
199.Iqbal, K, and Kelly, C. Treatment of rheumatoid arthritis-associated interstitial lung disease: a perspective review. Ther Adv Musculoskelet Dis. (2015) 7:247–67. doi: 10.1177/1759720X15612250
200.Matson, SM, Baqir, M, Moua, T, Marll, M, Kent, J, Iannazzo, NS, et al. Treatment outcomes for rheumatoid arthritis associated interstitial lung disease; a real-world, multisite study of the impact of immunosuppression on pulmonary function trajectory. Chest. (2022) S0012-3692:04205–2. doi: 10.1016/j.chest.2022.11.035
201.Narváez, J, Robles-Pérez, A, Molina-Molina, M, Vicens-Zygmunt, V, Luburich, P, Yañez, MA, et al. Real-world clinical effectiveness of rituximab rescue therapy in patients with progressive rheumatoid arthritis-related interstitial lung disease. Semin Arthritis Rheum. (2020) 50:902–10. doi: 10.1016/j.semarthrit.2020.08.008
202.Jani, M, Hirani, N, Matteson, EL, and Dixon, WG. The safety of biologic therapies in RA-associated interstitial lung disease. Nat Rev Rheumatol. (2014) 10:284–94. doi: 10.1038/nrrheum.2013.197
203.Mena-Vázquez, N, Godoy-Navarrete, FJ, Manrique-Arija, S, Aguilar-Hurtado, MC, Romero-Barco, CM, Ureña-Garnica, I, et al. Non-anti-TNF biologic agents are associated with slower worsening of interstitial lung disease secondary to rheumatoid arthritis. Clin Rheumatol. (2021) 40:133–42. doi: 10.1007/s10067-020-05227-9
204.Khanna, D, Lin, CJF, Furst, DE, Goldin, J, Kim, G, Kuwana, M, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. (2020) 8:963–74. doi: 10.1016/S2213-2600(20)30318-0
205.Manfredi, A, Cassone, G, Furini, F, Gremese, E, Venerito, V, Atzeni, F, et al. Tocilizumab therapy in rheumatoid arthritis with interstitial lung disease: a multicentre retrospective study. Intern Med J. (2020) 50:1085–90. doi: 10.1111/imj.14670
206.Vadillo, C, Nieto, MA, Romero-Bueno, F, Leon, L, Sanchez-Pernaute, O, Rodriguez-Nieto, MJ, et al. Efficacy of rituximab in slowing down progression of rheumatoid arthritis-related interstitial lung disease: data from the NEREA registry. Rheumatology (Oxford). (2020) 59:2099–108. doi: 10.1093/rheumatology/kez673
207.Maher, TM, Tudor, VA, Saunders, P, Gibbons, MA, Fletcher, SV, Denton, CP, et al. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the UK (RECITAL): a double-blind, double-dummy, randomised, controlled, phase 2b trial. Lancet Respir Med. (2022) 11:45–54. doi: 10.1016/S2213-2600(22)00359-9
208.Sendo, S, Saegusa, J, Yamada, H, Nishimura, K, and Morinobu, A. Tofacitinib facilitates the expansion of myeloid-derived suppressor cells and ameliorates interstitial lung disease in SKG mice. Arthritis Res Ther. (2019) 21:184. doi: 10.1186/s13075-019-1963-2
209.Wang, S, Liu, M, Li, X, Zhang, J, Wang, F, Zhang, C, et al. Canonical and noncanonical regulatory roles for JAK2 in the pathogenesis of rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis. FASEB J. (2022) 36:e22336. doi: 10.1096/fj.202101436R
210.d’Alessandro, M, Perillo, F, Metella Refini, R, Bergantini, L, Bellisai, F, Selvi, E, et al. Efficacy of baricitinib in treating rheumatoid arthritis: modulatory effects on fibrotic and inflammatory biomarkers in a real-life setting. Int Immunopharmacol. (2020) 86:106748. doi: 10.1016/j.intimp.2020.106748
211.Carrasco Cubero, C, Chamizo Carmona, E, and Vela, CP. Systematic review of the impact of drugs on diffuse interstitial lung disease associated with rheumatoid arthritis. Reumatol Clin (Engl Ed). (2020) 17:504–13. doi: 10.1016/j.reuma.2020.04.015
212.Kalyoncu, U, Bilgin, E, Erden, A, Satış, H, Tufan, A, Tekgöz, E, et al. Efficacy and safety of tofacitinib in rheumatoid arthritis-associated interstitial lung disease: TReasure real-life data. Clin Exp Rheumatol. (2022) 40:2071–7. doi: 10.55563/clinexprheumatol/9h6dtb
213.Mochizuki, T, Ikari, K, Yano, K, Sato, M, and Okazaki, K. Long-term deterioration of interstitial lung disease in patients with rheumatoid arthritis treated with abatacept. Mod Rheumatol. (2019) 29:413–7. doi: 10.1080/14397595.2018.1481566
214.Tardella, M, Di Carlo, M, Carotti, M, Giovagnoni, A, and Salaffi, F. Abatacept in rheumatoid arthritis-associated interstitial lung disease: short-term outcomes and predictors of progression. Clin Rheumatol. (2021) 40:4861–7. doi: 10.1007/s10067-021-05854-w
215.Fernández-Díaz, C, Loricera, J, Castañeda, S, López-Mejías, R, Ojeda-García, C, Olivé, A, et al. Abatacept in patients with rheumatoid arthritis and interstitial lung disease: a national multicenter study of 63 patients. Semin Arthritis Rheum. (2018) 48:22–7. doi: 10.1016/j.semarthrit.2017.12.012
216.Fernández-Díaz, C, Castañeda, S, Melero-González, RB, Ortiz-Sanjuán, F, Juan-Mas, A, Carrasco-Cubero, C, et al. Abatacept in interstitial lung disease associated with rheumatoid arthritis: national multicenter study of 263 patients. Rheumatology (Oxford). (2020) 59:3906–16. doi: 10.1093/rheumatology/keaa621
217.Xie, S, Li, S, Tian, J, and Li, F. Iguratimod as a new drug for rheumatoid arthritis: current landscape. Front Pharmacol. (2020) 11:73. doi: 10.3389/fphar.2020.00073
218.Shu, P, Shao, SQ, Cai, XN, Zhou, DM, Ma, H, Lu, L, et al. Iguratimod attenuates general disease activity and improves lung function in rheumatoid arthritis-associated interstitial lung disease patients. Eur Rev Med Pharmacol Sci. (2021) 25:4687–92. doi: 10.26355/eurrev_202107_26379
219.Flaherty, KR, Wells, AU, Cottin, V, Devaraj, A, Walsh, SLF, Inoue, Y, et al. Nintedanib in progressive Fibrosing interstitial lung diseases. N Engl J Med. (2019) 381:1718–27. doi: 10.1056/NEJMoa1908681
220.Matteson, EL, Kelly, C, Distler, JHW, Hoffmann-Vold, AM, Seibold, JR, Mittoo, S, et al. Nintedanib in patients with autoimmune disease-related progressive Fibrosing interstitial lung diseases: subgroup analysis of the INBUILD trial. Arthritis Rheumatol. (2022) 74:1039–47. doi: 10.1002/art.42075
221.Solomon, JJ, Danoff, SK, Woodhead, FA, Hurwitz, S, Maurer, R, Glaspole, I, et al. Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir Med. (2022) 161:A262–A2600. doi: 10.1016/j.chest.2021.12.293
222.Enomoto, N, Oyama, Y, Enomoto, Y, Yasui, H, Karayama, M, Kono, M, et al. Differences in clinical features of acute exacerbation between connective tissue disease-associated interstitial pneumonia and idiopathic pulmonary fibrosis. Chron Respir Dis. (2019) 16:147997231880947. doi: 10.1177/1479972318809476
223.Nakamura, K, Ohbe, H, Ikeda, K, Uda, K, Furuya, H, Furuta, S, et al. Intravenous cyclophosphamide in acute exacerbation of rheumatoid arthritis-related interstitial lung disease: a propensity-matched analysis using a nationwide inpatient database. Semin Arthritis Rheum. (2021) 51:977–82. doi: 10.1016/j.semarthrit.2021.07.008
224.Ota, M, Iwasaki, Y, Harada, H, Sasaki, O, Nagafuchi, Y, Nakachi, S, et al. Efficacy of intensive immunosuppression in exacerbated rheumatoid arthritis-associated interstitial lung disease. Mod Rheumatol. (2017) 27:22–8. doi: 10.3109/14397595.2016.1173816
225.Celli, BR, and Wedzicha, JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. (2019) 381:1257–66. doi: 10.1056/NEJMra1900500
226.Bluett, J, Jani, M, and Symmons, DPM. Practical Management of Respiratory Comorbidities in patients with rheumatoid arthritis. Rheumatol Ther. (2017) 4:309–32. doi: 10.1007/s40744-017-0071-5
227.Nagel, J, Jönsson, G, Nilsson, JÅ, Manuswin, C, Englund, M, Saxne, T, et al. Reduced risk of serious pneumococcal infections up to 10 years after a dose of pneumococcal conjugate vaccine in established arthritis. Vaccine. (2023) 41:504–10. doi: 10.1016/j.vaccine.2022.11.075
228.Naveen, R, Parodis, I, Joshi, M, Sen, P, Lindblom, J, Agarwal, V, et al. COVID-19 vaccination in autoimmune diseases (COVAD) study: vaccine safety and tolerance in rheumatoid arthritis. Rheumatology (Oxford). (2022):keac 624. doi: 10.1093/rheumatology/keac624
229.Landewé, RBM, Kroon, FPB, Alunno, A, Najm, A, Bijlsma, JW, Burmester, GRR, et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann Rheum Dis. (2022) 81:1628–39. doi: 10.1136/annrheumdis-2021-222006
230.King, CS, and Shlobin, OA. The trouble with group 3 pulmonary hypertension in interstitial lung disease: dilemmas in diagnosis and the conundrum of treatment. Chest. (2020) 158:1651–64. doi: 10.1016/j.chest.2020.04.046
231.Nathan, SD, and Hassoun, PM. Pulmonary hypertension due to lung disease and/or hypoxia. Clin Chest Med. (2013) 34:695–705. doi: 10.1016/j.ccm.2013.08.004
232.Andersen, CU, Mellemkjær, S, Hilberg, O, Nielsen-Kudsk, JE, Simonsen, U, and Bendstrup, E. Pulmonary hypertension in interstitial lung disease: prevalence, prognosis and 6 min walk test. Respir Med. (2012) 106:875–82. doi: 10.1016/j.rmed.2012.02.015
233.Dhont, S, Zwaenepoel, B, Vandecasteele, E, Brusselle, G, and De Pauw, M. Pulmonary hypertension in interstitial lung disease: an area of unmet clinical need. ERJ Open Res. (2022) 8:00272–2022. doi: 10.1183/23120541.00272-2022
234.Waxman, A, Restrepo-Jaramillo, R, Thenappan, T, Ravichandran, A, Engel, P, Bajwa, A, et al. Inhaled Treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. (2021) 384:325–34. doi: 10.1056/NEJMoa2008470
235.Amati, F, Spagnolo, P, Oldham, JM, Ryerson, CJ, Stainer, A, Gramegna, A, et al. Treatable traits in interstitial lung diseases: a call to action. Lancet Respir Med. (2023) 11:125–8. doi: 10.1016/S2213-2600(23)00002-4
236.Kozu, R, Shingai, K, Hanada, M, Oikawa, M, Nagura, H, Ito, H, et al. Respiratory impairment, limited activity, and pulmonary rehabilitation in patients with interstitial lung disease. Phys Ther Res. (2021) 24:9–16. doi: 10.1298/ptr.R0012
237.Kreuter, M, Bendstrup, E, Russell, AM, Bajwah, S, Lindell, K, Adir, Y, et al. Palliative care in interstitial lung disease: living well. Lancet Respir Med. (2017) 5:968–80. doi: 10.1016/S2213-2600(17)30383-1
238.Courtwright, AM, El-Chemaly, S, Dellaripa, PF, and Goldberg, HJ. Survival and outcomes after lung transplantation for non-scleroderma connective tissue-related interstitial lung disease. J Heart Lung Transplant. (2017) 36:763–9. doi: 10.1016/j.healun.2016.12.013
Keywords: progressive pulmonary fibrosis, biomarkers, immunology, precision medicine, rheumatoid arthritis, interstitial lung disease, clinical trials, lung ultrasonography
Citation: Stainer A, Tonutti A, De Santis M, Amati F, Ceribelli A, Bongiovanni G, Torrisi C, Iacopino A, Mangiameli G, Aliberti S and Selmi C (2023) Unmet needs and perspectives in rheumatoid arthritis-associated interstitial lung disease: A critical review. Front. Med. 10:1129939. doi: 10.3389/fmed.2023.1129939
Edited by:
Makon-Sébastien Njock, University of Liège, BelgiumReviewed by:
Paola Parronchi, University of Florence, ItalyChristian Ascoli, University of Illinois at Chicago, United States
Copyright © 2023 Stainer, Tonutti, De Santis, Amati, Ceribelli, Bongiovanni, Torrisi, Iacopino, Mangiameli, Aliberti and Selmi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria De Santis, maria.de_santis@hunimed.eu
†These authors share first authorship
 Anna Stainer1,2†
Anna Stainer1,2†  Antonio Tonutti
Antonio Tonutti Francesco Amati
Francesco Amati Angela Ceribelli
Angela Ceribelli Gabriele Bongiovanni
Gabriele Bongiovanni Giuseppe Mangiameli
Giuseppe Mangiameli Carlo Selmi
Carlo Selmi