Case report: Successful outcome of a young patient with rhabdomyolysis and shock caused by diquat poisoning
- 1Department of Intensive Care Unit, Guangzhou Twelfth People’s Hospital, Guangzhou, China
- 2Key Laboratory of Occupational Environment and Health, Guangzhou Twelfth People’s Hospital, Guangzhou, China
The widespread use of diquat as a substitute for paraquat has led to an increase in poisoning deaths. A successful case of diquat poisoning complicated with rhabdomyolysis and shock was lacking. A 13-year-old previously healthy girl ingested 40 ml of diquat solution in a suicide attempt. The concentration of diquat in serum was 436.2 ug/L at 10 h after poisoning. The clinical course was characterized by progressive multi-organ dysfunction, particularly rhabdomyolysis and shock. The main treatments included intensive hemoperfusion combined with continuous renal replacement therapy (CRRT), drainage, and activated carbon adsorption. Meanwhile, accurate dilatation under the model of pulse indicator continuous cardiac output (PICCO) was essential for the successful treatment of shock. The serum concentration of diquat declined to 20 ug/L after 96 h of treatments. The patient was discharged from the hospital after 3 weeks of treatment without obvious symptoms. So far, this was the first successful case of diquat poisoning complicated with rhabdomyolysis and shock, which would enrich the experience of diquat poisoning treatment.
Introduction
Pesticide poisoning is the leading cause of poisoning and accidental death in many developing countries. Diquat is a highly toxic bipyridine herbicide, and has led to remarkable increases in poisonings as a substitute for paraquat in recent years. The national poison data system of the United States revealed 2,128 cases of diquat poisoning between 1998 and 2013 (1). There was a clear correlation between the prognosis and intake, and the lethal dose for humans was 6–12 g (namely 56.10–112.20 ml diquat solution with 20% concentration), and there was no effective antidote (2). Acute diquat poisoning damaged the kidney, liver, and central nervous systems, and subsequent multiple-organ failure syndromes were the main cause of death (3). Diquat poisoning caused acute kidney injury (AKI) as much as 73.3%, which was higher than other types of pesticide poisoning (4–6). Rhabdomyolysis is rarely reported in diquat poisoning (7, 8), but it could largely aggravate kidney injury. Meanwhile, diquat poisoning with shock was extremely fatal (9), and no surviving cases had been reported so far (8, 10), indicating the need for more effective treatment options.
Hemodynamic monitoring could provide continuous and dynamic information about circulation, perfusion, and oxygenation in the tissues and organs, which could effectively prevent transfusion-associated circulatory overload (11). A previous study reported that invasive hemodynamic monitoring was used for the treatment of shock caused by organophosphorus poisoning (12). So far, the clinical experience of invasive hemodynamic monitoring in the treatment of shock caused by diquat poisoning is very lacking. Therefore, this work reported a severe case of diquat poisoning with rhabdomyolysis and shock, and highlighted the successful treatment using continuous renal replacement therapy (CRRT) and hemodynamic monitoring.
Clinical course
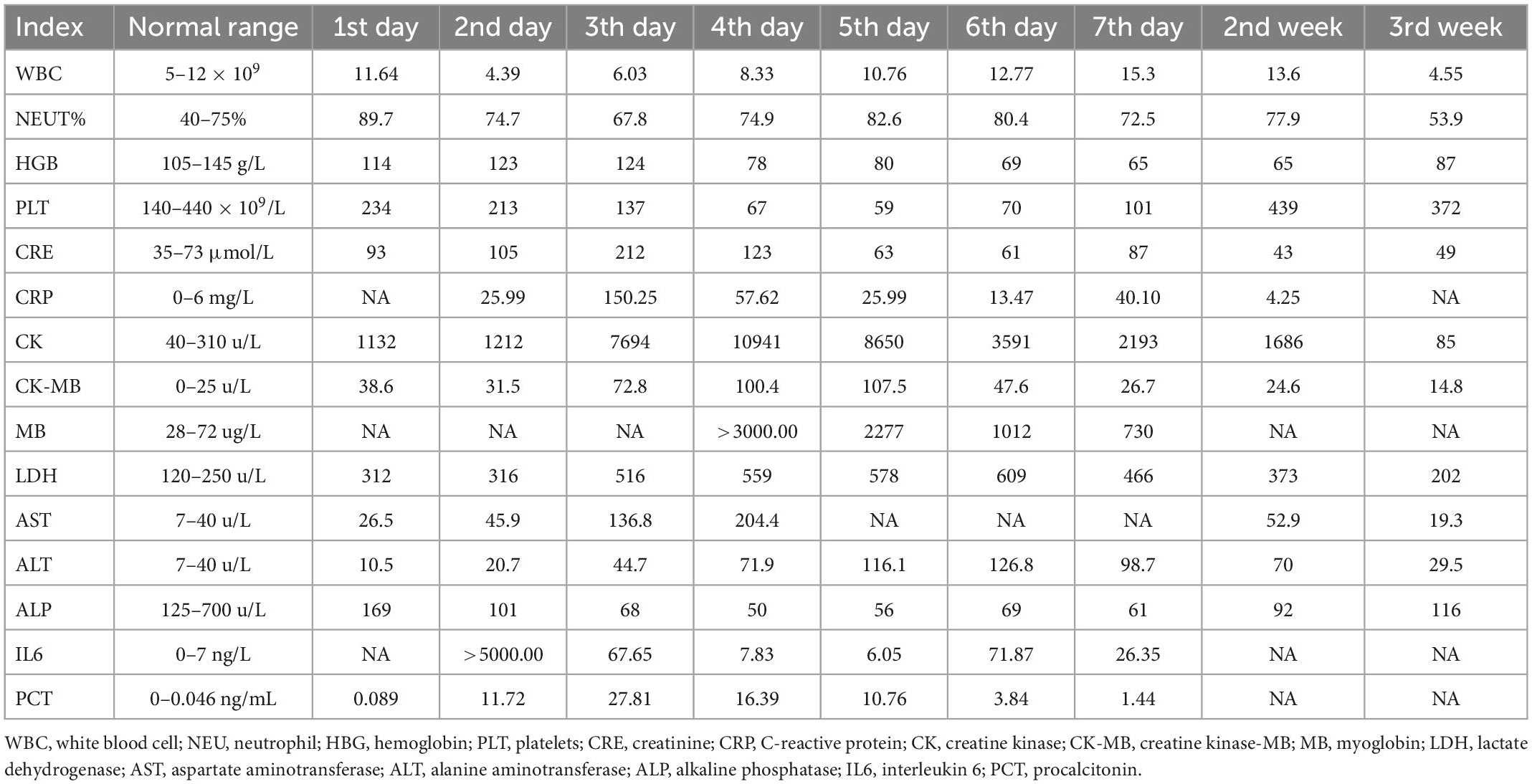
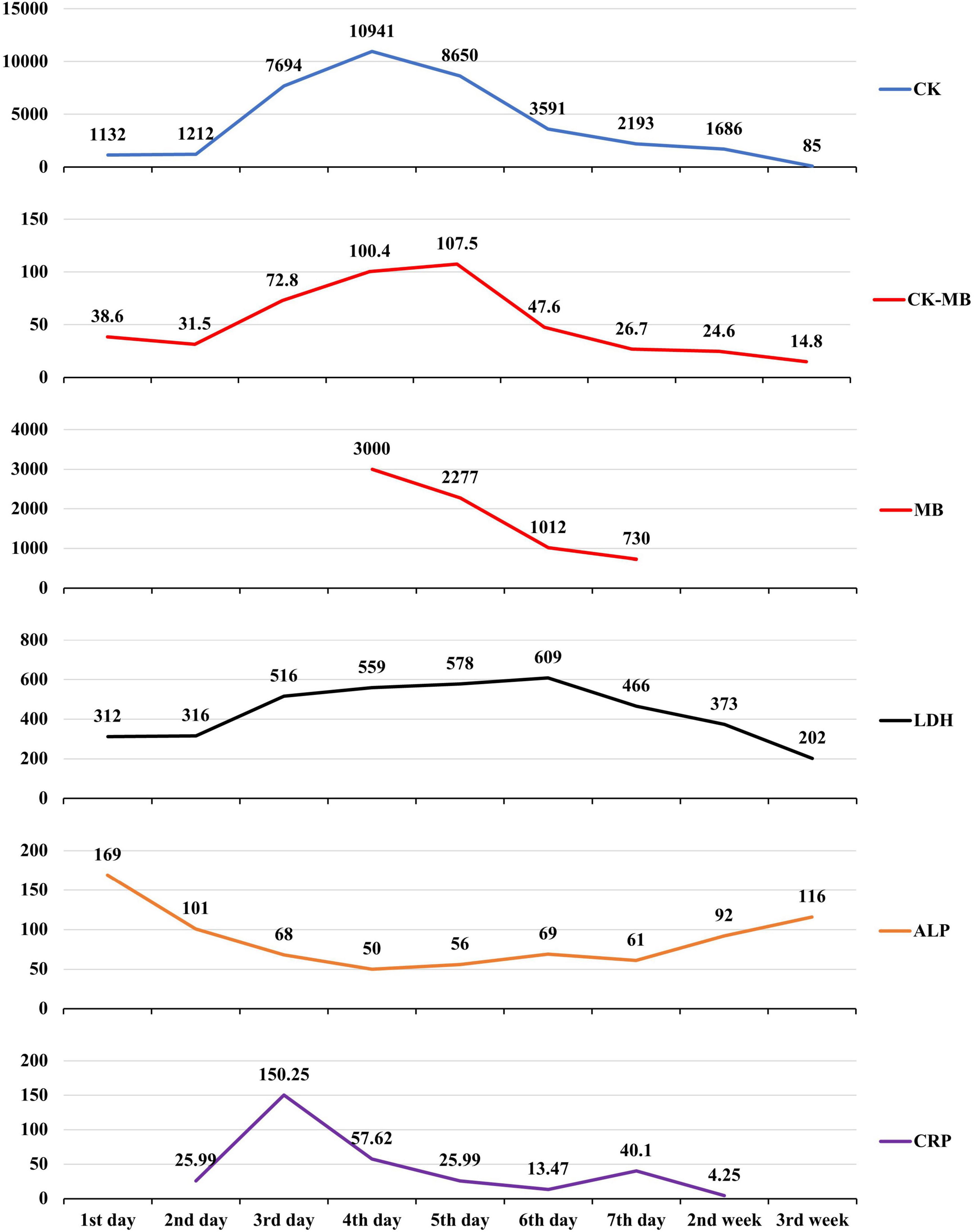
A 13-year-old previously healthy girl ingested 40 ml of diquat solution in a suicide attempt. The patient developed paroxysmal pain in the upper abdomen and a sore throat, accompanied by vomiting yellow-green stomach contents several times. After 10 h, she was transferred to the intensive care unit of our hospital for further treatment. The vital signs and physical examination showed no significant abnormalities, except slight tenderness in the upper abdomen (Figure 1A). The serum concentration of diquat was 436.2 ug/L, and urine fast screening of diquat was positive (Figure 1B). Many blood tests were abnormal, especially creatinine (93 μmol/L), creatine kinase (1132 u/L), creatine kinase MB isoenzyme (38.6 u/L), and lactate dehydrogenase (312 u/L). Immediately, she accepted the intensive hemoperfusion combined with continuous renal replacement therapy (CRRT) in the model of continuous venovenous hemodiafiltration (CVVHDF), activated carbon adsorption and Sivelestat (Shanghai Huilun Life Science & Technology Co., Ltd., Shanghai, China) for anti-inflammation, and other supportive treatments. In the afternoon, the patient developed dyspnea, irritability, and confusion, suggesting toxic encephalopathy, which was supported by the subsequent screen of computed tomography (CT) (Figure 1C). The patient was given nasal tracheal intubation with ventilator-assisted ventilation in the model of PSIMV (FiO2 45%, f 14 times/min, PC 16 cmH2O, PS 15 cmH2O, PEEP 3 cmH2O). On the 2nd day after admission, the patient developed an elevated body temperature, with a maximum body temperature of 40.1°C, accompanied by a heart rate of 145–179 beats/min. Blood pressure decreased progressively in the range of 55–68/28–35 mmHg, and urine volume also pronouncedly decreased. Procalcitonin 27.81 ng/ml. At the same time, hemodynamic detection using the model of pulse indicator continuous cardiac output (PICCO) showed CI 2.51 L/min/m2, SVRI 2465 dyn.s.m2/cm5, dPmax 1452 mmHg/s, GEDI 292 ml/m2, ITBI 364 ml/m2, ELWI 7 ml/kg, SVI 15 ml/m2, and GEF 23%. The above supported the diagnosis of hypovolemic shock, and anti-shock therapies, including dilatancy and fluid rehydration, were performed under PICCO monitoring immediately. Meanwhile, CRP 25.99 mg/L, IL-6 > 5000.0 ng/L, and PCT 11.72 ng/ml, indicating the diagnosis of systemic inflammatory response syndrome (SIRS). On the 3rd day after admission, the patient developed pain and swelling in both legs. The pronouncedly elevated levels of creatine kinase (the highest was 10941 u/L on the 4th day) and myoglobin (>3000.00 ug/L), which was diagnosed as rhabdomyolysis. After precise fluid rehydration and dilatation, the shock was corrected and urine volume recovered, and the myoenzyme spectrum decreased gradually (Table 1 and Figure 2). On the 5th day after admission, the serum concentration of diquat was cleared to less than 20 ug/L. On the 7th day after admission, the patient recovered well, including the spontaneous respiratory. Meanwhile, the indicators of auxiliary examination gradually stabilized. Three weeks after admission, the patient was discharged from the hospital without obvious discomfort.
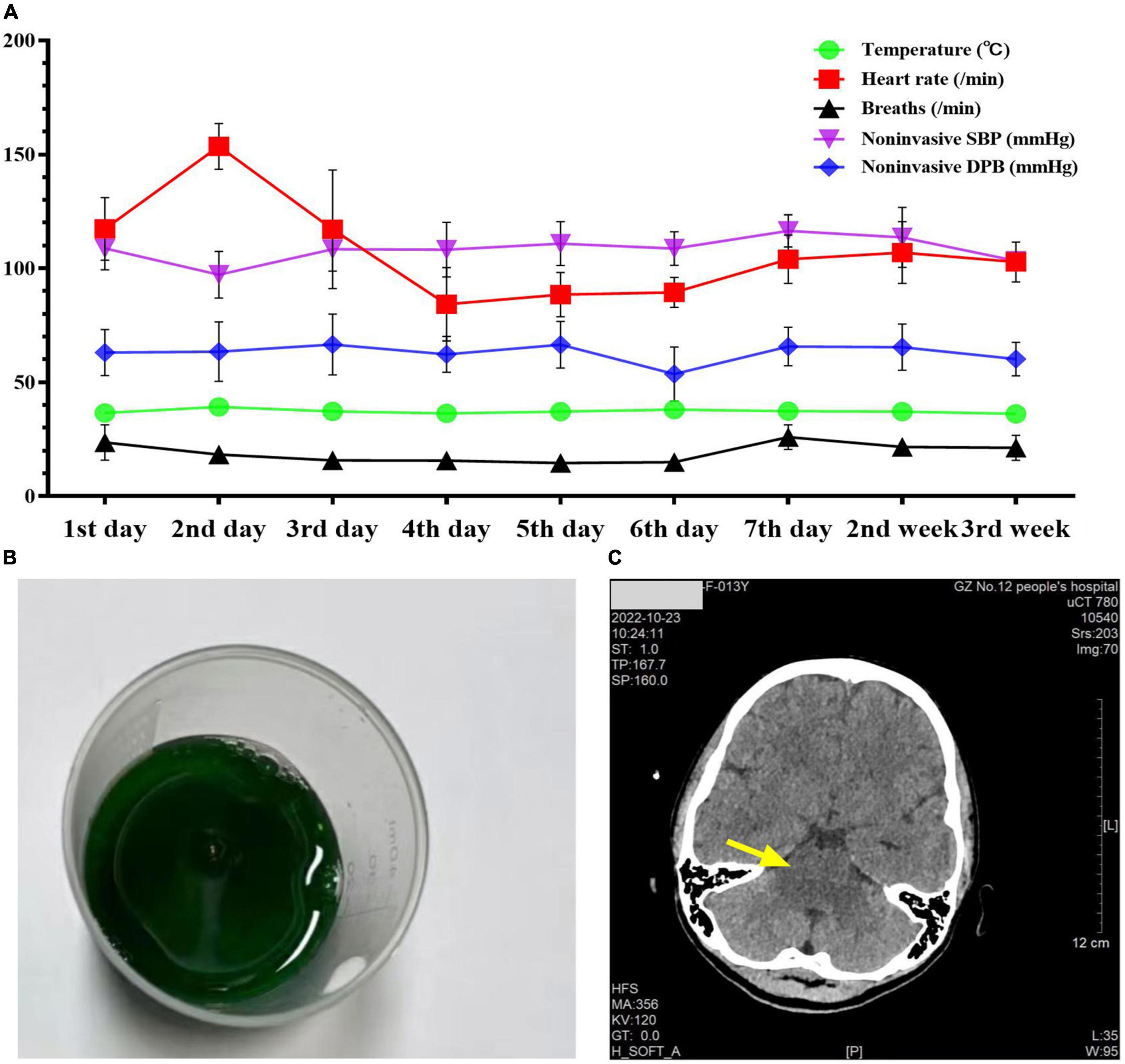
Figure 1. The patient’s vital signs, urine screening, and cranial CT examination during admission. Panel (A) was the changes in the patient’s vital signs during admission. Panel (B) was the positive urine fast screening of diquat (dark green). Panel (C) was the photograph of cranial CT examination on the 7th day after admission.
Discussion
Our institution is the Guangzhou regional center of poison treatment, and had treated dozens of diquat and paraquat poisonings in recent years. So far, this was the first successful outcome of diquat poisoning complicated with rhabdomyolysis and shock. Although the poison dose was not very high, the ingested dose was lethal for her underweight (height 1.60 m, weight 41.5 kg, body mass index 16.21). Meanwhile, underweight patients easily developed unfavorable mortality trends due to shock, which was probably related to malnutrition (13). Poison removal is critical to clinical treatment, but the intensity of hemoperfusion was still controversial and lack of systematic verification (14, 15). A prospective study found that continuous venous hemofiltration (CVVH) combined with hemoperfusion could significantly improve the 90-day survival of paraquat poisoning (16). The worsening condition was observed in this patient after the routine hemoperfusion. In order to promote the removal of poisons in the early stage of poisoning, an intensive model of CVVHDF was adopted in the early stage to remove poisons as soon as possible, thus reducing subsequent organ damage. The symptoms improved significantly using the intensive hemoperfusion strategy, which was similar to a previous study (17). In the present case, low blood pressure, elevated blood lactic acid, poor tissue perfusion, and maintenance of blood pressure with high doses of vasoactive drugs supported the diagnosis of circulatory failure (18). Diquat rapidly distributed throughout the body, and largely damaged tissues and cells, and subsequent systemic inflammatory response, resulting in reduced effective circulating blood volume (19), which probably explained the cause of shock. According to the previous report on diquat poisoning combined with rhabdomyolysis, the shock was the main cause of death due to the damage to myocardial function (8). Especially, the high level of IL6 indicated the development of systemic inflammatory response syndrome (SIRS) (20). Decreasing blood pressure might be related to the increased permeability of capillary caused by SIRS, and the transfer of extracellular fluid to the tissue space after rhabdomyolysis (21). Fluid resuscitation under the guidance of PICCO could accurately evaluate blood volume, regulate hemodynamics, and blood gas analysis, which effectively relieved symptoms of ischemia and hypoxia (22). Therefore, this case supported that invasive hemodynamic monitoring should be implemented as early as possible to guide precise treatment for shock. Diquat causes strong toxic reactions such as lipid peroxidation, damaged the fluidity and permeability of the cell membrane structure, and ultimately leads to cell rupture and death (10), which explained the cause of rhabdomyolysis. Yin et al. reported that creatine kinase isoenzyme and myoglobin were the important risk factors of AKI caused by pesticide poisoning (23). In this case, the serum creatinine rose to 212 umol/L on the third day after admission, indicating that the patient had acute renal function damage. However, the acute renal injury was effectively mitigated by continuous hemofiltration dialysis, and the antishock treatment also prevented renal function damage in the early stage. Sivelestat was a new drug for acute respiratory distress syndrome (ARDS) that blocked the systemic inflammatory reaction by targeting the inhibition of neutrophil elastase (24). The toxicological mechanism of bipyridine herbicides, such as paraquat and diquat, has not been clarified yet, but the currently recognized mechanism is the oxidative stress pathway. Diquat could greatly stimulate mitochondrial tissues to produce a large number of oxygen-free radicals, and also activate neutrophils to release a large number of inflammatory mediators (25). Early use of anti-inflammatory agents was recommended according to the latest guidelines for sepsis management. The use of Sivelestat aimed to reduce the neutrophil release of inflammatory mediators, thereby reducing systemic inflammatory response (26). The outcomes of Sivelestat in the treatment of diquat poisoning were positive, which yet still needed more clinical evidences to confirm.
In conclusion, the clinical treatment and management of diquat poisoning were complex, particularly complicated with rhabdomyolysis and shock. The successful outcomes of this patient highlighted that intensive CRRT and PICCO were effective measures for poisoning and shock. Additionally, adolescent mental health is also worth attracting more attention and assistance.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Guangzhou Twelfth People’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
YC and ZO conceptualized and wrote the draft in consultation with ZW. RZ and ST collected and collated the data. RF and ZL analyzed and visualized the data. All authors reviewed the manuscript and approved the submitted version.
Funding
This work was supported by the Major Science and Technology Project of Guangzhou Municipal Health Commission (Project Number: 2021A031003), Guangzhou Key Medical Discipline (2021–2023), and Key Research and Development Programme of Guangzhou Science and Technology Project (Project Number: 202206010061).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRRT, continuous renal replacement therapy; CVVHDF, continuous venovenous hemodiafiltration; CT, computed tomography; PSIMV, pressure support synchronized intermittent mandatory ventilation; PICCO, pulse indicator continuous cardiac output; SVRI, systemic vascular resistance index; dPmax, maximal left ventricular pressure rising rate; GEDI, whole heart diastolic end volume index; ITBI, intrathoracic blood volume index; ELWI, extravascular lung water index; SVI, stroke volume index; GEF, whole heart ejection fraction; ARDS, acute respiratory distress syndrome.
References
1. Fortenberry GZ, Beckman J, Schwartz A, Prado JB, Graham LS, Higgins S, et al. Magnitude and characteristics of acute paraquat- and diquat-related illnesses in the US: 1998-2013. Environ Res. (2016) 146:191–9. doi: 10.1016/j.envres.2016.01.003
2. Magalhaes N, Carvalho F, Dinis-Oliveira RJ. Human and experimental toxicology of diquat poisoning: toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum Exp Toxicol. (2018) 37:1131–60. doi: 10.1177/0960327118765330
3. Xing J, Chu Z, Han D, Jiang X, Zang X, Liu Y, et al. Lethal diquat poisoning manifesting as central pontine myelinolysis and acute kidney injury: a case report and literature review. J Int Med Res. (2020) 48:300060520943824. doi: 10.1177/0300060520943824
4. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. (2019) 394:1949–64. doi: 10.1016/S0140-6736(19)32563-2
5. Granata A, Distefano G, Pesce F, Battaglia Y, Suavo Bulzis P, Venturini M, et al. Performing an ultrasound-guided percutaneous needle kidney biopsy: an up-to-date procedural review. Diagnostics (Basel). (2021) 11:2186. doi: 10.3390/diagnostics11122186
6. Guck D, Hernandez R, Moore S, Van de Louw A, Haouzi P. Rapid glomerulotubular nephritis as an initial presentation of a lethal diquat ingestion. Case Rep Nephrol. (2021) 2021:4723092. doi: 10.1155/2021/4723092
7. Feng D, Fu L, Du X, Yao L. Acute diquat poisoning causes rhabdomyolysis. Am J Med Sci. (2022) 364:472–80. doi: 10.1016/j.amjms.2022.04.032
8. Yu G, Wang J, Jian T, Shi L, Zhao L, Li Y. Case series: diquat poisoning with acute kidney failure, myocardial damage, and rhabdomyolysis. Front Public Health. (2022) 10:991587. doi: 10.3389/fpubh.2022.991587
9. Jones GM, Vale JA. Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. J Toxicol Clin Toxicol. (2000) 38:123–8. doi: 10.1081/clt-100100926
10. Jovic-Stosic J, Babic G, Todorovic V. Fatal diquat intoxication. Vojnosanit Pregl. (2009) 66:477–81. doi: 10.2298/vsp0906477j
11. Sana N, Shaikh MS. Transfusion-associated circulatory overload (TACO): significance of appropriate pre-transfusion risk assessment to prevent fatal health outcomes. J Pak Med Assoc. (2021) 71:1049. doi: 10.47391/JPMA.04-532
12. Liu Y, Liu B, Zhou H, Wei LQ. Continuous levosimendan infusion for refractory cardiogenic shock complicating severe acute dichlorvos poisoning. Am J Med Sci. (2012) 344:166–70. doi: 10.1097/MAJ.0b013e318254490d
13. Girard L, Djemili F, Devineau M, Gonzalez C, Puech B, Valance D, et al. Effect of body mass index on the clinical outcomes of adult patients treated with venoarterial ECMO for cardiogenic shock. J Cardiothorac Vasc Anesth. (2022) 36:2376–84. doi: 10.1053/j.jvca.2021.11.012
14. Yaxley J, Scott T. Dialysis and extracorporeal therapies for enhanced elimination of toxic ingestions and poisoning. Ther Apher Dial. (2022) 26:865–78. doi: 10.1111/1744-9987.13843
15. Feinfeld DA, Rosenberg JW, Winchester JF. Three controversial issues in extracorporeal toxin removal. Semin Dial. (2006) 19:358–62. doi: 10.1111/j.1525-139X.2006.00187_1.x
16. Li A, Li W, Hao F, Wang H. Early stage blood purification for paraquat poisoning: a multicenter retrospective study. Blood Purif. (2016) 42:93–9. doi: 10.1159/000445991
17. Peng ZY, Chang P, Wang H, Cen ZR, Zhou J, Liu ZG. Intensive hemoperfusion and long-term hemofiltration for treatment of paraquat poisoning: a case report. Nan Fang Yi Ke Da Xue Xue Bao. (2015) 35:1515–8.
18. Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. task force of the european society of intensive care medicine. Intensive Care Med. (2014) 40:1795–815. doi: 10.1007/s00134-014-3525-z
19. Houze P, Baud FJ, Mouy R, Bismuth C, Bourdon R, Scherrmann JM. Toxicokinetics of paraquat in humans. Hum Exp Toxicol. (1990) 9:5–12. doi: 10.1177/096032719000900103
20. Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol. (2018) 10:a028456. doi: 10.1101/cshperspect.a028456
21. Tighe D, Moss R, Bennett D. Cell surface adrenergic receptor stimulation modifies the endothelial response to SIRS. systemic inflammatory response syndrome. New Horiz. (1996) 4:426–42.
22. Ni X, Liu XJ, Ding TT. The application of PiCCO-guided fluid resuscitation in patients with traumatic shock. Am Surg. (2022). doi: 10.1177/00031348221087898 [Epub ahead of print].
23. Yin YF, Pu WJ, Cai YX, Xie RN, Liu J. Risk factors associated with acute kidney injury caused by pesticide poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. (2021) 39:333–6. doi: 10.3760/cma.j.cn121094-20200909-00522
24. Lee JM, Yeo CD, Lee HY, Rhee CK, Kim IK, Lee DG, et al. Inhibition of neutrophil elastase contributes to attenuation of lipopolysaccharide-induced acute lung injury during neutropenia recovery in mice. J Anesth. (2017) 31:397–404.
25. Choi SE, Park YS, Koh HC. N/p53-activated inflammatory response involves in diquat-induced mitochondrial dysfunction and apoptosis. Environ Toxicol. (2018) 33:1005–18. doi: 10.1002/tox.22552
26. Aikawa N, Ishizaka A, Hirasawa H, Shimazaki S, Yamamoto Y, Sugimoto H, et al. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm Pharmacol Ther. (2011) 24:549–54. doi: 10.1016/j.pupt.2011.03.001
Keywords: diquat, multi-organ dysfunction, rhabdomyolysis, shock, pulse indicator continuous cardiac output
Citation: Chen Y, Ou Z, Zhang R, Long Z, Fu R, Tang S and Wang Z (2023) Case report: Successful outcome of a young patient with rhabdomyolysis and shock caused by diquat poisoning. Front. Med. 10:1116912. doi: 10.3389/fmed.2023.1116912
Received: 14 December 2022; Accepted: 16 January 2023;
Published: 03 February 2023.
Edited by:
Takeshi Wada, Hokkaido University, JapanReviewed by:
Yoshihiko Nakamura, Fukuoka University Hospital, JapanGuangcai Yu, Qilu Hospital, Shandong University, China
Copyright © 2023 Chen, Ou, Zhang, Long, Fu, Tang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Wang,  zhi_wang@outlook.com
zhi_wang@outlook.com
†These authors have contributed equally to this work
 Yunchao Chen1†
Yunchao Chen1†  Zejin Ou
Zejin Ou Zhi Wang
Zhi Wang