Carbonic anhydrase IX-related tumoral hypoxia predicts worse prognosis in breast cancer: A systematic review and meta-analysis
- 1Division of Head Neck and Breast Surgery, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 2School of Cancer Sciences, Wolfson Wohl Cancer Research Centre, University of Glasgow, Glasgow, United Kingdom
- 3Department of Immunology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Background: Tumoral hypoxia is associated with aggressiveness in many cancers including breast cancer. However, measuring hypoxia is complicated. Carbonic anhydrase IX (CAIX) is a reliable endogenous marker of hypoxia under the control of the master regulator hypoxia-inducible factor-1α (HIF-1α). The expression of CAIX is associated with poor prognosis in many solid malignancies; however, its role in breast cancer remains controversial.
Methods: The present study performed a meta-analysis to evaluate the correlation between CAIX expression and disease-free survival (DFS) and overall survival (OS) in breast cancer.
Results: A total of 2,120 publications from EMBASE, PubMed, Cochrane, and Scopus were screened. Of these 2,120 publications, 272 full texts were reviewed, and 27 articles were included in the meta-analysis. High CAIX was significantly associated with poor DFS (HR = 1.70, 95% CI = 1.39–2.07, p < 0.00001) and OS (HR = 2.02, 95% CI 1.40–2.91, p = 0.0002) in patients with breast cancer. When stratified by subtype, the high CAIX group was clearly associated with shorter DFS (HR = 2.09, 95% CI =1.11–3.92, p = 0.02) and OS (HR = 2.50, 95% CI =1.53–4.07, p = 0.0002) in TNBC and shorter DFS in ER+ breast cancer (HR = 1.81 95% CI =1.38–2.36, p < 0.0001).
Conclusion: High CAIX expression is a negative prognostic marker of breast cancer regardless of the subtypes.
Introduction
The incidence of breast cancer has increased in recent decades, with an estimated 13% of women developing breast cancer in their lifetime and over 40,000 deaths per year (1, 2). The survival depends on clinicopathological factors, such as tumor size, nodal status, evidence of distant metastasis as well as biological markers, including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status (3–5). The intrinsic breast cancer subtypes are currently significant prognostic and predictive markers. Five-year overall survival (OS) was the highest in the ER/PR-positive subtype (94%) as compared to the HER2-positive subtype (85%) and the triple-negative (TNBC) subtype (77%) (1). Breast cancer has distinct phenotypes as evidenced by patients who have a similar staging and molecular classification but have a different treatment response and prognosis (6–8). Thus, additional predictive and prognostic markers are warranted to improve the treatment and prognostic outcomes.
Tumoral hypoxia is a common characteristic of many solid tumors (9, 10). In breast cancer, median oxygen partial pressure is approximately 10 mmHg, which is less than that of the normal breast tissue (52–65 mmHg) (11, 12). Cancer cells adapt to survive under hypoxic conditions via hypoxia-inducible factor-1α (HIF-1α), leading to the transcription of targeted genes resulting in tumor progression and invasion (13). Subsequently, HIF-1α can trigger the transcription of targeted genes, leading to tumor progression and invasion (14).
The expression of carbonic anhydrase IX (CAIX) is targeted by the HIF-1α transcriptional activity and controls the pH between intracellular and extracellular compartments (15). It is mainly dependent on HIF-1α regulation; therefore, it can also be a marker of tumor hypoxia (16, 17). However, hypoxia is not an obligated factor, and the inactivation of the von Hippel–Lindau (VHL) gene can stabilize HIF-1α under a non-hypoxic condition and subsequently activated the CAIX overexpression (15, 18). CAIX catalyzes extracellular hydrating CO2 into HCO−3 and H+ and cooperates with other acid/base transporters to maintain extracellular acidosis and intracellular neutral/slight alkalosis (19). In contrast, CAIX-bound Cl−/HCO−3 exchangers (AEs) can import or provide or export HCO−3 from intracellular compartment during cell migration (20). CAIX expression mediates cancer cell growth, migration, and invasion (18) by directly binding to β-catenin, resulting in the disruption of the E-cadherin/cytoskeleton/β-catenin complex; and an acidic extracellular pH also suppresses the function of cytotoxic T-cells (21).
Many studies have shown that high CAIX expression was associated with adverse survival outcomes. In breast cancer, some studies evaluated the importance of CAIX expression in relation to survival; however, those results were controversial and mostly included a small number of patients. Ong et al. reported that CAIX expression was the independent prognostic factor for disease-free survival (DFS) and OS in TNBC. Similarly, Brennan et al. reported that high CAIX was associated with shorter OS, breast cancer-specific survival (BCSS), and relapse-free survival (RFS) (22, 23). In contrast, Currie et al. found no association between the level of CAIX and DFS and OS (24), while Pinheiro et al. reported that only a high CAIX expression was related to DFS but not to OS (25).
To address this issue, a meta-analysis was conducted to evaluate the prognostic value of CAIX in breast cancer and to determine the correlation between CAIX and breast cancer subtypes. To date, this is the first meta-analysis to focus on the prognostic role of CAIX in breast cancer. The meta-analysis revealed that a high CAIX protein expression was associated with unfavorable survival outcomes and could discriminate the prognosis in the ER-positive and TNBC subtypes.
Materials and methods
Search strategy
This study used EMBASE, PubMed, Cochrane, and Scopus electronic databases to search for articles. The keywords including [(Prognos*) OR (surviv*) OR (hazard) OR (disease-free) OR (“disease free”) OR (progression-free) OR (“progression-free”) OR (Kaplan–Meier) OR (“Kaplan Meier”) OR (predict*) OR (outcome) OR (efficacy) OR (effective*)] AND [(CAIX) OR (ca9) OR (“carbonic anhydrase IX”) OR (“carbonic anhydrase 9”) OR (“carbonic anhydrase-IX”) OR (“carbonic anhydrase-9”) OR (CA-IX) OR (ca-9) OR (G250)] AND [(breast cancer) OR (breast tumors*) OR (breast carcinoma)] were used.
Selection criteria
The inclusion criteria of the present study were as follows: (a) the patients in the study cohorts who were confirmed to have invasive breast cancer, regardless of the subtype, (b) CAIX expression which was detected by immunohistochemistry (IHC), (c) the studies that reported DFS or OS with hazards ratios (HRs) and 95% confidence intervals (CIs) or the Kaplan–Meier survival curves from which HRs and 95% CIs could be extracted, and (d) the studies that were published in English. The exclusion criteria for the present study were studies that failed to meet any of the inclusion criteria, were related to non-human studies, or contained duplicated and unavailable full texts.
Data extraction and quality assessment
The search with regard to data extraction and quality assessment was reviewed by three independent reviewers (WN, JP, and SY). The following information was extracted from each study: the first author’s name, year of publication, the total number of patients, the scoring method and cut-off level for high or low CAIX expression, breast cancer subtypes, HRs, 95% CIs of DFS and OS, and whether univariate or multivariate analysis was performed.
Statistical methods
Pooled HRs and their 95% CIs were used to determine the association between CAIX expression and survival. Heterogeneity among studies was assessed using the chi-squared test and I2. A p-values of <0.1 or an I2 statistic of >50% was indicative of significant heterogeneity between studies; in these cases, a random-effects model was used. The meta-analysis was performed with Review Manager 5.4 (RevMan the Cochrane Collaboration; Oxford, England). The p-values of <0.05 were considered statistically significant.
Results
Study selection and characteristics
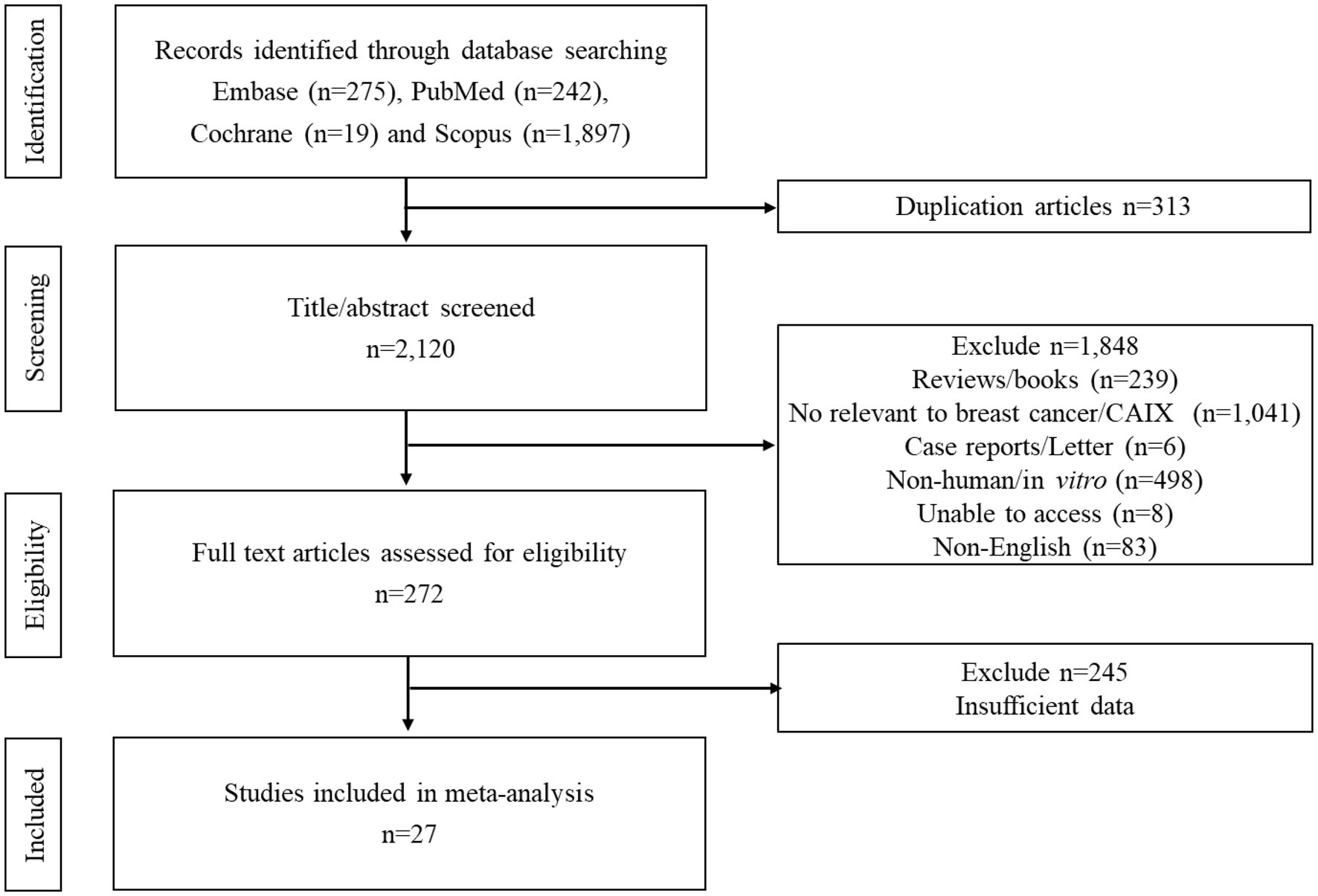
A PRISMA flow diagram for the process of study selection is summarized in Figure 1. Initially, 275 articles from EMBASE, 242 from PubMed, 19 from Cochrane, and 1,897 from Scopus were identified, and subsequently, 313 duplicated records were removed. A total of 2,120 papers were screened. A total of 1,848 studies were excluded based on the titles and abstracts resulting in 272 full texts being reviewed. Of these, 245 articles were excluded. Finally, 27 papers met the eligibility criteria (Figure 1; Table 1).
Study characteristics
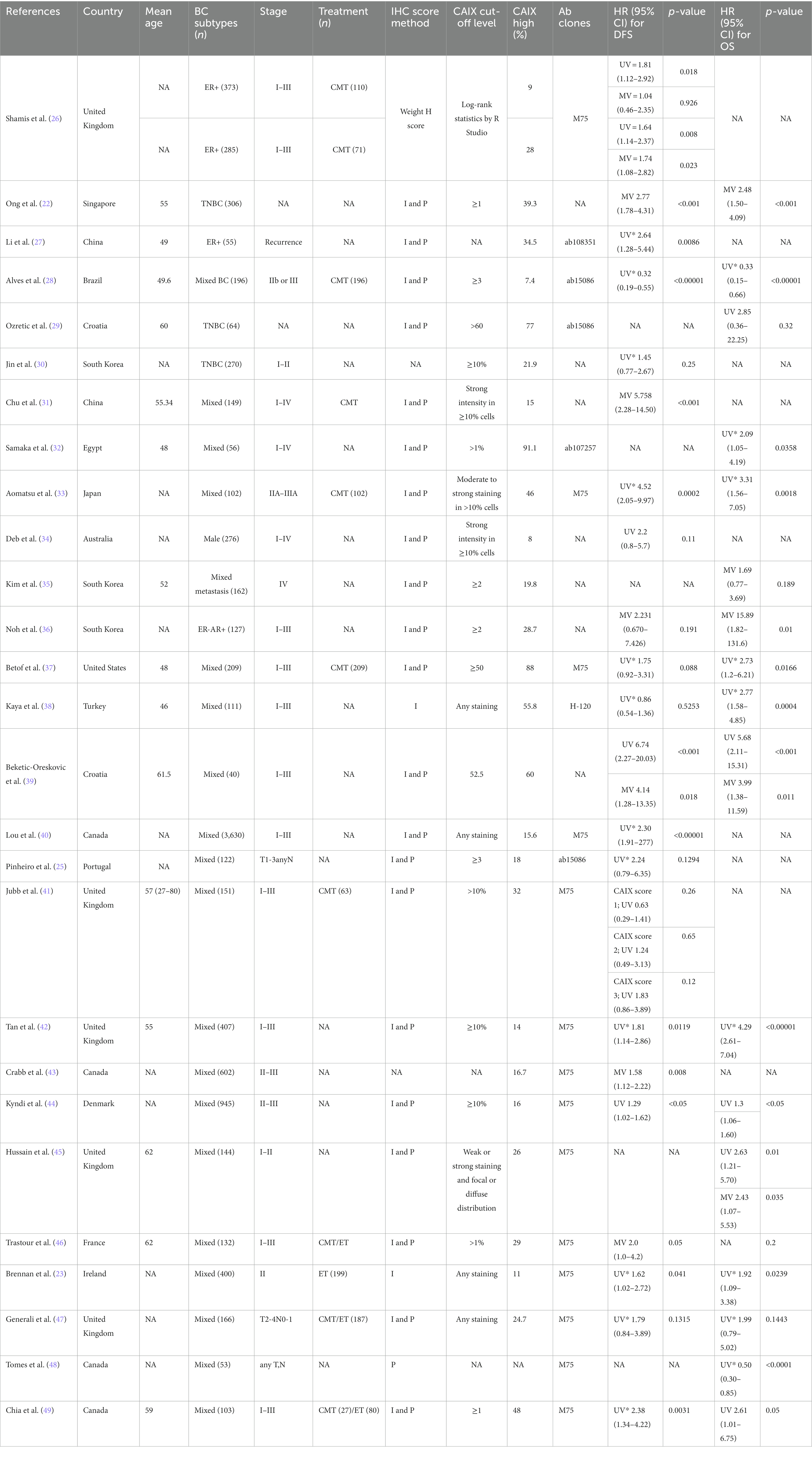
The 27 included studies were published between 2001 and 2022. DFS was reported in 22 articles, 10 of which provided HRs and 95% CIs, while the OS analysis was included in 16 articles, 7 of which provided HRs and 95% CIs (Table 1). Most of the articles (20 out of 27, 74%) were reported on mixed breast cancer subtypes and provided data on ER, PR, and/or HER2 staining, with survival analysis on all cases, regardless of the subtype. Three studies focused on TNBC, two articles on ER-positive (ER+), one on ER-negative (ER−), and one on male breast cancer. The mean age of patients was between 46 and 62 years. Fifty percent of the studies used the primary antibody clone M75 to detect the CAIX expression. In most studies (80%), the level of CAIX expression was determined by estimating both staining intensity and the percentage of tumor cells stained. The remaining studies (20%) used only intensity or percentage. The low–-high cutoff value varied across all studies. Overall, high CAIX expression in patients with breast cancer varied in each study, ranging from 8 to 91.1%. Most studies (45.5%, 12 out of 27 studies) did not report on the cellular location of CAIX expression. In 36% of studies, expression was reported in the cell membrane, in 9% of studies, CAIX expression was reported in the membrane and cytoplasm/nucleus, and in two studies, CAIX expression was reported in the exclusive cytoplasm or nuclear staining (9%).
High CAIX was associated with poor DFS in breast cancer
Twenty-two studies totaling 9,157 patients were analyzed for the effect of CAIX expression on DFS. Shamis et al. studied CAIX expression in two independent cohorts with specific HRs and 95% CIs and DFS in each cohort, and both cohorts were included in this meta-analysis (26). The study by Jubb et al. did not define the low/high cutoff for CAIX expression, but it provided the HR and 95% CI for each CAIX score of 1, 2, and 3 and compared each with that of the negative CAIX group (41). Hence, the HR and 95% CI for each CAIX score were included in the meta-analysis. High CAIX was significantly associated with poor DFS in patients with breast cancer (HR = 1.70, 95% CI = 1.39–2.07, p < 0.00001) with heterogeneity I2 = 83% (Figure 2).
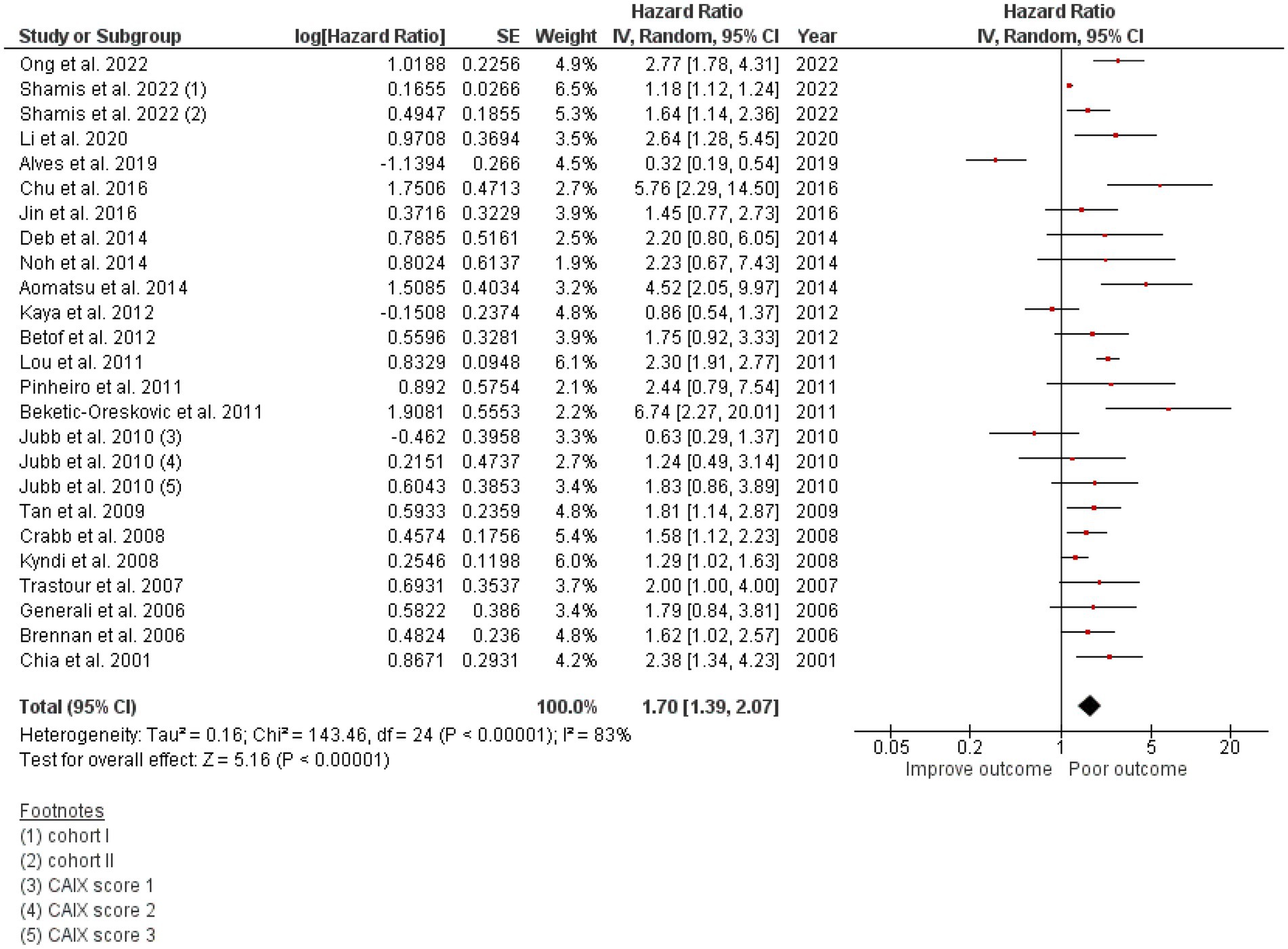
Figure 2. A Forest plot of HR and 95% CI for the association of CAIX with DFS of all patients with BC.
High CAIX was associated with poor OS in breast cancer
A total of 3,591 patients from the selected 17 studies were investigated for the association between CAIX expression and OS. High CAIX expression was statistically significantly associated with shorter OS (HR = 2.05, 95% CI 1.44–2.91, p < 0.0001) with heterogeneity I2 = 80% (Figure 3).
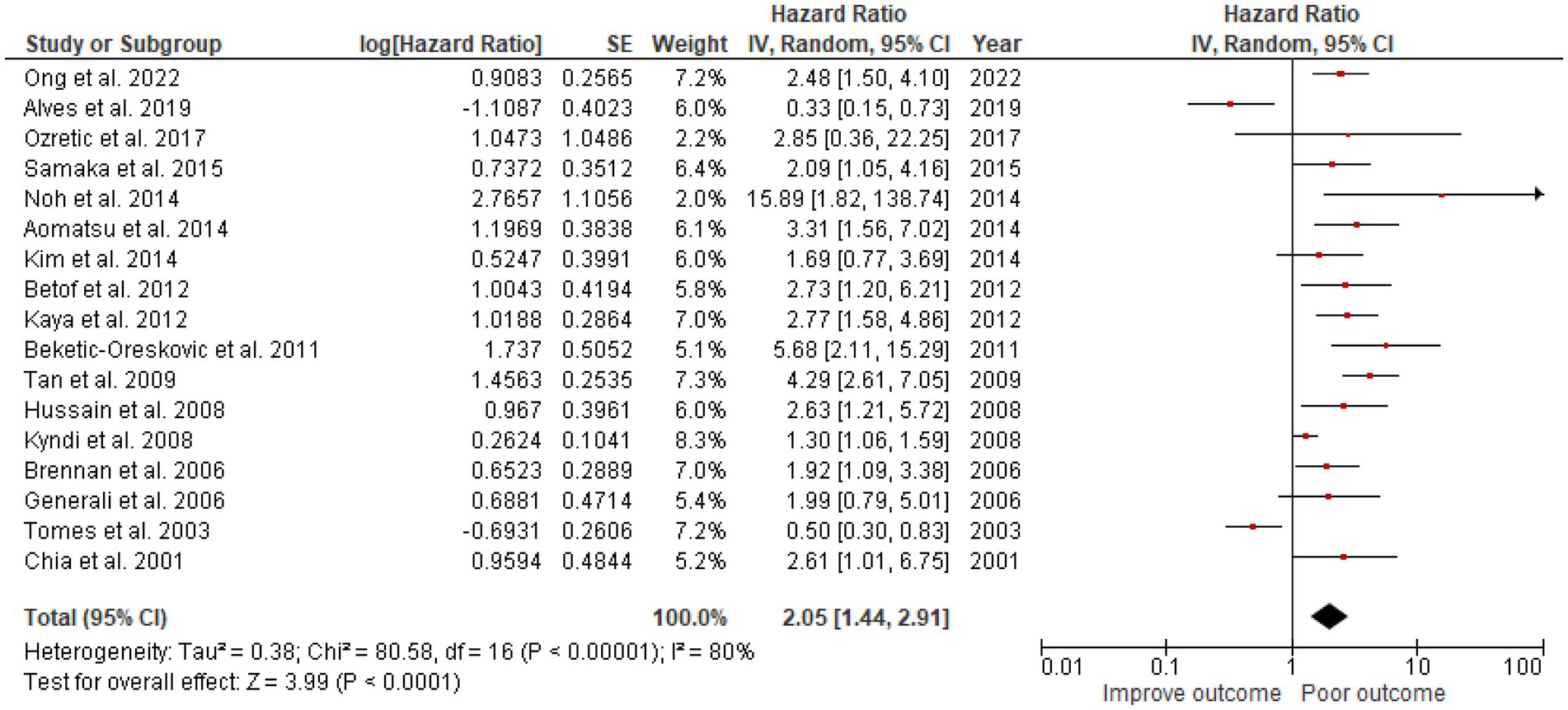
Figure 3. A Forest plot of HR and 95% CI for the association of CAIX with OS of all patients with BC.
High CAIX was associated with poor OS and DFS in ER+ and TNBC subtypes
Three articles focused on the CAIX expression in 640 TNBC cases. One study reported both DFS and OS, while the other two reported either DFS or OS, resulting in 576 TNBC cases included in the DFS analysis and 370 TNBC cases included in the OS analysis. Two articles focused on CAIX expression and DFS in ER+ breast cancer from 731 ER+ breast cancer cases. The results revealed that, when compared to patients with a low CAIX expression, patients with a high CAIX expression were clearly associated with shorter DFS in TNBC (HR = 2.09, 95% CI =1.11–3.92, p = 0.02) with heterogeneity I2 = 63% and OS (HR = 2.50, 95% CI =1.53–4.07, p = 0.0002) without heterogeneity I2 = 0%; and shorter DFS in ER+ breast cancer (HR = 1.81 95% CI =1.38–2.36, p < 0.0001) without heterogeneity I2 = 0% (Figure 4).
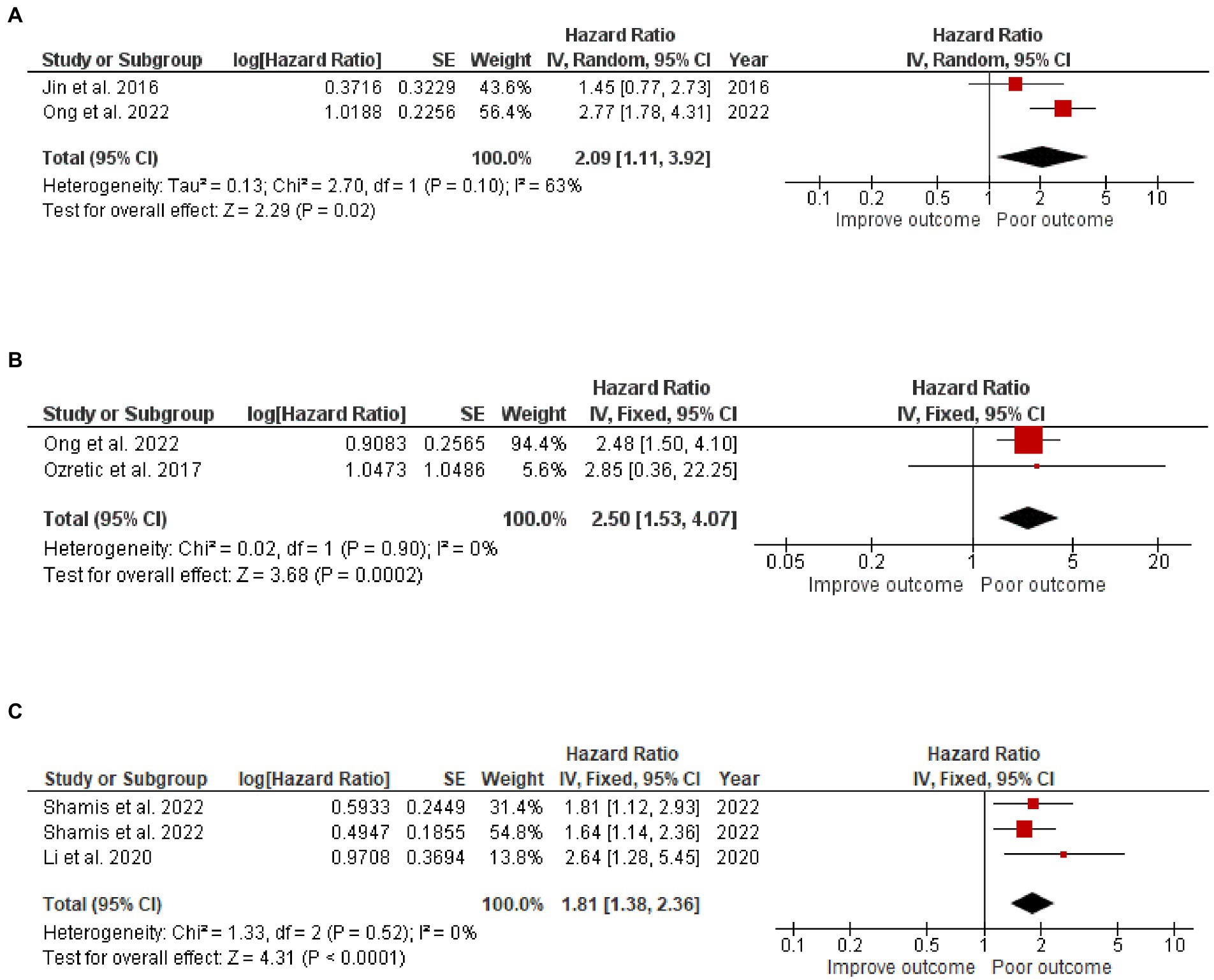
Figure 4. A Forest plot of HR and 95% CI for the association of CAIX with (A) DFS, (B) OS of patients with TNBC, and (C) DFS of patients with ER+ BC.
The antibody does not affect CAIX survival
The studies used a variety of CAIX antibodies for IHC. Twelve studies used an M75 antibody clone: 1 from BioScience, 1 from Novus Biologicals, and 1 from Bayer, but the other 9 could not be identified. The HR for DFS was 1.66 (95% CI: 1.35–2.0, p < 0.00001). Clones used in other studies were as follows: 6 studies used Abcam, 1 from Cell Marque, 1 from Novus Biologicals, and 2 from Santa Cruz Biotechnology (Table 1), which also demonstrated the effect of CAIX with HR for DFS 1.94 (95% CI: 1.06–3.57; p < 0.0001; Figure 5). There was no significant difference between the M75 antibody and other antibodies (p = 0.63; Figure 5). The HR for OS in the group stained with the M75 antibody was 2.01 (95% CI: 1.19–3.38; p = 0.009), and it was 2.10 (95% CI: 1.26–3.52; p = 0.002) for the other antibody group (Figure 6). There was no significant difference between the M75 antibody and the other antibodies in terms of OS (p = 0.90; Figure 6).
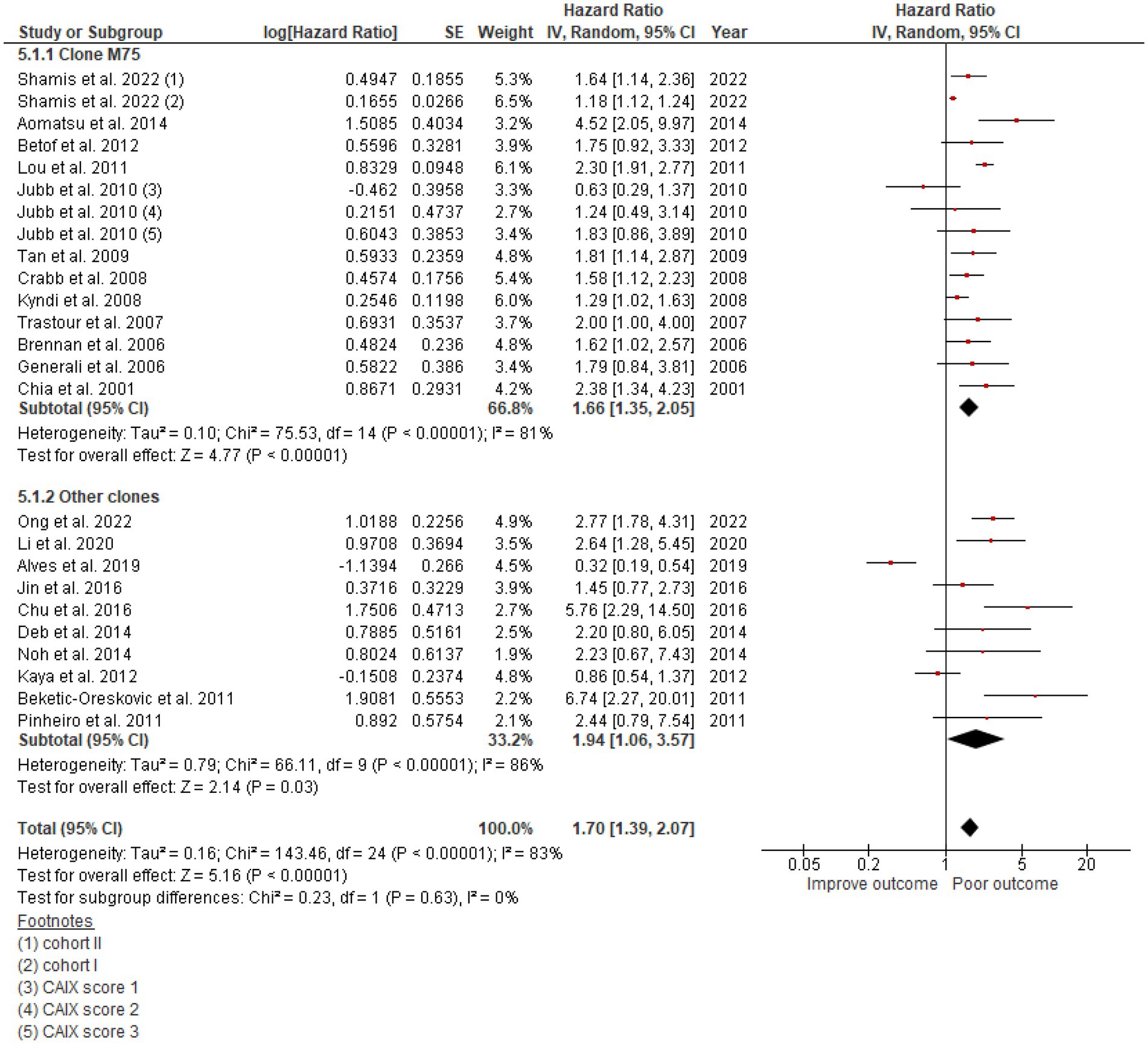
Figure 5. A Forest plot of HR and 95% CI for the association of CAIX expression with DFS in patients with BC stratified by the antibody clone.
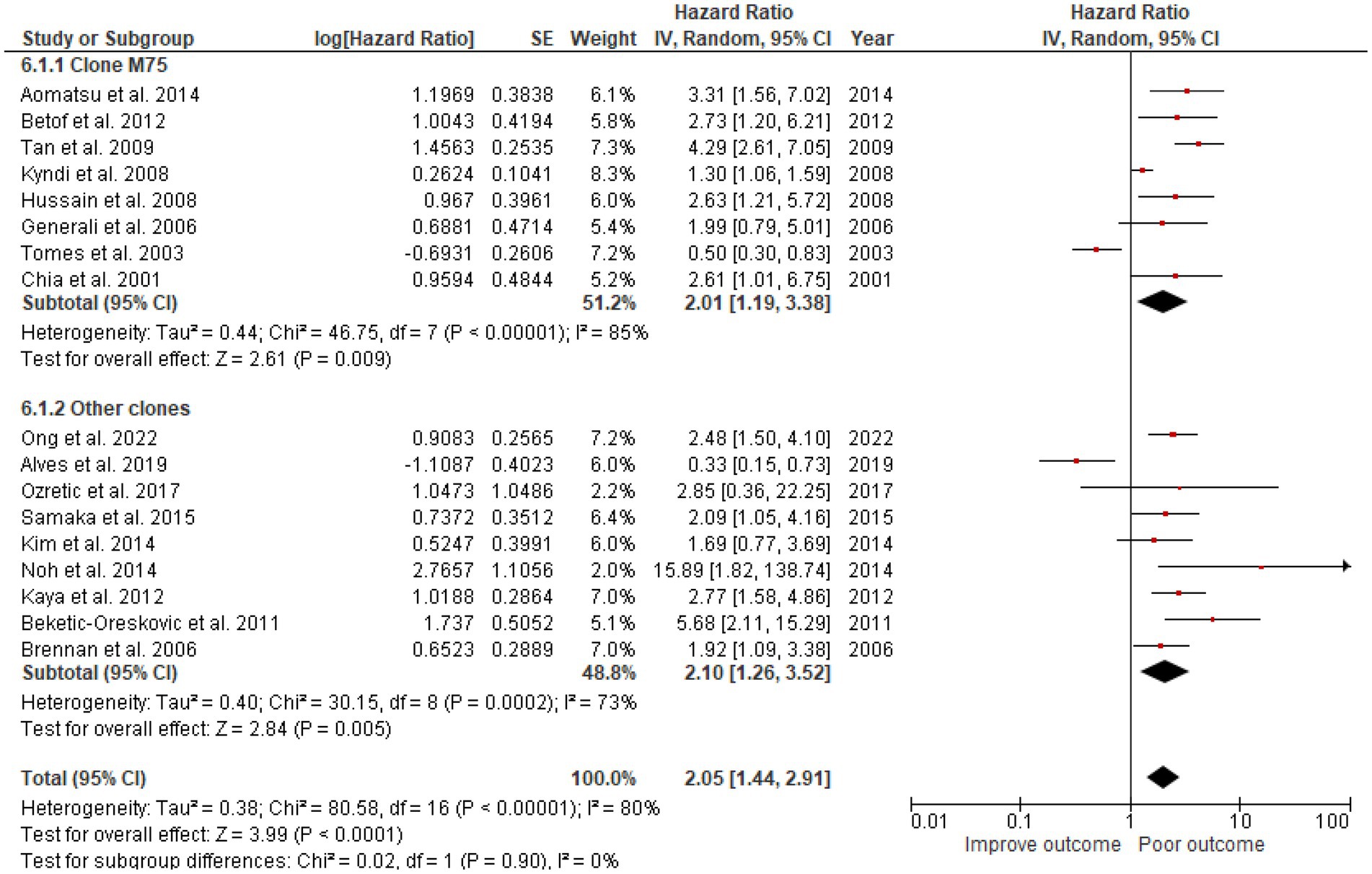
Figure 6. A Forest plot of HR and 95% CI for the association of CAIX expression with OS in patients with BC stratified by the antibody clone.
Discussion
This meta-analysis focused on the prognostic role of CAIX expression in breast cancer. Hypoxia, as determined by the CAIX expression, has been associated with poor survival outcomes, independent of other clinicopathological factors in many solid malignancies, including breast cancer (50). The current meta-analysis included a greater number of studies and confirmed a negative survival outcome in patients with breast cancer who had a high CAIX expression. To our knowledge, this is the first meta-analysis that has examined the CAIX expression exclusively in breast cancer. The results of this meta-analysis may lead to the use of CAIX expression as a prognostic marker, resulting in better treatment options for patients with breast cancer.
High CAIX was significantly associated with poor DFS (HR = 1.70, 95% CI = 1.39–2.07, p < 0.00001) and OS (HR = 2.02, 95% CI 1.40–2.91, p = 0.0002), despite the high heterogeneity of DFS, I2 = 83%, and OS, I2 = 81%. This heterogeneity could be explained by the bias in the scoring method and cutoff level as most of the studies determined the CAIX protein expression by the intensity and percentage of tumor cell staining and with individual cutoff levels. However, this meta-analysis did support the use of CAIX as a prognostic marker; therefore, the evaluation of CAIX expression should be considered in breast cancer.
Tumoral hypoxia has long been established as a factor in the progression and metastasis of cancer cells (51). CAIX protein expression is a reliable endogenous hypoxic marker as its expression is dependent on the HIF-1α activity (16). CAIX is a zinc metalloproteinase that is located at the transmembrane and acts to convert CO2 to HCO−3 and H+ (52). This process occurs extracellularly and results in an extracellular acidic pH. The cancer cells exploit the extracellular acidity to invade the stroma by promoting epithelial–mesenchymal transition (EMT) and cell motility as well as suppressing anti-tumor immunity by, for example, dysregulating cytotoxic T-cell functions while enhancing the function of M2 macrophages and myeloid-derived suppressor cells (MDSCs) (53, 54). These effects may explain the correlation between the increased expression of CAIX and poor survival outcomes.
Carbonic anhydrase IX is highly induced in a HIF-1-dependent manner and is constitutively expressed in VHL-defective cells. While CAXII is upregulated in VHL-defective renal tumors and induced hypoxia in tumor cells, its dependence on HIF is not well established (15). Additionally, it is well known that the tumor expression of HIF-1α and CAIX was correlated with poor patient survival, CAXII, which lacks the extracellular proteoglycan domain of CAIX implicated in cell adhesion, had a less obvious survival effect (17). CAXII expression is related to better survival statistics for patients (55–57). In breast cancer, there is a strong association between luminal cancers and CAXII expression. Moreover, CAXII is also a biomarker of favorable prognosis in lung (58) and brain (59) tumors but is associated with a poor prognosis in colorectal cancer (60).
Additionally, this meta-analysis clarified the importance of CAIX expression associated with survival outcomes in both ER+ and TNBC. Li et al. reported increased tamoxifen resistance in ER+ breast cancer with a high CAIX expression (27). Similarly, a study by Tan et al. demonstrated the adverse effect of CAIX expression on basal-like breast cancer subtypes by escalating the chemotherapy resistance (42). This may imply that CAIX overexpression is a hostile factor mediating treatment resistance. Thus, a combination of chemotherapy and CAIX inhibitors may be helpful in the prevention of chemoresistance. This meta-analysis had several limitations. The high degree of heterogeneity of the study indicated that we were unable to accurately define a CAIX expression scoring method and optimal threshold values. Further studies to standardize the IHC protocol for CAIX are needed. The publication bias might overestimate the survival outcome as articles reporting positive findings were selected.
Conclusion
Our results highlight the importance of a high CAIX expression being associated with poor DFS and OS in patients with breast cancer. This information may be useful for future studies, leading to the incorporation of CAIX inhibitors in treatment regimens for patients with breast cancer. High-quality studies with larger homogeneous samples are required to determine the prognostic role of CAIX in different breast cancer subtypes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WN and CT contributed to the framework and the overall perspective of the study design. The literature search was carried out by WN, SY, and JP. SY and JP extracted the data and assisted with quality control. JP carried out the statistical analysis. WN wrote the manuscript and created the tables and figures. The statistical analysis was supervised and verified by JE and CT. WN, JE, and CT contributed to the study’s quality assessment and manuscript revision. JQ checked and edited the English grammar. All authors contributed to the article and approved the submitted version.
Funding
This study received funding from the National Research Council of Thailand (NRCT) and Mahidol University (grant no. N42A650343).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.Giaquinto, AN, Sung, H, Miller, KD, Kramer, JL, Newman, LA, Minihan, A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. (2022) 72:524–41. doi: 10.3322/caac.21754
2.Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
3.Phung, MT, Tin Tin, S, and Elwood, JM. Prognostic models for breast cancer: a systematic review. BMC Cancer. (2019) 19:230. doi: 10.1186/s12885-019-5442-6
4.Yersal, O, and Barutca, S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol. (2014) 5:412–24. doi: 10.5306/wjco.v5.i3.412
5.Soerjomataram, I, Louwman, MWJ, Ribot, JG, Roukema, JA, and Coebergh, JWW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. (2008) 107:309–30. doi: 10.1007/s10549-007-9556-1
6.Schaafsma, E, Zhang, B, Schaafsma, M, Tong, C-Y, Zhang, L, and Cheng, C. Impact of Oncotype DX testing on ER+ breast cancer treatment and survival in the first decade of use. Breast Cancer Res. (2021) 23:74. doi: 10.1186/s13058-021-01453-4
7.Almstedt, K, Heimes, AS, Kappenberg, F, Battista, MJ, Lehr, HA, Krajnak, S, et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer. (2022) 173:10–9. doi: 10.1016/j.ejca.2022.06.012
8.Wang, C. A meta-analysis of efficacy and safety of PD-1/PD-L1 inhibitors in triple-negative breast cancer. J Oncol. (2022) 2022:1–7. doi: 10.1155/2022/2407211
9.Semenza, GL. Hypoxia and cancer. Cancer Metastasis Rev. (2007) 26:223–4. doi: 10.1007/s10555-007-9058-y
10.Vaupel, P, and Mayer, A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. (2007) 26:225–39. doi: 10.1007/s10555-007-9055-1
11.McKeown, SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. (2014) 87:20130676. doi: 10.1259/bjr.20130676
12.Godet, I, Doctorman, S, Wu, F, and Gilkes, DM. Detection of hypoxia in cancer models: significance, challenges, and advances. Cells. (2022) 11:686. doi: 10.3390/cells11040686
13.Ziello, JE, Jovin, IS, and Huang, Y. Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. (2007) 80:51–60.
14.Semenza, GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. (2003) 3:721–32. doi: 10.1038/nrc1187
15.Wykoff, CC, Beasley, NJ, Watson, PH, Turner, KJ, Pastorek, J, Sibtain, A, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. (2000) 60:7075–83.
16.Kaluz, S, Kaluzova, M, Liao, SY, Lerman, M, and Stanbridge, EJ. Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: a one transcription factor (HIF-1) show? Biochim Biophys Acta. (2009) 1795:162–72. doi: 10.1016/j.bbcan.2009.01.001
17.Pastorekova, S, Zatovicova, M, and Pastorek, J. Cancer-associated carbonic anhydrases and their inhibition. Curr Pharm Des. (2008) 14:685–98. doi: 10.2174/138161208783877893
18.Robertson, N, Potter, C, and Harris, AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. (2004) 64:6160–5. doi: 10.1158/0008-5472.CAN-03-2224
19.Queen, A, Bhutto, HN, Yousuf, M, Syed, MA, and Hassan, MI. Carbonic anhydrase IX: a tumor acidification switch in heterogeneity and chemokine regulation. Semin Cancer Biol. (2022) 86:899–913. doi: 10.1016/j.semcancer.2022.01.001
20.Becker, HM. Carbonic anhydrase IX and acid transport in cancer. Br J Cancer. (2020) 122:157–67. doi: 10.1038/s41416-019-0642-z
21.Svastová, E, Zilka, N, Zat'ovicová, M, Gibadulinová, A, Ciampor, F, Pastorek, J, et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp Cell Res. (2003) 290:332–45. doi: 10.1016/S0014-4827(03)00351-3
22.Ong, CHC, Lee, DY, Lee, B, Li, H, Lim, JCT, Lim, JX, et al. Hypoxia-regulated carbonic anhydrase IX (CAIX) protein is an independent prognostic indicator in triple negative breast cancer. Breast Cancer Res. (2022) 24:38. doi: 10.1186/s13058-022-01532-0
23.Brennan, DJ, Jirstrom, K, Kronblad, A, Millikan, RC, Landberg, G, Duffy, MJ, et al. CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res. (2006) 12:6421–31. doi: 10.1158/1078-0432.CCR-06-0480
24.Currie, MJ, Beardsley, BE, Harris, GC, Gunningham, SP, Dachs, GU, Dijkstra, B, et al. Immunohistochemical analysis of cancer stem cell markers in invasive breast carcinoma and associated ductal carcinoma in situ: relationships with markers of tumor hypoxia and microvascularity. Hum Pathol. (2013) 44:402–11. doi: 10.1016/j.humpath.2012.06.004
25.Pinheiro, C, Sousa, B, Albergaria, A, Paredes, J, Dufloth, R, Vieira, D, et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol. (2011) 26:1279–86. doi: 10.14670/HH-26.1279
26.Shamis, SAK, Quinn, J, Mallon, EEA, Edwards, J, and McMillan, DC. The relationship between the tumor cell expression of hypoxic markers and survival in patients with ER-positive invasive ductal breast cancer. J Histochem Cytochem. (2022) 70:479–94. doi: 10.1369/00221554221110280
27.Li, Y, Chen, X, Zhou, Z, Li, Q, Westover, KD, Wang, M, et al. Dynamic surveillance of tamoxifen-resistance in ER-positive breast cancer by CAIX-targeted ultrasound imaging. Cancer Med. (2020) 9:2414–26. doi: 10.1002/cam4.2878
28.Alves, W, Bonatelli, M, Dufloth, R, Kerr, LM, Carrara, GFA, da Costa, RFA, et al. CAIX is a predictor of pathological complete response and is associated with higher survival in locally advanced breast cancer submitted to neoadjuvant chemotherapy. BMC Cancer. (2019) 19:1173. doi: 10.1186/s12885-019-6353-2
29.Ozretic, P, Alvir, I, Sarcevic, B, Vujaskovic, Z, Rendic-Miocevic, Z, Roguljic, A, et al. Apoptosis regulator Bcl-2 is an independent prognostic marker for worse overall survival in triple-negative breast cancer patients. Int J Biol Markers. (2018) 33:109–15. doi: 10.5301/ijbm.5000291
30.Jin, MS, Lee, H, Park, IA, Chung, YR, Im, SA, Lee, KH, et al. Overexpression of HIF1α and CAXI predicts poor outcome in early-stage triple negative breast cancer. Virchows Arch. (2016) 469:183–90. doi: 10.1007/s00428-016-1953-6
31.Chu, CY, Jin, YT, Zhang, W, Yu, J, Yang, HP, Wang, HY, et al. CA IX is upregulated in CoCl2-induced hypoxia and associated with cell invasive potential and a poor prognosis of breast cancer. Int J Oncol. (2016) 48:271–80. doi: 10.3892/ijo.2015.3253
32.Samaka, RM, Abd El-Wahed, MM, Al Sharaky, DR, Shehata, MA, Hegazy, SE, and Aleskandarany, MA. Overexpression of carbonic anhydrase IX is a dismal prognostic marker in breast carcinoma in Egyptian patients. Appl Immunohistochem Mol Morphol. (2016) 24:405–13. doi: 10.1097/PAI.0000000000000208
33.Aomatsu, N, Yashiro, M, Kashiwagi, S, Kawajiri, H, Takashima, T, Ohsawa, M, et al. Carbonic anhydrase 9 is associated with chemosensitivity and prognosis in breast cancer patients treated with taxane and anthracycline. BMC Cancer. (2014) 14:400. doi: 10.1186/1471-2407-14-400
34.Deb, S, Johansson, I, Byrne, D, Nilsson, C, Constable, L, Fjällskog, ML, et al. Nuclear HIF1A expression is strongly prognostic in sporadic but not familial male breast cancer. Mod Pathol. (2014) 27:1223–30. doi: 10.1038/modpathol.2013.231
35.Kim, HM, Jung, WH, and Koo, JS. Site-specific metabolic phenotypes in metastatic breast cancer. J Transl Med. (2014) 12:354. doi: 10.1186/s12967-014-0354-3
36.Noh, S, Kim, JY, and Koo, JS. Metabolic differences in estrogen receptor-negative breast cancer based on androgen receptor status. Tumour Biol. (2014) 35:8179–92. doi: 10.1007/s13277-014-2103-x
37.Betof, AS, Rabbani, ZN, Hardee, ME, Kim, SJ, Broadwater, G, Bentley, RC, et al. Carbonic anhydrase IX is a predictive marker of doxorubicin resistance in early-stage breast cancer independent of HER2 and TOP2A amplification. Br J Cancer. (2012) 106:916–22. doi: 10.1038/bjc.2012.32
38.Kaya, AO, Gunel, N, Benekli, M, Akyurek, N, Buyukberber, S, Tatli, H, et al. Hypoxia inducible factor-1 alpha and carbonic anhydrase IX overexpression are associated with poor survival in breast cancer patients. J BUON. (2012) 17:663–8.
39.Beketic-Oreskovic, L, Ozretic, P, Rabbani, ZN, Jackson, IL, Sarcevic, B, Levanat, S, et al. Prognostic significance of carbonic anhydrase IX (CA-IX), endoglin (CD105) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in breast cancer patients. Pathol Oncol Res. (2011) 17:593–603. doi: 10.1007/s12253-010-9355-6
40.Lou, Y, McDonald, PC, Oloumi, A, Chia, S, Ostlund, C, Ahmadi, A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. (2011) 71:3364–76. doi: 10.1158/0008-5472.CAN-10-4261
41.Jubb, AM, Soilleux, EJ, Turley, H, Steers, G, Parker, A, Low, I, et al. Expression of vascular notch ligand delta-like 4 and inflammatory markers in breast cancer. Am J Pathol. (2010) 176:2019–28. doi: 10.2353/ajpath.2010.090908
42.Tan, EY, Yan, M, Campo, L, Han, C, Takano, E, Turley, H, et al. The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br J Cancer. (2009) 100:405–11. doi: 10.1038/sj.bjc.6604844
43.Crabb, SJ, Bajdik, CD, Leung, S, Speers, CH, Kennecke, H, Huntsman, DG, et al. Can clinically relevant prognostic subsets of breast cancer patients with four or more involved axillary lymph nodes be identified through immunohistochemical biomarkers? A tissue microarray feasibility study. Breast Cancer Res. (2008) 10:R6. doi: 10.1186/bcr1847
44.Kyndi, M, Sørensen, FB, Knudsen, H, Alsner, J, Overgaard, M, Nielsen, HM, et al. Carbonic anhydrase IX and response to postmastectomy radiotherapy in high-risk breast cancer: a subgroup analysis of the DBCG82 b and c trials. Breast Cancer Res. (2008) 10:R24. doi: 10.1186/bcr1981
45.Hussain, SA, Ganesan, R, Reynolds, G, Gross, L, Stevens, A, Pastorek, J, et al. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer. (2007) 96:104–9. doi: 10.1038/sj.bjc.6603530
46.Trastour, C, Benizri, E, Ettore, F, Ramaioli, A, Chamorey, E, Pouysségur, J, et al. HIF-1alpha and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int J Cancer. (2007) 120:1451–8. doi: 10.1002/ijc.22436
47.Generali, D, Berruti, A, Brizzi, MP, Campo, L, Bonardi, S, Wigfield, S, et al. Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res. (2006) 12:4562–8. doi: 10.1158/1078-0432.CCR-05-2690
48.Tomes, L, Emberley, E, Niu, Y, Troup, S, Pastorek, J, Strange, K, et al. Necrosis and hypoxia in invasive breast carcinoma. Breast Cancer Res Treat. (2003) 81:61–9. doi: 10.1023/A:1025476722493
49.Chia, SK, Wykoff, CC, Watson, PH, Han, C, Leek, RD, Pastorek, J, et al. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. (2001) 19:3660–8. doi: 10.1200/JCO.2001.19.16.3660
50.van Kuijk, SJ, Yaromina, A, Houben, R, Niemans, R, Lambin, P, and Dubois, LJ. Prognostic significance of carbonic anhydrase IX expression in cancer patients: a meta-analysis. Front Oncol. (2016) 6:69. doi: 10.3389/fonc.2016.00069
51.Muz, B, de la Puente, P, Azab, F, and Azab, AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. (2015) 3:83–92. doi: 10.2147/HP.S93413
52.Sedlakova, O, Svastova, E, Takacova, M, Kopacek, J, Pastorek, J, and Pastorekova, S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front Physiol. (2014) 4:400. doi: 10.3389/fphys.2013.00400
53.Huber, V, Camisaschi, C, Berzi, A, Ferro, S, Lugini, L, Triulzi, T, et al. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. (2017) 43:74–89. doi: 10.1016/j.semcancer.2017.03.001
54.Boedtkjer, E, and Pedersen, SF. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. (2020) 82:103–26. doi: 10.1146/annurev-physiol-021119-034627
55.Barnett, DH, Sheng, S, Charn, TH, Waheed, A, Sly, WS, Lin, CY, et al. Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res. (2008) 68:3505–15. doi: 10.1158/0008-5472.CAN-07-6151
56.Wykoff, CC, Beasley, N, Watson, PH, Campo, L, Chia, SK, English, R, et al. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am J Pathol. (2001) 158:1011–9. doi: 10.1016/S0002-9440(10)64048-5
57.Watson, PH, Chia, SK, Wykoff, CC, Han, C, Leek, RD, Sly, WS, et al. Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br J Cancer. (2003) 88:1065–70. doi: 10.1038/sj.bjc.6600796
58.Ilie, MI, Hofman, V, Ortholan, C, Ammadi, RE, Bonnetaud, C, Havet, K, et al. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int J Cancer. (2011) 128:1614–23. doi: 10.1002/ijc.25491
59.Nordfors, K, Haapasalo, J, Korja, M, Niemelä, A, Laine, J, Parkkila, AK, et al. The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an association of CA IX with poor prognosis. BMC Cancer. (2010) 10:148. doi: 10.1186/1471-2407-10-148
Keywords: breast cancer, carbonic anhydrase IX, meta-analysis, prognosis, survival
Citation: Numprasit W, Yangngam S, Prasopsiri J, Quinn JA, Edwards J and Thuwajit C (2023) Carbonic anhydrase IX-related tumoral hypoxia predicts worse prognosis in breast cancer: A systematic review and meta-analysis. Front. Med. 10:1087270. doi: 10.3389/fmed.2023.1087270
Edited by:
Noha Mousaad Elemam, University of Sharjah, United Arab EmiratesReviewed by:
Pawel Swietach, University of Oxford, United KingdomJaromir Pastorek, Slovak Academy of Sciences, Slovakia
Copyright © 2023 Numprasit, Yangngam, Prasopsiri, Quinn, Edwards and Thuwajit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chanitra Thuwajit, chanitra.thu@mahidol.ac.th
 Warapan Numprasit
Warapan Numprasit Supaporn Yangngam
Supaporn Yangngam Jaturawitt Prasopsiri
Jaturawitt Prasopsiri Jean A. Quinn2
Jean A. Quinn2  Joanne Edwards
Joanne Edwards Chanitra Thuwajit
Chanitra Thuwajit