Exome-wide analysis identify multiple variations in olfactory receptor genes (OR12D2 and OR5V1) associated with autism spectrum disorder in Saudi females
- 1Department of Clinical Pharmacy Research, Institute for Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 2Department of Genetic Research, Institute for Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 3College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 4Department of Neurology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Background: Autism Spectrum Disorder (ASD) is a multifactorial, neurodevelopmental disorder, characterized by deficits in communication, restricted and repetitive behaviors. ASD is highly heritable in Saudi Arabia; indecencies of affected individuals are increasing.
Objectives: To identify the most significant genes and SNPs associated with the increased risk of ASD in Saudi females to give an insight for early diagnosis.
Methods: Pilot case–control study mostly emphasized on the significant SNPs and haplotypes contributing to Saudi females with ASD patients (n = 22) compared to controls (n = 51) without ASD. With the use of allelic association analysis tools, 243,345 SNPs were studied systematically and classified according to their significant association. The significant SNPs and their genes were selected for further investigation for mapping of ASD candidate causal variants and functional impact.
Results: In females, five risk SNPs at p ≤ 2.32 × 10−05 was identified in association with autism. The most significant exonic variants at chromosome 6p22.1 with olfactory receptor genes (OR12D2 and OR5V1) clustered with high linkage disequilibrium through haplotyping analysis. Comparison between highly associated genes (56 genes) of male and female autistic patients with female autistic samples revealed that 39 genes are unique biomarkers for Saudi females with ASD.
Conclusion: Multiple variations in olfactory receptor genes (OR5V1 and OR12D2) and single variations on SPHK1, PLCL2, AKAP9 and LOC107984893 genes are contributing to ASD in females of Arab origin. Accumulation of these multiple predisposed coding SNPs can increase the possibility of developing ASD in Saudi females.
1. Introduction
Autism Spectrum Disorder (ASD) is a range of neurodevelopmental and neuropsychiatric disorders that start appearing from early childhood and lasts throughout the person’s life (1, 2). Autism is among the most heritable and severe form of ASD (3), characterized by deficits in communication as well as repetitive and restricted behaviors as reported in the Diagnostic and Statistical Manual, Fifth Edition (DSM-5) (4). The World Health Organization (WHO) revealed statistics of 1 in 160 children to be diagnosed with ASD (5). In 2017, the latest ASD statistics in Saudi Arabia revealed that one per 167 individual is affected by autism (6). ASD is commonly multifactorial and many studies suggested interactions between immunological, neurological, environmental and genetic factors (7, 8). Tremendous sex bias of ASD shows that males are more affected than females with a male to female ratio of 3:1 (9). Several studies investigated the genetic risk factors against ASD in females, yet the key factors remain unknown (10–12). This paper identifies some genetic variables susceptible to cause autism in Saudi females, addresses the correlation between some diseases and pathways and genetic variants in Saudi females.
2. Methodology
2.1. Sample collection
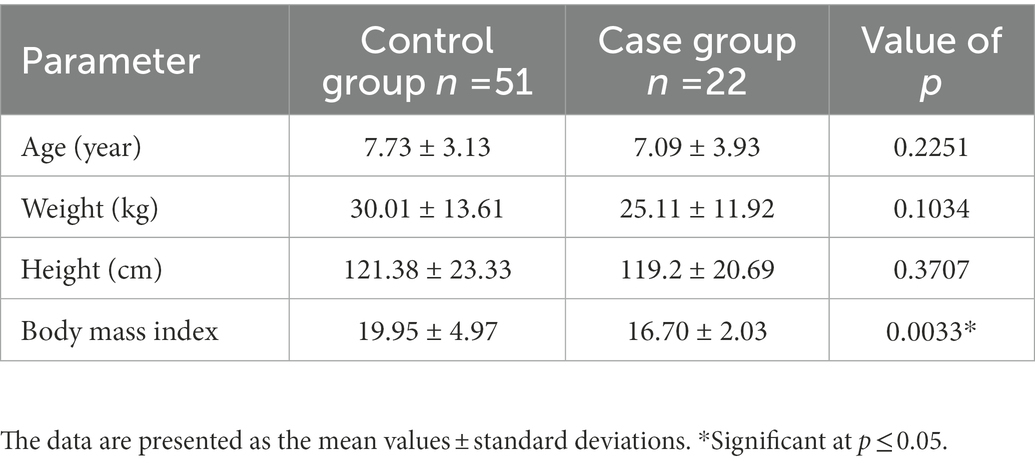
The present study is conducted in accordance with the Declaration of Helsinki and received approval from the Institutional Review Board (IRB) of Imam Abdulrahman Bin Faisal University (IRB-2016-13-152). Out of 73 female age matched samples were included, 22 were cases and 51 were controls (Table 1). The present study sheds light on potential genetic contributors to autism in Saudi female subjects. Buccal cell samples were collected from the study subjects upon receiving the signed informed consent. All the samples were collected from the King Fahad Hospital of the University, Al Khobar, Saudi Arabia.
2.2. DNA extraction and genotyping
To extract DNA from buccal cell samples, the Gentra Puregene Buccal Cell Kit (Qiagen, Hilden, Germany) was used. The buccal cells were collected by scraping the inside of the mouth 10 times with given sterile brush. DNA was extracted within 3 h from the collection, 300 μl Cell lysis solution was dispensed in a 1.5 ml tube, incubated at 65°C for 15 min, and 1.5 μl of proteinase K was added and incubated at 55°C for 60 min. Then we added 100 μl protein precipitation reagent and incubated for 5 min on ice and centrifuged (13,000–16,000 ×g for 3 min). Supernatant was mixed with 300 μl isopropanol and 0.5 μl glycogen, centrifuged for 5 min at 13,000–16,000 ×g. The supernatant was discarded, the DNA pellet was washed with 70% ethanol and suspended the DNA in TE buffer. The Human Exome Bead Chip Kit v1.0 and v1.1 Illumina (San Diego, CA, United States), which is constituted of 243,345 putative functional exonic markers, was used with Illumina iScan for the microarray genotyping. DNA processing was performed in accordance with the manufacturer’s protocol and all genotyping data were obtained from iScan control software (Illumina). DNA extraction and microarray genotyping and analysis took place in the genetics research laboratory of the Institute for Research and Medical Consultation, Imam Abdulrahman Bin Faial University, Dammam, Saudi Arabia. The procedures were executed between 2016 and 2019. The Infinium HTS workflow is a rapid 3 days work flow, in brief: The PicoGreen dsDNA quantification reagent was used to quantify double-stranded DNA samples. The quantified DNA samples were processed in 96 well plates. The quantified DNA samples were denatured and neutralized to prepare them for amplification. All the DNA samples were incubated uniformly to amplify, to generate a sufficient quantity of each individual DNA sample to be used in the Infinium HTS Assay. We Incubated the MSA3 plate with amplified DNA in the Illumina hybridization oven for 20–24 h at 37°C. Then to fragment the DNA, an endpoint fragmentation was used. A 100% 2-propanol and precipitation reagents were used to precipitate the DNA. Then, re-suspended the precipitated and fragmented DNA. The re-suspended DNA was dispensed onto bead chips and incubated for hybridization of each DNA sample to specific section of the bead chip. Afterwards, the bead chips were prepared for the staining process. Then the un-hybridized and non-specifically hybridized DNA samples were washed from the bead chips, added labeled nucleotides to extend primers hybridized to the sample, and stains the primers. For imaging the bead chip we followed the instructions in the System Guide for instrument to scan. Intensity files from iScan of the individual DNA samples from the exome chip were to perform the genotyping. Sample sheets with sample information, such as plate ID, cell ID, gender and so on were used for fetching the data from intensity files to perform the genotyping using GenomeStudio 2,0 software (Illumina.
2.3. Statistical and functional analysis
Initial quality check of call rate was fulfilled using GenomeStudio 2,0 software (Illumina). Only one control was eliminated from the analysis due to a call rate of < 0.98% and remaining samples were re-clustered. Using the Chi-square test with 1 degree of freedom (df), Hardy–Weinberg equilibrium (HWE) was tested individually for all the variants. Reference SNP ID numbers and gene names were acquired from SNP-Nexus (13) and Kaviar (14). To assess the outcomes of different alleles and haplotypes, 95% confidence interval, odds ratios and case–control association analyses were calculated using gPlink version 2.050 (15) and Haploview version 4.2 (16). The p values < 0.001 were regarded as significant. DAVID 6.7 (17) and Enricher (18) were utilized to annotate the highly significant remarkable (p < 1 × 10−05) genes for functional implications.
3. Results
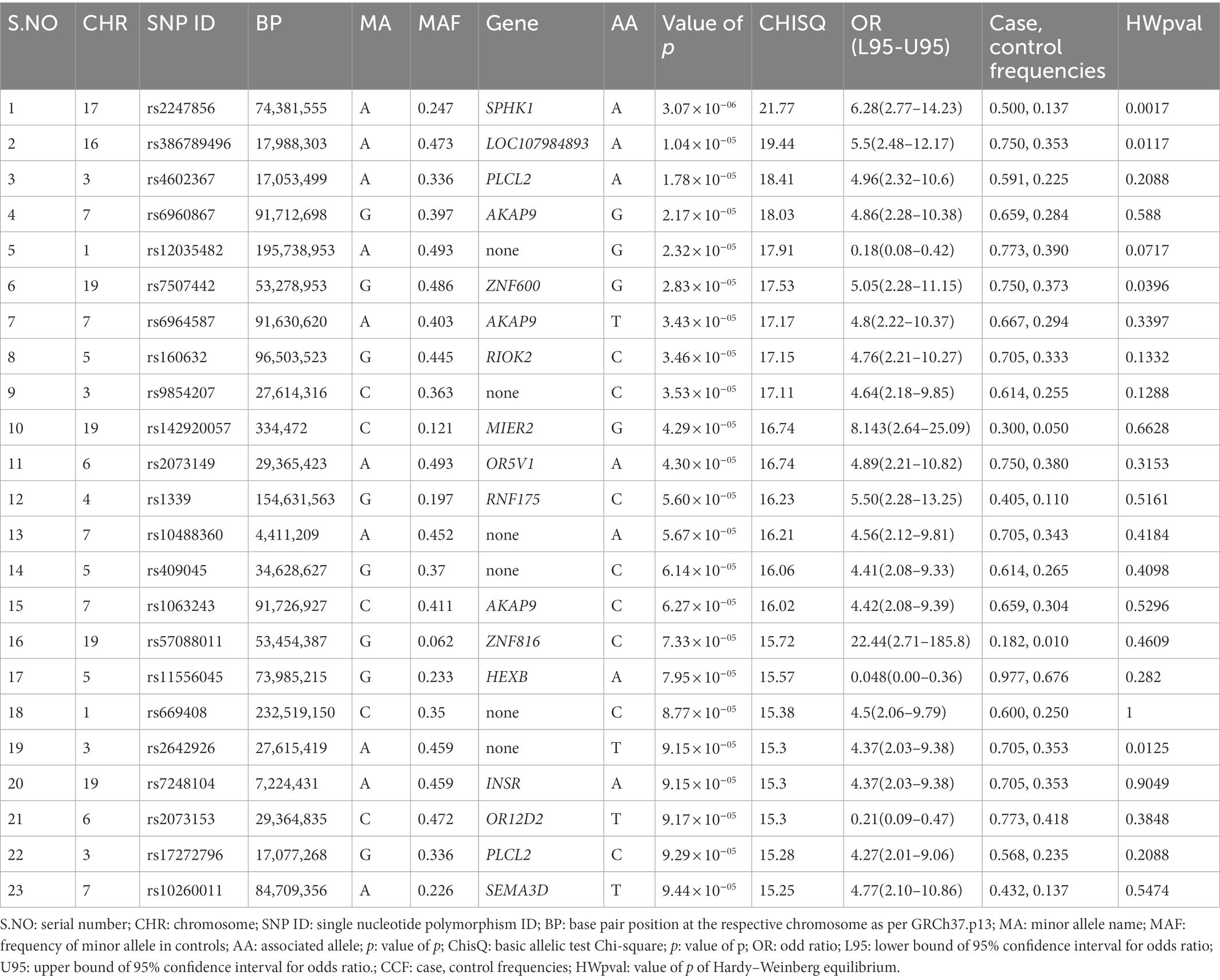
Genotyping (Illumina) data were submitted to the NCBI (National Center for Biotechnology Information) Gene Expression Omnibus (GEO) repository [GEO accession number: GSE221098; BioProject accession numbers: PRJNA912746; GEO accession numbers for individual samples: GSM6845201-GSM6845273].1 After filtering 243,345 SNPs according to their p values, 280 SNPs with p < 0.0001 were selected as significant (p < 9.44 × 10−05; Table 2). The most significant SNPs suggesting a correlation with autism were rs2247856 (p = 3.069 × 10−06 at SPHK1), rs386789496 (p = 1.036 × 10−05 at LOC107984893), rs4602367 (p = 1.783 × 10−05 at PLCL2), rs6960867 (p = 2.17 × 10−05 at AKAP9) and rs12035482 (p = 2.32 × 10−05) located on chromosome 17, 16, 3, 7 and 1, respectively, (Figure 1). All the significant SNPs of Saudi females autistic patients with p < 0.00018 are listed in Supplementary Table 1 in which all obey the hardy–Weinberg equilibrium.
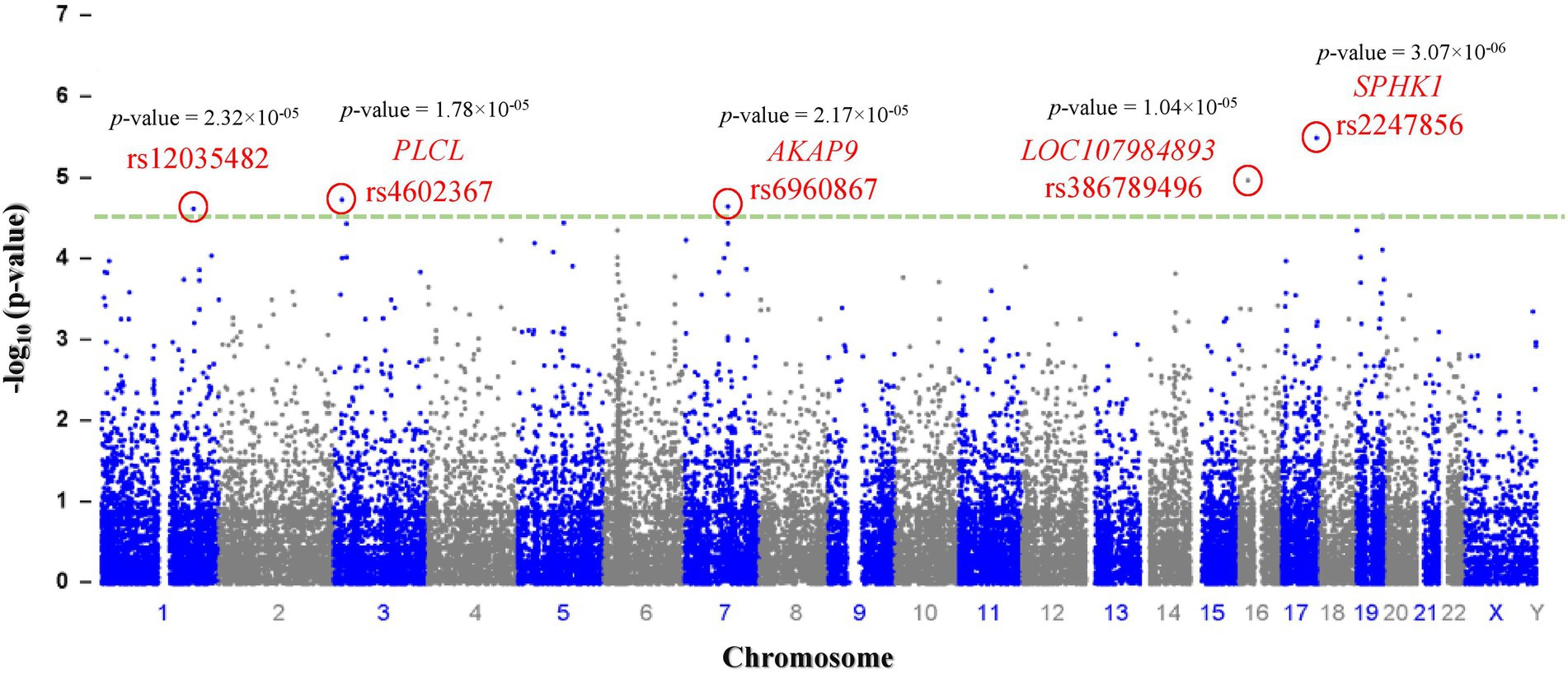
Figure 1. Manhattan plot: a total of (n = 243,345) SNPs are plotted according to p-values (y-axis) and their position in the genome (x-axis). The most significant candidate nucleotide variants rs2247856 (SPHK1), rs386789496 (LOC107984893), rs4602367 (PLCL), rs6960867 (AKAP9) and rs12035482 on chromosome 17, 16, 3, 7and 1 respectively, exceed the significance threshold line (p = 1.00 × 10–4.5 - green dash line) indicating a statistically significant correlation with autism.
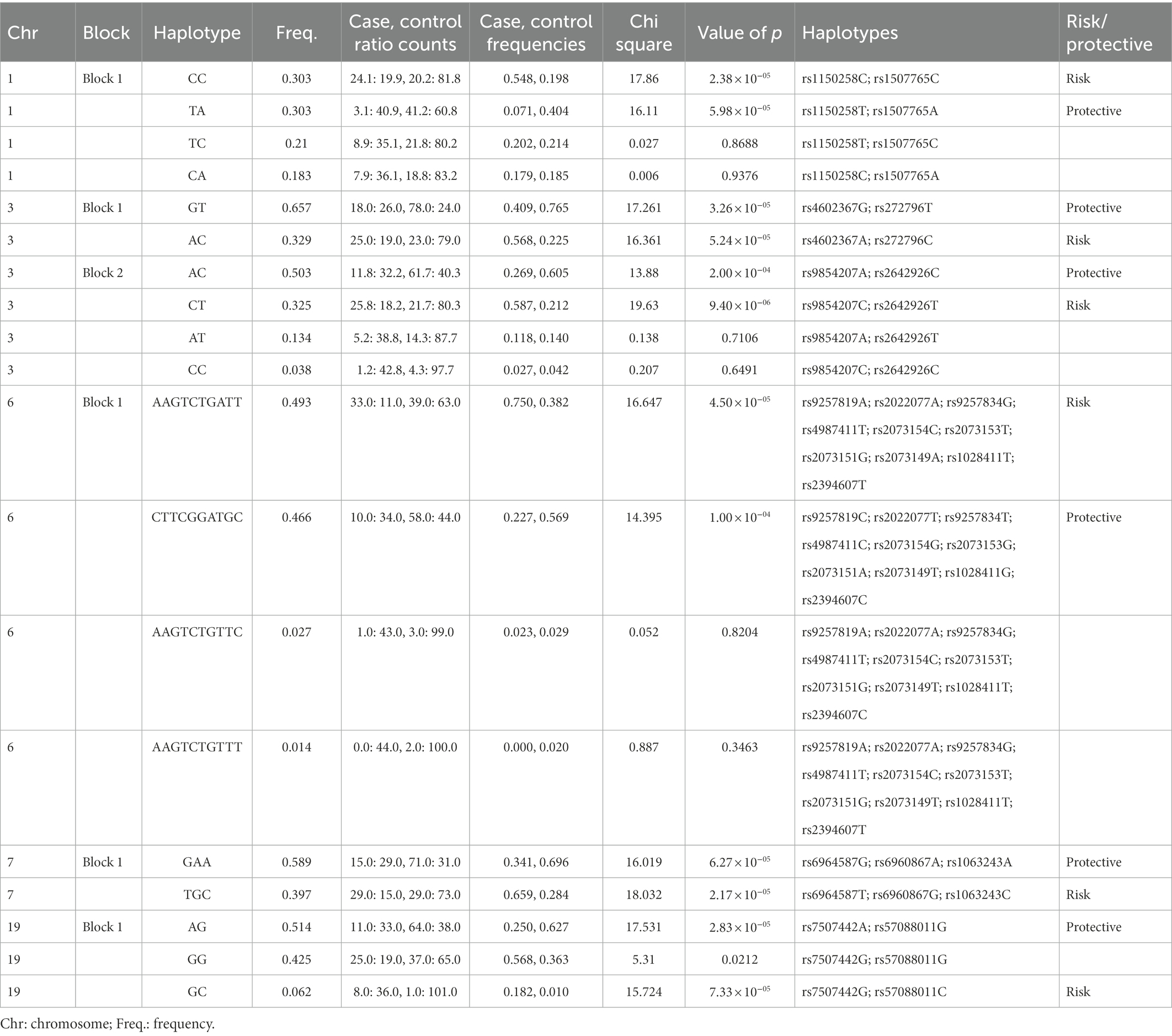
All association tests were screened using minor alleles’ frequency in controls, value of p of Hardy–Weinberg equilibrium and type 1 error rate to achieve the strongest genetic predisposition and imputed for linkage disequilibrium in HapMap SNPs in multiple chromosomes (Figure 2). The haplotype analysis implemented on SNPs with significance of p < 0.0001 were classified into protective (less probable to cause autism) and risk (more probable to cause autism; Table 3). Risk alleles are listed as the following: in Chromosome 1: IL24-rs1150258C; rs1507765C (value of p = 2.3773 × 10−5); Chromosome 3: PLCL2-rs4602367A; PLCL2-rs17272796C (value of p = 3.2579 × 10−5); rs9854207; rs2642926 (value of p = 9.399 × 10−6); Chromosome 6: OR5V1-rs9257819A; OR5V1-rs2022077A; OR12D2-rs9257834G; OR12D2-rs4987411T; OR12D2-rs2073154C; OR12D2-rs2073153T; OR12D2-rs2073151G; OR5V1-rs2073149A; OR5V1-rs1028411T; OR5V1-rs2394607T (value of p = 4.5015 × 10−5); Chromosome 7: AKAP9-rs6964587T; AKAP9-rs6960867G; AKAP9-rs1063243C (value of p = 2.1723 × 10−5) and Chromosome 19; ZNF600-rs7507442G; ZNF816-rs57088011C (value of p = 7.3276 × 10−5; Table 3; Figure 2). Whereas the alleles of protective haplotypes are: in Chromosome 1: IL24-rs1150258T; rs1507765A (value of p = 5.9759 × 10−5); Chromosome 3: PLCL2-rs4602367G; PLCL2-rs272796T (value of p = 3.2579 × 10−5); rs9854207A; rs2642926C (value of p = 2 × 10−4); Chromosome 6: OR5V1-rs9257819C; OR5V1-rs2022077T; OR12D2-rs9257834T; OR12D2-rs4987411C; OR12D2-rs2073154G; OR12D2-rs2073153G; OR12D2-rs2073151A; OR5V1-rs2073149T; OR5V1-rs1028411G; OR5V1-rs2394607C (value of p = 1 × 10−4); Chromosome 7: AKAP9-rs6964587G; AKAP9-rs6960867A; AKAP9-rs1063243A (value of p = 6.272 × 10−5) and Chromosome 19; ZNF600-rs7507442A; ZNF816-rs57088011G (value of p = 2.8266 × 10−5; Tables 3; Figure 2). Surprisingly, olfactory receptor family 23 subfamily D member 2 (OR12D2) and olfactory receptor family 5 subfamily V member 1 (OR5V1) located on chromosome 6 had multiple significant nucleotide variants in Saudi autistic females (Figure 2).
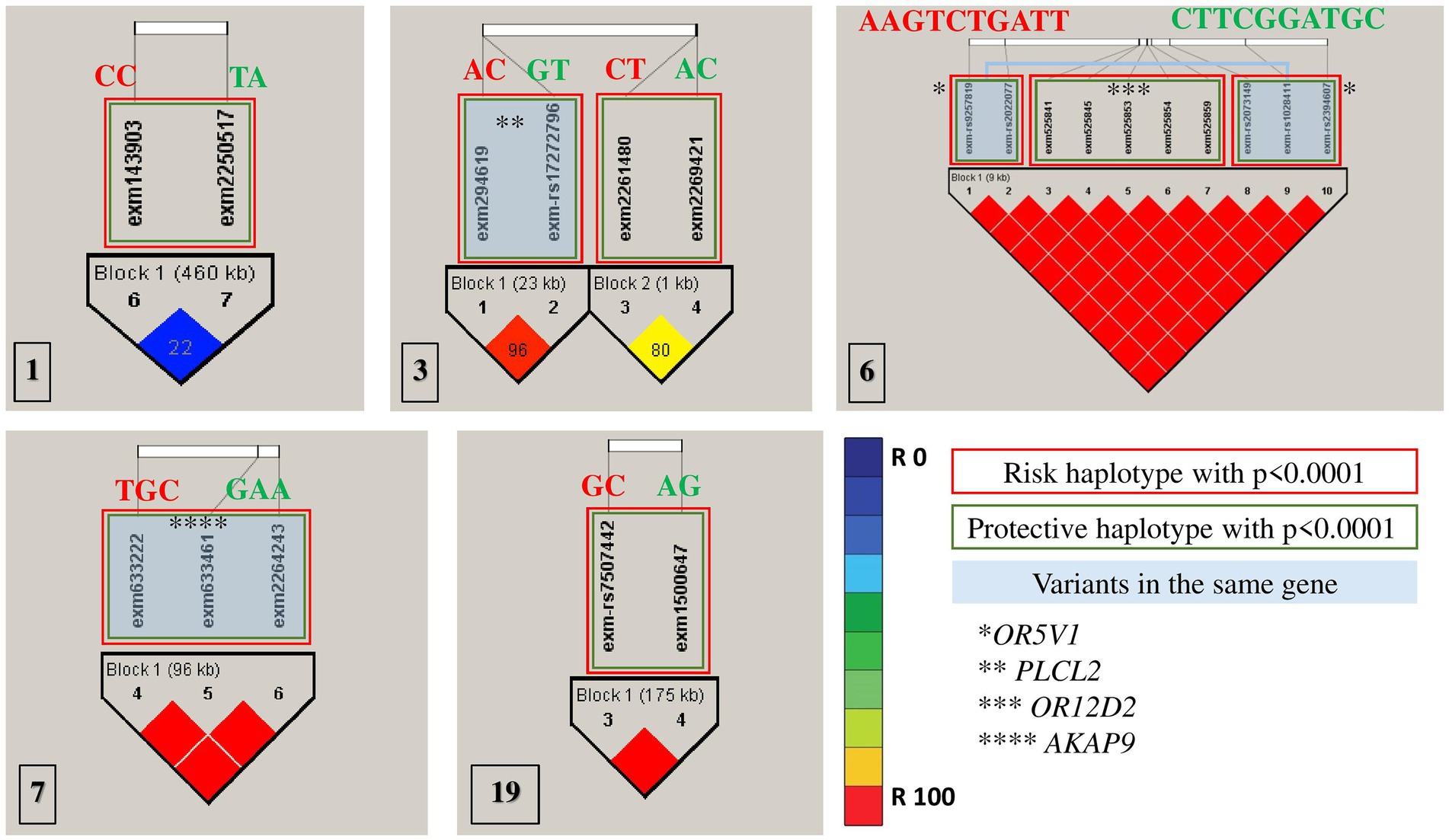
Figure 2. Haplotype blocks representing the linkage disequilibrium of chosen SNPs on chromosomes of autistic females in Saudi Arabia. The numbers at the bottom left of each picture correspond to the chromosome number. * refers to the genes’ names. Red rectangles are the most risk haplotypes, green rectangles are the most protective haplotypes and light blue rectangles highlights the SNPs which are located in the same gene. Further details are in Table 3.
After conducting functional enrichment analysis of females’ gene list, genes with SNPs p < 0.00018 have shown a link to certain diseases including systemic lupus erythematosus disease (SLE; 5 Genes; p = 0.004400769; BRD2, OR12D2, CR2, OR5V1, HLA-DOA), Amyotrophic Lateral Sclerosis (3 Genes; p = 0.046868501; CUBN, SIPA1L2, COMMD10), as well as some pathways like regulation of complement cascade (2 Genes; p = 0. 0.0018; CD55, CR2) vitamin B12 metabolism (2 Genes; p = 0.006683; CUBN, INSR), and female preferences for male odors (2 Genes; p = 0.008301262; OR12D2, OR5V1). The most significant SNPs with p < 0.0001 (Supplementary Table 1) associated with autism in Saudi females were subjected for the functional annotation, there were 27 DAVID IDs. Gene ontology enrichment analysis indicated the significant (p value = 0.0051; involved 6 genes MLXIPL, ZNF816, YEATS2, INSR, PROX2, and ZNF600) biological process, regulation of DNA-templated transcription (GO:0006355).
4. Discussion
This study evaluates the risk of genetic variation in ASD Saudi female subjects. The most significant SPHK1 SNP rs2247856 was reported recently as a significantly associated variant with Parkinson’s disease in both genders (19). GWAS catalog2 of rs2247856 reported the observed risk allele (rs2247856-A) of the present study with reticulocyte count, mean corpuscular volume, lymphocyte count, and reticulocyte fraction of red cells. However, no earlier reports on autism. No previous association was reported on rs386789496, rs6960867 and rs12035482. GWAS catalog of PLCL2 SNP rs4602367-A reported the association with rheumatoid arthritis (20). Even though, PLCL2 SNP rs4602367-A was not reported on autism, a recent study revealed the association of PLCL2 SNPs (rs6800583 and rs73139272) with autism (21).
Beginning with significant genes plotted in Manhattan plot, SPHK1 has the highest value of p of 3.069 × 10−6. SPHK1 is a key enzyme of sphingolipid metabolism which modulates cellular proliferation and pro-survival function (22). Since SPHK1 and SPHK2 phosphorylate sphingosine to sphingosine-1-phosphate (S1P) (23) (Figure 3), the presence of a high concentration of SPHK1 increases the production of S1P which when elevated can lead to autism according to Wu et al. (24). In addition, based on multiple logistic regression analysis, S1P alterations were considered significant biomarker predictor for autism (23). Similarly, dysregulation of S1P triggers the manifestation of psychiatric and neurological diseases such as Alzheimer’s disease (25), schizophrenia (26) Parkinson’s disease (27) and anxiety disorder (28). However, an experiment done on valproic acid rat model found that protein expression of SPHK1 wasn’t significant as it did not reach the significance level (24). Protein modeling of mutated SPHK1 denotes the damaging changes, which indicates the mutated SPHK1 protein can affect the Sphingolipid metabolism pathway (Figure 4). Some findings reported proteins encoded by AKAP9, another significant gene in the allelic association study, to be highly expressed in autism subjects (29). Yet, the mechanism of the association is still unknown.

Figure 3. Sphingolipid metabolism pathway illustrates the processes of sphingosine-1-phosphate (S1P) production. The last step is catalyzed by SPHK1, a significant protein-coding gene associated with autism in Saudi females.
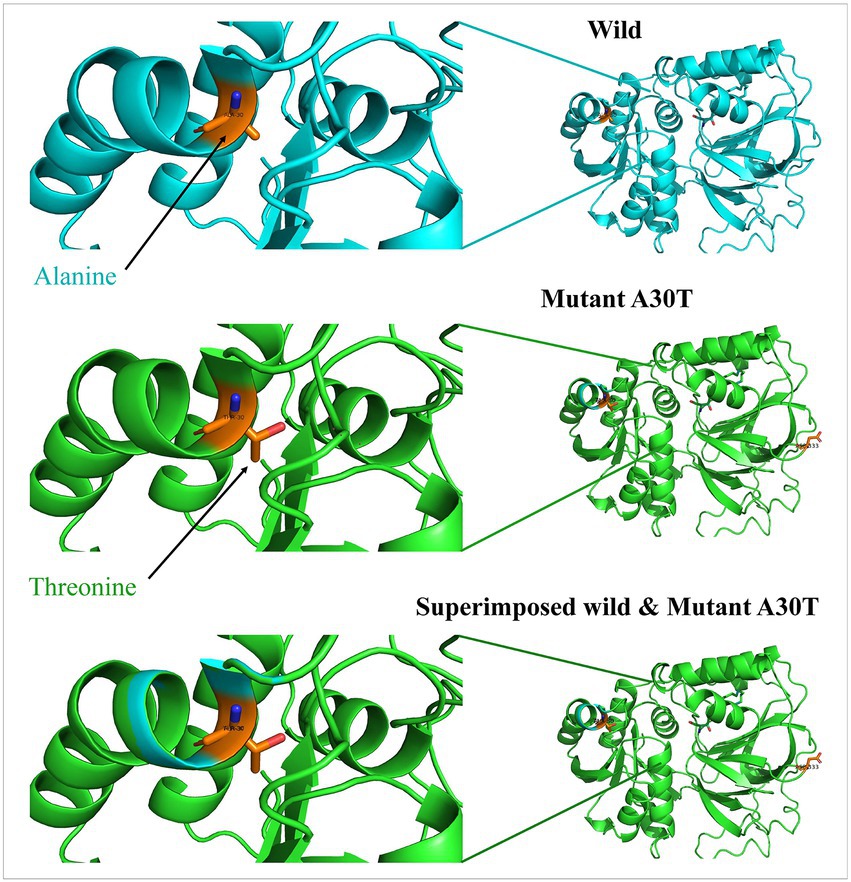
Figure 4. Protein models of SPHK1 wild-type and mutated proteins. Root mean square deviation (RMSD) for the superimposed: 0.001.
Significant SNP candidates for ASD etiology in females were perceived to be located at OR12D2 and OR5V1 genes on chromosome 6 having high linkage disequilibrium (Figure 2). Several studies have perceived sensory abnormalities in autism subjects including unusual odor perception (30, 31). Indeed, olfactory genes influence the neurodevelopment status in terms of social, emotional and behavioral functioning (30, 32). A study reported a link between a cluster of SNPs located within the olfactory receptor genes on chromosome 6p22.1 and social defects in ASD ( (33); Figure 5). Interestingly, Systemic Lupus Erythematosus (SLE), which is an autoimmune disease closely related to autism, is accompanied by variations in olfactory receptor genes (Figure 5). A research conducted in Egypt revealed that 7 out of 38 autoimmune ASD patients had a family history of SLE (34). The largest cohort study done on 719 SLE offspring reported a strong association between the two disorders (35). Further evidence supporting the relationship between the two disorders is found through the functional enrichment analysis suggesting that OR12D2 and OR5V1 are commonly affected genes in both SLE and female ASD patients. Large cluster of olfactory receptor genes on chromosome 6 is located in proximity to class 1 histocompatibility complex genes which mediate immunity (36). Another factor that attributes to the development of ASD in SLE’s offspring is the presence of the autoimmune antibodies in patients with SLE which attack the Ro60 protein bound to YRNA (37, 38). All these factors justify the reason behind the doubled risk of ASD in SLE patients’ offspring, giving that 21.4–26% of SLE offspring have autism (39). Current studies have emphasized on the potential role for the immune system in ASD, with immune-genetic abnormalities and the inappropriate response of the immune system to environmental challenges. A meta-analysis of 7 observational studies (25,005 ASD cases and 4,543,321 participants) was conducted assessing the relationships between maternal systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) and risk for ASD in offspring. The results showed that maternal RA was associated with an increased risk for ASDs, whereas maternal SLE was associated with an increased risk for ASD only in western population (40, 41).
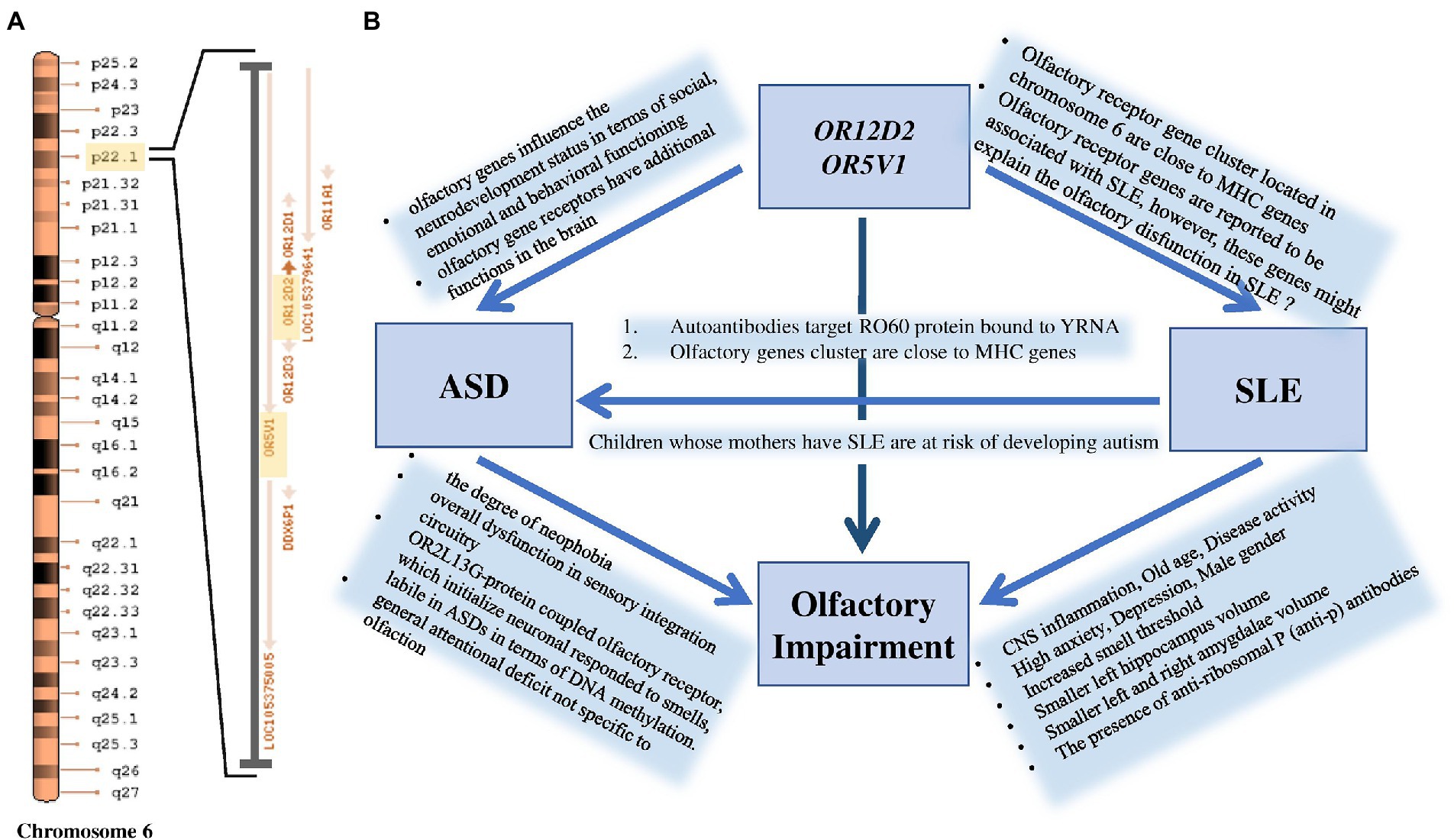
Figure 5. (A) Significant ASD variation at OR12D2 and OR5V1 genes are located within a cluster of olfactory receptor genes at the chromosomal region 6p22.1. (B) The figure shows that maternal SLE influences the manifestation of ASD in their offspring. Smell deficits and variations in olfactory receptor genes, particularly in OR12D2 and OR5V1, are common in both ASD and SLE patients.
Another medical condition that shares common genes with ASD is vitamin B12 (cobalamin) deficiency, which causes many neurological and psychiatric disorders. Cobalamin catalyzes the conversion of homocysteine to methionine (1, 42). A study conducted in Oman on 80 participants half of which are cases revealed an accumulation of homocysteine and reduced levels of methionine due to vitamin B12 insufficiency (43). Another study attributed the association between Vitamin B12 and autism to the role of vitamin B12 in the methylation cycle and genetic material biosynthesis (44). Biochemical abnormalities related with ASD consist of impaired methylation and sulphation capacities beside low glutathione (GSH) redox capacity. Possible managements for these abnormalities comprise cobalamin (B12). A systematic review of a total 17 studies was identified studies using vitamin B12 to manage ASD. The study found that generally; vitamin B12 seems to have evidence for efficacy in patients with ASD, especially in individuals who have been identified with unfavorable biochemical profiles. Initial clinical evidence proposes that vitamin B12, mostly subcutaneously injected, improves metabolic abnormalities in ASD alongside with clinical symptoms. Cobalamin is a promising supplement used in the management of ASD (45). The limitations of the current study should be acknowledged. First, the pilot study design nature and its relatively small sample size.
5. Conclusion
In summary, the findings of this study provide the first evidence for female-based genetic analysis in Saudi Arabia and assess the relationship between olfactory receptor genes and ASD. Furthermore, variations on olfactory receptor genes elucidate the impact of SLE in females and the inheritance of ASD. Future investigations with more representative samples that include experiments on rat models are needed to practically prove the association and enhance ASD managing choices.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: GEO database, under accession GSE221098.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of Imam Abdulrahman Bin Faisal University (IRB-2016-13-152). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
NA, AA, SA, JB: conceptualization, data curation, and investigation. MA, HA, SA, JB, and NA: formal analysis. NA: funding acquisition. NA, SA, and JB: methodology, project administration, resources, software, supervision, validation, and visualization. MA, HA, AA, SA, JB, and NA: writing—original draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University (Grant No: 2016-057-IRMC).
Acknowledgments
We express our sincere gratitude to the Dean of the Institute for Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia for her continuous encouragement and support. We also appreciate the technical assistance from Ranilo M. Tumbaga, Horace T. Pacifico, and Jee E. Aquino. We are grateful for all patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1051039/full#supplementary-material
Footnotes
References
1Almandil, NB, Alkuroud, D, AbdulAzeez, S, AlSulaiman, A, Elaissar, A, and Borgio, JF. Environmental and genetic factors in autism Spectrum disorders: special emphasis on data from Arabian studies. Int. J. Environ. Res. Public Health. (2019) 16:658. doi: 10.3390/ijerph16040658
2Werling, DM. The role of sex-differential biology in risk for autism spectrum disorder. Biol. Sex Differ. (2016) 7:58. doi: 10.1186/s13293-016-0112-8
3El-Fishawy, P. The genetics of autism: key issues, recent findings, and clinical implications. Psychiatr. Clin. (2010) 33:83–105. doi: 10.1016/j.psc.2009.12.002
4Grzadzinski, R, Huerta, M, and Lord, C. DSM-5 and autism spectrum disorders (ASDs): an opportunity for identifying ASD subtypes. Mol. Autism. (2013) 4:12. doi: 10.1186/2040-2392-4-12
5Who.int. (2018). Autism spectrum disorders. Available at: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders (Accessed July 14, 2019).
6Alnemary, FM, Aldhalaan, HM, Simon-Cereijido, G, and Alnemary, FM. Services for children with autism in the Kingdom of Saudi Arabia. Autism. (2017) 21:592–602. doi: 10.1177/1362361316664868
7Gottfried, C, Bambini-Junior, V, Francis, F, Riesgo, R, and Savino, W. The impact of neuroimmune alterations in autism spectrum disorder. Front. Psychol. (2015) 6:121. doi: 10.3389/fpsyt.2015.00121
8Lyall, K, Croen, L, Daniels, J, Fallin, MD, Ladd-Acosta, C, Lee, BK, et al. The changing epidemiology of autism spectrum disorders. Annu. Rev. Public Health. (2017) 38:81–102. doi: 10.1146/annurev-publhealth-031816-044318
9Loomes, R, Hull, L, and Mandy, WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry. (2017) 56:466–74. doi: 10.1016/j.jaac.2017.03.013
10Carney, RM, Wolpert, CM, Ravan, SA, Shahbazian, M, Ashley-Koch, A, Cuccaro, ML, et al. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr. Neurol. (2003) 28:205–11. doi: 10.1016/S0887-8994(02)00624-0
11Hu, VW, Sarachana, T, Sherrard, RM, and Kocher, KM. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol. Autism. (2015) 6:7. doi: 10.1186/2040-2392-6-7
12Woodbury-Smith, M, Deneault, E, Yuen, RK, Walker, S, Zarrei, M, Pellecchia, G, et al. Mutations in RAB39B in individuals with intellectual disability, autism spectrum disorder, and macrocephaly. Mol. Autism. (2017) 8:59. doi: 10.1186/s13229-017-0175-3
13Dayem Ullah, AZ, Lemoine, NR, and Chelala, C. SNPnexus: a web server for functional annotation of novel and publicly known genetic variants (2012 update). Nucleic Acids Res. (2012) 40:W65–70. doi: 10.1093/nar/gks364
14Glusman, G, Caballero, J, Mauldin, DE, Hood, L, and Roach, JC. Kaviar: an accessible system for testing SNV novelty. Bioinformatics. (2011) 27:3216–7. doi: 10.1093/bioinformatics/btr540
15Purcell, S, Neale, B, Todd-Brown, K, Thomas, L, Ferreira, MA, Bender, D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. (2007) 81:559–75. doi: 10.1086/519795
16Barrett, JC, Fry, B, Maller, JDMJ, and Daly, MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. (2004) 21:263–5. doi: 10.1093/bioinformatics/bth457
17Huang, DW, Sherman, BT, and Lempicki, RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. (2009) 4:44–57. doi: 10.1038/nprot.2008.211
18Chen, EY, Tan, CM, Kou, Y, Duan, Q, Wang, Z, Meirelles, GV, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. (2013) 14:128. doi: 10.1186/1471-2105-14-128
19Jo, S, Park, KW, Hwang, YS, Lee, SH, Ryu, H-S, and Chung, SJ. Microarray genotyping identifies new loci associated with dementia in Parkinson’s disease. Genes. (2021) 2021:1975. doi: 10.3390/genes12121975
20Ha, E, Bae, SC, and Kim, K. Large-scale meta-analysis across east Asian and European populations updated genetic architecture and variant-driven biology of rheumatoid arthritis, identifying 11 novel susceptibility loci. Ann. Rheum. Dis. (2021) 80:558–65. doi: 10.1136/annrheumdis-2020-219065
21Rao, S, Baranova, A, Yao, Y, Wang, J, and Zhang, F. Genetic relationships between attention-deficit/hyperactivity disorder, autism Spectrum disorder, and intelligence. Neuropsychobiology. (2022) 81:484–96. doi: 10.1159/000525411
22Maceyka, M, Sankala, H, Hait, NC, Le Stunff, H, Liu, H, Toman, R, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. (2005) 280:37118–29. doi: 10.1074/jbc.M502207200
23Wang, H, Liang, S, Wang, M, Gao, J, Sun, C, Wang, J, et al. Potential serum biomarkers from a metabolomics study of autism. J. Psychiatry Neurosci. (2016) 41:27–37. doi: 10.1503/jpn.140009
24Wu, H, Zhang, Q, Gao, J, Sun, C, Wang, J, Xia, W, et al. Modulation of sphingosine 1-phosphate (S1P) attenuates spatial learning and memory impairments in the valproic acid rat model of autism. Psychopharmacology. (2018) 235:873–86. doi: 10.1007/s00213-017-4805-4
25Asle-Rousta, M, Kolahdooz, Z, Oryan, S, Ahmadiani, A, and Dargahi, L. FTY720 (Fingolimod) attenuates Beta-amyloid peptide (Aβ 42)-induced impairment of spatial learning and memory in rats. J. Mol. Neurosci. (2013) 50:524–32. doi: 10.1007/s12031-013-9979-6
26Kucharska-Mazur, J, Tarnowski, M, Dołęgowska, B, Budkowska, M, Pędziwiatr, D, Jabłoński, M, et al. Novel evidence for enhanced stem cell trafficking in antipsychotic-naïve subjects during their first psychotic episode. J. Psychiatr. Res. (2014) 49:18–24. doi: 10.1016/j.jpsychires.2013.10.016
27Sivasubramanian, M, Kanagaraj, N, Dheen, ST, and Tay, SSW. Sphingosine kinase 2 and sphingosine-1-phosphate promotes mitochondrial function in dopaminergic neurons of mouse model of Parkinson’s disease and in MPP+-treated MN9D cells in vitro. Neuroscience. (2015) 290:636–48. doi: 10.1016/j.neuroscience.2015.01.032
28Jang, S, Kim, D, Lee, Y, Moon, S, and Oh, S. Modulation of sphingosine 1-phosphate and tyrosine hydroxylase in the stress-induced anxiety. Neurochem. Res. (2011) 36:258–67. doi: 10.1007/s11064-010-0313-1
29Poelmans, G, Franke, B, Pauls, DL, Glennon, JC, and Buitelaar, JK. AKAPs integrate genetic findings for autism spectrum disorders. Transl. Psychiatry. (2013) 3:e270. doi: 10.1038/tp.2013.48
30Almandil, NB, AlSulaiman, A, Aldakeel, SA, Alkuroud, DN, Aljofi, HE, Alzahrani, S, et al. Integration of Transcriptome and exome genotyping identifies significant variants with autism Spectrum disorder. Pharmaceuticals. (2022) 15:158. doi: 10.3390/ph15020158
31Boudjarane, MA, Grandgeorge, M, Marianowski, R, Misery, L, and Lemonnier, É. Perception of odors and tastes in autism spectrum disorders: a systematic review of assessments. Autism Res. (2017) 10:1045–57. doi: 10.1002/aur.1760
32Tonacci, A, Billeci, L, Tartarisco, G, Ruta, L, Muratori, F, Pioggia, G, et al. Olfaction in autism spectrum disorders: a systematic review. Child Neuropsychol. (2017) 23:1–25. doi: 10.1080/09297049.2015.1081678
33St Pourcain, B, Whitehouse, AO, Ang, WQ, Warrington, NM, Glessner, JT, Wang, K, et al. Common variation contributes to the genetic architecture of social communication traits. Mol. Autism. (2013) 4:34. doi: 10.1186/2040-2392-4-34
34Mostafa, GA, and Kitchener, N. Serum anti-nuclear antibodies as a marker of autoimmunity in Egyptian autistic children. Pediatr. Neurol. (2009) 40:107–12. doi: 10.1016/j.pediatrneurol.2008.10.017
35Vinet, É, Pineau, CA, Clarke, AE, Scott, S, Fombonne, É, Joseph, L, et al. Increased risk of autism spectrum disorders in children born to women with systemic lupus erythematosus: results from a large population-based cohort. Arthritis Rheum. (2015) 67:3201–8. doi: 10.1002/art.39320
36Ortega-Hernandez, OD, Kivity, S, and Shoenfeld, Y. Olfaction, psychiatric disorders and autoimmunity: is there a common genetic association? Autoimmunity. (2009) 42:80–8. doi: 10.1080/08916930802366140
37Kowalski, MP, and Krude, T. Functional roles of non-coding Y RNAs. Int. J. Biochem. Cell Biol. (2015) 66:20–9. doi: 10.1016/j.biocel.2015.07.003
38Wolin, SL, Belair, C, Boccitto, M, Chen, X, Sim, S, Taylor, DW, et al. Non-coding Y RNAs as tethers and gates: insights from bacteria. RNA Biol. (2013) 10:1602–8. doi: 10.4161/rna.26166
39Yengej, FAY, van Royen-Kerkhof, A, Derksen, RH, and Fritsch-Stork, RD. The development of offspring from mothers with systemic lupus erythematosus. A systematic review. Autoimmun. Rev. (2017) 16:701–11. doi: 10.1016/j.autrev.2017.05.005
40Enstrom, AM, Van de Water, JA, and Ashwood, P. Autoimmunity in autism. Curr. Opin. Investig. Drugs. (2009) 10:463–73.
41Zhu, Z, Tang, S, Deng, X, and Wang, Y. Maternal systemic lupus Erythematosus, rheumatoid arthritis, and risk for autism Spectrum disorders in offspring: a meta-analysis. J. Autism Dev. Disord. (2020) 50:2852–9. doi: 10.1007/s10803-020-04400-y
42Li, YJ, Ou, JJ, Li, YM, and Xiang, DX. Dietary supplement for core symptoms of autism spectrum disorder: where are we now and where should we go? Front. Psychol. (2017) 8:155. doi: 10.3389/fpsyt.2017.00155
43Al-Farsi, YM, Waly, MI, Deth, RC, Al-Sharbati, MM, Al-Shafaee, M, Al-Farsi, O, et al. Low folate and vitamin B12 nourishment is common in Omani children with newly diagnosed autism. Nutrition. (2013) 29:537–41. doi: 10.1016/j.nut.2012.09.014
44Zhang, Z, Yu, L, Li, S, and Liu, J. Association study of polymorphisms in genes relevant to vitamin B12 and Folate metabolism with childhood autism Spectrum disorder in a Han Chinese population. Med. Sci. Monit. (2018) 24:370–6. doi: 10.12659/MSM.905567
Keywords: autism spectrum disorder, Saudi females, coding variants, single nucleotide polymorphism, haplotyping
Citation: Almandil NB, Alismail MA, Alsuwat HS, AlSulaiman A, AbdulAzeez S and Borgio JF (2023) Exome-wide analysis identify multiple variations in olfactory receptor genes (OR12D2 and OR5V1) associated with autism spectrum disorder in Saudi females. Front. Med. 10:1051039. doi: 10.3389/fmed.2023.1051039
Edited by:
Thirumal Kumar D, Meenakshi Academy of Higher Education and Research, IndiaReviewed by:
Aliyah Almomen, King Saud University, Saudi ArabiaMahran Abdel-Rahman, Assiut University, Egypt
Aisha El-Turki, Brighton and Sussex Medical School, United Kingdom
Copyright © 2023 Almandil, Alismail, Alsuwat, AlSulaiman, AbdulAzeez and Borgio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noor B Almandil,  nbalmandil@iau.edu.sa
nbalmandil@iau.edu.sa
 Noor B. Almandil
Noor B. Almandil Maram Adnan Alismail
Maram Adnan Alismail Hind Saleh Alsuwat
Hind Saleh Alsuwat Abdulla AlSulaiman
Abdulla AlSulaiman Sayed AbdulAzeez
Sayed AbdulAzeez J. Francis Borgio
J. Francis Borgio