Postoperative Nausea and Vomiting in Female Patients Undergoing Breast and Gynecological Surgery: A Narrative Review of Risk Factors and Prophylaxis
- 1Department of Anesthesiology, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 2Department of Anesthesiology, Health Sciences Center, School of Medicine, Stony Brook University, New York, NY, United States
Postoperative nausea and vomiting (PONV) have been widely studied as a multifactorial entity, being of female gender the strongest risk factor. Reported PONV incidence in female surgical populations is extremely variable among randomized clinical trials. In this narrative review, we intend to summarize the incidence, independent predictors, pharmacological and non-pharmacological interventions for PONV reported in recently published clinical trials carried out in female patients undergoing breast and gynecologic surgery, as well as the implications of the anesthetic agents on the incidence of PONV. A literature search of manuscripts describing PONV management in female surgical populations (breast surgery and gynecologic surgery) was carried out in PubMed, MEDLINE, and Embase databases. Postoperative nausea and vomiting incidence were highly variable in patients receiving placebo or no prophylaxis among RCTs whereas consistent results were observed in patients receiving 1 or 2 prophylactic interventions for PONV. Despite efforts made, a considerable number of female patients still experienced significant PONV. It is critical for the anesthesia provider to be aware that the coexistence of independent risk factors such as the level of sex hormones (pre- and postmenopausal), preoperative anxiety or depression, pharmacogenomic pleomorphisms, and ethnicity further enhances the probability of experiencing PONV in female patients. Future RCTs should closely assess the overall risk of PONV in female patients considering patient- and surgery-related factors, and the level of compliance with current guidelines for prevention and management of PONV.
Introduction
Postoperative nausea and vomiting (PONV) are one of the main distressing symptoms commonly reported after surgery and prompt patients at risk to serious complications, such as gastric aspiration, psychological distress, wound dehiscence, deferred recovery, and prolonged discharge times. Female gender is considered an independent predictor of PONV, being a determinant factor when assessing its preoperative risk (1–3). The Society for Ambulatory Anesthesia (SAMBA) Guidelines for PONV management recommend a multimodal approach or combination therapy consisting of two or more interventions in patients with moderate and high risk of PONV, respectively (4, 5). Although the pathophysiology of PONV is multifactorial, PONV is more insidious in female surgical patients than in male, including elderly patients (6). Women also show a higher susceptibility to motion sickness during air, water, and terrestrial travel, which further increases their risk of PONV (1–3). Several studies have demonstrated an association between hormonal changes and PONV in females at a reproductive age (7–10). Nevertheless, current reports on the frequency of PONV during pre-ovulatory (proliferative) and post-ovulatory (luteal) phases of the menstrual cycle are controversial (7, 9–11).
Current literature describing PONV in female patients undergoing breast and/or gynecological surgery is highly variable in terms of incidence, predictors, risk stratification and management. Several reviews, protocols and guidelines have attempted to summarize PONV management in the general population,. We reviewed the most recent evidence on the impact of PONV occurrence after breast and gynecological surgery to summarize the reported specific considerations about the incidence, independent predictors, and perioperative management (pharmacological and non-pharmacological). Furthermore, we consider that this extensive review of the literature that we have carried out can provide us with a more precise view of some aspects of the clinical spectrum of PONV in female surgical patients that should require a systematic review and meta-analysis.
Objectives of the Review
To determine the incidence of PONV in female patients undergoing breast and/ or gynecological surgery.
• To identify independent predictors and risk factors for PONV in this subset of patients, although they apply to the female surgical population
• To evaluate the pharmacological and non-pharmacological strategies most currently used for the prophylactic and therapeutic management of PONV.
• To assess the influence of anesthetic agents on PONV occurrence and clarify the optimal anesthetic technique.
• To evaluate the efficacy of the most widely used risk-scoring systems in the risk stratification for PONV in the female surgical population.
Aims
To Provide updated knowledge to anesthesia providers about key elements that allow them to optimize the perioperative management of PONV in the female population.
Methods
The research question was formulated according to the PICO methodology. P = Women undergoing breast or gynecological surgery; I = Prevention and treatment of PONV; C = Premenopausal and postmenopausal adult female surgical patients; O = independent predictors and risk factors, risk stratification, available therapeutic strategies, anesthetic management in high-risk patients for PONV.
We performed an extensive literature search in PubMed, MEDLINE, and Embase databases of articles describing PONV management in female surgical populations (i.e., breast surgery and gynecologic surgery) published between January 1, 2011, and June 30, 2021, following the Preferred Reporting Items for Systematic Reviews and meta-Analysis (PRISMA) guidelines (Figure 1) (12). Initially, we use the following keywords and Medical Subject Headings (MeSH) terms: “postoperative nausea and vomiting,” “PONV,” “female gender,” “gynecological surgery,” “breast surgery” and their combinations were used. Thereafter, the following systematic search strategy was used: (PONV OR postoperative nausea and vomiting OR nausea and vomiting, postoperative OR postoperative vomiting OR vomiting, postoperative OR nausea, postoperative OR emesis, postoperative, postoperative, OR postoperative emesis OR postoperative nausea OR antiemetic effect OR complete response) AND (gynecological procedure OR gynecological surgery OR breast surgery OR mastectomy OR mammaplasty) AND (female OR woman). With the results of the initial electronic search, two authors hand-screened several to confirm the following eligibility criteria: Articles published in English language between January 1, 2011, and June 30, 2021, reporting PONV as a primary outcome and describing PONV management in female patients undergoing either breast or gynecologic surgery were included. In addition, our literature search included retrospective studies, systematic reviews, meta-analyses, and review articles from a cited reference search. We excluded conference abstracts and posters, reviews of non-primary research, case reports, series of case reports and articles published in other language other than English. All authors conducted the final review of all databases in July 2021.
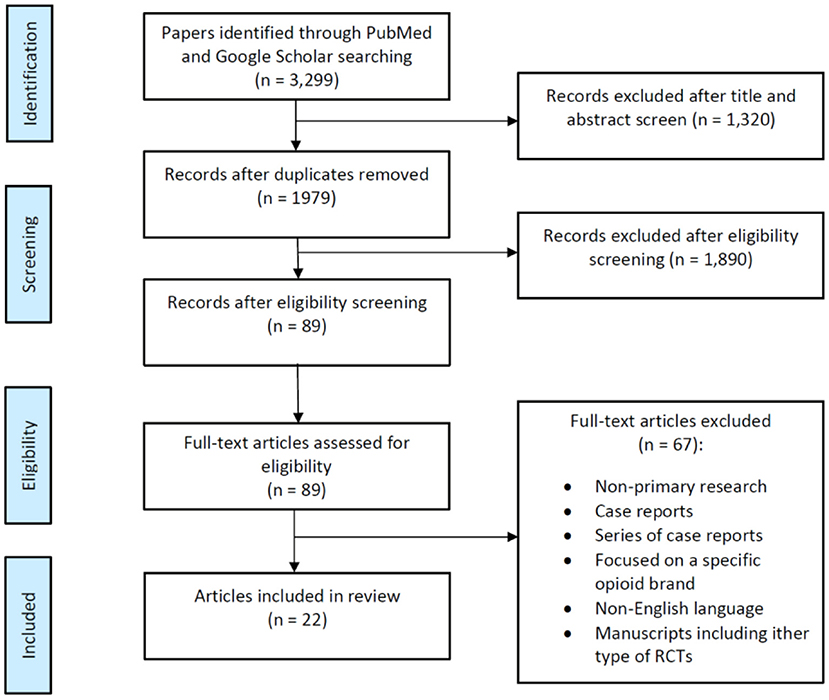
Figure 1. Flow diagram summarizing the selection of randomized clinical trials (RCTs) describing postoperative nausea and vomiting (PONV) in female surgical population.
Results
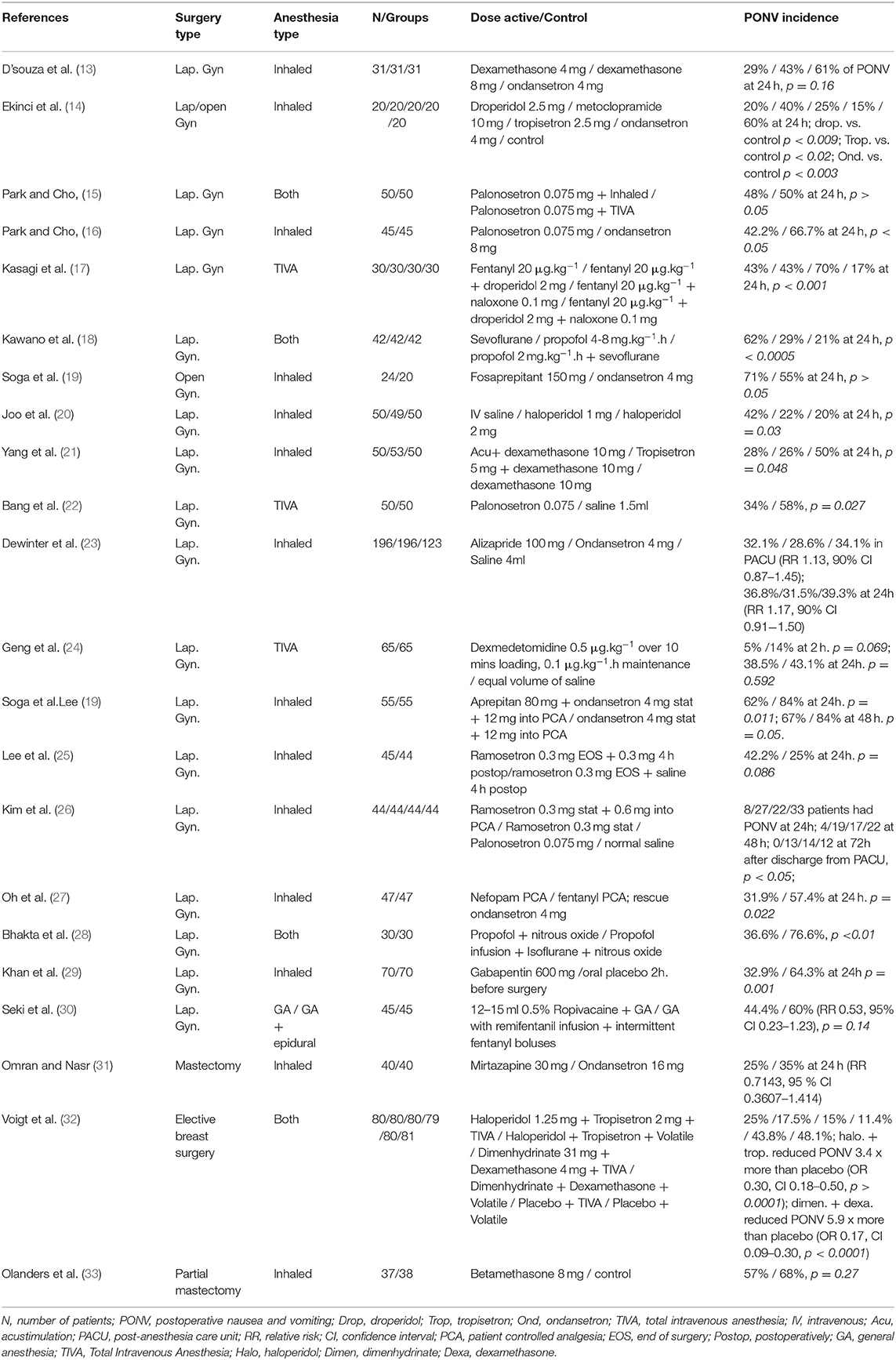
Our database search identified a total of 3,299 articles. After 1,320 duplicated articles were removed, 1,979 articles underwent title and abstract screening. Following this, we selected around 89 publications as reliable articles addressing exclusively PONV in females and screened for eligibility (Figure 1). Among these 89 articles, 67 were excluded for various reasons as shown in Figure 1, and we finally identified a total of 22 eligible publications with a significant number of patients and relevant compilation of demographic and clinical outcomes (Table 1). This is a narrative review; therefore no statistical analysis was performed.
Discussion
Incidence of PONV in Patients Undergoing Breast and Gynecological Surgery
There is sufficient documentation showing that women undergoing breast and gynecological surgery have a reported incidence of postoperative nausea and vomiting up to 80% to 95% within the first 24 h after surgery when they received insufficient or no prophylactic antiemetic therapy (34–36). Conversely, the occurrence of PONV in this subset of surgical patients can dramatically decrease after the systematic implementations of PONV guidelines (37).
Breast cancer surgery constitutes an additional risk factor for PONV in female surgical patients with a reported incidence of up to 30% to 68% within the first 24 h postoperatively in patients that received intraoperative prophylactic antiemetics (38–40), whereas in non-treated patients PONV frequency increases to 70%-80% of patients (41–43).
Gynecological surgery involves patients who are at high risk for PONV is associated with a higher incidence of PONV (female sex, non-smoking status, and requirement for postoperative opioids) (34). The incidence of PONV in the obstetric and gynecological surgical patients has ranged between 40–80%, especially in laparoscopic surgery (28, 44–46).
Specific Risk Factors for Postoperative Nausea and Vomiting in Female Surgical Populations
The multifactorial etiology of PONV has been widely studied with the subsequent identification of several independent predictors such as emetogenic factors (e.g., perioperative use of opioids, inhaled or balanced anesthesia, length of anesthesia) and patient-related risk factors (e.g., smoking status, female gender) (2). Being a female patient is the strongest predictor of PONV, followed by the antecedent of episodes of PONV and motion sickness (2, 3, 5). Other known PONV predictors in women are preoperative history of nausea and vomiting during pregnancy, female neonate, and premenstrual syndrome (2, 4). However, it is very important for the anesthesia providers to recognize the presence of other lesser known independent risk factors that enhance the frequency of PONV such as sex hormones levels, psychosocial factors, pharmacogenomic pleomorphism, and ethnicity.
Hormonal Status According to the Menstrual Cycle
Anecdotally, the incidence trend of emetic episodes increases after menarche and decreases through the menopausal transition (10, 47). Moreover, increased estrogen and progesterone levels during pregnancy have been associated with a prolonged gastrointestinal transit time and a reduction in the esophageal sphincter pressure (10). These facts suggest that cyclic variations in reproductive hormones in females may influence their susceptibility to nausea and motion sickness and therefore, to PONV (4, 6). Previous reports in women revealed that their hormonal status could play an important role in the occurrence of PONV within the first 5 days of the menstrual cycle (48, 49). Based on these assertions, a female patient undergoing major surgery under balanced or inhaled anesthesia, in which postoperative opioid use is expected (e.g., breast cancer surgery or laparoscopic gynecological surgery), is considered at high risk of PONV regardless of her age, smoking status or history of PONV and a multimodal prophylactic approach for PONV (>2 interventions) is highly recommended (5).
The correlation between the menstrual cycle phases and the frequency of PONV has been assessed by several authors, however, there is no firm evidence linking any specific phase of the cycle with a higher propensity for PONV. Nevertheless, an increased incidence of early PONV in women in the follicular and ovulatory stage, when levels of estrogen (estradiol) are higher, compared to those who were in the luteal phase has been reported by several studies (8, 9). Other researchers found a significant association between the ovulatory phase of the menstrual cycle and a higher incidence of early and late PONV when compared to the follicular and luteal phases. In addition, in the study of Zou et al., after multivariate logistic regression analysis showed that the phase of the menstrual cycle was an independent risk factor for early and late PONV (50). Conversely, other studies have concluded that changes in female hormones during the different stages of the menstrual cycle were not associated with an increased incidence of PONV (7, 51). The higher incidence of PONV in premenopausal women has been associated to high plasma levels of estrogen hormones, and to greater requirements of opioids (52, 53). The study conducted by Kudach et al. showed an equivalent rate of PONV in female patients up to ≥ 70 years, when the incidence of PONV was significantly lower (52). Therefore, we should consider these variables in female patients undergoing major surgery when assessing the PONV risk factors as described in the current consensus guidelines (4).
PONV Associated With Tumor Receptor Status in Breast Cancer Surgery
Estrogen and progesterone receptors in the breast tissue are affected by the level of sex hormones and are actively involved in the development of breast cancer; with the endogenous estrogen and progesterone binding specifically to estrogen receptors (ER) or progesterone-receptor (PR), and influencing tumor growth (54). In addition, elevated estrogen levels are also known to increase emesis, suggesting a potential interaction of the estrogen receptor (49). The higher incidence of PONV in premenopausal patients has been linked to elevated estrogen levels (estrone, estradiol, and dehydroepiandrosterone), hence, the higher frequency of PONV observed in postmenopausal women (>50 years) and positive-ER breast cancer also correlates with high estrogen levels (55) (Table 2).
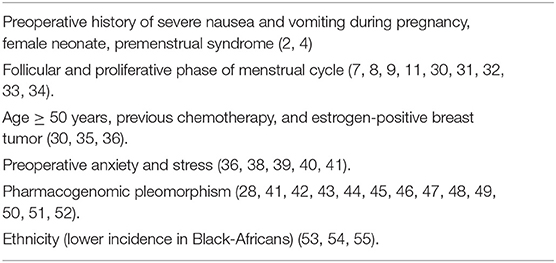
Table 2. Physiologic changes associated with an increased risk of postoperative nausea and vomiting in the female population (Independent risk factors).
Preoperative Psychosocial Factors Affecting Women Undergoing Breast and Gynecological Surgery
Preoperative psychological factors such as anxiety and distress may be associated with increased severity of PONV in women with breast cancer (56). Even conservative minor procedures, such as excisional breast biopsy and conservative lumpectomy can be very stressful for women. The onset of preoperative stress in these patients was associated to a variety of factors such as exposure to surgery and anesthesia, experiencing postoperative pain, appearance, scarring, and cancer diagnosis and prognosis (57). Response expectancies based on previous personal or vicarious experiences, have shown to determine immediate postoperative outcomes regarding pain, PONV and fatigue (56, 58–60). In addition, Montgomery et al. reported that anxiety and stress, as part of response expectancies, may have an important influence on late post-discharge nausea and vomiting occurrence (59).
Genomic Pleomorphisms and Ethnicity
Recent studies have demonstrat that previous history of chemotherapy-induced nausea and vomiting (CINV) may contribute to increase the risk of PONV (61). Conversely, there is also evidence showing that patients who have not presented PONV after general anesthesia do not experience CINV either because of different mechanisms including genetic predisposition (47, 61–65).
For instance, polymorphisms in the serotonin transport genes are associated with increased PONV in women with breast cancer, even before receiving chemotherapy (47), while there is a tendency for individuals categorized as CYP2D6 poor metabolizers to experience PONV (66). Moreover, polymorphisms in the serotonin receptor genes HTR3A and DRD3 are linked to a decrease rate of PONV, while on the contrary, HTR3B receptor gene polymorphism may contribute to an increase PONV (67–69). Therefore, pharmacogenomic variability in serotonin transport genes may explain the erratic incidence of PONV and the irregular response to antiemetic medication observed in around 30% of patients undergoing breast cancer surgery (69). Individual carriers of alleles to COMT, DRD3 and TPH genes show a tendency to low PONV frequency (69). Women presenting some genotypes such as Val/Val may experience higher pain intensity, and opioid requirements contributing to increase the occurrence of PONV (especially nausea), when compared with patients with heterozygous V/Met polymorphism (69). The Met/Met genotype has been related with an elevated density of mu receptors, which may explain the reduced levels of pain and opioid consumption observed in those patients (70, 71).
Several studies have demonstrated that ethnicity can be an independent risk factor for PONV, whose incidence shows variations in different ethnic groups, which have so far been more noticeable in Black patients. The effect of ethnicity on PONV could be influenced by pharmacogenomic and cultural factors (72–74). However, although more studies are lacking in various ethnic groups, the existing evidence would raise a question about the validity of the scoring systems derived predominantly from the ethnically Caucasian population and if ethnicity could be used to improve the predictability of PONV in the female surgical population.
Pharmacological Interventions for Postoperative Nausea and Vomiting in Female Surgical Populations
Postoperative nausea and vomiting persist as one of the commonest complications even though the use of many aggressive antiemetic prophylactic strategies has increased over the last twenty years (75). The growing implementation of the Enhance Recovery After Surgery (ERAS) protocols in most surgical procedures have allowed to tailor the pharmacologic treatment to the patient's risk level of PONV determined by the currently validated risk-scoring system and treatment guidelines (2, 4, 41).
Regarding the pharmacological management of PONV/PDNV, dexamethasone and 5-hydroxytryptamine-3 (5-HT3) receptor antagonists are the most common PONV prophylactic medications used among trials. Other pharmacological interventions can be used such as dopamine receptors antagonists, neurokinin-1 (NK-1) receptor antagonists, total intravenous anesthesia (TIVA), gabapentin, nefopam, midazolam, intranasal nicotine, and naloxone were also reported (4, 76). Moreover, there is limited data on non-pharmacological interventions such as the use of transcutaneous acupoint stimulation in female surgical populations (4).
Dexamethasone
The prophylactic effect of dexamethasone on PONV may vary based on dose administered and population-specific risk. Dexamethasone has proven its effectiveness at dosage of 4–12 mg IV usually combined with other antiemetics (13, 17, 20, 21, 32, 77). D'Souza et al. reported a significant reduction in PONV incidence at 3 and 24 h after the prophylactic administration of intravenous (IV) dexamethasone (4 mg) in comparison with IV ondansetron (4 mg) in patients undergoing laparoscopic gynecological surgery under inhaled anesthesia (22.6% vs. 51.6%, p = 0.03 and 29% vs. 61%, respectively; p = 0.02). Of note, authors excluded patients with past medical history of motion sickness from this trial (13). However, a higher dexamethasone dose (8 mg) was not associated with a significant reduction on PONV occurrence, being this outcome consistent with other published reports in similar surgical populations (13, 17, 20, 21).
In an interesting design, Kasagi et al. reported an important reduction in PONV incidence with the combination of droperidol, dexamethasone and naloxone in patients undergoing laparoscopic gynecological surgery under total intravenous anesthesia (TIVA) and who received patient-controlled analgesia (PCA) with fentanyl IV for the management of postoperative pain. Based on Apfel's score (34), more than a half of the patients included in this trial were at high risk of PONV. Then patients were randomly assigned to either one of four groups: droperidol (Dr), dexamethasone (Dx), naloxone (Nx) and combined therapy (Cm). There was a significant reduction in PONV occurrence in the group treated with combined therapy (Cm) (17).
A combination of prophylactic IV dexamethasone and IV haloperidol is also associated with a significant reduction of PONV incidence when compared to placebo in patients at high risk undergoing laparoscopic gynecological surgery under inhaled anesthesia (p = 0.003) (25). Likewise, Voigt et al. reported a 5.9 times reduction of PONV risk in patients undergoing elective breast surgery who received a prophylactic combination of dimenhydrinate and dexamethasone when compared to a control group (OR 0.17, CI 0.09–0.30; p < 0.0001) (32). Other dexamethasone combinations such as dexamethasone + IV tropisetron and dexamethasone + acupoint stimulation have been also associated with a significant reduction in PONV occurrence when compared to dexamethasone alone (p = 0.021) (21).
5-Hydroxytryptamine-3 (5-HT3) Receptor Antagonists
5-HT3 receptor antagonists have proved its effectiveness in PONV/PDNV prophylaxis and are the most recommended first-line prophylactic treatment for PONV (4, 5, 76, 78, 79). Recent clinical trials showed the efficacy of newer 5-HT3 receptor antagonist in reducing the incidence of PONV in gynecological and breast surgery (15, 16, 22, 80–82). In a prospective controlled trial comparing the effect of prophylactic palonosetron on PONV after gynecological laparoscopic procedures, Bang et al. reported a substantial reduction on PONV occurrence when compared to placebo (22). Moreover, Park et al. compared the PONV prophylactic effect of IV palonosetron (0.075 mg) with IV Ondansetron (8 mg) in patients with Apfel's score ≥2 finding a significant decrease in PONV incidence at 24 h in the palonosetron group when compared to ondansetron (42.2% vs. 66.7%, respectively; p < 0.05), although there were no significant differences between groups within the first 6 postoperative hours (15). The longer half-life of palonosetron compared to ondansetron could have influenced these outcomes (83). To our knowledge, no studies have been published describing the PONV incidence after postoperative day 1 in female patients receiving palonosetron or assessing cost-benefit of palonosetron administration on surgical patients at high-risk of PONV. Additionally, the effect of a prophylactic dose of palonosetron on the incidence of PONV is comparable to the results obtained with the administration of TIVA in this patient setting (16). In a recent report, Hong et al. compared the effectiveness of palonosetron (P) with the combination of midazolam-palonosetron (MP) in 104 patients undergoing breast cancer surgery. From 0 to 24 h after surgery with no intergroup statistical significance (42.3% and 48.1%) (81).
Ramosetron was also compared to palonosetron in female patients undergoing gynecological laparoscopic surgery in a study conducted by Kim et al. (26). They reported no significant differences on PONV occurrence in patients receiving one prophylactic IV dose of ramosetron (0.3 mg) when compared to 2 doses, one prophylactic and another dose 4 h after gynecological laparoscopic surgery (80).
Neurokinin-1 (NK-1) Receptor Antagonists
Aprepitant and fosaprepitant are the NK-1 receptor antagonists with long elimination half-life most studied as preventive treatment for PONV (84). An early study carried out by Gesztesi et al. revealed that NK-receptor antagonist CP-122,721 (200 mg PO), was more effective than ondansetron lowering the frequency of PONV in the first 24 following gynecological surgery (85). In a prospective study, Soga et al. compared the efficacy of fosaprepitant (150 mg IV) to ondansetron (4 mg) in 44 patients undergoing gynecological laparoscopic surgery under balanced general anesthesia and receiving epidural fentanyl in PCA pump for postoperative pain management. Even though complete response was similar between groups, there were no vomiting episodes reported in patients receiving fosaprepitant, whereas 4 patients experienced vomiting in the ondansetron group (0% vs. 20% respectively, p < 0.05) (19).
Moreover, the efficacy of oral aprepitant combined with IV ondansetron compared with ondansetron alone for PONV prophylaxis was studied by Ham et al. in patients with ≥2 risk factors for PONV and undergoing laparoscopic gynecological surgery (86). The occurrence of PONV at 24 h was significantly lower in the aprepitant +ondansetron group when compared to ondansetron group (62% vs. 84%, respectively; p = 0.011). However, this difference was not maintained at 48 h.
Dopamine Receptor Antagonists
Dopamine receptor antagonists (e.g., butyrophenones) have successfully been used for prevention and treatment of PONV in female surgical populations. However, effective doses are commonly linked to side effects such as sedation and extrapyramidal symptoms, hence limiting their perioperative use. Joo et al. randomized 150 female patients considered at high-risk of PONV and undergoing gynecological laparoscopic surgery into 3 groups: normal saline (Group H0), haloperidol 1 mg (H1), or haloperidol 2 mg (H2). The authors reported a significant reduction in PONV occurrence in both haloperidol groups when compared to normal saline (H1 = 29%, H2 = 24% and H0 = 54%, p = 0.001), although higher levels of sedation occurred in patients receiving 2 mg of haloperidol (H2 group) (20). Ekinci et al. compared the incidence of severe PONV in patients undergoing gynecologic procedures and receiving different prophylactic medications such as droperidol (2.5 mg), metoclopramide (10 mg), tropisetron (2.5 mg), ondansetron (4 mg), or saline (control group). The overall PONV incidence was 48%, being the lowest incidence of severe emesis observed in the ondansetron group compared to droperidol, metoclopramide, tropisetron, and saline (15%, 20%, 40%, 25%, and 60% respectively) (14). This finding correlates with previous reports describing the lack of efficacy of low metoclopramide doses for PONV prophylaxis (5).
To our knowledge, only one study has described the PONV incidence in patients receiving alizapride, another dopamine antagonist commonly used in oncology. Dewinter et al. found no significant differences in PONV occurrence after the administration of alizapride in patients at high risk of PONV undergoing laparoscopic gynecological surgery when compared to ondansetron (23).
Other Pharmacological Interventions
The impact of a single prophylactic dose of betamethasone on PONV in patients undergoing breast surgery was assessed by Olanders et al. Patients were randomized to receive IV betamethasone or no prophylaxis before surgery. The authors reported no significant intergroup differences in the overall PONV incidence. Nevertheless, severity of nausea between postoperative hours 4–12 was significantly lower in the group of patients receiving betamethasone (23% vs. 50%, p < 0.05) (33).
Considering that preoperative anxiety may play an important role in the onset of PONV, Omran et al. compared the PONV prophylactic effect of oral mirtazapine (30 mg), an antidepressant, to oral ondansetron (16 mg) in 80 patients undergoing mastectomy. Even though patients in the mirtazapine group experienced significantly lower preoperative anxiety levels, no differences were found in overall PONV incidence between groups (31).
In contrast, the short-acting benzodiazepine midazolam may be effective in diminishing PONV, especially when used combined with other antiemetics or as part of a multimodal antiemetic therapy in breast and other cancer-related surgeries (81, 82, 87, 88). A meta-analysis conducted by Grant et al. determined that midazolam was associated with a significant decrease in overall PONV rates as well as when used as rescue antiemetic medication (89). Similarly, Ahn et al. reported that patients medicated with midazolam presented lower incidence of PON, POV, and PONV (RR, 0.45; 95% CI, 0.36–0.57; I2 = 31%; NNT = 3; n = 7) (90). Although the exact mechanism for the antiemetic action of midazolam remains unknown, it has been proposed that midazolam may decrease dopamine synthesis and release by direct action on the chemoreceptor zone or by blocking adenosine reuptake (91). Although anxiolysis may contribute to the antiemetic effects of midazolam, binding to the Gamma-Aminobutyric Acid (GABA)-benzodiazepine complex reduces 5-HT3 release and dopaminergic neuronal activity (18, 92, 93).
Kamali et al. conducted a double blind randomized clinical trial to compare the effectiveness of ginger 1 mg (before and after anesthesia) with dexmedetomidine 25 mg (before surgery) in preventing PONV after hysterectomy (94). The group of patients treated with ginger showed a lower incidence of nausea (p = 0.02) and vomiting (p = 0.03) than the dexmedetomidine group within the first 2 h postoperatively. Beyond this timepoint, there were no differences between groups (94).
Non-Pharmacological Interventions for Postoperative Nausea and Vomiting in Female Surgical Populations
There are reports describing the use of acupoint electrical stimulation to reduce the incidence of PONV after breast surgery. However, its efficacy remains controversial (95–98). Küçük et al. studied the effect of acupressure on PONV occurrence after gynecological surgery (99). Patients were randomly allocated into three groups: to acupoint point P6 (both wrists) 1 h before surgery, to K-K9 point (both hands) 30 min before the end of surgery, and a control group (routine care). At 24 hours after surgery, patients in the K-K9 group experienced less nausea than the control group (p < 0.05), and less retching than patients in the P6 group (p < 0.05) (99). The results of a recent meta-analysis conducted by Sun et al. showed that non-needle acupoints stimulation also reduced the incidence of PONV in patients at moderate risk of PONV. However, the low quality and limited number of studies included in this meta-analysis did not allow for definite conclusions and recommendations (97).
Anesthetic Management and Postoperative Nausea and Vomiting in Female Surgical Populations
Even though female gender, non-smoking status, past medical history of PONV (or motion sickness) and postoperative use of opioids are recognized as the main risk factors for PONV (34), other secondary variables (e.g., age <50 years, gynecological surgery, laparoscopic surgery) should be considered when determining the overall individual PONV risk (5).
There is a weak association between intraoperative use of opioids and PONV occurrence. However, inhaled anesthesia (i.e., volatile anesthetics and/or nitrous oxide) is considered the main predictor of PONV related to the anesthetic management (level of evidence A1) (5). Inhaled general anesthesia is associated with increased incidence of early PONV (0–2 h after surgery) but has no impact in delayed PONV (2, 100).
Propofol infusion is widely known to improve PONV outcomes in female surgical patients when compared to balanced anesthesia (18, 28). Kawano et al. studied the incidence of PONV at 0–2 h and 0–24 h after gynecological laparoscopic surgery in 126 women. Patients were randomly assigned to receive general anesthesia with either sevoflurane (Group S), propofol (Group P), or a combination of propofol and sevoflurane (Group PS) (18). Immediately after surgery (0–2 h) and up to 24 h a significantly greater number of patients in the P and PS groups experienced a complete response when compared to group S (p = 0.001 and p < 0.0005 respectively). Nausea was also more frequent in the Group S than in the other two groups (Group S = 62%, Group P = 29% and Group PS = 21%; p < 0.0005) (18). Likewise, Bhakta et al. reported a significant reduction in postoperative emesis with the use of propofol infusion when compared to isoflurane anesthesia in patients undergoing gynecological laparoscopic surgery (28).
The use of inhalation anesthetic agents is associated with a dose-dependent rise in PONV prevalence (2, 100). In a retrospective study, Morita et al. reviewed 928 patients undergoing breast cancer surgery under inhalation anesthesia (101). Their results showed that the use of desflurane and the duration of anesthesia were independent risks factors for early PONV, whereas Apfel score and duration of anesthesia were considered by the authors independent risks factors for delayed PONV (101).
Dexmedetomidine as part of a TIVA approach or in combination with dexamethasone may improve PONV outcomes in female surgical populations (24, 102). In a randomized, controlled, double-blind trial, Kwak et al. demonstrated the efficacy of dexmedetomidine alone and in combination with dexamethasone to prevent PONV when compared to placebo after breast surgery (102). The incidence of PONV was significantly higher in the placebo group compared with the dexmedetomidine group and the dual group during both, at PACU stay (12%, 4%, and 3%, respectively) and within the first 24 h after surgery (70%, 20% and 12%, respectively). They concluded that dexmedetomidine alone or in combination with dexamethasone was equally effective in decreasing the occurrence of PONV in this subset of patients (102).
The antiemetic effect of dexmedetomidine may be mediated by a modulatory action on the post-synaptic α2A receptors acting as heteroreceptors, and reducing the release of 5-HT in the dorsal and median raphe nucleus located in cerebellum and mid-brain pons respectively (103). Other proposed antiemetic mechanisms of dexmedetomidine are the modulatory effect on dopamine release in the nucleus recumbens (104) and the suppression of histamine-mediated production of pro-inflammatory interleukine-6 (IL-6) (105).
The incidence of PONV was studied in patients undergoing laparoscopic gynecological surgery under an opioid-sparing anesthesia technique by Seki et al. They randomly assigned 90 patients to receive either general anesthesia alone (group G) or a combination of general anesthesia and epidural block with ropivacaine (group GE). All patients received PONV prophylaxis with dexamethasone and anesthesia maintenance with sevoflurane. Even though patients in the group G received more intra- and postoperative opioids, the authors found no significant difference when comparing PONV incidence among groups (RR:0.53, 95% CI: 0.23–1.23, p = 0.14) (30).
Other opioid-sparing analgesic approaches are the administration of nefopam, a centrally acting analgesic mostly used for neuropathic pain management, and gabapentin. Chung-Sik Oh et al. randomized 94 patients to receive either nefopam- or fentanyl-based PCA for pain management after gynecological laparoscopic surgery under total intravenous anesthesia (TIVA). The use of nefopam was associated with a significant decrease in PONV occurrence when compared to fentanyl (31.9% vs. 57.4% respectively, p = 0.022) (27). Likewise, Khan et al. reported a significant decreased PONV incidence after oral gabapentin (600 mg) compared to placebo in patients undergoing diagnostic gynecological laparoscopy surgery (32.9% vs. 64.3% respectively, p < 0.001) (29).
The development of Enhance Recovery After Surgery (ERAS) pathways for different surgical specialties, including cancer breast surgery, has led to a reduction in the prevalence of PONV, although the number of studies remains limited (106, 107). The growing use of multimodal perioperative analgesia strategies in ERAS protocols contributes to an effective management of postoperative pain with a considerable reduction in the amount of perioperative opioid use through the combination of non-opioid pharmacological management and regional anesthesia techniques, which consequently, decreased the prevalence of PONV (106, 108, 109). A recent retrospective study by Chiu et al. clearly showed a drastic reduction in PONV occurrence after the initiation of ERAS pathways for total mastectomy compared with a non-ERAS cohort (28% vs. 50%, respectively; p<0.001) (107). The use of regional nerve blocks (e.g., pectoral blocks or PECS, paravertebral, erector spinae plane block, and interfascial plane block) as a central component of multimodal opioid-free perioperative analgesia has had a significant impact on the frequency of PONV after breast surgery (110–114).
Conclusions and Future Directions
Despite the efforts made by health care providers and researchers to reduce the occurrence of PONV in female patients at high risk, breast and gynecological surgery constitute additional risks factors for PONV, with an incidence that reaches 30–68% in the first 24 postoperative hours even in patients who have received prophylactic antiemetic treatment. Even though published data is limited, other variables such as sex hormone levels (especially estrogens) in pre and post-menopausal women, preoperative psychosocial status, pharmacogenomic pleomorphisms, and ethnicity, which can be considered independent risk factors must be considered when assessing the risk of PONV in female surgical populations.
Overall risk stratification and increasing compliance with the consensus for PONV management may positively influence clinical outcomes. While novel drugs are continuously under research, future randomized clinical trials should aim to identify both pharmacological and non-pharmacological alternatives that could potentially decrease the current threshold of PONV incidence in female surgical patients.
Author Contributions
ME-V performed literature search, worked on the structural design and methodology, and wrote and authored the manuscript. JF-D and AU collaborated equally in developing the methodology, collecting the data, and writing the manuscript. SB provided the publication concept, editorial advice, and supervised the production. All authors reviewed the final manuscript before submission. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully acknowledge Sarah Kozik, Melanie Munoz, and Jillian Tishko for their writing and editing collaboration (they provided authorization to be named on this publication).
Abbreviations
ERAS, Enhance Recovery After Surgery; GA, general anesthesia; NIH, National Institutes of Health; PONV, postoperative nausea and vomiting; PON, postoperative nausea; POV, postoperative vomiting; TIVA, Total Intravenous Anesthesia.
References
1. Pierre S, Benais H, Pouymayou J. Apfel's simplified score may favourably predict the risk of postoperative nausea and vomiting. Can J Anaesth. (2002) 49:237. doi: 10.1007/BF03020521
2. Apfel C, Heidrich F, Jukar-Rao S, Jalota L, Hornuss C, Whelan R, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. (2012) 109:742–53. doi: 10.1093/bja/aes276
3. Smith CA, Ruth-Sahd L. Reducing the incidence of postoperative nausea and vomiting begins with risk screening: an evaluation of the evidence. J Perianesth Nurs. (2016) 31:158–71. doi: 10.1016/j.jopan.2015.03.011
4. Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. (2019) 131:411–48. doi: 10.1213/ANE.0000000000004833
5. Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. (2014) 118:85–113. doi: 10.1213/ANE.0000000000000002
6. Conti D, Ballo P, Boccalini R, Boccherini A, Cantini S, Venni A, et al. The effect of patient sex on the incidence of early adverse events in a population of elderly patients. Anaesth Intensive Care. (2014) 42:455–9. doi: 10.1177/0310057X1404200405
7. Lee J-W, Kim S-K, Kim D-C, Han YJ, Ko S-H. The effects of female hormones on postoperative nausea and vomiting. Korean J Anesthesiol. (2008) 54:58–62. doi: 10.4097/kjae.2008.54.1.58
8. Panditrao AM, Panditrao MM, Panditrao MM, Azher I. A prospective study to compare the incidence of post-operative nausea, vomiting (PONV) in female patients undergoing surgical procedures under general anaesthesia during proliferative and secretory phase of menstrual cycle. Int J Students' Res. (2011) 1:51–6. doi: 10.5549/IJSR.1.2.51-56
9. Šimurina T, Mraovic B, Skitarelić N, Andabaka T, Sonicki Z. Influence of the menstrual cycle on the incidence of nausea and vomiting after laparoscopic gynecological surgery: a pilot study. J Clin Anesth. (2012) 24:185–92. doi: 10.1016/j.jclinane.2011.07.011
10. Matchock RL, Levine ME, Gianaros PJ, Stern RM. Susceptibility to nausea and motion sickness as a function of the menstrual cycle. Women's Health Issues. (2008) 18:328–35. doi: 10.1016/j.whi.2008.01.006
11. Lee JW, Lee JS, Kim JH, Kim YJ, Woo JH, Kim DY, et al. Influence of the phase of menstrual cycle on postoperative nausea and vomiting after breast cancer surgery. Ewha Medical J. (2018) 41:19–23. doi: 10.12771/emj.2018.41.1.19
12. Deshpande S, van Asselt A, Tomini F, Armstrong N, Allen A, Noake C, et al. Preferred reporting items for systematic reviews and meta-analysis (PRISMA) checklist. Rapid fetal fibronectin testing to predict preterm birth in women with symptoms of premature labour: a systematic review and cost analysis. NIHR Journals Library. (2013).
13. D'souza N, Swami M, Bhagwat S. Comparative study of dexamethasone and ondansetron for prophylaxis of postoperative nausea and vomiting in laparoscopic gynecologic surgery. Int J Gynaecol Obstet. (2011) 113:124–7. doi: 10.1016/j.ijgo.2010.11.022
14. Ekinci O, Malat I, Işitmangil G, Aydin N. A randomized comparison of droperidol, metoclopramide, tropisetron, and ondansetron for the prevention of postoperative nausea and vomiting. Gynecol Obstet Invest. (2011) 71:59–65. doi: 10.1159/000320747
15. Park S, Cho E. A randomized, double-blind trial of palonosetron compared with ondansetron in preventing postoperative nausea and vomiting after gynaecological laparoscopic surgery. J Int Med Res. (2011) 39:399–407. doi: 10.1177/147323001103900207
16. Park S, Cho EJ. A randomized controlled trial of two different interventions for the prevention of postoperative nausea and vomiting: total intravenous anaesthesia using propofol and remifentanil versus prophylactic palonosetron with inhalational anaesthesia using sevoflurane-nitrous oxide. J Int Med Res. (2011) 39:1808–15. doi: 10.1177/147323001103900523
17. Kasagi Y, Hayashida M, Sugasawa Y, Kikuchi I, Yamaguchi K, Okutani R, et al. Antiemetic effect of naloxone in combination with dexamethasone and droperidol in patients undergoing laparoscopic gynecological surgery. J Anesth. (2013) 27:879–84. doi: 10.1007/s00540-013-1630-8
18. Kawano H, Ohshita N, Katome K, Kadota T, Kinoshita M, Matsuoka Y, et al. Effects of a novel method of anesthesia combining propofol and volatile anesthesia on the incidence of postoperative nausea and vomiting in patients undergoing laparoscopic gynecological surgery. Braz J Anesthesiol. (2016) 66:12–8. doi: 10.1016/j.bjane.2014.07.005
19. Soga T, Kume K, Kakuta N, Hamaguchi E, Tsutsumi R, Kawanishi R, et al. Fosaprepitant versus ondansetron for the prevention of postoperative nausea and vomiting in patients who undergo gynecologic abdominal surgery with patient-controlled epidural analgesia: a prospective, randomized, double-blind study. J Anesth. (2015) 29:696–701. doi: 10.1007/s00540-015-2006-z
20. Joo J, Park YG, Baek J, Moon YE. Haloperidol dose combined with dexamethasone for PONV prophylaxis in high-risk patients undergoing gynecological laparoscopic surgery: a prospective, randomized, double-blind, dose-response and placebo-controlled study. BMC Anesthesiol. (2015) 15:99. doi: 10.1186/s12871-015-0081-1
21. Yang X-Y, Xiao J, Chen Y-H, Wang Z-T, Wang H-L, He D-H, et al. Dexamethasone alone vs in combination with transcutaneous electrical acupoint stimulation or tropisetron for prevention of postoperative nausea and vomiting in gynaecological patients undergoing laparoscopic surgery. BJA. (2015) 115:883–9. doi: 10.1093/bja/aev352
22. Bang Y-S, Kim YU, Oh D, Shin EY, Park SK. A randomized, double-blind trial evaluating the efficacy of palonosetron with total intravenous anesthesia using propofol and remifentanil for the prevention of postoperative nausea and vomiting after gynecologic surgery. J Anesth. (2016) 30:935–40. doi: 10.1007/s00540-016-2249-3
23. Dewinter G, Teunkens A, Vermeulen K, Devroe S, Van Hemelrijck J, Meuleman C, et al. Alizapride and ondansetron for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic gynaecological surgery: a double-blind, randomised, placebo-controlled noninferiority study. EJA. (2016) 33:96–103. doi: 10.1097/EJA.0000000000000288
24. Geng Z-Y, Liu Y-F, Wang S-S, Wang D-X. Intra-operative dexmedetomidine reduces early postoperative nausea but not vomiting in adult patients after gynaecological laparoscopic surgery: a randomised controlled trial. EJA. (2016) 33:761–6. doi: 10.1097/EJA.0000000000000491
25. Lee S-H, Kim J-D, Park S-A, Oh C-S, Kim S-H. Effects of μ-Opioid receptor gene polymorphism on postoperative nausea and vomiting in patients undergoing general anesthesia with remifentanil: double blinded randomized trial. J Korean Med Sci. (2015) 30:651–7. doi: 10.3346/jkms.2015.30.5.651
26. Kim SH, Oh CS, Lee SJ. Efficacy of palonosetron and ramosetron on postoperative nausea and vomiting related to intravenous patient-controlled analgesia with opioids after gynecological laparoscopic surgery (double-blinded prospective randomized controlled trial). J Anesth. (2015) 29:585–92. doi: 10.1007/s00540-015-1981-4
27. Oh CS, Jung E, Lee SJ, Kim SH. Effect of nefopam- versus fentanyl-based patient-controlled analgesia on postoperative nausea and vomiting in patients undergoing gynecological laparoscopic surgery: a prospective double-blind randomized controlled trial. Curr Med Res Opin. (2015) 31:1599–607. doi: 10.1185/03007995.2015.1058251
28. Bhakta P, Ghosh BR, Singh U, Govind PS, Gupta A, Kapoor KS, et al. Incidence of postoperative nausea and vomiting following gynecological laparoscopy: A comparison of standard anesthetic technique and propofol infusion. Acta Anaesthesiologica Taiwanica. (2016) 54:108–13. doi: 10.1016/j.aat.2016.10.002
29. Khan MA, Siddiqi KJ, Khan MS. Prophylactic use of gabapentin to reduce postoperative nausea and vomiting in patients undergoing diagnostic gynecological laparoscopy. Anaesth Pain Intensive Care. (2017) 21:19–24.
30. Seki H, Furumoto K, Sato M, Kagoya A, Hashimoto H, Sekiguchi Y, et al. Effects of epidural anesthesia on postoperative nausea and vomiting in laparoscopic gynecological surgery: a randomized controlled trial. J Anesth. (2018) 32:608–15. doi: 10.1007/s00540-018-2525-5
31. Omran HASA, Nasr DAM. Effect of premedication with mirtazapine versus ondansetron on postoperative nausea and vomiting in breast surgery. Egpt J Anesth. (2011) 27:135–9. doi: 10.1016/j.egja.2011.06.003
32. Voigt M, Fröhlich CW, Waschke KF, Lenz C, Göbel U, Kerger H. Prophylaxis of postoperative nausea and vomiting in elective breast surgery. J Clin Anesth. (2011) 23:461–8. doi: 10.1016/j.jclinane.2011.01.005
33. Olanders KJ, Lundgren GA, Johansson AM. Betamethasone in prevention of postoperative nausea and vomiting following breast surgery. J Clin Anesth. (2014) 26:461–5. doi: 10.1016/j.jclinane.2014.02.006
34. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. (1999) 91:693–700. doi: 10.1097/00000542-199909000-00022
35. Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthe. (1997) 52:443–9. doi: 10.1111/j.1365-2044.1997.117-az0113.x
36. Krieser KA, Riley III JB, Baus JE, Hoffman JT, Sullivan JN. PONV Prophylaxis failure disproportionately affects female patients, despite intraoperative computerized decision support guidance. Graduate Med Edu Res J. (2020) 2:6. doi: 10.32873/unmc.dc.gmerj.2.1.091
37. Tabrizi S, Malhotra V, Turnbull ZA, Goode V. Implementation of postoperative nausea and vomiting guidelines for female adult patients undergoing anesthesia during gynecologic and breast surgery in an ambulatory setting. J Perianesth Nurs. (2019) 34:851–60. doi: 10.1016/j.jopan.2018.10.006
38. Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg. (1994) 78:7–16. doi: 10.1213/00000539-199401000-00004
39. Reihner E, Grunditz R, Giesecke K, Gustafsson L. Postoperative nausea and vomiting after breast surgery: efficacy of prophylactic ondansetron and droperidol in a randomized placebo-controlled study. Eur J Anaesthesiol. (2000) 17:197–203. doi: 10.1097/00003643-200003000-00012
40. Layeeque R, Siegel E, Kass R, Henry-Tillman RS, Colvert M, Mancino A, et al. Prevention of nausea and vomiting following breast surgery. Am J Surg. (2006) 191:767–72. doi: 10.1016/j.amjsurg.2005.07.040
41. ODDBY–MUHRBECK E, Jakobsson J, Andersson L, Askergren J. Postoperative nausea and vomiting. A comparison between intravenous and inhalation anaesthesia in breast surgery. Acta Anaesthesiologica Scandinavica. (1994) 38:52–6. doi: 10.1111/j.1399-6576.1994.tb03837.x
42. Hammas B, Thörn SE, Wattwil M. Superior prolonged antiemetic prophylaxis with a four-drug multimodal regimen–comparison with propofol or placebo. Acta Anaesthesiol Scand. (2002) 46:232–7. doi: 10.1034/j.1399-6576.2002.460302.x
43. Wattwil M, Thörn SE, Lövqvist Å, Wattwil L, Gupta A, Liljegren G. Dexamethasone is as effective as ondansetron for the prevention of postoperative nausea and vomiting following breast surgery. Acta Anaesthesiol Scand. (2003) 47:823–7. doi: 10.1034/j.1399-6576.2003.00172.x
44. Read M, James M. Immediate postoperative complications following gynaecological surgery. Obstetrician Gynaecologist. (2002) 4:29–35. doi: 10.1576/toag.2002.4.1.29
45. Tseng L-H, Liou S-C, Chang T-C, Tsai S-C, Soong Y-K, Wong S-Y, et al. Randomized blinded study of the incidence of postoperative nausea and vomiting in women after major gynecologic laparoscopic surgery. J Minim Invasive Gynecol. (2006) 13:413–7. doi: 10.1016/j.jmig.2006.05.003
46. Harmon D, Ryan M, Kelly A, Bowen M. Acupressure and prevention of nausea and vomiting during and after spinal anaesthesia for caesarean section. Br J Anaesth. (2000) 84:463–7. doi: 10.1093/oxfordjournals.bja.a013471
47. Wesmiller SW, Bender CM, Sereika SM, Ahrendt G, Bonaventura M, Bovbjerg DH, et al. Association between serotonin transport polymorphisms and postdischarge nausea and vomiting in women following breast cancer surgery. Oncol Nurs Forum. (2014) 41:195–202. doi: 10.1188/14.ONF.195-202
48. Pataky A, Kitz D, Andrews R, Lecky J. Nausea and vomiting following ambulatory surgery: are all procedures created equal? Anesth Analg. (1988) 67:163. doi: 10.1213/00000539-198802001-00163
49. Beattie WS, Lindblad T, Buckley DN, Forrest JB. The incidence of postoperative nausea and vomiting in women undergoing laparoscopy is influenced by the day of menstrual cycle. Can J Anaesthesia. (1991) 38:298–302. doi: 10.1007/BF03007618
50. Zou L, Miao S, Wang L, Wang G. Effects of menstrual cycle on nausea and vomiting after general anesthesia. J Anesth. (2020) 34:519–26. doi: 10.1007/s00540-020-02781-z
51. Eberhart L, Morin A, Georgieff M. The menstruation cycle in the postoperative phase. its effect of the incidence of nausea and vomiting. Anaesthesist. (2000) 49:532–5. doi: 10.1007/s001010070095
52. Kudach C, Dunwoody C, Wesmiller S. The relationship of age and postoperative pain in women after surgery for breast cancer. Pain Manag Nurs. (2018) 19:348–53. doi: 10.1016/j.pmn.2017.12.002
53. Medina-Diaz-Cortés G, Brancaccio-Pérez IV, Esparza-Estrada I, Barbosa-Camacho FJ, Fuentes-Orozco C, González-Hernández PG, et al. Differences in postoperative pain, nausea, and vomiting after elective laparoscopic cholecystectomy in premenopausal and postmenopausal mexican women. World J Surg. (2022) 46:356–61. doi: 10.1007/s00268-021-06367-y
54. Kakugawa Y, Minami Y, Tateno H, Inoue H, Fujiya T. Relation of serum levels of estrogen and dehydroepiandrosterone sulfate to hormone receptor status among postmenopausal women with breast cancer. Breast Cancer. (2007) 14:269–76. doi: 10.2325/jbcs.14.269
55. Bakshi SG, Jibhkate B, Sareen R, Badwe R. Nausea and vomiting after breast cancer surgery, and relationship with tumor receptor status. J Anesth. (2012) 26:187–95. doi: 10.1007/s00540-011-1274-5
56. Montgomery GH, Schnur JB, Erblich J, Diefenbach MA, Bovbjerg DH. Presurgery psychological factors predict pain, nausea, and fatigue one week after breast cancer surgery. J Pain Symptom Manage. (2010) 39:1043–52. doi: 10.1016/j.jpainsymman.2009.11.318
57. Montgomery GH, Bovbjerg DH. Presurgery distress and specific response expectancies predict postsurgery outcomes in surgery patients confronting breast cancer. Health Psychol. (2004) 23:381. doi: 10.1037/0278-6133.23.4.381
58. Schnur JB, Montgomery GH, Hallquist MN, Goldfarb AB, Silverstein JH, Weltz CR, et al. Anticipatory psychological distress in women scheduled for diagnostic and curative breast cancer surgery. Int J Behav Med. (2008) 15:21–8. doi: 10.1007/BF03003070
59. Kirsch I. Response expectancy as a determinant of experience and behavior. Am Psychol. (1985) 40:1189. doi: 10.1037/0003-066X.40.11.1189
60. Hofman M, Morrow GR, Roscoe JA, Hickok JT, Mustian KM, Moore DF, et al. Cancer patients' expectations of experiencing treatment-related side effects: a university of rochester cancer center-community clinical oncology program study of 938 patients from community practices. Cancer. (2004) 101:851–7. doi: 10.1002/cncr.20423
61. da Silva H, Sousa A, Guimarães G, Slullitel A, Ashmawi H. Does previous chemotherapy-induced nausea and vomiting predict postoperative nausea and vomiting? Acta Anaesthesiol Scand. (2015) 59:1145–53. doi: 10.1111/aas.12552
62. Oddby-Muhrbeck E, Öbrink E, Eksborg S, Rotstein S, Lönnqvist P. Is there an association between PONV and chemotherapy-induced nausea and vomiting? Acta Anaesthesiol Scand. (2013) 57:749–53. doi: 10.1111/aas.12053
63. Fasching P, Kollmannsberger B, Strissel P, Niesler B, Engel J, Kreis H, et al. Polymorphisms in the novel serotonin receptor subunit gene HTR3C show different risks for acute chemotherapy-induced vomiting after anthracycline chemotherapy. J Cancer Res Clin Oncol. (2008) 134:1079–86. doi: 10.1007/s00432-008-0387-1
64. Rueffert H, Thieme V, Wallenborn J, Lemnitz N, Bergmann A, Rudlof K, et al. Do variations in the 5-HT3A and 5-HT3B serotonin receptor genes (HTR3A and HTR3B) influence the occurrence of postoperative vomiting? Anesth Analg. (2009) 109:1442–7. doi: 10.1213/ane.0b013e3181b2359b
65. Zhang W, Yuan J, Kan Q, Zhang L, Chang Y, Wang Z. Study of the OPRM1 A118G genetic polymorphism associated with postoperative nausea and vomiting induced by fentanyl intravenous analgesia. Minerva Anestesiol. (2011) 77:33–9.
66. Wesmiller SW, Henker RA, Sereika SM, Donovan HS, Meng L, Gruen GS, et al. The association of CYP2D6 genotype and postoperative nausea and vomiting in orthopedic trauma patients. Biol Res Nurs. (2013) 15:382–9. doi: 10.1177/1099800412449181
67. Duncan NW, Wiebking C, Tiret B, Marjańska M, Hayes DJ, Lyttleton O, et al. Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. PLoS ONE. (2013) 8:e60312. doi: 10.1371/journal.pone.0060312
68. Laugsand EA, Fladvad T, Skorpen F, Maltoni M, Kaasa S, Fayers P, et al. Clinical and genetic factors associated with nausea and vomiting in cancer patients receiving opioids. Eur J Cancer. (2011) 47:1682–91. doi: 10.1016/j.ejca.2011.04.014
69. Wesmiller SW, Sereika SM, Bender CM, Bovbjerg D, Ahrendt G, Bonaventura M, et al. Exploring the multifactorial nature of postoperative nausea and vomiting in women following surgery for breast cancer. Autonomic Neurosci. (2017) 202:102–7. doi: 10.1016/j.autneu.2016.09.017
70. Candiotti KA, Yang Z, Buric D, Arheart K, Zhang Y, Rodriguez Y, et al. Catechol-o-methyltransferase polymorphisms predict opioid consumption in postoperative pain. Anesth Analg. (2014) 119:1194–200. doi: 10.1213/ANE.0000000000000411
71. Zubieta J-K. Mu Opioid Receptor Genetic Variation and Heroin Addiction. Biol Psychiatry. (2015) 78:439–40. doi: 10.1016/j.biopsych.2015.08.002
72. Ugochukwu O, Adaobi A, Ewah R, Obioma O. Postoperative nausea and vomiting in a gynecological and obstetrical population in South Eastern Nigeria. Pan Afr Med J. (2010) 7:6. doi: 10.4314/pamj.v7i1.69111
73. Rodseth RN, Gopalan PD, Cassimjee HM, Goga S. Reduced incidence of postoperative nausea and vomiting in black South Africans and its utility for a modified risk scoring system. Anesth Analg. (2010) 110:1591–4. doi: 10.1213/ANE.0b013e3181da9005
74. Alli A, Omar S, Tsang S, Naik B. The effect of ethnicity on the incidence of postoperative nausea and vomiting in moderate to high risk patients undergoing general anesthesia in South Africa: a controlled observational study. Middle East J Anaesthesiol. (2017) 24:119.
75. White PF, Sacan O, Nuangchamnong N, Sun T, Eng MR. The relationship between patient risk factors and early versus late postoperative emetic symptoms. Anesth Analg. (2008) 107:459–63. doi: 10.1213/ane.0b013e31817aa6e4
76. Elvir-Lazo OL, White PF, Yumul R, Eng HC. Management strategies for the treatment and prevention of postoperative/postdischarge nausea and vomiting: an updated review. F1000Res. (2020) 9:F1000. doi: 10.12688/f1000research.21832.1
77. Ormel G, Romundstad L, Lambert-Jensen P, Stubhaug A. Dexamethasone has additive effect when combined with ondansetron and droperidol for treatment of established PONV. Acta Anaesthesiol Scand. (2011) 55:1196–205. doi: 10.1111/j.1399-6576.2011.02536.x
78. Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. (2007) 105:1615–28. doi: 10.1213/01.ane.0000295230.55439.f4
79. Habib AS, Gan TJ. Evidence-based management of postoperative nausea and vomiting: a review. Can J Anesth. (2004) 51:326–41. doi: 10.1007/BF03018236
80. Lee B, Kim K, Suh DH, Shin H-J, No JH, Lee JR, et al. Efficacy of single-dose and 2-dose intravenous administration of ramosetron in preventing postoperative nausea and vomiting after laparoscopic gynecologic operation: A randomized, double-blind, placebo-controlled, phase 2 trial. Surg Laparosc Endosc Percutan Tech. (2017) 27:183–8. doi: 10.1097/SLE.0000000000000399
81. Hong J-M, Han Y-H, Lee D, Hwang BY, Baik J, Cho AR, et al. Comparison of efficacy between palonosetron-midazolam combination and palonosetron alone for prevention of postoperative nausea and vomiting in patients undergoing breast surgery and patient controlled analgesia: a prospective, randomized, double-blind study: A CONSORT-compliant study. Medicine. (2021) 100:26438. doi: 10.1097/MD.0000000000026438
82. Kim DS, Koo GH, Kang H, Baek CW, Jung YH, Woo YC, et al. The antiemetic effect of midazolam or/and ondansetron added to intravenous patient controlled analgesia in patients of pelviscopic surgery. Korean J Anesthesiol. (2012) 62:343. doi: 10.4097/kjae.2012.62.4.343
83. Stoltz R, Parisi S, Shah A, Macciocchi A. Pharmacokinetics, metabolism and excretion of intravenous [l4C]-palonosetron in healthy human volunteers. Biopharm Drug Dispos. (2004) 25:329–37. doi: 10.1002/bdd.410
84. Liu M, Zhang H, Du B-X, Xu F-Y, Zou Z, Sui B, et al. Neurokinin-1 receptor antagonists in preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Medicine. (2015) 94:e762. doi: 10.1097/MD.0000000000000762
85. Gesztesi Z, Scuderi PE, White PF, Wright W, Wender RH, D'Angelo R, et al. Substance P (Neurokinin-1) antagonist prevents postoperative vomiting after abdominal hysterectomy procedures. Anesthesiology. (2000) 93:931–7. doi: 10.1097/00000542-200010000-00009
86. Ham SY, Shim YH, Kim EH, Son MJ, Park WS, Lee JS. Aprepitant for antiemesis after laparoscopic gynaecological surgery: a randomised controlled trial. EJA. (2016) 33:90–5. doi: 10.1097/EJA.0000000000000242
87. Huh B, Jung S, White W, Jeon Y. Anti-emetic effect of midazolam added to morphine patient-controlled analgesia after total abdominal hysterectomy. Anaesth Intensive Care. (2010) 38:481–5. doi: 10.1177/0310057X1003800311
88. Ross JE, Vertosick E, Long M, Cansino C, Assel M, Twersky R. Midazolam's effect on post operative nausea and vomiting and discharge times in outpatients undergoing cancer related surgery. AANA J. (2019) 87:179.
89. Grant MC, Kim J, Page AJ, Hobson D, Wick E, Wu CL. The effect of intravenous midazolam on postoperative nausea and vomiting: a meta-analysis. Anesthe Analg. (2016) 122:656–63. doi: 10.1213/ANE.0000000000000941
90. Ahn EJ, Kang H, Choi GJ, Baek CW, Jung YH, Woo YC. The Effectiveness of Midazolam for Preventing Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis. Anesth Analg. (2016) 122:664–76. doi: 10.1213/ANE.0000000000001062
92. Florio TD. The use of midazolam for persistent postoperative nausea and vomiting. Anaesth Intensive Care. (1992) 20:383–6. doi: 10.1177/0310057X9202000324
93. Racke K, Reimann A, Schwörer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. (1995) 73:83–7. doi: 10.1016/0166-4328(96)00075-7
94. Kamali A, Beigi S, Shokrpour M, Pazuki S. The efficacy of ginger and doxedetomidine in reducing postoperative nausea and vomiting in patients undergoing abdominal hysterectomy. Altern Ther Health Med. (2020) 26:28–33.
95. Gu J. Wang G-n, Li M-m. Clinical observation of HANS for the prevention of postoperative nausea and vomiting after modified radical mastectomy Heilongjiang. Med J. (2009) 33:604–6.
96. Alcaraz R, Monerris M, Jiménez-Capel Y, Moret E, Janeiro M, Vila P. Effectiveness and safety of continuous stimulation without needles with STIPER® puncture in the prophylaxis of postoperative nausea and vomiting in elective breast surgery: 1AP7-6. (EJA). (2014) 31:23. doi: 10.1097/00003643-201406001-00065
97. Sun R, Dai W, Liu Y, Liu C, Liu Y, Gong Y, et al. Non-needle acupoint stimulation for prevention of nausea and vomiting after breast surgery: a meta-analysis. Medicine. (2019) 9:14713. doi: 10.1097/MD.0000000000014713
98. Ao L, Shi J, Bai Y, Zhang S, Gan J. Effects of transcutaneous electrical acupoint stimulation on perioperative immune function and postoperative analgesia in patients undergoing radical mastectomy: a randomized controlled trial. Exp Ther Med. (2021) 21:1. doi: 10.3892/etm.2021.9615
99. Küçük E, Bülbül T. The effects of acupressure on nausea, vomiting, and vital signs in patients undergoing gynecologic surgery: a randomized controlled trial. J Perianesth Nurs. (2021). doi: 10.1016/j.jopan.2020.10.017
100. Apfel C, Kranke P, Katz M, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. (2002) 88:659–68. doi: 10.1093/bja/88.5.659
101. Morita T, Yamamoto M, Sakamoto A. What are the factors affecting postoperative nausea and vomiting following breast cancer surgery with inhalation anesthesia? JNMS. (2020) 88:418–22. doi: 10.1272/jnms.JNMS.2021_88-510
102. Kwak H, Chang YJ, Lee KC, Jung WS, Kwon S, Jo YY. Antiemetic efficacy of dexmedetomidine versus dexmedetomidine-dexamethasone combination in patients undergoing breast surgery. J IntMed Res. (2019) 47:5060–9. doi: 10.1177/0300060519872031
103. Hopwood S, Stamford J. Noradrenergic modulation of serotonin release in rat dorsal and median raphe nuclei via α1 and α2A adrenoceptors. Neuropharmacol. (2001) 41:433–42. doi: 10.1016/S0028-3908(01)00087-9
104. Whittington RA, Virág L. Dexmedetomidine-induced decreases in accumbal dopamine in the rat are partly mediated via the locus coeruleus. Anesth Analge. (2006) 102:448–55. doi: 10.1213/01.ane.0000195234.07413.5a
105. Yang D, Hong JH. Dexmedetomidine modulates histamine-induced Ca2+ signaling and pro-inflammatory cytokine expression. Korean J Physiol Pharmacol. (2015) 19:413–20. doi: 10.4196/kjpp.2015.19.5.413
106. Dumestre DO, Webb CE, Temple-Oberle C. Improved recovery experience achieved for women undergoing implant-based breast reconstruction using an enhanced recovery after surgery model. Plast Reconstr Surg. (2017) 139:550–9. doi: 10.1097/PRS.0000000000003056
107. Chiu C, Aleshi P, Esserman LJ, Inglis-Arkell C, Yap E, Whitlock EL, et al. Improved analgesia and reduced post-operative nausea and vomiting after implementation of an enhanced recovery after surgery (ERAS) pathway for total mastectomy. BMC Anesthesiol. (2018) 18:41. doi: 10.1186/s12871-018-0505-9
108. Temple-Oberle C, Shea-Budgell MA, Tan M, Semple JL, Schrag C, Barreto M, et al. Consensus review of optimal perioperative care in breast reconstruction: enhanced recovery after surgery (ERAS) society recommendations. Plast Reconstr Surg. (2017) 139:1056e-71e. doi: 10.1097/PRS.0000000000003242
109. Astanehe A, Temple-Oberle C, Nielsen M, de Haas W, Lindsay R, Matthews J, et al. An enhanced recovery after surgery pathway for microvascular breast reconstruction is safe and effective. Prs-Glob Open. (2018) 6:1634. doi: 10.1097/GOX.0000000000001634
110. Tripathy S, Mandal I, Rao PB, Panda A, Mishra T, Kar M. Opioid-free anesthesia for breast cancer surgery: a comparison of ultrasound guided paravertebral and pectoral nerve blocks. a randomized controlled trial. J Anaesthesiol Clin Pharmacol. (2019) 35:475. doi: 10.4103/joacp.JOACP_364_18
111. Kim Y, Oh C, Youn S, Yun S, Park H, Lee W, et al. Thoracic interfascial plane block for multimodal analgesia after breast lumpectomy. Anesth Pain Med. (2019) 14:222–9. doi: 10.17085/apm.2019.14.2.222
112. Khemka R, Chakrborty A, Agrawal S, Ahmed R. Is COMBIPECS the answer to perioperative analgesia for breast surgery? a double blinded randomized controlled trial. Indian J Anaesth. (2019) 63:530. doi: 10.4103/ija.IJA_222_19
113. Bell A, Ali O, Robinson A, Aggarwal A, Blundell M, Townend A, et al. The role of pectoral nerve blocks in a day-case mastectomy service: a prospective cohort study. Ann Med Surg. (2019) 48:65–8. doi: 10.1016/j.amsu.2019.10.019
Keywords: postoperative nausea and vomiting, female gender, gynecological surgery, breast surgery, randomized clinical trial ERAS (Enhance Recovery After Surgery)
Citation: Echeverria-Villalobos M, Fiorda-Diaz J, Uribe A and Bergese SD (2022) Postoperative Nausea and Vomiting in Female Patients Undergoing Breast and Gynecological Surgery: A Narrative Review of Risk Factors and Prophylaxis. Front. Med. 9:909982. doi: 10.3389/fmed.2022.909982
Received: 31 March 2022; Accepted: 13 June 2022;
Published: 01 July 2022.
Edited by:
Somchai Amornyotin, Mahidol University, ThailandCopyright © 2022 Echeverria-Villalobos, Fiorda-Diaz, Uribe and Bergese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Echeverria-Villalobos, marco.echeverriavillalobos@osumc.edu
 Marco Echeverria-Villalobos
Marco Echeverria-Villalobos Juan Fiorda-Diaz
Juan Fiorda-Diaz Alberto Uribe
Alberto Uribe Sergio D. Bergese
Sergio D. Bergese