Case Report: Secondary Hemophagocytic Lymphohistiocytosis (sHLH) and Candida auris Fungemia in Post-acute COVID-19 Syndrome: A Clinical Challenge
- Department of Internal Medicine, Maulana Azad Medical College and Associated Lok Nayak Hopital, New Delhi, India
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes a disease (COVID-19) with multisystem involvement. The world is now entering a phase of post-COVID-19 manifestations in this pandemic. Secondary hemophagocytic lymphohistiocytosis (sHLH) is a life-threatening hyperinflammatory event triggered by viral infections, including SARS-CoV-2. Both Multisystem Inflammatory Syndrome-Adults (MIS-A) and Cytokine Storm Syndrome (CSS) are considered close differentials of sHLH and add to the spectrum of Post-acute COVID-19 syndrome (PACS). In this report, we presented the case of a middle-aged Asian man who was initially discharged upon recovery from severe COVID-19 infection after 17 days of hospitalization to a private institute and later came to our hospital 13 days post-discharge. Here, he was diagnosed with sHLH, occurring as an extension of CSS, with delayed presentation falling within the spectrum of PACS. The diagnosis of sHLH was made holistically with the HLH-2004 criteria. Our patient initially responded to intravenous immunoglobulin (IVIG) and dexamethasone, later complicated by disseminated Candida auris infection and had a fatal outcome. Though many cases of HLH during active COVID-19 and a few cases post COVID-19 recovery have been reported, based on H-score, which has limitations as a diagnostic tool. We report the first case report of post-COVID-19 sHLH using the HLH-2004 criteria, complicated by disseminated Candidemia, emphasizing that the care of patients with COVID-19 does not conclude at the time of hospital discharge. We highlight the importance of surveillance in the post-COVID phase for early detection of sHLH which may predispose to fatal opportunistic infections (OIs).
Introduction
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has overwhelmed healthcare facilities around the world. COVID-19 has an extended spectrum in the post-acute phase with an evolving list of complications. Prieto-Perez et al. first described post-COVID-19 secondary hemophagocytic lymphohistiocytosis (sHLH), indicating a systemic immune-inflammatory disease (1). HLH has been divided into primary and secondary subtypes. Primary HLH is familial, primarily seen in children, whereas sHLH is an adult entity due to underlying infection, autoimmune disease, malignancy, or “post-allogeneic” hematopoietic stem cell transplantation (2). HLH is a life-threatening hyperinflammatory state characterized by multiorgan dysfunction, adding to the spectrum of Post-Acute COVID-19 Syndrome (PACS) (3). Lledo et al. defined the PACS as a clinical entity when symptoms persist beyond four weeks (4). Similarly, the Center for Disease Control (CDC) has formulated “post-Covid conditions” to describe the health issues that persist more than four weeks after being infected with COVID-19 (5). Here, we present the first case report of post-COVID-19 sHLH occurring as an extension of the cytokine storm syndrome (CSS) falling under the timeline of PACS, who initially responded to dexamethasone and intravenous immunoglobulin (IVIG). However, his course was complicated by disseminated Candidemia and he succumbed to his illness. It is prudent to identify this fatal complication early to improve survival.
Case Report
A 36-year-old farmer, who had been admitted 29 days ago to another hospital with severe COVID-19 pneumonia, presented to our emergency 13 days after discharge with complaints of fever (101.4°F) for 5 days, generalized weakness and loss of appetite for 4 days, progressive exertional dyspnea, and occasional dry cough for 2 days. He had no history of orthopnea, paroxysmal nocturnal dyspnea, pedal edema, hemoptysis, chest pain, burning micturition, or bowel complaints. He had been discharged from the last admission on home-based intermittent oxygen therapy at 2-4 L/min, multivitamins, and oral pirfenidone (an anti-fibrotic agent for post-COVID-19 pulmonary fibrosis) 200 mg three times a day (TDS). He had no history of substance abuse or chronic illness, and his family history was insignificant.
He was conscious, oriented, and febrile (102.8°F/39.3°C). His oxygen saturation was 93% under ambient air, a respiratory rate of 15 breaths per min, blood pressure of 108/76 mmHg, and a pulse rate of 108 beats per min. On systemic examination, hepatomegaly was present 2 cm below the right subcostal margin in the mid-clavicular line and bilateral end-inspiratory fine crepitations were auscultated in the chest. The rest of the systemic examination was normal.
On investigation, his random blood sugar was 203 mg/dL, TruNat swab for SARS-CoV-2 was reported negative, and arterial blood gas analysis showed Type 1 respiratory failure. His ECG showed sinus tachycardia, and the bedside chest radiograph showed bilateral ground-glass opacities (Figure 1A). A provisional diagnosis of post-COVID-19 sequelae with bilateral lower respiratory tract infection (LRTI) with Type 1 respiratory failure was made. Further investigations were sent. He was treated with low flow oxygen support, antipyretics, injectable ceftriaxone, intravenous fluids, and supportive care. His preliminary blood investigations showed pancytopenia with reduced reticulocyte count and transaminitis [Hb-9.2 g/dL, TLC-3,400 cells/mm3, platelets-20,000 cells/mm3, corrected reticulocyte count < 0.5%, peripheral smear-pancytopenia, serum AST-4688 U/L, ALT-6680 U/L, serum bilirubin 0.8 mg/dL, and ALP-186 U/L]. Based on these, possible differentials were viral infection, post-acute COVID-19, steroid-induced immunosuppression, drug-induced liver injury (DILI) probably due to Pirfenidone, Multisystem Inflammatory Syndrome–Adult (MIS-A), or COVID-19 associated CSS. Biochemical analyses have been tabulated in Table 1. Serum serologies [for HIV 1 and 2, hepatitis A, B, C, D, and E, Epstein-Barr virus (EBV), parvovirus B19, herpes simplex virus (HSV), cytomegalovirus (CMV), dengue, HSV 1 and 2, VDRL, Rickettsia, Leptospira, and scrub typhus], peripheral blood smear for malarial antigens, and serum autoimmune profile (anti-nuclear antibodies via immunofluorescence assay) were sent.
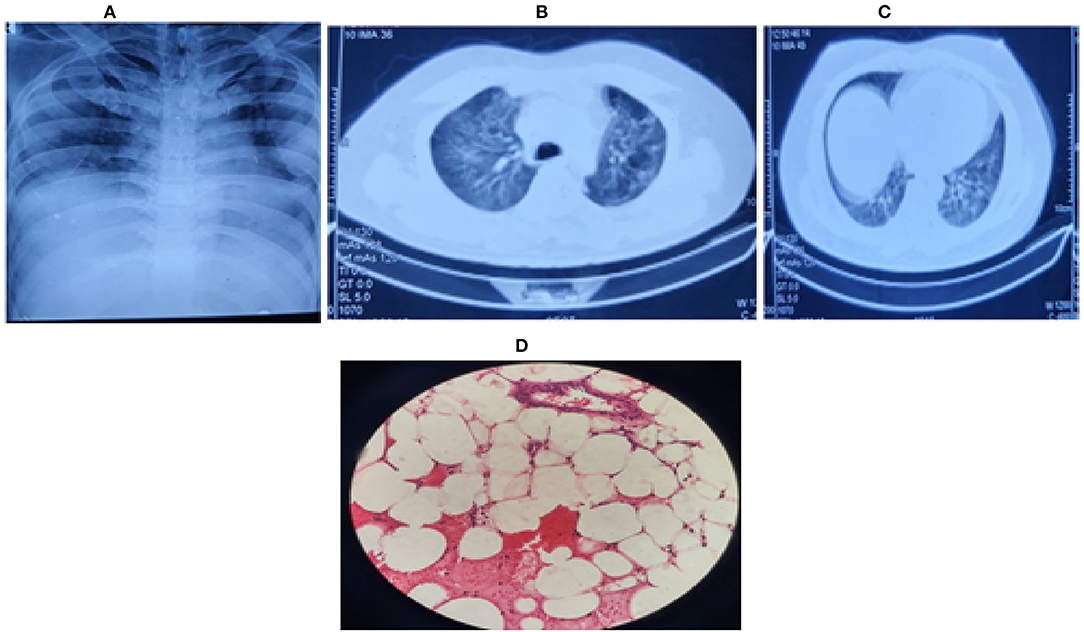
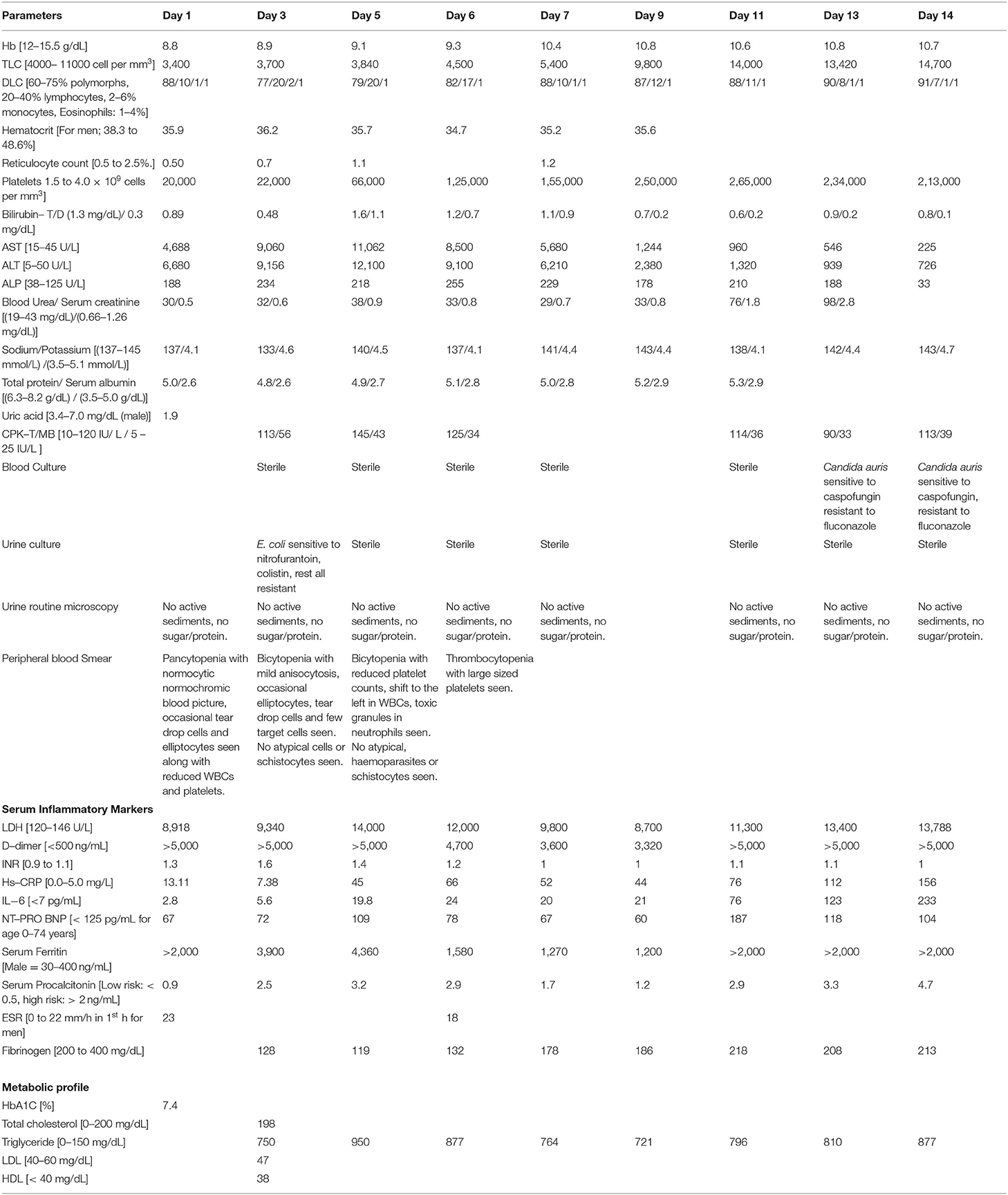
Figure 1. (A) Chest radiograph (bedside) on first day, showing bilateral middle zone haziness, (B) Contrast enhanced CT (CECT) chest on second day showing bilateral ground-glass opacities in lung parenchyma with (C) left upper lobe consolidation and air bronchogram. (D) Bone marrow biopsy showing hypocellular marrow (overall cellularity < 15%), lacunar spaces extensively replaced by fat cells; the residual cellularity mostly included lymphocytes, plasma cells, mast cells, and macrophages with only a few hematopoietic cells.
Further investigations revealed an elevated serum lactate dehydrogenase (8918 U/L), serum D-dimer (> 5,000 ng/mL), serum ferritin (> 2,000 ng/ml), INR (1.6), and serum fibrinogen levels (128 mg/mL), indicating disseminated intravascular coagulation (DIC) - like picture with a probably viral, drug-induced (pirfenidone, steroids), or autoimmune etiology. Antimicrobials were changed empirically to intravenous piperacillin-tazobactam (4.5 g QID) and doxycycline (100 mg BD) with ursodeoxycholic acid (300 mg TDS). In light of persistent pancytopenia, a bone marrow biopsy was performed under cover of platelets transfusion. Ultrasonography of the whole abdomen revealed hepatomegaly.
On the second day, his dyspnoea worsened, and because of raised D-dimer, a CT pulmonary angiography of the chest was done to rule out post-COVID-19 pulmonary embolism. However, it showed only a consolidation in the left upper lobe and bilateral ground-glass opacities with no evidence of pulmonary embolism (Figures 1B,C). Furthermore, his troponin I and T were negative, with normal creatine phosphokinase (CPK) and N terminal pro-B-type natriuretic peptide (NT Pro-BNP) levels. A transthoracic 2D-ECHO was reported normal. On the third day, serum procalcitonin was raised (2.5 ng/mL), but the blood culture was sterile, and urine culture after 48 h of incubation showed Escherichia coli susceptible to colistin which was initiated. Persistent fever prompted us to send repeated blood cultures, fungal cultures, urine routine microscopy, and cultures for the next 3 days. Sputum induction failed as the patient had a dry cough. Meanwhile, all viral serologies were reported negative. The patient persistently had transaminitis [AST-11,000 U/L; ALT-12000 U/L; INR-1.6 (refer to Table 1, day-5)], with no bleeding manifestations or altered sensorium throughout his hospital stay. Empirically vitamin K, rifaximin, and lactulose were added to the treatment. Meanwhile, his serum beta-D glucan level, galactomannan level, and autoimmune profile, including anti-nuclear antibodies (ANA), p-ANCA, c-ANCA, and thyroid profile, were reported normal.
After correlating pancytopenia, hepatomegaly, and DIC, with a consistently raised serum ferritin (> 4,000 ng/ml), serum triglycerides (950 mg/dL) and reduced serum fibrinogen levels (< 150 mg/dL) with an ESR of 23 mm (during the first hour) with his clinical presentation, we contemplated the possibility of secondary HLH (Hemophagocytic lymphohistiocytosis) as part of CSS due to COVID-19 infection. Meanwhile, bone marrow biopsy showed a hypocellular marrow for his age (overall cellularity < 15%), with a possibility of aplastic anemia, malignancy, or an invasive pathology (Figure 1D). After correlating the clinical, biochemical, radiological, and bone marrow reports, we diagnosed sHLH based on the HLH 2004 criteria and an H-Score of 256 (Table 2A. Our patient fulfilled six of the eight HLH-2004 criteria points with an H-score of 256, indicating a 99% probability of having HLH (Table 2A).
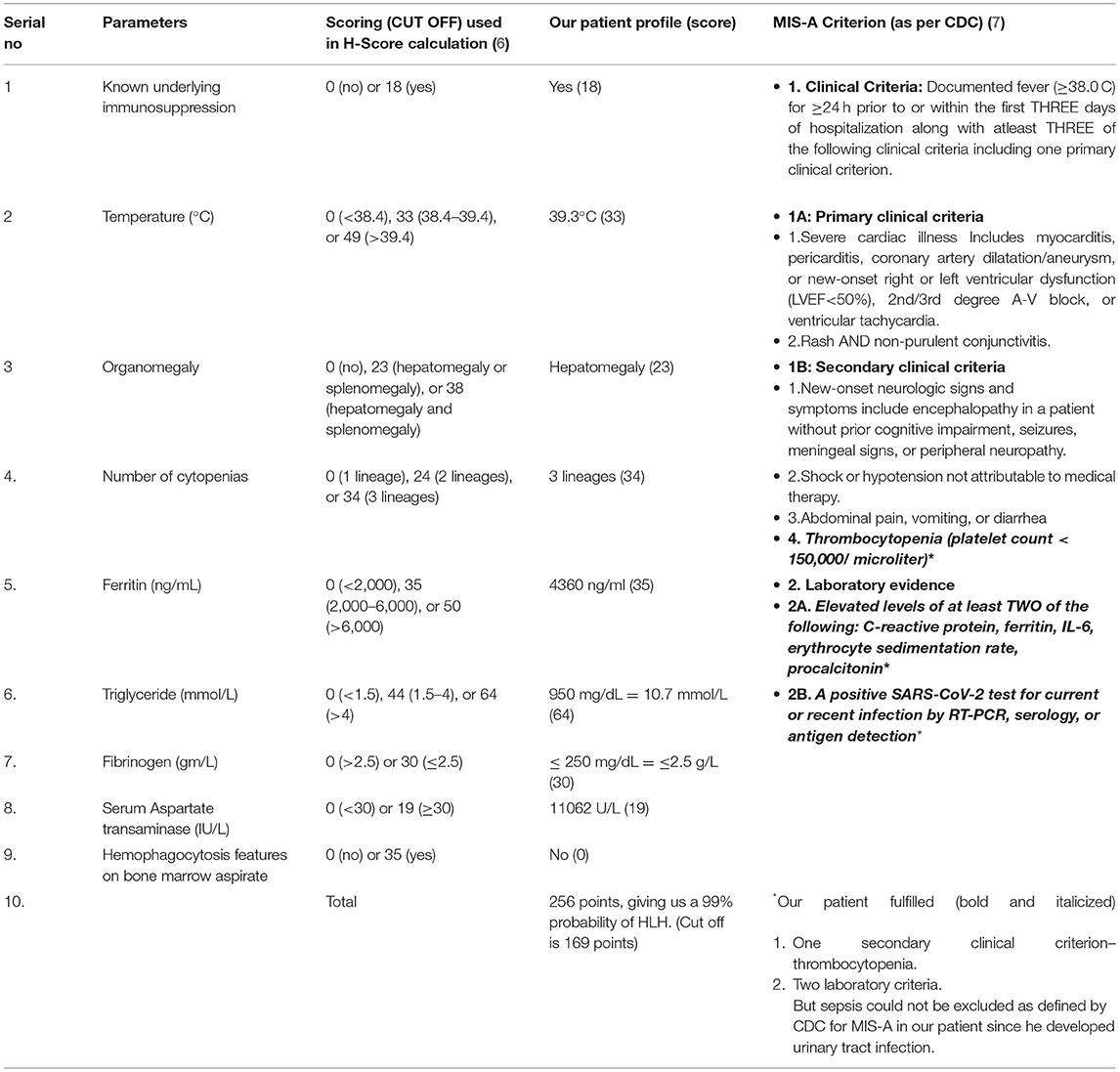
Table 2A. Calculation of H- Score (6) and Center for Disease Control (CDC) Multisystem Inflammatory Syndrome-Adults (MIS-A) criteria (7). H-Score calculation as per the criteria by Fardet et al. (6) An H-Score of 256 was found in our patient, giving us a 99% probability of hemophagocytic lymphohistiocytosis (HLH).
The following etiologies for sHLH were considered: a post-COVID-19 infection, DILI due to pirfenidone, or sepsis-induced HLH (compounded by steroid-induced immunosuppresion). The close differentials were CSS and MIS-A. Nevertheless, autoimmune disease, tuberculosis, and other viral etiologies had been ruled out. Hence, as per HLH treatment protocol, intravenous injection of 10 mg dexamethasone two times a day with 30 g IVIG per day was started.
Furthermore, serum soluble interleukin-2 receptor (sCD25) levels and flow cytometry for immunodeficiency profile were sent. From day six to ten, our patient responded clinically to this treatment. Also, no fever was documented, and no new lung crepitations were heard on auscultation. Moreover, improvements in the biochemical profile and inflammatory markers were seen. Repeated blood, fungal, and urine cultures were reported to be sterile (Table 1, from day 6 to 9).
Later, his sCD25 levels were reported positive, and serum flow cytometry showed a reduction in the percentages of T cells (CD3: 9.21% vs. 53.2% control), B cells (CD19: 1.32% vs. 7.19% control), CD20 cells (1.06% vs. 7.29%), NK cells (CD 56: 0.13% vs 13.54% control), and CD15 (0.26% vs. 11.71% control), along with a reduced activity of NK cells, and yet the CD4:CD8 ratio was maintained. This report suggested humoral and cell-mediated immunosuppression in our patient along with low NK cell activity, which was consistent with the diagnosis of sHLH.
On the eleventh day, an erythematous, maculopapular rash developed, along with fever (101.7°F) episodes. It was a non-palpable rash measuring 0.5 to 1 cm, on the flexor and extensor aspects of both arms, hands, face, and upper abdomen, suggesting a non-vasculitic but infective etiology (Figure 2). Hence, intravenous teicoplanin was added to cover methicillin- and vancomycin-resistant Staphylococcus aureus (MRSA and VRSA) along with the antifungal fluconazole. He also had raised inflammatory markers [serum procalcitonin, lactate dehydrogenase (LDH), D-dimer, C-reactive protein (CRP), and interleukin 6 (IL-6)] with deranged kidney functions tests (refer to Table 1), which raised the possibility of sepsis due to a new hospital-acquired infection in an immunosuppressed state. Blood culture on day thirteen grew Candida auris, sensitive to caspofungin. Hence, fluconazole was replaced with caspofungin. His fever subsided, but on day fourteen, his kidney function tests further worsened, and he developed respiratory distress with worsening hypoxemic respiratory failure. His ECG and cardiac biomarkers were normal with a negative TruNat for SARS-CoV-2. A CT angiography of the chest was planned to rule out pulmonary embolism, as inflammatory markers along with d-dimer were raised, but the patient had to be intubated because of worsening respiratory distress. His repeat blood culture on day fourteen was again positive for Candida auris, suggesting disseminated Candidemia. He finally succumbed to his illness on day fifteen of hospitalization, despite our best efforts.
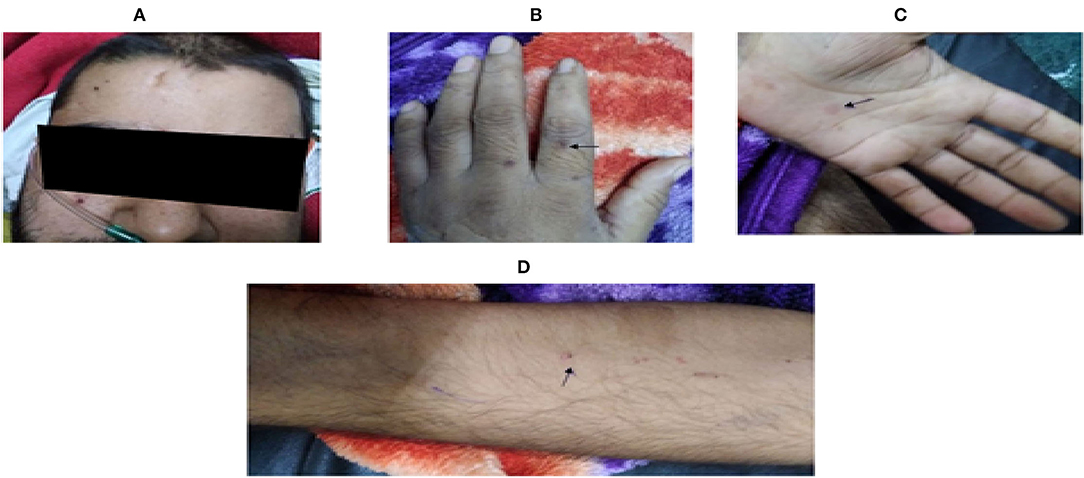
Figure 2. Showing skin lesions observed on eleventh day of hospital stay. (Arrow): Multiple, erythematous, and non-palpable maculopapular lesions over (A) face, (B,C) palm and dorsum of both hands, and (D) flexor aspect of arms.
The final diagnosis in this case was post-COVID-19 sequelae with superadded pneumonia and sepsis leading to secondary HLH with opportunistic disseminated candidiasis, causing multi-organ failure [acute kidney injury (AKI) with acute respiratory distress syndrome (ARDS)]. Written consent was taken from the patient's relative for using clinical data for this case report.
Discussion
Cytokine storm syndrome presents as multiorgan failure, rising inflammatory markers, and sHLH (20). CSS can be triggered by any infectious or rheumatological disease and cancer chemotherapy (20, 21). In the respiratory epithelial lining SARS-CoV-2 stimulates the proliferation of CD8+ T-cells and NK cells with cytokines converting histiocytes into activated macrophages that further release cytokines like IL-1, IL-6, and tumor necrosis factor-α, which stimulates CD8+ T-cells proliferation, maintaining the vicious cascade of cytokine storm (22). Similar to CSS, severe COVID-19 infection has also been associated with a dysregulated immune response. It leads to a hyperinflammatory state with elevation of IL-6, IL-8, IL1β, and ferritin (23). A study showed serum levels of IL-18, IFNγ, sFasL, and serum ICAM-1 were significantly elevated among patients with COVID-19 compared to those with classical HLH or MAS, which could guide to differentiate patients with SARS-CoV-2 induced immunosuppression (24). Our patient was previously admitted for severe COVID-19 infection and presented to us with features of sHLH, which could be attributed to an extension of CSS associated with COVID-19 due to a hyperinflammatory state.
Multisystem inflammatory syndrome is commonly seen among the pediatric population after COVID-19 infection (25). However, the CDC (7) has defined a criterion to diagnose MIS-A in adults as shown in Table 2A. MIS-A presents as a post-infectious syndrome with an antibody-mediated immune dysfunction and a varied clinical presentation, but the exact pathophysiology is unknown. (7, 25). The criteria include patients of age ≥ 21 years hospitalized for ≥ 24 hours or with an illness resulting in death with the exclusion of bacterial sepsis or worsened chronic medical condition.
Based on the proposed criteria for MIS-A (7), our patient presented within the proposed timeline of 12 weeks of recent COVID-19 infection. Nevertheless, our patient did not have MIS-A as he presented predominantly with respiratory complaints, later developed urosepsis, and lacked the required primary clinical criteria as highlighted in Table 2A. However, our patient fulfilled the laboratory criteria of a recent COVID-19 infection along with raised inflammatory markers and one of the secondary criteria; thrombocytopenia
Hemophagocytic lymphohistiocytosis is an uncommon condition characterized by immune dysfunction, persistent fever, and hemophagocytosis mediated by activated macrophages (26). HLH has been divided into primary and secondary subtypes. In both, there is immune dysregulation and cytokine storm leading to multiorgan dysfunction with fatal outcomes (27). Infection is the most common trigger for primary HLH, and malignancy is the most common cause in adults. Moreover, EBV is the most common infectious agent causing both primary and secondary HLH. (28, 29). Macrophage activation syndrome (MAS), a subtype of HLH, is characterized by excessive activation of macrophages leading to hyper-inflammation; and hyperferritinemia (30). Autoimmune and rheumatological conditions are associated with MAS (31).
There are case reports of SARS-CoV-2-induced sHLH diagnosed antemortem (11–15) and postmortem (1, 16–19) while the illness is/was ongoing, as shown in Table 2B. However, the literature is sparse about COVID-19 recovered cases developing sHLH (8–10), as shown in Table 2B. A study focusing on postmortem bone marrow biopsies of patients with COVID-19 showed histiocytic hyperplasia with hemophagocytosis (1). Elie Naous and colleagues have mentioned COVID-19-related HLH occuring 2 weeks after a documented clinical and biological recovery from COVID-19 illness in a 69-year-old woman (8). Our patient presented 4 weeks after severe COVID-19. He was initially suspected of having pirfenidone-induced liver injury with superadded lower respiratory tract infection, on a background of COVID-19 sequelae. Pirfenidone-related hepatotoxicity occurs with mild to moderate, asymptomatic serum transaminase elevations. The diagnosis of DILI is based on a high index of suspicion with the exclusion of other causes of liver dysfunction (32). Our patient was prescribed pirfenidone at another hospital as an off-label indication to prevent post-COVID-19 pulmonary fibrosis but this was stopped during the current hospitalization.
Hemophagocytic lymphohistiocytosis is a rare entity and a diagnosis of exclusion. In our case, after ruling out MIS-A and CSS, a diagnosis of HLH was contemplated because of raised inflammatory markers with persistent pancytopenia. We tried to eliminate most of the possible etiologies of sHLH and thus could attribute it to post COVID-19 infection. The probability of HLH was determined by the H-score given by Fardet et al.; our patient had a score of 256 points indicating a 99% probability of HLH (6). Previous case reports on post-COVID-19 HLH have relied mainly on H-score with scarce data on cytokine levels used in diagnosis for HLH (Table 2B) (8–10). The use of the H-Score for patients with COVID-19 has been questioned, and some authors recommend against using H-score. This is because of the low sensitivity of body temperature and the inability to distinguish between neutropenia and lymphocytopenia. Furthermore, there is a lack of published data on hypertriglyceridemia, splenomegaly, hepatomegaly, and marrow hemophagocytosis (16, 33). Most patients received steroids and had fatal outcomes despite treatment, as shown in Table 2B. Hence, we based our diagnosis of post-COVID-19 HLH on the HLH 2004 study criteria. This had a sensitivity of 91% and a specificity of 83.3% with a PPV of 97.6% and an NPV of 55.6% (34). The diagnosis of HLH needs any five out of the eight criterias: fever, splenomegaly, cytopenias affecting at least two of three lineages in the peripheral blood, hypertriglyceridemia (≥ 265 mg/dL) or hypofibrinogenemia (< 150 mg/dL), hemophagocytosis in bone marrow, spleen, or lymph nodes, low or absent NK-cell activity, hyperferritinemia (≥ 500 ng/mL), and raised sCD-25 levels (> 2SD) (26). Our patient met six of the eight criteria mentioned above. However, hemophagocytosis was not seen in the hypocellular bone marrow biopsy. Although hemophagocytosis is an essential component of HLH, it is not required for diagnosis. Bone marrow aspirates range from normocelluar to hypocellular to hypercellular in HLH. The prevalence of hemophagocytosis in patients with HLH ranges from 25 to 100% (35), which can also be caused by blood transfusions, infection, autoimmune disease, bone marrow failure, or red blood cell destruction (36–38). The presence of activated macrophages in the bone marrow or liver, as well as a comprehensive clinical examination, may help distinguish HLH from other causes of hemophagocytosis (39). As shown in Table 2B, most patients had hemophagocytosis, and 2 cases had no evidence of hemophagocytosis in the bone marrow. In our case, bone marrow was hypocellular for age and had < 15% cellularity. Autopsy evidence of HLH in patients with COVID-19 was first demonstrated by Prilutskiy et al. (Table 2B) (19).
In our case, MAS was ruled out after a negative autoimmune profile. Also, an extensive viral serology panel was negative; no apparent malignancy was detected on the CT scan or bone marrow biopsy and there was no blood transfusion. Hence, the clinical diagnosis of sHLH was attributed to COVID-19 based on the HLH-2004 criteria and raised H-score. Further, we considerd PACS in our patient as the patient presented to our hospital after 4 weeks of initial severe COVID-19 infection. Here, clinico-laboratory workup revealed sHLH after COVID-19 infection as a part of CSS. Admittedly, in this case report, considering sHLH as a delayed presentation of CSS due to COVID-19 or occurring as PACS is a debatable point.
There is also a possibility that sepsis could have triggered HLH in our patient. Although initial cultures were sterile, E. coli urosepsis was detected on the third day of admission, the very day we diagnosed him with HLH. A latent, persistent subclinical inflammatory state secondary to COVID-19 could have led to this presentation. Severe sepsis can present as the initial manifestation of HLH and hence, differentiating sepsis from HLH-sepsis overlap is quite challenging (40, 41). Clinical distinctions between severe sepsis and HLH might be ambiguous; prompt detection and management necessitate a high level of suspicion. More than 10% of patients with HLH die within 2 months of diagnosis due to bleeding in visceral organs, opportunistic infections due to neutropenia, or Multiple Organ Dysfunction Syndrome (MODS) (35). Our patient responded clinically and biochemically to dexamethasone and IVIG. However, due to immune dysfunction related to HLH, along with steroid-induced immunosuppression, he developed an opportunistic infection in the form of disseminated C. auris infection. As the initial fungal culture was sterile and the patient developed rashes on eleventh day, the temporality for causation of HLH by C. auris could not be established. C. auris has recently been linked to major outbreaks of invasive infections in healthcare facilities throughout the world (42). It is a multidrug-resistant pathogenic yeast that has high mortality rates (43). Globally, C. auris isolates are resistant to fluconazole in >90% of cases and amphotericin B in > 30% of cases (44). Our patient had C. auris on culture, resistant to fluconazole, but was sensitive to caspofungin. Despite treatment for HLH and candidemia, our patient developed ARDS, was intubated, and succumbed to the illness, thus highlighting the lethal sequence of events that challenged us in this case.
As this is a single case report, no consensus on guidelines and approach to management can be elucidated. More studies of such cases are needed to formulate strategies for the diagnosis and management of this fatal condition. Early detection of HLH among patients who have recovered from severe COVID-19 can be done by looking for the following pointers in patients (45): (1) ongoing remittent quotidian fever and being unresponsive to empiric antibiotic therapy; (2) hepatosplenomegaly; (3) falling ESR, leukopenia, and thrombocytopenia; (4) raised transaminases; (5) elevated CRP/ferritin/triglyceride; (6) hypofibrinogenemia.
Furthermore, with any of the above pointers, a panel of immunologic markers (elevated sCD25 and reduced NK cell cytotoxicity) should be ordered to detect HLH earlier, followed by guideline-directed treatment.
Learning Points
Secondary HLH occurring in ongoing illness among patients of COVID-19 is an uncommon finding. We wish to highlight the occurrence of sHLH as a delayed presentation of CSS within the time frame of PACS.
• Post-COVID HLH is likely under-recognized, and mortality remains high, especially in adults; thus, prompt diagnosis and treatment are essential.
• Clinical and laboratory surveillance is necessary for a more extended period in patients with severe COVID-19.
• For diagnosis of sHLH among patients with COVID-19, using the HLH-2004 criteria for diagnosis seems more reliable than H-score.
• Indiscriminate use of immunosuppressive agents and pirfenidone among patients with severe COVID-19 may predispose them to fatal OIs since the patient is already in an immunosuppressed state due to steroids, COVID-19, and sHLH per se.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors were involved in the management of the patient. SGau and GS collected all the clinically relevant data and completed the initial draft. SS and SGar had put their valuable input in editing and giving the shape to the final manuscript. All authors contributed to the article and approved the submitted version. Both SGau and SS made all the final changes and approved the author's proof.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Prieto-Pérez L, Fortes J, Soto C, Vidal-González Á, Alonso-Riaño M, Lafarga M, et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. (2020). 33:2139–46. doi: 10.1038/s41379-020-0613-1
2. Knight V, Heimall JR, Chong H, Nandiwada SL, Chen K, Lawrence MG, et al. A toolkit and framework for optimal laboratory evaluation of individuals with suspected primary immunodeficiency. J Allergy Clin Immunol Pract. (2021). 9:3293–307.e6. doi: 10.1016/j.jaip.2021.05.004
3. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021). 27:601–15. doi: 10.1038/s41591-021-01283-z
4. Lledó GM, Sellares J, Brotons C, Sans M, Antón JD, Blanco J, et al. Post-Acute COVID-19 Syndrome: A New Tsunami Requiring a Universal Case Definition. Clinical Microbiology Infection [Internet]. (2021). Available online at: https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(21)00661-3/fulltext (accessed March 3, 2022).
5. CDC. Healthcare Workers [Internet]. Centers for Disease Control and Prevention. (2020). Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html (accessed March 3, 2022).
6. Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis & rheumatology. (2014) 66:2613–20.
7. Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection? United Kingdom and United States, March–August (2020). MMWR. (2020). 69:1450–6. doi: 10.15585/mmwr.mm6940e1
8. Naous E, Nassani B-M, Yaghi C, Nasr F, Medlej R. Hemophagocytic lymphohistiocytosis, a new cause of death during “post-acute COVID-19 syndrome?” a case report. J Hematop. (2021). 1–5. doi: 10.1007/s12308-021-00452-w
9. Kalita P, Laishram D, Dey B, Mishra J, Barman B, Barman H. Secondary hemophagocytic lymphohistiocytosis in post-COVID-19 patients: a report of two cases. Cureus. (2021). 13:8. doi: 10.7759/cureus.17328
10. Wiseman D, Lin J, Routy J-P, Samoukovic G. Haemophagocytic lymphohistiocytosis in an adult with postacute COVID-19 syndrome. BMJ Case Rep. (2021). 14:e245031. doi: 10.1136/bcr-2021-245031
11. Dp V, H D, G Y, Sp V. Complicated case of COVID-19 disease with overlapping features of thrombotic thrombocytopenic purpura and haemophagocytic lymphohistiocytosis. BMJ Case Rep. (2021). 14:e242202. doi: 10.1136/bcr-2021-242202
12. Schnaubelt S, Tihanyi D, Strassl R, Schmidt R, Anders S, Laggner AN, et al. Hemophagocytic lymphohistiocytosis in COVID-19: Case reports of a stepwise approach. Medicine. (2021). 100:e25170. doi: 10.1097/MD.0000000000025170
13. von der Thüsen JH, van Bommel J, Kros JM, Verdijk RM, Lopuhaä B, Lam KH, et al. Case report: a fatal combination of hemophagocytic lymphohistiocytosis with extensive pulmonary microvascular damage in COVID-19 pneumonia. J Hematop. (2020). 1–5. doi: 10.1007/s12308-020-00423-7
14. Tholin B, Hauge MT, Aukrust P, Fehrle L, Tvedt TH. Hemophagocytic lymphohistiocytosis in a patient with COVID-19 treated with tocilizumab: a case report. J Med Case Rep. (2020). 14:187. doi: 10.1186/s13256-020-02503-9
15. Lima R, Filho CC, Ferreira Filho CM, Vaisman M, Cossenza A, Rebello CP, et al. Hemophagocytic syndrome and COVID-19. Respir Med Case Rep. (2020). 31:101162. doi: 10.1016/j.rmcr.2020.101162
16. Retamozo S, Brito-Zerón P, Sisó-Almirall A, Flores-Chávez A, Soto-Cárdenas M-J, Ramos-Casals M. Haemophagocytic syndrome and COVID-19. Clin Rheumatol. (2021). 40:1233–44. doi: 10.1007/s10067-020-05569-4
17. Núñez-Torrón C, Ferrer-Gómez A, Moreno Moreno E, Pérez-Mies B, Villarrubia J, Chamorro S, et al. Secondary haemophagocytic lymphohistiocytosis in COVID-19: correlation of the autopsy findings of bone marrow haemophagocytosis with HScore. J Clin Pathol. (2021) 0:1–7. doi: 10.1136/jclinpath-2020-207337
18. Meng M, Chen L, Zhang S, Dong X, Li W, Li R, et al. Risk factors for secondary hemophagocytic lymphohistiocytosis in severe coronavirus disease 2019 adult patients. BMC Infectious Diseases. (2021). 21:398. doi: 10.1186/s12879-021-06094-8
19. Prilutskiy A, Kritselis M, Shevtsov A, Yambayev I, Vadlamudi C, Zhao Q, et al. SARS-CoV-2 infection-associated hemophagocytic lymphohistiocytosis. Am J Clin Pathol. (2020). 154:466–74. doi: 10.1093/ajcp/aqaa124
20. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. (2020). 368:473–4. doi: 10.1126/science.abb8925
21. Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease (2019). Clin Immunol. (2020) 214:108393. doi: 10.1016/j.clim.2020.108393
22. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. (2020). 10:102–8. doi: 10.1016/j.jpha.2020.03.001
23. Min C-K, Cheon S, Ha N-Y, Sohn KM, Kim Y, Aigerim A, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. (2016). 6:25359. doi: 10.1038/srep25359
24. Kessel C, Vollenberg R, Masjosthusmann K, Hinze C, Wittkowski H, Debaugnies F, et al. Discrimination of COVID-19 from inflammation-induced cytokine storm syndromes using disease-related blood biomarkers. Arthritis Rheumatol. (2021). 73:1791–9. doi: 10.1002/art.41763
25. Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. (2020). 20:e276–88. doi: 10.1016/S1473-3099(20)30651-4
26. Henter J-I, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. (2007). 48:124–31. doi: 10.1002/pbc.21039
27. Hemophagocytic Lymphohistiocytosis Annual Annual Review of Pathology: Mechanisms of Disease [Internet]. Available online at: https://www.annualreviews.org/doi/full/10.1146/annurev-pathol-020117-043625 (accessed Nov 28, 2021).
28. Smith MC, Cohen DN, Greig B, Yenamandra A, Vnencak-Jones C, Thompson MA, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. (2014). 7:5738–49.
29. Allen CE, McClain K. Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematology. (2015) 2015:177–82. doi: 10.1182/asheducation-2015.1.177
30. Andersson U. Hyperinflammation: On the pathogenesis and treatment of macrophage activation syndrome. Acta Paediatr. (2021). 110:2717–22. doi: 10.1111/apa.15900
31. Lin C-I, Yu H-H, Lee J-H, Wang L-C, Lin Y-T, Yang Y-H, et al. Clinical analysis of macrophage activation syndrome in pediatric patients with autoimmune diseases. Clin Rheumatol. (2012). 31:1223–30. doi: 10.1007/s10067-012-1998-0
32. Verma N, Kumar P, Mitra S, Taneja S, Dhooria S, Das A, et al. Drug idiosyncrasy due to pirfenidone presenting as acute liver failure: case report and mini-review of the literature. Hepatol Commun. (2018). 2:142–7. doi: 10.1002/hep4.1133
33. Leverenz DL, Tarrant TK. Is the HScore useful in COVID-19? Lancet. (2020). 395:e83. doi: 10.1016/S0140-6736(20)31057-6
34. Croden J, Grossman J, Sun H. External validation of the HLH-2004 diagnostic criteria and H-score for diagnosis of hemophagocytic lymphohistiocytosis in adults. Blood. (2020). 136:44–5. doi: 10.1182/blood-2020-138589
35. Gupta A, Weitzman S, Abdelhaleem M. The role of hemophagocytosis in bone marrow aspirates in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. (2008). 50:192–4. doi: 10.1002/pbc.21441
36. Schaer DJ, Schaer CA, Schoedon G, Imhof A, Kurrer MO. Hemophagocytic macrophages constitute a major compartment of heme oxygenase expression in sepsis. Eur J Haematol. (2006). 77:432–6. doi: 10.1111/j.1600-0609.2006.00730.x
37. Biondi CS, Cotorruelo CM, Ensinck A, Racca LL, Racca AL. Use of the erythrophagocytosis assay for predicting the clinical consequences of immune blood cell destruction. Clin Lab. (2004). 50:265–70.
38. Kraus MD, Bartlett NL, Fleming MD, Dorfman DM. Splenic pathology in myelodysplasia: a report of 13 cases with clinical correlation. Am J Surg Pathol. (1998). 22:1255–66. doi: 10.1097/00000478-199810000-00011
39. Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. (2011). 118:4041–52. doi: 10.1182/blood-2011-03-278127
40. Hindi Z, Khaled AA, Abushahin A. Hemophagocytic syndrome masquerading as septic shock: an approach to such dilemma. SAGE Open Med Case Rep. (2017). 5:2050313X17746309. doi: 10.1177/2050313X17746309
41. Agarwal A, Agarwal A. Infection associated secondary hemophagocytic lymphohistiocytosis in sepsis syndromes - a tip of an iceberg. J Assoc Physicians India. (2016). 64:44–50.
42. Khan Z, Ahmad S. Candida auris: an emerging multidrug-resistant pathogen of global significance. CMRP. (2017). 7:240–8. doi: 10.1016/j.cmrp.2017.11.004
43. Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the prospective antifungal therapy (PATH) registry 2004-2008. PLoS ONE. (2014). 9:e101510. doi: 10.1371/journal.pone.0101510
44. Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. (2018). 73:891–9. doi: 10.1093/jac/dkx480
Keywords: post acute covid-19 syndrome (PACS), secondary hemophagocytic lymphohistiocytosis, Candida auris, macrophage activation syndrome (MAS), intravenous immunoglobulin, cytokine storm syndrome (CSS), multisystem inflammatory syndrome-adults (MIS-A), hyperferritinemia syndrome
Citation: Gautam S, Sharma G, Singla S and Garg S (2022) Case Report: Secondary Hemophagocytic Lymphohistiocytosis (sHLH) and Candida auris Fungemia in Post-acute COVID-19 Syndrome: A Clinical Challenge. Front. Med. 9:835421. doi: 10.3389/fmed.2022.835421
Received: 14 December 2021; Accepted: 28 March 2022;
Published: 17 June 2022.
Edited by:
Zhiliang Hu, Nanjing Second Hospital, ChinaReviewed by:
Carmen Silvia Valente Barbas, University of São Paulo, BrazilJohnson Liu, Maine Medical Center, United States
Jan Von Der Thüsen, Erasmus University Medical Center, Netherlands
Copyright © 2022 Gautam, Sharma, Singla and Garg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sumeet Singla, sumeetsingla555@gmail.com
 Sachin Gautam
Sachin Gautam Gaurav Sharma
Gaurav Sharma Sumeet Singla
Sumeet Singla Sandeep Garg
Sandeep Garg