Maintenance With Hypomethylating Agents After Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Systematic Review and Meta-Analysis
- 1Division of Hematology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 2Department of Pharmacology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 3Vancouver General Hospital, L/BMT Program of British Columbia, Vancouver, BC, Canada
- 4British Columbia Research Centre, Terry Fox Laboratory, Vancouver, BC, Canada
Introduction: Hypomethylating agents (HMAs) seem to have a range of properties favorable to post-allogeneic hematopoietic stem cell transplantation (allo-SCT) maintenance in acute myeloid leukemia (AML) patients.
Materials and Methods: The Embase, MEDLINE, and Cochrane Central Register of Controlled Trials databases were independently searched by two investigators to identify relevant studies published inception to 18 November 2021. These trials compared HMA maintenance to observation following allo-SCT for AML or myelodysplastic syndrome.
Results: The meta-analysis eligibility criteria were fulfilled by 14 studies. The overall survival and relapse-free survival of the HMA maintenance group were superior to the observation group, with a pooled risk ratio (RR) of 1.38 and 1.46, respectively. Moreover, the cumulative incidence of relapse was significantly lower in those who received HMAs. The HMA group also had lower non-relapse mortality compared with the observation group. Overall, the incidences of grades III–IV acute graft-vs.-host disease (GVHD) and chronic GVHD did not differ in both groups. However, when looking specifically at those receiving decitabine maintenance, the rate of chronic GVHD seemed to be lower compared with observation alone.
Conclusions: The current systematic review and meta-analysis illustrated that AML and MDS patients receiving HMA maintenance after allo-SCT had better outcomes in regards to OS, RFS, NRM, CIR as well as a reduced incidence of chronic GVHD.
Key Messages
• Role of HMAs Maintenance After Allo-SCT in AML Has Been Extensively Studied in Recent Years in Order to Improve Clinical Outcome.
• This Meta-Analysis Demonstrated Favorable Outcome of HMAs Maintenance in Term of Relapse Rate, non-Relapse Mortality, Relapse-Free Survival and Overall Survival.
• Decitabine Maintenance Resulted in Lower Chronic GVHD Rate Compared With Observation Strategy.
Introduction
Allogeneic stem cell transplantation is the mainstay treatment for AML stratified as intermediate or unfavorable risk as well as for high-risk MDS. This therapy has demonstrated superior efficacy over non-alloSCT approaches in regards to long-term clinical outcomes (1, 2). Nevertheless, even after allo-SCT, 35–45% of patients suffer from disease relapse, leading to dismal outcomes (3, 4).
Several strategies have been adopted to prolong disease-free survival. Based on the time of intervention, they can be categorized into either preemptive approaches—those commenced at the time of detection of minimal residual disease (MRD)—or prophylactic approaches—those initiated in the absence of detectable leukemia. In the case of the prophylactic approaches, both cellular and pharmacological maintenance strategies have been reported, including prophylactic donor leukocyte infusion (DLI), hypomethylating agents (HMAs), histone deacetylase inhibitors, FMS-like tyrosine kinase 3 (FLT3) inhibitors, or isocitrate dehydrogenase inhibitors (5–7). Notably, HMAs have generated considerable research interest in recent years due to their favorable side effect profile.
HMAs exhibit several properties that make them suitable for post-allo-SCT maintenance. They mediate a direct anti-leukemic effect in AML and MDS, regardless of their molecular mutation profile. Moreover, their abilities to induce a CD8+ tumor-specific T cell response, together with the expansion of regulatory T cells, lead to an epigenetically enhanced Graft vs. Leukemia (GVL) effect that is not counterbalanced by an increased risk of GVHD (8–12). Lastly, they are safe and well-tolerated by AML patients in remission (13).
Many studies have examined the use of azacitidine and decitabine as maintenance after allo-SCT for AML and MDS. Although the majority of the studies supported consideration of HMA maintenance therapy, the remainder did not demonstrate clear benefits (14–29). A recent systematic review explored the safety and efficacy of maintenance treatment following allo-SCT in AML and MDS. It demonstrated rates of 65.6 and 56.2% for the 2-year overall survival (OS) and the relapse-free survival (RFS), respectively, of HMA-treated patients. In addition, acute and chronic GVHD were found in 39.9 and 44.4%, of patients respectively. These results suggest that HMA maintenance could be employed to prolong RFS and OS (30). Nonetheless, the benefit of HMA maintenance after allo-SCT is still uncertain.
This meta-analysis was performed to review all relevant studies to compare the outcomes of patients undergoing allo-SCT for AML or MDS receiving HMA maintenance therapy with observation only.
Materials and Methods
Data Sources and Searches
The Embase, MEDLINE, and Cochrane Central Register of Controlled Trials databases were independently searched by two investigators (B.P., W.O.) to identify relevant studies published from inception to November 18, 2021. The search terms consisted of words associated with HMAs, acute myeloid leukemia, myelodysplastic syndrome, and stem cell transplantation. Supplementary Data 1 details the exhaustive search strategy lists. The study was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines (Supplementary Data 2).
Selection Criteria and Data Extraction
The inclusion criteria were as follows: (1) studies had to be either randomized controlled trials (RCTs) or cohort studies (prospective or retrospective); (2) the patients underwent allo-SCT for AML or MDS; (3) the studies compared two patient groups: one receiving an HMA post-allo-SCT, and the other being an observational group; and (4) the studies needed to report at least one of our primary outcomes of interest (OS, RFS, grades III–IV acute GVHD, and chronic GVHD). The secondary outcomes of interest were the CIR and NRM. Study eligibility was individually assessed by three investigators (B.P., W.O, S.K.); disagreements were resolved by consensus.
Two investigators (B.P., W.O.) utilized a standardized collection form to extract the baseline characteristic data of the patients in each group, along with details of the primary and secondary outcomes of interest. The extracted data was cross-checked to confirm its accuracy.
Definitions of Outcomes
The OS rate was defined as the time between the stem cell infusion and the time of death or last follow-up, while RFS was defined as the time interval from the stem cell infusion to the date of relapse or death from any cause. All causes of death (other than death from a relapse) were used to calculate the NRM rate.
Quality Assessment
Two investigators (B.P., W.O.) assessed the quality of each study using the Jadad scale for RCTs and the Newcastle–Ottawa scale for cohort studies (31, 32).
Statistical Analysis
The Mantel–Haenszel method was used to combine the effect estimates and 95% confidence intervals (CIs) of each study, and to calculate the pooled odds ratio (OR) with 95% CI (33). A random-effects model was preferred over a fixed-effects model because it was more likely that high heterogeneity would be found among the studies. Statistical heterogeneity was calculated using Cochran's Q test, estimated by the heterogeneity (I2) statistic. There were four heterogeneity levels: insignificant (I2 values of 0–25%), low (I2 values of 26–50%), moderate (I2 values of 51–75%), and high (I2 values of > 75%) (34). The presence of a publication bias was visualized by a funnel plot along with Egger's regression test. Due to a lack of clinical studies, a subgroup analysis based on the types of HMAs could not be performed. All statistical analyses were performed using the Review Manager (RevMan) software (version 5.3; The Cochrane Collaboration, Oxford, UK) and “meta” package version 5.1-0. This study was registered at www.inplasy.com as #INPLASY2021110078.
Results
Search Results
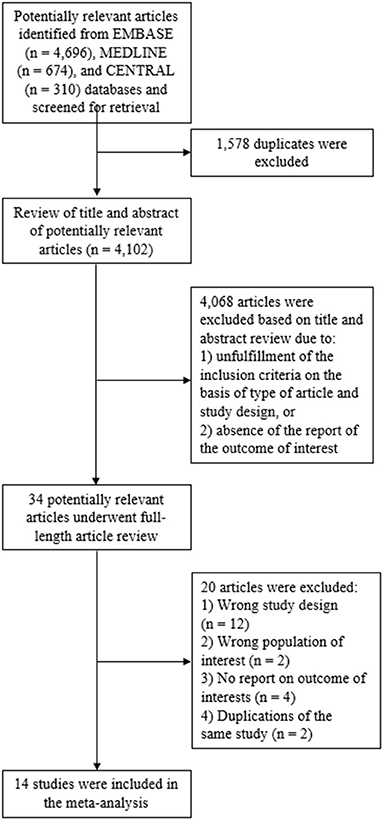
The systematic search of the Embase, MEDLINE and Cochrane Central Register of Controlled Trials databases identified 5,680 articles, from which 1,578 duplicates were removed. This resulted in 4,102 articles available for title and abstract review. Subsequently, 4,068 articles were excluded as the article type and study design did not fulfill the inclusion criteria, or there was no report on a primary outcome of interest. The remaining 34 articles underwent full-length review and 20 of those were excluded for the aforementioned reasons. Ultimately, the eligibility criteria for our meta-analysis were met by 14 studies: two RCTs, two prospective cohort study, and 10 retrospective cohort studies (14–22, 25, 27–29). Twelve of these compared azacitidine maintenance to observation, whereas two compared decitabine maintenance to observation. Figure 1 illustrates the full literature review and selection process.
Baseline Patient Characteristics
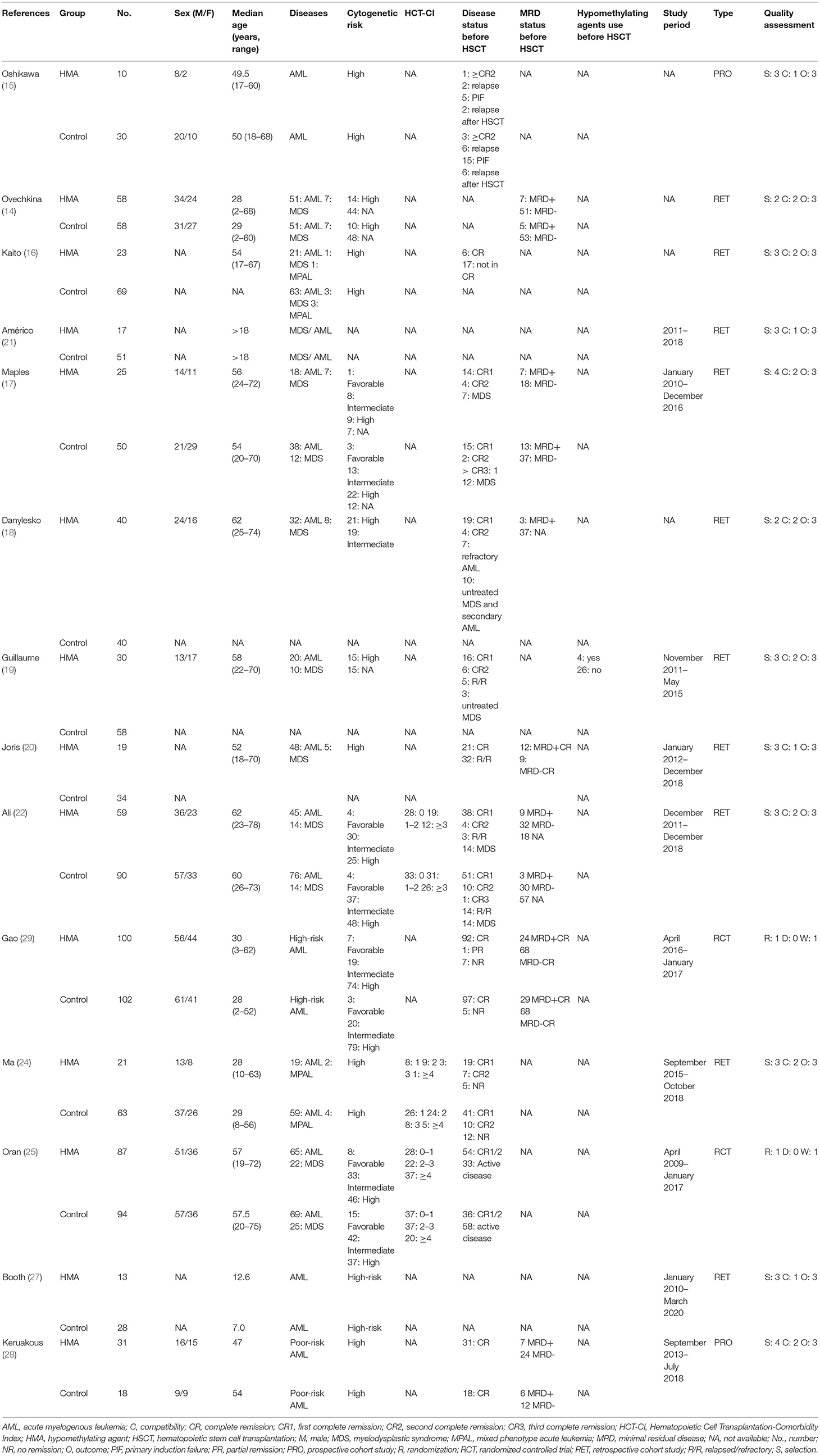
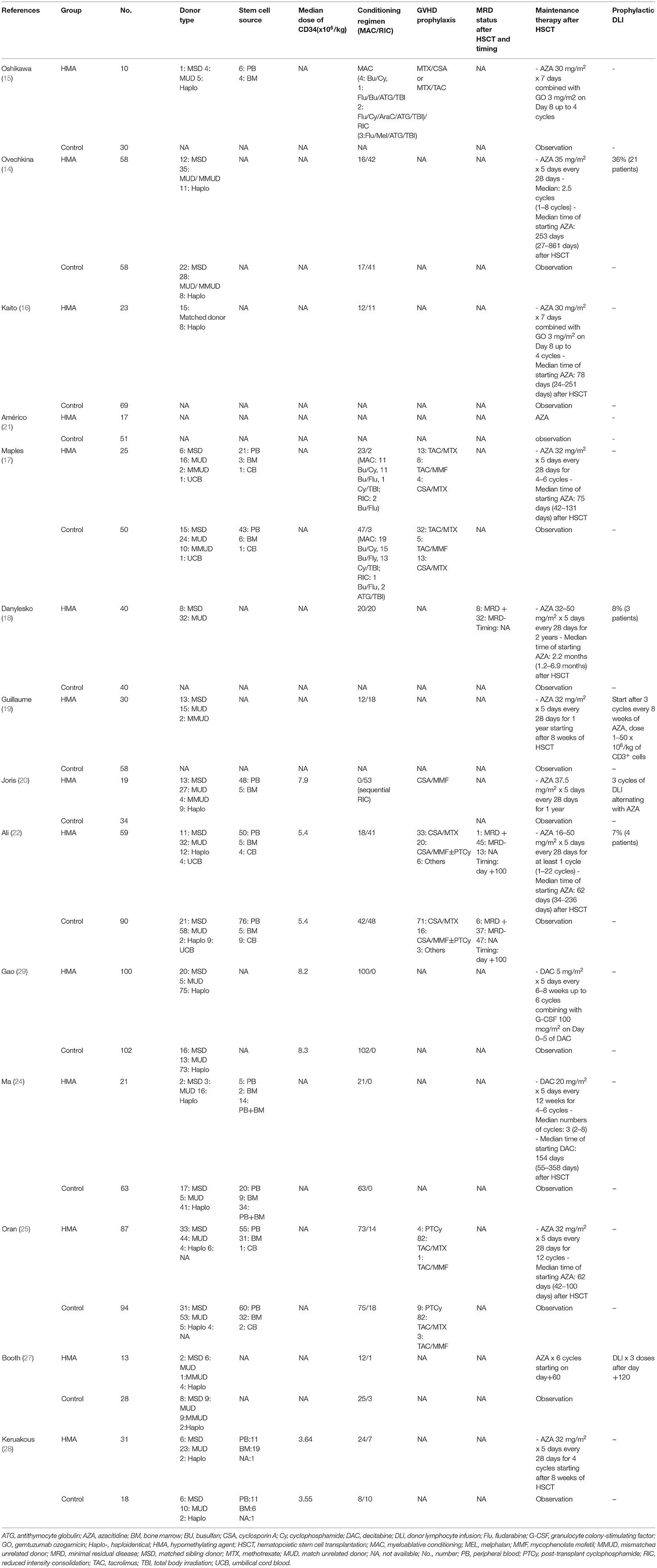
The 14 included studies were composed of 533 patients who received HMAs as maintenance, and another 784 patients who were observed post-allo-SCT. The age of the participants varied greatly (HMA group: 2 to 78 years; and observation group: 2 to 75 years). AML accounted for the largest proportion of the disease subtypes in the HMA group (84.8%), followed by MDS (14.6%) and mixed-phenotype leukemia (0.6%). These values were similar to the corresponding proportions found in the observation group (87.5, 11.2, and 1.3%, respectively). In both groups, matched unrelated donors and matched sibling donors were the most common donor sources for allo-SCT, accounting for 45.2 and 26.8%, respectively. In addition, myeloablative conditioning regimens (68.2%) were used more frequently compared to reduced-intensity conditioning regimens (31.8%). Details of the patient characteristics, such as disease status before allo-SCT, MRD status before allo-SCT cytogenetic risk, prior treatment and HMA before allo-SCT, comorbidities and performance status [Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI)], study period and quality assessment are summarized in Table 1. Details of the donor types, stem cell source, median dose of CD34+ stem cells, conditioning regimens, GVHD prophylaxis, MRD status after allo-SCT, maintenance protocols and prophylactic DLI are listed in Table 2.
HMA Maintenance Protocols After Allo-SCT
The median time of commencement of the HMAs varied between 56 and 154 days after allo-SCT. Twelve studies used azacitidine (14–22, 25, 27, 28), while two used decitabine (24, 29). The azacitidine dosage was 16–50 mg/m2 on Day 1 to Day 5 every 4 weeks for 1–22 cycles. A study from Oshikawa et al. and Kaito et al. combined azacitidine with gemtuzumab ozogamicin for the maintenance protocol (15, 16). Decitabine was administered at 5–20 mg/m2 on Day 1 to Day 5 every 6–12 weeks for 1–22 cycles. Some patients received prophylactic DLI in addition to HMA maintenance in five studies (14, 18–20, 22, 27).
Comparison of Clinical Outcomes of HMA and Observation Groups
The OS rates were reported as a 1-year rate in three studies (15–17), a 2-year rate in six studies (19–22, 28, 29), and a 3-year rate in three studies (14, 18, 24). The RFS rates were reported as a 1-year rate in two studies (15, 16), a 2-year rate in five studies (19–21, 27, 29), and a 3-year rate in two studies (18, 24). The OS of the HMA group was superior to that of the observation group, with a pooled RR of 1.38 (95% CI, 1.19–1.60; I2, 50%; Figure 2A) (14–22, 24, 28, 29). Similarly, a pooled meta-analysis found that the RFS was significantly better in patients who received HMAs, with a pooled RR of 1.46 (95% CI, 1.31–1.62; I2, 0%; Figure 2B) (15, 16, 18–21, 24, 27, 29). The patients receiving HMAs also had a lower NRM than those under observation (pooled RR, 0.36; 95% CI, 0.19–0.66; I2, 0%; Figure 3A) (14, 16, 18, 19, 28). Furthermore, the CIR was significantly higher for the observed patients (pooled RR, 0.69; 95% CI, 0.50–0.95; I2, 67%; Figure 3B) (14, 16, 18, 19, 21, 24, 25, 28, 29). However, the incidences of grades III–IV acute GVHD and chronic GVHD of the groups did not differ [pooled RR, 0.88; 95% CI, 0.30–2.60; I2, 604% (14, 17, 22, 24, 25); and pooled RR, 0.84; 95% CI, 0.58–1.23; I2, 65% (22, 24, 25, 29); Figures 4A,B, respectively].
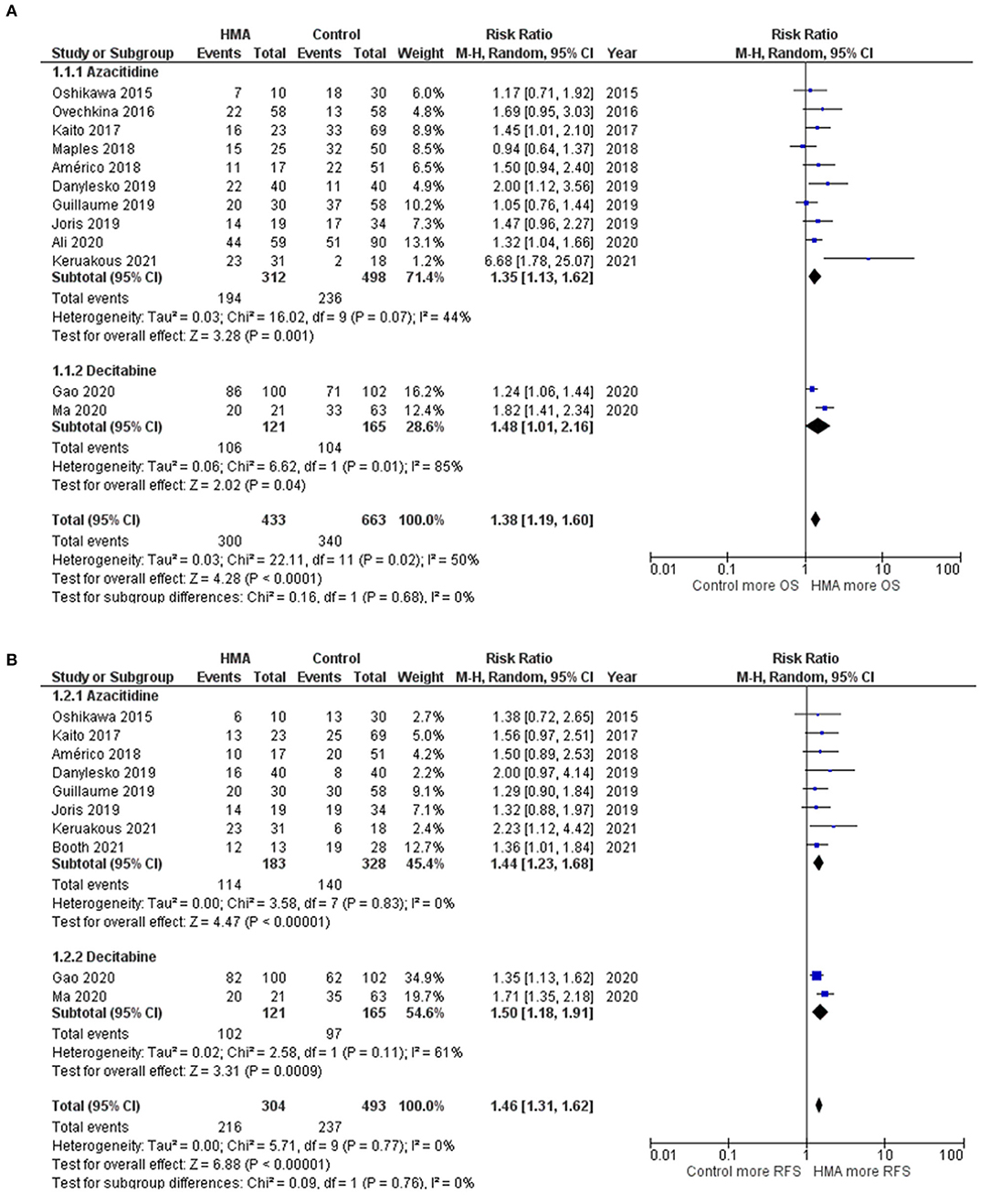
Figure 2. Forest plots of the meta-analysis of HMA maintenance compared with no HMA maintenance. (A) OS rate. (B) RFS rate.
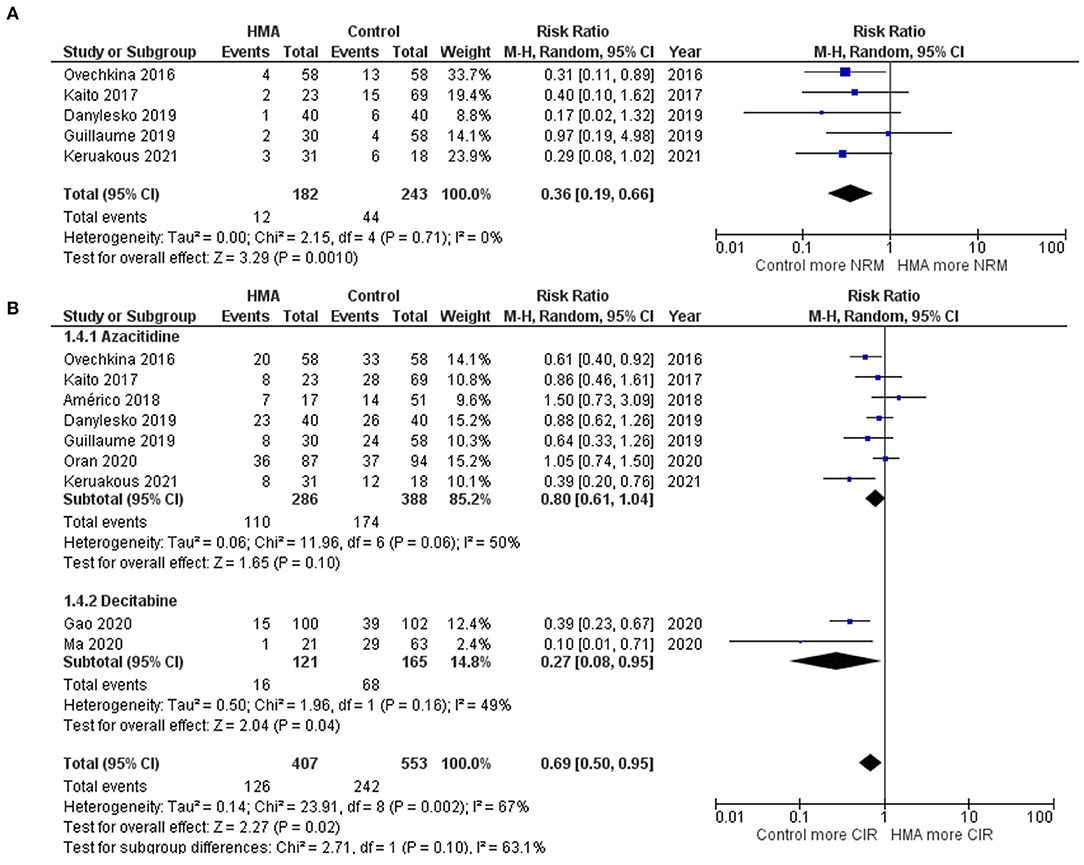
Figure 3. Forest plots of the meta-analysis of HMA maintenance compared with no HMA maintenance. (A) NRM rate. (B) CIR rate.
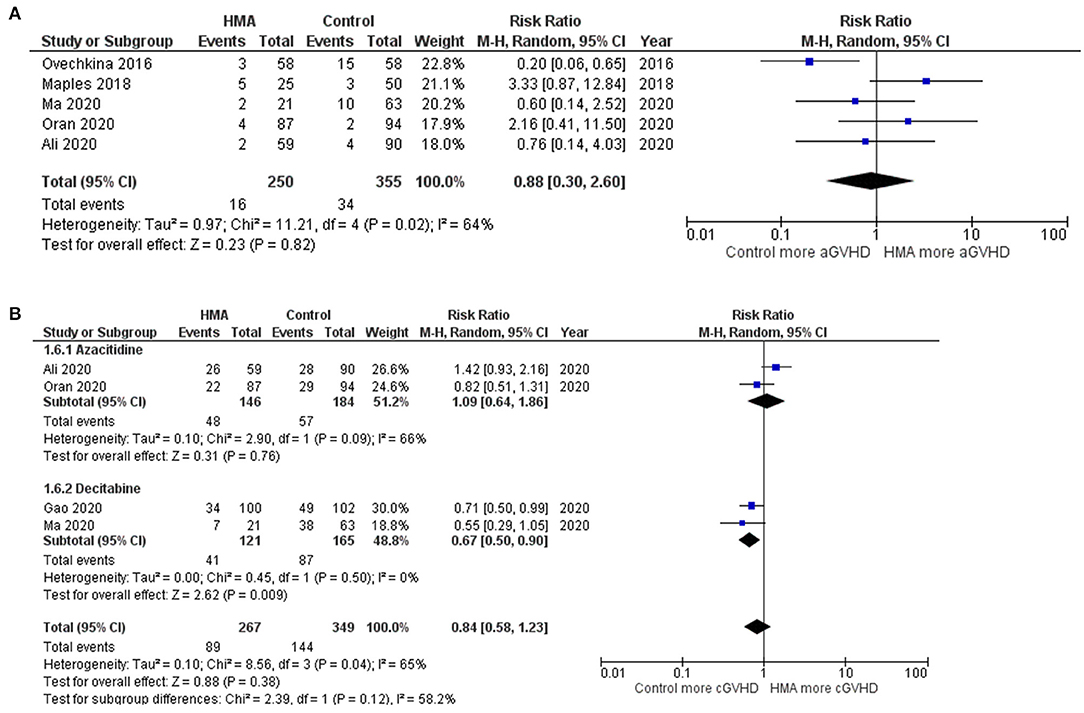
Figure 4. Forest plots of the meta-analysis of HMA maintenance compared with no HMA maintenance. (A) grade III–IV aGVHD rate. (B) cGVHD rate.
Because two studies combined gemtuzumab ozogamicin with HMA and five studies combined DLI with HMA, the efficacy of the HMAs was confirmed by conducting a sensitivity analysis that excluded those seven studies. As with the results of the full analysis, the OS, RFS, and NRM were found to be better for the HMA group than the observation group, whereas the CIR and the incidence of acute GVHD did not differ between the two groups (Supplementary Data 3). Nonetheless, the patients who received HMAs had a significantly lower incidence of chronic GVHD (pooled RR, 0.71; 95% CI, 0.55–0.91; I2, 0%) (24, 25, 29).
Funnel plots of the OS, RFS, NRM, CIR, grades III–IV acute GVHD, and chronic GVHD outcomes of the HMA and observation groups did not show a publication bias (Figure 5). Egger's regression test confirmed this (p = 0.1590, 0.2713, 0.8865, 0.1804, 0.3706, 0.8302 for OS, RFS, NRM, CIR, grades III–IV acute GVHD, and chronic GVHD; respectively).
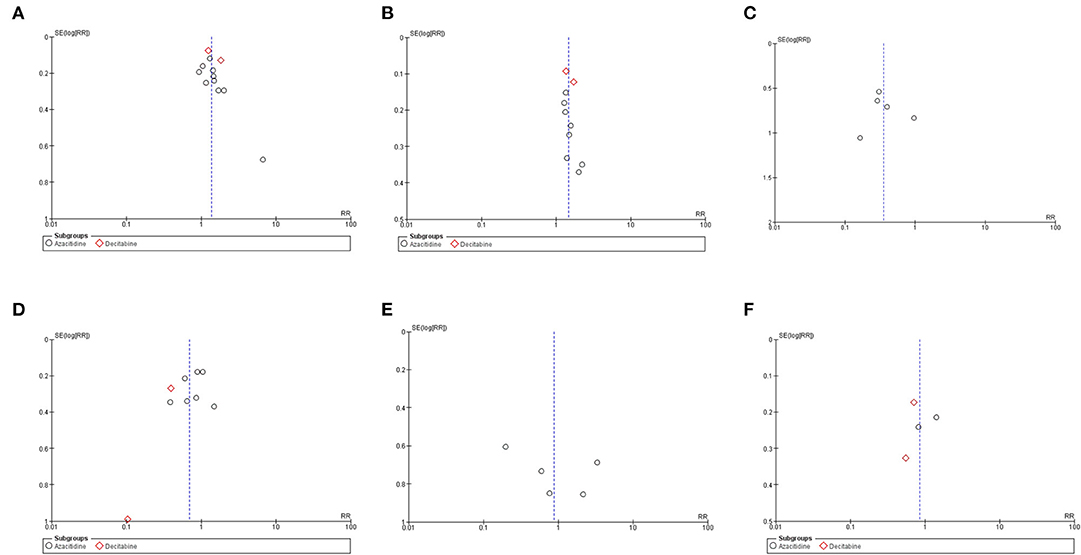
Figure 5. Funnel plots of the meta-analysis of HMA maintenance compared with no HMA maintenance. (A) OS rate. (B) RFS rate. (C) NRM rate. (D) CIR rate. (E) grade III–IV aGVHD rate. (F) cGVHD rate.
Subgroup Analysis Based on Study Design
A subgroup analysis based on the study design was performed. There is a trend for a significantly lower risk of chronic GVHD in randomized studies but not in observational studies. The CIR results appear to be similar between randomized studies and observational studies. In observational studies, other parameters which included OS, RFS were significantly better in HMAs arm. However, these parameters could not be analyzed in randomized studies subgroup due to limited number of studies (Supplementary Data 4).
Subgroup Analysis Based on Each HMA
Two HMAs were used in this meta-analysis: azacitidine and decitabine. The OS, RFS and NRM outcomes of the azacitidine group were significantly better than those of the observation group (Figures 2, 3) (14, 16–20, 22, 28). Likewise, the decitabine arm had superior OS, RFS and CIR outcomes to those of the observation arm (Figures 2, 3) (21, 24, 29). The incidence of grades III–IV acute GVHD and chronic GVHD in patients who received azacitidine were similar to those under observation (Figure 4) (14, 16, 22, 25). Interestingly, the rate of chronic GVHD in the decitabine group was significantly lower than in the observation group (pooled RR, 0.67; 95% CI, 0.50–0.90; I2, 0%; Figure 4B) (24, 29).
Subgroup Analysis of Patients Who Received HMAs in Combination With DLI
The work by Guillaume et al., Joris et al., and Booth et al. compared HMAs in combination with DLI and observation arms. Although the OS and RFS outcomes tended to be better for HMAs combined with DLI, only RFS rates were statistically significant (pooled RR, 1.20; 95% CI, 0.86–1.67; I2, 37%; and pooled RR, 1.33; 95% CI, 1.09–1.62; I2, 0%; Figure 6) (19, 20, 27).
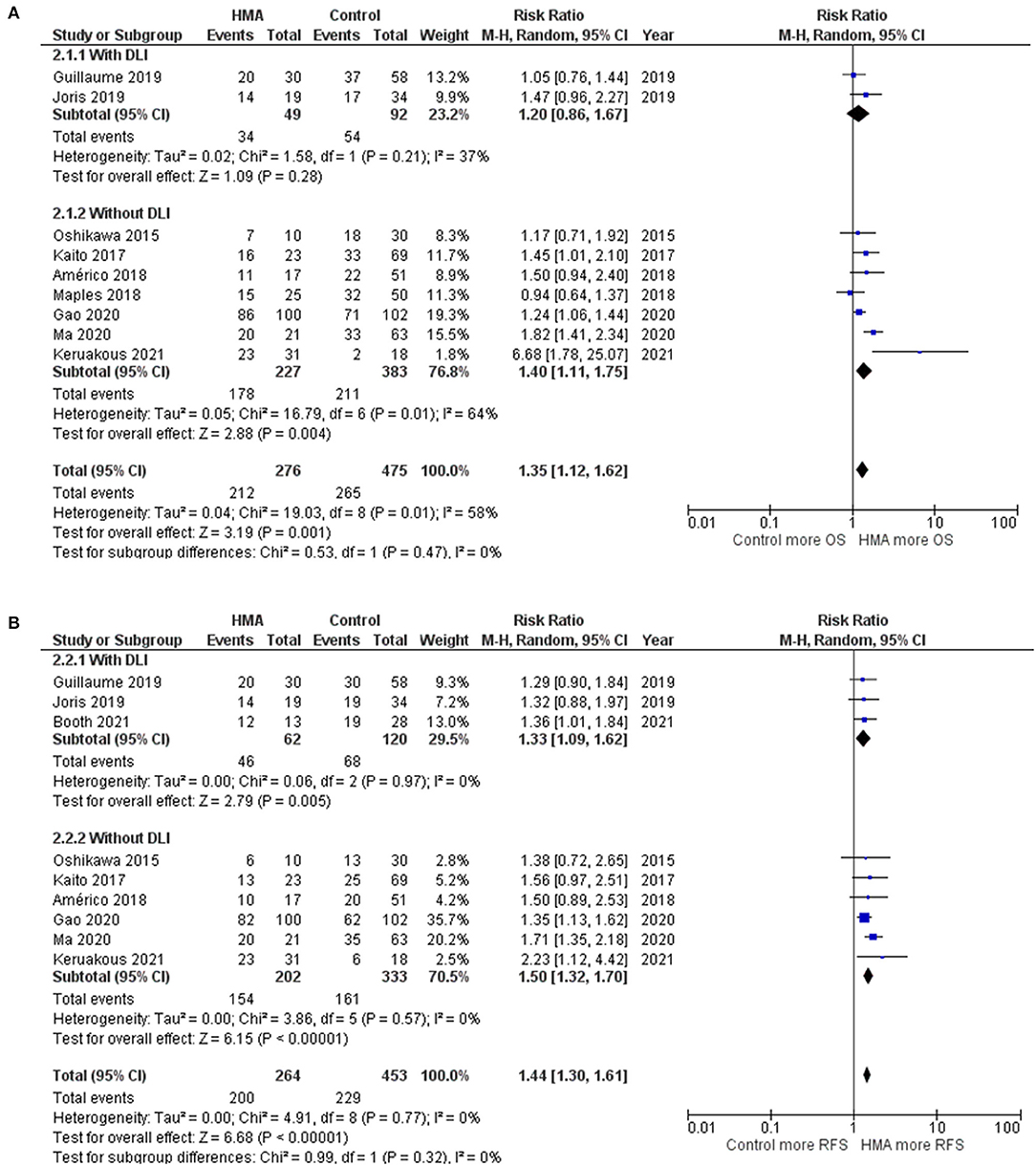
Figure 6. Subgroup analysis of studies with patients receiving HMA maintenance with DLI and without DLI (A) OS rate (B) RFS rate.
Sensitivity Analyses
In total, three sensitivity analyses were conducted. The first analysis was performed on studies which recruited only patients with AML. Only RFS and CIR were significantly better in HMAs arm. In addition, there was a trend of superior OS in HMAs group. Nevertheless, other parameters could not be evaluated due to limited number of studies (Supplementary Data 5). The second analysis was performed on studies with adult patients. The OS, RFS and NRM were significantly superior in HMAs arm while the other outcomes did not show significant superiority (Supplementary Data 6). The third analysis was performed on studies with low risk of bias by selecting only studies with a Newcastle-Ottawa scale of at least eight or Jadad score of at least two. Similar results were obtained compared to the main results (Supplementary Data 7).
Quality of Evidence Using Grading of Recommendations Assessment, Development, and Evaluation Approach
The quality of evidence generated by the current systematic review and meta-analysis is moderate.
Discussion
An earlier systemic review that focused on the safety and efficacy of HMAs as post-allo-SCT maintenance for AML and MDS found acceptable OS and RFS rates without a heightened GVHD rate (30). Unfortunately, a detailed analysis of the clinical outcomes of the control and treatment groups was not reported. A recent meta-analysis of HMAs and FLT3 Inhibitors as maintenance treatment for AML and MDS after allo-SCT showed a high percentage of OS and RFS (35). Due to the available limited studies comparing clinical outcomes between HMAs and observation arms, we focused on comparing the benefits of HMA maintenance following allo-SCTs with an observation approach and further analyzed the efficacy in each HMA subgroup. Notably, OS, RFS, NRM, and CIR were markedly improved with HMA maintenance. Our subgroup analysis demonstrated the advantages of both azacitidine and decitabine use in this setting. In terms of safety, the HMAs were not associated with a higher GVHD incidence. In the case of decitabine for post allo-SCT maintenance, the rate of chronic GVHD seemed to be lower than that of the observation arm. Previous studies showed that HMA maintenance had low rates of toxicities and infectious complications even if the treatment is given to elderly patients (36, 37). Furthermore, a prior study reported that 6.8% of post-allo-SCT patients experienced isolated extramedullary relapse which translate into dismal survival outcome (38). Accordingly, prophylaxis scheme post allo-SCT is a rational option to mitigate either isolated extramedullary or bone marrow relapse risk. Taken together, the use of HMAs is a feasible therapy for AML and MDS patients during the post-allo-SCT period, and it should be offered broadly to post allo-SCT patients.
Recently, an oral formulation of azacitidine (CC-486) was approved by the U.S. Food and Drug Administration for the continued treatment of adult AML. Based on data from the phase 3 QUAZAR AML-0001 clinical trial, the patients must have achieved first complete remission or must have an incomplete blood count recovery following intensive induction chemotherapy, and be unable to complete intensive curative therapy (39). The oral formulation of azacitidine may enhance patient convenience, eliminate injection-site reactions, and facilitate long-term administration. The application of this product in a post-allo-SCT setting has since been verified in a phase I/II study, which supports the promising clinical activity (40). A randomized, phase III trial to validate its efficacy is in development.
Although the present analysis confirms the usefulness of HMAs, several limitations are noted. First, variations in the disease status prior to transplantation, the difference in conditioning regimen, and the treatment and protocols of the studies (HMA dosage, number of cycles, and dates of administration) could lead to a diversity of clinical outcomes. Second, there was missing data on the European Group for Blood and Marrow Transplantation risk score and disease risk index. It is possible that older patients were selected to have less comorbidities, better performance status, and less prior treatment burden compared to the younger ones; which could be the confounding factors behind similar outcomes. Furthermore, the lack of comorbidity is an issue in identifying risk factors for NRM. Third, there is a recent trend in using MRD status in pre- and post-allo-SCT setting to classify MRD-positive patients who would benefit from HMAs maintenance after allo-SCT (41). However, MRD assessment data was scarce in published included trials precluding a subgroup analysis. In addition, the incorporation of other agents, such as gemtuzumab ozogamicin and G-CSF into each treatment protocol also impact outcomes of clinical trials. Large-scale randomized trials are warranted to clarify all of these unresolved issues.
Conclusion
The current systematic review and meta-analysis illustrated that the patients receiving HMA maintenance post-Allo-SCT had significantly better outcomes with regards to OS, RFS, NRM, and CIR. Furthermore, if decitabine was used for maintenance, the rate of chronic GVHD seemed to be lower than that of the observation arm. Further data, preferably from large prospective studies, is warranted to confirm the benefit of HMA-based maintenance after allo-SCT as well as describe the optimal agent, administration schedule, and the sub-groups of patients who benefit from such intervention.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The need for ethics approval by institutional board review was waived as this study did not directly involve human subjects.
Author Contributions
BP and WO collected the data. BP performed the statistical analyses. WO and SK drafted the manuscript and prepared the final version. FK made critical revisions. All authors designed the study, read, and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.801632/full#supplementary-material
References
1. Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. (2009) 301:2349–61. doi: 10.1001/jama.2009.813
2. Vasu S, Kohlschmidt J, Mrózek K, Eisfeld AK, Nicolet D, Sterling LJ, et al. Ten-year outcome of patients with acute myeloid leukemia not treated with allogeneic transplantation in first complete remission. Blood Adv. (2018) 2:1645–50. doi: 10.1182/bloodadvances.2017015222
3. Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert Rev Hematol. (2010) 3:429–41. doi: 10.1586/ehm.10.32
4. Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. (2015) 21:454–9. doi: 10.1016/j.bbmt.2014.11.007
5. Chen YB, McCarthy PL, Hahn T, Holstein SA, Ueda M, Kröger N, et al. Methods to prevent and treat relapse after hematopoietic stem cell transplantation with tyrosine kinase inhibitors, immunomodulating drugs, deacetylase inhibitors, and hypomethylating agents. Bone Marrow Transplant. (2019) 54:497–507. doi: 10.1038/s41409-018-0269-3
6. Lee CJ, Savani BN, Mohty M, Gorin NC, Labopin M, Ruggeri A, et al. Post-remission strategies for the prevention of relapse following allogeneic hematopoietic cell transplantation for high-risk acute myeloid leukemia: expert review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. (2019) 54:519–30. doi: 10.1038/s41409-018-0286-2
7. Rautenberg C, Germing U, Haas R, Kobbe G, Schroeder T. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: prevention, detection, and treatment. Int J Mol Sci. (2019) 20:228. doi: 10.3390/ijms20010228
8. Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood. (2012) 119:3361–9. doi: 10.1182/blood-2011-09-377044
9. Schroeder T, Fröbel J, Cadeddu RP, Czibere A, Dienst A, Platzbecker U, et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia. (2013) 27:1910–3. doi: 10.1038/leu.2013.64
10. Santourlidis S, Trompeter HI, Weinhold S, Eisermann B, Meyer KL, Wernet P, et al. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. J Immunol. (2002) 169:4253–61. doi: 10.4049/jimmunol.169.8.4253
11. Liu Y, Kuick R, Hanash S, Richardson B. DNA methylation inhibition increases T cell KIR expression through effects on both promoter methylation and transcription factors. Clin Immunol. (2009) 130:213–24. doi: 10.1016/j.clim.2008.08.009
12. Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, Caballero-Velazquez T, Blanco B, Herrero-Sánchez C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. (2010) 115:107–21. doi: 10.1182/blood-2009-03-210393
13. Griffin PT, Komrokji RS, De Castro CM, Rizzieri DA, Melchert M, List AF, et al. A multicenter, phase II study of maintenance azacitidine in older patients with acute myeloid leukemia in complete remission after induction chemotherapy. Am J Hematol. (2015) 90:796–9. doi: 10.1002/ajh.24087
14. Ovechkina V, Bondarenko S, Morozova EV, Moiseev I, Slesarchuk OA, Smirnova A, et al. Efficiency of 5-azacytidine administration after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia and myelodysplastic syndrome: P693. Bone Marrow Transplant. (2016) 51:S484.
15. Oshikawa G, Kakihana K, Saito M, Aoki J, Najima Y, Kobayashi T, et al. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br J Haematol. (2015) 169:756–9. doi: 10.1111/bjh.13248
16. Kaito S, Najima Y, Kishida Y, Nagata A, Konishi T, Yamada Y, et al. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk hematologic malignancies. Blood. (2017) 130:4517–7.
17. Maples KT, Sabo RT, McCarty JM, Toor AA, Hawks KG. Maintenance azacitidine after myeloablative allogeneic hematopoietic cell transplantation for myeloid malignancies. Leuk Lymphoma. (2018) 59:2836–41. doi: 10.1080/10428194.2018.1443334
18. Danylesko I, Shem-Tov N, Yerushalmi R, Nagler A, Shimoni A. Maintenance 5-azactidine may improve outcomes after allogeneic stem-cell transplantation in high-risk AML and MDS patients. Blood. (2019) 134:3302. doi: 10.1182/blood-2019-129966
19. Guillaume T, Malard F, Magro L, Labopin M, Tabrizi R, Borel C, et al. Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. (2019) 54:1815–26. doi: 10.1038/s41409-019-0536-y
20. Joris M, Lebon D, Charbonnier A, Morel P, Gruson B, Caulier A, et al. Immunomodulation with azacytidine and donor lymphocyte infusion following sequential conditioning allogenic stem cell transplantation improves outcome of unfavorable AML. Blood. (2019) 134 (Supplement_1):4600. doi: 10.1182/blood-2019-131314
21. Américo AD, Kerbauy MN, Correa da Silva C, Chapchap EC, Teixeira L, Pirse de Souza dos Santos F, et al. Maintenance azacitidine after hematopoietic stem cell transplantation for relapse prevention in acute myeloid leukemia and myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk. (2018) 18:S206. doi: 10.1016/j.clml.2018.07.059
22. Ali N, Tomlinson B, Metheny L, Goldstein SC, Fu P, Cao S, et al. Conditioning regimen intensity and low-dose azacitidine maintenance after allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Leuk Lymphoma. (2020) 61:2839–49. doi: 10.1080/10428194.2020.1789630
23. de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. (2010) 116:5420–31. doi: 10.1002/cncr.25500
24. Ma Y, Qu C, Dai H, Yin J, Li Z, Chen J, et al. Maintenance therapy with decitabine after allogeneic hematopoietic stem cell transplantation to prevent relapse of high-risk acute myeloid leukemia. Bone Marrow Transplant. (2020) 55:1206–8. doi: 10.1038/s41409-019-0677-z
25. Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. (2020) 4:5580–8. doi: 10.1182/bloodadvances.2020002544
26. Xuan L, Liu Q. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. (2021) 14:4. doi: 10.1186/s13045-020-01017-7
27. Booth N, Mirea L, Huschart E, Miller H, Salzberg D, Campbell C, et al. Azacitidine and prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in pediatric patients with high risk acute myeloid leukemia: a retrospective single-center cohort study. Bone Marrow Transplant. (2021) 56:167.
28. Keruakous AR, Holter-Chakrabarty J, Schmidt SA, Khawandanah MO, Selby G, Yuen C. Azacitidine maintenance therapy post-allogeneic stem cell transplantation in poor-risk acute myeloid leukemia. Hematol Oncol Stem Cell Ther. (2021) 38:4249–59. doi: 10.1016/j.hemonc.2021.03.001
29. Gao L, Zhang Y, Wang S, Kong P, Su Y, Hu J, et al. Effect of rhG-CSF Combined with decitabine prophylaxis on relapse of patients with high-risk MRD-negative AML after HSCT: an open-label, multicenter, randomized controlled trial. J Clin Oncol. (2020) 38:4249–59. doi: 10.1200/JCO.19.03277
30. Bewersdorf JP, Tallman MS, Cho C, Zeidan AM, Stahl M. Safety and efficacy of maintenance treatment following allogeneic hematopoietic cell transplant in acute myeloid leukemia and myelodysplastic syndrome—a systematic review and meta-analysis. Blood. (2020) 136:34–5. doi: 10.1182/blood-2020-136671
31. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
32. Wells GSB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis (2000). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
33. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Further Methods for Dichotomous Data. Introduction to Meta-Analysis. United Kingdom: John WIley & Sons, Ltd (2009).
34. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
35. Bewersdorf JP, Allen C, Mirza AS, Grimshaw AA, Giri S, Podoltsev NA, et al. Hypomethylating agents and FLT3 inhibitors as maintenance treatment for acute myeloid leukemia and myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation-A systematic review and meta-analysis. Transplant Cell Ther. (2021) 27:997.e1–11. doi: 10.1016/j.jtct.2021.09.005
36. El-Cheikh J, Massoud R, Fares E, Kreidieh N, Mahfouz R, Charafeddine M, et al. Low-dose 5-azacytidine as preventive therapy for relapse of AML and MDS following allogeneic HCT. Bone Marrow Transplant. (2017) 52:918–21. doi: 10.1038/bmt.2017.31
37. Marini C, Brissot E, Bazarbachi A, Duléry R, Sestili S, Battipaglia G, et al. Tolerability and efficacy of treatment with azacytidine as prophylactic or preemptive therapy for myeloid neoplasms after allogeneic stem cell transplantation. Clin Lymphoma Myeloma Leuk. (2020) 20:377–82. doi: 10.1016/j.clml.2019.10.011
38. Sakellari I, Gavriilaki E, Batsis I, Mallouri D, Gavriilaki M, Apostolou C, et al. Isolated extramedullary relapse as a poor predictor of survival after allogeneic hematopoietic cell transplantation for acute leukemia. Biol Blood Marrow Transplant. (2019) 25:1756–60. doi: 10.1016/j.bbmt.2019.05.018
39. Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. (2020) 383:2526–37. doi: 10.1056/NEJMoa2004444
40. de Lima M, Oran B, Champlin RE, Papadopoulos EB, Giralt SA, Scott BL, et al. Final analysis of the phase I/II study of CC-486 (oral azacitidine) maintenance therapy after allogeneic stem cell transplantation (Allo-SCT) in patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS). Blood. (2017) 130:4512. doi: 10.1016/j.bbmt.2015.11.784
Keywords: acute myeloid leukemia, azacitidine, decitabine, hypomethylating agent, maintenance, transplant
Citation: Kungwankiattichai S, Ponvilawan B, Roy C, Tunsing P, Kuchenbauer F and Owattanapanich W (2022) Maintenance With Hypomethylating Agents After Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Systematic Review and Meta-Analysis. Front. Med. 9:801632. doi: 10.3389/fmed.2022.801632
Received: 25 October 2021; Accepted: 10 January 2022;
Published: 15 February 2022.
Edited by:
Mutlu Arat, Istanbul Florence Nightingale Hospital, TurkeyReviewed by:
Jean El Cheikh, American University of Beirut Medical Center, LebanonIoanna Sakellari, G. Papanikolaou General Hospital, Greece
Copyright © 2022 Kungwankiattichai, Ponvilawan, Roy, Tunsing, Kuchenbauer and Owattanapanich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florian Kuchenbauer, fkuchenbauer@bccrc.ca; Weerapat Owattanapanich, weerapato36733@gmail.com
†These authors have contributed equally to this work
 Smith Kungwankiattichai
Smith Kungwankiattichai Ben Ponvilawan
Ben Ponvilawan Claudie Roy
Claudie Roy Pattaraporn Tunsing
Pattaraporn Tunsing Florian Kuchenbauer
Florian Kuchenbauer Weerapat Owattanapanich
Weerapat Owattanapanich