Preoperative plasma visfatin may have a dual effect on the occurrence of postoperative delirium
- 1Department of Anesthesiology, Peking University Third Hospital, Beijing, China
- 2Department of Anesthesiology, Beijing Jishuitan Hospital, Beijing, China
- 3Beijing Center of Quality Control and Improvement on Clinical Anesthesia, Beijing, China
Background: Visfatin is considered to be a “novel pro-inflammatory cytokine.” Neuroinflammatory response is one of the important mechanisms of postoperative delirium (POD). The relationship between preoperative plasma visfatin and POD is unclear.
Objective: To investigate the relationship between preoperative plasma visfatin concentrations and POD (primary outcome) in older hip fracture patients and to explore whether it affects POD through inflammatory factors.
Materials and methods: This prospective cohort study enrolled 176 elderly patients who were scheduled for hip fracture surgery. Preoperative plasma was collected on the morning of surgery, and visfatin levels were measured. Interleukin (IL)-1 and IL-6 were measured using patients’ plasma collected on the first day after surgery. We used the 3-min diagnostic interview for Confusion Assessment Method-defined delirium (3D-CAM) twice daily within the 2 days after surgery to assess whether POD had occurred. Restricted cubic splines and piecewise regression were used to explore the relationship between preoperative plasma visfatin concentrations and POD, and further mediation analysis was used to verify whether visfatin plays a role in POD through regulating inflammatory factors.
Results: The incidence of POD was 18.2%. A J-shaped association was observed between preoperative plasma visfatin levels and POD. The risk of POD decreased within the lower visfatin concentration range up to 37.87 ng/ml, with a hazard ratio of 0.59 per 5 ng/ml [odds ratio (OR) = 0.59, 95% confidence interval (CI) = 0.37–0.95], but the risk increased above this concentration (P for non-linearity < 0.001, with a hazard ratio of 1.116 per 10 ng/ml; OR = 1.10, 95% CI = 1.02–1.23). Mediation effect analysis showed that when the plasma visfatin concentration was higher than 37.87 ng/ml, the effect of visfatin on POD was mediated by IL-6 (p < 0.01). A significant indirect association with postoperative plasma IL-6 was observed between preoperative plasma visfatin and POD (adjusted β = 0.1%; 95% CI = 4.8∼38.9%; p < 0.01).
Conclusion: Visfatin is the protective factor in POD when the preoperative plasma visfatin concentration is below 37.87 ng/ml, but when it exceeds 37.87 ng/ml, the visfatin concentration is a risk factor for POD, which is mediated by postoperative plasma IL-6. The results suggest that preoperative visfatin may have a dual effect on the POD occurrence.
Clinical trial registration: [www.ClinicalTrials.gov], identifier [ChiCTR21 00052674].
Introduction
Hip fracture is a main disease that threaten the health and quality of life in older adults, and its incidence increases with population aging. Epidemiological data showed that one in five women and one in ten men have hip fractures globally per year, and most of them need surgery (1). Older adults undergoing hip fracture surgery have high disability and mortality rates, which become a serious medical, economic, and social problem (2).
Postoperative delirium (POD) is a common complication after hip fracture surgery in older patients (3). It is mainly manifested by changes in the level of consciousness, cognitive dysfunction, decreased attention, and disturbance of the sleep-wake cycle. The incidence rate is approximately 4–61% (4), which is depending on the type of surgery. Although it can occur at any time during a patient’s hospital stay, POD usually develops during the first few days after surgery. POD is associated with poor outcomes such as longer duration of hospitalization, increased mortality rates, and increased morbidity (5). Additionally, it can affect the recovery of physical function and the return to normal life after discharge from the hospital.
The mechanism of POD mainly includes five theories including the neuroinflammation theory (6). A lot of studies have shown that IL-1 and IL-6 levels in the peripheral circulation are related to POD occurrence (7, 8).
Visfatin is a multi-functional protein molecule that is present in many organs and tissues in the body including brain (such as cortex and hippocampus area) and lung (9). Visfatin can exert multiple insulin simulation effects, including enhanced glucose intake and increased triglyceride synthesis (10). Visfatin is also regarded as a new type of inflammatory cytokine. Studies have shown that visfatin can activate monocytes to produce inflammatory cytokines such as interleukin (IL)-1ß, IL-6, and tumor necrosis factor α (TNF-α) (11). Recently, some studies showed increased concentrations of blood visfatin were associated with cognitive impairment (12–14). Moreover, some evidences which suggest that decreased visfatin levels might lead to dementia and Alzheimer’s disease (15, 16). However, some other studies appeared that visfatin played an important role in cognitive function. One study showed visfatin reduced hippocampal CA1 cell death and protects against memory loss and cognitive decline in male rats (17). Another study showed that mice lacking visfatin appearing hippocampal and cortical atrophy, astrogliosis, microgliosis, and abnormal CA1 dendritic morphology with altered intrahippocampal connectivity and abnormal behavior; including hyperactivity, some defects in motor skills and memory impairment (18). All these results above suggested that there may be a complex relationship between visfatin and cognitive function. Currently, the relationship between blood visfatin levels and POD is unclear. We suspected that there is also a complex link between visfatin and POD.
Therefore, we conducted this prospective cohort study to identify the correlation between preoperative plasma visfatin and POD and to further explore whether it can affect POD through inflammation factors.
Materials and methods
Patients and setting
This study was approved by the Beijing Jishuitan Hospital Medical Science Research Ethics Committee (JLKS202103-35) and was conducted in accordance with the principles of the Declaration of Helsinki. Cerebrospinal fluid (CSF) samples were obtained for the purpose of laboratory research. All methods were performed in accordance with relevant guidelines and regulations. Written informed consent was obtained, and this study was registered at the Chinese Clinical Trial Registry (ChiCTR2100052674). All participants were recruited from Beijing Jishuitan Hospital (Beijing, China) from November 2021 to April 2022. Eligible patients who were willing to participate in the study met the entry criteria described below. The inclusion criteria were as follows: at least 65 years old; diagnosed with unilateral hip fracture; operation 48 h after admission; hospital admission for surgical treatment of hip fracture; and American Society of Anesthesiologists (ASA) physical status classification II to III. There were 468 patients recruited into this study (Figure 1). All patients received intravenous patient-controlled analgesia after surgery (Sufentanil 0.1 μg/kg, flurbiprofen axetil 200 mg, tropisetron hydrochloride 10 mg, and normal saline to a volume of 100 ml). The exclusion criteria were as follows: dementia; psychiatric patients; preoperative delirium; Parkinson’s disease; preoperative mini-mental state examination (MMSE) scores was lower than 17 for illiterate, 20 for individuals with 1–6 years of education, 24 for individuals with seven or more years of education; nerve block contraindications (needle insertion site infection, local anesthetic allergy, coagulation dysfunction, international normalized ratio > 1.4, platelet count < 80 × 109 μl); pathological fracture; metabolic bone disease; old fracture dementia; a stroke within 6 months; transferred to the intensive care unit (ICU) after surgery; difficulty communicating; and/or drug or alcohol abuse.
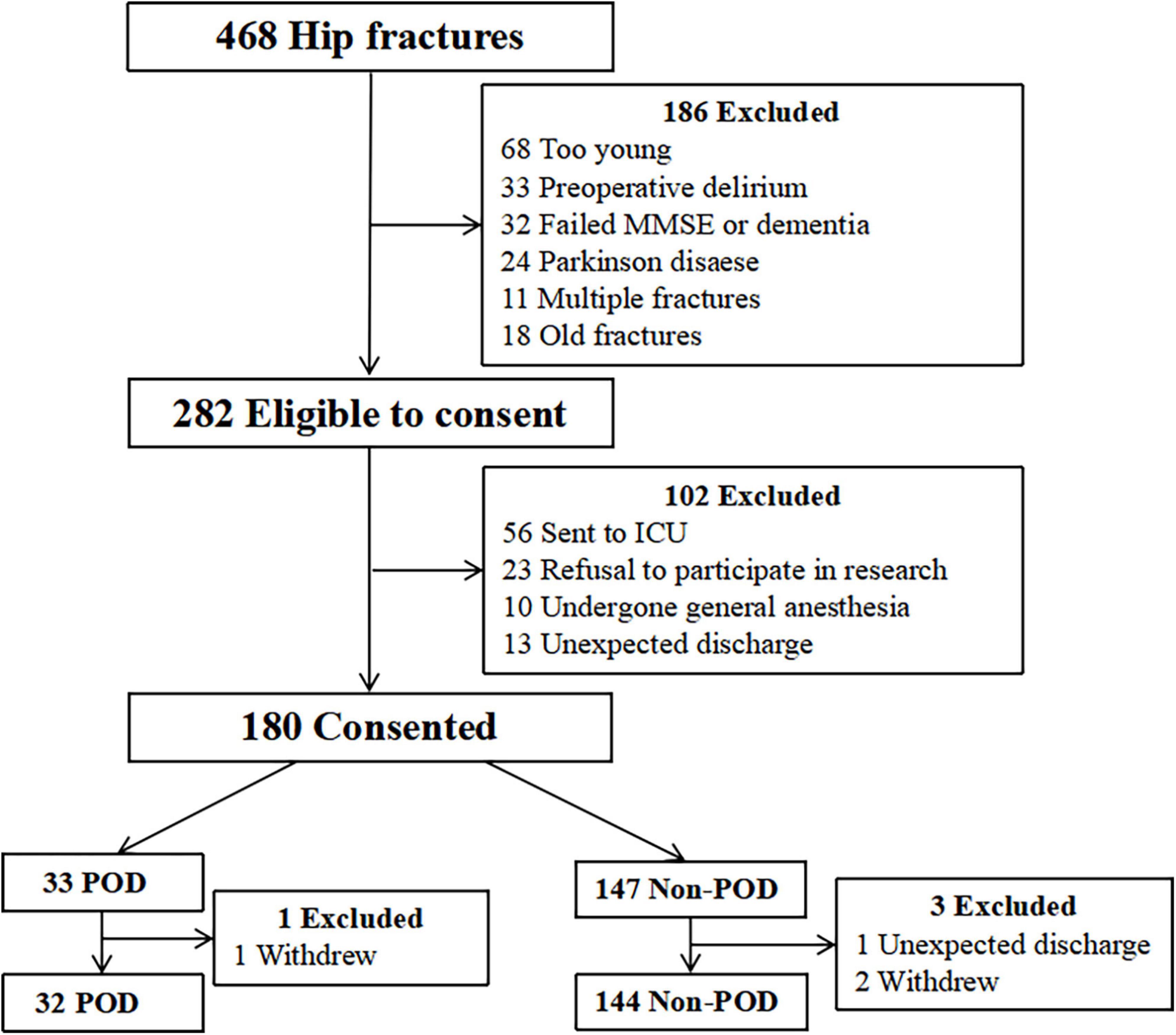
Figure 1. Study flow chart. Four hundred sixty-eight patients were initially screened for the study, and 176 patients were finally included in the data analysis. MMSE, mini-mental state examination; ICU, intensive care unit; POD, postoperative delirium.
Preoperative assessment
Baseline characteristics were collected 1 day before surgery, including patient demographic characteristics (e.g., sex, age, height, and weight), clinical characteristics (e.g., ASA grade, patient’s history of circulatory system, respiratory system, nervous system, and endocrine system), cognitive function status measured using the MMSE (19), and sleep quality measured using the Pittsburgh Sleep Quality Index (20). The age-adjusted Charlson comorbidity index (ACCI) scores (21) were calculated.
Blood sample collection and biochemical analysis
We collected 2 ml of blood in a vacutainer (BD Biosciences, San Jose, CA, USA), which used EDTAK2 as an anticoagulant, after preoperative radial artery catheterization, and the blood sample was taken at 6:00 a.m. on the first day after surgery. The samples were centrifuged at 4,000 rpm/min for 10 min, and the supernatant was removed and used for detection or it was aliquoted and frozen −80°C until analysis.
The visfatin plasma concentration was measured using the enzyme immunoassay method (RayBiotech, Norcross, GA, USA) that had a lower limit of detection of 0.778 ng/ml. Albumin, creatinine, thyroid stimulating hormone (TSH), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were tested using a blood biochemical analyzer (HITACHI, Tokyo, Japan); white blood cells (WBCs), red blood cells (RBCs), platelets, and hemoglobin levels were tested using a blood analyzer (SYSMEX, Kobe, Japan). IL-1β and IL-6 levels were also tested using an immunosorbent assay (Boster, Wuhan, China).
Anesthesia and analgesia
After admission, the geriatric doctor evaluated the pain of the patients before surgery. If the Numerical Rating Scale (NRS) score was greater than three, the patient would be given administrative drug Tylenine (containing oxycodone hydrochloride 5 mg and acetaminophen 325 mg) or Dolantin 50 mg according to their physical conditions.
Fascia iliaca block and subarachnoid spinal anesthesia was used. After entering the surgical theater, echocardiogram monitoring, invasive blood pressure through radial artery catheterization, and pulse oximetry monitoring were performed. All patients received ultrasound-guided fascia iliaca block before surgery and were administered 30 ml of 0.4% ropivacaine. For spinal anesthesia, the L2–3 or L3–4 levels was selected to puncture, and 8–10 mg of 0.3% ropivacaine was used, and no epidural catheter was placed.
All patients were treated with patient-controlled intravenous analgesia after surgery. The drug regimen was as follows: sufentanil 0.1 μg/kg, flurbiprofen axetil 200 mg, tropisetron hydrochloride 10 mg and normal saline to a volume of 100 ml. When the postoperative NRS pain scores was greater than three points or the patient actively requested additional analgesia because they were experiencing pain, the rescue analgesic drug Tylenine or Dolantin 50 mg was administered (22).
Delirium assessment
The 3-min diagnostic interview for Confusion Assessment Method (3D-CAM) is an efficient and reliable way to determine whether a patient has delirium. The 3D-CAM assessment can be completed in an average of 3 min, and it has excellent performance compared with other evaluation methods. Its sensitivity and specificity are about 84.6–87.2% and 96.7–97.4%, respectively (23). A geriatrician trained by a professional psychiatrist performed 3D-CAM assessments twice daily (morning and afternoon) on patients during the first two postoperative days (24).
Sample size
A piecewise regression model was used in the present study to examine the association between preoperative visfatin and POD. Five events per variable (EPV) is a widely used minimum criterion for sample size considerations in regression analysis. It was estimated that five variables could be included in the final model. For a given number of EPVs, 5 × EPV events are required in the analysis sample. For an EPV value of five, at least 25 events (development of POD) were required for the analysis. With an anticipated dropout rate of 10%, 28 POD patients were required. Additionally, a previous study showed that approximately 16% of patients who underwent hip replacement surgery developed POD, and thus, a minimum sample size of 176 was required for this study.
Statistical analysis
Analysis was performed using SPSS v.25.0 (IBM Corp., Armonk, NY, USA) and R statistical software (version 4.2.0, R Core Team, Vienna, Austria). The intergroup comparison for normally distributed measurement data was performed using an independent sample t-test, and the results are presented as the mean and standard deviation (SD). Measurement data with a skewed distribution was presented as the median (quartile), and the intergroup comparison was performed using the Mann–Whitney U-test. Categorical variables were described using the frequency (%), and the intergroup comparisons were described using Pearson chi-square or Fisher’s exact probability test. Logistic regression was used for multivariate analysis. The correlation between visfatin and IL-6 is based on the Pearson or Spearman analysis.
Restricted cubic splines were used to analyze the association between preoperative plasma visfatin and the risk of POD. When there was evidence of non-linearity, a piecewise regression model was next fitted. The package segmented (version 1.4-1) was used to fit piecewise regression models, while adjusting for covariates. Mediation analysis was using mediation package (25).
Results
Patients characteristics
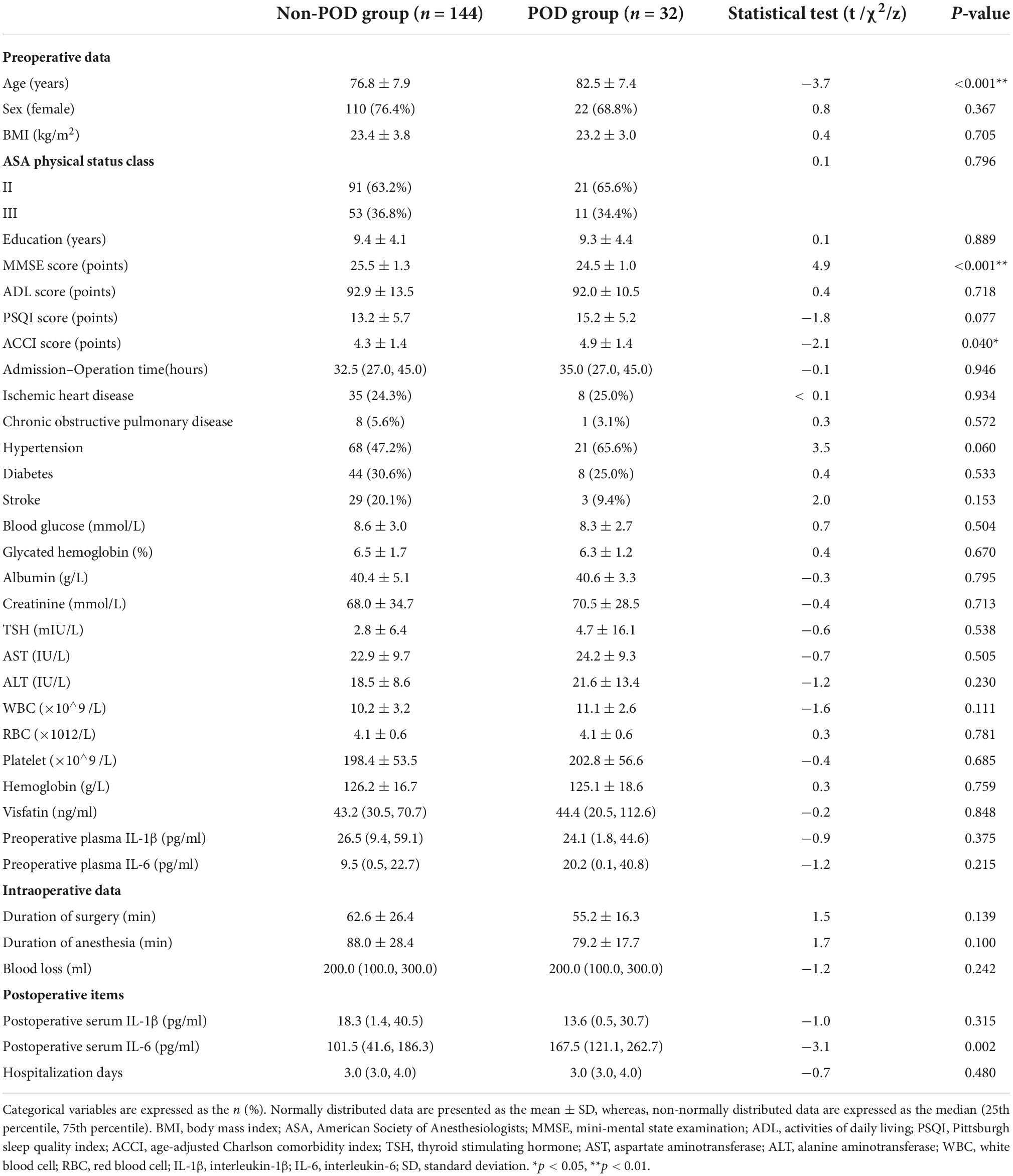
Among 176 patients who underwent hip fracture surgery, 18.2% (32 of 176) developed POD in our study population. The average age of patients in the POD group was 82.5 ± 7.4 years, which was significantly older than that of patients in the non-POD group (76.8 ± 7.9 years, p < 0.01). There were sixty-six (23.6%) and 10 (31.2%) men in the non-POD and POD groups, respectively, which was not significantly different (p > 0.05). Demographics such as gender, BMI, ASA, ADL, and education, did not show a group difference (p > 0.05). However, preoperative MMSE scores for patients in the POD group were significantly lower than those in the non-POD group (p < 0.01). Additionally, ACCI scores (p = 0.04) and postoperative plasma IL-6 levels (p = 0.002) were significantly higher in the POD group compared with that in the non-POD group. Other biochemical tests such as preoperative plasma glucose, creatinine, TSH, AST, ALT, WBC, RBC, and postoperative IL-1β levels showed no differences (p > 0.05). Additionally, preoperative plasma visfatin levels showed no difference between the two groups (Table 1).
Univariate logistic analysis showed that four factors, including age, preoperative MMSE scores, ACCI, and postoperative plasma IL-6, were statistically associated with POD in our research. Further multivariable analysis showed that three factors including age, low preoperative MMSE scores, and low postoperative plasma IL-6 levels were independent risk factors in POD among older hip fracture patients (Supplementary Table 1).
Among 32 POD patients, one patient had POD on the second day after operation (3.1%), and the other 31 patients had POD on the first day after operation (96.8%).
Correlation between preoperative plasma visfatin and postoperative delirium
Using R 4.2.0 software, we found the preoperative plasma visfatin concentrations had a non-linear relationship with the risk of POD, with an inflection point between 30 and 50 ng/ml (Figure 2). When the preoperative visfatin level was lower than that of the inflection point, the risk of POD decreases as the preoperative visfatin level increases. Above this inflection point, the risk of POD increases with increasing preoperative visfatin levels.
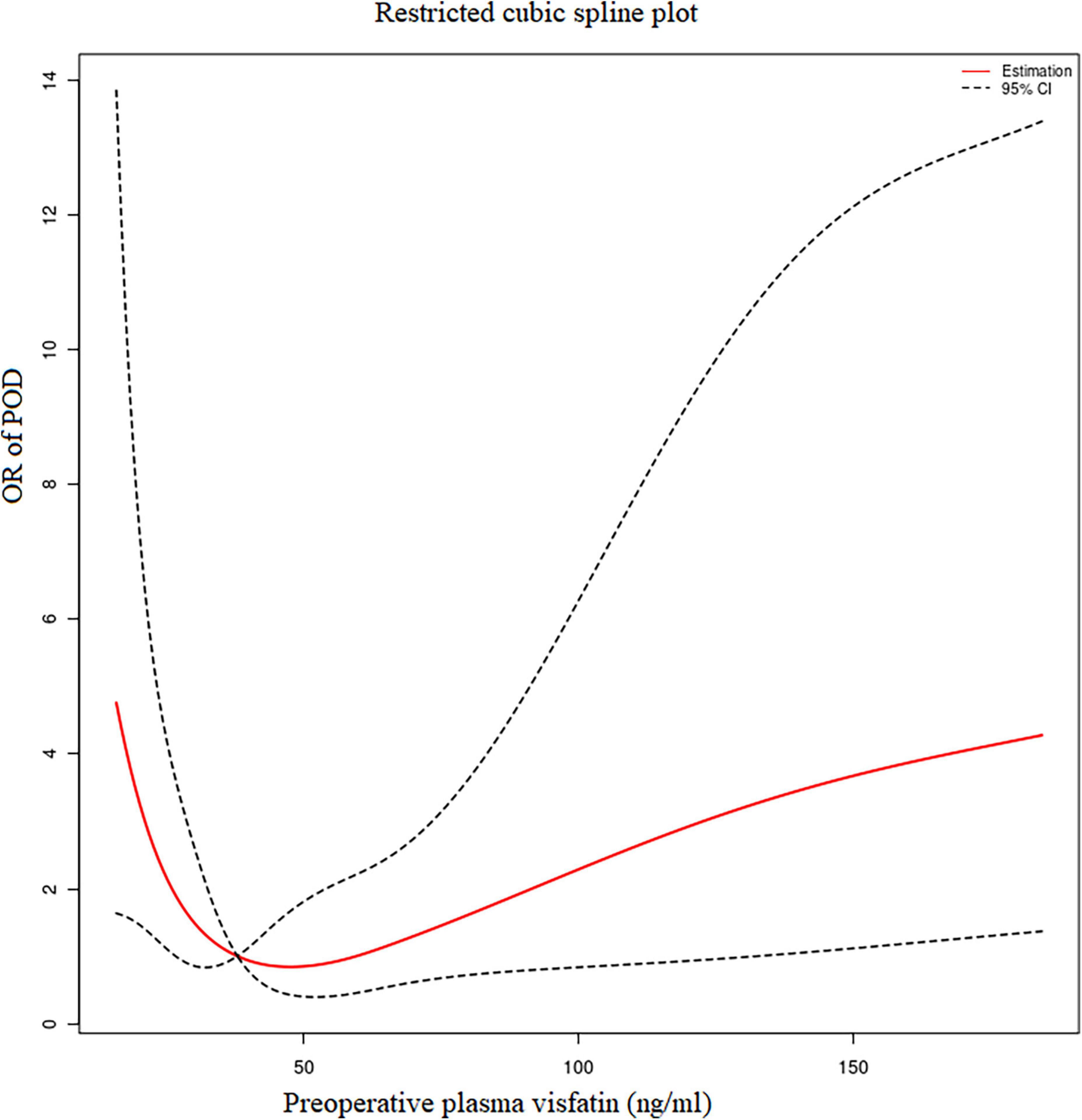
Figure 2. Restricted cubic spline analysis results. The relationship between the preoperative visfatin levels and OR of POD. OR, odd ratio; POD, postoperative delirium.
Break-point value by segmented regression
Segmented regression performed using R 4.2.0 software showed that the break-point value was 37.87 ng/ml. A decrease in the risk of POD was observed within the lower range until 37.87 ng/ml, with a hazard ratio of 0.59 per 5 ng/ml (OR = 0.59, 95% CI = 0.37–0.95), which increased thereafter (P for non-linearity < 0.001), with a hazard ratio of 1.116 per 10 ng/ml (OR = 1.10, 95% CI = 1.02–1.23).
Pearson correlation analysis of the relationship between visfatin and inflammatory factors
Pearson correlation analysis were used to analyze the correlation between visfatin and inflammatory factors. When the concentrations of preoperative plasma visfatin was lower than 37.87 ng/ml, there was no correlation between visfatin and IL-6 or IL-1 (Table 2), but when the preoperative plasma visfatin level was higher than 37.87 ng/ml, there was a positive correlation between concentrations of preoperative plasma visfatin and concentrations of postoperative plasma IL-6 (Table 3). Their relationship showed in a scatter plot (Figure 3).
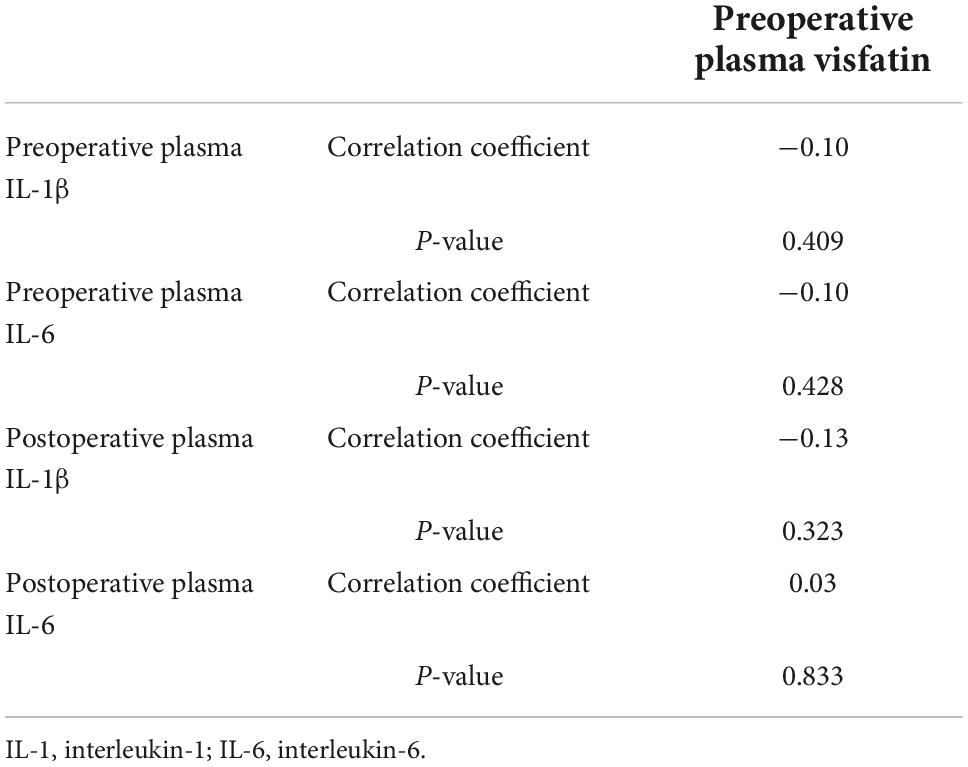
Table 2. Pearson correlation analysis between visfatin and inflammation factors when visfatin concentrations was lower than 37.87 ng/ml.
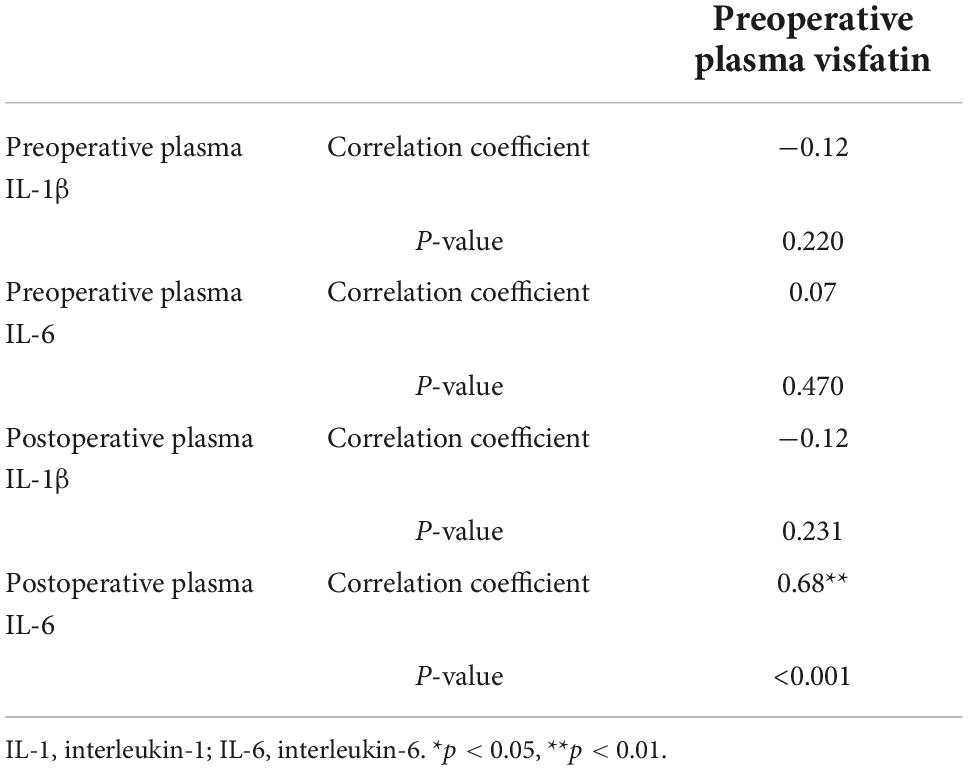
Table 3. Pearson correlation analysis between visfatin and inflammation factors when visfatin concentrations was above 37.87 ng/ml.
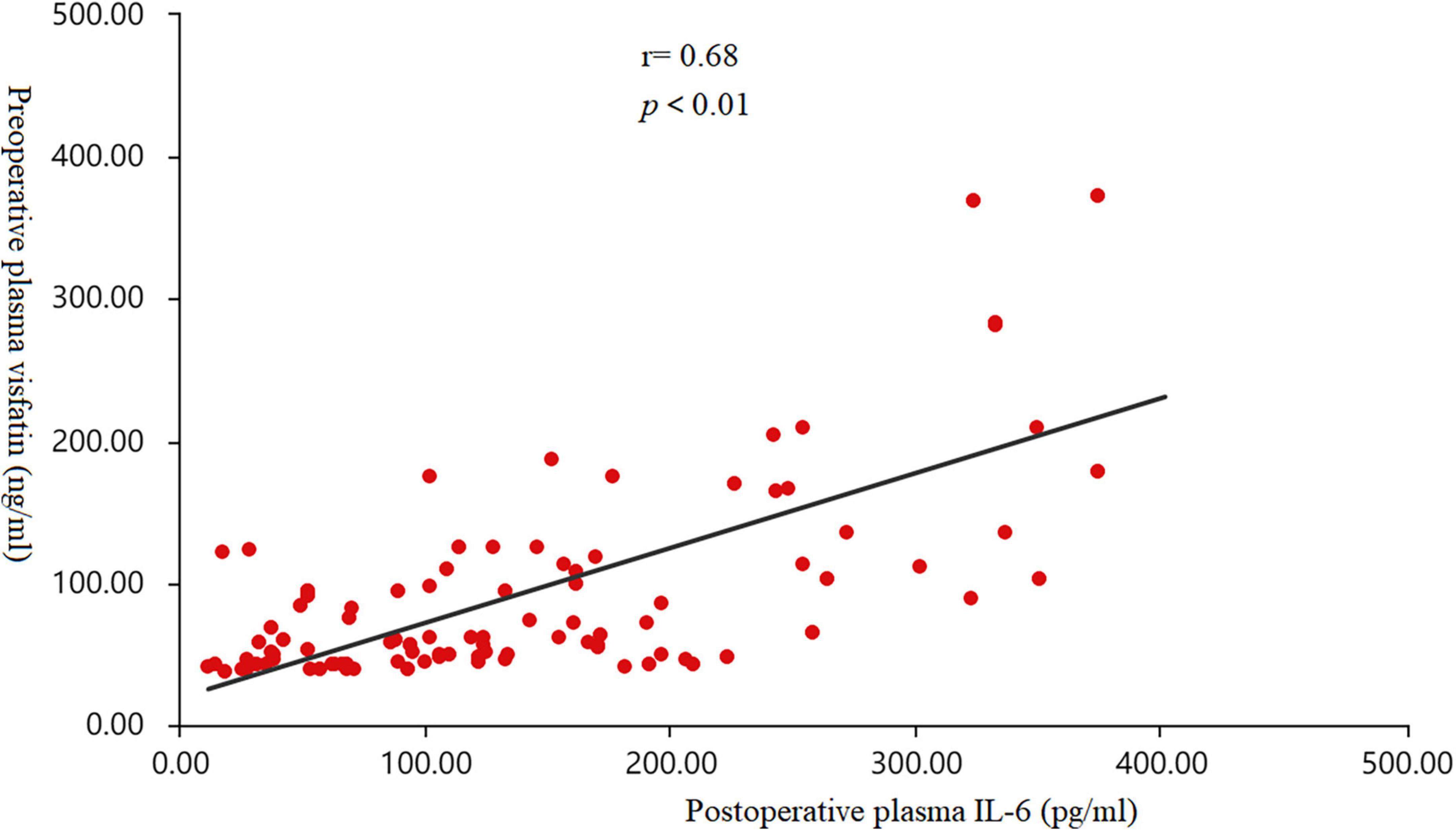
Figure 3. Scatter diagram. The correlation between the concentrations of preoperative plasma visfatin and postoperative plasma interleukin (IL-6) levels in patients with a plasma visfatin level of more than 37.87 ng/ml (r = 0.68, p < 0.01). IL-6, interleukin-6.
Mediation analysis of the relationship between visfatin, interleukin-6, and postoperative delirium
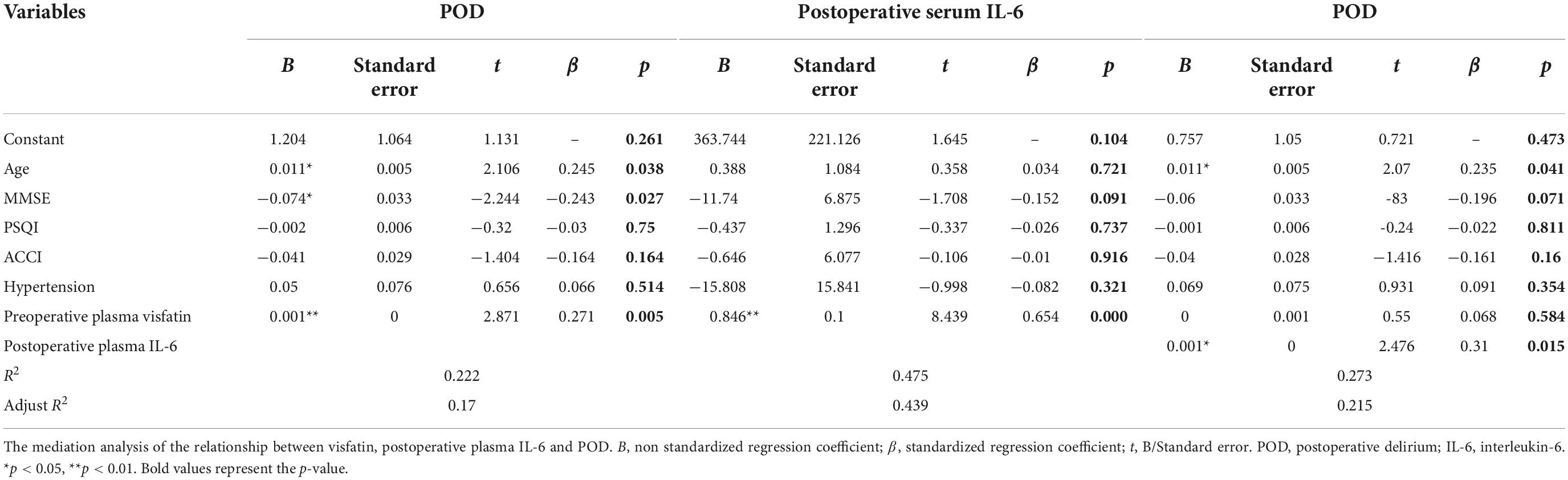
We further analyzed whether preoperative plasma visfatin can affect the occurrence of POD by regulating inflammatory using mediation analysis. When the concentration of visfatin was higher than 37.87 ng/ml, the total effect of preoperative plasma visfatin on POD (c) could be divided into direct effect (c’) and indirect effect (a and b), the indirect effect was exerted by affecting postoperative plasma IL-6. Here, we use an easy model to show the relationship between them (Figure 4). The coefficients of total effect, direct effect and indirect effect were showed in Table 4.
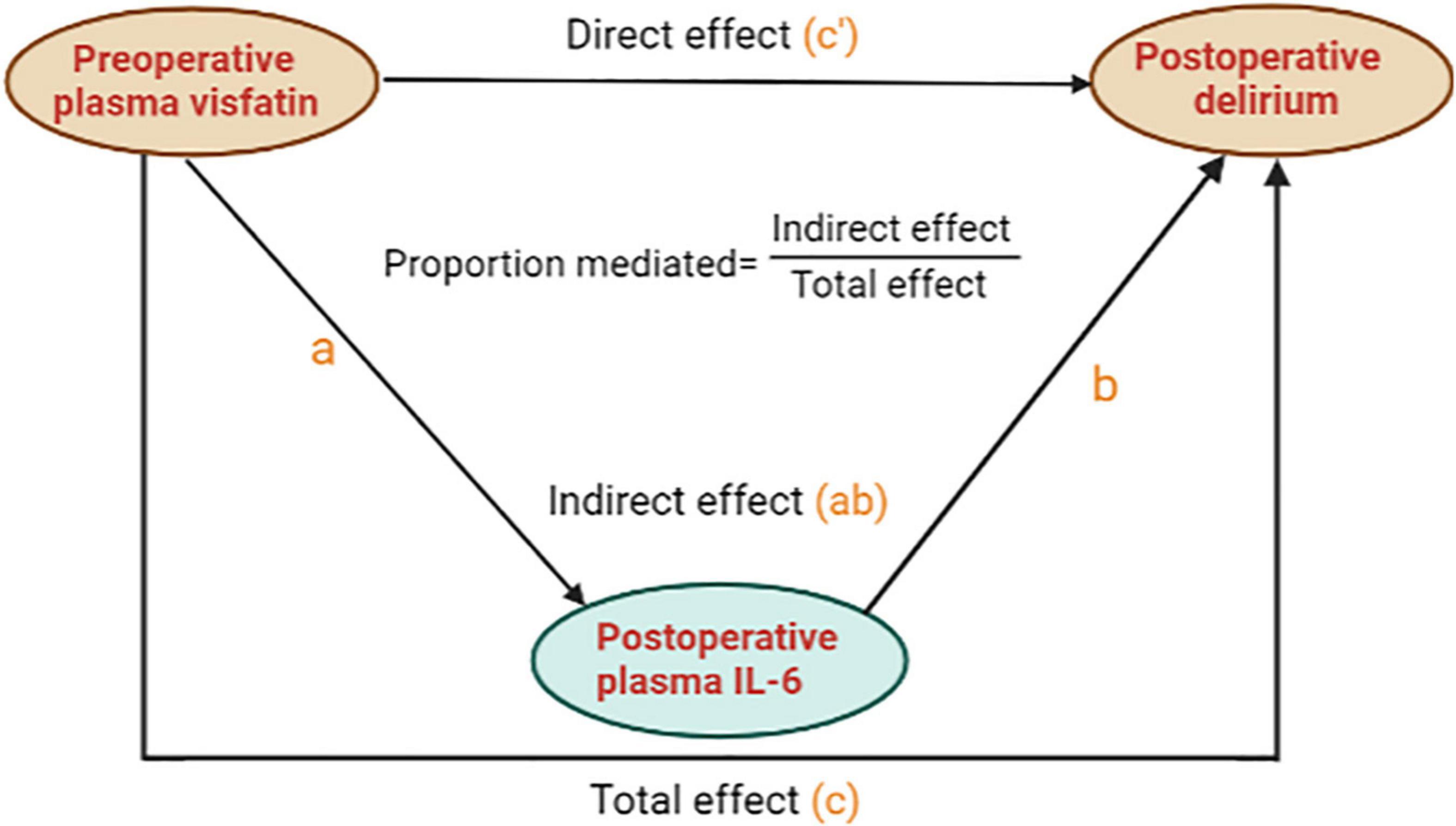
Figure 4. A mediation analysis model diagram. For brevity and clarity, a simplified version of the calculated model is presented. “c” means the total effect of preoperative plasma visfatin on postoperative delirium (POD) is represented by “c”, whereas, “c”’ means the direct effect of preoperative plasma visfatin on POD after controlling for the level of postoperative plasma interleukin (IL-6); “a” means the effect of preoperative plasma visfatin on the level of postoperative plasma IL-6; “b” means the effect of postoperative plasma IL-6 on POD, after controlling for preoperative plasma visfatin, the indirect effect of preoperative plasma visfatin on POD through postoperative plasma IL-6 can then be quantified as the product of “a” and “b”. IL-6, interleukin-6; POD, postoperative delirium.
Discussion
The results of our study show that visfatin has a dual effect on POD, when the concentration of preoperative visfatin is higher than 37.87 ng/ml, it may play a role by regulating the release of IL-6.
This study showed that increasing age, a low MMSE score, a high ACCI score, and high postoperative plasma IL-6 levels were independent risk factors for PODs. When the concentration of preoperative plasma visfatin was lower than 37.87 ng/ml, it was a negatively correlated with the risk of POD; when concentration was exceeded, it was positively correlated with the risk of POD, which was associated with postoperative plasma IL-6 levels.
Postoperative delirium is a serious complication, which can potentially lead to longer hospital stays and even permanent disabilities. Additionally, POD may increase the risk of delirium-related dementia. Many scholars have conducted researches to prevent and reduce the probability of POD occurrence. Some of our results are consistent with those of previous studies. Some studies showed that advanced age and multiple comorbidities are risk factors for POD (3, 26).
For the relationship between IL-6 and POD, many studies suggested that preoperative higher preoperative IL-6 blood levels were associated with POD occurrence (7, 27–29). However, our study did not show a difference in the preoperative plasma IL-6 concentration between the two groups, but the plasma IL-6 concentration on day one after surgery in the POD group was significantly higher than that in the non-POD group. This result may have occurred because we took blood samples from the patients on the morning of surgery instead of blood samples when the patients were just admitted to the hospital. After admission, the patients took non-steroidal anti-inflammatory drugs and painkillers, which reduced the inflammation, resulting in a decrease in the IL-6 level.
Visfatin is nicotinamide phosphoribosyltransferase (NAMPT), which includes extracellular NAMPT (eNAMPT) and intracellular NAMPT (iNAMPT). iNAMP is mainly involved in glucose metabolism, and eNAMPT regulates inflammatory factor expression (30). The relationship between the visfatin level in blood and cognitive function remains controversial. Some studies showed that elevated blood visfatin levels are associated with a cognitive decline (12, 14, 31). However, some studies indicated that loss of visfatin may harm neurons and impair cognitive function (18). To date, there have no relevant studies on POD, and our study is the first to explore the relationship between visfatin and POD.
In this study, we did not find a correlation between visfatin and IL-6 when the preoperative plasma visfatin concentration was less than 37.87 ng/ml. It is worth exploring the effect of visfatin on POD when below 37.87 ng/ml.
Visfatin plays a crucial role in glucose metabolism. Visfatin activates its target cells by binding to the insulin receptor (IR) and exerts multiple insulin-mimetic effects, including enhanced glucose uptake and increased triglyceride synthesis (10, 32–34). A secondary analysis indicated that type 2 diabetes was associated with POD occurrence (35). An observational study showed that the average blood glucose and blood glucose variability within 48 h of POD in patients after acute aortic separation are significantly higher than those of non-POD patients (36), which indicated that blood glucose fluctuations may cause cognitive dysfunction in older patients. A high basal blood glucose level may cause direct neuron damage, and compared with long-term blood sugar fluctuations, short-term (acute) blood glucose fluctuations may cause more damage to neurons and lead to cognitive dysfunction (37). We hypothesized that visfatin effects at lower levels may occur through regulation of glucose metabolism, thereby affecting POD occurrence. Unfortunately, in this study, we did not record patients’ blood glucose level at multiple time points during the perioperative period. We will next study the relationship between visfatin and the fluctuation of blood glucose.
Our study showed that an elevated visfatin concentration in the blood above a certain level will promote IL-6 expression and increase the risk of POD occurrence. Visfatin has a pro-inflammatory effect. Numerous studies have shown that visfatin activates monocytes to produce pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) (38–40). However, in our study, we did not detect other inflammatory factors such as TNF-α or C-reactive protein in patients’ blood samples, so it is not possible to determine whether visfatin affects POD occurrence by regulating other inflammatory factors. We will detect them in our future studies.
The main advantages of this study are as follows: our study is the first to explore the relationship between visfatin and POD. Our study exhibits that novel inflammatory factors “visfatin” has a dual effection on the occurrence of POD, which provides ideas for follow-up research and may provide some guidance for POD prediction. Furthermore, our study shows when visfatin concentration was higher than 37.87 ng/ml, it may affect POD through the medication effect on postoperative plasma IL-6. We demonstrated that there is a correlational relationship between high pre-operative visfatin levels, elevated post-operative levels of IL-6 and POD.
The main limitations of this study are as follows: (1) This study is a single center study with a relatively small sample size. In future studies, we will conduct a multi-center study; (2) the numbers of kinds of inflammatory factors detected in this study was too limited so we cannot make it clear that whether visfatin affect POD through other inflammatory factors (3) this study did not detect the central visfatin, and this issue will be investigated in our follow-up studies. (4) The patients in this study were discharged on the third day after surgery or transferred to the rehabilitation department for further treatment, so this study only evaluated the complications of 2 days after surgery. The follow-up study will observe and follow up the long-term prognosis of patients.
Conclusion
In summary, our study showed that there is a non-linear relationship between preoperative plasma visfatin and POD. Preoperative plasma visfatin has a dual effect on POD. Visfatin is the protective factor against POD when its preoperative plasma concentration is below 37.87 ng/ml. However, when it exceeds 37.87 ng/ml, the visfatin concentration becomes a risk factor for POD, which is mediated by postoperative plasma of IL-6.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Beijing Jishuitan Hospital Medical Science Research Ethics Committee (JLKS202103-35). The patients/participants provided their written informed consent to participate in this study.
Author contributions
NK, YY, NY, GW, and XG contributed to the study design. XG, YY, NY, and ZL obtained the funding. WZ, GW, and YY performed the anesthesia. NK and KZ collected the blood samples, contributed to the data collection, and responsible for data statistics. JH, YL, LC, and YL are laboratory technicians. YH, YS, and NK verified the underlying data. NK and YY drafted the manuscript. XG, KZ, YY, and NY reviewed the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82071189, 81971012, 81873726, 81901095, 81701052, and 81801070), Key Clinical Projects of Peking University Third Hospital (BYSYZD2019027), Beijing Jishuitan Hospital Science Foundation (ZR-202108), and Peking University “Clinical Medicine plus X” Youth Project (PKU2020LCXQ016).
Acknowledgments
We are thankful to all participants of this study. We thank all research coordinators for the clinical sample collection. We also thank Ellen Knapp, from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1024942/full#supplementary-material
References
1. Chen FP, Shyu YC, Fu TS, Sun CC, Chao AS, Tsai TL, et al. Secular trends in incidence and recurrence rates of hip fracture: A nationwide population-based study. Osteoporos Int. (2017) 28:811–8. doi: 10.1007/s00198-016-3820-3
2. Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. (1997) 7:407–13.
3. Wu J, Yin Y, Jin M, Li B. The risk factors for postoperative delirium in adult patients after hip fracture surgery: A systematic review and meta-analysis. Int J Geriatr Psychiatry. (2021) 36:3–14.
4. Austin CA, O’Gorman T, Stern E, Emmett D, Sturmer T, Carson S, et al. Association between postoperative delirium and long-term cognitive function after major nonemergent surgery. JAMA Surg. (2019) 154:328–34. doi: 10.1001/jamasurg.2018.5093
5. Maldonado JR. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. (2018) 33:1428–57.
6. Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: What we need to know and do. Br J Anaesth. (2017) 119:i115–25.
7. Liu X, Yu Y, Zhu S. Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLoS One. (2018) 13:e0195659. doi: 10.1371/journal.pone.0195659
8. Chen Y, Lu S, Wu Y, Shen Y, Zhao H, Ding S, et al. Change in serum level of interleukin 6 and delirium after coronary artery bypass graft. Am J Crit Care. (2019) 28:462–70.
9. Sonoli SS, Shivprasad S, Prasad CV, Patil AB, Desai PB, Somannavar MS. Visfatin–a review. Eur Rev Med Pharmacol Sci. (2011) 15:9–14.
10. Taskesen D, Kirel B, Us T. Serum visfatin levels, adiposity and glucose metabolism in obese adolescents. J Clin Res Pediatr Endocrinol. (2012) 4:76–81.
11. Heo YJ, Choi SE, Lee N, Jeon JY, Han SJ, Kim DJ, et al. Visfatin exacerbates hepatic inflammation and fibrosis in a methionine-choline-deficient diet mouse model. J Gastroenterol Hepatol. (2021) 36:2592–600. doi: 10.1111/jgh.15465
12. Huang HH, Chang JC, Liu HC, Yang ZY, Yang YJ, Chen LK, et al. Handgrip strength, tumor necrosis factor-alpha, interlukin-6, and visfatin levels in oldest elderly patients with cognitive impairment. Exp Gerontol. (2020) 142:111138. doi: 10.1016/j.exger.2020.111138
13. Kazem YM, Shebini SM, Moaty MI, Fouad S, Tapozada ST. Sleep deficiency is a modifiable risk factor for obesity and cognitive impairment and associated with elevated visfatin. Open Access Maced J Med Sci. (2015) 3:315–21. doi: 10.3889/oamjms.2015.063
14. Huang X, Wang C, Tian S, Huang R, Guo D, Zhang H, et al. Higher plasma level of nampt presaging memory dysfunction in chinese type 2 diabetes patients with mild cognitive impairment. J Alzheimers Dis. (2019) 70:303–14. doi: 10.3233/JAD-190269
15. Xing S, Hu Y, Huang X, Shen D, Chen C. Nicotinamide phosphoribosyltransferaserelated signaling pathway in early Alzheimer’s disease mouse models. Mol Med Rep. (2019) 20:5163–71. doi: 10.3892/mmr.2019.10782
16. Sharifipour E, Sharifimoghadam S, Hassanzadeh N, Ghasemian Mojarad N, Ghoreishi A, Hejazi SA, et al. Altered plasma visfatin levels and insulin resistance in patients with Alzheimer’s disease. Acta Neurol Belg. (2020) 120:901–6. doi: 10.1007/s13760-019-01084-9
17. Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F, et al. Visfatin reduces hippocampal CA1 cells death and improves learning and memory deficits after transient global ischemia/reperfusion. Neuropeptides. (2015) 49:63–8. doi: 10.1016/j.npep.2014.12.004
18. Stein LR, Wozniak DF, Dearborn JT, Kubota S, Apte RS, Izumi Y, et al. Expression of Nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. J Neurosci. (2014) 34:5800–15. doi: 10.1523/JNEUROSCI.4730-13.2014
19. Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: A prospective cohort study. Lancet Respir Med. (2018) 6:213–22. doi: 10.1016/S2213-2600(18)30062-6
20. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
21. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. (1994) 47:1245–51.
22. Yuan Y, Li Z, Yang N, Han Y, Ji X, Han D, et al. Exosome alpha-Synuclein release in plasma may be associated with postoperative delirium in hip fracture patients. Front Aging Neurosci. (2020) 12:67. doi: 10.3389/fnagi.2020.00067
23. Marcantonio ER, Ngo LH, O’Connor M, Jones RN, Crane PK, Metzger ED, et al. 3D-CAM: Derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: A cross-sectional diagnostic test study. Ann Intern Med. (2014) 161:554–61. doi: 10.7326/M14-0865
24. Prins TW, Scholtens IM, Bak AW, van Dijk JP, Voorhuijzen MM, Laurensse EJ, et al. Data on screening and identification of genetically modified papaya in food supplements. Data Brief. (2016) 9:43–6.
25. Fisher P, Cao W, Yu Q. Using SAS macros for multiple mediation analysis in R. J Open Res Softw. (2020) 8:22. doi: 10.5334/jors.277
26. Yang Y, Zhao X, Dong T, Yang Z, Zhang Q, Zhang Y. Risk factors for postoperative delirium following hip fracture repair in elderly patients: A systematic review and meta-analysis. Aging Clin Exp Res. (2017) 29:115–26. doi: 10.1007/s40520-016-0541-6
27. Noah AM, Almghairbi D, Evley R, Moppett IK. Preoperative inflammatory mediators and postoperative delirium: Systematic review and meta-analysis. Br J Anaesth. (2021) 127:424–34.
28. Capri M, Yani SL, Chattat R, Fortuna D, Bucci L, Lanzarini C, et al. Pre-Operative, high-IL-6 blood level is a risk factor of post-operative delirium onset in old patients. Front Endocrinol. (2014) 5:173. doi: 10.3389/fendo.2014.00173
29. Neerland BE, Hall RJ, Seljeflot I, Frihagen F, MacLullich AM, Raeder J, et al. Associations between delirium and preoperative cerebrospinal fluid C-reactive protein, interleukin-6, and interleukin-6 receptor in individuals with acute hip fracture. J Am Geriatr Soc. (2016) 64:1456–63. doi: 10.1111/jgs.14238
30. Carbone F, Liberale L, Bonaventura A, Vecchie A, Casula M, Cea M, et al. Regulation and function of extracellular nicotinamide phosphoribosyltransferase/visfatin. Compr Physiol. (2017) 7: 603–21.
31. Zeng M, Wei TF, Chen C, Shen C, Gao TY, Xie X, et al. Nicotinamide phosphoribosyltransferase inhibitor ameliorates mouse aging-induced cognitive impairment. J Cereb Blood Flow Metab. (2021) 41:2510–23. doi: 10.1177/0271678X211006291
32. Liang Z, Wu Y, Xu J, Fang Q, Chen D. Correlations of serum visfatin and metabolisms of glucose and lipid in women with gestational diabetes mellitus. J Diabetes Investig. (2016) 7:247–52.
33. Jian WX, Luo TH, Gu YY, Zhang HL, Zheng S, Dai M, et al. The visfatin gene is associated with glucose and lipid metabolism in a Chinese population. Diabet Med. (2006) 23:967–73. doi: 10.1111/j.1464-5491.2006.01909.x
34. Lee JO, Kim N, Lee HJ, Lee YW, Kim JK, Kim HI, et al. Visfatin, a novel adipokine, stimulates glucose uptake through the Ca2 +-dependent AMPK-p38 MAPK pathway in C2C12 skeletal muscle cells. J Mol Endocrinol. (2015) 54:251–62. doi: 10.1530/JME-14-0274
35. Liu K, Song Y, Yuan Y, Li Z, Wang X, Zhang W, et al. Type 2 diabetes mellitus with tight glucose control and poor pre-injury stair climbing capacity may predict postoperative delirium: A secondary analysis. Brain Sci. (2022) 12:951. doi: 10.3390/brainsci12070951
36. Yuan SJ, Shen J. Increased preoperative glucose variability is associated with adverse perioperative outcomes following orthopedic surgery in patients with type 2 diabetes mellitus. Curr Med Sci. (2020) 40:523–9. doi: 10.1007/s11596-020-2209-x
37. Lin YJ, Lin LY, Peng YC, Zhang HR, Chen LW, Huang XZ, et al. Association between glucose variability and postoperative delirium in acute aortic dissection patients: An observational study. J Cardiothorac Surg. (2021) 16:82. doi: 10.1186/s13019-021-01456-4
38. Liu SW, Qiao SB, Yuan JS, Liu DQ. Visfatin stimulates production of monocyte chemotactic protein-1 and interleukin-6 in human vein umbilical endothelial cells. Horm Metab Res. (2009) 41:281–6.
39. Gong YY, Peng HY. Correlation analysis of epicardial adipose tissue thickness, C-reactive protein, interleukin-6, visfatin, juxtaposed with another zinc finger protein 1, and type 2 diabetic macroangiopathy. Lipids Health Dis. (2021) 20:25. doi: 10.1186/s12944-021-01451-7
Keywords: hip fracture, visfatin, postoperative delirium, interleukin-6, mediation analysis
Citation: Kang N, Yang N, Zhao K, Li Z, Zhang W, Han Y, Liu K, Song Y, Chen L, Li Y, Hong J, Li Y, Guo X, Wang G and Yuan Y (2022) Preoperative plasma visfatin may have a dual effect on the occurrence of postoperative delirium. Front. Med. 9:1024942. doi: 10.3389/fmed.2022.1024942
Received: 09 September 2022; Accepted: 05 October 2022;
Published: 22 November 2022.
Edited by:
Kevin Nicholas Hascup, Southern Illinois University Carbondale, United StatesReviewed by:
Adam Lipson, Southern Illinois University School of Medicine, United StatesLei Zhao, Xuanwu Hospital, Capital Medical University, China
Najib Murr, North Oaks Health System, United States
Copyright © 2022 Kang, Yang, Zhao, Li, Zhang, Han, Liu, Song, Chen, Li, Hong, Li, Guo, Wang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangyang Guo, puthmzk@hsc.pku.edu.cn; Geng Wang, w_geng@163.com; Yi Yuan, julietyy@sina.com
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Ning Kang
Ning Kang Ning Yang
Ning Yang Kaixuan Zhao
Kaixuan Zhao Zhengqian Li
Zhengqian Li Wenchao Zhang
Wenchao Zhang Yongzheng Han
Yongzheng Han Kaixi Liu
Kaixi Liu Yanan Song1
Yanan Song1  Yue Li
Yue Li Jingshu Hong
Jingshu Hong Yitong Li
Yitong Li Xiangyang Guo
Xiangyang Guo Geng Wang
Geng Wang