Implementing HPV testing in 9 Latin American countries: The laboratory perspective as observed in the ESTAMPA study
- 1Early Detection, Prevention and Infections Branch, International Agency for Research on Cancer, Lyon, France
- 2Instituto de Enfermedades Infecciosas ANLIS-Malbrán, Buenos Aires, Argentina
- 3Instituto de Investigaciones en Microbiología, Universidad Nacional Autónoma de Honduras, Tegucigalpa, Honduras
- 4Grupo de Infección y Cáncer, Universidad de Antioquia, Medellín, Colombia
- 5SMS-Oncology, Amsterdam, Netherlands
- 6Instituto Nacional de Cancerología, Bogotá, Colombia
- 7Caja Costarricense de Seguro Social (CCSS), Región Pacífico Central, San José, Costa Rica
- 8Instituto de Investigaciones en Ciencias de la Salud, Universidad Nacional de Asunción, San Lorenzo, Paraguay
- 9Hospital Nacional Profesor Alejandro Posadas, El Palomar, Argentina
- 10Facultad de Medicina, Universidad Mayor, Real y Pontificia de San Francisco Xavier de Chuquisaca, Sucre, Bolivia
- 11Laboratorio de Biología Molecular, Departamento de Patología Clínica, Centro Hospitalario Pereira Rossell, Montevideo, Uruguay
- 12Instituto de Salud Pública de México, Morelos, Mexico
- 13Laboratorio de Patología Oncológica SAC, Lima, Peru
- 14Instituto de Patología y Biología Molecular, Lima, Peru
- 15Agencia Costarricense de Investigaciones Biomédicas (ACIB), Fundación Inciensa, San José, Guanacaste, Costa Rica
- 16Laboratorio Central de Salud Pública, Asunción, Paraguay
- 17Hospital de Clínicas José de San Martín, Buenos Aires, Argentina
- 18Clínica Angloamericana, Lima, Peru
- 19Liga contra el Cáncer, Lima, Peru
- 20Comisión Honoraria de Lucha Contra el Cáncer, Montevideo, Uruguay
- 21Pan American Health Organization (PAHO), Washington, DC, United States
- 22Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva, Switzerland
Background: Replacement of cytology screening with HPV testing is recommended and essential for cervical cancer elimination. HPV testing for primary screening was implemented in 12 laboratories within 9 Latin American countries, as part of the ESTAMPA cervical cancer screening study. Our observations provide information on critical operational aspects for HPV testing implementation in diverse resource settings.
Methods: We describe the implementation process of HPV testing in ESTAMPA, focusing on laboratory aspects. We assess the readiness of 12 laboratories to start HPV testing and their continuity capacity to maintain good quality HPV testing until end of recruitment or up to December 2021. Readiness was based on a checklist. Information from the study database; regular meetings and monitoring visits; and a questionnaire on laboratory operational aspects sent in May 2020 were used to assess continuity capacity. Compliance with seven basic requirements (readiness) and eight continuity requirements (continuity capacity) was scored (1 = compliant, 0 = not compliant) and totaled to classify readiness and continuity capacity as very limited, limited, moderate or high. Experiences, challenges, and enablers of the implementation process are also described.
Results: Seven of 12 laboratories had high readiness, three moderate readiness, and of two laboratories new to HPV testing, one had limited readiness and the other very limited readiness. Two of seven laboratories with high readiness also showed high continuity capacity, one moderate continuity capacity, and the other four showed limited continuity capacity since they could not maintain good quality HPV testing over time. Among three laboratories with moderate readiness, one kept moderate continuity capacity and two reached high continuity capacity. The two laboratories new to HPV testing achieved high continuity capacity. Based on gained expertise, five laboratories have become part of national screening programs.
Conclusion: High readiness of laboratories is an essential part of effective implementation of HPV testing. However, high readiness is insufficient to guarantee HPV testing high continuity capacity, for which a “culture of quality” should be established with regular training, robust monitoring and quality assurance systems tailored to local context. All efforts to strengthen HPV laboratories are valuable and crucial to guarantee effective implementation of HPV-based cervical screening.
Introduction
More than 600,000 new cases and 300,000 cervical cancer deaths occur every year; over 90% of these are in low-income and middle-income countries (LMIC) (1).
Cytology-based screening has successfully reduced cervical cancer rates in places where it has been systematically implemented (2). However, cytology has limited and variable sensitivity, requiring frequent repetition to reach an acceptable level of precancerous lesions detection (3, 4). Frequent cytology screening has not been feasible in most LMIC, where coverage is generally low, and follow-up of women with abnormal cytology and treatment of detected lesions is very limited (3, 5).
Persistent infection with high-risk HPV is the leading cause of cervical cancer (6). Several molecular techniques are available to detect HPV DNA and can be used in primary screening (7, 8). There is overwhelming worldwide evidence that HPV testing is more effective than cytology in identifying women at greater risk of precancerous cervical lesions (9–13). HPV testing is objective, can be automated, and can be done using self-collected samples with potential to increase screening coverage (14–17). In addition, the high negative predictive value of HPV testing allows extension of the screening interval (in comparison to the 3 years interval when using cytology) (18, 19), facilitating screening and treatment coverage in limited-resource settings.
In the World Health Assembly (20) adopted a global strategy for eliminating cervical cancer as a public health problem. In order to reach elimination by the end of the century, countries should achieve full vaccination of 90% of girls by age 15, screening of 70% of women twice by age 35 and 45 with a high-performance test, and treatment of 90% of women with cervical disease (precancer or invasive cervical cancer) (21). In 2021, WHO published cervical cancer screening and treatment guidelines recommending the use of HPV DNA detection in a screen and treat approach or a screen, triage and treat approach starting at age 30 every 5 to 10 years for the general population, and using HPV DNA detection in a screening, triage and treat approach starting at age 25 every 3 to 5 years for women living with HIV (22). As evidence-based cervical cancer screening and treatment interventions are available, it is time for country-driven implementation research to understand how to implement and scale-up HPV-based cervical screening (23, 24). In fact, reports from countries in Latin America that are replacing cytology with HPV testing at the national level or in pilots suggest that HPV-based cervical screening implementation is very challenging, and demands extensive planning of activities across the screening care continuum including preparation of laboratory facilities and training of personnel for HPV testing before scaling-up (25–29).
Several tools are available to support countries with the implementation of HPV testing within cervical cancer screening (30–33). In particular, the step-by-step guide on introducing and scaling up HPV within a comprehensive program of prevention and control of cervical cancer (33), intends to offer practical guidance to program managers once the decision to introduce HPV testing in their national cervical cancer prevention program has been made. Guidance covers three main domains: planning, implementation, and monitoring/scaling up of HPV testing in primary screening. Once planning is completed, the preparation of an implementation roadmap is recommended, to establish or strengthen quality management systems in laboratories, to define the procurement process and to use indicators to monitor the progress of the implementation. This is key to consolidate a screening platform for scaling up and final adoption of the screening strategy at national level. Using several features of this step-by-step guide, here we describe the HPV testing implementation process, including monitoring and evaluation of HPV testing performance over time of 12 laboratories in Latin America participating in the ESTAMPA study (NCT01881659) (34).
Materials and methods
ESTAMPA is a multicentric study of cervical cancer screening with HPV testing conducted in 12 study centers’ laboratories (SC1-12) in nine countries in Latin America. The study aims to evaluate the performance of different techniques and approaches to triage HPV positive women and to inform on how best to implement affordable and sustainable HPV-based screening programs in LMIC.
The study protocol has been previously published (34). Briefly, women aged 30 to 64 years old were invited for cervical cancer screening with HPV testing and, following country guidelines, also cytology. Women who consented to participate underwent pelvic examination, and samples were collected using Cervex brushes (Papette, Wallach, USA) that were washed in PreservCyt medium (PC) (Hologic, USA) for HPV testing and cytology.
Samples were tested for HPV DNA detection using either Digene HC2 HPV DNA test (QIAGEN, USA) or COBAS HPV test (Roche, Switzerland). After HPV testing, aliquots of PC were prepared for future molecular triage. Digene HC2 sample conversion kit was used for conversion of samples collected on PC before HC2 HPV testing. HPV (HC2 or COBAS) was done following manufacturer instructions and ESTAMPA standard operating procedures (SOPs). For quality control (QC) of HPV testing, in some laboratories, about 10% of samples tested with HC2 or COBAS were retested either at the same laboratory using a different HPV technique or at and international hub with COBAS. In one center, samples from the first 900 recruited women were stored in the Digene Standard Transport Medium (STM) and subsequently tested with HC2. In this center, a sub-study evaluating the impact of operational factors on HPV positivity of HPV assays including HC2, COBAS and APTIMA (Hologic, USA) was conducted and COBAS results were assumed as QC of HC2 and APTIMA (35).
Women with abnormal cytology or positive HPV results (including QC HPV test) were referred to colposcopy, 2-3 biopsies were collected from any observed lesions, and women diagnosed with cervical intraepithelial neoplasia (CIN) grade 2 or worse (CIN2 +) were treated with LLETZ. At this colposcopy visit, a cervical sample was collected for HPV testing and other molecular triage tests. Women with negative colposcopy or histology showing lesions less severe than CIN2 (< CIN2) were invited for a follow-up visit at 18 months for a second HPV screen to complete ascertainment of disease. Women positive for HPV at this visit were referred to colposcopy and clinically managed as needed.
Overall, women could have one, two, three or four HPV tests during the study, depending on their screening results and associated study visits (initial screening, colposcopy and 18months follow-up) and on the sample being selected for QC.
Steps to implement HPV testing in 12 ESTAMPA laboratories
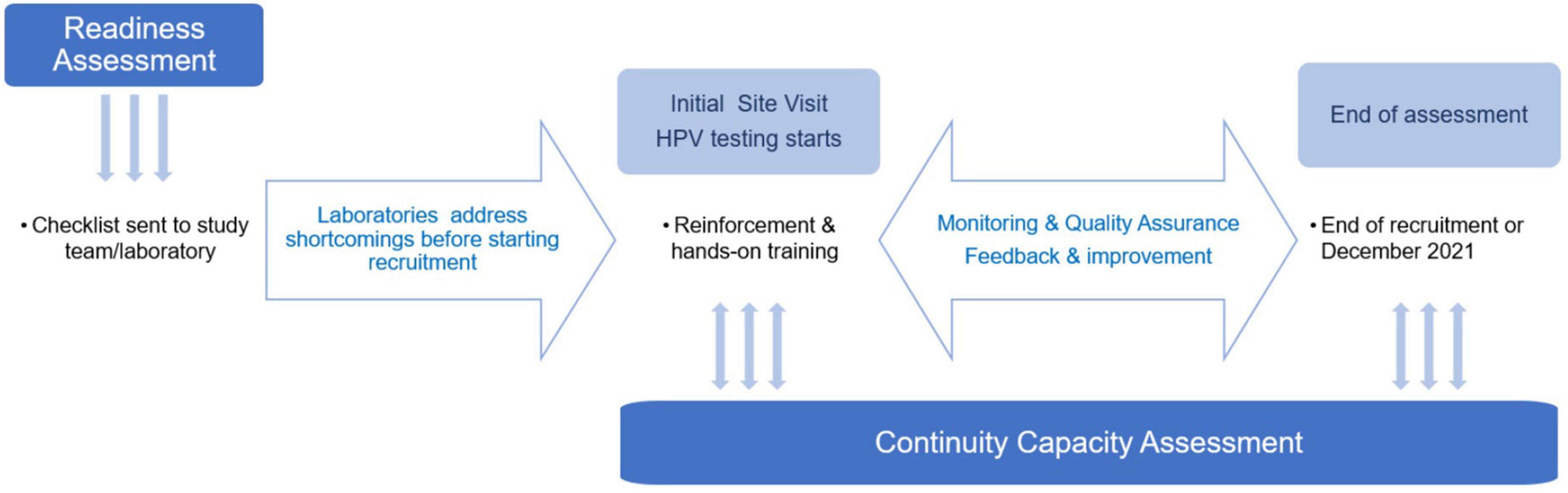
The main steps carried out to implement HPV testing in ESTAMPA laboratories (Figure 1) are described as follows.
(1) Assessment of readiness for HPV testing implementation in the laboratory. Before launching the study, local principal investigators willing to take part, were asked to identify a laboratory with adequate facilities as to carry out HPV testing for primary screening. Laboratory-based investigators could propose their own facilities or others thought more suitable for future participation in national HPV-based cervical screening program.
Next, a checklist (Supplementary Material 1) was used to assess the existing installed capacity of each laboratory. As the study was launched early on in the introduction of HPV testing in primary screening (December 2012), laboratories were asked about the availability of HPV equipment in the laboratory; whether collection medium and HPV reagents were registered in the country and if not, whether investigators had experience with importing and customs; and whether additional HPV testing consumables specifically selected for the study were available in the laboratory or the country.
Laboratories were also asked whether they had personnel to perform the test; and whether they had established logistics for transport and storage of samples, management of reagents validity and delivery of results. We did not request specific arrangements for external quality assurance (EQA) in this checklist, but we proposed different QA measures as the study progressed.
Based on this initial assessment, each study center developed a road map to address limitations and joined the project when ready to launch recruitment.
(2) Initial site visit to start recruitment and HPV testing. The study covered the cost of HPV reagents and additional consumables where needed. Procurement was done internationally or in-country, depending on availability of local distributors and registration of products. Once consumables were in place (usually for the first 500-1,000 tests) and laboratories were ready, a site visit by IARC researchers to launch the ESTAMPA study was organized.
During this initial visit, training on the study protocol, study’s SOPs and good clinical practices was given to all study personnel over 1-2 days, followed by real-life recruitment. In parallel, the selected HPV testing laboratory was visited to verify that conditions were ready to start testing. When enough samples for a full- or a half-testing batch (3-7 days depending on local arrangements) had been collected, additional training on the study protocol, laboratory SOPs and overall sample handling from reception at the laboratory to delivery of results, was provided to all laboratory personnel.
Next, using recently collected samples, hands-on training was given to 1-2 technicians on: (i) Running HPV testing if no previous experience, (ii) Performing conversion of samples collected in PC if HC2 testing was used, (iii) Preparing aliquots from PC vials, and (iv) Recording results on the study database. Lead investigators at each center made arrangements to repeat this training as needed when new laboratory personnel joined the study.
As the study progressed initial and monitoring site visits were done by a team mostly composed by local researchers selected from study centers already recruiting with support from ESTAMPA researchers.
(3) Assessment of continuity capacity of laboratories to perform good quality HPV testing. Laboratories’ continuity capacity was assessed throughout the study. Sources of information used for the assessment included: minutes from meetings with local investigators and laboratory managers, reports from monitoring visits, data provided by the study centralized web-system, and from a questionnaire on laboratory operational aspects (Supplementary Material 2) sent to laboratory managers in May 2020.
As laboratories joined the study, implementation of quality assurance processes was recommended, such as providing regular training to technicians on HPV testing and study’s SOPs, retesting of a subset of samples and annual subscription to EQA. The study covered the first year EQA fees for new laboratories which were encouraged to ensure funding for next subscriptions.
The centralized web-system specifically developed for the study allowed regular checking on the progress of recruitment, the number of HPV tests carried out (at enrolment, colposcopy, and 18 months), the speed of uploading results, and statistics on clinical activities. Thus, the web-system provided effective indicators of the HPV testing capacity of laboratories, such as the volume of HPV testing and the HPV positivity over time. As over 75% of samples tested in each laboratory were collected at the initial screening visit, the number of recruited women per study center was used as a proxy of HPV testing volume, and the positivity of HPV testing at the time of enrolment was used as a proxy of overall HPV positivity, independently of the HPV detection technique used (HC2, COBAS). Based on previously reported HPV prevalence among Latin American women (36–38), we assumed that HPV positivity values reported by laboratories should be between 12.5 and 15%. HPV positivity was regularly tabulated by center and was graphically assessed by plotting the HPV positivity with 95% confidence intervals (using exact binominal distribution and the R statistical package for computations). If any laboratory showed HPV positivity below 10% or above 16% in two consecutive runs, on any assessment, a full data inspection across the screening process (i.e., sample collection, HPV testing and laboratory activities, clinical management, data management) was done and whenever necessary was followed by a site visit to offer refresher training or suggest corrective measures.
The questionnaire sent to laboratory managers requested current details on HPV equipment status, annual subscriptions to EQA, training on HPV testing for current and new technicians, preparedness for procurement process of HPV and related laboratory supplies, availability of tracking systems for HPV reagents and laboratory supplies and for samples transport and storage and turnaround time of HPV results.
As recruitment progressed, many logistic activities were smoothly handled over to local investigators, including procurement of reagents and consumables if they became available in the country, and responsibility for maintaining adequate stocks. This transition facilitated implementation or expansion of HPV-based cervical screening programs.
All collected data were used to assess whether laboratories were able to continue performing good quality HPV testing over time, from the first batch of testing up until end of recruitment or December 2021.
Evaluation of laboratories’ readiness and continuity capacity for HPV testing implementation
Collected data from the readiness and continuity capacity assessments were summarized in six main domains: characteristics of the laboratory facility, personnel, procurement of reagents and consumables, tracking systems for reagents expiry and supplies, logistics for transport and storage and delivery of testing results. Responses to the initial checklist were classified into seven basic requirements (within the above six domains) and were used to assess the laboratory readiness for HPV implementation. Monitoring, and operational questionnaires classifying eight continuity requirements (within the six domains), were used to assess the continuity capacity of laboratories to perform good quality HPV testing over time.
Each requirement was scored as compliant (value = 1) or non-compliant (value = 0), and values were summed up to generate a total score (range = 0-7 for readiness and 0-8 for continuity capacity). Using the scores, the readiness of laboratories to start HPV testing at the time of initial assessment was classified as very limited (score ≤ 3), limited (score = 4-5), moderate (score = 6) and high (score = 7). Similarly, the continuity capacity of laboratories to perform HPV testing over time was classified as very limited (score ≤ 3), limited (score = 4-5), moderate (score = 6-7) and high (score = 8) (Tables 1, 2).
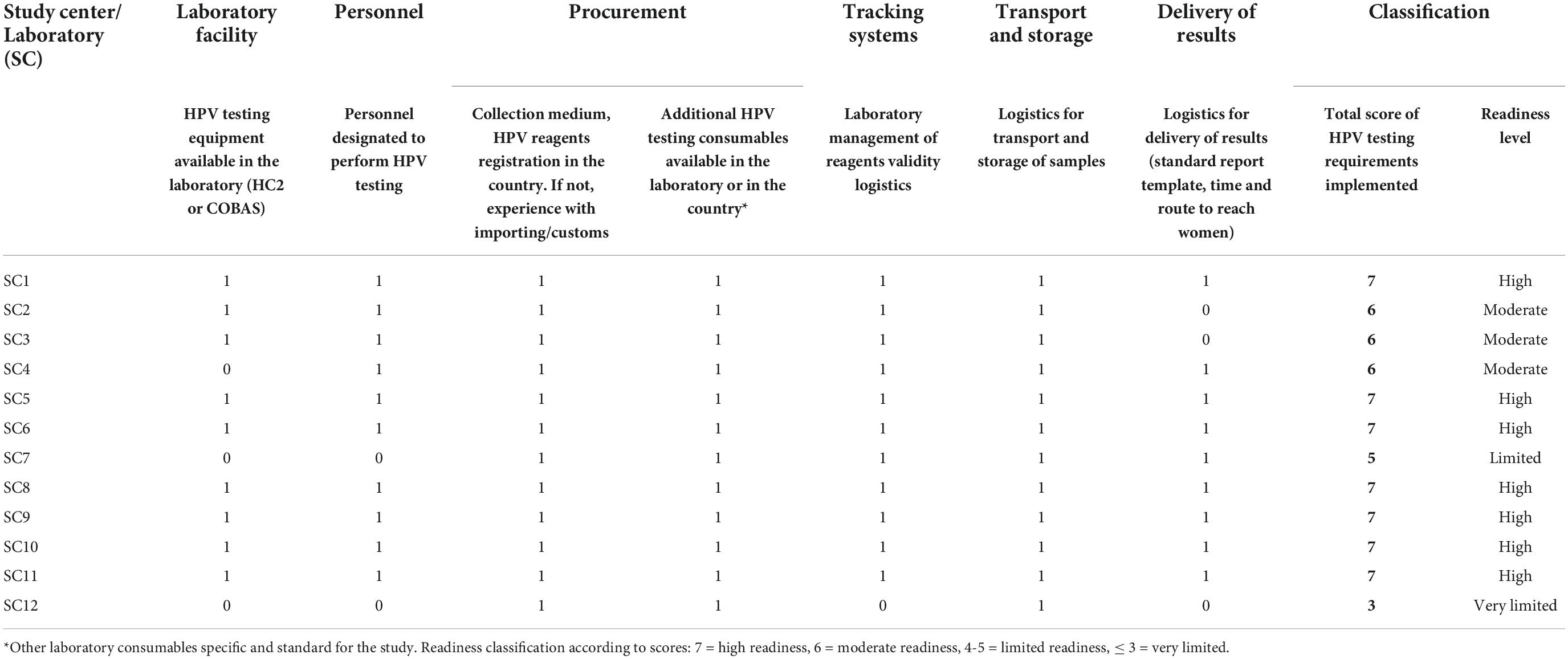
Table 1. Readiness of laboratories based on compliance (1 = complied, 0 = not complied) to 7 essential requirements.
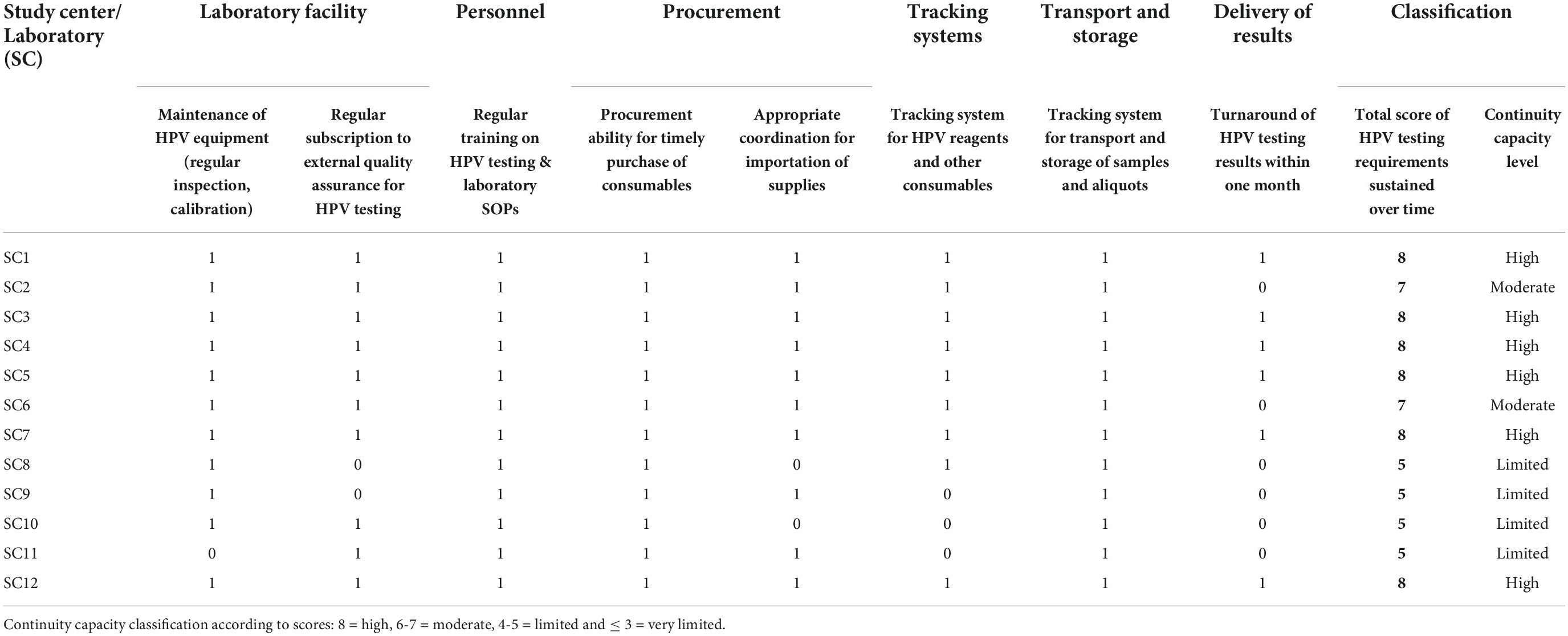
Table 2. Continuity capacity of laboratories based on compliance (1 = complied, 0 = not complied) to 8 continuity requirements.
Results
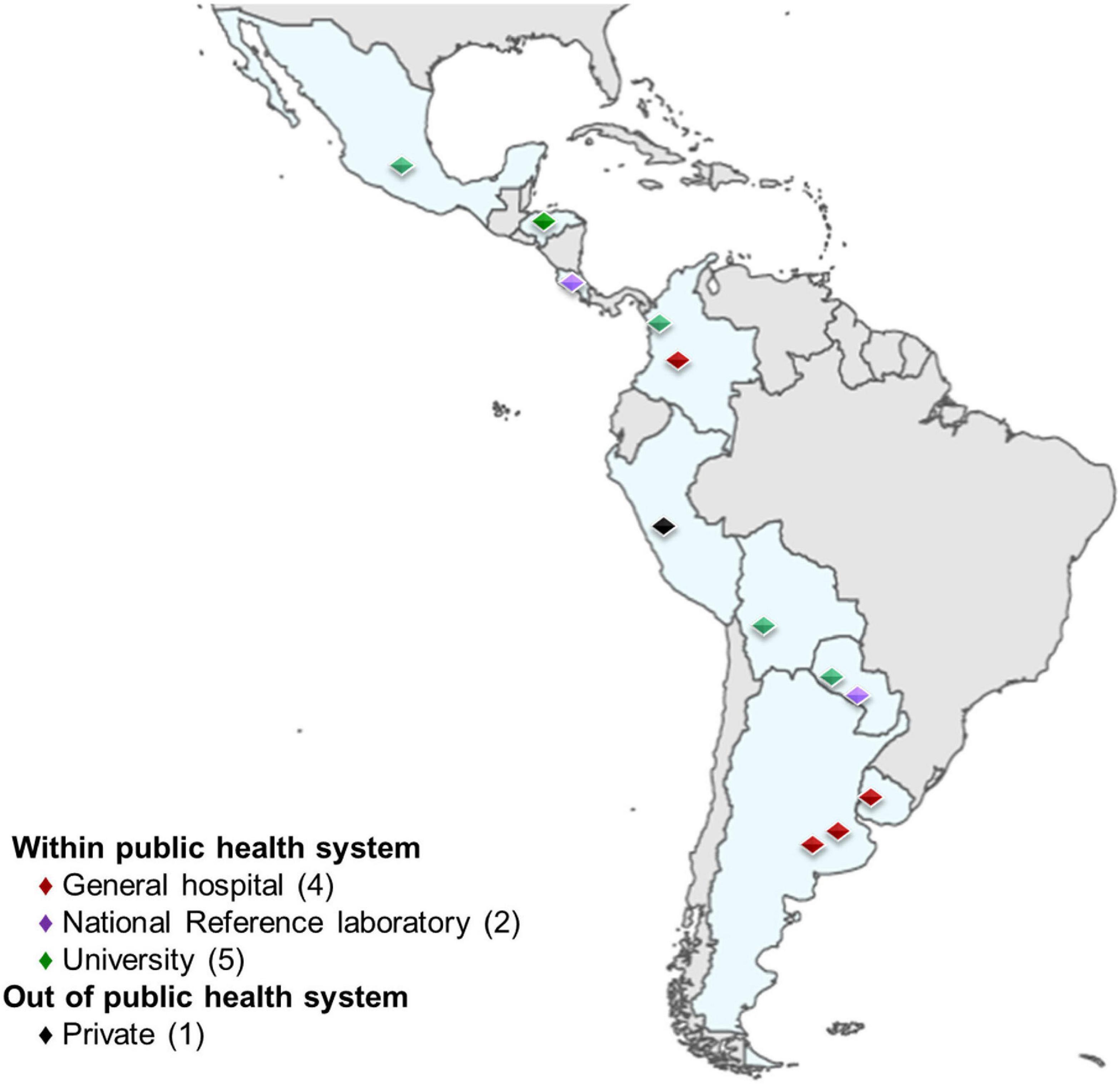
Twelve study centers with corresponding HPV laboratories, located in nine Latin American countries (Argentina, Bolivia, Colombia, Costa Rica, Honduras, Mexico, Paraguay, Peru and Uruguay) participated in the ESTAMPA study. Eleven laboratories were part of public systems (4 were based in general hospitals, 2 were national-referral laboratories, 5 were university-based), and one offered service under a private system. Six laboratories had staff with experience in detecting HPV for clinical diagnosis and research at the initial assessment. Two of the remaining laboratories had experience in detecting HPV for research only, and two had experience in HPV detection for diagnosis but not for research. One laboratory was set up in a regional hospital, but the study team was based on a high-level expertise (diagnostics and research) HPV reference laboratory. Finally, one laboratory did not have previous experience in HPV detection or any other molecular diagnostics (Figure 2).
Despite differences in recruitment targets (ranging from 500 to 10,000) and year when recruitment and HPV testing started (2012 to 2017), among 42,502 women with valid HPV results recruited until December 2021, the overall yearly HPV positivity consistently ranged between 12 and 16% over time (mean HPV positivity: 14.1%, 95CI: 13.8-14.4) (Supplementary Figure 1).
Compliance with readiness requirements
Table 1 summarizes the compliance with the seven readiness requirements per laboratory. Nine of the twelve laboratories had equipment for HPV detection (8 used HC2, 1 used Cobas). Procurement of equipment for the remaining three laboratories was dependent on when the local team agreed to participated in ESTAMPA and the availability of local funding. In study center 7 (SC7), the principal investigator who led an international reference HPV laboratory, obtained a Cobas machine under a loan for study purposes, which was installed in a regional hospital, as the national cervical screening program had plans to implement HPV testing there. The SC7 team trained, supervised, and supported the newly laboratory throughout the study. HC2 equipment was procured by the central team in 2014 for the laboratory working with study center 4 (SC4), while study center 12 (SC12) secured local funding for HC2 equipment, although political and administrative complexities meant final procurement did not happen until 2018. Regardless of previous experience on HPV detection techniques, all laboratories except the new ones, SC7 and SC12, had personnel allocated to perform HPV testing at the time of initial assessment.
As the study provided all relevant consumables at the start, centers did not need to engage in direct procurement activities. However, all teams and particularly laboratory leads demonstrated awareness of current local regulations for purchasing in-country, and the ability to deal with importation of non-registered in-country consumables or to negotiate prices of testing kits and machine loaning as SC7.
All laboratories had established logistics for transporting and storage samples (as per protocol), only (SC12) did not have a system to control the validity of laboratory reagents, and in addition to SC2 and SC3 did not have a system in place to return results to women, did not have a standard report template nor instructions on how to share results with participants.
In summary, seven laboratories (SC1, SC5, SC6, SC8, SC9, SC10, and SC11) had high readiness at initial assessment and three (SC2, SC3 and SC4) had moderate readiness with only one essential requirement not fulfilled. Of two laboratories totally new to HPV testing, the one (SC7) supported by a high-level laboratory had limited readiness and the other one (SC12) very limited readiness.
Compliance with continuity capacity requirements
Table 2 summarizes the compliance with the eight continuity capacity requirements per laboratory. All laboratories had regular maintenance including cleaning and calibration of their HPV testing equipment except for SC11 which was not serviced on time and testing had to stop, leading to severe delays in processing samples. Six laboratories (SC1, SC2, SC5, SC6, SC7, and SC10) that were already performing HPV testing (mainly for research) at initial assessment, had established EQA systems and maintained regular participation in either the College of Pathologists (CAP) scheme or the Quality Control for Molecular Diagnostics (QCMD). The study covered the first subscription payment for other four laboratories (SC3, SC4, SC11, and SC12), but only one (SC11) maintained the subscription using their own funds afterward, and two laboratories (SC8 and SC9) never subscribed to EQA during the assessment period. In parallel to these schemes, all laboratories were encouraged to repeat testing of 10% of samples, preferably with a different HPV detection platform or in a different laboratory.
All laboratories offered appropriate training in HPV testing and laboratory study SOPs to new laboratory personnel, even if they were not assigned to HPV testing and gained or improved the ability to ensure timely procurement of HPV reagents and other consumables.
Laboratories developed procurement plans, independently of whether the planning only involved preparing paperwork for customs clearance in advance or dealing with local distributors or purchasing the consumables (with study’s funds, health authorities funds or research granted competitive funds). However, two laboratories (SC8, SC10) had difficulties coordinating supplies’ importation all over recruitment.
Laboratory management systems (manual or digital) for tracking of reagents expiry and handling of samples (transport and storage) were incorporated or improved by all laboratories. However, three laboratories (SC9, SC10, and SC11) needed the central team to send regular reminders to verify expiration dates throughout the study.
Six laboratories struggled to consistently deliver results within one month. Four of them (SC8, SC9, SC10, and SC11) had additional challenges linked to procurement of consumables, while the delays for the other two laboratories (SC2 and SC6) were largely related to the study setting. SC2 recruited in a remote location, and samples were shipped by air only 1-2 times per month. SC6 recruited in several centers concurrently in order to reach its recruitment target of 10,000 women. This meant that samples arrived at both laboratories in large batches and accumulated, causing delays with processing and turnaround of HPV results. In such cases, different measures were applied when to guarantee adequate follow-up of screen-positives (e.g., testing in another laboratories, using cytology results for referral to or offering colposcopy to those with delayed results).
Overall, six laboratories (SC1, SC3, SC4, SC5, SC7, and SC12) showed high continuity capacity as they fulfilled all criteria; two (SC2 and SC6) had moderate continuity capacity having only not fulfilled one requirement and four (SC8, SC9, SC10 and SC11) showed limited continuity capacity.
Relation between readiness and continuity capacity
Figure 3 further depicts the relation between readiness and continuity capacity for HPV testing. Two (SC1, SC5) of seven laboratories (SC1, SC5, SC6, SC8, SC9, SC10, and SC11) with high readiness also showed high continuity capacity, one (SC6) showed moderate continuity capacity, and the other four (SC8, SC9, SC10, and SC11) were not able to maintain good-quality HPV testing over time after an excellent start and showed limited continuity capacity. Among three laboratories with moderate readiness, one (SC2) kept moderate continuity capacity and two (SC3 and SC4) reached high continuity capacity. The two laboratories new to molecular testing (SC7, SC12) with subsequent limited and very limited readiness, respectively, achieved high continuity capacity.
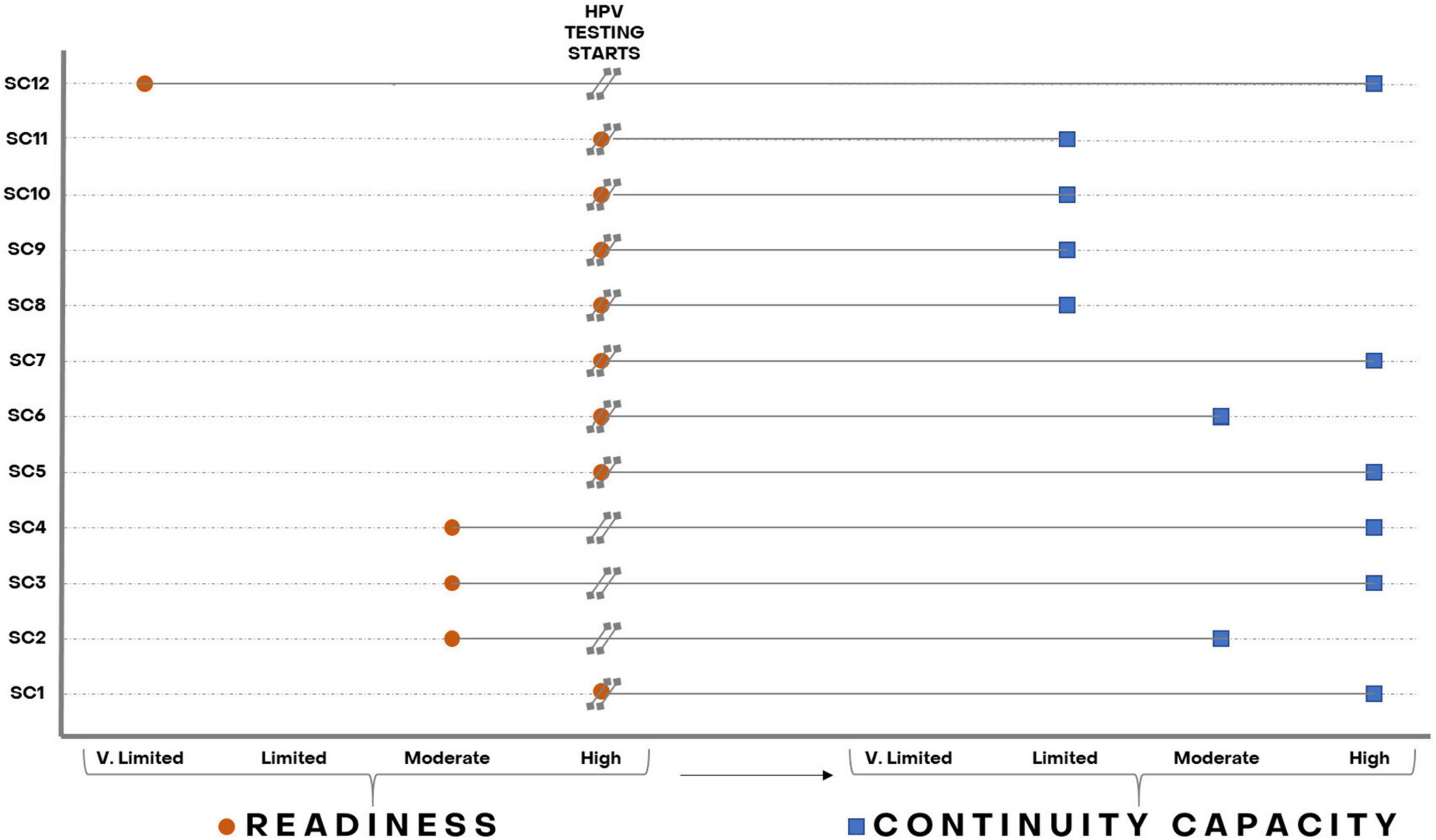
Figure 3. Laboratories’ readiness and continuity capacity to perform HPV testing throughout recruitment in the ESTAMPA study. Orange circles represent laboratories’ readiness and blue squares laboratories’ continuity capacity as assessed from the start of HPV testing up to December 2021.
Recruitment targets and laboratories’ participation in national screening programs by level of continuity capacity
Table 3 details the recruitment targets and their attainment status at the end of the assessment period, as proxy of HPV testing volume over the study, and whether laboratories is currently part or is considered to become part of the national screening program by level of continuity capacity. Among six study centers whose HPV laboratories showed high continuity capacity, SC5 reached its recruitment target, SC12, that is still recruiting, has achieved 64% of the target, SC4 and SC7 did not reach their targets, and SC1 and SC3 exceeded them. SC4 stopped after recruiting 88% of its target because of difficulties with the uptake of screening for several months, while SC7 stopped at 67% mainly due to termination of the loan of the COBAS machine to the study team and emerging priorities of the clinical team. Both SC2 and SC6 with moderate continuity capacity achieved their targets. SC9 only targeted 500 women and stopped at 82% after a slow recruitment pace over time which worsened with COVID pandemic restrictions. The SC8 team experienced sudden change of political and organization authorities who caused important disruptions to screening activities with unexpected disassembling of the study clinic. The SC10 highly experienced research laboratory faced study leadership instability leading to poor coordination of screening and laboratory activities. In view of these political and leadership issues, recruitment stopped at 14 and 17% attainment for SC8 and SC10 respectively. The only laboratory with direct HPV testing problems was SC11, where inadequate maintenance of the HPV equipment led to 31% of screened women (n = 497) having invalid HPV results. Immediate correcting measures to guarantee the safety of participants (e.g., multiple attempts to repeat sampling, recalling women directly to colposcopy) were implemented in these three SCs.
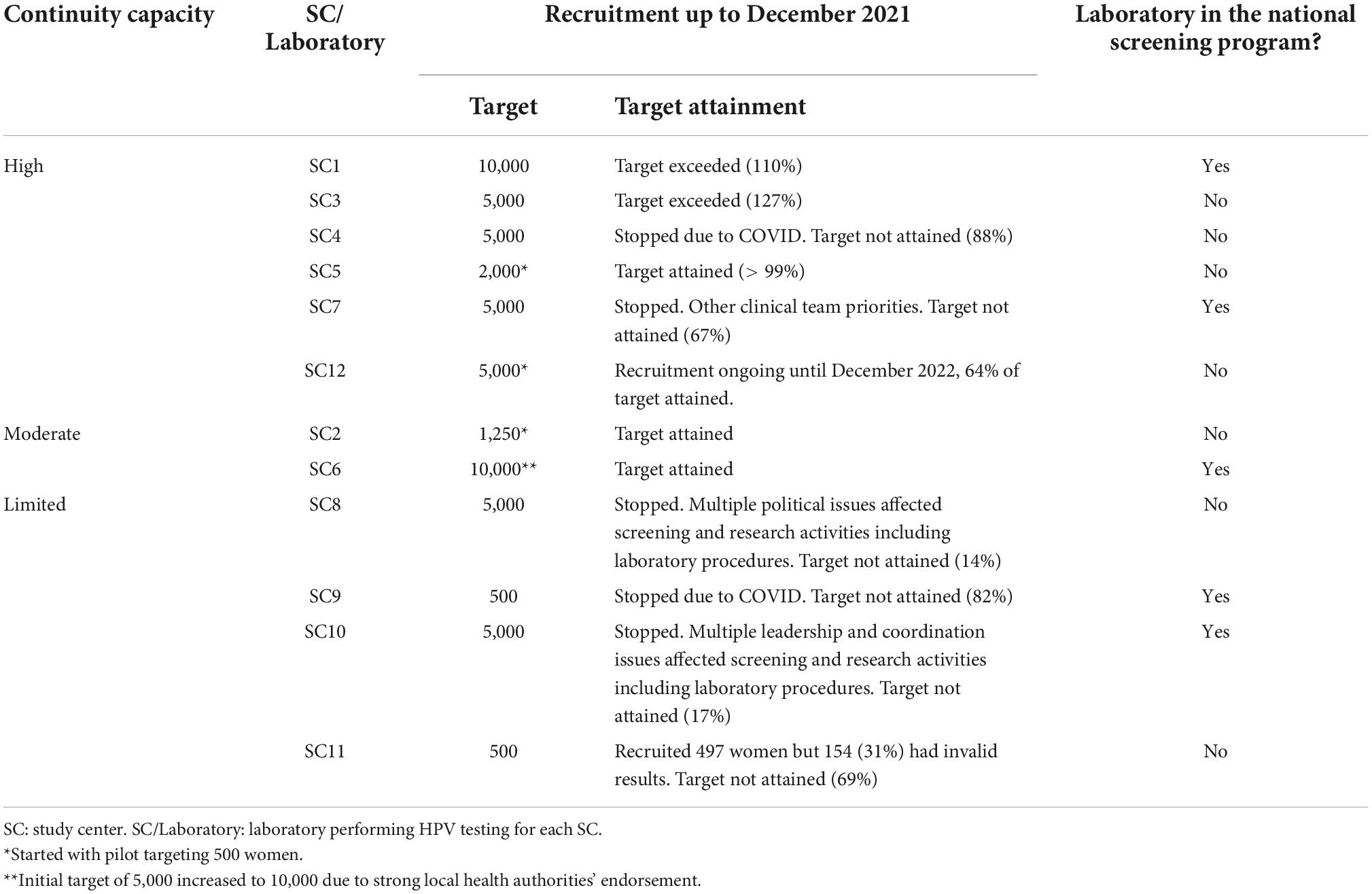
Table 3. Recruitment targets per study center (as proxy of HPV testing volume) and inclusion of laboratories in national screening programs by level of continuity capacity.
Five of the 12 laboratories are now or soon will be part of their national HPV-based screening program, including SC1 and SC7 with high continuity capacity, SC6 (moderate) and despite challenges during the study, SC9 and SC10 with limited continuity capacity based on their nature (national referral public health, highly experience research) and on the experience, increased installed capacity and lessons learnt throughout the study.
Discussion
The WHO recommends screening women with a high-performance test, such as HPV DNA testing, twice by age 35 and 45, and encourages countries to move from cytology- or VIA- to HPV-based cervical screening programs. However, most cervical screening worldwide is done with cytology and transition to HPV-based cervical screening should be carefully planned. There are several important factors to consider, such as the size of target population, the screening approach, women’s acceptance and participation in HPV testing, local availability of HPV testing platforms (equipment, consumables), laboratory installed capacity, availability of trained personnel, quality assurance, and other operational aspects.
In our study, two main factors enabled successful performance of HPV laboratories as part of HPV introduction: the initial assessment of existing laboratory capacity and the regular support offered to participating laboratories throughout the study. The initial assessment identified limitations in the laboratory’s ability to perform HPV testing and helped laboratory teams to develop a road map (staff training, equipment, procurement plan) to address shortcomings before launching the study. In all laboratories, rapid training of new technicians, regardless of their role, was provided in order to minimize disruption of activities. The simple hands-on processing of both HPV tests used in the study (HC2 and COBAS) also helped train new personnel. The training was usually completed within a week, even where staff had no previous HPV testing experience.
The ESTAMPA study aims to evaluate triage techniques for HPV positive women and contribute to the implementation of HPV-based cervical cancer screening in Latin America. Two baseline cervical samples were collected using a cytobrush washed in vials containing PreservCyt medium (PC) to assess the performance of different molecular triage techniques. HC2 testing is incompatible with PC and uses its own collection medium (Digene sample collection medium, STM). This meant that study centers using HC2 had to prepare samples for testing using a conversion kit, adding on average 1.5 full working days per testing batch of 88 samples. Additionally, all laboratories (independently of the HPV test used) were requested to produce aliquots from both PC vials for future evaluation of molecular triage techniques. This process could take up to one full working day. Additionally, screened positives attended a colposcopy visit at which another sample for HPV testing was collected, and those not receiving treatment based on colposcopy and histology results were recalled at 18 months and had another HPV test, and aliquots were produced from samples once HPV tested. In real-life settings, laboratories may have more capacity for HPV testing as these extra research activities will not be required.
The average HPV positivity over time was 14.1%, with some fluctuations related to debut and end of recruitment and testing at each center along the study. We detected HPV positivity values below 10% or above 16% (the inspection threshold) in some centers over time. This triggered an immediate evaluation of the entire screening process by using the study centralized web-system, followed by a site visit to reinforce training and ensure safety of participants as needed. In most cases, low or high HPV positivity was usually associated with characteristics of the included study population. For instance, one center reported a consistently high positivity (>16%), most likely due to the recruitment of a referral population (women with previous abnormal smears attending colposcopy) (data not shown). Assessments suggested HPV testing problems only in one laboratory, where immediate evaluation of the HPV equipment and a plan to maintain good cleaning and calibration of laboratory equipment were requested. This laboratory could not comply with these measures and the study had to stop before reaching the recruitment target.
Readiness for HPV testing was measured ahead of starting recruitment, and study teams whose laboratories were not fully ready to start, took several months (up to 3 years) to deal with shortcomings and they only started recruitment and testing once they fulfilled high readiness requirements. Once testing started, laboratories faced challenges in achieving high continuity capacity for HPV testing. In some study centers, the recruitment rate was unexpectedly low at times, leading to several weeks of delay before enough samples for a whole testing batch were collected, sometimes leading to expiry of reagents before HPV testing and impacting the turnaround of HPV results. Corrective measures such as testing half batches were introduced; however, sometimes, even half-batch testing was not achievable, particularly during the COVID pandemic, and inevitably led to wastage of reagents. In addition, as HPV testing had not been rolled out in almost all participating countries during the study, collection medium and HPV testing reagents needed to be imported as local procurement was not possible. Local principal investigators had to quickly learn regulations to import unregistered products, logistics associated with customs clearance, and different aspects of the procurement process, including price negotiation and management of expiry and stock levels of consumables. Laboratories managers were encouraged to subscribe to international EQA schemes. Six laboratories had yearly subscription to EQA schemes before the start of the study as required by local policies. The study covered the first-year fees for other four laboratories, but only one of them managed to continue with subscriptions afterward. Fees for the remaining two laboratories were not provided by the study nor covered by the laboratory. The main reason for not ensuring funds for EQA, in addition to limited resources, was the lack of a “culture of quality”, as managers did not understand the importance of maintaining regular EQA not only for HPV testing but for other laboratory activities.
We did not observe any relationship between laboratory high continuity capacity and attainment and size of recruitment goals. Of six laboratories with high continuity capacity, two exceeded their original recruitment targets of 10,000 and 5,000 by 10% and 27%, one reached its target (2,000), one is still recruiting (already 64% attained) and two aiming to recruit 5,000 had to stop at 67% and 88% attainment. The two with moderate continuity capacity reached their targets (1,250 and 10,000); and none of the four showing limited continuity capacity completed recruitment, two originally targeted 500 and the other two 5,000 women. Of importance, independently of the level of continuity capacity, five laboratories will become or are already part of their national screening program. For instance, one laboratory with high continuity capacity that stopped recruitment because the HPV testing platform loan ended, and the equipment was retrieved by the manufacturer company is now part of the national program though using a different HPV testing platform but maintaining the laboratory staff trained on HPV testing for the study. The incorporation of these five laboratories into national programs demonstrates that all efforts in training, continued support, and engagement with and between local and regional stakeholders during the study, are certainly contributing to the implementation of HPV-based screening programs in Latin America.
Most difficulties faced by laboratories reflect the early days of the HPV testing market. Currently, more than 250 HPV tests are available on the market, although most of them are not yet adequately validated (14, 39). It is important that manufacturers invest in validating their tests in line with consensus requirements that ensure safe use in clinical settings (40, 41). Notably, several adequately validated tests can be run in small batches or even individual samples and include internal (per sample) controls that can facilitate monitoring testing accuracy (14). Such tests are ideal for scenarios where the target screening population is small, or reaching screening coverage will take a long time, or where access to care is difficult and screening uptake is limited.
The main strengths of our results are: (1) The diversity of laboratories where HPV testing was performed, contributing with valuable information on the multiple challenges that countries may face during transition to, implementation and scale-up of HPV testing in primary screening; (2) The use of regular feedback given by the central coordinating team and most importantly through exchange of experiences and lessons through the strong ESTAMPA study network, which facilitated installing a “culture of quality” in laboratories, and (3) The demonstration that readiness and continuity capacity are good indicators of how HPV implementation is ongoing in terms of laboratory management and quality of HPV testing, and can be assessed using simple checklists and questionnaires. On the other hand, two limitations of our study are related to these simple tools. First, information provided by laboratory researchers or managers may be prone to reporting bias, and second, continuity capacity was assessed based on responses to a single-time questionnaire. Nevertheless, in our study, these limitations were mitigated by completing and verifying information through monitoring visits to all teams and their participating laboratories over time. However, it should be noted that in the context of a screening program, regular monitoring visits may not be feasible and other strategies may be needed to complete implementation assessment.
The 12 laboratories participating in ESTAMPA were essential for study centers screening and overall study achievements, despite several of them being new to HPV testing or to its use in cervical screening and having faced challenges to adopt the technique into daily activities. The strong support provided by the study network facilitated overcoming most difficulties leading to 42,502 HPV screened women across 12 study centers. We have described here the process of introducing HPV testing in 12 laboratories across Latin America, we further plan to use data per HPV testing batch to evaluate and compare HPV performance across laboratories, over study visits (enrolment, colposcopy, 18-months follow-up) and over time.
Conclusion
In summary, our assessments confirm that as high readiness is essential for successfully implementing HPV testing in laboratories; however, high readiness is not sufficient to guarantee HPV testing high continuity capacity. Several aspects to achieve this high continuity capacity should be considered:
(1) A “culture of quality” in laboratories and across the cervical cancer screening spectrum should be established, including regular training on SOPs, robust monitoring and quality assurance systems (with internal and external quality control measures) tailored to the installed capacity and local resources
(2) Using a friendly and tailored to local context database, if possible centralized and web-based, to allow regular monitoring of the overall screening process, including laboratory and clinical activities, and feedback for improvement
(3) Training on preparation of appropriate plans for HPV supplies procurement and stocking and on local regulations on products registration and importation of laboratory supplies should be provided to laboratory managers and leaders of cervical screening programs
(4) Strategies to guarantee reasonable turnaround of HPV results to women should be in place to ensure that any screened woman receives her HPV results and that screened positives receive adequate and timely treatment and follow-up
(5) Exchange of experiences and lessons learnt between multidisciplinary implementers from different settings and countries should be encouraged to apply most suitable implementation strategies according to context and define additional implementation research needs.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the International Agency for Research on Cancer (IEC Project 12-27-A7), the Pan American Health Organization (PAHO) Ethics Committee and Ethical Committees in each of the study participating centers. The informed consent include details on the background, procedures of the study, risks and benefits, statement of confidentiality, specimen use and study staff to contact. The study is considered minimal risk as the procedures are standard practice in cervical cancer screening programs. The patients/participants provided their written informed consent to participate in this study.
Authors contributions
MA and RH conceived the ESTAMPA study and are the principal investigators responsible for its overall conduction. MAP, AF, GS, LM, CT, LF, VV, GV, AC-V, GR, AC, and CW are the local principal investigators responsible for recruitment, clinical management, and data collection. MAP, AF, and GS conceptualized the laboratory aspects of the study and together with MLR, MH, LM, MRP, CT, PH-N, FD, JA-S, MZ, and VV coordinated all laboratory activities including training of new technicians as needed. JL, AP, MB, LM, PM, YC, RC, DC, LG, AR, MR, MLB, and LC performed HPV testing. JV and AB provided statistical support and together with MA, MH, MLR, EL, and AR centrally coordinated the study. MA, RH, NB, and SL contributed to the funding acquisition and local capacity building. MLR and MA wrote the manuscript. MH, AB, JV, SL, NB, and RH contributed to the finalization of the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
This project acknowledges the financial contribution from IARC/WHO; the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP/WHO), a cosponsored programme executed by the World Health Organization (WHO); the Pan American Health Organization (PAHO); the National Cancer Institute (NCI) UH2/3 CA202730; the NCI Center for Global Health; the National Council for Science and Technology (CONACYT) from Paraguay; the National Cancer Institute of Colombia; and all local collaborative institutions. The funders had no role in the design of the study, data collection, analysis, interpretation, writing, or the decision to publish the manuscript.
Acknowledgments
We are very grateful to all the women that participated in the study. We also would like to thank all multidisciplinary teams that conducted the study in Latin America and the many institutions that participated and supported the execution of the study. In particular, we would like to thank Raul Murillo (Instituto Nacional de Cancerología, Colombia, (INC)); Juan Mural (Hospital Nacional Profesor Alejandro Posadas, Argentina); Elena Kasamatsu (Universidad Nacional de Asunción, Paraguay (UNA)); Silvio Tatti (Hospital Clínicas José de San Martin, Argentina); María Luz Miraval (Instituto Nacional de Salud, Peru); Eduardo Payet (Instituto Nacional de Enfermedades Neoplásicas, Peru); Daniel Llanos and Hans Gonzales (Universidad Mayor, Real y Pontificia de San Francisco Xavier de Chuquisaca, Bolivia) for participating and supporting the implementation of the study; Joaquin Gonzalez (Instituto Nacional de Enfermedades Infecciosas-ANLIS “Malbrán”) for laboratory coordination at launching of the study as well as Pilar Hernández (INC) and Hernan Barrios (UNA), Yuly Salgado (INC), Sandra Martinez (INC) and Ivone Brito García (Instituto de Salud Pública de México) for their contribution to HPV screening. Finally, we would like to thank Sandrine Mace from the International Agency for Research on Cancer for her valuable contribution to study logistics, procurement, and study implementation.
Conflict of interest
Author MH was employed by SMS-Oncology, Amsterdam, Netherlands. Author FD was employed by Laboratorio de Patología Oncológica, SAC, Peru.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer or World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer or World Health Organization.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1006038/full#supplementary-material
References
1. Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. (2020) 8:e191–203.
2. Quinn M, Babb P, Jones J, Allen E. Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics. BMJ. (1999) 318:904–8. doi: 10.1136/bmj.318.7188.904
3. Lazcano-Ponce EC, Moss S, Alonso de Ruíz P, Salmerón Castro J, Hernández Avila M. Cervical cancer screening in developing countries: why is it ineffective? The case of Mexico. Arch Med Res. (1999) 30:240–50. doi: 10.1016/s0188-0128(99)00006-8
4. Almonte M, Murillo R, Sanchez GI, Jeronimo J, Salmeron J, Ferreccio C, et al. New paradigms and challenges in cervical cancer prevention and control in Latin America. Salud Publica Mex. (2010) 52:544–59. doi: 10.1590/s0036-36342010000600010
5. Gage JC, Ferreccio C, Gonzales M, Arroyo R, Huivin M, Robles SC. Follow-up care of women with an abnormal cytology in a low-resource setting. Cancer Detect Prev. (2003) 27:466–71. doi: 10.1016/j.cdp.2003.09.004
6. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. (1999) 189:12–9.
7. Herrero R, Ferreccio C, Salmeron J, Almonte M, Sanchez GI, Lazcano-Ponce E, et al. New approaches to cervical cancer screening in Latin America and the Caribbean. Vaccine. (2008) 26 Suppl. 11:L49–58.
8. Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. (2008) 26 Suppl. 10:K29–41. doi: 10.1016/j.vaccine.2008.06.019
9. Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. (2006) 119:1095–101. doi: 10.1002/ijc.21955
10. Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. (2011) 103:368–83.
11. Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. (2012) 30 Suppl. 5:F88–99.
12. Rijkaart DC, Berkhof J, Rozendaal L, van Kemenade FJ, Bulkmans NW, Heideman DA, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. (2012) 13:78–88. doi: 10.1016/S1470-2045(11)70296-0
13. Gilham C, Sargent A, Kitchener HC, Peto J. HPV testing compared with routine cytology in cervical screening: long-term follow-up of ARTISTIC RCT. Health Technol Assess. (2019) 23:1–44. doi: 10.3310/hta23280
14. Arbyn M, Simon M, Peeters E, Xu L, Meijer C, Berkhof J, et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin Microbiol Infect. (2021) 27:1083–95. doi: 10.1016/j.cmi.2021.04.031
15. Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. (2018) 363:k4823.
16. Cuzick J, Cadman L, Mesher D, Austin J, Ashdown-Barr L, Ho L, et al. Comparing the performance of six human papillomavirus tests in a screening population. Br J Cancer. (2013) 108:908–13. doi: 10.1038/bjc.2013.22
17. Campos NG, Alfaro K, Maza M, Sy S, Melendez M, Masch R, et al. The cost-effectiveness of human papillomavirus self-collection among cervical cancer screening non-attenders in El Salvador. Prev Med. (2020) 131:105931. doi: 10.1016/j.ypmed.2019.105931
18. Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. (2008) 337:a1754. doi: 10.1136/bmj.a1754
19. Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. (2014) 383:524–32. doi: 10.1016/S0140-6736(13)62218-7
20. World Health Assemby [WHA]. World Health Assembly Adopts Global Strategy to Accelerate Cervical Cancer Elimination. (2020). Available online at: https://www.who.int/news/item/19-08-2020-world-health-assembly-adopts-global-strategy-to-accelerate-cervical-cancer-elimination
21. World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Geneva: World Health Organization (2020).
22. World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention. 2nd ed. Geneva: World Health Organization (2021).
23. Broutet N, Jeronimo J, Kumar S, Almonte M, Murillo R, Huy NVQ, et al. Implementation research to accelerate scale-up of national screen and treat strategies towards the elimination of cervical cancer. Prev Med. (2022) 155:106906. doi: 10.1016/j.ypmed.2021.106906
24. Wentzensen N, Arbyn M. HPV-based cervical cancer screening- facts, fiction, and misperceptions. Prev Med. (2017) 98:33–5. doi: 10.1016/j.ypmed.2016.12.040
25. Maza M, Alfaro K, Garai J, Velado MM, Gage JC, Castle PE, et al. Cervical cancer prevention in El Salvador (CAPE)-An HPV testing-based demonstration project: changing the secondary prevention paradigm in a lower middle-income country. Gynecol Oncol Rep. (2017) 20:58–61. doi: 10.1016/j.gore.2017.02.011
26. Arrossi S, Thouyaret L, Laudi R, Marin O, Ramirez J, Paolino M, et al. Implementation of HPV-testing for cervical cancer screening in programmatic contexts: the Jujuy demonstration project in Argentina. Int J Cancer. (2015) 137:1709–18.
27. Lazcano-Ponce E, Lorincz AT, Torres L, Salmeron J, Cruz A, Rojas R, et al. Specimen self-collection and HPV DNA screening in a pilot study of 100,242 women. Int J Cancer. (2014) 135:109–16. doi: 10.1002/ijc.28639
28. Holme F, Jeronimo J, Maldonado F, Camel C, Sandoval M, Martinez-Granera B, et al. Introduction of HPV testing for cervical cancer screening in central America: the scale-up project. Prev Med. (2020) 135:106076. doi: 10.1016/j.ypmed.2020.106076
29. Alfaro K, Maza M, Felix JC, Gage JC, Castle PE, Alonzo TA, et al. Outcomes for step-wise implementation of a human papillomavirus testing-based cervical screen-and-treat program in El Salvador. JCO Glob Oncol. (2020) 6:1519–30. doi: 10.1200/GO.20.00206
30. World Health Organization. Human Papillomavirus Laboratory Manual. Geneva: World Health Organization (2009).
31. World Health Organization. Comprehensive Cervical Cancer Control: a Guide to Essential Practice. 2nd ed. Geneva: World Health Organization (2014).
32. Pan American Health Organization. Integrating HPV Testing in Cervical Cancer Screening Programs. A Manual for Program Managers. Washington, DC: Pan American Health Organization (2016).
33. World Health Organization. Introducing and Scaling up Testing for Human Papillomavirus as Part of a Comprehensive Programme for Prevention and Control of Cervical Cancer: a Step-by-Step Guide. Geneva: World Health Organization (2020).
34. Almonte M, Murillo R, Sanchez GI, Gonzalez P, Ferrera A, Picconi MA, et al. Multicentric study of cervical cancer screening with human papillomavirus testing and assessment of triage methods in Latin America: the ESTAMPA screening study protocol. BMJ Open. (2020) 10:e035796. doi: 10.1136/bmjopen-2019-035796
35. Robles C, Wiesner C, Martinez S, Salgado Y, Hernandez M, Lucas E, et al. Impact of operational factors on HPV positivity rates in an HPV-based screening study in Colombia. Int J Gynaecol Obstet. (2018) 143:44–51. doi: 10.1002/ijgo.12574
36. Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the international agency for research on cancer HPV prevalence surveys: a pooled analysis. Lancet. (2005) 366:991–8. doi: 10.1016/S0140-6736(05)67069-9
37. de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. (2007) 7:453–9.
38. Almonte M, Albero G, Molano M, Carcamo C, Garcia PJ, Perez G. Risk factors for human papillomavirus exposure and co-factors for cervical cancer in Latin America and the Caribbean. Vaccine. (2008) 26 Suppl. 11:L16–36. doi: 10.1016/j.vaccine.2008.06.008
39. Poljak M, Kocjan BJ, Ostrbenk A, Seme K. Commercially available molecular tests for human papillomaviruses (HPV): 2015 update. J Clin Virol. (2016) 76 Suppl. 1:S3–13.
40. Arbyn M, Depuydt C, Benoy I, Bogers J, Cuschieri K, Schmitt M, et al. VALGENT: a protocol for clinical validation of human papillomavirus assays. J Clin Virol. (2016) 76 Suppl. 1:S14–21.
Keywords: HPV testing, HPV testing implementation, readiness and continuity capacity, ESTAMPA study, cervical cancer screening, Latin America
Citation: Rol ML, Picconi MA, Ferrera A, Sánchez GI, Hernández MdlL, Lineros J, Peraza A, Brizuela M, Mendoza L, Mongelós P, Cabrera Y, Rodríguez de la Peña M, Correa RM, Terán C, Colque Reynaga D, García L, Ramírez AT, Hernández-Nevarez P, Doimi F, Ramón M, Arias-Stella J, Zúñiga M, Villagra V, Bobadilla ML, Cardinal L, Valls J, Lucas E, Baena A, Fleider L, Venegas G, Cruz-Valdez A, Rodríguez G, Calderón A, Wiesner C, Luciani S, Broutet N, Herrero R and Almonte M (2022) Implementing HPV testing in 9 Latin American countries: The laboratory perspective as observed in the ESTAMPA study. Front. Med. 9:1006038. doi: 10.3389/fmed.2022.1006038
Received: 28 July 2022; Accepted: 24 October 2022;
Published: 17 November 2022.
Edited by:
Redhwan Ahmed Al-Naggar, National University of Malaysia, MalaysiaReviewed by:
Raquel Ibañez, Catalan Institute of Oncology, SpainJosé Eduardo Levi, University of São Paulo, Brazil
Copyright © 2022 Rol, Picconi, Ferrera, Sánchez, Hernández, Lineros, Peraza, Brizuela, Mendoza, Mongelós, Cabrera, Rodríguez de la Peña, Correa, Terán, Colque Reynaga, García, Ramírez, Hernández-Nevarez, Doimi, Ramón, Arias-Stella, Zúñiga, Villagra, Bobadilla, Cardinal, Valls, Lucas, Baena, Fleider, Venegas, Cruz-Valdez, Rodríguez, Calderón, Wiesner, Luciani, Broutet, Herrero and Almonte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maribel Almonte, almontem@who.int
 Mary Luz Rol
Mary Luz Rol María Alejandra Picconi2
María Alejandra Picconi2  Annabelle Ferrera
Annabelle Ferrera Laura Mendoza
Laura Mendoza Yessy Cabrera
Yessy Cabrera María Ramón
María Ramón Michael Zúñiga
Michael Zúñiga Verónica Villagra
Verónica Villagra María Liz Bobadilla
María Liz Bobadilla Joan Valls
Joan Valls Laura Fleider
Laura Fleider Gino Venegas
Gino Venegas Rolando Herrero
Rolando Herrero Maribel Almonte
Maribel Almonte