Association Between Uric Acid and Worsening Peripheral Microangiopathy in Systemic Sclerosis
- 1Fourth Department of Internal Medicine, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 2First Department of Cardiology, Hippokration General Hospital, National and Kapodistrian University of Athens, Athens, Greece
- 3International Hellenic University, Thessaloniki, Greece
- 4Department of Rheumatology, Dudley Group of Hospitals, NS Foundation Trust, Dudley, United Kingdom
- 5Arthritis Research UK Centre for Epidemiology, University of Manchester, Manchester, United Kingdom
Objective: The key element in the pathogenesis of systemic sclerosis (SSc) is microcirculatory changes in several vascular beds. Uric acid is associated with endothelial dysfunction and therefore, microvascular damage. The aim of this study was to examine the association between uric acid (UA) and peripheral microvascular involvement in patients with SSc.
Methods: We included consecutive, consenting patients with SSc. Serum UA, urea and creatinine were measured, and glomerular filtration rate (GFR) was calculated with CKD-EPI. All participants underwent nailfold video-capillaroscopy (NVC) to evaluate the microcirculation.
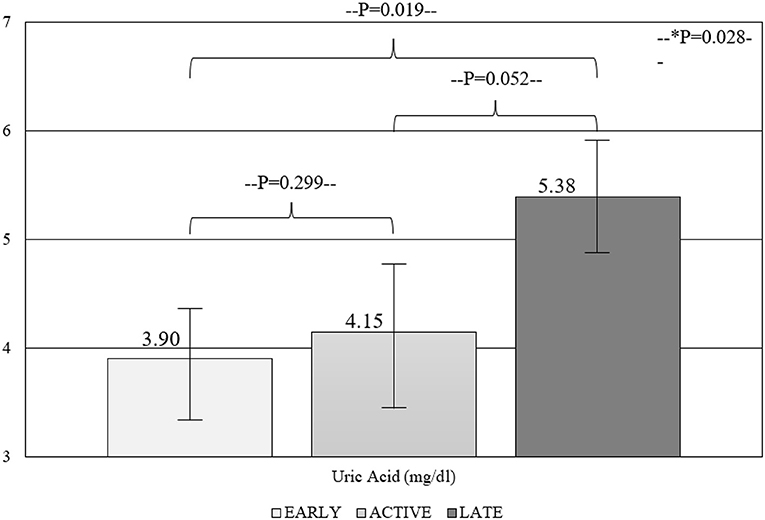
Results: A total of 64 patients (95.3% women) were included in the study. UA levels were significantly associated with the number of avascular areas (r = 0.290; p = 0.020), whereas no correlation was shown for the GFR (r = −0.065; p = 0.609). A significant trend of UA in the three capillaroscopic patterns was shown (3.90 ± 1.52 vs. 4.15 ± 0.98 vs. 5.38 ± 2.26; for early, active, and late patterns respectively, p = 0.028). Multivariate analysis showed that male gender (β = 3.049; 95% CI = 0.997–5.101) and UA (β = 0.352; 95% CI = 0.117–0.588) were independently associated with the number of avascular areas.
Conclusion: These data suggest that UA levels are significantly associated with the capillaroscopic patterns, reflecting a progressive microvasculopathy.
Introduction
In systemic sclerosis (SSc), inflammation and microvascular dysfunction appear to be the main events that progressively stimulate fibrotic process. The precise etiology of fibrotic changes remains partially understood and may include impaired communication between endothelial cells, epithelial cells and fibroblasts, lymphocyte activation, autoantibody production, inflammation, and connective tissue fibrosis (1). Alterations in microvasculature are considered the hallmark of SSc vascular involvement and occur in the first stages of the disease. Given the heterogenicity of clinical symptoms and organ involvement, there is an ongoing effort to establish biomarkers for the evaluation of microvasculopathy (2), which represents one of the earliest clinical manifestations of SSc presented in various guises such as Raynaud's phenomenon, digital ulcers, and pulmonary arterial hypertension (PAH) (3).
Uric acid (UA) is the final oxidation product of purine metabolism. Elevated serum UA levels have been associated with endothelial dysfunction, possibly by decreasing nitric oxide availability (4) and stimulating vascular smooth muscle cell proliferation leading to arterial stiffness, and gradually, widespread microvascular damage (5–7). UA levels have been found elevated in SSc patients and have been associated with the presence of vascular complications including pulmonary arterial hypertension (PAH) (8) digital ulcers (9) and abnormal findings in nailfold video capillaroscopy (NVC) (10); the latter is a non-invasive and reproducible imaging technique of the capillary vascular bed, used for the assessment of peripheral microvascular damage in SSc. It is extensively used in the differentiation between primary and secondary Raynaud's phenomenon in daily practice. “Abnormal nailfold capillaries” (when referring to the “scleroderma pattern”) are included in the 2013 American College of Rheumatology (ACR)/ European League Against Rheumatism (EULAR) classification criteria for SSc (11). Cumulative data suggest that NVC measurements can serve as a reliable marker of the extent and severity of microvasculopathy in different vascular districts such as pulmonary (12) and myocardial microcirculation (13) to the point that NVC is currently considered as a surrogate marker of SSc progression (14). However, the association between UA and NVC changes in SSc has not been well-established.
In this context, the aim of this study was to investigate the potential relationship between UA levels and microvascular alterations assessed by NVC in a large well-characterized cohort of SSc patients.
Materials and Methods
Study Participants and Inclusion / Exclusion Criteria
The study included consecutive patients with SSc attending the Scleroderma Clinic of the Fourth Department of Internal Medicine, Hippokration General Hospital, Thessaloniki, Greece, between March 2018 and September 2020, who were screened for the study. All patients satisfied the revised EULAR/ACR criteria for the diagnosis of SSc (11). The exclusion criteria included past diagnosis of cardiovascular disease defined as coronary heart disease, stroke, or peripheral vascular disease, diabetes mellites, as well as patients with carotid artery surgical procedures. Patients on diuretics were also excluded. The study had ethics approval from the Ethics Committee of the School of Medicine, Aristotle University of Thessaloniki and written informed consent was obtained from all participants according to the Declaration of Helsinki.
Protocol Overview
All participants underwent a thorough physical examination and demographic data were collected by a questionnaire. Complete medical history was also recorded which included the duration of the disease, existence of pulmonary hypertension, pulmonary fibrosis, or esophageal motility disorders, as documented by imaging or endoscopic examination, respectively, as well as medication and cardiovascular risk factors (smoking, hypertension).
Parameters of Interest and Definitions
Various hematological and biochemical laboratory parameters such as routine biochemistry and hematology, lipid and bone profile tests, inflammatory markers such as erythrocyte sedimentation rate (ESR) and Creactive protein (CRP), immunological markers such as antinuclear antibodies (ANA), anti-centromeric antibodies (ACA), and anti-topoisomerase IIa (anti-scl-70) antibodies were tested. Serum UA and creatinine levels were measured with photometric measurement of the solution and GRF was calculated using the CKD-EPI equation (15). NVC assessment and blood sampling were performed the same day. Blood pressure was recorded according to 2018 ESC/ESH Guidelines for the management of arterial hypertension, with a validated oscillometric device (16). Arterial hypertension was defined on the basis of the patients' history (self-reposted hypertension) and/or antihypertensive medication intake.
NVC Assessment
Study participants underwent NVC using an Optilia Digital Capillaroscope and a 200 × contact lens and the photos collected were analyzed with OptiPix Capillaroscopy (Sollentuna, Sweden) software system. Prior to performing the test, patients were placed in a quiet environment at a temperature between 20 and 25°C. A drop of cedar oil was placed on each fingernail prior to observation for better image analysis. The findings were classified in one of the following qualitative patterns: early, active, and late NVC pattern (17). The “early” pattern was characterized by a few enlarged or giant capillaries and relatively well-preserved capillary distribution; the “active” pattern was characterized by numerous giant capillaries, mildly disturbed capillary architecture, and moderate capillary loss; the “late” type was characterized by severe capillary loss with extensive vascular desertification, and disruption of normal capillary architecture. NVC parameters to be measured were capillary density (number of capillaries per 1 mm in the distal row of each finger), giant capillaries (homogeneously enlarged capillaries >50 μm), enlarged capillaries (>20 μm and ≤ 50 μm), micro-bleeding, oedema, avascular areas (the normal range adopted was 9 capillaries per linear millimeter), ramified capillaries, bushy and tortuous capillaries. Capillary's density was evaluated in the distal row of each finger, based on the number of capillaries per 1 mm, and the mean capillaroscopic skin ulcer risk index (CSURI) was automatically calculated with software image analysis.
Statistical Analysis
Statistical analysis was performed with Statistical Package for Social Sciences 22 (SPSS Inc, Chicago, IL, USA). Continuous variables were expressed as mean values ± standard deviation (SD) or median [interquartile range] according to the normality of distribution tested with the Kolmogorov-Smirnov or the Shapiro-Wilk test. Categorical variables were presented as absolute frequencies and percentages (n, %). Comparisons for continuous variables were performed with the student's t-test or the Mann-Whitney U test, according to the normality of the distribution. Multiple comparison analysis was performed with the ANOVA or the Kruskal-Wallis tests, according to normality. Chi-square or Fishers exact test was used for comparisons of categorical variables. Uni- and multivariable linear regression analysis was performed to explore the parameters possibly associated with the number of avascular areas. We first tested for univariate associations and included in the multivariable model only variables with associations of p < 0.2 in univariate analysis. We report β-coefficients with 95% confidence intervals (CI). P < 0.05 (two-tailed) were considered statistically significant for all comparisons.
Results
Patient Characteristics
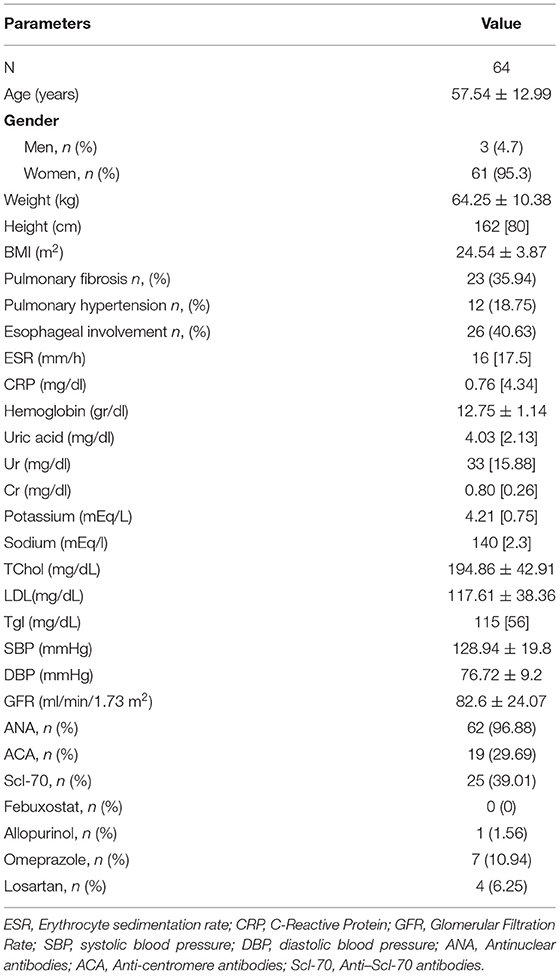
Table 1 depicts demographic, anthropometric, clinical, and laboratory characteristics of the study population. In total, 64 Caucasian patients with SSc (95.3% women) with mean age 57.54 ± 12.99 years were included in the study. The number of capillaries per mm were 6 [4], avascular areas were 2 [3] and CSURI index 3.25 [6.57].
Uric Acid and NVC Measurements
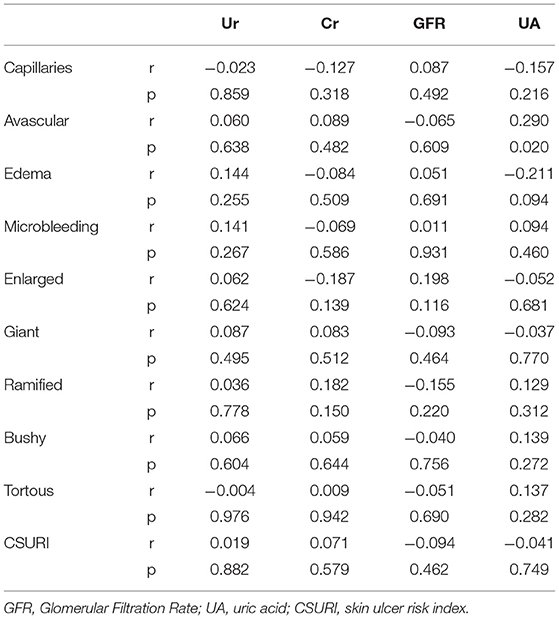
The correlation of UA, urea, creatinine, and GFR with the parameters of NVC is presented in Table 2. Serum UA levels were significantly associated with the number of avascular areas (r = 0.290; p = 0.020), whereas no correlation was shown for the eGFR (r = −0.065; p = 0.609). All the other NVC parameters measured showed no correlation with the levels of UA. There was no significant correlation with CSURI (r = −0.041; p = 0.749).
Within-groups comparisons revealed a significant trend of UA levels in the capillaroscopy patterns reflective of progressive micro-vasculopathy (3.90 ± 1.52 vs. 4.15 ± 0.98 vs. 5.38 ± 2.26; for early, active, and late patterns, respectively, p = 0.028) (Figure 1). Similarly, the comparison between different NVC pattern groups demonstrated higher UA levels in patients with late compared to early and active patterns (p = 0.019 and p = 0.052), while the degree of NVC changes was not associated with creatinine or eGFR levels.
Parameters Associated With Avascular Areas in NVC
In order to identify possible factors associated with the avascular areas in NVC, we have performed a uni- and multivariable linear regression analysis with a number of avascular areas being the dependent variable and several demographic, anthropometric, medical history, and laboratory parameters as the independent variables. As shown in Table 3, in multivariable analysis male gender (β = 3.049; 95% CI = 0.997–5.101) and UA (β = 0.352; 95% CI = 0.117–0.588) were shown to be independently associated with the number of avascular areas, whereas smoking was not.
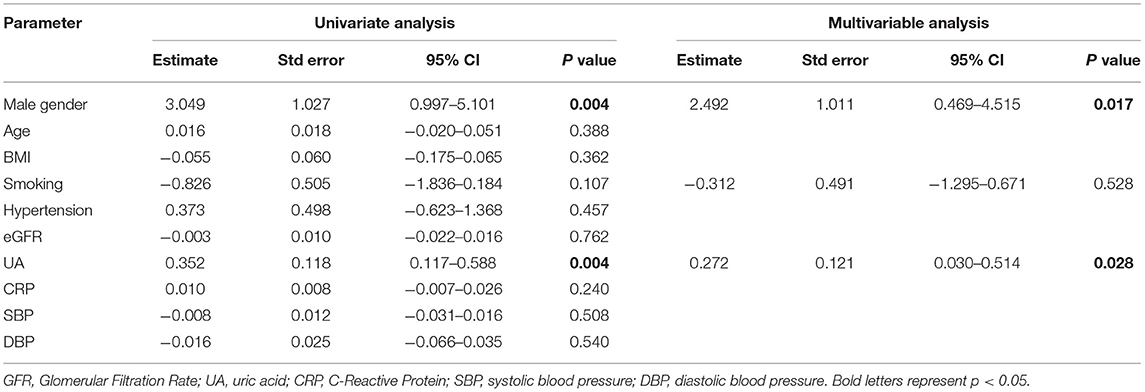
Table 3. Uni- and multivariable linear regression analysis of parameters possibly associated with the number of avascular areas as evidenced with the capillaroscopy.
Discussion
The main finding of our study is the positive correlation between NVC structural alterations and serum UA concentrations in patients with SSc. In particular advancing stages of SSc-related microangiopathy as determined by both NVC “scleroderma patterns” and the number of avascular areas are associated with higher UA levels, indicating UA as a potential biomarker of peripheral vascular damage in SSc.
This possibility may reflect biologically relevant metabolic procedures involved in the pathogenesis and progression of SSc-related microvasculopathy. For example degradation of UA by the oxidative action of the enzyme xanthine oxidoreductase leads to the production of reactive oxygen species and the initiation of several detrimental procedures such as increased cytokine production, inflammation, and endothelial activation all of which contribute to vascular injury (18). Taking into account that SSc is regarded as a disease of increased oxidative stress (19), upregulation of oxygen free radicals and low antioxidant defense capacity driven by increased UA levels (20) may play a crucial role in the pathophysiology of microangiopathy.
Thus, it is not surprising that previous reports have demonstrated significant associations between high UA levels and various aspects of SSc vasculopathy. In fact elevated UA levels do not only confer a diagnostic value in the identification of SSc individuals at early, asymptomatic stages of PAH as indicated by DETECT study (21), but they also serve as a useful biochemical tool for the assessment of PAH severity (22) and outcomes as they appear to be associated with survival in these patients (23). Besides pulmonary microvasculature, high UA levels were independently associated with the occurrence of digital ulcers in a cross-sectional study including 71 persons with SSc (9) providing another link between peripheral microangiopathy and oxidative stress in this condition. In addition, UA levels have been linked with the extent of renal microvasculopathy in SSc defined as increased intrarenal stiffness (10).
Our findings are in line with a previous small study (10) and confirm the positive correlation between UA levels and peripheral microvascular damage in SSc individuals. Furthermore, we demonstrated that UA levels are increasing in correlation with progressive stages of SSc microangiopathy, particularly in the presence of late (worse) NVC pattern suggesting that UA may contribute to the evolution of microcirculatory abnormalities in SSc. However, the cross-sectional design of all the aforementioned studies—including the current one—does not allow the establishment of any temporal relationships between UA and rarefaction of digital arteries in SSc. Whether such observations indicate a reverse causality, implying that elevated UA levels may not have a direct crucial effect on endothelial derangement and subsequent vascular injury, but they rather represent an easily measurable byproduct of oxidative stress remains to be determined in longitudinal or experimental studies.
On the other hand, the relationship between high UA and various markers of microvascular dysfunction in different vascular beds namely retinal arteriolar narrowing (24), microalbuminuria (25) and coronary flow reserve (26) has been described in previous studies especially in patients with higher cardiovascular risk profile. Considering the increased rate of cardiovascular events such as stoke and myocardial infarction among SSc patients (27) and the well-established contribution of hyperuricemia to the occurrence and development of cardiovascular disease in both rheumatic diseases and general population (28–30), the results of our study provide further insights in the complex association between micro- and macro vascular involvement in SSc. A growing amount of evidence point toward significant correlations between higher grades of SSc microangiopathy and indices of cardiovascular disease such as arterial stiffness (31) and cardiomyopathy (13, 32). Interestingly enough, a recent study by our group indicated that worsening phases of NVC patterns were associated with higher cardiovascular risk scores (33) suggesting that excessive capillary loss and macrovascular endothelial dysfunction may be closely interrelated and promote cardiovascular disease in this population. In this regard, the demonstrated association between progressive microvascular injury and higher UA levels may indicate UA as a marker of generalized, widespread vasculopathy in SSc including both micro- and microvasculature.
The main limitation of our study is the single-center, cross-sectional design which precludes any causal relationships between UA levels and vasculopathy as discussed above. However, NVC was performed in all fingers except thumbs and the acquisition of two adjacent images from each finger according to the updated European League against Rheumatism (EULAR) recommendations (34). We took particular care to include individuals with SSc, with a broad spectrum of disease-related visceral involvement as well as comorbidities representative of a real-life population with SSc. To the best of our knowledge, this is the largest study to investigate the association between UA and microvascular injury by detailed qualitative and semi-quantitative assessment based on a validated algorithm.
In conclusion, serum levels of UA are significantly associated with progressive micro-vasculopathy based on the qualitative NVC pattern. These results provide evidence that UA may be a significant mediator of the microvascular damage in these patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of School of Medicine, Aristotle University Thessaloniki, Greece. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
EP collected the data and drafted the paper with support from TD. SS contributed to the perception of the study and the editing of the paper. ET collected the data. AM verified the analytical methods and performed the analysis. GK and AG were involved in planning and supervised the work and critically reviewed the paper. TD supervised the project and contributed to drafting the paper. All authors discussed the results and contributed to the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abraham D, Distler O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther. (2007) 9 (Suppl. 2):S2. doi: 10.1186/ar2186
2. Ram Poudel D, George M, Dhital R, Karmacharya P, Sandorfi N, Derk CT. Mortality, length of stay and cost of hospitalization among patients with systemic sclerosis: results from the National Inpatient Sample. Rheumatology. (2018) 57:1611–22. doi: 10.1093/rheumatology/key150
3. Denton CP, Khanna D. Systemic sclerosis. Lancet. (2017) 390:1685–99. doi: 10.1016/S0140-6736(17)30933-9
4. Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. (2005) 25:39–42. doi: 10.1016/j.semnephrol.2004.09.007
5. Kim H, Kim SH, Choi AR, Kim S, Choi HY, Kim HJ, et al. Asymptomatic hyperuricemia is independently associated with coronary artery calcification in the absence of overt coronary artery disease: a single-center cross-sectional study. Medicine. (2017) 96:e6565. doi: 10.1097/MD.0000000000006565
6. Ding XH, Wang X, Cao R, Yang X, Xiao W, Zhang Y, et al. A higher baseline plasma uric acid level is an independent predictor of arterial stiffness: a community-based prospective study. Medicine. (2017) 96:e5957. doi: 10.1097/MD.0000000000005957
7. Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol. (2012) 59:235–42. doi: 10.1016/j.jjcc.2012.01.013
8. Dimitroulas T, Giannakoulas G, Dimitroula H, Sfetsios T, Parcharidou D, Karvounis H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int. (2011) 31:263–7. doi: 10.1007/s00296-010-1557-4
9. Kim E, Lee HN, Kim YK, Kim GT, So MW, Ahn E, et al. Increased serum uric acid levels are associated with digital ulcers in patients with systemic sclerosis. Rheumatol Int. (2019) 39:255–63. doi: 10.1007/s00296-019-04240-9
10. Gigante A, Barbano B, Barilaro G, Quarta S, Gasperini ML, Di Mario F, et al. Serum uric acid as a marker of microvascular damage in systemic sclerosis patients. Microvasc Res. (2016) 106:39–43. doi: 10.1016/j.mvr.2016.03.007
11. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. (2013) 72:1747–55. doi: 10.1136/annrheumdis-2013-204424
12. Minopoulou I, Theodorakopoulou M, Boutou A, Arvanitaki A, Pitsiou G, Doumas M, et al. Nailfold capillaroscopy in systemic sclerosis patients with and without pulmonary arterial hypertension: a systematic review and meta-analysis. J Clin Med. (2021) 10:1528. doi: 10.3390/jcm10071528
13. Zanatta E, Famoso G, Boscain F, Montisci R, Pigatto E, Polito P, et al. Nailfold avascular score and coronary microvascular dysfunction in systemic sclerosis: a newsworthy association. Autoimmun Rev. (2019) 18:177–83. doi: 10.1016/j.autrev.2018.09.002
14. Soulaidopoulos S, Triantafyllidou E, Garyfallos A, Kitas GD, Dimitroulas T. The role of nailfold capillaroscopy in the assessment of internal organ involvement in systemic sclerosis: a critical review. Autoimmun Rev. (2017) 16:787–95. doi: 10.1016/j.autrev.2017.05.019
15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
16. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. (2018) 36:1953–2041. doi: 10.1097/HJH.0000000000001940
17. Cutolo M, Sulli A, Smith V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol. (2010) 6:578–87. doi: 10.1038/nrrheum.2010.104
18. Sharaf El Din UAA, Salem MM, Abdulazim DO. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: a review. J Adv Res. (2017) 8:537–48. doi: 10.1016/j.jare.2016.11.004
19. Abdulle AE, Diercks GFH, Feelisch M, Mulder DJ, van Goor H. The role of oxidative stress in the development of systemic sclerosis related vasculopathy. Front Physiol. (2018) 9:1177. doi: 10.3389/fphys.2018.01177
20. Cruz-Domínguez MP, Montes-Cortes DH, Olivares-Corichi IM, Vera-Lastra O, Medina G, Jara LJ. Oxidative stress in Mexicans with diffuse cutaneous systemic sclerosis. Rheumatol Int. (2013) 33:2261–7. doi: 10.1007/s00296-013-2701-8
21. Coghlan JG, Denton CP, Grünig E, Bonderman D, Distler O, Khanna D, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. (2014) 73:1340–9. doi: 10.1136/annrheumdis-2013-203301
22. Wang J, Wang Y, Li X, Huang Y, Sun X, Wang Q, et al. Serum uric acid is associated with disease severity and may predict clinical outcome in patients of pulmonary arterial hypertension secondary to connective tissue disease in Chinese: a single-center retrospective study. BMC Pulm Med. (2020) 20:272. doi: 10.1186/s12890-020-01309-1
23. Simpson CE, Damico RL, Hummers L, Khair RM, Kolb TM, Hassoun PM, et al. Serum uric acid as a marker of disease risk, severity, and survival in systemic sclerosis-related pulmonary arterial hypertension. Pulm Circ. (2019) 9:2045894019859477. doi: 10.1177/2045894019859477
24. Yuan Y, Ikram MK, Jiang S, Lin H, Ren L, Yan H, et al. Hyperuricemia accompanied with changes in the retinal microcirculation in a Chinese high-risk population for diabetes. Biomed Environ Sci. (2011) 24:146–54. doi: 10.3967/0895-3988.2011.02.009
25. Oh CM, Park SK, Ryoo JH. Serum uric acid level is associated with the development of microalbuminuria in Korean men. Eur J Clin Invest. (2014) 44:4–12. doi: 10.1111/eci.12180
26. Kuwahata S, Hamasaki S, Ishida S, Kataoka T, Yoshikawa A, Orihara K, et al. Effect of uric acid on coronary microvascular endothelial function in women: association with eGFR and ADMA. J Atheroscler Thromb. (2010) 17:259–69. doi: 10.5551/jat.1594
27. Cen X, Feng S, Wei S, Yan L, Sun L. Systemic sclerosis and risk of cardiovascular disease: A PRISMA-compliant systemic review and meta-analysis of cohort studies. Medicine. (2020) 99:e23009. doi: 10.1097/MD.0000000000023009
28. Zhao L, Cao L, Zhao TY, Yang X, Zhu XX, Zou HJ, et al. Cardiovascular events in hyperuricemia population and a cardiovascular benefit-risk assessment of urate-lowering therapies: a systematic review and meta-analysis. Chin Med J. (2020) 133:982–93. doi: 10.1097/CM9.0000000000000682
29. Cox P, Gupta S, Zhao SS, Hughes DM. The incidence and prevalence of cardiovascular diseases in gout: a systematic review and meta-analysis. Rheumatol Int. (2021) 41:1209–19. doi: 10.1007/s00296-021-04876-6
30. Daoussis D, Kitas GD. Uric acid and cardiovascular risk in rheumatoid arthritis. Rheumatology. (2011) 50:1354–5. doi: 10.1093/rheumatology/keq388
31. Soulaidopoulos S, Pagkopoulou E, Katsiki N, Triantafyllidou E, Karagiannis A, Garyfallos A, et al. Arterial stiffness correlates with progressive nailfold capillary microscopic changes in systemic sclerosis: results from a cross-sectional study. Arthritis Res Ther. (2019) 21:253. doi: 10.1186/s13075-019-2051-3
32. Markusse IM, Meijs J, de Boer B, Bakker JA, Schippers HPC, Schouffoer AA, et al. Predicting cardiopulmonary involvement in patients with systemic sclerosis: complementary value of nailfold videocapillaroscopy patterns and disease-specific autoantibodies. Rheumatology. (2017) 56:1081–8. doi: 10.1093/rheumatology/kew402
33. Pagkopoulou E, Soulaidopoulos S, Triantafyllidou E, Arvanitaki A, Katsiki N, Loutradis C, et al. Peripheral microcirculatory abnormalities are associated with cardiovascular risk in systemic sclerosis: a nailfold video capillaroscopy study. Clin Rheumatol. (2021) 40:4957–68. doi: 10.1007/s10067-021-05795-4
Keywords: uric acid, nailfold video-capillaroscopy, microvasculopathy, systemic sclerosis, cardiovascular
Citation: Pagkopoulou E, Soulaidopoulos S, Triantafyllidou E, Malliari A, Kitas GD, Garyfallos A and Dimitroulas T (2021) Association Between Uric Acid and Worsening Peripheral Microangiopathy in Systemic Sclerosis. Front. Med. 8:806925. doi: 10.3389/fmed.2021.806925
Received: 01 November 2021; Accepted: 15 November 2021;
Published: 24 December 2021.
Edited by:
Eleni Gavriilaki, G. Papanikolaou General Hospital, GreeceReviewed by:
Panagiotis Dolgyras, G. Papanikolaou General Hospital, GreeceNikolaos Koletsos, University Hospital of Ioannina, Greece
Copyright © 2021 Pagkopoulou, Soulaidopoulos, Triantafyllidou, Malliari, Kitas, Garyfallos and Dimitroulas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theodoros Dimitroulas, dimitroul@hotmail.com
 Eleni Pagkopoulou1
Eleni Pagkopoulou1  Stergios Soulaidopoulos
Stergios Soulaidopoulos Alexandros Garyfallos
Alexandros Garyfallos Theodoros Dimitroulas
Theodoros Dimitroulas