Case Series: Video-Assisted Minimally Invasive Cardiac Surgery During Pregnancy
- 1Department of Anesthesiology, Guangdong Cardiovascular Institute & Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2College of Medicine, Shantou University, Shantou, China
- 3Department of Operation Room, Guangdong Cardiovascular Institute & Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 4Department of Anesthesiology, Linzhi People's Hospital, Linzhi, China
Surgical intervention is expected to improve maternal outcomes in pregnant patients with heart disease once the conservative treatment fails. For pregnant patients with heart disease, the risk of cardiac surgery under cardiopulmonary bypass (CPB) must be balanced due to the high fetal loss. The video-assisted minimally invasive cardiac surgery (MICS) has been progressively applied and shows advantages in non-pregnant patients over the years. We present five cases of pregnant women who underwent a video-assisted minimally invasive surgical approach for cardiac surgery and the management strategies. In conclusion, the video-assisted MICS is feasible and safe to pregnant patients, with good maternal and fetal outcomes under the multidisciplinary assessment and management.
Introduction
Heart diseases complicate 2–4% of pregnancies but account for up to 15% of maternal deaths (1). The cardiac potential to adapt hemodynamic change is impaired in women with structural heart disease, presented with reduced systolic and diastolic function (2). Once cardiac decompensation happens, cardiac surgery might be a solution for pregnant patients with structural heart diseases and compromised cardiac function. Maternal mortality after cardiac surgery during pregnancy is reported to be comparable to non-pregnant patients for about 11.2%, but the high fetal loss (33.1%) cannot be ignored (1). The management of these patients should be made with adequate multidisciplinary discussions, including cardiologists, anesthetists, and obstetricians, with aims to improve maternal and fetal outcomes.
The minimally invasive cardiac surgery (MICS) has been progressively applied in non-pregnant patients over the years and showed advantages, such as less transfusion rate and shorter postoperative ventilation support time as compared to that of mid-sternotomy approach thus, resulting to shorter ICU time and length of stay (3, 4). However, few MICS during pregnancy has been reported. This article presents a case series of five pregnant women who underwent a video-assisted MICS cardiac surgery during pregnancy in a tertiary medical center.
Case Series
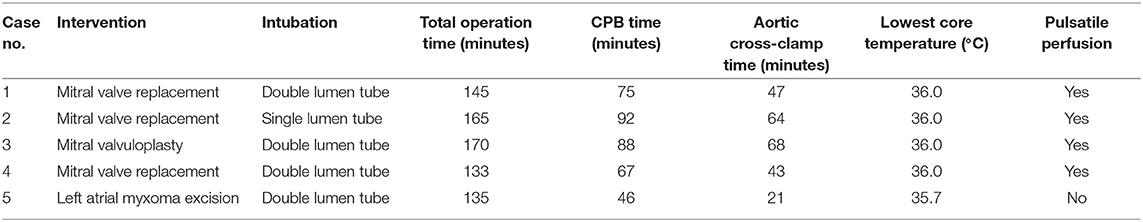
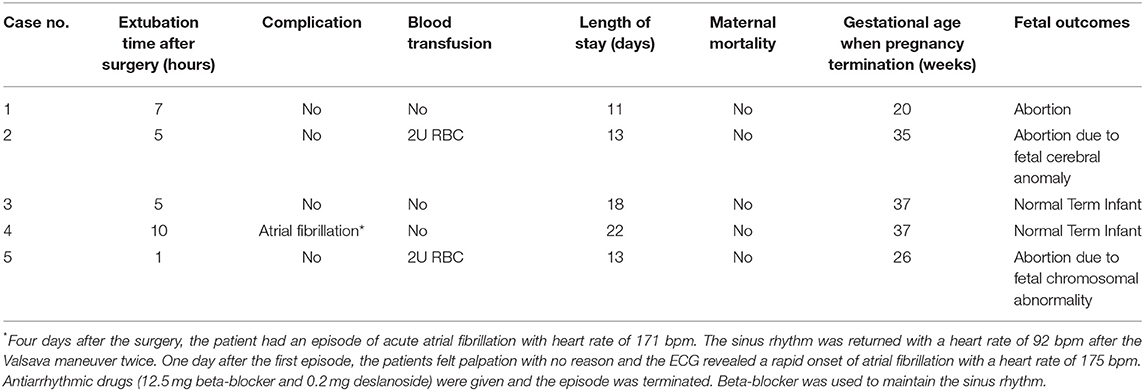
We retrospectively reviewed the records of all pregnancies with cardiac surgery in our hospital between 2019 and 2021. Only patients who underwent a video-assisted MICS (n = 5) were included. Informed consent has been obtained from all the patients. Baseline characteristics of all patients are shown in Table 1, and intraoperative and postoperative information of all patients are shown in Tables 2, 3.
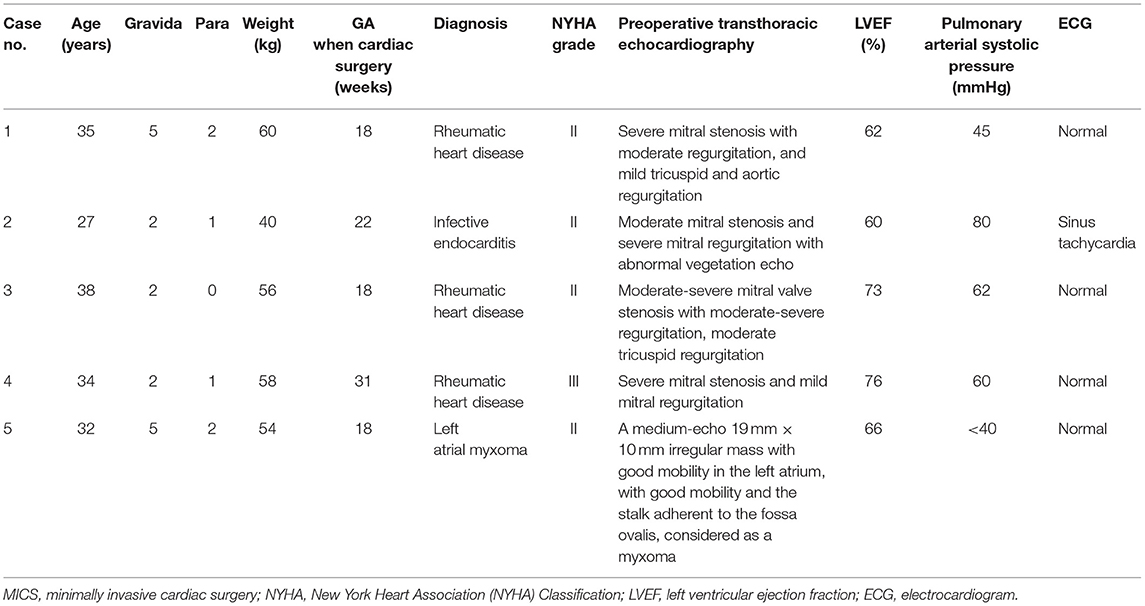
Table 1. Baseline characteristics of five patients undergoing minimally invasive cardiac surgery (MICS) during pregnancy.
Perioperative Management Strategy
Perioperative management was discussed by the multidisciplinary team that included cardiologists, cardiac surgeons, anesthesiologists, perfusionists, and gynecologists. The operative procedure was conducted under general anesthesia with 35 F left double-lumen intubation to allow single lung ventilation. A central venous catheter was placed on the right internal jugular vein. A 16 Fr venous cannula was placed in the superior cava vein through the right jugular vein for cardiopulmonary bypass (CPB) venous return. Transesophageal echocardiography was routinely set up for intraoperative monitoring. The patient was placed in a supine position with elevated right chest. Propofol and rocuronium were used for anesthetic induction. Sevoflurane, propofol, dexmedetomidine, and rocuronium were used for anesthetic maintenance with certain level of Nacrotrend values between 40 and 60. Sufentanil was intermittently given to ensure enough analgesia. Magnesium sulfate was used to inhibit uterine contraction. The fetal heart rate and uterine contraction were monitored by TEE and tocodynamometer. After heparinization, venous cannula and arterial cannula were placed in the right femoral vein and artery. A right anterolateral 4th intercostal 3.5 cm incision was made and the thoracoscopy was inserted via the 4th or 5th intercostal space. The high-flow, high-pressure normothermic CPB was then started. Vacuum-assisted venous drainage was also utilized (maximum negative pressure 20–40 mmHg). Cold Del Nido cardioplegia solution (blood and crystalloid mixed formula) was used as anterograde. During CPB, the hematocrit was maintained between 25 and 29%, as well as normothermia. Post-bypass transesophageal echocardiography has ensured a satisfying surgical outcome and fetal survival. An intercostal nerve block with 0.5% ropivacaine combined with intravenous analgesia, was used for postoperative multimodal analgesia.
The patient was transferred to the intensive care unit temporarily for monitoring. Fetal status was ensured by Doppler echography and uterine contraction was monitored by the tocodynamometer after the surgery. Atosiban was used postoperatively to inhibit uterine contraction. Warfarin and/or low molecular weight heparin were administrated for anticoagulation for those patients who underwent mechanical valve replacement.
Discussion
MICS During Pregnancy
Compared to standard sternotomy, the minimally invasive approach through thoracoscopy has been utilized in the past decades. However, there were few reports about occurrence of MICS with thoracoscopy during pregnancy. In one previous study, Nguyen et al. (5) reported a case of acute papillary muscle rupture during pregnancy. The minimally invasive mitral valve repair via the right thoracotomy was conducted, with main consideration on how morbidly obese this patient was. In our center, we conducted the MICS via video-assisted thoracoscopy, with the advantage of smaller operative incision, rather than the right thoracotomy. Also, favorable outcomes after the minimally invasive approach can be obtained more than that of standard sternotomy with less postoperative pain, faster recovery, less postoperative complication, and shorter length of stay in the hospital (6, 7). Qiu et al. (3) demonstrated that a full sternotomy was an independent risk factor for postoperative ventilation support. It was known that prolonged mechanical ventilation affects fetus morbidity and mortality in cardiac surgery during pregnancy (8). In our study, all fetuses remained alive after the cardiac surgery, supporting our supposition that the minimally invasive approach has its benefits to fetal survivals in pregnant women who underwent cardiac surgery under CPB. Also, sternal complications following a median sternotomy, including infection, sternal instability, and non-union, were reported by 1–8% worldwide (9). Sternal precautions were recommended for prevention of complication, which consisted of weight restrictions on the use of the upper limbs immediately after surgery for 6–12 weeks (10). This may interfere with normal maternal-infant bonding because motion restriction after median sternotomy may affect the mother in holding her child and in breastfeeding (5, 11). However, longer operation duration in the MICS should come into consideration for pregnant women as CPB time is reported as a risk factor for fetal mortality (12), but this may be solved by experienced surgeons. During MICS, single lung ventilation technique is required for a satisfactory field exposure. In our cases, we applied double lumen tube in four patients and single lumen tube in one patient. All surgical field remained satisfactory to the surgeons, including the one with single lumen intubation. Hypoxemia in the lung isolation after cardiopulmonary bypass (CPB) surgery might impair fetal oxygenation, and whether single lumen tube in MICS benefits to these patients still needs further investigation.
Cardiopulmonary Bypass During Pregnancy
Cardiopulmonary bypass can pose significant effects on both the fetus and the mother. Sustained uterine contraction during CPB is regarded as a risk factor to fetus survival. The cooling and rewarming process during CPB induces uterine contraction, especially after maternal hypothermia, which induces placental hypoperfusion and, consequently, fetal hypoxia. Hemodilution of progesterone during CPB also enhances uterine contraction (12, 13). In our study, we performed high perfusion pressure and normothermic CPB to ensure placental perfusion. It is thought that pulsatile perfusion can release endothelium-derived growth factors from the vascular endothelium and reduce uterine contractions, which may result to good fetal outcomes in pregnant women who undergo cardiac surgery (12, 14). In our study, non-pulsatile CPB was performed in two cases and both fetuses were alive after cardiac surgery, though 1 patient eventually has terminated pregnancy due to fetal chromosomal abnormality. Pulsatile perfusion was performed in three patients and one patient has terminated pregnancy due to fetal cerebral anomaly. In fact, there are few clinical data to support the advantage of pulsatile perfusion over non-pulsatile perfusion in pregnant women. In the cohort study of John et al. (15), there was a reported three fetal deaths among the 21 non-pulsatile CPB cases. Most of them (two fetal deaths) happened in women with other comorbidities. Further clinical research evidence is required to determine the beneficial application of pulsatile or non-pulsatile perfusion in cardiac surgery of pregnant women.
In the study of Jha et al. (1), the pooled rate of maternal complications was at 15%, maternal heart failure at 5.8%, and arrhythmia at 2.1%, respectively. In our study, only 1 patient experienced cardiovascular complication of acute onset of atrial fibrillation that requires treatment. Maternal mortality is comparable to that of CPB in non-pregnant women in the previous studies, with the estimated rate of 11% in the meta-analysis of Jha et al. (1). Maternal status with worse NYHA and emergency surgery contributed to unfavorable maternal outcomes in these patients. In our cases, all women survived and may benefit from good maternal NYHA status and semi-urgent surgery.
Management of Cardiac Surgery During Pregnancy
The decision to perform cardiac surgery during pregnancy should be thoroughly discussed within a multidisciplinary team of obstetricians, cardiologists, cardiac surgeons, anesthetists, and gynecologists. The Modified World Health Organization Classification of Cardiovascular Disease in Pregnancy is used as reference for risk stratification of maternal and neonatal complication (16). Compared to non-pregnant women, pregnant women are at higher risk of aspiration, difficult intubation, and thromboembolism, which made them require more attention in preoperative preparation (17). Once the patient is supine, the 15° position of left uterine displacement should be applied to avoid aortocaval compression after 18–20 weeks of gestation (18).
Pregnant patients are more sensitive to IV and inhalational medications. Propofol seems to be the preferred medication for induction in healthy pregnant patients. Mongardon et al. (19) demonstrated that the dose of propofol required in pregnant women for loss of consciousness is 8% less than in non-pregnant patients. Inhalation medication, such as desflurane and sevoflurane, inhibits myometrial contractions during the operation, which may be beneficial to pregnant women undergoing cardiac surgery (20). It suggested a more rapid onset of neuromuscular block with vecuronium and rocuronium in pregnant women (18).
Sympathomimetic agents, such as phenylephrine and norepinephrine, are safe to maintain blood pressure. In comparison with phenylephrine, ephedrine may act on fetal metabolism and be associated with neonatal acidosis and, therefore, should be considered as secondary choice of vasopressor in pregnancy (16, 21).
Intraoperative monitoring for both the mother and the fetus is critically significant to favorable maternal and fetal outcomes. If uterine contractions are detected, increase maternal intravascular volume may be helpful and tocolytic treatment can be administrated (22). Fetal bradycardia is an important indicator of fetal distress during CPB, which usually occurs at the initiation of CPB caused by a decrease in systemic vascular resistance, thereby affected by hemodilution, and the release of vasoactive substances. It has been reported that fetal heart rate (FHR) monitoring with an external cardiotocography reduces fetal mortality to 7.5% in cardiac surgeries with CPB (23). In our cases, the tocodynamometer combined with TEE were used to monitor uterine contraction and fetal heart rate (Figure 1). FHR was measured intraoperatively by Doppler echocardiography across the fetal blood flow, which was available in all pregnant women in our cases whose gestational age ranged from 18 to 31 weeks.
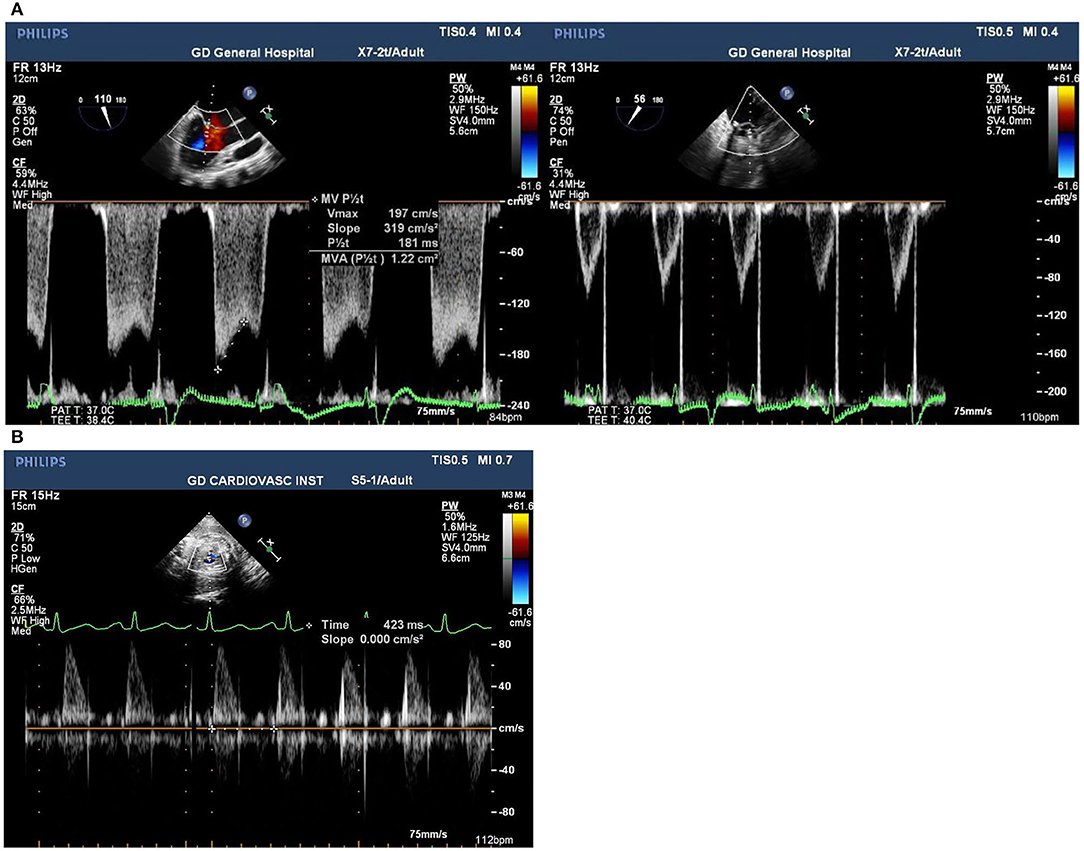
Figure 1. (A) Pre and post-surgical mitral valve view of transesophageal echocardiogram images of mitral valve stenosis in Case 1. (B) Postoperative transesophageal echocardiogram images of Doppler of fetal blood flow in Case 3 presenting the fetal heart rate at 141 bpm.
Commonly tocolytic drugs include magnesium, beta-adrenergic drugs, nitroglycerin, and prostaglandin inhibitors (22). In our case, we chose magnesium sulfate, which is mainly used for pre-eclampsia control, to inhibit uterine contraction by decreasing acetylcholine transmission in motor nerve terminals (24). Also, it was reported that antenatal usage of magnesium sulfate may contribute to fetal neuroprotection and may reduce the risk of cerebral palsy or even death (25). However, magnesium may potentiate the activity of both depolarizing and non-depolarizing neuromuscular blocking agents (NMBA). Consequently, the dose of NMBA should be reduced (24). In a case of left atrial myxoma resection reported by Alexis et al. (26), a low dose of nicardipine, a calcium channel blocker, was used to inhibit uterine contractility and may show an advantage to restore FHR.
For these patients, postoperative monitoring is pivotal along with the assessment of fetus by using Doppler ultrasound, as well checking of uterine contraction with a tocodynamometer. If necessary, tocolytic drugs should be administrated in case of a preterm labor. The left lateral position should be maintained to prevent aortocaval compression (18). Furthermore, postoperative analgesia is important for pain control and can reduce the risk of premature labor. NSAIDs should be avoided in women as prenatal exposure to NSAIDs after 30 weeks gestational age is associated with an increased risk of premature closure of the fetal ductus arteriosus and oligohydramnios (27). In our center, multimodal analgesia was performed in every patient including intercostal nerve block with 0.5% ropivacaine and intravenous analgesia with opioids, resulting all patients to report a pain score <3 on a numeric rating scale (NRS).
Conclusion
The video-assisted MICS is feasible and safe with good maternal and fetal outcomes, which may be progressively applied in patients in the need for cardiac surgery during pregnancy. The multidisciplinary team for decision in the management of these patients is of vital importance to favorable outcome for both the mother and the fetus.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Guangdong Provincial People's Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
NW, YY, and JH collected and organized the information of patients. AL wrote the first draft of the manuscript. JW, YY, JH, BL, and NW wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This grant of the study was from the Natural Science Foundation of Tibet Autonomous Region [XZ2020ZR-ZY55(Z)].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jha N, Jha AK, Chand Chauhan R, Chauhan NS. Maternal and fetal outcome after cardiac operations during pregnancy: a meta-analysis. Ann Thorac Surg. (2018) 106:618–26. doi: 10.1016/j.athoracsur.2018.03.020
2. Cornette J, Ruys TP, Rossi A, Rizopoulos D, Takkenberg JJ, Karamermer Y, et al. Hemodynamic adaptation to pregnancy in women with structural heart disease. Int J Cardiol. (2013) 168:825–31. doi: 10.1016/j.ijcard.2012.10.005
3. Qiu Z, Chen X, Xu Y, Huang F, Xiao L, Yang T, et al. Does full sternotomy have more significant impact than the cardiopulmonary bypass time in patients of mitral valve surgery? J Cardiothorac Surg. (2018) 13:29. doi: 10.1186/s13019-018-0719-4
4. Paparella D, Fattouch K, Moscarelli M, Santarpino G, Nasso G, Guida P, et al. Current trends in mitral valve surgery: a multicenter national comparison between full-sternotomy and minimally-invasive approach. Int J Cardiol. (2020) 306:147–51. doi: 10.1016/j.ijcard.2019.11.137
5. Nguyen S, Umana-Pizano JB, Donepudi R, Dhoble A, Nguyen TC. Minimally invasive mitral valve repair for acute papillary muscle rupture during pregnancy. Ann Thorac Surg. (2019) 107:e93–e5. doi: 10.1016/j.athoracsur.2018.06.048
6. Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. (2008) 34:943–52. doi: 10.1016/j.ejcts.2008.07.057
7. Zhang QL, Chen Q, Lin ZQ, Yu LL, Lin ZW, Cao H. Thoracoscope-assisted mitral valve replacement with a small incision in the right chest: a Chinese single cardiac center experience. Med Sci Monit. (2018) 24:1054–63. doi: 10.12659/MSM.905855
8. Kapoor MC. Cardiopulmonary bypass in pregnancy. Ann Card Anaesth. (2014) 17:33–9. doi: 10.4103/0971-9784.124133
9. Cahalin LP, Lapier TK, Shaw DK. Sternal precautions: is it time for change? Precautions versus restrictions - a review of literature and recommendations for revision. Cardiopulm Phys Ther J. (2011) 22:5–15. doi: 10.1097/01823246-201122010-00002
10. Balachandran S, Lee A, Royse A, Denehy L, El-Ansary D. Upper limb exercise prescription following cardiac surgery via median sternotomy: a web survey. J Cardiopulm Rehabil Prev. (2014) 34:390–5. doi: 10.1097/HCR.0000000000000053
11. Taksaudom N, Traisrisilp K, Kanjanavanit R. Left atrial myxoma in pregnancy: management strategy using minimally invasive surgical approach. Case Rep Cardiol. (2017) 2017:8510160. doi: 10.1136/bcr-2017-219624
12. Patel A, Asopa S, Tang AT, Ohri SK. Cardiac surgery during pregnancy. Tex Heart Inst J. (2008) 35:307–12.
13. Yuan SM. Indications for cardiopulmonary bypass during pregnancy and impact on fetal outcomes. Geburtshilfe Frauenheilkd. (2014) 74:55–62. doi: 10.1055/s-0033-1350997
14. Jahangiri M, Clarke J, Prefumo F, Pumphrey C, Ward D. Cardiac surgery during pregnancy: pulsatile or nonpulsatile perfusion? J Thorac Cardiovasc Surg. (2003) 126:894–5. doi: 10.1016/S0022-5223(03)00607-X
15. John AS, Gurley F, Schaff HV, Warnes CA, Phillips SD, Arendt KW, et al. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg. (2011) 91:1191–6. doi: 10.1016/j.athoracsur.2010.11.037
16. Carlier L, Devroe S, Budts W, Van Calsteren K, Rega F, Van de Velde M, et al. Cardiac interventions in pregnancy and peripartum - a narrative review of the literature. J Cardiothorac Vasc Anesth. (2020) 34:3409–19. doi: 10.1053/j.jvca.2019.12.021
17. Upadya M, Saneesh PJ. Anaesthesia for non-obstetric surgery during pregnancy. Indian J Anaesth. (2016) 60:234–41. doi: 10.4103/0019-5049.179445
18. Okeagu CN, Anandi P, Gennuso S, Hyatali F, Stark CW, Prabhakar A, et al. Clinical management of the pregnant patient undergoing non-obstetric surgery: review of guidelines. Best Pract Res Clin Anaesthesiol. (2020) 34:269–81. doi: 10.1016/j.bpa.2020.04.004
19. Mongardon N, Servin F, Perrin M, Bedairia E, Retout S, Yazbeck C, et al. Predicted propofol effect-site concentration for induction and emergence of anesthesia during early pregnancy. Anesth Analg. (2009) 109:90–5. doi: 10.1213/ane.0b013e3181a1a700
20. Yildiz K, Dogru K, Dalgic H, Serin IS, Sezer Z, Madenoglu H, et al. Inhibitory effects of desflurane and sevoflurane on oxytocin-induced contractions of isolated pregnant human myometrium. Acta Anaesthesiol Scand. (2005) 49:1355–9. doi: 10.1111/j.1399-6576.2005.00804.x
21. Veeser M, Hofmann T, Roth R, Klöhr S, Rossaint R, Heesen M. Vasopressors for the management of hypotension after spinal anesthesia for elective caesarean section. Systematic review and cumulative meta-analysis. Acta Anaesthesiol Scand. (2012) 56:810–6. doi: 10.1111/j.1399-6576.2011.02646.x
22. Chandrasekhar S, Cook CR, Collard CD. Cardiac surgery in the parturient. Anesth Analg. (2009) 108:777–85. doi: 10.1213/ane.0b013e31819367aa
23. Koh KS, Friesen RM, Livingstone RA, Peddle LJ. Fetal monitoring during maternal cardiac surgery with cardiopulmonary bypass. Can Med Assoc J. (1975) 112:1102–4.
24. Lu JF, Nightingale CH. Magnesium sulfate in eclampsia and pre-eclampsia: pharmacokinetic principles. Clin Pharmacokinet. (2000) 38:305–14. doi: 10.2165/00003088-200038040-00002
25. Magee LA, De Silva DA, Sawchuck D, Synnes A, von Dadelszen P. No. 376-Magnesium sulphate for fetal neuroprotection. J Obstet Gynaecol Can. (2019) 41:505–22. doi: 10.1016/j.jogc.2018.09.018
26. Alexis A, Origer P, Hacquebard JP, De Cannière D, Germay O, Vandenbossche JL, et al. Anesthetic management of a voluminous left atrial myxoma resection in a 19 weeks pregnant with atypical clinical presentation. Case Rep Anesthesiol. (2019) 2019:4181502. doi: 10.1155/2019/4181502
Keywords: minimally invasive cardiac surgery (MICS), video-assisted, pregnancy, cardiopulmonary bypass, perioperative management
Citation: Lu A, Ye Y, Hu J, Wei N, Wei J, Lin B and Wang S (2021) Case Series: Video-Assisted Minimally Invasive Cardiac Surgery During Pregnancy. Front. Med. 8:781690. doi: 10.3389/fmed.2021.781690
Received: 23 September 2021; Accepted: 09 November 2021;
Published: 22 December 2021.
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesReviewed by:
Zhengyuan Xia, The University of Hong Kong, Hong Kong SAR, ChinaLei Zhao, Xuanwu Hospital, Capital Medical University, China
Copyright © 2021 Lu, Ye, Hu, Wei, Wei, Lin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Wang, shengwang_gz@163.com
†These authors have contributed equally to this work and share first authorship
 Anyi Lu
Anyi Lu Yingxian Ye1†
Yingxian Ye1†