Association Between Perforating Scleral Vessel and Myopic Maculopathy: A Cross-Sectional Study of a Chinese Cohort
- Department of Ophthalmology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Purpose: To investigate the association between perforating scleral vessel (PSV) and different types of myopic maculopathy (MM) in a highly myopic population.
Methods: In total, 188 highly myopic eyes (117 participants) were enrolled. Each participant underwent detailed history taking and ocular examinations. Based on fundus photographs and optical coherence tomography, patients were subdivided into the non-MM group and MM group. Based on a new classification system (ATN), MM cases were classified as myopic atrophy maculopathy (MAM), myopic tractional maculopathy (MTM), and myopic neovascular maculopathy (MNM). The number of PSV and the macular choroidal thickness (mChT) were measured.
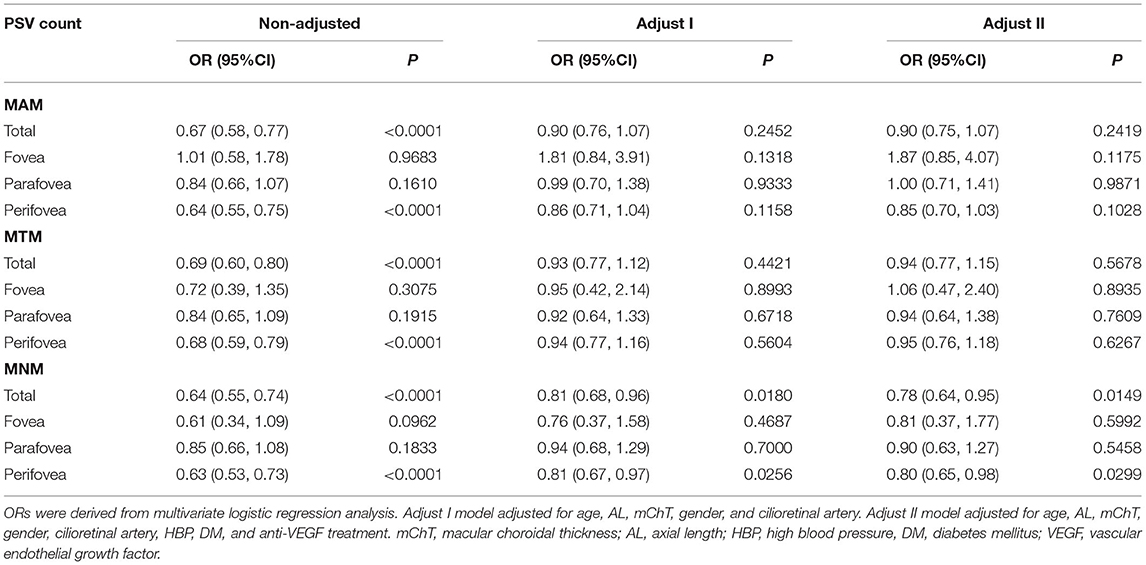
Results: Compared with non-MM group, MM group was characterized by relatively larger age (48.40 vs. 32.34; p < 0.001), longer axial length (AL, 29.72 vs. 27.75, p < 0.001), thinner mChT (52.90 vs. 122.52; p < 0.001), and lower PSV counts (6.73 vs. 9.47, p ≤ 0.001). The non-MM group had higher PSV counts in total area (0–9 mm, 9.47 vs. 6.73, p < 0.001) and perifovea area (3–9 mm, 7.25 vs. 4.71, p < 0.001) compared to the MM group. Univariate and multivariate analyses showed that PSV count had no association with MAM (p = 0.2419) and MTM (p = 0.5678). Total PSV count [odds ratio (OR) 0.78, 95% CI 0.64–0.95, p = 0.0149] and perifovea PSV count (OR 0.80, 95% CI 0.65–0.98, p = 0.0299) were both protective factors for MNM. The stratified analysis revealed that in groups with AL <28 mm, or mChT <50 μm, or mChT ≥100 μm, or eyes with cilioretinal artery, PSV count had no significant association with MNM.
Conclusion: Higher PSV counts in perifovea area (3–9 mm centered fovea) and total area (0–9 mm centered fovea) were protective factors for MNM, whereas PSV count had no association with MAM and MTM. These findings may provide novel insights into the mechanisms of pathologic myopia.
Introduction
Myopia causes a huge societal burden. Pathological myopia (PM) is especially prevalent in East Asian countries (1, 2). Myopic maculopathy (MM) is the main cause of vision loss in patients with PM (3, 4). In recent years, insights into the pathogenesis of high myopia due to scleral and choroidal levels have emerged. Recent studies show that perforating scleral vessel (PSV) may be anatomically associated with lacquer crack (Lc) (5), myopic choroidal neovascularization (mCNV) (6–9), and patchy atrophy (10). Previous researchers described these phenomena and calculated the probability of PSV occurrence under specific myopic complications. However, the association between PSV and MM has not been analyzed.
Choroidal and scleral thinning are typical characteristics of highly myopic eyes. Indocyanine green angiography showed that PSV was originated from the short posterior ciliary artery (SPCA), which was important to choroidal blood supply (7). Thus, PSV count may be significantly associated to myopic choroidal change and may influence PM progression. A new classification system for MM (ATN) that integrates atrophy (A), traction (T), and neovascular (N) has been recently proposed (11). Based on the ATN classification, we explored the relationship between PSV and MM in detail. Here, we analyzed the characteristics of a highly myopic adult cohort to determine if PSV count is a risk factor for the 3 MM types.
Methods
Study Design
We observed a series of patients presented at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, from August 2020 to March 2021 with high myopia in at least 1 eye. Ethical approval for the study was granted by the medical ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The study adhered to the Declaration of Helsinki guidelines. All participants gave written informed consent. The study was registered at http://www.chictr.org.cn (registration number ChiCTR2100043611).
History Taking and Ocular Examinations
All patients underwent a thorough history review and ocular examinations. Treatment history was recorded and classified based on the inclusion and exclusion criteria. Ocular examinations involved assessment of intraocular pressure (IOP) (NT-510, NIDEK CO., LTD., Japan), slit-lamp biomicroscopy (BP900, Haag-Streit International, Swiss), indirect ophthalmoscopy (YZ6H, 66Vision.Tech, China), axial length (AL) (AL-scan, NIDEK CO., LTD, Japan), color fundus photograph (AFC-210, Nidek Co., LTD., Japan), and spectral-domain optical coherence tomography (SD-OCT) (Spectralis OCT, Heidelberg Engineering, Germany). OCT-angiography (Spectralis OCT, Heidelberg Engineering, Germany and SVision Imaging, Henan, China), fluorescence, and indocyanine green angiography (Spectralis OCT, Heidelberg Engineering, Germany) were used to diagnose MNM where necessary.
Inclusion and Exclusion Criteria
Participants with an AL ≥26 mm were included in the study. Exclusion criteria were (1) an IOP >21 mmHg, (2) coexisting or history of severe ocular diseases, such as dense cataract, eye injury, glaucoma, diabetic retinopathy, or macular edema, (3) coexisting or history of severe systemic diseases other than well-controlled diabetes and hypertension, (4) history of pars plana vitrectomy, posterior scleral reinforcement, and laser photocoagulation treatment, (5) evidence of retinal pathology non-related to myopia, and (6) poor-quality images for MM or counting PSV grading.
Definition and Classification of MM
Based on the new classification and grading system for MM (ATN), MM was subdivided into 3 types: myopic atrophy maculopathy (MAM), myopic tractional maculopathy (MTM), and myopic neovascular maculopathy (MNM) (11). A scores were based on 5 levels: A0 (no myopic retinal lesions, A1 (tessellated fundus only), A2 (diffuse chorioretinal atrophy), A3 (patchy chorioretinal atrophy), and A4 (complete macular atrophy). MAM was defined as an A score of ≥2. T score had 6 levels: T0 (no macular schisis), T1 (inner or outer foveoschisis) T2, inner and outer foveoschisis, T3 (foveal retinal detachment), T4 (full-thickness macular hole [MH]), and T5 (MH and retinal detachment), and MTM were defined as T score ≥1. N score was divided into 4 levels (N0, no mCNV, N1 (macular LCs), N2a (active CNV), and N2s (scar or Fuch's spot), and MNM defined as an N score ≥1. The classification and grading of MM were done independently by two well-trained graders (JFS and HL). Disagreements were adjudicated by a retinal specialist (XFS).
Spectral-Domain OCT Imaging and Assessment
Macular-centered structural OCT images were obtained for each eye using enhanced depth imaging with 48 radial scans, a length of 9 mm, and an A-scan repeat time (ART) of 9. Macular choroidal thickness (mChT) was defined as the distance between the Bruch membrane and the choroid-sclera interface in the fovea. PSV was determined by 2 experienced retinal physicians (HMY and JFS) using the marking software (Heidelberg Engineering, Heidelberg, Germany). Based on the study by Giuffrè et al. (8), PSV was defined as (1) linear or wavy morphology in OCT images, (2) hyporeflective appearance, and (3) extension from the sclera through the choroid toward the retina. In OCT B-scan, the PSV mark was taken at the junction of the sclera and choroid, and at the corresponding site in the OCT en face image (Figure 1). The continuous 48 equally spaced radial OCT B-scans and three-dimensional picture were used to avoid duplicated counting by demonstrating how PSV passed through the sclera and entered the choroid (Figure 1). Since our study aimed to explore the maculopathy, PSVs within central 1, 3, and 9 mm diameter circles were counted.
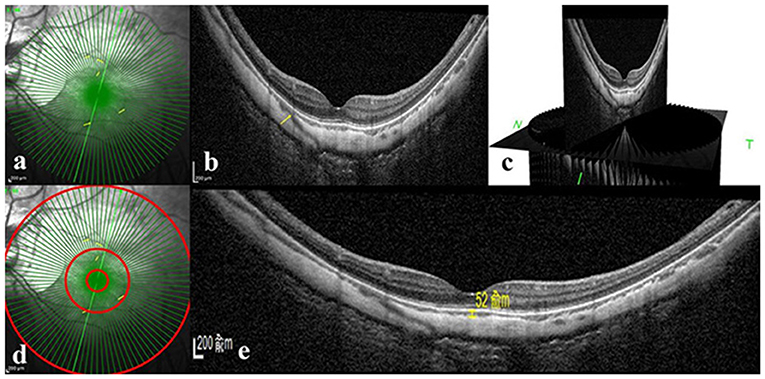
Figure 1. Perforating scleral vessel counting method and macular choroidal thickness assessment using spectral-domain OCT. (a) Structural OCT en face image of one patient. Enhanced depth OCT scanning was centered on the macular with 48 radical scans, a length of 9 mm and an A-scan repeat time of 9. (b) B-scan image corresponding to the scan line of the green arrow in part a. The yellow arrow in parts a and b indicates the corresponding position where PSV entered the choroid from the sclera. (c) Three-dimensional reconstruction picture of B-scan image based on Heidelberg software. (d) Counting of PSV in three different regions, such as 1 diameter circle, 3 diameter circle, and 9 diameter circle. (e) Structural OCT scan through the fovea. Macular choroidal thickness is measured in a 1:1 μm image using Heidelberg software (shown as a yellow line segment of 52 μm). OCT: optical coherence tomography.
Statistical Analysis
We first compared data distribution for each covariate between bilateral eyes, MM, and non-MM groups, among the MAM, MTM, and MNM groups. T-test (normal distribution) or Kruskal-Wallis rank-sum test (non-normal distribution) were used for continuous variables. Chi-square tests were used for categorical data (Tables 1–3). Univariate and multivariate logistic regression models were used to determine if PSV count correlated with MM (Table 4; Supplementary Table S1). Total PSV count was obtained in 3 different regions, separately. Stratified analysis was used to verify the relationships in different sub-groups (Table 5). All analyses were done on R (http://www.R-project.org) and EmpowerStats software (www.empowerstats.com, X&Y solutions, Inc. Boston, MA, USA).
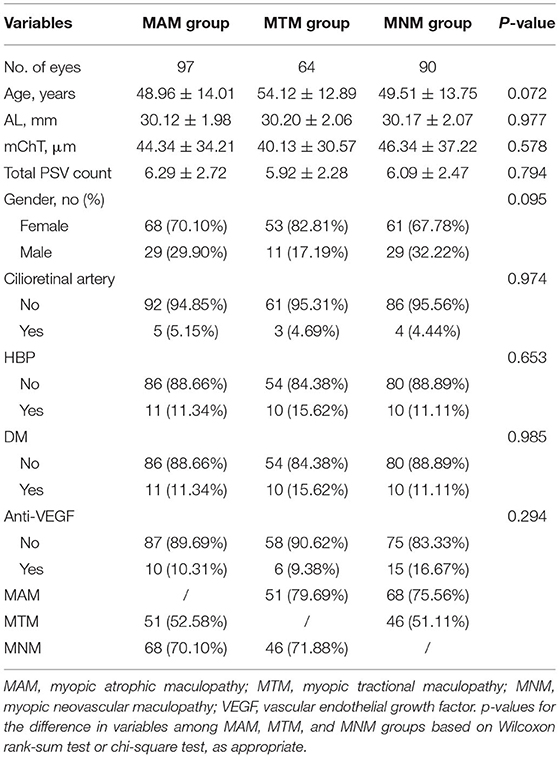
Table 2. Characteristics and comparison between highly myopic eyes and three different types of myopic maculopathy.
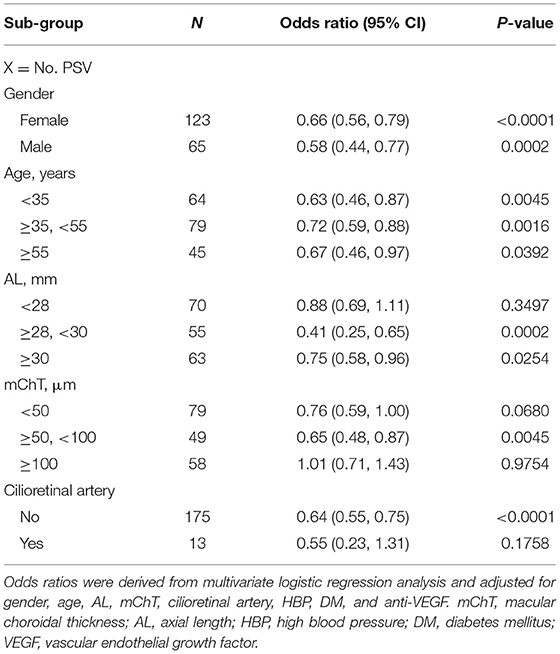
Table 5. Stratified analysis of the association between perforating scleral vessel and myopic neovascular maculopathy.
Results
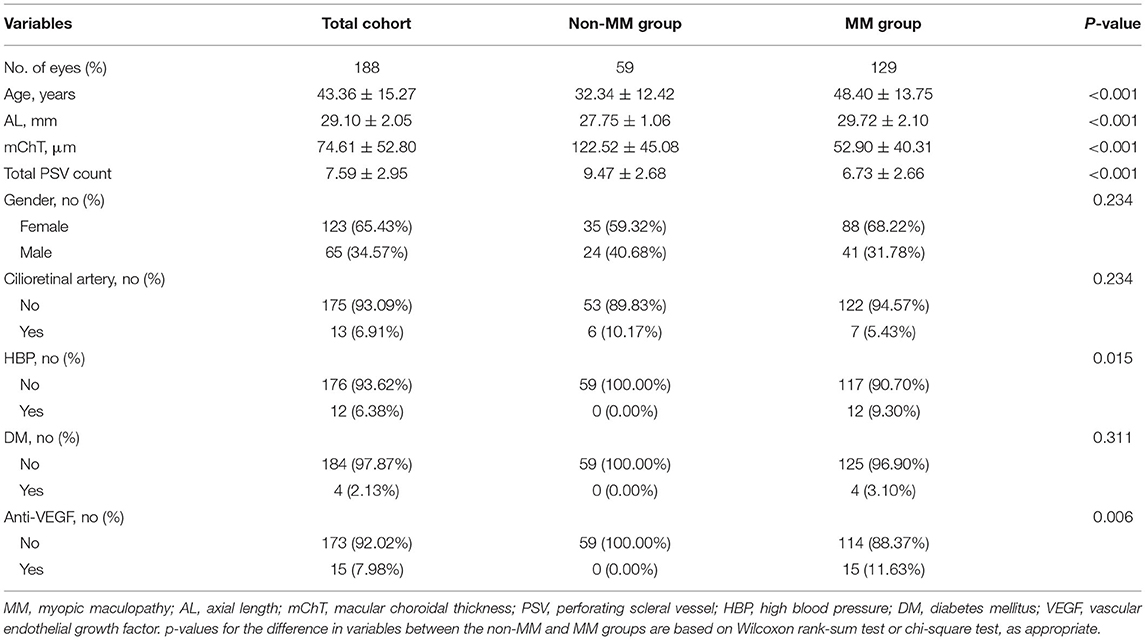
No significant differences in bilateral ocular biometrics, such as AL, mChT, and anti-vascular endothelial growth factor (VEGF) history, were revealed by the generalized equation regression models. Therefore, it was unnecessary to adjust for associations between the two eyes. General characteristics among participants with and without MM are shown in Table 1. The dataset was comprised of 188 eyes (117 participants). Mean age, mean AL, mean mChT, and mean PSV number were 43.36 ± 15.27 years, 29.10 ± 2.05 mm, 74.61 ± 52.80 μm, and 7.59 ± 2.95, respectively. Of the eligible eyes, 65 (34.57%) were male, 13 (6.91%) had cilioretinal artery, and 15 (7.98%) had a history of intravitreal anti-VEGF injection. In comparison with the non-MM group, MM group was characterized by older age (48.4 vs. 32.34, p < 0.001), longer AL (29.72 vs. 27.75, p < 0.001), thinner mChT (52.90 vs. 122.52, p < 0.001), and lower PSV count (6.73 vs. 9.47, p < 0.001). Other variables, such as gender, cilioretinal artery, and diabetes mellitus (DM), did not differ significantly between the non-MM group and MM group.
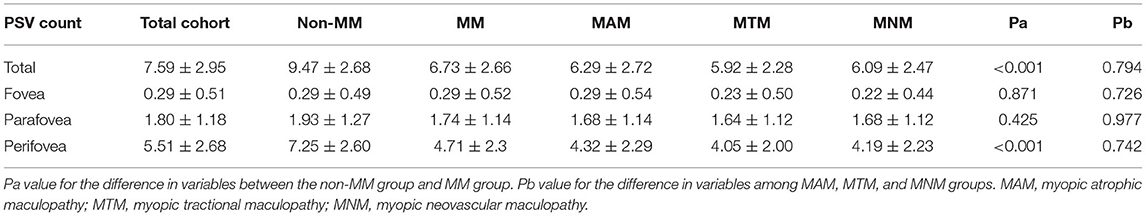
Myopic maculopathy was divided into MAM, MTM, and MNM (Table 2). The 3 groups did not differ significantly with regards to age, AL, mChT, total PSV count, gender, cilioretinal artery, high blood pressure (HBP), DM, and anti-VEGF treatment history. Venn diagram analysis was used to show the number and proportion of eyes in the 3 groups (Figure 2). Among them, the proportion of eyes that combined MAM, MTM, and MNM was the largest (22.87%), and the proportion of eyes that combined MTM and MNM was the least (1.60%). Figure 3 shows a representative left eye that combined 3 kinds of MMs, such as macular atrophy, foveoschisis, CNV, and Fuch's spot, from a patient aged 70–75 years old, with an AL of 29–30 mm. Table 3 describes PSV count in separate regions. In the total cohort, PSV count was 7.59 ± 2.95 in 0–9 mm total area, 0.29 ± 0.51 in 0–1 mm fovea area, 1.80 ± 1.18 in 1–3 mm parafovea area, and 5.51 ± 2.68 in 3–9 mm perifovea area. Among MAM, MTM, and MNM groups, PSV count in different regions had no significant difference. However, the non-MM group had higher PSV counts in total area (0–9 mm, 9.47 vs. 6.73, p < 0.001) and perifovea area (3–9 mm, 7.25 vs. 4.71, p < 0.001) relative to the MM group. Univariate and multivariate regression analyses revealed that there were no significant associations between PSV number in different regions with MAM (total p = 0.2419, fovea p = 0.1175, parafovea p = 0.9871, and perifovea p = 0.1028) and MTM (total p = 0.5678, fovea p = 0.8935, parafovea p = 0.7609, and perifovea p = 0.6267). Total PSV count [odds ratio (OR) 0.78, 95% CI 0.64–0.95, p = 0.0149] and perifovea PSV count (OR 0.80, 95% CI 0.65–0.98, p = 0.0299) were both protective factors for MNM. AL (OR 1.32, 95% CI 1.02–1.71, p = 0.0322) and mChT (OR 0.98, 95% CI 0.97–0.99, p = 0.0014) strongly correlated with MAM (Supplementary Table S1).
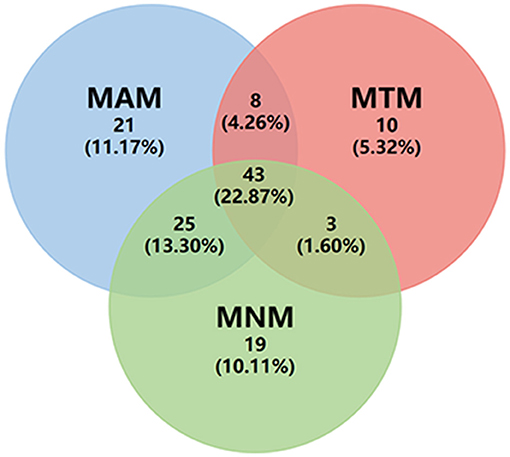
Figure 2. Venn diagram of eye count in three different types of myopic maculopathy. MAM, myopic atrophic maculopathy; MTM, myopic tractional maculopathy; MNM, myopic neovascular maculopathy.
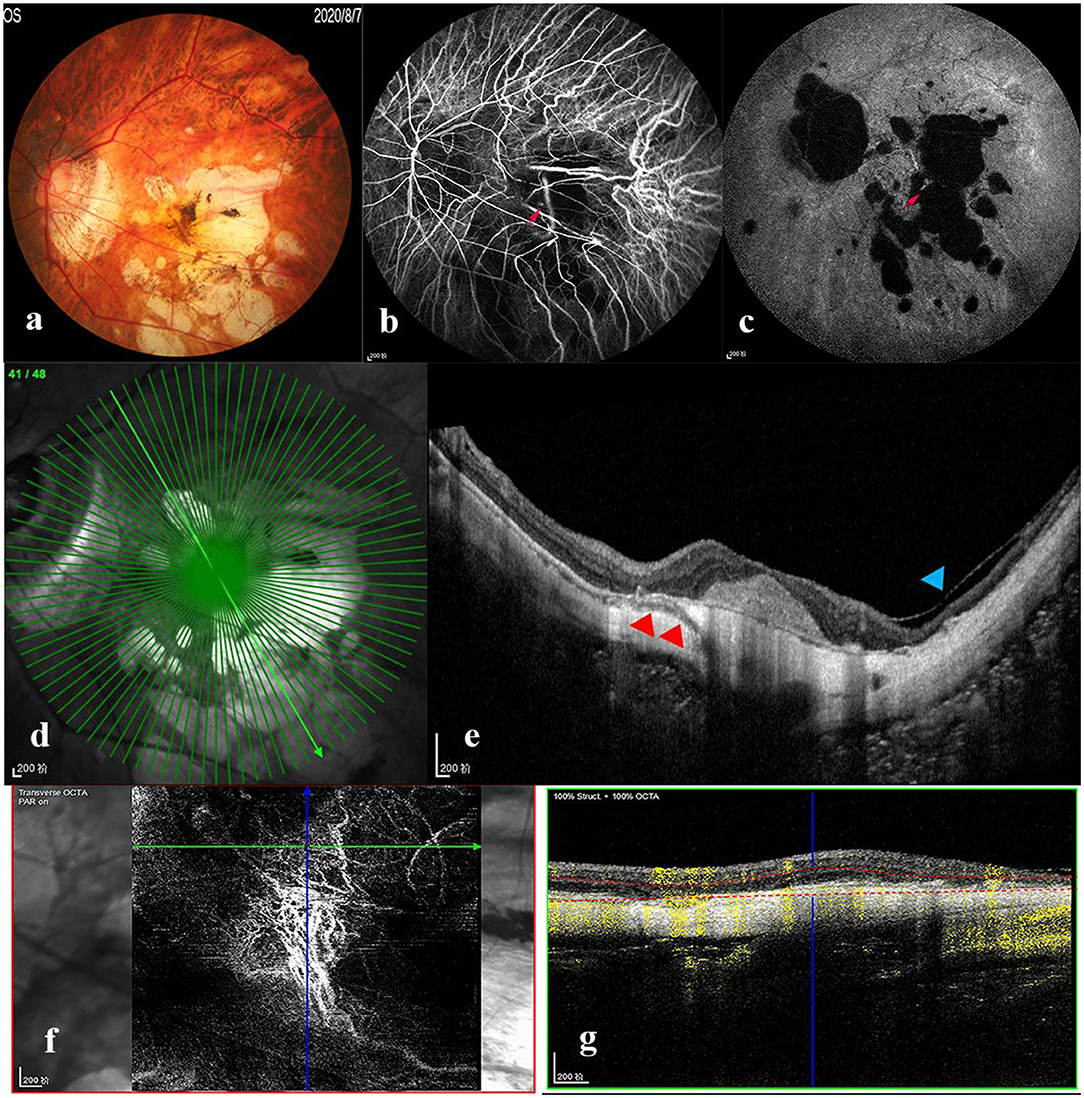
Figure 3. Multimode imaging of a patient who combined the three types of myopic maculopathy. Left eye images of a patient aged 70–75 years old, with an axial length of 29–30 mm. (a) Color fundus photograph showed macular atrophy (MAM) and Fuch's spot (MNM). Fluorescence leakage (red arrow) was seen in the early (b) and late (c) stages of fluorescence angiography. (d,e) OCT image demonstrated foveoschisis (MTM, blue arrowhead) and PSV (red arrowhead) beneath the choroidal neovascularization (CNV). (f,g) OCT-angiography showed CNV (MNM) formation. MAM, myopic atrophic maculopathy; MTM, myopic tractional maculopathy; MNM, myopic neovascular maculopathy; OCT, optical coherence tomography.
The stratified analysis demonstrated that regardless of subgroup, PSV count was negatively associated with MNM, with ORs ranging from 0.41 to 0.75 (Table 5). However, in patients with AL <28 mm, mChT <50 μm, mChT ≥100 μm, and eyes with cilioretinal artery, efficacies of PSV count were greatly attenuated (OR = 0.69–1.11, 0.59–1.00, 0.71–1.43, and 0.23–1.31, respectively).
Discussion
To our knowledge, this is the largest study demonstrating an association between PSV and 3 kinds of MMs. The highlight of this study was the quantitative analysis of PSV based on a large number of Chinese patients. We find that in groups with AL ≥28 mm or mChT 50–100 μm, or without cilioretinal artery, the greater number of PSV, the lower probability of MNM. If confirmed, these findings would help to identify individuals who are at high risk of developing MNM and determine the role of sclera and choroid in the pathophysiological mechanisms of myopia from the vascular perspective.
Differences between the non-MM and MM groups were consistent with previous studies. The prevalence of myopia was increased with age, and the progression of macular lesions in highly myopic eyes was correlated with age (2, 12). Other studies also reported higher AL and thinner mChT in PM groups compared to the simple myopia group (13, 14).
In our study, the stratified analysis revealed that clinical relevance between PSV and MNM was significant only in patients without cilioretinal artery, suggesting that cilioretinal arteries may protect from myopic progression, which is consistent with past findings. Zhu et al. (15) observed that adults with cilioretinal arteries had higher macular vessel density compared to those without. Meng et al. (16) found the presence of cilioretinal arteries may correlate with better visual function in myopic eyes.
Past studies reported that PSV may be an anatomical weakness in myopic eyes causing PM complications. PSV influences the formation of Lc and CNV (5–7, 9). In eyes with myopic patchy atrophy, PSV contributes to myopic morphological changes, such as sclera bowing and atrophy progression (10). However, in previous studies, only the probability of PSV occurrence under specific myopic complications was calculated, and factors, such as AL, mChT, gender, cilioretinal artery, illness, and treatment history, were not controlled for in statistical analyses. Hence, we performed a deeper analysis based on large sample size, and our conclusion was valid in certain groups based on stratified analysis.
The study by Giuffrè et al. (8) included PSV number per eye and PSV number per CNV as indicators of the relationship between PSV and CNV and found that the average PSV number per eye was 2.1 ± 1.0 and that PSV often coincided with CNV. However, the sample size of the study was quite small (41 eyes from 39 patients), and the scanning area was not specifically defined. The study by Rothenbuehler et al. (17) counted PSV in 22 emmetropic eyes based on 3D imaging and found the PSV for the central 1 mm diameter and 3 mm diameter to be 0.2 ± 0.5 (range 0–2) and 2.1 ± 1.8 (range 0–7), respectively, which is consistent with our data on highly myopic eyes without MM (0.29 ± 0.49 for central 1 mm diameter, 1.93 ± 1.27 central 3 mm diameter). In contrast, our study described PSV distribution better, using a larger scanning scope (9 mm diameter), and revealed the predictive effect of PSV on MM based on ATN staging.
For the association between PSV and MAM, Xie et al. showed that PSV was associated with sclera bowing and atrophy progression in eyes with patchy atrophy (10). Patchy atrophy, which rarely involved the fovea but always preferentially occurred inferior and temporal to the fovea, is one type of MAM lesion (A3 degree) (10, 18). Comparably, our study focused on the macular circle region for a large area (central 9 mm diameter) and all types of MAM lesions (not only patchy atrophy but also tessellated, diffuse, macular atrophy) were included. Additionally, our multivariate analysis showed that AL (OR 1.32, 95% CI 1.02–1.71, p = 0.0322) and mChT (OR 0.98, 95% CI 0.97–0.99, p = 0.0014) strongly correlated with MAM and attenuated the efficacy of PSV count (Supplementary Table S1).
The protective effect of PSV on MNM could be explained by vascular ischemic theory. AL elongation resulting in stretching and thinning of the choroid and sclera is a commonly accepted mechanism of PM. In high myopic eyes, the area of flow deficit in the choriocapillaris layer was significantly greater than that in emmetropes and mild or moderate myopic eyes (19, 20). Animal models revealed that choroidal thickness and choroidal blood perfusion are decreased in myopic eyes, and hypoxia-signaling is activated in the myopic sclera and these events can be targeted for myopia control (21, 22). PSV was confirmed to be SPCA, which mainly supplies choroid blood flow. Additionally, PSV may be crucial for oxygen diffusion to the sclera. Thus, metabolic insufficiency due to less PSV may cause hypoxia to the choroid and sclera, and trigger MNM.
The strength of our study is in its large sample size and rigorous statistical analyses. However, it has some limitations. First, due to poor penetration using the SD-OCT technique, it was not feasible to count PSVs in eyes with mChT ≥200 μm. Thus, our study population was mainly comprised of patients with thin choroids. Secondly, PSV counting was manual and may be inaccurate even counting was independently done by 2 researchers. Thirdly, we have not experimentally verified this finding, for instance, using animal models to verify differences between signaling pathways in PSV angiogenesis.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://www.medresman.org.cn.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XS and HY: conception or design of the work. HY, JS, HL, and ZW: data collection. HY and JS: data analysis and interpretation. HY: drafting the article. XS, JS, and HL: critical revision of the article. HY, JS, HL, ZW, and XS: final approval of the version. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of P.R. China (Grant No. 81974136) and the Health Commission of Hubei Province Scientific Research Project (Grant No. WJ2019M139).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.727680/full#supplementary-material
References
1. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. (2012) 379:1739–48. doi: 10.1016/S0140-6736(12)60272-4
2. Rudnicka AR, Kapetanakis VV, Wathern AK, Logan NS, Gilmartin B, Whincup PH, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. (2016) 100:882–90. doi: 10.1136/bjophthalmol-2015-307724
3. Ikuno Y. Overview of the complications of high myopia. Retina. (2017) 37:2347–51. doi: 10.1097/IAE.0000000000001489
4. Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. (2016) 52:156–87. doi: 10.1016/j.preteyeres.2015.12.001
5. Querques G, Corvi F, Balaratnasingam C, Casalino G, Parodi MB, Introini U, et al. Lacquer cracks and perforating scleral vessels in pathologic myopia: a possible causal relationship. Am J Ophthalmol. (2015) 160:759–66.e2. doi: 10.1016/j.ajo.2015.07.017
6. Louzada RN, Ferrara D, Novais EA, Moult E, Cole E, Lane M, et al. Analysis of scleral feeder vessel in myopic choroidal neovascularization using optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. (2016) 47:960–4. doi: 10.3928/23258160-20161004-11
7. Ishida T, Watanabe T, Yokoi T, Shinohara K, Ohno-Matsui K. Possible connection of short posterior ciliary arteries to choroidal neovascularisations in eyes with pathologic myopia. Br J Ophthalmol. (2019) 103:457–62. doi: 10.1136/bjophthalmol-2018-312015
8. Giuffre C, Querques L, Carnevali A, De Vitis LA, Bandello F, Querques G. Choroidal neovascularization and coincident perforating scleral vessels in pathologic myopia. Eur J Ophthalmol. (2017) 27:e39–45. doi: 10.5301/ejo.5000875
9. Sayanagi K, Hara C, Fukushima Y, Sakimoto S, Kawasaki R, Sato S, et al. Flow pattern and perforating vessels in three different phases of myopic choroidal neovascularization seen by swept-source optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. (2021) 259:2615–24. doi: 10.1007/s00417-021-05134-y
10. Xie S, Fang Y, Du R, Yokoi T, Takahashi H, Uramoto K, et al. Abruptly emerging vessels in eyes with myopic patchy chorioretinal atrophy. Retina. (2020) 40:1215–23. doi: 10.1097/IAE.0000000000002630
11. Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, Garcia-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. (2019) 69:80–115. doi: 10.1016/j.preteyeres.2018.10.005
12. Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: Aetiology and prevention. Prog Retin Eye Res. (2018) 62:134–49. doi: 10.1016/j.preteyeres.2017.09.004
13. Ye J, Wang M, Shen M, Huang S, Xue A, Lin J, et al. Deep retinal capillary plexus decreasing correlated with the outer retinal layer alteration and visual acuity impairment in pathological myopia. Invest Ophthalmol Vis Sci. (2020) 61:45. doi: 10.1167/iovs.61.4.45
14. Ye J, Shen M, Huang S, Fan Y, Yao A, Pan C, et al. Visual acuity in pathological myopia is correlated with the photoreceptor myoid and ellipsoid zone thickness and affected by choroid thickness. Invest Ophthalmol Vis Sci. (2019) 60:1714–23. doi: 10.1167/iovs.18-26086
15. Zhu X, Meng J, Wei L, Zhang K, He W, Lu Y. Cilioretinal arteries and macular vasculature in highly myopic eyes: an OCT angiography-based study. Ophthalmol Retina. (2020) 4:965–72. doi: 10.1016/j.oret.2020.05.014
16. Meng J, Wei L, Zhang K, He W, Lu Y, Zhu X. Cilioretinal arteries in highly myopic eyes: a photographic classification system and its association with myopic macular degeneration. Front Med. (2020) 7:595544. doi: 10.3389/fmed.2020.595544
17. Rothenbuehler SP, Maloca P, Scholl HPN, Gyger C, Schoetzau A, Kuske L, et al. Three-dimensional analysis of submacular perforating scleral vessels by enhanced depth imaging optical coherence tomography. Retina. (2018) 38:1231–7. doi: 10.1097/IAE.0000000000001686
18. Pedinielli A, Souied EH, Perrenoud F, Leveziel N, Caillaux V, Querques G. In vivo visualization of perforating vessels and focal scleral ectasia in pathological myopia. Invest Ophthalmol Vis Sci. (2013) 54:7637–43. doi: 10.1167/iovs.13-12981
19. Al-Sheikh M, Phasukkijwatana N, Dolz-Marco R, Rahimi M, Iafe NA, Freund KB, et al. Quantitative OCT angiography of the retinal microvasculature and the choriocapillaris in myopic eyes. Invest Ophthalmol Vis Sci. (2017) 58:2063–9. doi: 10.1167/iovs.16-21289
20. Su L, Ji YS, Tong N, Sarraf D, He X, Sun X, et al. Quantitative assessment of the retinal microvasculature and choriocapillaris in myopic patients using swept-source optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. (2020) 258:1173–80. doi: 10.1007/s00417-020-04639-2
21. Zhang S, Zhang G, Zhou X, Xu R, Wang S, Guan Z, et al. Changes in choroidal thickness and choroidal blood perfusion in guinea pig myopia. Invest Ophthalmol Vis Sci. (2019) 60:3074–83. doi: 10.1167/iovs.18-26397
Keywords: myopic maculopathy, perforating scleral vessels, optical coherence tomography, choroidal neovascularization, multivariate analysis
Citation: Yu H, Sun J, Luo H, Wang Z and Sun X (2022) Association Between Perforating Scleral Vessel and Myopic Maculopathy: A Cross-Sectional Study of a Chinese Cohort. Front. Med. 8:727680. doi: 10.3389/fmed.2021.727680
Received: 19 June 2021; Accepted: 01 December 2021;
Published: 06 January 2022.
Edited by:
Jiangyue Zhao, China Medical University, ChinaCopyright © 2022 Yu, Sun, Luo, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xufang Sun, sunxufang2016@163.com
 Huimin Yu
Huimin Yu Jinfu Sun
Jinfu Sun Huan Luo
Huan Luo Zhitao Wang
Zhitao Wang  Xufang Sun
Xufang Sun