Imprecision in the Era of Precision Medicine in Non-Small Cell Lung Cancer
- 1Royal Marsden Hospital, London, UK
- 2Department of Haematology-Oncology, National University Cancer Institute of Singapore, Singapore, Singapore
- 3The Institute of Cancer Research, London, UK
Over the past decade, major advances have been made in the management of advanced non-small cell lung cancer (NSCLC). There has been a particular focus on the identification and targeting of putative driver aberrations, which has propelled NSCLC to the forefront of precision medicine. Several novel molecularly targeted agents have now achieved regulatory approval, while many others are currently in late-phase clinical trial testing. These antitumor therapies have significantly impacted the clinical outcomes of advanced NSCLC and provided patients with much hope for the future. Despite this, multiple deficiencies still exist in our knowledge of this complex disease, and further research is urgently required to overcome these critical issues. This review traces the path undertaken by the different therapeutics assessed in NSCLC and the impact of precision medicine in this disease. We also discuss the areas of “imprecision” that still exist in NSCLC and the modern hypothesis-testing studies being conducted to address these key challenges.
Introduction
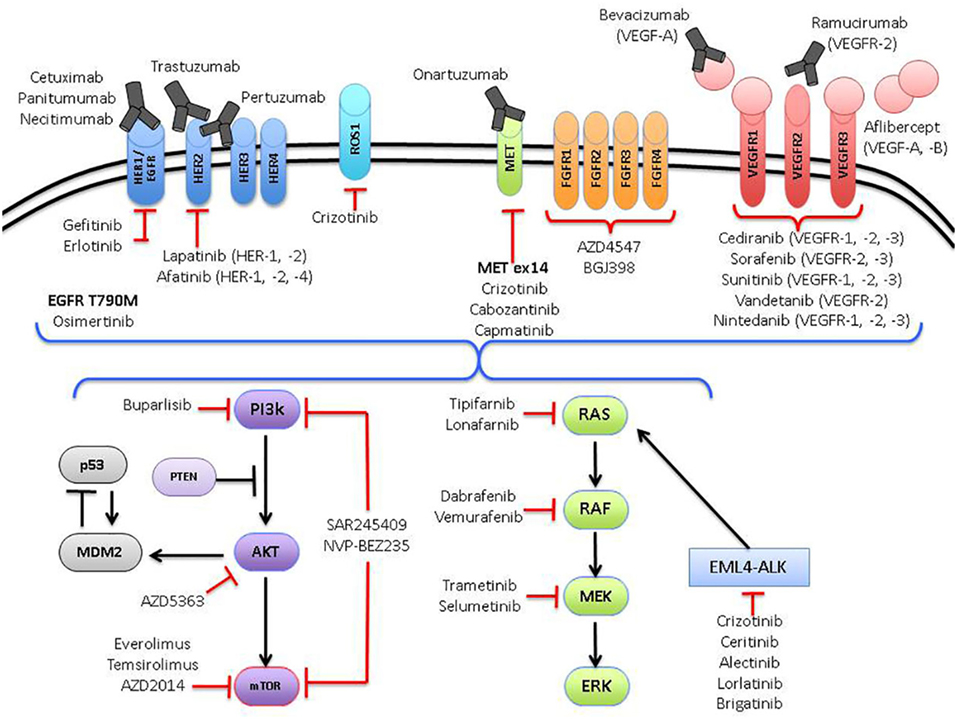
The management of advanced lung cancer has evolved dramatically over the past two decades. Back in the early 1990s, little was done to distinguish between the different histological subgroups of non-small cell lung cancer (NSCLC), with most trials focused on intensifying chemotherapy regimens and establishing the most effective treatments for advanced NSCLC (1), irrespective of histological subtype (2–4). Subsequently, subgroup analysis for a large randomized trial revealed critical differences in survival between patients with squamous and non-squamous histology treated with different chemotherapeutic agents (pemetrexed versus gemcitabine, in combination with cisplatin) (5). The development of tyrosine kinase inhibitors (TKIs) to epidermal growth factor receptor (EGFR) mutated NSCLC heralded the era of precision medicine in lung cancer. This prompted a paradigm shift toward the search for molecularly targeted agents against other putative driver aberrations in NSCLC and has led to the development of novel therapeutics matched against specific actionable aberrations, such as crizotinib (Pfizer) against ALK and ROS1 aberrations (6, 7) (Figure 1).
Despite these selected successes in NSCLC and the initial promise of individualizing treatments for all patients, the management of this disease for most remains generally imprecise. Current efforts are now focused on the matching of multiple actionable drivers with targeted agents in specific disease subgroups through large basket and umbrella adaptive trials. This article describes the current state of play in the development of molecularly targeted therapies for NSCLC and addresses the successes, pitfalls, and opportunities of precision medicine in this disease.
Food and Drug Administration (FDA)-Approved Molecularly Targeted Agents
EGFR Mutations
The initial rationale for targeting EGFR in NSCLC was based on the overexpression of EGFR in NSCLC (8) and its association with worse survival (9). Initial clinical trials (IDEAL 1 and 2) involving the EGFR TKI gefitinib (AstraZeneca) were promising (10, 11) and led to accelerated FDA approval (12). However, after the failure of the drug in a large randomized phase III study (ISEL trial) (13), FDA approval for gefitinib was withdrawn. Importantly, a subgroup of patients who were non-smokers and/or of Asian descent appeared to benefit from the drug. During the same period, another EGFR TKI, erlotinib (Roche), showed a survival benefit in an unselected population of patients with refractory NSCLC (BR.21 trial) (14), which subsequently led to FDA approval. Much time and effort was spent on studying EGFR alterations, using immunohistochemistry (IHC), gene amplification, and gene-copy number, with no clear correlation with efficacy. Sequencing of receptor tyrosine kinase genes revealed somatic mutations in EGFR and only tumors with these mutations responded to gefitinib, while wild-type tumors did not respond (15). It took another 5 years, before the landmark IPASS trial (16) and several other pivotal phase III randomized studies (17, 18) demonstrated the importance of EGFR mutations as a critical driver in NSCLC, and established EGFR TKIs as the standard-of-care first-line therapy for this subgroup of patients. Gefitinib had FDA approval reinstated for first line, after a phase IV study done in the Caucasian EGFR-mutated population demonstrated similar responses and survival to randomized studies (19).
Afatinib (Boehringer Ingelheim), a pan-HER family small-molecule inhibitor, binds irreversibly to EGFR and is considered a second-generation EGFR TKI. Phase III trials in Western population and the Asian populations (Lux-Lung 3 and Lux-Lung 6) demonstrated a progression-free survival (PFS) benefit over platinum-based doublet therapy (20, 21). While both trials did not demonstrate an overall survival (OS) benefit for afatinib over chemotherapy, a combined analysis of both trials revealed a statistically significant OS benefit for patients with exon 19 mutations in EGFR (22). Afatinib has also been combined with cetuximab (Merck), a chimeric monoclonal anti-EGFR antibody and has demonstrated promising clinical activity in EGFR-mutant NSCLC, albeit at the cost of high rates of diarrhea and skin rash (23). Erlotinib and gefitinib have been compared head-to-head in two randomized phase III studies and revealed no significant differences in response rates and survival, suggesting equivalence between the two drugs (24, 25).
Although response to initial therapy with EGFR TKI is common, resistance to therapy is invariable and is often due to secondary mutations in EGFR and amplification of MET. The most common secondary mutation in EGFR is the substitution of methionine for threonine (T790M) (26). Osimertinib (Astra Zeneca), a third-generation EGFR TKI with activity against T790M was FDA approved for use in patients with NSCLC EGFR mutations, who have progressed on prior EGFR therapy and harbor EGFR T790M mutations. In a phase III study, osimertinib demonstrated a PFS benefit of 5.7 months over platinum doublet therapy (27). The role of afatinib in inhibiting EGFR T790M-mutant NSCLC still remains unclear in the clinic and is probably now academic given the regulatory approval of osimertinib.
ALK Translocations
Inversion of the short arm of chromosome 2 leads to the joining of exons 1 to 13 of EML4 and exons 20 to 29 of ALK, resulting in the EML4–ALK chimeric protein, which is known to occur in approximately 4–7% of NSCLC (28, 29). ALK translocations are usually mutually exclusive to EGFR and KRAS mutations (30). Crizotinib (Pfizer) was initially developed as a MET inhibitor (31), but was also found to be a potent inhibitor of ALK signal transduction (32). Compared to EGFR TKIs, ALK-inhibitor trials have been conducted primarily in biomarker-selected studies, involving patients with ALK-translocated NSCLC. These early to late clinical trials have demonstrated clear survival benefit and have since obtained regulatory approval for routine clinical use. Crizotinib demonstrated a PFS benefit versus chemotherapy (docetaxel or pemetrexed) in patients previously treated with a platinum doublet chemotherapy (6). Benefit in a chemotherapy-naïve population was then subsequently proven in a trial of crizotinib versus platinum/pemetrexed (33). OS benefit was not demonstrated in either trial, likely due to a cross-over effect. Despite initial antitumor responses, resistance to crizotinib invariably develops, commonly in the gatekeeper mutation L1196M, or G1269A and G1202R (34), providing the rationale for the development of second-generation ALK-inhibitors.
Ceritinib (Novartis) is 20 times more potent than crizotinib and was developed in the clinic in a small, biomarker-driven phase I study of ALK-translocated NSCLC, in which 66% of patients were previously treated with crizotinib, demonstrating excellent response rates of 58% and a PFS of 7 months (35), leading to FDA approval of the drug after a phase I study (a first in the modern oncology era). The efficacy of ceritinib was proven further in a phase II study (36), and phase III studies are currently ongoing (NCT01828112, NCT01828099). It should be noted that while ceritinib has shown activity against the L1196M and G1269A resistance mutations, it is ineffective against G1202R mutations (35). Brain metastases are common in NSCLC, and are often a “sanctuary site” of disease progression for patients on TKI therapy. Crizotinib has only modest cerebrospinal fluid penetration, while another second-generation ALK-inhibitor, alectinib (Genentech) has comparatively much improved activity against brain metastases (37). Two phase II studies in crizotinib-resistant ALK-translocated NSCLC have demonstrated significant response and disease control (38, 39). Both were single-arm studies that included ALK-translocated NSCLC that that failed crizotinib therapy and demonstrated response rates of 50%, leading to FDA approval for alectinib. Preliminary data were presented for a first-line Japanese study (J-ALEX) comparing alectinib with crizotinib (40) and suggested an improved PFS for alectinib over crizotinib, with better tolerance. Final data from the J-ALEX study as well as the ALEX (global) study are awaited.
Lorlatinib (Pfizer) was developed to target the G1202R-mutated population, which is resistant to crizotinib, ceritinib, and alectinib. It has demonstrated antitumor activity in patients who have progressed on two or more prior ALK-inhibitors (41). Other ALK-inhibitors that are currently in clinical trials include brigatinib (Ariad), which also targets G1202R mutations (42) and ensartinib (X-396; Xcovery), a potent second-generation inhibitor with activity against L1196M and C1156Y mutations (43). Other resistance mechanisms to ALK-inhibitors include bypass signaling through HER3 and insulin-like growth factor-1 receptor pathways, and these will probably require combination strategies to overcome such complex networks of signaling resistance (44).
ROS1 Translocations
ROS1 is an insulin receptor family tyrosine kinase with translocation aberrations most commonly with CD74, and occurs in 1–2% of patients with NSCLC (45). Aberrant ROS1 kinase activity leads to downstream signaling of the PI3K and MAPK pathways (46). As ROS1 and ALK tyrosine kinase domains have a high degree of homology, crizotinib has been shown to also inhibit ROS1 effectively (45). Clinical activity in this subgroup of patients with crizotinib included response rates of over 70% and a median duration of response of 18 months (7, 47), leading to FDA approval of crizotinib in this subgroup of patients. Ceritinib appears to show clinical activity in ROS1-rearranged NSCLC upon progression on crizotinib (48).
Targeted Therapy in NSCLC Not Selected for Driver Mutations
Antiangiogenic Agents
High levels of vascular endothelial growth factor expression in NSCLC have been associated with a poorer prognosis, providing rationale for the use of antiangiogenic agents in this population. Several antiangiogenic agents have proven to be effective in the management of advanced NSCLC. Bevacizumab (Roche) was the first drug to show an OS benefit in combination with carboplatin and paclitaxel (E4599 trial) (49) and has been FDA approved for use in lung adenocarcinoma. However, there have been other negative phase III trials with the addition of bevacizumab to chemotherapy in the first line (50). Despite FDA approval, other drug approval bodies such as the National Institute for Clinical Excellence in the UK have not approved the use of this regimen (51). Ramucirumab (Lilly Oncology), a second-generation recombinant human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), was shown to improve OS when combined with docetaxel in the second-line setting in NSCLC (52) and is FDA approved for this indication. Nintedanib (Boehringer Ingelheim), a multikinase inhibitor against VEGFR, PDGFR, and FGFR showed a survival benefit in subset analysis in patients with lung adenocarcinoma (53) and is now approved for routine use in the United Kingdom. Despite much investment spent on the development of these novel antiangiogenic agents in NSCLC, including several others currently in clinical trials, including aflibercept (Sanofi) and bavituximab (Peregerine) (54), the discovery of predictive biomarkers of response for these inhibitors remains a challenge. Several putative predictive biomarkers, including circulating VEGF-A isoform, neurophilin-1 expression, and VEGFR-1 expression, have failed to predict the antitumor effects of bevacizumab in lung cancer and other tumor types (55, 56).
Anti-EGFR Antibodies
There have been mixed results with clinical trials assessing anti-EGFR antibodies in advanced NSCLC. Cetuximab demonstrated an OS benefit of 11.3 months when combined with cisplatin and vinorelbine chemotherapy versus 10.1 months with cisplatin and vinorelbine (HR 0.87, p = 0.044) in the first-line treatment of NSCLC in the FLEX trial (57). However, there was no PFS benefit, with both arms reporting a PFS of 4.8 months. This study was followed by a negative phase III study (BMS 099 trial) (58), when cetuximab was combined with a treatment regimen of either carboplatin and paclitaxel chemotherapy or docetaxel. Similar to the FLEX study, there was no PFS benefit observed in this trial. The OS benefit between the two arms was 9.7 versus 8.4 months, which was not statistically significant. Interestingly, the magnitude of benefit between the FLEX and BMS 099 studies was similar at around 1.2 months. Based on these data, both the FDA and the European Medicines Agency rejected the use of cetuximab in the first-line setting for metastatic NSCLC in combination with platinum-based chemotherapy, based on the lack of PFS benefit and marginal improvement in OS. Apart from cetuximab, the second-generation anti-EGFR antibody necitimumab (Lilly Oncology) has been assessed in combination with cisplatin and gemcitabine in squamous NSCLC in the SQUIRE study (59). The necitimumab combination extended OS modestly from 9.9 months to 11.5 months versus cisplatin and gemcitabine chemotherapy and has since been approved by the FDA for use in first-line squamous NSCLC.
Promising Targets in NSCLC
MET Aberrations
MET amplification has gained much interest as a putative mechanism of resistance to EGFR TKI therapy. However, MET overexpression and amplification may also occur de novo in 50% (60) and 5% (61) of NSCLC, respectively. Tivantinib (ArQule), a small-molecule TKI, was studied in a large randomized phase III study (62) in combination with erlotinib, in patients with advanced NSCLC who had failed 1–2 lines of standard therapy. There was no improvement in OS (8.5 versus 7.8 m, p = 0.81), although PFS improved from 1.9 to 3.6 m (p < 0.01). Tivantinib was later shown to have cytotoxic activity independent of MET inhibition through microtubule disruption, similar to vincristine (63, 64). Onartuzumab (Roche), a monoclonal antibody targeting MET showed promising results in a phase II study (65), but failed to show any benefit in a large randomized phase III study when combined with erlotinib, with a median PFS of 2.7 versus 2.6 m on both arms of the study (66, 67). This study highlighted the challenges of selecting patients with truly MET-addicted NSCLC. At current time, MET overexpression based on IHC does not appear to be sufficiently robust as a predictive biomarker of response (68). In contrast, the recent impressive responses observed with crizotinib and other MET inhibitors in patients with MET exon 14 skipping alterations, has renewed interest in the development of MET inhibitors in NSCLC (69, 70). MET exon 14 aberrations occur in approximately 3–4% of non-squamous NSCLC and are hypothesized to decrease MET degradation, transforming it into an oncogenic driver (69, 71). Capmatinib (INC280, Novartis), a small-molecule inhibitor of MET, has reported responses in a case-series in patients with MET exon 14 skipping mutations (69). MGCD265 (Glesatinib, Mirati Therapeutics), a small-molecule inhibitor of MET and Axl, is being investigated in NSCLC with genetic alterations in MET (72). Resistance to MET inhibition can occur through secondary mutations in the MET kinase domain, such as D1228N and Y1230C (73, 74). High MET amplification also appears to be a promising predictive biomarker of response to MET inhibitors, with early studies showing antitumor responses to crizotinib in this subgroup of patients (75). In order to optimize patient benefit and accelerate the path to drug approval, future trials should include molecular profiling designed to detect MET-driven NSCLC through MET amplification and exon 14 skipping alterations.
BRAF Mutations
These occur in about 2% of NSCLC, and like KRAS mutations, are more common in smokers (76). Similar to melanoma, the most common mutation is V600E in exon 15. BRAF inhibitors, such as vemurafenib (Genentech), which are approved for use in melanoma, have shown to have preliminary clinical activity in NSCLC as well (77). Of 20 patients treated, the objective response rate was 42%, median PFS was 7.3 months and 12-month OS was 66%.
HER2 Mutations
Compared to the more familiar HER2-amplification in breast and gastric cancer, HER2 mutations occur in about 1–2% of NSCLC, most commonly in exon 20. Trastuzumab (Roche), which is standard-of-care for HER2-amplified breast and gastric cancers, has failed to robustly demonstrate antitumor activity in HER2-mutated NSCLC (78). However, afatinib, an irreversible small-molecule TKI that inhibits HER1, 2, and 4, has been shown to have clinical activity in this subgroup of patients (79). Neratinib (Puma), a pan-HER inhibitor was evaluated in combination with temsirolimus in a phase I study, with two out of six HER2-mutated NSCLC demonstrating a partial response (80). A phase II trial evaluating neratinib in HER2-mutated NSCLC is currently ongoing (NCT1827267). Dacomitinib (Pfizer), also an irreversible pan-HER TKI, demonstrated an overall response of 12% in HER2-mutant NSCLC in a phase II study (81).
RET Translocation
RET translocation with genes KIF5B, CCDC6, and NCOA4 occurs in about 1% of adenocarcinoma NSCLC (82). Cabozantinib (Exelixis) a small-molecule inhibitor of RET, MET, AXL, and VEGFR2, has shown activity in RET-translocated NSCLC in a phase II trial (83). Case reports have also been reported of response to vandetanib (AstraZeneca) (84).
PI3K Pathway Aberrations
PIK3CA mutations have been described in 9% of squamous NSCLC (85) and are also postulated to occur as a resistance mechanism to EGFR inhibitors (86), while AKT mutations occur in about 5% of squamous NSCLC (87). In addition, PTEN loss occurs in approximately 20% of squamous NSCLC and 4% of lung adenocarcinoma (85). Several trials assessing mTOR, PI3K, and AKT inhibitors have been conducted to target this pathway in NSCLC. Unfortunately, most of these studies have been conducted in biomarker “unselected” populations, leading to negative results. Everolimus (Novartis), an inhibitor of mTORC1, had a response rate of 4.7%, with significant toxicities, including diarrhea (72%), rash (53%), and stomatitis (72%) (88). Another study combining everolimus with docetaxel in an unselected population had an ORR of 8%, which did not improve on the historical single agent response rates of docetaxel (89). Several novel TORC and PI3K inhibitors are currently in clinical trials, with early results already presented in abstract form. Buparlisib (Novartis) is a pan-PI3K inhibitor, which was assessed in a PIK3CA-activated [defined as PIK3CA mutation, PTEN mutation, or PTEN loss (less than 10% protein expression by IHC)] NSCLC population. The study reported a modest ORR of 3%, and a 12-week PFS of just 20%, leading to early termination of the study (90).
These negative findings have led to much discussion about whether such aberrations along the PI3K pathway are bona fide “driver” oncogenic mutations or simply “passenger” bystander mutations. However, AZD2014 (AstraZeneca), a dual TORC1 and TORC2 inhibitor, has reported early antitumor activity in patients with advanced squamous NSCLC, when combined with weekly paclitaxel, including those previously exposed to taxane chemotherapy (91). AZD5363, a potent catalytic inhibitor of all three isoforms of AKT (AKT1, 2, and 3), has demonstrated single agent activity in AKT E17K-mutated lung cancers, which occur in about 1% of NSCLC (92).
Targeting the PI3K pathway is more complex than inhibiting other pathways, probably because of the complex network of signaling pathways, including the disruption of negative feedback loops or development of signaling crosstalk with parallel resistance pathways.
FGFR1 Amplification
FGFR1 amplifications are seen almost exclusively in smokers and occur in about 25% of squamous NSCLC (93). BGJ398 (Novartis) is a pan-FGFR inhibitor and was tested in a phase I, biomarker-selected dose-escalation study of FGFR1-amplified squamous NSCLC, where only 12% achieved partial responses (94). AZD4547 (AstraZeneca), a FGFR1–3 inhibitor, was assessed in a biomarker-driven group of patients with squamous NSCLC with FGFR amplification. Again, only 7% of patients had partial responses (95). Several questions have been raised on the validity of FGFR amplification being chosen as the predictive biomarker for these drugs and if this is indeed a true oncogenic driver (96). Importantly, high-level clonal amplification of FGFR2 has been shown to have a differentially higher response to AZD4547 in gastric cancer (97), and this should now be assessed in lung cancer to allow for better patient selection in FGFR inhibitor trials.
KRAS Mutations
KRAS mutations comprise approximately 25% of NSCLC, especially in smokers (98). Drugging RAS has unfortunately largely failed to date (99) and efforts to target the pathway downstream of RAS has yielded only modest results. For example, in a trial of selumetinib (AstraZeneca) in combination with docetaxel, PFS was improved from 2.1 to 5.3 months, and a trend in OS improvement (9.4 versus 5.2 m) was observed (100). In another trial of selumetinib with erlotinib, the combination of the two drugs led to increased toxicity without any improvement in ORR and PFS (101). In addition, the MEK inhibitor trametinib (Novartis) did not show any benefit in PFS or ORR when compared to docetaxel in KRAS-mutated lung cancer (102). Chemotherapy remains the standard-of-care for first-line metastatic KRAS-mutated NSCLC. A novel RAF/MEK inhibitor, RO5126766, showed promise in a phase I expansion of KRAS-mutated NSCLC with preliminary results being recently presented, and mature results are awaited (103).
DDR2 Mutations
DDR2 mutations occur in approximately 4% of squamous cell NSCLC. DDR2 is a receptor tyrosine kinase that binds to collagen and promotes cellular proliferation. While the main target of dasatinib (Bristol-Myers Squibb) is BCR/ABL, it also inhibits DDR2 and appears to have early signals of antitumor activity in this subgroup of patients (104).
NTRK Translocation
NTRK translocation occurs in <1% of NSCLC and include rearrangements in NTRK1, NTRK2, and NTRK3. NTRK activation leads to downstream signaling through the MAPK and PI3K pathways. Entrectinib (RXDX-101) demonstrated a durable response in a patient with NTRK1 gene rearrangement (105) and trials investigating this drug are currently on going.
Modern Precision Medicine Trial Designs in NSCLC
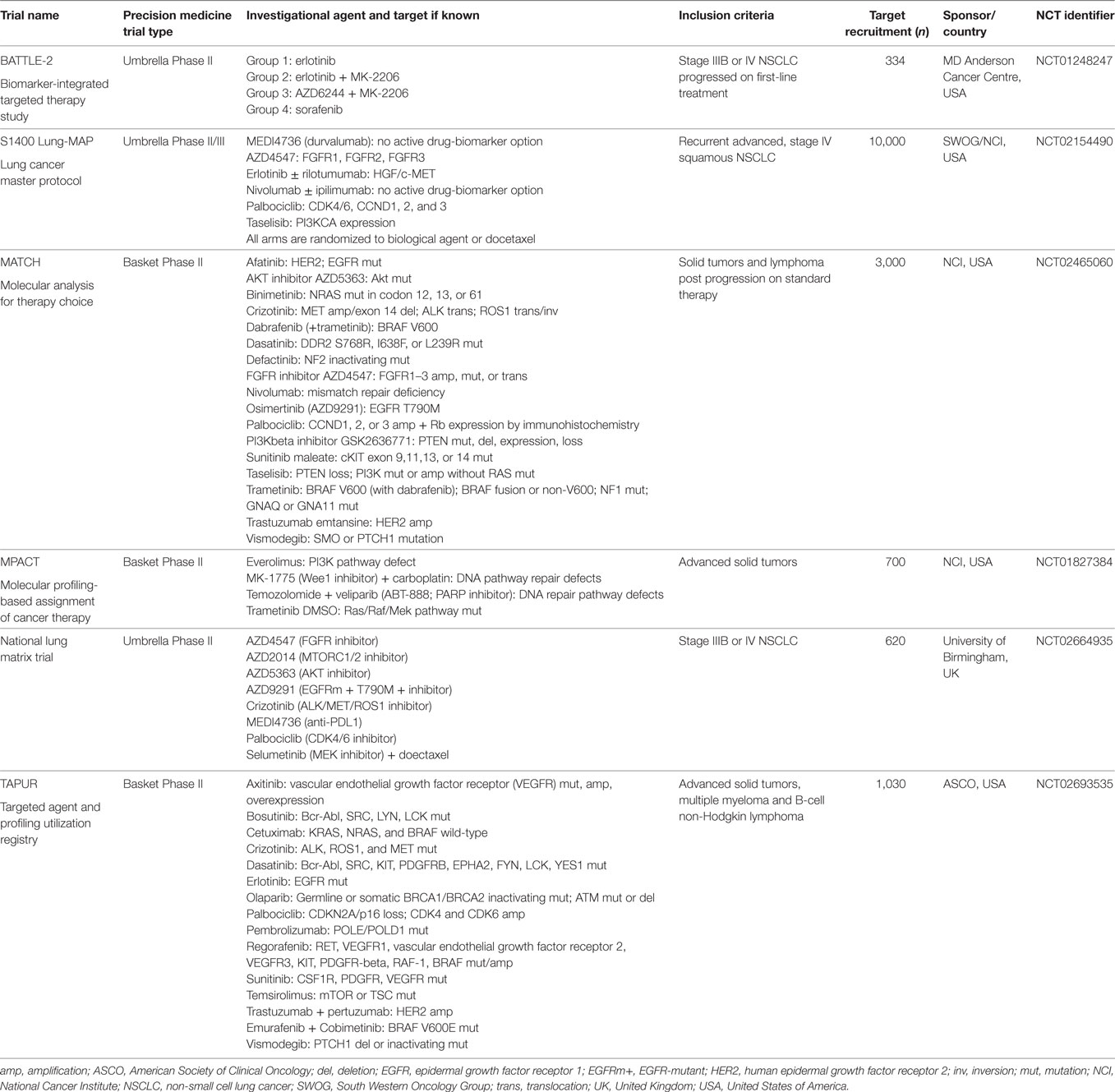
One of the main issues to address in NSCLC is that many driver aberrations only constitute a small percentage of the entire NSCLC population (Figure 2). Traditional registration trial strategies involving randomized, placebo-controlled, double-blind phase III clinical trials are, therefore, not optimal approaches and may even be considered unethical in view of the placebo control arms. Technical issues also arise as the detection of the multiple driver mutations are performed on different platforms. For example, EGFR mutations are usually detected by real-time polymerase chain reaction, while ALK rearrangements are detected by IHC and/or fluorescent in situ hybridization. Currently, a large number of driver aberrations in NSCLC can be assessed using large multiplexed next-generation sequencing (NGS) platforms. These are now increasingly being incorporated into clinical trials and daily clinical practice (106). Such an approach involving multiple NGS platforms abrogates issues associated with screening patients for “low frequency” genetic aberrations, especially if they are directly linked to a master protocol adaptive clinical trial. Umbrella trials assess multiple pre-specified genetic aberrations using NGS or other platforms and are matched to targeted agents, usually involving specific tumor types. Basket trials involve patients with a single or family of genetic abnormalities and are matched to targeted therapies, regardless of tumor origin (Table 1).

Figure 2. Relative frequency of genetic abnormalities in non-small cell lung cancer lung cancer. Note: some aberrations can occur concomitantly (not displayed in figure).
Umbrella Trials
BATTLE-2 Study
A Biomarker-Integrated Targeted Therapy Study in Previously Treated Patients with Advanced NSCLC (BATTLE-2) included patients with advanced NSCLC without sensitizing EGFR mutations and ALK fusion genes that progressed on at least one line of standard therapy (107). Two hundred patients were randomized into four arms: erlotinib, erlotinib + MK-2206 (AKT inhibitor; Merck), MK-2206 + selumetinib (MEK inhibitor), or sorafenib (Bayer), stratified for KRAS-mutation status. The median PFS was 2 months (95% CI, 1.9–2.8 months), median OS was 6.5 months (95% CI, 5.1–7.6 months), and 1-year survival was 28%. Only six partial responses and no complete responses were observed in this cohort of patients with a median of three prior lines of therapy. Importantly, there was no significant difference in PFS or OS between the different arms. Of note, KRAS-mutated patients had an improved PFS in the arms involving MK-2206 + selumetinib and sorafenib when compared with the erlotinib-containing arms.
Lung-MAP
A Biomarker-Driven Master Protocol for Previously Treated Squamous Cell Lung Cancer (Lung-MAP) is a study conducted in patients with squamous NSCLC, after developing disease progression on first-line platinum doublet therapy (NCT02154490) (108). Mandatory archival or fresh tumor biopsy samples must be provided for biomarker testing, which includes an NGS panel of over 200 genes (Foundation Medicine) and IHC for patient allocation to different rational therapies. Five different arms targeting PD-L1, PI3K, CDK4/6, FGFR, and c-Met pathways, involve the investigational agents durvalumab (AstraZeneca), taselisib (Genentech), palbociclib (Pfizer), AZD4547, and rilotumumab (Amgen) + erlotinib, respectively, with a standard arm of docetaxel chemotherapy. After results of rilotumumab in gastric cancer showed poor efficacy and increased toxicities, the sub-study of Lung-MAP with rilotumumab + erlotinib was withdrawn. All sub-studies included 1:1 randomization to investigational agent or docetaxel. This study is currently recruiting, and the expected accrual is 10,000 patients across the United States.
MATRIX Trial
The National Lung Matrix trial is non-randomized multi-arm study in the United Kingdom sponsored by University of Birmingham and Cancer Research UK (NCT02664935) (109). This study involved eight investigational arms—AZD5363 (AKT inhibitor), AZD 4547 (FGFR inhibitor), AZD2014 (mTORC1/2 inhibitor), palbocilib (CDK4/6 inhibitor; Pfizer), crizotinib, AZD9291 (third-generation EGFR inhibitor), selumetinib (MEK inhibitor) + docetaxel, and durvalumab (anti PD-L1 monoclonal antibody). Biomarker testing involves a multiplex NGS panel (Illumina) that includes various actionable mutations, which determines the allocation of patients to the appropriate investigational arms.
Basket Trials
One of the first basket studies reported was in non-melanoma patients with BRAF V600 mutations treated with vemurafenib (77). The results of the NSCLC cohort of this study have been described in a previous section of this article.
MATCH Trial
The NCI Molecular Analysis for Therapy Choice (MATCH) trial (NCT02465060) is a study that utilizes somatic genomic screening to assign patients with specific molecular aberrations to matched targeted therapy, regardless of the primary tumor site (110). This study is coordinated by the ECOG-ACRIN Cancer Research Group and involves 1,059 sites across the United States with a target recruitment of 3,000 patients. All patients must have advanced solid tumors refractory to standard therapy, undergo a mandatory fresh biopsy prior to enrolling onto the study, and to undergo a biopsy upon progression of disease. The molecular profiling assays include a targeted Ampliseq panel of 143 genes and other assays, such as IHC. The latest protocol involves 24 arms and includes agents, which have either attained FDA approval or completed trials to achieve recommended phase 2 dose (RP2D). FDA-approved drug arms include afatinib, crizotinib, osimertinib, dabrafenib (Novartis), trametinib (Novartis), ado-trastuzumab emtansine (Roche), vismodegib (Genentech), sunitinib (Pfizer), dasatinib, palbocilib, and nivolumab (Bristol-Myers Squibb). Other investigational drugs include taselisib (PI3K inhibitor), GSK2636771 (PI3K inhibitor; Glaxo-Smith Kline), defactinib (FAK inhibitor; Verastem), AZD4547 (FGFR inhibitor), AZD5363 (AKT inhibitor), and binimetinib (MEK inhibitor; Array Biopharma).
Molecular Profiling-Based Assignment of Cancer Therapy (MPACT) Trial
The MPACT study (NCT01827384), which is sponsored by the NCI aims to recruit 700 patients across three sites in the United States. Similar to the MATCH study, patients with advanced cancers, including NSCLC refractory to standard therapy will undergo a fresh biopsy to identify mutations in one of three pathways—MAPK, PI3K, or DNA repair. Patients with no identifiable mutations will be excluded from the study. The four treatment arms comprise veliparib (PARP inhibitor; Abbvie) + temozolomide, AZD-1775 (Wee1 inhibitor; Astra Zeneca) + carboplatin, everolimus (mTOR inhibitor), and trametinib (MEK inhibitor). The major difference between MATCH and MPACT is that patients in MPACT are randomized in a 2:1 fashion to either a “matched” arm or another arm based on their biomarker analysis. Biomarker analysis is performed on a 20-gene panel, and an informatics system, GeneMed, assists in streamlining the annotation of sequencing data, facilitating review of variant mutations, and identifying actionable mutations (110).
TAPUR Trial
Testing the Use of FDA-Approved Drugs That Target a Specific Abnormality in a Tumor Gene in People with Advanced Stage Cancer (TAPUR) is a trial sponsored by the American Society of Clinical Oncology with a plan to enroll 1,030 patients with advanced solid tumors refractory to standard therapy. All patients will need to harbor at least one somatic genomic variant that can be targeted by one of the drugs in the 15 arms of the study.
Combination Treatment Strategies and Precision Medicine
Combining various anticancer agents with different mechanisms of action and minimal overlapping toxicities has been a principle applied to the management of NSCLC with varying degrees of success. In the chemotherapy era, the addition of platinum chemotherapy to other agents showed clear benefit of combination therapy (111). However, there was a limit to the number of chemotherapeutic agents that could be combined simultaneously, with triplet therapies not showing an incremental benefit over doublet regimens due to increasing toxicity (112). Combination regimen with targeted agents has innumerable permutations and is an area of active research, with many trials being conducted. A major challenge with combination targeted therapy has been synergistic toxicity, particularly involving horizontal blockade of parallel signaling pathways. These toxicities prevent dose escalation of drugs to single agent RP2Ds, leading to subtherapeutic doses and lack of target modulation due to poor pharmacokinetic exposures. While a few positive trials have emerged, many more studies have been negative (113).
Combination Therapy with EGFR Inhibitors
During the early development of EGFR inhibitors, four large randomized phase III trials were conducted combining erlotinib and gefitinib with first-line chemotherapy in unselected patients with NSCLC. All these combination trials failed to show a survival benefit and were associated with increased toxicities (114–117). Intercalated erlotinib and chemotherapy (platinum given on day 1, gemcitabine on day 1 and 8, and erlotinib day 15–28) showed an OS benefit of 3.1 months (18.3 versus 15.2 months) in the FAST-ACT2 study in an unselected population, but subgroup analysis demonstrated that the benefit was only in the EGFR-mutated population (118). The combination of pemetrexed and gefitinib has demonstrated a PFS benefit of 4.9 months (15.8 versus 10.9 months) in a phase II study of EGFR-mutated NSCLC (119). Combining chemotherapy upon progression on EGFR TKI therapy also did not demonstrate a benefit in the phase III IMPRESS trial (120). Combination of bevacizumab with erlotinib in an EGFR-mutated population demonstrated a PFS benefit of 6.3 months (16 versus 9.7 months), with OS data pending (121). The rational combination of cetuximab and afatinib appear to combine with favorable response rates, albeit with higher toxicity (23). The insulin growth factor-1 receptor monoclonal antibody figitumumab (Pfizer) did not demonstrate a survival benefit and also had significantly higher toxicities when combined with erlotinib (122).
Immune Checkpoint Inhibition and Precision Medicine
Immune checkpoint inhibition has transformed the current management landscape of NSCLC and the incorporation of this group of agents into NSCLC management is rapidly evolving. Pembrolizumab (Merck), nivolumab (Bristol-Myers Squibb), and atezolizumab (Roche) have been FDA approved for use in NSCLC. It is beyond the scope of this review to discuss immunotherapeutic strategies in detail. Cumulative data suggest PD-L1 expressing tumors benefit from both PD-1 and PD-L1 antibodies. However, several uncertainties exist, including the definition of PD-L1 positivity and variation in results observed between PD-L1 IHC assays used by different pharmaceutical companies. Currently, nivolumab is FDA approved for use in the second-line treatment of NSCLC after failure of platinum doublet therapy without biomarker selection, while pembrolizumab is FDA-approved for use in the first-line setting for tumors that express PD-L1 in at least 50% of cells. This in itself highlights the discrepancies in current clinical practice in the management of NSCLC with PD-1 inhibitors. The debate surrounding biomarker selection for immunotherapies rages on, with other novel promising predictive biomarkers of response emerging (123).
Conclusion
The management of advanced NSCLC continues to evolve due to rapid recent advances made in precision medicine. The ultimate goal remains the identification of molecular subgroups of patients with driver aberrations who may benefit from molecularly targeted therapies that provide long-term control of NSCLC with minimal toxicities. It is now clear that there is unlikely to be a single “magic bullet” for NSCLC, and there is still a large proportion of patients with unknown or complex multiple drivers, and those harboring known driver aberrations, which are currently still not druggable. Moving forward, we will need to focus on innovative biomarker-driven trial designs with greater collaborations between academic and industry partners. There is, therefore, still much work to be done before we can truly achieve precision medicine in NSCLC.
Author Contributions
RS: conception or design of the work; acquisition, analysis, and interpretation of data for the work; drafting the work; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MC-P: acquisition, analysis, and interpretation of data for the work; drafting the work; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. DC: acquisition, analysis, and interpretation of data for the work; drafting the work; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TY: conception of the work; interpretation of data for the work; revising it critically for important intellectual content; and final approval of the version.
Conflict of Interest Statement
TY has received research support from AstraZeneca and Merck, and has served on Advisory Boards and received travel support from Pfizer and Bristol-Myers Squibb. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
The Drug Development Unit of the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research is supported in part by a programme grant from Cancer Research UK. Support is also provided by the Experimental Cancer Medicine Centre (to The Institute of Cancer Research) and the National Institute for Health Research Biomedical Research Centre (jointly to the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research).
Abbreviations
CRUK, cancer research United Kingdom; EGFR, epidermal growth factor receptor; EMA, European medicines agency; FDA, food and drug administration; FISH, fluorescent in situ hybridization; IHC, immunohistochemistry; NGS, next generation sequencing; NICE, national institute for clinical excellence; NSCLC, non-small cell lung cancer; OS, overall survival; PCR, polymerase chain reaction; PFS, progression-free survival; RP2D, recommended phase 2 dose; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
References
1. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small cell lung cancer collaborative group. BMJ (1995) 311:899–909. doi: 10.1136/bmj.311.7010.899
2. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med (2002) 346:92–8. doi:10.1056/NEJMoa011954
3. Fossella F, Pereira JR, Von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol (2003) 21:3016–24. doi:10.1200/JCO.2003.12.046
4. Delbaldo C, Michiels S, Syz N, Soria JC, Le Chevalier T, Pignon JP. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. JAMA (2004) 292:470–84. doi:10.1001/jama.292.4.470
5. Scagliotti GV, Parikh P, Von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol (2008) 26:3543–51. doi:10.1200/JCO.2007.15.0375
6. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med (2013) 368:2385–94. doi:10.1056/NEJMoa1214886
7. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med (2014) 371:1963–71. doi:10.1056/NEJMoa1406766
8. Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol (1995) 19:183–232. doi:10.1016/1040-8428(94)00144-I
9. Volm M, Rittgen W, Drings P. Prognostic value of ERBB-1, VEGF, cyclin A, FOS, JUN and MYC in patients with squamous cell lung carcinomas. Br J Cancer (1998) 77:663–9. doi:10.1038/bjc.1998.106
10. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol (2003) 21:2237–46. doi:10.1200/JCO.2003.10.038
11. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA (2003) 290:2149–58. doi:10.1001/jama.290.16.2149
12. Cohen MH, Williams GA, Sridhara R, Chen G, Mcguinn WD Jr, Morse D, et al. United States food and drug administration drug approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res (2004) 10:1212–8. doi:10.1158/1078-0432.CCR-03-0564
13. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, Von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa survival evaluation in lung cancer). Lancet (2005) 366:1527–37. doi:10.1016/S0140-6736(05)67625-8
14. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med (2005) 353:123–32. doi:10.1056/NEJMoa050753
15. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (2004) 304:1497–500. doi:10.1126/science.1099314
16. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med (2009) 361:947–57. doi:10.1056/NEJMoa0810699
17. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med (2010) 362:2380–8. doi:10.1056/NEJMoa0909530
18. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol (2011) 12:735–42. doi:10.1016/S1470-2045(11)70184-X
19. Douillard JY, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer (2014) 110:55–62. doi:10.1038/bjc.2013.721
20. Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol (2013) 31:3327–34. doi:10.1200/JCO.2012.44.2806
21. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol (2014) 15:213–22. doi:10.1016/S1470-2045(13)70604-1
22. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol (2015) 16:141–51. doi:10.1016/S1470-2045(14)71173-8
23. Janjigian YY, Smit EF, Groen HJ, Horn L, Gettinger S, Camidge DR, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov (2014) 4:1036–45. doi:10.1158/2159-8290.CD-14-0326
24. Yang JJ, Zhou Q, Yan HH, Zhang XC, Chen HJ, Tu HY, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer (2017) 116:568–74. doi:10.1038/bjc.2016.456
25. Urata Y, Katakami N, Morita S, Kaji R, Yoshioka H, Seto T, et al. Randomized phase III study comparing gefitinib with erlotinib in patients with previously treated advanced lung adenocarcinoma: WJOG 5108L. J Clin Oncol (2016) 34:3248–57. doi:10.1200/JCO.2015.63.4154
26. Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med (2005) 352:786–92. doi:10.1056/NEJMoa044238
27. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med (2017) 16:629–40. doi:10.1056/NEJMoa1612674
28. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature (2007) 448:561–6. doi:10.1038/nature05945
29. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol (2009) 27:4247–53. doi:10.1200/JCO.2009.22.6993
30. Solomon B, Varella-Garcia M, Camidge DR. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol (2009) 4:1450–4. doi:10.1097/JTO.0b013e3181c4dedb
31. Zou HY, Li Q, Lee JH, Arango ME, Mcdonnell SR, Yamazaki S, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res (2007) 67:4408–17. doi:10.1158/0008-5472.CAN-06-4443
32. Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, Mcdonnell SR, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther (2007) 6:3314–22. doi:10.1158/1535-7163.MCT-07-0365
33. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med (2014) 371:2167–77. doi:10.1056/NEJMoa1408440
34. Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med (2010) 363:1734–9. doi:10.1056/NEJMoa1007478
35. Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med (2014) 370:1189–97. doi:10.1056/NEJMoa1311107
36. Crino L, Ahn MJ, De Marinis F, Groen HJ, Wakelee H, Hida T, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol (2016) 34:2866–73. doi:10.1200/JCO.2015.65.5936
37. Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, Azada MC, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol (2014) 15:1119–28. doi:10.1016/S1470-2045(14)70362-6
38. Ou SH, Ahn JS, De Petris L, Govindan R, Yang JC, Hughes B, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol (2016) 34:661–8. doi:10.1200/JCO.2015.63.9443
39. Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol (2016) 17:234–42. doi:10.1016/S1470-2045(15)00488-X
40. Nokihara H, Hida T, Konda M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in ALK-inhibitor naïve ALK-positive non-small cell lung cancer: primary results from the J-Alex study. J Clin Oncol (2016) 34(Suppl):abstr 9008.
41. Solomon BJ, Bauer TM, Felip E, Besse B, James LP, Clancy JS, et al. Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). J Clin Oncol (2016) 34(Suppl):abstr 9009.
42. Kim D-W, Tiseo M, Ahn M-J, Reckamp KL, Hansen KH, Kim S-W, et al. Brigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): first report of efficacy and safety from a pivotal randomized phase (ph) 2 trial (ALTA). J Clin Oncol (2016) 34(Suppl):abstr 9007.
43. Horn L, Wakelee H, Blumenschein G, Reckamp K, Waqar S, Carter CA, et al. Phase I/II trial of X-396 in patients with ALK + non-small cell lung cancer: correlation with plasma and tissue genotyping and response to therapy. Ann Oncol (2016) 27:1210D. doi:10.1093/annonc/mdw383.10
44. Isozaki H, Ichihara E, Takigawa N, Ohashi K, Ochi N, Yasugi M, et al. Non-small cell lung cancer cells acquire resistance to the ALK inhibitor alectinib by activating alternative receptor tyrosine kinases. Cancer Res (2016) 76:1506–16. doi:10.1158/0008-5472.CAN-15-1010
45. Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, Mcdonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol (2012) 30:863–70. doi:10.1200/JCO.2011.35.6345
46. Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta (2009) 1795:37–52. doi:10.1016/j.bbcan.2008.07.006
47. Mazieres J, Zalcman G, Crino L, Biondani P, Barlesi F, Filleron T, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol (2015) 33:992–9. doi:10.1200/JCO.2014.58.3302
48. Subbiah V, Hong DS, Meric-Bernstam F. Clinical activity of ceritinib in ROS1-rearranged non-small cell lung cancer: bench to bedside report. Proc Natl Acad Sci U S A (2016) 113:1419–20. doi:10.1073/pnas.1522052113
49. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med (2006) 355:2542–50. doi:10.1056/NEJMoa061884
50. Reck M, Von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol (2010) 21:1804–9. doi:10.1093/annonc/mdq020
51. NICE. (2008). Available from: https://www.nice.org.uk/guidance/ta148
52. Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet (2014) 384:665–73. doi:10.1016/S0140-6736(14)60845-X
53. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol (2014) 15:143–55. doi:10.1016/S1470-2045(13)70586-2
54. Chu BF, Otterson GA. Incorporation of antiangiogenic therapy into the non-small-cell lung cancer paradigm. Clin Lung Cancer (2016) 17:493–506. doi:10.1016/j.cllc.2016.05.020
55. Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol (2010) 11:1172–83. doi:10.1016/S1470-2045(10)70232-1
56. Boro A, Arlt MJ, Lengnick H, Robl B, Husmann M, Bertz J, et al. Prognostic value and in vitro biological relevance of neuropilin 1 and neuropilin 2 in osteosarcoma. Am J Transl Res (2015) 7:640–53.
57. Pirker R, Pereira JR, Szczesna A, Von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet (2009) 373:1525–31. doi:10.1016/S0140-6736(09)60569-9
58. Lynch TJ, Patel T, Dreisbach L, Mccleod M, Heim WJ, Hermann RC, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol (2010) 28:911–7. doi:10.1200/JCO.2009.21.9618
59. Thatcher N, Hirsch FR, Luft AV, Szczesna A, Ciuleanu TE, Dediu M, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol (2015) 16:763–74. doi:10.1016/S1470-2045(15)00021-2
60. Guo B, Cen H, Tan X, Liu W, Ke Q. Prognostic value of MET gene copy number and protein expression in patients with surgically resected non-small cell lung cancer: a meta-analysis of published literatures. PLoS One (2014) 9:e99399. doi:10.1371/journal.pone.0099399
61. Kubo T, Yamamoto H, Lockwood WW, Valencia I, Soh J, Peyton M, et al. MET gene amplification or EGFR mutation activate MET in lung cancers untreated with EGFR tyrosine kinase inhibitors. Int J Cancer (2009) 124:1778–84. doi:10.1002/ijc.24150
62. Scagliotti G, Von Pawel J, Novello S, Ramlau R, Favaretto A, Barlesi F, et al. Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol (2015) 33:2667–74. doi:10.1200/JCO.2014.60.7317
63. Basilico C, Pennacchietti S, Vigna E, Chiriaco C, Arena S, Bardelli A, et al. Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clin Cancer Res (2013) 19:2381–92. doi:10.1158/1078-0432.CCR-12-3459
64. Katayama R, Aoyama A, Yamori T, Qi J, Oh-Hara T, Song Y, et al. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res (2013) 73:3087–96. doi:10.1158/0008-5472.CAN-12-3256
65. Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH Jr, Blumenschein GR Jr, et al. Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol (2013) 31:4105–14. doi:10.1200/JCO.2012.47.4189
66. Spigel DR, Edelman MJ, Mok T, O’Byrne K, Paz-Ares L, Yu W, et al. Treatment rationale study design for the Met lung trial: a randomized, double-blind phase III study of onartuzumab (MetMAb) in combination with erlotinib versus erlotinib alone in patients who have received standard chemotherapy for stage IIIB or IV Met-positive non-small-cell lung cancer. Clin Lung Cancer (2012) 13:500–4.
67. Spigel DR, Edelman MJ, O’Byrne K, Paz-Ares L, Shames DS, Yu W, et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. J Clin Oncol (2014) 32(Suppl):5s; abstr 8000.
68. Koeppen H, Yu W, Zha J, Pandita A, Penuel E, Rangell L, et al. Biomarker analyses from a placebo-controlled phase II study evaluating erlotinib ± onartuzumab in advanced non-small cell lung cancer: MET expression levels are predictive of patient benefit. Clin Cancer Res (2014) 20:4488–98. doi:10.1158/1078-0432.CCR-13-1836
69. Frampton GM, Ali SM, Rosenzweig M, Chmielecki J, Lu X, Bauer TM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov (2015) 5:850–9. doi:10.1158/2159-8290.CD-15-0285
70. Drilon AE, Camidge DR, Ou S-HI, Clark JW, Socinski MA, Weiss J, et al. Efficacy and safety of crizotinib in patients (pts) with advanced MET exon 14-altered non-small cell lung cancer (NSCLC). J Clin Oncol (2016) 34(Suppl):abstr 108.
71. Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol (2016) 34:721–30. doi:10.1200/JCO.2015.63.4600
72. Rybkin II, Kio EA, Masood A, Shum MK, Halmos B, Blakely CM, et al. Amethyst NSCLC trial: phase 2, parallel-arm study of receptor tyrosine kinase inhibitor MGCD265, in patients with advanced or metastatic non-small cell lung cancer with activating genetic alterations in mesenchymal-epithelial transition factor. J Clin Oncol (2016) 34(Suppl):abstr TS9099.
73. Heist RS, Sequist LV, Borger D, Gainor JF, Arellano RS, Le LP, et al. Acquired resistance to crizotinib in NSCLC with MET exon 14 skipping. J Thorac Oncol (2016) 11:1242–5. doi:10.1016/j.jtho.2016.06.013
74. Ou SI, Young L, Schrock AB, Johnson A, Klempner SJ, Zhu VW, et al. Emergence of preexisting MET Y1230C mutation as a resistance mechanism to crizotinib in NSCLC with MET exon 14 skipping. J Thorac Oncol (2017) 12:137–40. doi:10.1016/j.jtho.2016.09.119
75. Camidge DR, Ou S-HI, Shapiro G, Otterson GA, Villaruz LC, Villalona-Calero MA, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). J Clin Oncol (2014) 32(Suppl):5s; abstr 8001.
76. Villaruz LC, Socinski MA, Abberbock S, Berry LD, Johnson BE, Kwiatkowski DJ, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the lung cancer mutation consortium. Cancer (2015) 121:448–56. doi:10.1002/cncr.29042
77. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med (2015) 373:726–36. doi:10.1056/NEJMoa1502309
78. Gatzemeier U, Groth G, Butts C, Van Zandwijk N, Shepherd F, Ardizzoni A, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol (2004) 15:19–27. doi:10.1093/annonc/mdh031
79. De Greve J, Teugels E, Geers C, Decoster L, Galdermans D, De Mey J, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer (2012) 76:123–7. doi:10.1016/j.lungcan.2012.01.008
80. Gandhi L, Bahleda R, Tolaney SM, Kwak EL, Cleary JM, Pandya SS, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol (2014) 32:68–75. doi:10.1200/JCO.2012.47.2787
81. Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O’Connell J, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol (2015) 26:1421–7. doi:10.1093/annonc/mdv186
82. Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol (2012) 30:4352–9. doi:10.1200/JCO.2012.44.1477
83. Drilon A, Rekhtman N, Arcila M, Wang L, Ni A, Albano M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol (2016) 17:1653–60. doi:10.1016/S1470-2045(16)30562-9
84. Falchook GS, Ordóñez NG, Bastida CC, Stephens PJ, Miller VA, Gaido L, et al. Effect of the RET inhibitor vandetanib in a patient with RET fusion-positive metastatic non-small-cell lung cancer. J Clin Oncol (2016) 34:141–4. doi:10.1200/JCO.2013.50.5016
85. Spoerke JM, O’Brien C, Huw L, Koeppen H, Fridlyand J, Brachmann RK, et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res (2012) 18:6771–83. doi:10.1158/1078-0432.CCR-12-2347
86. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med (2011) 3:75ra26. doi:10.1126/scitranslmed.3002003
87. Malanga D, Scrima M, De Marco C, Fabiani F, De Rosa N, De Gisi S, et al. Activating E17K mutation in the gene encoding the protein kinase AKT1 in a subset of squamous cell carcinoma of the lung. Cell Cycle (2008) 7:665–9. doi:10.4161/cc.7.5.5485
88. Soria JC, Shepherd FA, Douillard JY, Wolf J, Giaccone G, Crino L, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol (2009) 20:1674–81. doi:10.1093/annonc/mdp060
89. Ramalingam SS, Owonikoko TK, Behera M, Subramanian J, Saba NF, Kono SA, et al. Phase II study of docetaxel in combination with everolimus for second- or third-line therapy of advanced non-small-cell lung cancer. J Thorac Oncol (2013) 8:369–72. doi:10.1097/JTO.0b013e318282709c
90. Vansteenkiste JF, Canon JL, Braud FD, Grossi F, De Pas T, Gray JE, et al. Safety and efficacy of buparlisib (BKM120) in patients with PI3K pathway-activated non-small cell lung cancer: results from the phase II BASALT-1 study. J Thorac Oncol (2015) 10:1319–27. doi:10.1097/JTO.0000000000000607
91. Krebs MG, Spicer J, Steele N, Talbot DC, Brada M, Wilson RH, et al. TAX-TORC: the novel combination of weekly paclitaxel and the dual mTORC1/2 inhibitor AZD2014 for the treatment of squamous NSCLC. 17th World Conference on Lung Cancer. Vienna (2016). ID4803 p.
92. Hyman DM, Smyth L, Bedard PL, Oza A, Dean E, Armstrong A, et al. AZD5363, a catalytic pan-Akt inhibitor, in Akt1 E17K mutation positive advanced solid tumors. Mol Cancer Ther (2015) 14:B109–109. doi:10.1158/1535-7163.TARG-15-B109
93. Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature (2012) 489:519–25. doi:10.1038/nature11404
94. Nogova L, Sequist LV, Cassier PA, Hidalgo M, Delord J-P, Schuler MH, et al. Targeting FGFR1-amplified lung squamous cell carcinoma with the selective pan-FGFR inhibitor BGJ398. J Clin Oncol (2014) 32(Suppl):5s; abstr 8034.
95. Paik PK, Shen R, Ferry D, Soria J-C, Mathewson A, Kilgour E, et al. A phase 1b open-label multicenter study of AZD4547 in patients with advanced squamous cell lung cancers: preliminary antitumor activity and pharmacodynamic data. J Clin Oncol (2014) 32(Suppl):5s; abstr 8035.
96. Weeden CE, Solomon B, Asselin-Labat ML. FGFR1 inhibition in lung squamous cell carcinoma: questions and controversies. Cell Death Discov (2015) 1:15049. doi:10.1038/cddiscovery.2015.49
97. Pearson A, Smyth E, Babina IS, Herrera-Abreu MT, Tarazona N, Peckitt C, et al. High-level clonal FGFR amplification and response to FGFR inhibition in a translational clinical trial. Cancer Discov (2016) 6:838–51. doi:10.1158/2159-8290.CD-15-1246
98. Ahrendt SA, Decker PA, Alawi EA, Zhu Yr YR, Sanchez-Cespedes M, Yang SC, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer (2001) 92:1525–30. doi:10.1002/1097-0142(20010915)92:6<1525::AID-CNCR1478>3.0.CO;2-H
100. Janne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol (2013) 14:38–47. doi:10.1016/S1470-2045(12)70489-8
101. Carter CA, Rajan A, Keen C, Szabo E, Khozin S, Thomas A, et al. Selumetinib with and without erlotinib in KRAS mutant and KRAS wild-type advanced nonsmall-cell lung cancer. Ann Oncol (2016) 27:693–9. doi:10.1093/annonc/mdw008
102. Blumenschein GR Jr, Smit EF, Planchard D, Kim DW, Cadranel J, De Pas T, et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC). Ann Oncol (2015) 26:894–901. doi:10.1093/annonc/mdv072
103. Harris SJ, Luken MJ, Perez DR, Lopez RP, Parmar M, Prathapan V, et al. Updated efficacy and safety results from the phase I study of intermittent dosing of the dual MEK/RAF inhibitor, RO5126766 in patients (pts) with RAS/RAF mutated advanced solid tumours. J Clin Oncol (2016) 34(Suppl):abstr 2582.
104. Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov (2011) 1:78–89. doi:10.1158/2159-8274.CD-11-0005
105. Farago AF, Le LP, Zheng Z, Muzikansky A, Drilon A, Patel M, et al. Durable clinical response to entrectinib in NTRK1-rearranged non-small cell lung cancer. J Thorac Oncol (2015) 10:1670–4. doi:10.1097/01.JTO.0000473485.38553.f0
106. Sequist LV, Heist RS, Shaw AT, Fidias P, Rosovsky R, Temel JS, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol (2011) 22:2616–24. doi:10.1093/annonc/mdr489
107. Papadimitrakopoulou V, Lee JJ, Wistuba II, Tsao AS, Fossella FV, Kalhor N, et al. The BATTLE-2 study: a biomarker-integrated targeted therapy study in previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol (2016) 34:3638–47. doi:10.1200/JCO.2015.66.0084
108. Steuer CE, Papadimitrakopoulou V, Herbst RS, Redman MW, Hirsch FR, Mack PC, et al. Innovative clinical trials: the LUNG-MAP study. Clin Pharmacol Ther (2015) 97:488–91. doi:10.1002/cpt.88
109. Middleton G, Crack LR, Popat S, Swanton C, Hollingsworth SJ, Buller R, et al. The national lung matrix trial: translating the biology of stratification in advanced non-small-cell lung cancer. Ann Oncol (2015) 26:2464–9. doi:10.1093/annonc/mdv394
110. Do K, O’Sullivan Coyne G, Chen AP. An overview of the NCI precision medicine trials-NCI MATCH and MPACT. Chin Clin Oncol (2015) 4:31. doi:10.3978/j.issn.2304-3865.2015.08.01
111. Le Chevalier T, Brisgand D, Douillard JY, Pujol JL, Alberola V, Monnier A, et al. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small-cell lung cancer: results of a European multicenter trial including 612 patients. J Clin Oncol (1994) 12:360–7. doi:10.1200/JCO.1994.12.2.360
112. Azim HA Jr, Elattar I, Loberiza FR Jr, Azim H, Mok T, Ganti AK. Third generation triplet cytotoxic chemotherapy in advanced non-small cell lung cancer: a systematic overview. Lung Cancer (2009) 64:194–8. doi:10.1016/j.lungcan.2008.08.011
113. Sundar R, Valeri N, Harrington KJ, Yap TA. Combining molecularly targeted agents: is more always better? Clin Cancer Res (2016) 23:1123–5. doi:10.1158/1078-0432.CCR-16-2399
114. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 1. J Clin Oncol (2004) 22:777–84. doi:10.1200/JCO.2004.08.001
115. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 2. J Clin Oncol (2004) 22:785–94. doi:10.1200/JCO.2004.07.215
116. Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol (2005) 23:5892–9. doi:10.1200/JCO.2005.02.840
117. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol (2007) 25:1545–52. doi:10.1200/JCO.2005.05.1474
118. Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, Ladrera G, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol (2013) 14:777–86. doi:10.1016/S1470-2045(13)70254-7
119. Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH, et al. Randomized phase II trial of gefitinib with and without pemetrexed as first-line therapy in patients with advanced nonsquamous non-small-cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol (2016) 34:3258–66. doi:10.1200/JCO.2016.66.9218
120. Soria JC, Wu YL, Nakagawa K, Kim SW, Yang JJ, Ahn MJ, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol (2015) 16:990–8. doi:10.1016/S1470-2045(15)00121-7
121. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol (2014) 15:1236–44. doi:10.1016/S1470-2045(14)70381-X
122. Scagliotti GV, Bondarenko I, Blackhall F, Barlesi F, Hsia TC, Jassem J, et al. Randomized, phase III trial of figitumumab in combination with erlotinib versus erlotinib alone in patients with nonadenocarcinoma nonsmall-cell lung cancer. Ann Oncol (2015) 26:497–504. doi:10.1093/annonc/mdu517
Keywords: precision medicine, lung cancer, targeted therapy, imprecision, clinical trials
Citation: Sundar R, Chénard-Poirier M, Collins DC and Yap TA (2017) Imprecision in the Era of Precision Medicine in Non-Small Cell Lung Cancer. Front. Med. 4:39. doi: 10.3389/fmed.2017.00039
Received: 19 December 2016; Accepted: 22 March 2017;
Published: 10 April 2017
Edited by:
Diego Luigi Cortinovis, San Gerardo Hospital, ItalyReviewed by:
Yanis Boumber, Fox Chase Cancer Center, USAAlessandro Laganà, Icahn School of Medicine at Mount Sinai, USA
Copyright: © 2017 Sundar, Chénard-Poirier, Collins and Yap. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy A. Yap, TYap@mdanderson.org
 Raghav Sundar
Raghav Sundar Maxime Chénard-Poirier1,3
Maxime Chénard-Poirier1,3
 Dearbhaile Catherine Collins
Dearbhaile Catherine Collins Timothy A. Yap
Timothy A. Yap